Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Equillium, Inc. | eq-ex992_9.htm |
| 8-K - FORM 8-K - Equillium, Inc. | eq-8k_20210212.htm |

Exhibit 99.1 John Koreth, Alison W. Loren, Ryotaro Nakamura, Marco Mielcarek, Trent Wang, George L. Chen, Sarah Anand, Joseph A. Pidala, Edmund K. Waller, Gabrielle Meyers, Daanish Hoda, Mark Schroeder, Jeremy Pantin, Nosha Farhadfar, Cherie Ng, Lisette M. Acevedo, Maple Fung, Joel Rothman, Stephen Connelly, Krishna R. Polu, Corey Cutler Interim results from the EQUATE Study: Preliminary safety and efficacy of itolizumab, a novel targeted anti-CD6 therapy, in newly diagnosed severe acute graft-versus-host disease

Research Support: BMS, Miltenyi, Clinigen, Amgen, Regeneron Advisory Board: Therakos, Cugene, Regeneron Consulting: EMD Serono/Merck, Biolojic Design, Gentibio, Moderna Therapeutics, Equillium, Inc Investigational use of Itolizumab (EQ001) for aGVHD Disclosures 2
CD6 is a co-stimulatory receptor expressed on CD4 and CD8 effector T cells (Teff) Multiple CD6 ligands, including activated leukocyte cell adhesion molecule (ALCAM), expressed on both APCs and tissues, including the skin and GI tract CD6 and ALCAM both overexpressed during GI inflammation CD6-ALCAM pathway modulates both T cell activity and trafficking Prior studies performed at DFCI validated CD6 as a target relevant to GVHD pathogenesis CD6-ALCAM Pathway Central to Immune Inflammation Consuegra-Fernandez et al., Cell Mol Immunol 2018; Ma et al., J Crohns & Colitis 2019; Soiffer et al., JCO 1992 3 CD6high High proliferation Pathogenic phenotypes Pro-inflammatory cytokines CD6low/- Low proliferation Regulatory phenotype Anti-inflammatory cytokines
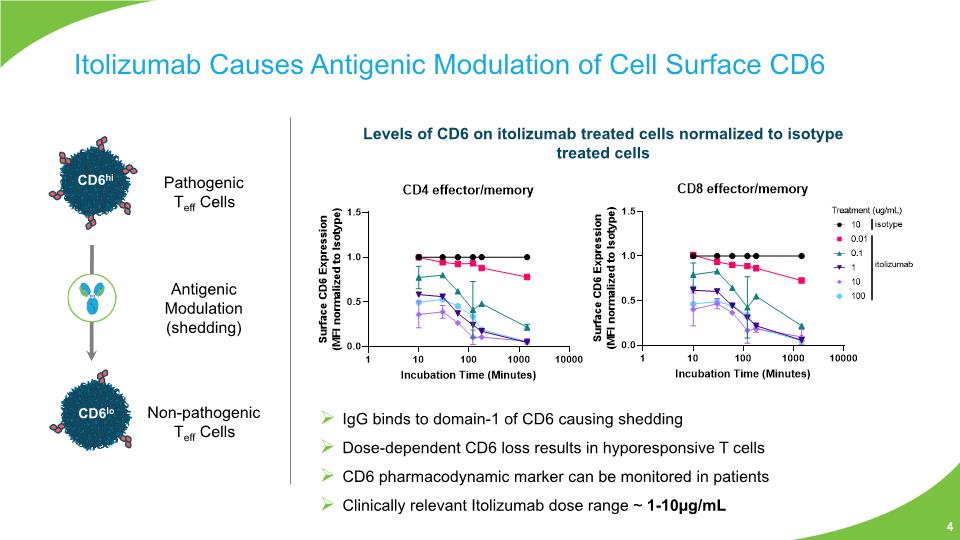
Itolizumab Causes Antigenic Modulation of Cell Surface CD6 Levels of CD6 on itolizumab treated cells normalized to isotype treated cells IgG binds to domain-1 of CD6 causing shedding Dose-dependent CD6 loss results in hyporesponsive T cells CD6 pharmacodynamic marker can be monitored in patients Clinically relevant Itolizumab dose range ~ 1-10µg/mL Pathogenic Teff Cells Non-pathogenic Teff Cells Antigenic Modulation (shedding) 4
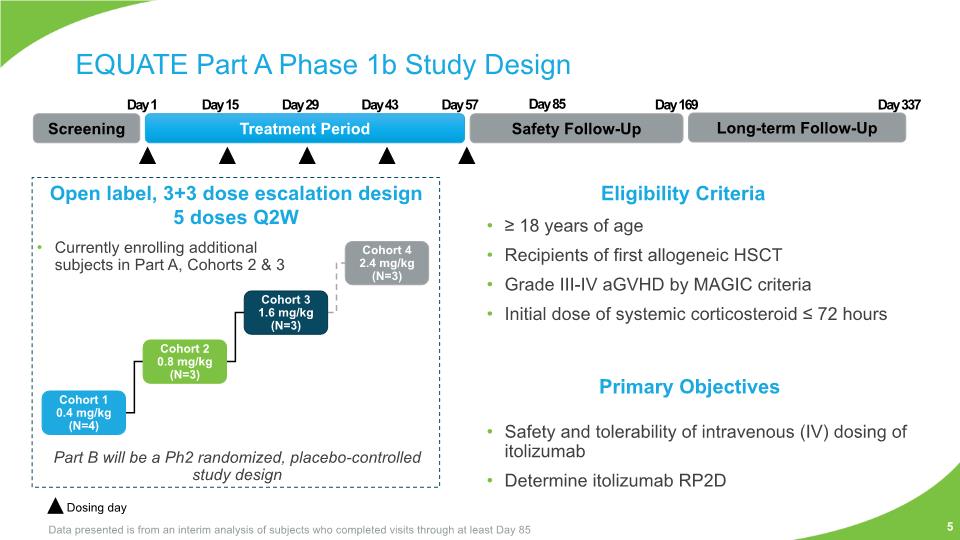
≥ 18 years of age Recipients of first allogeneic HSCT Grade III-IV aGVHD by MAGIC criteria Initial dose of systemic corticosteroid ≤ 72 hours Safety and tolerability of intravenous (IV) dosing of itolizumab Determine itolizumab RP2D EQUATE Part A Phase 1b Study Design Screening Treatment Period Safety Follow-Up Long-term Follow-Up Day 1 Day 15 Day 29 Day 43 Day 57 Day 169 Day 337 Open label, 3+3 dose escalation design 5 doses Q2W Eligibility Criteria Currently enrolling additional subjects in Part A, Cohorts 2 & 3 Dosing day Primary Objectives Data presented is from an interim analysis of subjects who completed visits through at least Day 85 Part B will be a Ph2 randomized, placebo-controlled study design 5 Day 85
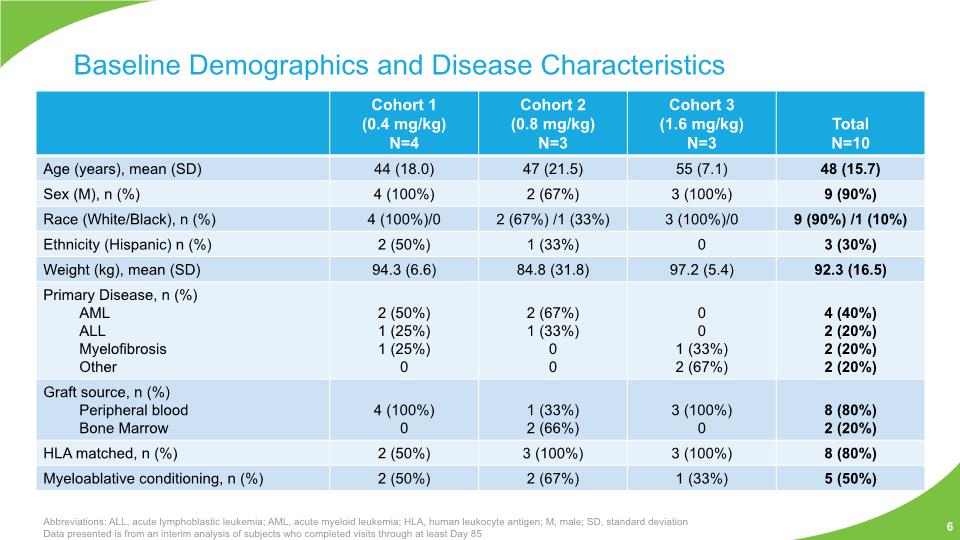
Baseline Demographics and Disease Characteristics Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; HLA, human leukocyte antigen; M, male; SD, standard deviation Data presented is from an interim analysis of subjects who completed visits through at least Day 85 6
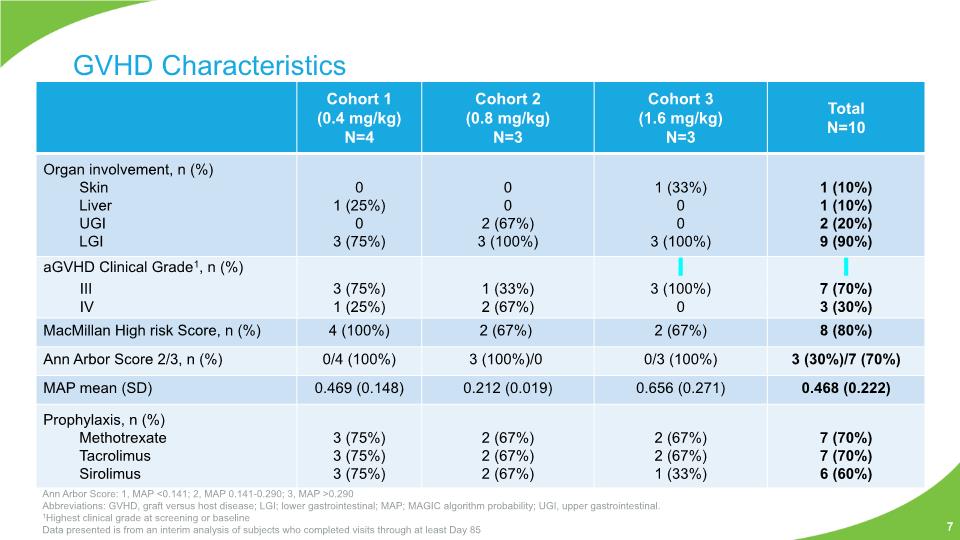
GVHD Characteristics Ann Arbor Score: 1, MAP <0.141; 2, MAP 0.141-0.290; 3, MAP >0.290 Abbreviations: GVHD, graft versus host disease; LGI; lower gastrointestinal; MAP; MAGIC algorithm probability; UGI, upper gastrointestinal. 1Highest clinical grade at screening or baseline Data presented is from an interim analysis of subjects who completed visits through at least Day 85 7
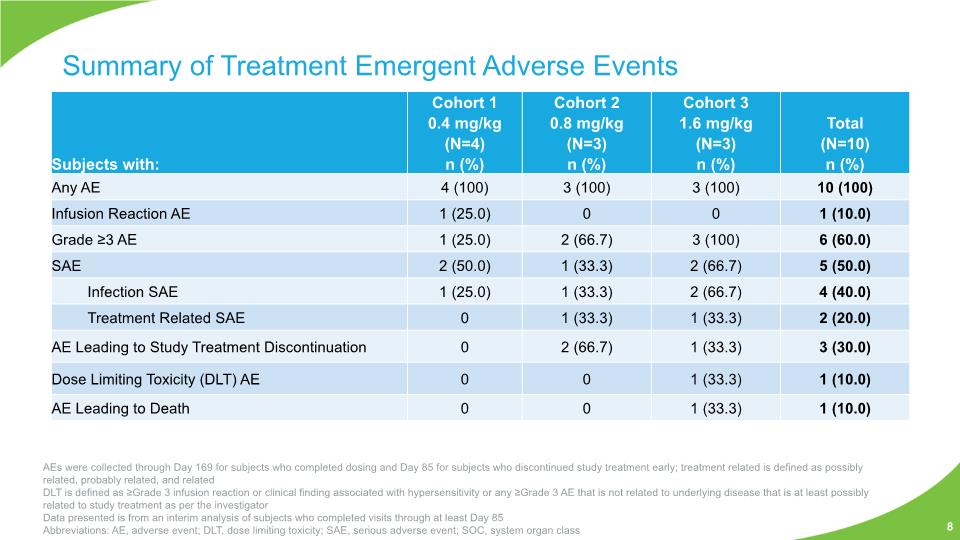
Summary of Treatment Emergent Adverse Events 8 AEs were collected through Day 169 for subjects who completed dosing and Day 85 for subjects who discontinued study treatment early; treatment related is defined as possibly related, probably related, and related DLT is defined as ≥Grade 3 infusion reaction or clinical finding associated with hypersensitivity or any ≥Grade 3 AE that is not related to underlying disease that is at least possibly related to study treatment as per the investigator Data presented is from an interim analysis of subjects who completed visits through at least Day 85 Abbreviations: AE, adverse event; DLT, dose limiting toxicity; SAE, serious adverse event; SOC, system organ class
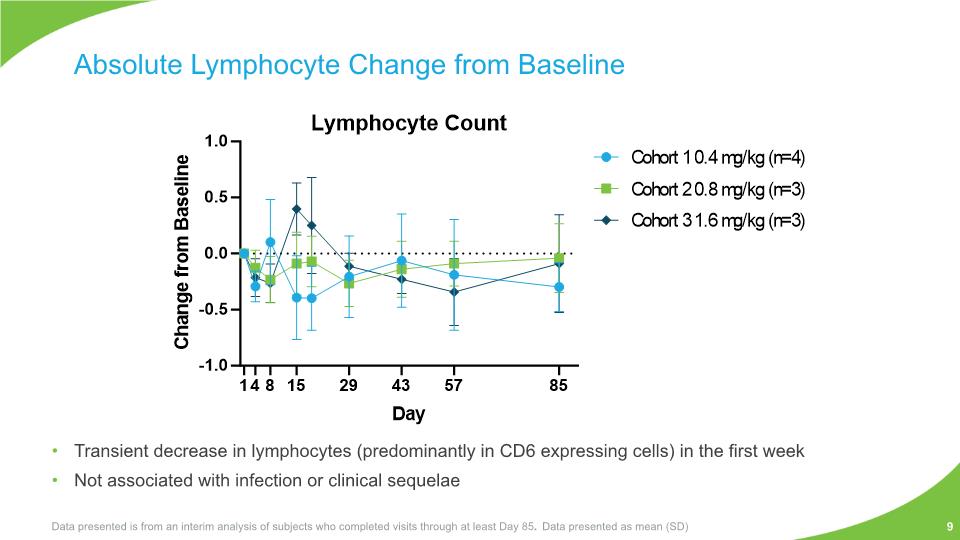
Transient decrease in lymphocytes (predominantly in CD6 expressing cells) in the first week Not associated with infection or clinical sequelae Absolute Lymphocyte Change from Baseline Data presented is from an interim analysis of subjects who completed visits through at least Day 85. Data presented as mean (SD) 9
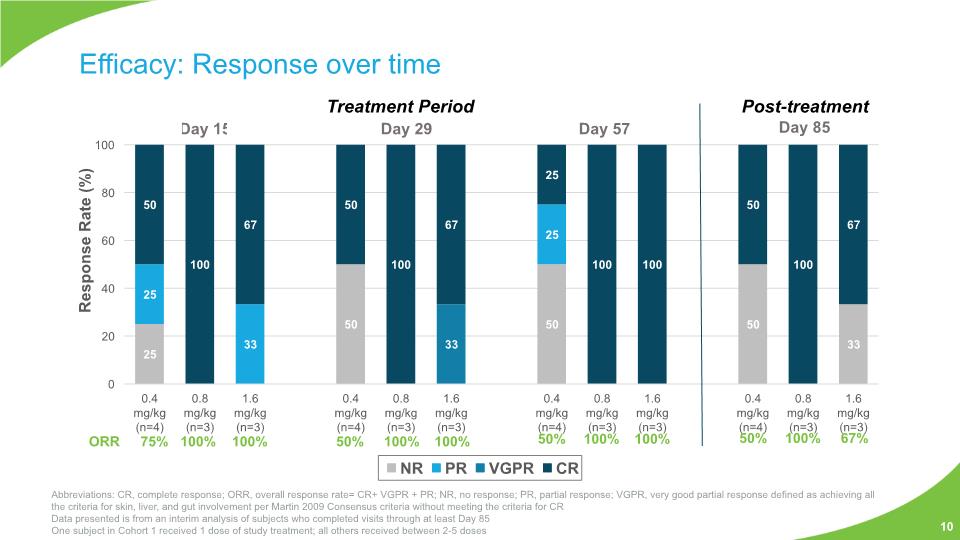
Efficacy: Response over time 10 Abbreviations: CR, complete response; ORR, overall response rate= CR+ VGPR + PR; NR, no response; PR, partial response; VGPR, very good partial response defined as achieving all the criteria for skin, liver, and gut involvement per Martin 2009 Consensus criteria without meeting the criteria for CR Data presented is from an interim analysis of subjects who completed visits through at least Day 85 One subject in Cohort 1 received 1 dose of study treatment; all others received between 2-5 doses ORR 75% 100% 100% 50% 100% 100% 50% 100% 100% 50% 100% 67% Post-treatment Treatment Period
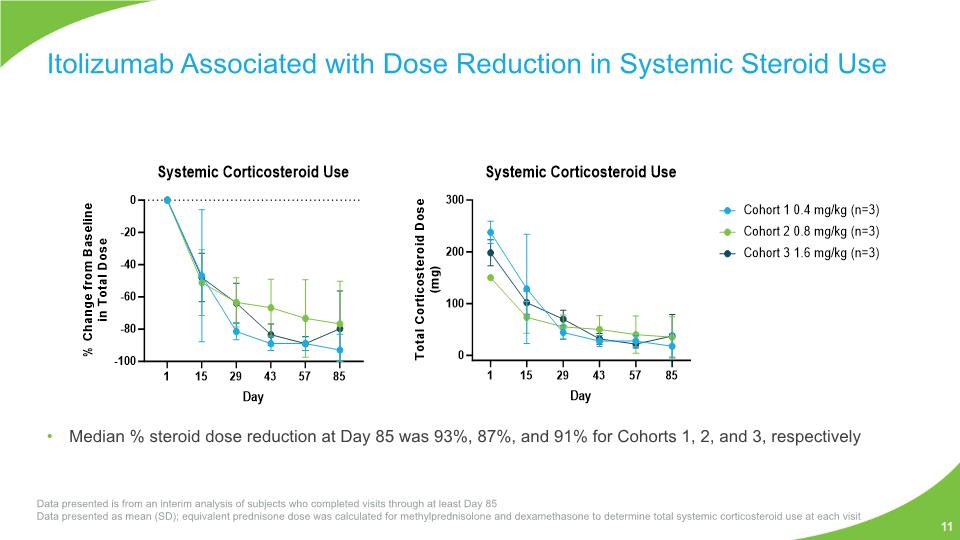
Itolizumab Associated with Dose Reduction in Systemic Steroid Use 11 Data presented is from an interim analysis of subjects who completed visits through at least Day 85 Data presented as mean (SD); equivalent prednisone dose was calculated for methylprednisolone and dexamethasone to determine total systemic corticosteroid use at each visit Median % steroid dose reduction at Day 85 was 93%, 87%, and 91% for Cohorts 1, 2, and 3, respectively
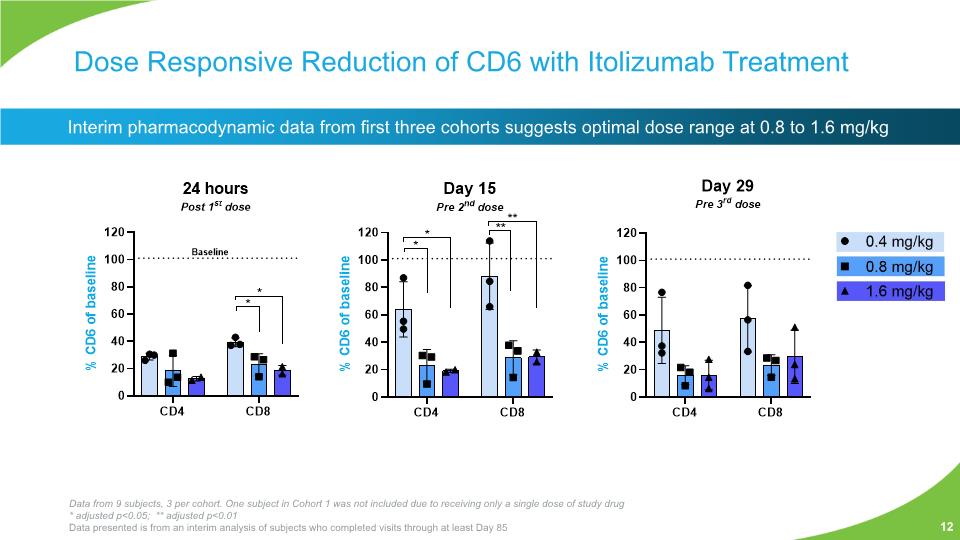
Dose Responsive Reduction of CD6 with Itolizumab Treatment Interim pharmacodynamic data from first three cohorts suggests optimal dose range at 0.8 to 1.6 mg/kg 12 Data from 9 subjects, 3 per cohort. One subject in Cohort 1 was not included due to receiving only a single dose of study drug * adjusted p<0.05; ** adjusted p<0.01 Data presented is from an interim analysis of subjects who completed visits through at least Day 85
The CD6-ALCAM pathway modulates activity and trafficking of Teff cells CD6high Teff cells are known to be more pathogenic, showing increased proliferation and cytokine secretion Treatment with itolizumab leads to antigenic modulation of CD6 and inhibition of Teff cell responses Itolizumab was generally well tolerated across all doses in high risk aGVHD patients Lymphocyte count changes are monitored carefully and have been manageable Only one acute infusion reaction was noted early in the study (Cohort 1, first subject dosed) Direct drug toxicities have not been noted; though relationship to infection in this vulnerable population will need continued assessment Key findings for itolizumab Dose-dependent reduction of CD6 expression on CD4+ and CD8+ T-cells is consistent with proposed MoA Strong response for higher dose level cohorts (0.8 and 1.6 mg/kg) with ORR of 100% (N=6) at Day 29, most have been complete responses (one VGPR) Clinical responses have been sustained through Day 57 – all subjects in CR Reduction in baseline corticosteroid use at Day 29 was ~40 - 80% Summary and Conclusions 13 Itolizumab demonstrates a favorable benefit-risk profile that supports further characterization of safety and efficacy in future studies
Thank you to our patients, sites, and advisors! Leslie Kean, MD, PhD Jerome Ritz, MD Robert Soiffer, MD Jamie Ferrara, MD EQUATE DSMC Committee 14 For more information: clinicaltrials@equilliumbio.com
