Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Alkermes plc. | alks-ex991_7.htm |
| 8-K - 8-K - Alkermes plc. | alks-8k_20210211.htm |

Fourth Quarter and Year-End 2020 Financial Results & Business Update February 11, 2021 Exhibit 99.2

Forward-Looking Statements and Non-GAAP Financial Information Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the company’s expectations with respect to its future financial and operating performance, business plans or prospects, including potential growth of revenue from its commercial products, potential diversification of its product portfolio, therapeutic areas that the company may pursue and the company’s plans to manage for growth and long-term profitability through execution of its Value Enhancement Plan, including its commitment to profitability targets, optimization of its cost structure and exploration of strategic opportunities; the potential therapeutic and commercial value of the company’s marketed and development products; the company’s expectations and assumptions regarding the future impacts of COVID-19 on its business; the company’s timelines, plans and expectations for development activities relating to the company’s products and product development candidates in both neuroscience and oncology, including (i) for nemvaleukin alfa (“nemvaleukin”), plans to initiate additional studies with IV nemvaleukin, select additional tumor types to pursue, and explore strategic collaborations and (ii) for ALKS 1140, plans to begin phase 1 first-in-human trials; and the company’s expectations concerning future regulatory activities and interactions, including expected timing of the U.S. Food and Drug Administration’s (“FDA”) target Prescription Drug User Fee Act (“PDUFA”) action date for the new drug application (“NDA”) for LYBALVITM and plans to advance discussions on registration plans for nemvaleukin with regulatory agencies. The company cautions that forward-looking statements are inherently uncertain. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: the impacts of the ongoing COVID-19 pandemic and continued efforts to mitigate its spread on the company’s business, results of operations or financial condition; the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation and other patent litigation, related to any of the company’s products, which may lead to competition from generic drug manufacturers; data from clinical trials may be interpreted by the FDA in different ways than the company interprets it; the FDA may not agree with the company’s regulatory approval strategies or components of the company’s NDAs, including clinical trial designs, conduct and methodologies, manufacturing processes and facilities, or the adequacy of the data or other information included in the company’s regulatory submissions to support the FDA’s requirements for approval; the FDA or regulatory authorities outside the U.S. may make adverse decisions regarding the company’s products, including with respect to the NDA for LYBALVI; the company’s development activities may not be completed on time or at all; the results of the company’s development activities may not be positive, or predictive of real-world results or of results in subsequent trials, and preliminary or interim results of the company’s development activities may not be predictive of final results of such activities, results of future preclinical or clinical trials or real-world results; the company and its licensees may not be able to successfully commercialize their products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended Dec. 31, 2020 and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov, and on the company’s website at www.alkermes.com in the ‘Investors – SEC filings’ section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Non-GAAP Financial Measures: This presentation includes information about certain financial measures that are not prepared in accordance with generally accepted accounting principles in the U.S. (GAAP), including non-GAAP net income and non-GAAP earnings per share. These non-GAAP measures are not based on any standardized methodology prescribed by GAAP and are not necessarily comparable to similar measures presented by other companies. Reconciliations of these non-GAAP financial measures to the most directly comparable GAAP financial measures can be found in the Alkermes plc Current Report on Form 8-K filed with the SEC on Feb. 11, 2021. Note Regarding Trademarks: The company is the owner of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, ARISTADA INITIO® , LYBALVITM and VIVITROL®. VUMERITY® is a registered trademark of Biogen MA Inc., used by Alkermes under license. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto. 2

Introduction Sandy Coombs, VP, Investor Relations Corporate Update Richard Pops, Chief Executive Officer Q4 & FY 2020 Financial Results; 2021 Financial Expectations Iain Brown, Chief Financial Officer Q4 & FY 2020 Commercial Review Todd Nichols, Chief Commercial Officer R&D Pipeline Update Richard Pops, Chief Executive Officer Agenda

Execution Against Our Strategic Priorities Commercial Execution VIVITROL® and ARISTADA® Strong performance in a complex environment Adapted commercial strategy in response to COVID-19 Prepared for synergistic launch of LYBALVI™ (ALKS 3831) within psychiatry portfolio Supported launch of VUMERITY® Advancement of Highest Potential R&D Programs Completed successful Advisory Committee meeting for LYBALVI* Advanced nemvaleukin alfa (ALKS 4230) development program Observed anti-tumor activity in monotherapy and combination settings with intravenous administration Accelerated patient enrollment and expanded clinical trial network globally Nominated first clinical candidate from HDAC** inhibitor program Efficient Management of Operating Structure and Strong Governance Adapted cost structure in response to COVID-19-related disruptions Announced Value Enhancement Plan Commitment to profitability targets Focus on strategic opportunities Continued Board refreshment Appointed two new independent directors Announced upcoming retirement of two long-serving directors 2020 Key Accomplishments *NDA resubmission under review following FDA Complete Response Letter and records requests relating to manufacturing of LYBALVI. **HDAC: histone deacetylase 4
Focus on Value Creation in 2021: Three Key Components Grow and Diversify Commercial Revenues Demonstrate Value of R&D Investments Manage for Growth & Long-Term Profitability Drive VIVITROL® and ARISTADA® net sales Support VUMERITY® growth Launch LYBALVI™ (PDUFA* June 1, 2021) Nemvaleukin alfa Determine registration pathway Demonstrate anti-tumor activity Explore strategic collaboration ALKS 1140 (CoREST**-selective HDAC inhibitor) Initiate phase 1/FIH study Investor Day Provide update on pipeline platforms and programs Operationalize commitment to profitability targets Optimize cost structure and drive operating leverage Explore strategic opportunities to maximize value and enhance profitability 1 2 3 *Prescription Drug User Fee Act **Co-repressor of repressor element-1 silencing transcription factor 5
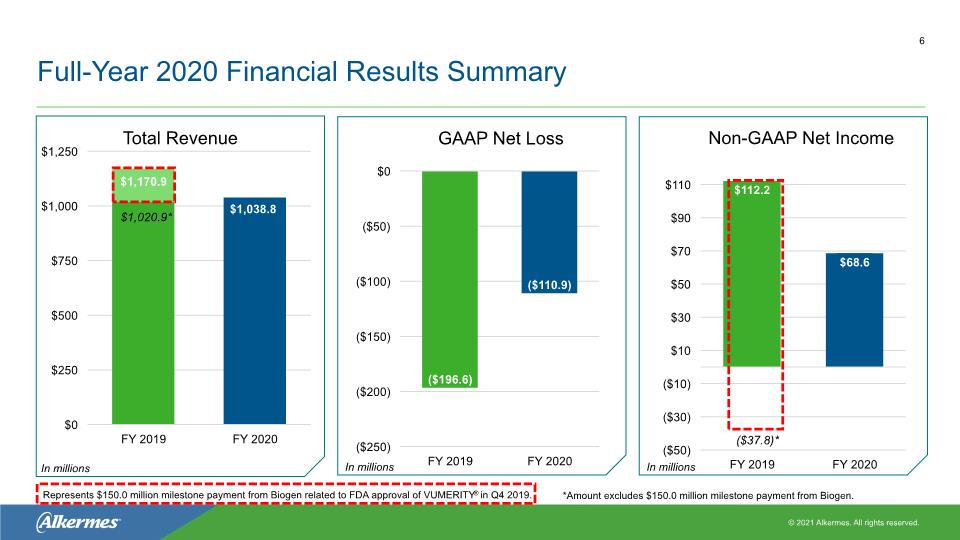
Full-Year 2020 Financial Results Summary 6 In millions In millions In millions Represents $150.0 million milestone payment from Biogen related to FDA approval of VUMERITY® in Q4 2019. ($37.8)* $1,020.9* *Amount excludes $150.0 million milestone payment from Biogen.
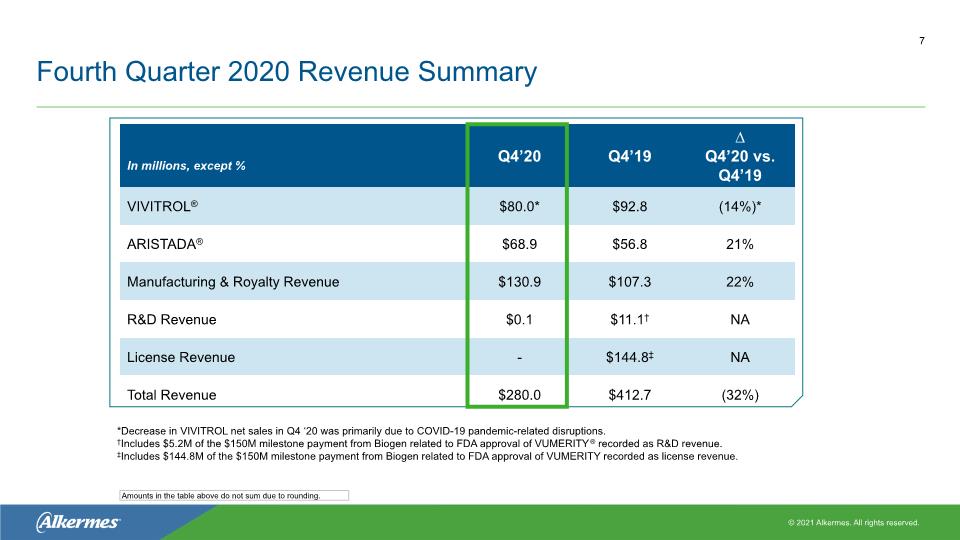
Fourth Quarter 2020 Revenue Summary 7 Amounts in the table above do not sum due to rounding. *Decrease in VIVITROL net sales in Q4 ‘20 was primarily due to COVID-19 pandemic-related disruptions. †Includes $5.2M of the $150M milestone payment from Biogen related to FDA approval of VUMERITY® recorded as R&D revenue. ‡Includes $144.8M of the $150M milestone payment from Biogen related to FDA approval of VUMERITY recorded as license revenue.
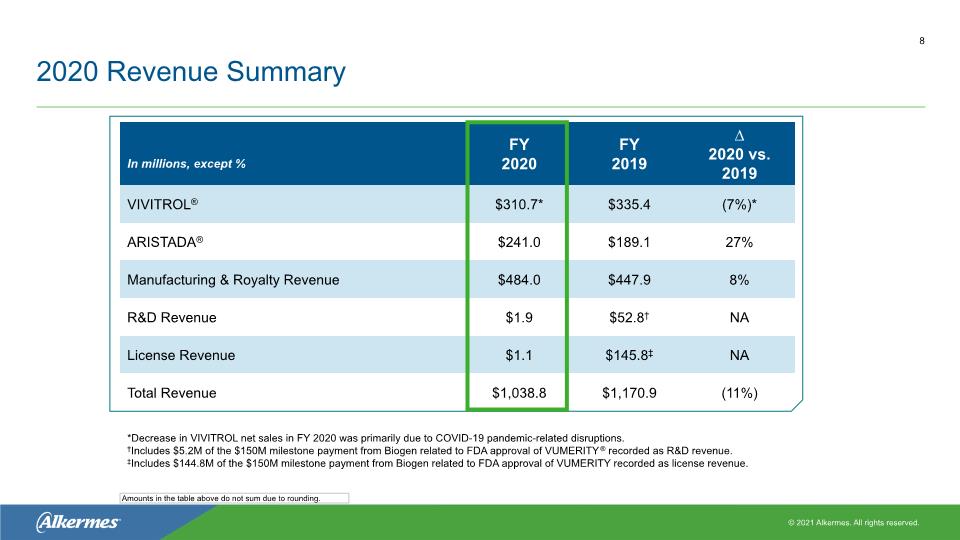
2020 Revenue Summary 8 *Decrease in VIVITROL net sales in FY 2020 was primarily due to COVID-19 pandemic-related disruptions. †Includes $5.2M of the $150M milestone payment from Biogen related to FDA approval of VUMERITY® recorded as R&D revenue. ‡Includes $144.8M of the $150M milestone payment from Biogen related to FDA approval of VUMERITY recorded as license revenue. Amounts in the table above do not sum due to rounding.
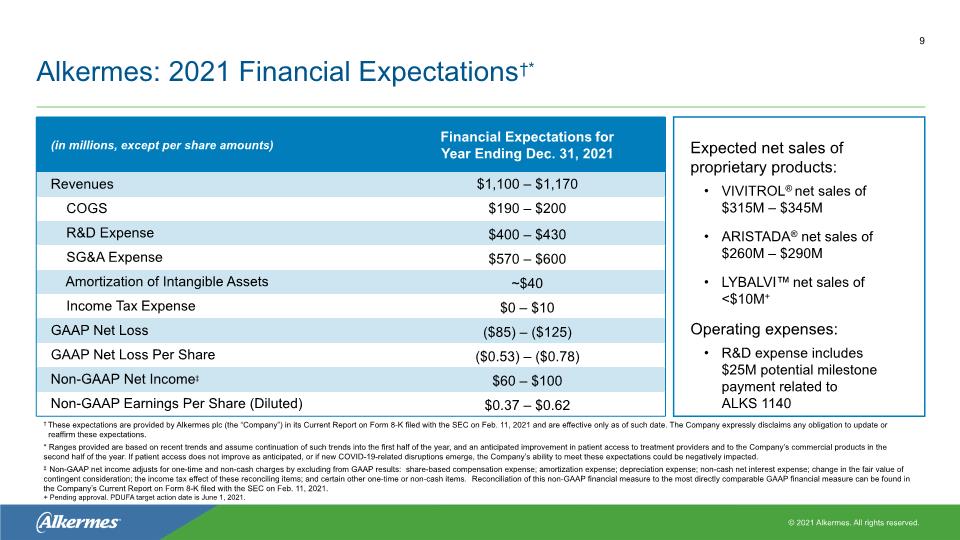
9 † These expectations are provided by Alkermes plc (the “Company”) in its Current Report on Form 8-K filed with the SEC on Feb. 11, 2021 and are effective only as of such date. The Company expressly disclaims any obligation to update or reaffirm these expectations. * Ranges provided are based on recent trends and assume continuation of such trends into the first half of the year, and an anticipated improvement in patient access to treatment providers and to the Company’s commercial products in the second half of the year. If patient access does not improve as anticipated, or if new COVID-19-related disruptions emerge, the Company’s ability to meet these expectations could be negatively impacted. ‡ Non-GAAP net income adjusts for one-time and non-cash charges by excluding from GAAP results: share-based compensation expense; amortization expense; depreciation expense; non-cash net interest expense; change in the fair value of contingent consideration; the income tax effect of these reconciling items; and certain other one-time or non-cash items. Reconciliation of this non-GAAP financial measure to the most directly comparable GAAP financial measure can be found in the Company’s Current Report on Form 8-K filed with the SEC on Feb. 11, 2021. + Pending approval. PDUFA target action date is June 1, 2021. Expected net sales of proprietary products: VIVITROL® net sales of $315M – $345M† ARISTADA® net sales of $260M – $290M LYBALVI™ net sales of <$10M+ Operating expenses: R&D expense includes $25M potential milestone payment related to ALKS 1140 Alkermes: 2021 Financial Expectations†*
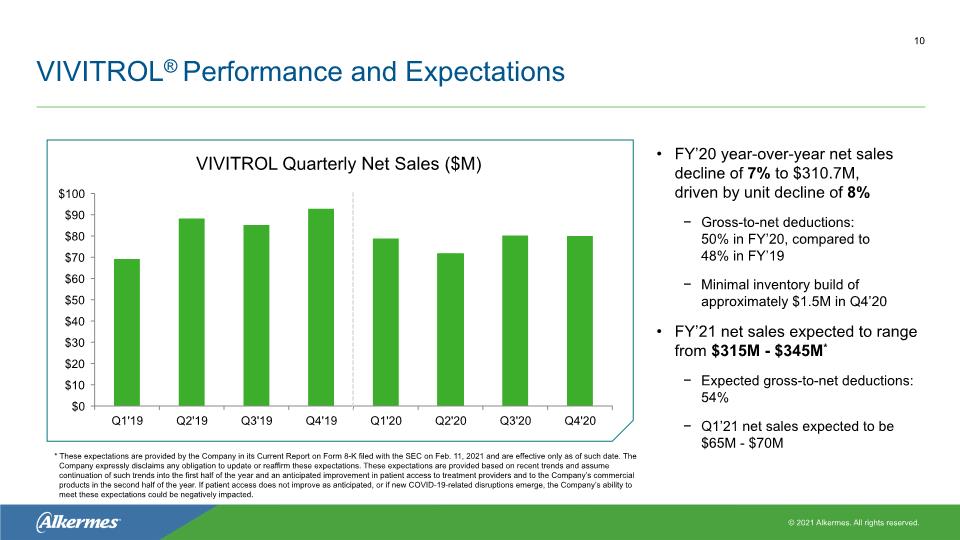
VIVITROL® Performance and Expectations FY’20 year-over-year net sales decline of 7% to $310.7M, driven by unit decline of 8% Gross-to-net deductions: 50% in FY’20, compared to 48% in FY’19 Minimal inventory build of approximately $1.5M in Q4’20 FY’21 net sales expected to range from $315M - $345M* Expected gross-to-net deductions: 54% Q1’21 net sales expected to be $65M - $70M VIVITROL Quarterly Net Sales ($M) 10 * These expectations are provided by the Company in its Current Report on Form 8-K filed with the SEC on Feb. 11, 2021 and are effective only as of such date. The Company expressly disclaims any obligation to update or reaffirm these expectations. These expectations are provided based on recent trends and assume continuation of such trends into the first half of the year and an anticipated improvement in patient access to treatment providers and to the Company’s commercial products in the second half of the year. If patient access does not improve as anticipated, or if new COVID-19-related disruptions emerge, the Company’s ability to meet these expectations could be negatively impacted.
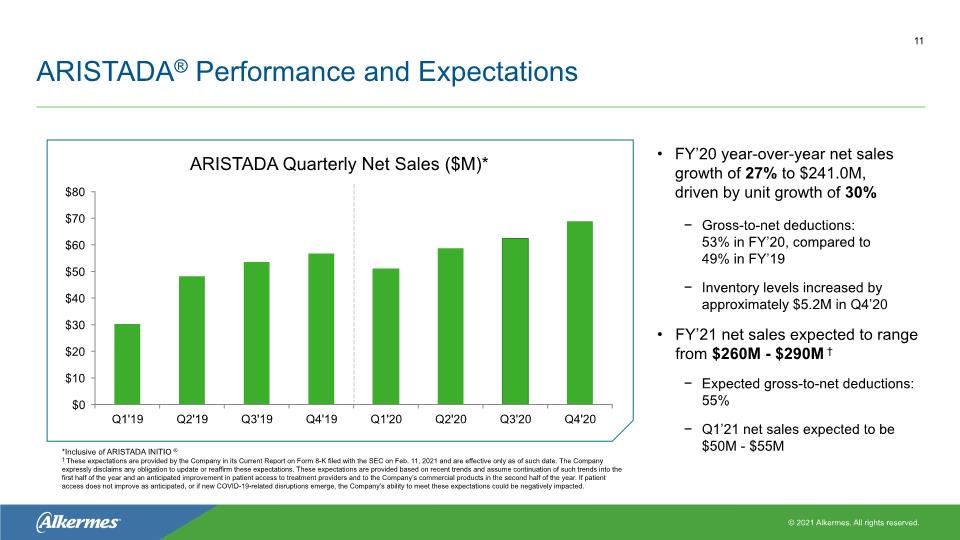
ARISTADA® Performance and Expectations FY’20 year-over-year net sales growth of 27% to $241.0M, driven by unit growth of 30% Gross-to-net deductions: 53% in FY’20, compared to 49% in FY’19 Inventory levels increased by approximately $5.2M in Q4’20 FY’21 net sales expected to range from $260M - $290M † Expected gross-to-net deductions: 55% Q1’21 net sales expected to be $50M - $55M ARISTADA Quarterly Net Sales ($M)* 11 *Inclusive of ARISTADA INITIO® † These expectations are provided by the Company in its Current Report on Form 8-K filed with the SEC on Feb. 11, 2021 and are effective only as of such date. The Company expressly disclaims any obligation to update or reaffirm these expectations. These expectations are provided based on recent trends and assume continuation of such trends into the first half of the year and an anticipated improvement in patient access to treatment providers and to the Company’s commercial products in the second half of the year. If patient access does not improve as anticipated, or if new COVID-19-related disruptions emerge, the Company’s ability to meet these expectations could be negatively impacted.
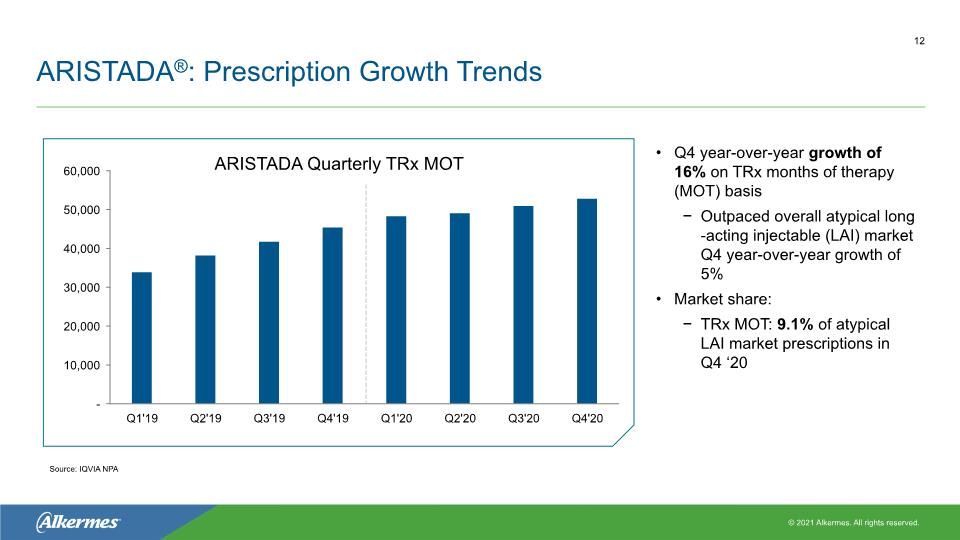
ARISTADA®: Prescription Growth Trends Q4 year-over-year growth of 16% on TRx months of therapy (MOT) basis Outpaced overall atypical long-acting injectable (LAI) market Q4 year-over-year growth of 5% Market share: TRx MOT: 9.1% of atypical LAI market prescriptions in Q4 ‘20 ARISTADA Quarterly TRx MOT Source: IQVIA NPA 12
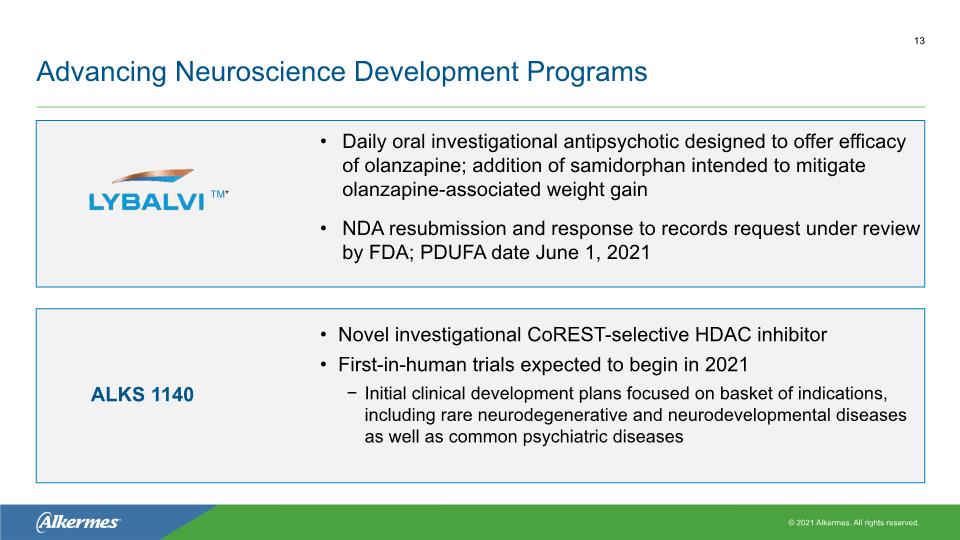
Advancing Neuroscience Development Programs Daily oral investigational antipsychotic designed to offer efficacy of olanzapine; addition of samidorphan intended to mitigate olanzapine-associated weight gain NDA resubmission and response to records request under review by FDA; PDUFA date June 1, 2021 Novel investigational CoREST-selective HDAC inhibitor First-in-human trials expected to begin in 2021 Initial clinical development plans focused on basket of indications, including rare neurodegenerative and neurodevelopmental diseases as well as common psychiatric diseases 13 ALKS 1140 TM*
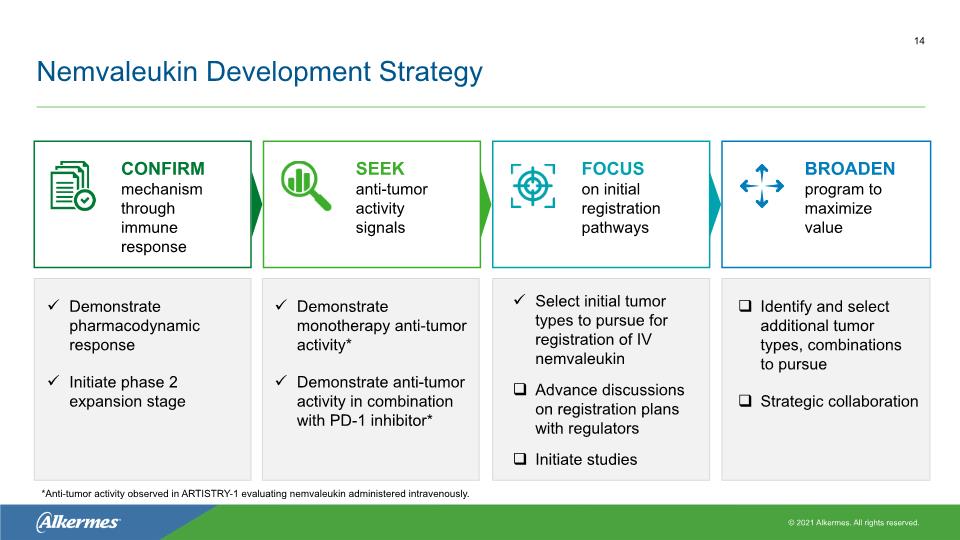
Nemvaleukin Development Strategy Demonstrate pharmacodynamic response Initiate phase 2 expansion stage Demonstrate monotherapy anti-tumor activity* Demonstrate anti-tumor activity in combination with PD-1 inhibitor* Select initial tumor types to pursue for registration of IV nemvaleukin Advance discussions on registration plans with regulators Initiate studies Identify and select additional tumor types, combinations to pursue Strategic collaboration CONFIRM mechanism through immune response SEEK anti-tumor activity signals FOCUS on initial registration pathways BROADEN program to maximize value 14 *Anti-tumor activity observed in ARTISTRY-1 evaluating nemvaleukin administered intravenously.

www.alkermes.com 15
