Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - CASSAVA SCIENCES INC | sava-20210208xex99_2.htm |
| 8-K - 8-K - CASSAVA SCIENCES INC | sava-20210208x8k.htm |
Exhibit 99.1

CASSAVA sciences We Focus on Alzheimer’s disease February 2021 CASSAVA sciences 1

Forward-Looking Statements & Safe Harbor This presentation contains forward-looking statements, including statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995, relating to: our strategy and plans; the treatment of Alzheimer’s disease; the status of current and future clinical studies with simufilam, including the interpretation of a 6-month interim analysis of open-label study results; changes to the open-label study, including future interim analysis; our intention to initiate a Cognition Maintenance Study and a Phase 3 clinical program with simufilam in 2021; results of our EOP2 meeting with FDA and the timing of further announcements; our ability to manufacture drug supply for a Phase 3 program and to enter into a long-term commercial drug supply agreement; the timing of validation studies with SavaDx; our ability to expand therapeutic indications for simufilam outside of Alzheimer’s disease; expected cash use in future periods; plans to publish results of a Phase 2b study in a peer-reviewed journal; verbal commentaries made by our employees; and potential benefits, if any, of the our product candidates. These statements may be identified by words such as “may,” “anticipate,” “believe,” “could,” “expect,” “forecast,” “intend,” “plan,” “possible,” “potential,” and other words and terms of similar meaning. Drug development and commercialization involve a high degree of risk, and only a small number of research and development programs result in commercialization of a product. Our clinical results from earlier-stage clinical trials may not be indicative of full results or results from later-stage or larger scale clinical trials and do not ensure regulatory approval. You should not place undue reliance on these statements or any scientific data we present or publish. Such statements are based largely on our current expectations and projections about future events. Such statements speak only as of the date of this presentation and are subject to a number of risks, uncertainties and assumptions, including, but not limited to, those risks relating to the ability to conduct or complete clinical studies on expected timelines, to demonstrate the specificity, safety, efficacy or potential health benefits of our product candidates, potential health benefits, if any, of changes in levels of biomarkers, the severity and duration of health care precautions given the COVID-19 pandemic, any unanticipated impacts of the pandemic on our business operations, including those described in the section entitled “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2019 and future reports to be filed with the SEC. The foregoing sets forth many, but not all, of the factors that could cause actual results to differ from expectations in any forward-looking statement. In light of these risks, uncertainties and assumptions, the forward-looking statements and events discussed in this presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Except as required by law, we disclaim any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. For further information regarding these and other risks related to our business, investors should consult our filings with the SEC, which are available on the SEC's website at www.sec.gov. This presentation may also contain statistical data and drug information based on independent industry publications or other publicly available information. We have not independently verified the accuracy or completeness of the data contained in these publicly available sources of data and information. Accordingly, we make no representations as to the accuracy or completeness of such data or information. You are cautioned not to give undue weight to such data. The content of this presentation is solely our responsibility and does not necessarily represent the official views of the National Institutes of Health (NIH). CASSAVA sciences 2

Cassava Sciences Highlights Our goal is to defeat Alzheimer's disease. Alzheimer’s disease is one of the greatest unmet medical needs, with no disease-modifying medicines. Our scientific approach is unique, our clinical data is highly differentiated. Our science programs have been developed with scientific and financial support from the National Institutes of Health (NIH). We are developing simufilam, a proprietary drug candidate to treat Alzheimer's disease and SavaDx, a blood-based investigational diagnostic. Simufilam is Phase 3 ready in 2021. Key drivers of our clinical development program: A decade of research in basic biology Clear scientific rationale Published pre-clinical results Well-understood mechanism of action Clean safety profile Evidence of target engagement in patients Unprecedented CSF biomarker data Phase 2b clinical results Early data on cognition and behavior Successful End-of-Phase 2 meeting with FDA CASSAVA sciences 3

Meet the Team Remi Barbier - Chairman, President & CEO Nadav Friedmann, PhD/MD - CMO, Board member Eight FDA drug approvals prior to Cassava Sciences. Jim Kupiec, MD – Chief Clinical Development Officer Two FDA drug approvals prior to Cassava Sciences. Eric Schoen - Chief Financial Officer Lindsay H. Burns, PhD - SVP Neuroscience Michael Zamloot - SVP Technical Operations Four FDA drug approvals prior to Cassava Sciences. Independent Directors Sanford Robertson Founding Partner - Francisco Partners Founder & Chairman - Robertson, Stephens & Company Robert Gussin, PhD Formerly, Johnson & Johnson, Chief Scientific Officer and Corporate VP, Science and Technology Patrick Scannon, MD/PhD Formerly, Founder & CSO/CMO - XOMA Corporation Michael O’Donnell Partner, Morrison & Foerster LLP CASSAVA sciences 4
Introduction to Simufilam is our proprietary, small molecule (oral) drug candidate to treat Alzheimer’s disease (AD) and other neurodegenerative diseases. Simufilam binds a single target, has a dual mechanism of action: Reduces neurodegeneration and neuroinflammation. Published preclinical data and mechanism of action studies support simufilam’s potential as a disease-modifying drug for AD that also provides symptomatic improvement. CASSAVA sciences 5
Clinical Development 2017: simufilam is safe, well-tolerated in human volunteers. 2019: positive results on CSF biomarkers of disease in an open-label Phase 2a study of simufilam in AD patients. 2020: positive results on CSF biomarkers of disease in a double-blind, randomized, placebo-controlled Phase 2b study of simufilam in AD patients. 2021: positive results on cognition in a 6-month interim analysis of an on-going, open-label study in AD patients. We plan to initiate a Phase 3 study of simufilam in Alzheimer's disease in 2nd half 2021. CASSAVA sciences 6

Science & Technology Lindsay Burns, PhD – SVP Neuroscience Nadav Friedmann, PhD/MD – Chief Medical Officer Jim Kupiec, MD - Chief Clinical Development Officer CASSAVA sciences 7
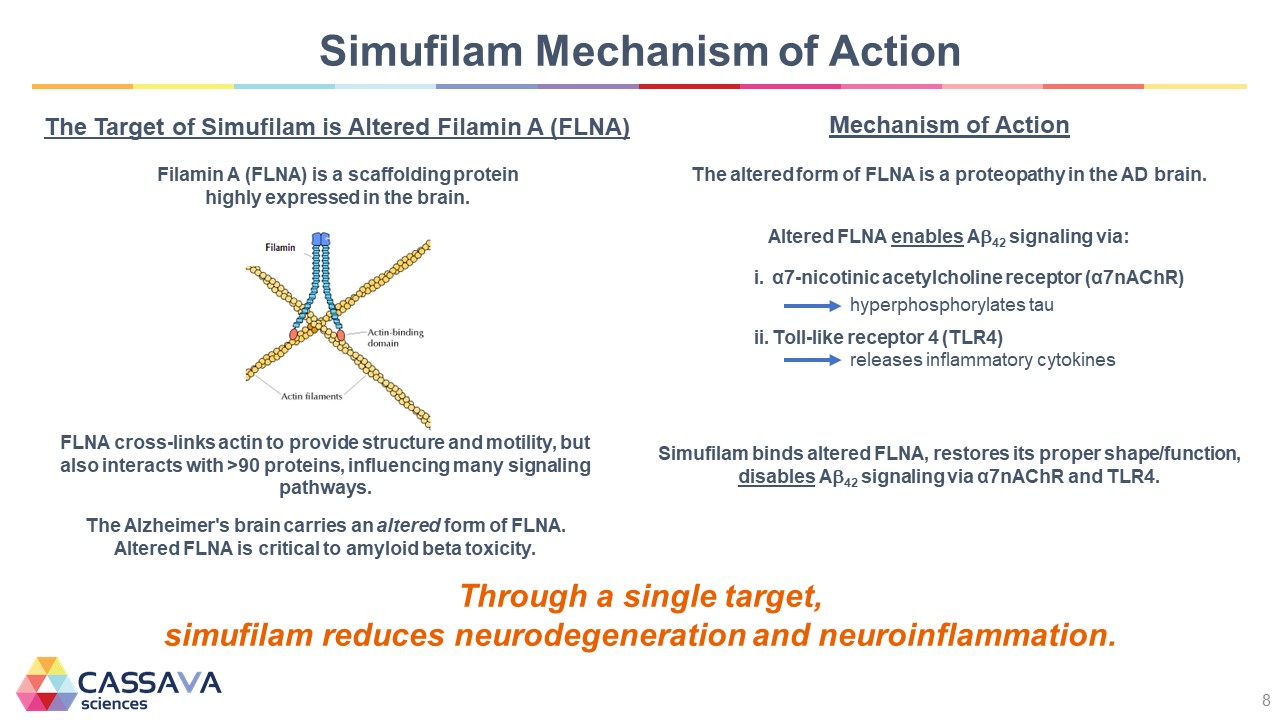
Simufilam Mechanism of Action The Target of Simufilam is Altered Filamin A (FLNA) Filamin A (FLNA) is a scaffolding protein highly expressed in the brain. FLNA cross-links actin to provide structure and motility, but also interacts with >90 proteins, influencing many signaling pathways. The Alzheimer's brain carries an altered form of FLNA. Altered FLNA is critical to amyloid beta toxicity. Mechanism of Action The altered form of FLNA is a proteopathy in the AD brain. Altered FLNA enables A42 signaling via: α7-nicotinic acetylcholine receptor (α7nAChR) hyperphosphorylates tau Toll-like receptor 4 (TLR4) releases inflammatory cytokines Simufilam binds altered FLNA, restores its proper shape/function, disables A42 signaling via α7nAChR and TLR4. Through a single target, simufilam reduces neurodegeneration and neuroinflammation. CASSAVA sciences 8
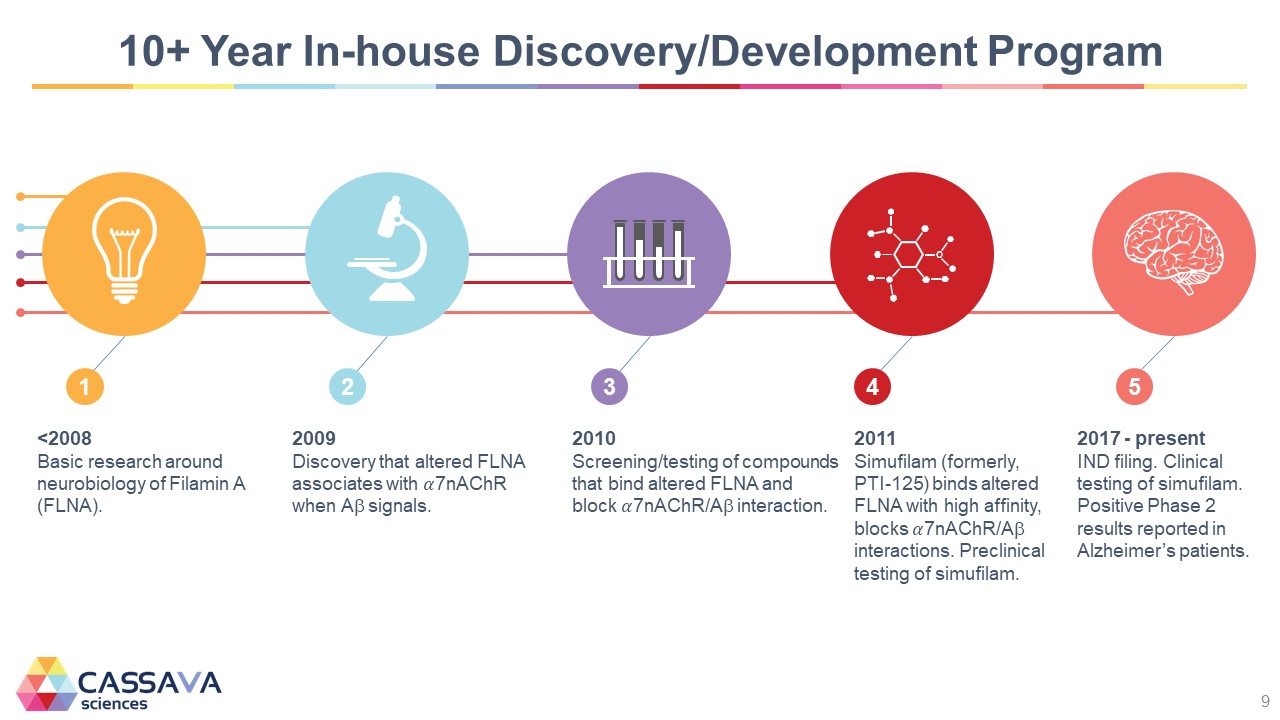
10+ Year In-house Discovery/Development Program 1 <2008 Basic research around neurobiology of Filamin A (FLNA). 2 2009 Discovery that altered FLNA associates with ��7nAChR when A signals. 3 2010 Screening/testing of compounds that bind altered FLNA and block ��7nAChR/A interaction. 4 2011 Simufilam (formerly, PTI-125) binds altered FLNA with high affinity, blocks ��7nAChR/A interactions. Preclinical testing of simufilam. 5 2017 - present IND filing. Clinical testing of simufilam. Positive Phase 2 results reported in Alzheimer’s patients. CASSAVA sciences 9
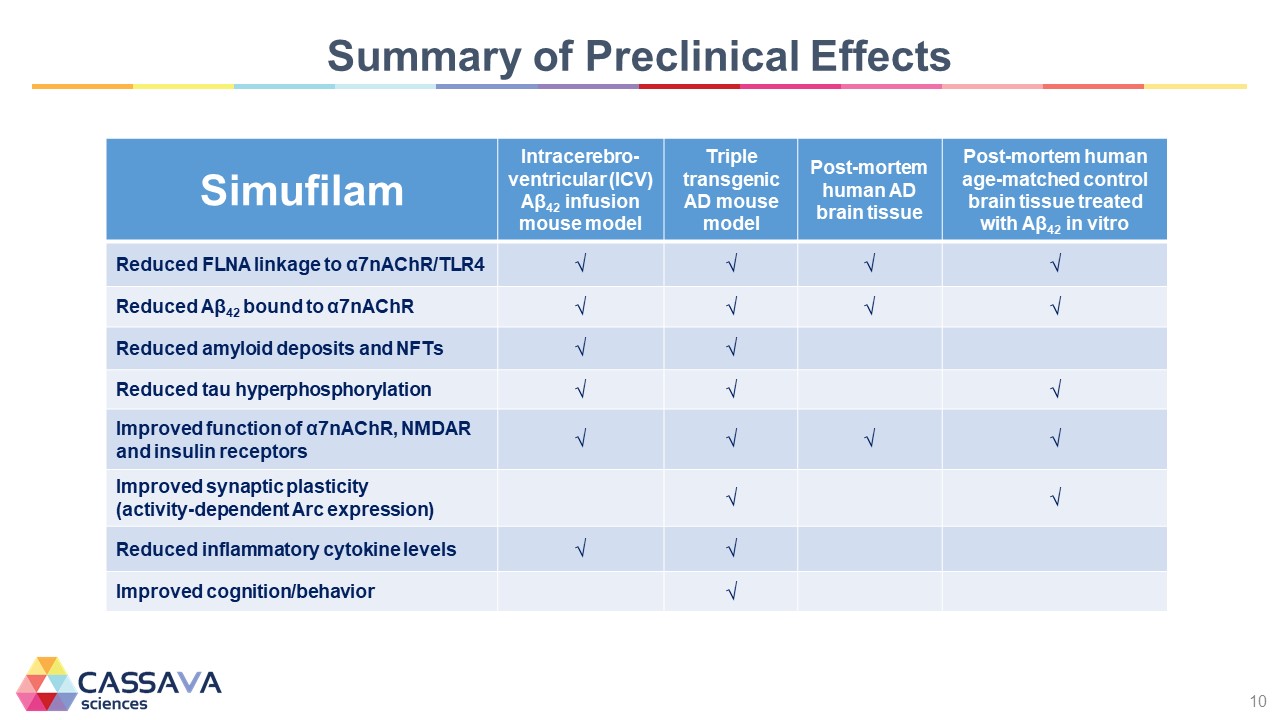
Summary of Preclinical Effects Simufilam Intracerebro-ventricular (ICV) Aβ42 infusion mouse model Triple transgenic AD mouse model Post-mortem human AD brain tissue Post-mortem human age-matched control brain tissue treated with Aβ42 in vitro Reduced FLNA linkage to α7nAChR/TLR4 √ √ √ √ Reduced Aβ42 bound to α7nAChR √ √ √ √ Reduced amyloid deposits and NFTs √ √ Reduced tau hyperphosphorylation √ √ √ Improved function of α7nAChR, NMDAR and insulin receptors √ √ √ √ Improved synaptic plasticity (activity-dependent Arc expression) √ √ Reduced inflammatory cytokine levels √ √ Improved cognition/behavior √ CASSAVA sciences 10
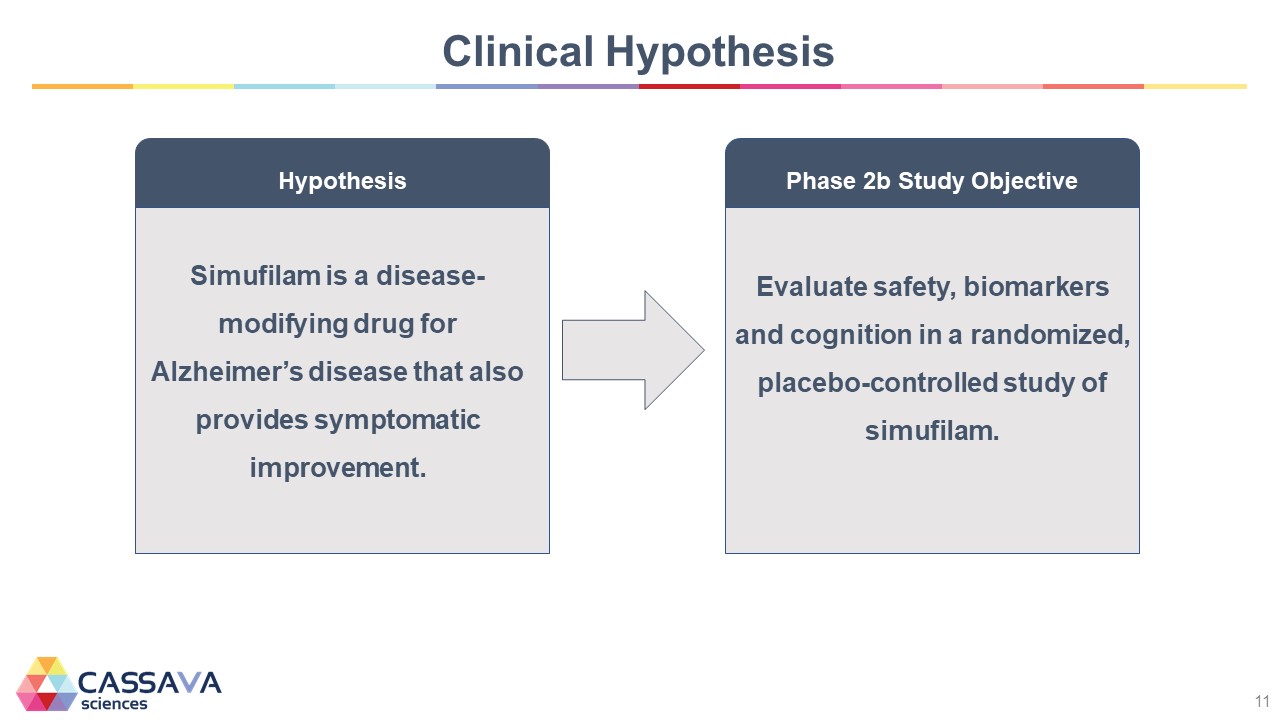
Clinical Hypothesis Hypothesis Simufilam is a disease-modifying drug for Alzheimer’s disease that also provides symptomatic improvement. Phase 2b Study Objective Evaluate safety, biomarkers and cognition in a randomized, placebo-controlled study of simufilam. CASSAVA sciences 11
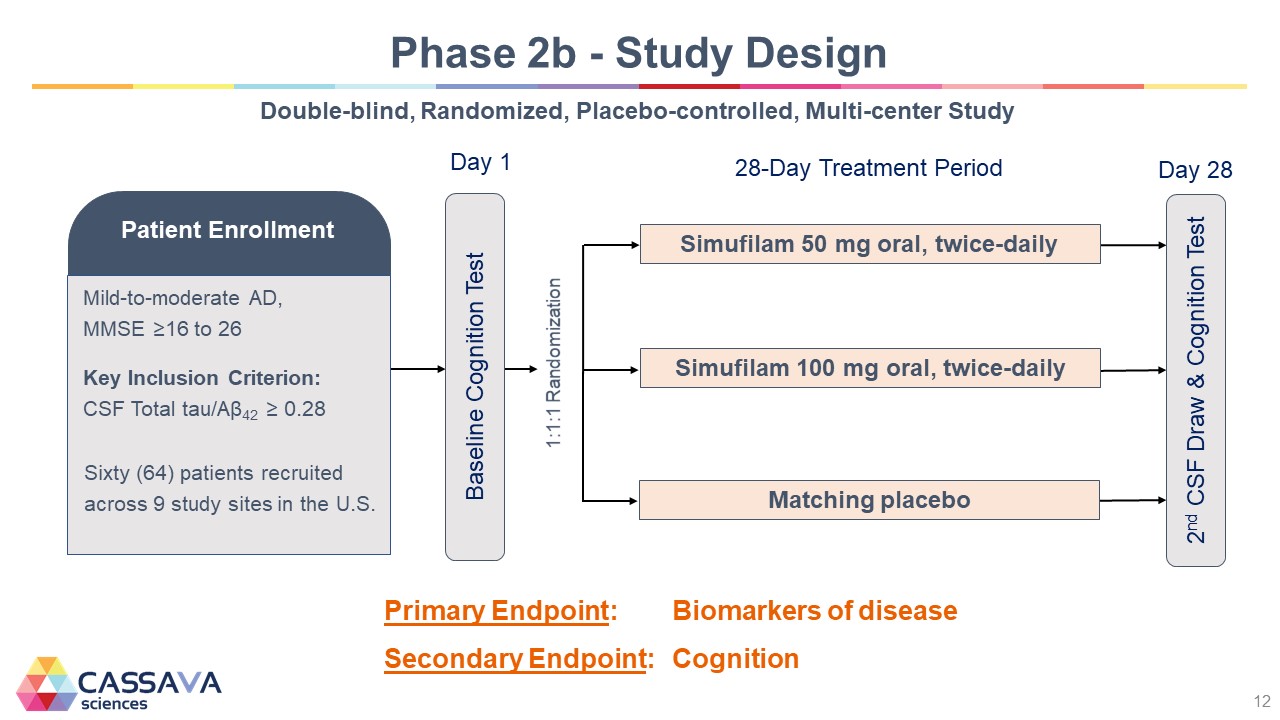
Phase 2b - Study Design Double-blind, Randomized, Placebo-controlled, Multi-center Study Patient Enrollment Mild-to-moderate AD, MMSE ≥16 to 26 Key Inclusion Criterion: CSF Total tau/Aβ42 ≥ 0.28 Sixty (64) patients recruited across 9 study sites in the U.S. Day 1 Baseline Cognition Test 1:1:1 Randomization 28-Day Treatment Period Simufilam 50 mg oral, twice-daily Simufilam 100 mg oral, twice-daily Matching placebo Day 28 2nd CSF Draw & Cognition Test Primary Endpoint: Biomarkers of disease Secondary Endpoint: Cognition CASSAVA sciences 12

Phase 2b Results – Safety & Baseline Simufilam was safe and well-tolerated No serious adverse events No drug-related patient discontinuation No drug-related adverse events Common, non-persistent side-effects observed in placebo & drug groups Baseline characteristics were well-balanced between treatment groups, assigned through (1:1:1) randomization. CASSAVA sciences 13
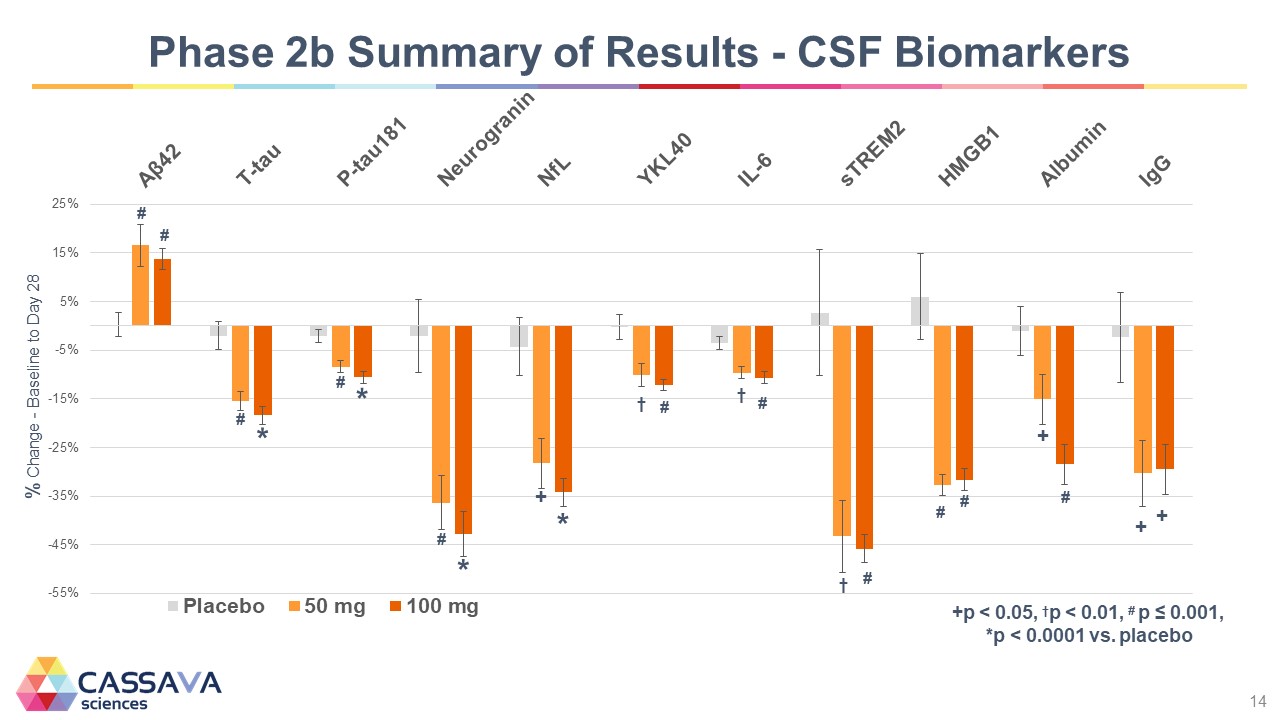
Phase 2b Summary of Results - CSF Biomarkers % Change - Baseline to Day 28 -55% -45% -35% -25% -15% -5% 5% 15% 25% Placebo 50 mg 100 mg +p < 0.05, tp < 0.01,*p < 0.001, *p < 0.0001 vs. placebo CASSAVA sciences 14
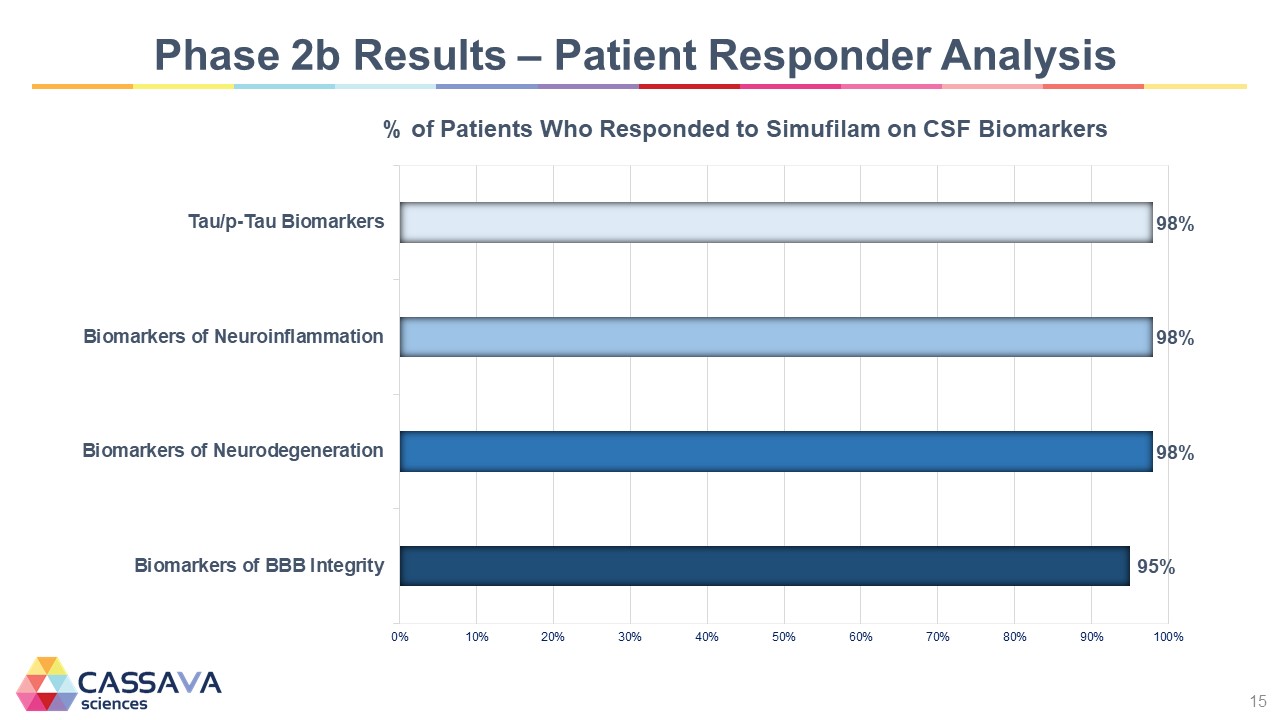
Phase 2b Results – Patient Responder Analysis % of Patients Who Responded to Simufilam on CSF Biomarkers % of Patients Who Responded to Simufilam on CSF Biomarkers Tau/p-Tau Biomarkers 98% Biomarkers of Neuroinflammation 98% Biomarkers of Neurodegeneration 98% Biomarkers of BBB Integrity 95% 0% 10% 20% 30% a0% 50% 60% 70% 30% 90% 100% CASSAVA sciences 15
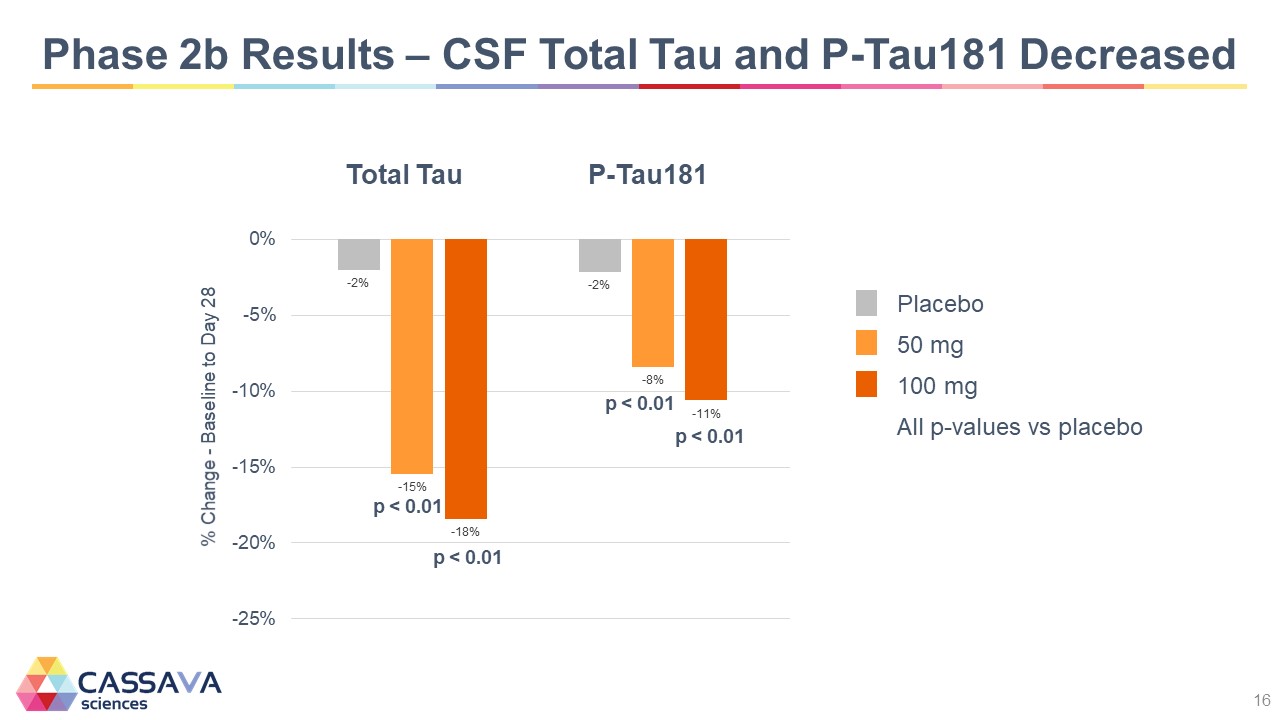
Phase 2b Results – CSF Total Tau and P-Tau181 Decreased Total Tau P-Tau181 % Change - Baseline to Day 28 -25% -20% -15% -10% -5% 0% -2% -15% p < 0.01 -18% p < 0.01 P-Tau181 -2% -8% p < 0.01 -11% p < 0.01 Placebo 50 mg 100 mg All p-values vs placebo CASSAVA sciences 16
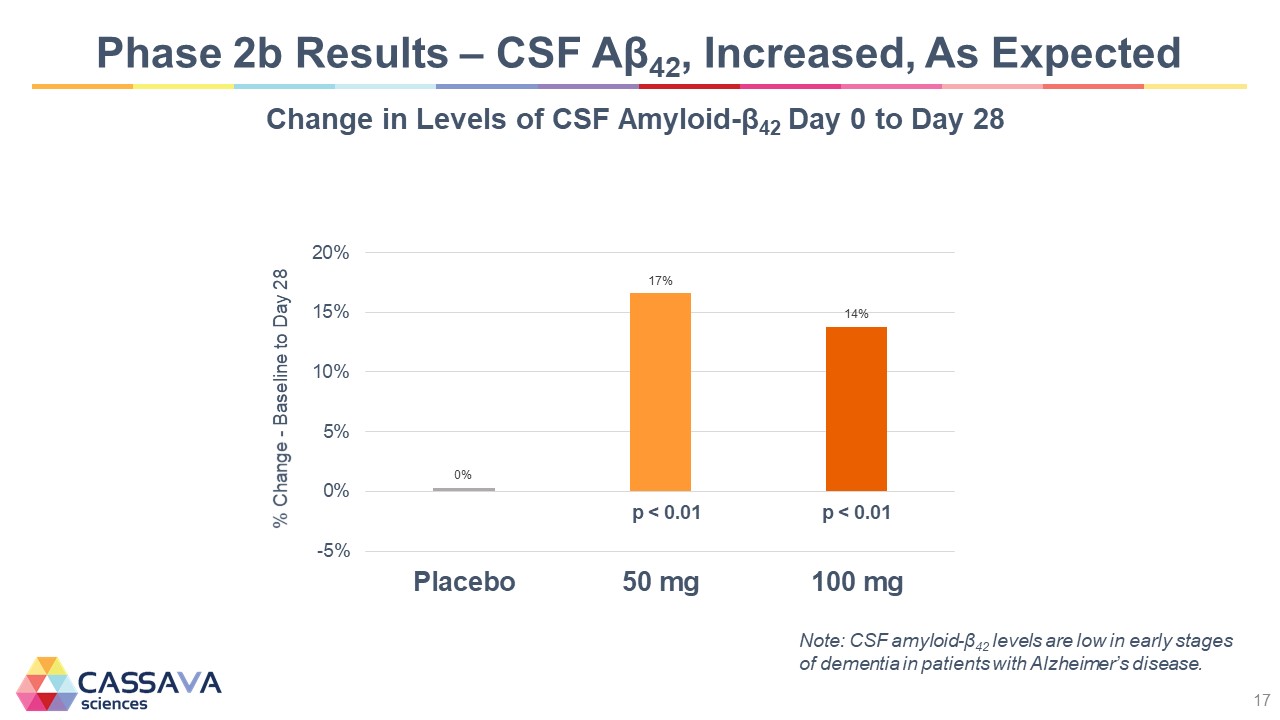
Phase 2b Results – CSF Aβ42, Increased, As Expected Change in Levels of CSF Amyloid-β42 Day 0 to Day 28 % Change - Baseline to Day 28 20% 15% 10% 5% 0% -5% Placebo 17% p < 0.01 p < 0.01 50 mg 100 mg Note: CSF amyloid-β42 levels are low in early stages of dementia in patients with Alzheimer’s disease. CASSAVA sciences 17
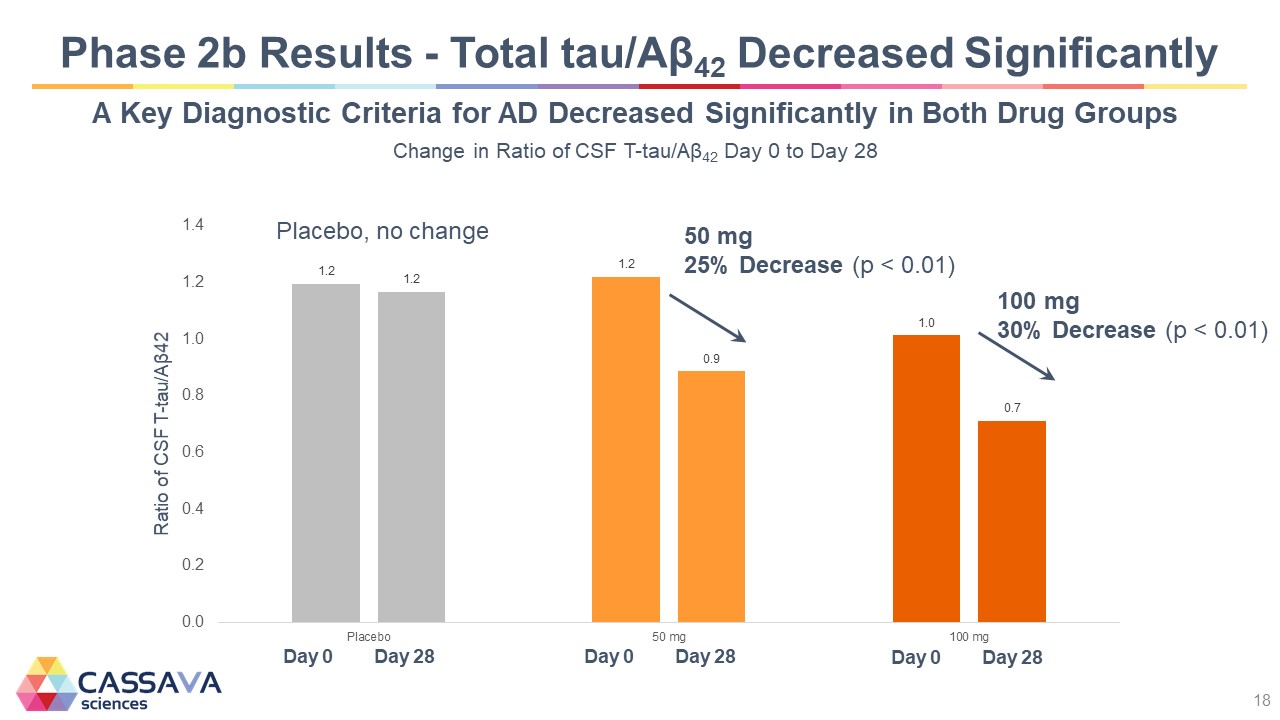
Phase 2b Results - Total tau/Aβ42 Decreased Significantly A Key Diagnostic Criteria for AD Decreased Significantly in Both Drug Groups Change in Ratio of CSF T-tau/Aβ42 Day 0 to Day 28 Ratio of CSF T-tau/Aß42 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 Placebo, no change Day 0 1.2 Day 28 1.2 50 mg 25% Decrease (p < 0.01) 50 mg Day 0 1.2 Day 28 0.9 100 mg 30% Decrease (p < 0.01) Day 0 1.0 Day 28 0.7 CASSAVA sciences 18
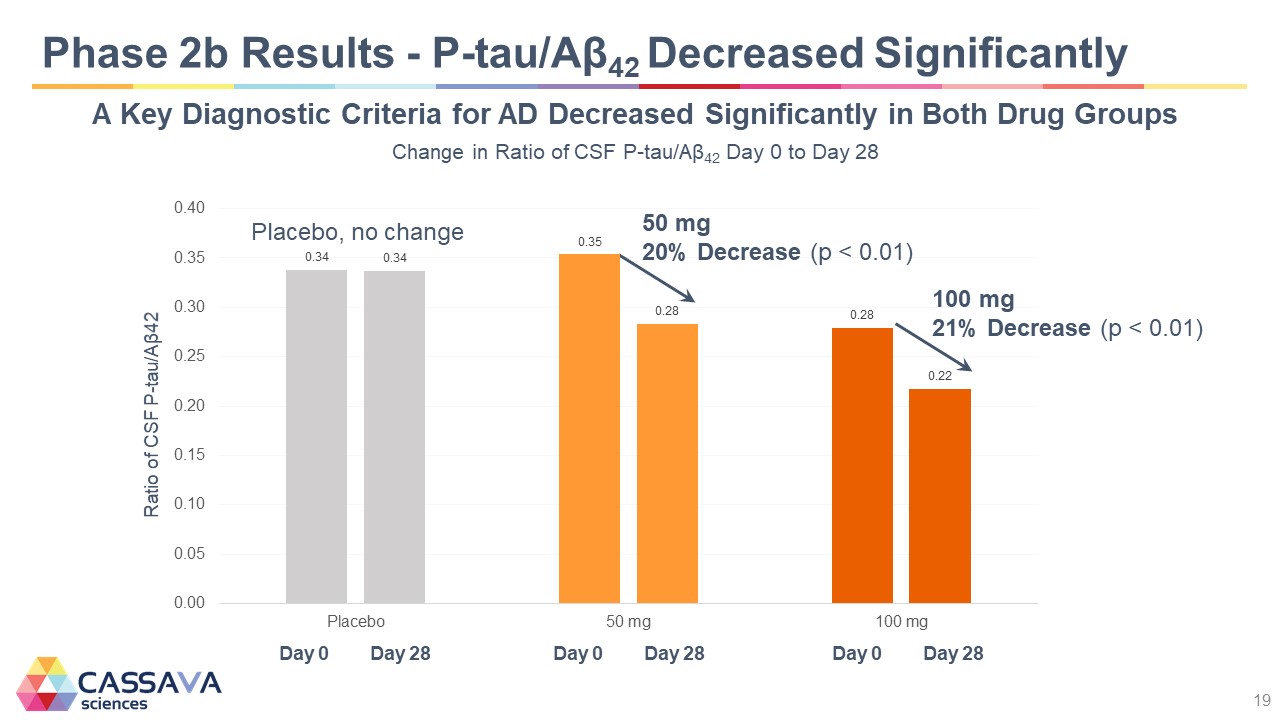
Phase 2b Results - P-tau/Aβ42 Decreased Significantly A Key Diagnostic Criteria for AD Decreased Significantly in Both Drug Groups Change in Ratio of CSF P-tau/Aβ42 Day 0 to Day 28 Ratio of CSF P-tau/Aβ42 0.00 0.05 0.10 0.15 0.20 0.25 0.30 0.35 0.40 Placebo, no change Day 0 0.34 Day 28 0.34 50 mg 20% Decrease (p < 0.01) Day 0 0.35 Day 28 0.28 100 mg 21% Decrease (p < 0.01) Day 0 0.28 Day 28 0.22 CASSAVA sciences 19
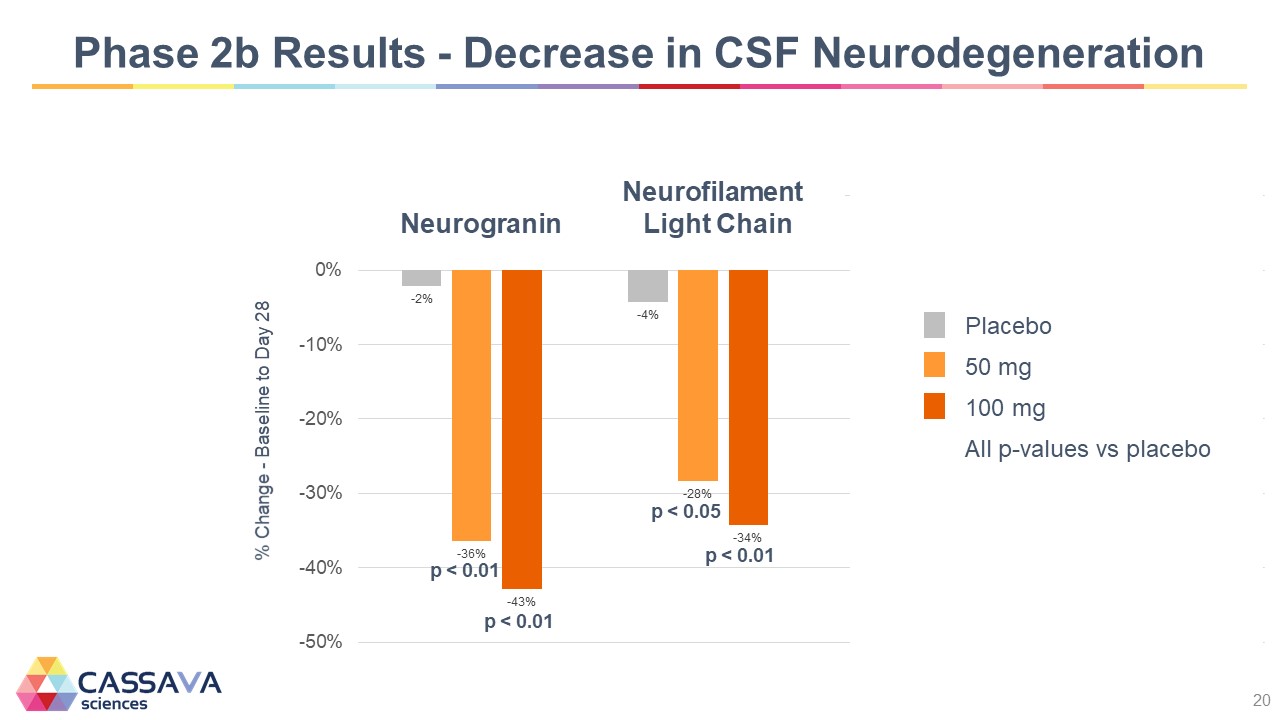
Phase 2b Results - Decrease in CSF Neurodegeneration Neurofilament % Change - Baseline to Day 28 -50% -40% -30% -20% -10% 0% -2% -36% p < 0.01 -43% p < 0.01 Neurogranin Light Chain -4% -28% p < 0.05 -34% p < 0.01 Placebo 50 mg 100 mg All p-values vs placebo CASSAVA sciences 20
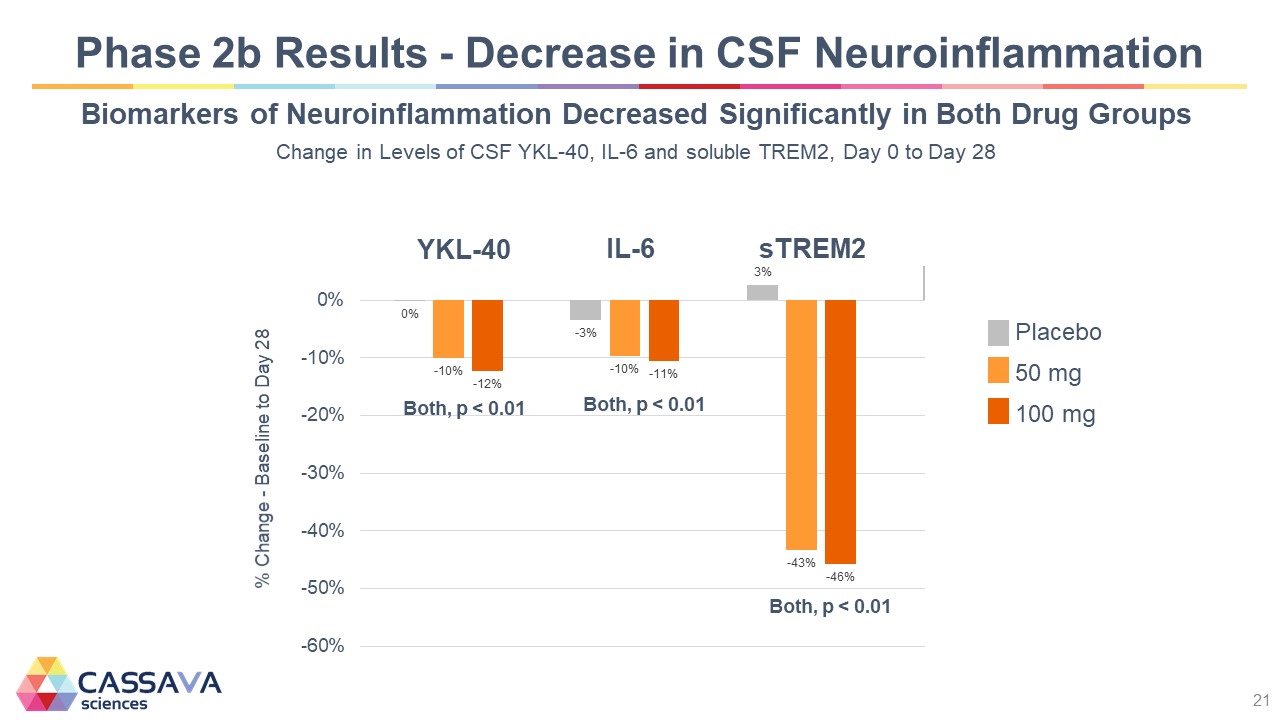
Phase 2b Results - Decrease in CSF Neuroinflammation Biomarkers of Neuroinflammation Decreased Significantly in Both Drug Groups Change in Levels of CSF YKL-40, IL-6 and soluble TREM2, Day 0 to Day 28 % Change - Baseline to Day 28 -60% -50% -40% -30% -20% -10% 0% YKL-40 0% -10% -12% Both, p < 0.01 IL-6 -% -10% -11% Both, p < 0.01 sTREM2 3% -43% -46% Both, p < 0.01 Placebo 50 mg 100 mg CASSAVA sciences 21
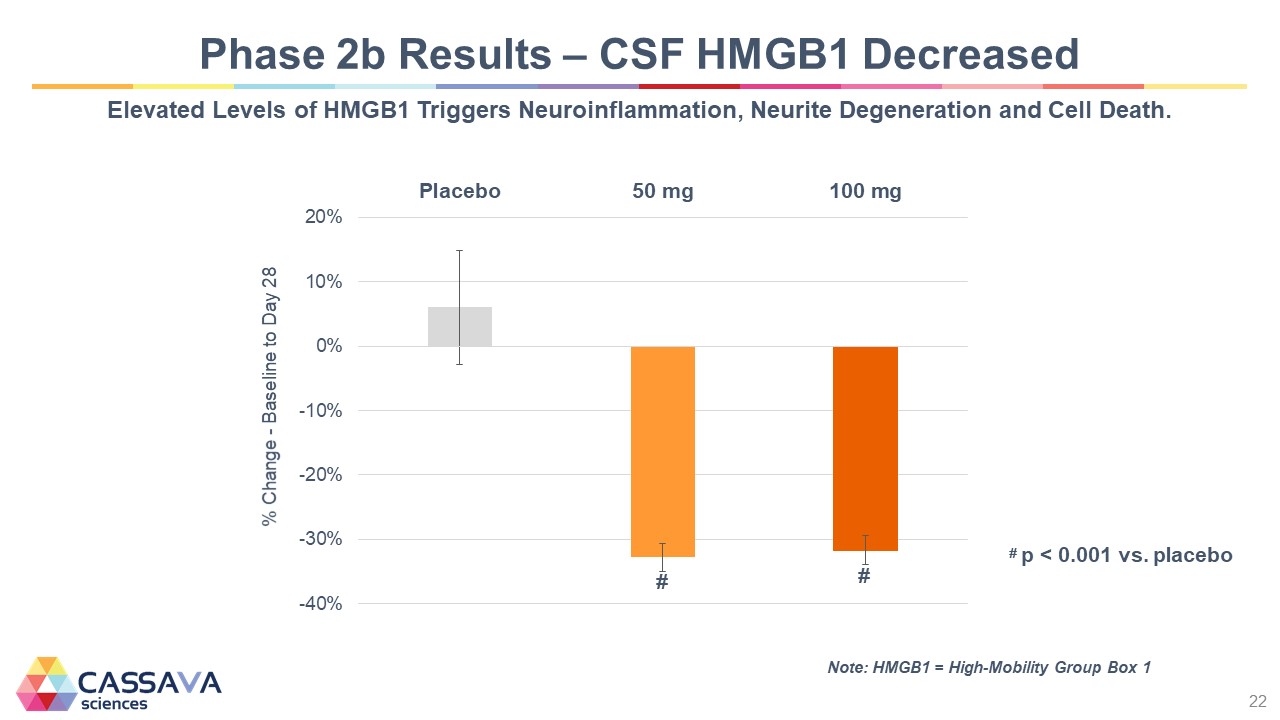
Phase 2b Results – CSF HMGB1 Decreased Elevated Levels of HMGB1 Triggers Neuroinflammation, Neurite Degeneration and Cell Death. % Change - Baseline to Day 28 -40% -30% -20% -10% 0% 10% 20% Placebo 50 mg 100 mg # p < 0.001 vs. placebo Note: HMGB1 = High-Mobility Group Box 1 CASSAVA sciences 22
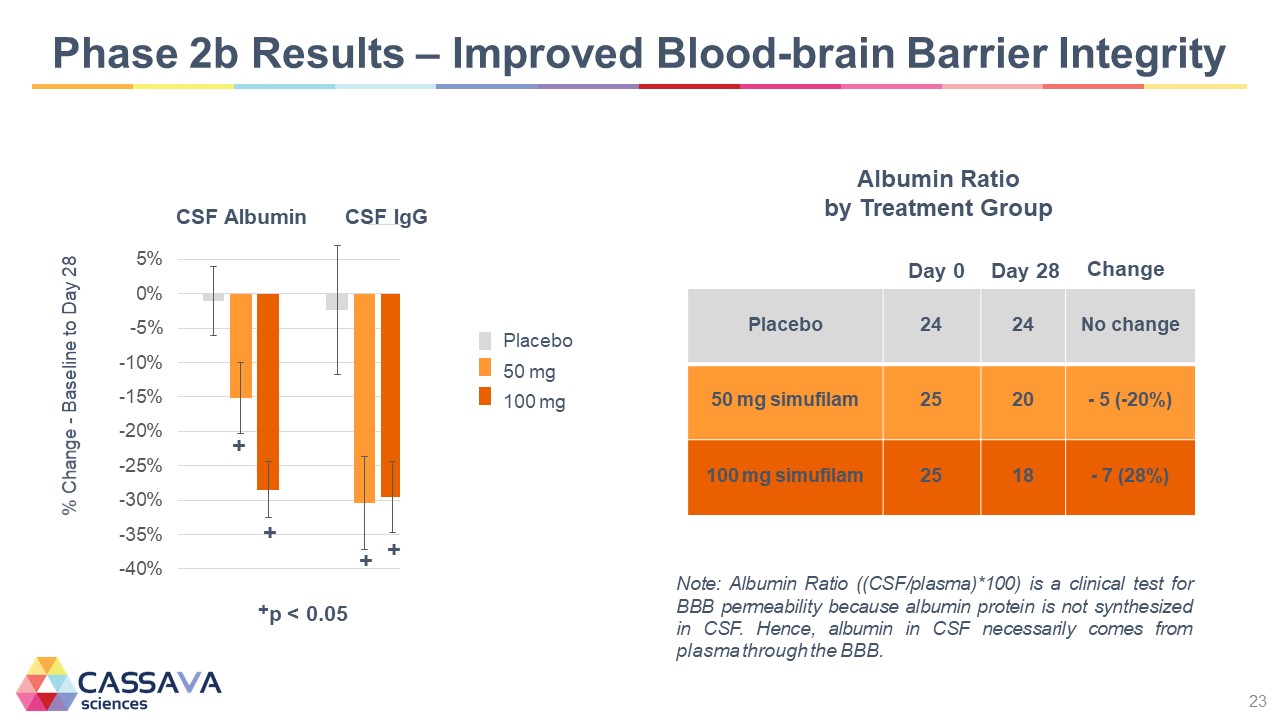
Phase 2b Results – Improved Blood-brain Barrier Integrity % Change - Baseline to Day 28 -40% -35% -30% -25% -20% -15% -10% -5% 0% 5% CSF Albumin + + CSF IgG + + Placebo 50mg 100 mg +p <0.005 Albumin Ratio by Treatment Group Day 0 Day 28 Change Placebo 24 24 No change 50 mg simufilam 25 20 - 5 (-20%) 100 mg simufilam 25 18 - 7 (28%) Note: Albumin Ratio ((CSF/plasma)*100) is a clinical test for BBB permeability because albumin protein is not synthesized in CSF. Hence, albumin in CSF necessarily comes from plasma through the BBB. CASSAVA sciences 23
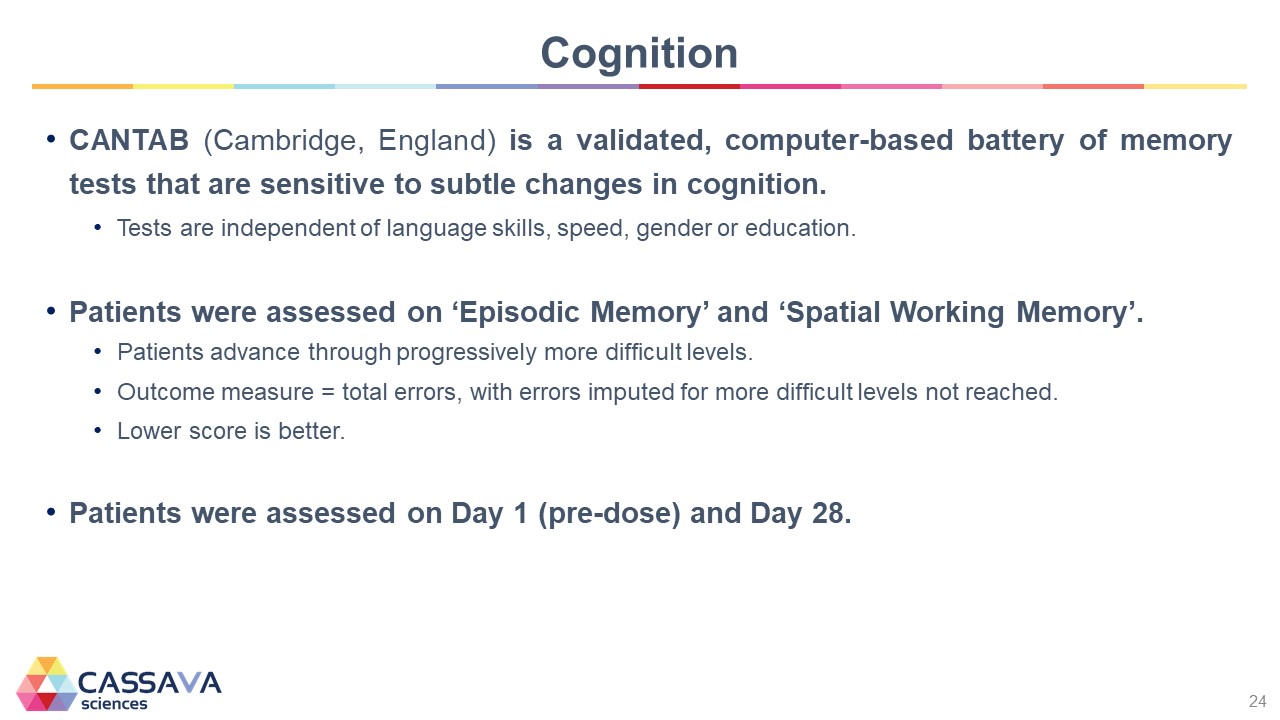
Cognition CANTAB (Cambridge, England) is a validated, computer-based battery of memory tests that are sensitive to subtle changes in cognition. Tests are independent of language skills, speed, gender or education. Patients were assessed on ‘Episodic Memory’ and ‘Spatial Working Memory’. Patients advance through progressively more difficult levels. Outcome measure = total errors, with errors imputed for more difficult levels not reached. Lower score is better. Patients were assessed on Day 1 (pre-dose) and Day 28. CASSAVA sciences 24
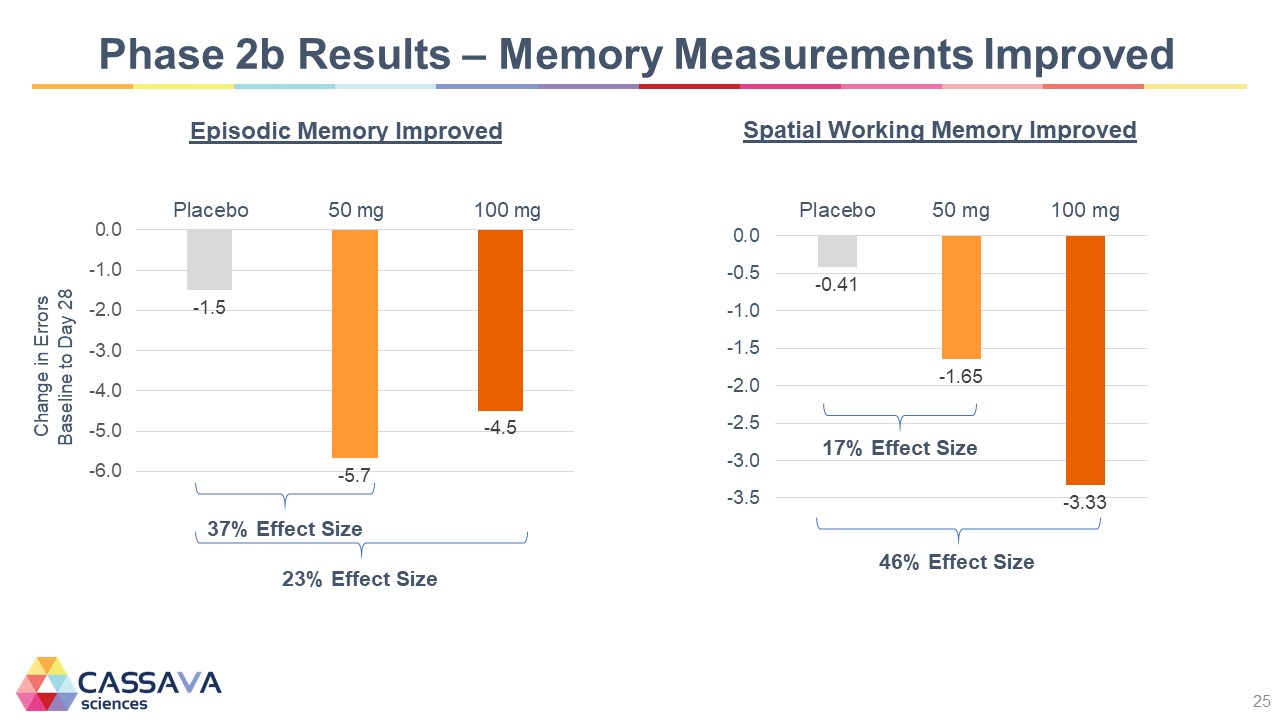
Phase 2b Results – Memory Measurements Improved Change in Errors Baseline to Day 28 -6.0 -5.0 -4.0 -3.0 -2.0 -1.0 0.0 Episodic Memory Improved Placebo -1.5 50 mg -5.7 100 mg -4.5 37% Effect Size 23% Effect Size Spatial Working Memory Improved -3.5 -3.0 -2.5 -2.0 -1.5 -1.0 -0.5 0.0 Placebo -0.41 50 mg -1.65 17% Effect Size 100 mg -3.3 46% Effect Size CASSAVA sciences 25
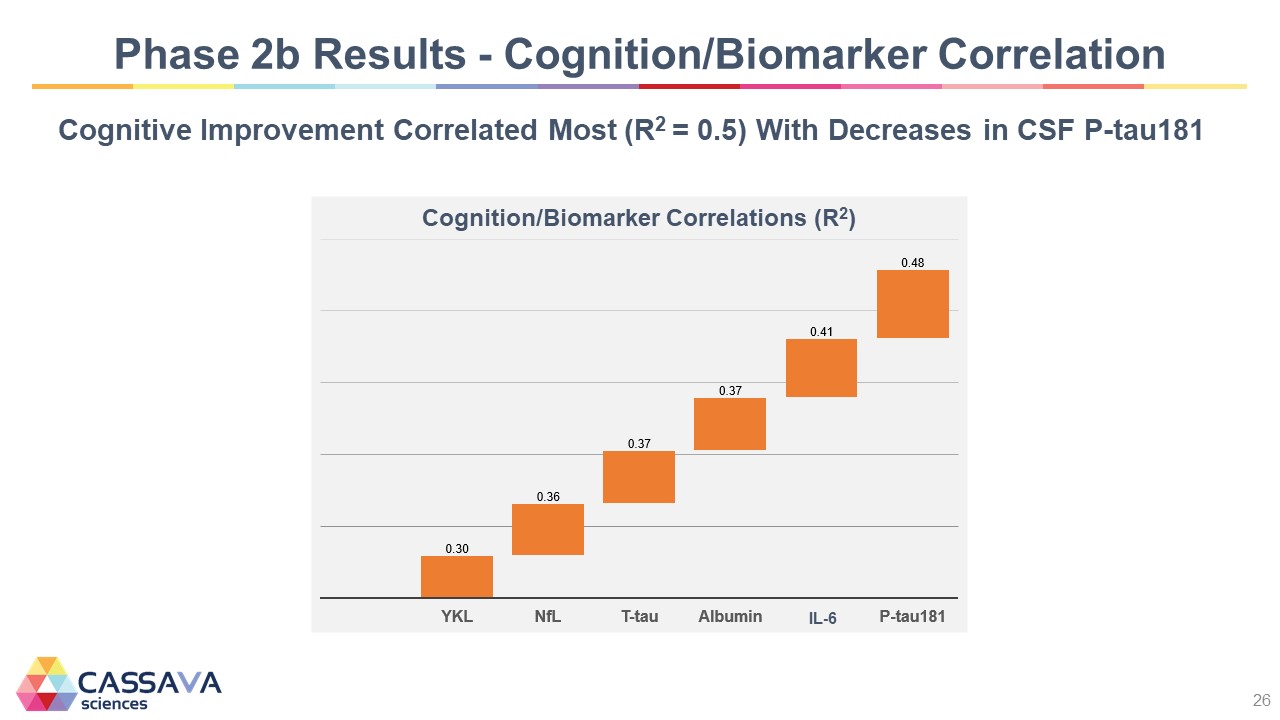
Phase 2b Results - Cognition/Biomarker Correlation Cognitive Improvement Correlated Most (R2 = 0.5) With Decreases in CSF P-tau181 Cognition/Biomarker Correlations (R2) YKL 0.30 NfL 0.36 T-tau 0.37 Albumin 0.37 IL-6 0.41 P-tau181 0.48 CASSAVA sciences 26
Phase 2b Study Conclusions Simufilam showed promising treatment effects in a placebo-controlled study in patients with mild-to-moderate Alzheimer’s disease. Simufilam improved a panel of validated biomarkers of disease pathology, neuroinflammation and BBB integrity. Simufilam appeared to enhance cognition. Phase 2b data replicate prior clinical results and are consistent with published preclinical data and mechanism of action studies. CASSAVA sciences 27
Open-label Study We are conducting an on-going one-year, open-label safety study of simufilam, with scientific and financial support from the National Institutes of Health (NIH). Patients are evaluated for safety, cognition and behavior. Cognition is evaluated on ADAS-Cog11. AD-behavior is evaluated on NPI (Neuropsychiatric Inventory). Total target enrollment is increased, up to 150 patients with mild-to-moderate AD. ≈ 80 patients enrolled as of February 2021. Interim Analyses planned at 6 and 12 months. CASSAVA sciences 28
First Interim Analysis, Open-label Study First interim analysis is in first 50 patients who’ve completed 6 months of treatment. Simufilam improves cognition and behavior in Alzheimer’s Disease. Cognition scores improved by 1.6 points on ADAS-Cog11, a 10% mean improvement from baseline to month 6. Dementia-related behavior, such as anxiety, delusions and agitation, improved by 1.3 points on NPI, a 29% mean improvement from baseline to month 6. Alzheimer’s is a progressive disease. Over time, a patient’s cognition will always worsen. “Experience based on longitudinal studies of ambulatory patients with mild to moderate Alzheimer’s disease suggest that scores on ADAS-cog decline by 6 - 12 points per year”, according to FDA’s Prescription Information sheet for ARICEPT® (donepezil), a drug approved for the treatment of dementia of the Alzheimer’s type. Second interim analysis (12 months) is expected mid-2021. CASSAVA sciences 29
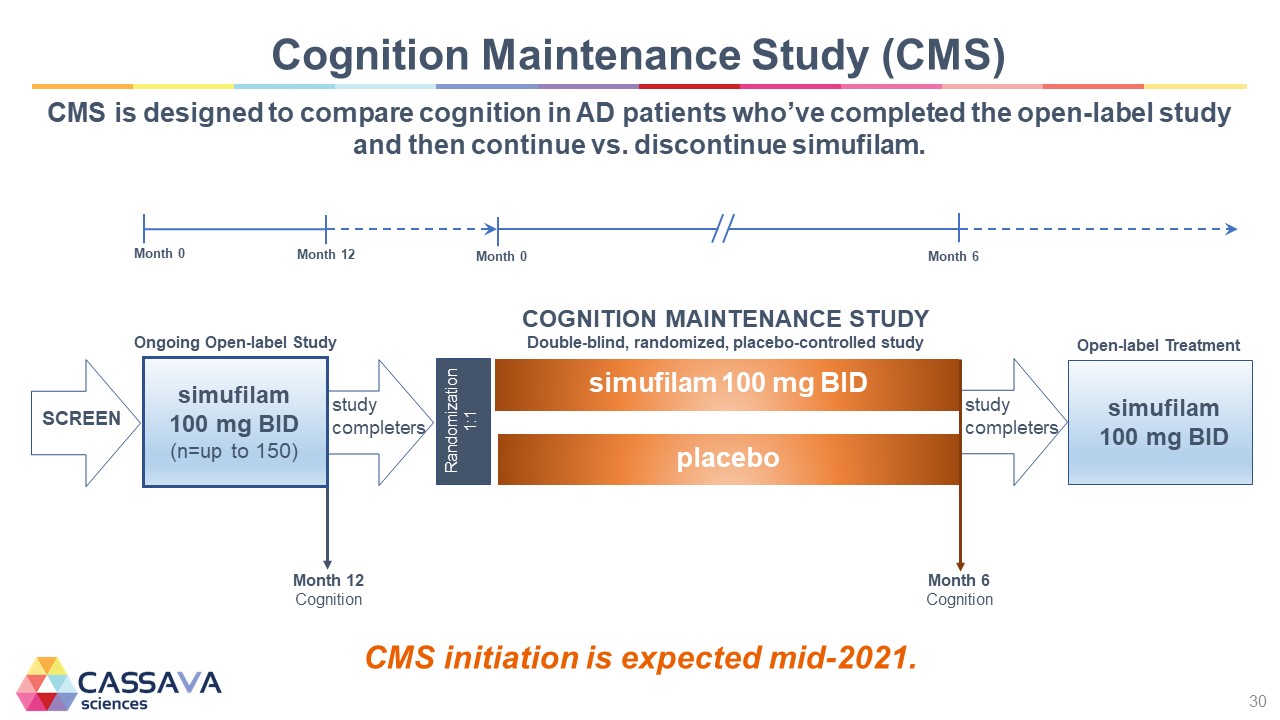
Cognition Maintenance Study (CMS) CMS is designed to compare cognition in AD patients who’ve completed the open-label study and then continue vs. discontinue simufilam. Month 0 Month 12 Month 0 Month 6 SCREEN Ongoing Open-label Study simufilam 100 mg BID (n=up to 150) Month 12 Cognition study completers Randomization 1:1 COGNITION MAINTENANCE STUDY Double-blind, randomized, placebo-controlled study simufilam 100 mg BID placebo Month 6 cognition study completers Open-label Treatment simufilam 100 mg BID CMS initiation is expected mid-2021. CASSAVA sciences 30
Regulatory Status End-of-phase 2 (EOP2) meeting was held with FDA January 14, 2021. EOP2 meeting objectives were to gain general agreement around a Phase 3 clinical program and to identify outstanding data requirements, if any, to support the statutory requirements for a 505(b)(1) NDA submission and marketing approval of simufilam for the treatment of mild-to-moderate Alzheimer’s disease. Announcement regarding results of EOP2 meeting expected in February 2021, pending official FDA meeting minutes. CASSAVA sciences 31

Phase 3 Development Goals The Phase 3 program consists of two Phase 3 studies to evaluate simufilam in patients with mild-to-moderate Alzheimer’s disease dementia. First Phase 3 study is designed to show disease-modifying effects in AD. Goal is to show slower decline in AD patients treated with simufilam compared to placebo. 18-month randomized, controlled study. Second Phase 3 study is designed to show symptomatic relief of AD. Goal is to show symptomatic improvement in AD patients taking simufilam vs placebo. 6-month randomized, controlled study. We are on-track to initiate the Phase 3 program in the 2nd half 2021. CASSAVA sciences 32

SavaDx: Our Investigational Diagnostic for Alzheimer’s The underlying science for simufilam supports the development of a diagnostic technology to detect Alzheimer’s disease with a simple blood test, called SavaDx. Goal is to detect Alzheimer’s disease before the appearance of memory loss. SavaDx development plan benefits from long-term scientific & financial support from NIH. CASSAVA sciences 33

Financials Eric Schoen - Chief Financial Officer CASSAVA sciences 34
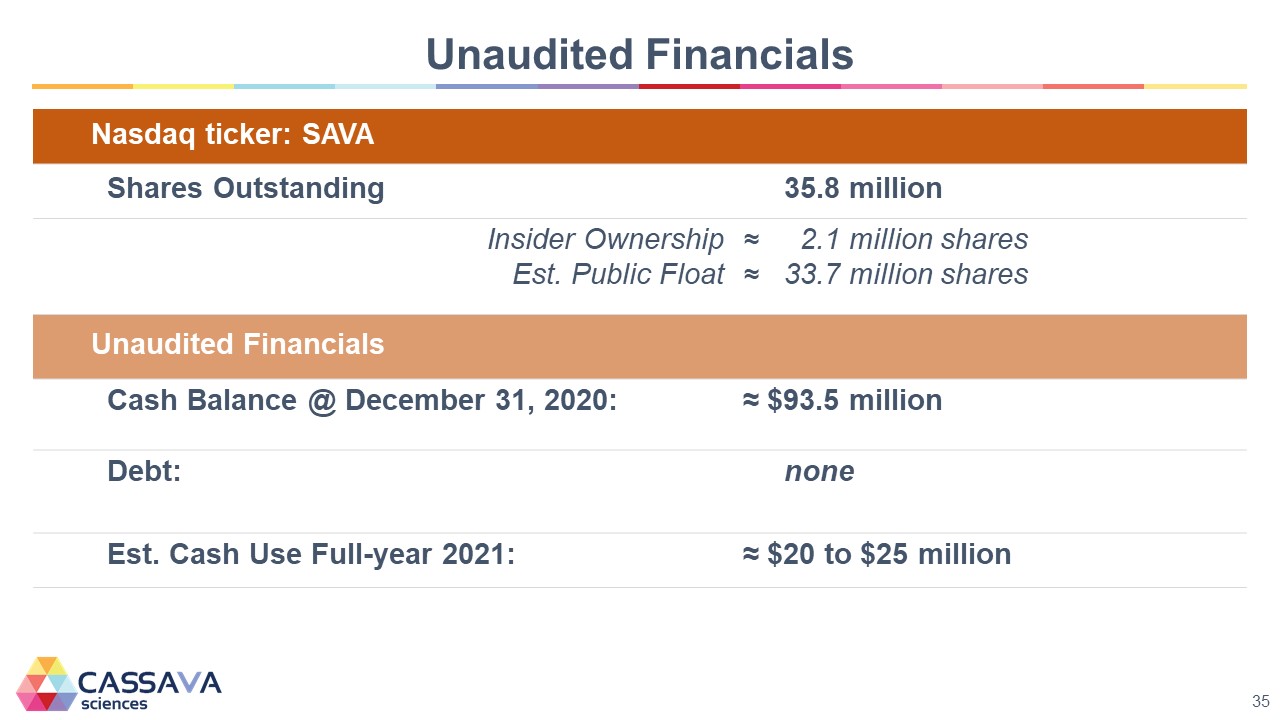
Unaudited Financials Nasdaq ticker: SAVA Shares Outstanding 35.8 million Insider Ownership Est. Public Float ≈ 2.1 million shares ≈ 33.7 million shares Unaudited Financials Cash Balance @ December 31, 2020: ≈ $93.5 million Debt: none Est. Cash Use Full-year 2021: ≈ $20 to $25 million CASSAVA sciences 35
Intellectual Property Simufilam is a novel molecule. Cassava Sciences owns composition of matter claims on simufilam and other novel, filamin-binding molecules. Cassava Sciences’ patent protection for simufilam includes six issued patents and currently runs through 2033. Cassava Sciences owns exclusive, worldwide rights to simufilam and related technologies, without milestone or royalty obligations to any third party. There is no patent protection for SavaDx, which is protected by trade secrets, know-how and other proprietary rights technology. CASSAVA sciences 36

Expected 2021 Milestones Our goal is to initiate a Phase 3 study of simufilam in Alzheimer's disease 2nd half 2021. End-of-phase 2 (EOP2) meeting with FDA to gain general agreement around a Phase 3 clinical development program in Alzheimer’s disease dementia – completed Jan 2021 Results of interim analysis (6-month) of open-label study - completed Feb 2021 Results of EOP2 meeting with FDA. Results of interim analysis (12-month) of ongoing open-label study in Alzheimer’s. Long-term supply agreement with contract manufacturer for simufilam. Manufacture large-scale Phase 3 clinical trial supplies (drug substance + oral tablets). Initiate Cognition Maintenance Study (CMS) mid-2021. Complete patient enrollment of on-going, open-label study of simufilam. Publication of Phase 2b results in peer-reviewed technical journal. Initiate validation study with SavaDx. CASSAVA sciences 37

Thank you! CASSAVA sciences 38

Appendix: Key Publications Journal of Prevention of Alzheimer’s Disease 2020; DOI: 10.14283 PTI-125 Reduces Biomarkers of Alzheimer’s Disease In Patients: http://link.springer.com/article/10.14283/jpad.2020.6 Neuroimmunology and Neuroinflammation 2017;4:263-71: Altered filamin A enables amyloid beta induced tau hyperphosphorylation and neuroinflammation in Alzheimer’s disease: http://nnjournal.net/article/view/2313 Neurobiology of Aging (Volume 55) July 2017, Pages 99—114) PTI-125 binds and reverses an altered conformation of filamin A to reduce Alzheimer's disease pathogenesis: http://www.neurobiologyofaging.org/article/S0197-4580(17)30087-8/ Alzheimer's & Dementia Volume 8, Issue 4, Supplement, 1 July 2012, Pages p259-p260 PTI-125 reduces amyloid-related Alzheimer's pathogenesis by targeting filamin A: https://www.sciencedirect.com/science/article/pii/S1552526012008242 Journal of Neuroscience 18 July 2012, 32 (29) 9773-9784 Reducing amyloid-related Alzheimer's disease pathogenesis by a small molecule targeting filamin A http://www.jneurosci.org/content/32/29/9773.short CASSAVA sciences 39
