Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OCULAR THERAPEUTIX, INC | tm213633d1_8k.htm |
Exhibit 99.1

OTX - TIC, AN INTRACAMERAL HYDROGEL - BASED TRAVOPROST IMPLANT TO TREAT PATIENTS WITH GLAUCOMA & OCULAR HYPERTENSION PHASE 1 TRIAL UPDATE MICHAEL GOLDSTEIN, MD, MBA PRESIDENT, OPHTHALMOLOGY & CHIEF MEDICAL OFFICER GLAUCOMA 360 | VIRTUAL | JANUARY , 2021 (NASDAQ: OCUL)

FINANCIAL DISCLOSURE Sponsorship for the clinical trial: Ocular Therapeutix, Inc. The author(s) do have financial interest in this presentation: Dr. Goldstein is an employee of Ocular Therapeutix 2

Any statements in this presentation about future expectations, plans, and prospects for the Company, including the commercialization of DEXTENZA®, ReSure® Sealant, or any of the Company’s product candidates ; the commercial launch of, and effectiveness of reimbursement codes for DEXTENZA, the conduct of post - approval studies of DEXTENZA, the development and regulatory status of the Company’s product candidates, such as the Company’s development of and prospects for approvability of DEXTENZA for additional indications including allergic conjunctivitis, OTX - DED for the short - term treatment of the signs and symptoms of dry eye disease, OTX - CSI for the chronic treatment of dry eye disease, OTX - TIC for the treatment of primary open - angle glaucoma or ocular hypertension, OTX - TKI for the treatment of retinal diseases including wet AMD, and OTX - AFS as an extended - delivery formulation of the VEGF trap aflibercept for the treatment of retinal diseases including wet AMD ; the ongoing development of the Company’s extended - delivery hydrogel depot technology ; the size of potential markets for our product candidates ; the potential utility of any of the Company’s product candidates ; the potential benefits and future operation of the collaboration with Regeneron Pharmaceuticals, including any potential future payments thereunder ; projected net product revenue, unit sales and other financial and operational metrics of DEXTENZA ; the expected impact of the COVID - 19 pandemic on the Company and its operations ; the sufficiency of the Company’s cash resources and other statements containing the words "anticipate," "believe," "estimate," "expect," "intend", "goal," "may", "might," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," and similar expressions, constitute forward - looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 . Actual results may differ materially from those indicated by such forward - looking statements as a result of various important factors . Such forward - looking statements involve substantial risks and uncertainties that could cause the Company’s clinical development programs, future results, performance or achievements to differ significantly from those expressed or implied by the forward - looking statements . Such risks and uncertainties include, among others, the timing and costs involved in commercializing DEXTENZA, ReSure Sealant or any product candidate that receives regulatory approval, including the conduct of post - approval studies, the ability to retain regulatory approval of DEXTENZA, ReSure Sealant or any product candidate that receives regulatory approval, the ability to maintain reimbursement codes for DEXTENZA, the initiation, timing and conduct of clinical trials, availability of data from clinical trials and expectations for regulatory submissions and approvals, the Company’s scientific approach and general development progress, the availability or commercial potential of the Company’s product candidates, the Company’s ability to generate its projected net product revenue and unit sales on the timeline expected, if at all, the sufficiency of cash resources, the Company’s existing indebtedness, the ability of the Company’s creditors to accelerate the maturity of such indebtedness upon the occurrence of certain events of default, the outcome of the Company’s ongoing legal proceedings, the severity and duration of the COVID - 19 pandemic including its effect on the Company’s and relevant regulatory authorities’ operations, any additional financing needs or other actions and other factors discussed in the “Risk Factors” section contained in the Company’s quarterly and annual reports on file with the Securities and Exchange Commission . In addition, the forward - looking statements included in this presentation represent the Company’s views as of the date of this presentation . The Company anticipates that subsequent events and developments will cause the Company’s views to change . However, while the Company may elect to update these forward - looking statements at some point in the future, the Company specifically disclaims any obligation to do so except as required by law . These forward - looking statements should not be relied upon as representing the Company’s views as of any date subsequent to the date of this presentation . FORWARD LOOKING STATEMENTS 3
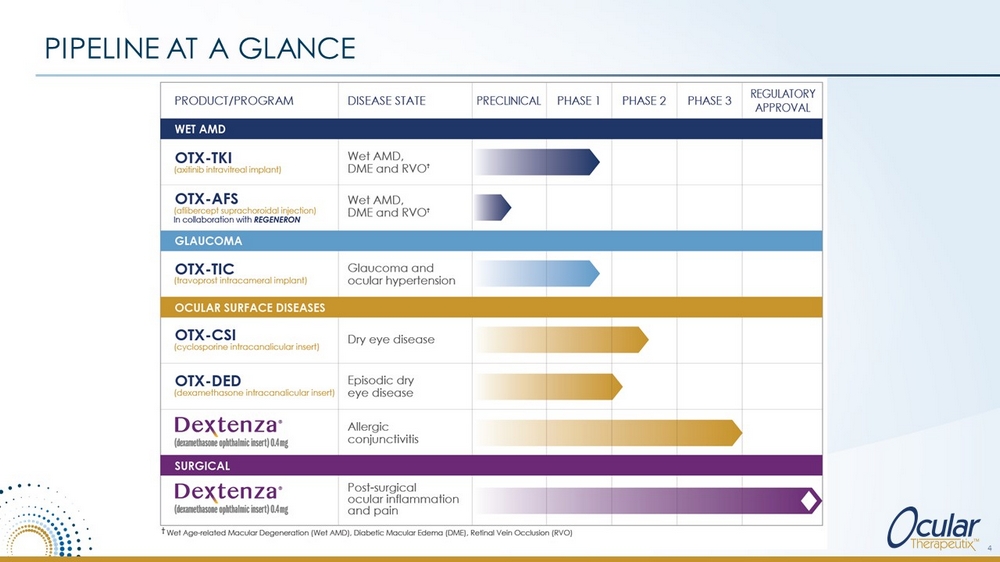
PIPELINE AT A GLANCE 4

Factors for Consideration in Designing a Long Duration Intracameral Implant: DRUG DELIVERY TO THE INTRACAMERAL SPACE □ Clinically - meaningful decrease in IOP Well - tolerated with clinically - meaningful efficacy □ Duration of therapy 4 months or more □ Bioresorbable Duration of drug and duration of carrier vehicle □ Implant location and movement Limited movement and cosmetically invisible, but able to be monitored □ Corneal health Gentle to the endothelium 5
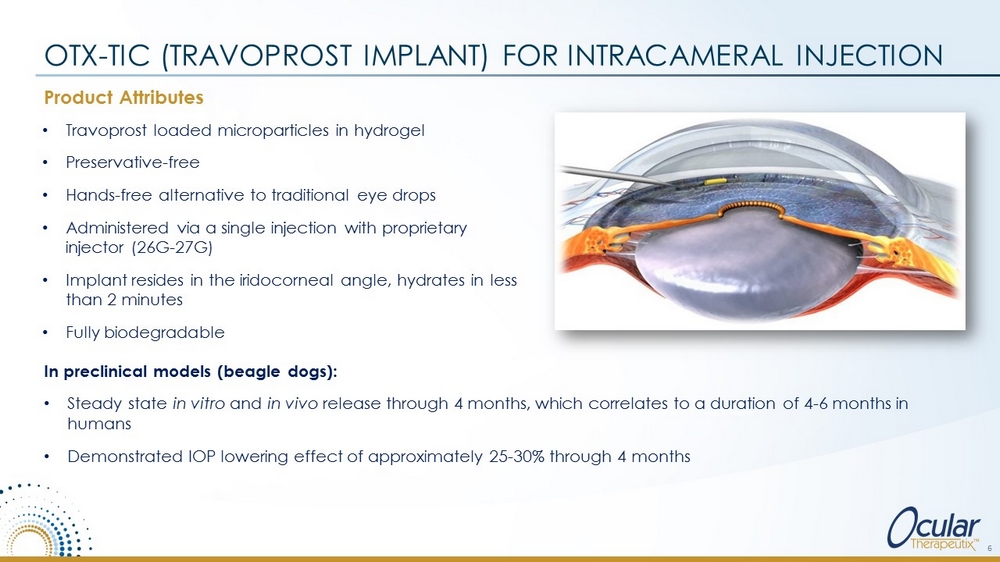
Product Attributes OTX - TIC (TRAVOPROST IMPLANT) FOR INTRACAMERAL INJECTION Video Courtesy Dr. Thomas R. Walters • Travoprost loaded microparticles in hydrogel • Preservative - free • Hands - free alternative to traditional eye drops • Administered via a single injection with proprietary injector (26G - 27G) • Implant resides in the iridocorneal angle, hydrates in less than 2 minutes • Fully biodegradable 6 In preclinical models (beagle dogs): • Steady state in vitro and in vivo release through 4 months, which correlates to a duration of 4 - 6 months in humans • Demonstrated IOP lowering effect of approximately 25 - 30% through 4 months
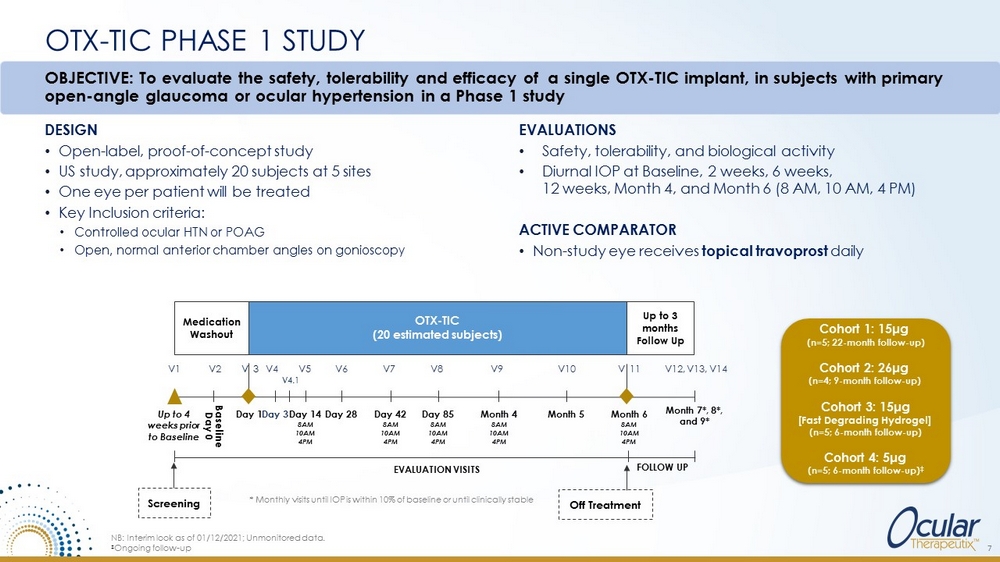
OTX - TIC PHASE 1 STUDY DESIGN • Open - label, proof - of - concept study • US study, approximately 20 subjects at 5 sites • One eye per patient will be treated • Key Inclusion criteria: • Controlled ocular HTN or POAG • Open, normal anterior chamber angles on gonioscopy EVALUATIONS • Safety, tolerability, and biological activity • Diurnal IOP at Baseline, 2 weeks, 6 weeks, 12 weeks, Month 4, and Month 6 (8 AM, 10 AM, 4 PM) ACTIVE COMPARATOR • Non - study eye receives topical travoprost daily * Monthly visits until IOP is within 10% of baseline or until clinically stable Up to 4 weeks prior to Baseline Day 1 EVALUATION VISITS FOLLOW UP Month 7*, 8*, and 9* Medication Washout Up to 3 months Follow Up OTX - TIC (20 estimated subjects) Screening Off Treatment Baseline Day 0 V1 V2 V 3 Day 3 V4 V 11 V12, V13, V14 Day 14 8AM 10AM 4PM V5 Day 42 8AM 10AM 4PM V7 Day 28 V6 Day 85 8AM 10AM 4PM V8 Month 6 8AM 10AM 4PM Month 4 8AM 10AM 4PM V9 Month 5 V10 V4.1 OBJECTIVE: To evaluate the safety, tolerability and efficacy of a single OTX - TIC implant, in subjects with primary open - angle glaucoma or ocular hypertension in a Phase 1 study NB: Interim look as of 01/12/2021; Unmonitored data. ‡ Ongoing follow - up 7 Cohort 1: 15 μ g (n=5; 22 - month follow - up) Cohort 2: 26 μ g (n=4; 9 - month follow - up) Cohort 3: 15 μ g [Fast Degrading Hydrogel] (n=5; 6 - month follow - up) Cohort 4: 5 μ g (n=5; 6 - month follow - up) ‡
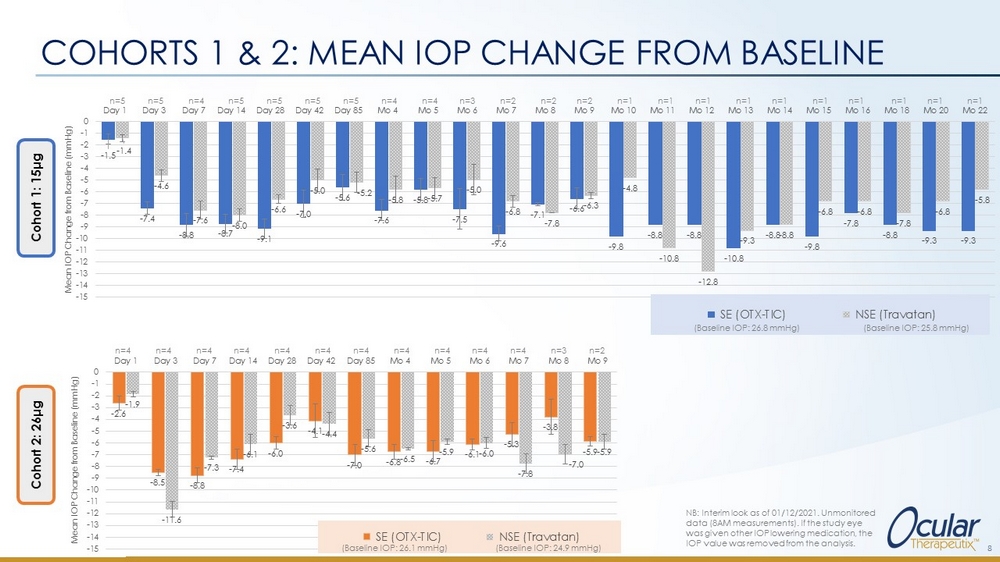
COHORTS 1 & 2: MEAN IOP CHANGE FROM BASELINE 8 NB: Interim look as of 01/12/2021. Unmonitored data (8AM measurements). If the study eye was given other IOP lowering medication, the IOP value was removed from the analysis. - 1.5 - 7.4 - 8.8 - 8.7 - 9.1 - 7.0 - 5.6 - 7.6 - 5.8 - 7.5 - 9.6 - 7.1 - 6.6 - 9.8 - 8.8 - 8.8 - 10.8 - 8.8 - 9.8 - 7.8 - 8.8 - 9.3 - 9.3 - 1.4 - 4.6 - 7.6 - 8.0 - 6.6 - 5.0 - 5.2 - 5.8 - 5.7 - 5.0 - 6.8 - 7.8 - 6.3 - 4.8 - 10.8 - 12.8 - 9.3 - 8.8 - 6.8 - 6.8 - 7.8 - 6.8 - 5.8 -15 -14 -13 -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Day 1 Day 3 Day 7 Day 14 Day 28 Day 42 Day 85 Mo 4 Mo 5 Mo 6 Mo 7 Mo 8 Mo 9 Mo 10 Mo 11 Mo 12 Mo 13 Mo 14 Mo 15 Mo 16 Mo 18 Mo 20 Mo 22 Mean IOP Change from Baseline (mmHg) SE (OTX-TIC) NSE (Travatan) n=5 n=5 n=4 n=5 n=5 n=5 n=5 n=4 n=4 n=3 n=2 n=2 n=2 n=1 n=1 n=1 n=1 n=1 n=1 n=1 n=1 n=1 n=1 (Baseline IOP: 26.8 mmHg) (Baseline IOP: 25.8 mmHg) - 2.6 - 8.5 - 8.8 - 7.4 - 6.0 - 4.1 - 7.0 - 6.8 - 6.7 - 6.1 - 5.3 - 3.8 - 5.9 - 1.9 - 11.6 - 7.3 - 6.1 - 3.6 - 4.4 - 5.6 - 6.5 - 5.9 - 6.0 - 7.8 - 7.0 - 5.9 -15 -14 -13 -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Day 1 Day 3 Day 7 Day 14 Day 28 Day 42 Day 85 Mo 4 Mo 5 Mo 6 Mo 7 Mo 8 Mo 9 Mean IOP Change from Baseline (mmHg) SE (OTX-TIC) NSE (Travatan) n=4 n=4 n=4 n=4 n=4 n=4 n=4 n=4 n=4 n=4 n=4 n=3 n=2 (Baseline IOP: 26.1 mmHg) (Baseline IOP: 24.9 mmHg) Cohort 1: 15 μ g Cohort 2: 26 μ g
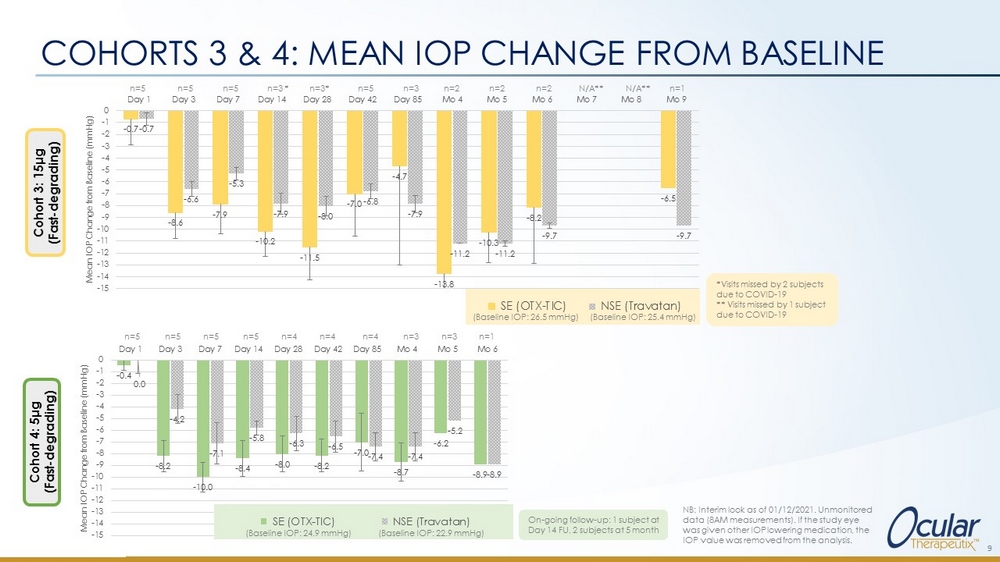
COHORTS 3 & 4: MEAN IOP CHANGE FROM BASELINE 9 NB: Interim look as of 01/12/2021. Unmonitored data (8AM measurements). If the study eye was given other IOP lowering medication, the IOP value was removed from the analysis. - 0.7 - 8.6 - 7.9 - 10.2 - 11.5 - 7.0 - 4.7 - 13.8 - 10.3 - 8.2 - 6.5 - 0.7 - 6.6 - 5.3 - 7.9 - 8.0 - 6.8 - 7.9 - 11.2 - 11.2 - 9.7 - 9.7 -15 -14 -13 -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Day 1 Day 3 Day 7 Day 14 Day 28 Day 42 Day 85 Mo 4 Mo 5 Mo 6 Mo 7 Mo 8 Mo 9 Mean IOP Change from Baseline (mmHg) SE (OTX-TIC) NSE (Travatan) n=5 n=5 n=5 n=3 * n=3* n= 5 n=3 n=2 n=2 n=2 N/A** N/A** n =1 (Baseline IOP: 26.5 mmHg) (Baseline IOP: 25.4 mmHg) - 0.4 - 8.2 - 10.0 - 8.4 - 8.0 - 8.2 - 7.0 - 8.7 - 6.2 - 8.9 0.0 - 4.2 - 7.1 - 5.8 - 6.3 - 6.5 - 7.4 - 7.4 - 5.2 - 8.9 -15 -14 -13 -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Day 1 Day 3 Day 7 Day 14 Day 28 Day 42 Day 85 Mo 4 Mo 5 Mo 6 Mean IOP Change from Baseline (mmHg) SE (OTX-TIC) NSE (Travatan) n=5 n=5 n=5 n=5 n=4 n=4 n=4 n=3 n=3 n=1 Cohort 3: 15 μ g (Fast - degrading) Cohort 4: 5 μ g (Fast - degrading) (Baseline IOP: 24.9 mmHg) (Baseline IOP: 22.9 mmHg) *Visits missed by 2 subjects due to COVID - 19 ** Visits missed by 1 subject due to COVID - 19 On - going follow - up: 1 subject at Day 14 FU, 2 subjects at 5 month
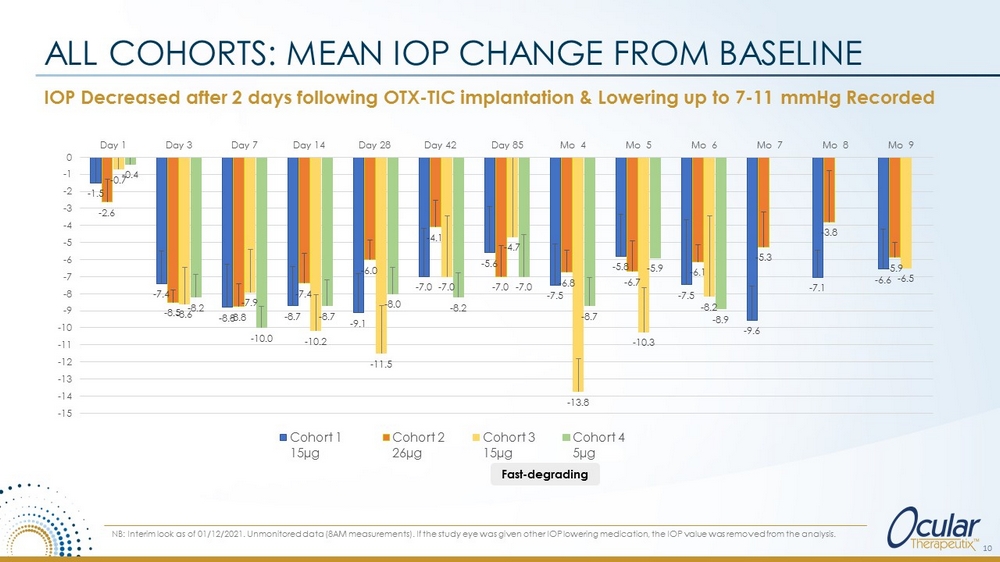
IOP Decreased after 2 days following OTX - TIC implantation & Lowering up to 7 - 11 mmHg Recorded ALL COHORTS: MEAN IOP CHANGE FROM BASELINE 10 NB: Interim look as of 01/12/2021. Unmonitored data (8AM measurements). If the study eye was given other IOP lowering medication, the IOP value was removed from the analysis. - 1.5 - 7.4 - 8.8 - 8.7 - 9.1 - 7.0 - 5.6 - 7.5 - 5.8 - 7.5 - 9.6 - 7.1 - 6.6 - 2.6 - 8.5 - 8.8 - 7.4 - 6.0 - 4.1 - 7.0 - 6.8 - 6.7 - 6.1 - 5.3 - 3.8 - 5.9 - 0.7 - 8.6 - 7.9 - 10.2 - 11.5 - 7.0 - 4.7 - 13.8 - 10.3 - 8.2 - 6.5 - 0.4 - 8.2 - 10.0 - 8.7 - 8.0 - 8.2 - 7.0 - 8.7 - 5.9 - 8.9 -15 -14 -13 -12 -11 -10 -9 -8 -7 -6 -5 -4 -3 -2 -1 0 Day 1 Day 3 Day 7 Day 14 Day 28 Day 42 Day 85 Mo 4 Mo 5 Mo 6 Mo 7 Mo 8 Mo 9 Cohort 1 15µg Cohort 2 26µg Cohort 3 15µg F2 Cohort 4 5µg F3 Fast - degrading
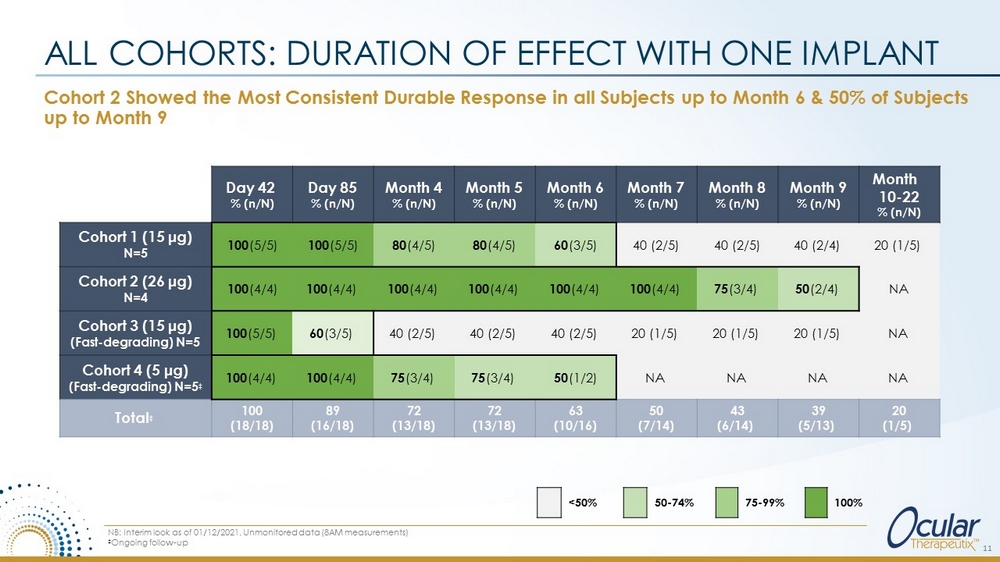
Cohort 2 Showed the Most Consistent Durable Response in all Subjects up to Month 6 & 50% of Subjects up to Month 9 ALL COHORTS: DURATION OF EFFECT WITH ONE IMPLANT 11 NB: Interim look as of 01/12/2021. Unmonitored data (8AM measurements) ‡ Ongoing follow - up Day 42 % (n/N) Day 85 % (n/N) Month 4 % (n/N) Month 5 % (n/N) Month 6 % (n/N) Month 7 % (n/N) Month 8 % (n/N) Month 9 % (n/N) Month 10 - 22 % (n/N) Cohort 1 (15 μg) N=5 100 (5/5) 100 (5/5) 80 (4/5) 80 (4/5) 60 (3/5) 40 (2/5) 40 (2/5) 40 (2/4) 20 (1/5) Cohort 2 (26 μg) N=4 100 (4/4) 100 (4/4) 100 (4/4) 100 (4/4) 100 (4/4) 100 (4/4) 75 (3/4) 50 (2/4) NA Cohort 3 (15 μ g) (Fast - degrading) N=5 100 (5/5) 60 (3/5) 40 (2/5) 40 (2/5) 40 (2/5) 20 (1/5) 20 (1/5) 20 (1/5) NA Cohort 4 (5 μ g) (Fast - degrading) N=5 ‡ 100 (4/4) 100 (4/4) 75 (3/4) 75 (3/4) 50 (1/2) NA NA NA NA Total ‡ 100 (18/18) 89 (16/18) 72 (13/18) 72 (13/18) 63 (10/16) 50 (7/14) 43 (6/14) 39 (5/13) 20 (1/5) <50% 50 - 74% 75 - 99% 100%
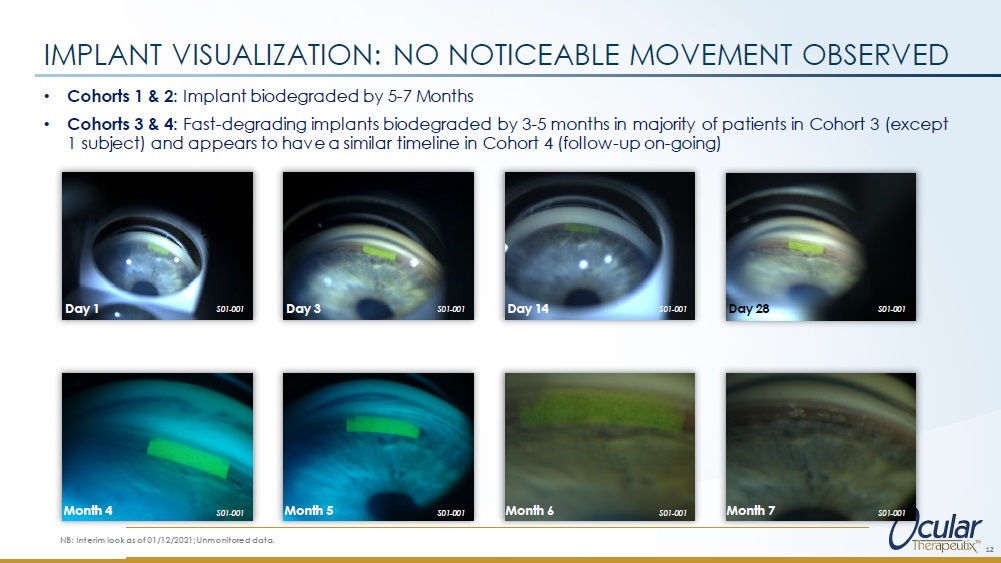
• Cohorts 1 & 2: Implant biodegraded by 5 - 7 Months • Cohorts 3 & 4: Fast - degrading implants biodegraded by 3 - 5 months in majority of patients in Cohort 3 (except 1 subject) and appears to have a similar timeline in Cohort 4 (follow - up on - going) IMPLANT VISUALIZATION: NO NOTICEABLE MOVEMENT OBSERVED Day 28 12 NB: Interim look as of 01/12/2021; Unmonitored data.
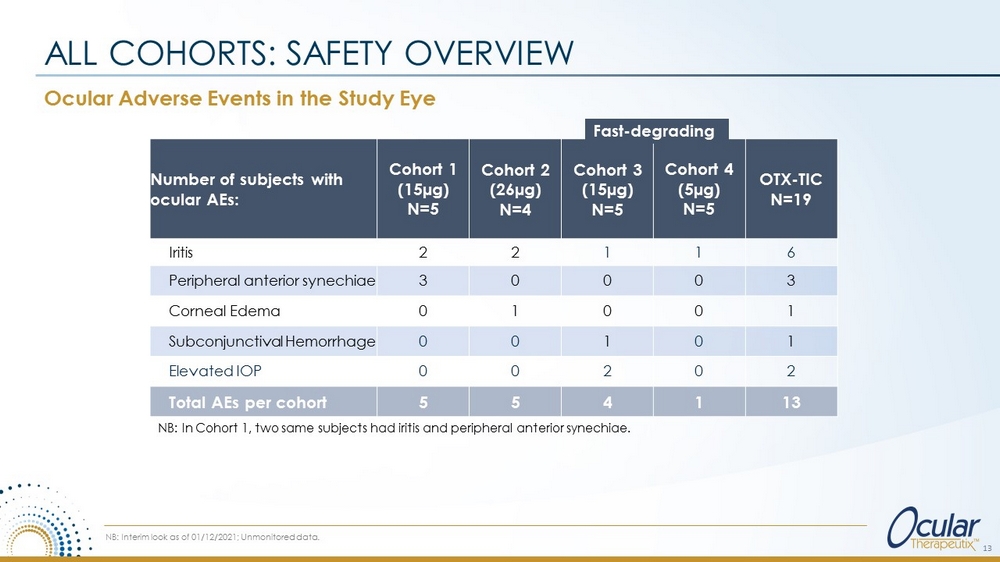
Ocular Adverse Events in the Study Eye ALL COHORTS: SAFETY OVERVIEW NB: In Cohort 1, two same subjects had iritis and peripheral anterior synechiae. NB: Interim look as of 01/12/2021; Unmonitored data. Number of subjects with ocular AEs: Cohort 1 (15µg) N=5 Cohort 2 (26µg) N=4 Cohort 3 (15µg) N=5 Cohort 4 (5µg) N=5 OTX - TIC N=19 Iritis 2 2 1 1 6 Peripheral anterior synechiae 3 0 0 0 3 Corneal Edema 0 1 0 0 1 Subconjunctival Hemorrhage 0 0 1 0 1 Elevated IOP 0 0 2 0 2 Total AEs per cohort 5 5 4 1 13 13 Fast - degrading
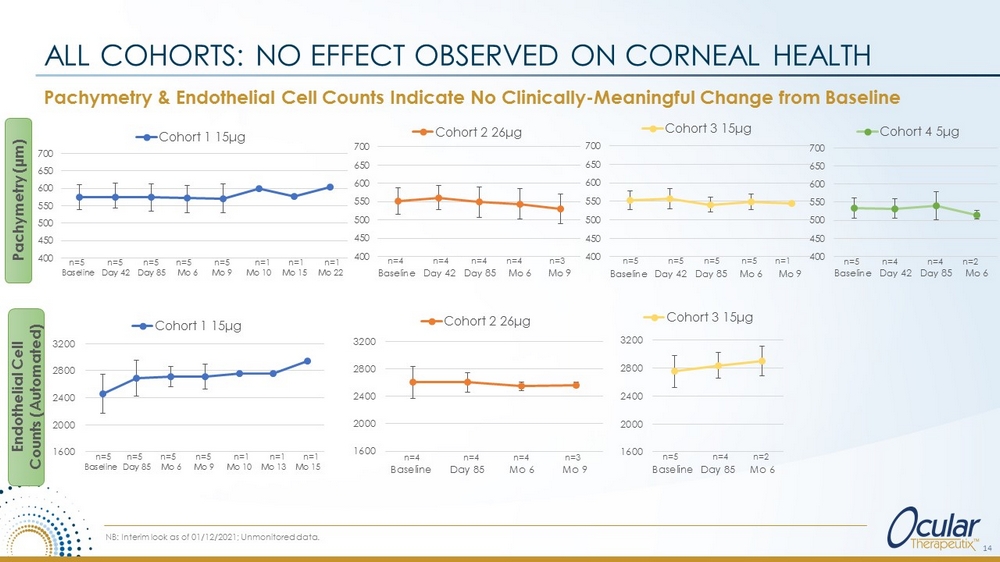
Pachymetry & Endothelial Cell Counts Indicate No Clinically - Meaningful Change from Baseline ALL COHORTS: NO EFFECT OBSERVED ON CORNEAL HEALTH Pachymetry (µm) Endothelial Cell Counts (Automated) 1600 2000 2400 2800 3200 Baseline Day 85 Mo 6 Mo 9 Mo 10 Mo 13 Mo 15 Cohort 1 15 μ g 1600 2000 2400 2800 3200 Baseline Day 85 Mo 6 Cohort 3 15 μ g 1600 2000 2400 2800 3200 Baseline Day 85 Mo 6 Mo 9 Cohort 2 26 μ g n=5 n=5 n= 5 n= 5 n=1 n=1 n=1 n=5 n=4 n=2 n=4 n=4 n=4 n=3 14 NB: Interim look as of 01/12/2021; Unmonitored data. 400 450 500 550 600 650 700 Baseline Day 42 Day 85 Mo 6 Mo 9 Mo 10 Mo 15 Mo 22 Cohort 1 15 μ g 400 450 500 550 600 650 700 Baseline Day 42 Day 85 Mo 6 Mo 9 Cohort 3 15 μ g 400 450 500 550 600 650 700 Baseline Day 42 Day 85 Mo 6 Mo 9 Cohort 2 26 μ g n=5 n=5 n= 5 n= 5 n= 5 n=1 n=1 n=1 n=5 n=5 n=5 n=5 n=1 n=4 n=4 n=4 n=4 n= 3 400 450 500 550 600 650 700 Baseline Day 42 Day 85 Mo 6 Cohort 4 5 μ g n=5 n=4 n=4 n=2

OTX - TIC shows Promise as a Sustained - Release Therapy with a Long Duration of Action CONCLUSIONS □ Clinically - meaningful decrease in IOP Mean IOP values were decreased in patients receiving both OTX - TIC as early as two days following administration, and mean IOP decrease was comparable to topical travoprost therapy □ Duration of therapy Many subjects exhibited duration of IOP - lowering effect of 6+ months in Cohorts 1 and 2, and between 3 - 6 months in Cohorts 3 and 4 (fast degrading implant) with a single implant: Longest and most consistent IOP lowering in Cohort 2 □ Bioresorbable Implant biodegraded in 5 - 7 months (Cohorts 1 & 2); Fast degrading implants biodegraded in 3 - 5 months (Cohorts 3 & 4) □ Implant location and movement Implant was not observed to move at slit lamp and was visible at all exams in all patients using gonioscopy □ Corneal health Endothelial cell counts, pachymetry assessments, and slit lamp examinations indicate no changes from baseline NB: Interim look as of 01/12/2021; Unmonitored data. NEXT STEPS: • Ongoing Study; Continued long - term evaluation in 3 subjects in Cohort 4 • Phase II Trial Planning Initiated; Planned start - up mid - year 2021 15

THANK YOU TRANSFORMING DRUG DELIVERY LEVERAGING A NOVEL TECHNOLOGY PLATFORM (NASDAQ: OCUL) 16
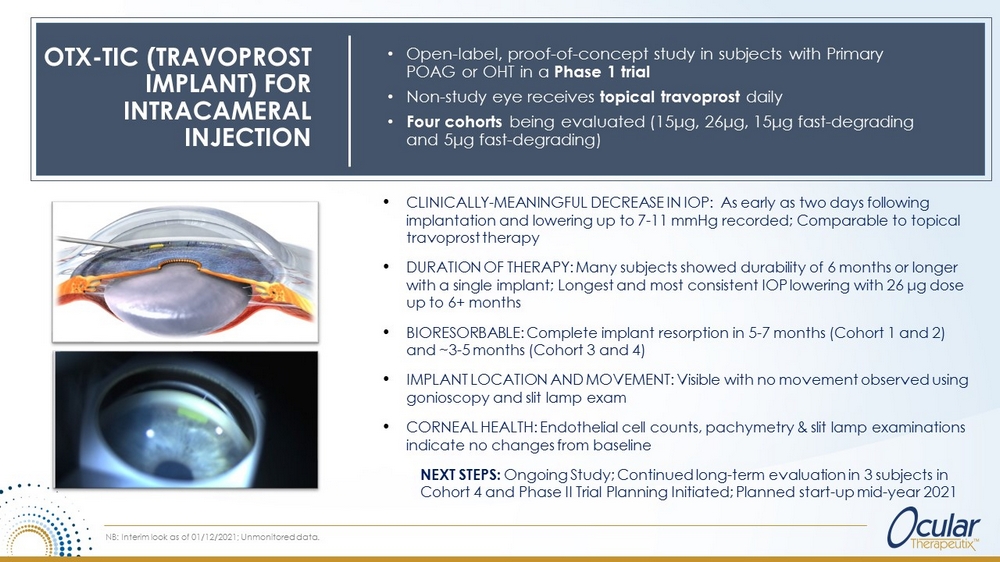
OTX - TIC (TRAVOPROST IMPLANT) FOR INTRACAMERAL INJECTION • Open - label, proof - of - concept study in subjects with Primary POAG or OHT in a Phase 1 trial • Non - study eye receives topical travoprost daily • Four cohorts being evaluated (15μg, 26μg, 15μg fast - degrading and 5μg fast - degrading) • CLINICALLY - MEANINGFUL DECREASE IN IOP: As early as two days following implantation and lowering up to 7 - 11 mmHg recorded; C omparable to topical travoprost therapy • DURATION OF THERAPY: Many subjects showed durability of 6 months or longer with a single implant; Longest and most consistent IOP lowering with 26 μ g dose up to 6+ months • BIORESORBABLE: Complete implant resorption in 5 - 7 months (Cohort 1 and 2) and ~3 - 5 months (Cohort 3 and 4) • IMPLANT LOCATION AND MOVEMENT: Visible with no movement observed using gonioscopy and slit lamp exam • CORNEAL HEALTH: Endothelial cell counts, pachymetry & slit lamp examinations indicate no changes from baseline NEXT STEPS: Ongoing Study; Continued long - term evaluation in 3 subjects in Cohort 4 and Phase II Trial Planning Initiated; Planned start - up mid - year 2021 NB: Interim look as of 01/12/2021; Unmonitored data.
