Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - PTC THERAPEUTICS, INC. | tmb-20210204xex99d1.htm |
| 8-K - 8-K - PTC THERAPEUTICS, INC. | tmb-20210204x8k.htm |
Exhibit 99.2
| Translarna™ Study 045 and Clinical Update Stuart W. Peltz, Ph.D. Chief Executive Officer |
| Forward Looking Statement This presentation contains forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 . All statements contained in this presentation, other than statements of historic fact, are forward -looking statements, including statements regarding: the future expectations, plans and prospects for PTC, including with respect to the commercialization of its products and product candid ates; PTC's plans for interactions with the FDA; the clinical utility and potential advantages of Translarna; PTC's strategy, future operations, fu ture financial position, future revenues, projected costs; and the objectives of management. Other forward -looking statements may be identified by the words "guidance", "plan," "anticipate," "believe," "estimate," "expect," "intend," "may," "target," "potential," "will," "would," "could," "should," "c ontinue," and similar expressions. PTC's actual results, performance or achievements could differ materially from those expressed or implied by forward -looking statements it makes as a result of a variety of risks and uncertainties, including those related to: the outcome of pricing, coverage and reimbursemen t negotiations with third party payors for PTC's products or product candidates that PTC commercializes or may commercialize in the future; PTC's abili ty to support a re- submission of its Translarna NDA for the treatment of nonsense mutation Duchenne muscular dystrophy ( nmDMD) to the FDA, and PTC's ability to perform any necessary clinical trials, non-clinical studies, and CMC assessments or analyses at significant cost; PTC's ability to maintain its marketing authorization of Translarna for the treatment of nmDMD in the European Economic Area (EEA), including whether the European Medicines Agency (EMA) determines in future annual renewal cycles that the benefit -risk balance of Translarna authorization supports renew al of such authorization; PTC's ability to enroll, fund, complete and timely submit to the EMA the results of Study 041, a randomized, 1 8-month, placebo-controlled clinical trial of Translarna for the treatment of nmDMD followed by an 18-month open-label extension, which is a specific obligation to continued marketing authorization in the EEA; whether regulators will agree with PTC’s characterization of the results of PTC’s clinica l trials including demonstration of meaningful clinical benefit of its products and product candidates; significant business effects, including the effects of industry, market, economic, political or regulatory conditions; changes in tax and other laws, regulations, rates and policie s; the eligible patient base and commercial potential of PTC's products and product candidates; PTC's scientific approach and general development progress ; and the factors discussed in the "Risk Factors" section of PTC's most recent Quarterly Report on Form 10 -Q and Annual Report on Form 10-K, as well as any updates to these risk factors filed from time to time in PTC's other filings with the SEC. You are urged to carefully consider all such factors. As with any pharmaceutical under development, there are significant risks in the development, regulatory approval and commerc ialization of new products. There are no guarantees that any product will receive or maintain regulatory approval in any territory, or prove to be commercially successful, including Translarna. The forward-looking statements contained herein represent PTC's views only as of the date of this presentation and PTC does not undertake or plan to update or revise any such forward-looking statements to reflect actual results or changes in plans, prospects, assumptions, esti mates or projections, or other circumstances occurring after the date of this presentation except as required by law. 2 |
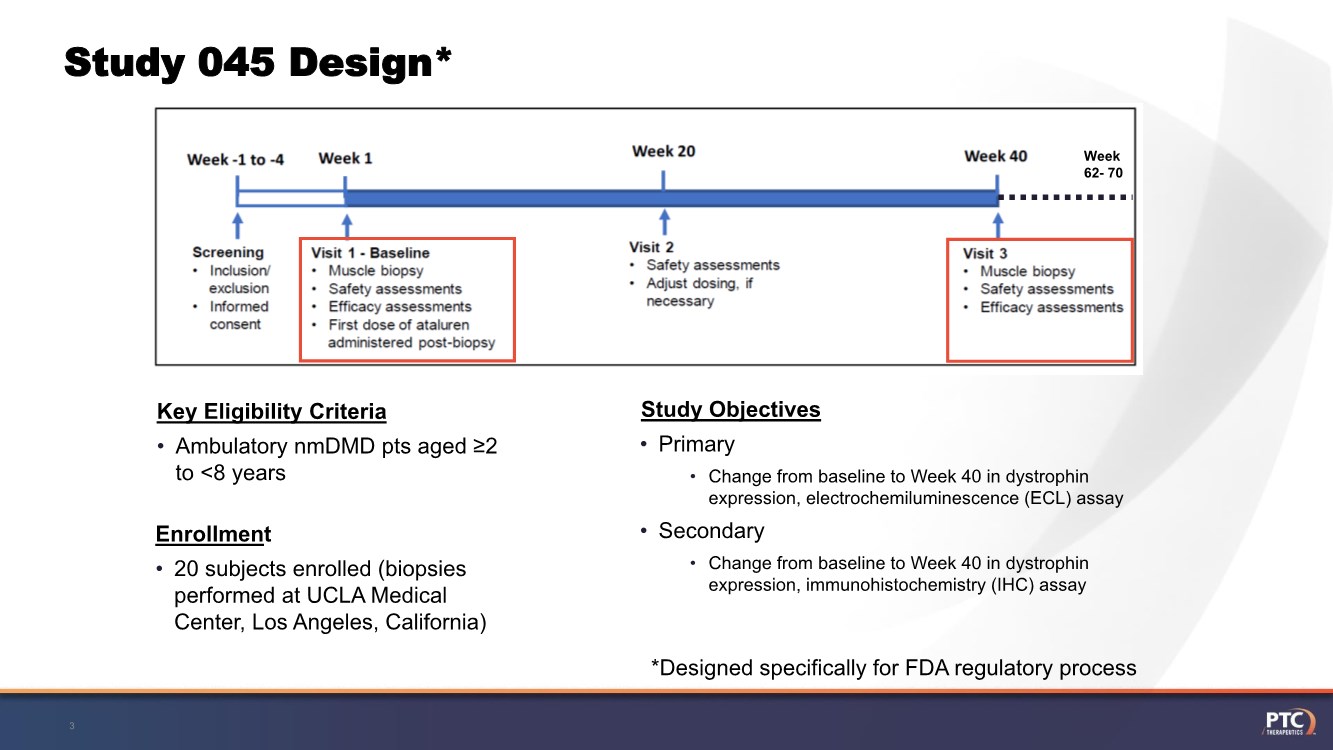
| Study 045 Design* 3 • Ambulatory nmDMD pts aged ≥2 to <8 years Key Eligibility Criteria • Primary • Change from baseline to Week 40 in dystrophin expression, electrochemiluminescence (ECL) assay • Secondary • Change from baseline to Week 40 in dystrophin expression, immunohistochemistry (IHC) assay Study Objectives • 20 subjects enrolled (biopsies performed at UCLA Medical Center, Los Angeles, California) Enrollment Week 62- 70 *Designed specifically for FDA regulatory process |
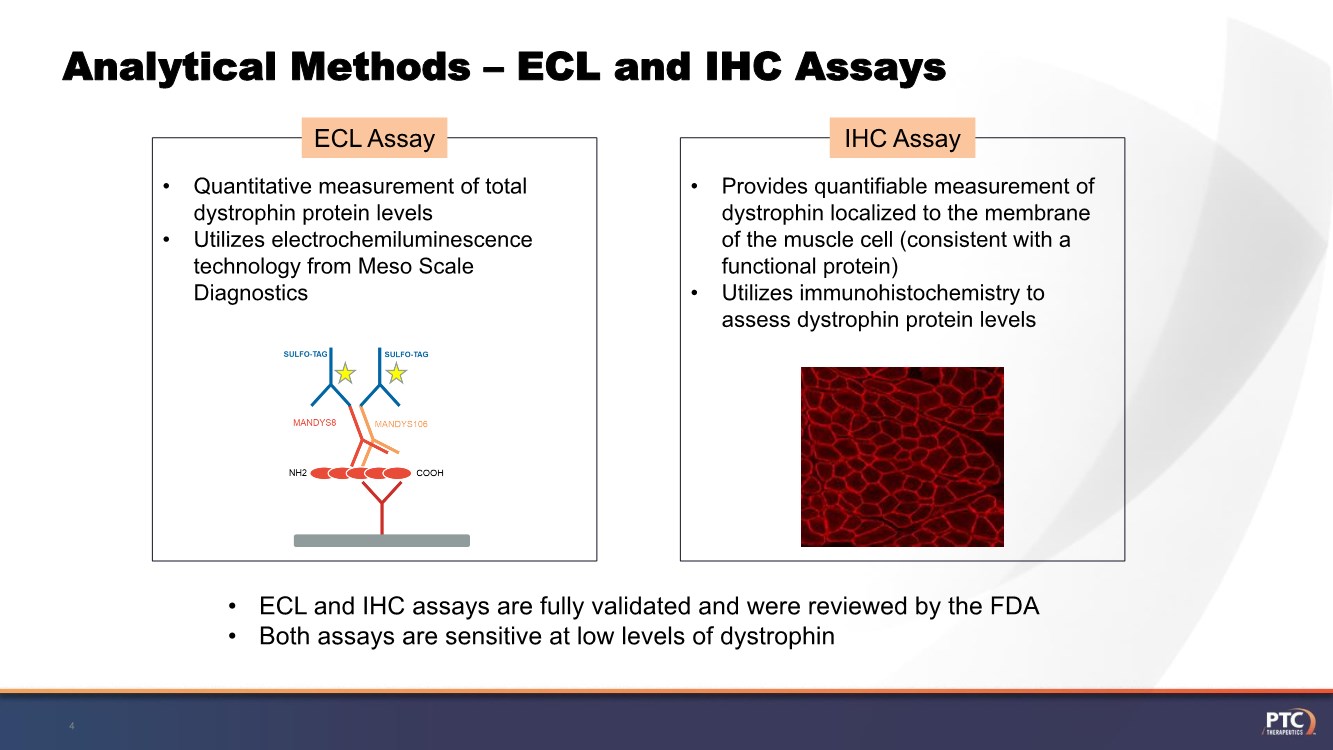
| Analytical Methods – ECL and IHC Assays 4 • Quantitative measurement of total dystrophin protein levels • Utilizes electrochemiluminescence technology from Meso Scale Diagnostics ECL Assay • Provides quantifiable measurement of dystrophin localized to the membrane of the muscle cell (consistent with a functional protein) • Utilizes immunohistochemistry to assess dystrophin protein levels IHC Assay • ECL and IHC assays are fully validated and were reviewed by the FDA • Both assays are sensitive at low levels of dystrophin |
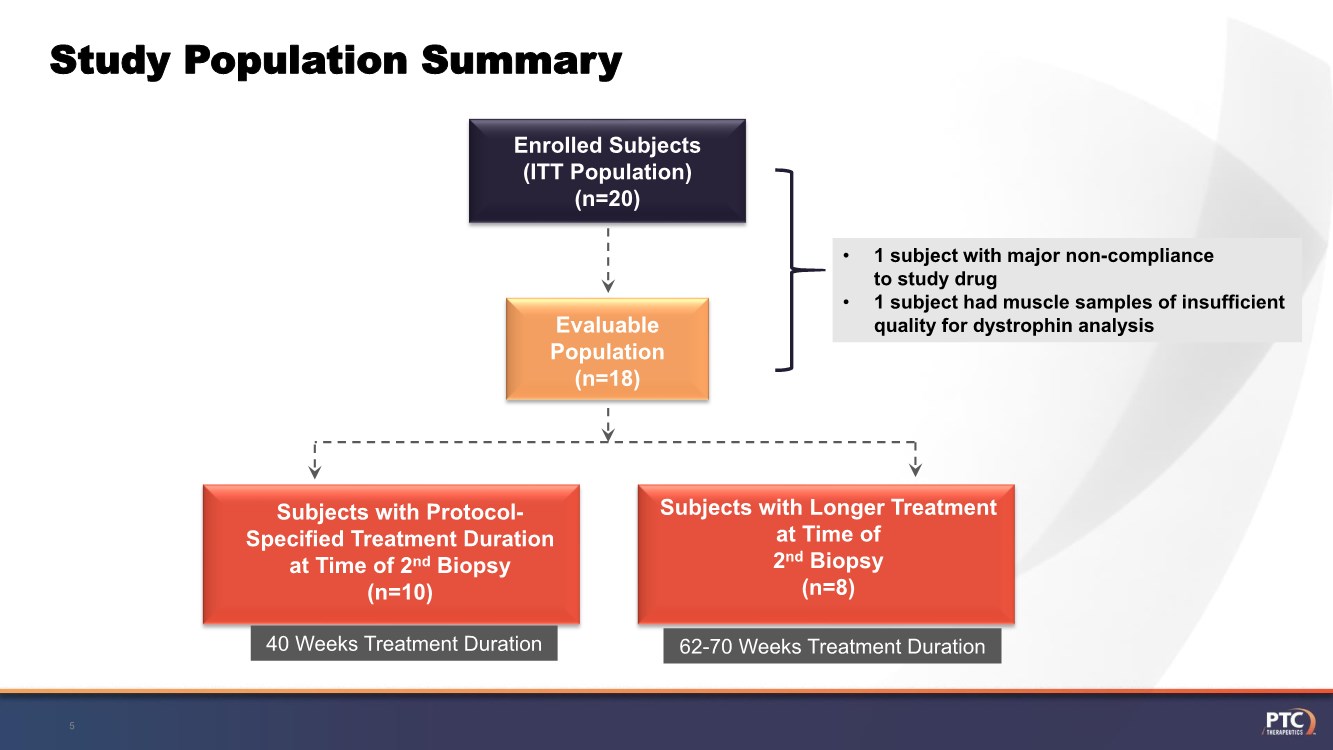
| Study Population Summary 5 Text Here Text Here Text Here Enrolled Subjects (ITT Population) (n=20) Evaluable Population (n=18) Subjects with Longer Treatment at Time of 2nd Biopsy (n=8) Text Here Text Here Subjects with Protocol- Specified Treatment Duration at Time of 2nd Biopsy (n=10) • 1 subject with major non-compliance to study drug • 1 subject had muscle samples of insufficient quality for dystrophin analysis 40 Weeks Treatment Duration 62-70 Weeks Treatment Duration |
| Translarna™ Treatment Resulted in Increased Dystrophin Expression on both ECL and IHC Assays *Exploratory; not adjusted for multiplicity 6 Mean % Change from Baseline ITT Population (n=20) Evaluable Population (n=18) ECL Assay IHC Assay ECL Assay* IHC Assay* 6.56% (p=0.24) 4.91% (p=0.11) 9.20% (p=0.19) 7.00% (p=0.04) Primary endpoint – ECL Assay ITT Population Secondary endpoint – IHC Assay ITT Population |
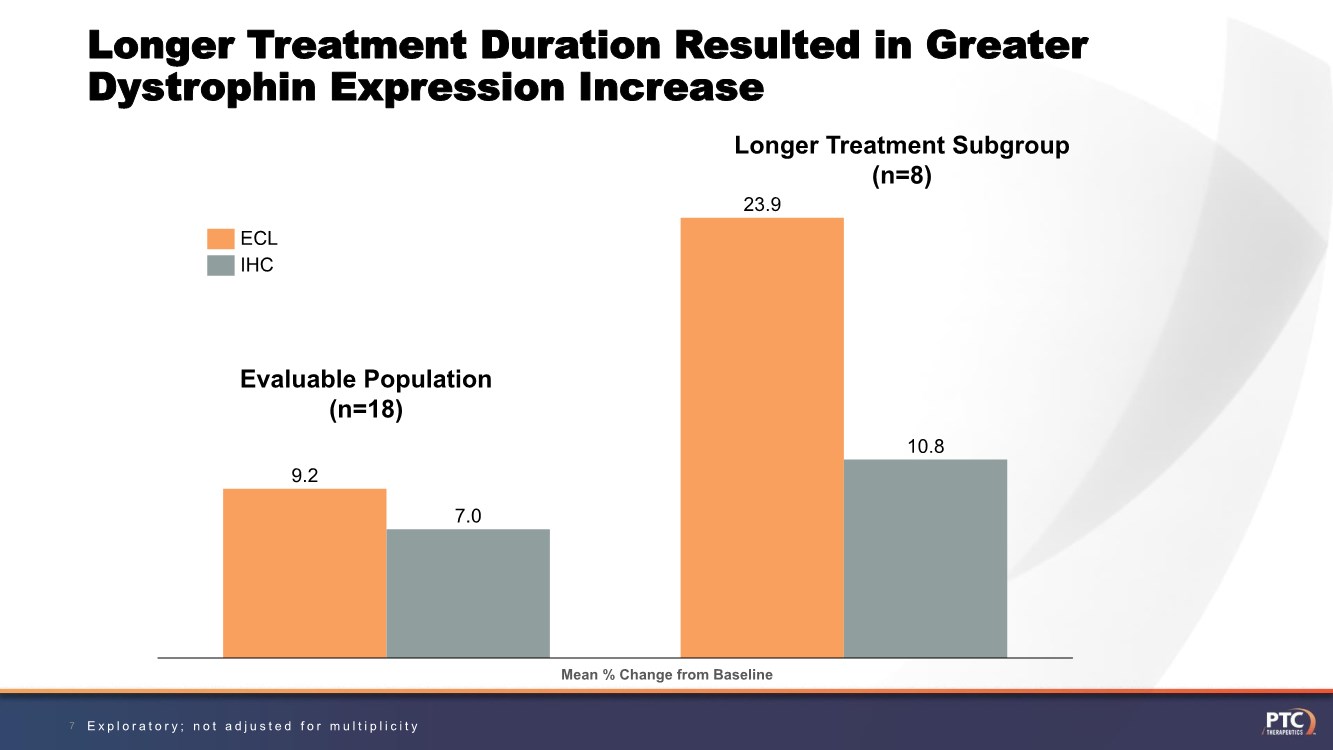
| Longer Treatment Duration Resulted in Greater Dystrophin Expression Increase 7 9.2 23.9 7.0 10.8 ECL IHC Evaluable Population (n=18) Longer Treatment Subgroup (n=8) Mean % Change from Baseline Exploratory; not adjusted for multiplicity |
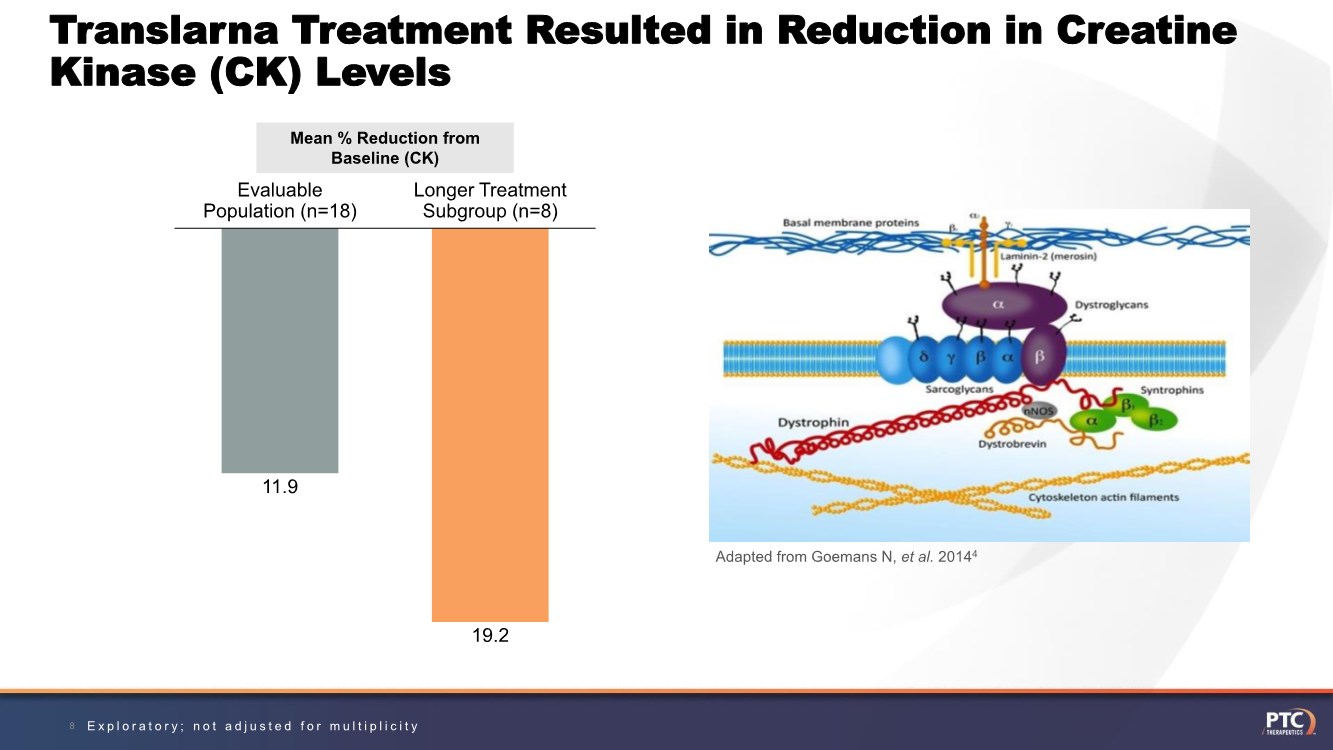
| Translarna Treatment Resulted in Reduction in Creatine Kinase (CK) Levels 8 Mean % Reduction from Baseline (CK) 11.9 19.2 Evaluable Population (n=18) Longer Treatment Subgroup (n=8) Adapted from Goemans N, et al. 20144 Exploratory; not adjusted for multiplicity |
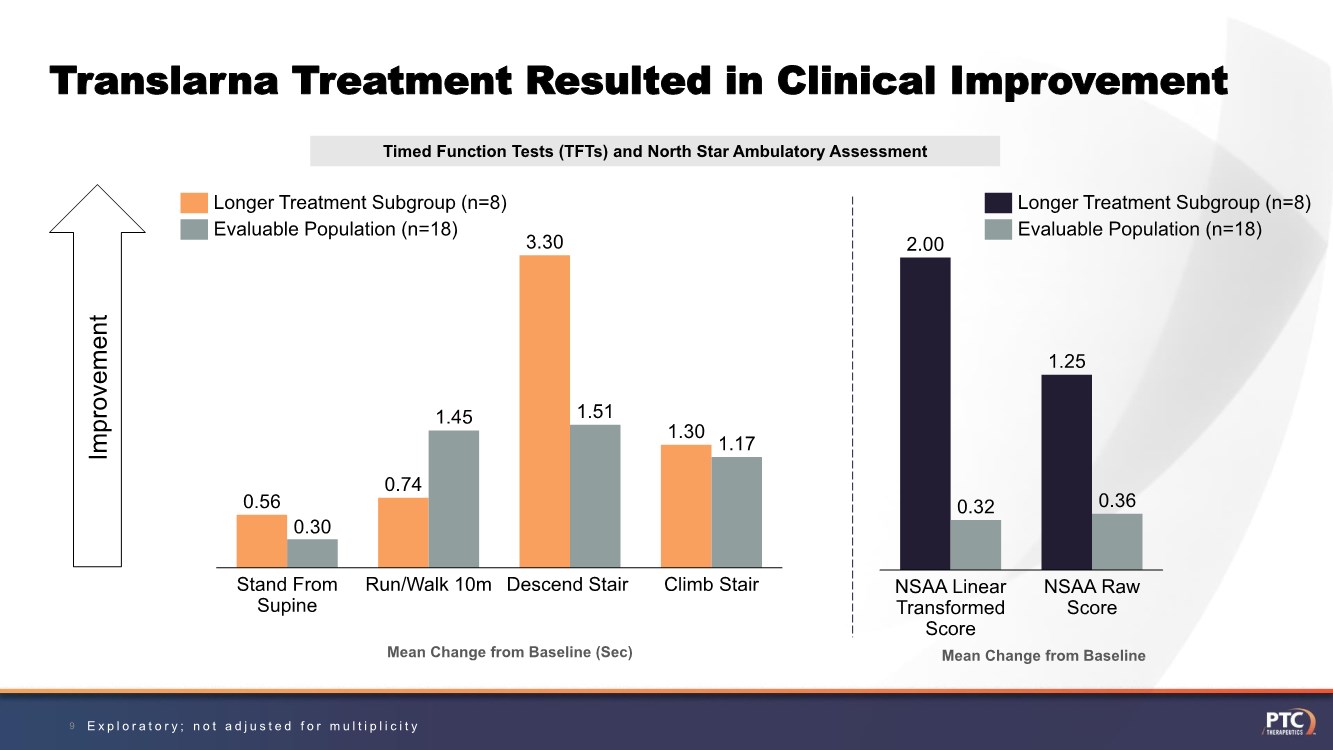
| Translarna Treatment Resulted in Clinical Improvement 9 Timed Function Tests (TFTs) and North Star Ambulatory Assessment Mean Change from Baseline (Sec) 0.56 0.74 3.30 1.30 0.30 1.45 1.51 1.17 Descend Stair Stand From Supine Run/Walk 10m Climb Stair Longer Treatment Subgroup (n=8) Evaluable Population (n=18) Improvement 2.00 1.25 0.32 0.36 NSAA Linear Transformed Score NSAA Raw Score Longer Treatment Subgroup (n=8) Evaluable Population (n=18) Mean Change from Baseline Exploratory; not adjusted for multiplicity |
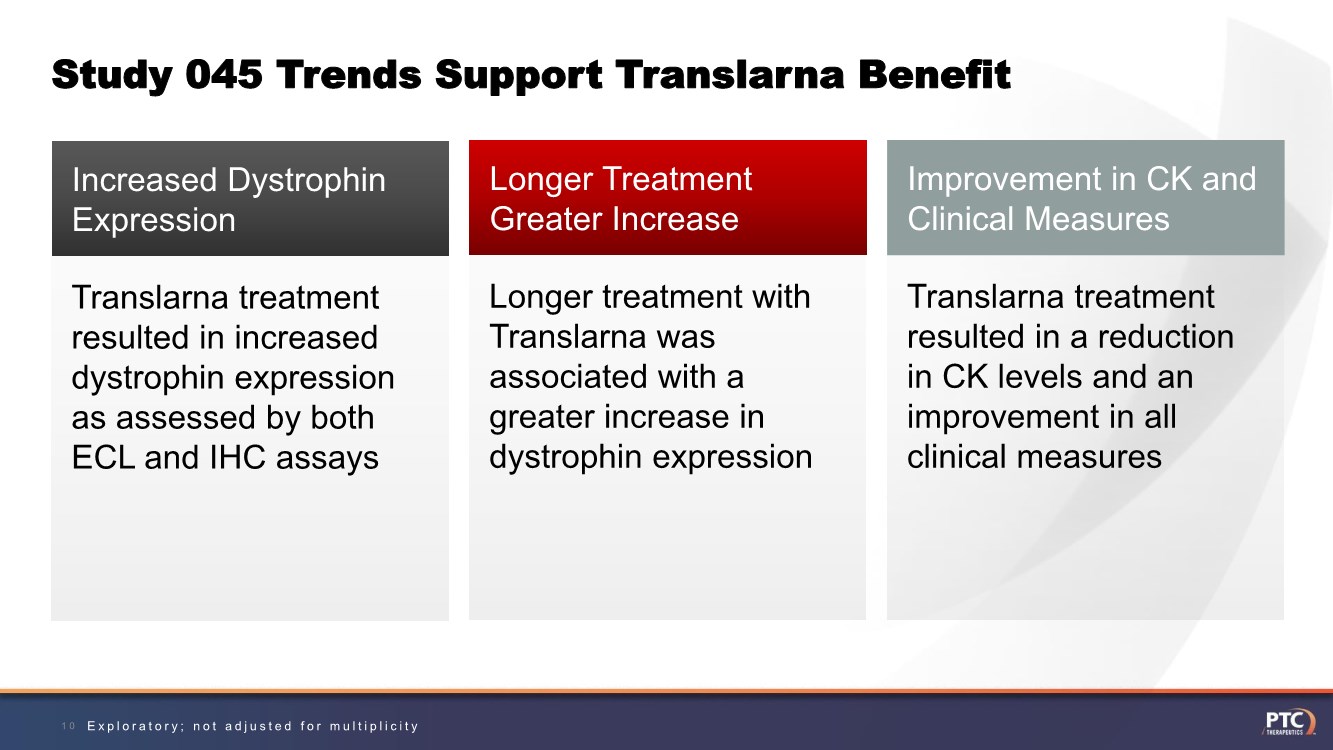
| Study 045 Trends Support Translarna Benefit 10 Translarna treatment resulted in increased dystrophin expression as assessed by both ECL and IHC assays Increased Dystrophin Expression Longer treatment with Translarna was associated with a greater increase in dystrophin expression Longer Treatment Greater Increase Translarna treatment resulted in a reduction in CK levels and an improvement in all clinical measures Improvement in CK and Clinical Measures Exploratory; not adjusted for multiplicity |
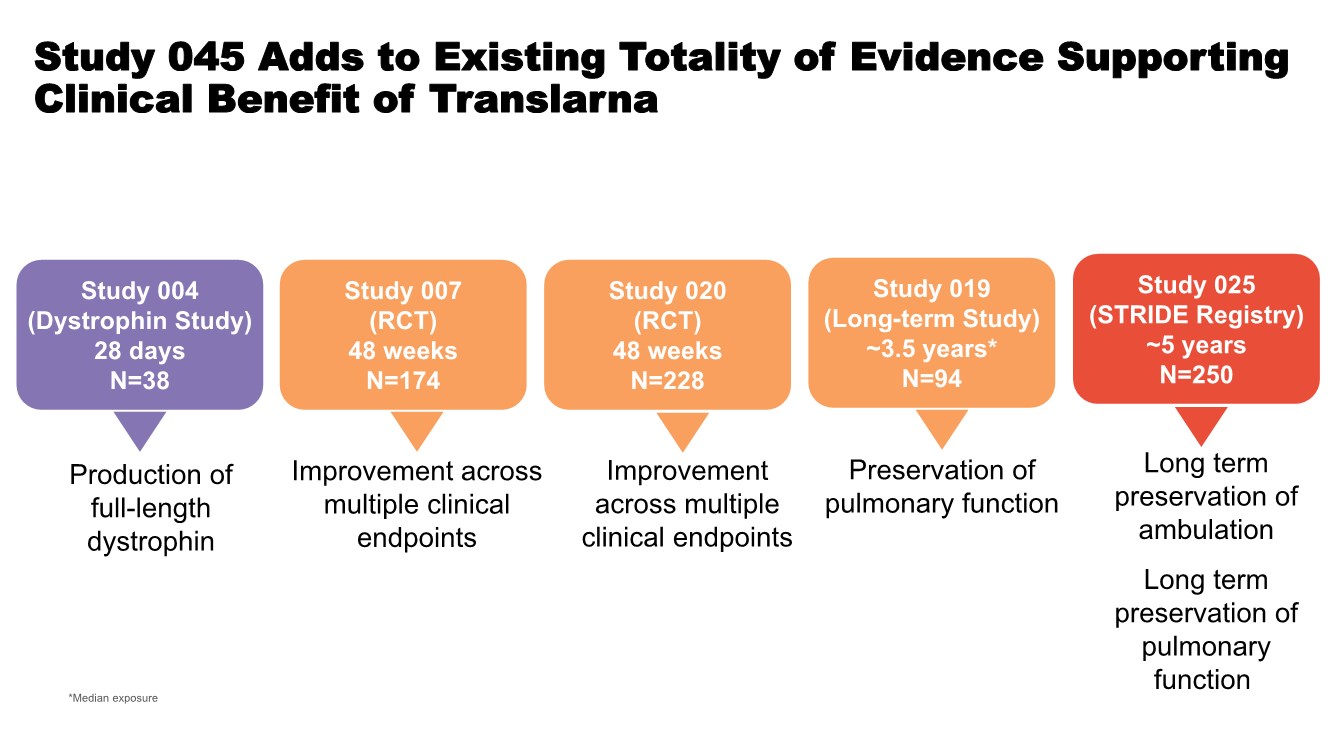
| Study 045 Adds to Existing Totality of Evidence Supporting Clinical Benefit of Translarna *Median exposure Study 019 (Long-term Study) ~3.5 years* N=94 Preservation of pulmonary function Production of full-length dystrophin Study 004 (Dystrophin Study) 28 days N=38 Study 007 (RCT) 48 weeks N=174 Study 020 (RCT) 48 weeks N=228 Improvement across multiple clinical endpoints Improvement across multiple clinical endpoints Study 025 (STRIDE Registry) ~5 years N=250 Long term preservation of ambulation Long term preservation of pulmonary function |
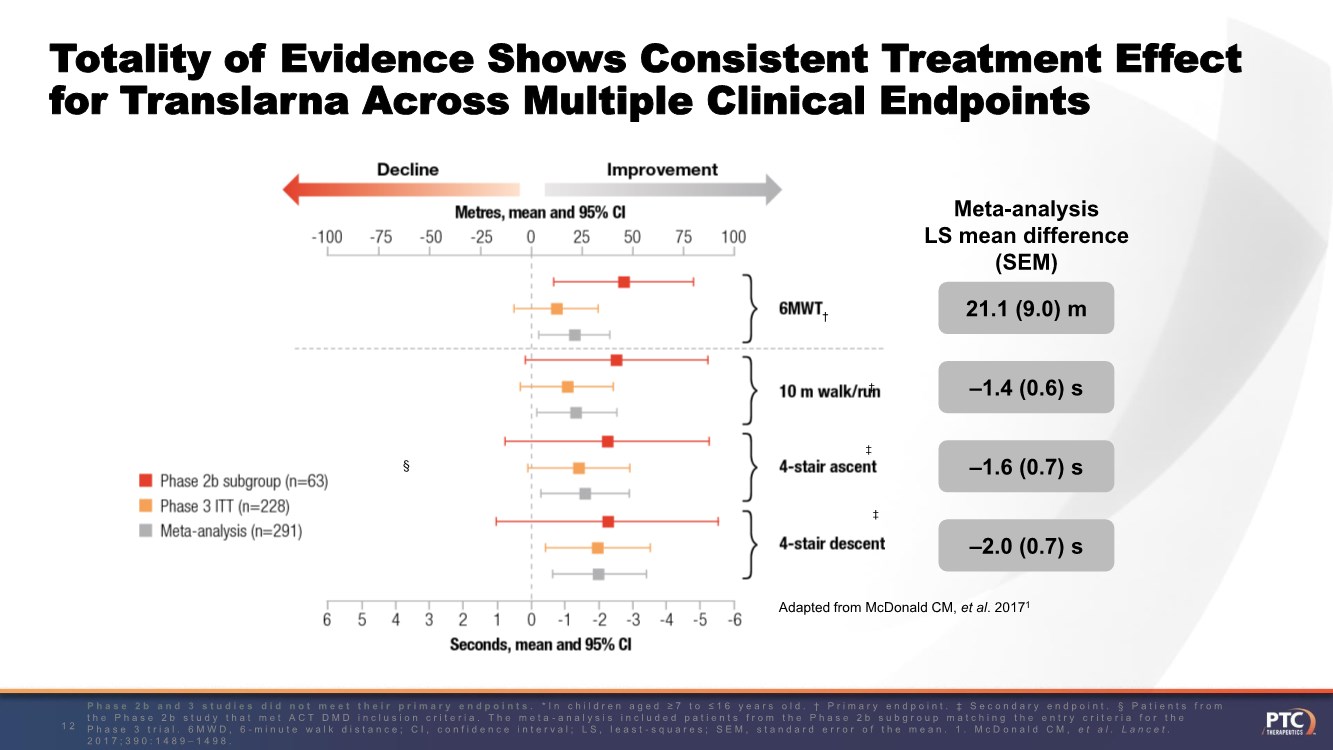
| Totality of Evidence Shows Consistent Treatment Effect for Translarna Across Multiple Clinical Endpoints Phase 2b and 3 studies did not meet their primary endpoints. *In children aged ≥7 to ≤16 years old. † Primary endpoint. ‡ Secondary endpoint. § Patients from the Phase 2b study that met ACT DMD inclusion criteria. The meta - analysis included patients from the Phase 2b subgroup matching the entry criteria for the Phase 3 trial. 6MWD, 6 - minute walk distance; CI, confidence interval; LS, least - squares; SEM, standard error of the mean. 1. McD o n a l d C M , et al. Lancet. 2017;390:1489 – 1 4 9 8 . 12 † ‡ § ‡ ‡ Adapted from McDonald CM, et al. 20171 21.1 (9.0) m ‒1.4 (0.6) s ‒1.6 (0.7) s ‒2.0 (0.7) s Meta-analysis LS mean difference (SEM) |
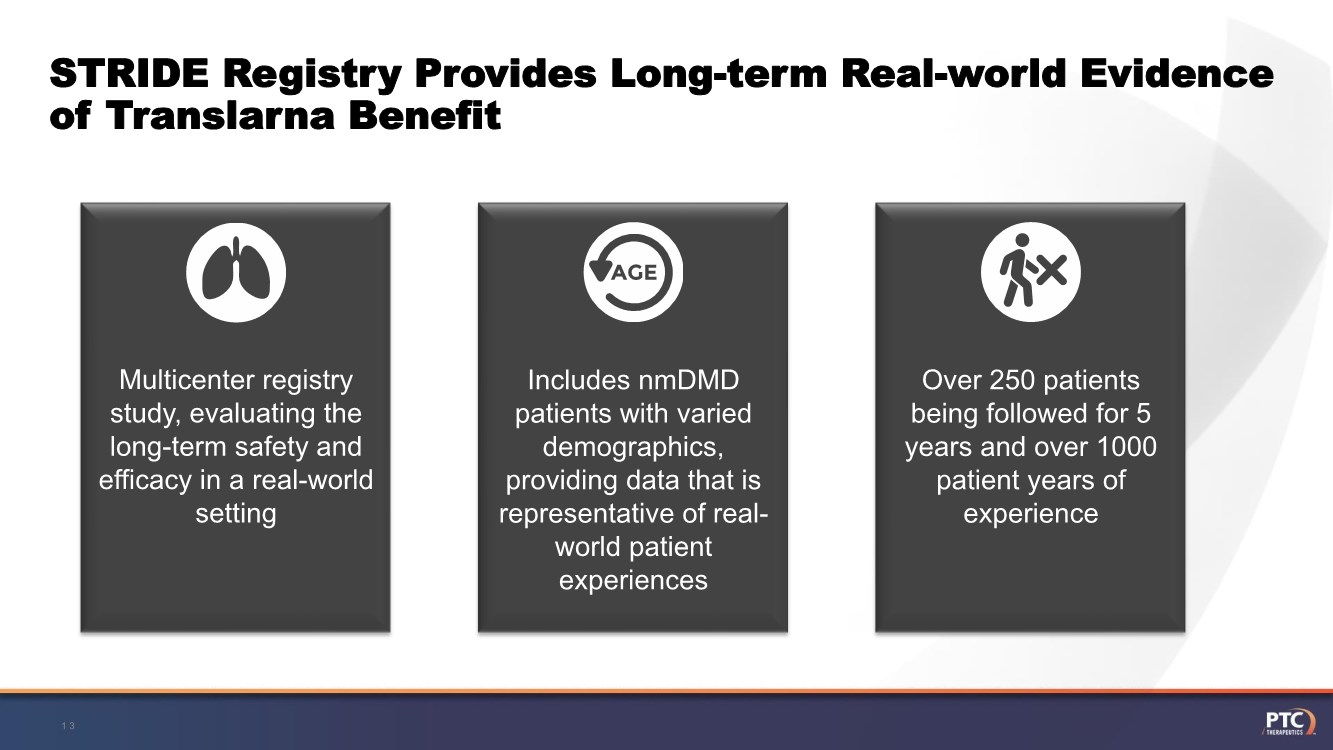
| STRIDE Registry Provides Long-term Real-world Evidence of Translarna Benefit 13 Multicenter registry study, evaluating the long-term safety and efficacy in a real-world setting Includes nmDMD patients with varied demographics, providing data that is representative of real- world patient experiences Over 250 patients being followed for 5 years and over 1000 patient years of experience |
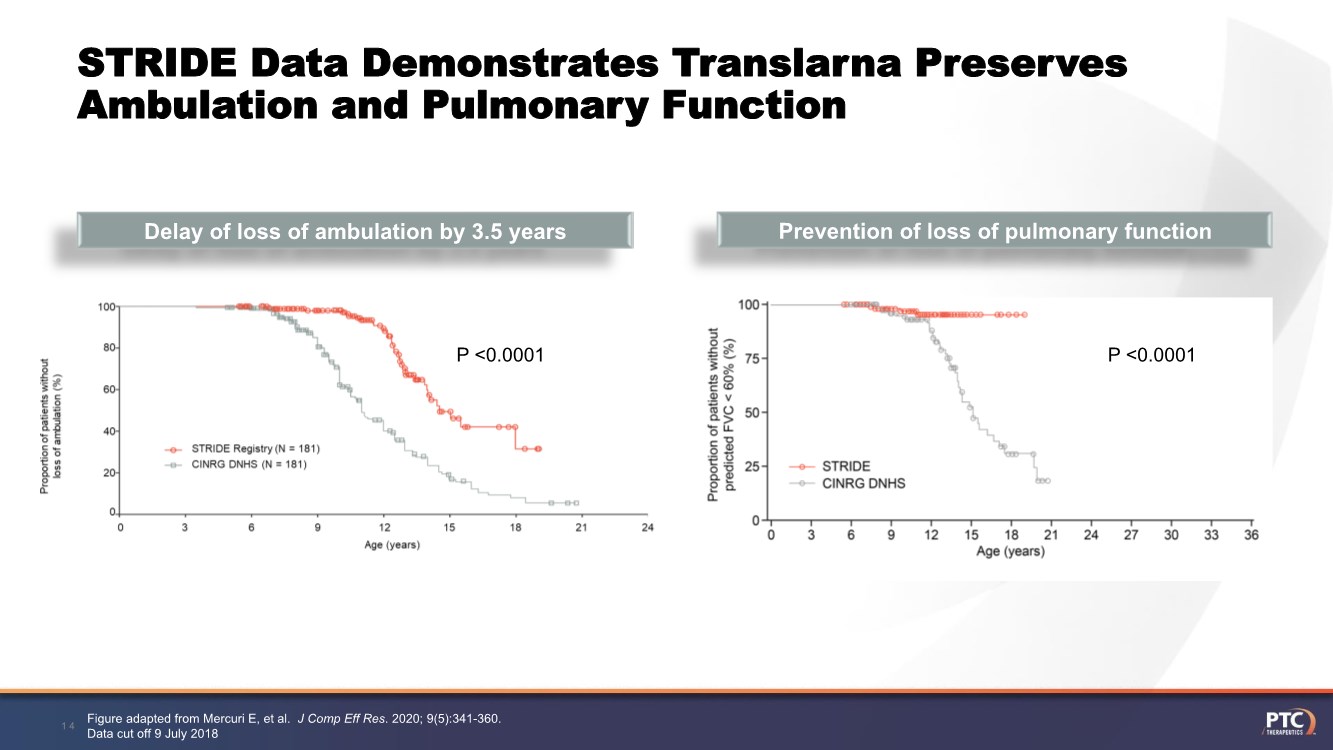
| STRIDE Data Demonstrates Translarna Preserves Ambulation and Pulmonary Function Figure adapted from Mercuri E, et al. J Comp Eff Res. 2020; 9(5):341-360. Data cut off 9 July 2018 14 Prevention of loss of pulmonary function Delay of loss of ambulation by 3.5 years P <0.0001 P <0.0001 |
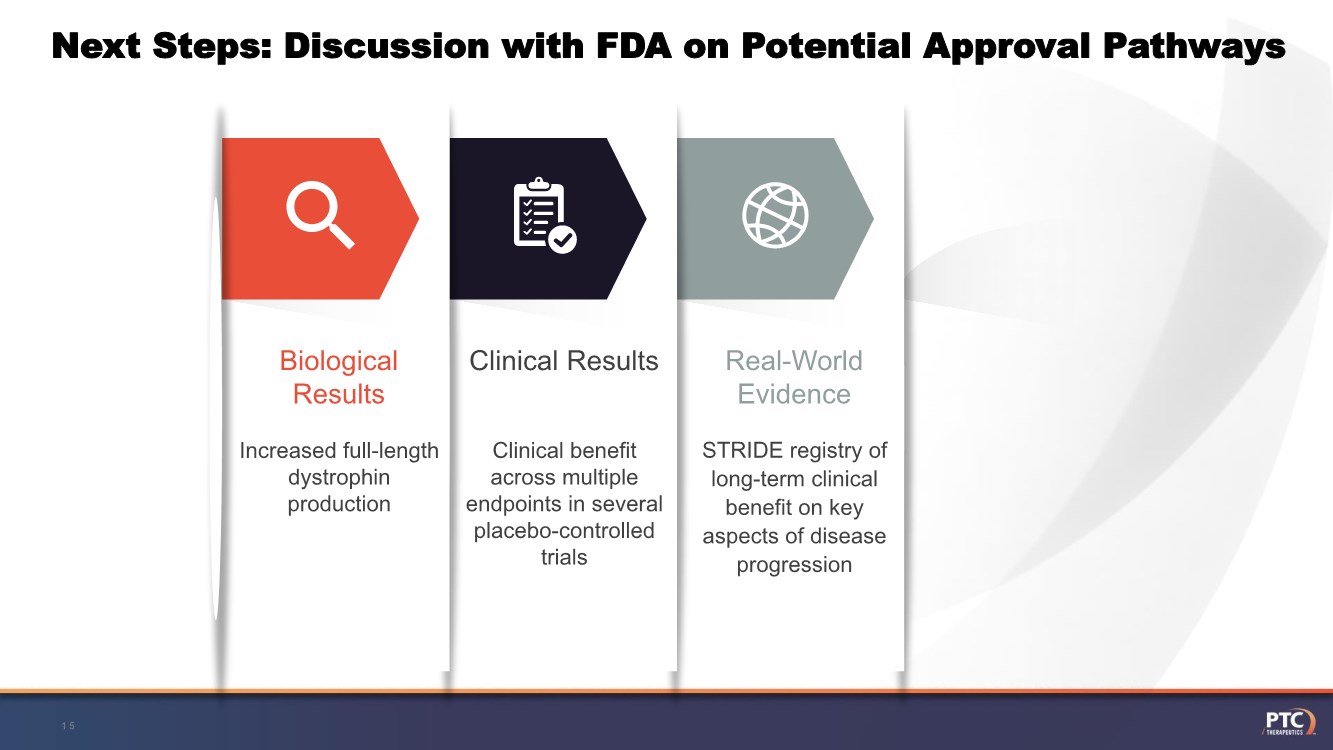
| Next Steps: Discussion with FDA on Potential Approval Pathways 15 STRIDE registry of long-term clinical benefit on key aspects of disease progression Real-World Evidence Clinical benefit across multiple endpoints in several placebo-controlled trials Clinical Results Increased full-length dystrophin production Biological Results |
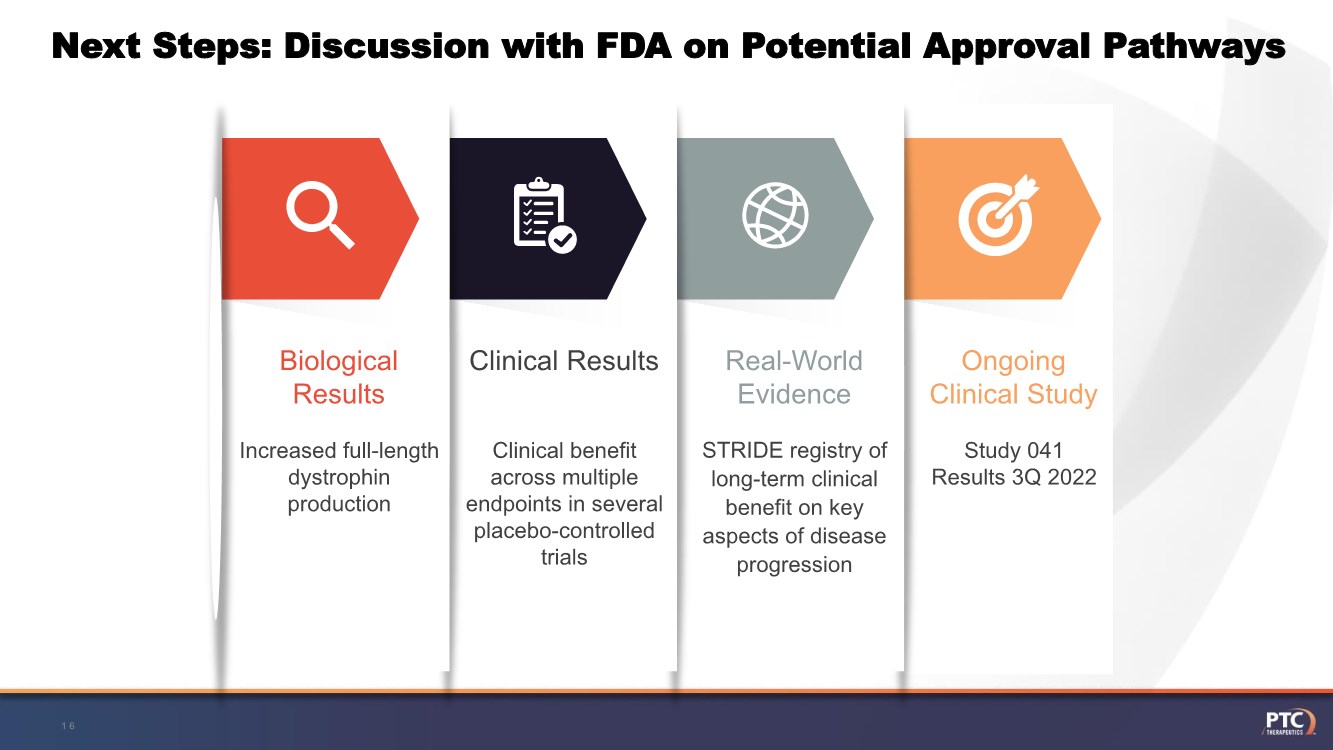
| Next Steps: Discussion with FDA on Potential Approval Pathways 16 Study 041 Results 3Q 2022 Ongoing Clinical Study STRIDE registry of long-term clinical benefit on key aspects of disease progression Real-World Evidence Clinical benefit across multiple endpoints in several placebo-controlled trials Clinical Results Increased full-length dystrophin production Biological Results |