Attached files
| file | filename |
|---|---|
| EX-23.2 - EX-23.2 - Apria, Inc. | d62545dex232.htm |
| EX-23.1 - EX-23.1 - Apria, Inc. | d62545dex231.htm |
| EX-10.36 - EX-10.36 - Apria, Inc. | d62545dex1036.htm |
Table of Contents
As filed with the Securities and Exchange Commission on February 9, 2021.
Registration No. 333-252146
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2 to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Apria, Inc.
(Exact Name of Registrant as Specified in its Charter)
| Delaware | 8082 | 82-4937641 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
7353 Company Drive
Indianapolis, Indiana 46237
Telephone: (800) 990-9799
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Raoul Smyth
Executive Vice President, General Counsel and Secretary
Apria, Inc.
7353 Company Drive
Indianapolis, Indiana 46237
Telephone: (800) 990-9799
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| Edgar J. Lewandowski William R. Golden III Simpson Thacher & Bartlett LLP 425 Lexington Avenue New York, New York 10017 Telephone: (212) 455-2000 |
Michael Kaplan Deanna L. Kirkpatrick Davis Polk & Wardwell LLP 450 Lexington Avenue New York, New York 10017 Telephone: (212) 450-4000 |
Approximate date of commencement of the proposed sale of the securities to the public: As soon as practicable after the Registration Statement is declared effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. ☐
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.:
| Large accelerated filer | ☐ | Accelerated filer | ☐ | |||
| Non-accelerated filer | ☒ | Smaller reporting company | ☐ | |||
| Emerging growth company | ☐ | |||||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
CALCULATION OF REGISTRATION FEE
|
| ||||||||
| Title of Each Class of Securities to be Registered |
Amount to be Registered(1) |
Proposed Maximum Aggregate Offering Price |
Proposed Maximum Aggregate Offering Price(1)(2) |
Amount of Registration Fee(3) | ||||
| Common Stock, par value $0.01 per share |
8,625,000 | $21.00 | $181,125,000 | $19,760.74 | ||||
|
| ||||||||
|
| ||||||||
| (1) | Includes 1,125,000 shares of common stock that are subject to the underwriters’ option to purchase additional shares. |
| (2) | Estimated solely for the purpose of determining the amount of the registration fee in accordance with Rule 457(a) under the Securities Act of 1933. |
| (3) | Previously paid. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to completion, dated February 9, 2021
Preliminary Prospectus
7,500,000 Shares

Apria, Inc.
Common Stock
This is the initial public offering of shares of common stock of Apria, Inc. No public market currently exists for our common stock. The selling stockholders are offering 7,500,000 shares of common stock. We will not receive any proceeds from the sale of shares of common stock by the selling stockholders. We anticipate that the initial public offering price will be between $19.00 and $21.00 per share. We intend to list our shares of common stock on the Nasdaq Global Select Market (“Nasdaq”) under the symbol “APR.”
After the completion of this offering, affiliates of The Blackstone Group Inc. will continue to own a majority of the voting power of shares eligible to vote in the election of our directors. As a result, we will be a “controlled company” within the meaning of the corporate governance standards of Nasdaq. See “Management—Controlled Company Exception” and “Principal and Selling Stockholders.”
Investing in shares of our common stock involves risks. See “Risk Factors” beginning on page 23 to read about factors you should consider before buying shares of our common stock.
| Per Share |
Total | |||||||
| Initial public offering price |
$ | $ | ||||||
| Underwriting discounts and commissions(1) |
$ | $ | ||||||
| Proceeds, before expenses, to selling stockholders |
$ | $ | ||||||
| (1) | See “Underwriting” for additional information regarding underwriting compensation. |
To the extent that the underwriters sell more than 7,500,000 shares of our common stock, the underwriters have the option to purchase up to an additional 1,125,000 shares of our common stock from the selling stockholders at the initial public offering price less the underwriting discounts and commissions, within 30 days from the date of this prospectus.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of our common stock against payment in New York, New York on or about , 2021.
Joint Book-Running Managers
| Citigroup | Goldman Sachs & Co. LLC |
| BofA Securities | J.P. Morgan |
| UBS Investment Bank |
Co-Managers
| Piper Sandler |
| Citizens Capital Markets | Fifth Third Securities | TD Securities |
| Academy Securities
|
Blaylock Van, LLC | Penserra Securities LLC | Stern |
The date of this prospectus is , 2021.
Table of Contents
Neither we nor the selling stockholders, nor the underwriters have authorized anyone to provide you with information different from that contained in this prospectus, any amendment or supplement to this prospectus or any free writing prospectus prepared by us or on our behalf. Neither we nor the selling stockholders, nor the underwriters take any responsibility for, or can provide any assurance as to the reliability of, any information other than the information in this prospectus, any amendment or supplement to this prospectus or any free writing prospectus prepared by us or on our behalf. We, the selling stockholders and the underwriters are offering to sell, and seeking offers to buy, shares of our common stock only in jurisdictions where offers and sales are permitted. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of shares of our common stock. Our business, financial condition, results of operations and prospects may have changed since that date.
Through and including , 2021 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
For investors outside the United States: Neither we nor the selling stockholders, nor the underwriters have done anything that would permit our initial public offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the shares of our common stock and the distribution of this prospectus outside of the United States.
i
Table of Contents
About This Prospectus
Financial Statement Presentation
This prospectus includes certain historical consolidated financial and other data for Apria Healthcare Group Inc. (“Apria Healthcare Group”) and its subsidiaries. At or prior to the completion of this offering, we will undertake certain reorganization transactions (the “pre-IPO reorganization transactions”) so that Apria, Inc. will directly or indirectly own all of the equity interests in Apria Healthcare Group and become the holding company of our business.
Apria, Inc. will be the financial reporting entity following this offering. Other than the balance sheets as of September 30, 2020, December 31, 2019 and December 31, 2018, financial information of Apria, Inc. has not been included in this prospectus as since its formation on March 22, 2018 it has not entered into any business transactions or activities, has no capitalization, and had no assets or liabilities during the periods presented in this prospectus.
Certain Definitions
As used in this prospectus, unless otherwise noted or the context requires otherwise:
| • | “Apria,” the “Company,” “we,” “us” and “our” refer (1) prior to the consummation of this offering and the pre-IPO reorganization transactions, to Apria Healthcare Group, the existing holding company of our business, and its consolidated subsidiaries and (2) after the consummation of this offering and the pre-IPO reorganization transactions, to Apria, Inc. and its consolidated subsidiaries, including Apria Healthcare Group. |
| • | “Blackstone” or “Sponsor” refer to investment funds associated with, or managed or designated by, The Blackstone Group Inc., which funds are our current majority owners, and their permitted successors and assigns. |
| • | “CBP” and “DMEPOS CBP” refers to the DMEPOS competitive bidding program. |
| • | “CMS” refers to the Centers for Medicare and Medicaid Services. |
| • | “DMEPOS” refers to Medicare durable medical equipment, prosthetics, orthotics and supplies. |
| • | “GAAP” refers to generally accepted accounting principles in the United States of America. |
| • | “Medicare patients” refers to Medicare patients other than those participating in Medicare through the Medicare Advantage program. |
| • | “Payors” refers to third-party healthcare payors, including government and commercial payors. |
| • | “pre-IPO owners” refer to our Sponsor together with other owners of Apria Healthcare Group prior to this offering. |
| • | “The Joint Commission” refers to a nationally recognized, independent organization that develops standards for various healthcare industry segments and monitors compliance with those standards through voluntary surveys of participating providers. |
Unless indicated otherwise, the information included in this prospectus assumes no exercise by the underwriters of their option to purchase up to an additional 1,125,000 shares of common stock from us and the selling stockholders and that the shares of common stock to be sold in this offering are sold at $20.00 per share of common stock, which is the midpoint of the price range indicated on the front cover of this prospectus.
ii
Table of Contents
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information you should consider before investing in shares of our common stock. You should read this entire prospectus carefully, including the section entitled “Risk Factors” and the financial statements and the related notes thereto included elsewhere in this prospectus, before you decide to invest in shares of our common stock.
Apria
We are a leading provider of integrated home healthcare equipment and related services in the United States. We offer a comprehensive range of products and services for in-home care and delivery across three core service lines: (1) home respiratory therapy (including home oxygen and non-invasive ventilation (“NIV”) services); (2) obstructive sleep apnea (“OSA”) treatment (including continuous positive airway pressure (“CPAP”) and bi-level positive airway pressure devices, and patient support services); and (3) negative pressure wound therapy (“NPWT”). Additionally, we supply a wide range of home medical equipment and other products and services to help improve the quality of life for patients with home care needs. Our revenues are generated through fee-for-service and capitation arrangements with Payors for equipment, supplies, services and other items we rent or sell to patients. Through our offerings, we also provide patients with a variety of clinical and administrative support services and related products and supplies, most of which are prescribed by a physician as part of a care plan. We are focused on being the industry’s highest-quality provider of home healthcare equipment and related services, while maintaining our commitment to being a low-cost operator. We offer a compelling value proposition to patients, providers and Payors by allowing patients to receive necessary care and services in the comfort of their own home, while, at the same time, reducing the costs of treatment. We generated over $1 billion of net revenue in 2019, of which approximately 80% was from home respiratory therapy and OSA treatment, service categories in which we believe we have a leading market position.
We believe our integrated product and service offerings, combined with our national scale and strong reputation, provide us with a strategic advantage in being a preferred home healthcare provider for patients, providers and Payors. Our Payors include substantially all of the national and regional insurers, managed care organizations and government Payors in the United States. We benefit from long-standing relationships with a community of providers and referral sources for post-acute services across the acuity spectrum because of the consistency and reliability of our high quality clinical support, our national distribution footprint and our breadth of Payor relationships.
Our product and service offerings are distinguished by the complexity and sophistication required in their clinical delivery, logistical coordination and payment arrangements. We offer patients and providers differentiated clinical service, leveraging our protocols and expertise to improve outcomes across our service lines. With an expansive network of delivery technicians and therapists that is not readily replicated, we are able to provide home healthcare therapies that require high-touch service, providing a bridge from the acute care setting to the home. Our services include:
| • | providing in-home delivery, set-up and maintenance of equipment and supplies; |
| • | educating patients and caregivers about health conditions or illnesses and providing instructions about home safety, self-care and the proper use of equipment; |
| • | therapy compliance monitoring and intervention to enhance compliance; |
| • | clinical monitoring of complex respiratory service patients’ individualized treatment plans; |
| • | reporting patient progress and status to the physician, national and regional insurers and/or managed care organizations; and |
| • | processing claims to Payors on behalf of patients. |
1
Table of Contents
In 2019, we served nearly 2 million patients, made nearly 2.4 million deliveries and conducted over 744,000 clinician interactions with our patients. In addition, in 2019, we generated $1.1 billion of net revenue, $15.6 million in net income, $174.0 million of Adjusted EBITDA and $80.5 million of Adjusted EBITDA less Patient Equipment Capex. Through various strategic and operational initiatives, we have improved profitability despite reimbursement rate pressure, improving our Adjusted EBITDA margin by 110 basis points from 2017 through 2019 on a basis that excludes the impact of new accounting policies adopted in 2018 and 2019. For reconciliations of Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex to net income, the most directly comparable financial measure prepared in accordance with GAAP, see “—Summary Historical Financial and Other Data.”
Strong Industry Fundamentals Support Our Business Model
The U.S. home healthcare market comprises a broad range of products and services—including respiratory therapy, OSA therapy, negative pressure wound therapy, home medical equipment, infusion therapy, home healthcare nursing, orthotics and prosthetics, diabetic supplies and general medical supplies. CMS estimates that the total revenue of the U.S. home healthcare market was $108.9 billion in 2019, and forecasts this market to grow at a compound annual growth rate (“CAGR”) of approximately 7% between 2020 and 2028. We operate in the durable medical equipment sub-segment of the home healthcare industry. CMS forecasts the durable medical equipment sub-segment to grow at a CAGR of approximately 6% between 2019 and 2028. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025. In 2018, industry analysts estimated that the market size for respiratory devices and OSA devices was approximately $6.2 billion and approximately $2.2 billion, respectively, in North America. The U.S. market for negative pressure wound therapy devices is expected by industry analysts to grow at a CAGR of approximately 5% between 2018 to 2023, and in 2018, the global market size of negative pressure wound therapy devices was estimated by industry analysts to be approximately $2.1 billion. Our sub-segment of the U.S. home healthcare industry is highly fragmented. The five largest players in this sub-segment accounted for approximately 50% of this sub-segment’s revenues in 2019. The remaining market revenues are attributable to thousands of other companies that primarily operate in local markets or regions.
We expect to benefit from the following continuing trends within the home healthcare market:
| • | aging population; |
| • | rising incidence of chronic diseases; |
| • | continued shift toward home healthcare driven by the compelling economic value proposition to key stakeholders and technological developments making remote monitoring more feasible; |
| • | increased prevalence of in-home treatments and preference for in-home care where available; and |
| • | consolidation of the highly fragmented market to the benefit of national players. |
Our Value Proposition
We believe we offer a compelling value proposition for patients, providers and Payors.
Value Proposition for Patients: We are committed to improving the experience and clinical outcome for each patient we serve. We believe our patients prefer the convenience and typical cost advantages of home healthcare over institutional care and the greater independence, increased responsibility and improved responsiveness to treatment that comes with it. By providing in-home delivery and equipment set-up, patient and caregiver education, as well as patient monitoring and compliance services, we enable the patient to move from
2
Table of Contents
or avoid an acute care setting and remain in or return to the home, which is the lowest cost, and overwhelmingly the patient-preferred, setting.
Value Proposition for Providers: Physicians, hospital professionals and other providers refer patients to us because of the consistent, high quality and reliable services we offer them and their patients. We seek to be a trusted advocate and provider of patient clinical needs in patients’ homes, while fostering lasting doctor-patient relationships. Additionally, the reliability of our clinical support, quality equipment and data collection facilitate a clinically adept transition to a lower-cost setting, which we believe benefits healthcare professionals, and helps to put them at ease regarding their patients’ ongoing treatments. We believe our services improve patient compliance and clinical outcomes, reduce hospital re-admissions and enable hospital providers to have greater control over timely patient discharges.
Value Proposition for Payors: We offer Payors access to an extensive national footprint, national logistics systems, respiratory clinical expertise, competitive pricing, alternative payment arrangements, including fee-for-service and capitation for defined patient populations, and our ability to connect electronically with Payors’ systems. We seek to effectively manage the transition from the acute care setting to a low-cost home setting with a high reliability of clinical support, data collection and quality equipment to lower readmission rates.
Our Competitive Strengths
National Scale with Local Presence. Our platform combines local market presence with the advantages and efficiencies of national scale, clinical expertise and reputation. As one of the largest providers of home healthcare services in the United States, we enjoy long-tenured relationships with the majority of the major commercial Payors, who value an expansive geographic footprint that can service their entire patient populations, in addition to our reputation for reliable and quality care.
Expansive Offering with Exposure to Attractive Product Markets. We seek to offer high quality, clinically appropriate care across a broad spectrum of services and treatments amenable to the home setting. Our extensive offering helps make us an attractive and convenient choice for our patients, providers and Payors. With top two market positions in the United States in home respiratory therapy, OSA treatment and NPWT, we are aligned with large and growing addressable markets across our core service lines.
Strong Relationships with Payors and Referral Sources Developed with Differentiated Sales Model. We enjoy deep and long-standing relationships with national and regional insurers and managed care organizations, many of whom we have been contracted with for over 20 years. We believe Payors value the breadth of our entire platform, including our geographic reach and the range of conditions our service offerings cover through both our fee-for-service and capitation arrangements. Furthermore, we believe they value our flexibility in payment arrangements and competence in managing value as well as volume under capitation for defined patient populations.
Leveraging our broad market access to patients through our centralized Payor relationships, we cultivate individual relationships with thousands of local referral sources, including hospitals, outpatient facilities, physicians and sleep centers, through a field sales force of approximately 380 in-market sales representatives and approximately 200 front-line managers. We believe this differentiated approach of combining Company-wide Payor relationships with in-market referral relationships enhances the efficiency and productivity of our marketing efforts.
Leadership in Clinical Delivery. We specialize in certain complex home healthcare services and treatments that require deep technical and clinical expertise in addition to frequent patient interaction. We believe we are distinguished from e-commerce and logistics platforms by our ability to meet patient needs directly through our
3
Table of Contents
highly trained technicians and therapists who deliver and provide these services, as well as our ability to bill a patient’s insurance provider. We utilize differentiated clinical expertise and protocols to drive optimal outcomes across our core service lines and all of these operations are accredited by The Joint Commission.
Leading Revenue Cycle Management. We manage medical claims and patient collections on a single operating platform that allows for capitation and fee-for-service arrangements. We believe we lead the industry in (1) ease of use for our key referral sources, (2) regulatory compliance and (3) revenue cycle management and billing and collections efficiency. We offer several advantages to our key referral sources and Payors, which we believe improve our ability to win new business and capture share from our competitors.
Strong and Experienced Management Team. We are led by a team of talented industry veterans, comprising individuals with long tenures at Apria and deep knowledge of our history and operations, as well as individuals who joined us more recently and bring fresh perspectives, insights and best practices developed through experience in other industries and at other companies. With an average of over 20 years of industry experience and over ten years with Apria, our senior leadership team has expertise spanning nearly every segment of the healthcare industry.
Growth Strategy
Our goal is to be the market leader in the provision of high quality, cost-efficient home healthcare services, creating value for all stakeholders—patients, providers and Payors. We seek to achieve this goal through the following growth strategies:
Maintain leadership in markets with favorable industry dynamics. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025. With a national distribution platform that is difficult to replicate, deep and long-standing relationships with national and regional insurers and managed care organizations and a reputation for quality and reliability of service, we believe we are well positioned to maintain leadership in these attractive and growing markets. Industry groups suggest that approximately 80% of moderate and severe sleep-disordered breathing cases remain undiagnosed. We have been educating primary care physicians about the proper diagnosis and treatment of OSA. As the first line of care, these primary care physicians are more likely to encounter undiagnosed instances of OSA. In addition to traditional solutions, we coordinate an end-to-end virtual solution that includes home sleep testing (rather than testing at an offsite sleep clinic) which is convenient for the patient and safer, especially in the current pandemic environment. We believe that facilitating the diagnosis and treatment of this condition will lead to increased growth in OSA sales and increase revenue synergies as newly acquired OSA patients will have access to our other product and service lines when clinically indicated.
Enable the transition to value-based healthcare. Government and commercial Payors are increasingly seeking ways to shift from traditional fee-for-service to a value-based model. We believe the ability to transition patients from the acute care setting to the home, as well as to prevent unnecessary readmissions, represents a critical part of this effort. As a leading provider of home healthcare equipment and related services, we believe we will increasingly benefit from this ongoing paradigm shift in the industry. In addition, we believe our demonstrated expertise in non-traditional payment models, such as capitation arrangements, will position us well to take advantage of this trend.
Expand product and service offerings. We continue to focus on expanding into new product lines and services both organically and through strategic acquisition to continue to grow and diversify our revenue with a patient-centric view centered on the long term value of each patient. For example, we are pursuing products and services related to the treatment of diabetes and the provision of diabetic supplies. Technological advances in continuous glucose monitoring and insulin pump delivery (creating an “artificial pancreas”) have changed the
4
Table of Contents
diabetes treatment paradigm and enabled providers to better treat patients in the home healthcare setting. Moreover, diabetic patients often suffer from many co-morbidities, including OSA, respiratory ailments and heart disease, many of which we already treat through our sleep and respiratory product lines. We believe we are well positioned to leverage our national platform and scalable infrastructure to help diabetic patients benefit from new diabetes treatment technologies as well as treatments for co-morbidities.
Leverage patient interactions. Our business model provides for frequent interaction with our patients and a direct entry point into the home, allowing our technicians, therapists and customer service agents to assess whether the patients are in need of additional services, supplies or convenience items. Where appropriate, our technicians, therapists and customer service agents can assist our patients in obtaining these additional services, supplies or convenience items through Apria. In addition, existing patients and potential patients may utilize our e-commerce platform to access additional items that are not covered by Payors and are purchased directly by patients.
Grow e-commerce through a patient-centric approach. Our e-commerce platform is primarily focused on supplies, accessories and additional items that are not covered by Payors and are purchased directly by patients. E-commerce is a convenient way to provide products and services to our existing patients and also serves as an additional acquisition channel for new patients. Through direct-to-patient marketing focused on service and clinical support, we believe we can continue to grow volume through our e-commerce channel. For example, we frequently acquire patients to meet a single product need even though they typically have additional home healthcare needs. Through patient interaction and education, they can discover and access other products and services that we offer through e-commerce that can address their needs. In addition, we believe the broader home healthcare trends discussed below will also enable us to grow our revenue and product and service offerings through e-commerce. Given that the supplies and accessories that are its primary focus are less capital intensive, we believe growing our e-commerce channel represents an attractive opportunity to increase cash flows and profitability.
Grow with new home healthcare trends. Telemedicine and remote provider care have been gaining traction in recent years and have been significantly accelerated given the recent COVID-19 pandemic. We believe that these trends will help accelerate growth in home healthcare and we are well positioned to benefit from these trends given our national footprint and scalable infrastructure. Moreover, due to the impacts of the COVID-19 pandemic, we expect accelerated and sustainable demand for home healthcare solutions including respiratory and other medical conditions that we treat regularly. These trends in telemedicine and the emphasis on treating patients in the home, outside the traditional medical clinic and hospital, will enable us to grow our revenue and product and service offerings further.
Strategic acquisitions. We believe there is also opportunity to accelerate our growth rate, market share gains and expand into new product markets through strategic acquisitions. We will continue to evaluate such opportunities through a disciplined approach, seeking only acquisitions that will complement our existing operations and businesses and/or create synergies. The various markets we participate in remain highly fragmented and ripe for consolidation and we believe that our nationally integrated platform, scale and strong reputation position us well to be a consolidator in our industry. We have a relatively unburdened balance sheet with low debt levels and meaningful debt capacity for acquisitions. Moreover, as a public company, we will have greater access to capital markets and we will be able to use our stock as an acquisition currency or to raise additional capital for strategic acquisitions.
Continue to capture efficiencies through scale and operational improvement. Our existing distribution network of approximately 275 branch locations can reach more than 90% of the U.S. population across both high-density urban markets and rural markets. Coupled with scalable technology and centralized operations, including revenue cycle management, we believe we can continue to grow beyond our nearly 2 million patients
5
Table of Contents
served in 2019 without significant incremental capital investment in infrastructure. As we maintain our strong position in growing product markets, we believe the ability to scale our operations and leverage fixed costs represents a significant opportunity for growth in profitability. In addition, we believe we can continue to improve the cost-efficiency of our operations and support functions through new and recently completed technology initiatives, such as robotic desktop automation (“RDA”) to simplify agent workflow, robotic process automation technology (“RPA”) for certain repetitive and volume staff tasks and automating activities such as new employee onboarding and equipment provisioning, and enhanced workflow to improve ease of use and direct activity to the appropriately skilled staff. We have also invested in DMEhub, a cloud-based e-prescribing platform that allows providers to submit medical equipment orders more efficiently and accurately, which we believe drives ease of use, enables more efficient order processing and reduces administrative burden.
Continue to improve cash profile. We continually evaluate opportunities to enhance our cash flow and return on investment. We see opportunities in this regard both to increase our exposure to less capital intensive products and to benefit from recent investment in our longer-lived, complex equipment fleet. Patients on OSA therapy and NPWT require periodic replenishment of supplies and accessories in order to remain in compliance with their prescribed therapies. These supplies are less capital intensive and we believe represent a multi-billion dollar market with an attractive growth profile. The opportunity to increase our exposure to this market is enhanced by our growing e-commerce distribution channel, which is primarily focused on these supplies and accessories, offering patients speed and convenience and helping us to continue to grow our volume.
Our Strategic Transformations
Since we were acquired by Blackstone in 2008, the home healthcare industry has experienced a number of significant changes, including reimbursement, regulatory and technological changes. We have continually worked to adapt our business and organizational structure to best meet the current needs of patients, providers and Payors.
Our most recent business initiative, which we call Simplify, focused on retaining clear customer ownership at our local branch level with support from our scalable national platform to greatly improve the patient and referral source experience. With a goal of optimizing the end-to-end customer experience while enabling us to increase our growth rate at lower cost, we evaluated local, regional and centralized processes to foster local branch customer ownership, supported by regional shared services where scale, consistency and process expertise is needed, and our national platform where scale can be leveraged. This transformation has led to significant improvement in profitability and lower operating costs and has positioned us well for future growth. Our patient-centric growth plan begins with an improved customer experience, which we believe will support increased cross-sell opportunities and growth across product lines to the over 2 million unique patients we serve annually.
In addition to Simplify, we have also made other substantial changes to our business since our acquisition by Blackstone:
| • | we brought on a management team of individuals with extensive expertise and experience in the workings of our industry and regulatory environment and others who specialize in designing and developing scalable business infrastructures and business transformation; |
| • | we improved our business and revenue mix by growing our relationships with commercial Payors; |
| • | we expanded our service offerings to include more complex respiratory services, including NIV therapy, and we shifted our focus to three core service lines including less capital intensive products and services which complement our core offerings; |
| • | we invested in patient monitoring and outcomes data collection to differentiate our service offerings and provide enhanced value to patients, providers and Payors; |
6
Table of Contents
| • | we improved sales productivity through changes in the sales force hiring profile and implementation of a new sales customer relationship management system; |
| • | we formed strategic relationships with our suppliers to help optimize equipment costs and provide state-of-the art patient equipment; |
| • | we streamlined our management structure and further optimized our branch network by reducing our locations open to the public from over 400 to approximately 275 without materially reducing the coverage of our service areas or the quality of our service; |
| • | we built a scalable infrastructure with data analytics and forecasting capabilities to more efficiently leverage the favorable trends of an aging population and the paradigm shift in healthcare from the acute setting to the home; and |
| • | we consolidated regional and branch level billing, collections and aspects of the customer service function, reorganized their work flows and adopted new technology and processes to help us improve productivity, monitor performance and more effectively manage important aspects of our business in real-time. |
Our Sponsor
Blackstone (NYSE: BX) is one of the world’s leading investment firms. Blackstone’s asset management businesses include investment vehicles focused on real estate, private equity, public debt and equity, growth equity, opportunistic, non-investment grade credit, real assets and secondary funds, all on a global basis. Through its different businesses, Blackstone had total assets under management of approximately $584 billion as of September 30, 2020.
After the completion of this offering, our Sponsor will beneficially own approximately 72.0% of our common stock (or 68.8% if the underwriters exercise their option to purchase additional shares in full). As a result, we will be a “controlled company” within the meaning of the Nasdaq corporate governance standards. Under these corporate governance standards, a company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance standards, including the requirements (1) that a majority of our board of directors consist of independent directors, (2) that our board of directors have a compensation committee that is comprised entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities and (3) that our director nominations be made, or recommended to our full board of directors, by our independent directors or by a nominations committee that is comprised entirely of independent directors and that we adopt a written charter or board resolution addressing the nominations process. For at least some period following this offering, we intend to utilize these exemptions. As a result, immediately following this offering, we do not expect a majority of our directors will have been affirmatively determined to be independent or that our compensation committee or nominating and corporate governance committee of the board will be comprised entirely of directors who have been affirmatively determined to be independent. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of these corporate governance requirements. In the event that we cease to be a “controlled company” and our common stock continues to be listed on Nasdaq, we will be required to comply with these provisions within the applicable transition periods.
7
Table of Contents
Summary Risk Factors
An investment in shares of our common stock involves substantial risks and uncertainties that may adversely affect our business, financial condition and results of operations and cash flows. Some of the more significant challenges and risks relating to an investment in our Company include, among other things, the following:
| • | the recent coronavirus (COVID-19) pandemic and the global attempt to contain it may harm our business, results of operations and ability to execute on our business plan; |
| • | our capitation arrangements may prove unprofitable if actual utilization rates exceed our assumptions; |
| • | our Payor contracts, including those with organizations that represent a significant portion of our business, are subject to renegotiation or termination which could result in a decrease in our revenue and profits; |
| • | we depend on reimbursements by Payors, which could lead to delays and uncertainties in the reimbursement process; |
| • | possible changes in the mix of patients and products and services provided, as well as Payor mix and payment methodologies, could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity; |
| • | if we are unable to provide consistently high quality of care, our business will be adversely impacted; |
| • | our reliance on relatively few vendors for the majority of our patient equipment and supplies and excise taxes which are to be imposed on certain manufacturers of such items could adversely affect our ability to operate; |
| • | the home healthcare industry is highly competitive and fragmented, with limited barriers to entry which may make it susceptible to vertical integration by manufacturers, Payors, providers (such as hospital systems) or disruptive new entrants; |
| • | we may be adversely affected by consolidation among health insurers and other industry participants; |
| • | there is an inherent risk of liability in the provision of healthcare services; damage to our reputation or our failure to adequately insure against losses, including from substantial claims and litigation, could have an adverse impact on our operations, financial condition, or prospects; |
| • | the current economic downturn, deepening of the economic downturn, continued deficit spending by the federal government or state budget pressures may result in a reduction in payments and covered services; |
| • | changes in home healthcare technology and/or product and therapy innovations may make the services we currently provide obsolete or less competitive; |
| • | reductions in Medicare, Medicaid and commercial Payor reimbursement rates could have a material adverse effect on our results of operations and financial condition; |
| • | if we fail to comply with applicable laws and regulations, we could suffer penalties or be required to make significant changes to our operations; |
| • | we have been, are and could become the subject of federal and state investigations and compliance reviews; |
| • | if we fail to maintain required licenses, certifications, or accreditation, or if we do not fully comply with requirements to provide notice to or obtain approval from regulatory authorities due to changes in our ownership structure or operation, it could adversely impact our operations; |
8
Table of Contents
| • | a cyber-attack, a security breach, or the improper disclosure or use of protected health information could cause a loss of confidential data, give rise to remediation and other expenses, expose us to liability under the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), consumer protection, common law or other legal theories, subject us to litigation and federal and state governmental inquiries, damage our reputation, and otherwise be disruptive to our business; and |
| • | our Sponsor and its affiliates control us and their interests may conflict with ours or yours in the future. |
Please see “Risk Factors” for a discussion of these and other factors you should consider before making an investment in shares of our common stock.
Our Organizational Structure
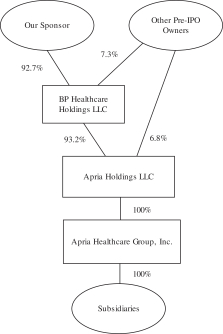
Existing Organizational Structure
The following diagram depicts our current organizational structure and equity ownership. Apria, Inc. is not pictured, as it was incorporated in connection with this offering and does not have any outstanding shares of capital stock. We currently conduct our business through Apria Healthcare Group and its subsidiaries. As described below, Apria, Inc. will become the holding company for Apria Healthcare Group. This diagram is provided for illustrative purposes only and does not show all of our legal entities.
Organizational Structure Following This Offering
At or prior to the completion of this offering, we will undertake the pre-IPO reorganization transactions so that Apria, Inc. will, through a newly formed direct subsidiary of Apria, Inc. (“Intermediate Holdco”), own all of the equity interests in Apria Healthcare Group and become the holding company of our business. More specifically, a newly formed direct subsidiary of Intermediate Holdco will merge with and into Apria Healthcare Group, with Apria Healthcare Group surviving. As a result, Apria Healthcare Group will become an indirect
9
Table of Contents
wholly owned subsidiary of Apria, Inc. Our pre-IPO owners, through their ownership of Apria Holdings LLC as the 100% direct owner of Apria Healthcare Group prior to such transaction, will receive an aggregate of 35,210,915 shares of newly issued common stock of Apria, Inc., assuming an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus. However, the precise number of shares of common stock issued to our pre-IPO owners, and accordingly the total number of our outstanding shares of stock, will differ if the actual initial offering price per share differs from this assumed price. For example, if the initial offering price of common stock in this offering is (i) $19.00 per share, which is the low point of the price range indicated on the front cover of this prospectus, we would issue 35,210,795 shares of common stock to our pre-IPO owners and (ii) $21.00 per share, which is the high point of the price range indicated on the front cover of this prospectus, we would issue 35,211,020 shares of common stock to our pre-IPO owners.
In connection with this offering, the stock appreciation rights (“SARs”) that are issued by Apria Healthcare Group will remain outstanding and will continue to vest and settle pursuant to their original terms, but will be amended to track the shares of Apria, Inc. common stock rather than the shares of common stock of Apria Healthcare Group. Following the completion of this pre-IPO reorganization, the SARs outstanding at Apria, Inc. will be 3,766,228, assuming an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus. However, the precise number of SARs in Apria, Inc. issued to our pre-IPO owners will differ if the actual initial offering price per share differs from this assumed price in order to preserve the value due to each holder. For example, if the initial offering price of common stock in this offering is (i) $19.00 per share, which is the low point of the price range indicated on the front cover of this prospectus, we would issue 3,766,397 SARs to our pre-IPO owners and (ii) $21.00 per share, which is the high point of the price range indicated on the front cover of this prospectus, we would issue 3,766,045 SARs to our pre-IPO owners.
Prior to the consummation of this offering, Apria Healthcare Group will be converted into a Delaware limited liability company.
Apria Holdings LLC, an entity controlled by our Sponsor, will be the direct and indirect 100% owner of Apria, Inc. and Apria Healthcare Group immediately prior to and immediately following the consummation of the merger. For this reason, the merger will be accounted for as a reorganization of entities under common control. As a result, the consolidated financial statements of Apria, Inc. will recognize the assets and liabilities received in the merger at their historical carrying amounts, as reflected in the historical consolidated financial statements of Apria Healthcare Group, the accounting predecessor. Following the consummation of these transactions in accordance with a master reorganization agreement, the form of which has been filed as an exhibit to the registration statement of which this prospectus forms a part, the selling stockholders will complete the offer and sale of Apria, Inc.’s common stock to investors in this offering.
We believe there are financing benefits in having an organizational structure with a holding company issuer above the entities that are subject to debt covenants and such structures are frequently used by corporate issuers. We do not expect that there will be any material benefit or detriment to our stockholders from our organizational structure after giving effect to the pre-IPO reorganization transactions, as all stockholders, including our pre-IPO owners and public stockholders, will hold their respective interest in a single class of our common stock and will be entitled to the same relative benefits or detriments.
The following diagram depicts our organizational structure and equity ownership immediately following
the pre-IPO reorganization transactions and this offering. This diagram is provided for illustrative purposes only and does not show all of our legal entities or ownership percentages of such entities.
10
Table of Contents
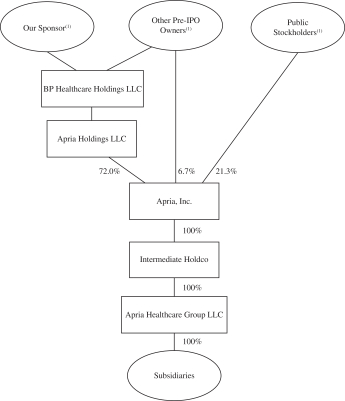
| (1) | After the completion of this offering, our Sponsor will beneficially own 72.0% of our outstanding common stock (or 68.8% if the underwriters exercise their option to purchase additional shares in full), our other pre-IPO owners will beneficially own 6.7% of our outstanding common stock (or 6.7% if the underwriters exercise their option to purchase additional shares in full) and public stockholders will beneficially own 21.3% of our outstanding common stock (or 24.5% if the underwriters exercise their option to purchase additional shares in full) assuming an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus. For additional information, see “Principal and Selling Stockholders.” |
In connection with this offering we intend to enter into (i) a stockholders agreement which among other rights, will provide our Sponsor with the right to require us to nominate a number of individuals designated by our Sponsor for election as our directors for as long as it retains significant ownership of us and (ii) a registration rights agreement, which will provide our Sponsor an unlimited number of “demand” registration rights and customary “piggyback” registration rights. The stockholders agreement will require us to nominate a number of individuals designated by our Sponsor (the “Sponsor Directors”) based on the beneficial ownership of our pre-IPO owners and their affiliates of our common stock entitled to vote generally in the election of our directors. The number of such Sponsor Directors will be the lowest whole number that is greater than 50% of the total number of directors comprising our board of directors if our pre-IPO owners and their affiliates beneficially own at least 50% of the shares of our outstanding common stock (and any securities convertible into, or exchangeable or exercisable for, such shares) entitled to vote generally in the election of our directors as of the record date for such meeting and such number will generally decrease proportionally as the beneficial ownership of our pre-IPO owners and their affiliates decrease, except that our Sponsor will continue to have the right to nominate the lowest whole number of directors that is at least 10% of the total number of directors for so long as our pre-IPO owners and their affiliates own at least 5% of our outstanding common stock. Accordingly, our Sponsor will have the right to designate 10% of the board of directors even though the pre-IPO owners and their affiliates may own less than 10% of the outstanding common stock (so long as they own at least 5%). After the completion of this offering, we expect that our Sponsor will have the right, but be under no obligation, to designate five members of our nine member board of directors as their nominees for election at our first meeting of stockholders
11
Table of Contents
following this offering (or such greater or lesser number of directors that would constitute the lowest whole number of directors that is greater than 50% of the total number of directors then comprising the board). See “Certain Relationships and Related Person Transactions” for additional information.
Apria, Inc. was incorporated in Delaware on March 22, 2018. Our principal executive offices are located at 7353 Company Drive, Indianapolis, Indiana 46237 and our telephone number is (800) 990-9799.
Recent Developments
Preliminary Estimated Unaudited Financial Results of Operations for the Year Ended December 31, 2020
The data presented below reflects our preliminary estimated unaudited financial results for the year ended December 31, 2020 based upon information available to us as of the date of this prospectus. In preparing our preliminary estimated unaudited financial results, we have utilized accounting estimates in a manner consistent with the description in Note 1 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus. However, this data is not a comprehensive statement of our financial results for the year ended December 31, 2020, and our actual results may differ materially from this preliminary estimated data, as our closing process and related audit have not been completed. During the preparation of our financial statements and related notes additional adjustments to the preliminary estimated financial information presented below may be identified. Any such adjustments may be material. Our independent registered public accounting firm, Deloitte & Touche LLP, has not audited, reviewed, compiled or performed any procedures with respect to this preliminary financial data and, accordingly, Deloitte & Touche LLP does not express an opinion or any other form of assurance with respect thereto.
Based upon such preliminary estimated financial results, we expect various key metrics for the year ended December 31, 2020, to be between the ranges set out in the following table, as compared to the year ended December 31, 2019:
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | |||||||||||
| (Estimated) | ||||||||||||
| (in thousands) | Low | High | ||||||||||
| Net revenues: |
||||||||||||
| Home respiratory therapy |
$ | 451,726 | $ | 453,826 | $ | 435,680 | ||||||
| OSA treatment |
452,407 | 454,407 | 438,572 | |||||||||
| NPWT |
42,766 | 42,966 | 42,122 | |||||||||
| Other equipment and services |
156,818 | 157,518 | 172,501 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net revenues |
$ | 1,103,717 | $ | 1,108,717 | $ | 1,088,875 | ||||||
| Net income |
$ | 41,956 | $ | 44,156 | $ | 15,622 | ||||||
| EBITDA |
$ | 183,378 | $ | 186,378 | $ | 138,991 | ||||||
| Adjusted EBITDA |
$ | 223,858 | $ | 226,858 | $ | 173,972 | ||||||
| Adjusted EBITDA less Patient Equipment Capex |
$ | 131,223 | $ | 134,223 | $ | 80,523 | ||||||
As of December 31, 2020, we expect cash and cash equivalents to be approximately $195 million and approximately $401 million of debt to be outstanding under the Term Loan A Facility after giving effect to the Incremental Term Loans. This amount is not presented net of unamortized debt issuance costs.
Estimated net income for the year ended December 31, 2020 of $42.0 million to $44.2 million is significantly greater than our net income for the nine months ended September 30, 2020 of $20.3 million
12
Table of Contents
primarily due to incurrence of one-time costs in the first nine months of the year for (x) an estimated probable loss recorded in relation to a series of civil investigative demands and (y) a special recognition bonus for front-line and other non-leadership employees. These costs were recorded in selling, distribution and administrative expenses in the first nine months of 2020.
EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex are non-GAAP measures and should not be considered in isolation, or as a substitute for our results as reported under GAAP. See “—Summary Historical Financial and Other Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Non-GAAP Financial Information” for discussion on how we define and calculate EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex and why we believe these measures are important.
The following table reconciles net income, the most directly comparable GAAP measure, to EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex:
| Year Ended December 31, | ||||||||||||
| 2020 | 2019 | |||||||||||
| (Estimated) | ||||||||||||
| (in thousands) | Low | High | ||||||||||
| Net income |
$ | 41,956 | $ | 44,156 | $ | 15,622 | ||||||
| Interest expense, net and other |
5,810 | 5,810 | 3,666 | |||||||||
| Income tax expense |
20,382 | 21,182 | 8,127 | |||||||||
| Depreciation and amortization |
115,230 | 115,230 | 111,576 | |||||||||
|
|
|
|
|
|
|
|||||||
| EBITDA |
$ | 183,378 | $ | 186,378 | $ | 138,991 | ||||||
| Strategic transformation initiatives: |
||||||||||||
| Simplify(a) |
1,159 | 1,159 | 11,775 | |||||||||
| Financial system(b) |
1,846 | 1,846 | — | |||||||||
| Other initiatives(c) |
465 | 465 | 834 | |||||||||
| Stock-based compensation and other(d) |
4,839 | 4,839 | 9,024 | |||||||||
| Legal settlement(e) |
28,891 | 28,891 | 12,200 | |||||||||
| Offering costs(f) |
3,280 | 3,280 | 1,148 | |||||||||
|
|
|
|
|
|
|
|||||||
| Adjusted EBITDA |
$ | 223,858 | $ | 226,858 | $ | 173,972 | ||||||
|
|
|
|
|
|
|
|||||||
| Patient Equipment Capex |
(92,635 | ) | (92,635 | ) | (93,449 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Adjusted EBITDA less Patient Equipment Capex |
$ | 131,223 | $ | 134,223 | $ | 80,523 | ||||||
|
|
|
|
|
|
|
|||||||
| (a) | Simplify represents one-time advisory fees and implementation costs associated with a key 2019 business transformation initiative focused on shifting to a patient-centric platform and optimizing end-to-end customer service. |
| (b) | Costs associated with the implementation of a new financial system. |
| (c) | Other initiatives include one-time third-party logistics advisory costs associated with a 24-month initiative launched in January 2018 designed to modify the branch network in order to reduce branch operating costs while maintaining or improving patient service levels, one-time costs associated with customer service initiatives, and one-time costs associated with implementation of an electronic sales, service and rental agreement. |
| (d) | Stock-based compensation has historically been granted to certain of our employees in the form of profit interest units of our parent and stock appreciation rights (“SARs”). For time-based vesting awards, we recognize a non-cash compensation expense based on the fair value of the awards determined at the date of grant over the requisite service period. In 2019, all outstanding performance Class B units were modified to |
13
Table of Contents
| accelerate vesting resulting in $7.0 million stock compensation expense. Other compensation includes long-term incentive compensation. |
| (e) | Represents the increase in the settlement amount in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York offset by a one-time unrelated $3.0 million recovery in 2020. See “Business—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York” and Note 8 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus for additional information. |
| (f) | Offering costs represent one-time costs relating to preparation for our initial public offering and accelerated implementation of new accounting standards. |
Settlement with SDNY Office
On December 18, 2020, a federal judge approved a civil and administrative settlement Apria recently entered into with the United States and state Medicaid programs, in a complaint filed by three relators under the qui tam provisions of the False Claims Act (“FCA”), 31 U.S.C. § 3729 et seq., as well as comparable state false claims laws, in connection with the rental of non-invasive ventilators (“NIVs”). Apria also entered into separate settlements to resolve the relators’ claims brought on behalf of the States of California and Illinois related to NIV covered by private insurers. The matter had been pending since 2017.
The government had alleged that Apria violated the FCA by submitting false claims seeking reimbursement for NIVs which were not being used, or not being used sufficiently, by patients, for NIVs which were being used pursuant to physician orders on a device setting which was available from other less expensive devices, and for improperly waiving co-pays to induce beneficiaries to rent NIVs. To resolve any potential liability, Apria agreed to enter a civil settlement agreement and to pay $40 million to the federal government and the states. Apria also agreed with the California Department of Insurance to pay $500,000 to resolve claims asserted by the relators under the California Insurance Frauds Prevention Act, Cal. Ins. Code § 1871 et seq. Apria separately agreed with the relators to settle all remaining claims from their complaint, including: (1) claims for retaliation in violation of federal and state laws; (2) claims for attorneys’ fees and costs available under federal and state law; and (3) claims under the Illinois Insurance Claims Fraud Prevention Act, 740 Ill. Comp. Stat. 92/1 et seq. Apria did not admit that any of its conduct was illegal or otherwise improper.
As part of the federal and state Medicaid settlement, Apria also entered into a five-year corporate integrity agreement (the “Corporate Integrity Agreement” or “CIA”) with the Office of Inspector General of the U.S. Department of Health and Human Services (“HHS OIG”). The CIA requires Apria to maintain its ongoing corporate compliance program and obligates Apria to implement or continue, as applicable, a set of defined corporate integrity activities for a period of five years from the effective date of the CIA. Among other things, the CIA requires Apria to impose certain oversight obligations on Apria’s board of directors; provide certain management certifications; continue or implement, as applicable, certain compliance training and education; and engage an Independent Review Organization to perform certain reviews. The CIA also includes certain reporting, certification, record retention, and notification requirements. In the event of a breach of the CIA, Apria could become liable for payment of certain stipulated penalties or could be excluded from participation in federal healthcare programs.
Credit Facility Amendment and Dividend
On December 11, 2020, we entered into an amendment (the “Credit Facility Amendment”) to our credit agreement to incur $260.0 million of incremental term loans (the “Incremental Term Loans”). Net proceeds from the Incremental Term Loans were used to fund a $200.3 million dividend payment to our stockholders and a $9.7 million distribution to SARs holders declared and paid in December 2020, with the remaining proceeds used to pay fees and expenses in connection with the Credit Facility Amendment and for general corporate purposes.
14
Table of Contents
The Offering
| Common stock offered by the selling stockholders |
7,500,000 shares. |
| Option to purchase additional shares from the selling stockholders |
The selling stockholders have granted the underwriters an option for a period of 30 days to purchase up to 1,125,000 additional shares of common stock from the selling stockholders. |
| Common stock outstanding after giving effect to this offering |
35,210,915 shares assuming an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus. See “—Our Organizational Structure—Organizational Structure Following This Offering.” |
| Use of proceeds |
We will not receive any proceeds from the sale of the shares of common stock offered by the selling stockholders (including any sales pursuant to the underwriters’ option to purchase additional shares from the selling stockholders). |
| Dividend policy |
We have no current plans to pay dividends on our common stock following this offering. Any decision to declare and pay dividends in the future will be made at the sole discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions and other factors that our board of directors may deem relevant. Because we are a holding company and have no direct operations, we will only be able to pay dividends from funds we receive from our subsidiaries. In addition, our ability to pay dividends will be limited by covenants in our existing indebtedness and may be limited by the agreements governing any indebtedness we or our subsidiaries may incur in the future. See “Dividend Policy.” |
| Risk factors |
See “Risk Factors” for a discussion of risks you should carefully consider before deciding to invest in our common stock. |
| Proposed trading symbol |
“APR” |
In this prospectus, unless otherwise indicated, the number of shares of common stock outstanding and the other information based thereon is based on 35,210,915 shares outstanding as of the date of this prospectus, after giving effect to the pre-IPO reorganization transactions and this offering, and does not reflect:
| • | 2,594,224 shares that would be issuable upon settlement of outstanding stock appreciation rights granted pursuant to the 2015 Plan (as defined herein) (“SARs”) if such SARs were vested and exercised at the time of this offering (assuming an offering price of $20.00 per share of common stock, which is the midpoint of the price range set forth on the cover of this prospectus). At the time of this offering, there will be 3,766,228 SARs outstanding under the 2015 Plan with a weighted average strike price of $6.22. See “Executive Compensation—Long-Term Equity Incentive Compensation—Stock Appreciation Rights of Apria Healthcare Group”; |
15
Table of Contents
| • | Awards granted under the 2019 LTIP (as defined herein) with a maximum value of $4.4 million (or 220,000 shares assuming an offering price of $20.00 per share of common stock, which is the midpoint of the price range set forth on the cover of this prospectus, which such shares will be issued pursuant to the Apria, Inc. 2021 Omnibus Incentive Plan (the “Omnibus Incentive Plan”)). See “Executive Compensation—Long-Term Cash Incentive Compensation—2019 LTIP”; and |
| • | 3,912,324 shares of common stock that may be granted under the Omnibus Incentive Plan. See “Executive Compensation—Equity Incentive Plans—Omnibus Incentive Plan,” including: |
| • | The award described under “Executive Compensation—Long-Term Equity Incentive Compensation—Chief Financial Officer RSU Award” with a value of $3.2 million (or 159,977 shares), assuming an offering price of $20.00 per share of common stock, which is the midpoint of the price range set forth on the cover of this prospectus. |
16
Table of Contents
Summary Historical Financial and Other Data
We derived the summary statement of income data for the years ended December 31, 2019, 2018 and 2017 and the summary balance sheet data as of December 31, 2019 and 2018 from our audited consolidated financial statements included elsewhere in this prospectus. We derived the summary balance sheet data as of December 31, 2017 from our audited consolidated financial statements that are not included in this prospectus. The summary statement of income data for the nine months ended September 30, 2020 and 2019 and the summary balance sheet data as of September 30, 2020 were derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus. The unaudited condensed consolidated financial statements have been prepared on the same basis as our audited consolidated financial statements and, in the opinion of our management, reflect all normal recurring adjustments necessary for the fair statement of our consolidated results for these periods. The results for any interim period are not necessarily indicative of the results that may be expected for the full year. Our historical results are not necessarily indicative of the results expected for any future period.
You should read the summary historical financial data below, together with the consolidated financial statements and related notes thereto appearing elsewhere in this prospectus, as well as “Selected Historical Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the other financial information included elsewhere in this prospectus.
On January 1, 2019, we adopted Financial Accounting Standards Board (“FASB”) Accounting Standards Update (“ASU”) No. 2016-02, Leases (“Topic 842”), using the modified retrospective transition method. For lessor accounting, upon adoption the provision for doubtful accounts associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now charged to net rental revenue instead of general and administrative expense. For lessee accounting, upon adoption total assets and total liabilities increased $74.4 million as of January 1, 2019.
17
Table of Contents
The comparative information for periods prior to adoption has not been restated and continues to be reported under the accounting standards in effect for those periods.
| Nine Months Ended September 30, |
Year Ended December 31, | |||||||||||||||||||
| (in thousands, except share and per share data) | 2020 | 2019 | 2019 | 2018 | 2017 | |||||||||||||||
| Summary Statement of Income Data: |
||||||||||||||||||||
| Net revenues: |
||||||||||||||||||||
| Fee-for-service arrangements(1)(2) |
$ | 646,630 | $ | 644,624 | $ | 870,344 | $ | 898,622 | $ | 866,397 | ||||||||||
| Capitation |
168,298 | 163,086 | 218,531 | 212,261 | 207,413 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total net revenues |
814,928 | 807,710 | 1,088,875 | 1,110,883 | 1,073,810 | |||||||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Cost of net revenues |
||||||||||||||||||||
| Product and supply costs |
141,563 | 154,591 | 206,067 | 218,099 | 200,621 | |||||||||||||||
| Patient equipment depreciation |
75,840 | 72,588 | 97,386 | 108,340 | 101,724 | |||||||||||||||
| Home respiratory therapists costs |
12,848 | 14,844 | 19,560 | 20,371 | 20,802 | |||||||||||||||
| Other |
13,669 | 13,043 | 17,701 | 13,276 | 9,398 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total cost of net revenues |
243,920 | 255,066 | 340,714 | 360,086 | 332,545 | |||||||||||||||
| Provision for doubtful accounts(1)(2) |
— | — | — | 31,719 | 42,672 | |||||||||||||||
| Selling, distribution and administrative |
534,110 | 537,546 | 720,746 | 698,681 | 672,442 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
778,030 | 792,612 | 1,061,460 | 1,090,486 | 1,047,659 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating income |
36,898 | 15,098 | 27,415 | 20,397 | 26,151 | |||||||||||||||
| Interest expense |
4,047 | 3,345 | 5,112 | 1,338 | 1,246 | |||||||||||||||
| Interest income and other |
(451 | ) | (1,552 | ) | (1,446 | ) | (897 | ) | (486 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Income before income taxes |
33,302 | 13,305 | 23,749 | 19,956 | 25,391 | |||||||||||||||
| Income tax expense (benefit) |
13,034 | 3,465 | 8,127 | 6,829 | (71,560 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net income(3)(4) |
$ | 20,268 | $ | 9,840 | $ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic and diluted earnings per share: |
||||||||||||||||||||
| Net income per share |
$ | 20.42 | $ | 9.91 | $ | 15.74 | $ | 13.22 | $ | 97.66 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Weighted average shares outstanding: |
||||||||||||||||||||
| Basic and diluted |
992,719 | 992,719 | 992,719 | 992,719 | 992,719 | |||||||||||||||
| Pro Forma Earnings Per Share Information(5): |
||||||||||||||||||||
| Pro forma basic earnings per share: |
$ | 0.58 | $ | 0.44 | ||||||||||||||||
| Pro forma diluted earnings per share |
$ | 0.54 | $ | 0.42 | ||||||||||||||||
| Pro forma basic weighted average shares outstanding |
35,210,915 | 35,210,915 | ||||||||||||||||||
| Pro forma diluted weighted average shares outstanding |
37,525,139 | 37,565,139 | ||||||||||||||||||
18
Table of Contents
| September 30, | December 31, | |||||||||||||||
| (in thousands) | 2020 | 2019 | 2018 | 2017 | ||||||||||||
| Summary Balance Sheet Data: |
||||||||||||||||
| Total assets |
$ | 645,051 | $ | 617,153 | $ | 576,222 | $ | 643,553 | ||||||||
| Total liabilities(3)(6) |
488,812 | 482,637 | 290,970 | 298,049 | ||||||||||||
| Total stockholders’ equity(7) |
156,239 | 134,516 | 285,252 | 345,504 | ||||||||||||
| Nine Months Ended September 30, |
Year Ended December 31, | |||||||||||||||||||
| (in thousands) | 2020 | 2019 | 2019 | 2018 | 2017 | |||||||||||||||
| Operational and Other Data: |
||||||||||||||||||||
| EBITDA(8) |
$ | 123,813 | $ | 98,348 | $ | 138,991 | $ | 145,386 | $ | 145,448 | ||||||||||
| Adjusted EBITDA(8) |
162,765 | 118,963 | 173,972 | 155,727 | 153,340 | |||||||||||||||
| Adjusted EBITDA less Patient Equipment Capex(8) |
99,283 | 45,562 | 80,523 | 45,579 | 3,417 | |||||||||||||||
| (1) | The decrease in net revenue from fee-for-service arrangements for the year ended December 31, 2019 was primarily driven by the impact of adopting Topic 842. Upon adoption the provision for doubtful accounts associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now charged to net rental revenue instead of general and administrative expense. |
| (2) | On January 1, 2018, we adopted Topic 606, using the modified retrospective method. Upon adoption, patient co-pay and deductible amounts that were previously included in the provision for doubtful accounts related to implicit price concessions, which were $9.5 million for the year ended December 31, 2018, are now presented as an offsetting adjustment to the related fee-for-service sale net revenues. The comparative information for periods prior to adoption has not been restated and continues to be reported under the accounting standards in effect for those periods. |
| (3) | For the nine months ended September 30, 2020 and year ended December 31, 2019, net income reflects an expense of $32.5 million and $12.2 million, respectively, representing the estimated probable loss in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York. See “Business—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York” and Note 8 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus for additional information. |
| (4) | For the year ended December 31, 2017, net income reflects the net valuation allowance release of $105.1 million on the basis of our reassessment of the amount of deferred tax assets that are more likely than not to be realized, offset by a charge to tax expense of $24.2 million due to the re-measurement of deferred tax assets and liabilities resulting from the decrease in federal corporate tax rate as a result of the enactment of the Tax Cuts and Jobs Act in December 2017. |
| (5) | Unaudited basic pro forma earnings per share is computed using historical net income for the period divided by the pro forma weighted average number of common shares outstanding during the period after giving effect to the pre-IPO reorganization transactions. Pro forma diluted earnings per share is computed using the weighted average number of common shares and the effect of dilutive equity awards after giving effect to the pre-IPO reorganization transactions. |
| (6) | On June 21, 2019, we entered into a credit agreement with Citizens Bank and a syndicate of lenders for both a Term Loan A Facility (the “TLA”) of $150.0 million and a Revolving Credit Facility (the “Revolver” and together with the TLA and the Credit Facility Amendment, the “Credit Facility”) of $100.0 million. Proceeds from the TLA were used to fund the dividend payment to common stockholders and SARs holders in June 2019. |
| (7) | In June 2019, we declared and paid a $175.0 million dividend to common stockholders and SARs holders. In June 2018, we declared $75.0 million in dividends paid to common stockholders on July 5, 2018. |
19
Table of Contents
| (8) | EBITDA is a non-GAAP measure that represents net income for the period before the impact of interest income, interest expense, income taxes, and depreciation and amortization. EBITDA is widely used by securities analysts, investors and other interested parties to evaluate the profitability of companies. EBITDA eliminates potential differences in performance caused by variations in capital structures, tax positions, the cost and age of tangible assets and the extent to which intangible assets are identifiable. Adjusted EBITDA is a non-GAAP measure that represents EBITDA before certain items that impact comparison of the performance of our businesses either period-over-period or with other businesses. We use Adjusted EBITDA as a key profitability measure to assess the performance of our businesses. We believe that Adjusted EBITDA should, therefore, be made available to securities analysts, investors and other interested parties to assist in their assessment of the performance of our businesses. Adjusted EBITDA less Patient Equipment Capex is a non-GAAP measure that represents Adjusted EBITDA less purchases of patient equipment net of dispositions (“Patient Equipment Capex”). For purposes of this metric, Patient Equipment Capex is measured as the value of the patient equipment received less the net book value of dispositions of patient equipment during the accounting period. This metric is useful in evaluating the financial performance of the Company as the business requires significant capital expenditures to maintain its patient equipment fleet due to asset replacement and contractual commitments. We believe that Adjusted EBITDA less Patient Equipment Capex should, therefore, be made available to securities analysts, investors, and other interested parties to assist in their assessment of the performance of our businesses. |
Below, we have provided a reconciliation of EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex to our net income, the most directly comparable financial measure calculated and presented in accordance with GAAP. EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex should not be considered alternatives to net income or any other measure of financial performance calculated and presented in accordance with GAAP. Our EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex may not be comparable to similarly titled measures of other organizations because other organizations may not calculate EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex in the same manner as we calculate these measures.
Our uses of EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex have limitations as analytical tools, and you should not consider them in isolation or as a substitute for analysis of our results as reported under GAAP. Some of these limitations are:
| • | although depreciation and amortization are noncash charges, the assets being depreciated and amortized may have to be replaced in the future, EBITDA and Adjusted EBITDA do not reflect capital expenditure requirements for such replacements or other contractual commitments; |
| • | EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex do not reflect changes in, or cash requirements for, our working capital needs; |
| • | EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex do not reflect the interest expense or the cash requirements necessary to service interest or principal payments on our indebtedness; and |
| • | other companies, including companies in our industry, may calculate EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex measures differently, which reduces their usefulness as a comparative measure. |
EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex exclude items that can have a significant effect on our profit or loss and should, therefore, be used in conjunction with, not as substitutes for, profit or loss for the period. We compensate for these limitations by separately monitoring net income from continuing operations for the period.
20
Table of Contents
The following table reconciles net income, the most directly comparable GAAP measure, to EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex:
| Nine Months Ended September 30, |
Year Ended December 31, | |||||||||||||||||||
| (in thousands) | 2020 | 2019 | 2019 | 2018 | 2017 | |||||||||||||||
| Net income |
$ | 20,268 | $ | 9,840 | $ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||||||
| Interest expense, net and other |
3,596 | 1,793 | 3,666 | 441 | 760 | |||||||||||||||
| Income tax expense (benefit) |
13,034 | 3,465 | 8,127 | 6,829 | (71,560 | ) | ||||||||||||||
| Depreciation and amortization |
86,915 | 83,250 | 111,576 | 124,989 | 119,297 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| EBITDA |
$ | 123,813 | $ | 98,348 | $ | 138,991 | $ | 145,386 | $ | 145,448 | ||||||||||
| Strategic transformation initiatives: |
||||||||||||||||||||
| Branch network optimization(a) |
$ | — | $ | 566 | $ | 570 | $ | 1,367 | $ | — | ||||||||||
| Customer service optimization(b) |
99 | 238 | 264 | 789 | — | |||||||||||||||
| E-commerce platform(c) |
— | — | — | 342 | 432 | |||||||||||||||
| Simplify(d) |
1,159 | 10,926 | 11,775 | — | — | |||||||||||||||
| Financial systems(e) |
1,414 | — | — | — | — | |||||||||||||||
| eImpact(f) |
— | — | — | — | 3,742 | |||||||||||||||
| Technology investments(g) |
— | — | — | — | 1,997 | |||||||||||||||
| Stock-based compensation and other(h) |
1,929 | 8,057 | 9,024 | 1,621 | 1,721 | |||||||||||||||
| Legal expense(i) |
32,525 | — | 12,200 | — | — | |||||||||||||||
| Offering costs(j) |
1,826 | 828 | 1,148 | 6,222 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Adjusted EBITDA |
$ | 162,765 | $ | 118,963 | $ | 173,972 | $ | 155,727 | $ | 153,340 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Patient Equipment Capex |
(63,482 | ) | (73,401 | ) | (93,449 | ) | (110,148 | ) | (149,923 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Adjusted EBITDA less Patient Equipment Capex |
$ | 99,283 | $ | 45,562 | $ | 80,523 | $ | 45,579 | $ | 3,417 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (a) | Branch network optimization represents one-time, third-party logistics advisory costs associated with a 24-month initiative launched in January 2018 designed to modify the branch network in order to reduce branch operating costs while maintaining or improving patient service levels. |
| (b) | Customer service optimization primarily represents one-time costs associated with customer service initiatives, as well as evaluating a strategic partnership expansion for certain aspects of customer service. |
| (c) | E-commerce platform primarily represents one-time costs associated with developing and launching our e-commerce platform. |
| (d) | Simplify represents one-time advisory fees and implementation costs associated with a key 2019 business transformation initiative focused on shifting to a patient-centric platform and optimizing end-to-end customer service. |
| (e) | Costs associated with the implementation of a new financial system. |
| (f) | eImpact represents one-time advisory and implementation fees incurred in 2016 and 2017 as part of a 24-month cost saving initiative focused on identifying opportunities to improve branch operational and sales effectiveness, optimize training, reduce corporate overhead and eliminate unnecessary expenses. |
| (g) | Technology investments represent various one-time costs associated with order-to-cash process improvements, patient monitoring, implementation of driver productivity and safety tools, and offshoring of certain revenue management capabilities. |
| (h) | Stock-based compensation has historically been granted to certain of our employees in the form of profit interest units of our parent and stock appreciation rights (“SARs”). For time-based vesting awards, we recognize a non-cash compensation expense based on the fair value of the awards |
21
Table of Contents
| determined at the date of grant over the requisite service period. In June 2019, all outstanding performance Class B units were modified to accelerate vesting resulting in $7.0 million stock compensation expense. Other compensation includes long-term incentive compensation. |
| (i) | Legal expense represents an accrual of the estimated probable loss in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York. See “Business—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York” and Note 8 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus for additional information. |
| (j) | Offering costs represent one-time costs relating to preparation for our initial public offering and accelerated implementation of new accounting standards. |
22
Table of Contents
An investment in shares of our common stock involves risks. You should carefully consider the following information about these risks, together with the other information contained in this prospectus, before investing in shares of our common stock.
Risks Related to Our Business and Operations
The recent coronavirus (COVID-19) pandemic and the global attempt to contain it may harm our business, results of operations and ability to execute on our business plan.
The global spread of the novel strain of coronavirus (“COVID-19”) and the various efforts to contain it have created significant volatility, uncertainty, and economic disruption. In response to government mandates, healthcare advisories, and in order to respond to employee, customer, and supplier concerns, we have altered certain aspects of our operations, including acquisition and distribution of personal protective equipment (PPE) to our patient-facing employees, accelerated capital expenditures of certain products and relocation of significant portions of our workforce to “work-from-home” status. Our workforce has had to spend a significant amount of time working from home, which impacts their productivity. While many of our operations can be performed remotely, there is no guarantee that we have been (or will be) as effective given that our team is dispersed, many employees may have additional personal needs to attend to (such as looking after children as a result of school closures or family who become sick), and employees may become sick themselves and unable to work. While the impact of the COVID-19 pandemic did not have a material adverse impact on our consolidated operating results for the nine months ended September 30, 2020, we have experienced declines in net revenues in certain services associated with elective medical procedures and the disruption in physician practices (such as commencement of new CPAP services, ventilation therapy and negative pressure wound therapy), and such declines may continue during the duration of the COVID-19 pandemic. These declines were offset by an increase in net revenue related to increased demand for certain respiratory products (such as oxygen), increased sales in our resupply businesses and the one-time sale of certain respiratory equipment to hospitals. There is no guarantee that these offsetting increases in revenue will continue during the duration of the COVID-19 pandemic. In response, we have instituted temporary cost mitigation measures such as reduced hours and furloughs for a small subset of our impacted workforce. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Impact of COVID-19 Pandemic.” Similarly, our suppliers and vendors have had their operations altered. COVID-19 has impacted manufacturing in the regions where some of our vendors manufacture their products. While the global closures and limitations on movement related to COVID-19 pandemic are expected to be temporary, and while such closures, limitations, and related impacts have not materially disrupted our supply chain to date, such supply chain disruption remains possible and the financial impact of any such disruption cannot be estimated at this time. Should such closures and limitations on movement continue for an extended period of time, the impact on our supply chain could materially and adversely affect our business and results of operations. To the extent the resulting economic disruption is severe, we could see some of our suppliers and vendors go out of business, resulting in supply and/or service constraints as well as increased costs or delays in meeting the needs of our patients. We may also see a slowdown in cash collections if our customers are unable to pay their medical bills as a result of worsening economic conditions.
The full extent to which the COVID-19 pandemic and the various responses to it impact our business, operations and financial results will depend on numerous other evolving factors that we are not able to predict, including:
| • | the duration and scope of the pandemic; |
| • | governmental, business and individuals’ actions that have been and continue to be taken in response to the pandemic; |
| • | the availability of, and cost to access, the capital markets; |
| • | our ability to pursue, conduct diligence regarding, finance and integrate acquisitions; |
23
Table of Contents
| • | our ability to comply with financial and operating covenants in our debt and operating lease agreements; |
| • | potential for intangible asset impairment charges; |
| • | the increased cost of and loss of efficiency associated with additional precautions and personal protective equipment required to engage in in-person interactions with and provide care to patients infected or potentially infected with COVID-19; |
| • | the effect on our patients and physician/facility referral sources, and the demand for and ability to pay for healthcare services; |
| • | disruptions or restrictions on our employees’ ability to travel and to work, including as a result of their health and well-being; |
| • | availability of third-party providers to whom we outsource portions of our internal business functions, including billing, collections, administrative and information systems and other services; and |
| • | increased cybersecurity risks as a result of remote working conditions. |
During the COVID-19 pandemic, we may not be able to provide the same level of service and products that our patients and physicians/facility referral sources are used to, which could negatively impact their perception of our products and/or services. Furthermore, given increased government expenditures associated with its response to the COVID-19 pandemic, we could see increased government obligations which could negatively impact reimbursement rates, and accordingly, our results of operations.
We will continue to actively monitor the issues raised by the COVID-19 pandemic and may take further actions that alter our business operations, as may be required by federal, state, or local authorities, or that we determine are in the best interests of our patients, employees, customers, and stockholders. It is not clear what the potential effects any such alterations or modifications may have on our business, including the effects on our customers, suppliers or vendors, or on our financial results.
The potential effects of the COVID-19 pandemic could also heighten the risks disclosed in many of our risk factors described below, including as a result of, but not limited to, the factors described above. Because the COVID-19 pandemic is unprecedented and continuously evolving, the other potential impacts to our risk factors that are further described below are uncertain.
Our capitation arrangements may prove unprofitable if actual utilization rates exceed our assumptions.
From time to time, we enter into capitation arrangements with commercial Payors pursuant to which they agree to pay us a set amount (on a per member per month basis for a defined patient population) without regard to the actual services provided. Capitation revenues represented approximately 20% of our total net revenues for 2019. We negotiate the contractual rates in these arrangements with Payors based on assumptions regarding average expected utilization of services. If actual utilization rates exceed our assumptions, the profitability of such arrangements may be diminished. Moreover, we may be obligated to perform under such capitation arrangements even if the contractual reimbursement rates are insufficient to cover our costs based on actual levels of utilization.
Our Payor contracts, including those with organizations that represent a significant portion of our business, are subject to renegotiation or termination which could result in a decrease in our revenue and profits.
From time to time, our Payor contracts are amended (sometimes through unilateral action by Payors regarding payment policy), renegotiated, subjected to a bidding process with our competitors, or terminated altogether. Sometimes in the renegotiation process, certain lines of business may not be renewed or a Payor may enlarge its provider network or otherwise adversely change the way it conducts its business with us. In other cases, a Payor may reduce its provider network in exchange for lower payment rates. Our revenue from a Payor
24
Table of Contents
may also be adversely affected if the Payor alters its utilization management expectations and/or administrative procedures for payments and audits, changes its order of preference among the providers to which it refers business or imposes a third-party administrator, network manager or other intermediary. Any reduction in our projected revenues as a result of these or other factors could also lead to impairment of the value of our intangible assets which would result in a decrease in these assets on our balance sheet. We cannot assure you that our Payor contracts will not be terminated or altered in ways that are unfavorable to us as a result of renegotiation or such administrative changes. Terminations or alterations of contracts, particularly those that are concentrated with organizations that represent a significant portion of our business, such as Kaiser Foundation Health Plan, Inc., which represented approximately 23% of our revenues for the year ended December 31, 2019, could have a material effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. Payors may also decide to refer business to their owned provider subsidiaries. Some Payors have developed or acquired, or may in the future develop or acquire, an ownership interest in our competitors or administrative intermediaries. These activities could materially reduce our revenue from these Payors.
Possible changes in the mix of patients and products and services provided, as well as Payor mix and payment methodologies, could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our revenues are determined by a number of factors, including our mix of patients, the rates of payment among Payors and the mix of our products and services provided. A shift towards Payors with lower prices, or from higher gross margin products to lower gross margin products, would reduce our gross margins. Changes in the mix of our patients, products and services provided, payment methodologies or the Payor mix among Medicare, Medicaid and commercial Payors could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
We depend on reimbursements by Payors, which could lead to delays and uncertainties in the reimbursement process.
We receive a substantial portion of our payments for our products and services from Payors, including Medicare and Medicaid, national and regional insurers and managed care organizations. For the year ended December 31, 2019, approximately 19% and 1% of our net revenues were reimbursed by the Medicare and state Medicaid programs, respectively.
The reimbursement process is complex and can involve lengthy delays. Payors continue their efforts to control expenditures for healthcare products and services, including proposals to revise reimbursement policies. While we generally recognize revenue on the date of delivery of equipment to the patient, or as a result of entering into a contract in the case of capitation revenue without regard to the actual services provided, there can be delays before we receive actual payment for these products and services. In addition, Payors may disallow, in whole or in part, requests for reimbursement based on determinations that certain amounts are not reimbursable under plan coverage, that products or services provided were not medically necessary, or that additional supporting documentation is necessary, for one or more reasons, such as retroactive membership status change. Recoupments and retroactive adjustments may change amounts realized from Payors. Certain Payors have filing deadlines and will not pay claims submitted after such deadlines. We are subject to audits of our reimbursement claims under Medicare, Medicaid, and other governmental programs and may be required to repay these agencies if found that we were incorrectly reimbursed. Delays and uncertainties in the reimbursement process may adversely affect our financial position or results and increase the overall costs of our collection efforts.
Risks associated with collecting reimbursement from Payors and the inability to monitor and manage accounts receivable successfully, including estimating the collectability of certain accounts receivable, could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
25
Table of Contents
If we are unable to provide consistently high quality of care, our business will be adversely impacted.
Providing quality patient care is the cornerstone of our business. We believe that hospitals, physicians and other referral sources refer patients to us in large part because of our reputation for delivering quality care. Clinical quality is becoming increasingly important within our industry. Medicare imposes a financial penalty upon hospitals that have excessive rates of patient readmissions within 30 days from hospital discharge. We believe this provides a competitive advantage to home healthcare providers who can differentiate themselves based upon quality, particularly by achieving low patient acute care hospitalization readmission rates and by implementing disease management programs designed to be responsive to the needs of patients served by referring hospitals. We are focused on improving our patient outcomes. If we should fail to attain our goals regarding patient acute care hospitalization readmission rates and other quality metrics, we expect our ability to generate referrals would be adversely impacted, which could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Further, we may need to increase costs, including for clinical labor, to provide our services at appropriate quality levels, which could lead to lower margins.
Our failure to establish and maintain relationships with hospital and physician referral sources may cause our revenue to decline.
We do not have contracts or exclusive arrangements with most hospitals or physicians. Instead, we attempt to work closely with hospitals and physicians to accept discharges and referrals of their patients who require our services. Therefore, our success is significantly dependent on referrals from hospital and physician sources. If we are unable to successfully establish new referral sources and maintain strong relationships with our current referral sources, or if efforts to increase the skill level and effectiveness of our sales force fail, our revenues may decline. In addition, our relationships with referral sources are subject to federal and state healthcare laws such as the federal Anti-Kickback Statute (“AKS”) and the Stark Law, and compliance with these laws limits the scope of our relationships with our referral sources.
Our failure to successfully design, modify and implement technology and other process changes to maximize productivity and ensure compliance could ultimately have a significant negative impact on our results of operations and financial condition.
We have identified a number of areas throughout our operations where we have modified or intend to modify the current processes or systems in order to attain a higher level of productivity or ensure compliance. The ultimate cost savings expected from the successful design and implementation of such initiatives will be necessary to help offset the impact of Medicare and Medicaid reimbursement reductions and continued downward pressure on pricing. Additionally, Medicare and Medicaid often change their documentation requirements. CMS has taken steps to support electronic data interchange processes, as well as to implement electronic clinical templates and suggested clinical data elements for documenting face-to-face encounters, detailed written orders and written orders prior to delivery, and laboratory test results for certain DMEPOS items. The standards and rules for healthcare transactions, code sets and unique identifiers also continue to evolve, such as ICD-10 and HIPAA 5010 and other data security requirements. Moreover, government programs and/or commercial Payors may have difficulty administering new standards and rules for healthcare transactions and this may adversely affect timelines of payment or payment error rates. Our failure to successfully design and implement system or process modifications could have a significant impact on our business, results of operations and financial condition. The implementation of new standards and rules may require us to make substantial investments. Further, the implementation of these system or process changes could have a disruptive effect on related transaction processing and operations. If our implementation efforts related to systems development are unsuccessful, we may need to write off amounts that we have capitalized related to systems development projects. Additionally, if systems development implementations do not occur, we may need to incur additional costs to support our existing systems.
26
Table of Contents
Our failure to maintain controls and processes over billing and collections, including impacts from the outsourcing or offshoring of parts of our billing and collections activities, estimating the collectability of our accounts receivable or the deterioration of the financial condition of our Payors, could have a significant negative impact on our results of operations and financial condition.
The collection of accounts receivable is one of our most significant challenges and requires constant focus and involvement by management and ongoing enhancements to information systems and billing center operating procedures. Further, some of our patients and Payors may experience financial difficulties, or may otherwise not pay accounts receivable when due, resulting in increased write-offs. The COVID-19 pandemic may exacerbate many of these conditions. See “—The recent coronavirus (COVID-19) pandemic and the global attempt to contain it may harm our business, results of operations and ability to execute on our business plan.” There can be no assurance that we will be able to maintain our current levels of collectability in future periods or accurately estimate the collectability of accounts receivable. If we are unable to properly bill and collect our accounts receivable, our results will be adversely affected. From time to time, we are involved in disputes with various parties, including Payors and their intermediaries regarding their performance of various contractual or regulatory obligations. These disputes may lead to legal and other proceedings and may cause us to incur costs or experience delays in collections, increases in accounts receivable or loss of revenue. In addition, in the event such disputes are not resolved in our favor and/or result in the termination of our relationships with such parties, there may be an adverse impact on our financial condition and results of operations.
In addition, we have an outsourcing strategy in place with respect to certain billing, collection, administrative and information systems and other services. When permitted, we currently have several offshore and domestic business process outsourcing and services firms to assist with implementing this strategy. There is intense competition around the world for skilled technical professionals and we expect that competition to increase, which could result in our outsourcing strategy not achieving its intended benefits. Operations in other parts of the world involve certain regional geopolitical risks that are different as compared to operating in the United States, including the possibility of civil unrest, terrorism, and substantial regulation by the individual governments. These factors may cause disruptions in our business processes, which could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. We also may experience negative reactions from some patients, providers and Payors, as a result of the actual or perceived disruption caused by the outsourcing of portions of our business operations.
Our reliance on relatively few vendors for the majority of our patient equipment and supplies and which are to be imposed on certain manufacturers of such items could adversely affect our ability to operate.
We currently rely on a relatively small number of vendors to provide us with the majority of our patient equipment and supplies, with five of our vendors providing approximately 81% of our total service equipment and supply purchases in the year ended December 31, 2019. From time to time, we also enter into certain exclusive arrangements with a given vendor for the provision of patient equipment and supplies. Further, some of our supply agreements contain pricing scales that depend on meeting certain order volumes. Our inability to procure certain equipment and supplies, including as a result of failure to maintain and renew certain agreements and access arrangements, could have a materially adverse effect on our results of operations. We often use vendors selectively for quality and cost reasons. Significant price increases, or disruptions in the ability to obtain such equipment and supplies from existing vendors, may force us to increase our prices (which we may be unable to do) or reduce our margins and could force us to use alternative vendors. In response to the declaration of a public health emergency due to the COVID-19 pandemic, CMS temporarily suspended all accreditation and reaccreditation activities for DMEPOS suppliers. However, as of July 6, 2020, CMS resumed all accreditation and reaccreditation activities, including surveys, which may be conducted on-site, virtually or a combination of both depending on each state’s reopening plan. Similarly, CMS-approved accreditation organizations, such as The Joint Commission, temporarily suspended survey and review activities, but resumed such activities in June 2020.
27
Table of Contents
Any change in the existing vendors we use could cause delays in the delivery of products and possible losses in revenue, which could adversely affect our results of operations. In addition, alternative vendors may not be available, or may not provide their products and services at similar or favorable prices. If we cannot obtain the patient equipment and supplies we currently use, or alternatives at similar or favorable prices, our ability to provide such products may be severely impacted, which could have an adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
We rely on third parties for certain technology, software, information systems and products.
For certain of our technology, software, information systems and product needs, we rely upon third-party contractors to assist us. If we are unable to utilize such technology, software, information systems or products, we could incur unanticipated expenses, suffer disruptions in service, experience regulatory issues and lose revenue from the operation of such business. Further, some of our technology, software, information systems and products contain components that are developed by third parties. We may not be able to replace the functions provided by these third-party components or products if they become obsolete, defective or incompatible with future versions of our products or with our services and solutions, or if they are not adequately maintained or updated.
We believe we have all the necessary licenses from third parties to use technology, software and intellectual property assets that we do not own. A third party could, however, allege that we are infringing its rights, which may deter our ability to obtain licenses on commercially reasonable terms from the third party, if at all, or cause the third party to commence litigation against us. In addition, we may find it necessary to initiate litigation against a third party to protect our trade secrets, to enforce our intellectual property rights and to determine the scope and validity of any proprietary rights of others. Any such litigation, or the failure to obtain any necessary licenses or other rights, could materially and adversely affect our business. Alternate sources for the technology, software, information systems and products currently provided by third parties to us may not be available to us in a timely manner, and may not provide us with the same functions as currently provided to us or may be more expensive than products we currently use or sell.
Further, the risk of intellectual property infringement claims against us may increase as we expand our business to include more third-party systems and products and continue to incorporate third-party components, software and/or other intellectual property into the products we sell. Also, individuals and firms have purchased intellectual property assets in order to assert claims of infringement against technology providers and customers that use such technology. Any infringement action brought against us or our providers could be costly to defend or lead to an expensive settlement or judgment against us.
Changes or disruption in supplies provided by third parties could adversely affect our business.
As a medical device distributor, we rely on device manufacturers and suppliers to maintain compliance with all applicable regulatory requirements and to deliver products on schedule or in accordance with our expectations. There is a risk that a supplier or manufacturer fails to comply with applicable regulations, including the FDA pre- or post-approval inspections and current Good Manufacturing Practice (“cGMP”) requirements (e.g., violations that could render a product adulterated or misbranded), which could result in the FDA taking administrative or other legal action, as described above. An unfavorable resolution or outcome of any administrative or legal action against a manufacturer or supplier or any other matter that may arise out of any FDA inspection of their facilities could significantly and adversely affect our business. In March 2020, the FDA temporarily paused on-site routine surveillance inspections due to the COVID-19 pandemic. In a guidance issued in August 2020, the FDA explained that it resumed prioritized domestic inspections, such as pre-approval and surveillance inspections and that, for the foreseeable future, such prioritized domestic inspections would be pre-announced. The FDA has continued, on a case-by case basis, to conduct “mission-critical” inspections based on its evaluation of a number of factors related to the public health benefit of U.S. patients having access to the product subject to inspection (e.g., whether the product may have received breakthrough therapy designation or is
28
Table of Contents
used to diagnose, treat, or prevent a serious disease or medical condition for which there is no other appropriate substitute). Failure by any such supplier to meet its contractual obligations or to comply with applicable laws or regulations could delay or prevent the manufacture, commercialization, or distribution of our products, and could also result in non-compliance or reputational harm.
Our reliance on outside manufacturers and suppliers also subjects us to risks that could harm our business, including: a risk that we may not be able to obtain adequate supply in a timely manner or on commercially reasonable terms; a risk that we may have difficulty timely locating and qualifying alternative suppliers or manufacturers; a risk that there may be fluctuations in demand for products that affect our manufacturers’ or suppliers’ ability to deliver products in a timely manner; a risk that the manufacturers or suppliers with which we contract may fail to comply with regulatory requirements, be subject to lengthy compliance, validation or qualification periods, or make errors in manufacturing products or components that could negatively impact our product; and a risk that the manufacturers or suppliers with which we contract may encounter financial hardships unrelated to our demand for products or components, which could inhibit their ability to fulfill our orders and meet our requirements. In addition, given the rapid and evolving nature of the pandemic, COVID-19 could negatively affect our suppliers or manufacturers by interrupting, slowing, or rendering our supply chains inoperable. The COVID-19 pandemic also could result in a requirement to utilize alternative, and potentially more expensive, sources of materials or products, or an inability to find such alternative sources of materials or products. It is uncertain how the COVID-19 pandemic will affect our operations generally if these impacts persist or exacerbate over an extended period of time. These impacts could have a material adverse effect on our business and operations.
Identifying and qualifying additional or replacement suppliers, if required, may not be accomplished quickly and could involve significant additional costs. Any interruption or delay in the supply of products, or our inability to obtain products from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demands of our customers and cause providers to refer patients to our competitors and could therefore have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our operations involve the storage, transportation and provision of compressed and liquid oxygen, which carries an inherent risk of rupture or other accidents with the potential to cause substantial loss.
Our operations are subject to the many hazards inherent in the storage, transportation and provision of medical gas products and compressed and liquid oxygen, including ruptures, leaks and fires. These risks could result in substantial losses due to personal injury or loss of life, severe damage to and destruction of property and equipment and pollution or other environmental damage and may result in curtailment or suspension of our related operations. If a significant accident or event occurs, it could adversely affect our business, financial position and results of operations. Additionally, corrective action plans, fines or other sanctions may be levied by government regulators who oversee the storage, transportation and provision of hazardous materials such as compressed or liquid oxygen.
Our business operations are labor intensive. Difficulty in hiring enough additional management and other employees, increasing costs of compensation or employee benefits, and the potential impact of unionization and organizing activities could have an adverse effect on our costs and results of operations.
The success of our business depends upon our ability to attract and retain highly motivated, well-qualified management and other employees. The payment of salaries and benefits to our approximately 6,500 employees is one of our most significant expenses. In addition, we face significant competition in the recruitment of qualified employees, which has in the past resulted in salary and wage increases for certain employee groups. If we are unable to recruit or retain a sufficient number of qualified employees, or if the costs of compensation or employee benefits increase substantially, our ability to deliver services effectively could suffer and our profitability would likely be adversely affected. In addition, union organizing activities have occurred in the past
29
Table of Contents
and may occur in the future, and the adverse impact of unionization and organizing activities on our costs and operating results could be substantial.
We are highly dependent upon senior management; our failure to attract and retain key members of senior management could have a material adverse effect on us.
We are highly dependent on the performance and continued efforts of our senior management team. Our future success is dependent on our ability to continue to attract and retain qualified executive officers and senior management. Any inability to manage our operations effectively could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
We may be required to take significant write downs in connection with impairment of our intangible or other long-lived assets.
Intangible and other long-lived assets comprise a significant portion of our total assets. Intangible assets include trade names, capitated relationships, Payor relationships and accreditations with commissions. An impairment review of indefinite-lived intangible assets is conducted at least once a year in connection with the annual audit and if events or changes in circumstances indicate that their carrying value may not be recoverable. Intangible assets with a finite life and other long-lived assets are tested for recoverability whenever changes in circumstances indicate that their carrying value may not be fully recoverable.
Depending on the future business performance of our reporting unit and other events, we may be required to recognize increased levels of future intangible amortization, or incur further charges to recognize the impairment of our assets. Such charges may be significant and may be further adversely impacted by the COVID-19 pandemic. See “—The recent coronavirus (COVID-19) pandemic and the global attempt to contain it may harm our business, results of operations and ability to execute on our business plan.”
Limitations on the availability of capital or other financing sources on reasonable terms, as well as losses due to existing bad or uncollectible debts, could have an adverse impact on our operations, financial condition, or prospects.
Our business requires significant liquidity to fund labor costs, including salaries, bonuses, benefits and travel-related expenses, product and supply costs, third-party customer service, billing and collections and logistics costs and patient equipment capital expenditures. Limitations on the availability of capital or other financing sources, including deferred payment arrangements with key suppliers, could have an adverse impact on our business. In addition, we may be required to seek new or additional equity or debt financing sources. In the event that additional financing is required from outside sources, we may not be able to raise it on terms acceptable to us or at all. Furthermore, as we shift our Payor mix toward commercial Payor plans that tend to have higher deductibles, there is a subsequent increase in patient co-pay responsibility and deductible volume which may cause us to be unable to collect on certain debts that individual patients incur. If we are unable to collect on these debts, raise additional capital or access other financing sources on reasonable terms, our operations, financial condition or prospects may be materially and adversely affected.
We are subject to risks associated with our incurrence of debt.
On June 21, 2019, we entered into the Credit Facility, including the TLA of $150.0 million and Revolver of $100.0 million. Net proceeds from the TLA, together with cash on hand, were used to fund the $175.0 million dividend payment to our stockholders and SARs holders declared and paid in June 2019. Accordingly, we did not retain any of the proceeds from the TLA. The TLA matures on June 21, 2024 and we were required to make quarterly principal payments on the TLA beginning June 30, 2020. On December 11, 2020, we entered into the Credit Facility Amendment to incur $260.0 million of Incremental Term Loans. Net proceeds from the Incremental Term Loans were used to fund the $200.3 million dividend payment to our stockholders and
30
Table of Contents
$9.7 million distribution to SARs holders declared and paid in December 2020, with the remaining proceeds used to pay fees and expenses in connection with the Credit Facility Amendment and for general corporate purposes. We expect to refinance, renew or replace the Credit Facility prior to its maturity in June 2024 or to repay it with cash from operations. An inability to refinance our Credit Facility prior to its maturity could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. See “Management’s Discussion and Analysis of Financial Conditions and Results of Operations—Liquidity and Capital Resources—Long-Term Debt” for more information on our Credit Facility.
Although we currently believe that we will be able to obtain any necessary amendment or refinancing of our Credit Facility at a reasonable cost, there can be no assurance that we will succeed in obtaining such amendment or refinancing on favorable terms, if at all, which could significantly increase our future interest expense and adversely impact our liquidity and results of operations.
Further, an increase to our level of indebtedness could:
| • | require us to dedicate a portion of our cash flow from operations to payments on our indebtedness, which could reduce the availability of cash flow to fund acquisitions, start-ups, working capital, capital expenditures and other general corporate purposes; |
| • | limit our ability to borrow money or sell stock for working capital, capital expenditures, debt service requirements and other purposes; |
| • | limit our flexibility in planning for, and reacting to, changes in our industry or business; |
| • | make us more vulnerable to unfavorable economic or business conditions; and |
| • | limit our ability to make acquisitions or take advantage of other business opportunities. |
In the event we incur additional indebtedness, the risks described above could increase.
Risks Related to Our Industry and Competition
The home healthcare industry is highly competitive and fragmented, with limited barriers to entry which may make it susceptible to vertical integration by manufacturers, Payors, providers (such as hospital systems) or disruptive new entrants.
The home healthcare industry is intensely competitive and highly fragmented, as are each of the service line markets in which we compete. There are a large number of providers, some of which are national providers, but most of which are either regional or local providers, including hospital systems, physician specialists and sleep labs. Furthermore, other types of healthcare providers, including industrial gas manufacturers, home healthcare agencies and health maintenance organizations, have entered and may continue to enter the market to compete with our various service lines. Some of our competitors may now or in the future have greater financial or marketing resources than we do, which may increase pricing pressure and limit our ability to maintain or increase our market share. In addition, in certain markets, competitors may have more effective sales and marketing activities or other products and services that are or perceived to be superior to our own. For example, in the NPWT market, the leading provider who directly competes with our services, we believe, maintains the majority of the market share in the United States. There are relatively few barriers to entry in local home healthcare markets. Hospitals and health systems are routinely looking to provide coverage and better control of post-acute healthcare services, including home healthcare services of the types we provide. Hospitals and/or physician groups who accept capitation amounts that include payment for our services could seek reduced payment arrangements as compared to Payors.
These trends may continue as new payment models evolve, including bundled payment models, shared savings programs, value-based reimbursement and other payment systems.
31
Table of Contents
Further, certain manufacturers or Payors may choose to compete with us in the future, including by integrating vertically with companies in our industry, which could generate new synergies and put us at a competitive disadvantage. A number of manufacturers of home respiratory equipment currently provide equipment directly to patients on a limited basis. Such manufacturers have the ability to provide their equipment at prices below those charged by us, and there can be no assurance that such direct-to-patient sales efforts will not increase in the future or that such manufacturers will not seek reimbursement contracts directly with our commercial Payors, who could seek to provide equipment directly to patients from the manufacturer. In addition, pharmacy benefit managers, including CVS Health Corporation and the OptumRx business of UnitedHealth Group Incorporated, could enter the home healthcare market and compete with us. Large technology companies, such as Amazon.com, Inc. and Alphabet Inc., have disrupted other supply businesses and entered the healthcare market, in the case of Amazon.com, Inc. and their new pharmacy offerings, or publicly stated their interest in doing so. In the event such companies enter the home healthcare market, we may experience a loss of referrals or revenue. Similarly, disruptive entrants may adopt a more efficient business model and cause further price reduction, or significant e-commerce competitors could limit our ability to expand our e-commerce business.
We cannot assure you that these and other industry changes and the competitive nature of the home healthcare environment will not adversely affect our business, financial condition, results of operations, cash flow, capital resources, liquidity, or prospects.
We may be adversely affected by consolidation among health insurers and other industry participants.
In recent years, a number of health insurers have merged or increased efforts to consolidate with other non-governmental Payors. Insurers are also increasingly pursuing alignment initiatives with healthcare providers. Consolidation within the health insurance industry may result in insurers having increased negotiating leverage and competitive advantages, such as greater access to performance and pricing data. Our ability to negotiate prices and favorable terms with health insurers in certain markets could be affected negatively as a result of this consolidation. In addition, the shift toward value-based payment models could be accelerated if larger insurers, including those engaging in consolidation activities, find these models to be financially beneficial. There can be no assurance that we will be able to negotiate favorable terms with Payors and otherwise respond effectively to the impact of increased consolidation in the Payor industry or vertical integration efforts.
There is an inherent risk of liability in the provision of healthcare services; damage to our reputation or our failure to adequately insure against losses, including from substantial claims and litigation, could have an adverse impact on our operations, financial condition, or prospects.
There is an inherent risk of liability in the provision of healthcare services. As participants in the healthcare industry, we are and expect to be periodically subject to lawsuits, some of which may involve large claims and significant costs to defend, such as mass tort or other class actions. In that case, the coverage under our insurance programs may not be adequate to protect us. Our insurance policies are subject to annual renewal and our insurance premiums could be subject to material increases in the future. We cannot be assured that we will be able to maintain this insurance on acceptable terms in the future, or at all. A successful claim in excess of, or not covered by, our insurance policies could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. Even where our insurance is adequate to cover claims against us, damage to our reputation in the event of a judgment against us, or continued increases in our insurance costs, could have an adverse effect on our business, financial condition, results of operations, cash flow, capital resources, liquidity, or prospects.
Any economic downturn, deepening of an economic downturn, continued deficit spending by the federal government or state budget pressures may result in a reduction in payments and covered services.
Adverse economic or political developments in the United States, including a slowdown of economic growth, disruptions in financial markets, economic downturns, inflation, elevated unemployment levels, sluggish
32
Table of Contents
or uneven economic recovery, government deficit reduction, natural and other disasters and public health crises, could lead to a reduction in federal government expenditures, including governmentally funded programs in which we participate, such as Medicare and Medicaid. In addition, if at any time the federal government is not able to meet its debt payments unless the federal debt ceiling is raised, and legislation increasing the debt ceiling is not enacted, the federal government may stop or delay making payments on its obligations, including funding for government programs in which we participate, such as Medicare and Medicaid. Failure of the government to make payments under these programs could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. The COVID-19 pandemic may exacerbate many of these conditions. See “—The recent coronavirus (COVID-19) pandemic and the global attempt to contain it may harm our business, results of operations and ability to execute on our business plan.” Further, any failure by Congress to complete the federal budget process and fund government operations may result in a federal government shutdown, potentially causing us to incur substantial costs without reimbursement under the Medicare program, which could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. For example, the failure of the 2011 Joint Select Committee to meet its deficit reduction goal resulted in an automatic across-the-board reduction in Medicare payments of 2% beginning April 1, 2013. The 2% reduction in Medicare payments has been extended several times by Congress, although in response to COVID-19 the reduction was suspended through December 31, 2020. As part of the recent COVID-19 relief package, Congress extended the payment reduction suspension through March 31, 2021.
In addition, sustained unfavorable economic conditions may affect the number of patients enrolled in managed care programs and the profitability of managed care companies, which could result in reduced payment rates and could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Turmoil in the financial markets, including in the capital and credit markets, and any uncertainty over its breadth, depth and duration may put pressure on the global economy and could have a negative effect on our business. Further, historical worldwide financial and credit turmoil could reduce the availability of liquidity and credit to fund the continuation and expansion of business operations worldwide. The shortage of liquidity and credit combined with substantial losses in worldwide equity markets could cause an economic recession in the United States or worldwide. If financial markets in the United States, Europe and Asia experience extreme disruption, including, among other things, extreme volatility in security prices, severely diminished liquidity and credit availability, rating downgrades of certain investments and declining valuations of others, governments may take unprecedented actions intended to address extreme market conditions that may include severely restricted credit and declines in real estate values.
Changes in home healthcare technology and/or product and therapy innovations may make the equipment and services we currently provide obsolete or less competitive.
We evaluate changes in home healthcare technology and product innovation on an ongoing basis, for purposes of determining the feasibility of replacing or supplementing items currently included in the equipment and services that we offer to our patients. We consider a variety of factors, including overall quality, functional reliability, availability of supply, Payor reimbursement policies, product features, labor costs associated with the technology, acquisition, repair and ownership costs and overall patient and referral source demand, as well as patient therapeutic and lifestyle benefits. Manufacturers continue to invest in research and development to introduce new products to the marketplace. It is possible that major changes in available technology, Payor benefit or coverage policies related to those changes, or the preferences of patients and referral sources, may cause our current product offerings to become less competitive or obsolete, and it will be necessary for us to adapt to those changes. It is also possible that product innovation may reduce clinical support requirements, thereby reducing our clinical value for some portion of the patient population. Furthermore, if the reimbursement model for certain of our therapies should change, certain products may be offered in alternative channels such as pharmacy, retail, or e-commerce, increasing competitive pressures. Such unanticipated changes could cause us to
33
Table of Contents
incur increased capital expenditures and accelerated write-offs, and could force us to alter our sales, operations, and marketing strategies.
Expansion of group purchasing organizations (“GPO”) or provider networks and the multi-tiered costing structure may place us at a competitive disadvantage.
The medical products industry is subject to a multi-tiered costing structure, which can vary by manufacturer and/or product. Under this structure, certain institutions can obtain more favorable prices for medical products than we are able to obtain. The multi-tiered costing structure continues to expand as many large integrated healthcare providers and others with significant purchasing power, such as GPOs, demand more favorable pricing terms. Additionally, the formation of provider networks and GPOs may shift purchasing decisions to entities or persons with whom we do not have a historical relationship. This may threaten our ability to compete effectively, which could in turn negatively impact our financial results. Although we are seeking to obtain similar terms from manufacturers to obtain access to lower prices demanded by GPO contracts or other contracts, and to develop relationships with provider networks and new GPOs, we cannot assure you that such terms will be obtained or contracts will be executed.
Risks Related to Regulation and Litigation
Our failure to comply with regulatory requirements or receive regulatory clearances or approvals for the Company’s products or operations in the United States could adversely affect our business.
The medical gas products we manufacture and distribute and certain other products we distribute are subject to extensive regulation by the Food and Drug Administration (“FDA”) and other federal and state governing authorities. Compliance with FDA, state, and other requirements regarding production, safety, quality, and good manufacturing regulations is costly and time-consuming, and while we seek to be in full compliance, instances of non-compliance could arise from time to time. We cannot be assured that any of our medical gases will be certified by the FDA. We have applied for, and received, designated gas certifications for our medical gas products. We may not be successful in receiving certification in the future. Other potential product manufacturing-related risks include difficulties or delays in product manufacturing, sales, or marketing, which could affect future results through regulatory actions, shutdowns, approval delays, withdrawals, recalls, penalties, supply disruptions or shortages, reputational harm, product liability, and/or unanticipated costs.
Failure to comply with applicable regulatory requirements could result in administrative enforcement action by the FDA or state agencies, which may include any of the following: adverse publicity; warning or untitled letters; fines; injunctions; consent decrees; civil money penalties; recalls; termination of distribution or seizure of our products; operating restrictions or partial suspension or total shutdown of production; delays in the introduction of products into the market; withdrawals or suspensions of current medical gas certifications or drug approvals, resulting in prohibitions on sales of our products; and criminal prosecution. There is also a risk that we may not adequately implement sustainable processes and procedures to maintain regulatory compliance and to address future regulatory agency findings, should they occur. The FDA may change its policies, adopt additional regulations or revise existing regulations, each of which could prevent or delay certification of our medical gases, or could impact our ability to market a device that was previously certified or cleared by the FDA. Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Reductions in Medicare, Medicaid and commercial Payor reimbursement rates and/or exclusion from markets or product lines, including due to the DMEPOS CBP, could have a material adverse effect on our results of operations and financial condition.
We have faced, and expect to continue to face, pricing pressures due to reductions in provider reimbursement for our products and services. For the year ended December 31, 2019, we derived approximately
34
Table of Contents
20% of our net revenues from Medicare and Medicaid reimbursements. There are increasing pressures on Medicare, and state Medicaid programs, to control healthcare costs and to reduce or limit reimbursement rates for home medical equipment and other products. Consistently, legislation enacted by Congress has included provisions that directly impact reimbursement for the products and services we provide, as well as the cost of providing those services, including legislation that requires the DMEPOS CBP to award contracts based on price and capacity to fulfill service requirements. These legislative provisions have had and may continue to have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity. See “Business—Government Regulation.”
The competitive bidding process has historically put downward pressure on the amounts we are reimbursed in the markets in which we operate, as well as in areas that are not subject to the DMEPOS CBP. We continue to monitor developments regarding the DMEPOS CBP. In March 2019, CMS announced plans to consolidate the competitive bidding areas (“CBAs”) included in the Round 1 2017 and Round 2 Recompete DMEPOS CBPs into a single round of competition, referred to by CMS as “Round 2021.” Round 2021 contracts are scheduled to become effective on January 1, 2021, and to extend through December 31, 2023. The bid window for Round 2021 closed on September 18, 2019.
On April 9, 2020, CMS announced that, due to the COVID-19 pandemic, CMS removed the NIV product category from Round 2021 of the DMEPOS CBP. CMS noted that the reasons for this change included not only the agency’s ongoing concerns regarding the COVID-19 pandemic, but also the President’s exercise of the Defense Production Act for the production of ventilators, public concern regarding access to ventilators, and the fact that the NIV product category would be new to the DMEPOS CBP. A NIV is a ventilator used with a non-invasive interface (e.g., mask) in contrast to an invasive interface (e.g., tracheostomy tube). Because NIVs have been removed from Round 2021 of the DMEPOS CBP, any Medicare-enrolled DMEPOS supplier can furnish NIV under the Medicare program.
On October 27, 2020, CMS announced further revisions to Round 2021 of the DMEPOS CBP. Namely, only two out of the original 16 product categories, off-the-shelf (“OTS”) back braces and OTS knee braces, will be included in Round 2021 of the DMEPOS CBP. All other product categories were removed from Round 2021, at least in part because “the payment amounts did not achieve expected savings.” Accordingly, there are no longer any products in our product lines included on the list of products subject to Round 2021 of the DMEPOS CBP.
Following the expiration of all previous DMEPOS CBP contracts on December 31, 2018, CMS implemented new DMEPOS payment policies during the temporary gap in the DMEPOS CBP for DMEPOS items that are paid based on information from the DMEPOS CBP. From January 1, 2019 through December 31, 2020, CMS established separate fee schedule adjustment methodologies for such DMEPOS items for three geographic areas: (1) other non-CBAs (those that are not defined as rural or non-contiguous), (2) rural/non-contiguous areas, and (3) former CBAs. For other non-CBAs, the payment amounts for such DMEPOS items have been adjusted based on regional averages of the single payment amounts (“SPAs”) that apply to the DMEPOS CBP (referred to as the “Adjusted Fee Schedule”). Because the SPAs generated from the DMEPOS CBP competitions expired on January 1, 2019, the Adjusted Fee Schedule amount was increased by 1.6% on January 1, 2020, which is the percentage change in the Consumer Price Index for all Urban Consumers (“CPI-U”) for the 12-month period ending June 30, 2020 and will be increased again by 0.6% on January 1, 2021. For rural/non-contiguous areas, the payment amounts for such DMEPOS items were based on a blended rate of 50% of the non-Adjusted Fee Schedule amount and 50% of the Adjusted Fee Schedule amount. The Adjusted Fee Schedule amount of the blended rate was increased by the percentage change in the CPI-U (1.6%) on January 1, 2020, and will be increased again by 0.6% on January 1, 2021. For former CBAs, the payment amounts for such DMEPOS items are based on the lower of the supplier’s charge for the item or fee schedule amounts that are based on the SPAs that were in effect in the CBA before the CBP contract ended, increased by the projected percentage change in the CPI-U. Accordingly, for 2019, the fee schedule amounts were based on the SPAs in effect on December 31, 2018 for each specific CBA, increased by 2.5% (the projected percentage change in the
35
Table of Contents
CPI-U for the 12-month period ending January 1, 2019), and for 2020, the fee schedule amounts increased by 2.4% (the projected percentage change in the CPI-U for the 12-month period ending January 1, 2020). For 2021, the fee schedule amounts will be increased by 0.6% (the projected percentage change in the CPI-U for the 12-month period ending January 1, 2021).
The Coronavirus Aid, Relief, and Economic Security (“CARES”) Act introduced a new blended rate for such DMEPOS items furnished in other non-CBAs (those that are not defined as rural or non-contiguous) that is based on 25% of the non-Adjusted Fee Schedule amount and 75% of the Adjusted Fee Schedule amount, effective March 6, 2020 through the end of the COVID-19 pandemic. For rural and non-contiguous areas, the payment amount for such DMEPOS items will continue to be based on a blended rate of 50% of the non-Adjusted Fee Schedule amount and 50% of the Adjusted Fee Schedule amount until December 31, 2020 or the end of the COVID-19 pandemic, whichever is later.
On November 4, 2020, CMS issued a proposed rule establishing the methodologies for adjusting the fee schedule payment amounts for such DMEPOS items furnished in non-CBAs on or after April 1, 2021 or the date immediately following the duration of the public health emergency period (which has recently been extended through April 20, 2021), whichever is later. CMS proposes to pay 100% of the Adjusted Fee Schedule amount in other non-CBAs. Under the proposal, CMS would continue paying suppliers higher rates (e.g., at the 50/50 blended rate) for furnishing such DMEPOS items in rural and non-contiguous areas as compared to in other non-CBAs, informed by stakeholder input indicating higher costs in these areas, greater travel distances and costs in certain non-CBAs compared to CBAs, the unique logistical challenges and costs of furnishing items to beneficiaries in the non-contiguous areas, significantly lower volume of items furnished in these areas versus CBAs, and concerns about financial incentives for suppliers in surrounding urban areas to continue including outlying rural areas in their service areas. For such DMEPOS items that originally were included in Round 2021 but for which contracts were not awarded, CMS is considering whether to simply extend application of the current fee schedule adjustment rules for non-CBAs, CBAs, and former CBAs until new SPAs are calculated for the items in a future round of the DMEPOS CBP. We believe that this proposal does not include NIVs, since they were not included in previous rounds of the DMEPOS CBP and therefore would continue to be paid based on the Medicare DMEPOS fee schedule.
National and regional insurers and managed care organizations also regularly attempt to seek reductions in the prices at which we provide products and services to them and their members, including through direct contracts with healthcare providers, increased oversight and greater enrollment of patients in managed care programs and preferred provider organizations. These commercial Payors are increasingly demanding discounted fee structures, including setting reimbursement rates based on Medicare fee schedules or requiring healthcare providers or suppliers to assume a greater degree of financial risk related to patient care. We have a large number of contractual arrangements with commercial Payors (which includes all Payors other than government Payors) through various national and regional insurers and managed care organizations, which represented approximately 80% of our net revenue in 2019. Most of our commercial Payor contracts have two to five-year terms with an automatic extension unless it otherwise terminates. Most of our contracts are based on price–we generally do not have contracted volume guarantees. We expect that we will continue to maintain our contracts with commercial Payors and enter into more of these contractual arrangements as market conditions evolve. However, there can be no assurance that we will retain or obtain these contracts, or that such plans will not attempt to further reduce the rates they pay to providers. Reimbursement rates under such contracts may not remain at current levels and may not be sufficient to cover the costs of caring for patients enrolled in such programs, which would have a negative impact on our pricing flexibility, changes in Payor mix and growth in operating expenses. Contracts with commercial Payors that generated approximately 42% of our net revenue in 2019 are scheduled to expire in 2021. Increased pricing pressure from commercial Payors could result in us lowering our prices, which could adversely impact our financial condition and results of operations.
We cannot predict the full extent to which reimbursement for our products and cost of operations may be affected by federal and/or state legislative efforts or by initiatives, including future rounds of the CBP, to reduce
36
Table of Contents
costs for national and regional insurers and managed care organizations. If we are unable to successfully reduce our costs or increase our volumes, our results would be adversely affected. For example, we may be unable to continue to provide services directly to patients of certain Payors or through contractual arrangements with Payors. This would have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
For further information, see “Business—Government Regulation—Medicare and Medicaid Revenues” and “Business—Government Regulation—Medicare Reimbursement.”
If we fail to comply with applicable laws and regulations, we could suffer penalties or be required to make significant changes to our operations.
The healthcare sector is heavily regulated, and we are required to comply with extensive and complex laws and regulations at the federal, state and local government levels relating to, among other things:
| • | billing and coding for services, including documentation of care, appropriate treatment of overpayments and credit balances, and the submission of false statements or claims; |
| • | relationships and arrangements with physicians, hospitals, vendors, and other referral sources and referral recipients, including self-referral restrictions, prohibitions on kickbacks and other non-permitted forms of remuneration, and prohibitions on the payment of inducements to Medicare and Medicaid beneficiaries in order to influence their selection of a provider; |
| • | the necessity, appropriateness, and adequacy of medical care, equipment, and personnel and conditions of coverage and payment for products and services; |
| • | licensure, certification, and enrollment in government programs, including requirements affecting the operation, establishment, and addition of products and services; |
| • | anti-competitive conduct; and |
| • | confidentiality, privacy, data breaches, identity theft, and security issues associated with the maintenance of health-related and other personal information and medical records. |
These federal, state and local laws and regulations are stringent and frequently changing, requiring compliance with burdensome and complex billing and payment, substantiation, and record-keeping requirements. For example, the Durable Medical Equipment Medicare Administrative Contractor (“DME MAC”) Supplier Manuals provide that clinical information from the “patient’s medical record” is required to justify the medical necessity for the provision of Medicare DMEPOS items. Although we have implemented policies and procedures that are designed to meet Medicare’s documentation requirements, an auditor for at least one of the DME MACs has taken the position that, among other things, the “patient’s medical record” refers not to documentation maintained by the DMEPOS supplier but instead to documentation maintained by the patient’s physician, healthcare facility or other clinician, and that clinical information created by the DMEPOS supplier’s personnel and confirmed by the patient’s physician is not sufficient to establish medical necessity. It may be difficult, and sometimes impossible, for us to obtain documentation from other healthcare providers. While we have taken this position into account in refining our policies and procedures, if these or related positions adopted by auditors or regulatory authorities are broadly adopted in administering the Medicare program, it could result in our making refunds and other payments to Medicare and our future revenues from Medicare may be reduced.
In addition, if we fail to comply with applicable laws and regulations, we could suffer civil or criminal penalties, including fines, damages, recoupment of overpayments, loss of licenses needed to operate, loss of enrollment and approvals necessary to participate in Medicare, Medicaid and other government and third-party healthcare programs, and exclusion from participation in Medicare, Medicaid and other government healthcare programs. Investors, officers, and managing employees associated with entities found to have committed healthcare fraud also may be excluded from participation in government healthcare programs. Enforcement
37
Table of Contents
officials have numerous mechanisms to combat fraud and abuse. Many of these laws and regulations are complex, broad in scope, and have few or narrowly structured exceptions and safe harbors. Further, these laws and regulations are subject to continuing and evolving interpretation by regulatory agencies and courts. New interpretations of existing requirements, new laws or regulations or the enforcement of existing or new laws and regulations, could subject our current practices to allegations of impropriety or illegality or require us to make changes in our operations, facilities, equipment, personnel, services, capital expenditure programs, or operating expenses to comply with evolving requirements. We cannot assure you that we will make any such changes quickly enough or in a cost-efficient manner. Furthermore, suits filed under the federal False Claims Act (“FCA”), some of which are known as qui tam actions, can be brought by any individual on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in any amounts ultimately paid by the entity to the government in the form of fines or settlements. The frequency of filing qui tam actions has increased significantly in recent years, causing greater numbers of medical device, pharmaceutical and healthcare companies to have to defend FCA actions. When an entity is determined to have violated the federal FCA, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties for each separate false claim. Various states have also enacted laws modeled after the federal FCA, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, that apply regardless of Payor.
Any enforcement action against us, even if we successfully defend against it, could cause our reputation to suffer, or cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. To avoid sanctions and resolve expensive enforcement actions, we may be required to enter into settlement agreements with the government. Typically, such settlement agreements require substantial payments to the government in exchange for the government’s release of its claims and may also require us to enter into a corporate integrity agreement that imposes extensive ongoing compliance obligations.
We cannot assure you that current or future legislative initiatives, government regulation or judicial or regulatory interpretations thereof will not have a material adverse effect on us. We cannot assure you that a review of our business by judicial, regulatory or accreditation authorities will not subject us to fines or penalties, require us to expend significant amounts, reduce the demand for our services or otherwise adversely affect our operations.
For further information, see “—We have been, are and could become the subject of federal and state investigations and compliance reviews” below. In addition, see “Business—Government Regulation” for a description of the extensive government regulation to which we are subject, including numerous laws directed at regulating reimbursement of our products and services under various government programs and preventing fraud and abuse.
We have been, are and could become the subject of federal and state investigations and compliance reviews.
Our operations, including our billing practices and our arrangements with healthcare providers, are subject to extensive federal and state laws and audits, inquiries, and investigations from government agencies. For example, in August 2017, we entered into a settlement agreement resolving an investigation conducted by the Commonwealth of Massachusetts regarding our billing practices for services provided to MassHealth (Massachusetts Medicaid) members. Among other things, we were required to develop a compliance and monitoring program to oversee design and implementation of written policies and procedures focused on compliance with certain MassHealth billing practices and to institute and complete training for all of our employees who have any involvement with billing MassHealth members. Violation of the terms of the settlement agreement could result in the imposition of penalties and sanctions, including disqualification from MassHealth programs. We have actively monitored the billing practices at issue in this matter, submitted periodic reports to the Massachusetts Attorney General’s office, and conducted training as required by the settlement agreement. This matter was closed in August 2020.
38
Table of Contents
Additionally, in connection with the settlement agreements resolving the investigation conducted by the SDNY Office regarding CIDs, we were required to enter into a five-year Corporate Integrity Agreement. The CIA provides that Apria will, among other things, impose certain oversight obligations on its board of directors, provide certain management certifications, and continue or implement, as applicable certain compliance training and education. The CIA also requires Apria to engage independent third parties to review compliance with the CIA, as well as certain reporting, certification, record retention and notification requirements. Failure to comply with the obligations under the CIA could have material consequences for Apria including monetary penalties or exclusion from participation in federal healthcare programs. See “Summary—Recent Developments—Settlement with SDNY Office.” Applicable laws may be directed at payments for the products and services we provide, conduct of our operations, preventing fraud and abuse, and billing and reimbursement from government programs such as Medicare and Medicaid and from commercial Payors. These laws may have related rules and regulations that are subject to interpretation and may not provide definitive guidance as to their application to our operations, including our arrangements with hospitals, physicians, and other healthcare providers.
Federal and state governments have contracted with private entities to audit and recover revenue resulting from payments made in excess of those permitted by federal and state benefit program rules. These entities include, but are not limited to, Recovery Audit Contractors that are responsible for auditing Medicare claims, Unified Program Integrity Contractors that are responsible for the identification of suspected fraud through medical record review and Medicaid Integrity Contractors, that are responsible for auditing Medicaid claims. We believe audits, inquiries, and investigations from these contractors and others will occur from time to time in the ordinary course of our business. We also may be subject to increased audits from commercial Payors and pursuant to federal, civil, and criminal statutes that relate to our billings to commercial Payors. Our efforts to be responsive to these audits, inquiries, and investigations may result in substantial costs and divert management’s time and attention away from the operation of our business. Moreover, an adverse outcome with respect to any audit, inquiry or investigation may result in damage to our reputation, or in fines, penalties or other sanctions imposed on us. Such pending or future audits, inquiries, or investigations, or the public disclosure of such matters, could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Federal and state laws are broadly worded and may be interpreted or applied by prosecutorial, regulatory, or judicial authorities in ways that we cannot predict. Additionally, in many instances, there are only limited publicly-available guidelines and methodologies for determining errors with certain audits. As a result, there can be a significant lack of clarity regarding required documentation and audit methodology. The clarity and completeness of each patient medical file, some of which is the work product of physicians not employed by us, is essential to successfully challenging any payment denials. For example, certain provisions under CMS guidance manuals, local coverage determinations, and the DME MAC Supplier Manuals provide that clinical information from the “patient’s medical record” is required to justify the initial and ongoing medical necessity for the provision of DMEPOS. Some DME MACs, CMS staff and other government contractors have taken the position, that the “patient’s medical record” refers not to documentation maintained by the DMEPOS supplier but instead to documentation maintained by the patient’s physician, healthcare facility or other clinician, and that clinical information created by the DMEPOS supplier’s personnel and confirmed by the patient’s physician is not sufficient to establish medical necessity. If treating physicians do not adequately document, among other things, their diagnoses and plans of care, the risks that the Company will be subject to audits and payment denials are likely to increase. Moreover, auditors’ interpretations of these policies are inconsistent and subject to individual
interpretation, leading to significant increases in individual supplier and industry-wide perceived error rates. High error rates could lead to further audit activity and regulatory burdens, and could result in our making significant refunds and other payments to Medicare and other government programs. Accordingly, our future revenues and cash flows from government healthcare programs may be reduced. Commercial Payors also may conduct audits and may take legal action to recover alleged overpayments. We could be adversely affected in some of the markets in which we operate if the auditing Payor alleges substantial overpayments were made to us due to coding errors or lack of documentation to support medical necessity determinations. We cannot currently
39
Table of Contents
predict the adverse impact these measures might have on our financial condition and results of operations, but such impact could be material.
Moreover, provisions of the Patient Protection and Affordable Care Act of 2010 (the “PPACA”) implemented by CMS require that overpayments be reported and returned within 60 days of the date on which the overpayment is “identified.” Any overpayment retained after this deadline may be considered an “obligation” for purposes of the False Claims Act, liability for which can result in the imposition of substantial fines and penalties. CMS currently requires a six-year “lookback period,” for reporting and returning overpayments.
Accordingly, our arrangements and business practices may be the subject of government scrutiny or be found to violate applicable laws. If federal or state government officials challenge our operations or arrangements with third parties that we have structured based upon our interpretation of these laws, rules, and regulations, such a challenge could potentially disrupt our business operations and we may incur substantial defense costs, even if we successfully defend our interpretation of these laws, rules, and regulations. If the government or third parties successfully challenge our interpretation, such a challenge may have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Ongoing federal and state health reform initiatives could adversely affect our operations and business condition.
Economic, political, and regulatory influences at both the federal and state level are continuously causing fundamental changes in the U.S. healthcare industry. In 2010, Congress enacted significant reforms to the U.S. healthcare system. These reforms, contained primarily in the PPACA and its companion act, the Health Care Education and Reconciliation Act of 2010 (collectively, the “Health Reform Laws”), significantly altered the U.S. healthcare system. Since their passage in 2010, the Health Reform Laws have faced various and ongoing legal challenges to repeal or modify those laws or delay the implementation of certain aspects of those laws.
Consequently, the core tenets of the Health Reform Laws currently remain in effect, but with several exceptions. The individual mandate penalty was reduced to zero through the Tax Cuts and Jobs Act of 2017, signed into law in December 2017 and effective January 1, 2019. In addition, the Bipartisan Budget Act of 2018, enacted in February 2018, eliminated the Independent Payment Advisory Board, which was a 15-member panel of healthcare experts created by the Health Reform Laws and tasked with making annual cost-cutting recommendations for the Medicare program if Medicare spending exceeded a specified growth rate. In December of 2019, Congress passed the Further Consolidated Appropriations Act, 2020 (Pub. Law 116-94) that repealed several provisions included in the Health Reforms Laws to pay for increased federal spending associated with those laws. Specifically, Congress: (i) repealed the Medical Device Excise Tax, which imposed a 2.3% excise tax on manufacturers, producers and importers of certain medical devices, effective in 2020; (ii) repealed the so-called “Cadillac Tax,” which imposed an excise tax of 40% on premiums of employer-sponsored insurance for individuals and families that exceeded a certain minimum threshold, effective in 2020; and (iii) repealed the health insurance tax, which applies to most fully insured plans, effective in 2021.
The Health Reform Laws also are the subject of ongoing litigation. In particular, a collection of 20 state governors and state attorneys general (subsequently two states have dropped out) filed a lawsuit against the federal government in the Northern District of Texas seeking to enjoin the entire Health Reform Laws following the elimination of the individual mandate penalty in 2019. The District Court ruled that without the penalty the individual mandate was unconstitutional and that all other provisions of the Health Reform Laws should be overturned as well. The U.S. Court of Appeals for the 5th Circuit affirmed the trial court’s decision; however, instead of deciding whether the rest of the PPACA must be struck down, the 5th Circuit sent the case back to the trial court for additional analysis. In March of 2020, the United States Supreme Court granted cert in the case, and on November 10, 2020 heard oral arguments. We are unable to predict the ultimate outcome of the lawsuit but note its potential impact on the Health Reform Laws moving forward.
40
Table of Contents
We anticipate that federal and state governments will continue to review and assess alternative healthcare delivery systems and payment methodologies, and that public debate regarding these issues will continue in the future. Changes in the law or new interpretations of existing laws can have a substantial effect on permissible activities, the relative costs associated with doing business in the healthcare industry, and the amount of reimbursement available from government and other Payors. If the Health Reform Laws are repealed or modified, or if implementation of certain aspects of the Health Reform Laws continues to be delayed, such repeal, modification, or delay may have a material and adverse impact on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
We may be adversely affected by Congress’ elimination of the PPACA’s individual mandate penalty.
The provisions of the PPACA that penalized Americans if they failed to maintain a basic level of health insurance coverage, commonly referred to as the law’s “individual mandate penalty,” were effectively repealed through the Tax Cuts and Jobs Act of 2017, when Congress reduced the penalty to zero dollars. The elimination of the individual mandate penalty may have the ongoing effect of increasing instability and financial disruption in the market for health insurance in 2021 and beyond, as a population of patients who may previously have obtained coverage because they were required to under the PPACA may choose now to enroll in less expensive and less robust insurance products or to drop their coverage altogether. The repeal became effective January 1, 2019 but such choices may continue to materialize as more patients are made aware of the elimination of the individual mandate penalty. At the same time as a result of a changing administration and changes in Congress, it is possible that the individual mandate could be reinstated. These and other risks and uncertainties resulting from the elimination of the individual mandate penalty may have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
If we fail to maintain required licenses, certifications, or accreditation, or if we do not fully comply with requirements to provide notice to or obtain approval from regulatory authorities due to changes in our ownership structure or operation, it could adversely impact our operations.
We are required to maintain state and/or federal licenses and certifications for our operations and facilities. In addition, certain employees, primarily those with clinical expertise in pharmacy, respiratory therapy, and nutrition, are required to maintain licenses in the states in which they practice. From time to time, we may become subject to new or different licensing requirements due to legislative or regulatory requirements or the development of or changes to our business. Accurate licensure is also a critical threshold issue for Medicare enrollment and for participation in the Medicare DMEPOS CBP. We are subject to periodic inspection by governmental and other authorities to assure continued compliance with the various standards necessary for licensing and certifications, some of which are complex and may be unclear or subject to varying interpretation. In response to the declaration of a public health emergency due to the COVID-19 pandemic, CMS instituted flexibilities related to the DMEPOS supplier standards and temporarily suspended all DMEPOS provider enrollment site visits in order to ease provider burden during the COVID-19 pandemic. However, as of July 6, 2020, CMS has resumed all DMEPOS provider enrollment site visits, and is no longer waiving certain DMEPOS supplier standards. Although we believe we have the right systems in place to monitor licensure and certification, our failure, or the failure of one or more of our clinicians, to maintain appropriate licensure or certification for our operations, facilities, and clinicians could result in interruptions in our operations and our ability to service patients, refunds to state and/or federal Payors, and the imposition of sanctions or fines, which could have an adverse and material impact on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Accreditation is required by most major commercial Payors and is a mandatory requirement for all Medicare DMEPOS suppliers. In response to the declaration of a public health emergency due to the COVID-19 pandemic, CMS temporarily suspended all accreditation and reaccreditation activities for DMEPOS suppliers. However, as of July 6, 2020, CMS resumed all accreditation and reaccreditation activities, including surveys, which may be conducted on-site, virtually or a combination of both depending on each state’s reopening plan.
41
Table of Contents
We and all of our branch locations are currently accredited by The Joint Commission. If we or any of our branch locations should lose accreditation, or if any of our new branch locations are unable to become accredited, our failure to maintain our accreditation or become accredited could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
The requirements for licensure and certification may include notification or approval in the event of a transfer or change of ownership or certain other changes. Agencies or commercial Payors with which we have contracts may have similar requirements and some of those processes may be complex. Failure to provide required notifications or obtain the requisite approvals could result in the delay or inability to complete an acquisition or transfer, loss of licensure, lapses in reimbursement or other penalties. While we make reasonable efforts to substantially comply with these requirements, if we are found to have failed to comply in some material respect, it could have an adverse or material impact on our business and our financial conditions.
A recall of any of our products, either voluntarily or at the direction of the FDA or another governmental authority, or the discovery of serious safety issues with our products that leads to corrective actions being taken, could have a significant adverse impact on our business.
The FDA has authority to request the recall of medical gas products or medical devices in the event a product presents a risk of illness or injury or gross consumer deception, such as due to a material deficiency or defect in design, labeling or manufacture of a product, and a cessation of distribution and/or recall is necessary to protect the public health and welfare. If after providing the Company or the manufacturer with an opportunity to consult with the agency, the FDA finds that there is a reasonable probability that a device intended for human use would cause serious, adverse health consequences or death, the FDA has the power to mandate a recall of medical devices. Manufacturers may also, under their own initiative, recall a product if any material deficiency in a drug is identified or withdraw a product for other reasons. Any major recall would divert management attention and financial resources from the operation of our business, could cause the price of our stock to decline and expose us to product liability or other claims and harm our reputation with customers. If we do not adequately address problems associated with our drugs or devices, we may face additional regulatory enforcement action, FDA warning letters, product seizure, injunctions, administrative penalties, civil money penalties or criminal fines. We may also be required to bear other costs or take other actions that may have a negative impact on our sales, or significant adverse publicity of regulatory consequences, which could harm our business.
We may be subject to fines, penalties, or injunctions if we are determined to be promoting the use of our products for unapproved or “off-label” uses, resulting in damage to our reputation and our business.
Our promotional materials and training methods must comply with the FDA and other applicable laws and regulations, including the prohibition of the promotion of a medical gas or medical device for a use that has not been cleared or approved by the FDA. If the FDA determines that our promotional materials or training constitutes promotion of an off-label use that is either false or misleading, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, which could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Corporate officers may be subject to a misdemeanor penalty (and possible subsequent felony) under the Federal Food, Drug and Cosmetic Act (“FFDCA”) for alleged violations of the Act.
The Park Doctrine, as established by the U.S. Supreme Court, provides that a responsible corporate official can be held liable for a first time misdemeanor (and possible subsequent felony) for a company’s alleged violations of the FFDCA without proof that the corporate official participated in, had intent or negligence, or was even aware of the violations. In pursuing such a charge, the government need only demonstrate that the official was in a position of authority to prevent or correct the alleged violation. Under Section 303(f)(1) of the FFDCA, a person who violates a requirement relating to a device can be liable for a civil penalty for all violations
42
Table of Contents
adjudicated in a single proceeding, except for those relating to cGMPs and medical device reporting violations that do not constitute a significant or knowing departure from requirements or a risk to public health; filth violations in devices that are not otherwise defective; and minor violations relating to device tracking and correction and removal reporting requirements if the person shows substantial compliance with such provisions. The FDA may use misdemeanor prosecution as an enforcement tool and will refer the prosecutions to the Department of Justice (“DOJ”). Once a person has been convicted of a misdemeanor under the FFDCA, any subsequent violation is a felony, even without proof that the responsible corporate official acted with the intent to defraud or mislead. In some cases, a misdemeanor conviction of an individual may serve as the basis for debarment by the FDA. The maximum civil money penalty amounts are periodically adjusted for inflation. If the DOJ were to pursue such a misdemeanor or felony prosecution against a responsible corporate official of the Company, it could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Our medical gas facilities and operations are subject to extensive regulation by federal and state authorities and there can be no assurance that our medical gas facilities will achieve and maintain compliance with such regulations.
We have a number of medical gas facilities in several states. These facilities are subject to federal and state regulatory requirements. Our medical gas facilities and operations are subject to extensive regulation by the FDA and other federal and state authorities. The FDA regulates medical gases, including medical oxygen, pursuant to its authority under the FFDCA. Among other requirements, the FDA’s cGMP regulations impose certain quality control, documentation, and recordkeeping requirements on the receipt, processing, and distribution of medical gas. Further, in each state where we operate medical gas facilities, we are subject to regulation under state health and safety laws, which vary from state to state. The FDA and state authorities conduct periodic, unannounced inspections at medical gas facilities to assess compliance with the cGMP and other regulations. For further information, see “—Risks Related to Our Business and Operation—Changes or disruption in supplies provided by third parties could adversely affect our business” above.
We expend significant time, money, and resources in an effort to achieve substantial compliance with the cGMP regulations and other federal and state law requirements at each of our medical gas facilities. There can be no assurance, however, that these efforts will be successful and that our medical gas facilities will achieve and maintain compliance with federal and state laws and regulations. Our failure to achieve and maintain regulatory compliance at our medical gas facilities could result in enforcement action, including warning letters, fines, product recalls or seizures, temporary or permanent injunctions, or suspensions in operations at one or more locations, as well as civil or criminal penalties, all of which could materially harm our business, financial condition, results of operations, cash flow, capital resources, and liquidity.
A cyber-attack, a security breach or the improper disclosure of protected health information (“PHI”) could cause a loss of confidential data, give rise to remediation and other expenses, expose us to liability under HIPAA and HITECH, consumer protection, common law or other legal theories, subject us to litigation and federal and state governmental inquiries, damage our reputation, and otherwise be disruptive to our business.
We rely extensively on our information technology (“IT”) systems to bill patients and Payors, to manage clinical and financial data, to communicate with our consumers, Payors, vendors and other third parties, and to summarize and analyze our operating results. Although we have implemented various policies, procedures and other security measures to protect our IT systems and data and face ongoing cyber-attacks and threats, there can be no assurance that we will not be subject to a cyber-attack, a security breach or the improper disclosure or use of PHI in the future. Such attacks or breaches could result in loss of protected patient medical data or other information subject to privacy laws or disrupt our information technology systems or business, potentially exposing us to regulatory action, litigation and liability.
43
Table of Contents
The healthcare industry has been and continues to be a target for cyber-attacks and the number of threats has only increased during the COVID-19 pandemic. Numerous federal agencies that monitor and regulate internet and cyber-crime have issued guidance, alerts and directives warning of software vulnerabilities that require immediate patching, malicious actors targeting healthcare related systems and nation state sponsored hacking designed to steal valuable information, including COVID-19 vaccine and treatment research.
As required by HIPAA, the U.S. Department of Health and Human Services (“HHS”) has issued privacy and security regulations that extensively regulate the disclosure and use of PHI and require covered entities, including healthcare providers and health plans, and vendors known as “business associates,” to implement administrative, physical and technical safeguards to protect the security of PHI. Covered entities and/or their business associates must report breaches of unsecured PHI without unreasonable delay to the HHS Office for Civil Rights (“OCR”), under certain circumstances to affected individuals and, in the case of larger breaches, the media. Violations of the HIPAA privacy and security regulations may result in significant criminal and civil penalties. The HIPAA privacy, security, and breach notification regulations have imposed, and will continue to impose, significant compliance costs on our operations.
HHS’s goal, as directed by The Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”), was to improve the nation’s healthcare system by enabling health information to follow the patient wherever and whenever it is needed. The HHS Office of the National Coordinator for Health Information Technology (“ONC”) and OCR worked together on a number of projects to ensure that this electronic exchange of health information was built on a foundation of privacy and security. OCR introduced revised HIPAA regulations to expand individuals’ rights to access their information and restrict certain disclosures of PHI to health plans, extend the applicability of certain of the Privacy and Security Rules’ requirements to the business associates of covered entities, establish new limitations on the use and disclosure of PHI for marketing and fundraising purposes, and prohibit the sale of PHI without patient authorization. In addition, the rules were designed to strengthen and expand OCR’s ability to enforce HIPAA’s Privacy and Security provisions and to strengthen the privacy and security of health information, and were integral in broadening the use of health IT in healthcare today.
Furthermore, under the administrative simplification provisions in HIPAA and the PPACA, CMS set national standards that require the use of uniform electronic transactions, establish code sets, and define unique identifiers to standardize business practices to simplify communications with insurers for healthcare claims and simplify payment transactions submitted or received electronically. CMS administers the Compliance Review Program to ensure compliance among covered entities with HIPAA Administrative Simplification rules for electronic healthcare transactions that include imposing civil money penalties on covered entities that violate any HIPAA Administrative Simplification requirements.
The 21st Century Cures Act gave broad authority to the ONC to require that all electronic health information be accessible without special effort by the user. The regulations that were finalized in June 2020 require the use of open or standardized Application Programming Interfaces (“APIs”) so that individuals can access their health information securely and easily through apps on their phones and other devices. The ONC Rules also prohibit “information blocking,” defined as a practice that is likely to interfere with access, exchange, or use of electronic health information. The ONC rules apply to healthcare providers and may impact our operations and IT interfaces and systems as interoperability continues to evolve and as the definition of information blocking is more clearly defined through HHS OIG rulemaking, oversight, and enforcement. In December 2020, the ONC issued Proposed Modifications to the HIPAA Privacy Rule to Support, and Remove Barriers to, Coordinated Care and Individual Engagement (this proposed rule was published in the Federal Register on January 21, 2021). These proposed HIPAA modifications are meant to streamline patient access and improve information sharing through standardized APIs and the use of third-party mobile applications. In order to do so, OCR has proposed modifications to the individuals’ right of access to their protected health information, putting patients in charge of their health records and giving patients and their families more control over their healthcare choices. While these HIPAA Privacy Rule modifications are still in the proposed phase of
44
Table of Contents
rulemaking, in order to remain HIPAA compliant when the new rules are finalized and implemented, healthcare providers may need to modernize their information technology capabilities and update internal policies and procedures, as well as business associate agreements.
In addition, various federal and state legislative and regulatory bodies, or self-regulatory organizations, may expand current laws or regulations, enact new laws or regulations or issue revised rules or guidance regarding privacy, data protection and consumer protection. For instance, the California Consumer Privacy Act (“CCPA”) became effective on January 1, 2020 and on June 1, 2020, the California Attorney General’s office released the third set of CCPA proposed regulations. The CCPA gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used by requiring covered businesses to provide new disclosures to California consumers (as that term is broadly defined) and provide such consumers new ways to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Despite its broad scope, the CCPA does create certain exemptions designed around HIPAA. For example, CCPA does not apply to medical information as defined in the California Confidentiality of Medical Information Act (“CCMIA”) or PHI as defined by HIPAA, which is collected by a covered entity or business associate. In addition, in November 2020, Californians approved Proposition 24, that was also known as the California Privacy Rights Act (the “CPRA”). The CPRA modifies and expands the CCPA and established a new California Privacy Protection Agency. While the CPRA extended the current CCPA exemption of employment and business-to-business data until January 1, 2023, it also established January 1, 2023 as the new compliance date for most of the other substantive provisions that companies doing business in California must be prepared to meet. In addition to applying to businesses that buy and sell personal information the CPRA applies to businesses that buy, sell or share personal information and sets forth a new category of “sensitive personal information” that includes, genetic data; biometric or health information; and sex life or sexual orientation information. In addition to the modifications that enhance individuals’ rights under the CCPA, the CPRA added five more rights, including the authority for the State to regulate the requirement for businesses to conduct risk assessments and cybersecurity audits. There is still a significant amount of uncertainty with respect to the CPRA’s three-year compliance roll-out that may increase our compliance costs and potential liability. Other states, such as New York, Massachusetts, North Dakota, Hawaii, and Maryland have all considered laws that would give citizens increased control over their personal data. With respect to data safeguards laws, there are a number of states that have passed legislation, most notably New York’s Stop Hacks and Improve Electronic Data Security Act (the “SHIELD Act”), signed into law in July 2019, that requires any person or business owning or licensing computerized data that includes the private information of a resident of New York to implement and maintain reasonable safeguards to protect the security, confidentiality, and integrity of the private information. While entities subject to HIPAA are deemed to be in compliance with the security provisions of the SHIELD Act, the SHIELD Act does require such entities to provide notice to the New York Attorney General in the event of a breach of personal information.
There are other similar state privacy laws and data safeguards laws and there have been many efforts to propose similar comprehensive privacy laws at the federal level. Despite the increased interest in data protection, the legal paradigms governing the security and privacy of personal data are complex and technical, and lack uniformity at the federal level. There remains no single federal law that comprehensively regulates the collection and use of personal data.
In the absence of comprehensive federal legislation, there remains a patchwork of numerous laws and legislative and regulatory initiatives at the federal and state levels addressing privacy and security concerns. These laws vary and may impose additional obligations or penalties. For example, various state laws and regulations may require us to notify affected individuals in the event of a data breach involving individually identifiable information (even if no health-related information is involved). The Federal Trade Commission (“FTC”) and many state attorneys general are interpreting existing federal and state consumer protection laws to impose evolving standards for the online collection, use, dissemination and security of health-related and other personal information. Courts may also adopt the standards for fair information practices promulgated by the
45
Table of Contents
FTC, which concern consumer notice, choice, security and access. Consumer protection laws require us to publish statements that describe how we handle personal information and choices individuals may have about the way we handle their personal information. If such information that we publish is considered untrue, it may be subject to government claims of unfair or deceptive trade practices, which could lead to significant liabilities and consequences. Furthermore, according to the FTC, violating consumers’ privacy rights or failing to take appropriate steps to keep consumers’ personal information secure may constitute unfair acts or practices in or affecting commerce in violation of Section 5 of the FTC Act.
Under the Federal CAN-SPAM Act, the Telephone Consumer Protection Act of 1991 (“TCPA”) and the Telemarketing Sales Rule and Medicare regulations, we are limited in the ways in which we can market our products and services by use of email, text or telephone marketing. The CAN-SPAM Act also prohibits and protects consumers against all auto-dialed or pre-recorded calls or text messages to an individual’s cell phone. The actual or perceived improper making of telephone calls or sending of text messages may subject us to potential risks, including liabilities or claims relating to consumer protection laws. Numerous class-action suits under federal and state laws have been filed in recent years against companies that conduct SMS texting programs, with many resulting in multi-million-dollar settlements to the plaintiffs. Any future such litigation against us could be costly and time-consuming to defend. For example, the TCPA, a federal statute that protects consumers from unwanted telephone calls, faxes and text messages, restricts telemarketing and the use of automated SMS text messages without proper consent. Additionally state regulators may determine that telephone calls to our patients are subject to state telemarketing regulations. If we do not comply with existing or new laws and regulations related to telephone contacts or patient health information, we could be subject to criminal or civil sanctions. New health information standards, whether implemented pursuant to HIPAA, HITECH, the PPACA, congressional action or otherwise, could have a significant effect on the manner in which we handle healthcare-related data and communications with Payors, and the cost of complying with these standards could be significant. The scope and interpretation of the laws that are or may be applicable to the delivery of consumer phone calls, emails and text messages are continuously evolving and developing. If we do not comply with these laws or regulations or if we become liable under these laws or regulations, we could face direct liability, could be required to change some portions of our business model, and could face negative publicity and our business, financial condition and results of operations could be adversely affected. Even an unsuccessful challenge of our phone, email or SMS text practices by consumers, regulatory authorities or other third parties could result in negative publicity and could require a costly response from and defense by us.
Compliance with changes in privacy and information security laws and with rapidly evolving industry standards may result in our incurring significant expense due to increased investment in technology and the development of new operational processes. Further, to the extent we fail to comply with one or more federal and/or state privacy and security requirements or if we are found to be responsible for the non-compliance of our vendors, we could be subject to substantial fines or penalties, damage to our reputation, as well as third-party claims which could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Risks Related to this Offering and Ownership of our Common Stock
Our Sponsor and its affiliates control us and their interests may conflict with ours or yours in the future.
Immediately following this offering, our Sponsor and its affiliates will beneficially own approximately 72.0% of our common stock (or 68.8% if the underwriters exercise their option to purchase additional shares in full) assuming an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus. For additional information see “Principal and Selling Stockholders.” Moreover, under our amended and restated bylaws and the stockholders’ agreement with our Sponsor and its affiliates that will be in effect by the completion of this offering, for so long as our pre-IPO owners and their affiliates retain significant ownership of us, we will agree to nominate to our board individuals designated by our Sponsor, whom we refer to as the “Sponsor Directors.” Even when our Sponsor and its affiliates cease to own shares of our stock
46
Table of Contents
representing a majority of the total voting power, for so long as our Sponsor continues to own a significant percentage of our stock, our Sponsor will still be able to significantly influence the composition of our board of directors and the approval of actions requiring stockholder approval through their voting power. Accordingly, for such period of time, our Sponsor will have significant influence with respect to our management, business plans and policies, including the appointment and removal of our officers. In particular, for so long as our Sponsor continues to own a significant percentage of our stock, our Sponsor will be able to cause or prevent a change of control of our company or a change in the composition of our board of directors and could preclude any unsolicited acquisition of our company. The concentration of ownership could deprive you of an opportunity to receive a premium for your shares of common stock as part of a sale of our company and ultimately might affect the market price of our common stock. Additionally, our Sponsor may assign its rights under the stockholders’ agreement to a third party without our consent, for example, in connection with a privately negotiated sale of all or a portion of our Sponsor’s holdings of our common stock to a third party. Such third party would then have the right to designate individuals to be nominated to our board, as well as other rights under the stockholders’ agreement. The interests of our Sponsor or any such third party with respect to such rights may conflict with our interests or your interests in the future.
Our Sponsor and its affiliates engage in a broad spectrum of activities, including investments in the healthcare services industry. In the ordinary course of their business activities, our Sponsor and its affiliates may engage in activities where their interests conflict with our interests or those of our stockholders. Our amended and restated certificate of incorporation will provide that none of our Sponsor, any of its affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his or her director and officer capacities) or his or her affiliates, will have any duty to refrain from engaging, directly or indirectly, in the same business activities or similar business activities or lines of business in which we operate. Our Sponsor also may pursue acquisition opportunities that may be complementary to our business, and, as a result, those acquisition opportunities may not be available to us. In addition, our Sponsor may have an interest in our pursuing acquisitions, divestitures and other transactions that, in its judgment, could enhance its investment, even though such transactions might involve risks to us and our stockholders.
We are a holding company with no operations of our own and we are accordingly dependent upon distributions from our subsidiaries to pay taxes and pay dividends.
We are a holding company and our operations are conducted entirely through our subsidiaries. Our ability to generate cash to pay applicable taxes at assumed tax rates and pay cash dividends we declare, if any, is dependent on the earnings and the receipt of funds from Apria Healthcare Group and its subsidiaries via dividends or intercompany loans. Deterioration in the financial condition, earnings or cash flow of Apria Healthcare Group and its subsidiaries for any reason could limit or impair their ability to pay such distributions. Additionally, to the extent that we need funds and our subsidiaries are restricted from making such distributions under applicable law or regulation or under the terms of our financing arrangements, or are otherwise unable to provide such funds, it could materially adversely affect our liquidity and financial condition.
Upon the listing of our shares on Nasdaq, we will be a “controlled company” within the meaning of the rules of Nasdaq and, as a result, will qualify for, and intend to rely on, exemptions from certain corporate governance requirements. You will not have the same protections afforded to stockholders of companies that are subject to such requirements.
After completion of this offering, our Sponsor will continue to control a majority of the combined voting power of all classes of our stock entitled to vote generally in the election of directors. As a result, we will be a “controlled company” within the meaning of the corporate governance standards of Nasdaq. Under these rules, a company of which more than 50% of the voting power in the election of directors is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance
47
Table of Contents
requirements. For example, controlled companies, within one year of the date of the listing of their common stock:
| • | are not required to have a board that is composed of a majority of “independent directors,” as defined under the Nasdaq rules; |
| • | are not required to have a compensation committee that is composed entirely of independent directors or have a written charter addressing the committee’s purpose and responsibilities; and |
| • | are not required to have director nominations be made, or recommended to the full board of directors, by its independent directors or by a nominations committee that is composed entirely of independent directors, and to adopt a written charter or a board resolution addressing the nominations process. |
For at least some period following this offering, we intend to utilize these exemptions. As a result, immediately following this offering, we do not expect a majority of our directors will have been affirmatively determined to be independent or that our compensation committee or nominating and corporate governance committee of the board will be comprised entirely of directors who have been determined to be independent. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of the corporate governance requirements of Nasdaq.
Our internal controls over financial reporting currently do not meet all of the standards contemplated by Section 404 of the Sarbanes-Oxley Act, and failure to achieve and maintain effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act could have a material adverse effect on our business and the market price of the common stock.
As a public company, we will have significant requirements for enhanced financial reporting and internal controls. The process of designing and implementing effective internal controls is a continuous effort that will require us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company. If we are unable to establish or maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations on a timely basis, result in material misstatements in our consolidated financial statements and harm our operating results. Our internal controls over financial reporting currently do not meet all of the standards contemplated by Section 404 of the Sarbanes-Oxley Act that eventually we will be required to meet. Because currently we do not have comprehensive documentation of our internal controls and have not yet tested our internal controls in accordance with Section 404, we cannot conclude in accordance with Section 404 that we do not have a material weakness in our internal controls or a combination of significant deficiencies that could result in the conclusion that we have a material weakness in our internal controls. Beginning with our second annual report on Form 10-K, our independent registered public accounting firm will be required to attest to the effectiveness of our internal controls over financial reporting on an annual basis. If we are not able to complete our initial assessment of our internal controls and otherwise implement the requirements of Section 404 in a timely manner or with adequate compliance, our independent registered public accounting firm may not be able to certify as to the adequacy of our internal controls over financial reporting.
Matters impacting our internal controls may cause us to be unable to report our financial information on a timely basis and thereby subject us to adverse regulatory consequences, including sanctions by the SEC or violations of applicable stock exchange listing rules, which may result in a breach of the covenants under existing or future financing arrangements. There also could be a negative reaction in the financial markets due to a loss of investor confidence in us and the reliability of our financial statements. Confidence in the reliability of our financial statements also could suffer if we or our independent registered public accounting firm were to report a material weakness in our internal controls over financial reporting. This could materially adversely affect us and lead to a decline in the market price of our common stock.
48
Table of Contents
Because we have no current plans to pay dividends on our common stock following this offering, you may not receive any return on your investment unless you sell your common stock for a price greater than that which you paid for it.
Although we paid dividends to our pre-IPO owners prior to this offering, we have no current plans to pay dividends on our common stock following this offering. The declaration, amount and payment of any future dividends on shares of common stock will be at the sole discretion of our board of directors. Our board of directors may take into account general and economic conditions, our financial condition and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions and implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as our board of directors may deem relevant. In addition, our ability to pay dividends is limited by our existing indebtedness and may be limited by covenants of other indebtedness we or our subsidiaries incur in the future. As a result, you may not receive any return on an investment in our common stock unless you sell your common stock for a price greater than that which you paid for them.
You may be diluted by the future issuance of additional common stock in connection with our incentive plans, acquisitions or otherwise.
After this offering we will have approximately 964.8 million shares of common stock authorized but unissued. Our amended and restated certificate of incorporation will authorize us to issue these shares of common stock and options, rights, warrants and appreciation rights relating to common stock for the consideration and on the terms and conditions established by our board of directors in its sole discretion, whether in connection with acquisitions or otherwise. Additionally:
| • | 2,594,224 shares will be issuable upon settlement of outstanding SARs granted pursuant to the 2015 Plan if such SARs were vested and exercised at the time of this offering (assuming an offering price of $20.00 per share of common stock, which is the midpoint of the price range set forth on the cover of this prospectus). At the time of this offering, there will be 3,766,228 SARs outstanding under the 2015 Plan with a weighted average strike price of $6.22. See “Executive Compensation—Long-Term Equity Incentive Compensation—Stock Appreciation Rights of Apria Healthcare Group”; |
| • | awards will be granted under the 2019 LTIP (as defined herein) with a maximum value of $4.4 million (or 220,000 shares assuming an offering price of $20.00 per share of common stock, which is the midpoint of the price range set forth on the cover of this prospectus, which such shares will be issued pursuant to Omnibus Incentive Plan). See “Executive Compensation—Long-Term Cash Incentive Compensation—2019 LTIP”; and |
| • | we have reserved 3,912,324 shares for issuance under our Omnibus Incentive Plan. See “Executive Compensation—Equity Incentive Plans—Omnibus Incentive Plan.” |
Any common stock that we issue, including under our Omnibus Incentive Plan, the 2015 Plan, the 2019 LTIP or other equity incentive plans that we may adopt in the future, would dilute the percentage ownership held by the investors who purchase common stock in this offering.
If we or our pre-IPO owners sell additional shares of our common stock after this offering or are perceived by the public markets as intending to sell them, the market price of our common stock could decline.
The sale of substantial amounts of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock. These sales, or the possibility that these sales may occur, also might make it more difficult for you to sell your common stock in the future at a time and at a price that you deem appropriate, if at all. Upon completion of this offering, we will have a total of 35,210,915 shares of our common stock outstanding. Of the outstanding shares, the 7,500,000 shares sold in this offering (or 8,625,000 shares of our common stock if the underwriters exercise their option to purchase additional shares in full), assuming an offering price of $20.00 per share of common stock, which is the
49
Table of Contents
midpoint of the range on the front cover of this prospectus, will be freely tradable without restriction or further registration under the Securities Act of 1933 (the “Securities Act”), except that any shares held by our affiliates, as that term is defined under Rule 144 of the Securities Act, may be sold only in compliance with the limitations described in “Shares Eligible for Future Sale.”
The remaining outstanding 27,710,915 shares of common stock held by our pre-IPO owners and management after this offering will be subject to certain restrictions on resale. We, our officers, directors, director nominee and certain holders (including the selling stockholders) of our outstanding shares of common stock immediately prior to this offering, including our Sponsor, that collectively will own 26,540,051 shares following this offering, will sign lock-up agreements with the underwriters that will, subject to certain customary exceptions, restrict the sale of the shares of our common stock held by them for 180 days following the date of this prospectus. Citigroup Global Markets Inc. and Goldman Sachs & Co. LLC may, in their sole discretion, release all or any portion of the shares of common stock subject to lock-up agreements. See “Underwriting” for a description of these lock-up agreements. See “Principal and Selling Stockholders” and “Shares Eligible for Future Sale—Lock-Up Arrangements.”
Upon the expiration of the lock-up agreements described above, all of such shares will be eligible for resale in the public market, subject, in the case of shares held by our affiliates, to volume, manner of sale and other limitations under Rule 144. We expect that our Sponsor will continue to be considered an affiliate following the expiration of the lock-up period based on its expected share ownership and its board nomination rights. Certain other of our stockholders may also be considered affiliates at that time. However, subject to the expiration or waiver of the 180-day lock-up period, the holders of these shares of common stock will have the right, subject to certain exceptions and conditions, to require us to register their shares of common stock under the Securities Act, and they will have the right to participate in future registrations of securities by us. Registration of any of these outstanding shares of common stock would result in such shares becoming freely tradable without compliance with Rule 144 upon effectiveness of the registration statement. See “Shares Eligible for Future Sale.”
We intend to file one or more registration statements on Form S-8 under the Securities Act to register 7,698,552 shares of our common stock or securities convertible into or exchangeable for shares of our common stock issued pursuant to our Omnibus Incentive Plan and the 2015 Plan. Any such Form S-8 registration statements will automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
As restrictions on resale end, the market price of our shares of common stock could drop significantly if the holders of these restricted shares sell them or are perceived by the market as intending to sell them. These factors could also make it more difficult for us to raise additional funds through future offerings of our shares of common stock or other securities or to use our shares of common stock as consideration for acquisitions of other businesses, investments or other corporate purposes.
Anti-takeover provisions in our organizational documents and Delaware law might discourage or delay acquisition attempts for us that you might consider favorable.
Our amended and restated certificate of incorporation and amended and restated bylaws that will become effective immediately prior to the consummation of this offering will contain provisions that may make the merger or acquisition of our company more difficult without the approval of our board of directors. Among other things, these provisions:
| • | provide that our board of directors will be divided into three classes, as nearly equal in size as possible with terms of the directors of only one class expiring in any given year; |
| • | provide for the removal of directors (other than directors elected by a separate vote of the holders of any preferred stock) only for cause and only upon the affirmative vote of the holders of at least |
50
Table of Contents
| 66 2/3% in voting power of the outstanding shares of our capital stock entitled to vote at any time our Sponsor beneficially owns less than 30% of the total voting power of all then outstanding shares of our capital stock entitled to vote generally in the election of directors and provide that specified directors designated pursuant to the stockholders agreement may not be removed without cause without the consent of the specified designating party; |
| • | provide that, subject to the rights of the holders of any preferred stock and the rights granted pursuant to the stockholders agreement, vacancies and newly created directorships may be filled only by the remaining directors at any time our Sponsor beneficially owns less than 30% of the total voting power of all then outstanding shares of our capital stock entitled to vote generally in the election of directors; |
| • | would allow us to authorize the issuance of one or more series of preferred stock in connection with a stockholder rights plan or otherwise, the terms of which may be established by our board and the shares of which may be issued without stockholder approval, and which may include super voting, special approval, dividend, or other rights, powers or preferences superior to the rights of the holders of common stock; |
| • | prohibit stockholder action by written consent at any time our Sponsor beneficially owns less than 30% of the total voting power of all then outstanding shares of our capital stock entitled to vote generally in the election of directors, and require that, at any time our Sponsor beneficially own at least 30% of the total voting power of all then outstanding shares of our capital stock entitled to vote generally in the election of directors, any action by consent of stockholders in lieu of a meeting may only be taken with the consent of the holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote were presented and voted and the consent of the stockholder designated pursuant to our stockholders agreement; |
| • | provide for certain limitations on convening special stockholder meetings; |
| • | provide that the board of directors is expressly authorized to make, alter, or repeal our bylaws and that our stockholders may only amend our bylaws with the approval of 66 2/3% or more of all of the outstanding shares of our capital stock entitled to vote at any time our Sponsor beneficially owns less than 30% of the total voting power of all then outstanding shares of our capital stock entitled to vote generally in the election of directors; |
| • | provide that certain provisions of our amended and restated certificate of incorporation may be amended only by the affirmative vote of the holders of at least 66 2/3% in voting power of the outstanding shares of our capital stock entitled to vote thereon at any time our Sponsor beneficially owns less than 30% of the total voting power of all then outstanding shares of our capital stock entitled to vote generally in the election of directors; |
| • | establish advance notice requirements for nominations for elections to our board or for proposing matters that can be acted upon by stockholders at stockholder meetings; and |
| • | provide that, subject to the rights of holders of preferred stock and the terms of our stockholders agreement, the total numbers of directors shall be determined exclusively by resolution adopted by the Board. |
We have opted out of Section 203 of the General Corporation Law of the State of Delaware (the “DGCL”); however, our amended and restated certificate of incorporation contains similar provisions providing that we may not engage in certain “business combinations” with any “interested stockholder” for a three-year period following the time that the stockholder became an interested stockholder, unless the transaction fits within an enumerated exception, such as board approval of the business combination or the transaction that resulted in a person becoming an interested stockholder prior to the time such person became an interested stockholder. Our amended and restated certificate of incorporation provides that our Sponsor and its affiliates, and any of their respective direct or indirect transferees, and any group as to which such persons are a party, do not constitute
51
Table of Contents
“interested stockholders” for purposes of this provision. See “Description of Capital Stock—Business Combinations.” These anti-takeover provisions and other provisions under our amended and restated certificate of incorporation, amended and restated by laws or Delaware law could discourage, delay or prevent a transaction involving a change in control of our company, including actions that our stockholders may deem advantageous, or negatively affect the trading price of our common stock. These provisions could also discourage proxy contests and make it more difficult for you and other stockholders to elect directors of your choosing and to cause us to take other corporate actions you desire.
Our amended and restated certificate of incorporation will designate the Court of Chancery of the State of Delaware and the federal district courts of the United States of America as the sole and exclusive forums for certain types of actions and proceedings that may be initiated by our stockholders, which could limit our stockholders’ ability to obtain a favorable judicial forum for disputes with the Company or the Company’s directors, officers or other employees.
Our amended and restated certificate of incorporation will provide that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for any (1) derivative action or proceeding brought on behalf of our Company, (2) action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee or stockholder of our Company to the Company or the Company’s stockholders, (3) action asserting a claim against the Company or any current or former director or officer of the Company arising pursuant to any provision of the DGCL or our amended and restated certificate of incorporation or our amended and restated bylaws, or (4) action asserting a claim against us or any director or officer of the Company governed by the internal affairs doctrine. Our amended and restated certificate of incorporation further provides that, unless we consent in writing to the selection of an alternative forum, to the fullest extent permitted by law, the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the federal securities laws of the United States of America. Our amended and restated certificate of incorporation will provide that, to the fullest extent permitted by law, any person or entity purchasing or otherwise acquiring any interest in any shares of our capital stock shall be deemed to have notice of and to have provided consent to the forum provisions in our amended and restated certificate of incorporation. These choice-of-forum provisions may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable or convenient for disputes with the Company or the Company’s directors, officers, other employees or stockholders, which may discourage such lawsuits. Alternatively, if a court were to find these provisions of our amended and restated certificate of incorporation inapplicable or unenforceable with respect to one or more of the specified types of actions or proceedings, we may incur additional costs associated with resolving such matters in other jurisdictions, which could materially and adversely affect our business, financial condition and results of operations and result in a diversion of the time and resources of our management and board of directors.
General Risk Factors
Natural disasters or other catastrophic events could materially disrupt and have a negative effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Natural disasters, such as hurricanes or earthquakes, could disrupt our ability to do business. For example, such events could result in physical damage to one or more of our properties, the temporary closure of one or more of our locations, the temporary inability to process payroll or process claims, a negative effect in our ability to comply with certain licensing requirements, and/or a delay in the delivery of products and the provision of our service offerings. These events could also reduce demand for our products and service offerings, or make it difficult or impossible to receive products from suppliers. We may be required to suspend operations in some of our branch locations, which could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
52
Table of Contents
Future acquisitions or growth initiatives may be unsuccessful and could expose us to unforeseen liabilities.
Our strategic growth plan may involve acquisition of other companies. Any such acquisitions would involve a number of risks and uncertainties, including: difficulties related to combining previously separate businesses into a single unit, including patient transitions, product and service offerings, distribution and operational capabilities and business cultures; loss of patients, providers and Payors and other general business disruption; assumption of liabilities of an acquired business, including unforeseen or contingent liabilities or liabilities in excess of the amounts estimated; and obtaining necessary regulatory licenses and Payor-specific approvals, which may impact the timing of when we are able to bill and collect for services rendered.
The failure to effectively integrate an acquired business in a cost-effective manner could have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources, liquidity or prospects. In addition, we may not be able to realize the potential cost savings, synergies and revenue enhancements that we anticipate from any acquisitions, either in the amount or within the time frame that we expect, and the costs of achieving these benefits may be higher than, and the timing may differ from, what we expect. If we fail to realize anticipated cost savings, synergies or revenue enhancements, our financial results will be adversely affected.
We will incur increased costs and become subject to additional regulations and requirements as a result of becoming a public company, which could lower our profits, make it more difficult to run our business or divert management’s attention from our business.
As a public company, we will be required to commit significant resources and management time and attention to the requirements of being a public company, which will cause us to incur significant legal, accounting and other expenses that we have not incurred as a private company, including costs associated with public company reporting requirements. We also will incur costs associated with the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”) and related rules implemented by the SEC and Nasdaq, and compliance with these requirements will place significant demands on our legal, accounting and finance staff and on our accounting, financial and information systems. In addition, we might not be successful in implementing these requirements. The expenses incurred by public companies generally for reporting and corporate governance purposes have been increasing. We expect these rules and regulations to increase our legal and financial compliance costs and to make some activities more time-consuming and costly, although we are currently unable to estimate these costs with any degree of certainty. These laws and regulations also could make it more difficult or costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. These laws and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as our executive officers. Furthermore, if we are unable to satisfy our obligations as a public company, we could be subject to delisting of our common stock, fines, sanctions and other regulatory action and potentially civil litigation.
If securities or industry analysts do not publish research or reports about our business, or if they downgrade their recommendations regarding our common stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If any of the analysts who cover us downgrade our common stock or publish inaccurate or unfavorable research about our business, our common stock price may decline. If analysts cease coverage of us or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause our common stock price or trading volume to decline and our common stock to be less liquid.
53
Table of Contents
There may not be an active trading market for shares of our common stock, which may cause shares of our common stock to trade at a discount from their initial offering price and make it difficult to sell the shares of common stock you purchase.
Prior to this offering, there has not been a public trading market for shares of our common stock. It is possible that after this offering an active trading market will not develop or continue or, if developed, that any market will be sustained which would make it difficult for you to sell your shares of common stock at an attractive price or at all. The initial public offering price per share of common stock will be determined by agreement among us, the selling stockholders and the representatives of the underwriters, and may not be indicative of the price at which shares of our common stock will trade in the public market after this offering.
The market price of shares of our common stock may be volatile or may decline regardless of our operating performance, which could cause the value of your investment to decline.
Even if a trading market develops, the market price of our common stock may be highly volatile and could be subject to wide fluctuations. Securities markets worldwide experience significant price and volume fluctuations. This market volatility, as well as general economic, market or political conditions, could reduce the market price of shares of our common stock regardless of our operating performance. In addition, our operating results could be below the expectations of public market analysts and investors due to a number of potential factors, including variations in our quarterly operating results or dividends, if any, to stockholders, additions or departures of key management personnel, failure to meet analysts’ earnings estimates, publication of research reports about our industry, litigation and government investigations, changes or proposed changes in laws or regulations or differing interpretations or enforcement thereof affecting our business, adverse market reaction to any indebtedness we may incur or securities we may issue in the future, changes in market valuations of similar companies or speculation in the press or investment community, announcements by our competitors of significant contracts, acquisitions, dispositions, strategic partnerships, joint ventures or capital commitments, adverse publicity about the industries we participate in or individual scandals, and in response the market price of shares of our common stock could decrease significantly. You may be unable to resell your shares of common stock at or above the initial public offering price.
Stock markets have recently experienced extreme price and volume fluctuations. In the past, following periods of volatility in the overall market and the market price of a company’s securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management’s attention and resources.
54
Table of Contents
This prospectus contains forward-looking statements that reflect our current views with respect to, among other things, our operations and financial performance. Forward-looking statements include all statements that are not historical facts. In some cases, you can identify these forward-looking statements by the use of words such as “outlook,” “believes,” “expects,” “potential,” “continues,” “may,” “will,” “should,” “could,” “seeks,” “predicts,” “intends,” “trends,” “plans,” “estimates,” “anticipates” or the negative version of these words or other comparable words. Such forward-looking statements are subject to various risks and uncertainties. Accordingly, there are or will be important factors that could cause actual outcomes or results to differ materially from those indicated in these statements. These factors include but are not limited to those described under “Risk Factors.” These factors should not be construed as exhaustive and should be read in conjunction with the other cautionary statements that are included in this prospectus. We undertake no obligation to publicly update or review any forward-looking statement, whether as a result of new information, future developments or otherwise, except as required by law.
This prospectus includes market and industry data and forecasts that we have derived from independent consultant reports, publicly available information, various industry publications, other published industry sources and our internal data and estimates. Independent consultant reports, industry publications and other published industry sources generally indicate that the information contained therein was obtained from sources believed to be reliable.
Although we believe that these third-party sources are reliable, we do not guarantee the accuracy or completeness of this information, and neither we nor the underwriters have independently verified this information. Some market data and statistical information are also based on our good faith estimates, which are derived from management’s knowledge of our industry and such independent sources referred to above. Certain market, ranking and industry data included elsewhere in this prospectus, including the size of certain markets and our size or position and the positions of our competitors within these markets, including our services relative to our competitors, are based on estimates of our management. These estimates have been derived from our management’s knowledge and experience in the markets in which we operate, as well as information obtained from surveys, reports by market research firms, our customers, distributors, suppliers, trade and business organizations and other contacts in the markets in which we operate and have not been verified by independent sources. Unless otherwise noted, all of our market share and market position information presented in this prospectus is an approximation. Our market share and market position in each of our lines of business, unless otherwise noted, is based on our sales relative to the estimated sales in the markets we served. References herein to our being a leader in a market or product category refer to our belief that we have a leading market share position in each specified market, unless the context otherwise requires. As there are no publicly available sources supporting this belief, it is based solely on our internal analysis of our sales as compared to our estimates of sales of our competitors. In addition, the discussion herein regarding our various end markets is based on how we define the end markets for our products, which products may be either part of larger overall end markets or end markets that include other types of products and services.
Our internal data and estimates are based upon information obtained from trade and business organizations and other contacts in the markets in which we operate and our management’s understanding of industry conditions. Although we believe that such information is reliable, we have not had this information verified by any independent sources.
55
Table of Contents
TRADEMARKS, SERVICE MARKS AND TRADE NAMES
We own or have rights to trademarks, service marks or trade names that we use in connection with the operation of our business. In addition, our names, logos and website domain names and addresses are our service marks or trademarks. We do not intend our use or display of other companies’ trademarks, service marks, copyrights or trade names to imply a relationship with, or endorsement or sponsorship of us by, any other companies. The trademarks we own or have the right to use include, among others, Apria. We also own or have the rights to copyrights that protect the content of our literature, be it in print or electronic form.
Solely for convenience, certain trademarks, service marks and trade names referred to in this prospectus are used without the ™ or ® symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent under applicable law, our rights or the rights of the applicable licensors to these trademarks, service marks and trade names. All trademarks, service marks and trade names appearing in this prospectus are the property of their respective owners.
56
Table of Contents
Table of Contents
We have no current plans to pay dividends on our common stock following this offering. Any decision to declare and pay dividends in the future will be made at the sole discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions and other factors that our board of directors may deem relevant. Because we are a holding company and have no direct operations, we will only be able to pay dividends from funds we receive from our subsidiaries. In addition, our ability to pay dividends will be limited by covenants in our existing indebtedness and may be limited by the agreements governing any indebtedness we or our subsidiaries may incur in the future.
58
Table of Contents
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2020:
| • | on a historical basis; and |
| • | on an as adjusted basis to reflect (x) the Credit Facility Amendment and cash dividend and distribution to SARs holders effected on December 15, 2020 and (y) the pre-IPO reorganization transactions described under “Summary—Our Organizational Structure.” |
The information below is illustrative only and our capitalization following this offering will be adjusted based on the actual terms of this offering. Cash and cash equivalents are not components of our total capitalization. You should read this table together with the other information contained in this prospectus, including “Use of Proceeds,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical financial statements and related notes thereto included elsewhere in this prospectus.
| September 30, 2020 | ||||||||
| Actual | As Adjusted | |||||||
| (In thousands, except share and per share amounts) |
||||||||
| Cash and cash equivalents(1)(2) |
$ | 140,125 | $ | 186,774 | ||||
|
|
|
|
|
|||||
| Term Loan A(3) |
$ | 146,250 | $ | 406,250 | ||||
| Apria, Inc. preferred stock, par value $0.01 per share, 100,000,000 shares authorized, zero shares issued and outstanding on an as adjusted basis |
— | — | ||||||
| Apria, Inc. common stock, par value $0.01 per share, 1,000,000,000 shares authorized, and 35,210,915 outstanding on an as adjusted basis |
— | 352 | ||||||
| Additional paid-in capital(4)(5) |
1,162,542 | 954,390 | ||||||
| Accumulated deficit |
(1,006,303 | ) | (1,006,303 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
156,239 | (51,561 | ) | |||||
|
|
|
|
|
|||||
| Total capitalization |
$ | 302,489 | $ | 354,689 | ||||
|
|
|
|
|
|||||
| (1) | Cash and cash equivalents on as adjusted basis reflects the proceeds from the Incremental Term Loans, the $200.3 million distribution to our stockholders and the $9.7 million distribution to SARs holders declared and paid in December 2020, along with payments for accrued transaction costs through September 30, 2020 and the cash settlement of the Tranche I RSUs (as defined herein). |
| (2) | Cash and cash equivalents on an as adjusted basis reflects the payment of approximately $1.6 million in respect of the Tranche I RSUs (as defined herein). In 2018, our Board of Directors determined it was appropriate to enter into a letter agreement with our chief financial officer (“CFO”) that provides for an additional long-term equity incentive award in the form of restricted stock units (“RSUs”), which will become granted by the Company pursuant to the Omnibus Incentive Plan upon the occurrence of this offering. The total value of RSUs granted to the CFO will be equal to 0.4% of the amount by which the equity value of the Company exceeds negative $36.181 million upon the occurrence of this offering. Assuming an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus, the total value to the CFO with respect to the RSUs is $3.2 million. The RSUs vest in tranches, with Tranche I RSUs vesting immediately upon the closing of the transaction and the remaining RSUs vesting in two equal tranches upon the six month and first year anniversary following the transaction, subject to the CFO’s continued employment. Each tranche of RSUs can be settled in either cash or shares of our common stock at the election of the CFO. It is our expectation that the Tranche I RSUs will be settled in cash and paid upon the closing of this offering. The RSUs will be accounted for as liability classified stock-based compensation. For further description of the RSUs, see “Executive and Director Compensation—Long-Term Cash Incentive Compensation”. |
59
Table of Contents
| (3) | Reflects the principal amount of outstanding debt under the Term Loan A Facility after giving effect to the Incremental Term Loans and is not presented net of unamortized debt issuance costs. |
| (4) | Additional paid-in capital on as adjusted basis is reduced by the $200.3 million dividend payment to our stockholders and a $9.7 million distribution to SARs holders declared and paid in December 2020. Given our accumulated deficit, the Company accounted for the dividend and distribution through additional paid-in capital by analogy to SAB Topic 3-C (codified in FASB ASC Topic 480). |
| (5) | Additional paid-in capital on as adjusted basis reflects the reclassification of the 2019 LTIP (as defined herein) from a liability to equity classified award. In connection with this offering, pursuant to the terms of the 2019 LTIP, the 2019 LTIP will be settled according to the existing vesting schedule in shares of our common stock with the number of shares determined by dividing the actual amount of each participant’s earned award by the volume-weighted average price of a share of our common stock over the 20 trading days following this offering, beginning on the first trading date after the date of this offering. For further description of the 2019 LTIP, see “Executive and Director Compensation—Long-Term Cash Incentive Compensation.” |
60
Table of Contents
If you invest in shares of our common stock in this offering, your investment will be immediately diluted to the extent of the difference between the initial public offering price per share of common stock and the as adjusted net tangible book value per share of common stock after this offering. Dilution results from the fact that the per share offering price of the shares of common stock is substantially in excess of the as adjusted net tangible book value per share attributable to our pre-IPO owners.
Our net tangible book value as of September 30, 2020 was approximately $94.6 million, or $2.69 per share of common stock. Net tangible book value represents the amount of total tangible assets less total liabilities, and net tangible book value per share of common stock represents net tangible book value divided by the number of shares of common stock outstanding upon consummation of the pre-IPO reorganization transactions.
We will not receive any proceeds from the sale of common stock by the selling stockholders in this offering. Consequently, this offering will not result in any change to our net tangible book value per share, prior to giving effect to the payment of estimated fees and expenses in connection with this offering. Purchasing shares of common stock in this offering will result in net tangible book value dilution to new investors of $17.31 per share. The following table illustrates this per share dilution to new investors:
| Assumed initial public offering price per share of common stock |
$ | 20.00 | ||||||
| Net tangible book value per share of common stock as of September 30, 2020 |
$ | 2.69 | ||||||
|
|
|
|||||||
| As adjusted net tangible book value per share of common stock after the offering |
$ | 2.69 | ||||||
|
|
|
|||||||
| Dilution per share of common stock to investors in this offering |
$ | 17.31 | ||||||
|
|
|
The following table summarizes, as of September 30, 2020, the average price per share paid by pre-IPO owners and by investors in this offering. As the table shows, new investors purchasing shares in this offering will pay an average price per share lower than our pre-IPO owners paid. The table below reflects an assumed initial public offering price of $20.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, for shares purchased in this offering and excludes underwriting discounts and commissions and estimated offering expenses payable by us:
| Shares of Common Stock Purchased |
Total Consideration |
Average Price Per Share of Common Stock |
||||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||||
| (In thousands) |
||||||||||||||||||||
| Pre-IPO owners |
27,710,915 | 78.7% | $ | 676,908 | (1) | 81.9 | % | $ | 24.43 | |||||||||||
| Investors in this offering |
7,500,000 | 21.3% | $ | 150,000 | 18.1 | % | $ | 20.00 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
35,210,915 | 100.0% | $ | 826,908 | 100.0 | % | $ | 23.48 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | New investors purchasing shares in this offering will pay an average price per share lower than the price paid by our pre-IPO owners. The total consideration paid by our pre-IPO owners has not been adjusted for returns on such consideration arising from distributions. |
Each $1.00 increase in the assumed offering price of $20.00 per share would increase total consideration paid by investors in this offering by $7.5 million, assuming the number of shares offered by us remains the same and after deducting estimated underwriting discounts and commissions. A $1.00 decrease in the assumed initial public offering price per share would result in equal changes in the opposite direction.
61
Table of Contents
The dilution information above is for illustrative purposes only. Our net tangible book value following the consummation of this offering is subject to adjustment based on the actual initial public offering price of our shares and other terms of this offering determined at pricing.
62
Table of Contents
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
We derived the selected statement of income data for the years ended December 31, 2019, 2018 and 2017 and the selected balance sheet data as of December 31, 2019 and 2018 from our audited consolidated financial statements included elsewhere in this prospectus. We derived the selected statement of income data for the years ended December 31, 2016 and 2015 and balance sheet data as of December 31, 2017, 2016 and 2015, from our consolidated financial statements that are not included in this prospectus. The selected statement of income data for the nine months ended September 30, 2020 and 2019 and the selected balance sheet data as of September 30, 2020 were derived from our unaudited condensed consolidated financial statements included elsewhere in this prospectus. The unaudited condensed consolidated financial statements have been prepared on the same basis as our audited consolidated financial statements and, in the opinion of our management, reflect all normal recurring adjustments necessary for the fair statement of our consolidated results for these periods. The results for any interim period are not necessarily indicative of the results that may be expected for the full year. Our historical results are not necessarily indicative of the results expected for any future period.
You should read the selected historical financial data below, together with the consolidated financial statements and related notes thereto appearing elsewhere in this prospectus, as well as “Summary—Summary Historical Financial and Other Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the other financial information included elsewhere in this prospectus.
On January 1, 2019, we adopted FASB ASU No. 2016-02, Leases (“Topic 842”), using the modified
retrospective transition method. For lessor accounting, upon adoption the provision for doubtful accounts
associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now charged to net rental revenue instead of general and administrative expense. For lessee accounting, upon adoption total assets and total liabilities increased $74.4 million as of January 1, 2019.
The comparative information for periods prior to adoption has not been restated and continues to be reported under the accounting standards in effect for those periods.
| Nine Months Ended September 30, |
Year Ended December 31, | |||||||||||||||||||||||||||
| (in thousands, except per share data) |
2020 | 2019 | 2019 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||||||||
| Statement of Income Data: |
||||||||||||||||||||||||||||
| Total net revenues(1)(2) |
$ | 814,928 | $ | 807,710 | $ | 1,088,875 | $ | 1,110,883 | $ | 1,073,810 | $ | 1,061,258 | $ | 1,094,434 | ||||||||||||||
| Operating income(1) |
36,898 | 15,098 | 27,415 | 20,397 | 26,151 | 7,500 | 21,870 | |||||||||||||||||||||
| Net income(3)(4)(5) |
20,268 | 9,840 | 15,622 | 13,127 | 96,951 | 9,731 | 20,001 | |||||||||||||||||||||
| Basic and diluted earnings per share |
$ | 20.42 | $ | 9.91 | $ | 15.74 | $ | 13.22 | $ | 97.66 | $ | 9.80 | $ | 20.92 | ||||||||||||||
| Pro Forma Earnings Per Share Information:(6) |
||||||||||||||||||||||||||||
| Pro forma basic earnings per share |
$ | 0.58 | $ | 0.44 | ||||||||||||||||||||||||
| Pro forma diluted earnings per share |
$ | 0.54 | $ | 0.42 | ||||||||||||||||||||||||
| Pro forma basic weighted average shares outstanding |
35,210,915 | 35,210,915 | ||||||||||||||||||||||||||
| Pro forma diluted weighted average shares outstanding |
37,525,139 | 37,565,139 | ||||||||||||||||||||||||||
63
Table of Contents
| September 30, | December 31, | |||||||||||||||||||||||
| 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | |||||||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||||||
| Total assets(2) |
$ | 645,051 | $ | 617,153 | $ | 576,222 | $ | 643,553 | $ | 535,675 | $ | 525,797 | ||||||||||||
| Total liabilities(2)(3)(7) |
488,812 | 482,637 | 290,970 | 298,049 | 288,843 | 290,078 | ||||||||||||||||||
| Total stockholders’ equity(8) |
156,239 | 134,516 | 285,252 | 345,504 | 246,832 | 235,719 | ||||||||||||||||||
| (1) | On January 1, 2018, we adopted Topic 606, using the modified retrospective method. Upon adoption, patient co-pay and deductible amounts that were previously included in the provision for doubtful accounts related to implicit price concessions, which were $9.5 million for the year ended December 31, 2018, are now presented as an offsetting adjustment to the related fee-for-service sale net revenues. The comparative information for periods prior to adoption has not been restated and continues to be reported under the accounting standards in effect for those periods. |
| (2) | On January 1, 2019, we adopted Topic 842, using the modified retrospective transition method. For lessor accounting, upon adoption the provision for doubtful accounts associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now charged to net rental revenue instead of general and administrative expense. For lessee accounting, upon adoption total assets and total liabilities increased $74.4 million as of January 1, 2019. The comparative information for periods prior to adoption has not been restated and continues to be reported under the accounting standards in effect for those periods. |
| (3) | For the nine months ended September 30, 2020 and year ended December 31, 2019, net income reflects an expense of $32.5 million and $12.2 million, respectively, representing the estimated probable loss in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York. See “Business—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York” and Note 8 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus for additional information. |
| (4) | For the year ended December 31, 2017, net income reflects the net valuation allowance release of $105.1 million on the basis of our reassessment of the amount of deferred tax assets that are more likely than not to be realized, offset by a charge to tax expense of $24.2 million due to the re-measurement of deferred tax assets and liabilities resulting from the decrease in federal corporate tax rate as a result of the enactment of the Tax Cuts and Jobs Act in December 2017. |
| (5) | For the year ended December 31, 2015, $20.0 million relates to income from continuing operations, which excludes $0.8 million of income from operations of discontinued component Home Infusion Segment included in net income. |
| (6) | Unaudited basic pro forma earnings per share is computed using historical net income for the period divided by the pro forma weighted average number of common shares outstanding during the period after giving effect to the pre-IPO reorganization transactions. Pro forma diluted earnings per share is computed using the weighted average number of common shares and the effect of dilutive equity awards after giving effect to the pre-IPO reorganization transactions. |
| (7) | On June 21, 2019, we entered the Credit Facility. Proceeds from the TLA were used to fund the dividend payment to common stockholders and SARs holders. |
| (8) | In June 2019, we declared and paid a $175.0 million dividend to common stockholders and SARs holders. In June 2018, we declared $75.0 million in dividends paid to common stockholders on July 5, 2018. |
64
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with the “Selected Historical Consolidated Financial Data” and our consolidated financial statements and related notes thereto included elsewhere in this prospectus. In addition to historical information, this discussion contains forward-looking statements that involve risks, uncertainties and assumptions that could cause actual results to differ materially from management’s expectations. Factors that could cause such differences are discussed in “Forward-Looking Statements” and “Risk Factors.”
Our Company
We are a leading provider of integrated home healthcare equipment and related services in the United States. We offer a comprehensive range of products and services for in-home care and delivery across three core service lines: (1) home respiratory therapy (including home oxygen and NIV services); (2) OSA treatment (including CPAP and bi-level positive airway pressure devices, and patient support services); and (3) NPWT. Additionally, we supply a wide range of home medical equipment and other products and services to help improve the quality of life for patients with home care needs. Our revenues are generated through fee-for-service and capitation arrangements with Payors for equipment, supplies, services and other items we rent or sell to patients. Through our offerings, we also provide patients with a variety of clinical and administrative support services and related products and supplies, most of which are prescribed by a physician as part of a care plan. We are focused on being the industry’s highest-quality provider of home healthcare equipment and related services, while maintaining our commitment to being a low-cost operator. We offer a compelling value proposition to patients, providers and Payors by allowing patients to receive necessary care and services in the comfort of their own home, while, at the same time, reducing the costs of treatment. We generated over $1 billion of net revenue in 2019, of which approximately 80% was from home respiratory therapy and OSA treatment, service categories in which we believe we have a leading market position.
We believe our integrated product and service offerings, combined with our national scale and strong reputation, provide us with a strategic advantage in being a preferred home healthcare provider for patients, providers and Payors. Our Payors include substantially all of the national and regional insurers, managed care organizations and government Payors in the United States. We benefit from long-standing relationships with a community of providers and referral sources for post-acute services across the acuity spectrum because of the consistency and reliability of our high quality clinical support, our national distribution footprint and our breadth of Payor relationships.
Our product and service offerings are distinguished by the complexity and sophistication required in their clinical delivery, logistical coordination and payment arrangements. We offer patients and providers differentiated clinical service, leveraging our protocols and expertise to improve outcomes across our service lines. With an expansive network of delivery technicians and therapists that is not readily replicated, we are able to provide home healthcare therapies that require high-touch service, providing a bridge from the acute care setting to the home. In 2019, we served nearly 2 million patients, made over 2.4 million deliveries and conducted over 744,000 clinician interactions with our patients.
The healthcare sector is heavily regulated, and our business is accordingly subject to extensive government regulation, including numerous laws directed at regulating reimbursement of our products and services under various government programs and preventing fraud and abuse. For additional information, see “Business—Government Regulation.”
65
Table of Contents
Trends and Factors Affecting our Future Performance
Significant trends and factors that we believe may affect our future performance include:
| • | Growing addressable markets. We are aligned with large and growing addressable markets across our core service lines. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025. |
| • | Aging population. As the baby boomer population ages and life expectancy increases, the elderly—who comprise a large portion of our patients—will represent a higher percentage of the overall population. The CMS Office of the Actuary projects that the number of Medicare beneficiaries will grow, on average, by 2.5% annually over the period from 2020 to 2028 and the U.S. Census Bureau projects that the United States population aged 65 and over will grow substantially from 15.2% of the population in 2016 to 20.6% of the population by 2030. This demographic trend has resulted in patient volume growth in the United States and is expected to continue to drive volume growth. |
| • | Rising incidence of chronic diseases. Increasing obesity rates, the clinical consequences of the high prevalence of smoking from earlier decades, the current under-diagnosis of certain health conditions and higher diagnosis rates for a number of chronic health conditions, such as chronic obstructive pulmonary disease, OSA, diabetes and others, have collectively driven growth in the industry. We believe that in the United States 1 in 15 adults has moderate to severe obstructive sleep apnea, while 1 in 10 adults has diabetes, with asthma similarly prevalent among adults. We believe that patients with these chronic conditions will increasingly be treated in their homes instead of in the hospital setting and utilize the services that we provide. |
| • | Transition to value-based healthcare. Government and commercial Payors are increasingly seeking ways to shift from traditional fee-for-service to a value-based model. We believe that the ability to transition patients from the acute care setting to the home represents a critical part of this effort. As a leading provider of home healthcare services, we believe that we will increasingly benefit from this paradigm shift in the industry. In addition, we believe our demonstrated expertise in non-traditional payment models, such as capitation arrangements, will position us well to take advantage of this trend. |
| • | Increased prevalence of and preference for in-home treatments. Improved technology has resulted in a wider variety of treatments being administered in patient homes, and medical advancements have also made medical equipment more simple, adaptable and cost-effective for use in the home. According to CMS estimates, between 2020 and 2028, U.S. home healthcare spending is projected to increase to $201.3 billion, growing at a CAGR of approximately 7%. |
| • | Consolidation of the highly fragmented market to the benefit of national players. Between 2012 and 2018, the overall number of DMEPOS suppliers that bill Medicare more than $10 million annually fell from 73 to 57, and between 2013 and 2020, the total number of DMEPOS suppliers that bill Medicare fell from 8,837 to 6,152, a decline of approximately 30%. We attribute this decline to the inability of smaller regional players to make the significant capital investment and achieve the scale required to effectively compete in the economic environment that followed the implementation of the CBP. We believe that the home healthcare market will continue to favor larger, financially stable, national players with consistent, scalable, high quality service. |
| • | Decreasing price and reimbursement levels. We expect to continue to face pricing pressures from Medicare and Medicaid, as a result of programs such as the DMEPOS CBP or government sequestrations, as well as from our managed care Payors as they seek to lower costs by obtaining more favorable pricing from providers such as us. In addition to the pricing reductions, such changes could cause us to provide reduced levels of certain products and services in the future, resulting in corresponding reductions in revenue. |
| • | Cost containment efforts of Payors. The consolidation of our managed care Payors into larger purchasing groups, such as group purchasing organizations and integrated delivery networks, has |
66
Table of Contents
| increased their negotiating and purchasing power. This trend, in turn, has resulted in increasing pricing pressure on us due to the consolidation of healthcare facilities, purchasing groups and U.S.-based insurance Payors, pricing concessions and other cost containment efforts, such as the CBP. Contracts with Payors that generated approximately 42% of our revenue in 2019 will expire in 2021. |
Certain additional items may impact the comparability of the historical results presented below with our future performance, such as:
| • | Cost of being a public company. To operate as a public company, we will be required to continue to implement changes in certain aspects of our business and develop, manage, and train management level and other employees to comply with ongoing public company requirements. We will also incur new expenses as a public company, including public reporting obligations, proxy statements, stockholder meetings, stock exchange fees, transfer agent fees, SEC and Financial Industry Regulatory Authority filing fees and offering expenses. |
| • | Investment in longer-lived respiratory equipment. We have made significant capital investments in ventilation products with longer useful lives than traditional home oxygen equipment. We expect this legacy investment will continue to generate revenue in future periods without the need for significant incremental capital, enhancing our cash flows. |
Impact of COVID-19 Pandemic
Our priorities during the COVID-19 pandemic are protecting the health and safety of our employees (including patient-facing employees providing respiratory and other services), maximizing the availability of our services and products to support patient health needs, and the operational and financial stability of our business.
In response to the COVID-19 pandemic and the National Emergency Declaration, dated March 13, 2020, we activated certain business interruption protocols, including acquisition and distribution of personal protective equipment (PPE) to our patient-facing employees, accelerated capital expenditures of certain products and relocation of significant portions of our workforce to “work-from-home” status.
While the impact of the COVID-19 pandemic, the National Emergency Declaration and the various state and local government imposed stay-at-home restrictions did not have a material adverse impact on our consolidated operating results for the nine months ended September 30, 2020, we have experienced declines in net revenues in certain services associated with elective medical procedures and the disruption in physician practices (such as commencement of new CPAP services, ventilation therapy and negative pressure wound therapy) and such declines may continue during the duration of the COVID-19 pandemic. Offsetting these declines in net revenue, we have experienced an increase in net revenue related to increased demand for certain respiratory products (such as oxygen), particularly in the fourth quarter of 2020, increased sales in our resupply businesses (primarily as a result of the increased ability to contact patients at home as a result of state and local government imposed stay-at-home orders) and the one-time sale of certain respiratory equipment (primarily ventilators) to hospitals. In response, we have instituted temporary cost mitigation measures such as reduced hours and furloughs for a small subset of our impacted workforce. In addition, as part of the CARES Act (discussed in more detail in the Medicare Reimbursement section below), we experienced an increase in Medicare reimbursement rates which will last through the later of April 20, 2021 or the end of the public health emergency and a suspension of Medicare sequestration through December 31, 2020 (resulting in a 2% increase in Medicare payments to all providers) resulting in a temporary increase in net revenues for certain products and services. The Consolidated Appropriations Act, 2021, signed into law on December 27, 2020, extends the suspension of Medicare sequestration through March 31, 2021. Recent regulatory guidance from CMS expanding telemedicine and reducing documentation requirements during the emergency period is also expected to result in increased net revenues for certain products and services. We have not experienced a significant slowdown in cash collections, and as a result our cash flow from operations has not been materially adversely impacted to date.
67
Table of Contents
We are closely monitoring the impact of COVID-19 pandemic on our business. It is difficult to predict the future impact of COVID-19 may have on our business, results of operations, financial position and cash flows. For additional information on risk factors that could impact our results, please refer to “Risk Factors” in this prospectus.
Components of Operating Results
Net Revenues. Revenues are recognized under fee-for-service and capitation arrangements for equipment, supplies, services and other items we rent or sell to patients. Fee-for-service is a payment model where we are paid for our service to provide equipment, supplies and other items. Capitation is a payment arrangement where a set amount is paid per member per month for a defined patient population, based on a negotiated contractual rate derived using average expected utilization of services.
Revenue generated from equipment that we rent to patients is recognized over the noncancelable rental period and commences on delivery of the equipment to the patients. Revenue related to sales of equipment and supplies is recognized on the date of delivery to the patients. Capitation revenue is recognized as a result of entering into a contract with a third party to stand ready to provide its members certain services without regard to the actual services provided; therefore, revenue is recognized over the period that the beneficiaries are entitled to healthcare services. Due to the nature of our industry and the reimbursement environment in which we operate, certain estimates are required to record total net revenues.
Cost of Net Revenues and Gross Margin.
Cost of Net Revenues. We incur product and supply costs, depreciation of patient equipment, home respiratory therapists costs and other costs in connection with providing our services:
| • | Product and supply costs are comprised primarily of the cost of supplies, equipment and accessories provided to patients. |
| • | Patient equipment depreciation is provided using the straight-line method over the estimated useful lives of the equipment, which range from 1 to 10 years. Patient equipment depreciation is classified in our consolidated statements of income within costs of net revenues as the equipment is rented to patients as part of our primary operations. Patient equipment is generally placed for rent; however, it could also be sold to customers. Upon a sale, we record the proceeds of the sale within net revenue and the cost related to the carrying net book value as other costs within cost of net revenues in our consolidated statements of income. |
| • | Home respiratory therapists costs are comprised primarily of employee salary and benefit costs or contract fees paid to respiratory therapists and other related professionals who are deployed to service a patient. |
Gross Margin. Gross margin is gross profit expressed as a percentage of net revenues. Our gross margin is impacted by Payor and product mix, fluctuations in pricing of supplies, equipment and accessories, as well as changes in reimbursement rates.
Provision for Doubtful Accounts. Prior to the adoption of Topic 842, the provision for doubtful accounts is based on our estimate of the net realizable value of accounts receivable. Significant estimates and judgments are required in determining the provision for doubtful accounts. Upon adoption of Topic 842, we have elected to record a reserve for expected credit losses as part of net rental revenue adjustments in order to report rental revenue at an expected collectable amount based on the total portfolio of operating lease receivables for which collectability has been deemed probable.
Selling, Distribution and Administrative. Selling, distribution and administrative expenses are comprised of expenses incurred in support of our operations and administrative functions and includes labor costs, such as
68
Table of Contents
salaries, bonuses, commissions, benefits and travel-related expenses for our employees, facilities rental costs, third-party revenue cycle management costs and corporate support costs including finance, information technology, legal, human resources, procurement, and other administrative costs. Distribution expenses represents the cost incurred to coordinate and deliver products and services to the patients. Included in distribution expenses are leasing, maintenance, licensing and fuel costs for the vehicle fleet; salaries, benefits and other costs related to drivers and dispatch personnel; and amounts paid to couriers and other third-party logistics and shipping vendors.
Income Tax Expense (Benefit). Our provision for income taxes is based on expected income, permanent book/tax differences and statutory tax rates in the various jurisdictions in which we operate. Significant estimates and judgments are required in determining the provision for income taxes.
Seasonality
Our business is sensitive to seasonal fluctuations. Our patients are generally responsible for a greater percentage of the cost of their treatment or therapy during the early months of the year due to co-insurance, co-payments and deductibles, and therefore may defer treatment and services of certain therapies until meeting their annual deductibles. In addition, changes to employer insurance coverage often go into effect at the beginning of each calendar year which may impact eligibility requirements and delay or defer treatment or recognition of revenues. These factors may lead to lower total revenues and cash flow in the early part of the year and higher total revenues and cash flow in the latter half of the year. Additionally, the increased incidence of respiratory infections during the winter season may result in initiation of additional respiratory services such as oxygen therapy for certain patient populations. Our quarterly operating results may fluctuate significantly in the future depending on these and other factors.
Key Performance Metrics
We regularly review key performance metrics to evaluate our business, measure our performance, identify trends in our business, prepare financial projections and make strategic decisions.
EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex. We use the non-GAAP financial information of EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex as key profitability measures to evaluate the business. Refer to the “Non-GAAP financial information” section for further detail, including a table reconciling each of such measures to net income, the most directly comparable GAAP measure. The below table sets forth net income, EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex for each of the nine months ended September 30, 2020 and 2019 and for the years ended December 31, 2019, 2018 and 2017:
| Nine Months Ended September 30, |
Year Ended December 31, | |||||||||||||||||||
| (in thousands) | 2020 | 2019 | 2019 | 2018 | 2017 | |||||||||||||||
| Net income |
$ | 20,268 | $ | 9,840 | $ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||||||
| EBITDA |
$ | 123,813 | $ | 98,348 | $ | 138,991 | $ | 145,386 | $ | 145,448 | ||||||||||
| Adjusted EBITDA |
$ | 162,765 | $ | 118,963 | $ | 173,972 | $ | 155,727 | $ | 153,340 | ||||||||||
| Adjusted EBITDA less Patient Equipment Capex |
$ | 99,283 | $ | 45,562 | $ | 80,523 | $ | 45,579 | $ | 3,417 | ||||||||||
69
Table of Contents
Results of Operations
Comparison of Nine Months Ended September 30, 2020 and Nine Months Ended September 30, 2019.
The following table summarizes our consolidated results of operations:
| Nine Months Ended September 30, | ||||||||||||||||
| (dollar amounts in thousands) | 2020 | 2019 | Change $ | Change % | ||||||||||||
| Net revenues |
$ | 814,928 | $ | 807,710 | $ | 7,218 | 0.9 | % | ||||||||
| Cost of net revenues, including related depreciation |
243,920 | 255,066 | (11,146 | ) | (4.4 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
571,008 | 552,644 | 18,364 | 3.3 | % | |||||||||||
| Selling, distribution and administrative |
534,110 | 537,546 | (3,436 | ) | (0.6 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
778,030 | 792,612 | (14,582 | ) | (1.8 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating income |
36,898 | 15,098 | 21,800 | 144.4 | % | |||||||||||
| Interest expense |
4,047 | 3,345 | 702 | 21.0 | % | |||||||||||
| Interest income and other |
(451 | ) | (1,552 | ) | (1,101 | ) | (70.9 | )% | ||||||||
| Income tax expense |
13,034 | 3,465 | 9,569 | NM | * | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income |
$ | 20,268 | $ | 9,840 | $ | 10,428 | 106.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| * | NM Not meaningful |
Net Revenues. Net revenues for the nine months ended September 30, 2020 were $814.9 million compared to $807.7 million for the nine months ended September 30, 2019, an increase of $7.2 million or 0.9%. The increase in net revenues for the nine months ended September 30, 2020 was primarily due to higher reimbursement levels driven by revenue cycle management improvements and growth in OSA treatment and ventilation therapy. The increase was partially offset by a decrease in net revenues from enteral nutrition as part of our strategic deemphasis of the product, a decrease in oxygen volume, and reduced demand for certain products and services associated with elective medical procedures and the disruption in physician practices (such as commencement of new CPAP services, ventilation therapy and negative pressure wound therapy) during the COVID-19 pandemic. The increase in net revenues was also due to increased Medicare reimbursement rates from the CARES Act and the temporary suspension of Medicare sequestration, partially offset by reductions in commercial Payor reimbursement rates. Our core services comprise total net revenues as follows:
| Nine Months Ended September 30, | ||||||||||||||||
| (dollar amounts in thousands) | 2020 | 2019 | Change $ | Change % | ||||||||||||
| Home respiratory therapy |
$ | 335,277 | $ | 325,422 | $ | 9,855 | 3.0 | % | ||||||||
| OSA treatment |
330,966 | 319,971 | 10,995 | 3.4 | % | |||||||||||
| NPWT |
30,751 | 30,345 | 406 | 1.3 | % | |||||||||||
| Other equipment and services |
117,934 | 131,972 | (14,038 | ) | (10.6 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net revenues |
$ | 814,928 | $ | 807,710 | $ | 7,218 | 0.9 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Net revenues for the nine months ended September 30, 2020 increased primarily due to the following:
| • | Home respiratory therapy. Net revenues increased 3.0% primarily due to increased volume of patients requiring ventilation therapy and higher reimbursement levels, partially offset by decreased volume of oxygen therapy. Net revenues also increased due to increased Medicare reimbursement rates from the CARES Act and the temporary suspension of Medicare sequestration partially offset by a reduction in commercial Payor reimbursement rates. |
| • | OSA treatment. Net revenues increased 3.4% primarily due to organic growth in equipment and related supplies, as well as higher reimbursement levels, despite experiencing a reduction in demand from new |
70
Table of Contents
| patient starts during the COVID-19 pandemic. The increase in net revenues was offset by reductions in commercial Payor reimbursement rates which were in turn partially offset by increased Medicare reimbursement rates from the CARES Act and the temporary suspension of Medicare sequestration. |
| • | NPWT. Net revenues increased 1.3% due to higher reimbursement levels and increased volume resulting from the deliberate focus of our NPWT sales force on acute referral sources, where most wound treatment begins, despite experiencing a reduction in demand associated with elective medical procedures during the COVID-19 pandemic. The increase was partially offset by a reduction in reimbursement rates. |
| • | Other equipment and services. Net revenues decreased 10.6% primarily due to a decrease in enteral nutrition as part of our strategic deemphasis of the product in 2019 and a decrease in other respiratory therapy volume partially due to a reduction in demand associated with elective medical procedures and the disruption in physician practices during the COVID-19 pandemic. |
Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 21% and 1%, respectively, of total net revenues for the nine months ended September 30, 2020. Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 19% and 1%, respectively, of total net revenues for the nine months ended September 30, 2019.
Cost of Net Revenues and Gross Margin.
Cost of Net Revenues. Cost of net revenues for the nine months ended September 30, 2020 was $243.9 million compared to $255.1 million for the nine months ended September 30, 2019, a decrease of $11.1 million or 4.4%. The decrease in cost of net revenues for the nine months ended September 30, 2020 was primarily due to lower product and supply costs and lower home respiratory therapists costs offset by increased patient equipment depreciation as described below. Our cost of net revenues was as follows:
| Nine Months Ended September 30, | ||||||||||||||||
| (dollar amounts in thousands) | 2020 | 2019 | Change $ | Change % | ||||||||||||
| Product and supply costs |
$ | 141,563 | $ | 154,591 | $ | (13,028 | ) | (8.4 | )% | |||||||
| Patient equipment depreciation |
75,840 | 72,588 | 3,252 | 4.5 | % | |||||||||||
| Home respiratory therapists costs |
12,848 | 14,844 | (1,996 | ) | (13.4 | )% | ||||||||||
| Other |
13,669 | 13,043 | 626 | 4.8 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cost of net revenues |
$ | 243,920 | $ | 255,066 | $ | (11,146 | ) | (4.4 | )% | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
Product and supply costs decreased primarily due to a decrease in enteral nutrition costs as part of our strategic deemphasis of the product in 2019 as well as reduced demand in certain services associated with elective medical procedures and the disruption in physician practices during the COVID-19 pandemic. The decrease was partially offset by increased product and supply costs to support increased volume in OSA treatment.
Patient equipment depreciation costs increased primarily as a result of equipment purchases to support increased volume in OSA treatment.
Home respiratory therapists costs decreased primarily due to reduced demand in certain services associated with elective medical procedures and the disruption in physician practices during the COVID-19 pandemic.
Other costs increased as a result of a $1.9 million insurance settlement in the prior year, partially offset by a decrease in volume of sales or title transfers of patient equipment driven by contractual requirements.
Gross margin. Gross margin for the nine months ended September 30, 2020 was 70.1% compared to 68.4% for the nine months ended September 30, 2019, an increase of 1.7%. The gross margin increase was driven by
71
Table of Contents
higher reimbursement levels due to revenue cycle management improvements, increased Medicare reimbursement rates from the CARES Act, the temporary suspension of Medicare sequestration and reduced demand for certain products and services associated with elective medical procedures and the disruption in physician practices under capitation arrangements during the COVID-19 pandemic. The increase was partially offset by increased patient equipment depreciation and reductions in commercial Payor reimbursement rates.
Selling, Distribution and Administrative. Selling, distribution and administrative expenses for the nine months ended September 30, 2020 were $534.1 million compared to $537.5 million for the nine months ended September 30, 2019, a decrease of $3.4 million or 0.6%. Selling, distribution and administrative expenses for the nine months ended September 30, 2020 were 65.5% of total net revenues compared to 66.6% of total net revenues for the nine months ended September 30, 2019. The decrease was primarily due to a reduction in operating costs as a result of our 2019 business transformation initiative to shift to a patient-centric platform and optimize end-to-end customer service, including a reduction in the associated $9.8 million one-time costs of the initiative, as well as, a reduction in costs in response to the COVID-19 pandemic. It is difficult to ascertain with precision what portion of the decrease is attributable to the COVID-19 pandemic as compared to the impact of the business improvements. The reduction in costs also includes a $6.6 million decrease in stock compensation expense related to the accelerated vesting of outstanding performance Class B profit interest units in 2019. The reductions were partially offset by increased one-time legal expenses of $32.5 million, representing the change in the estimated probable loss in relation to a series of civil investigative demands, higher incentive compensation based on performance, a special recognition bonus for employees that do not otherwise participate in the Company’s variable compensation programs and an increase in other one-time costs primarily associated with this offering and implementation of a new financial system.
Income Tax Expense. Income tax expense for the nine months ended September 30, 2020 was $13.0 million compared to $3.5 million for the nine months ended September 30, 2019, an increase of $9.6 million. Our effective tax rate was 39.1% and 26.0% for the nine months ended September 30, 2020 and 2019, respectively. The change in income tax expense is primarily a result of increased taxable operating income. For the nine months ended September 30, 2020, the Company’s effective tax rate differed from federal and state statutory rates primarily due to non-deductible expenses for tax purposes.
Comparison of Years Ended December 31, 2019 and December 31, 2018.
The following table summarizes our consolidated results of operations:
| Year Ended December 31, | ||||||||||||||||
| (dollar amounts in thousands) | 2019 | 2018 | Change $ | Change % | ||||||||||||
| Net revenues(1) |
$ | 1,088,875 | $ | 1,110,883 | $ | (22,008 | ) | (2.0 | )% | |||||||
| Cost of net revenues, including related depreciation |
340,714 | 360,086 | (19,372 | ) | (5.4 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
748,161 | 750,797 | (2,636 | ) | (0.4 | )% | ||||||||||
| Provision for doubtful accounts(1) |
— | 31,719 | (31,719 | ) | (100.0 | )% | ||||||||||
| Selling, distribution and administrative |
720,746 | 698,681 | 22,065 | 3.2 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
1,061,460 | 1,090,486 | (29,026 | ) | (2.7 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating income |
27,415 | 20,397 | 7,018 | 34.4 | % | |||||||||||
| Interest expense |
5,112 | 1,338 | 3,774 | 282.1 | % | |||||||||||
| Interest income and other |
(1,446 | ) | (897 | ) | (549 | ) | 61.2 | % | ||||||||
| Income tax expense |
8,127 | 6,829 | 1,298 | 19.0 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income |
$ | 15,622 | $ | 13,127 | $ | 2,495 | 19.0 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Upon adoption the provision for doubtful accounts associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now treated as net rental revenue instead of general and administrative expense. |
72
Table of Contents
Net Revenues. Net revenues for the year ended December 31, 2019 were $1,088.9 million compared to $1,110.9 million for the year ended December 31, 2018, a decrease of $22.0 million or 2.0%. The decrease in net revenues for the year ended December 31, 2019 was primarily driven by the impact of adopting Topic 842. Upon adoption the provision for doubtful accounts associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now treated as net rental revenue instead of general and administrative expense. Our core services comprise total net revenues as follows:
| Year Ended December 31, | ||||||||||||||||
| (dollar amounts in thousands) | 2019 | 2018 | Change $ | Change % | ||||||||||||
| Home respiratory therapy |
$ | 435,680 | $ | 441,190 | $ | (5,510 | ) | (1.2 | )% | |||||||
| OSA treatment |
438,572 | 430,354 | 8,218 | 1.9 | % | |||||||||||
| NPWT |
42,122 | 41,383 | 739 | 1.8 | % | |||||||||||
| Other equipment and services |
172,501 | 197,956 | (25,455 | ) | (12.9 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net revenues |
$ | 1,088,875 | $ | 1,110,883 | $ | (22,008 | ) | (2.0 | )% | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
Net revenues for the year ended December 31, 2019 decreased primarily due to the following:
| • | Home respiratory therapy. Net revenues decreased 1.2% primarily due to reserves for expected credit losses of $19.9 million being recorded to net rental revenue under the adoption of Topic 842 in 2019, as well as reductions in the reimbursement rates of oxygen therapy. The decrease was partially offset by increased volume of patients requiring ventilation therapy, partially offset by decreased volume of oxygen therapy, each driven by the focus of our sales efforts towards physicians treating ventilation patients. |
| • | OSA treatment. Net revenues increased 1.9% primarily due to increased volume as a result of the rising diagnoses of OSA across the industry and the related increase in equipment rentals and supplies partially offset by reductions in reimbursement rates and reserves for expected credit losses of $10.0 million being recorded to net rental revenue under the adoption of Topic 842 in 2019. |
| • | NPWT. Net revenues increased 1.8% due to increased volume resulting from the deliberate focus of our NPWT sales force on acute referral sources, where most wound treatment begins. The increased volume was partially offset by reductions in reimbursement rates and reserves for expected credit losses of $1.3 million being recorded to net rental revenue under the adoption of Topic 842 in 2019. |
| • | Other equipment and services. Net revenues decreased 12.9% primarily due to a decrease in enteral nutrition as part of our strategic deemphasis of the product in 2019, a decrease in respiratory medications and other respiratory therapy volume due to our strategic shift towards patients requiring ventilation therapy as well as reserves for expected credit losses of $3.3 million being recorded to net rental revenue under the adoption of Topic 842 in 2019. |
Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 19% and 1%, respectively, of total net revenues for the year ended December 31, 2019. Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 18% and 1%, respectively, of total net revenues for the year ended December 31, 2018.
73
Table of Contents
Cost of Net Revenues and Gross Margin.
Cost of Net Revenues. Cost of net revenues for the year ended December 31, 2019 were $340.7 million compared to $360.1 million for the year ended December 31, 2018, a decrease of $19.4 million or 5.4%. Our cost of net revenues was as follows:
| Year Ended December 31, | ||||||||||||||||
| (dollar amounts in thousands) | 2019 | 2018 | Change $ | Change % | ||||||||||||
| Product and supply costs |
$ | 206,067 | $ | 218,099 | $ | (12,032 | ) | (5.5 | )% | |||||||
| Patient equipment depreciation |
97,386 | 108,340 | (10,954 | ) | (10.1 | )% | ||||||||||
| Home respiratory therapists costs |
19,560 | 20,371 | (811 | ) | (4.0 | )% | ||||||||||
| Other |
17,701 | 13,276 | 4,425 | 33.3 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cost of net revenues |
$ | 340,714 | $ | 360,086 | $ | (19,372 | ) | (5.4 | )% | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
Product and supply costs decreased primarily due to a decrease in enteral nutrition costs as part of our strategic deemphasis of the product in 2019. The decrease was partially offset by increased product and supply costs to support increased volume in OSA treatment and increased patient equipment dispositions cost.
Patient equipment depreciation costs decreased primarily as a result of a contractual change in the timing of when title to certain patient equipment is transferred to the patient. The decrease was partially offset by the related increase in patient equipment costs included in other costs and increased disposition costs included in product and supply costs. Patient equipment depreciation costs also decreased due to oxygen therapy equipment becoming fully depreciated without replacement.
Gross margin. Gross margin for the year ended December 31, 2019 was 68.7% compared to 67.6% for the year ended December 31, 2018, an increase of 1.1%. The gross margin increase was driven by favorable product mix and lower patient equipment depreciation. The favorability was partially offset by a 1.0% reduction due to reserves for expected credit losses being recorded to net rental revenue under the adoption of Topic 842 in 2019 and lower reimbursement rates.
Provision for Doubtful Accounts. Provision for doubtful accounts for the year ended December 31, 2019 was $0.0 million compared to $31.7 million for the year ended December 31, 2018, a decrease of $31.7 million or 100.0%. The provision for doubtful accounts, expressed as a percentage of total net revenues, was 0.0% and 2.9% for the years ended December 31, 2019 and December 31, 2018, respectively. The decrease in the provision for doubtful accounts for the year ended December 31, 2019 was due to the adoption of Topic 842. Upon adoption the provision for doubtful accounts associated with rental revenue of $34.5 million for the year ended December 31, 2019 is now charged to net rental revenue instead of general and administrative expense.
Selling, Distribution and Administrative. Selling, distribution and administrative expenses for the year ended December 31, 2019 were $720.7 million compared to $698.7 million for the year ended December 31, 2018, an increase of $22.1 million or 3.2%. Selling, distribution and administrative expenses for the year ended December 31, 2019 were 66.2% of total net revenues compared to 62.9% of total net revenues for the year ended December 31, 2018.
The increase was primarily a result of increased one-time costs associated with our strategic transformation initiatives of $10.1 million, an accrual related to the CID legal matter of $12.2 million and stock compensation expense of $7.4 million, partially offset by a decrease in costs directly attributable to the initial public offering of $5.1 million. The increase was also offset by the continued favorable outcome of improved productivity initiatives and related reductions in operating costs associated with the strategic transformation.
74
Table of Contents
Comparison of Years Ended December 31, 2018 and December 31, 2017.
The following table summarizes our consolidated results of operations:
| Year Ended December 31, | ||||||||||||||||
| (dollar amounts in thousands) | 2018 | 2017 | Change $ | Change % | ||||||||||||
| Net revenues |
$ | 1,110,883 | $ | 1,073,810 | $ | 37,073 | 3.5 | % | ||||||||
| Cost of net revenues, including related depreciation |
360,086 | 332,545 | 27,541 | 8.3 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
750,797 | 741,265 | 9,532 | 1.3 | % | |||||||||||
| Provision for doubtful accounts |
31,719 | 42,672 | (10,953 | ) | (25.7 | )% | ||||||||||
| Selling, distribution and administrative |
698,681 | 672,442 | 26,239 | 3.9 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
1,090,486 | 1,047,659 | 42,827 | 4.1 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating income |
20,397 | 26,151 | (5,754 | ) | (22.0 | )% | ||||||||||
| Interest expense |
1,338 | 1,246 | 92 | 7.4 | % | |||||||||||
| Interest income and other |
(897 | ) | (486 | ) | (411 | ) | 84.6 | % | ||||||||
| Income tax expense (benefit) |
6,829 | (71,560 | ) | 78,389 | (109.5 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income |
$ | 13,127 | $ | 96,951 | $ | (83,824 | ) | (86.5 | )% | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
Net Revenues. Net revenues for the year ended December 31, 2018 were $1,110.9 million compared to $1,073.8 million for the year ended December 31, 2017, an increase of $37.1 million or 3.5%. The increase was primarily due to volume growth in home respiratory therapy and OSA treatment as described below. The increase in net revenues for the year ended December 31, 2018 was partially offset by the impact of adopting Topic 606. Upon adoption, patient co-pay and deductible amounts that were previously included in the provision for doubtful accounts in selling, general and administrative costs related to implicit price concessions, which were $9.5 million for the year ended December 31, 2018, are now presented as an offsetting adjustment to the related fee-for-service sale net revenues.
Our core services comprise total net revenues as follows:
| Year Ended December 31, | ||||||||||||||||
| (dollar amounts in thousands) | 2018 | 2017 | Change $ | Change % | ||||||||||||
| Home respiratory therapy |
$ | 441,190 | $ | 432,416 | $ | 8,774 | 2.0 | % | ||||||||
| OSA treatment |
430,354 | 400,672 | 29,682 | 7.4 | % | |||||||||||
| NPWT |
41,383 | 42,013 | (630 | ) | (1.5 | )% | ||||||||||
| Other equipment and services |
197,956 | 198,709 | (753 | ) | (0.4 | )% | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net revenues |
$ | 1,110,883 | $ | 1,073,810 | $ | 37,073 | 3.5 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Net revenues for the year ended December 31, 2018 increased primarily due to the following:
| • | Home respiratory therapy. Net revenues increased 2.0% primarily due to increased volume offset by a reduction in the reimbursement rates of oxygen therapy and implicit price concessions under the adoption of Topic 606 of $2.3 million. The increase in volume was primarily the result of an increase in patients requiring ventilation therapy, which was driven by the focus of our sales efforts towards physicians treating ventilation patients. |
| • | OSA treatment. Net revenues increased 7.4% primarily due to increased volume as a result of the rising diagnoses of OSA across the industry and the related increase in equipment rentals and supplies. The increase was partially offset by implicit price concessions under the adoption of Topic 606 of $3.9 million. |
| • | NPWT. Net revenues decreased 1.5% primarily due to implicit price concessions under the adoption of Topic 606 of $1.1 million. |
75
Table of Contents
| • | Other equipment and services. Net revenues decreased 0.4% primarily due to implicit price concessions under the adoption of Topic 606 of $2.2 million, as well as a decrease in respiratory medications and other respiratory equipment volume. This was driven by our strategic decision to focus on ventilation patients, thereby driving lower sales and rental of other respiratory equipment such as nebulizers. The decrease was partially offset by increased enteral nutrition volume due to the relaunch of the product. |
Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 18% and 1%, respectively, of total net revenues for the year ended December 31, 2018. Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 18% and 2%, respectively, of total net revenues for the year ended December 31, 2017.
Cost of Net Revenues and Gross Margin.
Cost of Net Revenues. Cost of net revenues for the year ended December 31, 2018 was $360.1 million compared to $332.5 million for the year ended December 31, 2017, an increase of $27.5 million or 8.3%. Our cost of net revenues was as follows:
| Year Ended December 31, |
||||||||||||||||
| (dollar amounts in thousands) | 2018 | 2017 | Change $ | Change % | ||||||||||||
| Product and supply costs |
$ | 218,099 | $ | 200,621 | $ | 17,478 | 8.7 | % | ||||||||
| Patient equipment depreciation |
108,340 | 101,724 | 6,616 | 6.5 | % | |||||||||||
| Home respiratory therapists costs |
20,371 | 20,802 | (431 | ) | (2.1 | )% | ||||||||||
| Other |
13,276 | 9,398 | 3,878 | 41.3 | % | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cost of net revenues |
$ | 360,086 | $ | 332,545 | $ | 27,541 | 8.3 | % | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
Product and supply costs increased due to additional costs to support increased OSA treatment and enteral nutrition volume due to product relaunch and patient equipment reserves due to a payor contractual change and obsolete equipment. The increase was partially offset by lower lease expenses associated with patient services equipment based on a change in strategy to purchase equipment.
Patient equipment depreciation costs increased as a result of equipment purchases to support volume growth in OSA treatment and ventilation therapy, as well as a change from leasing to purchasing patient services equipment. Historically, expenses associated with leasing this service equipment were included within product and supply costs, whereas depreciation expense is included within patient equipment depreciation.
Other costs increased as a result of an increase in sales or title transfers of patient equipment driven by equipment not being returned by patients and contractual requirements. Costs related to the carrying net book value are included within other costs.
Gross margin. Gross margin for the year ended December 31, 2018 was 67.6% compared to 69.0% for the year ended December 31, 2017, a decrease of 1.4%. The decrease in gross margin was primarily driven by implicit price concessions, higher depreciation costs, a reduction in reimbursement rates and patient equipment reserves (as referenced above), partially offset by the favorable shift from leasing to purchasing patient equipment. Upon adoption of Topic 606, patient co-pay and deductible amounts that were previously included in the provision for doubtful accounts related to implicit price concessions are now presented as an offsetting adjustment to the related fee-for-service sale net revenues. The adoption of Topic 606 resulted in a decrease in gross margin of 0.3% in 2018.
Provision for Doubtful Accounts. Provision for doubtful accounts for the year ended December 31, 2018 was $31.7 million compared to $42.7 million for the year ended December 31, 2017, a decrease of $11.0 million
76
Table of Contents
or 25.7%. The provision for doubtful accounts, expressed as a percentage of total net revenues, was 2.9% and 4.0% for the year ended December 31, 2018 and 2017, respectively. The decrease in the provision for doubtful accounts during the year ended December 31, 2018 was primarily due to the adoption of Topic 606. Upon adoption, patient co-pay and deductible amounts that were previously included in the provision for doubtful accounts related to implicit price concessions are now presented as an offsetting adjustment to the related fee-for-service sale net revenues.
Selling, Distribution and Administrative Expenses. Selling, distribution and administrative expenses for the year ended December 31, 2018 were $698.7 million compared to $672.4 million for the year ended December 31, 2017, an increase of $26.2 million or 3.9%. Selling, distribution and administrative expenses for the year ended December 31, 2018 were 62.9% of total net revenues compared to 62.6% for the year ended December 31, 2017.
The overall increase was primarily a result of increased variable costs associated with revenue growth across our core service lines and an increase in one-time costs directly attributable to the offering of $6.2 million. The increase was offset by the favorable outcome of improved productivity initiatives and related reductions in headcount, lower facilities costs associated with strategic transformation and a decrease in incentive compensation based on certain performance metrics.
Income Tax Expense (Benefit). Income tax expense for the year ended December 31, 2018 was $6.8 million compared to an income tax benefit of $(71.6) million for the year ended December 31, 2017, a change of $78.4 million. Our effective tax rate was 34.2% and (281.8)% for the years ended December 31, 2018 and 2017, respectively. The $78.4 million change was primarily due to the release of the $105.1 million valuation allowance against the net deferred tax assets. This benefit was partially offset by a charge to tax expense of $24.2 million, due to the re-measurement of deferred tax assets and liabilities resulting from the decrease in the federal corporate tax rate as a result of the enactment of the Tax Cuts and Jobs Act in December 2017. The change was also partially offset by a $4.7 million decrease in income tax expense calculated at statutory rates and a $2.1 million increase due to state taxes and other non-deductible items.
Impact of Inflation and Changing Prices
We experience pricing pressures in the form of continued reductions in reimbursement rates, particularly from managed care organizations and from governmental Payors such as Medicare and Medicaid. We are also impacted by rising costs for certain inflation-sensitive operating expenses such as labor and employee benefits, facility and equipment leases, and vehicle fuel.
Liquidity and Capital Resources
Our principal source of liquidity is our operating cash flow, which is supplemented by extended payment terms from our suppliers and our Revolver, which provides for revolving credit of up to $100.0 million, subject to availability. Our principal liquidity requirements are labor costs, including salaries, bonuses, benefits and travel-related expenses, product and supply costs, third-party customer service, billing and collections and logistics costs and patient equipment capital expenditures. Our future capital expenditure requirements will depend on many factors, including our revenue growth rates. Our capital expenditures are made in advance of patients beginning service. Certain operating costs are incurred at the beginning of the equipment rental period and during initial patient set up. We may be required to seek additional equity or debt financing. In the event that additional financing is required from outside sources, we may not be able to raise it on terms acceptable to us or at all. If we are unable to raise additional capital when desired, our business, results of operations, and financial condition would be materially and adversely affected. We believe that our operating cash flow, together with our existing cash, cash equivalents, and Revolver, will continue to be sufficient to fund our operations and growth strategies for at least the next 12 months.
Apria, Inc. is a holding company and our operations will be conducted entirely through our subsidiaries. Our ability to generate cash to pay applicable taxes at assumed tax rates and pay cash dividends we declare, if any, is
77
Table of Contents
dependent on the earnings and the receipt of funds from Apria Healthcare Group and its subsidiaries via dividends or intercompany loans. Deterioration in the financial condition, earnings or cash flow of Apria Healthcare Group and its subsidiaries for any reason could limit or impair their ability to pay such distributions. Additionally, the terms of our financing arrangements, including the TLA and the Revolver, contain covenants that may restrict Apria Healthcare Group and its subsidiaries from paying such distributions, subject to certain exceptions. See “Risk Factors—We are a holding company with no operations of our own and we are accordingly dependent upon distributions from our subsidiaries to pay taxes and pay dividends.”
On December 11, 2020, we entered into the Credit Facility Amendment to incur $260.0 million of Incremental Term Loans. Net proceeds from the Incremental Term Loans were used to fund a $200.3 million dividend payment to our stockholders and $9.7 million distribution to SARs holders declared and paid in December 2020, with the remaining proceeds used to pay fees and expenses in connection with the Credit Facility Amendment and for general corporate purposes. We also declared and paid a $175.0 million dividend to common stockholders and SARs holders payable in June 2019 and a $75.0 million dividend to common stockholders in July 2018. We have no current plans to pay dividends on our common stock following this offering.
As permitted under the CARES Act, we have elected to defer certain portions of employer-paid FICA taxes otherwise payable from March 27, 2020 to January 1, 2021, which will be paid in two equal installments on December 31, 2021 and December 31, 2022. The amount deferred at September 30, 2020 was $9.5 million.
Cash Flow. The following table presents selected data from our consolidated statement of cash flows:
| Nine Months Ended September 30, | Year Ended December 31, | |||||||||||||||||||
| (in thousands) | 2020 | 2019 | 2019 | 2018 | 2017 | |||||||||||||||
| Net cash provided by operating activities |
$ | 158,939 | $ | 83,850 | $ | 161,850 | $ | 159,578 | $ | 155,345 | ||||||||||
| Net cash used in investing activities |
(72,532 | ) | (68,266 | ) | (92,713 | ) | (97,469 | ) | (105,919 | ) | ||||||||||
| Net cash used in financing activities |
(20,973 | ) | (51,141 | ) | (59,116 | ) | (109,802 | ) | (20,108 | ) | ||||||||||
| Net increase (decrease) in cash, cash equivalents and restricted cash |
65,434 | (35,557 | ) | 10,021 | (47,693 | ) | 29,318 | |||||||||||||
| Cash, cash equivalents and restricted cash at beginning of period |
74,691 | 64,670 | 64,670 | 112,363 | 83,045 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Cash, cash equivalents and restricted cash at end of period |
$ | 140,125 | $ | 29,113 | $ | 74,691 | $ | 64,670 | $ | 112,363 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Comparison of Nine Months Ended September 30, 2020 and September 30, 2019. Net cash provided by operating activities for the nine months ended September 30, 2020 was $158.9 million compared to $83.9 million for the nine months ended September 30, 2019, an increase of $75.1 million. The increase in net cash provided by operating activities was primarily the result of the following:
| • | $10.4 million increase in net income; |
| • | $4.1 million increase in non-cash items; and |
| • | $60.6 million increase in cash provided by the change in operating assets and liabilities, due primarily to the increase of cash provided by legal reserve of $32.5 million and accrued expenses of $15.3 million (which includes the deferral of $9.5 million employer-paid FICA taxes as permitted under the CARES Act) and the decrease of cash used in accounts payable of $9.2 million and accrued payroll and related taxes and benefits of $5.8 million, partially offset by cash used for prepaid expenses and other assets of $6.9 million. |
Net cash used in investing activities for the nine months ended September 30, 2020 was $72.5 million, compared to $68.3 million for the nine months ended September 30, 2019. The primary use of funds in the nine months ended September 30, 2020 was $85.8 million to purchase patient equipment and property, equipment and
78
Table of Contents
improvements, which was partially offset by proceeds from the sale of patient equipment and other of $13.3 million. The primary use of funds in the nine months ended September 30, 2019 was $85.2 million to purchase patient equipment and property, equipment and improvements, which was partially offset by proceeds from the sale of patient equipment and other of $16.9 million.
Net cash used in financing activities for the nine months ended September 30, 2020 was $21.0 million compared to $51.1 million for the nine months ended September 30, 2019. Net cash used in financing activities for the nine months ended September 30, 2020 primarily reflected payments on asset financing of $17.2 million and payments on long-term debt of $3.8 million. Net cash used in financing activities for the nine months ended September 30, 2019 primarily reflected cash dividends paid to common stockholders and SARs holders of $175.0 million, payments on asset financing of $23.4 million and payments of deferred financing costs related to the credit agreement with Citizens Bank of $2.8 million, offset by borrowings on the TLA of $150.0 million.
Comparison of Years Ended December 31, 2019 and December 31, 2018. Net cash provided by operating activities for the year ended December 31, 2019 was $161.9 million compared to $159.6 million for the year ended December 31, 2018, an increase of $2.3 million. The increase in net cash provided by operating activities was primarily the result of the following:
| • | $2.5 million increase in net income; |
| • | $3.9 million decrease in non-cash items; and |
| • | $3.7 million decrease in cash used in the change in operating assets and liabilities, due primarily to the increase of cash provided by accounts receivable and accrued payroll and related taxes and benefits offset by the cash used for accounts payable. |
Net cash used in investing activities for the year ended December 31, 2019 was $92.7 million, compared to $97.5 million for the year ended December 31, 2018. The primary use of funds in 2019 was $114.5 million to purchase patient equipment and property, equipment and improvements, which was partially offset by proceeds from the sale of patient equipment and other of $21.8 million. The primary use of funds in 2018 was $122.1 million to purchase patient equipment and property, equipment and improvements, which was partially offset by proceeds from sale of patient equipment and other of $21.9 million and proceeds from the settlement of corporate-owned life insurance of $3.5 million.
Net cash used in financing activities for the year ended December 31, 2019 was $59.1 million compared to $109.8 million for the year ended December 31, 2018. Net cash used in financing activities for the year ended December 31, 2019 primarily reflected cash dividends paid to our stockholders of $175.0 million, payments on asset financing of $31.4 million and payments of deferred financing costs related to the new credit agreement with Citizens Bank of $2.8 million, offset by borrowings on the TLA of $150.0 million. Net cash used in financing activities for the year ended December 31, 2018 primarily reflected cash dividends paid to our stockholders of $75.0 million and payments on asset financing of $33.4 million.
Comparison of Years Ended December 31, 2018 and December 31, 2017. Net cash provided by operating activities for the year ended December 31, 2018 was $159.6 million compared to $155.3 million for the year ended December 31, 2017, an increase of $4.2 million. The increase in net cash provided by operating activities was primarily the result of the following:
| • | $11.1 million decrease in net income, after excluding the $72.7 million change in deferred income taxes which is a non-cash item; |
| • | $10.2 million increase in non-cash items, excluding the $72.7 million change in deferred income taxes; and |
| • | $5.2 million decrease in cash used in the change in operating assets and liabilities, due primarily to the decrease of cash used in accrued expenses and changes in accounts receivable offset by the increase in |
79
Table of Contents
| cash used for prepaid expenses and other assets, increase in cash used in accrued payroll and related taxes and benefits, and decrease in cash provided by the changes in accounts payable. |
Net cash used in investing activities for the year ended December 31, 2018 was $97.5 million compared to $105.9 million for the year ended December 31, 2017. The primary use of funds in the year ended December 31, 2018 was $122.1 million to purchase patient equipment and property, equipment and improvements, which was partially offset by proceeds from the sale of patient equipment and other of $21.9 million and proceeds from the settlement of corporate-owned life insurance of $3.5 million. The primary use of funds in the year ended December 31, 2017 was $126.4 million to purchase patient equipment and property, equipment and improvements, which was partially offset by proceeds from sale of patient equipment and other of $20.5 million.
Net cash used in financing activities for the year ended December 31, 2018 was $109.8 million compared to $20.1 million for the year ended December 31, 2017. Net cash used in financing activities for the year ended December 31, 2018 primarily reflected cash dividends paid to our stockholders of $75.0 million and payments on asset financing of $33.4 million. For the year ended December 31, 2017, net cash used in financing activities primarily reflected payments on asset financing of $20.4 million.
Non-GAAP Financial Information.
EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex. EBITDA is a non-GAAP measure that represents net income for the period before the impact of interest income, interest expense, income taxes, and depreciation and amortization. EBITDA is widely used by securities analysts, investors and other interested parties to evaluate the profitability of companies. EBITDA eliminates potential differences in performance caused by variations in capital structures, tax positions, the cost and age of tangible assets and the extent to which intangible assets are identifiable. Adjusted EBITDA is a non-GAAP measure that represents EBITDA before certain items that impact comparison of the performance of our businesses either period-over-period or with other businesses. We use Adjusted EBITDA as a key profitability measure to assess the performance of our businesses. We believe that Adjusted EBITDA should, therefore, be made available to securities analysts, investors and other interested parties to assist in their assessment of the performance of our businesses.
Adjusted EBITDA less Patient Equipment Capex is a non-GAAP measure that represents Adjusted EBITDA less purchases of patient equipment net of dispositions (“Patient Equipment Capex”). For purposes of this metric, Patient Equipment Capex is measured as the value of the patient equipment received less the net book value of dispositions of patient equipment during the accounting period. We use Adjusted EBITDA less Patient Equipment Capex as a key profitability measure to assess the performance of our businesses because our businesses require significant capital expenditures to maintain its patient equipment fleet due to asset replacement and contractual commitments. We believe that Adjusted EBITDA less Patient Equipment Capex should, therefore, be made available to securities analysts, investors and other interested parties to assist in their assessment of the performance of our businesses.
Below, we have provided a reconciliation of EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex to our net income, the most directly comparable financial measure calculated and presented in accordance with GAAP. EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex should not be considered alternatives to net income or any other measure of financial performance calculated and presented in accordance with GAAP. Our EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex may not be comparable to similarly titled measures of other organizations because other organizations may not calculate EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex in the same manner as we calculate these measures.
80
Table of Contents
Our uses of EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex have limitations as analytical tools, and you should not consider them in isolation or as a substitute for analysis of our results as reported under GAAP. Some of these limitations are:
| • | although depreciation and amortization are non-cash charges, the assets being depreciated and amortized may have to be replaced in the future, EBITDA and Adjusted EBITDA do not reflect capital expenditure requirements for such replacements or other contractual commitments; |
| • | EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex do not reflect changes in, or cash requirements for, our working capital needs; |
| • | EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex do not reflect the interest expense or the cash requirements necessary to service interest or principal payments on our indebtedness; and |
| • | other companies, including companies in our industry, may calculate EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex measures differently, which reduces their usefulness as a comparative measure. |
EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex exclude items that can have a significant effect on our profit or loss and should, therefore, be used in conjunction with, not as substitutes for, profit or loss for the period. We compensate for these limitations by separately monitoring net income from continuing operations for the period.
The following table reconciles net income, the most directly comparable GAAP measure, to EBITDA, Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex:
| Nine Months Ended September 30, | Year Ended December 31, | |||||||||||||||||||
| (in thousands) | 2020 | 2019 | 2019 | 2018 | 2017 | |||||||||||||||
| Net income |
$ | 20,268 | $ | 9,840 | $ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||||||
| Interest expense, net and other |
3,596 | 1,793 | 3,666 | 441 | 760 | |||||||||||||||
| Income tax expense (benefit) |
13,034 | 3,465 | 8,127 | 6,829 | (71,560 | ) | ||||||||||||||
| Depreciation and amortization |
86,915 | 83,250 | 111,576 | 124,989 | 119,297 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| EBITDA |
$ | 123,813 | $ | 98,348 | $ | 138,991 | $ | 145,386 | $ | 145,448 | ||||||||||
| Strategic transformation initiatives: |
||||||||||||||||||||
| Branch network optimization(a) |
$ | — | $ | 566 | $ | 570 | $ | 1,367 | $ | — | ||||||||||
| Customer service optimization(b) |
99 | 238 | 264 | 789 | — | |||||||||||||||
| E-commerce platform(c) |
— | — | — | 342 | 432 | |||||||||||||||
| Simplify(d) |
1,159 | 10,926 | 11,775 | — | — | |||||||||||||||
| Financial systems(e) |
1,414 | — | — | — | — | |||||||||||||||
| eImpact(f) |
— | — | — | — | 3,742 | |||||||||||||||
| Technology investments(g) |
— | — | — | — | 1,997 | |||||||||||||||
| Stock-based compensation and other(h) |
1,929 | 8,057 | 9,024 | 1,621 | 1,721 | |||||||||||||||
| Legal expense(i) |
32,525 | — | 12,200 | — | — | |||||||||||||||
| Offering costs(j) |
1,826 | 828 | 1,148 | 6,222 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Adjusted EBITDA |
$ | 162,765 | $ | 118,963 | $ | 173,972 | $ | 155,727 | $ | 153,340 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Patient Equipment Capex |
(63,482 | ) | (73,401 | ) | (93,449 | ) | (110,148 | ) | (149,923 | ) | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Adjusted EBITDA less Patient Equipment Capex |
$ | 99,283 | $ | 45,562 | $ | 80,523 | $ | 45,579 | $ | 3,417 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (a) | Branch network optimization represents one-time, third-party logistics advisory costs associated with a 24-month initiative launched in January 2018 designed to modify the branch network in order to reduce branch operating costs while maintaining or improving patient service levels. |
81
Table of Contents
| (b) | Customer service optimization primarily represents one-time costs associated with customer service initiatives, as well as evaluating a strategic partnership expansion for certain aspects of customer service. |
| (c) | E-commerce platform primarily represents one-time costs associated with developing and launching our e-commerce platform. |
| (d) | Simplify represents one-time advisory fees and implementation costs associated with a key 2019 business transformation initiative focused on shifting to a patient-centric platform and optimizing end-to-end customer service. |
| (e) | Costs associated with the implementation of a new financial system. |
| (f) | eImpact represents one-time advisory and implementation fees incurred in 2016 and 2017 as part of a 24-month cost saving initiative focused on identifying opportunities to improve branch operational and sales effectiveness, optimize training, reduce corporate overhead and eliminate unnecessary expenses. |
| (g) | Technology investments represent various one-time costs associated with order-to-cash process improvements, patient monitoring, implementation of driver productivity and safety tools, and offshoring of certain revenue management capabilities. |
| (h) | Stock-based compensation has historically been granted to certain of our employees in the form of profit interest units of our parent and stock appreciation rights (“SARs”). For time-based vesting awards, we recognize a non-cash compensation expense based on the fair value of the awards determined at the date of grant over the requisite service period. In June 2019, all outstanding performance Class B units were modified to accelerate vesting resulting in $7.0 million stock compensation expense. Other compensation includes long-term incentive compensation. |
| (i) | Legal expense represents an accrual of the estimated probable loss in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York. See “Business—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York” and Note 8 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus for additional information. |
| (j) | Offering costs represent one-time costs relating to preparation for our initial public offering and accelerated implementation of new accounting standards. |
Contractual Obligations. The following table summarizes the long-term cash payment obligations to which we are contractually bound as of September 30, 2020. The years presented below represent 12-month periods ending September 30. The table does not reflect the $260.0 million of Incremental Term Loans we incurred on December 11, 2020. See “Summary—Recent Developments—Credit Facility Amendment and Dividend.”
| (in thousands) |
Less than 1 Year |
1-3 Years | 3-5 Years | More than 5 Years |
Totals | |||||||||||||||
| TLA(1) |
$ | 7,500 | $ | 26,250 | $ | 112,500 | $ | — | $ | 146,250 | ||||||||||
| Expected Interest(2)(3) |
3,855 | 7,050 | 2,215 | — | 13,120 | |||||||||||||||
| Operating Leases |
26,818 | 29,447 | 8,365 | 224 | 64,854 | |||||||||||||||
| Purchase Obligations(4) |
15,009 | 3,716 | — | — | 18,725 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total Contractual Obligations |
$ | 53,182 | $ | 66,463 | $ | 123,080 | $ | 224 | $ | 242,949 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | Represents the principal amount of indebtedness under the TLA. See Note 4 in our unaudited condensed consolidated financial statements included elsewhere in this prospectus for additional information. |
| (2) | Consists of interest payable under the Credit Facility. Interest payments for future periods were estimated using the interest rate of 2.18%, which was assumed for the remainder of the loan period until maturity. The actual amounts of interest and fee payments under the Credit Facility will ultimately depend on the amount of debt and letters of credit outstanding and the interest rates in effect during each period. We are also required to pay customary letter of credit fees equal to the applicable rate based on the total net leverage ratio and certain agency fees. |
| (3) | The credit agreement permits the interest rate to be selected at our option at either Adjusted LIBOR or Alternative Base Rate plus their respective applicable margin. Adjusted LIBOR is the rate for Eurodollar deposits for the applicable interest period while the Alternate Base Rate is the highest of (i) the |
82
Table of Contents
| Administrative Agent’s “Prime Rate”, (ii) the Federal Funds Effective Rate plus 0.50%, and (iii) one-month Adjusted LIBOR plus 1.00%. Additionally, the margin applied to both the TLA and Revolver is determined based on total net leverage ratio. Total net leverage ratio is defined as net debt, which represents indebtedness minus up to $25.0 million in cash and cash equivalents over consolidated EBITDA as defined under the credit agreement. There are additional margin and commitment fees payable on the TLA and available Revolver, based on the total net leverage ratio, including applicable margin fees ranging from 2.00% to 2.75% for Adjusted LIBOR Loans or 1.00% to 1.75% for Alternative Base Rate Loans and commitment fees ranging from 0.20% to 0.35%. As of September 30, 2020, there were no borrowings under the Revolver outstanding. |
| (4) | The purchase obligations primarily relate to amounts we expect to pay under extended payment term agreements for patient services equipment, as well as non-cancelable cash agreements for our software as a service contract. |
Accounts Receivable. Accounts receivable decreased to $71.8 million as of September 30, 2020 from $84.1 million at December 31, 2019, a decrease of $12.3 million. Days sales outstanding (calculated as of each period-end by dividing accounts receivable, net by the rolling average of total net revenues) were 30 days at September 30, 2020 compared to 35 days at December 31, 2019. The decrease in 2020 accounts receivable was primarily due to higher reimbursement levels driven by revenue cycle management improvements and increased estimated reserves for expected credit losses due to economic conditions related to the COVID-19 pandemic.
Accounts receivable before allowance for doubtful accounts decreased to $84.1 million as of December 31, 2019 from $117.7 million at December 31, 2018, a decrease of $33.6 million. Days sales outstanding (calculated as of each period-end by dividing accounts receivable, less allowance for doubtful accounts, by the rolling average of total net revenues) were 35 days at December 31, 2019 compared to 40 days at December 31, 2018. The decrease in 2019 accounts receivable was primarily due to the adoption of Topic 842. Upon adoption, the allowance for doubtful accounts associated with rental revenue is now presented as an offsetting reduction to the related accounts receivable. The decrease was also due to the result of improved accounts receivable collections driven by revenue cycle management improvements.
Unbilled Receivables. Included in accounts receivable are earned but unbilled receivables of $17.1 million and $24.1 million at September 30, 2020 and December 31, 2019, respectively. Delays, ranging from a single day to several weeks, between the date of service and billing can occur due to delays in obtaining certain required Payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in our analysis of historical performance and collectability. Efficiencies gained from a transformation initiative to transition billing processes to our localized branch locations contributed to the decrease in unbilled receivables in 2020, as well as relaxed documentation and authorization guidelines during the COVID-19 pandemic from Medicare and other Payors.
Included in accounts receivable are earned but unbilled receivables of $24.1 million and $17.7 million at December 31, 2019 and December 31, 2018, respectively. Delays, ranging from a single day to several weeks, between the date of service and billing can occur due to delays in obtaining certain required Payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in our analysis of historical performance and collectability. The increase in unbilled receivables in 2019 is mainly due to a transformation initiative to transition billing processes to our localized branch locations.
Inventories and Patient Equipment. Inventories consist primarily of respiratory supplies and items used in conjunction with patient equipment. Patient equipment consists of respiratory and home medical equipment that is provided to in-home patients for the course of their care plan, normally on a rental basis, and subsequently returned to us for redistribution after cleaning and maintenance is performed. We maintain inventory and patient equipment at levels we believe will provide for the needs of our patients.
83
Table of Contents
Long-Term Debt. On November 2, 2018, we entered into a senior secured asset-based revolving credit facility (the “ABL Facility”), with Wells Fargo Bank, N.A., an administrative agent and a syndicate of financial institutions and institutional lenders. The ABL Facility replaced the prior secured asset-based revolving credit facility dated May 2, 2014, which provided for a revolving credit financing of up to $100.0 million.
On June 21, 2019, we entered into a credit agreement with Citizens Bank and a syndicate of lenders for both a Term Loan A Facility of $150.0 million and a Revolving Credit Facility of $100.0 million. The Revolver replaced the prior ABL Facility which provided for revolving credit financing of up to $125.0 million. Proceeds from the TLA were used to fund a dividend payment to common stockholders and SARs holders. On December 11, 2020, we entered into the Credit Facility Amendment to incur $260.0 million of Incremental Term Loans. Net proceeds from the Incremental Term Loans were used to fund a $200.3 million dividend payment to our stockholders and $9.7 million distribution to SARs holders declared and paid in December 2020, with the remaining proceeds used to pay fees and expenses in connection with the Credit Facility Amendment and for general corporate purposes.
The credit agreement permits the interest rate to be selected at our option at either Adjusted LIBOR or Alternative Base Rate plus their respective applicable margin. Adjusted LIBOR is the rate for Eurodollar deposits for the applicable interest period while the Alternate Base Rate is the highest of (i) the Administrative Agent’s “Prime Rate”, (ii) the Federal Funds Effective Rate plus 0.50%, and (iii) one-month Adjusted LIBOR plus 1.00%. Furthermore, Adjusted LIBOR is subject to a 0.50% per annum floor and the Alternative Base Rate is subject to a 1.50% per annum floor. Additionally, the margin applied to both the TLA and Revolver is determined based on total net leverage ratio. Total net leverage ratio is defined as net debt, which represents indebtedness minus up to $25.0 million in cash and cash equivalents over consolidated EBITDA as defined under the credit agreement. The following is a summary of the additional margin and commitment fees payable on both the TLA and available Revolver:
| Level |
Total Net Leverage Ratio |
Applicable Margin for Adjusted LIBOR Loans |
Applicable Margin for Alternative Base Rate Loans |
Commitment Fee |
||||||||||
| I |
Greater than or equal to 3.00x | 2.75 | % | 1.75 | % | 0.35 | % | |||||||
| II |
Greater than or equal to 2.50x but less than 3.00x | 2.50 | % | 1.50 | % | 0.30 | % | |||||||
| III |
Greater than or equal to 1.50x but less than 2.50x | 2.25 | % | 1.25 | % | 0.25 | % | |||||||
| IV |
Less than 1.50x | 2.00 | % | 1.00 | % | 0.20 | % | |||||||
The TLA matures on June 21, 2024 and we are required to make quarterly principal payments on the TLA beginning June 30, 2020. Upon entering into the Credit Facility Amendment the amount of those quarterly principal payments was adjusted to account for the Interim Term Loans. We expect to refinance, renew or replace the TLA prior to its maturity in June 2024 or to repay it with cash from operations. The table below is a summary of the expected principal repayments each year:
| (in thousands) | ||||
| Years Ending December 31 |
||||
| 2020 |
$ | 8,958 | ||
| 2021 |
20,833 | |||
| 2022 |
36,458 | |||
| 2023 |
41,667 | |||
| 2024 |
302,083 | |||
The credit agreement encompassing the TLA and Revolver permits, subject to certain exceptions, an increase in our TLA or our Revolver, as well as incurs additional indebtedness, as long as it does not exceed a total net leverage ratio of 3.00x. The credit agreement requires mandatory prepayments upon the occurrence of certain events, such as dispositions and casualty events, subject to certain exceptions. The TLA or Revolver may be voluntarily prepaid at any time without any premium or penalty.
84
Table of Contents
Apria Healthcare Group’s assets and equity interests of Apria Healthcare Group and all present and future wholly owned direct domestic subsidiaries of Apria Healthcare Group, with certain exceptions, are pledged as collateral for the TLA and Revolver. The credit agreement contains a financial covenant requiring us to maintain a total net leverage ratio less than 3.50x. The credit agreement also contains negative covenants that, among other things, restrict, subject to certain exceptions, the ability of Apria Healthcare Group and its restricted subsidiaries to incur additional indebtedness and guarantee indebtedness, create or incur liens, engage in mergers or consolidations, dispose of assets, pay dividends and distributions or repurchase capital stock, repay certain indebtedness, make investments and engage in certain transactions with affiliates.
As of September 30, 2020, no amounts were outstanding under the Revolver, there were $15.9 million outstanding letters of credit, and additional availability under the Revolver net of letters of credit outstanding was $84.1 million. We were in compliance with all debt covenants set forth in the TLA and Revolver as of September 30, 2020.
In accordance with ASU 2015-03, Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs, we record origination and other expenses related to certain debt issuance cost as a direct deduction from the carrying amount of the debt liability. These expenses are deferred and amortized using the straight-line method over the stated life, which approximates the effective interest rate method. The unamortized debt issuance costs related to the ABL Facility dated November 2, 2018 were expensed as a result of the new credit agreement. Amortization of deferred debt issuance costs are classified within interest expense in our consolidated statements of income and was $0.4 million, $1.0 million, $1.1 million, $0.4 million, and $0.3 million for the nine months ended September 30, 2020 and 2019 and for the years ended December 31, 2019, 2018 and 2017, respectively.
Interest expense, excluding deferred debt issuance costs discussed above, was $3.7 million, $2.4 million, $4.0 million, $0.9 million and $0.9 million for the nine months ended September 30, 2020 and 2019 and for the years ended December 31, 2019, 2018 and 2017, respectively. Interest paid on debt totaled $3.7 million, $2.6 million, $4.2 million, $1.0 million and $1.0 million for the nine months ended September 30, 2020 and 2019 and for the years ended December 31, 2019, 2018 and 2017, respectively.
Off-Balance Sheet Arrangements
We did not have any off-balance sheet arrangements, as defined in Item 303(a)(4)(ii) of Regulation S-K during the periods presented.
Commitments and Contingencies
From time to time we enter into certain types of contracts that contingently require us to indemnify parties against third-party claims. The contracts primarily relate to: (i) certain asset purchase agreements, under which we may provide customary indemnification to the seller of the business being acquired; (ii) certain real estate leases, under which we may be required to indemnify property owners for environmental and other liabilities, and other claims arising from our use of the applicable premises; and (iii) certain agreements with our officers, directors and employees, under which we may be required to indemnify such persons for liabilities arising out of their relationship with us. In addition, we issued certain letters of credit under our Credit Facility as described under “Liquidity and Capital Resources—Long-Term Debt” above.
The terms of such obligations vary by contract and in most instances a specific or maximum dollar amount is not explicitly stated therein. Generally, amounts under these contracts cannot be reasonably estimated until a specific claim is asserted. Consequently, no liabilities have been recorded for these obligations on our balance sheets for any of the periods presented.
85
Table of Contents
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with GAAP. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenue and expenses and related disclosures of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue recognition, collectability of accounts receivable, reserves related to insurance and litigation, intangible assets, stock-based compensation, income taxes and contingencies. We base these estimates on our historical experience and various other assumptions that we believe to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results experienced may vary materially and adversely from our estimates. To the extent there are material differences between our estimates and the actual results, our future results of operations may be affected.
We consider the accounting policies that govern revenue recognition and the determination of the net realizable value of accounts receivable to be the most critical in relation to our consolidated financial statements. These policies require the most complex and subjective judgments of management. Additionally, the accounting policies related to long-lived assets, stock-based compensation, income taxes and self-insurance reserves require significant judgment.
Fee-for-Service Net Revenues. Revenues are recognized under fee-for-service arrangements for equipment we rent to patients and sales of equipment, supplies and other items we sell to patients.
Rental revenues – In accordance with Topic 842, revenue generated from equipment that is rented to patients is recognized over the noncancelable rental period, typically one month, and commences on delivery of the equipment to the patients. The portfolio of lease contracts is evaluated at lease commencement and the start of each monthly renewal period to determine if it is reasonably certain that the monthly renewal or purchase options would be exercised. The exercise of monthly renewal or purchase options by a patient has historically not been reasonably certain to occur at lease commencement or subsequent monthly renewal.
Revenues are recorded at amounts estimated to be received under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare, Medicaid and patients. Rental revenue, less estimated adjustments, is recognized as earned on a straight-line basis over the non-cancellable lease term. Rental of patient equipment is billed on a monthly basis beginning on the date the equipment is delivered. Since deliveries can occur on any day during a month, the amount of billings that apply to the next month are deferred.
Our lease agreements generally contain lease and non-lease components. Non-lease components primarily relate to supplies. The transaction price is allocated to the separate lease and non-lease components that qualify as performance obligations using the stand-alone selling price.
Sale revenues – Revenue related to sales of equipment and supplies is recognized on the date of delivery as this is when control of the promised goods is transferred to patients and is presented net of applicable sales taxes. Revenues are recorded only to the extent it is probable that a significant reversal will not occur in the future as amounts may include implicit price concessions under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare, Medicaid and patients. The sales transaction price is determined based on contractually agreed-upon rates, adjusted for estimates of variable consideration. The expected value method is used in determining the variable consideration as part of determining the sales transaction price using historical reimbursement experience, historical sales returns, and other operating trends. Payment terms and conditions vary by contract. The timing of revenue recognition, billing, and cash collection generally results in billed and unbilled accounts receivable.
Capitation Revenues. Revenues are recognized under capitation arrangements with third-party payors for services and equipment for which we stand ready to provide to the members of these payors without regard to the
86
Table of Contents
actual services provided. The stand ready obligation generally extends beyond one year. Revenue is recognized over the month that the members are entitled to healthcare services using the contractual rate for each covered member. The actual number of covered members may vary each month. As a practical expedient, no disclosures have been made related to the amount of variable consideration expected to be recognized in future periods under these capitation arrangements. Capitation payments are typically received in the month members are entitled to healthcare services.
Realizable Value of Accounts Receivable. Due to the nature of our industry and the reimbursement environment in which we operate, certain estimates are required to record total net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain Payors may result in adjustments to amounts originally recorded. Such adjustments are typically identified and recorded at the point of cash application, claim denial or account review.
We perform periodic analyses to evaluate accounts receivable balances to ensure that recorded amounts reflect estimated net realizable value. Specifically, we consider historical realization data, accounts receivable aging trends, other operating trends, and the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care Payor claims processing procedures and system changes.
Additionally, focused reviews of certain large and/or problematic Payors are performed. Due to continuing changes in the healthcare industry and third-party reimbursement, it is possible that our estimate could change in the near term, which could have an impact on operations and cash flows.
Prior to adoption of Topic 842, accounts receivable was reduced by a separate allowance for doubtful accounts which provided for those accounts from which payment was not expected to be received, although services were provided and revenue was earned. Upon determination that an account was uncollectible, it was written off and charged to the allowance. Upon adoption of Topic 842, we have elected to record a reserve for expected credit losses as part of net rental revenue adjustments in order to report rental revenue at an expected collectable amount based on the total portfolio of operating lease receivables for which collectability has been deemed probable.
Indefinite-Lived Intangible Assets and Long-Lived Assets. Indefinite-lived intangible assets are not amortized but instead tested at least annually for impairment or more frequently when events or changes in circumstances indicate that the assets might be impaired. We perform the annual test for impairment for indefinite-lived intangible assets as of the first day of the fourth quarter. The fair value of the trade name is determined using a relief-from-royalty method under the income approach, which uses projected revenue allocable to the trade name and an assumed royalty rate. We assessed qualitative factors and determined it was more likely than not that the fair value of the accreditations with commissions is greater than its carrying amount.
We first assess qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If, based on a review of qualitative factors, it is more likely than not that the fair value is less than its carrying amount, we will use a quantitative approach, and calculate the fair value and compare it to its carrying amount. If the fair value exceeds the carrying amount, there is no indication of impairment. If the carrying amount exceeds the fair value, an impairment loss is recorded equal to the difference.
We performed an assessment of qualitative factors and determined that no events or circumstances existed that would lead to a determination that it is more likely than not that the fair value of indefinite-lived assets were less than the carrying amount. As such, a quantitative analysis was not required to be performed.
Long-lived assets, including property and equipment and purchased definite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an
87
Table of Contents
asset or asset group may not be recoverable. Significant judgment is required in determining whether a potential indicator of impairment of long-lived assets exists and in estimating future cash flows for any necessary impairment tests. Recoverability of assets to be held and used is measured by the comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
We did not record any impairment charges related to indefinite-lived intangible assets or long-lived assets for the nine months ended September 30, 2020 and 2019. We did not record any impairment charges related to indefinite-lived intangible assets or long-lived assets for the years ended December 31, 2019, 2018 and 2017.
Leases. We determine if an arrangement is a lease at commencement and perform an evaluation to determine whether the lease should be classified as an operating or finance lease. Operating leases are included in operating lease right-of-use (ROU) assets, current portion of operating lease liabilities and operating lease liabilities, less current portion, on the consolidated balance sheet. ROU assets represent our right to use an underlying asset for the lease term and lease liabilities represent our obligation to make lease payments arising from the lease. Operating lease ROU assets and the related liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. As our leases do not provide an implicit rate, we use its incremental borrowing rate (IBR) based on the information available at the lease commencement date in determining the present value of future lease payments. We use market rates from recent secured financing to determine the IBR.
The operating lease ROU asset also includes any lease payments made to the lessor at or before the commencement date and is adjusted by any lease incentives received. Variable lease payments are not included in the operating lease liability as they cannot be reasonably estimated and are recognized in the period in which the obligation for those payments is incurred. Lease terms may include options to extend or terminate the lease and are included only when it is reasonably certain that we will exercise that option. For all asset classes, leases with a lease term of twelve months or less at the lease commencement date are not recorded on the consolidated balance sheet, as permitted by the short-term lease exception. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
Our lease agreements do not contain any material residual value guarantees or material restrictive covenants. We do not have any material subleases. We do not have any leases classified as a finance leasing arrangement. As such, all leases are classified as operating leases. See further discussion at Note 8 – Leases.
Stock-Based Compensation. Stock-based compensation has historically been granted to certain of our employees in the form of profit interest units of our parent entity and SARs. We account for our stock-based awards in accordance with provisions of Accounting Standards Codification 718, “Compensation—Stock Compensation.”
We recognize compensation expense in respect of the profit interest units and SARs based on the fair value of the awards as measured on the grant date. Fair value is not subsequently remeasured unless the conditions on which the award was granted are modified. As our equity is not publicly traded, we must estimate the fair value of our equity instruments, as discussed in “Equity Valuations” below and in the audited consolidated financial statements included elsewhere in this prospectus. To the extent applicable, following the closing of this offering, the fair value of our equity instruments for purposes of determining stock-based compensation will be the closing price of shares as reported on the applicable date.
Generally, the compensation expense for each separately vesting portion of the awards is recognized on a straight-line basis over the vesting period for that portion of the award subject to continued service. Compensation expense for awards containing performance conditions is recognized only to the extent that it is probable the performance conditions will be made.
88
Table of Contents
We expect to continue to grant stock-based compensation in the future, and, to the extent that we do, our stock-based compensation expense in future periods will likely increase.
Equity Valuations. The fair value of our equity instruments has historically been determined based upon information available at the time of grant. Given the absence of a public trading market for our equity and in accordance with the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately Held Company Equity Securities Issued as Compensation, management has exercised reasonable judgment and considered numerous objective and subjective factors to determine the best estimate of the fair value of our equity instruments at each grant date.
These factors included:
| • | our operating and financial performance; |
| • | current business conditions and projections; |
| • | the likelihood of achieving a liquidity event for the underlying equity instruments, such as an initial public offering or sale of our company, given prevailing market conditions; |
| • | the lack of marketability of our shares; and |
| • | the market performance of comparable publicly traded companies. |
In valuing our equity instruments, we determined the equity value of our business using a weighted blend of the income and market approaches. The income approach estimates the fair value of a company based on the present value of such company’s future estimated cash flows and the residual value of such company beyond the forecast period. These future values are discounted to their present values to reflect the risks inherent in such company achieving these estimated cash flows.
Significant inputs of the income approach (in addition to our estimated future cash flows themselves) include the long-term growth rate assumed in the residual value, discount rate and normalized long-term operating margin. The terminal value was calculated to estimate our value beyond the forecast period by applying valuation metrics to the final year of our forecasted revenue and discounting that value to the present value using the same weighted average cost of capital applied to the forecasted periods.
Income Taxes. Our provision for income taxes is based on expected income, permanent book/tax differences and statutory tax rates in the various jurisdictions in which we operate. Significant estimates and judgments are required in determining the provision for income taxes.
Deferred income tax assets and liabilities are computed for differences between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized.
In determining the necessity and amount of a valuation allowance, all available information (both positive and negative) is considered and analysis is performed to determine the appropriate weight that should be afforded to available objective and subjective evidence.
For the year ended December 31, 2017, we recorded a net valuation allowance release of $105.1 million on the basis of management’s reassessment of the amount of its deferred tax assets that are more likely than not to be realized. As of December 31, 2017, we evaluated all of the positive and negative evidence related to the valuation allowance, including three years of cumulative pre-tax income and projected income, and determined that there was sufficient positive evidence to conclude that it is more likely than not that net deferred taxes of $50.7 million were realizable. We therefore reduced the valuation allowance accordingly.
89
Table of Contents
Self-Insurance. Coverage for certain employee medical claims and benefits, as well as workers’ compensation, professional and general liability, and vehicle liability are self-insured. Amounts accrued for costs of workers’ compensation, medical, professional and general liability, and vehicle liability are classified as current or long-term liabilities based upon an estimate of when the liability will ultimately be paid.
Legal Reserves. We are involved in various legal proceedings, claims, and litigation that arise in the ordinary course of business. We investigate these matters as they arise and reserves for potential loss in accordance with ASC No. 450, Contingencies. Significant judgment is required in the determination of both the probability of loss and whether the amount of the loss can be reasonably estimated. Estimates are subjective and are made in consultation with internal and external legal counsel. See further discussion at Note 8 – Commitments and Contingencies to our unaudited condensed consolidated financial statements included elsewhere in this prospectus.
Accounting Pronouncements Not Yet Adopted
Recently issued accounting pronouncements that may be relevant to our operations but have not yet been adopted are outlined in Note 2 – Recent Accounting Pronouncements to our consolidated financial statements included elsewhere in this prospectus.
Quantitative and Qualitative Disclosures About Market Risk
Our exposure to market risk relates to fluctuations in interest rates from borrowings under the Credit Facility. Our letter of credit fees and interest accrued on our debt borrowings carry a floating interest rate which is tied either Adjusted LIBOR or Alternative Base Rate plus their respective applicable margin and therefore are exposed to changes in interest rates. As of September 30, 2020, there was $146.3 million outstanding on the TLA, no amounts outstanding under the Revolver, $15.9 million outstanding letters of credit, and additional availability under the Revolver, net of letters of credit outstanding, was $84.1 million.
90
Table of Contents
Apria
We are a leading provider of integrated home healthcare equipment and related services in the United States. We offer a comprehensive range of products and services for in-home care and delivery across three core service lines: (1) home respiratory therapy (including home oxygen and NIV services); (2) OSA treatment (including CPAP and bi-level positive airway pressure devices, and patient support services); and (3) NPWT. Additionally, we supply a wide range of home medical equipment and other products and services to help improve the quality of life for patients with home care needs. Our revenues are generated through fee-for-service and capitation arrangements with Payors for equipment, supplies, services and other items we rent or sell to patients. Through our offerings, we also provide patients with a variety of clinical and administrative support services and related products and supplies, most of which are prescribed by a physician as part of a care plan. We are focused on being the industry’s highest-quality provider of home healthcare equipment and related services, while maintaining our commitment to being a low-cost operator. We offer a compelling value proposition to patients, providers and Payors by allowing patients to receive necessary care and services in the comfort of their own home, while, at the same time, reducing the costs of treatment. We generated over $1 billion of net revenue in 2019, of which approximately 80% was from home respiratory therapy and OSA treatment, service categories in which we believe we have a leading market position.
We believe our integrated product and service offerings, combined with our national scale and strong reputation, provide us with a strategic advantage in being a preferred home healthcare provider for patients, providers and Payors. Our Payors include substantially all of the national and regional insurers, managed care organizations and government Payors in the United States. We benefit from long-standing relationships with a community of providers and referral sources for post-acute services across the acuity spectrum because of the consistency and reliability of our high quality clinical support, our national distribution footprint and our breadth of Payor relationships.
Our product and service offerings are distinguished by the complexity and sophistication required in their clinical delivery, logistical coordination and payment arrangements. We offer patients and providers differentiated clinical service, leveraging our protocols and expertise to improve outcomes across our service lines. With an expansive network of delivery technicians and therapists that is not readily replicated, we are able to provide home healthcare therapies that require high-touch service, providing a bridge from the acute care setting to the home. In 2019, we served nearly 2 million patients, made over 2.4 million deliveries and conducted over 744,000 clinician interactions with our patients.
We believe that we are well positioned to capitalize on a number of opportunities for organic growth. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025. The U.S. market for NPWT devices is expected by industry analysts to grow at a CAGR of approximately 5% between 2018 to 2023. With top two market positions in the United States in home respiratory therapy, OSA treatment and NPWT, we believe this industry tailwind represents an attractive embedded opportunity for growth. Moreover, we believe the home healthcare industry, which plays a critical role in the transition of patients from an acute care setting to a lower cost home setting, is at the center of a paradigm shift by Payors from the traditional fee-for-service model to value-based reimbursement. As one of the industry leaders, we expect to increasingly benefit from this dynamic. We will also seek to capture efficiencies through increasing scale and operational improvements. Our existing distribution network can reach over 90% of the U.S. population and has substantial presence in both high-density urban markets and rural markets. Coupled with scalable technology and centralized operations, including customer service and revenue cycle management, we believe we can continue to grow our patient base without significant incremental capital investment in our infrastructure. We will also continue to focus on improving the cost-efficiency of our operations through various new technology initiatives and data analytics capabilities. Finally, we believe we can continue to enhance our cash profile through increased focus on less capital intensive products, such as sleep supplies and NPWT, and by benefitting from our investment in longer-lived, ventilation therapy equipment.
91
Table of Contents
In 2019, we generated $1.1 billion of net revenue, $15.6 million in net income, $174.0 million of Adjusted EBITDA and $80.5 million of Adjusted EBITDA less Patient Equipment Capex. Through various strategic and operational initiatives, we have improved profitability despite reimbursement rate pressure, improving our Adjusted EBITDA margin by 110 basis points from 2017 through 2019 on a basis that excludes the impact of new accounting policies adopted in 2018 and 2019. For reconciliations of Adjusted EBITDA and Adjusted EBITDA less Patient Equipment Capex to net income, the most directly comparable financial measure prepared in accordance with GAAP, see “Summary—Summary Historical Financial and Other Data.”
Industry Overview
The U.S. home healthcare market comprises a broad range of products and services—including respiratory therapy, OSA therapy, negative pressure wound therapy, home medical equipment, infusion therapy, home healthcare nursing, orthotics and prosthetics, diabetic supplies and general medical supplies. CMS estimates that the total revenue of the U.S. home healthcare market was $108.9 billion in 2019, and forecasts this market to grow at a CAGR of approximately 7% between 2020 and 2028. CMS forecasts the durable medical equipment sub-segment to grow at a CAGR of approximately 6% between 2019 and 2028. This growth is forecasted despite the various cost control programs that have been implemented in the Medicare sector. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025. In 2018, industry analysts estimated that the market size for respiratory devices and OSA devices was approximately $6.2 billion and approximately $2.2 billion, respectively, in North America. The U.S. market for negative pressure wound therapy devices is expected by industry analysts to grow at a CAGR of approximately 5% between 2018 to 2023, and in 2018, the global market size of negative pressure wound therapy devices was estimated by industry analysts to be approximately $2.1 billion. Our sub-segment of the U.S. home healthcare industry is highly fragmented. The five largest players in this sub-segment accounted for approximately 50% of this sub-segment’s revenues in 2019. The remaining market revenues are attributable to thousands of other companies that primarily operate in local markets or regions.
We expect to benefit from the following continuing trends within the home healthcare market:
| • | Aging Population. Favorable demographic trends have resulted in patient volume growth in the United States and are expected to continue to drive volume growth. As the baby boomer population ages and life expectancy increases, the elderly—who comprise a large portion of our patients—will represent a higher percentage of the overall population. The CMS Office of the Actuary projects that the number of Medicare beneficiaries will grow, on average, by 2.5% annually over the period from 2020 to 2028 and the U.S. Census Bureau projects that the United States population aged 65 and over will grow substantially from 15.2% of the population in 2016 to 20.6% of the population by 2030. |
| • | Rising Incidence of Chronic Diseases. Increasing obesity rates, the clinical consequences of the high prevalence of smoking from earlier decades, the current under-diagnosis of certain health conditions and higher diagnosis rates for a number of chronic health conditions, such as chronic obstructive pulmonary disease, OSA, diabetes and others, have collectively driven growth in the industry. We believe that in the United States 1 in 15 adults has moderate to severe obstructive sleep apnea, while 1 in 10 adults has diabetes, with asthma similarly prevalent among adults. We believe that patients with these chronic conditions will increasingly be treated in their homes instead of in the hospital setting and utilize the services that we provide. |
| • | Continued Shift Toward Home Healthcare Driven by the Compelling Economic Value Proposition to Key Stakeholders. Between 2019 and 2028, the nation’s healthcare spending is projected to increase to $6.2 trillion, growing at an average annual rate of 5.5%, according to CMS. In August 2020, CMS published national healthcare expenditure data which showed that durable medical equipment spending grew 25.6% for all Payors from 2012 to 2018. Despite multiple initiatives designed to reduce durable |
92
Table of Contents
| medical equipment spending in Medicare, which translated to a lower growth rate than the national average for all Payors, the rising cost of healthcare has caused many managed care Payors to look for ways to contain costs and home healthcare is increasingly sought out as an attractive, cost-effective, clinically appropriate alternative to expensive facility-based care. |
| • | Increased Prevalence of In-home Treatments and Preference for In-home Care Where Available. Improved technology has resulted in a wider variety of treatments being administered in patient homes. These improvements have allowed for earlier patient discharge and have lengthened the portion of the recuperation period spent outside of an institutional setting. In addition, medical advancements have also made medical equipment more simple, adaptable and cost-effective for use in the home. As technology continues to improve, we believe that these trends will likely continue. Furthermore, as the availability of in-home care increases, we believe that many patients will opt for in-home treatments since patients generally prefer the convenience and typical cost advantages of home healthcare over institutional care and the greater independence, increased responsibility and improved responsiveness to treatment that comes with it. According to CMS estimates, between 2020 and 2028, U.S. home healthcare spending is projected to increase to $201.3 billion, growing at a CAGR of approximately 7%. |
| • | Consolidation of the Highly Fragmented Market to the Benefit of National Players. According to HME News, between 2012 and 2018, the overall number of DMEPOS suppliers that bill Medicare more than $10 million annually fell from 73 to 57, and between 2013 and 2020, the total number of DMEPOS suppliers that bill Medicare fell from 8,837 to 6,152, a decline of approximately 30%. We attribute this decline to the inability of smaller local and regional players to make the significant capital investment and achieve the scale required to effectively compete in the economic environment that followed the implementation of the CBP. We believe that the home healthcare market will continue to favor larger, financially stable, national players with consistent, scalable, high quality service. |
Our Value Proposition
We believe we offer a compelling value proposition for patients, providers and Payors.
Value Proposition for Patients. We are committed to improving the experience and clinical outcome for each patient we serve. We believe our patients prefer the convenience and typical cost advantages of home healthcare over institutional care and the greater independence, increased responsibility and improved responsiveness to treatment that comes with it. By providing in-home delivery and equipment set-up, patient and caregiver education, as well as patient monitoring and compliance services, we enable the patient to move from or avoid an acute care setting and remain in or return to the home, which is the lowest cost, and overwhelmingly the patient-preferred, setting.
Value Proposition for Providers. Physicians, hospital professionals and other providers refer patients to us because of the consistent, high quality and reliable services we offer them and their patients. We seek to be a trusted advocate and provider of patient clinical needs in patients’ homes, while fostering lasting doctor-patient relationships. Additionally, the reliability of our clinical support, quality equipment and data collection facilitate a clinically adept transition to a lower-cost setting, which we believe benefits healthcare professionals, and helps to put them at ease regarding their patients’ ongoing treatments. We believe our services improve patient compliance and clinical outcomes, reduce hospital re-admissions and enable hospital providers to have greater control over timely patient discharges.
Value Proposition for Payors. We offer Payors access to an extensive national footprint, national logistics systems, respiratory clinical expertise, competitive pricing, alternative payment arrangements, including fee-for-service and capitation for defined patient populations, and our ability to connect electronically with Payors’ systems. We seek to effectively manage the transition from the acute care setting to a low-cost home setting with a high reliability of clinical support, data collection and quality equipment to lower readmission rates.
93
Table of Contents
Our Strategic Transformations
Since we were acquired by Blackstone in 2008, the home healthcare industry has experienced a number of significant changes, including reimbursement, regulatory and technological changes. We have continually worked to adapt our business and organizational structure to best meet the current needs of patients, providers and Payors.
Our most recent business initiative, which we call Simplify, focused on retaining clear customer ownership at our local branch level with support from our scalable national platform to greatly improve the patient and referral source experience. With a goal of optimizing the end-to-end customer experience while enabling us to increase our growth rate at lower cost, we evaluated local, regional and centralized processes to foster local branch customer ownership, supported by regional shared services where scale, consistency and process expertise is needed, and our national platform where scale can be leveraged. This transformation has led to significant improvement in profitability and lower operating costs and has positioned us well for future growth. Our patient-centric growth plan begins with an improved customer experience, which we believe will support increased cross-sell opportunities and growth across product lines to the over 2 million unique patients we serve annually.
In addition to Simplify, we have also made other substantial changes to our business since our acquisition by Blackstone:
| • | we brought on a management team of individuals with extensive expertise and experience in the workings of our industry and regulatory environment and others who specialize in designing and developing scalable business infrastructures and business transformation; |
| • | we improved our business and revenue mix by growing our relationships with commercial Payors; |
| • | we expanded our service offerings to include more complex respiratory services, including NIV therapy, and we shifted our focus to three core service lines including less capital intensive products and services which complement our core offerings; |
| • | we invested in patient monitoring and outcomes data collection to differentiate our service offerings and provide enhanced value to patients, providers and Payors; |
| • | we improved sales productivity through changes in the sales force hiring profile and implementation of a new sales customer relationship management system; |
| • | we formed strategic relationships with our suppliers to help optimize equipment costs and provide state-of-the art patient equipment; |
| • | we streamlined our management structure and further optimized our branch network by reducing our locations open to the public from over 400 to approximately 275 without materially reducing the coverage of our service areas or the quality of our service; |
| • | we built a scalable infrastructure with data analytics and forecasting capabilities to more efficiently leverage the favorable trends of an aging population and the paradigm shift in healthcare from the acute setting to the home; and |
| • | we consolidated regional and branch level billing, collections and aspects of the customer service function, reorganized their work flows and adopted new technology and processes to help us improve productivity, monitor performance and more effectively manage important aspects of our business in real-time. |
As result of these strategic transformations and future initiatives, we are well positioned to compete and win in the current industry environment and to successfully navigate any future changes.
Our Competitive Strengths
National Scale with Local Presence. Our platform combines local market presence with the advantages and efficiencies of national scale, clinical expertise and reputation. As one of the largest providers of home healthcare
94
Table of Contents
services in the United States, we enjoy long-tenured relationships with the majority of the major commercial Payors, who value an expansive geographic footprint that can service their entire patient populations, in addition to our reputation for reliable and quality care. We also offer alternative reimbursement arrangements such as capitations for defined patient populations. As a result, we are able to be a preferred provider for integrated healthcare systems and other major commercial Payors, including national single-source arrangements. We also benefit from the cost advantages and efficiencies of a scalable, centralized infrastructure. Our consolidated customer service and billing centers work with local branch locations to ensure seamless service to meet local customer requirements. Our revenue cycle management process utilizes centralized operations and technology to manage claims and patient collections on a single platform. At the same time, with approximately 275 branch locations and a substantial presence in high-density urban population centers, as well as a presence in non-urban markets, we have built an in-market network of last-mile logistics operations, delivery technicians and therapists that enables us to deliver high-touch, sophisticated clinical support and service in a fast and reliable manner. We manage and measure speed of delivery through a variety of means, depending on the type of product and patient-specific needs, including expedited order processing and delivery times (typically less than four hours) for patients discharging from the hospital to their homes. We believe this combination of national scale and local presence is not readily replicated by new entrants, and anticipate that industry trends toward continued consolidation of a highly fragmented home healthcare services market will inure to our benefit.
Expansive Offering with Exposure to Attractive Product Markets. We seek to offer high quality, clinically appropriate care across a broad spectrum of services and treatments amenable to the home setting. Our extensive offering helps make us an attractive and convenient choice for our patients, providers and Payors. With top two market positions in the United States in home respiratory therapy, OSA treatment and NPWT, we are aligned with large and growing addressable markets across our core service lines. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025.
Strong Relationships with Payors and Referral Sources Developed with Differentiated Sales Model. We enjoy deep and long-standing relationships with national and regional insurers and managed care organizations, many of whom we have been contracted with for over 20 years. We believe Payors value the breadth of our entire platform, in both the geographic markets we serve and the range of conditions our service offerings cover, and our flexibility in payment arrangements, including capitation for defined patient populations, demonstrating our competence in managing value as volume, as well as varying fee-for-service arrangements. We employ a centralized managed care sales function to handle Payor arrangements. Our team has negotiated over 1,600 contracts with national and regional commercial Payors, including fee-for-service as well as capitated arrangements. The depth and diversity of our contractual relationships with commercial Payors (which includes all Payors other than government Payors), which represented approximately 80% of our net revenue in 2019, enhances the stability of our pricing by reducing our dependence on any single contract. At the same time, we believe our critical mass with the traditional Medicare and Medicaid population, which represented approximately 20% of 2019 net revenue, enhances our ability to strategically rebalance our exposure to commercial and government Payors depending on market and reimbursement conditions.
Leveraging our broad market access to patients through our centralized Payor relationships, we cultivate individual relationships with thousands of local referral sources, including hospitals, outpatient facilities, physicians and sleep centers, through a field sales force of approximately 380 in-market sales representatives and approximately 200 front-line managers. We believe this differentiated approach of combining Company-wide Payor relationships with in-market referral relationships enhances the efficiency and productivity of our marketing efforts.
Leadership in Clinical Delivery. We specialize in certain complex home healthcare services and treatments that require deep technical and clinical expertise in addition to frequent patient interaction. We believe we are distinguished from e-commerce and logistics platforms by our ability to meet patient needs directly through our
95
Table of Contents
highly trained technicians and therapists who deliver and provide these services, as well as our ability to bill a patient’s insurance provider. We utilize differentiated clinical expertise and protocols to drive optimal outcomes across our core service lines and all of these operations are accredited by The Joint Commission. For example, our Premium Care SleepTM program (“Premium Care SleepTM”) is a fully-integrated sleep management program designed to conveniently provide OSA patients with the tools they need to improve their sleep. Through Premium Care SleepTM, we monitor the OSA treatment compliance of approximately 168,000 sleep patients on a nightly basis during the critical first 90 days of their treatment and proactively reach out to patients who may need help to improve their compliance through clinical intervention and other support from Apria. We believe this program has resulted in industry-leading sleep therapy compliance rates.
Leading Revenue Cycle Management. We manage medical claims and patient collections on a single operating platform that allows for capitation and fee-for-service arrangements. We believe we lead the industry in (1) ease of use for our key referral sources, (2) regulatory compliance and (3) revenue cycle management and billing and collections efficiency. Our process offers several advantages to our key referral sources and Payors, which we believe improve our ability to win new business and capture share from our competitors. We offer referral sources and Payors ease of use, speed, consistency, stability and predictability. Service preferences can be customized at the individual prescribing physician level, and standards for qualification and service can be customized to the requirements of each Payor. The integration of our processes, systems and our overall billing and collections capability allow us to effectively and efficiently bill, collect and manage our revenue. Almost all claims are billed electronically and almost all cash is posted automatically.
Strong and Experienced Management Team. We are led by a team of talented industry veterans, comprising individuals with long tenures at Apria and deep knowledge of our history and operations, as well as individuals who joined us more recently and bring fresh perspectives, insights and best practices developed through experience in other industries and at other companies. Together, they have led our strategic transformation and successfully navigated a challenging reimbursement environment. With an average of over 20 years of industry experience and over ten years with Apria, our senior leadership team has expertise spanning nearly every segment of the healthcare industry, including managed care, group purchasing organizations, manufacturing, supply chain, procurement, home healthcare, acute care, skilled nursing and long-term care pharmacy. They possess in-depth knowledge of our industry, markets, regulatory environment and service offerings.
Growth Strategy
Our goal is to be the market leader in the provision of high quality, cost-efficient home healthcare services, creating value for all stakeholders—patients, providers and Payors. We seek to achieve this goal through the following growth strategies:
Maintain leadership in markets with favorable industry dynamics. The broader U.S. markets for respiratory devices and OSA devices, which align with our home respiratory and OSA treatment product lines, two of our core product lines and which represent over 80% of 2019 net revenue, are expected by industry analysts to grow at CAGRs of approximately 6% and approximately 8%, respectively, between 2018 and 2025. With a national distribution platform that is difficult to replicate, deep and long-standing relationships with national and regional insurers and managed care organizations and a reputation for quality and reliability of service, we believe we are well positioned to maintain leadership in these attractive and growing markets. Industry groups suggest that approximately 80% of moderate and severe sleep-disordered breathing cases remain undiagnosed. We have been educating primary care physicians about the proper diagnosis and treatment of OSA. As the first line of care, these primary care physicians are more likely to encounter undiagnosed instances of OSA. In addition to traditional solutions, we coordinate an end-to-end virtual solution that includes home sleep testing (rather than testing at an offsite sleep clinic) which is convenient for the patient and safer, especially in the current pandemic environment. We believe that facilitating the diagnosis and treatment of this condition will lead to increased growth in OSA sales and increase revenue synergies as newly acquired OSA patients will have access to our other product and service lines when clinically indicated.
96
Table of Contents
Enable the transition to value-based healthcare. Government and commercial Payors are increasingly seeking ways to shift from traditional fee-for-service to a value-based model. We believe the ability to transition patients from the acute care setting to the home, as well as to prevent unnecessary readmissions, represents a critical part of this effort. As a leading provider of home healthcare equipment and related services, we believe we will increasingly benefit from this ongoing paradigm shift in the industry. In addition, we believe our demonstrated expertise in non-traditional payment models, such as capitation arrangements, will position us well to take advantage of this trend.
Expand product and service offerings. We continue to focus on expanding into new product lines and services both organically and through strategic acquisition to continue to grow and diversify our revenue with a patient-centric view centered on the long term value of each patient. For example, we are pursuing products and services related to the treatment of diabetes and the provision of diabetic supplies. Technological advances in continuous glucose monitoring and insulin pump delivery (creating an “artificial pancreas”) have changed the diabetes treatment paradigm and enabled providers to better treat patients in the home healthcare setting. Moreover, diabetic patients often suffer from many co-morbidities, including OSA, respiratory ailments and heart disease, many of which we already treat through our sleep and respiratory product lines. We believe we are well positioned to leverage our national platform and scalable infrastructure to help diabetic patients benefit from new diabetes treatment technologies as well as treatments for co-morbidities.
Leverage patient interactions. Our business model provides for frequent interaction with our patients and a direct entry point into the home, allowing our technicians, therapists and customer service agents to assess whether the patients are in need of additional services, supplies or convenience items. Where appropriate, our technicians, therapists and customer service agents can assist our patients in obtaining these additional services, supplies or convenience items through Apria. In addition, existing patients and potential patients may utilize our e-commerce platform to access additional items that are not covered by Payors and are purchased directly by patients.
Grow e-commerce through a patient-centric approach. Our e-commerce platform is primarily focused on supplies, accessories and additional items that are not covered by Payors and are purchased directly by patients. E-commerce is a convenient way to provide products and services to our existing patients and also serves as an additional acquisition channel for new patients. Through direct-to-patient marketing focused on service and clinical support, we believe we can continue to grow volume through our e-commerce channel. For example, we frequently acquire patients to meet a single product need even though they typically have additional home healthcare needs. Through patient interaction and education, they can discover and access other products and services that we offer through e-commerce that can address their needs. In addition, we believe the broader home healthcare trends discussed below will also enable us to grow our revenue and product and service offerings through e-commerce. Given that the supplies and accessories that are its primary focus are less capital intensive, we believe growing our e-commerce channel represents an attractive opportunity to increase cash flows and profitability.
Grow with new home healthcare trends. Telemedicine and remote provider care have been gaining traction in recent years and have been significantly accelerated given the recent COVID-19 pandemic. We believe that these trends will help accelerate growth in home healthcare and we are well positioned to benefit from these trends given our national footprint and scalable infrastructure. Moreover, due to the impacts of the COVID-19 pandemic, we expect accelerated and sustainable demand for home healthcare solutions including respiratory and other medical conditions that we treat regularly. These trends in telemedicine and the emphasis on treating patients in the home, outside the traditional medical clinic and hospital, will enable us to grow our revenue and product and service offerings further.
Strategic acquisitions. We believe there is also opportunity to accelerate our growth rate, market share gains and expand into new product markets through strategic acquisitions. We will continue to evaluate such opportunities through a disciplined approach, seeking only acquisitions that will complement our existing operations and businesses and/or create synergies. The various markets we participate in remain highly
97
Table of Contents
fragmented and ripe for consolidation and we believe that our nationally integrated platform, scale and strong reputation position us well to be a consolidator in our industry. We have a relatively unburdened balance sheet with low debt levels and meaningful debt capacity for acquisitions. Moreover, as a public company, we will have greater access to capital markets and we will be able to use our stock as an acquisition currency or to raise additional capital for strategic acquisitions.
Continue to capture efficiencies through scale and operational improvement. Our existing distribution network of approximately 275 branch locations can reach more than 90% of the U.S. population across both high-density urban markets and rural markets. Coupled with scalable technology and centralized operations, including revenue cycle management, we believe we can continue to grow beyond our nearly 2 million patients served in 2019 without significant incremental capital investment in infrastructure. As we maintain our strong position in growing product markets, we believe the ability to scale our operations and leverage fixed costs represents a significant opportunity for growth in profitability. In addition, we believe we can continue to improve the cost-efficiency of our operations and support functions through new and recently completed technology initiatives, such as RDA to simplify agent workflow, RPA for certain repetitive and volume staff tasks and automating activities such as new employee onboarding and equipment provisioning, and enhanced workflow to improve ease of use and direct activity to the appropriately skilled staff. We have also invested in DMEhub, a cloud-based e-prescribing platform that allows providers to submit medical equipment orders more efficiently and accurately, which we believe drives ease of use, enables more efficient order processing and reduces administrative burden.
Continue to improve cash profile. We continually evaluate opportunities to enhance our cash flow and return on investment. We see opportunities in this regard both to increase our exposure to less capital intensive products and to benefit from recent investment in our longer-lived, complex equipment fleet. Patients on OSA therapy and NPWT require periodic replenishment of supplies and accessories in order to remain in compliance with their prescribed therapies. These supplies are less capital intensive and we believe represent a multi-billion dollar market with an attractive growth profile. The opportunity to increase our exposure to this market is enhanced by our growing e-commerce distribution channel, which is primarily focused on these supplies and accessories, offering patients speed and convenience and helping us to continue to grow our volume.
Service Lines
Our business activities comprise a single operating segment focused on three core service lines: (1) home respiratory therapy (including oxygen and NIV services); (2) OSA treatment (including CPAP and bi-level positive airway pressure devices, and patient support services); and (3) NPWT. Additionally, we supply a wide range of home medical equipment, other products and services to help improve the quality of life for patients with home care needs. Through our offerings, we also provide a broad range of home healthcare products and services, and provide patients with a variety of clinical and administrative support services and supplies, most of which are prescribed by a physician as part of a care plan. We provide substantial benefits to both patients and Payors by allowing patients to receive necessary care and services in the comfort of their own home while reducing the cost of treatment. For example, on average, it costs approximately $50 per day to create an in-home hospital room versus approximately $2,271 per day for in-patient hospital care, according to the Medicare Payment Advisory Commission and Henry J. Kaiser Family Foundation. Our services include:
| • | providing in-home delivery, set-up and maintenance of equipment and/or supplies; |
| • | educating patients and caregivers about health conditions or illnesses and providing instructions about home safety, self-care and the proper use of equipment; |
| • | monitoring therapy compliance and intervening to enhance compliance; |
| • | clinical monitoring of complex respiratory service patients’ individualized treatment plans; |
98
Table of Contents
| • | reporting patient progress and status to the physician, national and regional insurers and/or managed care organizations; and |
| • | processing claims to Payors on behalf of patients. |
The following table sets forth a summary of total net revenues by service line, expressed as percentages of total net revenues:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Home respiratory therapy |
40.0 | % | 39.7 | % | 40.3 | % | ||||||
| OSA treatment |
40.3 | 38.7 | 37.3 | |||||||||
| NPWT |
3.9 | 3.7 | 3.9 | |||||||||
| Other equipment and services |
15.8 | 17.9 | 18.5 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total |
100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
|
|
|||||||
Home Respiratory Therapy. ($435.7 million, $441.2 million and $432.4 million, or 40.0%, 39.7% and 40.3%, of our net revenues for the years ended December 31, 2019, 2018 and 2017, respectively).
We are one of the largest providers of home respiratory therapies in the United States, which includes the supply of stationary and portable home oxygen equipment and non-invasive ventilators. Our distribution model allows our respiratory care practitioners to educate, on a timely and efficient basis, newly-diagnosed patients about their condition, the equipment their physician has prescribed for them, and the long-term importance of complying with the physician’s order. Our services offer a compelling relative cost advantage to our patients and Payors. Patients utilize our products to treat a variety of conditions, including chronic obstructive pulmonary diseases, such as emphysema and chronic bronchitis (collectively, the third leading cause of death in the United States), neuromuscular disorders and restrictive thoracic disorder.
We employ a nationwide clinical staff of approximately 450 respiratory care professionals, including home respiratory therapists who perform patient assessments and set-ups for highly complex clinical equipment, and provide direct patient care, monitoring and 24-hour support services under physician-directed treatment plans and in accordance with our proprietary program.
OSA Treatment. ($438.6 million, $430.4 million and $400.7 million, or 40.3%, 38.7% and 37.3%, of our net revenues for the years ended December 31, 2019, 2018 and 2017, respectively).
We are one of the largest providers of OSA therapy devices, including CPAP and bi-level positive airway pressure devices, and patient support services in the United States. The incidence and diagnosis of OSA continues to increase in the United States. We believe that the strength of our position in this market is partly due to our significant presence in the commercial Payor market, since the initial diagnosis of OSA typically occurs among adults between the ages of 35 and 65 rather than the population served by Medicare. To manage our significant new and recurring patient volumes in a cost-effective, clinically sound manner, we developed an innovative care model, which includes initial delivery and instructional services, an ongoing customer service relationship, and offering sleep supplies to patients for convenience purposes and continuing to provide such supplies to the patients into the future. We believe that an increasing focus on our user-friendly e-commerce platform will help contribute to this provision of sleep supplies. In addition, we operate a comprehensive patient compliance model to ensure that commercially insured patients and Medicare patients adhere to their therapy according to their physician’s prescription and the established guidelines for reimbursement, and a Payor compliance and monitoring model. The patient compliance model includes both one-on-one patient education and teaching performed in group settings, as well as remote monitoring technologies.
NPWT. ($42.1 million, $41.4 million and $42.0 million, or 3.9%, 3.7% and 3.9%, of our net revenues for the years ended December 31, 2019, 2018 and 2017, respectively).
99
Table of Contents
We provide NPWT pumps, supplies, and the dressings needed for patients’ individualized therapy. The most common types of wounds treated with NPWT are pressure ulcers, diabetic ulcers, post-surgical wounds, burns or other wounds developed as a result of traumatic injury. Our strength comes from partnering with manufacturers who are proven leaders in wound care to provide state-of-the-art pumps, supplies and dressings with an individualized patient care model to help promote wound healing while minimizing dressing-related pain and trauma. In order to facilitate a streamlined care transition, we leverage our national distribution network to deliver the initial kit either before the patient is transitioned from the hospital or shortly after they return home. We continue to provide supplies to the patient to ensure continuity in therapy as prescribed by their physician. This service integrates well with our goal of being the home healthcare provider that helps transition patients from the hospital setting to home healthcare.
Other Equipment and Services. ($172.5 million, $198.0 million and $198.7 million, or 15.8%, 17.9% and 18.5%, of our net revenues for the years ended December 31, 2019, 2018 and 2017, respectively).
In addition to the core services described above, we also supply a wide range of home medical equipment, other respiratory services and other products to help improve the quality of life for patients with home care needs. Our integrated service approach allows patients, hospital and physician referral sources and managed care organizations accessing any of our service offerings to also access needed home medical equipment and other respiratory services through a single source. The use of home medical equipment provides a significant relative cost advantage to our patients and Payors.
Patients and Payors
Patients and Payors rely on the equipment and high quality services that we provide in managing patients with chronic conditions.
Patients. In contrast to many healthcare services which are single transaction in nature, Apria develops long-term relationships with its patients through the provision of recurring services over time to treat chronic conditions. Home healthcare patients are generally diagnosed at a point in their disease progression where the proper use of durable medical equipment allows them to manage their diseases at home over an extended period of time. For respiratory therapy and OSA treatment, these therapies are often provided for multiple years, during which time Apria provides continued equipment servicing and certain supplies, such as sleep supplies, used in conjunction with the equipment. While the length of care for NPWT is normally shorter in duration, it is typically longer than one month and often extends for multiple months. In addition to providing equipment and ongoing service, Apria also bills and collects payments from the Payor source, on behalf of the patients. Starting at the patient’s initial admission and extending through the length of service, Apria collects the necessary paperwork and information needed for reimbursement from the patient’s Payor source. In order to meet any established utilization management guidelines required for ongoing reimbursement, Apria also coordinates with the patient and their Payor throughout the patient’s time as a customer.
Commercial Payors. National and regional insurers and managed care organizations continue to represent a significant portion of our business and we have long-standing relationships with most of the commercial Payors that we contract with. For the year ended December 31, 2019, approximately 80% of our revenue was from commercial Payors (which includes all Payors other than government Payors), including approximately 23% from Kaiser Foundation Health Plan, Inc. No other third-party Payor accounts for more than 10% of our revenues for the year ended December 31, 2019. Most of our commercial Payor contracts have two to five year terms with an automatic extension unless it otherwise terminates. Most of our commercial Payor contracts are based on price – we generally do not have contracted volume. We believe that our long-term relationships with national and regional insurers and managed care organizations provides considerable stability to our business. We have more than 1,600 agreements in place with various commercial Payors, from large nationals to regional and local Payors. For the year ended December 31, 2019, approximately 50% of our revenues were from commercial Payors with whom we have been contracted for over 20 years.
100
Table of Contents
Medicare. For the year ended December 31, 2019, approximately 19% of our revenue was from servicing patients with traditional Medicare coverage. While in previous rounds of the DMEPOS CBP we had CBP contracts in many of the CBAs, in recent years, we have reduced our reliance on Medicare business in response to the implementation of the DMEPOS CBP, which is burdensome and does not reflect the true cost of equipment and services provided. The next round of the CBP, beginning on January 1, 2021, was originally expected to include a number of products in our product lines. However, CMS announced on October 27, 2020 that only two out of the original 16 product categories will be included in Round 2021 of the DMEPOS CBP. Accordingly, none of our products will be subject to the DMEPOS CBP for the three-year period in which Round 2021 is in effect, and we are not restricted from supplying our products in any regions of the country during this period. We have maintained critical mass with the traditional Medicare population to allow for future flexibility should improvements to the DMEPOS CBP be made that provide opportunities for growth in this segment.
Sales and Marketing
Our sales and marketing strategy is tailored around our Payors and referral sources. In order to effectively serve our Payors and referral sources, we have a managed care sales team and a field sales team. As of December 31, 2019, we employed approximately 25 managed care sales professionals whose primary responsibilities are to build and maintain existing referral relationships with Payors and negotiate Payor pricing and contract terms. We also employed approximately 380 sales professionals whose primary responsibilities are to obtain new referrals and to maintain existing referral relationships for all of our service lines. We provide our sales professionals with the necessary clinical and technical training to represent our service offerings. Our sales structure and strategies are designed to adapt to changing market factors and will continue to adjust as further changes in the industry occur. While in most cases the ultimate referral decision is made by an individual referral source, our relationships with commercial Payors are important to ensure broad market access given that most of our patients are their members.
Marketing Initiatives. We have developed and put into practice several marketing initiatives to enhance the attractiveness of our service offerings, including:
| • | Comprehensive, Patient-Centric Clinical and Therapy Management Programs. We offer a number of clinical management programs, such as Premium Care SleepTM, which are designed to help improve patients’ quality of life and clinical outcomes, and to reduce costs for providers and Payors through reduced hospital and emergency admissions. |
| • | Patient Satisfaction and Complaint Resolution Process. We have a centralized patient satisfaction survey function that periodically conducts patient surveys and targeted member satisfaction studies for key national and regional insurers and managed care organizations as specified by various contractual arrangements. We also have a centralized team that administers a portal where patients’ complaints can be addressed. This team routes patient complaints to specific subject matter experts throughout the Company to resolve complaints. This process has enriched our data collection and analysis which is used to better manage resolutions and capture trends, allowing us to focus our improvement efforts. We believe that both centralized processes afford us visibility to centralized performance improvement data and trends that enable us to amend policies and procedures as necessary to meet the needs of patients. |
Organization and Operations
Organization. Our approximately 6,500 employees deliver home healthcare products and services to patients in their homes and to other care sites with the support of our approximately 275 branch locations, six regional distribution and repair centers, consolidated billing team, delivery fleet and qualified delivery professionals and clinical employees. Our home respiratory therapy, OSA treatment, NPWT and other equipment and service lines are organized into geographic zones and markets that provide management oversight.
Corporate Compliance. As a leader in the home healthcare industry, we have a robust compliance program that is designed to further our commitment to providing quality home healthcare products and services while
101
Table of Contents
maintaining high standards of ethical and legal conduct. Our enterprise-wide corporate compliance program applies to all operating divisions and is grounded in existing laws, rules and regulations and guidelines for healthcare organizations issued by the HHS OIG. We believe that it is essential to operate our business with integrity and in full compliance with applicable laws. Our corporate compliance program is headed by an experienced corporate compliance officer who reports directly to the board of directors and our Chief Executive Officer, for compliance purposes. The program also includes a written code of ethical business conduct that employees receive as part of their initial orientation process and which management reviews with employees under their supervision through a periodic review and attestation process. The program is designed to accomplish the goals described above through employee education, a confidential disclosure program, written policy guidelines, excluded parties, checks, periodic reviews, frequent reinforcement, compliance audits, a formal disciplinary component and other programs. Compliance oversight is provided by our corporate compliance committee, which meets quarterly and consists of senior and mid-level management personnel from various functional disciplines. In addition to updates provided to the board of directors during its regular meetings, a written compliance program report is submitted annually to the board of directors for review and discussion. On December 18, 2020, a federal judge approved a civil and administrative settlement Apria entered into with the United States and state Medicaid programs. As part of the settlement, Apria also entered into a five-year Corporate Integrity Agreement, under which we agreed to undertake certain obligations designed to promote compliance with federal healthcare programs. In addition, Apria entered into settlements to resolve claims brought under the laws of the States of California and Illinois on behalf of private insurers. For further information, see “Summary—Recent Developments—Settlement with SDNY Office.”
Operating Systems and Controls. Our operating and field information operating systems, policies and procedures for proper contract administration, accurate order entry and pricing, billing and collections, and inventory and patient equipment management enable us to monitor operational performance. Our information services department works closely with all of the operating areas of our business to ensure that our information technology policies, procedures and functions are compliant with government regulations and Payor requirements and to support their business improvement initiatives with technological solutions.
We have established performance indicators which measure operating results against expected thresholds for the purpose of allowing all levels of management to identify and modify areas requiring improvement and to monitor the resulting progress. Operating models with strategic targets have been developed to move us toward more effective management of the sales, customer service, accounts receivable, clinical and distribution areas of our business.
Receivables Management. We operate in an environment with complex requirements governing billing and reimbursement for our products and services. We have ongoing initiatives focused specifically on accounts receivables management such as system enhancements, process refinements and organizational changes.
We are continuing to expand our use of technology in areas such as web portals/electronic ordering, electronic claims submission and electronic funds transfer with managed care organizations to more efficiently process business transactions. This use of technology can improve the initial patient admission process, expedite claims processing and reduce the administrative costs associated with this activity for both us and our providers and Payors. We now submit almost all of our home respiratory and other equipment and services claims electronically. We have also developed and are continuing to develop internal expertise with the unique reimbursement requirements of certain large Payors and continue to negotiate simplifications in the claims submission process in an effort to reduce subsequent denials and shorten related collection periods. Our policy is to collect co-payments from the patient or applicable secondary Payor. In the absence of a secondary Payor, we generally require the co-payment at the time the patient is initially established with the product/service. Subsequent months’ co-payments are billed to the patient. We are also seeking to streamline related processes in order to improve the co-payment collection and initial claim denial rates. Certain accounts receivable administrative functions continue to be administered by third-party providers while others have been incorporated into our existing customer service and billing centers. We have established policies and procedures and provide training for all contracted service providers to perform effectively on our behalf.
102
Table of Contents
Suppliers. Virtually all products used in our business are purchased from third parties. We have a number of key supplier relationships.
We believe that we are not wholly dependent upon any single product supplier and that our product needs can be met by several similar suppliers if any one supplier became unable to continue our current business relationship. Our sourcing strategy leverages relationships with industry-leading suppliers but maintains competition and standardization that allows us to source from any supplier in a given category.
Nationwide Accreditation. All of our operations, including our branch locations, are accredited by The Joint Commission. As the home healthcare industry has grown and accreditation has become a mandatory requirement for Medicare DMEPOS providers, the need for objective quality measurements has increased. Accreditation by The Joint Commission entails a lengthy voluntary review process that is conducted every three years. Accreditation is also widely considered a prerequisite for entering into contracts with managed care organizations.
Essential Care Model. We have developed the Essential Care Model, a proprietary model that outlines Apria’s product formulary and defines the base-line levels of services, supplies and products offered in conjunction with key prescribed home healthcare equipment and therapies. The Essential Care Model is used to establish consistent and clear base-line expectations for patients, providers and Payors.
Outsourced Activities
We engage third-party providers for certain billing, collections, administrative and information systems and other services. As part of our outsourcing strategy, we seek to minimize the risk of business disruption by contracting with multiple vendors across multiple locations.
Competition
The segment of the healthcare market in which we operate is highly fragmented and highly competitive. In each of our service lines there are a limited number of national providers and numerous regional and local providers. Ultimately, our services are provided to an individual patient that is usually referred to Apria by a third-party healthcare professional. The competitive factors most important in the referral process are: reputation with referral sources, including local physicians and hospital-based professionals; accessibility and an efficient, responsive referral process; speed and quality of services; overall ease of doing business; quality of patient care and associated services; range of home healthcare products and services; and being an in-network provider for the commercial Payor(s) specific to an individual patient.
We compete with other large national providers, including Lincare Holdings, Inc., AdaptHealth, Rotech and Aerocare. Other types of healthcare providers, including individual hospitals and hospital systems, physicians and physician groups, home healthcare agencies, health maintenance organizations and managed services intermediaries have entered and may continue to enter the market to compete with our various service lines.
Government Regulation
We are subject to extensive government regulation, including numerous federal, state and local laws directed at regulating reimbursement of our products and services under various government programs and preventing fraud and abuse, as more fully described below. We maintain certain safeguards intended to reduce the likelihood that we will engage in conduct or enter into arrangements in violation of these restrictions. Legal department personnel review and approve written contracts, such as agreements with physicians, subject to these laws. We also maintain various educational and audit programs designed to keep our managers and employees updated and informed regarding developments on these topics and to reinforce to employees our policy of strict compliance in this area. Notwithstanding these measures, violations of these laws and regulations may still occur,
103
Table of Contents
which could subject us to civil and criminal enforcement actions; licensure revocation, suspension, or non-renewal; severe fines and penalties; the repayment of amounts previously paid to us; and even the termination of our ability to provide services under certain government programs such as Medicare and Medicaid. See “Risk Factors—Risks Related to Our Business and Industry— Reductions in Medicare, Medicaid and commercial Payor reimbursement rates and/or exclusion from markets or product lines, including due to the DMEPOS CBP, could have a material adverse effect on our results of operations and financial condition.”
Medicare and Medicaid Revenues. For the year ended December 31, 2019, approximately 19% and 1% of our net revenues were reimbursed by the Medicare and state Medicaid programs, respectively. With the exception of Kaiser Foundation Health Plan, Inc., which represented approximately 23% of our revenues during the period, no other third-party Payor represented more than 10% of our total net revenues for the year ended December 31, 2019. The majority of our revenues are derived from rental income on equipment rented and related services provided to patients, and the sales of equipment, supplies, pharmaceuticals and other items we sell to patients for patient care under fee-for-service arrangements.
Medicare Reimbursement. There are a number of historic and ongoing legislative and regulatory activities in Congress and at CMS that affect or may affect Medicare reimbursement policies for products and services we provide. Specifically, a number of important legislative changes that affect our business were included in the Medicare Prescription Drug, Improvement and Modernization Act of 2003 (the “MMA”), the Deficit Reduction Act of 2005 (the “DRA”) and the Medicare Improvements for Patients & Providers Act of 2008 (the “MIPPA”). The MMA, DRA and MIPPA and their implementing regulations and guidelines contain numerous provisions that were significant to us when enacted and continue to have an impact on our operations today, as described below. In addition, more recent legislative changes that will have an ongoing effect on our business were included in the PPACA, the Medicare Access and CHIP Reauthorization Act of 2015 (the “MACRA”), the 21st Century Cures Act and the Bipartisan Budget Act of 2018 (the “BBA”).
DMEPOS Competitive Bidding. A significant regulatory activity affecting Medicare reimbursement is the DMEPOS CBP, which was mandated by Congress through the MMA. The DMEPOS CBP impacts the Medicare reimbursement amounts for suppliers of certain DMEPOS items, and in the past, included some DMEPOS items that we provide to our patients.
The first round of the DMEPOS CBP was implemented briefly in 2008, but temporarily delayed and revised through the MIPPA. Subsequently, round one rebid contracts were fully implemented in January 1, 2011 in nine CBAs for nine product categories. CMS is required by law to recompete contracts under the DMEPOS CBP at least once every three years. Accordingly, the round one rebid contracts went through two re-competition processes since the original contract period started in 2011, for contract periods running from January 1, 2014 through December 31, 2016 (in nine CBAs for six product categories) and from January 1, 2017 through December 31, 2018 (in 13 CBAs for eight product categories). CMS implemented round two of the DMEPOS CBP on July 1, 2013 in 91 CBAs for eight product categories. Round two contracts went through one re-competition process, for contract periods running from July 1, 2016 through December 31, 2018 (in 117 CBAs for seven product categories). Cumulatively, in round one, round two, round one recompete, round two recompete and round one 2017, we were offered contracts for a substantial majority of the CBAs and product categories for which we submitted bids for oxygen, CPAP and NPWT.
In general, when a DMEPOS CBP competition round becomes effective in a CBA, beneficiaries under the Medicare fee-for-service program must obtain competitively bid DMEPOS items from a contract supplier. However, suppliers who are not awarded contracts through a DMEPOS CBP competition round, but who are furnishing competitively bid DMEPOS items at the time a DMEPOS CBP competition round begins, may continue furnishing certain items to beneficiaries if the supplier chooses to become a “grandfathered” supplier. The decision to become a grandfathered supplier applies to all items within a DMEPOS CBP product category that the supplier furnished prior to implementation of the DMEPOS CBP competition round. There are specific payment and policy requirements for grandfathered suppliers under each category of DMEPOS (e.g., capped
104
Table of Contents
rental, inexpensive and routinely purchased, oxygen and oxygen equipment), as well as specific beneficiary transition rules that a non-contract supplier must comply with, depending on whether the non-contract supplier chooses to be a grandfathered supplier or not.
In January 2017, CMS announced plans to consolidate all rounds and areas of the DMEPOS CBP into a single round of competition that would go into effect on January 1, 2019 in 141 CBAs for 11 product categories. However, CMS subsequently rescinded the originally published guidance. The then-existing DMEPOS CBP round one and round two contracts expired on December 31, 2018. In March 2019, CMS announced that it would consolidate all rounds and areas of the DMEPOS CBP into a single round of competition that would not go into effect until January 1, 2021 (“Round 2021”). Since that time, and as a result, there has been a temporary gap in the DMEPOS CBP. Since January 1, 2019, and through December 31, 2020, any Medicare-enrolled DMEPOS supplier has been able to furnish DMEPOS equipment and services in all areas of the country.
Round 2021 contracts are scheduled to become effective on January 1, 2021, and to extend through December 31, 2023. The bid window for Round 2021 closed on September 18, 2019. For each CBA included in Round 2021, providers submitted bids in September 2019 to CMS offering to supply certain covered items of DME in the CBA at certain prices. A number of products in our product lines originally were included on the list of products subject to Round 2021, including oxygen and oxygen equipment, CPAP devices and RADs, nebulizers and NPWT pumps. Although NIVs were originally included in the list of products subject to Round 2021, on April 9, 2020, CMS announced that the NIV product category has been removed from Round 2021 due to the COVID-19 pandemic. By removing NIVs from Round 2021, any Medicare-enrolled DMEPOS supplier can furnish any of the types of ventilators covered under the Medicare program. On October 27, 2020, CMS announced further revisions to Round 2021 of the DMEPOS CBP. Namely, only two out of the original 16 product categories, OTS back braces and OTS knee braces, are going to be included in Round 2021 of the DMEPOS CBP. All other product categories were removed from Round 2021. Accordingly, there are no longer any products in our product lines included on the list of products subject to Round 2021 of the DMEPOS CBP.
Competitive bidding contracts are expected to be re-bid at least every three years. Although none of our products are subject to Round 2021 of the DMEPOS CBP, we cannot guarantee that our products will be excluded from any subsequent rounds of the DMEPOS CBP. Our exclusion from certain markets or product lines could materially adversely affect our financial condition and results of operations. Further, the competitive bidding process has historically put downward pressure on the amount we are reimbursed in the markets in which we operate, as well as in areas that are not subject to the DMEPOS CBP. The rates required to win future competitive bids could continue to depress reimbursement rates. While we cannot predict the outcome of the DMEPOS CBP on our business in the future nor the Medicare payment rates that will be in effect in future years for the items subjected to competitive bidding, the program may materially adversely affect our financial condition and results of operations.
Medicare Fee Schedule for DMEPOS and CPI Adjustments. DMEPOS items that are not subject to the CBP are paid for under the Medicare DMEPOS fee schedule. The fee schedule amounts are calculated on a statewide basis. Further, the fee schedule amounts are updated annually by the percentage increase in the CPI-U and adjusted by the change in economy-wide productivity that is equal to the 10-year moving average of changes in the annual economy-wide private non-farm business Multi-Factor Productivity (“MFP”). For 2020, the CPI-U percentage increase is 1.6% and the MFP adjustment is 0.7%, resulting in a net increase of 0.9% for the update factor that is applied to the DMEPOS fee schedule amounts. For 2021, the CPI-U percentage increase is 0.6% and the MFP adjustment is 0.4%, resulting in a net increase of 0.2% for the update factor that is applied to the DMEPOS fee schedule amounts.
Effective January 1, 2016, DMEPOS items that are subject to CBP but furnished in all non-CBAs have experienced reductions in the Medicare DMEPOS fee schedule. The fee schedules for those items in the non-CBAs have been adjusted based on regional averages of the SPAs that apply to the DMEPOS CBP (referred to as the “Adjusted Fee Schedule”). Fee schedule amounts that are adjusted using information from the DMEPOS
105
Table of Contents
CBP are not subject to the annual CPI-U adjustments discussed above, but are updated when information from the DMEPOS CBP is updated.
However, the 21st Century Cures Act, enacted in December 2016, included a provision to roll back the full application of the Adjusted Fee Schedule amount to the non-CBAs that was effective from July 1, 2016 through December 31, 2016. Effective from January 1, 2017, non-CBA rates were set at 100% of the Adjusted Fee Schedule amount, based on the regional competitive bidding rates. In May 2018, CMS issued an interim final rule that resumed the transition period for phasing in adjustments to the Adjusted Fee Schedule amount in rural areas and non-contiguous areas (Alaska, Hawaii and U.S. territories) not subject to the DMEPOS CBP from June 1, 2018 through December 31, 2018. For this 7-month time period, DMEPOS items furnished in rural and non-contiguous areas were paid for based on a blended rate of 50% of the non-Adjusted Fee Schedule amount and 50% of the Adjusted Fee Schedule amount. Other non-CBAs (those that are not defined as rural or non-contiguous) were not impacted by this interim final rule and DMEPOS items furnished in these areas were paid at 100% of the Adjusted Fee Schedule amount.
During the temporary gap in the DMEPOS CBP, from January 1, 2019 through December 31, 2020, CMS established separate fee schedule adjustment methodologies for three geographic areas: (1) other non-CBAs (those that are not defined as rural or noncontiguous), (2) rural/non-contiguous areas, and (3) former CBAs. For other non-CBAs, the payment amounts for DMEPOS items were based on 100% of the Adjusted Fee Schedule amount. Because the SPAs generated from the DMEPOS CBP competitions expired on January 1, 2019, the Adjusted Fee Schedule amount was increased by the percentage change in the CPI-U (1.6%) on January 1, 2020, and will be increased again by 0.6% on January 1, 2021. For rural/non-contiguous areas, the payment amounts for DMEPOS items were based on a blended rate of 50% of the non-Adjusted Fee Schedule amount and 50% of the Adjusted Fee Schedule amount. The Adjusted Fee Schedule amount of the blended rate also was increased by the percentage change in the CPI-U (1.6%) on January 1, 2020, and will be increased again by 0.6% on January 1, 2021. For former CBAs, the payment amount for DMEPOS items are based on the lower of the supplier’s charge for the item or fee schedule amounts that are based on the SPAs that were in effect in the CBA before the CBP contract ended, increased by the projected percentage change in the CPI-U. Accordingly, for 2019, the fee schedule amounts were based on the SPAs in effect on December 31, 2018 for each specific CBA, increased by 2.5% (the projected percentage change in the CPI-U for the 12-month period ending January 1, 2019), and for 2020, the fee schedule amounts increased by 2.4% (the projected percentage change in the CPI-U for the 12-month period ending January 1, 2020). For 2021, the fee schedule amounts will be increased by 0.6% (the projected percentage change in the CPI-U for the 12-month period ending January 1, 2021).
The CARES Act introduced a new blended rate for DMEPOS items furnished in other non-CBAs (those that are not defined as rural or non-contiguous) that is based on 25% of the non-Adjusted Fee Schedule amount and 75% of the Adjusted Fee Schedule amount, effective March 6, 2020 through the end of the COVID-19 pandemic. For rural and non-contiguous areas, the payment amount for DMEPOS items will continue to be based on a blended rate of 50% of the non-Adjusted Fee Schedule amount and 50% of the Adjusted Fee Schedule amount until December 31, 2020 or the end of the COVID-19 pandemic, whichever is later.
On November 4, 2020, CMS issued a proposed rule establishing the methodologies for adjusting the fee schedule payment amounts for such DMEPOS items furnished in non-CBAs on or after April 1, 2021 or the date immediately following the duration of the public health emergency period (which has recently been extended through April 20, 2021), whichever is later. CMS proposes to pay 100% of the Adjusted Fee Schedule amount in other non-CBAs. Under the proposal, CMS would continue paying suppliers higher rates (e.g., at the 50/50 blended rate) for furnishing such DMEPOS items in rural and non-contiguous areas as compared to in other non-CBAs, informed by stakeholder input indicating higher costs in these areas, greater travel distances and costs in certain non-CBAs compared to CBAs, the unique logistical challenges and costs of furnishing items to beneficiaries in the non-contiguous areas, significantly lower volume of items furnished in these areas versus CBAs, and concerns about financial incentives for suppliers in surrounding urban areas to continue including outlying rural areas in their service areas. For such DMEPOS items that originally were included in Round 2021
106
Table of Contents
but for which contracts were not awarded, CMS is considering whether to simply extend application of the current fee schedule adjustment rules for non-CBAs, CBAs, and former CBAs until new SPAs are calculated for the items once competitive bidding of the items in a future round of the DMEPOS CBP. We believe that this proposal does not include NIVs, since they were not included in previous rounds of the DMEPOS CBP and therefore would continue to be paid based on the Medicare DMEPOS fee schedule.
We furnish DMEPOS items in both rural and non-contiguous areas, as well as other non-CBAs. While some relief has been provided for rural and non-contiguous areas and, recently, but only on a temporary basis, to other non-CBAs through the CARES Act, the overall impact has been a reduction in payments for DMEPOS items in these areas that reflect competitively bid prices. See “Risk Factors–Risks Related to Our Business and Industry– Reductions in Medicare, Medicaid and commercial Payor reimbursement rates and/or exclusion from markets or product lines, including due to the DMEPOS CBP, could have a material adverse effect on our results of operations and financial condition.”
Reimbursement for Capped Rentals and Oxygen Equipment. Medicare covers certain DMEPOS items, including CPAP and RAD under its category for “capped rentals.” In general, items in this category are rented to Medicare beneficiaries, and Medicare payment is based on a monthly rental payment that covers the cost of the base device and any necessary maintenance and service of the device. DMEPOS suppliers may bill Medicare separately for any related accessories (e.g., masks, filters, humidifiers) that are used with the capped rental device. The rental period for these items is limited to 13 months of continuous use, after which time the Medicare monthly payment for the base equipment ceases and the Medicare beneficiary takes ownership of the device. After the capped rental period ends, Medicare continues to pay for replacement of the accessories that are used with the beneficiary-owned device. At the end of the five-year useful life of the equipment, the beneficiary may obtain replacement equipment and, if he or she can be requalified for the Medicare benefit, a new maximum 13-month payment and five-year useful life cycle would begin.
Medicare reimbursement for oxygen and oxygen equipment is limited to a maximum of 36 months within a 60-month service period. The supplier that billed Medicare for the 36th month of service continues to be responsible for the beneficiary’s oxygen therapy needs for months 37 through 60, and generally there is no additional reimbursement for oxygen-generating portable equipment for these later months. CMS does not separately reimburse DMEPOS suppliers for any oxygen tubing, cannulas and supplies that may be required for the beneficiary. The DMEPOS supplier is required to keep the equipment provided in working order and in some cases, CMS will provide reimbursement for repair costs. At the end of the five-year useful life of the equipment, the beneficiary may obtain replacement equipment and, if he or she can be requalified for the Medicare benefit, a new maximum 36-month payment cycle would begin for the next 60 months of service. Unlike capped rental items, the oxygen equipment always remains the property of the DMEPOS supplier. Note that, for 2019, CMS added a new oxygen payment class and set the rental payment for portable liquid oxygen equivalent to the rental payment made for portable concentrators and transfilling equipment. CMS also added a new payment class for high-flow portable liquid oxygen contents when a patient’s prescribed flow rate exceeds 4 liters per minute. This new high-flow oxygen content class allows for the continuation of high-flow oxygen volume adjustment payments beyond the initial 36 months of continuous use. CMS implemented these changes in a budget neutral manner.
Reimbursement for Inhalation and Infusion Therapy Drugs. Inhalation therapy drugs are typically paid under the Medicare Part B fee schedule. Inhalation drugs have been paid based on the Average Sales Price (“ASP”) plus 6% for the drug since January 1, 2005. Effective January 1, 2017, the payment amount for infusion therapy drugs furnished through DME also began to be based on ASP plus 6%, rather than the historical payment amount based on 95% of the manufacturers’ Average Wholesale Price (“AWP”). This change in payment for infusion therapy drugs followed reports from the HHS OIG that found that the then-current AWP methodology may have resulted in excessive reimbursement for certain drugs while underpaying for other drugs. Inhalation and infusion therapy drugs are not subject to the DMEPOS CBP. However, the durable medical equipment used to deliver the inhalation and infusion therapy drugs (e.g., nebulizers, external infusion pumps and supplies) have been subject to various competition rounds under the DMEPOS CBP.
107
Table of Contents
Reimbursement for Non-Invasive Pressure Support Ventilators. For patients experiencing chronic respiratory failure, we offer NIV therapy. Medicare pays for NIV therapy under the DME benefit category for items requiring frequent and substantial servicing, and payments may continue until medical necessity ends (rather than being capped after a certain period of time). CMS and its contractors have expressed concerns about the recent substantial increase in Medicare billing for non-invasive pressure support ventilators. As described by the HHS OIG in a September 2016 data brief, ventilator technology has evolved so that it is possible for a single device to treat numerous respiratory conditions by operating in several different modes. According to the HHS OIG, this creates an opportunity for abuse if DMEPOS suppliers were to bill Medicare for the device as if it were being used as a ventilator, when use of a lower cost device (e.g., CPAP, RAD) is indicated based on the beneficiary’s medical condition. The HHS OIG’s data brief examined the results of prepayment reviews conducted by two of the DME MACs that resulted in the denial of more than 90% of claims for NIVs. The primary reason cited for many of these denials was insufficient clinical documentation. Following the release of the HHS OIG’s 2016 data brief, in which the HHS OIG recommended that CMS monitor providers with the largest market shares of ventilator beneficiaries, CMS consolidated billing codes for ventilators effective January 1, 2016 and decreased the reimbursement amount for non-invasive pressure support ventilators. On July 22, 2020, CMS conducted a virtual Medicare Evidence Development & Coverage Advisory Committee (“MEDCAC”) meeting to review the evidence specific to the home use of noninvasive positive pressure ventilation by patients with chronic respiratory failure consequent to chronic obstructive pulmonary disease (“COPD”). Devices to be considered are home mechanical ventilators, bi-level positive airway pressure (BPAP) devices, and CPAP devices. Following the MEDCAC panel, an informal Technical Expert Panel (“TEP”) led by the American College of Chest Physicians (“CHEST”) with representatives from the American Association for Respiratory Care (“AARC”), the American Thoracic Society (“ATS”), and the American Academy of Sleep Medicine (“AASM”) was convened to develop recommendations to inform CMS coverage policy regarding the use of at-home NIV devices for patients in five diagnostic categories. The five categories include: (1) Bilevel Transition from CPAP When Therapeutic Benefit Was Not Achieved; (2) Severe COPD; (3) Hypoventilation Syndrome; (4) Thoracic Restrictive Disorders; and (5) Complex/Central Sleep Apnea. A final document with the TEP’s findings will be presented to CMS and submitted for peer-reviewed publication. Together, the efforts of the MEDCAC panel and the TEP are likely to be used by CMS to shape a Medicare national coverage determination (“NCD”) related to the use of at-home NIV devices in COPD patients in the future.
Claims Auditing and Monitoring and Medical Necessity Documentation Requirements. As a Medicare provider, we are subject to extensive government regulation, including laws and regulations directed at ascertaining the appropriateness of reimbursement, preventing fraud and abuse, and otherwise regulating reimbursement under the Medicare program. The federal government has contracted with private entities to audit and recover revenue resulting from payments made in excess of those permitted by federal and state benefit program rules. These entities include but are not limited to comprehensive error rate testing program contractors that are responsible for measuring improper payments on Medicare fee-for-service claims and Unified Program Integrity Contractors (UPICs) that are responsible for the identification of suspected fraud through medical record review.
In order to ensure that Medicare beneficiaries only receive medically necessary items and services, the Medicare program has adopted a number of documentation requirements. Additionally, to ensure compliance with Medicare, the DME MACs conduct pre- and post-payment audits or other types of inquiries, and request patient records and other documentation, to support claims we submit for payment for services and products we render to Medicare beneficiaries. These audits typically involve a complex medical review, by Medicare, or its designated contractors and representatives, of documentation supporting the services and products provided. In connection with a Medicare request for supporting documentation, we are obligated to procure and submit the underlying medical records retained by various clinical providers, medical facilities and prescribers. Obtaining these medical records in connection with a claims audit may be challenging and, in any event, all of these records are subject to further examination, interpretation and dispute by the auditing authority. Under standard Medicare procedures, we are entitled to demonstrate the sufficiency of documentation and the establishment of medical necessity and we have the right to appeal any adverse determinations. If a determination is made that our records
108
Table of Contents
or the patients’ medical records are insufficient to meet medical necessity or Medicare coverage or reimbursement requirements for the claims, we could be subject to denials or overpayment demands for claims submitted for Medicare reimbursement. In the rare event that such an audit results in major discrepancies of claims records which lacked medical necessity, Medicare may be entitled to take additional corrective measures, including extrapolation of audit results across a wider population of claims, submission of recoupment demands for claims other than those examined in the audit, or placing the provider on a full pre-payment review.
Face-to-Face Provisions for DMEPOS. In November 2012, CMS issued a final rule that implemented a provision of the Social Security Act (“SSA”) establishing requirements for a face-to-face encounter and written orders prior to delivery for certain items of DMEPOS. As written, the final rule would have required a physician to document that the physician, or a nurse practitioner, physician assistant, or clinical nurse specialist, had a face-to-face encounter with the beneficiary prior to issuing a written order to the beneficiary for certain DMEPOS items. The MACRA eliminated this face-to-face requirement as originally written, and revised the requirement to be that a physician, nurse practitioner, physician assistant, or clinical nurse specialist must document they have written an order for a DMEPOS item pursuant to a face-to-face encounter with the beneficiary, but that encounter could have occurred anytime within the six months before the order was written for the DMEPOS item.
In response to the COVID-19 pandemic, CMS has exercised enforcement discretion with respect to the clinical conditions and face-to-face encounter requirements established under certain national and local coverage determinations applicable to certain items and supplies, including respiratory items (oxygen, CPAP, RADs, nebulizers) and infusion pumps. Accordingly, on an interim basis, requirements related to face-to-face or in-person encounters for evaluations, assessments, certifications or other implied face-to-face services would not apply. This enforcement discretion is temporary, as it is only in effect for the duration of the COVID-19 pandemic, and it is subject to ongoing and evolving interpretation by CMS and the DME MACs. As such, we may be required to modify our policies and procedures to remain in compliance with these CMS requirements.
Prior Authorization Rules for DMEPOS. In December 2015, CMS issued a final rule to require prior authorization (“PA”) by Medicare for certain DMEPOS items that the agency characterizes as frequently subject to unnecessary utilization. The final rule specifies a master list of DMEPOS items that potentially could be subject to PA and CMS will update this master list annually. The first two DMEPOS items requiring PA are two types of power wheelchairs (single and multiple power options) and in March 2017 the DME MACs began accepting PA requests for these items. The PA final rule did not create any new clinical documentation requirements; instead, the same information necessary to support Medicare payment was required, but simply prior to the DMEPOS item being furnished to the Medicare beneficiary.
CMS has established a list of DMEPOS items frequently subject to unnecessary utilization. Items on this list could be subject to PA as a condition of Medicare payment. Since 2012, CMS also has maintained a list of categories of DMEPOS items that require face-to-face encounters with practitioners and written orders before the DMEPOS supplier may furnish the items to Medicare beneficiaries. In a final rule published in November 2019, CMS combined these two lists to create a single, unified “master list” that CMS will use to identify DMEPOS items for which face-to-face encounters, written orders prior to delivery, and/or PA will be required. This expanded master list increases the number of DMEPOS items potentially eligible to be subject to prior authorization, face-to-face encounters, and written order prior to delivery requirements as a condition of payment.
While the current list of DMEPOS items requiring PA does not affect any of our products other than support surfaces, CMS may include products from our product lines on the required PA list in future phases of the PA process. If any of our products are ultimately subject to PA, it could reduce the number of our patients qualified to come on service using their Medicare benefits, it could delay the start of those patients’ service while we wait for PA to be received, and/or it could decrease our sales productivity. As a result, this could adversely affect our business, financial condition, results of operations, cash flow, capital resources and liquidity.
109
Table of Contents
Reimbursement Under Medicare Part C (Medicare Advantage). We maintain contracts to provide home respiratory, home medical equipment and related services to a significant number of managed care companies that maintain Medicare Advantage plans nationwide. While Medicare Advantage plans are required to cover all benefits to which beneficiaries are entitled under the Medicare Parts A and B programs, these plans have flexibility to negotiate and set payment rates that differ from the Medicare rates. Further, the DMEPOS CBP only applies to the Medicare fee-for-service program and therefore does not apply to Medicare Advantage plans. Enrollment in Medicare Advantage plans continues to grow and these plans are likely to continue to be attractive alternatives to traditional Medicare fee-for-service for those beneficiaries who choose them and we intend to continue to contract with these plans.
We cannot estimate the combined possible impact of all legislative, regulatory, and contemplated reimbursement changes that could have a material adverse effect on our business, financial condition and results of operations. Moreover, our estimates of the impact of certain of these changes appearing in this “Government Regulation” section are based on a number of assumptions and there can be no assurance that the actual impact was not or will not be different from our estimates.
Medicaid Reimbursement. State Medicaid programs implement reimbursement policies for the products and services we provide. Such policies may or may not be similar to those of the Medicare program. Budget pressures on state Medicaid programs often result in pricing and coverage changes that may have a detrimental impact on our operations. States sometimes have adopted alternative pricing methods for certain drugs and home medical equipment under their Medicaid programs that reduce the level of reimbursement received by us, without a corresponding offset or increase to compensate for the service costs incurred.
The 21st Century Cures Act accelerated the implementation of the omnibus spending bill passed in December 2015 that requires state Medicaid agencies to match Medicare reimbursement rates for certain durable medical equipment items, including oxygen, to be effective beginning January 1, 2018. Through passage of the 21st Century Cures Act, Congress added section 1903(i)(27) to the SSA, which prohibits federal Medicaid reimbursement to states for certain durable medical equipment expenditures that are, in the aggregate, in excess of what Medicare would have paid for such items. As such, a state’s Medicaid expenditures for DME items that are subject to this provision will be determined in the aggregate and any expenditures in excess of what Medicare would have paid for such items in the aggregate, either on a fee schedule basis or under its competitive bidding process, are not eligible for federal financial participation. CMS issued guidance through a federal register notice published on November 28, 2017 and in a state Medicaid director letter dated December 27, 2017 regarding state implementation of this Medicaid program requirement. Unfortunately, most states did not take the appropriate action and this became effective on January 1, 2018. States then worked to enact changes to their fee schedules. In addition, states considered whether to make such changes retroactive to January 1, 2018. The impact of this Medicaid program requirement has varied by state, depending on how much the state’s Medicaid fee-for-service rate differs from the applicable Medicare rate.
On January 30, 2020, CMS, on behalf of the Trump Administration, announced a new opportunity called the Healthy Adult Opportunity (“HAO”) to support states with greater flexibility to improve the health of their Medicaid populations. According to the Trump Administration, the HAO “emphasizes the concept of value-based care while granting states with extensive flexibility to administer and design their programs within a defined budget.” The Trump Administration has viewed HAO as a unique opportunity for states to enhance their Medicaid program’s integrity and potentially increase enrollment opportunities for previously ineligible recipients (about 2 million people in non-expansion states could theoretically be eligible under the criteria CMS outlined. CMS states that other low-income adults, children, pregnant women, elderly adults and people with disabilities will not be affected by this initiative); however, this in all likelihood will be subject to highly tailored and nuanced state specifications for this targeted population. As a part of HOA, CMS encourages states to implement evidence-based payment and delivery system reforms, including a combination of fee-for-service and managed care delivery systems that can be altered over the course of the demonstration. As this program can yield enrollment opportunities for previously ineligible enrollees, and can lead to alternate payment
110
Table of Contents
methodologies, any adoption by individual states must be reviewed. It is likely that, consistent with President Biden’s January 28, 2021 Executive Order on Strengthening Medicaid and the Affordable Care Act, the incoming Biden Administration will significantly modify or repeal the HAO.
Historically, when states have adopted alternative pricing methodologies, we have sometimes elected to stop accepting new Medicaid patient referrals for the affected drugs and/or home medical equipment. We continuously evaluate the possibility of stopping or reducing our Medicaid business in certain states with reimbursement policies that make it difficult for us to conduct our operations profitably. We cannot currently predict the adverse impact, if any, that any such reduction in our Medicaid business might have on our business, financial condition and results of operations, but such impact could be material. In addition, we cannot predict whether states may consider adopting additional reimbursement reductions or whether any such changes could have a material adverse effect on our business, financial condition and results of operations.
HIPAA / HITECH / Federal and State Consumer Protection and Privacy and Security Requirements. HIPAA is comprised of a number of components pertaining to the privacy and security of certain PHI, as well as the standard formatting of certain electronic health transactions. HITECH (enacted under the American Recovery and Reinvestment Act of 2009) further limited how covered entities, business associates and business associate subcontractors may use and disclose PHI and the security measures that must be taken in connection with protecting that information and related systems. In addition, the PPACA requires healthcare providers, health plans, payors, and other HIPAA-covered entities to use the electronic standard transactions, operating rules, code sets and unique identifiers that have been adopted through regulation by the Secretary. Under the 21st Century Cures Act, Congress authorized ONC to engage in rulemaking that would drive interoperability and provide timely access to health information through standardized application programming interfaces (APIs) to seamlessly coordinate care, improve outcomes and reduce the cost of care. CMS also published new regulations under their authority to regulate managed care plans and healthcare providers participating in Medicare and Medicaid programs that enable better patient access to their health information and reduce the burden on payers and providers. Due to the public health emergency and in response to the healthcare industry’s need to focus on responding to COVID-19, both CMS and ONC exercised their enforcement discretion to allow for flexibility with respect to compliance and applicability of their respective Interoperability and Patient Access rules. Then, in October 2020, ONC published an Interim Final Rule further extending the applicability date for the Information Blocking provisions until April 2021 and laying out a compliance timeline that extends through December 31. 2023. We are subject to HIPAA as a covered entity. HIPAA also applies to business associates of covered entities, which are individuals and entities that provide services for or on behalf of those covered entities. We enter into contracts with our business associates that provide services to us to require those business associates to safeguard PHI in accordance with the requirements of HIPAA and HITECH and sometimes enter into contracts as the business associate of another covered entity. In addition, we may be considered an “actor” or will participate in a health information exchange or network under the ONC and CMS Interoperability Rules and we will likely be required to comply with the new regulatory framework that is emerging around value-based payments and patient-centered care.
Numerous other federal and state laws that protect the confidentiality, privacy, availability, integrity and security of PHI and healthcare related data also apply to us. In many cases, these laws are more restrictive than, and not preempted by, the HIPAA and HITECH rules and requirements, and may be subject to varying interpretation by courts and government agencies, creating complex compliance issues for us and potentially exposing us to additional expenses, adverse publicity and liability.
Further, federal and state consumer laws are being applied increasingly by the FTC and state attorneys general, to regulate the collection, use and disclosure of personal information or patient health information, and to ensure that appropriate data safeguards are implemented by business and organizations that are maintaining personal information about individuals. For example, the CCPA, which became effective on January 1, 2020, gives California residents expanded rights to access and delete their personal information, opt out of certain personal information sharing and receive detailed information about how their personal information is used by
111
Table of Contents
requiring covered businesses to provide new disclosures to California consumers (as that term is broadly defined) and provide such consumers new ways to opt-out of certain sales of personal information. The CCPA provides for civil penalties for violations, as well as a private right of action for data breaches that is expected to increase data breach litigation. Additional requirements resulting from the approval of the CPRA are also on the horizon, with a January 1, 2023 compliance deadline that both amends and expands the CCPA even before many of its key provisions have become effective. Similarly, other states including New York, Massachusetts, North Dakota, Hawaii, and Maryland also are considering laws that would give citizens increased control over their personal data. Courts also may adopt the standards for fair information practices promulgated by the FTC that concern consumer notice, choice, security and access.
New information standards, whether implemented pursuant to federal or state laws, could have a significant effect on the manner in which we must handle healthcare related data, and the cost of complying with such standards could be significant. We have implemented various compliance measures in connection with the HIPAA, HITECH and 21st Century Cures Act rules and requirements, and other federal and state privacy and security rules and requirements, but we may be required to take additional steps, including costly system purchases and modifications, to comply with these rules and requirements as they may evolve over time. We face potential administrative, civil and criminal sanctions if we do not comply with the existing or new laws and regulations dealing with the privacy and security of PHI and patient information. Imposition of any such sanctions could have a material adverse effect on our operations. Similarly, if we, or any of our business associates, experience a breach of PHI, the breach reporting requirements required by HITECH and state laws could result in substantial financial liability and reputational harm.
Enforcement of Healthcare Fraud and Abuse Laws. The federal government, and its various state counterparts, have made policy decisions to continue increasing the financial resources allocated to enforcing healthcare fraud and abuse laws. Commercial Payors also have increased their level of scrutiny of healthcare claims, in an effort to identify and prosecute fraudulent and abusive practices in the healthcare industry. Violation of these federal and state laws can result in the imposition of criminal and civil monetary penalties as well as exclusion from participation in federal and state healthcare programs. Exclusion for a minimum of five years is mandatory for a conviction, and the presence of aggravating circumstances in a case can lead to an even longer period of exclusion. The federal government also has the discretion to exclude providers for certain conduct even absent a criminal conviction. Such conduct includes, but is not limited to, participation in a fraud scheme, payment or receipt of kickbacks and/or failing to provide services of a quality that meets professionally recognized standards. The HHS OIG also has authority under certain circumstances to suspend or terminate Medicare billing privileges during the course of an investigation.
We seek to structure our business operations, our financial relationships with referral sources, our billing and documentation practices and other practices to comply with the laws discussed below. However, we cannot assure you that a federal or state agency charged with enforcement of these various laws might not assert a contrary position or that new federal or state laws might not be enacted that would cause these arrangements to become illegal or result in the imposition of penalties on us or certain of our facilities and operations. If any of those allegations were successfully asserted against us or even if there is an assertion of a potential violation, there could be a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Anti-Kickback Prohibitions. As a provider of services under the Medicare and Medicaid programs, we must comply with the AKS. The AKS prohibits the offer or receipt of any bribe, kickback, or rebate in return for the referral of patients, products, or services covered by federal healthcare programs. Federal healthcare programs have been defined to include plans and programs that provide healthcare benefits funded by the United States government, including Medicare, Medicaid and TRICARE (formerly known as the Civilian Health and Medical Program of the Uniformed Services), among others. Some courts and the HHS OIG have interpreted the AKS to
112
Table of Contents
cover any arrangement where even one purpose of the remuneration is to influence referrals. Violations of the AKS may result in civil and criminal penalties and exclusion from participation in federal healthcare programs.
Despite the breadth of the AKS’s prohibitions, there are only a limited number of statutory exceptions that protect various common business transactions and arrangements from prosecution. In addition, the HHS OIG has published safe harbor regulations that outline other types of arrangements that also are deemed protected from prosecution under the AKS, provided all applicable criteria are met. In 2020, OIG published a final rule, “Revisions to the Safe Harbors Under the Anti-Kickback Statute and Civil Monetary Penalty Rules Regarding Beneficiary Inducements,” that implements seven new safe harbors, modifies four existing safe harbors, and codifies one new exception. The failure to meet all of the applicable safe harbor criteria does not necessarily mean that the particular arrangement in question violates the AKS; rather, these arrangements could be subject to greater scrutiny by enforcement agencies. A determination that a financial arrangement violates the AKS could subject us to liability under the SSA, including civil and criminal penalties, as well as exclusion from participation in federal healthcare programs such as Medicare and Medicaid.
In order to obtain additional clarification on the AKS, a provider can obtain written interpretative advisory opinions from the HHS OIG regarding existing or contemplated transactions. Advisory opinions are binding as to HHS but only with respect to the requesting party or parties. The advisory opinions are not binding as to other governmental agencies (e.g., the DOJ) and certain matters (e.g., whether certain payments made in conjunction with conduct seeking to meet certain safe harbor protections are at fair market value) are not within the purview of an advisory opinion.
Certain states in which we operate have enacted statutes and regulations similar to the AKS that prohibit some direct or indirect payments if those payments are designed to induce or encourage the referral of patients to a particular provider. Most states have anti-kickback statutes that prohibit kickbacks relating to the state’s Medicaid program, but some state anti-kickback statutes are broader and apply not only to the federal and state healthcare programs but also to other Payor sources (e.g., national and regional insurers and managed care organizations). These state laws may contain exceptions and/or safe harbors that are different from those at the federal level and may vary widely from state to state. A number of states in which we operate also have laws that prohibit fee-splitting arrangements between healthcare providers, if such arrangements are designed to induce or encourage the referral of patients to a particular provider. Possible sanctions for violations of these laws include exclusion from state-funded healthcare programs, loss of licensure and civil and criminal penalties. These laws vary from state to state, often are vague and often have been subject to only limited court and/or regulatory agency interpretation.
Physician Self-Referral Prohibitions. The federal physician self-referral law, commonly referred to as the “Stark Law,” prohibits a physician (and certain other healthcare professionals) from making a referral of Medicare and Medicaid patients for a designated health service to an entity, such as us, if the physician or a member of the physician’s immediate family has a financial relationship with the entity, unless an exception applies. The term “designated health services” includes several services commonly performed or supplied by us, including durable medical equipment and pharmacy items and services. In addition, the term “financial relationship” is broadly defined to include any ownership or investment interest, or compensation arrangement, pursuant to which a physician receives remuneration from the provider at issue. The Stark Law prohibition applies regardless of the reasons for the financial relationship and the referral; and, therefore, unlike the AKS, an intent to violate the law is not required. Violations of the Stark Law may result in loss of Medicare and Medicaid reimbursement, civil penalties and exclusion from participation in the Medicare and Medicaid programs. The Stark Law contains a number of statutory and regulatory exceptions intended to protect certain types of transactions and business arrangements from penalty. In order to qualify an arrangement under a particular Stark Law exception, compliance with all of the exception’s requirements is necessary. Since the Stark Law was enacted in 1989, there have continued to be ongoing changes and clarifications to a number of the provisions in the legislation and regulations. In 2019, the 2020 Physician Fee Schedule final rule finalized changes to the Stark advisory opinion process, amending the process such that HHS will not pursue sanctions against any party to an
113
Table of Contents
arrangement that CMS determines is “indistinguishable in all material aspects” from an arrangement described in a favorable advisory opinion. The final rule allows individuals and entities to rely on advisory opinions as “non-binding guidance.” In addition, in 2020, CMS issued a final rule, “Modernizing and Clarifying the Physician Self-Referral Regulations,” which creates new permanent exceptions to the Stark Law for value-based arrangements as well as provides additional guidance on several key requirements that must be met in order for physicians and healthcare providers to comply with the Stark Law. For example, compensation provided to a physician by another healthcare provider generally must be at fair market value, and the final rule provides guidance on how to determine if compensation meets this requirement. The Stark Law has also been subject to varying, and sometimes contradictory, decisions by the courts.
In addition, a number of the states in which we operate have similar prohibitions against physician self-referrals, which are not limited to just the federal healthcare program. These state prohibitions may differ from the Stark Law’s prohibitions and exceptions may apply to a broader or narrower range of services, arrangements and financial relationships and may apply to other healthcare professionals in addition to physicians. Violations of these state laws may result in prohibition of payment for services rendered, loss of licenses, fines and criminal penalties. State statutes and regulations also may require physicians and/or other healthcare professionals to disclose to patients any financial relationships the physicians and/or healthcare professionals have with healthcare providers who are recommended to patients. These laws vary from state to state, often are vague, and in many cases, have not been interpreted by courts or regulatory agencies.
False Claims Act. The federal FCA imposes civil and criminal liability on individuals or entities that submit false or fraudulent claims for payment to the government. Violations of the FCA may result in treble damages, civil monetary penalties and exclusion from the Medicare, Medicaid and other federally funded healthcare programs. If certain criteria are satisfied, the FCA allows a private individual to bring a qui tam suit on behalf of the government and, if the case is successful, to share in any recovery. FCA suits brought directly by the government or private individuals against healthcare providers, like us, are increasingly common and are expected to increase. For those individuals or entities that are presently subject to FCA qui tam suits, audits, denials of claims, or other audit or enforcement actions based exclusively on allegations of noncompliance with guidance documents, a 2020 HHS final rule, titled “Good Guidance Practices,” provides a new basis to rebut these allegations.
The federal government has used the FCA to prosecute a wide variety of alleged false claims and other frauds allegedly perpetrated against Medicare, Medicaid and other federal and state funded healthcare programs. In addition, violation of other statutes (such as the AKS) can be considered to trigger violations of the FCA.
On December 18, 2020, a federal judge approved a civil and administrative settlement Apria recently entered into with the United States and state Medicaid programs, in a complaint filed by three relators under the qui tam provisions of the FCA, 31 U.S.C. § 3729 et seq., as well as comparable state false claims laws, in connection with the rental of NIVs. Apria also entered into separate settlements to resolve the relators’ claims brought on behalf of the States of California and Illinois related to NIV covered by private insurers. The matter had been pending since 2017.
The government had alleged that Apria violated the FCA by submitting false claims seeking reimbursement for NIVs which were not being used, or not being used sufficiently, by patients, for NIVs which were being used pursuant to physician orders on a device setting which was available from other less expensive devices, and for improperly waiving co-pays to induce beneficiaries to rent NIVs. To resolve any potential liability, Apria agreed to enter a civil settlement agreement and to pay $40 million to the federal government and the states. Apria also agreed with the California Department of Insurance to pay $500,000 to resolve claims asserted by the relators under the California Insurance Frauds Prevention Act, CAL. INS. CODE § 1871 et seq. Apria separately agreed with the relators to settle all remaining claims from their complaint, including: (1) claims for retaliation in violation of federal and state laws; (2) claims for attorneys’ fees and costs available under federal and state law; and (3) claims under the Illinois Insurance Claims Fraud Prevention Act, 740 ILL. COMP. STAT. 92/1 et seq. Apria did not admit that any of its conduct was illegal or otherwise improper.
114
Table of Contents
As part of the federal and state Medicaid settlement, Apria also entered into a five-year CIA with the HHS OIG. The CIA requires Apria to maintain its ongoing corporate compliance program and obligates Apria to implement or continue, as applicable, a set of defined corporate integrity activities for a period of five years from the effective date of the CIA. Among other things, the CIA requires Apria to impose certain oversight obligations on Apria’s board of directors; provide certain management certifications; continue or implement, as applicable, certain compliance training and education; and engage an Independent Review Organization to perform certain reviews. The CIA also includes certain reporting, certification, record retention, and notification requirements. In the event of a breach of the CIA, Apria could become liable for payment of certain stipulated penalties or could be excluded from participation in federal healthcare programs.
In addition to federal enforcement of the FCA, a number of states have enacted false claims acts that are similar to the FCA. Generally, these state laws allow for the recovery of money that was fraudulently obtained by a healthcare provider from the state, such as Medicaid funds provided by the state, or in some cases, from private Payors and to assess fines and penalties. As a result of these state laws, there has been a corresponding increase in state-initiated false claims enforcement efforts. See “—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York” below for more information.
Other Fraud and Abuse Laws. In addition to the laws described above, various other laws and regulations prohibit fraud and abuse in the healthcare industry and provide for significant penalties. For example, the knowing and willful defrauding of, or attempt to defraud, a healthcare benefit program, including both governmental and private healthcare programs and plans, may result in criminal penalties. Further, the payment of inducements to Medicare and Medicaid beneficiaries intended to influence those beneficiaries to order or receive services from a particular provider or practitioner may result in civil penalties. Federal enforcement officials have numerous enforcement mechanisms to combat fraud and abuse, including an incentive program under which individuals can receive up to $1,000 for providing information on Medicare fraud and abuse that leads to the recovery of at least $100 of Medicare funds. In addition, federal enforcement officials have the ability to exclude from Medicare and Medicaid any investors, officers and managing employees associated with business entities that have committed healthcare fraud.
There have also been new statutes enacted to prevent fraud and abuse in healthcare, as well as existing statutes used in new ways to target healthcare fraud. In addition, in recent years the DOJ has started using the Travel Act as a basis for prosecuting healthcare fraud defendants based on violations of state anti-kickback or anti-bribery laws.
In August 2017, we entered into a settlement agreement resolving an investigation conducted by the Commonwealth of Massachusetts regarding our billing practices for services provided to MassHealth (Massachusetts Medicaid) members. Among other things, we were required to execute an assurance of discontinuance, to pay a settlement in the amount of approximately $765,000, to develop a compliance and monitoring program to oversee design and implementation of written policies and procedures focused on compliance with MassHealth billing practices and to institute and complete training for all of our employees who have any involvement with billing MassHealth members. Violation of the terms of the settlement agreement could result in the imposition of penalties and sanctions, including disqualification from MassHealth programs. We have actively monitored the billing practices at issue in this matter, submitted periodic reports to the Massachusetts Attorney General’s office, and conducted training as required by the settlement agreement. This matter was closed in August 2020.
Marketing Laws. Because of the products and services that we provide to patients, we are subject to certain federal and state laws and regulations regarding our marketing activities and the nature of our interactions with physicians and other healthcare providers. These laws may require us to comply with certain codes of conduct, limit or report certain marketing expenses and disclose certain physician and other provider arrangements. Violations of these laws and regulations, to the extent they are applicable, could subject us to civil and criminal fines and penalties, as well as possible exclusion from participation in federal healthcare programs, such as
115
Table of Contents
Medicare and Medicaid. From time to time, we may be the subject of investigations or audits, or be a party to litigation which alleges violations of these laws and regulations. If any of those allegations were successfully asserted against us, there could be a material adverse effect on our business, financial condition and results of operations.
Corporate Compliance Program. We have developed a corporate compliance program in an effort to monitor compliance with federal and state laws and regulations applicable to healthcare organizations and to implement policies, procedures and processes designed to ensure that our employees act in compliance with all applicable laws, regulations and company policies. The HHS OIG has issued a series of compliance program guidance documents in which it has set out the elements of an effective compliance program. The HHS OIG has followed the model of the U.S. Sentencing Guidelines, which are used by federal judges in determining sentences in federal criminal cases. The guidelines are advisory, not mandatory, and with respect to corporations state that having an effective ethics and compliance program may be a relevant mitigating factor in determining sentencing. To comply with the U.S. Sentencing Guidelines, a corporate compliance program must be reasonably designed, implemented and enforced such that it is generally effective in preventing and detecting criminal conduct. The U.S. Sentencing Guidelines also state that corporations should take certain steps such as periodic monitoring and responding appropriately to detected criminal conduct.
Our compliance program has been structured to include these elements. The primary compliance program components recommended by the HHS OIG, all of which we have attempted to implement, include: formal policies and written procedures; designation of a compliance officer; education and training programs; auditing, monitoring and risk assessments; a process for responding appropriately to detected misconduct; open lines of communication; and discipline and accountability. We conduct routine compliance auditing and monitoring, including checks of relevant governmental exclusion and debarment lists, and we audit compliance with our corporate compliance program on a regular basis. From time to time, issues identified through our compliance program may trigger internal or external reviews related to our compliance with federal and state healthcare laws and regulations. These internal and external reviews may result in changes to our coding, billing and claims adjudication process for our claims.
While we have attempted to develop our corporate compliance program to be consistent with these guidelines, we cannot be certain that a court, or the HHS OIG, would agree. Although we believe our approach to compliance reflects a reasonable and accepted approach in the healthcare industry, we cannot assure you that our corporate compliance program will prevent, detect and/or rectify all compliance issues in all markets we serve and in all applicable time periods. If we fail to prevent, detect and/or rectify such issues, depending on the nature and scope of such issues, we could face future claims for recoupment of overpayments, civil fines and other penalties, or other material adverse consequences.
In addition, pursuant to the settlement agreement with the Commonwealth of Massachusetts, we have implemented a specialized compliance program that includes the appointment of a compliance officer, training and policies and procedures focused on issues pertaining to limitations on direct billing of MassHealth patients. This compliance program concluded in August 2020.
Finally, pursuant to the December 2020 settlement agreement with the United States and state Medicaid programs, we also have entered into a five-year CIA with HHS OIG. Under this CIA, we agreed to undertake certain obligations designed to promote compliance with federal healthcare programs. For further information, see “—Enforcement of Healthcare Fraud and Abuse Laws—False Claims Act” above.
Facility and Clinician Licensure. Various federal and state authorities and clinical practice boards regulate the licensure of our facilities and clinical specialists working for us, either directly as employees or on a per diem or contractual basis. Regulations and requirements vary from state to state. We are committed to complying with all applicable licensing requirements and maintain centralized functions to manage over 2,900 facility licenses and/or permits that are required to operate our business.
116
Table of Contents
Healthcare Reform. Economic, political and regulatory influences are continuously causing fundamental changes in the healthcare industry in the United States. In 2010, the U.S. Congress enacted and President Obama signed into law, significant reforms to the U.S. healthcare system. These reforms, contained in the Health Reform Laws, significantly altered the U.S. healthcare system by authorizing, among many other things: (i) increased access to health insurance benefits for the uninsured and underinsured populations; (ii) new facilitators and providers of health insurance, as well as new health insurance purchasing access points (i.e., exchanges); (iii) incentives for certain employer groups to purchase health insurance for their employees; (iv) opportunities for subsidies to certain qualifying individuals to help defray the cost of premiums and other out-of-pocket costs associated with the purchase of health insurance, and over the longer term; and (v) mechanisms to foster alternative payment and reimbursement methodologies focused on outcomes, quality and care coordination. In addition, certain states in which we operate are periodically considering various healthcare reform proposals.
Since their passage in 2010, the Health Reform Laws have triggered many changes to the U.S. healthcare system, some of which took effect (e.g., the subsequently eliminated individual mandate penalty) while others have continued to be delayed and subsequently repealed (e.g., the medical device tax). The Health Reform Laws also have faced several challenges and remain subject to ongoing efforts to repeal or modify the laws or delay the implementation of certain aspects of these laws. For example, President Trump issued an Executive Order 13765 (Minimizing the Economic Burden of the Patient Protection and Affordable Care Act Pending Repeal) on January 20, 2017 granting authority to certain executive departments and agencies to minimize the economic burden of the PPACA. However, President Biden revoked this Executive Order on January 28, 2021 (as part of President Biden’s Executive Order on Strengthening Medicaid and the Affordable Care Act), and directed heads of departments to “consider whether to suspend, revise, or rescind — and, as applicable, publish for notice and comment proposed rules suspending, revising, or rescinding” actions taken by the Trump Administration which may hinder the operation of the Health Reform Laws.
Consequently, the core tenets of the Health Reform Laws remain in effect with several exceptions. The individual mandate penalty was eliminated beginning in 2019 through the Tax Cuts and Jobs Act of 2017. In addition, on December 20, 2019, the Further Consolidated Appropriations Act, 2020 H.R. 1865 (Pub.L. 116-94), was signed into law which repealed several provisions that were included in the Health Reform Laws to pay for the increased federal spending associated with the Health Reform Laws. Specifically, Congress: (i) repealed the Medical Device Excise Tax, which imposed a 2.3% excise tax on manufacturers, producers and importers of certain medical devices; (ii) repealed the health insurance tax, which applies to most fully insured plans, beginning in 2021; and (iii) repealed the so-called Cadillac Tax, which imposed an excise tax of 40% on premiums for employer-sponsored individuals and families that exceeded a certain minimum threshold. Prior to these changes Congress had passed a short-term spending bill as part of the Continuing Appropriations Act of 2018 that delayed the implementation of these provisions and eliminated the Independent Payment Advisory Board, which was a 15-member panel of healthcare experts created by the Health Reform Laws and tasked with making annual cost-cutting recommendations for Medicare if Medicare spending exceeded a specified growth rate.
The Health Reform Laws also are the subject of ongoing litigation. In particular, a collection of twenty (20) state governors and state attorneys general (subsequently two states have dropped out) filed a lawsuit against the federal government in the Northern District of Texas seeking to enjoin the entire Health Reform Laws following the elimination of the individual mandate penalty in 2019. The District Court ruled that without the penalty the individual mandate was unconstitutional and that all other provisions of the Health Reform Laws should be overturned as well. The U.S. Court of Appeals for the 5th Circuit affirmed the trial court’s decision; however, instead of deciding whether the rest of the PPACA must be struck down, the 5th Circuit sent the case back to the trial court for additional analysis. In March of 2020 the United States Supreme Court granted cert in the case and heard oral arguments on November 10, 2020. We are unable to predict the ultimate outcome of the lawsuit but note its potential impact on the Health Reform Laws moving forward.
The Trump Administration has made a number of changes that have affected the individual and small group exchange markets, including modifications to the open enrollment periods, funding cuts to patient support resources, including the patient navigator program, and failing to issue cost-sharing reduction payments to
117
Table of Contents
insurers participating in the exchanges. In June 2018, the Trump Administration published a final rule that allows small businesses and self-employed individuals to band together to create associations that are considered “employers” under the Employee Retirement Income Security Act such that these associations are eligible to access large group health plans, which are typically less expensive and are not subject to as many of the consumer protections imposed by the PPACA on small group and individual health plans. In addition, the Trump Administration published a final rule which makes short term, limited duration plans more accessible, providing individuals with another product offering that is generally less expensive but has fewer protections than under the PPACA plans. This final rule combined with the association health plan final rule, may increase instability in the healthcare exchanges by siphoning off potentially healthier people from the risk pool. However, in 2021 President Biden issued an Executive Order on Strengthening Medicaid and the Affordable Care Act, directing heads of departments to review and potentially revoke or revise these Trump-era actions.
In light of the ongoing efforts to alter the Health Reform Laws, we are unable at this time to predict the full impact that potential changes will have on our business, including provisions in the Health Reform Laws related to Medicare payments, mechanisms to foster alternative payment and reimbursement methodologies focused on outcomes, quality and care coordination, Medicare enrollment and claims submission requirements and revisions to other federal healthcare laws such as the AKS, the Stark Law and the FCA. We anticipate, however, that federal and state governments will continue to review and assess alternative healthcare delivery systems and payment methodologies, and that public debate regarding these issues will continue in the future. Changes in the law or new interpretations of existing laws can have a substantial effect on permissible activities, the relative costs associated with doing business in the healthcare industry, and the amount of reimbursement available from government and other Payors. If the Health Reform Laws are repealed or modified, or if implementation of certain aspects of the Health Reform Laws continues to be delayed, such repeal, modification, or delay may materially adversely impact our business, financial condition, results of operations, cash flow, capital resources and liquidity. In addition, the potential proposals for alternative legislation to replace the Health Reform Laws may have an adverse impact on our business.
FDA. Apria is governed by various regulations that affect the manufacture, distribution, importation, marketing and sale of medical gases and related oxygen medical devices and supplies. Our medical gas facilities and operations are subject to extensive regulation by the FDA and other federal and state authorities. The FDA regulates medical gases, including medical oxygen, pursuant to its authority under the FFDCA. Among other requirements, the FDA’s cGMP regulations impose certain quality control, documentation and recordkeeping requirements on the receipt, processing and distribution of medical gas. Further, in each state where we operate medical gas facilities, we are subject to regulation under state health and safety laws, which vary from state to state. The FDA and state authorities conduct periodic, unannounced inspections at medical gas facilities to assess compliance with cGMPs and other regulations. We expend significant time, money and resources in an effort to achieve substantial compliance with cGMP regulations and other federal and state law requirements at each of our medical gas facilities. There can be no assurance, however, that these efforts will be successful and that our medical gas facilities will achieve and maintain compliance with federal and state laws and regulations. Our failure to achieve and maintain regulatory compliance at our medical gas facilities could result in enforcement action, including warning letters, fines, product recalls or seizures, temporary or permanent injunctions, or suspensions in operations at one or more locations, as well as civil or criminal penalties, all of which could have a material adverse effect on our business, financial condition and results of operations.
Our facilities must comply with applicable federal and state laws, regulations and licensing standards. For example, all of our locations that fill and distribute medical oxygen containers must register with the FDA as a medical gas manufacturer, and these registered locations must comply with all applicable Company and cGMP policies and practices. Regulations are subject to change as a result of legislative, administrative or judicial action, which may further increase our costs or reduce sales. From time to time, we may undertake field corrective actions to correct product issues that may arise. These actions are necessary to ensure the products we distribute adhere to high standards of quality and safety. Additionally, we have policies and procedures in place that address the process for taking field corrective actions should we become aware of any issue related to the
118
Table of Contents
medical oxygen products that we fill and distribute. We continue to operate these programs to ensure compliance with applicable regulations and actively monitor proposed changes in the FDA’s regulation of medical gases and related products, particularly those which could have a material adverse effect on the products we manufacture or distribute, or us as a whole.
We have facilities in certain states that manufacture medical gases, including medical oxygen. In the United States, medical gases, which are products that are recognized by an official pharmacopoeia or formulary, or intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease, are regulated as finished pharmaceuticals (drugs). The production, distribution and sale of medical gases and medical devices are regulated by the U.S. federal government under FFDCA as well as by various state and local laws and other regulations. Before a medical gas can be marketed in the United States, the FDA must approve a request to certify the product as a “designated medical gas.” The FDA reviews requests for certification to confirm that the gas to which the request applies is a designated medical gas and contains the information required by regulation. Certification of a designated medical gas has the effect of a drug approval under section 505 of the FFDCA (for gases intended for human use). If changes are made to the original certification request, such as information about the requestor or the formulation of a medical gas, an amendment may be submitted to the FDA explaining the change. Apria has obtained this certification, and in accordance with cGMPs, we verify the reliability of the medical gas supplier’s analytical methodology to ensure the bulk oxygen we receive has been tested in conformance with cGMP requirements. In addition, all bulk oxygen deliveries must be accompanied with a certificate of analysis that meets cGMP specifications. Each delivery of bulk oxygen provided to our bulk stand tanks must be tested by trained Apria personnel in accordance with the mandated analytical methodology defined by cGMPs. We also conduct primary source verification of the FDA registration and state licensure held by each medical gas supplier that provides bulk oxygen to any of our facilities.
The FDA requires entities that manufacture or engage in certain processing operations related to medical gases (e.g., combining gases or transfilling a gas from one container to another) to comply with all establishment and drug registration and listing requirements and to follow cGMPs regarding quality, personnel, facilities, equipment design and calibration, production, testing processes, container and closure specifications, labeling requirements and records and complaint management. The FDA periodically inspects manufacturing facilities to assess compliance with cGMPs, which impose extensive procedural and record keeping requirements. While the FDA temporarily paused on-site routine surveillance inspections starting in March 2020 due to the COVID-19 pandemic, in a guidance issued in August 2020, the FDA explained that it resumed prioritized domestic inspections, such as pre-approval and surveillance inspections, as of July 2020, and that, for the foreseeable future, such prioritized domestic inspections would be pre-announced. The FDA has continued, on a case-by case basis, to conduct “mission-critical” inspections based on its evaluation of a number of factors related to the public health benefit of U.S. patients having access to the product subject to inspection (e.g., whether the product may have received breakthrough therapy designation or is used to diagnose, treat, or prevent a serious disease or medical condition for which there is no other appropriate substitute). Since January 2020, no Apria facilities have been subject to inspection by the FDA, and we are not aware of any planned FDA inspections of any Apria facility. Quality control and manufacturing procedures must continue to conform to cGMPs after certification as a designated medical gas. Additionally, under the FDA’s regulations, we are subject to ongoing post-market requirements. For example, drug manufacturers must report adverse reactions (any undesirable experience associated with the use of a drug, including serious drug side effects, product use errors, product quality problems and therapeutic failures), provide updated safety and efficacy information and comply with requirements concerning advertising and promotional materials and activities. Any post-market regulatory obligations, and the cost of complying with such obligations, could expand in the future.
As a medical device distributor, we must rely on device manufacturers and suppliers to comply with regulatory requirements and adhere to the FDA’s cGMP and other quality requirements. We cannot predict whether any issues may arise out of any FDA inspection of their sites or regulation of their operations.
In the United States, the FFDCA, FDA regulations and other federal and state statutes and regulations govern, among other things, medical device design and development, preclinical and clinical testing, premarket
119
Table of Contents
clearance or approval, registration and listing, manufacturing, labeling, storage, advertising and promotion, sales and distribution and post-market surveillance. Failure to comply with applicable requirements may subject a company to a variety of administrative or legal sanctions, such as warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties and criminal prosecution. Unlike manufacturers of medical devices, distributors are generally only required to comply with certain post-market requirements for medical devices, including reporting of complaints and adverse events related to device malfunctions, serious injuries or deaths associated with the medical device. In May 2020, the FDA issued guidance on temporary exceptions for post-market adverse event reporting for companies experiencing reduced workforces due to employee absenteeism as a result of COVID-19. Although continued, regular post-market adverse event reporting is encouraged during the pandemic, the FDA intends to exercise enforcement discretion if certain reports are not submitted to the FDA within the timeframes typically required by statute and regulation, provided that any delayed reports are submitted within six months of the restoration of adverse event reporting processes to their pre-pandemic state. This temporary guidance is limited to the duration of the COVID-19 pandemic, or another period of time as determined by the FDA, after which post-market adverse event reporting requirements will apply in full force and effect.
We distribute an array of “legend devices” or medical devices that are regulated by the FDA and which only can be dispensed pursuant to a valid prescription from an appropriately licensed healthcare provider or restricted to use by a prescribing healthcare provider. Examples of such legend devices include CPAPs, ventilators and concentrators. Note that, due to the COVID-19 pandemic, the FDA has issued guidance on temporary exceptions to the prescription requirement for ventilators in order to expand access to the devices. For example, the FDA is temporarily allowing NIV patient interfaces capable of prescribed breath to be used for patients requiring such ventilatory support. Under the same guidance, CPAP machines may be used to support patients with respiratory insufficiency under appropriate monitoring. These legend devices are commercially available finished medical devices and are not manufactured to our particular specifications. From time to time, for business and competitive reasons, we have entered into primary source agreements with manufacturers of these legend devices. Generally, we do not believe that such agreements pose a material risk that we will be unable to obtain needed devices in the event the manufacturer is disabled from providing a device or in the event we are prohibited from distributing devices in its inventory due to regulatory issues encountered by the manufacturer. However, these potential risks, and the resulting shortages of product that may occur, can interfere with our business.
For example, Apria used to distribute a NPWT device manufactured by Smith & Nephew, Inc. (“Smith & Nephew”), the Renasys Negative Pressure Wound Therapy (the “Renasys Device”). In 2014, the FDA issued a warning letter to Smith & Nephew relating to quality system processes and procedures at its Saint Petersburg, Florida facility, at which the Renasys Device is manufactured (“Smith & Nephew Warning Letter”). The Smith & Nephew Warning Letter stated that Smith & Nephew made two modifications to the Renasys Device which, allegedly, required the submission of a new 510(k) premarket notification prior to introducing any modified devices into interstate commerce. The Smith & Nephew Warning Letter alleged that the products were adulterated and misbranded and the FDA requested that Smith & Nephew immediately cease activities that result in the misbranding or adulteration of the products, such as the commercial distribution of the device for the uses discussed in the letter. We worked with the FDA and Smith & Nephew to avoid disruption in the continuity of care for its patients. Ultimately, however, we terminated our relationship with Smith & Nephew.
Fair Debt Collection Practices Act. Some of our operations may be subject to compliance with certain provisions of the Fair Debt Collection Practices Act and comparable statutes in many states. Under the Fair Debt Collection Practices Act, a third-party collection company is restricted in the methods it uses to contact consumer debtors and elicit payments with respect to placed accounts. Requirements under state collection agency statutes vary, with most requiring compliance similar to that required under the Fair Debt Collection Practices Act. We believe we are in substantial compliance with the Fair Debt Collection Practices Act and comparable state statutes where applicable. If our collection practices are viewed as inconsistent with these standards, we may be subject to damages and penalties.
120
Table of Contents
Federal CAN-SPAM Act, Telephone Consumer Protection Act and Telemarketing Sales Rule. Some of our operations may be subject to compliance with certain provisions of the Federal CAN-SPAM Act, the Telephone Consumer Protection Act of 1991 (“TCPA”) and the Telemarketing Sales Rule and Medicare regulations. Under such regulations, companies are restricted in the methods used to contact consumers by email, telephone and text and through the use of automated “auto-dialer” type devices. Numerous class-action suits under federal and state laws have been filed in recent years against companies that conduct SMS texting programs, with many resulting in multi-million-dollar settlements to the plaintiffs. Requirements under state telephone contact laws will vary, with most requiring compliance similar to that required under the TCPA. We believe we are in substantial compliance with the federal regulations we are subject to, as well as comparable state equivalents where applicable. The scope and interpretation of the laws that are or may be applicable to the delivery of consumer phone calls, emails and text messages are continuously evolving and developing. If we do not comply with these laws or regulations or if we become liable under these laws or regulations, we could face direct liability, could be required to change some portions of our business model, could face negative publicity and our business, financial condition and results of operations could be adversely affected.
Antitrust Laws. The federal government and most states have enacted antitrust laws that prohibit certain types of conduct deemed to be anti-competitive. These laws prohibit price fixing, market allocation, bid-rigging, concerted refusal to deal, market monopolization, price discrimination, tying arrangements, acquisitions of competitors and other practices that have, or may have, an adverse effect on competition. Violations of federal or state antitrust laws can result in various sanctions, including criminal and civil penalties. Antitrust enforcement in the healthcare sector is currently a priority of the FTC and the DOJ. We believe we are in compliance with such federal and state laws, but courts or regulatory authorities may reach a determination in the future that could adversely affect our operations.
Environmental Matters. We are subject to federal, state and local laws and regulations relating to hazardous materials, pollution and the protection of the environment. Such regulations include those governing emissions to air, discharges to water, storage, treatment and disposal of wastes, including medical waste, remediation of contaminated sites and protection of worker health and safety. These laws and regulations frequently change and have become increasingly stringent over time. Non-compliance with these laws and regulations may result in significant fines or penalties or limitations on our operations or claims for remediation costs, as well as alleged personal injury or property damages. We believe our current operations are in substantial compliance with all applicable environmental, health and safety requirements and that we maintain all material permits required to operate our business.
Certain environmental laws and regulations impose strict, and under certain circumstances joint and several, liability for investigation and remediation of the release of regulated substances into the environment. Such liability can be imposed on current or former owners or operators of contaminated sites, or on persons who dispose or arrange for disposal of wastes at a contaminated site. Based on available information, we do not believe that any known compliance obligations, releases or investigations under environmental laws or regulations will have a material adverse effect on our business, financial condition and results of operations. However, there can be no guarantee that these releases or newly-discovered information, more stringent enforcement of or changes in environmental requirements, or our inability to enforce available indemnification agreements will not result in significant costs.
Legal Proceedings
Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York. On December 18, 2020, a federal judge approved a civil and administrative settlement Apria recently entered into with the United States and state Medicaid programs, in a complaint filed by three relators under the qui tam provisions of the FCA, 31 U.S.C. § 3729 et seq., as well as comparable state false claims laws, in connection with the rental of NIVs. Apria also entered into separate settlements to resolve the relators’ claims brought on behalf of the States of California and Illinois related to NIV covered by private insurers. The matter had been pending since 2017.
121
Table of Contents
The government had alleged that Apria violated the FCA by submitting false claims seeking reimbursement for NIVs which were not being used, or not being used sufficiently, by patients, for NIVs which were being used pursuant to physician orders on a device setting which was available from other less expensive devices, and for improperly waiving co-pays to induce beneficiaries to rent NIVs. To resolve any potential liability, Apria agreed to enter a civil settlement agreement and to pay $40 million to the federal government and the states. Apria also agreed with the California Department of Insurance to pay $500,000 to resolve claims asserted by the relators under the California Insurance Frauds Prevention Act, CAL. INS. CODE § 1871 et seq. Apria separately agreed with the relators to settle all remaining claims from their complaint, including: (1) claims for retaliation in violation of federal and state laws; (2) claims for attorneys’ fees and costs available under federal and state law; and (3) claims under the Illinois Insurance Claims Fraud Prevention Act, 740 ILL. COMP. STAT. 92/1 et seq. Apria did not admit that any of its conduct was illegal or otherwise improper.
As part of the federal and state Medicaid settlement, Apria also entered into a five-year corporate integrity agreement (the “CIA”) with the HHS OIG. The CIA requires Apria to maintain its ongoing corporate compliance program and obligates Apria to implement or continue, as applicable, a set of defined corporate integrity activities for a period of five years from the effective date of the CIA. Among other things, the CIA requires Apria to impose certain oversight obligations on Apria’s board of directors; provide certain management certifications; continue or implement, as applicable, certain compliance training and education; and engage an Independent Review Organization to perform certain reviews. The CIA also includes certain reporting, certification, record retention, and notification requirements. In the event of a breach of the CIA, Apria could become liable for payment of certain stipulated penalties or could be excluded from participation in federal healthcare programs.
Other Litigation. We are engaged in the defense of certain other claims and lawsuits arising out of the ordinary course and conduct of our business, the outcomes of which are not determinable at this time. Insurance policies covering such potential losses, where such coverage is cost effective, are maintained. In the opinion of management, any liability that might be incurred upon the resolution of these claims and lawsuits will not, in the aggregate, have a material adverse effect on our business, financial condition, results of operations, cash flow, capital resources and liquidity.
Intellectual Property
Apria has registered the Apria trademark and numerous related trade names. We also hold numerous other trade names and copyrights which are not material in nature. We do not own or have a license or other rights under any patents that are material to our business.
Seasonality
Our business is sensitive to seasonal fluctuations. Our patients are generally responsible for a greater percentage of the cost of their treatment or therapy during the early months of the year due to co-insurance, co- payments and deductibles, and therefore may defer treatment and services of certain therapies until they have met their annual deductibles. In addition, changes to employer insurance coverage often go into effect at the beginning of each calendar year which may impact eligibility requirements and delay or defer treatment or recognition of revenues. These factors may lead to lower total revenues and cash flow in the early part of the year and higher total revenues and cash flow in the latter half of the year. Additionally, the increased incidence of respiratory infections during the winter season may result in initiation of additional respiratory services such as oxygen therapy for certain patient populations. Our quarterly operating results may fluctuate significantly in the future depending on these and other factors.
Properties
We lease our headquarters, located in Indianapolis, Indiana, which consists of approximately 83,000 square feet of office space. The lease expires on March 31, 2026.
122
Table of Contents
We have approximately 275 branch locations that are capable of reaching over 90% of the U.S. population, as well as regional distribution and repair centers, customer service and billing centers, a national pharmacy and a biomedical center for the repair, maintenance and distribution of patient equipment. The regional facilities are typically located in light industrial areas and generally range from 15,000 to 88,000 square feet. The typical branch location facility is a combination warehouse and office and can range from 1,000 to 20,000 square feet. We lease substantially all of our facilities with lease terms of ten years or less.
Human Capital Resources
As of September 30, 2020, we had approximately 6,350 employees, of which approximately 6,050 were full-time and approximately 300 were part-time and per diem. Our human capital resources objectives include attracting and retaining highly motivated, well-qualified employees. Our compensation program is designed to attract, retain and motivate highly qualified employees and executives. We use a mix of competitive salaries and other benefits to attract and retain employees and executives. We believe that our employee relations are good, and we are committed to inclusion and policies and procedures to maintain a safe work environment. The health and safety of our employees, patients and customers are of primary concern. During the COVID-19 pandemic, we have taken significant steps to protect our workforce including but not limited to, working remotely, introducing contact-free operational procedures, procuring personal protective equipment, communicating hygiene and cleaning protocols, and implementing mandatory face-covering usage, self-monitoring processes, and social distancing protocols consistent with guidelines issued by federal, state and local law.
123
Table of Contents
Directors and Executive Officers
The following table sets forth the names, ages and positions of our directors, director nominee and executive officers as of the date of this prospectus.
| Name |
Age |
Position | ||
| Executive Officers |
||||
| Daniel J. Starck |
54 |
Director and Chief Executive Officer | ||
| Debra L. Morris |
62 |
Executive Vice President, Chief Financial Officer | ||
| Robert P. Walker |
54 |
Executive Vice President, Managed Care | ||
| Raoul Smyth |
66 | Executive Vice President, General Counsel and Secretary | ||
| Angela Fyfe |
57 |
Executive Vice President, Enterprise Services | ||
| Mark E. Litkovitz |
57 | Executive Vice President, Chief Information Officer | ||
| Celina M. Scally |
50 | Senior Vice President, Chief Human Resources Officer | ||
| Directors |
||||
| John G. Figueroa |
58 | Director and Chairman of the Board | ||
| Michael Audet |
34 | Director | ||
| John R. Murphy |
70 | Director | ||
| Norman C. Payson, M.D. |
72 | Director | ||
| Devon Rinker |
32 | Director Nominee | ||
| Neil P. Simpkins |
54 | Director | ||
| Lynn Shapiro Snyder |
64 |
Director | ||
| Daniel J. Starck |
54 | Director and Chief Executive Officer | ||
| Mike S. Zafirovski |
67 | Director |
Daniel J. Starck has served as our Chief Executive Officer since February 2015. Prior to assuming this role, Mr. Starck served as the Chief Executive Officer of our home respiratory therapy and home medical equipment segment since he joined the Company in April 2012 and became one of our Directors in December 2013. Prior to joining us, from 2006 to 2012, Mr. Starck served as Chief Executive Officer of CorVel Corporation (“CorVel”), an Orange County, California-based national provider of industry-leading workers’ compensation solutions for employers, third-party administrators, insurance companies and government agencies seeking to control costs and promote positive outcomes. Mr. Starck joined CorVel in 2006 as President and Chief Operating Officer after serving Apria and a predecessor company in a series of progressively more responsible operations roles from 1992 to 2006. Mr. Starck holds a B.S. in Business from San Diego State University in California.
Debra L. Morris has served as our Executive Vice President, Chief Financial Officer since March 2013. Prior to joining the Company, Ms. Morris served as Chief Financial Officer—Americas for SITEL Worldwide Corporation from February 2010 to February 2013. Prior to that she served as a Partner of Tatum LLC from 2004 to 2010 and as a Director from 2008 to 2010 and provided interim and permanent CFO services for companies contracted with Tatum LLC including LifeMasters Supported Selfcare and RelaDyne. From 1999 to 2002 she was Chief Financial Officer of Caliber Collision Centers. Ms. Morris spent the earlier part of her career in progressively more responsible roles with CB Richard Ellis, including as Executive Vice President—Global Marketing and Integration and Executive Vice President—Global Chief Accounting Officer. Ms. Morris currently serves as a member of the Board of Directors of Rexford Industrial Realty, Inc. Ms. Morris holds a B.S. in Business Administration from Colby Sawyer College in New London, New Hampshire.
Robert P. Walker has served as our Executive Vice President, Managed Care since April 2013. Prior to his assuming this role, Mr. Walker served as our Vice President of Sales from 1998 through 2002 and was later Chief Sales Officer and President for the oncology laboratory division at Poplar Healthcare from 2010 to 2013. Mr. Walker served on the boards of Valet Living, LLC and Evident, LLC and was Chairman-elect of the
124
Table of Contents
Oklahoma State Alumni Association. He currently serves on the board of University FanCards LLC. Mr. Walker holds a B.S. in Marketing and Management from Oklahoma State University.
Raoul Smyth has served as our Executive Vice President, General Counsel and Secretary since 2015 and has been with the Company since 1996. Prior to assuming his current role, Mr. Smyth worked in Apria’s Legal Department in progressively more responsible roles. Prior to joining us, Mr. Smyth was a partner at a large Dallas law firm and served as the Chief Executive Officer and Managing Partner of Smyth & Devereux, P.C., a law firm with offices in Dallas and Los Angeles. He holds a B.A. from the University of North Texas and received both his J.D. and M.S. in Foreign Service (focusing on international business) from Georgetown University in Washington, D.C.
Angela Fyfe serves as our Executive Vice President, Enterprise Services, which includes customer service, field shared services, marketing and product management. Ms. Fyfe brings over 25 years of executive experience in diverse industries including healthcare, financial services, and retail, serving in a variety of leadership positions within operations, customer care, sales, marketing, and client services. Ms. Fyfe joined Apria in 2016 as the Vice President, Customer Care Center Customer Service Operations and prior to joining Apria, she was the Chief Operating Officer for LIFENEXUS, a healthcare technology company that filed for Chapter 7 bankruptcy protection in January 2016. Ms. Fyfe earned her B.A. in marketing at the University of Tulsa and her M.B.A. from the University of Phoenix.
Mark E. Litkovitz has served as our Executive Vice President, Chief Information Officer since January 2014. Prior to assuming this role, Mr. Litkovitz led multiple areas of our IT organization since April 2009. Mr. Litkovitz previously spent 24 years in IT services, primarily with Perot Systems. Mr. Litkovitz holds a B.S. in Computer Science from the University of Akron in Ohio.
Celina M. Scally has served as our Senior Vice President, Chief Human Resources Officer since 2013 and has been with the Company since 1990. In her current role, Ms. Scally is responsible for managing all aspects of the Company’s human resources department. Prior to assuming this role, Ms. Scally served the Company in progressively more responsible roles from roles in the field organization in both billing and customer service, to Director, Customer Service and Training & Development to Vice President, Organizational Effectiveness & Change Management. Ms. Scally holds a Bachelor of Science in Business from the University of Phoenix.
John G. Figueroa first became one of our directors in November 2012 when he joined us as Chairman of our board of directors and also served in the dual roles as Chief Executive Officer of the Company and of our home infusion therapy segment from November of 2012 until January 2014. Since June 2020, Mr. Figueroa has served as the Chairman and Chief Executive Officer of CarepathRX, a company providing innovative pharmacy solutions to hospital health systems and served as Chief Executive Officer of Genoa Healthcare, the leading behavioral health specialty pharmacy in the United States from 2014 to 2018. Prior to his appointment to these roles, Mr. Figueroa served as Chief Executive Officer and board member of Cincinnati, Ohio-based Omnicare, Inc., a Fortune 500 healthcare services company that provides pharmaceuticals and related services to long-term care facilities and specialized drugs for complex disease states. Before that, Mr. Figueroa served as President of McKesson Corporation’s U.S. Pharmaceutical Group from 2006 to 2010, after holding progressively more responsible operations and sales positions in the company’s Supply Solutions, Pharmaceutical and Health Systems groups from 1997 through early 2006. He spent the initial years of his career in various sales and operations roles for Baxter Healthcare Corporation’s Hospitex and Medical Surgical divisions. Mr. Figueroa currently serves as a director and the Chairman of the Compensation Committee and member of the Governance Committee of Reliance Steel & Aluminum Co., and on the Executive Committee for the board at Pepperdine University Graziadio School of Business. Mr. Figueroa holds B.A.s in English Literature and Political Science from the University of California at Los Angeles and received his M.B.A. from Pepperdine University in California.
Michael Audet became one of our directors in June 2019. Mr. Audet is a Managing Director at Blackstone in Strategic Partners. Since initially joining Blackstone in 2010, Mr. Audet previously worked in Blackstone’s
125
Table of Contents
private equity business where he was involved in the execution of Blackstone’s investments in Paysafe, First Eagle Asset Management, Tradesmen International, Summit Materials, and Apria Healthcare. Mr. Audet received an AB in mathematics from Dartmouth College, where he graduated summa cum laude and was elected to Phi Beta Kappa, and a Masters of Business Administration with Distinction from Harvard Business School. He is also a Co-Chair of the New Wave Steering Committee for Film at Lincoln Center.
John R. Murphy became one of our directors in August 2019. Mr. Murphy currently serves on the Board of Directors of O’Reilly Automotive, Inc., Alight Solutions LLC and Summit Materials, Inc. Previously, he served on the Board of Directors of DJO Global, Inc., Graham Packaging, Inc. and Accuride Corporation, Inc. He previously served as Interim Chief Financial Officer of Summit Materials, Inc. in 2013, Senior Vice President and Chief Financial Officer of Smurfit-Stone Container Corporation from 2009 to 2010, and Chief Financial Officer, then President and Chief Operating Officer, then President and Chief Executive Officer with Accuride Corporation, Inc. from 1998 to 2008. Mr. Murphy holds a Bachelor of Science in Accounting from Pennsylvania State University and a Master of Business Administration from the University of Colorado, and is a Certified Public Accountant.
Norman C. Payson, M.D. first became one of our directors in 2006 and served as our Executive Chairman of the Board of Directors and Chief Executive Officer from October 2008 until November 2012 when he retired as Executive Chairman and as Chief Executive Officer and became a Senior Advisor to us while remaining a member of our Board of Directors. He was Chief Executive Officer of Oxford Health Plans from 1998 through 2002. Dr. Payson co-founded Healthsource, Inc., a large health plan operating in 15 states, in 1985 and served as its Chief Executive Officer from 1985 through 1997. He currently serves as Chairman of the board of directors of Access Clinical Partners, an integrated urgent care health network operating in ten states. He is also Chairman Emeritus of the board of directors of City of Hope, a not-for-profit tertiary cancer hospital and research center. Dr. Payson previously served as a member of the board of directors of Evolent Health Holdings Inc. (NYSE: EVH) from December 2013 to June 2019 and as a consultant of Evolent Health Holdings Inc. from March 2014 through December 2020. He is also a board member of Progyny, a fertility benefits provider and also serves as a Director of Smile Brands, one of the nation’s leading Dental Support Organizations with over 350 locations across 15 states. Dr. Payson is an Emeritus Board Member of Geisel School of Medicine at Dartmouth, as well as a member of the Mailman School of Public Health at Columbia HPM National Advisory Board, and a member of the USC Schaeffer Center Advisory Board. Dr. Payson holds a B.S. from the Massachusetts Institute of Technology in Cambridge, Massachusetts and received his medical degree from Dartmouth Medical School in New Hampshire.
Devon Rinker is a nominee to our board of directors and a Principal in Blackstone’s Private Equity Group. Since joining Blackstone in 2017, Mr. Rinker has been involved with Blackstone’s investments in HealthMarkets, NCR, SERVPRO, and Exeter Finance. He previously served as a director on the board of directors of HealthMarkets. Before joining Blackstone, Mr. Rinker was an Associate at KKR, where he evaluated and executed private equity investments in the technology, media and communications industries. Prior to that, he worked in investment banking at Evercore Partners. Mr. Rinker received a B.S. in Commerce from the University of Virginia, where he graduated with distinction and Beta Gamma Sigma, and an M.B.A. from Harvard Business School.
Neil P. Simpkins became one of our directors immediately after the completion of our acquisition by private investment funds affiliated with our Sponsor in October 2008. He is a Senior Managing Director of Blackstone’s Corporate Private Equity Group. Since joining Blackstone in 1998, Mr. Simpkins has led the acquisitions of TRW Automotive, Vanguard Health Systems, TeamHealth, Gates Industrial Corporation plc, Change Healthcare and Summit Materials. Before joining Blackstone, Mr. Simpkins was a Principal at Bain Capital. While at Bain Capital, Mr. Simpkins was involved in the execution of investments in the consumer products, industrial, healthcare and information industries. Prior to joining Bain Capital, Mr. Simpkins was a consultant at Bain & Company in the Asia Pacific region and in London. He currently serves as a director of Change Healthcare Inc., Gates Industrial Corporation plc and TeamHealth Holdings, Inc.
126
Table of Contents
Lynn Shapiro Snyder became one of our directors in August 2019. Ms. Snyder currently is a senior Member of Epstein Becker Green (“EBG”) in the Health Care and Life Sciences and Litigation practices in the firm’s Washington, DC office, and she is a Strategic Advisor with EBG Advisors, Inc. Ms. Snyder has over 40 years of experience at EBG, advising a wide variety of clients about federal, state, and international health law/policy/commercial issues, including Commercial Payer, Medicare, Medicaid, TRICARE, VA, compliance and managed care issues. Ms. Snyder is also Founder and President of the Women Business Leaders of the U.S. Health Care Industry Foundation (“The WBL Foundation”). Ms. Snyder currently is a board member of the following companies: The Trustmark Mutual Holding Company on the Audit and Compensation Committees, EBG as the Vice Chair of Finance Committee; and The WBL Foundation as the Chairperson of the Board. She is a graduate of Franklin & Marshall College and George Washington University National Law Center.
Mike S. Zafirovski became one of our directors in October 2011. Mr. Zafirovski is the founder and president of The Zaf Group LLC, which he established in November 2012. Since October 2011, Mr. Zafirovski has served as an Executive Advisor to The Blackstone Group Inc. and serves on the boards of certain Blackstone portfolio companies. Previously, he served as director, President and Chief Executive Officer of Nortel Networks Corporation from November 2005 to August 2009. Prior to that, Mr. Zafirovski was director, President and Chief Operating Officer of Motorola, Inc. (“Motorola”) from July 2002 to January 2005, and remained a consultant to and a director of Motorola until May 2005. He served as Executive Vice President and President of the Personal Communications Sector (mobile devices) of Motorola from June 2000 to July 2002. Prior to joining Motorola, Mr. Zafirovski spent nearly 25 years with General Electric Company, where he served in management positions, including 13 years as President and Chief Executive Officer of five businesses in the industrial and financial services arenas, his most recent being President and Chief Executive Officer of GE Lighting from July 1999 to May 2000. Mr. Zafirovski also serves on the board of Stericycle, Inc. and served on the board of the Boeing Company for 16 years until retiring in May 2020. Mr. Zafirovski holds a B.A. in Mathematics from Edinboro University in Pennsylvania.
There are no family relationships among any of our executive officers and directors.
Composition of the Board of Directors After this Offering
Our business and affairs are managed under the direction of our board of directors. In connection with this offering, we will amend and restate our certificate of incorporation to provide for a classified board of directors, with three directors in class I (expected to be Dr. Payson and Messrs. Simpkins and Starck), three directors in class II (expected to be Messrs. Audet, Figueroa and Zafirovski) and three directors in class III (expected to be Messrs. Murphy and Rinker and Ms. Snyder). See “Description of Capital Stock—Anti-Takeover Effects of our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws and Certain Provisions of Delaware Law—Classified Board of Directors.” In addition, we intend to enter into a stockholders agreement with certain affiliates of our Sponsor in connection with this offering. This agreement will grant our Sponsor the right to designate nominees to our board of directors subject to the maintenance of certain ownership requirements in us. See “Certain Relationships and Related Person Transactions—Stockholders Agreement.”
Director Independence
Our board of directors has affirmatively determined that each of Messrs. Figueroa, Audet, Murphy, Rinker, Simpkins and Zafirovski, Dr. Payson and Ms. Snyder qualify as independent directors under the Nasdaq listing standards.
Background and Experience of Directors
When considering whether directors and nominees have the experience, qualifications, attributes or skills, taken as a whole, to enable our board of directors to satisfy its oversight responsibilities effectively in light of our
127
Table of Contents
business and structure, the board of directors focuses primarily on each person’s background and experience as reflected in the information discussed in each of the directors’ individual biographies set forth above. We believe that our directors provide an appropriate mix of experience and skills relevant to the size and nature of our business. In particular, the members of our board of directors considered the following important characteristics, among others:
| • | Mr. Figueroa has significant management and operational experience from his service in various senior management roles, including as our former Chief Executive Officer and the Chief Executive Officer of Omnicare, Inc. |
| • | Mr. Audet has significant financial and investment experience from his involvement in Blackstone, including as Managing Director in Strategic Partners. |
| • | Mr. Murphy has significant executive and financial experience from his service in various senior management roles, including as former interim Chief Financial Officer of Summit Materials, Inc. and President and Chief Executive Officer of Accuride Corporation. |
| • | Dr. Payson has significant executive experience from his service in various senior management roles, including as our former Chief Executive Officer and the Chief Executive Officer of Oxford Health Plans. |
| • | Mr. Rinker has significant financial and business experience from his service at Blackstone, including as Principal in the Private Equity Group. |
| • | Mr. Simpkins has significant financial and business experience, including as a Senior Managing Director in the Corporate Private Equity Group at Blackstone and former Principal at Bain Capital. |
| • | Ms. Snyder has significant industry experience from her role as Senior Member of Epstein Becker Green’s Health Care and Life Sciences and Litigation practice. |
| • | Mr. Starck has extensive business and industry experience as well as his experience leading Apria as Chief Executive Officer since February 2015 and his former role as the Chief Executive Officer of CorVel Corporation. |
| • | Mr. Zafirovski has extensive experience in executive management through his role as Executive Advisor at Blackstone and as a director of certain of Blackstone’s portfolio companies as well as his former role as Chief Operating Officer of Motorola, Inc. |
Controlled Company Exception
After the completion of this offering, our Sponsor will continue to hold more than a majority of the voting power of our common stock eligible to vote in the election of our directors. As a result, we will be a “controlled company” within the meaning of the Nasdaq corporate governance standards. Under these corporate governance standards, a company of which more than 50% of the voting power is held by an individual, group or another company is a “controlled company” and may elect not to comply with certain corporate governance standards, including the requirements (1) that a majority of our board of directors consist of independent directors, (2) that our board of directors have a compensation committee that is comprised entirely of independent directors with a written charter addressing the committee’s purpose and responsibilities and (3) that our director nominations be made, or recommended to our full board of directors, by our independent directors or by a nominations committee that is comprised entirely of independent directors and that we adopt a written charter or board resolution addressing the nominations process. For at least some period following this offering, we intend to utilize these exemptions. As a result, immediately following this offering, we do not expect a majority of our directors will have been affirmatively determined to be independent or that our compensation committee or nominating and corporate governance committee of the board will be comprised entirely of directors who have been determined to be independent. Accordingly, you will not have the same protections afforded to stockholders of companies that are subject to all of these corporate governance requirements. In the event that we cease to be a “controlled company” and our shares continue to be listed on Nasdaq, we will be required to comply with these provisions within the applicable transition periods.
128
Table of Contents
Board Committees
We anticipate that, prior to the completion of this offering, our board of directors will establish the following committees: an audit committee, a compensation committee, a compliance committee and a nominating and corporate governance committee. The composition and responsibilities of each committee are described below. Our board of directors may also establish from time to time any other committees that it deems necessary or desirable. Members serve on these committees until their resignation or until otherwise determined by our board.
Audit Committee
Upon completion of this offering, we expect our audit committee will consist of Mr. Audet, Mr. Murphy and Mr. Zafirovski, with Mr. Murphy serving as chair. Our audit committee will be responsible for, among other things:
| • | selecting and hiring our independent auditors, and approving the audit and non-audit services to be performed by our independent auditors; |
| • | assisting the board of directors in evaluating the qualifications, performance and independence of our independent auditors; |
| • | assisting the board of directors in monitoring the quality and integrity of our financial statements and our accounting and financial reporting; |
| • | assisting the board of directors in monitoring our compliance with legal and regulatory requirements; |
| • | reviewing the adequacy and effectiveness of our internal control over financial reporting processes; |
| • | assisting the board of directors in monitoring the performance of our internal audit function; |
| • | monitoring the performance of our internal audit function; |
| • | reviewing with management and our independent auditors our annual and quarterly financial statements; |
| • | establishing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls or auditing matters and the confidential, anonymous submission by our employees of concerns regarding questionable accounting or auditing matters; and |
| • | preparing the audit committee report that the rules and regulations of the SEC require to be included in our annual proxy statement. |
The SEC rules and Nasdaq rules require us to have one independent audit committee member upon the listing of our common stock on Nasdaq, a majority of independent directors within 90 days of the effective date of the registration statement and all independent audit committee members within one year of the effective date of the registration statement. Mr. Murphy qualifies as an independent director under the Nasdaq listing standards and the independence standards of Rule 10A-3 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”).
Compensation Committee
Upon completion of this offering, we expect our compensation committee will consist of Mr. Figueroa, Mr. Rinker and Mr. Simpkins, with Mr. Simpkins serving as chair. The compensation committee will be responsible for, among other things:
| • | reviewing and approving corporate goals and objectives relevant to the compensation of our CEO, evaluating our CEO’s performance in light of those goals and objectives, and, either as a committee or |
129
Table of Contents
| together with the other independent directors (as directed by the board of directors), determining and approving, or making recommendations to the board of directors with respect to, our CEO’s compensation level based on such evaluation; |
| • | reviewing and approving, or making recommendations to the board of directors with respect to, the compensation of our other executive officers, including annual base salary, bonus and equity-based incentives and other benefits; |
| • | reviewing and recommending the compensation of our directors; |
| • | reviewing and discussing annually with management our “Compensation Discussion and Analysis” disclosure required by SEC rules; |
| • | preparing the compensation committee report required by the SEC to be included in our annual proxy statement; and |
| • | reviewing and making recommendations with respect to our equity compensation plans. |
Compliance Committee
Upon completion of this offering, we expect our compliance committee will consist of Mr. Rinker, Ms. Snyder and Mr. Zafirovski, with Ms. Snyder serving as chair. The compliance committee will be responsible for, among other things, assisting the board of directors in the review and oversight of matters related to compliance with federal health care program requirements and overseeing monitoring of internal and external audits and investigations.
Nominating and Corporate Governance Committee
Upon completion of this offering, we expect our nominating and corporate governance committee will consist of Mr. Figueroa, Dr. Payson and Mr. Simpkins, with Mr. Figueroa serving as chair. The nominating and corporate governance committee is responsible for, among other things:
| • | assisting our board of directors in identifying prospective director nominees and recommending nominees to the board of directors; |
| • | overseeing the evaluation of the board of directors and management; |
| • | reviewing developments in corporate governance practices and developing and recommending a set of corporate governance guidelines; and |
| • | recommending members for each committee of our board of directors. |
Compensation Committee Interlocks and Insider Participation
None of our executive officers serves as a member of the board of directors or compensation committee (or other committee performing equivalent functions) of any entity that has one or more executive officers serving on our board of directors or compensation committee.
Code of Ethics
We will adopt a new Code of Business Conduct and Ethics that applies to all of our officers, directors and employees, including our principal executive officer, principal financial officer, principal accounting officer and controller, or persons performing similar functions, which will be posted on our website. Our Code of Business Conduct and Ethics is a “code of ethics,” as defined in Item 406(b) of Regulation S-K. We will make any legally required disclosures regarding amendments to, or waivers of, provisions of our code of ethics on our website. The information contained on, or accessible from, our website is not part of this prospectus by reference or otherwise.
130
Table of Contents
EXECUTIVE AND DIRECTOR COMPENSATION
Director Compensation
Director Compensation for 2020
The following table provides summary information for fiscal year 2020 concerning the compensation of the current members of our Board of Directors. The compensation paid to Mr. Starck, our Chief Executive Officer, is presented in the Summary Compensation Table below and the related explanatory tables. Our Chief Executive Officer does not receive additional compensation for his services as a director.
| Name |
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1) |
Total ($) |
|||||||||
| John G. Figueroa |
200,000 | — | 200,000 | |||||||||
| Norman C. Payson, M.D. |
100,000 | — | 100,000 | |||||||||
| Mike S. Zafirovski |
100,000 | — | 100,000 | |||||||||
| John R. Murphy |
100,000 | 74,980 | 174,980 | |||||||||
| Lynn Shapiro Snyder |
100,000 | 74,980 | 174,980 | |||||||||
| Neil P. Simpkins |
— | — | — | |||||||||
| Michael Audet |
— | — | — | |||||||||
| (1) | Amounts included in this column reflect the aggregate grant date fair value of 648 stock appreciation rights (“SARs”) granted on May 12, 2020, with a vesting start date of May 12, 2020 to each of Mr. Murphy and Ms. Shapiro Snyder, respectively, calculated in accordance with FASB ASC Topic 718, utilizing the assumptions discussed in Note 5 to our consolidated financial statements for the nine months ended September 30, 2020 included elsewhere in this prospectus. As of December 31, 2020, the aggregate number of outstanding unvested SARs held by each of Mr. Murphy and Ms. Shapiro Snyder was 1,296 SARs. |
Description of Director Compensation. Our employee director and directors that are employees of our Sponsor receive no additional compensation for serving as directors on our board of directors. As such, none of our directors, other than Messrs. Figueroa, Payson, Murphy and Zafirovski and Ms. Shapiro Snyder, received compensation for the year ended December 31, 2020. Messrs. Payson, Murphy and Zafirovski and Ms. Shapiro Snyder were entitled to an annual cash retainer of $100,000, payable quarterly, for their service as a director during 2020. Mr. Figueroa was entitled to an annual cash retainer of $200,000 for his service as chairman of our Board of Directors during 2020. All of our directors are reimbursed for their reasonable out-of-pocket expenses related to their service as directors. In addition, Mr. Murphy and Ms. Shapiro Snyder were each granted SARs in 2019 and 2020. For a description of the vesting and other terms of the SARs granted to our directors, see “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards in 2020 Table—Terms of Equity Awards—Stock Appreciation Rights of Apria Healthcare Group” below. Except as specifically set forth herein, the description of the terms of the 2015 Plan, the number of shares of Apria, Inc. subject to each SAR and the strike price of each SAR described in this “Executive and Director Compensation” section have not been adjusted to give effect to the assumption of the 2015 Plan by Apria, Inc. and the equitable adjustment associated therewith.
On December 4, 2020, our Board of Directors declared, and on December 11, 2020 paid, a $201.79 per share dividend to common stockholders (the “2020 Dividend”). Under the terms of the 2015 Plan, an adjustment to the outstanding SARs was determined to be equitable and necessary in order to prevent the dilution or enlargement of benefits under the 2015 Plan. Holders of vested SARs were entitled to an immediate cash payment per SAR equal to the lower of (i) the per share dividend amount and (ii) an amount equal to the difference between $495.13 and the strike price of such vested SAR. Accordingly, Mr. Murphy and Ms. Shapiro Snyder each received a cash payment of $16,949 with respect to their vested SARs and the strike price for each vested SAR was reduced by an amount
131
Table of Contents
equal to the per share dividend amount less the cash payment per vested SAR. No cash payment was made with respect to their unvested SARs, but the strike price for each of their unvested SARs was reduced by the per share dividend amount. In accordance with FASB ASC Topic 718, changes to the SARs associated with the 2020 Dividend were accounted for as modifications. As such, the fair value of each award immediately before and after the modifications were compared and no incremental stock compensation was recognized.
We anticipate that we will review our director compensation program in connection with this offering and make such changes as we determine are necessary or appropriate for our status as a public company.
Equity Units of BP Healthcare and Holdings. In connection with Dr. Payson’s prior service as our Chief Executive Officer, he purchased 10,000,000 Class A-2 Units of BP Healthcare Holdings LLC (“BP Healthcare”) and was also granted 38,697,318 Class B Units of BP Healthcare. In addition, we granted Dr. Payson an additional 3,830,365 Class B Units of BP Healthcare in connection with entering into a services agreement with him in November 2012, in order to retain his services following his retirement as our Chief Executive Officer. Following this offering, Dr. Payson’s BP Healthcare Units will remain outstanding. In connection with Mr. Figueroa’s appointment as our Chief Executive Officer in 2012, he purchased 1,000,000 Class A-2 Units of Apria Holdings LLC (“Holdings”) and was also granted 12,257,169 Class B Units of Holdings. In connection with joining our Board of Directors in 2011, Mr. Zafirovski purchased 1,000,000 Class A-2 Units of Holdings and was also granted 5,030,651 Class B Units of Holdings. All of their respective Class A-2 Units were fully vested when purchased and all of their respective Class B Units are now fully vested (other than Mr. Zafirovski’s 3,353,767 target-based vesting Class B Units, which expired unvested).
Termination, Clawback and Restrictive Covenants. With respect to the Class B Units granted to Dr. Payson in 2012, if his services as a director are terminated by the Company for “cause” (as defined in his applicable subscription agreement) or by Dr. Payson when grounds exist for a termination for cause, then all of such vested Class B Units will be forfeited. If Mr. Zafirovski’s service as director is (1) terminated by us for “cause” (as defined in the applicable subscription agreement) or he voluntarily resigns where grounds for cause exist or (2) he breaches any of the restrictive covenants set forth below, then we have the right to purchase all of his vested Class B Units at the lesser of fair market value thereof and cost, which means that such vested Class B Units will be effectively forfeited.
In certain circumstances the Company has the right to “clawback” and recover any gains Mr. Figueroa may have realized with respect to his Class B Units if he is terminated for “cause” (as such term was defined in his employment agreement) or he voluntarily resigns where grounds for cause exist and the Company notifies him of such fact within 30 days of his resignation. Similarly, the Company has the right to “clawback” and recover any gains Mr. Zafirovski may have realized with respect to his Class B Units if he violates any of the restrictive covenants described below or we discover after a termination of employment that grounds existed for “cause” (as defined in his applicable subscription agreement).
As a condition of receiving their Class B Units, each of Dr. Payson, Mr. Figueroa and Mr. Zafirovski agreed to certain restrictive covenants, including confidentiality of information, non-disparagement (in the case of Messrs. Figueroa and Zafirovski), non-solicitation and non-competition covenants. The confidentiality covenant has an indefinite term, the non-disparagement covenant has an indefinite term (24 months in the case of Mr. Figueroa), and the non-solicitation and non-competition covenants each have a term effective both during the term of the director’s term of service and for 12 (24 months in the case of Mr. Figueroa) and 24 months, respectively, following a termination of his services.
Conversion of Holdings Units. In connection with this offering, we expect that all Holdings Units held by our directors will be surrendered in exchange for vested shares of our common stock. The number of vested shares of common stock delivered will be determined in a manner intended to replicate the economic value associated with their Holdings Units, based upon the valuation derived from the initial public offering price.
132
Table of Contents
The following table sets forth the number and value of vested shares of our common stock that each of Messrs. Figueroa and Zafirovski will receive in exchange for their Holdings Units, based on the initial public offering price of $20.00 per share.
| Name |
Common Stock Received in Exchange for Vested Class A-2 Units |
Common Stock Received in Exchange for Vested Class B Units |
||||||||||||||
| (#) | ($) | (#) | ($) | |||||||||||||
| John G. Figueroa |
45,871 | $ | 917,420 | 562,132 | $ | 11,242,640 | ||||||||||
| Mike S. Zafirovski |
45,871 | $ | 917,420 | 76,904 | $ | 1,538,080 | ||||||||||
133
Table of Contents
Executive Compensation
Compensation Discussion and Analysis
Introduction. Our executive compensation plan is designed to attract and retain individuals qualified to manage and lead our Company and to also motivate them to contribute to the achievement of our financial goals and ultimately create and grow our equity value.
Our named executive officers for 2020 were:
| • | Daniel J. Starck, our Chief Executive Officer; |
| • | Debra L. Morris, our Executive Vice President and Chief Financial Officer; |
| • | Raoul Smyth, our Executive Vice President, General Counsel and Secretary; |
| • | Robert P. Walker, our Executive Vice President, Managed Care; and |
| • | Mark Litkovitz, our Executive Vice President, Chief Information Officer. |
Executive Compensation Objectives and Philosophy. Our primary executive compensation objectives are to:
| • | attract, retain and motivate leaders who are capable of advancing our mission and strategy and, ultimately, creating and maintaining our long-term equity value. Such leaders must engage in a collaborative approach and possess the ability to execute our strategy in an industry characterized by competitiveness and a challenging business environment; |
| • | reward senior management in a manner aligned with our financial performance; and |
| • | align senior management’s interests with our equity owners’ long-term interests through equity participation and ownership. |
To achieve our objectives, we have delivered executive compensation through a combination of the following components:
| • | Base salary; |
| • | Annual cash bonuses; |
| • | Long-term incentive compensation; |
| • | Broad-based employee benefits; and |
| • | Severance benefits. |
We provide competitive base salaries and other benefits, including severance benefits, to attract and retain senior management talent. We have also used annual cash incentive compensation and long-term cash and equity incentives to ensure a performance-based delivery of pay that aligns as closely as possible the rewards of our named executive officers with the long-term interests of our equity-owners while enhancing executive retention.
We anticipate that we will continue to review our executive compensation programs in connection with this offering and make such changes as are determined to be necessary or appropriate for our status as a public company. As we gain experience as a public company, we expect that the specific direction, emphasis and components of our executive compensation program will continue to evolve.
134
Table of Contents
Compensation Determination Process.
Role of Board and Management. Prior to this offering, executive compensation and related decisions have been made by the Board of Directors of our subsidiary, Apria Healthcare Group. In connection with this offering, our Board of Directors will establish a compensation committee that will assume responsibility for establishing, maintaining and administering our compensation and benefit policies. Except where the context requires otherwise, the terms “Board” or “Board of Directors” as used in this “Executive Compensation” section refer to the Board of Directors of Apria Healthcare Group.
Over the course of the year, Mr. Starck participates in discussions and deliberations with our Board of Directors regarding the determinations of annual cash incentive awards for our executives other than for himself. Specifically, he makes recommendations to our Board of Directors regarding the performance targets to be used under our annual executive bonus plan and the amounts of annual cash incentive awards and any discretionary bonus amounts to be made to our senior executives. Mr. Starck also makes recommendations to our Board of Directors regarding base salary adjustments for other executives based on their annual review of each executive’s performance. Our Board of Directors considers these recommendations and may exercise discretion in modifying them.
Employment Agreements. For retention purposes, we entered into an employment agreement with Mr. Starck. A full description of the material terms of Mr. Starck’s employment agreement is presented below in the narrative section following the “Grants of Plan-Based Awards in 2020” table.
Compensation Elements.
The following is a discussion and analysis of each component of our executive compensation program.
Base Salary. Base salary compensates executives for performing requirements of their positions and provides executives with a level of cash income predictability and stability with respect to a portion of their total compensation. Base salaries may be adjusted annually and, in certain circumstances, adjusted mid-year to deal with competitive pressures or changes in job responsibilities. In 2020, there were no changes to the base salaries of our named executive officers.
Bonuses.
2020 Annual Cash Incentive Compensation. Annual cash incentive awards are available to our named executive officers under our annual executive bonus plan in order to motivate our executive officers to achieve short-term performance goals and tie a portion of their cash compensation to performance.
Executive Bonus Plan. Under our Executive Bonus Plan for 2020 (the “2020 Bonus Plan”), each named executive officer who participated in the 2020 Bonus Plan was eligible to earn a cash incentive award based on achievement of performance targets for 2020. These performance targets were determined by our Board of Directors early in the year, after taking into consideration Mr. Starck’s recommendations and our budget for the year. The potential amount of the cash incentive award was based on a percentage of the executive officer’s base salary during 2020. The following table illustrates the 2020 potential target and maximum cash incentive awards expressed as a percentage of their base salaries for our named executive officers who were eligible to receive a 2020 bonus under the 2020 Bonus Plan:
| Target Bonus Opportunity (% of Base Salary) |
Maximum Bonus Opportunity (% of Base Salary) |
|||||||
| Daniel J. Starck |
100 | % | 200 | % | ||||
| Debra L. Morris |
100 | % | 150 | % | ||||
| Raoul Smyth |
75 | % | 125 | % | ||||
| Robert P. Walker |
100 | % | 150 | % | ||||
| Mark Litkovitz |
75 | % | 125 | % | ||||
135
Table of Contents
In 2020, the cash incentive award opportunities were based on an EBIT target which accounted for 60% of the total award opportunity and a free cash flow target, which accounted for 40% of the total award opportunity. The Board of Directors has reserved the ability to adjust the actual fiscal year results to exclude the effects of extraordinary, unusual or infrequently occurring events. For purposes of the 2020 Bonus Plan, EBIT is calculated as net income for the period before the impact of interest income, interest expense and income taxes. The EBIT target excludes expenses incurred in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York (See “Business—Legal Proceedings—Civil Investigative Demand Issued by the United States Attorney’s Office for the Southern District of New York”) and expenses associated with the preparation to be a public company. Free cash flow is calculated as net cash provided by operating activities excluding term loan interest payments less net cash used in investing activities. The free cash flow target excludes payments for expenses related to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York and payments for expenses associated with the preparation to be a public company.
Under our 2020 Bonus Plan, no cash incentive award will be made with respect to any performance metric unless our actual EBITDA less Capex is at least $63.7 million. For the 2020 Bonus Plan, EBITDA less Capex is calculated as EBITDA (as defined in the section entitled “Summary Historical Financial and Other Data”) less capital expenditures net of dispositions. For purposes of this metric “Capex” is defined as the value of the capital equipment received less the net book value of dispositions of capital equipment during the accounting period. The EBITDA less Capex target excludes expenses incurred in relation to a series of civil investigative demands from the United States Attorney’s Office for the Southern District of New York and expenses associated with the preparation to be a public company.
For 2020, the named executive officers’ target incentive opportunities were: $594,500 for Mr. Starck, $400,000 for Ms. Morris, $252,450 for Mr. Smyth, $290,700 for Mr. Walker and $240,975 for Mr. Litkovitz. We have not yet calculated our actual performance for 2020. We expect to do so, and determine the 2020 Bonus Plan awards earned by each of our named executive officers, in the first quarter of 2021.
Other Cash Bonuses. From time to time, we may award sign-on and discretionary bonuses. Sign-on bonuses are used only when necessary to attract highly skilled officers to the Company. Generally, they are used to incentivize candidates to leave their current employers or may be used to offset the loss of unvested compensation they may forfeit as a result of leaving their current employers.
Long-Term Cash Incentive Compensation.
2019 LTIP. In December 2019, our Board of Directors approved the Amended and Restated Apria Healthcare Group, Inc. Long-Term Incentive Plan (2019 – 2021 With Successive Annual Extension Options) (which will be assumed by the Company in connection with this offering and renamed the Second Amended and Restated Apria, Inc. Long-Term Incentive Plan (2019-2021 With Successive Annual Extension Options), the “2019 LTIP”) and awarded long-term cash incentive awards to our named executive officers under the 2019 LTIP for the performance period beginning January 1, 2019 and ending December 31, 2021. The purpose of the 2019 LTIP is to incentivize free cash flow improvement and the transformational changes needed to position the Company properly for the future.
In June 2020, our Board of Directors awarded additional long-term cash incentive awards to our named executive officers under the 2019 LTIP for the performance period beginning January 1, 2020 and ending December 31, 2022. Mr. Starck was awarded an award with a target value of $200,000 and Ms. Morris was awarded an award with a target value of $100,000. Messrs. Smyth, Walker and Litkovitz were each awarded an award with a target value of $75,000.
The 2019 LTIP award opportunities are based on the achievement of specified cumulative free cash flow targets during the applicable performance periods. Based on achievement of the cumulative performance goals a
136
Table of Contents
participant can earn between 50% of their target award amount for threshold achievement and 200% of their target award amount for maximum achievement. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards in 2020 Table—Terms of Long-Term Cash Incentive Awards—2019 LTIP Awards” below for a discussion of the vesting and other terms of these awards.
Following this offering, pursuant to the terms of the 2019 LTIP, the 2019 LTIP awards will be settled in shares of our common stock issued pursuant to our Omnibus Incentive Plan (as described below), with the number of shares determined by dividing the actual amount of each executive’s award by the volume-weighted average price of a share of our common stock over the 20 trading days following this offering, beginning on the first trading date after the date of this offering. The shares in respect of the 2019 LTIP awards will be delivered no later than March 15th of the year following the final year of the applicable performance period, in all cases subject to the applicable participant remaining employed on the applicable payment date. Notwithstanding the foregoing, in the event that an executive was terminated prior to this offering, payments will be made in a lump sum as soon as administratively practicable following this offering and if an executive is terminated without cause following this offering, any remaining payments will be made in a lump sum as soon as administratively practicable following such termination.
Long-Term Equity Incentive Compensation.
Holdings Units. Prior to this offering, the board of directors of Holdings, our parent entity prior to this offering, determined to grant to our management employees, including our named executive officers, long-term incentive awards that were designed to promote our interests by providing our management employees with the opportunity to acquire equity interests as an incentive for the recipient to remain in our service. The Holdings board of directors granted these long-term incentive awards to our named executive officers in the form of Class B Units and Class C Units of Holdings (together, “Holdings Units”).
Class B and Class C Units of Holdings are limited liability company profits interests having economic characteristics similar to SARs and representing the right to share in any increase in the equity value of Holdings that exceeds specified thresholds. For a Class B Unit, the threshold is the value of a Class A Unit of Holdings on the grant date which was generally $1.00. Therefore, a Class B Unit generally has a value at any given time equal to the value of a Class A Unit minus $1.00. For a Class C Unit, the threshold was $2.00. Therefore, a Class C Unit has a value at any given time equal to the value of a Class A Unit minus $2.00. Certain Class B Units have a higher threshold representing the estimated fair value of a Class A Unit on the applicable grant date.
Generally, the Class B Units of Holdings were divided into a time-vesting portion (2/3 of the Class B Units granted) and a performance-vesting portion (1/3 of the Class B Units granted). All Class C Units are performance-vesting. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards in 2020 Table—Terms of Equity Awards—Holdings Units” below for a discussion of the vesting and other terms of these equity units.
As a condition to receiving their Holdings Units, each named executive officer was required to enter into a subscription agreement with us and to become a party to the Holdings limited liability company agreement, as well as a securityholders agreement. These agreements generally governed the named executive officer’s rights with respect to the Holdings Units.
Class B Units. The Class B Units of Holdings granted to Ms. Morris and Messrs. Smyth, Walker and Litkovitz between 2009 and 2013 were divided into a time-vesting portion (2/3 of the Class B Units granted) and a performance-vesting portion (1/3 of the Class B Units granted). The Class B Units of Holdings granted to Mr. Starck in 2012 were 50% time-vesting and 50% performance-vesting. Mr. Starck’s Class B Units also contained a catchup provision that provided for the opportunity to participate in the increase in the equity value of Holdings prior to the date his Class B Units were granted.
137
Table of Contents
Class C Units. All Class C Units of Holdings granted to Ms. Morris and Messrs. Smyth, Walker and Litkovitz were performance-vesting and had the same vesting terms as the performance-vesting Class B Units. The performance-vesting Class B and Class C Units vested only if Blackstone received cash proceeds (not subject to any clawback, indemnity or similar contractual obligation) in respect of 25% (100% for Mr. Starck’s performance-vesting Class B Units) of its units equal to 200% of its aggregate capital contributions for such units.
All time-vesting Class B Units are fully vested and, on June 21, 2019, our Board of Directors determined it was appropriate to accelerate the vesting of all outstanding performance-vesting Class B Units. In addition, as a result of the 2020 Dividend described above, all outstanding Class C Units fully vested in accordance with their terms.
Call Option. If (1) the named executive officer’s employment is terminated by us for “cause” (as defined in the applicable subscription agreement) or the named executive officer voluntarily resigns where grounds for cause exist or (2) the named executive officers breaches any of the restrictive covenants set forth below, then we have the right to purchase all of the named executive officer’s vested Class B or Class C Units at the lesser of fair market value thereof and cost, which means that such vested Class B or Class C Units will be effectively forfeited.
Restrictive Covenants. As a condition of receiving the Class B and Class C Units, our named executive officers agreed to certain restrictive covenants, including confidentiality of information, noncompetition, non-solicitation and non-disparagement covenants. The confidentiality and non-disparagement covenants have an indefinite term, whereas the non-competition covenant has a term of one year and the non-solicitation covenant has a term of two years.
Clawback. The Company has the right to “clawback” and recover any gains the named executive officer may have realized with respect to his or her Class B Units or Class C Units if he or she violates any of the restrictive covenants described above or we discover after a termination of employment that grounds existed for “cause”.
Conversion of Holdings Units. In connection with this offering, we expect that all vested Holdings Units will be surrendered in exchange for vested shares of our common stock. The number of vested shares of common stock delivered will be determined in a manner intended to replicate the respective economic value associated with the Class B and Class C Units of Holdings, as applicable, based upon the valuation derived from the initial public offering price.
The following table sets forth the number and value of vested shares of our common stock that each of our named executive officers will receive in exchange for their vested Class B Units and vested Class C Units, in each case, based on the initial public offering price of $20.00 per share.
| Name | Common Stock Received in Exchange for Vested Class B Units |
Common Stock Received in Exchange for Vested Class C Units |
||||||||||||||
| (#) | ($) | (#) | ($) | |||||||||||||
| Daniel J. Starck |
360,426 | $ | 7,208,520 | — | $ | — | ||||||||||
| Debra. L. Morris |
39,931 | $ | 798,620 | 13,310 | $ | 266,200 | ||||||||||
| Raoul Smyth |
6,655 | $ | 133,100 | 2,218 | $ | 44,360 | ||||||||||
| Robert P. Walker |
26,621 | $ | 532,420 | 8,873 | $ | 177,460 | ||||||||||
| Mark Litkovitz |
10,648 | $ | 212,960 | 3,549 | $ | 70,980 | ||||||||||
Stock Appreciation Rights of Apria Healthcare Group. In 2015, our Board of Directors approved the Apria Healthcare Group Inc. 2015 Stock Plan (which will be assumed by Apria, Inc. and renamed the “Apria, Inc. 2015 Stock Plan,” the “2015 Stock Plan”) pursuant to which SARs were granted to employees of Apria Healthcare
138
Table of Contents
Group and its affiliates, including our named executive officers. All SARs granted under the 2015 Plan are time-vesting. See “Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards in 2020 Table—Terms of Equity Awards—Stock Appreciation Rights of Apria Healthcare Group” below for a discussion of the vesting and other terms of the SARs.
In 2020, in order to motivate continued performance and enhance retention, we granted additional SARs to each of the named executive officers in the following amounts: Mr. Starck, 3,450 SARs; Ms. Morris, 1,724 SARs; and Messrs. Smyth, Walker and Litkovitz were each granted 648 SARs, respectively.
2020 Dividend. As described above, on December 4, 2020, our Board of Directors declared, and on December 11, 2020 paid the 2020 Dividend, a $201.79 per share dividend to common stockholders. Under the terms of the 2015 Plan, an adjustment to the outstanding SARs was determined to be equitable and necessary in order to prevent the dilution or enlargement of benefits under the 2015 Plan. Holders of vested SARs were entitled to an immediate cash payment per SAR equal to the lower of (i) the per share dividend amount and (ii) an amount equal to the difference between $495.13 and the strike price of such vested SAR. Accordingly, in addition to certain strike price reductions, the named executive officers received the following cash payments with respect to their vested SARs: Mr. Starck, $2,198,152; Ms. Morris, $1,021,363; Mr. Smyth, $469,731; Mr. Walker, $708,174; and Mr. Litkovitz, $381,930. For holders of vested SARs who received the cash payment in clause (ii) above, the strike price of each such vested SAR was reduced by an amount equal to the per share dividend amount less the cash payment per vested SAR. With respect to outstanding unvested SARs, we reduced the per share exercise prices of such SARs by the per share dividend amount; provided, that with respect to unvested SARs for which such strike price reduction would have resulted in the strike price for such unvested SAR being less than $73.34, the strike price for such unvested SAR was reduced to $73.34 and the holder of each such unvested SAR is entitled to receive a cash payment equal to the difference between the per share dividend amount and the strike price reduction when such unvested SAR vests. Accordingly, in addition to certain strike price reductions, the named executive officers will be entitled to receive the following cash payments with respect to their unvested SARs: Mr. Starck, $116,325; Ms. Morris, $116,325; Mr. Smyth $29,068; Mr. Walker, $116,325; and Mr. Litkovitz, $29,068. In accordance with FASB ASC Topic 718 changes to the SARs associated with the 2020 Dividend were accounted for as modifications. As such, the fair value of each award immediately before and after the modifications were compared and no incremental stock compensation was recognized.
Chief Financial Officer RSU Award. In 2018, our Board of Directors determined it was appropriate to enter into a letter agreement with Ms. Morris that provides for an additional long-term equity incentive award in the form of restricted stock units (“RSUs”) upon the occurrence of an initial public offering or in connection with a change of control (each, a “Qualifying Transaction”). Accordingly, pursuant to the terms of the letter agreement, as may be amended from time to time, immediately prior to the closing of this offering Ms. Morris will be granted a number of RSUs equal to 0.4% of the amount by which the equity value of the Company exceeds negative $36.181 million. The RSUs will be granted under our Omnibus Incentive Plan. Subject to Ms. Morris’ continued employment through each applicable vesting date, the RSUs will vest as follows:
| • | 50% of the then-outstanding RSUs (rounded down to the nearest whole share) will vest upon the closing of the Qualifying Transaction (the “Tranche I RSUs”); |
| • | 25% of the then-outstanding RSUs (rounded down to the nearest whole share) will vest six months following the Qualifying Transaction; and |
| • | the remaining then-outstanding RSUs will vest on the first anniversary following the Qualifying Transaction. |
In the event of Ms. Morris’ termination of employment as a result of her death, by the Company without “cause” (as defined in her executive severance agreement) or by Ms. Morris for “good reason” (as defined in her executive severance agreement, except that “good reason” will not include a relocation of her current office location), all of the then-outstanding RSUs will immediately vest. If her employment is terminated for any other
139
Table of Contents
reason, all of the then-outstanding unvested RSUs will be immediately forfeited. The RSUs will be settled in either cash or shares of our common stock at Ms. Morris’ election. In the event that Ms. Morris elects for the RSUs to be settled in cash, the Company will deliver the cash in respect of the Tranche I RSUs upon the closing of the Qualifying Transaction and the cash in respect of the remaining RSUs will be delivered upon the applicable vesting dates. In the event Ms. Morris elects for the RSUs to be settled in shares of common stock, shares of common stock underlying vested RSUs will be delivered as soon as reasonably practicable, but in no event will any shares be delivered prior to the date of the first open trading window of the Company that is at least six months following the closing of the Qualifying Transaction. It is our expectation that the Tranche I RSUs will be settled in cash.
Broad-based Employee Benefits. We provide to all of our employees, including our named executive officers, broad-based benefits that are intended to attract and retain employees while providing them with retirement and health and welfare security. Broad-based employee benefits include:
| • | a 401(k) savings plan; |
| • | paid vacation, sick time and holidays; |
| • | medical, dental, vision, life and accident insurance, disability coverage, dependent care and healthcare flexible spending accounts; and |
| • | employee assistance program benefits. |
Under our 401(k) savings plan, we match a portion of the funds set aside by the employee. The maximum match available under the 401(k) savings plan is 25% of the first 8% of the employee’s eligible contributions per pay period. For participants who became an employee before January 1, 2007, all matching contributions by us vest 20% after one year of service, 40% after two years of service and are fully vested after three years of service. For participants who became an employee on or after January 1, 2007, all matching contributions by us become fully vested after three years of service. In addition, we may make non-elective contributions under the 401(k) savings plan, subject to statutory limitations imposed by Internal Revenue Code of 1986, as amended (the “Code”).
At no cost to the employee, we also provide $50,000 in basic life and accident insurance coverage insurance. The employee may also select supplemental life and accident insurance, for a premium to be paid by the employee.
Supplemental Executive Benefits. We provide a nonqualified deferred compensation plan, which is also intended to attract and retain executives. The nonqualified deferred compensation plan is intended to promote retention by providing to participants a long-term savings opportunity on a tax-efficient basis. Under the nonqualified deferred compensation plan, participants may defer certain portions of their salary, annual bonus and annual 401(k) savings plan refund offset amount, as more fully explained in the narrative following the Nonqualified Deferred Compensation for 2020 Table below. In 2020, the Company matched 100% of participant contributions to the nonqualified deferred compensation plan up to 4% of the participant’s salary. For plan year 2021, the Company will again match 100% of a participant’s salary contributions to the nonqualified deferred compensation plan up to 4% of the participant’s salary. Participants are 100% vested in matching contributions if they remain employed at the end of the applicable year.
Severance Arrangements and Non-Competition Agreements. Our Board of Directors believes that severance arrangements are necessary to attract and retain the talent necessary for our long-term success. Our Board of Directors views our severance arrangements as recruitment and retention devices that help secure the continued employment and dedication of our named executive officers, including when we are considering strategic alternatives.
Each of our named executive officers has a severance arrangement with us, either as part of their employment agreement or as a separate severance agreement. Under the terms of these severance arrangements,
140
Table of Contents
each of the named executive officers is entitled to severance benefits if he or she is terminated by us without cause or by him or her as a result of constructive termination or for good reason, as applicable. See “—Potential Payments Upon Termination or Change-in-Control—Severance Arrangements” below for descriptions of these arrangements.
In December 2020, in recognition of his service and contributions to the Company we entered into a letter agreement with Mr. Smyth governing the terms of his retirement, which agreement is described under “Potential Payments Upon Termination or Change-in-Control—Raoul Smyth Retirement Agreement” below.
Actions Take in Connection with This Offering
Post-IPO Long-Term Incentive Plan. In connection with this offering, our Board of Directors expects to adopt, and we expect our stockholders to approve, our Omnibus Incentive Plan, which will allow us to implement a new market-based long-term incentive program to align our executive compensation package with similarly situated public companies. See “—Equity Incentive Plans—Omnibus Incentive Plan” below for additional details.
141
Table of Contents
Summary Compensation Table
The following table provides summary information concerning compensation to or on behalf of our named executive officers for services rendered to us during the fiscal years indicated below.
| Name and Principal Position |
Year | Salary ($)(1) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($)(2) |
Non-Equity Incentive Plan Compensation ($)(3) |
Change in Pension Value and Nonqualified Deferred Compensation Earnings ($) |
All Other Compensation ($)(4) |
Total ($)(5) |
|||||||||||||||||||||||||||
| Daniel J. Starck, |
2020 | 594,500 | — | — | 399,200 | — | — | 16,552 | 1,010,252 | |||||||||||||||||||||||||||
| Chief Executive Officer |
2019 | 594,500 | — | 4,751,871 | 399,200 | 620,658 | — | — | 6,366,229 | |||||||||||||||||||||||||||
| Debra L. Morris, |
2020 | 400,000 | — | — | 199,484 | — | — | 20,875 | 620,359 | |||||||||||||||||||||||||||
| EVP CFO |
2019 | 400,000 | — | 350,966 | 199,600 | 408,800 | — | 43,517 | 1,402,883 | |||||||||||||||||||||||||||
| Raoul Smyth, |
2020 | 336,600 | — | — | 74,980 | — | — | 4,875 | 416,455 | |||||||||||||||||||||||||||
| EVP GC |
2019 | 336,600 | — | 58,494 | 74,980 | 259,855 | — | 13,175 | 743,104 | |||||||||||||||||||||||||||
| Robert P. Walker, |
2020 | 290,700 | — | — | 74,980 | — | — | 2,683 | 368,363 | |||||||||||||||||||||||||||
| EVP Managed Care |
2019 | 290,700 | — | 233,977 | 74,980 | 297,095 | — | 2,598 | 899,350 | |||||||||||||||||||||||||||
| Mark Litkovitz, EVP CIO |
2020 | 321,300 | — | — | 74,980 | — | — | 16,822 | 413,102 | |||||||||||||||||||||||||||
| (1) | Amounts included in this column reflect the named executive officer’s annual base salary earned during the fiscal year taking into account increases, if any, in base salary during the course of the year and are not reduced to reflect the named executive officer’s election, if any, to defer receipt of salary into our nonqualified deferred compensation plan. |
| (2) | Amounts included in this column reflect the aggregate grant date fair value of SARs granted in 2020, calculated in accordance with FASB ASC Topic 718, utilizing the assumptions discussed in Note 5 to our unaudited condensed consolidated financial statements for the nine months ended September 30, 2020 included elsewhere in this prospectus. |
| (3) | The amounts reported in the “Non-Equity Incentive Plan Compensation” column for 2020 are not calculable as of the date of this prospectus. 2020 Bonus Plan Awards, if any, are expected to be determined in the first quarter of 2021. See “—Compensation Discussion and Analysis—Compensation Elements—Bonuses—2020 Annual Cash Incentive Compensation.” |
| (4) | Amount reported for Mr. Starck reflects rent reimbursement for an apartment near the Company’s former headquarters and an associated tax gross-up. Amounts reported for the other named executive officers reflect Company matching contributions to our 401(k) plan and Company matching contributions to our nonqualified deferred compensation plan as follows: $16,000 for Ms. Morris and $12,852 for Mr. Litkovitz. |
| (5) | Total amounts reported do not include 2020 Bonus Plan Awards, if any, for the named executive officers, which are expected to be determined in the first quarter of 2021. |
142
Table of Contents
Grants of Plan-Based Awards in 2020
The following table provides supplemental information relating to grants of plan-based awards made to our named executive officers during 2020.
| Name |
Award Type |
Grant Date |
Estimated Future Payouts Under Non-Equity Incentive Plan Awards ($) |
All Other Option Awards: Number of Securities Underlying Options (#)(3) |
Exercise or Base Price of Option Awards ($/Sh)(3) |
Grant Date Fair Value of Stock and Option Awards ($)(4) |
||||||||||||||||||||||||
| Threshold | Target | Maximum | ||||||||||||||||||||||||||||
| D. Starck |
2020 Bonus Plan(1) | 118,900 | 594,500 | 1,189,000 | ||||||||||||||||||||||||||
| 2019 LTIP Award(2) | 100,000 | 200,000 | 400,000 | |||||||||||||||||||||||||||
| SARs | 5/12/2020 | 3,450 | 390.50 | 399,200 | ||||||||||||||||||||||||||
| D. Morris |
2020 Bonus Plan(1) | 80,000 | 400,000 | 600,000 | ||||||||||||||||||||||||||
| 2019 LTIP Award(2) | 50,000 | 100,000 | 200,000 | |||||||||||||||||||||||||||
| SARs | 5/12/2020 | 1,724 | 390.50 | 199,484 | ||||||||||||||||||||||||||
| R. Smyth |
2020 Bonus Plan(1) | 50,490 | 252,450 | 420,750 | ||||||||||||||||||||||||||
| 2019 LTIP Award(2) | 37,500 | 75,000 | 150,000 | |||||||||||||||||||||||||||
| SARs | 5/12/2020 | 648 | 390.50 | 74,980 | ||||||||||||||||||||||||||
| R. Walker |
2020 Bonus Plan(1) | 58,140 | 290,700 | 436,050 | ||||||||||||||||||||||||||
| 2019 LTIP Award(2) | 37,500 | 75,000 | 150,000 | |||||||||||||||||||||||||||
| SARs | 5/12/2020 | 648 | 390.50 | 74,980 | ||||||||||||||||||||||||||
| M. Litkovitz |
2020 Bonus Plan(1) | 48,195 | 240,975 | 401,625 | ||||||||||||||||||||||||||
| 2019 LTIP Award(2) | 37,500 | 75,000 | 150,000 | |||||||||||||||||||||||||||
| SARs | 5/12/2020 | 648 | 390.50 | 74,980 | ||||||||||||||||||||||||||
| (1) | Reflects possible payouts under our 2020 Bonus Plan. See “Compensation Discussion and Analysis—Compensation Elements—Bonuses—2020 Annual Cash Incentive Compensation” for a discussion of threshold, target and maximum cash incentive compensation payouts. |
| (2) | Reflects potential future payouts under our 2019 LTIP. See “Compensation Discussion and Analysis—Compensation Elements— Long-Term Cash Incentive Compensation.” |
| (3) | Reflects the number of SARs granted during 2020. The exercise price was determined by our Board of Directors to be the fair market value of our common stock as of the date of grant based on the most recent valuation prior to the date of grant. In connection with the 2020 Dividend, the exercise prices of these SARs were reduced to $188.71. See “—Compensation Discussion and Analysis—Long-Term Equity Incentive Compensation—2020 Dividend.” |
| (4) | Amounts reported reflect the grant date fair value of the SARs granted in 2020, calculated in accordance with FASB ASC Topic 718. |
Narrative Disclosure to Summary Compensation Table and Grants of Plan-Based Awards in 2020
Daniel J. Starck Employment Agreement.
We entered into an employment agreement, dated March 14, 2012, and amended on December 5, 2012, with Mr. Starck pursuant to which Mr. Starck serves as our Chief Executive Officer. Mr. Starck’s employment with us is on an “at-will” basis. Mr. Starck’s employment agreement contains the terms summarized below:
Compensation Arrangements.
| • | initial base salary at the annual rate of $580,000, subject to merit-based wage adjustments consistent with other senior executives under our wage administration policy; |
| • | target annual bonus award of 100% of base salary (and a maximum bonus award of 200% of base salary); |
| • | eligibility for equity award grants; |
| • | participation in our employee benefit plans; and |
| • | reimbursement of reasonable and customary business expenses. |
Termination Provisions. Generally, either party may terminate Mr. Starck’s employment agreement at any time, but Mr. Starck must provide 45 days advance written notice to Apria of his resignation. See “—Potential Payments Upon Termination or Change-in-Control—Severance Arrangements” below for a description of the payments and benefits Mr. Starck is entitled to in connection with a termination of his employment.
143
Table of Contents
Terms of Long-Term Cash Incentive Awards.
2019 LTIP Awards. Thirty-three and one-third percent of the awards vest on December 31st of the first year of the performance period and, subject to continued employment, an additional 8 1/3% will vest on the last day of each fiscal quarter thereafter such that 100% of the award will be vested on the last day of the performance period. See “—Potential Payments Upon Termination or Change-in-Control—2019 LTIP Awards” below for a description of the potential vesting that each of the named executive officers may be entitled to in connection with certain terminations of employment or a change of control.
Terms of Outstanding Equity Awards.
Stock Appreciation Rights of Apria Healthcare Group. The SARs granted to our named executive officers vest 20% on the first anniversary of the vesting start date, and then in increments equal to 5% of the initial grant on the last day of each succeeding three-month period thereafter for four years. All vested SARs will become exercisable in connection with this offering.
Upon the exercise of a SAR, we will pay to the participant an amount equal to the number of shares of common stock subject to the SAR that is being exercised multiplied by the excess, if any, of the fair market value of one share of common stock on the exercise date over the strike price, less an amount equal to any federal, state, local and non-U.S. income and employment taxes required to be withheld. Such amount may be in (w) cash, (x) shares of common stock valued at fair market value, (y) shares of common stock or units of capital stock of one of our majority-owned subsidiaries or (z) any combination thereof. Any fractional shares of common stock may be settled in cash, at our election. For the avoidance of doubt, it is our expectation that all SARs will generally be settled in shares of our common stock.
Any portion of an executive’s SARs that is vested upon termination of employment by us without “cause” (as defined in the 2015 Plan) and not due to the executive’s death or disability will generally remain exercisable during the period beginning on the date of a liquidity event, and ending on the earlier of (x) 60 days following the termination of employment and (y) the tenth anniversary of the date of grant. This period is shortened to 30 days if the executive resigns and no grounds for a termination by us for cause exists, and is extended to 12 months if employment is terminated due to death or disability. Vested SARs will immediately terminate if the executive’s employment is terminated by us for cause, if the executive resigns during or following the existence of grounds for a termination by us with cause or by the executive prior to the second anniversary of the vesting start date, if there is a restrictive covenant violation or if the executive engages in competitive activity. See “—Potential Payments Upon Termination or Change-in-Control—Equity Awards” below for a description of the potential vesting that each of the named executive officers may be entitled to in connection with a change of control.
Call Option. If (1) the named executive officer is terminated by us for cause or voluntarily resigns where grounds for cause exist or (2) the named executive officer voluntarily resigns (other than as a result of a “constructive termination” (as defined in the award agreement)) prior to the second anniversary of the vesting start date, then we have the right to purchase any or all of the shares acquired upon exercise of vested SARs for the lesser of (a) fair market value and (b) cost. If the named executive officer terminates employment voluntarily (other than as a result of a constructive termination) after the second anniversary of the vesting start date, then we have the right to purchase any or all of the shares acquired upon exercise of vested SARs for the fair market value.
Restrictive Covenants. As a condition of receiving the SARs, our named executive officers agreed to certain restrictive covenants, including confidentiality of information, noncompetition, non-solicitation and non-disparagement covenants. The confidentiality and non-disparagement covenants have an indefinite term, whereas the non-competition covenant has a term of one year and the non-solicitation covenant has a term of two years.
Clawback. The Company has the right to “clawback” and recover any gains the named executive officer may have realized with respect to his or her SARs if he or she is terminated for cause, violates any of the restrictive covenants described above or we discover after a termination of employment that grounds existed for cause.
144
Table of Contents
Treatment of SARs. In connection with this offering, it is expected that the SARs will remain outstanding and will continue to vest and settle pursuant to their original terms, but will be equitably adjusted to track the shares of our common stock rather than the shares of common stock of Apria Healthcare Group.
145
Table of Contents
Outstanding Equity Awards at 2020 Fiscal-Year End
The following table provides information regarding outstanding equity awards made to our named executive officers as of December 31, 2020.
| Name |
Grant Date | Time- Vesting Start Date |
Number of securities underlying unexercised options (#) exercisable(1) |
Number of securities underlying unexercised options (#) unexercisable(1) |
Option Exercise Price ($)(2) |
Option Expiration Date |
||||||||||||||||||
| D. Starck |
6/5/2015 | 5/21/2015 | 7,347 | $ | 293.33 | 6/5/2025 | ||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 1,837 | $ | 165.35 | 6/5/2025 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,904 | $ | 293.33 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,269 | $ | 165.35 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,057 | $ | 73.34 | 3/9/2027 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 863 | $ | 293.33 | 10/8/2029 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 2,587 | $ | 188.71 | 10/8/2029 | |||||||||||||||||||
| 5/12/2020 | 5/12/2020 | 3,450 | $ | 188.71 | 5/12/2030 | |||||||||||||||||||
| D. Morris |
2/20/2015 | 1/1/2014 | 997 | $ | 293.33 | 2/20/2025 | ||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 1,225 | $ | 293.33 | 6/5/2025 | |||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 306 | $ | 165.35 | 6/5/2025 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,904 | $ | 293.33 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,269 | $ | 165.35 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,057 | $ | 73.34 | 3/9/2027 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 431 | $ | 293.33 | 10/8/2029 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 1,294 | $ | 188.71 | 10/8/2029 | |||||||||||||||||||
| 5/12/2020 | 5/12/2020 | 1,724 | $ | 188.71 | 5/12/2030 | |||||||||||||||||||
| R. Smyth |
6/5/2015 | 5/21/2015 | 245 | $ | 293.33 | 6/5/2025 | ||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 61 | $ | 165.35 | 6/5/2025 | |||||||||||||||||||
| 8/13/2015 | 8/13/2015 | 1,148 | $ | 293.33 | 8/13/2025 | |||||||||||||||||||
| 8/13/2015 | 8/13/2015 | 383 | $ | 165.35 | 8/13/2025 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 476 | $ | 293.33 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 317 | $ | 165.35 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 264 | $ | 73.34 | 3/9/2027 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 162 | $ | 293.33 | 10/8/2029 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 486 | $ | 188.71 | 10/8/2029 | |||||||||||||||||||
| 5/12/2020 | 5/12/2020 | 648 | $ | 188.71 | 5/12/2030 | |||||||||||||||||||
| R. Walker |
2/20/2015 | 1/1/2014 | 499 | $ | 293.33 | 2/20/2025 | ||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 245 | $ | 293.33 | 6/5/2025 | |||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 61 | $ | 165.35 | 6/5/2025 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,904 | $ | 293.33 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,269 | $ | 165.35 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 1,057 | $ | 73.34 | 3/9/2027 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 162 | $ | 293.33 | 10/8/2029 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 486 | $ | 188.71 | 10/8/2029 | |||||||||||||||||||
| 5/12/2020 | 5/12/2020 | 648 | $ | 188.71 | 5/12/2030 | |||||||||||||||||||
| M. Litkovitz |
2/20/2015 | 1/1/2014 | 1,097 | $ | 293.33 | 2/20/2025 | ||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 245 | $ | 293.33 | 6/5/2025 | |||||||||||||||||||
| 6/5/2015 | 5/21/2015 | 61 | $ | 165.35 | 6/5/2025 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 476 | $ | 293.33 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 317 | $ | 165.35 | 3/9/2027 | |||||||||||||||||||
| 3/9/2017 | 3/1/2017 | 264 | $ | 73.34 | 3/9/2027 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 162 | $ | 293.33 | 10/8/2029 | |||||||||||||||||||
| 10/8/2019 | 8/15/2019 | 486 | $ | 188.71 | 10/8/2029 | |||||||||||||||||||
| 5/12/2020 | 5/12/2020 | 648 | $ | 188.71 | 5/12/2030 | |||||||||||||||||||
| (1) | Reflects time-vesting SARs that vest 20% on the first anniversary of the vesting start date, and then in increments equal to 5% of the initial grant on the last day of each succeeding three-month period thereafter for four years. Amounts reported as exercisable reflect time-vested SARs as of December 31, 2020 that were subject to a restriction on exercise that will lapse in connection with this offering. |
| (2) | In connection with the 2020 Dividend, the exercise prices for certain vested SARs and all unvested SARs were reduced. See “—Compensation Discussion and Analysis—Long-Term Equity Incentive Compensation—2020 Dividend.” |
146
Table of Contents
Option Exercises and Stock Vested in 2020
The following table provides information regarding the number of equity units that vested for our named executive officers during 2020.
| Name |
Option Awards | Stock Awards | ||||||||||||||
| # of Shares Acquired on Exercise (#) |
Value Realized on Exercise ($) |
# of Shares or Units Acquired on Vesting (#)(1) |
Value Realized on Vesting ($)(2) |
|||||||||||||
| D. Starck |
— | — | — | — | ||||||||||||
| D. Morris |
— | — | 290,230 | 266,200 | ||||||||||||
| R. Smyth |
— | — | 48,372 | 44,360 | ||||||||||||
| R. Walker |
— | — | 193,487 | 177,460 | ||||||||||||
| M. Litkovitz |
— | — | 77,395 | 70,980 | ||||||||||||
| (1) | Amounts reported for Ms. Morris and Messrs. Smyth, Walker and Litkovitz reflect the vesting of all their respective outstanding Class C units in connection with the 2020 Dividend. |
| (2) | Based on the appreciation in the equity value of Holdings since the date of grant through the date of vesting. The equity value of Holdings on the vesting date is based upon the assumed initial public offering price of $20.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus. |
Pension Benefits for 2020
We do not offer pension benefits to our named executive officers.
Nonqualified Deferred Compensation for 2020
The following table provides information regarding activity in our nonqualified deferred compensation plan for the named executive officers during 2020.
| Name |
Executive Contributions in Last FY(1) ($) |
Registrant Contributions in last FY(2) ($) |
Aggregate Earnings in Last FY ($)(3) |
Aggregate Withdrawals / Distributions ($) |
Aggregate Balance at Last FYE(4) ($) |
|||||||||||||||
| D. Starck |
— | — | — | — | — | |||||||||||||||
| D. Morris |
16,000 | 16,000 | 17,968 | — | 321,993 | |||||||||||||||
| R. Smyth |
— | — | 39,939 | — | 499,447 | |||||||||||||||
| R. Walker |
— | — | — | — | — | |||||||||||||||
| M. Litkovitz |
16,065 | 12,852 | 2,627 | 31,544 | ||||||||||||||||
| (1) | The amounts in the “Executive Contributions in Last FY” column were reported in the Summary Compensation Table in the Salary column. No amounts are reflected with respect to annual bonus deferrals since such amounts are not calculable until the 2020 Bonus Plan Awards are determined in the first quarter of 2021. |
| (2) | This column contains matching contributions by us with respect to the last fiscal year under our nonqualified deferred compensation plan and were reported in the Summary Compensation Table in the All Other Compensation column. |
| (3) | Because amounts included in this column do not include above-market or preferential earnings, none of these amounts are included under the “Change in Pension Value and Nonqualified Deferred Compensation Earnings” column of the Summary Compensation Table. |
| (4) | The following amounts reported in the “Aggregate Balance at Last Fiscal Year End” column were reported as compensation in the Summary Compensation Table for fiscal 2019: Ms. Morris $162,564; and Mr. Smyth $20,889. As noted above, the amounts reported in this column do not reflect annual bonus deferrals, which are not calculable as of the date of this prospectus. |
Under our nonqualified deferred compensation plan, the participants may defer up to 50% of their salary, up to 100% of their annual bonus and 100% of their annual 401(k) savings plan refund offset amount, the latter of
147
Table of Contents
which is an amount equal to their refund (if any) from our 401(k) savings plan. Returns on deferrals in an individual’s account under the nonqualified deferred compensation plan are credited or debited based on the performance of hypothetical measurement funds selected by the individual, which selection can be changed not less frequently than monthly, from a menu of options offered in connection with the plan. The hypothetical measurement funds that may be selected by the individual mirror the investment alternatives available in our 401(k) savings plan. In 2020, the Company matched 100% of participant contributions to the nonqualified deferred compensation plan up to 4% of the participant’s salary. Participants are 100% vested in matching contributions if they remain employed at the end of the applicable year.
An individual may choose to receive distributions in either a lump sum or in annual installments at death, retirement, or termination of employment with us or, in the event of an in-service distribution, at a date specified by the individual at least three years after the end of the year in which the deferral is made. An individual may also receive a distribution if he or she experiences an unforeseeable financial emergency, as defined in the nonqualified deferred compensation plan.
148
Table of Contents
Potential Payments Upon Termination or Change-in-Control
The following table describes the potential payments and benefits that would have been payable to our named executive officers under existing plans and contractual arrangements assuming (1) a termination of employment and (2) a change of control occurred on December 31, 2020.
The amounts shown in the table do not include payments and benefits to the extent they are provided generally to all salaried employees upon termination of employment and do not discriminate in scope, terms or operation in favor of the named executive officers. These include accrued salary, distributions of plan balances under our 401(k) savings plan and distributions of plan balances under the non-qualified deferred compensation plan. Furthermore, the amounts shown in the table do not include amounts that may have been payable to a named executive officer upon the sale or purchase of his or her vested equity pursuant to the exercise of put or call rights, which rights (other than certain limited call rights) expire in connection with this offering.
| Termination |
D. Starck ($) |
D. Morris ($) |
R. Smyth ($) |
R. Walker ($) |
M. Litkovitz ($) |
|||||||||||||||
| Termination—Without Cause or With Good Reason |
||||||||||||||||||||
| Cash Severance Payments(1) |
2,103,657 | 806,755 | 336,600 | 536,623 | 321,300 | |||||||||||||||
| Health Plan Continuation(2) |
47,552 | 14,584 | 1,092 | 23,776 | 14,584 | |||||||||||||||
| Total |
2,151,209 | 821,339 | 337,692 | 560,399 | 335,584 | |||||||||||||||
|
Change-in-Control—Without Termination |
||||||||||||||||||||
| Acceleration of SARs(3) |
3,932,525 | 2,360,213 | 787,590 | 1,379,388 | 787,590 | |||||||||||||||
| Acceleration of 2019 LTIP(4) |
600,000 | 300,000 | 225,000 | 225,000 | 225,000 | |||||||||||||||
| Total |
4,532,525 | 2,660,213 | 1,012,590 | 1,604,388 | 1,012,590 | |||||||||||||||
|
Change-in-Control—With Termination—Without Cause or With Good Reason |
||||||||||||||||||||
| Cash Severance Payments(1) |
2,103,657 | 806,755 | 336,600 | 536,623 | 321,300 | |||||||||||||||
| Health Continuation(2) |
47,552 | 14,584 | 1,092 | 23,776 | 14,584 | |||||||||||||||
| Acceleration of SARs(3) |
3,932,525 | 2,360,213 | 787,590 | 1,379,388 | 787,590 | |||||||||||||||
| Acceleration of 2019 LTIP(4) |
600,000 | 300,000 | 225,000 | 225,000 | 225,000 | |||||||||||||||
| Total |
6,683,734 | 3,481,552 | 1,350,282 | 2,164,787 | 1,348,474 | |||||||||||||||
| (1) | Cash severance payment includes the following: |
| • | Mr. Starck—two times the sum of (1) his annual base salary rate ($594,500) and (2) the average of his two most annual cash incentive awards ($457,328); |
| • | Ms. Morris—one times the sum of (1) her annual base salary rate ($400,000) and (2) the average of her two most annual cash incentive awards ($406,755); |
| • | Mr. Smyth—one times the sum of his annual base salary rate ($336,600); |
| • | Mr. Walker—one times the sum of (1) his annual base salary rate ($290,700) and (2) the average of his two most annual cash incentive awards ($245,923); and |
| • | Mr. Litkovitz—one times the sum of his annual base salary rate ($321,300). |
| (2) | Reflects the cost of providing the named executive officer with continued medical, dental and vision insurance under COBRA for a period of 24 months for Mr. Starck and for a period of twelve months for Ms. Morris and Messrs. Walker, Smyth and Litkovitz, assuming 2021 rates. |
| (3) | Upon a change in control during the executive’s continued employment, all unvested SARs will become fully vested. The amounts reported reflect this “spread” value, if any, for the unvested SARs, in each case representing the difference between the assumed initial public offering price of $20.00 per share, which is the midpoint of the price range set forth on the cover page of this prospectus, and the applicable exercise price, and includes the applicable cash payments associated with the 2020 Dividend that become payable upon vesting of the unvested SARs. |
149
Table of Contents
| (4) | The amounts reported reflect accelerated cash payments that would have become payable in connection with a change in control with respect to the vested portion of the 2019 LTIP awards assuming maximum achievement of the cumulative free cash flow goals through December 31, 2020. |
Severance Arrangements.
Mr. Starck. Pursuant to the terms of Mr. Starck’s employment agreement, either party may terminate Mr. Starck’s employment at any time. If Mr. Starck’s employment is terminated without “good reason” (as defined in his employment agreement) by Mr. Starck, then Mr. Starck must give us at least 45 days advance written notice.
If Mr. Starck’s employment is terminated by us without “cause” (as defined in his employment agreement) or terminated with good reason by Mr. Starck, he will be entitled to receive a severance payment equal in the aggregate to two times the sum of:
| • | his annual base salary at the rate in effect at the time of termination; |
| • | the average of his two most recent annual bonuses, if any, received prior to termination; and |
| • | an amount equal to the annual cost of providing him with a continuation of medical, dental and vision insurance under the Consolidated Omnibus Budget Reconciliation Act (“COBRA”). |
The amounts payable to Mr. Starck upon a termination of employment described above shall be paid in periodic installments over a period of 24 months provided that Mr. Starck executes a release of all claims to us. Such payments are also contingent upon Mr. Starck’s continued compliance with certain non-solicitation, non-disparagement and confidentiality covenants contained in his employment agreement. The confidentiality covenant has an indefinite term, whereas the non-solicitation covenant has a term of two years and the non-disparagement covenant has a term of one year.
Ms. Morris and Messrs. Smyth, Walker and Litkovitz. Pursuant to the terms of their respective executive severance agreements with us, either party may terminate the named executive officer’s employment at any time. If the named executive officer’s employment is terminated by us without “cause” (as defined in the executive severance agreement) or with “good reason” (as defined in the executive severance agreement) by the named executive officer, then the terminating party must give the other party at least 30 days advance written notice.
If the named executive officer’s employment is terminated by us without cause or terminated with good reason by the named executive officer, the named executive officer will be entitled to receive a severance payment equal in the aggregate to the sum of:
| • | the executive’s annual base salary at the rate in effect at the time of termination; |
| • | the average of the executive’s two most recent annual bonuses, if any, received prior to termination (other than for Messrs. Smyth and Litkovitz); and |
| • | an amount equal to the annual cost of providing the executive with a continuation of medical, dental and vision insurance under COBRA. |
The amounts payable to the named executive officer upon a termination of employment described above shall be paid in periodic installments over a period of 12 months provided that the named executive officer executes a release of all claims to us. Such payments are also contingent upon the named executive officer’s continued compliance with certain non-competition, non-solicitation and confidentiality covenants contained in their respective severance agreement. The confidentiality covenant has an indefinite term, whereas the non-competition and non-solicitation covenants each have a term of one year.
Raoul Smyth Retirement Agreement.
In December 2020, we entered into a letter agreement with Mr. Smyth governing the terms of his retirement. Pursuant to the agreement, Mr. Smyth is expected to retire at the close of business on March 31, 2021. Subject to
150
Table of Contents
his continued employment through December 31, 2020, or in the event of Mr. Smyth’s earlier termination of employment by us for reasons other than for cause or by Mr. Smyth for good reason (each as defined in Mr. Smyth’s severance agreement), Mr. Smyth will be entitled to (i) a full bonus in respect of 2020 based on actual performance and (ii) a pro-rata payment of awards under the 2019 LTIP with respect to the 2019-2021 and 2020-2022 performance periods as and when provided for retired employees under such plan. In addition, subject to his continued employment through the retirement date, or in the event of Mr. Smyth’s earlier termination of employment by us for reasons other than for cause or by Mr. Smyth for good reason on or following January 1, 2021, but prior to the retirement date, Mr. Smyth will be entitled to a pro-rata bonus in respect of 2021 based on actual performance.
Further, on the earlier to occur of (x) December 31, 2020 or (y) Mr. Smyth’s termination of employment by us for reasons other than cause or by Mr. Smyth for good reason, Mr. Smyth’s SARs will become fully vested; provided that no SARs will be exercisable until the date on which the SARs would have vested in accordance with the applicable award agreement had Mr. Smyth continued employment with us (the “Exercise Eligibility Date”). Additionally, we have agreed to extend the exercisability of Mr. Smyth’s vested SARs to the later of the 60th day following (i) a Liquidity Event (as defined in the 2015 Plan) and (ii) the Exercise Eligibility Date, but in no event later than the 10th anniversary of the original date of grant of the SARs. Further, in the event of a change of control prior to the 10th anniversary of the original grant date, Mr. Smyth’s unvested SARs will become vested and payable in accordance with the 2015 Plan; provided that with respect to the SARs granted to Mr. Smyth in 2017, the requirement for Mr. Smyth to continue employment for nine months following the change of control will be waived such that the SARs would be converted into a fixed payment right on the date of the change of control.
Long-Term Cash Incentive Awards.
2019 LTIP Awards. Upon a termination of employment by the Company for cause (as defined in the 2019 LTIP), the executive will forfeit all rights to receive any payment. Upon a voluntary termination of employment, other than a bona fide “Retirement” (as defined in the Company’s nonqualified deferred compensation plan), the named executive officer will forfeit all rights to receive any payment. Upon a termination of employment by the Company without cause, or if the executive retires as provided above, the vested percentage will be calculated assuming the executive remained employed through the end of the fiscal quarter of the executive’s termination and such executive will receive a payout in respect of the vested portion of his or her award, at such times and in the same form as the other participants in the 2019 LTIP. In the event of a change in control prior to the end of the applicable performance period, the vested portion of the 2019 LTIP award becomes payable in cash based on actual performance through the last day of the most recently completed fiscal quarter prior to the date of such change in control; provided that the vested portion shall increase by an additional 25% on the date of the change in control subject to the executive’s active employment through such date or the executive’s termination of employment prior to the change in control by the Company without Cause or due to the executive’s Retirement.
Equity Awards.
Stock Appreciation Rights of Apria Healthcare Group. In the event of a change in control during the executive’s continued employment, all unvested SARs will become fully vested. In addition, with respect to the SARs granted in 2017, such vested SARs would have been converted into a fixed cash payment right equal to the excess of (x) the fair market value of one share of common stock over (y) the strike price, with such amounts payable in respect of each such SAR on the earliest of (i) the regularly scheduled vesting date (but contingent on the executive’s continued employment through such date), (ii) the date that is nine months following the change of control (but contingent on the executive’s continued employment through such date), (iii) the date, on or following the change of control, of the executive’s termination by the Company without cause or by the executive as a result of a constructive termination and (iv) the tenth anniversary of the date of grant. In connection with this offering, our Board of Directors amended the terms of the SARs granted in 2017 to be consistent with the other SARs previously granted, such that in the event of a change in control during the executive’s continued employment, all unvested SARs granted in 2017 will become fully vested.
151
Table of Contents
Equity Incentive Plans
Omnibus Incentive Plan. In connection with this offering, our Board of Directors expects to adopt, and we expect our stockholders to approve, our Omnibus Incentive Plan prior to the completion of the offering. The term “Board of Directors” as used in this “Omnibus Incentive Plan” section refers to the Board of Directors of Apria, Inc.
Purpose. The purpose of our Omnibus Incentive Plan is to provide a means through which to attract and retain key personnel and to provide a means whereby our directors, officers, employees, consultants and advisors can acquire and maintain an equity interest in us, or be paid incentive compensation, including incentive compensation measured by reference to the value of our shares of common stock, thereby strengthening their commitment to our welfare and aligning their interests with those of our stockholders.
Eligibility. Eligible participants are any (i) individual employed by us or any of our subsidiaries; provided, however, that no employee covered by a collective bargaining agreement will be eligible to receive awards under our Omnibus Incentive Plan unless and to the extent that such eligibility is set forth in such collective bargaining agreement or in an agreement or instrument relating thereto; (ii) of our directors or officers or of any of our subsidiaries; or (iii) of our consultants or advisors or of any of our subsidiaries who may be offered securities registrable pursuant to a registration statement on Form S-8 under the Securities Act, who, in the cases of clauses (i) through (iii) above, has entered into an award agreement or who has received written notification from the Committee or its designee that they have been selected to participate in our Omnibus Incentive Plan.
Administration. Our Omnibus Incentive Plan will be administered by the compensation committee of our Board of Directors, or such other committee of our Board of Directors to which it has properly delegated power, or if no such committee or subcommittee exists, our Board of Directors (such administering body referred to herein, for purposes of this description of the Omnibus Incentive Plan, as the “Committee”). Except to the extent prohibited by applicable law, the Committee may allocate all or any portion of its responsibilities and powers to any one or more of its members and may delegate all or any part of its responsibilities and powers to any person or persons selected by it in accordance with the terms of our Omnibus Incentive Plan. The Committee is authorized to: (i) designate participants; (ii) determine the type or types of awards to be granted to a participant; (iii) determine the number of shares of common stock to be covered by, or with respect to which payments, rights or other matters are to be calculated in connection with, awards; (iv) determine the terms and conditions of any award; (v) determine whether, to what extent and under what circumstances awards may be settled in, or exercised for, cash, shares of common stock, other securities, other awards or other property, or canceled, forfeited or suspended and the method or methods by which awards may be settled, exercised, canceled, forfeited or suspended; (vi) determine whether, to what extent, and under what circumstances the delivery of cash, shares of common stock, other securities, other awards, or other property and other amounts payable with respect to an award will be deferred either automatically or at the election of the participant or of the Committee; (vii) interpret, administer, reconcile any inconsistency in, correct any defect in, and/or supply any omission in our Omnibus Incentive Plan and any instrument or agreement relating to, or award granted under, our Omnibus Incentive Plan; (viii) establish, amend, suspend, or waive any rules and regulations and appoint such agents as the Committee may deem appropriate for the proper administration of our Omnibus Incentive Plan; (ix) adopt sub-plans; and (x) make any other determination and take any other action that the Committee deems necessary or desirable for the administration of our Omnibus Incentive Plan. Unless otherwise expressly provided in our Omnibus Incentive Plan, all designations, determinations, interpretations and other decisions under or with respect to our Omnibus Incentive Plan or any award or any documents evidencing awards granted pursuant to our Omnibus Incentive Plan are within the sole discretion of the Committee, may be made at any time, and are final, conclusive and binding upon all persons or entities, including, without limitation, us, any participant, any holder or beneficiary of any award and any of our stockholders.
Awards Subject to our Omnibus Incentive Plan. Our Omnibus Incentive Plan provides that the total number of shares of common stock that may be issued under our Omnibus Incentive Plan is 3,912,324, or the “Absolute
152
Table of Contents
Share Limit”; provided, however, that the Absolute Share Limit shall be increased on the first day of each fiscal year beginning with the 2022 fiscal year in an amount equal to the least of (x) 978,081 shares of common stock, (y) 2.5% of the total number of shares of common stock outstanding on the last day of the immediately preceding fiscal year, and (z) a lower number of shares of common stock as determined by our Board of Directors. Of this amount, the maximum number of shares of common stock for which incentive stock options may be granted is 3,912,324; and during a single fiscal year, each non-employee director shall be granted a number of shares of common stock subject to awards, taken together with any cash fees paid to such non-employee director during the fiscal year, equal to a total value of $1,000,000 or such lower amount as determined by our Board of Directors. In addition to the Absolute Share Limit, an additional number of shares of common stock not to exceed the quotient of (x) $4,400,000, divided by (y) the volume-weighted average price of a share of common stock over the 20 trading days following the initial public offering of common stock, beginning on the first trading date after the date on which this offering occurs, may be issued in satisfaction of the Company’s obligations under the 2019 LTIP. Except for shares of common stock reserved for issuance in order to satisfy the Company’s obligations under the 2019 LTIP or “Substitute Awards” (as described below), to the extent that an award expires or is canceled, forfeited, terminated, settled in cash, or otherwise is settled without issuance to the participant of the full number of shares of common stock to which the award related, the unissued shares will again be available for grant under our Omnibus Incentive Plan. Shares of common stock withheld in payment of the exercise price, or taxes relating to an award, and shares equal to the number of shares surrendered in payment of any exercise price, or taxes relating to an award, shall be deemed to constitute shares not issued; provided, however, that such shares shall not become available for issuance if either: (i) the applicable shares are withheld or surrendered following the termination of our Omnibus Incentive Plan or (ii) at the time the applicable shares are withheld or surrendered, it would constitute a material revision of our Omnibus Incentive Plan subject to stockholder approval under any then-applicable rules of the national securities exchange on which the common stock is listed. No award may be granted under our Omnibus Incentive Plan after the tenth anniversary of the Effective Date (as defined in our Omnibus Incentive Plan), but awards granted before then may extend beyond that date. Awards may, in the sole discretion of the Committee, be granted in assumption of, or in substitution for, outstanding awards previously granted by an entity directly or indirectly acquired by us or with which we combine, or Substitute Awards, and such Substitute Awards will not be counted against the Absolute Share Limit, except that Substitute Awards intended to qualify as “incentive stock options” will count against the limit on incentive stock options described above.
Performance Conditions. All awards granted under our Omnibus Incentive Plan will vest and become exercisable in such manner and on such date or dates or upon such event or events as determined by the Committee, including, without limitation, attainment of Performance Conditions. For purposes of this proxy statement, “Performance Conditions” means specific levels of performance of Apria (and/or one or more of its subsidiaries, divisions or operational and/or business units, product lines, brands, business segments, administrative departments, or any combination of the foregoing), which may be determined in accordance with GAAP or on a non-GAAP basis on, without limitation, the following measures: (i) net earnings, net income (before or after taxes), or consolidated net income; (ii) basic or diluted earnings per share (before or after taxes); (iii) net revenue or net revenue growth; (iv) gross revenue or gross revenue growth, gross profit or gross profit growth; (v) net operating profit (before or after taxes); (vi) return measures (including, but not limited to, return on investment, assets, capital, employed capital, invested capital, equity, or sales); (vii) cash flow measures (including, but not limited to, operating cash flow, free cash flow, or cash flow return on capital), which may be but are not required to be measured on a per share basis; (viii) actual or adjusted earnings before or after interest, taxes, depreciation, and/or amortization (including EBIT and EBITDA); (ix) gross or net operating margins; (x) productivity ratios; (xi) share price (including, but not limited to, growth measures and total stockholder return); (xii) expense targets or cost reduction goals, general and administrative expense savings; (xiii) operating efficiency; (xiv) objective measures of customer/client satisfaction; (xv) working capital targets; (xvi) measures of economic value added or other ‘value creation’ metrics; (xvii) enterprise value; (xviii) sales; (xix) stockholder return; (xx) customer/client retention; (xxi) competitive market metrics; (xxii) employee retention; (xxiii) objective measures of personal targets, goals, or completion of projects (including, but not limited to, succession and hiring projects, completion of specific acquisitions, dispositions, reorganizations, or other corporate transactions or capital-raising transactions, expansions
153
Table of Contents
of specific business operations, and meeting divisional or project budgets); (xxiv) comparisons of continuing operations to other operations; (xxv) market share; (xxvi) cost of capital, debt leverage, year-end cash position or book value; (xxvii) strategic objectives; or (xxviii) any combination of the foregoing. Any one or more of the aforementioned performance criteria may be stated as a percentage of another performance criteria, or used on an absolute or relative basis to measure the performance of one or more of Apria or its subsidiaries as a whole or any divisions or operational and/or business units, product lines, brands, business segments, or administrative departments of Apria and/or one or more of its subsidiaries or any combination thereof, as the Committee may deem appropriate, or any of the above performance criteria may be compared to the performance of a selected group of comparison companies, or a published or special index that the Committee, in its sole discretion, deems appropriate, or as compared to various stock market indices.
Options. Under our Omnibus Incentive Plan, the Committee may grant non-qualified stock options and incentive stock options with terms and conditions determined by the Committee that are not inconsistent with our Omnibus Incentive Plan; provided, that all stock options granted under our Omnibus Incentive Plan are required to have a per share exercise price that is not less than 100% of the fair market value of our shares of common stock underlying such stock options on the date such stock options are granted (other than in the case of options that are Substitute Awards), and all stock options that are intended to qualify as incentive stock options must be granted pursuant to an award agreement expressly stating that the options are intended to qualify as incentive stock options, and will be subject to the terms and conditions that comply with the rules as may be prescribed by Section 422 of the Code. The maximum term for stock options granted under our Omnibus Incentive Plan will be ten years from the initial date of grant, or with respect to any stock options intended to qualify as incentive stock options, such shorter period as prescribed by Section 422 of the Code. However, if a non-qualified stock option would expire at a time when trading of our shares of common stock is prohibited by our insider trading policy (or “blackout period” imposed by us), the term will automatically be extended to the 30th day following the end of such period. The purchase price for the shares of common stock as to which a stock option is exercised may be paid to us, to the extent permitted by law (i) in cash, check, cash equivalent and/or shares of common stock valued at the fair market value at the time the option is exercised; provided, that such shares of common stock are not subject to any pledge or other security interest and have been held by the participant for at least six months (or such other period as established from time to time by the Committee in order to avoid adverse accounting treatment applying generally accepted accounting principles or (ii) by such other method as the Committee may permit in its sole discretion, including, without limitation: (a) in other property having a fair market value on the date of exercise equal to the exercise price, (b) if there is a public market for the shares of common stock at such time, by means of a broker-assisted “cashless exercise” pursuant to which we are delivered (including telephonically to the extent permitted by the Committee) a copy of irrevocable instructions to a stockbroker to sell the shares of common stock otherwise issuable upon the exercise of the option and to deliver promptly to us an amount equal to the exercise price or (c) a “net exercise” procedure effected by withholding the minimum number of shares of common stock otherwise issuable in respect of an option that is needed to pay the exercise price. Any fractional shares of common stock shall be settled in cash.
Stock Appreciation Rights. The Committee may grant SARs under our Omnibus Incentive Plan, with terms and conditions determined by the Committee that are not inconsistent with our Omnibus Incentive Plan. The Committee may also award SARs independent of any option. The maximum term for SARs granted under our Omnibus Incentive Plan will be ten years from the date of grant. Generally, each SAR will entitle the participant upon exercise to an amount (in cash, shares of common stock or a combination of cash and shares, as determined by the Committee) equal to the product of (i) the excess of (a) the fair market value on the exercise date of one share of common stock over (b) the strike price per share of common stock covered by the SAR, times (ii) the number of shares of common stock covered by the SAR, less any taxes required to be withheld. The strike price per share of common stock covered by a SAR will be determined by the Committee at the time of grant but in no event may such amount be less than 100% of the fair market value of an share of common stock on the date the SAR is granted (other than in the case of SARs granted in substitution of previously granted awards).
154
Table of Contents
Restricted Stock and Restricted Stock Units. The Committee may grant restricted shares of our shares of common stock or RSUs, representing the right to receive, upon vesting and the expiration of any applicable restricted period, one share of common stock for each RSU, or, in the sole discretion of the Committee, the cash value thereof (or any combination thereof). As to restricted shares of our shares of common stock, subject to the other provisions of our Omnibus Incentive Plan, the holder will generally have the rights and privileges of a stockholder as to such restricted shares of common stock, including, without limitation, the right to vote such restricted shares of common stock.
Other Equity-Based Awards and Other Cash-Based Awards. The Committee may grant other equity-based or cash-based awards under our Omnibus Incentive Plan, with terms and conditions determined by the Committee that are not inconsistent with our Omnibus Incentive Plan.
Effect of Certain Events on Our Omnibus Incentive Plan and Awards. In the event of (i) any dividend (other than regular cash dividends) or other distribution (whether in the form of cash, shares of common stock, other securities or other property), recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, split-off, spin-off, combination, repurchase or exchange of shares of common stock or other securities, issuance of warrants or other rights to acquire shares of common stock or other securities, or other similar corporate transaction or event that affects the shares of common stock (including a “Change in Control,” as defined in our Omnibus Incentive Plan); or (ii) unusual or nonrecurring events affecting us, including changes in applicable rules, rulings, regulations, or other requirements, that the Committee determines, in its sole discretion, could result in substantial dilution or enlargement of the rights intended to be granted to, or available for, participants (any event in (i) or (ii), an “Adjustment Event”), the Committee will, in respect of any such Adjustment Event, make such proportionate substitution or adjustment, if any, as it deems equitable, to any or all of (a) the Absolute Share Limit, or any other limit applicable under our Omnibus Incentive Plan with respect to the number of awards which may be granted thereunder; (b) the number of our shares of common stock or other of our securities (or number and kind of other securities or other property) which may be issued in respect of awards or with respect to which awards may be granted under our Omnibus Incentive Plan or any sub-plan; and (c) the terms of any outstanding award, including, without limitation, (x) the number of our shares of common stock or other of our securities (or number and kind of other securities or other property) subject to outstanding awards or to which outstanding awards relate; (y) the exercise price or strike price with respect to any award; or (z) any applicable performance measures; provided, that in the case of any “equity restructuring,” (within the meaning of the FASB ASC Topic 718 (or any successor pronouncement thereto)) the Committee will make an equitable or proportionate adjustment to outstanding awards to reflect such equity restructuring. In connection with any Adjustment Event, the Committee may, in its sole discretion, provide for any one or more of the following: (i) substitution or assumption of awards, acceleration of the exercisability of, lapse of restrictions on, or termination of, awards or a period of time for participants to exercise outstanding awards prior to the occurrence of such event (and any such award not so exercised will terminate upon the occurrence of such event); and (ii) subject to any limitations or reductions as may be necessary to comply with Section 409A of the Code, cancellation of any one or more outstanding awards and payment to the holders of such awards that are vested as of such cancellation (including, without limitation, any awards that would vest as a result of the occurrence of such event but for such cancellation or for which vesting is accelerated by the Committee in connection with such event) the value of such awards, if any, as determined by the Committee (which value, if applicable, may be based upon the price per share of common stock received or to be received by other holders of our shares of common stock in such event), including, without limitation, in the case of stock options and SARs, a cash payment equal to the excess, if any, of the fair market value of the shares of common stock subject to the option or SAR over the aggregate exercise price or strike price thereof, or, in the case of restricted stock, RSUs, or other equity-based awards that are not vested as of such cancellation, a cash payment or equity subject to deferred vesting and delivery consistent with the vesting restrictions applicable to such award prior to cancellation of the underlying shares in respect thereof.
Nontransferability of Awards. No award will be permitted to be assigned, alienated, pledged, attached, sold or otherwise transferred or encumbered by a participant other than by will or by the laws of descent and
155
Table of Contents
distribution and any such purported assignment, alienation, pledge, attachment, sale, transfer or encumbrance will be void and unenforceable against us or any of our subsidiaries. However, the Committee may, in its sole discretion, permit awards (other than incentive stock options) to be transferred, including transfers to a participant’s family members, any trust established solely for the benefit of a participant or such participant’s family members, any partnership or limited liability company of which a participant, or such participant and such participant’s family members, are the sole member(s), and a beneficiary to whom donations are eligible to be treated as “charitable contributions” for tax purposes.
Amendment and Termination. Our Board of Directors may amend, alter, suspend, discontinue or terminate our Omnibus Incentive Plan or any portion thereof at any time; provided, that no such amendment, alteration, suspension, discontinuance or termination may be made without stockholder approval if (i) such approval is required under applicable law; (ii) it would materially increase the number of securities which may be issued under our Omnibus Incentive Plan (except for adjustments in connection with certain corporate events); or (iii) it would materially modify the requirements for participation in our Omnibus Incentive Plan; provided, further, that any such amendment, alteration, suspension, discontinuance or termination that would materially and adversely affect the rights of any participant or any holder or beneficiary of any award will not to that extent be effective without such individual’s consent.
The Committee may, to the extent consistent with the terms of any applicable award agreement, waive any conditions or rights under, amend any terms of, or alter, suspend, discontinue, cancel or terminate, any award
granted or the associated award agreement, prospectively or retroactively (including after a Termination); provided, that, except as otherwise permitted in our Omnibus Incentive Plan, any such waiver, amendment, alteration, suspension, discontinuance, cancellation or termination that would materially and adversely affect the rights of any participant with respect to such award will not to that extent be effective without such individual’s consent; provided, further, that without stockholder approval, except as otherwise permitted in our Omnibus Incentive Plan, (i) no amendment or modification may reduce the exercise price of any option or the strike price of any SAR; (ii) the Committee may not cancel any outstanding option or SAR and replace it with a new option or SAR (with a lower exercise price or strike price, as the case may be) or other award or cash payment that is greater than the value of the cancelled option or SAR; and (iii) the Committee may not take any other action which is considered a “repricing” for purposes of the stockholder approval rules of any securities exchange or inter-dealer quotation system on which our securities are listed or quoted.
Dividends and Dividend Equivalents. The Committee in its sole discretion may provide as part of an award dividends or dividend equivalents, on such terms and conditions as may be determined by the Committee in its sole discretion. Any dividends payable in respect of restricted stock awards that remain subject to vesting conditions shall be retained by the Company and delivered to the participant within 15 days following the date on which such restrictions on such restricted stock awards lapse and, if such restricted stock is forfeited, the participant shall have no right to such dividends. Dividends attributable to RSUs shall be distributed to the participant in cash or, in the sole discretion of the Committee, in shares of common stock having a fair market value equal to the amount of such dividends, upon the settlement of the RSUs and, if such RSUs are forfeited, the participant shall have no right to such dividends.
Clawback/Repayment. All awards are subject to reduction, cancellation, forfeiture or recoupment to the extent necessary to comply with (i) any clawback, forfeiture or other similar policy adopted by our Board of Directors or the Committee and as in effect from time to time and (ii) applicable law. To the extent that a participant receives any amount in excess of the amount that the participant should otherwise have received under the terms of the award for any reason (including, without limitation, by reason of a financial restatement, mistake in calculations or other administrative error), the participant will be required to repay us any such excess amount.
Detrimental Activity. If a participant has engaged in any detrimental activity, as defined in our Omnibus Incentive Plan, as determined by the Committee, the Committee may, in its sole discretion, provide for one or
156
Table of Contents
more of the following: (i) cancellation of any or all of such participant’s outstanding awards or (ii) forfeiture and repayment to us on any gain realized on the vesting, exercise or settlement of any awards previously granted to such participant.
Apria, Healthcare Group 2015 Stock Plan. On February 20, 2015, we adopted the Apria Healthcare Group Inc. 2015 Stock Plan, which we amended effective as of March 9, 2017, referred to herein as the 2015 Plan. In connection with the pre-IPO reorganization transactions, the 2015 Plan will be assumed by Apria, Inc. and renamed the “Apria, Inc. 2015 Stock Plan.”
Following this offering and in connection with the assumption and equitable adjustment of the 2015 Plan by Apria, Inc., the number of shares of Apria, Inc. common stock subject to the 2015 Plan will be 3,766,228, which is the total number of outstanding SARs granted under the 2015 Plan prior to the offering which will remain outstanding in accordance with the terms of the 2015 Plan. No new equity-based awards will be granted under the 2015 Plan. New equity-based awards will be granted under the Omnibus Incentive Plan, which we intend to adopt in connection with this offering. The following description sets forth the material terms of the 2015 Plan, which is incorporated herein by reference.
Purpose. The purpose of the 2015 Plan is to provide a means through which Apria Healthcare Group and its affiliates may attract and retain key personnel and to provide a means whereby directors, officers, employees, consultants and advisors of Apria Healthcare Group and its affiliates can acquire and maintain an equity interest in Apria Healthcare Group, or be paid incentive compensation, including incentive compensation measured by reference to the value of shares of common stock, thereby strengthening their commitment to the welfare of Apria Healthcare Group and its affiliates and aligning their interests with those of Apria Healthcare Group’s stockholders.
Awards. Awards granted under the 2015 Plan (“Awards”) may include grants of SARs, phantom stock units and other stock-based awards.
Eligibility. Awards may be granted to employees, directors, officers, consultants or advisors of Apria Healthcare Group or its affiliates who are selected by the compensation committee (for purposes of this description of the 2015 Plan, the “Committee”) of the board of directors of Apria Healthcare Group (for purposes of this description of the 2015 Plan, the “Board”) or a sub-committee thereof or such other committee thereof to which the Board has delegated power to act under or pursuant to the provisions of 2015 Plan and if no such committee has been created, the Board to participate in the 2015 Plan.
Administration. The 2015 Plan is administered by the Committee, which may delegate its duties and powers in whole or in part to any sub-committee or to any employee or group of employees of Apria Healthcare Group or an affiliate; provided that such delegation and grants are consistent with applicable law and guidelines established by the Board from time to time. The Committee is authorized to correct any defect, supply any omission or reconcile any inconsistency in the 2015 Plan or any award agreement in the manner and to the extent the Committee deems necessary or desirable without the consent of any participant. Any decision of the Committee in the interpretation and administration of the 2015 Plan, as described herein, lies within its sole and absolute discretion and will be final, conclusive and binding on all parties concerned (including, but not limited to, participants and their beneficiaries or successors). The Committee has the full power and authority in its sole discretion to make any determinations that it deems necessary or desirable for the administration of the 2015 Plan. In particular, the Committee has the authority in its sole discretion to exercise all of the powers granted to it under the 2015 Plan; to construe and interpret the 2015 Plan and any award agreement; to amend the 2015 Plan to reflect changes in applicable law; to grant Awards and determine who will receive Awards, when such Awards will be granted, and establish the terms and conditions of such Awards consistent with the provisions of the 2015 Plan, including to determine the number of shares to be covered by each such Award so granted; to waive any such terms and conditions at any time (including, without limitation, accelerating or waiving any vesting conditions); to establish, amend and rescind any rules and regulations relating to the
157
Table of Contents
2015 Plan; and to make any other determinations that it deems necessary or desirable for the administration of the 2015 Plan.
Awards may, in the discretion of the Committee, be made under the 2015 Plan in assumption of, or in substitution for, outstanding awards previously granted by Apria Healthcare Group or its affiliates or a company acquired by Apria Healthcare Group or with which Apria Healthcare Group combines. The number of shares of Apria Healthcare Group stock underlying such substitute awards will not be counted against the aggregate number of shares of Apria Healthcare Group stock available for Awards under the 2015 Plan.
Stock Appreciation Rights. The Committee may grant SARs. The strike price will not be less than 100% of the Fair Market Value (as defined in the 2015 Plan) of a share of Apria Healthcare Group common stock on the date of grant. SARs granted under the 2015 Plan are exercisable at such time and upon such terms and conditions as determined by the Committee, but in no event are SARs exercisable more than ten years after the date it is granted.
SARs may be exercised by delivery of written or electronic notice of exercise to Apria Healthcare Group in accordance with the terms of the Award, specifying the number of SARs to be exercised and the date on which such SARs were awarded.
Upon the exercise of a SAR, Apria Healthcare Group Inc. shall pay to the participant an amount equal to the number of shares of common stock subject to the SAR that is being exercised multiplied by the excess, if any, of the Fair Market Value of one (1) share of common stock on the exercise date over the strike price, less an amount equal to any federal, state, local and non-U.S. income and employment taxes required to be withheld. As determined by the Committee, Apria Healthcare Group shall pay such amount in (x) cash, (y) shares of common stock valued at Fair Market Value or (z) any combination thereof. Any fractional shares of common stock may be settled in cash, at the Committee’s election. For the avoidance of doubt, it is the expectation of the Committee that all SARs will generally be settled in shares of common stock.
Phantom Stock Units. The Committee may grant phantom stock units. Phantom stock units granted under the 2015 Plan are subject to restrictions or, as applicable, a period of time within which performance is measured for purposes of determining whether an Award has been earned (the “Restricted Period”). The Restricted Period with respect to phantom stock units shall lapse in such manner and on such date or dates determined by the Committee, and the Committee shall determine the treatment of the unvested portion of phantom stock units upon termination of employment or service of the participant granted the applicable Award.
Unless otherwise provided by the Committee in an Award agreement, upon the expiration of the Restricted Period with respect to any outstanding phantom stock units, Apria Healthcare Group shall deliver to the participant, or his or her beneficiary, without charge, one (1) share of common stock (or other securities or other property, as applicable) for each such outstanding phantom stock unit; provided, however, that the Committee may, in its sole discretion, elect to (i) pay cash or part cash and part common stock in lieu of delivering only shares of common stock in respect of such phantom stock units or (ii) defer the delivery of shares of common stock (or cash or part common stock and part cash, as the case may be) beyond the expiration of the Restricted Period if such extension would not cause adverse tax consequences under Section 409A of the Code. If a cash payment is made in lieu of delivering shares of common stock, the amount of such payment shall be equal to the Fair Market Value of the shares of common stock as of the date on which the Restricted Period lapsed with respect to such phantom stock units. To the extent provided in an Award agreement, the holder of outstanding phantom stock units shall be entitled to be credited with dividend equivalent payments (upon the payment by Apria Healthcare Group of dividends on shares of common stock) either in cash or, at the sole discretion of the Committee, in shares of common stock having a Fair Market Value equal to the amount of such dividends (and interest may, at the sole discretion of the Committee, be credited on the amount of cash dividend equivalents at a rate and subject to such terms as determined by the Committee), which accumulated dividend equivalents (and interest thereon, if applicable) shall be payable at the same time as the underlying phantom stock units are settled
158
Table of Contents
following the release of restrictions on such phantom stock units, and, if such phantom stock units are forfeited, the participant shall have no right to such dividend equivalent payments.
Other Stock-Based Awards. The Committee, in its sole discretion, may grant Awards at a future date, or other Awards with respect to the value of shares of Apria Healthcare Group stock under the 2015 Plan, alone or in tandem with other Awards, in such amounts as the Committee shall from time to time in its sole discretion determine. Such other stock-based awards will be evidenced by an Award agreement, and subject to such conditions not inconsistent with the 2015 Plan as may be reflected in the applicable Award agreement.
Changes in Capital Structure and Similar Events. In the event of either (1) any dividend or other distribution, recapitalization, stock split, reverse stock split, reorganization, merger, consolidation, split-up, split-off, spin-off, combination, repurchase or exchange of shares of common stock or other securities of Apria Healthcare Group, issuance of warrants or other rights to acquire shares of common stock or other securities of Apria Healthcare Group, or other similar corporate transaction or event (including, without limitation, a change of control) that affects the shares of common stock, or (2) unusual or nonrecurring events (including, without limitation, a change of control) affecting Apria Healthcare Group or any affiliate, or the financial statements of Apria Healthcare Group or any affiliate, or changes in applicable rules, rulings, regulations or other requirements of any governmental body or securities exchange or inter-dealer quotation system, accounting principles or law, the Committee will adjust the 2015 Plan and outstanding Awards as it deems necessary, in good faith, to prevent dilution or enlargement of rights immediately resulting from such event or transaction (or in order to preserve the economic value of the outstanding Awards and the value that may be delivered pursuant to outstanding Awards under the 2015 Plan) with such adjustments to be made in such manner as the Committee may determine in its sole discretion and without liability to any person will make such substitution or adjustment, if any, as it deems to be equitable, as to (i) the number of shares of Apria Healthcare Group stock or other securities of Apria Healthcare Group with respect to which Awards have or may be granted under the 2015 Plan, (ii) the terms of any outstanding Award, including (A) the number of shares of Apria Healthcare Group stock or other securities of Apria Healthcare Group subject to outstanding Awards or to which outstanding Awards relate and (B) the strike price of any SAR and/or (iii) any applicable performance measures.
The Committee may provide for a substitution or assumption of Awards (or awards of an acquiring company), accelerate the exercisability of, lapse of restrictions on, or termination of, Awards or providing for a period of time for participants to exercise outstanding Awards prior to the occurrence of such event (and any such Award not so exercised shall terminate upon the occurrence of such event); and cancelling any one or more outstanding Awards and causing to be paid to the holders holding vested Awards (including any Awards that would vest as a result of the occurrence of such event but for such cancellation) the value of such Awards, if any, as determined by the Committee (which if applicable may be based upon the price per share of common stock received or to be received by other stockholders of Apria Healthcare Group in such event), including without limitation, in the case of an outstanding SAR, a cash payment in an amount equal to the excess, if any, of the Fair Market Value (as of a date specified by the Committee) of the shares of common stock subject to such SAR over the aggregate strike price of such SAR, respectively (it being understood that, in such event, any SAR having a per share strike price equal to, or in excess of, the Fair Market Value of a share of common stock subject thereto may be canceled and terminated without any payment or consideration therefor); provided, however, that in the case of any “equity restructuring” (within the meaning of the FASB Accounting Standards Codification Topic 718 (or any successor pronouncement thereto)), the Committee shall make an equitable or proportionate adjustment to outstanding Awards to reflect such equity restructuring.
Amendment and Termination. The Board may amend, alter, suspend, discontinue or terminate the 2015 Plan, but not without the approval of the shareholders of Apria Healthcare Group if (i) such approval is necessary to comply with any regulatory requirement applicable to the 2015 Plan (including, without limitation, as necessary to comply with any rules or regulations of any securities exchange or inter-dealer quotation system on which the securities of Apria Healthcare Group may be listed or quoted) or for changes in GAAP to new accounting standards or (ii) after a Public Offering (as defined in the 2015 Plan), (A) it would materially increase the number
159
Table of Contents
of securities which may be issued under the 2015 Plan or (B) it would materially modify the requirements for participation in the 2015 Plan, except as may be permitted as described under the section “Changes in Capital Structure and Similar Events” above.
Clawback/Forfeiture. Certain award agreements issued pursuant to the 2015 Plan contain “clawback” provisions, as described under the section “Terms of Equity Awards” above.
160
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PERSON TRANSACTIONS
The agreements described in this section, or forms of such agreements as they will be in effect at the time of this offering, are filed as exhibits to the registration statement of which this prospectus forms a part, and the following descriptions are qualified by reference thereto.
Stockholders Agreement
In connection with this offering, we intend to enter into a stockholders’ agreement with our Sponsor. This agreement will require us to nominate a number of individuals designated by our Sponsor for election as our directors at any meeting of our stockholders, each a “Sponsor Director,” such that, upon the election of each such individual and each other individual nominated by or at the direction of our board of directors or a duly-authorized committee of the board, as a director of our company, the number of Sponsor Directors serving as directors of our company will be equal to: (1) if our pre-IPO owners and their affiliates together continue to beneficially own at least 50% of the outstanding shares of our common stock (and any securities convertible into, or exchangeable or exercisable for, such shares) entitled to vote generally in the election of our directors as of the record date for such meeting, the lowest whole number that is greater than 50% of the total number of directors comprising our board of directors; (2) if our pre-IPO owners and their affiliates together continue to beneficially own at least 40% (but less than 50%) of the outstanding shares of our common stock (and any securities convertible into, or exchangeable or exercisable for, such shares) entitled to vote generally in the election of our directors as of the record date for such meeting, the lowest whole number that is at least 40% of the total number of directors comprising our board of directors; (3) if our pre-IPO owners and their affiliates together continue to beneficially own at least 30% (but less than 40%) of the outstanding shares of our common stock (and any securities convertible into, or exchangeable or exercisable for, such shares) entitled to vote generally in the election of our directors as of the record date for such meeting, the lowest whole number that is at least 30% of the total number of directors comprising our board of directors; (4) if our pre-IPO owners and their affiliates together continue to beneficially own at least 20% (but less than 30%) of the outstanding shares of our common stock (and any securities convertible into, or exchangeable or exercisable for, such shares) entitled to vote generally in the election of our directors as of the record date for such meeting, the lowest whole number that is at least 20% of the total number of directors comprising our board of directors; and (5) if our pre-IPO owners and their affiliates together continue to beneficially own at least 5% (but less than 20%) of the outstanding shares of our common stock (and any securities convertible into, or exchangeable or exercisable for, such shares) entitled to vote generally in the election of our directors as of the record date for such meeting, the lowest whole number that is at least 10% of the total number of directors comprising our board of directors. For so long as the stockholders’ agreement remains in effect, Sponsor Directors may be removed only with the consent of our Sponsor. In the case of a vacancy on our board created by the removal or resignation of a Sponsor Director, the stockholders’ agreement will require us to nominate an individual designated by our Sponsor for election to fill the vacancy.
The above-described provisions of the stockholders’ agreement will remain in effect until our Sponsor is no longer entitled to nominate a Sponsor Director pursuant to the stockholders’ agreement, unless our Sponsor requests that they terminate at an earlier date. The stockholders agreement also requires us to cooperate our Sponsor in connection with certain future pledges, hypothecations, grants of security interest in or transfers (including to a third party investor) of any or all of the shares of our common stock held by our Sponsor, including to banks or financial institutions as collateral or security for loans, advances or extensions of credit. The agreement will also permit our Sponsor to assign its rights and obligations under the agreement, in whole or in part, without our prior written consent.
Registration Rights Agreement
In connection with this offering, we expect to enter into a registration rights agreement with our Sponsor. This agreement will provide to our Sponsor an unlimited number of “demand” registration rights and customary “piggyback” registration rights. The registration rights agreement will also provide that we will pay certain expenses relating to such registrations and indemnify our Sponsor against (or make contributions in respect of) certain liabilities which may arise under the Securities Act.
161
Table of Contents
Other Transactions
In connection with our acquisition by Blackstone in 2008, we succeeded to and assumed the rights and obligations under a transaction and management fee agreement with Blackstone Management Partners (“BMP”). In 2014, we paid a lump sum termination fee and BMP is no longer obligated to provide specified transaction and management services and we are no longer obligated to pay specified ongoing fees. However, our obligations with respect to indemnification, release and limitation of liability and certain other matters survived such termination.
In connection with the completed divestiture of the Home Infusion Segment to a subsidiary of CVS Caremark Corporation, we entered into a nominee agreement with Apria Holdings LLC, a stockholder controlled by our Sponsor, which allows us to facilitate certain payments as part of the transaction. We had net inflow/(outflow) of $0.0 million, $(0.8) million and $0.2 million in restricted cash on behalf of the Sponsor for the years ended December 31, 2019, 2018 and 2017, respectively. As of December 31, 2019, all contingent payments related to the nominee agreement have been received.
Commercial Transactions with Sponsor Portfolio Companies
Our Sponsor and its affiliates have ownership interests in a broad range of companies. We have entered and may in the future enter into commercial transactions in the ordinary course of our business with some of these companies, including the sale of goods and services and the purchase of goods and services. Our Sponsor holds an interest in the following companies that we have agreements with:
| • | Change Healthcare performs various revenue and payment cycle management functions. We paid approximately $3.8 million, $3.3 million and $4.2 million to Change Healthcare for the years ended December 31, 2019, 2018 and 2017, respectively. |
| • | JDA Software provides software solutions for supply chain planning optimization. We paid approximately $0.0 million, $0.6 million and $1.0 million to JDA Software for the years ended December 31, 2019, 2018 and 2017, respectively. |
| • | Equity Healthcare LLC (“Equity Healthcare”) provides certain negotiating, monitoring and other services in connection with our health benefit plans. Because of the combined purchasing power of its client participants, Equity Healthcare is able to negotiate pricing terms for providers that are believed to be more favorable than the companies could obtain for themselves on an individual basis. We paid approximately $0.1 million to Equity Healthcare for each of the years ended December 31, 2019, 2018 and 2017. |
| • | BREIT Industrial Canyon PA1W01 LLC (“BREIT Industrial Canyon”) is a subsidiary of Blackstone Real Estate Income Trust, Inc., a non-exchange traded, perpetual life real estate investment trust externally managed by an affiliate of the Sponsor. In May 2018, we began paying BREIT Industrial Canyon, which had acquired a property for which we are in a lease agreement through October 2023. We paid approximately $0.8 million and $0.5 million to BREIT Industrial Canyon for the years ended December 31, 2019 and 2018, respectively. |
| • | RGIS, LLC (“RGIS”) provides certain inventory services. We paid approximately $0.0 million, $0.1 million, and $0.0 million to RGIS for the years ended December 31, 2019, 2018 and 2017, respectively. |
| • | Mphasis (“Mphasis”) provides certain technology related services. There were no amounts paid to Mphasis for the years ended December 31, 2019, 2018 and 2017, respectively. |
| • | Alight Solutions (“Alight”) provides certain consulting services in connection with deployment of a new financial management system, beginning in December 2019. There were no amounts paid to Alight by for the years ended December 31, 2019, 2018 and 2017, respectively. |
| • | Intelenet Global Services (“Intelenet”) performs certain functions related to billing, collections and other administrative and clerical services. In October 2018, an affiliate of the Sponsor sold Intelenet. Through the October 2018 transaction date, we paid approximately $1.9 million and $7.8 million to Intelenet for the years ended December 31, 2018 and 2017, respectively. |
162
Table of Contents
| • | Optiv Inc. (“Optiv”) supplies us with certain IT-related services. In March 2017, an affiliate of the Sponsor sold Optiv. Through the date of the March 2017 transaction, we paid approximately $0.1 million to Optiv for the year ended December 31, 2017. |
Statement of Policy Regarding Transactions with Related Persons
Prior to the completion of this offering, our board of directors will adopt a written statement of policy regarding transactions with related persons, which we refer to as our “related person policy.” Our related person policy requires that a “related person” (as defined in paragraph (a) of Item 404 of Regulation S-K) must promptly disclose to our general counsel any “related person transaction” (defined as any transaction that is anticipated would be reportable by us under Item 404(a) of Regulation S-K in which we were or are to be a participant and the amount involved exceeds $120,000 and in which any related person had or will have a direct or indirect material interest) and all material facts with respect thereto. Our general counsel will then promptly communicate that information to our board of directors. No related person transaction entered into following this offering will be executed without the approval or ratification of our board of directors or a duly authorized committee of our board of directors. It is our policy that directors interested in a related person transaction will recuse themselves from any vote on a related person transaction in which they have an interest.
Indemnification of Directors and Officers
Our amended and restated bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by the DGCL. In addition, our amended and restated certificate of incorporation will provide that our directors will not be liable for monetary damages for breach of fiduciary duty to the fullest extent permitted by the DGCL.
There is no pending litigation or proceeding naming any of our directors or officers to which indemnification is being sought, and we are not aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
We intend to enter into indemnification agreements with our directors and executive officers. These agreements will require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. Insofar as indemnification for liabilities arising under the Securities Act, may be permitted to directors or executive officers, we have been informed that, in the opinion of the SEC, such indemnification is against public policy and is therefore unenforceable.
163
Table of Contents
PRINCIPAL AND SELLING STOCKHOLDERS
The following table sets forth information regarding the beneficial ownership of shares of our common stock immediately after the pre-IPO reorganization transactions and this offering by (1) the selling stockholders, (2) each person known to us to beneficially own more than 5% of our outstanding common stock, (3) each of our directors, director nominee and named executive officers and (4) all of our directors, director nominee and executive officers as a group.
The amounts and percentages of shares beneficially owned are reported on the basis of SEC regulations governing the determination of beneficial ownership of securities. Under SEC rules, a person is deemed to be a “beneficial owner” of a security if that person has or shares voting power or investment power, which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which that person has a right to acquire beneficial ownership within 60 days. Securities that can be so acquired are deemed to be outstanding for purposes of computing such person’s ownership percentage, but not for purposes of computing any other person’s percentage. Under these rules, more than one person may be deemed to be a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Except as otherwise indicated in the footnotes below, each of the beneficial owners has, to our knowledge, sole voting and investment power with respect to the indicated shares. Unless otherwise noted, the address of each beneficial owner is 7353 Company Drive, Indianapolis, Indiana 46237.
Immediately prior to the consummation of this offering, there will be 51 holders of record of our common stock.
| Common Stock Beneficially Owned Prior to this Offering |
Shares to be Sold in the Offering |
Percentage of Shares Beneficially Owned After the Offering |
||||||||||||||||||||||
| Name of Beneficial Owner |
Number(1) | % | Assuming Underwriters’ Option is Not Exercised |
Assuming Underwriters’ Option is Exercised in Full |
Assuming Underwriters’ Option is Not Exercised |
Assuming Underwriters’ Option is Exercised in Full |
||||||||||||||||||
| Principal Stockholders: |
||||||||||||||||||||||||
| Our Sponsor(1) |
32,837,042 | 93.3 | 7,500,000 | 8,625,000 | 72.0 | 68.8 | ||||||||||||||||||
| Directors, Director Nominee and Named Executive Officers: |
||||||||||||||||||||||||
| John G. Figueroa |
608,003 | 1.7 | — | — | 1.7 | 1.7 | ||||||||||||||||||
| Michael Audet(3) |
— | — | — | — | — | — | ||||||||||||||||||
| John R. Murphy |
— | — | — | — | — | — | ||||||||||||||||||
| Norman C. Payson, M.D.(2) |
— | — | — | — | — | — | ||||||||||||||||||
| Devon Rinker(3) |
— | — | — | — | — | — | ||||||||||||||||||
| Neil P. Simpkins(3) |
— | — | — | — | — | — | ||||||||||||||||||
| Lynn Shapiro Snyder |
— | — | — | — | — | — | ||||||||||||||||||
| Daniel J. Starck |
360,426 | 1.0 | — | — | 1.0 | 1.0 | ||||||||||||||||||
| Mike S. Zafirovski |
122,775 | * | — | — | * | * | ||||||||||||||||||
| Debra L. Morris |
53,241 | * | — | — | * | * | ||||||||||||||||||
| Mark E. Litkovitz |
14,197 | * | — | — | * | * | ||||||||||||||||||
| Raoul Smyth |
8,873 | * | — | — | * | * | ||||||||||||||||||
| Robert P. Walker |
35,494 | * | — | — | * | * | ||||||||||||||||||
| Directors, director nominee and executive officers as a group (15 persons) |
1,203,009 | 3.4 | — | — | 3.4 | 3.4 | ||||||||||||||||||
164
Table of Contents
| * | Represents less than one percent. |
| (1) | Reflects shares held directly by Apria Holdings LLC. The controlling member of Apria Holdings LLC is BP Healthcare Holdings LLC. The controlling member of BP Healthcare Holdings LLC is Blackstone Capital Partners V L.P. The general partner of Blackstone Capital Partners V L.P. is Blackstone Management Associates V L.L.C. The sole member of Blackstone Management Associates V L.L.C. is BMA V L.L.C. The managing member of BMA V L.L.C. is Blackstone Holdings III L.P. The general partner of Blackstone Holdings III L.P. is Blackstone Holdings III GP L.P. The general partner of Blackstone Holdings III GP L.P. is Blackstone Holdings III GP Management L.L.C. The sole member of Blackstone Holdings III GP Management L.L.C. is The Blackstone Group Inc. The sole holder of the Class C common stock of The Blackstone Group Inc. is Blackstone Group Management L.L.C. Blackstone Group Management L.L.C. is wholly owned by Blackstone’s senior managing directors and controlled by its founder, Stephen A. Schwarzman. Each of the Blackstone entities described in this footnote and Stephen A. Schwarzman (other than to the extent it or he directly holds securities as described herein) may be deemed to beneficially own the securities directly or indirectly controlled by such Blackstone entities or him, but each disclaims beneficial ownership of such securities. The address of each of such Blackstone entities and Mr. Schwarzman is c/o The Blackstone Group Inc., 345 Park Avenue, New York, New York 10154. |
| (2) | Dr. Payson is a member of BP Healthcare Holdings LLC (described in footnote (1) above), but has no individual investment or voting control over the shares beneficially owned by BP Healthcare Holdings LLC. As a member of BP Healthcare Holdings LLC, Dr. Payson has an indirect interest in shares beneficially owned by BP Healthcare Holdings LLC and is expected to receive a portion of the cash proceeds from the offering and sale of shares that are beneficially owned by BP Healthcare Holdings LLC. |
| (3) | Mr. Audet and Mr. Simpkins are employees of affiliates of our Sponsor and are members of the board of directors of BP Healthcare Holdings LLC (described in footnote (1) above), but each disclaims beneficial ownership of shares beneficially owned by our Sponsor and its affiliates. Mr. Rinker is also an employee of an affiliate of our Sponsor, but disclaims beneficial ownership of shares beneficially owned by our Sponsor and its affiliates. The address for each of Mr. Audet, Mr. Simpkins and Mr. Rinker are c/o The Blackstone Group Inc., 345 Park Avenue, New York, New York 10154. |
The foregoing table assumes an offering price of $20.00 per share of common stock, which is the midpoint of the range on the front cover of this prospectus. However, the precise number of common stock issued to our pre-IPO owners in connection with our pre-IPO reorganization transactions will differ from that presented in the table above if the actual initial public offering price per share differs from this assumed price.
165
Table of Contents
For example, if the initial offering price per share of common stock in this offering is $19.00, which is the low point of the price range indicated on the front cover of this prospectus, the beneficial ownership of common stock of the identified holders would be as follows:
| Common Stock Beneficially Owned Prior to this Offering |
Percentage of Shares Beneficially Owned After the Offering |
|||||||||||||||
| Name of Beneficial Owner |
Number(1) | % | Assuming Underwriters’ Option is Not Exercised |
Assuming Underwriters’ Option is Exercised in Full |
||||||||||||
| Principal Stockholders: |
||||||||||||||||
| Our Sponsor(1) |
32,837,274 | 93.3 | 72.0 | 68.8 | ||||||||||||
| Directors, Director Nominee and Named Executive Officers: |
||||||||||||||||
| John G. Figueroa |
608,002 | 1.7 | 1.7 | 1.7 | ||||||||||||
| Michael Audet |
— | — | — | — | ||||||||||||
| John R. Murphy |
— | — | — | — | ||||||||||||
| Norman C. Payson, M.D. |
— | — | — | — | ||||||||||||
| Devon Rinker |
— | — | — | — | ||||||||||||
| Neil P. Simpkins |
— | — | — | — | ||||||||||||
| Lynn Shapiro Snyder |
— | — | — | — | ||||||||||||
| Daniel J. Starck |
360,425 | 1.0 | 1.0 | 1.0 | ||||||||||||
| Mike S. Zafirovski |
122,775 | * | * | * | ||||||||||||
| Debra L. Morris |
53,241 | * | * | * | ||||||||||||
| Mark E. Litkovitz |
14,197 | * | * | * | ||||||||||||
| Raoul Smyth |
8,873 | * | * | * | ||||||||||||
| Robert P. Walker |
35,494 | * | * | * | ||||||||||||
| Directors, director nominee and executive officers as a group (15 persons) |
1,203,007 | 3.4 | 3.4 | 3.4 | ||||||||||||
| * | Represents less than one percent. |
166
Table of Contents
Conversely, if the initial offering price per share of common stock in this offering is $21.00, which is the high point of the price range indicated on the front cover of this prospectus, the beneficial ownership of common stock of the identified holders would be as follows:
| Common Stock Beneficially Owned Prior to this Offering |
Percentage of Shares Beneficially Owned After the Offering |
|||||||||||||||
| Name of Beneficial Owner |
Number(1) | % | Assuming Underwriters’ Option is Not Exercised |
Assuming Underwriters’ Option is Exercised in Full |
||||||||||||
| Principal Stockholders: |
||||||||||||||||
| Our Sponsor(1) |
32,836,830 | 93.3 | 72.0 | 68.8 | ||||||||||||
| Directors, Director Nominee and Named Executive Officers: |
||||||||||||||||
| John G. Figueroa |
608,005 | 1.7 | 1.7 | 1.7 | ||||||||||||
| Michael Audet |
— | — | — | — | ||||||||||||
| John R. Murphy |
— | — | — | — | ||||||||||||
| Norman C. Payson, M.D. |
— | — | — | — | ||||||||||||
| Devon Rinker |
— | — | — | — | ||||||||||||
| Neil P. Simpkins |
— | — | — | — | ||||||||||||
| Lynn Shapiro Snyder |
— | — | — | — | ||||||||||||
| Daniel J. Starck |
360,427 | 1.0 | 1.0 | 1.0 | ||||||||||||
| Mike S. Zafirovski |
122,775 | * | * | * | ||||||||||||
| Debra L. Morris |
53,241 | * | * | * | ||||||||||||
| Mark E. Litkovitz |
14,197 | * | * | * | ||||||||||||
| Raoul Smyth |
8,873 | * | * | * | ||||||||||||
| Robert P. Walker |
35,494 | * | * | * | ||||||||||||
| Directors, director nominee and executive officers as a group (15 persons) |
1,203,012 | 3.4 | 3.4 | 3.4 | ||||||||||||
| * | Represents less than one percent. |
167
Table of Contents
In connection with this offering, we will amend and restate our certificate of incorporation and our bylaws. The following is a description of the material terms of, and is qualified in its entirety by, our amended and restated certificate of incorporation and amended and restated bylaws, each of which will be in effect upon the consummation of this offering, the forms of which are filed as exhibits to the registration statement of which this prospectus forms a part. Under “Description of Capital Stock,” “we,” “us,” “our” and “our company” refer to Apria, Inc. and not to any of its subsidiaries.
Our purpose is to engage in any lawful act or activity for which corporations may now or hereafter be organized under the DGCL. Upon the consummation of this offering, our authorized capital stock will consist of 1,000,000,000 shares of common stock, par value $0.01 per share, and 100,000,000 shares of preferred stock, par value $0.01 per share. No shares of preferred stock will be issued or outstanding immediately after the public offering contemplated by this prospectus. Unless our board of directors determines otherwise, we will issue all shares of our capital stock in uncertificated form.
Common Stock
Holders of our common stock are entitled to one vote for each share held of record on all matters on which stockholders are entitled to vote generally, including the election or removal of directors elected by our stockholders generally. Holders of our common stock, however, are not entitled to vote upon any amendment to our amended and restated certificate of incorporation that relates solely to the terms of one or more outstanding series of preferred stock if the holders of one or more series of our preferred stock are entitled to vote as a separate class on such amendment under our amended and restated certificate of incorporation or applicable law. The holders of our common stock do not have cumulative voting rights in the election of directors. Upon our liquidation, dissolution, or winding up and after payment in full of all amounts required to be paid to creditors and to the holders of preferred stock having liquidation preferences, if any, the holders of our common stock will be entitled to receive pro rata our remaining assets available for distribution. Holders of our common stock do not have preemptive, subscription, redemption, or conversion rights under our amended and restated certificate of incorporation. The common stock will not be subject to further calls or assessment by us. There will be no redemption or sinking fund provisions applicable to the common stock. All shares of our common stock that will be outstanding at the time of the completion of the offering will be fully paid and non-assessable. The rights, powers, preferences, and privileges of holders of our common stock will be subject to those of the holders of any shares of our preferred stock that we may authorize and issue in the future.
Preferred Stock
Our amended and restated certificate of incorporation authorizes our board of directors to establish one or more series of preferred stock (including convertible preferred stock). Unless required by law or by any stock exchange, and subject to the terms of our amended and restated certificate of incorporation, the authorized shares of preferred stock will be available for issuance without further action by holders of our common stock. Our board of directors is authorized to determine, with respect to any series of preferred stock, the powers (including voting powers), preferences and relative participating, optional or other special rights, and the qualifications, limitations, or restrictions thereof, including, without limitation:
| • | the designation of the series; |
| • | the number of shares of the series, which our board of directors may, except where otherwise provided in the preferred stock designation, increase (but not above the total number of authorized shares of the class) or decrease (but not below the number of shares then outstanding); |
| • | whether dividends, if any, will be cumulative or non-cumulative and the dividend rate of the series; |
| • | the dates at which dividends, if any, will be payable on shares of such series; |
168
Table of Contents
| • | the redemption rights and price or prices, if any, for shares of the series; |
| • | the terms and amounts of any sinking fund provided for the purchase or redemption of shares of the series; |
| • | the amounts payable on shares of the series in the event of any voluntary or involuntary liquidation, dissolution or winding-up of our affairs or other event; |
| • | whether the shares of the series will be convertible into shares of any other class or series, or any other security, of us or any other entity, and, if so, the specification of the other class or series or other security, the conversion price or prices or rate or rates, any rate adjustments, the date or dates as of which the shares will be convertible, and all other terms and conditions upon which the conversion may be made; |
| • | restrictions on the issuance of shares of the same series or of any other class or series of our capital stock; and |
| • | the voting rights, if any, of the holders of the series. |
We could issue a series of preferred stock that could, depending on the terms of the series, impede or discourage an acquisition attempt or other transaction that some, or a majority, of the holders of our common stock might believe to be in their best interests or in which the holders of our common stock might receive a premium over the market price of the shares of our common stock. Additionally, the issuance of preferred stock may adversely affect the rights of holders of our common stock by restricting dividends on the common stock, diluting the voting power of the common stock, or subordinating the rights of the common stock to distributions to the holders of preferred stock upon a liquidation, dissolution or winding up or other event. As a result of these or other factors, the issuance of preferred stock could have an adverse impact on the market price of our common stock.
Dividends
The DGCL permits the directors, subject to any restriction in the certificate of incorporation, to declare and pay dividends out of the corporation’s “surplus” or, if there is no “surplus,” out of its net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year. “Surplus” is defined as the excess of the net assets of the corporation over the amount determined to be the capital of the corporation. The capital of the corporation is typically an amount equal to (and cannot be less than) the aggregate par value of all issued shares of capital stock. Net assets is calculated to be the amount by which the fair value of the total assets of the corporation exceeds its total liabilities, and capital and surplus are not liabilities for such purpose. The DGCL also provides that dividends may not be paid out of net profits if, after the payment of the dividend, the remaining capital would be less than the capital represented by the outstanding stock of all classes having a preference upon the distribution of assets. Declaration and payment of any dividend will be subject to the discretion of our board of directors.
We have no current plans to pay dividends on our common stock following this offering. Any decision to declare and pay dividends in the future will be made at the sole discretion of our board of directors and will depend on, among other things, our results of operations, cash requirements, financial condition, contractual restrictions and other factors that our board of directors may deem relevant. Because we are a holding company and have no direct operations, we will only be able to pay dividends from funds we receive from our subsidiaries. In addition, our ability to pay dividends will be limited by covenants in our existing indebtedness and may be limited by the agreements governing any indebtedness we or our subsidiaries may incur in the future. See “Dividend Policy.”
169
Table of Contents
Annual Stockholder Meetings
Our amended and restated bylaws provide that annual stockholder meetings will be held at a date, time, and place, if any, as exclusively selected by our board of directors. To the extent permitted under applicable law, we may conduct meetings by remote communications, including by webcast.
Anti-Takeover Effects of Our Amended and Restated Certificate of Incorporation and Amended and Restated Bylaws and Certain Provisions of Delaware Law
Our amended and restated certificate of incorporation, amended and restated bylaws, and the DGCL contain provisions which are summarized in the following paragraphs and that are intended to enhance the likelihood of continuity and stability in the composition of our board of directors. These provisions are intended to avoid costly takeover battles, reduce our vulnerability to a hostile or abusive change of control and enhance the ability of our board of directors to maximize stockholder value in connection with any unsolicited offer to acquire us. However, these provisions may have an anti-takeover effect and may delay, deter or prevent a merger or acquisition of the Company by means of a tender offer, a proxy contest or other takeover attempt that a stockholder might consider in its best interest, including those attempts that might result in a premium over the prevailing market price for the shares of common stock held by stockholders.
Authorized but Unissued Capital Stock
Delaware law does not require stockholder approval for any issuance of shares that are authorized and available for issuance. However, the listing requirements of Nasdaq, which would apply so long as our common stock remains listed on Nasdaq, require stockholder approval of certain issuances equal to or exceeding 20% of the then outstanding voting power of our capital stock or then outstanding number of shares of common stock. These additional shares may be used for a variety of corporate purposes, including future public offerings, to raise additional capital, or to facilitate acquisitions.
Our board of directors may generally issue shares of one or more series of preferred stock on terms calculated to discourage, delay or prevent a change of control of the Company or the removal of our management. Moreover, our authorized but unissued shares of preferred stock will be available for future issuances in one or more series without stockholder approval and could be utilized for a variety of corporate purposes, including future offerings to raise additional capital, to facilitate acquisitions and employee benefit plans.
One of the effects of the existence of authorized and unissued and unreserved common stock or preferred stock may be to enable our board of directors to issue shares to persons friendly to current management, which issuance could render more difficult or discourage an attempt to obtain control of the Company by means of a merger, tender offer, proxy contest or otherwise, and thereby protect the continuity of our management and possibly deprive our stockholders of opportunities to sell their shares of common stock at prices higher than prevailing market prices.
Classified Board of Directors
Our amended and restated certificate of incorporation provides that, subject to the right of holders of any series of preferred stock, our board of directors will be divided into three classes of directors, as nearly equal in number as possible, and with the directors serving staggered three-year terms, with only one class of directors being elected at each annual meeting of stockholders. As a result, approximately one-third of our board of directors will be elected each year. The classification of directors will have the effect of making it more difficult for stockholders to change the composition of our board of directors. Our amended and restated certificate of incorporation and amended and restated bylaws provide that, subject to any rights of holders of preferred stock to elect additional directors under specified circumstances and the terms of our stockholders agreement, the number of directors will be fixed from time to time exclusively pursuant to a resolution adopted by the board of directors.
170
Table of Contents
Business Combinations
We have opted out of Section 203 of the DGCL; however, our amended and restated certificate of incorporation contains similar provisions providing that we may not engage in certain “business combinations” with any “interested stockholder” for a three-year period following the time that the stockholder became an interested stockholder, unless:
| • | prior to such time, our board of directors approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder; |
| • | upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of our voting stock outstanding at the time the transaction commenced, excluding certain shares; or |
| • | at or subsequent to that time, the business combination is approved by our board of directors and by the affirmative vote of holders of at least 66 2/3% of our outstanding voting stock that is not owned by the interested stockholder. |
Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who owns 15% or more of our outstanding voting stock or is an affiliate or associate of us and was the owner of 15% or more of our outstanding voting stock at the date of termination, and their affiliates and associates. For purposes of this section only, “voting stock” has the meaning given to it in Section 203 of the DGCL.
Under certain circumstances, this provision will make it more difficult for a person who would be an “interested stockholder” to effect various business combinations with us for a three-year period. This provision may encourage companies interested in acquiring us to negotiate in advance with our board of directors because the stockholder approval requirement would be avoided if our board of directors approves either the business combination or the transaction that results in the stockholder becoming an interested stockholder. These provisions also may have the effect of preventing changes in our board of directors and may make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
Our amended and restated certificate of incorporation provides that our Sponsor and its affiliates, and any of their respective direct or indirect transferees, and any group as to which such persons are a party, do not constitute “interested stockholders” for purposes of this provision.
Removal of Directors; Vacancies and Newly Created Directorships
Under the DGCL, unless otherwise provided in our amended and restated certificate of incorporation, directors serving on a classified board may be removed by the stockholders only for cause. Our amended and restated certificate of incorporation provides that the directors divided into classes may be removed with or without cause upon the affirmative vote of a majority in voting power of all outstanding shares of stock entitled to vote generally in the election of directors, voting together as a single class; provided, however, at any time when our Sponsor and its affiliates beneficially own, in the aggregate, less than 30% of the total voting power of all then outstanding shares of our stock entitled to vote generally in the election of directors, directors may only be removed for cause, and only upon the affirmative vote of holders of at least 66 2/3% of the total voting power of all the then outstanding shares of stock entitled to vote generally in the election of directors, voting together as a single class; provided, further, however, that specified directors designated pursuant to the stockholders agreement may not be removed without cause without the consent of the designating party. In addition, our amended and restated certificate of incorporation provides that, subject to the rights granted to one or more series of preferred stock then outstanding or the rights granted under the stockholders agreement with our Sponsor, any newly created directorship on the board of directors that results from an increase in the number of directors and any vacancies on our board of directors will be filled only by the affirmative vote of a majority of the remaining directors, even if less than a quorum, by a sole remaining director or by the stockholders; provided, however, at
171
Table of Contents
any time when our Sponsor and its affiliates beneficially own, in the aggregate, less than 30% of the total voting power of all then outstanding shares of stock of the Company entitled to vote generally in the election of directors, any newly created directorship on the board of directors that results from an increase in the number of directors and any vacancy occurring in the board of directors may only be filled by a majority of the directors then in office, although less than a quorum, or by a sole remaining director (and not by the stockholders) (other than directors elected by the holders of any series of preferred stock, by voting separately as a series or together with one or more series, as the case may be).
No Cumulative Voting
Under Delaware law, the right to vote cumulatively does not exist unless the certificate of incorporation specifically authorizes cumulative voting. Our amended and restated certificate of incorporation does not authorize cumulative voting. Therefore, stockholders holding a majority in voting power of the shares of our stock entitled to vote generally in the election of directors will be able to elect all of our directors.
Special Stockholder Meetings
Our amended and restated certificate of incorporation provides that special meetings of our stockholders may be called at any time only by or at the direction of the board of directors or the chairman of the board of directors; provided, however, at any time when our Sponsor and its affiliates beneficially own, in the aggregate, at least 30% of the total voting power of all then outstanding shares of stock entitled to vote generally in the election of directors, special meetings of our stockholders shall also be called by the board of directors or the chairman of the board of directors at the request of our Sponsor and its affiliates. Our amended and restated bylaws prohibit the conduct of any business at a special meeting other than as specified in the notice for such meeting. These provisions may have the effect of deterring, delaying, or discouraging hostile takeovers, or changes in control or management of the Company.
Director Nominations and Stockholder Proposals
Our amended and restated bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors. In order for any matter to be “properly brought” before a meeting, a stockholder will have to comply with advance notice requirements and provide us with certain information. Generally, to be timely, a stockholder’s notice must be received at our principal executive offices not less than 90 days nor more than 120 days prior to the first anniversary date of the immediately preceding annual meeting of stockholders. Our amended and restated bylaws also specify requirements as to the form and content of a stockholder’s notice. These provisions will not apply to our Sponsor and its affiliates so long as the stockholders agreement remains in effect. Our amended and restated bylaws allow the chairman of the meeting at a meeting of the stockholders to adopt rules and regulations for the conduct of meetings that may have the effect of precluding the conduct of certain business at a meeting if the rules and regulations are not followed. These provisions may also defer, delay, or discourage a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to influence or obtain control of the Company.
Stockholder Action by Written Consent
Our amended and restated certificate of incorporation will provide that any action required to be taken at any annual or special meeting of the stockholders may be taken without a meeting, without prior notice, and without a vote if a consent or consents in writing, setting forth the action so taken, is or are signed by the stockholder designated pursuant to our stockholders agreement and holders of outstanding stock having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares of our stock entitled to vote thereon were present and voted. Our amended and restated certificate of incorporation will prohibit stockholder action by written consent in lieu of a meeting of stockholders at any time
172
Table of Contents
our Sponsors and its affiliates own, in the aggregate, less than 30% in voting power of our stock entitled to vote generally in the election of directors; provided that any action required or permitted to be taken by the holders of preferred stock, voting separately as a series or separately as a class with one or more other such series, may be taken without a meeting, without prior notice and without a vote, to the extent expressly so provided by the applicable certificate of designation relating to such series of preferred stock.
Supermajority Provisions
Our amended and restated certificate of incorporation and amended and restated bylaws will provide that the board of directors is expressly authorized to make, alter, amend, change, add to, rescind, or repeal, in whole or in part, our bylaws without a stockholder vote in any matter not inconsistent with the laws of the State of Delaware or our amended and restated certificate of incorporation. For as long as our Sponsor and its affiliates beneficially own, in the aggregate, at least 30% of the total voting power of all then outstanding shares of our stock entitled to vote generally in the election of directors, any amendment, alteration, change, addition, or repeal of our bylaws by our stockholders requires the affirmative vote of a majority in voting power of the outstanding shares of our stock present in person or represented by proxy at the meeting and entitled to vote on such amendment, alteration, rescission or repeal. At any time when our Sponsor and its affiliates beneficially own, in the aggregate, less than 30% in voting power of our stock entitled to vote generally in the election of directors, any amendment, alteration, rescission, or repeal of our bylaws by our stockholders requires the affirmative vote of the holders of at least 66 2/3% of the total voting power of all then outstanding shares of our stock entitled to vote thereon, voting together as a single class.
The DGCL provides generally that the affirmative vote of a majority of the outstanding shares entitled to vote thereon, voting together as a single class, is required to amend a corporation’s certificate of incorporation, unless the certificate of incorporation requires a greater percentage.
Our amended and restated certificate of incorporation will provide that at any time when our Sponsor and its affiliates beneficially own, in the aggregate, less than 30% in voting power of our stock entitled to vote generally in the election of directors, the following provisions in our amended and restated certificate of incorporation may be amended, altered, repealed or rescinded only by the affirmative vote of the holders of at least 662⁄3% in voting power of all the then outstanding shares of our stock entitled to vote thereon, voting together as a single class:
| • | the provision requiring a 662⁄3% supermajority vote for stockholders to amend our amended and restated bylaws; |
| • | the provisions providing for a classified board of directors (the election and term of our directors); |
| • | the provisions regarding resignation and removal of directors; |
| • | the provisions regarding competition and corporate opportunities; |
| • | the provisions regarding entering into business combinations with interested stockholders; |
| • | the provisions regarding stockholder action by written consent; |
| • | the provisions regarding calling special meetings of stockholders; |
| • | the provisions regarding filling vacancies on our board of directors and newly created directorships; |
| • | the provisions eliminating monetary damages for breaches of fiduciary duty by a director; |
| • | the provisions regarding forum selection; and |
| • | the amendment provision requiring that the above provisions be amended only with a 66 2/3% supermajority vote. |
The combination of the classification of our board of directors, the lack of cumulative voting and the supermajority voting requirements will make it more difficult for our existing stockholders to replace our board
173
Table of Contents
of directors as well as for another party to obtain control of us by replacing our board of directors. Because our board of directors has the power to retain and discharge our officers, these provisions could also make it more difficult for existing stockholders or another party to effect a change in management.
These provisions may have the effect of deterring hostile takeovers or delaying or preventing changes in control of us or our management, such as a merger, reorganization or tender offer. These provisions are intended to enhance the likelihood of continued stability in the composition of our board of directors and its policies and to discourage certain types of transactions that may involve an actual or threatened acquisition of the Company. These provisions are designed to reduce our vulnerability to an unsolicited acquisition proposal. The provisions are also intended to discourage certain tactics that may be used in proxy fights. However, such provisions could have the effect of discouraging others from making tender offers for our shares and, as a consequence, they also may inhibit fluctuations in the market price of our shares that could result from actual or rumored takeover attempts. Such provisions may also have the effect of preventing changes in management.
Dissenters’ Rights of Appraisal and Payment
Under the DGCL, with certain exceptions, our stockholders will have appraisal rights in connection with a merger or consolidation in which we are a constituent entity. Pursuant to the DGCL, stockholders who properly request and perfect appraisal rights in connection with such merger or consolidation will have the right to receive payment of the fair value of their shares as determined by the Delaware Court of Chancery.
Stockholders’ Derivative Actions
Under the DGCL, any of our stockholders may bring an action in our name to procure a judgment in our favor, also known as a derivative action, provided that the stockholder bringing the action is a holder of our shares at the time of the transaction to which the action relates or such stockholder’s stock thereafter devolved by operation of law. To bring such an action, the stockholder must otherwise comply with Delaware law regarding derivative actions.
Exclusive Forum
Our amended and restated certificate of incorporation will provide that unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware shall, to the fullest extent permitted by law, be the sole and exclusive forum for any (i) derivative action or proceeding brought on behalf of our Company, (ii) action asserting a claim of breach of a fiduciary duty owed by any current or former director, officer, employee or stockholder of our Company to the Company or the Company’s stockholders, (iii) action asserting a claim against the Company or any current or former director, officer, employee or stockholder of the Company arising pursuant to any provision of the DGCL or our amended and restated certificate of incorporation or our amended and restated bylaws, or (iv) action asserting a claim against the Company or any director, officer, employee or stockholder of the Company governed by the internal affairs doctrine of the law of the State of Delaware. Our amended and restated certificate of incorporation will further provide that to the fullest extent permitted by law the federal district courts of the United States of America will be the exclusive forum for the resolution of any complaint asserting a cause of action arising under the U.S. federal securities laws. Any person or entity purchasing or otherwise acquiring or holding any interest in shares of capital stock of the Company shall be deemed to have notice of and provided consent to the forum provisions in our amended and restated certificate of incorporation.
Conflicts of Interest
Delaware law permits corporations to adopt provisions renouncing any interest or expectancy in certain opportunities that are presented to the corporation or its officers, directors or stockholders. Our amended and restated certificate of incorporation will, to the maximum extent permitted from time to time by Delaware law,
174
Table of Contents
renounce any interest or expectancy that we have in, or right to be offered an opportunity to participate in, specified business opportunities that are from time to time presented to our officers, directors or stockholders or their respective affiliates, other than those officers, directors, stockholders or affiliates who are our or our subsidiaries’ employees. Our amended and restated certificate of incorporation will provide that, to the fullest extent permitted by law, none of our Sponsor or any of its affiliates or any director who is not employed by us (including any non-employee director who serves as one of our officers in both his or her director and officer capacities) or his or her affiliates will have any duty to refrain from (i) engaging in a corporate opportunity in the same or similar lines of business in which we or our affiliates now engage or propose to engage or (ii) otherwise competing with us or our affiliates. In addition, to the fullest extent permitted by law, in the event that our Sponsor or any non-employee director acquires knowledge of a potential transaction or other business opportunity which may be a corporate opportunity for itself, himself or herself or its, his or her affiliates or for us or our affiliates, such person will have no duty to communicate or offer such transaction or business opportunity to us or any of our affiliates and they may take any such opportunity for themselves or offer it to another person or entity. Our amended and restated certificate of incorporation will not renounce our interest in any business opportunity that is expressly offered to a non-employee director solely in his or her capacity as a director or officer of the Company. To the fullest extent permitted by law, no business opportunity will be deemed to be a potential corporate opportunity for us unless we would be permitted to undertake the opportunity under our amended and restated certificate of incorporation, we have sufficient financial resources to undertake the opportunity and the opportunity would be in line with our business.
Limitations on Liability and Indemnification of Officers and Directors
The DGCL authorizes corporations to limit or eliminate the personal liability of directors to corporations and their stockholders for monetary damages for breaches of directors’ fiduciary duties, subject to certain exceptions. Our amended and restated certificate of incorporation includes a provision that eliminates the personal liability of directors for monetary damages to the corporation or its stockholders for any breach of fiduciary duty as a director, except to the extent such exemption from liability or limitation thereof is not permitted under the DGCL. The effect of these provisions is to eliminate the rights of us and our stockholders, whether directly or through a suit brought derivatively on our behalf, to recover monetary damages from a director for breach of fiduciary duty as a director, including breaches resulting from grossly negligent behavior. However, exculpation does not apply to any director if the director has breached such director’s duty of loyalty, acted in bad faith, knowingly or intentionally violated the law, authorized illegal dividends, redemptions or repurchases or derived an improper benefit from his or her actions as a director.
Our amended and restated bylaws generally provide that we must indemnify and advance expenses to our directors and officers to the fullest extent authorized by the DGCL. We also are expressly authorized to carry directors’ and officers’ liability insurance providing indemnification for our directors, officers and certain employees for some liabilities. We believe that these indemnification and advancement provisions and insurance are useful to attract and retain qualified directors and executive officers.
The limitation of liability, indemnification and advancement provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duty. These provisions also may have the effect of reducing the likelihood of derivative litigation against directors and officers, even though such an action, if successful, might otherwise benefit us and our stockholders. In addition, your investment may be adversely affected to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
There is currently no pending material litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought.
175
Table of Contents
Transfer Agent and Registrar
The transfer agent and registrar for shares of our common stock will be Broadridge Corporate Issuer Solutions, Inc.
Listing
We intend to list our common stock on Nasdaq under the symbol “APR.”
176
Table of Contents
CERTAIN UNITED STATES FEDERAL INCOME AND ESTATE
TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following is a summary of certain United States federal income and estate tax consequences of the purchase, ownership and disposition of our common stock as of the date hereof. Except where noted, this summary deals only with common stock that is held as a capital asset by a non-U.S. holder (as defined below).
A “non-U.S. holder” means a beneficial owner of our common stock (other than an entity treated as a partnership for United States federal income tax purposes) that is not, for United States federal income tax purposes, any of the following:
| • | an individual citizen or resident of the United States; |
| • | a corporation (or any other entity treated as a corporation for United States federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; |
| • | an estate the income of which is subject to United States federal income taxation regardless of its source; or |
| • | a trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable United States Treasury regulations to be treated as a United States person. |
This summary is based upon provisions of the Code, and regulations, rulings and judicial decisions as of the date hereof. Those authorities may be changed, perhaps retroactively, so as to result in United States federal income and estate tax consequences different from those summarized below. This summary does not address all aspects of United States federal income and estate taxes and does not deal with foreign, state, local or other tax considerations that may be relevant to non-U.S. holders in light of their particular circumstances. In addition, it does not represent a detailed description of the United States federal income and estate tax consequences applicable to holders that are subject to special treatment under the United States federal income tax laws (including if such holder is a United States expatriate, foreign pension fund, “controlled foreign corporation,” “passive foreign investment company” or a partnership or other pass-through entity for United States federal income tax purposes). We cannot make assurances that a change in law will not alter significantly the tax considerations that we describe in this summary.
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) holds our common stock, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. Any partnership holding our common stock, or a partner in any such partnership, should consult its own tax advisors.
Any person considering the purchase of our common stock should consult its own tax advisors concerning the particular United States federal income and estate tax consequences to it of the purchase, ownership and disposition of our common stock, as well as the consequences to such person arising under other United States federal tax laws and the laws of any other taxing jurisdiction.
Dividends
In the event that we make a distribution of cash or other property (other than certain pro rata distributions of our stock) in respect of our common stock, the distribution generally will be treated as a dividend for United States federal income tax purposes to the extent it is paid from our current or accumulated earnings and profits, as determined under United States federal income tax principles. Any portion of a distribution that exceeds our current and accumulated earnings and profits generally will be treated first as a tax-free return of
177
Table of Contents
capital, causing a reduction in the adjusted tax basis of a non-U.S. holder’s common stock, and to the extent the amount of the distribution exceeds a non-U.S. holder’s adjusted tax basis in our common stock, the excess will be treated as gain from the disposition of our common stock (the tax treatment of which is discussed below under “Gain on Disposition of Common Stock”).
Dividends paid to a non-U.S. holder generally will be subject to withholding of United States federal income tax at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. However, dividends that are effectively connected with the conduct of a trade or business by the non-U.S. holder within the United States (and, if required by an applicable income tax treaty, are attributable to a United States permanent establishment) are not subject to the withholding tax, provided certain certification and disclosure requirements are satisfied. Instead, such dividends are subject to United States federal income tax on a net income basis in the same manner as if the non-U.S. holder were a United States person as defined under the Code. Any such effectively connected dividends received by a foreign corporation may be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty.
A non-U.S. holder who wishes to claim the benefit of an applicable treaty rate and avoid backup withholding, as discussed below, for dividends will be required (a) to provide the applicable withholding agent with a properly executed IRS Form W-BEN or Form W-8BEN-E (or other applicable form) certifying under penalty of perjury that such holder is not a United States person as defined under the Code and is eligible for treaty benefits or (b) if our common stock is held through certain foreign intermediaries, to satisfy the relevant certification requirements of applicable United States Treasury regulations. Special certification and other requirements apply to certain non-U.S. holders that are pass-through entities rather than corporations or individuals.
A non-U.S. holder eligible for a reduced rate of United States federal withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by timely filing an appropriate claim for refund with the IRS.
Gain on Disposition of Common Stock
Subject to the discussion of backup withholding below, any gain realized by a non-U.S. holder on the sale or other disposition of our common stock generally will not be subject to United States federal income tax unless:
| • | the gain is effectively connected with a trade or business of the non-U.S. holder in the United States (and, if required by an applicable income tax treaty, is attributable to a United States permanent establishment of the non-U.S. holder); |
| • | the non-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of that disposition, and certain other conditions are met; or |
| • | we are or have been a “United States real property holding corporation” for United States federal income tax purposes and certain other conditions are met. |
A non-U.S. holder described in the first bullet point immediately above will be subject to tax on the gain derived from the sale or other disposition of our common stock in the same manner as if the non-U.S. holder were a United States person as defined under the Code. In addition, if any non-U.S. holder described in the first bullet point immediately above is a foreign corporation, the gain realized by such non-U.S. holder may be subject to an additional “branch profits tax” at a 30% rate or such lower rate as may be specified by an applicable income tax treaty. An individual non-U.S. holder described in the second bullet point immediately above will be subject to a 30% (or such lower rate as may be specified by an applicable income tax treaty) tax on the gain derived from the sale or other disposition, which gain may be offset by United States source capital losses even though the individual is not considered a resident of the United States.
Generally, a corporation is a “United States real property holding corporation” if the fair market value of its United States real property interests equals or exceeds 50% of the sum of the fair market value of its worldwide
178
Table of Contents
real property interests and its other assets used or held for use in a trade or business (all as determined for United States federal income tax purposes). We believe we are not and do not anticipate becoming a “United States real property holding corporation” for United States federal income tax purposes.
Federal Estate Tax
Common stock held by an individual non-U.S. holder at the time of death will be included in such holder’s gross estate for United States federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Information Reporting and Backup Withholding
Distributions paid to a non-U.S. holder and the amount of any tax withheld with respect to such distributions generally will be reported to the IRS. Copies of the information returns reporting such distributions and any withholding may also be made available to the tax authorities in the country in which the non-U.S. holder resides under the provisions of an applicable income tax treaty.
A non-U.S. holder will not be subject to backup withholding on dividends received if such holder certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that such holder is a United States person as defined under the Code), or such holder otherwise establishes an exemption.
Information reporting and, depending on the circumstances, backup withholding will apply to the proceeds of a sale or other disposition of our common stock made within the United States or conducted through certain United States-related financial intermediaries, unless the beneficial owner certifies under penalty of perjury that it is a non-U.S. holder (and the payor does not have actual knowledge or reason to know that the beneficial owner is a United States person as defined under the Code), or such owner otherwise establishes an exemption.
Backup withholding is not an additional tax and any amounts withheld under the backup withholding rules will be allowed as a refund or a credit against a non-U.S. holder’s United States federal income tax liability provided the required information is timely furnished to the IRS.
Additional Withholding Requirements
Under Sections 1471 through 1474 of the Code (such Sections commonly referred to as “FATCA”), a 30% United States federal withholding tax applies to any dividends paid on our common stock paid to (i) a “foreign financial institution” (as specifically defined in the Code) which does not provide sufficient documentation, typically on IRS Form W-8BEN-E, evidencing either (x) an exemption from FATCA, or (y) its compliance (or deemed compliance) with FATCA (which may alternatively be in the form of compliance with an intergovernmental agreement with the United States) in a manner which avoids withholding, or (ii) a “non-financial foreign entity” (as specifically defined in the Code) which does not provide sufficient documentation, typically on IRS Form W-8BEN-E, evidencing either (x) an exemption from FATCA, or (y) adequate information regarding certain substantial United States beneficial owners of such entity (if any). If a dividend payment is both subject to withholding under FATCA and subject to the withholding tax discussed above under “Dividends,” the withholding under FATCA may be credited against, and therefore reduce, such other withholding tax. If withholding under FATCA is imposed, a beneficial owner that is not a foreign financial institution generally may obtain a refund of any amounts withheld by filing a United States federal income tax return (which may entail significant administrative burden). Holders should consult their own tax advisors regarding these requirements and whether they may be relevant to their ownership and disposition of our common stock.
179
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for shares of our common stock. We cannot predict the effect, if any, future sales of shares of common stock, or the availability for future sale of shares of common stock, will have on the market price of shares of our common stock prevailing from time to time. The sale of substantial amounts of shares of our common stock in the public market, or the perception that such sales could occur, could harm the prevailing market price of shares of our common stock and could impair our future ability to raise capital through the sale of our equity or equity-related securities at a time and price that we deem appropriate. See “Risk Factors—Risks Related to this Offering and Ownership of our Common Stock—If we or our pre-IPO owners sell additional shares of our common stock after this offering or are perceived by the markets as intending to sell them, the market price of our common stock could decline.”
Upon completion of this offering, we will have a total of 35,210,915 shares of our common stock outstanding. Of the outstanding shares, the 7,500,000 shares sold in this offering (or 8,625,000 shares if the underwriters exercise their option to purchase additional shares in full) will be freely tradable without restriction or further registration under the Securities Act, except that any shares held by our affiliates, as that term is defined under Rule 144 of the Securities Act, may be sold only in compliance with the limitations described below. The remaining outstanding 27,710,915 shares of common stock held by our pre-IPO owners and management after this offering (or 26,585,915 shares if the underwriters exercise their option to purchase additional shares in full) will be deemed restricted securities under Rule 144 and may be sold in the public market only if registered or if they qualify for an exemption from registration, including the exemption pursuant to Rule 144 which we summarize below.
We intend to file one or more registration statements on Form S-8 under the Securities Act to register 7,698,552 shares of our common stock or securities convertible into or exchangeable for shares of our common stock issued pursuant to our Omnibus Incentive Plan and the 2015 Plan. Any such Form S-8 registration statements will automatically become effective upon filing. Accordingly, shares registered under such registration statements will be available for sale in the open market.
Registration Rights
In connection with this offering, we intend to enter into a registration rights agreement that will provide our Sponsor an unlimited number of “demand” registrations and customary “piggyback” registration rights. The registration rights agreement also provides that we will pay certain expenses relating to such registrations and indemnify the registration rights holders against certain liabilities which may arise under the Securities Act. Securities registered under any such registration statement will be available for sale in the open market unless restrictions apply. See “Certain Relationships and Related Person Transactions—Registration Rights Agreement.”
Lock-Up Agreements
We, our officers, directors, director nominees and certain of our pre-IPO owners have agreed that, subject to enumerated exceptions, for a period of 180 days from the date of this prospectus, we and they will not, without the prior written consent of Citigroup Global Markets Inc. and Goldman Sachs & Co. LLC, offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of any shares of our stock, or any options or warrants to purchase any shares of our stock, or any securities convertible into, exchangeable for or that represent the right to receive shares of our stock. Citigroup Global Markets Inc. and Goldman Sachs & Co. LLC in their sole discretion may release any of the securities subject to these lock-up agreements at any time, which, in the case of officers and directors, shall be with notice.
Rule 144
In general, under Rule 144, as currently in effect, a person who is not deemed to be our affiliate for purposes of Rule 144 or to have been one of our affiliates at any time during the three months preceding a sale and who
180
Table of Contents
has beneficially owned the shares of common stock proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares of common stock without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares of common stock proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person is entitled to sell those shares of common stock without complying with any of the requirements of Rule 144. In general, six months after the effective date of the registration statement of which this prospectus forms a part, under Rule 144, as currently in effect, our affiliates or persons selling shares of common stock on behalf of our affiliates are entitled to sell, within any three-month period, a number of shares of common stock that does not exceed the greater of (1) 1% of the number of shares of common stock then outstanding and (2) the average weekly trading volume of the shares of common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. Sales under Rule 144 by our affiliates or persons selling shares of common stock on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
181
Table of Contents
Citigroup Global Markets Inc. and Goldman Sachs & Co. LLC are acting as representatives of the underwriters named below. Subject to the terms and conditions stated in the underwriting agreement dated the date of this prospectus, each underwriter named below has severally agreed to purchase, and the selling stockholders have severally agreed to sell to that underwriter, the number of shares set forth opposite the underwriter’s name.
| Underwriter |
Number of Shares |
|||
| Citigroup Global Markets Inc. |
||||
| Goldman Sachs & Co. LLC |
||||
| BofA Securities, Inc. |
||||
| J.P. Morgan Securities LLC |
||||
| UBS Securities LLC |
||||
| Piper Sandler & Co. |
||||
| Citizens Capital Markets, Inc. |
||||
| Fifth Third Securities, Inc. |
||||
| TD Securities (USA) LLC |
||||
| Academy Securities, Inc. |
||||
| Blaylock Van, LLC. |
||||
| Penserra Securities LLC |
||||
| Stern Brothers & Co. |
||||
|
|
|
|||
| Total |
7,500,000 | |||
|
|
|
|||
The underwriting agreement provides that the obligations of the underwriters to purchase the shares included in this offering are subject to approval of legal matters by counsel and to other conditions. The underwriters are obligated to purchase all the shares (other than those covered by the underwriters’ option to purchase additional shares described below) if they purchase any of the shares. The offering of the shares by the underwriters is subject to receipt and acceptance and subject to the underwriters’ right to reject any order in whole or in part.
Shares sold by the underwriters to the public will initially be offered at the initial public offering price set forth on the cover of this prospectus. Any shares sold by the underwriters to securities dealers may be sold at a discount from the initial public offering price not to exceed $ per share. If all the shares are not sold at the initial offering price, the underwriters may change the offering price and the other selling terms. The representatives have advised us and the selling stockholders that the underwriters do not intend to make sales to discretionary accounts.
If the underwriters sell more shares than the total number set forth in the table above, the selling stockholders have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to 1,125,000 additional shares at the public offering price less the underwriting discount. To the extent the option is exercised, each underwriter must purchase a number of additional shares approximately proportionate to that underwriter’s initial purchase commitment. Any shares issued or sold under the option will be issued and sold on the same terms and conditions as the other shares that are the subject of this offering.
We, our officers, directors, director nominee and the selling stockholders, representing substantially all of our outstanding shares as of the date of this prospectus, have agreed that, subject to enumerated exceptions, for a period of 180 days from the date of this prospectus, we and they will not, without the prior written consent of Citigroup Global Markets Inc. and Goldman Sachs & Co. LLC, offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of any shares of our stock, or any options or warrants to purchase any shares of our stock, or any securities convertible into, exchangeable for or that represent the right to receive shares of our stock. Citigroup Global Markets Inc. and Goldman Sachs & Co. LLC in their sole discretion may release any of the securities subject to these lock-up agreements at any time, which, in the case of officers and directors, shall be with notice.
182
Table of Contents
Prior to this offering, there has been no public market for our shares. Consequently, the initial public offering price for the shares was determined by negotiations among the selling stockholders and the representatives. Among the factors considered in determining the initial public offering price were our results of operations, our current financial condition, our future prospects, our markets, the economic conditions in and future prospects for the industry in which we compete, our management, and currently prevailing general conditions in the equity securities markets, including current market valuations of publicly traded companies considered comparable to our company. We cannot assure you, however, that the price at which the shares will sell in the public market after this offering will not be lower than the initial public offering price or that an active trading market in our shares will develop and continue after this offering.
We intend to list our shares on Nasdaq under the symbol “APR.”
The following table shows the underwriting discounts and commissions that the selling stockholders are to pay to the underwriters in connection with this offering. These amounts are shown assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares as described above.
| Paid by Selling Stockholders | ||||||||
| No Exercise | Full Exercise | |||||||
| Per share |
$ | $ | ||||||
| Total |
$ | $ | ||||||
We and the selling stockholders estimate that our respective portions of the total expenses of this offering other than the underwriting discounts and commissions will be $ and $ . We have also agreed to reimburse the underwriters for certain FINRA-related expenses incurred by them in connection with the offering in an amount up to $60,000.
In connection with the offering, the underwriters may purchase and sell shares in the open market. Purchases and sales in the open market may include short sales, purchases to cover short positions, which may include purchases pursuant to the underwriters’ option to purchase additional shares, and stabilizing purchases.
| • | Short sales involve secondary market sales by the underwriters of a greater number of shares than they are required to purchase in the offering. |
| • | “Covered” short sales are sales of shares in an amount up to the number of shares represented by the underwriters’ option to purchase additional shares. |
| • | “Naked” short sales are sales of shares in an amount in excess of the number of shares represented by the underwriters’ option to purchase additional shares. |
| • | Covering transactions involve purchases of shares either pursuant to the underwriters’ option to purchase additional shares or in the open market in order to cover short positions. |
| • | To close a naked short position, the underwriters must purchase shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering. |
| • | To close a covered short position, the underwriters must purchase shares in the open market or must exercise the option to purchase additional shares. In determining the source of shares to close the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the underwriters’ option to purchase additional shares. |
| • | Stabilizing transactions involve bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum. |
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased shares sold by or for the account of such underwriter in stabilizing or short covering transactions.
183
Table of Contents
Purchases to cover short positions and stabilizing purchases, as well as penalty bids and other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the shares. They may also cause the price of the shares to be higher than the price that would otherwise exist in the open market in the absence of these transactions. The underwriters may conduct these transactions on Nasdaq, in the over-the-counter market or otherwise. If the underwriters commence any of these transactions, they may discontinue them at any time.
The underwriters are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their respective affiliates have in the past performed commercial banking, investment banking and advisory services for us from time to time for which they have received customary fees and reimbursement of expenses and may, from time to time, engage in transactions with and perform services for us in the ordinary course of their business for which they may receive customary fees and reimbursement of expenses. In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (which may include bank loans and/or credit default swaps) for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities and instruments. Such investments and securities activities may involve securities and/or instruments of ours or our affiliates. The underwriters and their affiliates may also make investment recommendations and/or publish or express independent research views in respect of such securities or financial instruments and may hold, or recommend to clients that they acquire, long and/or short positions in such securities and instruments. Affiliates of BofA Securities, Inc., J.P. Morgan Securities LLC, Citizens Capital Markets, Inc., Fifth Third Securities, Inc. and TD Securities (USA) LLC are arrangers and/or lenders under the Credit Facility.
We and the selling stockholders have agreed to indemnify the several underwriters against certain liabilities, including liabilities under the Securities Act, or to contribute to payments the underwriters may be required to make because of any of those liabilities.
Notice to Prospective Investors in the European Economic Area
In relation to each member state of the European Economic Area (each, a “relevant state”), no shares of common stock have been offered or will be offered pursuant to this offering to the public in that relevant member state prior to the publication of a prospectus in relation to the shares that has been approved by the competent authority in that relevant member state or, where appropriate, approved in another relevant state and notified to the competent authority in that relevant state, all in accordance with the Prospectus Regulation, except that offers of shares may be made to the public in that relevant state at any time under the following exemptions under the Prospectus Regulation:
| • | to any legal entity which is a qualified investor as defined under the Prospectus Regulation; |
| • | to fewer than 150 natural or legal persons (other than qualified investors as defined under the Prospectus Regulation); or |
| • | in any other circumstances falling within Article 1(4) of the Prospectus Regulation. |
provided that no such offer of shares shall require the Issuer or any representative to publish a prospectus pursuant to Article 3 of the Prospectus Regulation or supplement a prospectus pursuant to Article 23 of the Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to any shares in any relevant state means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “Prospectus Regulation” means Regulation (EU) 2017/1129.
184
Table of Contents
We have not authorized and do not authorize the making of any offer of shares through any financial intermediary on their behalf, other than offers made by the underwriters with a view to the final placement of the shares as contemplated in this prospectus. Accordingly, no purchaser of the shares, other than the underwriters, is authorized to make any further offer of the shares on behalf of us or the underwriters.
Notice to Prospective Investors in the United Kingdom
In relation to the United Kingdom, no shares of common stock have been offered or will be offered pursuant to this offering to the public in the United Kingdom prior to the publication of a prospectus in relation to the shares that either (i) has been approved by the Financial Conduct Authority, or (ii) is to be treated as if it had been approved by the Financial Conduct Authority in accordance with the transitional provision in Regulation 74 of the Prospectus (Amendment etc.) (EU Exit) Regulations 2019, except that offers of shares may be made to the public in the United Kingdom at any time under the following exemptions under the UK Prospectus Regulation:
| • | to any legal entity which is a qualified investor as defined in Article 2 of the UK Prospectus Regulation; |
| • | to fewer than 150 natural or legal persons (other than qualified investors as defined in Article 2 of the UK Prospectus Regulation); or |
| • | in any other circumstances falling within section 86 of the Financial Services and Markets Act 2000 (“FSMA”), |
provided that no such offer of shares shall require the Issuer or any representative to publish a prospectus pursuant to section 85 of the FSMA or supplement a prospectus pursuant to Article 23 of the UK Prospectus Regulation.
For the purposes of this provision, the expression an “offer to the public” in relation to any shares in any relevant state means the communication in any form and by any means of sufficient information on the terms of the offer and any shares to be offered so as to enable an investor to decide to purchase or subscribe for any shares, and the expression “UK Prospectus Regulation” means Regulation (EU) 2017/1129 as it forms part of domestic law by virtue of the European Union (Withdrawal) Act 2018.
We have not authorized and do not authorize the making of any offer of shares through any financial intermediary on their behalf, other than offers made by the underwriters with a view to the final placement of the shares as contemplated in this prospectus. Accordingly, no purchaser of the shares, other than the underwriters, is authorized to make any further offer of the shares on behalf of us or the underwriters.
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors” (as defined in Article 2 of the UK Prospectus Regulation) (i) who have professional experience in matters relating to investments falling within Article 19(5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Order, and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”) or otherwise in circumstances which have not resulted and will not result in an offer to the public of the shares in the United Kingdom within the meaning of the FSMA.
Any person in the United Kingdom that is not a relevant person should not act or rely on the information included in this document or use it as basis for taking any action. In the United Kingdom, any investment or investment activity that this document relates to may be made or taken exclusively by relevant persons.
Notice to Prospective Investors in Canada
The shares may be sold only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the
185
Table of Contents
Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the shares must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws.
Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor.
Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriters’ conflicts of interest in connection with this offering.
Notice to Prospective Investors in France
Neither this prospectus nor any other offering material relating to the shares described in this prospectus has been submitted to the clearance procedures of the Autorité des Marchés Financiers or by the competent authority of another member state of the European Economic Area and notified to the Autorité des Marchés Financiers.
The shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France. Neither this prospectus nor any other offering material relating to the shares has been or will be:
| • | released, issued, distributed or caused to be released, issued or distributed to the public in France; or |
| • | used in connection with any offer for subscription or sale of the shares to the public in France. |
Such offers, sales and distributions will be made in France only:
| • | to qualified investors (investisseurs qualifiés) and/or to a restricted circle of investors (cercle restreint d’investisseurs), in each case investing for their own account, all as defined in, and in accordance with, Article L.411-2, D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the French Code monétaire et financier; |
| • | to investment services providers authorized to engage in portfolio management on behalf of third parties; or |
| • | in a transaction that, in accordance with article L.411-2-II-1° -or-2° -or 3° of the French Code monétaire et financier and article 211-2 of the General Regulations (Règlement Général) of the Autorité des Marchés Financiers, does not constitute a public offer (appel public à l’épargne). |
The shares may be resold directly or indirectly, only in compliance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French Code monétaire et financier.
Notice to Prospective Investors in Hong Kong
The shares may not be offered or sold in Hong Kong by means of any document other than (i) in circumstances which do not constitute an offer to the public within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong), or (ii) to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder, or (iii) in other circumstances which do not result in the document being a “prospectus” within the meaning of the Companies Ordinance (Cap. 32, Laws of Hong Kong) and no advertisement, invitation or document relating to the shares may be issued or may be in the possession of any person for the purpose of issue (in each case whether in Hong Kong or elsewhere), which is
186
Table of Contents
directed at, or the contents of which are likely to be accessed or read by, the public in Hong Kong (except if permitted to do so under the laws of Hong Kong) other than with respect to shares which are or are intended to be disposed of only to persons outside Hong Kong or only to “professional investors” within the meaning of the Securities and Futures Ordinance (Cap. 571, Laws of Hong Kong) and any rules made thereunder.
Notice to Prospective Investors in Japan
The shares offered in this prospectus have not been and will not be registered under the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948, as amended) and, accordingly, will not be offered or sold, directly or indirectly, in Japan, or for the benefit of any Japanese Person or to others for re-offering or resale, directly or indirectly, in Japan or to any Japanese Person, except in compliance with all applicable laws, regulations and ministerial guidelines promulgated by relevant Japanese governmental or regulatory authorities in effect at the relevant time. For the purposes of this paragraph, “Japanese Person” will mean any person resident in Japan, including any corporation or other entity organized under the laws of Japan.
Notice to Prospective Investors in Singapore
This prospectus has not been registered as a prospectus with the Monetary Authority of Singapore. Accordingly, this prospectus and any other document or material in connection with the offer or sale, or invitation for subscription or purchase, of the shares may not be circulated or distributed, nor may the shares be offered or sold, or be made the subject of an invitation for subscription or purchase, whether directly or indirectly, to persons in Singapore other than (i) to an institutional investor (as defined in Section 4A of the Securities and Futures Act, Chapter 289 of Singapore (the “SFA”)) pursuant to Section 274 of the SFA, (ii) to a relevant person (as defined in Section 275(2) of the SFA) pursuant to Section 275(1) of the SFA, or any person pursuant to Section 275(1A) of the SFA, and in accordance with the conditions specified in Section 275 of the SFA or (iii) otherwise pursuant to, and in accordance with the conditions of, any other applicable provision of the SFA, in each case subject to compliance with conditions set forth in the SFA.
Where the shares are subscribed or purchased under Section 275 of the SFA by a relevant person which is:
| • | a corporation (which is not an accredited investor (as defined in Section 4A of the SFA)) the sole business of which is to hold investments and the entire share capital of which is owned by one or more individuals, each of whom is an accredited investor; or |
| • | a trust (where the trustee is not an accredited investor) whose sole purpose is to hold investments and each beneficiary of the trust is an individual who is an accredited investor |
shares, debentures and units of shares and debentures of that corporation or the beneficiaries’ rights and interest (howsoever described) in that trust will not be transferred within six months after that corporation or that trust has acquired the shares pursuant to an offer made under Section 275 of the SFA except:
| • | to an institutional investor or to a relevant person, or to any person arising from an offer referred to in Section 275(1A) or Section 276(4)(i)(B) of the SFA; |
| • | where no consideration is or will be given for the transfer; |
| • | where the transfer is by operation of law; or |
| • | as specified in Section 276(7) of the SFA. |
Notice to Prospective Investors in Australia
No prospectus or other disclosure document (as defined in the Corporations Act 2001 (Cth) of Australia (“Corporations Act”)) in relation to the shares has been or will be lodged with the Australian Securities &
187
Table of Contents
Investments Commission (“ASIC”). This document has not been lodged with ASIC and is only directed to certain categories of exempt persons. Accordingly, if you receive this document in Australia:
| a) | you confirm and warrant that you are either: |
| i. | a “sophisticated investor” under section 708(8)(a) or (b) of the Corporations Act; |
| ii. | a “sophisticated investor” under section 708(8)(c) or (d) of the Corporations Act and that you have provided an accountant’s certificate to us which complies with the requirements of section 708(8)(c)(i) or (ii) of the Corporations Act and related regulations before the offer has been made; |
| iii. | a person associated with the company under section 708(12) of the Corporations Act; or |
| iv. | a “professional investor” within the meaning of section 708(11)(a) or (b) of the Corporations Act, and to the extent that you are unable to confirm or warrant that you are an exempt sophisticated investor, associated person or professional investor under the Corporations Act any offer made to you under this document is void and incapable of acceptance; and |
| b) | you warrant and agree that you will not offer any of the common shares for resale in Australia within 12 months of the common shares being issued unless any such resale offer is exempt from the requirement to issue a disclosure document under section 708 of the Corporations Act. |
Notice to Prospective Investors in Chile
The shares are not registered in the Securities Registry (Registro de Valores) or subject to the control of the Chilean Securities and Exchange Commission (Superintendencia de Valores y Seguros de Chile). This prospectus and other offering materials relating to the offer of the shares do not constitute a public offer of, or an invitation to subscribe for or purchase, the shares in the Republic of Chile, other than to individually identified purchasers pursuant to a private offering within the meaning of Article 4 of the Chilean Securities Market Act (Ley de Mercado de Valores) (an offer that is not “addressed to the public at large or to a certain sector or specific group of the public”).
Notice to Prospective Investors in Switzerland
The shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering or marketing material relating to the shares or the offering may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering or marketing material relating to the offering, the Company, or the shares have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of shares will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA), and the offer of shares has not been and will not be authorized under the Swiss Federal Act on Collective Investment Schemes (“CISA”). The investor protection afforded to acquirers of interests in collective investment schemes under the CISA does not extend to acquirers of shares.
Notice to Prospective Investors in the Dubai International Financial Centre
This prospectus relates to an Exempt Offer in accordance with the Offered Securities Rules of the Dubai Financial Services Authority (“DFSA”). This prospectus is intended for distribution only to persons of a type specified in the Offered Securities Rules of the DFSA. It must not be delivered to, or relied on by, any other person. The DFSA has no responsibility for reviewing or verifying any documents in connection with Exempt
188
Table of Contents
Offers. The DFSA has not approved this prospectus nor taken steps to verify the information set forth herein and has no responsibility for the prospectus. The shares to which this prospectus relates may be illiquid and/or subject to restrictions on their resale. Prospective purchasers of the shares offered should conduct their own due diligence on the shares. If you do not understand the contents of this prospectus you should consult an authorized financial advisor.
189
Table of Contents
The validity of the shares of common stock will be passed upon for us by Simpson Thacher & Bartlett LLP, New York, New York. Certain legal matters in connection with this offering will be passed upon for the underwriters by Davis Polk & Wardwell LLP, New York, New York. An investment vehicle comprised of selected partners of Simpson Thacher & Bartlett LLP, members of their families, related persons and others owns an interest representing less than 1% of the capital commitments of funds affiliated with The Blackstone Group Inc.
The financial statements of Apria, Inc. as of December 31, 2019 and 2018, included in this prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein. Such financial statements have been included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
The financial statements of Apria Healthcare Group Inc. as of December 31, 2019 and 2018, and for each of the three years in the period ended December 31, 2019, included in this prospectus have been audited by Deloitte & Touche LLP, an independent registered public accounting firm, as stated in their report appearing herein. Such financial statements have been included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus, filed as part of the registration statement, does not contain all of the information set forth in the registration statement and its exhibits and schedules, portions of which have been omitted as permitted by the rules and regulations of the SEC. For further information about us and shares of our common stock, we refer you to the registration statement and to its exhibits and schedules. Statements in this prospectus about the contents of any contract, agreement or other document are not necessarily complete and in each instance we refer you to the copy or form of such contract, agreement or document filed as an exhibit to the registration statement. You may inspect these reports and other information without charge at a website maintained by the SEC. The address of this site is http://www.sec.gov.
We maintain an internet site at http://www.apria.com. The information on, or accessible from, our website is not part of this prospectus by reference or otherwise.
Upon completion of this offering, we will become subject to the informational requirements of the Exchange Act and will be required to file reports and other information with the SEC. You will be able to inspect these reports and other information without charge at the SEC’s website. We intend to make available to our common stockholders annual reports containing consolidated financial statements audited by an independent registered public accounting firm.
190
Table of Contents
| Audited Balance Sheets of Apria, Inc. |
||||
| F-2 | ||||
| Balance Sheets as of December 31, 2019 and December 31, 2018 |
F-3 | |||
| F-4 | ||||
| Unaudited Balance Sheets of Apria, Inc. |
||||
| Unaudited Balance Sheets as of September 30, 2020 and December 31, 2019 |
F-5 | |||
| F-6 | ||||
| Audited Consolidated Financial Statements of Apria Healthcare Group Inc. |
||||
| F-7 | ||||
| Consolidated Balance Sheets as of December 31, 2019 and December 31, 2018 |
F-8 | |||
| F-9 | ||||
| F-10 | ||||
| F-11 | ||||
| F-13 | ||||
| Unaudited Condensed Consolidated Financial Statements of Apria Healthcare Group Inc. |
||||
| Unaudited Condensed Consolidated Balance Sheets as of September 30, 2020 and December 31, 2019 |
F-36 | |||
| F-37 | ||||
| F-38 | ||||
| F-39 | ||||
| Notes to Unaudited Condensed Consolidated Financial Statements |
F-41 | |||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors of
Apria, Inc.
Lake Forest, California
Opinion on the Financial Statement
We have audited the accompanying balance sheets of Apria, Inc. (the “Company”) as of December 31, 2019 and 2018 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements presents fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ DELOITTE & TOUCHE LLP
Costa Mesa, California
August 6, 2020
We have served as the Company’s auditor since 2018.
F-2
Table of Contents
Table of Contents
NOTES TO BALANCE SHEETS
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Incorporation—Apria, Inc. (the “Company”) was incorporated as a Delaware corporation on March 22, 2018.
The Company has not commenced operations, nor has the Company entered into any contracts. The principal activity of the Company is intended to be that of a holding company.
Basis of Preparation—Balance sheets are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The financial statements are prepared in U.S. dollars which is considered to be the functional currency of the Company.
An income statement, cash flow statement and statement of changes in equity have not been presented as the Company has had no activity since incorporation.
| 2. | STOCKHOLDERS’ EQUITY |
The Corporation is authorized to issue 1,000 shares of common stock, par value $0.01 per share.
As of December 31, 2019 and 2018, the Company has not issued any shares of its common stock and does not have any assets or liabilities.
| 3. | SUBSEQUENT EVENTS |
Subsequent events have been evaluated through August 6, 2020, the date these financial statements were issued.
F-4
Table of Contents
BALANCE SHEETS (UNAUDITED)
| September 30, 2020 |
December 31, 2019 |
|||||||
| ASSETS |
||||||||
| TOTAL ASSETS |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| LIABILITIES |
||||||||
| TOTAL LIABILITIES |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
| STOCKHOLDERS’ EQUITY |
||||||||
| Common stock |
— | — | ||||||
|
|
|
|
|
|||||
| TOTAL STOCKHOLDERS’ EQUITY |
— | — | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
See notes to unaudited balance sheets.
F-5
Table of Contents
NOTES TO UNAUDITED BALANCE SHEETS
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Incorporation—Apria, Inc. (the “Company”) was incorporated as a Delaware corporation on March 22, 2018.
The Company has not commenced operations, nor has the Company entered into any contracts. The principal activity of the Company is intended to be that of a holding company.
Basis of Preparation—The balance sheets are prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). The financial statements are prepared in U.S. dollars which is considered to be the functional currency of the Company. The unaudited balance sheets have been prepared on the same basis as the audited balance sheet and, in the opinion of management, reflects all normal recurring adjustments necessary for the fair statement of the results for the period. Interim period results are not necessarily indicative of the results that may be expected for the full fiscal year.
An income statement, cash flow statement and statement of changes in equity have not been presented as the Company has had no activity since incorporation.
| 2. | STOCKHOLDERS’ EQUITY |
The Corporation is authorized to issue 1,000 shares of common stock, par value $0.01 per share.
As of September 30, 2020, the Company has not issued any shares of its common stock and does not have any assets or liabilities.
| 3. | SUBSEQUENT EVENTS |
Subsequent events have been evaluated through December 3, 2020, the date these financial statements were issued.
F-6
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Apria Healthcare Group Inc.
Lake Forest, California
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Apria Healthcare Group Inc. and subsidiaries (the “Company”) as of December 31, 2019 and 2018, the related consolidated statements of income, stockholder’s equity, and cash flows, for each of the three years in the period ended December 31, 2019, and the related notes to the financial statements (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Change in Accounting Principle
As discussed in Note 2 to the financial statements, effective January 1, 2019, the Company adopted FASB Accounting Standards Update 2016-02, Leases (Topic 842), using the modified retrospective transition method.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB and in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Deloitte & Touche LLP
Costa Mesa, California
August 6, 2020
We have served as the Company’s auditor since 1998.
F-7
Table of Contents
CONSOLIDATED BALANCE SHEETS
| December 31, | ||||||||
| (in thousands, except per share data) | 2019 | 2018 | ||||||
| ASSETS | ||||||||
| CURRENT ASSETS |
||||||||
| Cash and cash equivalents |
$ | 74,691 | $ | 63,890 | ||||
| Accounts receivable, less allowance for doubtful accounts of $0 and $20,131 as of December 31, 2019 and 2018, respectively |
84,071 | 97,527 | ||||||
| Inventories |
6,849 | 7,165 | ||||||
| Prepaid expenses and other current assets |
17,344 | 19,675 | ||||||
|
|
|
|
|
|||||
| TOTAL CURRENT ASSETS |
182,955 | 188,257 | ||||||
| NONCURRENT RESTRICTED CASH |
— | 780 | ||||||
| PATIENT EQUIPMENT, less accumulated depreciation of $322,153 and $314,830 as of December 31, 2019 and 2018, respectively |
232,656 | 236,592 | ||||||
| PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET |
26,436 | 27,578 | ||||||
| INTANGIBLE ASSETS, NET |
62,071 | 62,645 | ||||||
| OPERATING LEASE RIGHT-OF-USE ASSETS |
65,332 | — | ||||||
| DEFERRED INCOME TAXES, NET |
34,699 | 44,089 | ||||||
| OTHER ASSETS |
13,004 | 16,281 | ||||||
|
|
|
|
|
|||||
| TOTAL ASSETS |
$ | 617,153 | $ | 576,222 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| CURRENT LIABILITIES |
||||||||
| Accounts payable |
$ | 122,819 | $ | 148,593 | ||||
| Accrued payroll and related taxes and benefits |
48,651 | 40,722 | ||||||
| Other accrued liabilities |
27,834 | 33,820 | ||||||
| Deferred revenue |
24,860 | 25,470 | ||||||
| Current portion of operating lease liabilities |
24,546 | — | ||||||
| Current portion of long-term debt |
3,750 | — | ||||||
|
|
|
|
|
|||||
| TOTAL CURRENT LIABILITIES |
252,460 | 248,605 | ||||||
| LONG-TERM DEBT, less current portion |
144,034 | — | ||||||
| OPERATING LEASE LIABILITIES, less current portion |
40,287 | — | ||||||
| OTHER NONCURRENT LIABILITIES |
45,856 | 42,365 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES |
482,637 | 290,970 | ||||||
| COMMITMENTS AND CONTINGENCIES (NOTE 9) |
||||||||
| STOCKHOLDERS’ EQUITY |
||||||||
| Common stock, $0.01 par value: 1,100,000 shares authorized; 992,719 shares issued as of December 31, 2019 and 2018 |
— | — | ||||||
| Additional paid-in capital |
1,161,087 | 1,327,445 | ||||||
| Accumulated deficit |
(1,026,571 | ) | (1,042,193 | ) | ||||
|
|
|
|
|
|||||
| TOTAL STOCKHOLDERS’ EQUITY |
134,516 | 285,252 | ||||||
|
|
|
|
|
|||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
$ | 617,153 | $ | 576,222 | ||||
|
|
|
|
|
|||||
See notes to consolidated financial statements.
F-8
Table of Contents
CONSOLIDATED STATEMENTS OF INCOME
| Year Ended December 31, | ||||||||||||
| (in thousands, except per share data) | 2019 | 2018 | 2017 | |||||||||
| Net revenues: |
||||||||||||
| Fee for service arrangements |
$ | 870,344 | $ | 898,622 | $ | 866,397 | ||||||
| Capitation |
218,531 | 212,261 | 207,413 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL NET REVENUES |
1,088,875 | 1,110,883 | 1,073,810 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses: |
||||||||||||
| Cost of net revenues |
||||||||||||
| Product and supply costs |
206,067 | 218,099 | 200,621 | |||||||||
| Patient equipment depreciation |
97,386 | 108,340 | 101,724 | |||||||||
| Home respiratory therapists costs |
19,560 | 20,371 | 20,802 | |||||||||
| Other |
17,701 | 13,276 | 9,398 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL COST OF NET REVENUES |
340,714 | 360,086 | 332,545 | |||||||||
| Provision for doubtful accounts |
— | 31,719 | 42,672 | |||||||||
| Selling, distribution and administrative |
720,746 | 698,681 | 672,442 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL COSTS AND EXPENSES |
1,061,460 | 1,090,486 | 1,047,659 | |||||||||
|
|
|
|
|
|
|
|||||||
| OPERATING INCOME |
27,415 | 20,397 | 26,151 | |||||||||
| Interest expense |
5,112 | 1,338 | 1,246 | |||||||||
| Interest income and other |
(1,446 | ) | (897 | ) | (486 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| INCOME BEFORE INCOME TAXES |
23,749 | 19,956 | 25,391 | |||||||||
| Income tax expense (benefit) |
8,127 | 6,829 | (71,560 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| NET INCOME |
$ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||
|
|
|
|
|
|
|
|||||||
| Basic and diluted earnings per share: |
||||||||||||
| Net income attributable to common stockholders |
$ | 15.74 | $ | 13.22 | $ | 97.66 | ||||||
|
|
|
|
|
|
|
|||||||
| Weighted average shares outstanding: |
||||||||||||
| Basic and diluted |
992,719 | 992,719 | 992,719 | |||||||||
See notes to consolidated financial statements.
F-9
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
| (in thousands, except for share data) | Shares of Common Stock |
Additional Paid In Capital |
Accumulated Deficit |
Total Stockholders’ Equity |
||||||||||||
| Balance as of December 31, 2016 |
992,719 | $ | 1,399,103 | $ | (1,152,271 | ) | $ | 246,832 | ||||||||
| Stock-based compensation |
— | 1,721 | — | 1,721 | ||||||||||||
| Net income |
— | — | 96,951 | 96,951 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2017 |
992,719 | $ | 1,400,824 | $ | (1,055,320 | ) | $ | 345,504 | ||||||||
| Dividends |
— | (75,000 | ) | — | (75,000 | ) | ||||||||||
| Stock-based compensation |
— | 1,621 | — | 1,621 | ||||||||||||
| Net income |
— | — | 13,127 | 13,127 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2018 |
992,719 | $ | 1,327,445 | $ | (1,042,193 | ) | $ | 285,252 | ||||||||
| Dividends |
— | (175,000 | ) | — | (175,000 | ) | ||||||||||
| Stock-based compensation |
— | 8,642 | — | 8,642 | ||||||||||||
| Net income |
— | — | 15,622 | 15,622 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of December 31, 2019 |
992,719 | $ | 1,161,087 | $ | (1,026,571 | ) | $ | 134,516 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
See notes to consolidated financial statements.
F-10
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| OPERATING ACTIVITIES |
||||||||||||
| Net income |
$ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||
| Items included in net income not requiring cash: |
||||||||||||
| Provision for doubtful accounts |
— | 31,719 | 42,672 | |||||||||
| Depreciation |
111,001 | 124,214 | 118,680 | |||||||||
| Amortization of intangible assets |
574 | 775 | 617 | |||||||||
| Non-cash lease expense |
27,596 | — | — | |||||||||
| Deferred income taxes |
9,390 | 6,615 | (72,712 | ) | ||||||||
| Stock-based compensation |
8,642 | 1,621 | 1,721 | |||||||||
| Amortization of deferred debt issuance costs |
1,107 | 378 | 263 | |||||||||
| Loss (gain) on sale of patient equipment and other |
4,130 | 1,041 | (7,753 | ) | ||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Accounts receivable |
13,456 | (22,012 | ) | (31,808 | ) | |||||||
| Inventories |
315 | 739 | (1,289 | ) | ||||||||
| Prepaid expenses and other assets |
2,332 | (1,089 | ) | 5,142 | ||||||||
| Accounts payable |
(15,086 | ) | 1,819 | 10,192 | ||||||||
| Accrued payroll and related taxes and benefits |
7,929 | (2,424 | ) | 1,477 | ||||||||
| Operating lease liabilities |
(29,494 | ) | — | — | ||||||||
| Income taxes payable |
(1,448 | ) | 96 | 83 | ||||||||
| Deferred revenue |
(610 | ) | 741 | 2,222 | ||||||||
| Accrued expenses |
6,394 | 2,218 | (11,113 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES |
161,850 | 159,578 | 155,345 | |||||||||
|
|
|
|
|
|
|
|||||||
| INVESTING ACTIVITIES |
||||||||||||
| Purchases of patient equipment and property, equipment and |
(114,485 | ) | (122,123 | ) | (126,408 | ) | ||||||
| Proceeds from sale of patient equipment and other |
21,772 | 21,857 | 20,532 | |||||||||
| Proceeds from the settlement of corporate-owned life insurance |
— | 3,505 | — | |||||||||
| Cash paid for acquisition |
— | (708 | ) | (43 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| NET CASH USED IN INVESTING ACTIVITIES |
(92,713 | ) | (97,469 | ) | (105,919 | ) | ||||||
|
|
|
|
|
|
|
|||||||
Continued on following page.
F-11
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS (CONTINUED)
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| FINANCING ACTIVITIES |
||||||||||||
| Payments on asset financing |
(31,353 | ) | (33,361 | ) | (20,351 | ) | ||||||
| Borrowings on debt |
150,000 | — | — | |||||||||
| Proceeds from revolving credit facility |
5,000 | 7,000 | — | |||||||||
| Disbursements to revolving credit facility |
(5,000 | ) | (7,000 | ) | — | |||||||
| Payments of deferred financing costs |
(2,763 | ) | (587 | ) | — | |||||||
| Payment of dividend |
(175,000 | ) | (75,000 | ) | — | |||||||
| Proceeds from related-party payable |
— | — | 9,440 | |||||||||
| Disbursements related to related-party payable |
— | (854 | ) | (9,197 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| NET CASH USED IN FINANCING ACTIVITIES |
(59,116 | ) | (109,802 | ) | (20,108 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| NET INCREASE (DECREASE) IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH |
10,021 | (47,693 | ) | 29,318 | ||||||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH AT BEGINNING OF PERIOD |
64,670 | 112,363 | 83,045 | |||||||||
|
|
|
|
|
|
|
|||||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH AT END OF PERIOD |
$ | 74,691 | $ | 64,670 | $ | 112,363 | ||||||
|
|
|
|
|
|
|
|||||||
SUPPLEMENTAL DISCLOSURES—See Note 5 – Debt and Note 7 – Income Taxes for a discussion of cash paid for interest and income taxes, respectively.
NONCASH INVESTING AND FINANCING TRANSACTIONS—Purchases of patient equipment and property, equipment and improvements exclude purchases that remain unpaid at the end of the respective period. Such amounts are then included in the following period’s purchases. Unpaid purchases were $59.8 million, $73.8 million and $82.7 million as of December 31, 2019, 2018 and 2017, respectively. Unpaid purchases include $24.4 million, $32.4 million and $34.8 million of patient equipment and property, equipment and improvements acquired under extended payment terms as of December 31, 2019, 2018 and 2017, respectively.
See notes to consolidated financial statements.
F-12
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation—The accompanying consolidated financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America and are presented in U.S. dollars. These consolidated financial statements include the accounts of Apria Healthcare Group Inc. (the “Company”) and its subsidiaries. The Company had no items of other comprehensive income; as such, its comprehensive income is the same as the net income for all periods presented. Intercompany transactions and accounts have been eliminated in consolidation.
Company Background—The Company operates in the home health care segment of the health care industry, providing a variety of high-quality clinical patient care management programs, related products and supplies as prescribed by a physician and/or authorized by a case manager as part of a care plan. Essentially all products and services offered by the Company are provided through the Company’s network of approximately 315 locations (as of December 31, 2019), which are located throughout the United States. The Company provides services and products in one operating segment: home respiratory therapy/home medical equipment. The Company provides patients in their homes with products and services which are primarily paid for by a third-party payor, such as Medicare, Medicaid, a managed care plan or another third-party insurer. Sales are primarily derived from referral sources such as hospital discharge planners, medical groups or independent physicians.
On October 28, 2008, the Company was acquired by a wholly owned affiliate of BP Healthcare Holdings LLC (“Buyer” or “BP Healthcare”). Buyer is controlled by private investment funds affiliated with The Blackstone Group Inc. (the “Sponsor”).
Use of Accounting Estimates—The preparation of the consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the consolidated financial statements and accompanying notes. Actual results could differ from those estimates. Among the significant estimates affecting the consolidated financial statements are those related to revenue recognition and the resulting accounts receivable, self-insurance reserves, long-lived assets, stock-based compensation and income taxes.
Fee-for-Service Net Revenues—Revenues are recognized under fee-for-service arrangements for equipment the Company rents to patients and sales of equipment, supplies and other items the Company sells to patients.
F-13
Table of Contents
Rental and sale net revenues under fee-for-service arrangements disaggregated by each core service line item were:
| Year Ended December 31, | ||||||||||||||||||||||||||||||||||||
| 2019 | 2018 | 2017 | ||||||||||||||||||||||||||||||||||
| (dollars in thousands) |
Rental | Sale | Total Fee- For- Service |
Rental | Sale | Total Fee- For- Service |
Rental | Sale | Total Fee- For- Service |
|||||||||||||||||||||||||||
| Home respiratory therapy |
$ | 385,429 | $ | 2,241 | $ | 387,670 | $ | 390,835 | $ | 2,520 | $ | 393,355 | $ | 378,573 | $ | 4,238 | $ | 382,811 | ||||||||||||||||||
| Obstructive sleep apnea treatment |
84,225 | 262,789 | 347,014 | 92,857 | 252,872 | 345,729 | 92,034 | 230,501 | 322,535 | |||||||||||||||||||||||||||
| Negative pressure wound therapy |
29,832 | 2,677 | 32,509 | 28,228 | 2,217 | 30,445 | 28,665 | 3,378 | 32,043 | |||||||||||||||||||||||||||
| Other equipment and services |
43,317 | 59,834 | 103,151 | 52,115 | 76,978 | 129,093 | 50,806 | 78,202 | 129,008 | |||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| Total |
$ | 542,803 | $ | 327,541 | $ | 870,344 | $ | 564,035 | $ | 334,587 | $ | 898,622 | $ | 550,078 | $ | 316,319 | $ | 866,397 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||||
| 62.4 | % | 37.6 | % | 100.0 | % | 62.8 | % | 37.2 | % | 100.0 | % | 63.5 | % | 36.5 | % | 100.0 | % | |||||||||||||||||||
Rental revenues—In accordance with Financial Accounting Standards Board (FASB) Accounting Standards Update (ASU) No. 2016-02, Leases (Topic 842), revenue generated from equipment that the Company rents to patients is recognized over the noncancelable rental period, typically one month, and commences on delivery of the equipment to the patients. The Company evaluates the portfolio of lease contracts at lease commencement and the start of each monthly renewal period to determine if it is reasonably certain that the monthly renewal or purchase options would be exercised. The exercise of monthly renewal or purchase options by a patient has historically not been reasonably certain to occur at lease commencement or subsequent monthly renewal.
Revenues are recorded at amounts estimated to be received under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare, Medicaid and patients. Rental revenue, less estimated adjustments, is recognized as earned on a straight-line basis over the non-cancellable lease term. Rental of patient equipment is billed on a monthly basis beginning on the date the equipment is delivered. Since deliveries can occur on any day during a month, the amount of billings that apply to the next month are deferred.
The Company’s lease agreements generally contain lease and non-lease components. Non-lease components primarily relate to supplies. The Company allocates the transaction price to the separate lease and non-lease components that qualify as performance obligations using the stand-alone selling price.
Sale revenues—Revenue related to sales of equipment and supplies is recognized on the date of delivery as this is when control of the promised goods is transferred to patients and is presented net of applicable sales taxes. Revenues are recorded only to the extent it is probable that a significant reversal will not occur in the future as amounts may include implicit price concessions under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare, Medicaid and patients. The Company determines the sales transaction price based on contractually agreed-upon rates, adjusted for estimates of variable consideration. The Company uses the expected value method in determining the variable consideration as part of determining the sales transaction price using historical reimbursement experience, historical sales returns, and other operating trends. Payment terms and conditions vary by contract. The timing of revenue recognition, billing, and cash collection generally results in billed and unbilled accounts receivable.
Capitation Revenues—Revenues are recognized under capitation arrangements with third-party payors for services and equipment for which the Company stands ready to provide to the members of these payors
F-14
Table of Contents
without regard to the actual services provided. The stand ready obligation generally extends beyond one year. Revenue is recognized over the month that the members are entitled to health care services using the contractual rate for each covered member. The actual number of covered members may vary each month. As a practical expedient, no disclosures have been made related to the amount of variable consideration expected to be recognized in future periods under these capitation arrangements. Capitation payments are typically received in the month members are entitled to health care services. Contracts with a single national payor constituted 86%, 85% and 84% of the total capitation revenue for the years ended December 31, 2019, 2018 and 2017, respectively.
Concentration of Credit Risk—Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 19% and 1%, respectively, of total net revenues for the year ended December 31, 2019. Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 18% and 1%, respectively, of total net revenues for the year ended December 31, 2018. Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 18% and 2%, respectively, of total net revenues for the year ended December 31, 2017. Contracts with a single national payor constituted 23%, 21% and 22% of total net revenues for the year ended December 31, 2019, 2018 and 2017, respectively. As of December 31, 2019, 2018 and 2017, Medicare represented greater than 10% of net accounts receivable. No other third-party payor constituted more than 10% of the Company’s total net revenues for any period presented.
Cash and Cash Equivalents—Cash is maintained with various financial institutions. These financial institutions are located throughout the United States and the Company’s cash management practices limit exposure to any one institution. Management considers all highly liquid instruments purchased with an original maturity of less than three months to be cash equivalents.
Restricted Cash—Noncurrent restricted cash is related to amounts required to be held for payment of benefits under the Company’s nonqualified deferred compensation plan and was $0.0 million and $0.8 million as of December 31, 2019 and 2018, respectively.
Accounts Receivable—Included in accounts receivable are earned but unbilled receivables of $24.1 million and $17.7 million as of December 31, 2019 and 2018, respectively. Delays ranging from a day up to several weeks between the date of service and billing can occur due to delays in obtaining certain required payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in the analysis of historical performance and collectability.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record total net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Such adjustments are typically identified and recorded at the point of cash application, claim denial or account review.
Management performs periodic analyses to evaluate accounts receivable balances to ensure that recorded amounts reflect estimated net realizable value. Specifically, management considers historical realization data, accounts receivable aging trends, other operating trends, and the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care payor claims processing procedures and system changes.
Additionally, focused reviews of certain large and/or problematic payors are performed. Due to continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on operations and cash flows.
Prior to adoption of Topic 842, accounts receivable was reduced by a separate allowance for doubtful accounts which provided for those accounts from which payment was not expected to be received, although services were provided and revenue was earned. Upon determination that an account was uncollectible, it
F-15
Table of Contents
was written off and charged to the allowance. Upon adoption of Topic 842, the Company has elected to record a reserve for expected credit losses as part of net rental revenue adjustments in order to report rental revenue at an expected collectable amount based on the total portfolio of operating lease receivables for which collectability has been deemed probable.
The following table summarizes activity for the allowance for doubtful accounts for the years ended December 31, 2019, 2018 and 2017:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (in thousands) | ||||||||||||
| Balance beginning of the year |
$ | 20,131 | $ | 37,709 | $ | 34,735 | ||||||
| Provision for doubtful accounts |
— | 31,719 | 42,672 | |||||||||
| Deductions |
(20,131 | ) | (49,297 | ) | (39,698 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Balance end of the year |
$ | — | $ | 20,131 | $ | 37,709 | ||||||
|
|
|
|
|
|
|
|||||||
Inventories—Inventories are stated at the lower of cost (approximate costs determined on the first-in, first-out basis) or net realizable value and consist primarily of respiratory supplies and items used in conjunction with patient equipment.
Patient Equipment—Patient equipment is stated at cost less depreciation and consists of medical equipment rented to patients on a month-to-month basis. Depreciation is provided using the straight-line method over the estimated useful lives of the equipment, which range from 1 to 10 years. Patient equipment depreciation is classified in the Company’s consolidated statements of income within costs of net revenues as the equipment is rented to patients as part of the Company’s primary operations. Depreciation expense for patient equipment was $97.4 million, $108.3 million and $101.7 million for the years ended December 31, 2019, 2018 and 2017, respectively.
Patient equipment is generally placed for rent; however, it could also be sold to customers. Once returned to the Company, patient equipment is assessed and repaired as necessary. Patient equipment is typically leased to subsequent patients if its condition is suitable. Upon a sale, the Company records the proceeds of the sale within net revenues and the costs related to the carrying net book value as other costs within cost of net revenues in the Company’s consolidated statements of income.
Given rental income is generated from such products, purchases of patient equipment are considered an investing activity when paid soon before or after purchase while other payments made are considered a financing activity within the consolidated statements of cash flows. Certain unpaid purchases are secured by a security interest in $17.9 million, $45.5 million and $50.4 million of patient equipment as of December 31, 2019, 2018 and 2017, respectively. The net loss (gain) from the sale of patient equipment is reported as an adjustment to net income within cash provided by operating activities in the consolidated statements of cash flows.
Property, Equipment and Improvements—Property, equipment and improvements are stated at cost less depreciation. Depreciation is provided using the straight-line method over the estimated useful lives of the assets, which range from 1 to 15 years or, for leasehold improvements, the shorter of the useful life of the asset or the remaining life of the related lease.
Capitalized Software—Capitalized software costs related to internally developed and purchased software are included in property, equipment and improvements in the consolidated balance sheets and are amortized using the straight-line method over the estimated useful lives of the assets, which range from three to five years. Capitalized costs include direct costs of materials and services incurred in developing or obtaining internal-use software and payroll and benefit costs for employees directly involved in the development of internal-use software. Capitalization of such costs ceases when the project is substantially complete and ready for its intended purpose. Costs incurred during the preliminary and post-implementation stages, as
F-16
Table of Contents
well as software maintenance and training costs, are expensed in the period in which they are incurred. Additions to capitalized internally developed software totaled $5.2 million and $5.4 million for the years ended December 31, 2019 and 2018, respectively. Amortization expense for internally developed software was $5.7 million, $7.2 million and $7.3 million for the years ended December 31, 2019, 2018 and 2017, respectively.
Indefinite-Lived Intangible Assets and Long-Lived Assets—Indefinite-lived intangible assets are not amortized but instead tested at least annually for impairment or more frequently when events or changes in circumstances indicate that the assets might be impaired. The Company performs the annual test for impairment for indefinite-lived intangible assets as of the first day of the fourth quarter.
The Company will first assess qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If, based on a review of qualitative factors, it is more likely than not that the fair value is less than its carrying amount, the Company will use a quantitative approach, and calculate the fair value and compare it to its carrying amount. If the fair value exceeds the carrying amount, there is no indication of impairment. If the carrying amount exceeds the fair value, an impairment loss is recorded equal to the difference.
The Company performed an assessment of qualitative factors and determined that no events or circumstances existed that would lead to a determination that it is more likely than not that the fair value of indefinite-lived assets were less than the carrying amount. As such, a quantitative analysis was not required to be performed.
Long-lived assets, including property and equipment and purchased definite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Significant judgment is required in determining whether a potential indicator of impairment of long-lived assets exists and in estimating future cash flows for any necessary impairment tests. Recoverability of assets to be held and used is measured by the comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
The Company did not record any impairment charges related to indefinite-lived intangible assets or long-lived assets for the years ended December 31, 2019, 2018 and 2017.
Fair Value of Financial Instruments—Management is required to disclose the estimated fair value of certain assets and liabilities of financial instruments. Financial instruments are generally defined as cash, evidence of ownership interest in an entity or a contractual obligation that both conveys to one entity a right to receive cash or other financial instruments from another entity and imposes on the other entity the obligation to deliver cash or other financial instruments to the first entity. The carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, and accrued expenses approximate fair value due to their short maturity. The carrying amounts of the Company’s long-term debt, including the Term Loan A Facility and Revolving Credit Facility as of December 31, 2019, approximate fair value due to the variable rate nature of the agreements. All debt classifications represent Level 2 fair value measurements.
Leases—The Company determines if an arrangement is a lease at commencement and performs an evaluation to determine whether the lease should be classified as an operating or finance lease. Operating leases are included in operating lease right-of-use (ROU) assets, current operating lease liability and noncurrent operating lease liability on the consolidated balance sheet. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and the related liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate (IBR) based on the information available at the lease commencement date in determining the present value
F-17
Table of Contents
of future lease payments. The Company used market rates from recent secured financing to determine the IBR.
The operating lease ROU asset also includes any lease payments made to the lessor at or before the commencement date and is adjusted by any lease incentives received. Variable lease payments are not included in the operating lease liability as they cannot be reasonably estimated and are recognized in the period in which the obligation for those payments is incurred. Lease terms may include options to extend or terminate the lease and are included only when it is reasonably certain that the Company will exercise that option. For all asset classes, leases with a lease term of twelve months or less at the lease commencement date are not recorded on the consolidated balance sheet, as permitted by the short-term lease exception. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
The Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants. The Company does not have any material subleases. The Company does not have any leases classified as a finance leasing arrangement. As such, all leases are classified as operating leases. See further discussion at Note 8 – Leases.
Product and Supply Costs—Product and supply costs presented within total cost of net revenues are comprised primarily of the cost of supplies, equipment and accessories provided to patients.
Contract Costs—The Company pays sales commissions on fee-for-service arrangements in an effort to increase the volume of serviced patients. The Company elected to use the practical expedient to expense sales commissions as incurred since the amortization period would otherwise be less than one year. These costs are included in selling, distribution and administrative expense in the consolidated statements of income.
Home Respiratory Therapists Costs—Home respiratory therapists costs presented within total cost of net revenues are comprised primarily of employee salary and benefit costs or contract fees paid to respiratory therapists and other related professionals who are deployed to service a patient. Home respiratory therapy personnel are also engaged in a number of administrative and marketing tasks and, accordingly, these costs are classified within selling, distribution and administrative expenses and were $15.5 million, $12.7 million and $16.1 million for the years ended December 31, 2019, 2018 and 2017, respectively.
Distribution Expenses—Distribution expenses are included in selling, distribution and administrative expenses and totaled $128.0 million, $126.3 million and $119.5 million for the years ended December 31, 2019, 2018 and 2017, respectively. Such expense represents the cost incurred to coordinate and deliver products and services to the patients. Included in distribution expenses are leasing, maintenance, licensing and fuel costs for the vehicle fleet; salaries and other costs related to drivers and dispatch personnel; and amounts paid to courier and other outside shipping vendors. Such expenses fall within the definition of “shipping and handling” costs and are classified within selling, distribution and administrative expenses and may not be comparable to other companies.
Self-Insurance—Coverage for certain employee medical claims and benefits, as well as workers’ compensation, professional and general liability, and vehicle liability are self-insured. Amounts accrued for costs of workers’ compensation, medical, professional and general liability, and vehicle liability are classified as current or long-term liabilities based upon an estimate of when the liability will ultimately be paid.
Amounts accrued as current liabilities within other accrued liabilities are as follows:
| December 31, | ||||||||
| (in thousands) | 2019 | 2018 | ||||||
| Workers’ compensation |
$ | 4,963 | $ | 6,190 | ||||
| Professional and general liability/vehicle |
2,745 | 2,464 | ||||||
| Medical insurance |
2,413 | 1,962 | ||||||
F-18
Table of Contents
Amounts accrued as long-term liabilities within other noncurrent liabilities are as follows:
| December 31, | ||||||||
| (in thousands) | 2019 | 2018 | ||||||
| Workers’ compensation |
$ | 17,793 | $ | 21,198 | ||||
| Professional and general liability/vehicle |
5,281 | 5,409 | ||||||
Related-Party Payable—Related-party payable represents amounts held by the Company on behalf of the Sponsor in accordance with a nominee agreement.
Stock-Based Compensation—The Company accounts for its stock-based awards in accordance with provisions of FASB Accounting Standards Codification (ASC) No. 718, Compensation—Stock Compensation. The Company has two types of compensation awards, profit interest units and stock appreciation rights (“SARs”). The Company recognizes compensation expense in respect of the profit interest units and SARs based on the fair value of the awards as measured on the grant date. Fair value is not subsequently remeasured unless the conditions on which the award was granted are modified. The determination of fair value at grant date requires the use of estimates, which are based on management’s judgment. Generally, compensation expense for each separately vesting portion of the awards is recognized on a straight-line basis over the vesting period for that portion of the award subject to continued service. Compensation expense for awards containing performance conditions is recognized only to the extent that it is probable the performance conditions will be satisfied.
Legal Reserves—The Company is involved in various legal proceedings, claims, and litigation that arise in the ordinary course of business. The Company investigates these matters as they arise and reserves for potential loss in accordance with ASC No. 450, Contingencies. Significant judgment is required in the determination of both the probability of loss and whether the amount of the loss can be reasonably estimated. Estimates are subjective and are made in consultation with internal and external legal counsel. See further discussion at Note 9 – Commitments and Contingencies.
Dividend—In June 2019, the Company declared and paid a $175.0 million dividend to common stockholders and SARs unit-holders. On June 19, 2018, the Company declared a $75.0 million dividend, which was paid to common stockholders on July 5, 2018.
Income Taxes—The Company’s provision for income taxes is based on expected income, permanent book/tax differences and statutory tax rates in the various jurisdictions in which the Company operates. Significant management estimates and judgments are required in determining the provision for income taxes.
Deferred income tax assets and liabilities are computed for differences between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized.
Business Segments—The Company has evaluated segment reporting in accordance with FASB ASC No. 280, Segment Reporting. The Company’s chief executive officer is its chief operating decision maker. The chief operating decision maker reviews financial information about the business at the enterprise-wide consolidated level when allocating the resources of the Company and assessing business performance. Accordingly, the Company has determined that its business activities comprise a single operating and reporting segment, the home respiratory therapy/home medical equipment segment. Through its single segment, the Company focuses on three core service lines: home respiratory therapy (including home oxygen and non-invasive ventilation services), obstructive sleep apnea treatment (including continuous positive airway pressure and bi-level positive airway pressure devices, and patient support services) and negative pressure wound therapy. Additionally, the Company supplies a wide range of home medical equipment and other products and services to help improve the quality of life for patients with home care needs.
F-19
Table of Contents
Net revenues for each core service line were:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (in thousands) | ||||||||||||
| Home respiratory therapy |
$ | 435,680 | $ | 441,190 | $ | 432,416 | ||||||
| Obstructive sleep apnea treatment |
438,572 | 430,354 | 400,672 | |||||||||
| Negative pressure wound therapy |
42,122 | 41,383 | 42,013 | |||||||||
| Other equipment and services |
172,501 | 197,956 | 198,709 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net revenues |
$ | 1,088,875 | $ | 1,110,883 | $ | 1,073,810 | ||||||
|
|
|
|
|
|
|
|||||||
Earnings per share—Basic income per share represents net income divided by the weighted average number of shares outstanding during the period. Diluted income per share represents net income divided by the weighted-average number of shares outstanding plus potential dilutive shares. For the years ended December 31, 2019, 2018 and 2017, the Company determined that there were no potential dilutive shares to be included in the calculation of diluted income per share as a result of its stock-based awards.
The computation of net income per share is presented below:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| (in thousands, except per share data) | ||||||||||||
| Net income attributable to common shareholders |
$ | 15,622 | $ | 13,127 | $ | 96,951 | ||||||
| Weighted average number of shares outstanding |
992,719 | 992,719 | 992,719 | |||||||||
| Dilutive effect of stock-based awards |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted weighted average number of shares outstanding |
992,719 | 992,719 | 992,719 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic and diluted net income per share |
$ | 15.74 | $ | 13.22 | $ | 97.66 | ||||||
| 2. | RECENT ACCOUNTING PRONOUNCEMENTS |
Recently Adopted Accounting Pronouncements
In November 2016, the FASB issued ASU No. 2016-18, Statement of Cash Flows (Topic 230): Restricted Cash, which amends FASB ASC No. 230, Statement of Cash Flows, to clarify guidance on the classification and presentation of restricted cash in the statement of cash flows. ASU No. 2016-18 is effective for fiscal years beginning after December 15, 2017, and interim periods therein, and must be applied retrospectively to all periods presented. The Company adopted this ASU on January 1, 2018. Prior to the adoption of this ASU, the Company’s consolidated statements of cash flows excluded restricted cash from the beginning and ending balances of cash and cash equivalents.
The following table provides a reconciliation of cash, cash equivalents and restricted cash reported on the consolidated balance sheets that sum to amounts reported in the consolidated statements of cash flows:
| (in thousands) | December 31, | |||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Cash and cash equivalents |
$ | 74,691 | $ | 63,890 | $ | 111,509 | ||||||
| Restricted cash |
— | 780 | 854 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cash, cash equivalents and restricted cash |
$ | 74,691 | $ | 64,670 | $ | 112,363 | ||||||
|
|
|
|
|
|
|
|||||||
In August 2016, the FASB issued ASU No. 2016-15, Statement of Cash Flows (Topic 230): Classification of Certain Cash Receipts and Cash Payments, which amends FASB ASC No. 230, Statement of Cash Flows, to add or clarify guidance on certain cash receipts and payments in the statement of cash flows.
F-20
Table of Contents
ASU No. 2016-15 is effective for fiscal years beginning after December 15, 2017, including interim periods therein, and must be applied retrospectively to all periods presented unless retrospective application would be impractical. The Company adopted this ASU for the year ended December 31, 2018. As such, any cash proceeds from the settlement of corporate-owned life insurance policies would be classified in investing activities in the Company’s consolidated statements of cash flows.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842), which results in lessees recognizing a ROU asset and corresponding lease liability on the consolidated balance sheet for most leases. Lessor accounting will remain substantially similar to the current accounting; however, certain refinements were made to conform the standard to the recently issued revenue recognition guidance in ASU 2014-09, Revenue from Contracts with Customers (Topic 606), specifically related to the allocation and recognition of contract consideration earned from lease and non-lease revenue components. In July 2018, the FASB issued ASU 2018-11, Leases (Topic 842), Targeted Improvements, which allows entities to elect not to recast the comparative periods presented in transition. ASU No. 2016-02 is effective for fiscal years beginning after December 15, 2018 and interim periods therein. The Company adopted this ASU effective January 1, 2019 using the modified retrospective transition method. By electing this transition method, the Company was not required to recast its comparative financial statements or provide disclosures required by the new standard for comparative periods. There were no cumulative adjustments recorded to accumulated deficit as a result of the adoption.
Lessor Accounting
The Company determined that its lessor accounting would not substantially change from its current accounting. The Company elected to use the portfolio approach and recognizes equipment rental revenue over the noncancelable lease term, which is typically one month, less estimated adjustments. The impact on rental revenue as a result of the adoption did not have a material impact on the Company’s consolidated financial results; however, what was previously included in the provision for doubtful accounts associated with rental revenue will be charged to net rental revenue instead of general and administrative expense upon adoption. This reclassification resulted in decreased net rental revenue and decreased provision for doubtful accounts of $34.5 million for the year ended December 31, 2019.
Lessee Accounting
The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification determines whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease. A lessee is also required to record a right-of-use asset and a lease liability for all leases with a term of greater than 12 months, regardless of lease classification.
The Company elected the package of practical expedients, which allowed the carryforward of historical lease identification, lease classification and initial direct costs. In addition, the Company elected the practical expedient to combine lease and non-lease components for all its real estate leases into a single component. The Company did not elect the use of the hindsight practical expedient.
Additionally, the Company elected the practical expedient to not record leases with an initial term of twelve months or less on the consolidated balance sheets for all asset classes. As these leases are considered short-term in nature, they are expensed on a straight-line basis over the lease term, which commences on the date the Company has the right to control the property.
F-21
Table of Contents
The following table summarizes the impact of the adoption of the new Topic 842 lease accounting guidance on the Company’s consolidated balance sheet as of January 1, 2019.
| (in thousands) | Balances at December 31, 2018 |
Adjustments from Adoption of Topic 842(1) |
Balances at January 1, 2019 |
|||||||||
| Total Assets |
$ | 576,222 | 74,425 | 650,647 | ||||||||
| Total Liabilities |
290,970 | 74,425 | 365,395 | |||||||||
| (1) | Adjustments includes the reclassification of deferred rent and deferred lease incentives into right-of-use assets and the reclassification of prepaid rent to operating lease liabilities as of January 1, 2019. |
See additional disclosures at Note 8 – Leases.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (Topic 606), and related amendments, which require an entity to recognize the amount of revenue for which it expects to be entitled for the transfer of promised goods or services to customers. The provisions of this ASU supersede most current revenue recognition guidance, including industry-specific guidance. In August 2015, the FASB issued ASU No. 2015-14, Revenue from Contracts with Customers (Topic 606): Deferral of the Effective Date, to defer the effective date of ASU No. 2014-09 for one year. As such, the standard is effective for annual and interim reporting periods beginning after December 15, 2017. The standard permits two methods of adoption: retrospectively to each prior reporting period presented (full retrospective method), or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (the modified retrospective method).
On January 1, 2018, the ASU was adopted. The Company’s revenues impacted by this ASU include its fee-for-service sale and capitation revenues. The Company used the modified retrospective method for contracts not yet complete as of January 1, 2018. The comparative information for the year end December 31, 2017 has not been restated and continues to be reported under the accounting standards in effect for that period.
Upon adoption, patient co-pay and deductible amounts that were previously included in the provision for doubtful accounts related to implicit price concessions, which were $9.5 million for the year ended December 31, 2018, are now presented as an adjustment to the related fee-for-service sale net revenues. The Company did not have a cumulative effect of initially applying the new standard as there were no changes in the timing of revenue recognition upon adoption. There were also no effects on net cash provided by operating activities, net cash used in investing activities or net cash used in financing activities for the year ended December 31, 2019 and 2018.
Recently Issued Accounting Pronouncements
In November 2018, the FASB issued ASU No. 2018-19, Codification Improvements to Topic 326, Financial Instruments-Credit Losses, which is an amendment to ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The amendment clarifies that receivables arising from operating leases are not within the scope of ASU No. 2016-13 and impairment of receivables arising from operating leases should be accounted for in accordance with ASU No. 2016-02, Leases (Topic 842). This ASU is effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. Early adoption is permitted. The adoption of this ASU is not expected to have a material impact on the Company’s consolidated financial statements.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement that is a Service Contract, which amends FASB ASC No. 350-40, Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement, to align the accounting for the implementation costs incurred in a cloud computing arrangement that is a service contract with the guidance
F-22
Table of Contents
on capitalizing costs associated with developing or obtaining internal-use software in that implementation costs of a cloud computing arrangement that is a service contract should be capitalized. For nonpublic entities, ASU No. 2018-15 is effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2019. Early adoption is permitted and can be applied using either a retrospective or prospective approach. When a prospective transition is chosen, entities must apply the transition requirements to any eligible costs incurred after adoption. The adoption of ASU No. 2018-15 is not expected to have a material effect on the Company’s consolidated financial statements.
| 3. | PROPERTY, EQUIPMENT AND IMPROVEMENTS |
Property, equipment and improvements consist of the following:
| (in thousands) | December 31, | |||||||
| 2019 | 2018 | |||||||
| Leasehold improvements |
$ | 21,627 | $ | 25,039 | ||||
| Equipment and furnishings |
11,876 | 11,503 | ||||||
| Information systems—hardware |
25,192 | 24,803 | ||||||
| Information systems—software |
83,757 | 76,330 | ||||||
|
|
|
|
|
|||||
| 142,452 | 137,675 | |||||||
| Less accumulated depreciation |
(116,016 | ) | (110,097 | ) | ||||
|
|
|
|
|
|||||
| $ | 26,436 | $ | 27,578 | |||||
|
|
|
|
|
|||||
Depreciation expense for property, equipment and improvements was $13.6 million, $15.9 million and $17.0 million for the years ended December 31, 2019, 2018 and 2017, respectively. Depreciation expense for property, equipment and improvements is recorded in selling, distribution, and administrative expenses in the consolidated statements of income.
| 4. | INTANGIBLE ASSETS |
Intangible assets consist of the following:
| December 31, 2019 | December 31, 2018 | |||||||||||||||||||||||||||
| (dollars in thousands) | Average Life in Years |
Gross Carrying Amount |
Accumulated Amortization |
Net Book Value |
Gross Carrying Amount |
Accumulated Amortization |
Net Book Value |
|||||||||||||||||||||
| Intangible assets subject to amortization: |
||||||||||||||||||||||||||||
| Capitated relationships |
20.0 | $ | 4,400 | $ | (2,686 | ) | $ | 1,714 | $ | 4,400 | $ | (2,492 | ) | $ | 1,908 | |||||||||||||
| Payor relationships |
20.0 | 7,600 | (4,243 | ) | 3,357 | 7,600 | (3,863 | ) | 3,737 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Subtotal |
12,000 | (6,929 | ) | 5,071 | 12,000 | (6,355 | ) | 5,645 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Intangible assets not subject to amortization: |
||||||||||||||||||||||||||||
| Trade names |
— | 50,000 | — | 50,000 | 50,000 | — | 50,000 | |||||||||||||||||||||
| Accreditations with commissions |
— | 7,000 | — | 7,000 | 7,000 | — | 7,000 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Subtotal |
57,000 | — | 57,000 | 57,000 | — | 57,000 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total |
$ | 69,000 | $ | (6,929 | ) | $ | 62,071 | $ | 69,000 | $ | (6,355 | ) | $ | 62,645 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
Amortization expense was $0.6 million, $0.8 million and $0.6 million the years ended December 31, 2019, 2018 and 2017, respectively.
F-23
Table of Contents
Estimated amortization expense for each of the fiscal years ending December 31 is presented below:
| (in thousands) | ||||
| Years Ending December 31 |
||||
| 2020 |
$ | 574 | ||
| 2021 |
574 | |||
| 2022 |
574 | |||
| 2023 |
574 | |||
| 2024 |
574 | |||
| Thereafter |
2,201 | |||
| 5. | DEBT |
Long-term debt consists of the following:
| (in thousands) | December 31, | |||||||
| 2019 | 2018 | |||||||
| Term Loan A |
$ | 150,000 | $ | — | ||||
| Less: Current portion |
(3,750 | ) | — | |||||
| Less: Unamortized debt issuance costs |
(2,216 | ) | — | |||||
|
|
|
|
|
|||||
| Total long-term debt |
$ | 144,034 | $ | — | ||||
|
|
|
|
|
|||||
On November 2, 2018, the Company entered into a senior secured asset-based revolving credit facility (the “ABL Facility”), with Wells Fargo Bank, N.A., an administrative agent and a syndicate of financial institutions and institutional lenders. The ABL Facility replaced the prior secured asset-based revolving credit facility dated May 2, 2014, which provided for a revolving credit financing of up to $100.0 million.
On June 21, 2019, Apria entered into a credit agreement with Citizens Bank and a syndicate of lenders for both a Term Loan A Facility (the “TLA”) of $150.0 million and a Revolving Credit Facility (the “Revolver”) of $100.0 million. The Revolver replaced the prior ABL Facility which provided for revolving credit financing of up to $125.0 million. Proceeds from the TLA were used to fund the dividend payment to common stockholders and SARs unit-holders.
The credit agreement permits the interest rate to be selected at the Company’s option at either Adjusted LIBOR or Alternative Base Rate plus their respective applicable margin. Adjusted LIBOR is the rate for Eurodollar deposits for the applicable interest period while the Alternate Base Rate is the highest of (i) the Administrative Agent’s “Prime Rate”, (ii) the Federal Funds Effective Rate plus 0.50%, and (iii) one-month Adjusted LIBOR plus 1.00%. Additionally, the margin applied to both the TLA and Revolver is determined based on total net leverage ratio. Total net leverage ratio is defined as net debt, which represents indebtedness minus up to $25.0 million in cash and cash equivalents over consolidated EBITDA as defined under the credit agreement. The following is a summary of the additional margin and commitment fees payable on both the TLA and available Revolver:
| Level |
Total Net Leverage Ratio |
Applicable Margin for Adjusted LIBOR Loans |
Applicable Margin for Alternative Base Rate Loans |
Commitment Fee |
||||||||||
| I |
Greater than or equal to 3.00x |
2.75 | % | 1.75 | % | 0.35 | % | |||||||
| II |
Greater than or equal to 2.50x but less than 3.00x |
2.50 | % | 1.50 | % | 0.30 | % | |||||||
| III |
Greater than or equal to 1.50x but less than 2.50x |
2.25 | % | 1.25 | % | 0.25 | % | |||||||
| IV |
Less than 1.50x |
2.00 | % | 1.00 | % | 0.20 | % | |||||||
F-24
Table of Contents
The TLA matures on June 21, 2024 and the Company is required to make quarterly principal payments on the TLA beginning September 30, 2020. The table below is a summary of the expected principal repayments each year:
| (in thousands) | ||||
| Years Ending December 31 |
||||
| 2020 |
$ | 3,750 | ||
| 2021 |
7,500 | |||
| 2022 |
11,250 | |||
| 2023 |
15,000 | |||
| 2024 |
112,500 | |||
The credit agreement encompassing the TLA and Revolver permits the Company, subject to certain exceptions, to increase its TLA or its Revolver, as well as incur additional indebtedness, as long as it does not exceed the total net leverage ratio of 3.00x. The credit agreement requires mandatory prepayments upon the occurrence of certain events, such as dispositions and casualty events, subject to certain exceptions. The TLA or Revolver may be voluntarily prepaid by the Company at any time without any premium or penalty.
The assets of the Company and equity interest of all present and future wholly owned direct domestic subsidiaries, with certain exceptions, are pledged as collateral for the TLA and Revolver. The credit agreement contains a financial covenant requiring the Company to maintain a total net leverage ratio less than 3.50x.
As of December 31, 2019, no amounts were outstanding under the Revolver, there were $17.8 million outstanding letters of credit, and additional availability under the Revolver net of letters of credit outstanding was $82.2 million. The Company was in compliance with all debt covenants set forth in the TLA and Revolver as of December 31, 2019.
In accordance with ASU 2015-03, Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs, the Company records origination and other expenses related to certain debt issuance cost as a direct deduction from the carrying amount of the debt liability. These expenses are deferred and amortized using the straight-line method over the stated life, which approximates the effective interest rate method. The unamortized debt issuance costs related to the ABL Facility dated November 2, 2018 were expensed as a result of the new credit agreement. Amortization of deferred debt issuance costs are classified within interest expense in the Company’s consolidated statements of income and was $1.1 million, $0.4 million, and $0.3 million for the years ended December 31, 2019, 2018 and 2017, respectively.
Interest expense, excluding deferred debt issuance costs discussed above, was $4.0 million, $0.9 million and $0.9 million for the years ended December 31, 2019, 2018 and 2017, respectively. Interest paid on debt totaled $4.2 million, $1.0 million and $1.0 million for the years ended December 31, 2019, 2018 and 2017, respectively. The interest rate as of December 31, 2019 was 3.70%.
| 6. | STOCK-BASED COMPENSATION |
Profit Interest Units—BP Healthcare and its subsidiary, Apria Holdings LLC (“Holdings”), granted equity units to certain employees, Board members and a member of a subsidiary’s Board of Directors for purposes of retaining them and enabling such individuals to participate in the long-term growth and financial success of the Company. Profit interest units are measured at the grant date, based on the calculated fair value of the award, and are recognized as an expense over the employee’s requisite service period. These equity awards were issued in exchange for services to be performed.
Profit interest units are composed of Class B and Class C units related to Holdings. Holdings also has 3,575,000 outstanding in Class A-2 units, which are senior in liquidation rights to Class B units, whereas Class B units are senior in liquidation rights to Class C units. Class B units have a strike price ranging from $0.00 - $0.63, and the holders of those units only share in the value of Holdings to the extent it exceeds the per unit strike price.
F-25
Table of Contents
A portion of the units vested over a specified period of time, generally five years, based on continued service, and a portion of the units vest based on performance/market conditions and will generally vest if affiliates of the Sponsor receive a specific return in respect of their capital investment. Certain units will become fully vested on an accelerated basis either upon a change in control (including an initial public offering) while the management employee who was granted the units continues to provide services to the Company or its subsidiaries, or if affiliates of the Sponsor receive a specific return in respect of their capital investment.
On June 21, 2019, the Board of Directors of Holdings approved the modification of all outstanding performance Class B units to accelerate vesting, resulting in stock compensation expense for the year ended December 31, 2019 of $7.0 million based on the fair value of the vested awards on the date of modification. Expense related to profit interest units held by unit-holders continuing to perform services for the Company is recorded within selling, distribution and administrative expenses in the consolidated statements of income and was $7.0 million, $0.0 million and $0.3 million for the years ended December 31, 2019, 2018, and 2017, respectively. The total fair market value of units vested was $7.0 million, $0.1 million and $0.3 million for the years ended December 31, 2019, 2018, and 2017, respectively.
The Company uses the income approach and the guideline approach to estimate enterprise value, which is utilized to assess the fair value of each instrument. No units were granted in 2019, 2018 or 2017, as profit interest units are no longer granted.
There are no stated contractual lives for the units.
The following table summarizes activity for all profit interest units for the period from December 31, 2018 to December 31, 2019:
| Class B Units | Weighted- Average Exercise Price |
Aggregate Intrinsic Value ($000s) |
Class C Units |
Weighted- Average Exercise Price |
Aggregate Intrinsic Value ($000s) |
|||||||||||||||||||
| Outstanding as of December 31, 2018 |
88,124,940 | $ | 0.01 | 3,023,230 | — | |||||||||||||||||||
| Granted |
— | — | — | — | ||||||||||||||||||||
| Forfeited |
(116,090 | ) | — | (174,138 | ) | — | ||||||||||||||||||
| Exercised |
— | — | — | — | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Outstanding as of December 31, 2019 |
88,008,850 | $ | 0.01 | $ | 44,222 | 2,849,092 | — | $ | 773 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Vested units as of December 31, 2019 |
88,008,850 | — | ||||||||||||||||||||||
The following table summarizes activity for unvested profit interest units for the period from December 31, 2018 to December 31, 2019:
| Class B Units | Weighted- Average Grant-Date Fair Value |
Class C Units | Weighted- Average Grant-Date Fair Value |
|||||||||||||
| Unvested as of December 31, 2018 |
5,985,281 | $ | 0.36 | 3,023,230 | $ | 0.22 | ||||||||||
| Granted |
— | — | — | — | ||||||||||||
| Vested |
(5,985,281 | ) | 0.36 | — | — | |||||||||||
| Forfeited |
— | — | (174,138 | ) | 0.28 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Unvested as of December 31, 2019 |
— | — | 2,849,092 | $ | 0.22 | |||||||||||
Stock Appreciation Rights—In 2015, the Company’s Board of Directors approved a stock plan that provides for the grant of 27,689 stock appreciation rights to directors, officers, employees, consultants and
F-26
Table of Contents
advisers (and prospective directors, officers, employees, consultants, and advisers) of the Company and its affiliates. The plan mandates a maximum award term of 10 years and that SARs be granted with a strike price not less than the fair market value determined as of the date of grant. SARs are measured at the grant date, based on the calculated fair value of the award and are recognized as an expense over the employee’s requisite service period. These equity awards are issued in exchange for services to be performed. The Company’s Board of Directors approved an additional 42,516 SARs and 60,000 SARs available for grant under the 2015 stock plan in March 2017 and October 2019, respectively.
SARs granted under the plan generally vest over 60 months from the date of grant based on continued service. All unvested SARs are forfeited upon employee termination. In the event of a change in capital structure or similar event, SARs may be modified.
Expense related to SARs held by unit-holders continuing to perform services for the Company is recorded within selling, distribution and administrative expenses in the consolidated statements of income and were $1.6 million, $1.6 million and $1.4 million for the years ended December 31, 2019, 2018 and 2017, respectively. As of December 31, 2019, total unrecognized compensation cost related to unvested SARs was $4.8 million, which is expected to be expensed over a weighted-average period of 3.5 years.
The Company has estimated the grant-date fair value of SARs using the Black-Scholes valuation model. The following assumptions were used in estimating the value and determining the related stock-based compensation attributable to the current period.
| 2019 | 2018 | 2017 | ||||||||||
| Expected asset volatility(1) |
45.0% | — | 45.0% | |||||||||
| Risk free interest rate(2) |
1.8% | — | 1.2% | |||||||||
| Expected life(3) |
6.35 years | — | 6.35 years | |||||||||
| Dividend(4) |
— | — | — | |||||||||
| (1) | Volatility is estimated as the average of the historical volatilities of the publicly traded comparable companies. |
| (2) | The risk-free interest rate is interpolated from the United States Constant Maturity Treasury curve with a term matching the expected life. |
| (3) | The expected life assumes the expected exercise date is the midpoint of vesting start date and the contractual expiration date of each tranche. |
| (4) | The dividend assumed is zero. |
The contractual lives for the units are 10 years. The Company accounts for forfeitures when they occur, and ultimately stock-based compensation is only recognized for awards that vest.
The following table summarizes activity for all SARs for the period from December 31, 2018 to December 31, 2019:
| Stock Appreciation Rights |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Term |
Aggregate Intrinsic Value ($000s) |
|||||||||||||
| Outstanding as of December 31, 2018 |
65,043 | $ | 335.21 | |||||||||||||
| Granted |
24,486 | 390.50 | ||||||||||||||
| Forfeited |
(3,099 | ) | 207.67 | |||||||||||||
| Redeemed |
— | — | ||||||||||||||
|
|
|
|||||||||||||||
| Outstanding as of December 31, 2019 |
86,430 | $ | 305.36 | 7.4 | $ | 6,731 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Vested units as of December 31, 2019 |
44,270 | |||||||||||||||
As a result of the dividend declared on June 21, 2019 to common stockholders and vested SARs unit-holders, the exercise price of the unvested SARs was adjusted to maintain the same valuation before and after the modification.
F-27
Table of Contents
The following table summarizes the activity for unvested shares for the period from December 31, 2018 to December 31, 2019:
| Stock Appreciation Rights |
Weighted- Average Grant-Date Fair Value |
|||||||
| Unvested as of December 31, 2018 |
31,876 | $ | 131.44 | |||||
| Granted |
24,486 | 115.71 | ||||||
| Vested |
(11,103 | ) | 127.49 | |||||
| Forfeited |
(3,099 | ) | 135.00 | |||||
| Redeemed |
— | — | ||||||
|
|
|
|||||||
| Unvested as of December 31, 2019 |
42,160 | $ | 123.08 | |||||
|
|
|
|||||||
The weighted-average grant-date fair value of options granted was $136.09 for the year ended December 31, 2017.
As of December 31, 2019, there were 43,775 SARs available for future grants under the plan.
| 7. | INCOME TAXES |
Income tax expense (benefit) consists of the following:
| Year Ended December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| Current |
||||||||||||
| Federal |
$ | — | $ | — | $ | — | ||||||
| State |
(1,263 | ) | 214 | 1,152 | ||||||||
|
|
|
|
|
|
|
|||||||
| (1,263 | ) | 214 | 1,152 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred |
||||||||||||
| Federal |
7,022 | 4,754 | (55,455 | ) | ||||||||
| State |
2,368 | 1,861 | (17,257 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| 9,390 | 6,615 | (72,712 | ) | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 8,127 | $ | 6,829 | $ | (71,560 | ) | ||||||
|
|
|
|
|
|
|
|||||||
The current income tax expense (benefit) includes a tax expense (benefit) of $(1.4) million, $0.1 million and $0.0 million relating to changes in the Company’s accruals for uncertain tax positions for the years ended December 31, 2019, 2018 and 2017, respectively.
F-28
Table of Contents
A reconciliation of the differences between income tax expense computed at the federal statutory rate and the provision for income taxes consist of the following:
| Year Ended December 31, | ||||||||||||
| 2019 | 2018 | 2017 | ||||||||||
| Income tax expense at statutory rate |
21 | % | 21 | % | 35 | % | ||||||
| Non-deductible expenses |
6 | 6 | 1 | |||||||||
| Federal rate change on deferred taxes |
— | — | 95 | |||||||||
| State taxes, net of federal benefit and state loss carryforwards |
9 | 8 | 2 | |||||||||
| Stock-based compensation |
4 | — | — | |||||||||
| Change in federal and state valuation allowance |
— | — | (414 | ) | ||||||||
| Change in liability for unrecognized tax benefits |
(5 | ) | — | — | ||||||||
| Other |
(1 | ) | (1 | ) | (1 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Effective tax rate |
34 | % | 34 | % | (282 | )% | ||||||
|
|
|
|
|
|
|
|||||||
Significant components of deferred tax assets and liabilities are as follows:
| December 31, | ||||||||
| (in thousands) | 2019 | 2018 | ||||||
| Deferred tax assets: |
||||||||
| Accounts receivable |
$ | 8,712 | $ | 8,884 | ||||
| Overpayment reserve |
736 | 999 | ||||||
| Accruals |
10,317 | 9,743 | ||||||
| Accrued vacation |
3,425 | 3,438 | ||||||
| Asset valuation reserves |
735 | 1,344 | ||||||
| Net operating loss carryforward and tax credits |
66,190 | 61,795 | ||||||
| Tax deductible goodwill |
1,669 | 3,940 | ||||||
| Intangible assets |
395 | 666 | ||||||
| Tax benefits related to unrecognized state tax benefits and interest accrued |
67 | 384 | ||||||
| Other, net |
4,367 | 4,596 | ||||||
|
|
|
|
|
|||||
| 96,613 | 95,789 | |||||||
| Less: valuation allowance |
(1,566 | ) | (1,641 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax assets, net |
95,047 | 94,148 | ||||||
| Deferred tax liabilities: |
||||||||
| Trade names and other indefinite-lived intangibles |
(14,591 | ) | (14,491 | ) | ||||
| Tax over book depreciation |
(42,150 | ) | (31,769 | ) | ||||
| Deferred expenses |
(616 | ) | (631 | ) | ||||
| Definite-lived intangibles |
(1,334 | ) | (1,485 | ) | ||||
| Other, net |
(1,657 | ) | (1,683 | ) | ||||
|
|
|
|
|
|||||
| Total deferred tax liabilities |
(60,348 | ) | (50,059 | ) | ||||
|
|
|
|
|
|||||
| Deferred income taxes, net |
$ | 34,699 | $ | 44,089 | ||||
|
|
|
|
|
|||||
Deferred income taxes arise from temporary differences between the carrying amounts of assets and liabilities for tax and financial reporting purposes and tax losses and credit carryforwards. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized.
A valuation allowance of $1.6 million was recorded against the gross deferred tax asset balance as of December 31, 2019 and 2018, related to the future realization of state net operating losses. For the year
F-29
Table of Contents
ended December 31, 2017 we recorded a net valuation allowance release of $105.1 million on the basis of management’s reassessment of the amount of its deferred tax assets that are more likely than not to be realized.
As of each reporting date, management considers new evidence, both positive and negative, that could affect its view of the future realization of deferred tax assets. As of December 31, 2019, management evaluated all of the positive and negative evidence, including its three years of cumulative pretax income and its projected income, and determined that there is sufficient positive evidence to conclude that it is more likely than not that net deferred taxes of $34.7 million are realizable.
On December 22, 2017, the Tax Cuts and Jobs Act was signed into legislation. The Act reduces the corporate tax rate to 21%, effective January 1, 2018. Consequently, we recorded a tax expense of $24.2 million due to the re-measurement of deferred tax assets and liabilities resulting from the decrease in the Federal corporate tax rate in the year ended December 31, 2017. The Act also allows for additional bonus depreciation that provides for full expensing of qualified property placed in service after September 27, 2017. Under Staff Accounting Bulletin 118, issued by the SEC on December 22, 2017, our accounting for the Tax Act was considered complete as of December 31, 2017.
The Company recognizes tax benefits related to positions taken, or expected to be taken, on its tax returns, only if the positions are “more-likely-than-not” sustainable. Once this threshold has been met, the Company’s measurement of its expected tax benefits is recognized in its financial statements.
A reconciliation of the beginning and ending balances of the gross liability for unrecognized tax benefits as of December 31, 2019, 2018 and 2017 is as follows:
| December 31, | ||||||||||||
| (in thousands) | 2019 | 2018 | 2017 | |||||||||
| Total gross unrecognized tax benefits as of January 1 |
$ | 1,439 | $ | 1,439 | $ | 1,439 | ||||||
| Reductions due to lapse in statute of limitations |
(704 | ) | — | — | ||||||||
|
|
|
|
|
|
|
|||||||
| Total gross unrecognized tax benefits as of December 31 |
$ | 735 | $ | 1,439 | $ | 1,439 | ||||||
|
|
|
|
|
|
|
|||||||
Total gross unrecognized tax benefits of $0.7 million are reflected in the Company’s December 31, 2019 balance sheet as follows: (a) $0.1 million included in income taxes payable and other non-current liabilities and (b) $0.6 million included in deferred income taxes.
The amount of unrecognized tax benefits, including accrued interest, penalties and federal tax benefit, which, if ultimately recognized, could affect the effective tax rate in a future period are $0.7 million, $1.8 million and $1.8 million as of December 31, 2019, 2018 and 2017, respectively.
The Company does not expect any material changes to its tax uncertainties within the 12-month rolling period ending December 31, 2020.
Interest expense and penalties related to unrecognized tax benefits are recognized as part of the provision for income taxes. Gross interest and penalties of $0.1 million, $0.9 million and $0.8 million are provided for within the liability for unrecognized tax benefits as of December 31, 2019, 2018 and 2017, respectively.
As of December 31, 2019, federal net operating loss carryforwards of approximately $272.4 million are available to offset future federal taxable income. Net operating loss carryforwards of $177.1 million will expire in varying amounts during the Company’s 2034 and 2037 tax years. Due to the enactment of the Tax Cuts and Jobs Act, federal net operating losses generated beginning in 2018 and thereafter are carried forward indefinitely. Therefore, the remaining $95.3 million of net operating loss carryforwards will not expire.
The Company files federal and state income tax returns in jurisdictions with varying statutes of limitations expiration dates. The Company’s calendar 2015 through 2019 tax years generally remain subject to examination by tax authorities. The Internal Revenue Service is currently examining the calendar 2017 tax year.
F-30
Table of Contents
Net income tax payments were $0.1 million, $0.1 million and $0.2 million for the years ended December 31, 2019, 2018 and 2017, respectively.
| 8. | LEASES |
The Company leases all its facilities. The Company’s real estate lease portfolio primarily consists of modified gross leases, in which the Company pays a share of the operating costs, and triple-net leases, in which the Company pays all of the operating costs. Operating costs that are the responsibility of the Company include taxes, maintenance, insurance and other allowable expenses. These expenses are considered variable costs as they are not tied to an index or rate. In addition, delivery vehicles and office equipment are leased under operating leases. Lease terms are generally five years or less with renewal options for additional periods and often contain early termination clauses. Rents are generally increased annually by amounts stated within individual agreements, subject to certain maximum amounts defined within individual agreements.
The Company also leases certain patient equipment. Lease terms are generally twelve months or less with renewal options for additional periods. The Company also has short-term patient equipment leases with certain suppliers that are entirely variable based on equipment usage or a percentage of net revenues collected for specific products. Patient equipment lease expense is recorded in product and supply costs in the consolidated statements of income in the period incurred. The Company uses the portfolio approach to review patient equipment leases.
All of the Company’s leases are classified as operating leases. The leases do not include options to purchase the underlying assets that the Company is reasonably certain to exercise. The components of lease assets and liabilities are included on the consolidated balance sheet.
Significant components of lease expense for the year ended December 31, 2019 are as follows:
| (in thousands) | Year Ended December 31, 2019 |
|||
| Operating lease expense |
$ | 39,297 | ||
| Variable lease expense |
21,196 | |||
| Short-term lease expense |
665 | |||
|
|
|
|||
| Total lease expense |
$ | 61,158 | ||
|
|
|
|||
The following table summarizes supplemental cash flow information related to our operating leases:
| (in thousands) | Year Ended December 31, 2019 |
|||
| Cash paid for amounts included in the measurement of operating lease liabilities |
$ | 33,692 | ||
| Right-of-use assets obtained in exchange for new operating lease liabilities |
16,036 | |||
As of December 31, 2019, the weighted average remaining lease term is three years and the weighted average discount rate used to determine the operating lease liability is 5.48%.
F-31
Table of Contents
Future minimum lease payments, by year and in the aggregate, required under noncancelable operating leases consist of the following as of December 31, 2019:
| (in thousands) | Operating Leases |
|||
| 2020 |
$ | 27,391 | ||
| 2021 |
20,968 | |||
| 2022 |
12,495 | |||
| 2023 |
6,634 | |||
| 2024 |
2,498 | |||
| Thereafter |
309 | |||
|
|
|
|||
| Total future minimum lease payments |
70,295 | |||
| Less: imputed interest |
(5,462 | ) | ||
|
|
|
|||
| Present value of future minimum lease liabilities |
64,833 | |||
| Less: current lease liabilities |
(24,546 | ) | ||
|
|
|
|||
| Long-term operating lease liabilities |
$ | 40,287 | ||
|
|
|
|||
Future minimum lease payments, by year and in the aggregate, required under noncancelable operating leases, based on the former accounting guidance for leases, consist of the following as of December 31, 2018:
| (in thousands) | Operating Leases |
|||
| 2019 |
$ | 37,811 | ||
| 2020 |
33,015 | |||
| 2021 |
23,071 | |||
| 2022 |
11,662 | |||
| 2023 |
3,778 | |||
| Thereafter |
246 | |||
|
|
|
|||
| $ | 109,583 | |||
|
|
|
|||
| 9. | COMMITMENTS AND CONTINGENCIES |
Litigation—Since December 2017, the Company has been engaged in responding to a series of related civil investigative demands (“CIDs”) from the United States Attorney’s Office for the Southern District of New York (the “SDNY Office”) concerning an investigation into “the sales, marketing, and/or patient service practices at Apria Healthcare in relation to the rental of non-invasive ventilators (“NIV”) that may violate the Anti-Kickback Statute, 42 U.S.C. Section 1320a-7b(b), and/or the False Claims Act, 31 U.S.C. Sections 3729 et seq.”
The government lawyers with the SDNY Office handling this matter have informed the Company’s counsel that the investigation is the result of a qui tam lawsuit and have suggested a framework for settling the claims the government is asserting. While the Company believes it has meritorious arguments and would pursue a vigorous defense if the matter were to proceed to litigation, the Company recorded a reserve in the amount of $12.2 million as a noncurrent liability on the consolidated balance sheet as of December 31, 2019, representing its estimated probable loss as of such date. The accrual is included within selling, distribution and administrative expenses in the consolidated statement of income for the year ended December 31, 2019. As a result of discussions and developments through the date as of which subsequent events have been evaluated, the Company expects to increase this accrual on its consolidated balance sheet as of June 30, 2020 to $25.0 million. See Note 12 – Subsequent Events.
F-32
Table of Contents
If the Company determines not to settle the matter, then it expects it will proceed to litigation. The Company’s management cannot provide any assurances as to the timing or outcome of the investigations and any subsequent litigation or of any qui tam lawsuit.
While it is reasonably possible that the Company may incur a loss in excess of the amount accrued, given the stage of the matter and aforementioned uncertainties, management cannot estimate the possible loss or range of loss in excess of the amount accrued that may result from the proceedings described above and, therefore, has not recorded any related accruals beyond the amount it proposes to offer in settlement. If a judge, jury or administrative agency were to determine that practices occurred or payments were made in violation of the Anti-Kickback Statute, that false claims were submitted to federal health care programs with the requisite level of scienter, or that there were overpayments by the government that the Company knowingly concealed or knowingly and improperly avoided refunding to the Government, the Company could face claims for treble damages, criminal, civil and administrative penalties, claims for refunds, fines and sanctions, in amounts that would be material to its business, results of operations and financial condition, including the exclusion of the Company from participation in government health care programs.
In addition to the matter referenced in this note, the Company is engaged in the defense of certain claims and lawsuits arising out of the ordinary course and conduct of its business, the outcomes of which are not determinable at this time. Insurance policies covering such potential losses, where such coverage is cost effective, are maintained. In the opinion of management, any liability that might be incurred upon the resolution of these claims and lawsuits will not, in the aggregate, have a material effect on the Company’s financial condition or results of operations, cash flows and liquidity.
Supplier Concentration—Currently, approximately 81% of purchases for patient equipment and supplies are from five vendors. Although there are a limited number of suppliers, management believes that other vendors could provide similar products on comparable terms. However, a change in suppliers could cause delays in service delivery and possible losses of revenue, which could adversely affect the Company’s consolidated financial condition or operating results. In addition, from time to time, the Company enters into exclusive arrangements with certain suppliers to provide patient equipment and supplies.
Purchase Obligations—In April 2009, the Company entered into a ten-year information technology services agreement to outsource certain information systems functions. Effective March 2016, the agreement was extended to April 1, 2022. If the Company terminated the agreement, the required obligation to the vendor would be approximately $3.2 million for services during the 120-day cancellation notice period.
Noncancelable Contractual Obligations—Future payments due under noncancelable contractual obligations related to software license and maintenance agreements consist of the following at December 31, 2019:
| (in thousands) | Noncancelable Contractual Obligations |
|||
| 2020 |
$ | 2,604 | ||
| 2021 |
959 | |||
| 2022 |
530 | |||
| 2023 |
— | |||
| 2024 |
— | |||
| Thereafter |
— | |||
|
|
|
|||
| $ | 4,093 | |||
|
|
|
|||
Guarantees and Indemnities—From time to time, certain types of contracts are entered into that contingently require indemnification of parties against third-party claims. These contracts primarily relate to (i) certain asset purchase agreements, under which indemnification may be provided to the seller of the
F-33
Table of Contents
business being acquired; (ii) certain real estate leases, which may require indemnification to property owners for environmental or other liabilities and other claims arising from use of the applicable premises; and (iii) certain agreements with officers, directors, and employees, which may require indemnification of such persons for liabilities arising out of their relationship with the Company.
The terms of such obligations vary by contract, and in most instances, a specific or maximum dollar amount is not explicitly stated therein. Generally, amounts under these contracts cannot be reasonably estimated until a specific claim is asserted. Consequently, no liabilities have been recorded for these obligations on the consolidated balance sheets for any of the periods presented.
| 10. | EMPLOYEE BENEFIT PLANS |
401(k) Savings Plan—The Company has a 401(k)-defined contribution plan, whereby eligible employees may contribute up to 35% of their annual base earnings. The Company has a discretionary match based on the Company’s performance. Total expenses related to the defined contribution plan were $2.0 million, $1.0 million and $2.0 million for the years ended December 31, 2019, 2018 and 2017, respectively.
Deferred Compensation Plan—A nonqualified deferred compensation plan is available for approximately 90 employees. The plan provides participants with the advantages of pre-tax contributions and tax-deferred compounding of interest. Plan assets, which represent the fair market value of the investments, were $5.0 million and $4.4 million, and plan liabilities were $4.7 million and $3.6 million as of December 31, 2019 and 2018, respectively.
| 11. | CERTAIN RELATIONSHIPS AND RELATED-PARTY TRANSACTIONS |
Nominee Agreement—On January 16, 2014, pursuant to the terms of a Stock Purchase Agreement, the Company completed the divesture (the “Transaction”) of its Home Infusion Segment to a subsidiary of CVS Caremark Corporation. In connection with the Transaction, the Company entered into a nominee agreement with Holdings, which allows the Company to facilitate certain payments related to the Transaction. As of December 31, 2019, all contingent payments related to the nominee agreement have been received. The Company held $0.0 million in restricted cash on behalf of the Sponsor as of December 31, 2019 and 2018.
Change Healthcare—In December 1999, the Company entered into an agreement with Change Healthcare, a company affiliated with the Sponsor since 2011, to perform various revenue and payment cycle management functions. The Company paid Change Healthcare approximately $3.8 million, $3.3 million and $4.2 million for the years ended December 31, 2019, 2018 and 2017, respectively. Amounts included in accounts payable were $0.3 million and $0.6 million as of December 31, 2019 and 2018, respectively.
Mphasis—In September 2019, the Company entered into an eighty-seven-month agreement with Mphasis, a Company affiliated with the Sponsor since 2016, to perform various technology related services previously performed by another outsource vendor. The Company has the right to terminate for convenience with a minimum three-month notice. There were no amounts paid for the year ended December 31, 2019 and no amounts due as of December 31, 2019.
Alight Solutions—In December 2019, the Company entered into an agreement with Alight Solutions, a Company affiliated with the Sponsor since 2017, to perform services related to the deployment of a new financial management system. There were no amounts paid for the year ended December 31, 2019 and no amounts due as of December 31, 2019.
Intelenet Global Services Agreement—In May 2009, the Company entered into the Master Services Agreement (the “Intelenet Agreement”) with Intelenet Global Services Private Limited (“Intelenet”), an Indian company affiliated with the Sponsor, regarding the outsourcing of certain functions related to billing, collections and other administrative and clerical services. In July 2011, Intelenet was sold to Serco Group PLC, an international services company, until an affiliate of the Sponsor repurchased Intelenet in 2015.
F-34
Table of Contents
In June 2018, it was announced that an affiliate of the Sponsor agreed to sell Intelenet to Teleperformance, a business process outsourcing company. The transaction closed at the beginning of October 2018. Through the October 2018 transaction date, the Company paid Intelenet approximately $1.9 million for the year ended December 31, 2018 and $7.8 million for the year ended December 31, 2017. As of December 31, 2018, there were no amounts included in accounts payable related to service periods prior to the October 2018 transaction date.
| 12. | SUBSEQUENT EVENTS |
The Company evaluated subsequent events through March 25, 2020, the date on which the December 31, 2019 financial statements were originally issued, and has updated such evaluation for disclosure purposes through August 6, 2020, the date on which the December 31, 2019 financial statements were reissued.
F-35
Table of Contents
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
| (in thousands, except share and per share data) | Pro Forma September 30, 2020 |
September 30, 2020 |
December 31, 2019 |
|||||||||
| ASSETS | ||||||||||||
| CURRENT ASSETS |
||||||||||||
| Cash and cash equivalents |
$ | 140,125 | $ | 140,125 | $ | 74,691 | ||||||
| Accounts receivable, net |
71,777 | 71,777 | 84,071 | |||||||||
| Inventories |
6,417 | 6,417 | 6,849 | |||||||||
| Prepaid expenses and other current assets |
21,729 | 21,729 | 17,344 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL CURRENT ASSETS |
240,048 | 240,048 | 182,955 | |||||||||
| PATIENT EQUIPMENT, less accumulated depreciation of $341,516 and $322,153 as of September 30, 2020 and December 31, 2019, respectively |
220,298 | 220,298 | 232,656 | |||||||||
| PROPERTY, EQUIPMENT AND IMPROVEMENTS, NET |
24,248 | 24,248 | 26,436 | |||||||||
| INTANGIBLE ASSETS, NET |
61,640 | 61,640 | 62,071 | |||||||||
| OPERATING LEASE RIGHT-OF-USE ASSETS |
60,081 | 60,081 | 65,332 | |||||||||
| DEFERRED INCOME TAXES, NET |
22,550 | 22,550 | 34,699 | |||||||||
| OTHER ASSETS |
16,186 | 16,186 | 13,004 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL ASSETS |
$ | 645,051 | $ | 645,051 | $ | 617,153 | ||||||
|
|
|
|
|
|
|
|||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||||||
| CURRENT LIABILITIES |
||||||||||||
| Accounts payable |
$ | 90,748 | $ | 90,748 | $ | 122,819 | ||||||
| Accrued payroll and related taxes and benefits |
49,267 | 49,267 | 48,651 | |||||||||
| Legal reserve |
44,725 | 44,725 | — | |||||||||
| Other accrued liabilities |
28,454 | 28,454 | 27,834 | |||||||||
| Deferred revenue |
26,177 | 26,177 | 24,860 | |||||||||
| Current portion of operating lease liabilities |
24,629 | 24,629 | 24,546 | |||||||||
| Current portion of long-term debt |
7,500 | 7,500 | 3,750 | |||||||||
| Dividend and Distribution Payable |
210,000 | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL CURRENT LIABILITIES |
481,500 | 271,500 | 252,460 | |||||||||
| LONG-TERM DEBT, less current portion |
136,904 | 136,904 | 144,034 | |||||||||
| OPERATING LEASE LIABILITIES, less current portion |
36,068 | 36,068 | 40,287 | |||||||||
| LEGAL RESERVE |
— | — | 12,200 | |||||||||
| OTHER NONCURRENT LIABILITIES |
44,340 | 44,340 | 33,656 | |||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL LIABILITIES |
698,812 | 488,812 | 482,637 | |||||||||
| COMMITMENTS AND CONTINGENCIES (Note 8) |
||||||||||||
| STOCKHOLDERS’ EQUITY |
||||||||||||
| Common stock, $0.01 par value: 1,100,000 shares authorized; 992,719 shares issued as of September 30, 2020 and December 31, 2019 |
— | — | — | |||||||||
| Additional paid-in capital |
952,542 | 1,162,542 | 1,161,087 | |||||||||
| Accumulated deficit |
(1,006,303 | ) | (1,006,303 | ) | (1,026,571 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| TOTAL STOCKHOLDERS’ EQUITY (DEFICIT) |
(53,761 | ) | 156,239 | 134,516 | ||||||||
|
|
|
|
|
|
|
|||||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ EQUITY |
$ | 645,051 | $ | 645,051 | $ | 617,153 | ||||||
|
|
|
|
|
|
|
|||||||
See notes to unaudited condensed consolidated financial statements.
F-36
Table of Contents
APRIA HEALTHCARE GROUP INC.
CONDENSED CONSOLIDATED STATEMENTS OF INCOME
(UNAUDITED)
| Nine Months Ended September 30, |
||||||||
| (in thousands, except share and per share data) | 2020 | 2019 | ||||||
| Net revenues: |
||||||||
| Fee-for-service arrangements |
$ | 646,630 | $ | 644,624 | ||||
| Capitation |
168,298 | 163,086 | ||||||
|
|
|
|
|
|||||
| TOTAL NET REVENUES |
814,928 | 807,710 | ||||||
|
|
|
|
|
|||||
| Costs and expenses: |
||||||||
| Cost of net revenues: |
||||||||
| Product and supply costs |
141,563 | 154,591 | ||||||
| Patient equipment depreciation |
75,840 | 72,588 | ||||||
| Home respiratory therapists costs |
12,848 | 14,844 | ||||||
| Other |
13,669 | 13,043 | ||||||
|
|
|
|
|
|||||
| TOTAL COST OF NET REVENUES |
243,920 | 255,066 | ||||||
| Selling, distribution and administrative |
534,110 | 537,546 | ||||||
|
|
|
|
|
|||||
| TOTAL COSTS AND EXPENSES |
778,030 | 792,612 | ||||||
|
|
|
|
|
|||||
| OPERATING INCOME |
36,898 | 15,098 | ||||||
| Interest expense |
4,047 | 3,345 | ||||||
| Interest income and other |
(451 | ) | (1,552 | ) | ||||
|
|
|
|
|
|||||
| INCOME BEFORE INCOME TAXES |
33,302 | 13,305 | ||||||
| Income tax expense |
13,034 | 3,465 | ||||||
|
|
|
|
|
|||||
| NET INCOME |
$ | 20,268 | $ | 9,840 | ||||
|
|
|
|
|
|||||
| Basic and diluted earnings per share: |
||||||||
| Net income attributable to common stockholders |
$ | 20.42 | $ | 9.91 | ||||
|
|
|
|
|
|||||
| Weighted average shares outstanding: |
||||||||
| Basic and diluted |
992,719 | 992,719 | ||||||
|
|
|
|
|
|||||
See notes to unaudited condensed consolidated financial statements.
F-37
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(UNAUDITED)
| (in thousands, except share data) | Shares of Common Stock |
Additional Paid In Capital |
Accumulated Deficit |
Total Stockholders’ Equity |
||||||||||||
| Balance as of December 31, 2019 |
992,719 | $ | 1,161,087 | $ | (1,026,571 | ) | $ | 134,516 | ||||||||
| Stock-based compensation |
— | 1,455 | — | 1,455 | ||||||||||||
| Net income |
— | — | 20,268 | 20,268 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of September 30, 2020 (unaudited) |
992,719 | $ | 1,162,542 | $ | (1,006,303 | ) | $ | 156,239 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (in thousands, except share data) | Shares of Common Stock |
Additional Paid In Capital |
Accumulated Deficit |
Total Stockholders’ Equity |
||||||||||||
| Balance as of December 31, 2018 |
992,719 | $ | 1,327,445 | $ | (1,042,193 | ) | $ | 285,252 | ||||||||
| Dividends |
— | (175,000 | ) | — | (175,000 | ) | ||||||||||
| Stock-based compensation |
— | 8,057 | — | 8,057 | ||||||||||||
| Net income |
— | — | 9,840 | 9,840 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance as of September 30, 2019 (unaudited) |
992,719 | $ | 1,160,502 | $ | (1,032,353 | ) | $ | 128,149 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
See notes to unaudited condensed consolidated financial statements.
F-38
Table of Contents
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| Nine Months Ended September 30, |
||||||||
| (in thousands) | 2020 | 2019 | ||||||
| OPERATING ACTIVITIES |
||||||||
| Net income |
$ | 20,268 | $ | 9,840 | ||||
| Items included in net income not requiring cash: |
||||||||
| Depreciation |
86,484 | 82,819 | ||||||
| Amortization of intangible assets |
431 | 431 | ||||||
| Non-cash lease expense |
20,416 | 20,713 | ||||||
| Deferred income taxes |
12,149 | 5,163 | ||||||
| Stock-based compensation |
1,455 | 8,057 | ||||||
| Amortization of deferred debt issuance costs |
370 | 983 | ||||||
| Loss on sale of patient equipment and other |
3,318 | 2,400 | ||||||
| Changes in operating assets and liabilities: |
||||||||
| Accounts receivable |
12,294 | 13,114 | ||||||
| Inventories |
432 | (293 | ) | |||||
| Prepaid expenses and other assets |
(7,567 | ) | (667 | ) | ||||
| Accounts payable |
(17,711 | ) | (26,941 | ) | ||||
| Accrued payroll and related taxes and benefits |
616 | (5,208 | ) | |||||
| Income taxes payable |
9 | (763 | ) | |||||
| Operating lease liabilities |
(19,300 | ) | (22,241 | ) | ||||
| Deferred revenue |
1,317 | 344 | ||||||
| Legal reserve |
32,525 | — | ||||||
| Accrued expenses |
11,433 | (3,901 | ) | |||||
|
|
|
|
|
|||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES |
158,939 | 83,850 | ||||||
|
|
|
|
|
|||||
| INVESTING ACTIVITIES |
||||||||
| Purchases of patient equipment and property, equipment and improvements |
(85,847 | ) | (85,194 | ) | ||||
| Proceeds from sale of patient equipment and other |
13,315 | 16,928 | ||||||
|
|
|
|
|
|||||
| NET CASH USED IN INVESTING ACTIVITIES |
(72,532 | ) | (68,266 | ) | ||||
|
|
|
|
|
|||||
| FINANCING ACTIVITIES |
||||||||
| Payments on asset financing |
(17,223 | ) | (23,385 | ) | ||||
| Proceeds from borrowings on long-term debt |
— | 150,000 | ||||||
| Payments on long-term debt |
(3,750 | ) | — | |||||
| Proceeds from ABL revolver |
— | 5,000 | ||||||
| Disbursements on ABL revolver |
— | (5,000 | ) | |||||
| Payment of dividend |
— | (175,000 | ) | |||||
| Debt issuance costs |
— | (2,756 | ) | |||||
|
|
|
|
|
|||||
| NET CASH USED IN FINANCING ACTIVITIES |
(20,973 | ) | (51,141 | ) | ||||
|
|
|
|
|
|||||
| NET INCREASE (DECREASE) IN CASH, CASH EQUIVALENTS AND RESTRICTED CASH |
65,434 | (35,557 | ) | |||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH AT BEGINNING OF PERIOD |
74,691 | 64,670 | ||||||
|
|
|
|
|
|||||
| CASH, CASH EQUIVALENTS AND RESTRICTED CASH AT END OF PERIOD |
$ | 140,125 | $ | 29,113 | ||||
|
|
|
|
|
|||||
F-39
Table of Contents
SUPPLEMENTAL DISCLOSURES—See Note 4 – Debt and Note 6 – Income Taxes for a discussion of cash paid for interest and income taxes, respectively.
NONCASH INVESTING AND FINANCING TRANSACTIONS—Purchases of patient equipment and property, equipment and improvements exclude purchases that remain unpaid at the end of the respective period. Such amounts are then included in the following period’s purchases. Unpaid purchases were $45.3 million and $67.9 million as of September 30, 2020 and 2019, respectively. Unpaid purchases include $17.3 million and $29.1 million of patient equipment and property, equipment and improvements acquired under extended payment terms as of September 30, 2020 and 2019, respectively.
See notes to unaudited condensed consolidated financial statements.
F-40
Table of Contents
APRIA HEALTHCARE GROUP INC.
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 1. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation—The accompanying unaudited condensed consolidated financial statements and related notes have been prepared in accordance with accounting principles generally accepted in the United States of America and are presented in U.S. dollars. These unaudited condensed consolidated financial statements include the accounts of Apria Healthcare Group Inc. (the “Company”) and its subsidiaries. The Company had no items of other comprehensive income; as such, its comprehensive income is the same as the net income for all periods presented. Intercompany transactions and accounts have been eliminated in consolidation. The unaudited condensed consolidated financial statements have been prepared on the same basis as the audited consolidated financial statements and, in the opinion of management, reflect all normal recurring adjustments necessary for the fair statement of the consolidated results for these periods. Interim period results are not necessarily indicative of the results that may be expected for the full fiscal year.
Company Background—The Company operates in the home health care segment of the health care industry, providing a variety of high-quality clinical patient care management programs, related products and supplies as prescribed by a physician and/or authorized by a case manager as part of a care plan. Essentially all products and services offered by the Company are provided through the Company’s network of approximately 310 locations, which are located throughout the United States. The Company provides services and products in one operating segment: home respiratory therapy/home medical equipment. The Company provides patients in their homes with products and services which are primarily paid for by a third-party payor, such as Medicare, Medicaid, a managed care plan or another third-party insurer. Sales are primarily derived from referral sources such as hospital discharge planners, medical groups or independent physicians.
On October 28, 2008, the Company was acquired by a wholly owned affiliate of BP Healthcare Holdings LLC (“Buyer” or “BP Healthcare”). Buyer is controlled by private investment funds affiliated with The Blackstone Group Inc. (the “Sponsor”).
Use of Accounting Estimates—The preparation of the unaudited condensed consolidated financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the amounts reported in the unaudited condensed consolidated financial statements and accompanying notes. Actual results could differ from those estimates. Among the significant estimates affecting the unaudited condensed consolidated financial statements are those related to revenue recognition and the resulting accounts receivable, self-insurance reserves, long-lived assets, stock-based compensation, legal reserves and income taxes.
Unaudited Pro Forma Information—In December 2020, the Company amended its existing credit facility to borrow $260.0 million in incremental term loans. Net proceeds were used to fund a $200.3 million dividend payment to our stockholders and $9.7 million distribution to SARs unit-holders, with the remaining proceeds used to pay fees and expenses in connection with the Credit Facility Amendment and for general corporate purposes. Pro forma balance sheet amounts reflect the dividend and distribution payable of $210.0 million.
Fee-for-Service Net Revenues—Revenues are recognized under fee-for-service arrangements for equipment the Company rents to patients and sales of equipment, supplies and other items the Company sells to patients.
F-41
Table of Contents
Rental and sale net revenues under fee-for-service arrangements disaggregated by each core service line item were:
| Nine Months Ended September 30, | ||||||||||||||||||||||||
| 2020 | 2019 | |||||||||||||||||||||||
| (dollars in thousands) | Rental | Sale | Total Fee- For-Service |
Rental | Sale | Total Fee- For-Service |
||||||||||||||||||
| Home respiratory therapy |
$ | 294,874 | $ | 2,654 | $ | 297,528 | $ | 287,915 | $ | 1,518 | $ | 289,433 | ||||||||||||
| Obstructive sleep apnea treatment |
63,285 | 199,564 | 262,849 | 61,479 | 190,548 | 252,027 | ||||||||||||||||||
| Negative pressure wound therapy |
21,324 | 1,605 | 22,929 | 21,261 | 1,910 | 23,171 | ||||||||||||||||||
| Other equipment and services |
30,530 | 32,794 | 63,324 | 32,441 | 47,552 | 79,993 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Total |
$ | 410,013 | $ | 236,617 | $ | 646,630 | $ | 403,096 | $ | 241,528 | $ | 644,624 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| 63.4% | 36.6% | 100.0% | 62.5% | 37.5% | 100.0% | |||||||||||||||||||
Rental revenues—Revenue generated from equipment that the Company rents to patients is recognized over the noncancelable rental period, typically one month, and commences on delivery of the equipment to the patients. The Company evaluates the portfolio of lease contracts at lease commencement and the start of each monthly renewal period to determine if it is reasonably certain that the monthly renewal or purchase options would be exercised. The exercise of monthly renewal or purchase options by a patient has historically not been reasonably certain to occur at lease commencement or subsequent monthly renewal.
Revenues are recorded at amounts estimated to be received under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare, Medicaid and patients. Rental revenue, less estimated adjustments, is recognized as earned on a straight-line basis over the non-cancellable lease term. Rental of patient equipment is billed on a monthly basis beginning on the date the equipment is delivered. Since deliveries can occur on any day during a month, the amount of billings that apply to the next month are deferred.
The Company’s lease agreements generally contain lease and non-lease components. Non-lease components primarily relate to supplies. The Company allocates the transaction price to the separate lease and non-lease components that qualify as performance obligations using the stand-alone selling price.
Sale revenues—Revenue related to sales of equipment and supplies is recognized on the date of delivery as this is when control of the promised goods is transferred to patients and is presented net of applicable sales taxes. Revenues are recorded only to the extent it is probable that a significant reversal will not occur in the future as amounts may include implicit price concessions under reimbursement arrangements with third-party payors, including private insurers, prepaid health plans, Medicare, Medicaid and patients. The Company determines the sales transaction price based on contractually agreed-upon rates, adjusted for estimates of variable consideration. The Company uses the expected value method in determining the variable consideration as part of determining the sales transaction price using historical reimbursement experience, historical sales returns, and other operating trends. Payment terms and conditions vary by contract. The timing of revenue recognition, billing, and cash collection generally results in billed and unbilled accounts receivable.
Capitation Revenues—Revenues are recognized under capitation arrangements with third-party payors for services and equipment for which the Company stands ready to provide to the members of these payors without regard to the actual services provided. The stand ready obligation generally extends beyond one year. Revenue is recognized over the month that the members are entitled to health care services using the contractual rate for each covered member. The actual number of covered members may vary each month. As a practical expedient, no disclosures have been made related to the amount of variable consideration expected to be recognized in future periods under these capitation arrangements. Capitation payments are typically received in the month members are entitled to health care services. Contracts with a single national payor constituted 86% of the total capitation revenue for the nine months ended September 30, 2020 and 2019.
F-42
Table of Contents
Concentration of Credit Risk—Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 21% and 1%, respectively, of total net revenues for the nine months ended September 30, 2020. Revenues reimbursed under arrangements with Medicare and Medicaid were approximately 19% and 1%, respectively, of total net revenues for the nine months ended September 30, 2019. Contracts with two national payors each represented more than 10% of the Company’s total net revenues constituting 23% and 11% of total net revenues for the nine months ended September 30, 2020 and 23% and 10% of total net revenues for the nine months ended September 30, 2019. As of September 30, 2020 and December 31, 2019, Medicare represented greater than 10% of net accounts receivable.
Cash and Cash Equivalents—Cash is maintained with various financial institutions. These financial institutions are located throughout the United States and the Company’s cash management practices limit exposure to any one institution. Management considers all highly liquid instruments purchased with an original maturity of less than three months to be cash equivalents.
Accounts Receivable—Included in accounts receivable are earned but unbilled receivables of $17.1 million and $24.1 million as of September 30, 2020 and December 31, 2019, respectively. Delays ranging from a day up to several weeks between the date of service and billing can occur due to delays in obtaining certain required payor-specific documentation from internal and external sources. Earned but unbilled receivables are aged from date of service and are considered in the analysis of historical performance and collectability.
Due to the nature of the industry and the reimbursement environment in which the Company operates, certain estimates are required to record total net revenues and accounts receivable at their net realizable values. Inherent in these estimates is the risk that they will have to be revised or updated as additional information becomes available. Specifically, the complexity of many third-party billing arrangements and the uncertainty of reimbursement amounts for certain services from certain payors may result in adjustments to amounts originally recorded. Such adjustments are typically identified and recorded at the point of cash application, claim denial or account review.
Management performs periodic analyses to evaluate accounts receivable balances to ensure that recorded amounts reflect estimated net realizable value. Specifically, management considers historical realization data, accounts receivable aging trends, other operating trends, and the extent of contracted business and business combinations. Also considered are relevant business conditions such as governmental and managed care payor claims processing procedures and system changes.
Additionally, focused reviews of certain large and/or problematic payors are performed. Due to continuing changes in the health care industry and third-party reimbursement, it is possible that management’s estimates could change in the near term, which could have an impact on operations and cash flows.
The Company records a reserve for expected credit losses as part of net rental revenue adjustments in order to report rental revenue at an expected collectable amount based on the total portfolio of operating lease receivables for which collectability has been deemed probable.
Inventories—Inventories are stated at the lower of cost (approximate costs determined on the first-in, first-out basis) or net realizable value and consist primarily of respiratory supplies and items used in conjunction with patient equipment.
Patient Equipment—Patient equipment is stated at cost less depreciation and consists of medical equipment rented to patients on a month-to-month basis. Depreciation is provided using the straight-line method over the estimated useful lives of the equipment, which range from 1 to 10 years. Patient equipment depreciation is classified in the Company’s unaudited condensed consolidated statements of income within costs of net revenues as the equipment is rented to patients as part of the Company’s primary operations.
Patient equipment is generally placed for rent; however, it could also be sold to customers. Once returned to the Company, patient equipment is assessed and repaired as necessary. Patient equipment is typically leased to subsequent patients if its condition is suitable. Upon a sale, the Company records the proceeds of the sale within net revenues and the costs related to the carrying net book value as other costs within cost of net revenues in the Company’s unaudited condensed consolidated statements of income.
F-43
Table of Contents
Given rental income is generated from such products, purchases of patient equipment are considered an investing activity when paid soon before or after purchase, while other payments made are considered a financing activity within the unaudited condensed consolidated statements of cash flows. Certain unpaid purchases are secured by a security interest in $5.6 million and $17.9 million of patient equipment as of September 30, 2020 and December 31, 2019, respectively. The net loss from the sale of patient equipment is reported as an adjustment to net income within cash provided by operating activities in the unaudited condensed consolidated statements of cash flows.
Property, Equipment and Improvements—Property, equipment and improvements are stated at cost less depreciation. Depreciation is provided using the straight-line method over the estimated useful lives of the assets, which range from 1 to 15 years or, for leasehold improvements, the shorter of the useful life of the asset or the remaining life of the related lease.
Capitalized Software—Capitalized software costs related to internally developed and purchased software are included in property, equipment and improvements in the unaudited condensed consolidated balance sheets and are amortized using the straight-line method over the estimated useful lives of the assets, which range from three to five years. Capitalized costs include direct costs of materials and services incurred in developing or obtaining internal-use software and payroll and benefit costs for employees directly involved in the development of internal-use software. Capitalization of such costs ceases when the project is substantially complete and ready for its intended purpose. Costs incurred during the preliminary and post-implementation stages, as well as software maintenance and training costs, are expensed in the period in which they are incurred. Additions to capitalized internally developed software totaled $3.8 million and $4.1 million for the nine months ended September 30, 2020 and 2019, respectively. Amortization expense for internally developed software was $4.4 million for the nine months ended September 30, 2020 and 2019.
Indefinite-Lived Intangible Assets and Long-Lived Assets—Indefinite-lived intangible assets are not amortized but instead tested at least annually for impairment or more frequently when events or changes in circumstances indicate that the assets might be impaired. The Company performs the annual test for impairment for indefinite-lived intangible assets as of the first day of the fourth quarter.
The Company will first assess qualitative factors to determine whether it is more likely than not that the fair value is less than its carrying amount. If, based on a review of qualitative factors, it is more likely than not that the fair value is less than its carrying amount, the Company will use a quantitative approach, and calculate the fair value and compare it to its carrying amount. If the fair value exceeds the carrying amount, there is no indication of impairment. If the carrying amount exceeds the fair value, an impairment loss is recorded equal to the difference.
Long-lived assets, including property and equipment and purchased definite-lived intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset or asset group may not be recoverable. Significant judgment is required in determining whether a potential indicator of impairment of long-lived assets exists and in estimating future cash flows for any necessary impairment tests. Recoverability of assets to be held and used is measured by the comparison of the carrying amount of an asset to future undiscounted net cash flows expected to be generated by the asset. If such an asset is considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell.
The Company did not record any impairment charges related to indefinite-lived intangible assets or long-lived assets for the nine months ended September 30, 2020 and 2019.
Fair Value of Financial Instruments—Management is required to disclose the estimated fair value of certain assets and liabilities of financial instruments. Financial instruments are generally defined as cash, evidence of ownership interest in an entity or a contractual obligation that both conveys to one entity a right to receive cash or other financial instruments from another entity and imposes on the other entity the obligation to deliver cash or other financial instruments to the first entity. The carrying amounts of cash and
F-44
Table of Contents
cash equivalents, accounts receivable, accounts payable, and accrued expenses approximate fair value due to their short maturity. The carrying amounts of the Company’s long-term debt, including the Term Loan A Facility and Revolving Credit Facility, as of September 30, 2020, approximate fair value due to the variable rate nature of the agreements. All debt classifications represent Level 2 fair value measurements.
Leases—The Company determines if an arrangement is a lease at commencement and performs an evaluation to determine whether the lease should be classified as an operating or finance lease. Operating leases are included in operating lease right-of-use (ROU) assets, current portion of operating lease liabilities and operating lease liabilities, less current portion, on the unaudited condensed consolidated balance sheets. ROU assets represent the Company’s right to use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising from the lease. Operating lease ROU assets and the related liabilities are recognized at the commencement date based on the present value of lease payments over the lease term. As the Company’s leases do not provide an implicit rate, the Company uses its incremental borrowing rate (IBR) based on the information available at the lease commencement date in determining the present value of future lease payments. The Company used market rates from recent secured financing to determine the IBR.
The operating lease ROU asset also includes any lease payments made to the lessor at or before the commencement date and is adjusted by any lease incentives received. Variable lease payments are not included in the operating lease liability as they cannot be reasonably estimated and are recognized in the period in which the obligation for those payments is incurred. Lease terms may include options to extend or terminate the lease and are included only when it is reasonably certain that the Company will exercise that option. For all asset classes, leases with a lease term of twelve months or less at the lease commencement date are not recorded on the unaudited condensed consolidated balance sheets, as permitted by the short-term lease exception. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
The Company’s lease agreements do not contain any material residual value guarantees or material restrictive covenants. The Company does not have any material subleases. The Company does not have any leases classified as a finance leasing arrangement. As such, all leases are classified as operating leases. See further discussion at Note 7 – Leases.
Product and Supply Costs—Product and supply costs presented within total cost of net revenues are comprised primarily of the cost of supplies, equipment and accessories provided to patients.
Contract Costs—The Company pays sales commissions on fee-for-service arrangements in an effort to increase the volume of serviced patients. The Company elected to use the practical expedient to expense sales commissions as incurred since the amortization period would otherwise be less than one year. These costs are included in selling, distribution and administrative expense in the unaudited condensed consolidated statements of income.
Home Respiratory Therapists Costs—Home respiratory therapists costs presented within total cost of net revenues are comprised primarily of employee salary and benefit costs or contract fees paid to respiratory therapists and other related professionals who are deployed to service a patient. Home respiratory therapy personnel are also engaged in a number of administrative and marketing tasks and, accordingly, these costs are classified within selling, distribution and administrative expenses and were $11.1 million for the nine months ended September 30, 2020 and 2019.
Distribution Expenses—Distribution expenses totaled $87.0 million and $97.1 million for the nine months ended September 30, 2020 and 2019, respectively. Such expense represents the cost incurred to coordinate and deliver products and services to the patients. Included in distribution expenses are leasing, maintenance, licensing and fuel costs for the vehicle fleet; salaries and other costs related to drivers and dispatch personnel; and amounts paid to courier and other outside shipping vendors. Such expenses fall within the definition of “shipping and handling” costs and are classified within selling, distribution and administrative expenses and may not be comparable to other companies.
F-45
Table of Contents
Self-Insurance—Coverage for certain employee medical claims and benefits, as well as workers’ compensation, professional and general liability, and vehicle liability are self-insured. Amounts accrued for costs of workers’ compensation, medical, professional and general liability, and vehicle liability are classified as current or long-term liabilities based upon an estimate of when the liability will ultimately be paid.
Amounts accrued as current liabilities within other accrued liabilities are as follows:
| (in thousands) | September 30, 2020 |
December 31, 2019 |
||||||
| Workers’ compensation |
$ | 4,800 | $ | 4,963 | ||||
| Professional and general liability/vehicle |
3,071 | 2,745 | ||||||
| Medical insurance |
2,279 | 2,413 | ||||||
Amounts accrued as long-term liabilities within other noncurrent liabilities are as follows:
| (in thousands) | September 30, 2020 |
December 31, 2019 |
||||||
| Workers’ compensation |
$ | 17,741 | $ | 17,793 | ||||
| Professional and general liability/vehicle |
6,180 | 5,281 | ||||||
Stock-Based Compensation—The Company accounts for its stock-based awards in accordance with provisions of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) No. 718, Compensation—Stock Compensation. The Company has two types of compensation awards, profit interest units and stock appreciation rights (“SARs”). The Company recognizes compensation expense in respect of the profit interest units and SARs based on the fair value of the awards as measured on the grant date. Fair value is not subsequently remeasured unless the conditions on which the award was granted are modified. The determination of fair value at grant date requires the use of estimates, which are based on management’s judgment. Generally, compensation expense for each separately vesting portion of the awards is recognized on a straight-line basis over the vesting period for that portion of the award subject to continued service. Compensation expense for awards containing performance conditions is recognized only to the extent that it is probable the performance conditions will be satisfied.
Legal Reserves—The Company is involved in various legal proceedings, claims, and litigation that arise in the ordinary course of business. The Company investigates these matters as they arise and reserves for potential loss in accordance with ASC No. 450, Contingencies. Significant judgment is required in the determination of both the probability of loss and whether the amount of the loss can be reasonably estimated. Estimates are subjective and are made in consultation with internal and external legal counsel. See further discussion at Note 8 – Commitments and Contingencies and Note 10 – Subsequent Events.
Dividend—In June 2019, the Company declared and paid a $175.0 million dividend to common stockholders and SARs unit-holders. See subsequent events disclosure updates at Note 10 – Subsequent Events.
Income Taxes—The Company’s provision for income taxes is based on expected income, permanent book/tax differences and statutory tax rates in the various jurisdictions in which the Company operates. Significant management estimates and judgments are required in determining the provision for income taxes.
Deferred income tax assets and liabilities are computed for differences between the carrying amounts of assets and liabilities for financial statement and tax purposes. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized.
On March 27, 2020, the Coronavirus Aid, Relief and Economic Security (CARES) Act was enacted and signed into U.S. law to provide economic relief to individuals and businesses facing economic hardship as a
F-46
Table of Contents
result of the COVID-19 public health emergency. The CARES Act includes, among other things, provisions relating to payroll tax credits and deferrals, net operating loss carryback periods, alternative minimum tax credits refunds, modifications to the net interest deduction limitations, and technical corrections to tax depreciation methods for qualified improvement property. As permitted under the CARES Act, the Company has elected to defer certain portions of employer-paid FICA taxes otherwise payable from March 27, 2020 to January 1, 2021, which will be paid in two equal installments on December 31, 2021 and December 31, 2022. The amount deferred as of September 30, 2020 was $9.5 million and is included in other noncurrent liabilities on the unaudited condensed consolidated balance sheet.
Business Segments—The Company has evaluated segment reporting in accordance with FASB ASC No. 280, Segment Reporting. The Company’s chief executive officer is its chief operating decision maker. The chief operating decision maker reviews financial information about the business at the enterprise-wide consolidated level when allocating the resources of the Company and assessing business performance. Accordingly, the Company has determined that its business activities comprise a single operating and reporting segment, the home respiratory therapy/home medical equipment segment. Through its single segment, the Company focuses on three core service lines: home respiratory therapy (including home oxygen and non-invasive ventilation services), obstructive sleep apnea treatment (including continuous positive airway pressure and bi-level positive airway pressure devices, and patient support services) and negative pressure wound therapy. Additionally, the Company supplies a wide range of home medical equipment and other products and services to help improve the quality of life for patients with home care needs.
Net revenues for each core service line were:
| Nine Months Ended September 30, |
||||||||
| (in thousands) | 2020 | 2019 | ||||||
| Home respiratory therapy |
$ | 335,277 | $ | 325,422 | ||||
| Obstructive sleep apnea treatment |
330,966 | 319,971 | ||||||
| Negative pressure wound therapy |
30,751 | 30,345 | ||||||
| Other equipment and services |
117,934 | 131,972 | ||||||
|
|
|
|
|
|||||
| Net revenues |
$ | 814,928 | $ | 807,710 | ||||
|
|
|
|
|
|||||
Earnings per share—Basic income per share represents net income divided by the weighted-average number of shares outstanding during the period. Diluted income per share represents net income divided by the weighted-average number of shares outstanding plus potential dilutive shares. For the nine months ended September 30, 2020 and 2019, the Company determined that there were no potential dilutive shares to be included in the calculation of diluted income per share as a result of its stock-based awards.
The computation of net income per share is presented below:
| Nine Months Ended September 30, |
||||||||
| (in thousands, except share and per share data) | 2020 | 2019 | ||||||
| Net income attributable to common shareholders |
$ | 20,268 | $ | 9,840 | ||||
| Weighted average number of shares outstanding |
992,719 | 992,719 | ||||||
| Dilutive effect of stock-based awards |
— | — | ||||||
|
|
|
|
|
|||||
| Diluted weighted average number of shares outstanding |
992,719 | 992,719 | ||||||
|
|
|
|
|
|||||
| Basic and diluted net income per share |
$ | 20.42 | $ | 9.91 | ||||
F-47
Table of Contents
| 2. | RECENT ACCOUNTING PRONOUNCEMENTS |
Recently Adopted Accounting Pronouncements
In November 2018, the FASB issued Accounting Standards Update (ASU) No. 2018-19, Codification Improvements to Topic 326, Financial Instruments-Credit Losses, which is an amendment to ASU No. 2016-13, Financial Instruments-Credit Losses (Topic 326): Measurement of Credit Losses on Financial Instruments. The amendment clarifies that receivables arising from operating leases are not within the scope of ASU No. 2016-13 and impairment of receivables arising from operating leases should be accounted for in accordance with ASU No. 2016-02, Leases (Topic 842). This ASU is effective for fiscal years beginning after December 15, 2019, and interim periods within those fiscal years. The adoption of this ASU as of January 1, 2020 did not have a material effect on the Company’s unaudited condensed consolidated financial statements.
In August 2018, the FASB issued ASU No. 2018-15, Intangibles—Goodwill and Other—Internal-Use Software (Subtopic 350-40): Customer’s Accounting for Implementation Costs Incurred in a Cloud Computing Arrangement that is a Service Contract, which amends FASB ASC No. 350-40, Customer’s Accounting for Fees Paid in a Cloud Computing Arrangement, to align the accounting for the implementation costs incurred in a cloud computing arrangement that is a service contract with the guidance on capitalizing costs associated with developing or obtaining internal-use software in that implementation costs of a cloud computing arrangement that is a service contract should be capitalized. ASU No. 2018-15 is effective for fiscal years beginning after December 15, 2019, and interim periods within fiscal years beginning after December 15, 2019, using either a retrospective or prospective approach. When a prospective transition is chosen, entities must apply the transition requirements to any eligible costs incurred after adoption. The adoption of ASU No. 2018-15 on a prospective basis as of January 1, 2020 did not have a material effect on the Company’s unaudited condensed consolidated financial statements.
| 3. | INTANGIBLE ASSETS |
Intangible assets consist of the following:
| September 30, 2020 | December 31, 2019 | |||||||||||||||||||||||||||
| (dollars in thousands) | Average Life in Years |
Gross Carrying Amount |
Accumulated Amortization |
Net Book Value |
Gross Carrying Amount |
Accumulated Amortization |
Net Book Value |
|||||||||||||||||||||
| Intangible assets subject to amortization: |
||||||||||||||||||||||||||||
| Capitated relationships |
20.0 | $ | 4,400 | $ | (2,832 | ) | $ | 1,568 | $ | 4,400 | $ | (2,686 | ) | $ | 1,714 | |||||||||||||
| Payor relationships |
20.0 | 7,600 | (4,528 | ) | 3,072 | 7,600 | (4,243 | ) | 3,357 | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Subtotal |
12,000 | (7,360 | ) | 4,640 | 12,000 | (6,929 | ) | 5,071 | ||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Intangible assets not subject to amortization: |
||||||||||||||||||||||||||||
| Trade names |
— | 50,000 | — | 50,000 | 50,000 | — | 50,000 | |||||||||||||||||||||
| Accreditations with commissions |
— | 7,000 | — | 7,000 | 7,000 | — | 7,000 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Subtotal |
57,000 | — | 57,000 | 57,000 | — | 57,000 | ||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Total |
$ | 69,000 | $ | (7,360 | ) | $ | 61,640 | $ | 69,000 | $ | (6,929 | ) | $ | 62,071 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
Amortization expense was $0.4 million for the nine months ended September 30, 2020 and 2019.
F-48
Table of Contents
Estimated amortization expense for each of the fiscal years ending December 31 is presented below:
| (in thousands) | ||||
| Years Ending December 31 |
||||
| 2020 |
$ | 574 | ||
| 2021 |
574 | |||
| 2022 |
574 | |||
| 2023 |
574 | |||
| 2024 |
574 | |||
| Thereafter |
2,201 | |||
| 4. | DEBT |
Long-term debt consists of the following:
| (in thousands) | September 30, 2020 |
December 31, 2019 |
||||||
| Term Loan A |
$ | 146,250 | $ | 150,000 | ||||
| Less: Current Portion |
(7,500 | ) | (3,750 | ) | ||||
| Less: Unamortized debt issuance costs |
(1,846 | ) | (2,216 | ) | ||||
|
|
|
|
|
|||||
| Total long-term debt |
$ | 136,904 | $ | 144,034 | ||||
|
|
|
|
|
|||||
On November 2, 2018, the Company entered into a senior secured asset-based revolving credit facility (the “ABL Facility”), with Wells Fargo Bank, N.A., an administrative agent and a syndicate of financial institutions and institutional lenders. The ABL Facility replaced the prior secured asset-based revolving credit facility dated May 2, 2014, which provided for a revolving credit financing of up to $100.0 million.
On June 21, 2019, Apria entered into a credit agreement with Citizens Bank and a syndicate of lenders for both a Term Loan A Facility (the “TLA”) of $150.0 million and a Revolving Credit Facility (the “Revolver”) of $100.0 million. The Revolver replaced the prior ABL Facility which provided for revolving credit financing of up to $125.0 million. Proceeds from the TLA were used to fund the 2019 dividend payment to common stockholders and SARs unit-holders.
The credit agreement permits the interest rate to be selected at the Company’s option at either Adjusted LIBOR or Alternative Base Rate plus their respective applicable margin. Adjusted LIBOR is the rate for Eurodollar deposits for the applicable interest period while the Alternate Base Rate is the highest of (i) the Administrative Agent’s “Prime Rate”, (ii) the Federal Funds Effective Rate plus 0.50%, and (iii) one-month
Adjusted LIBOR plus 1.00%. Additionally, the margin applied to both the TLA and Revolver is determined based on total net leverage ratio. Total net leverage ratio is defined as net debt, which represents indebtedness minus up to $25.0 million in cash and cash equivalents over consolidated EBITDA as defined under the credit agreement. The following is a summary of the additional margin and commitment fees payable on both the TLA and available Revolver:
| Level |
Total Net Leverage Ratio |
Applicable Margin for Adjusted LIBOR Loans |
Applicable Margin for Alternative Base Rate Loans |
Commitment Fee |
||||||||||
| I |
Greater than or equal to 3.00x |
2.75 | % | 1.75 | % | 0.35 | % | |||||||
| II |
Greater than or equal to 2.50x but less than 3.00x |
2.50 | % | 1.50 | % | 0.30 | % | |||||||
| III |
Greater than or equal to 1.50x but less than 2.50x |
2.25 | % | 1.25 | % | 0.25 | % | |||||||
| IV |
Less than 1.50x |
2.00 | % | 1.00 | % | 0.20 | % | |||||||
F-49
Table of Contents
The TLA matures on June 21, 2024 and the Company is required to make quarterly principal payments on the TLA beginning June 30, 2020. The table below is a summary of the expected principal repayments each year:
| (in thousands) | ||||
| Years Ending December 31 |
||||
| 2020 |
$ | 5,625 | ||
| 2021 |
7,500 | |||
| 2022 |
13,125 | |||
| 2023 |
15,000 | |||
| 2024 |
108,750 | |||
The credit agreement encompassing the TLA and Revolver permits the Company, subject to certain exceptions, to increase its TLA or its Revolver, as well as incur additional indebtedness, as long as it does not exceed the total net leverage ratio of 3.00x. The credit agreement requires mandatory prepayments upon the occurrence of certain events, such as dispositions and casualty events, subject to certain exceptions. The TLA or Revolver may be voluntarily prepaid by the Company at any time without any premium or penalty.
The assets of the Company and equity interest of all present and future wholly owned direct domestic subsidiaries, with certain exceptions, are pledged as collateral for the TLA and Revolver. The credit agreement contains a financial covenant requiring the Company to maintain a total net leverage ratio less than 3.50x.
As of September 30, 2020, no amounts were outstanding under the Revolver, there were $15.9 million outstanding letters of credit, and additional availability under the Revolver net of letters of credit outstanding was $84.1 million. The Company was in compliance with all debt covenants set forth in the TLA and Revolver as of September 30, 2020.
In accordance with ASU 2015-03, Interest—Imputation of Interest (Subtopic 835-30): Simplifying the Presentation of Debt Issuance Costs, the Company records origination and other expenses related to certain debt issuance cost as a direct deduction from the carrying amount of the debt liability. These expenses are deferred and amortized using the straight-line method over the stated life, which approximates the effective interest rate method. The unamortized debt issuance costs related to the ABL Facility dated November 2, 2018 were expensed in the nine months ended September 30, 2019 as a result of the new credit agreement. Amortization of deferred debt issuance costs are classified within interest expense in the Company’s unaudited condensed consolidated statements of income and was $0.4 million and $1.0 million for the nine months ended September 30, 2020 and 2019, respectively.
Interest expense, excluding deferred debt issuance costs discussed above, was $3.7 million and $2.4 million for the nine months ended September 30, 2020 and 2019, respectively. The interest rate was 2.15% and 3.70% as of September 30, 2020 and December 31, 2019, respectively.
Interest paid on debt totaled $3.7 million and $2.6 million for the nine months ended September 30, 2020 and 2019, respectively. See subsequent events disclosure at Note 10 – Subsequent Events.
| 5. | STOCK-BASED COMPENSATION |
Profit Interest Units—BP Healthcare and its subsidiary, Apria Holdings LLC (“Holdings”), granted equity units to certain employees, Board members and a member of a subsidiary’s Board of Directors for purposes of retaining them and enabling such individuals to participate in the long-term growth and financial success of the Company. Profit interest units are measured at the grant date, based on the calculated fair value of the award, and are recognized as an expense over the employee’s requisite service period. These equity awards were issued in exchange for services to be performed.
Profit interest units are composed of Class B and Class C units related to Holdings. Holdings also has 3,575,000 outstanding in Class A-2 units, which are senior in liquidation rights to Class B units, whereas
F-50
Table of Contents
Class B units are senior in liquidation rights to Class C units. Class B units have a strike price ranging from $0.00 - $0.63, and the holders of those units only share in the value of Holdings to the extent it exceeds the per unit strike price.
A portion of the units vested over a specified period of time, generally five years, based on continued service, and a portion of the units vest based on performance/market conditions and will generally vest if affiliates of the Sponsor receive a specific return in respect of their capital investment. Certain units will become fully vested on an accelerated basis either upon a change in control (including an initial public offering) while the management employee who was granted the units continues to provide services to the Company or its subsidiaries, or if affiliates of the Sponsor receive a specific return in respect of their capital investment.
On June 21, 2019, the Board of Directors of Holdings approved the modification of all outstanding performance Class B units to accelerate vesting. Based on the fair value of the vested awards on the date of modification, stock compensation expense was $7.0 million for the nine months ended September 30, 2019. Expense related to profit interest units is recorded within selling, distribution and administrative expenses in the unaudited condensed consolidated statements of income. There was no expense related to profit interest units for the nine months ended September 30, 2020.
The Company uses the income approach and the guideline approach to estimate enterprise value, which is utilized to assess the fair value of each instrument. No units were granted in 2020 or 2019, as profit interest units are no longer granted.
There are no stated contractual lives for the units.
The following table summarizes activity for all profit interest units for the period from December 31, 2019 to September 30, 2020:
| Class B Units |
Weighted- Average Exercise Price |
Aggregate Intrinsic Value ($000s) |
Class C Units |
Weighted- Average Exercise Price |
Aggregate Intrinsic Value ($000s) |
|||||||||||||||||||
| Outstanding as of December 31, 2019 |
88,008,850 | $ | 0.01 | 2,849,092 | — | |||||||||||||||||||
| Granted |
— | — | — | — | ||||||||||||||||||||
| Forfeited |
— | — | — | — | ||||||||||||||||||||
| Exercised |
— | — | — | — | ||||||||||||||||||||
|
|
|
|
|
|||||||||||||||||||||
| Outstanding as of September 30, 2020 |
88,008,850 | $ | 0.01 | $ | 65,210 | 2,849,092 | — | $ | 1,452 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Vested units as of September 30, 2020 |
88,008,850 | — | ||||||||||||||||||||||
There were 2,849,092 unvested Class C units with a weighted-average grant-date fair value of $0.22 as of September 30, 2020 and December 31, 2019.
Stock Appreciation Rights—In 2015, the Company’s Board of Directors approved a stock plan that provides for the grant of 27,689 stock appreciation rights to directors, officers, employees, consultants and advisers (and prospective directors, officers, employees, consultants, and advisers) of the Company and its affiliates. The plan mandates a maximum award term of 10 years and that SARs be granted with a strike price not less than the fair market value determined as of the date of grant. SARs are measured at the grant date, based on the calculated fair value of the award and are recognized as an expense over the employee’s requisite service period. These equity awards are issued in exchange for services to be performed. The Company’s Board of Directors approved an additional 42,516 SARs and 60,000 SARs available for grant under the 2015 stock plan in March 2017 and October 2019, respectively.
SARs granted under the plan generally vest over 60 months from the date of grant based on continued service. All unvested SARs are forfeited upon employee termination. In the event of a change in capital structure or similar event, SARs may be modified.
F-51
Table of Contents
Expense related to SARs held by unit-holders continuing to perform services for the Company is recorded within selling, distribution and administrative expenses in the unaudited condensed consolidated statements of income and was $1.5 million and $1.1 million for the nine months ended September 30, 2020 and 2019, respectively. As of September 30, 2020, total unrecognized compensation cost related to unvested SARs was $5.6 million, which is expected to be expensed over a weighted-average period of 3.7 years.
The Company has estimated the grant-date fair value of SARs using the Black-Scholes valuation model. The following assumptions were used in estimating the value and determining the related stock-based compensation attributable to the current period.
| 2020 | ||||
| Expected asset volatility(1) |
45.0 | % | ||
| Risk free interest rate(2) |
1.83 | % | ||
| Expected life(3) |
6.35 years | |||
| Dividend(4) |
— | |||
| (1) | Volatility is estimated as the average of the historical volatilities of the publicly traded comparable companies. |
| (2) | The risk-free interest rate is interpolated from the United States Constant Maturity Treasury curve with a term matching the expected life. |
| (3) | The expected life assumes the expected exercise date is the midpoint of vesting start date and the contractual expiration date of each tranche. |
| (4) | The dividend assumed is zero. |
The contractual lives for the units are 10 years. The Company accounts for forfeitures when they occur, and ultimately stock-based compensation is only recognized for awards that vest.
The following table summarizes activity for all SARs for the period from December 31, 2019 to September 30, 2020:
| Stock Appreciation Rights |
Weighted- Average Exercise Price |
Weighted- Average Remaining Contractual Term |
Aggregate Intrinsic Value ($000s) |
|||||||||||||
| Outstanding as of December 31, 2019 |
86,430 | $ | 305.36 | 7.4 | ||||||||||||
| Granted |
20,407 | 390.50 | ||||||||||||||
| Forfeited |
(724 | ) | 279.48 | |||||||||||||
|
|
|
|||||||||||||||
| Outstanding as of September 30, 2020 |
106,113 | $ | 321.91 | 7.2 | $ | 25,973 | ||||||||||
|
|
|
|
|
|
|
|||||||||||
| Vested units as of September 30, 2020 |
56,323 | |||||||||||||||
The following table summarizes the activity for unvested shares for the period from December 31, 2019 to September 30, 2020:
| Stock Appreciation Rights |
Weighted- Average Grant-Date Fair Value |
|||||||
| Unvested as of December 31, 2019 |
42,160 | $ | 123.08 | |||||
| Granted |
20,407 | 115.71 | ||||||
| Vested |
(12,053 | ) | 123.47 | |||||
| Forfeited |
(724 | ) | 125.76 | |||||
|
|
|
|||||||
| Unvested as of September 30, 2020 |
49,790 | $ | 119.93 | |||||
|
|
|
|||||||
There were no SARs granted in the nine months ended September 30, 2019.
F-52
Table of Contents
As of September 30, 2020, there were 24,092 SARs available for future grants under the plan.
| 6. | INCOME TAXES |
The Company’s effective tax rate was 39.1% for the nine months ended September 30, 2020 compared to 26.0% for the nine months ended September 30, 2019. For the nine months ended September 30, 2020, the Company’s effective tax rate differed from federal and state statutory rates primarily due to non-deductible expenses for tax purposes. For the nine months ended September 30, 2019, the Company’s effective tax rate differed from federal and state statutory rates primarily due to non-deductible expenses for tax purposes offset by excess tax benefits related to equity-based compensation and the release of effectively settled unrecognized tax benefits.
Deferred income taxes arise from temporary differences between the carrying amounts of assets and liabilities for tax and financial reporting purposes and tax losses and credit carryforwards. Deferred income tax assets are required to be reduced by a valuation allowance when it is determined that it is more likely than not that all or a portion of a deferred tax asset will not be realized.
As of each reporting date, management considers new evidence, both positive and negative, that could affect its view of the future realization of deferred tax assets. As of September 30, 2020, management evaluated all of the positive and negative evidence related to its valuation allowance, including its three years of cumulative pretax income and its projected income, and determined that there is sufficient positive evidence to conclude that it is more likely than not that net deferred taxes of $22.6 million and $34.7 million as of September 30, 2020 and December 31, 2019, respectively, are realizable.
The Company accounts for its tax uncertainties under generally accepted accounting principles. For the nine months ended September 30, 2020, no material changes occurred with respect to the Company’s tax uncertainties that would require disclosure.
The Company does not expect any material changes to its tax uncertainties within the 12-month rolling period ending September 30, 2021.
Net income tax payments were $1.7 million and $0.1 million for the nine months ended September 30, 2020 and 2019, respectively.
| 7. | LEASES |
The Company leases all its facilities. The Company’s real estate lease portfolio primarily consists of modified gross leases, in which the Company pays a share of the operating costs, and triple-net leases, in which the Company pays all of the operating costs. Operating costs that are the responsibility of the Company include taxes, maintenance, insurance and other allowable expenses. These expenses are considered variable costs as they are not tied to an index or rate. In addition, delivery vehicles and office equipment are leased under operating leases. Lease terms are generally five years or less with renewal options for additional periods and often contain early termination clauses. Rents are generally increased annually by amounts stated within individual agreements, subject to certain maximum amounts defined within individual agreements.
The Company also leases certain patient equipment. Lease terms are generally twelve months or less with renewal options for additional periods. The Company also has short-term patient equipment leases with certain suppliers that are entirely variable based on equipment usage or a percentage of net revenues collected for specific products. Patient equipment lease expense is recorded in product and supply costs in the unaudited condensed consolidated statements of income in the period incurred. The Company uses the portfolio approach to review patient equipment leases.
All of the Company’s leases are classified as operating leases. The leases do not include options to purchase the underlying assets that the Company is reasonably certain to exercise. The components of lease assets and liabilities are included on the unaudited condensed consolidated balance sheets.
F-53
Table of Contents
Significant components of lease expense were:
| Nine Months Ended September 30, |
||||||||
| (in thousands) | 2020 | 2019 | ||||||
| Operating lease expense |
$ | 27,962 | $ | 30,787 | ||||
| Variable lease expense |
17,392 | 15,280 | ||||||
| Short-term lease expense |
904 | 459 | ||||||
|
|
|
|
|
|||||
| Total lease expense |
$ | 46,258 | $ | 46,526 | ||||
|
|
|
|
|
|||||
The following table summarizes supplemental information related to our operating leases:
| Nine Months Ended September 30, |
||||||||
| (dollars in thousands) | 2020 | 2019 | ||||||
| Cash paid for amounts included in the measurement of operating lease liabilities |
$ | 21,794 | $ | 25,272 | ||||
| Right-of-use assets obtained in exchange for new operating lease liabilities |
15,165 | 12,959 | ||||||
| Weighted average remaining lease term |
3.0 years | 3.1 years | ||||||
| Weighted average discount rate |
4.9 | % | 5.4 | % | ||||
| 8. | COMMITMENTS AND CONTINGENCIES |
Litigation—Since December 2017, the Company has been engaged in responding to a series of related civil investigative demands (“CIDs”) from the United States Attorney’s Office for the Southern District of New York (the “SDNY Office”) concerning an investigation into “the sales, marketing, and/or patient service practices at Apria Healthcare in relation to the rental of non-invasive ventilators (“NIV”) that may violate the Anti-Kickback Statute, 42 U.S.C. Section 1320a-7b(b), and/or the False Claims Act, 31 U.S.C. Sections 3729 et seq.”
The government lawyers with the SDNY Office handling this matter have informed the Company’s counsel that the investigation is the result of a qui tam lawsuit and have suggested a framework for settling the claims the government is asserting. On September 15, 2020, the Company reached an agreement in principle to settle all claims arising out of or relating to the subject matter prior to December 31, 2019 and are conditioned on resolution under terms satisfactory to the Company of certain other claims. While the Company believes it has meritorious arguments and would pursue a vigorous defense if the matter were to proceed to litigation, the Company recorded a reserve of $44.7 million as a current liability and $12.2 million as a noncurrent liability on the consolidated balance sheets as of September 30, 2020 and December 31, 2019, respectively, representing its estimated probable loss as of such date. The accrual is included within selling, distribution and administrative expenses in the unaudited condensed consolidated statements of income and was $32.5 million and $0.0 million for the nine months ended September 30, 2020 and 2019, respectively.
If the Company determines not to settle the matter, then it expects it will proceed to litigation. The Company’s management cannot provide any assurances as to the timing or outcome of the investigations and any subsequent litigation or of any qui tam lawsuit.
While it is reasonably possible that the Company may incur a loss in excess of the amount accrued, given the stage of the matter and aforementioned uncertainties, management cannot estimate the possible loss or range of loss in excess of the amount accrued that may result from the proceedings described above and, therefore, has not recorded any related accruals beyond the amount it proposes to offer in settlement. If a judge, jury or administrative agency were to determine that practices occurred or payments were made in violation of the Anti-Kickback Statute, that false claims were submitted to federal health care programs with
F-54
Table of Contents
the requisite level of scienter, or that there were overpayments by the government that the Company knowingly concealed or knowingly and improperly avoided refunding to the Government, the Company could face claims for treble damages, criminal, civil and administrative penalties, claims for refunds, fines and sanctions, in amounts that would be material to its business, results of operations and financial condition, including the exclusion of the Company from participation in government health care programs.
In addition to the matter referenced in this note, the Company is engaged in the defense of certain claims and lawsuits arising out of the ordinary course and conduct of its business, the outcomes of which are not determinable at this time. Insurance policies covering such potential losses, where such coverage is cost effective, are maintained. In the opinion of management, any liability that might be incurred upon the resolution of these claims and lawsuits will not, in the aggregate, have a material effect on the Company’s financial condition or results of operations, cash flows and liquidity.
Supplier Concentration—Currently, approximately 77% of purchases for patient equipment and supplies are from five vendors. Although there are a limited number of suppliers, management believes that other vendors could provide similar products on comparable terms. However, a change in suppliers could cause delays in service delivery and possible losses of revenue, which could adversely affect the Company’s consolidated financial condition or operating results. In addition, from time to time, the Company enters into exclusive arrangements with certain suppliers to provide patient equipment and supplies.
Purchase Obligations—In April 2009, the Company entered into a ten-year information technology services agreement to outsource certain information systems functions. Effective February 2020, the agreement was amended a second time to extend the agreement through January 2024. If the Company terminated the agreement, the required obligation to the vendor would be approximately $4.8 million for services during the 120-day cancellation notice period plus termination fees.
Guarantees and Indemnities—From time to time, certain types of contracts are entered into that contingently require indemnification of parties against third-party claims. These contracts primarily relate to (i) certain asset purchase agreements, under which indemnification may be provided to the seller of the business being acquired; (ii) certain real estate leases, which may require indemnification to property owners for environmental or other liabilities and other claims arising from use of the applicable premises; and (iii) certain agreements with officers, directors, and employees, which may require indemnification of such persons for liabilities arising out of their relationship with the Company.
The terms of such obligations vary by contract, and in most instances, a specific or maximum dollar amount is not explicitly stated therein. Generally, amounts under these contracts cannot be reasonably estimated until a specific claim is asserted. Consequently, no liabilities have been recorded for these obligations on the unaudited condensed consolidated balance sheets for any of the periods presented.
| 9. | CERTAIN RELATIONSHIPS AND RELATED-PARTY TRANSACTIONS |
Change Healthcare— In December 1999, the Company entered into an agreement with Change Healthcare, a company affiliated with the Sponsor since 2011, to perform various revenue and payment cycle management functions. The Company paid Change Healthcare approximately $2.3 million and $2.8 million for the nine months ended September 30, 2020 and 2019, respectively. Amounts included in accounts payable were $0.5 million and $0.3 million as of September 30, 2020 and December 31, 2019, respectively.
BREIT Industrial Canyon PA1W01 LLC—In May 2018, the Company began paying BREIT Industrial Canyon, a subsidiary of Blackstone Real Estate Income Trust, Inc., which is a non-exchange traded, perpetual life real estate investment trust externally managed by an affiliate of the Sponsor, which had acquired a property for which the Company is in a lease agreement through October 2023. The Company paid BREIT Industrial Canyon approximately $0.5 million and $0.7 million for the nine months ended September 30, 2020 and 2019, respectively. The discounted operating lease liability for the remaining non-cancelable lease term is $2.0 million and $2.4 million as of September 30, 2020 and December 31, 2019, respectively.
F-55
Table of Contents
Mphasis—In September 2019, the Company entered into an eighty-seven-month agreement with Mphasis, a Company affiliated with the Sponsor since 2016, to perform various technology related services previously performed by another outsource vendor. The Company has the right to terminate for convenience with a minimum three-month notice. If the Company terminated the agreement, the required obligation to the vendor would be approximately $1.2 million for services during the three-month cancellation notice period. The Company paid Mphasis approximately $2.5 million for the nine months ended September 30, 2020. Amounts included in accounts payable were $0.9 million and $0.0 million as of September 30, 2020 and December 31, 2019, respectively.
Alight Solutions—In December 2019, the Company entered into an agreement with Alight Solutions, a Company affiliated with the Sponsor since 2017, to perform services related to the deployment of a new financial management system. The Company paid Alight approximately $0.5 million for the nine months ended September 30, 2020. Amounts included in accounts payable were $0.5 million and $0.0 million as of September 30, 2020 and December 31, 2019, respectively.
| 10. | SUBSEQUENT EVENTS |
The Company evaluated subsequent events through December 3, 2020, the date on which the September 30, 2020 financial statements were originally issued and has updated such evaluation for disclosure purposes through December 31, 2020, the date on which the September 30, 2020 financial statements were reissued.
Settlement with SDNY Office—On December 18, 2020, a federal judge approved a civil and administrative settlement Apria recently entered into with the United States and state Medicaid programs in a complaint filed by three relators under the qui tam provisions of the False Claims Act (“FCA”), 31 U.S.C. § 3729 et seq., as well as comparable state false claims laws, in connection with the rental of non-invasive ventilators (“NIVs”). Apria also entered into separate settlements to resolve the relators’ claims brought on behalf of the States of California and Illinois related to NIV covered by private insurers. The matter has been pending since 2017.
The government had alleged that Apria violated the FCA by submitting false claims seeking reimbursement for NIVs which were not being used, or not being used sufficiently, by patients, for NIVs which were being used pursuant to physician orders on a device setting which was available from other less expensive devices, and for improperly waiving co-pays to induce beneficiaries to rent NIVs. To resolve any potential liability, Apria agreed to enter a civil settlement agreement and to pay $40 million to the federal government and the states. Apria also agreed with the California Department of Insurance to pay $500,000 to resolve claims asserted by the relators under the California Insurance Frauds Prevention Act, CAL. INS. CODE § 1871 et seq. Apria separately agreed with the relators to settle all remaining claims from their complaint, including: (1) claims for retaliation in violation of federal and state laws; (2) claims for attorneys’ fees and costs available under federal and state law; and (3) claims under the Illinois Insurance Claims Fraud Prevention Act, 740 ILL. COMP. STAT. 92/1 et seq. Apria did not admit that any of its conduct was illegal or otherwise improper.
As part of the federal and state Medicaid settlement, Apria also entered into a five-year corporate integrity agreement (the “Corporate Integrity Agreement” or “CIA”) with the Office of Inspector General of the U.S. Department of Health and Human Services (“HHS OIG”). The CIA requires Apria to maintain its ongoing corporate compliance program and obligates Apria to implement or continue, as applicable, a set of defined corporate integrity activities for a period of five years from the effective date of the CIA. Among other things, the CIA requires Apria to impose certain oversight obligations on Apria’s board of directors; provide certain management certifications; continue or implement, as applicable, certain compliance training and education; and engage an Independent Review Organization to perform certain reviews. The CIA also includes certain reporting, certification, record retention, and notification requirements. In the event of a breach of the CIA, Apria could become liable for payment of certain stipulated penalties or could be excluded from participation in federal healthcare programs.
F-56
Table of Contents
Credit Facility Amendment and Dividend—On December 11, 2020, we entered into an amendment to our credit agreement to incur $260.0 million of incremental term loans. Net proceeds from the incremental term loans were used to fund a $200.3 million dividend payment to our stockholders and a $9.7 million distribution to SARs holders declared and paid in December 2020, with the remaining proceeds used to pay fees and expenses in connection with the credit facility amendment and for general corporate purposes.
* * * * * *
F-57
Table of Contents

Table of Contents
7,500,000 Shares

Apria, Inc.
Common Stock
Preliminary Prospectus
Joint Book-Running Managers
Citigroup
Goldman Sachs & Co. LLC
BofA Securities
J.P. Morgan
UBS Investment Bank
Co-Managers
Piper Sandler
Citizens Capital Markets
Fifth Third Securities
TD Securities
Academy Securities
Blaylock Van, LLC
Penserra Securities LLC
Stern
Through and including the 25th day after the date of this prospectus, all dealers that effect transactions in shares of our common stock, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligations to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
, 2021
Table of Contents
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
| ITEM 13. | OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION. |
The following table sets forth the expenses payable by the Registrant expected to be incurred in connection with the issuance and distribution of the shares of common stock being registered hereby (other than underwriting discounts and commissions). All of such expenses are estimates, other than the filing and listing fees payable to the Securities and Exchange Commission, the Financial Industry Regulatory Authority, Inc. and the Nasdaq.
| Filing Fee—Securities and Exchange Commission |
$ | 25,093 | ||
| Fee—Financial Industry Regulatory Authority, Inc. |
14,850 | |||
| Listing Fee—Nasdaq |
170,000 | |||
| Fees and Expenses of Counsel |
3,000,000 | |||
| Printing Expenses |
300,000 | |||
| Fees and Expenses of Accountants |
1,157,000 | |||
| Transfer Agent and Registrar’s Fees |
10,000 | |||
| Miscellaneous Expenses |
809,000 | |||
|
|
|
|||
| Total |
$ | 5,485,943 | ||
|
|
|
| ITEM 14. | INDEMNIFICATION OF DIRECTORS AND OFFICERS. |
Section 102(b)(7) of the Delaware General Corporation Law, or DGCL, allows a corporation to provide in its certificate of incorporation that a director of the corporation will not be personally liable to the corporation or its stockholders for monetary damages for breach of fiduciary duty as a director, except where the director breached the duty of loyalty, failed to act in good faith, engaged in intentional misconduct or knowingly violated a law, authorized the payment of a dividend or approved a stock redemption or repurchase in violation of Delaware corporate law or obtained an improper personal benefit. Our amended and restated certificate of incorporation will provide for this exculpation against liability for breach of fiduciary duty to the fullest extent permitted by law.
Section 145 of the DGCL, or Section 145, provides, among other things, that a Delaware corporation may indemnify any person who was, is or is threatened to be made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative (other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director, employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful. Section 145 also provides that a Delaware corporation may indemnify any persons who were or are a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or enterprise or is or was serving at the request of such corporation as a director, officer, employee or agent of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection with the defense or settlement of such action or suit, provided
II-1
Table of Contents
such person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests, provided further that no indemnification is permitted without judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Section 145 further provides that, where directors and specified officers are successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify them against the expenses they have actually and reasonably incurred.
Section 145 provides that expenses (including attorneys’ fees) incurred by a current officer or director of the corporation in defending against any civil, criminal, administrative or investigative action, suit or proceeding may be paid by the corporation in advance of the final disposition of the action, suit or proceeding upon receipt of an undertaking if it shall ultimately be determined that the person is not entitled to be indemnified by the corporation as authorized under Section 145. Such expenses incurred by a former officer or director or other employees and agents of the corporation or by persons serving at the request of the corporation in specified capacities for other enterprises may be paid upon terms and conditions as the corporation deems appropriate.
Section 145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation or enterprise, against any liability asserted against such person and incurred by such person in any such capacity, or arising out of his or her status as such, whether or not the corporation would otherwise have the power to indemnify him or her under Section 145.
Our amended and restated bylaws will provide that we must indemnify our directors and officers to the fullest extent authorized by the DGCL (subject to certain limited circumstances) and must also pay expenses incurred by them in defending any such proceeding in advance of its final disposition upon delivery of an undertaking, by or on behalf of an indemnified person, to repay all amounts so advanced if it should be determined ultimately that such person is not entitled to be indemnified under our amended and restated bylaws or otherwise.
The rights to indemnification and advancement set forth above shall not be exclusive of any other right which an indemnified person may have or hereafter acquire under any statute, provision of our amended and restated certificate of incorporation, our amended and restated bylaws, agreement, vote of stockholders or disinterested directors or otherwise.
We expect to maintain standard policies of insurance that provide coverage (1) to our directors and officers against loss arising from claims made by reason of breach of duty or other wrongful act and (2) to us with respect to indemnification payments that we may make to such directors and officers.
We intend to enter into indemnification agreements with our directors and executive officers. These agreements will require us to indemnify these individuals to the fullest extent permitted under Delaware law against liabilities that may arise by reason of their service to us, and to advance expenses incurred as a result of any proceeding against them as to which they could be indemnified. Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors or executive officers, we have been informed that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy and is therefore unenforceable
The proposed form of Underwriting Agreement to be filed as Exhibit 1.1 to this Registration Statement provides for indemnification to our directors and officers by the underwriters against certain liabilities.
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES.
In connection with the certain pre-IPO reorganization transactions to be effected at or prior to the completion of this offering, the Registrant expects to issue up to 35,211,020 shares of common stock to the
II-2
Table of Contents
pre-IPO owners of Apria Healthcare Group. Such securities will be issued in reliance on the exemption contained in Section 4(a)(2) of the Securities Act, as transactions by issuers not involving a public offering. No general solicitation or underwriters will be involved in such issuances.
ITEM 16. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a) Exhibits. See the Exhibit Index immediately preceding the signature pages hereto, which is incorporated by reference as if fully set forth herein.
(b) Financial Statement Schedules. None.
ITEM 17. UNDERTAKINGS
| (1) | The undersigned Registrant hereby undertakes to provide to the underwriter at the closing specified in the underwriting agreements certificates in such denominations and registered in such names as required by the underwriter to permit prompt delivery to each purchaser. |
| (2) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue. |
| (3) | The undersigned Registrant hereby undertakes that: |
| (A) | For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| (B) | For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| (4) | The undersigned Registrant undertakes that in a primary offering of securities of the undersigned Registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (A) | Any preliminary prospectus or prospectus of the undersigned Registrant relating to the offering required to be filed pursuant to Rule 424 under the Securities Act; |
| (B) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned Registrant or used or referred to by the undersigned Registrant; |
II-3
Table of Contents
| (C) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned Registrant or its securities provided by or on behalf of the undersigned Registrant; and |
| (D) | Any other communication that is an offer in the offering made by the undersigned Registrant to the purchaser. |
II-4
Table of Contents
EXHIBIT INDEX
II-5
Table of Contents
| ** | Previously filed. |
II-6
Table of Contents
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Indianapolis, State of Indiana, on the 9th day of February, 2021.
| APRIA, INC. | ||
| By: | /s/ Daniel J. Starck | |
| Name: Daniel J. Starck | ||
| Title: Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, as amended, this Registration Statement has been signed by the following persons in the capacities indicated on the 9th day of February, 2021.
| Signature |
Title | |
| /s/ Daniel J. Starck Daniel J. Starck |
Chief Executive Officer and Director (principal executive officer) | |
| /s/ Debra L. Morris Debra L. Morris |
Chief Financial Officer (principal financial officer and principal accounting officer) | |
| * John G. Figueroa |
Director and Chairman of the Board of Directors | |
| * Michael Audet |
Director | |
| * John R. Murphy |
Director | |
| * Norman C. Payson M.D. |
Director | |
| * Neil P. Simpkins |
Director | |
| * Lynn Shapiro Snyder |
Director | |
| * Mike S. Zafirovski |
Director | |
| * By: | /s/ Debra L. Morris | |
| Debra L. Morris | ||
| Attorney-in-fact | ||
II-7
