Attached files
| file | filename |
|---|---|
| 8-K - 8-K - iTeos Therapeutics, Inc. | d107242d8k.htm |

Exhibit 99.1 Pioneering Novel IO Therapies Focused on Key Mechanisms of Immunosuppression JANUARY 2021Exhibit 99.1 Pioneering Novel IO Therapies Focused on Key Mechanisms of Immunosuppression JANUARY 2021

Disclaimer This Presentation has been prepared by iTeos Therapeutics, Inc. (“we,” “us,” our “our”) and contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and future conditions. All statements, other than statements of historical facts, contained in this Presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates, including our clinical trials of Inupadenant (EOS-850), our clinical trials of EOS-448 and of our research and development programs; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; our ability to manufacture our product candidates, including Inupadenant and EOS-448, or any other product candidate in conformity with the Food and Drug Administration’s requirements and to scale up manufacturing of our product candidates to commercial scale, if approved; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; and our plans to develop and commercialize our current product candidates and any future product candidates and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. These statements are based on management’s current expectations and beliefs and are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: uncertainties inherent in clinical studies and the availability and timing of data from ongoing clinical studies; whether interim results from a clinical trial will be predictive of the final results of a trial; that the results from our clinical trials for Inupadenant and EOS-448 may not support further development and marketing approval; the risk that we may be unable to gain approval for our product candidates on a timely basis, if at all; the risk that the current COVID-19 pandemic will impact our clinical trials and operations; and other risks set forth under the caption ‘Risk Factors’ in our most recent Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, as filed with the SEC on November 12, 2020, and in our future filings with the SEC available at the SEC’s website at www.sec.gov. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. You should not place undue reliance on any forward-looking statements, which speak only as of the date they are made. Certain information contained in this Presentation and statements made orally during this Presentation relate to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party studies, publications, surveys and other data to be reliable as of the date of the Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent sources has evaluated the reasonableness or accuracy of the Company’s internal estimates or research and no reliance should be made on any information or statements made in this Presentation relating to or based on such internal estimates and research. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. 2Disclaimer This Presentation has been prepared by iTeos Therapeutics, Inc. (“we,” “us,” our “our”) and contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and future conditions. All statements, other than statements of historical facts, contained in this Presentation, including statements regarding our strategy, future financial condition, future operations, projected costs, prospects, plans, objectives of management and expected market growth, are forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as ‘‘aim,’’ ‘‘anticipate,’’ ‘‘assume,’’ ‘‘believe,’’ ‘‘contemplate,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘design,’’ ‘‘due,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘goal,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘objective,’’ ‘‘plan,’’ ‘‘predict,’’ ‘‘positioned,’’ ‘‘potential,’’ ‘‘seek,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include statements about the initiation, timing, progress and results of our current and future clinical trials and current and future preclinical studies of our product candidates, including our clinical trials of Inupadenant (EOS-850), our clinical trials of EOS-448 and of our research and development programs; our ability to successfully establish or maintain collaborations or strategic relationships for our product candidates; our ability to manufacture our product candidates, including Inupadenant and EOS-448, or any other product candidate in conformity with the Food and Drug Administration’s requirements and to scale up manufacturing of our product candidates to commercial scale, if approved; our financial performance; the effect of the COVID-19 pandemic, including mitigation efforts and economic effects, on any of the foregoing or other aspects of our business operations, including but not limited to our preclinical studies and future clinical trials; and our plans to develop and commercialize our current product candidates and any future product candidates and the implementation of our business model and strategic plans for our business, current product candidates and any future product candidates. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. These statements are based on management’s current expectations and beliefs and are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward-looking statements. Such risks and uncertainties include, among others: uncertainties inherent in clinical studies and the availability and timing of data from ongoing clinical studies; whether interim results from a clinical trial will be predictive of the final results of a trial; that the results from our clinical trials for Inupadenant and EOS-448 may not support further development and marketing approval; the risk that we may be unable to gain approval for our product candidates on a timely basis, if at all; the risk that the current COVID-19 pandemic will impact our clinical trials and operations; and other risks set forth under the caption ‘Risk Factors’ in our most recent Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, as filed with the SEC on November 12, 2020, and in our future filings with the SEC available at the SEC’s website at www.sec.gov. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. You should not place undue reliance on any forward-looking statements, which speak only as of the date they are made. Certain information contained in this Presentation and statements made orally during this Presentation relate to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party studies, publications, surveys and other data to be reliable as of the date of the Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, no independent sources has evaluated the reasonableness or accuracy of the Company’s internal estimates or research and no reliance should be made on any information or statements made in this Presentation relating to or based on such internal estimates and research. While we may elect to update these forward-looking statements at some point in the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe the expectations reflected in such forward-looking statements are reasonable, we can give no assurance that such expectations will prove to be correct. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. 2

iTeos Made Progress in 2020 Building the Foundation to Support the Evolution of our Pipeline • Growing track record in immuno-oncology drug discovery and development relying on our deep expertise in the biology of the tumor microenvironment • Inupadenant (EOS-850),an A receptor antagonist, and EOS-448, an IgG1 2A antibody directed against TIGIT being developed in multiple indications and combinations. • Both programs discovered internally with global rights retained by iTeos • Well capitalized with approximately $340MM of cash on the balance sheet as of September 30, 2020 • Have added key personnel to accelerate development activities. Significantly enhanced our research and drug development capabilities, particularly in clinical development, regulatory affairs and CMC in order to bring the next generation of immunotherapies to patients. 3iTeos Made Progress in 2020 Building the Foundation to Support the Evolution of our Pipeline • Growing track record in immuno-oncology drug discovery and development relying on our deep expertise in the biology of the tumor microenvironment • Inupadenant (EOS-850),an A receptor antagonist, and EOS-448, an IgG1 2A antibody directed against TIGIT being developed in multiple indications and combinations. • Both programs discovered internally with global rights retained by iTeos • Well capitalized with approximately $340MM of cash on the balance sheet as of September 30, 2020 • Have added key personnel to accelerate development activities. Significantly enhanced our research and drug development capabilities, particularly in clinical development, regulatory affairs and CMC in order to bring the next generation of immunotherapies to patients. 3

Pipeline of Promising Immuno-Oncology Product Candidates Program Trial Design Indications Preclinical Phase 1 Phase 1b/2a Phase 2/3 Initiation Data Adenosine A Receptor Antagonist 2A Expansion initiated Updated results 2Q Monotherapy Solid Tumors 2Q 2020 2021 Anti-PD-1-Resistant + pembrolizumab Initiated 3Q 2020 Melanoma Inupadenant Safety 2Q 2021 Castrate-Resistant + pembrolizumab Initiated 3Q 2020 Prostate Cancer + paclitaxel- Triple-Negative Initiated 4Q 2020 Safety 4Q 2021 carboplatin Breast Cancer Anti-TIGIT mAb Fc�� R-Engaging Presentation of initial Dose Finding, PK/PD Solid Tumors Initiated 1Q 2020 results 2Q 2021 + IMID Multiple Myeloma Initiation mid-2021 Mid 2022 EOS-448 + pembrolizumab Solid Tumors Initiation mid-2021 Mid 2022 + Inupadenant Solid Tumors Initiation mid-2021 Mid 2022 Preclinical Pipeline Candidate Oncology Adenosine pathway inhibitor selection 2021 4Pipeline of Promising Immuno-Oncology Product Candidates Program Trial Design Indications Preclinical Phase 1 Phase 1b/2a Phase 2/3 Initiation Data Adenosine A Receptor Antagonist 2A Expansion initiated Updated results 2Q Monotherapy Solid Tumors 2Q 2020 2021 Anti-PD-1-Resistant + pembrolizumab Initiated 3Q 2020 Melanoma Inupadenant Safety 2Q 2021 Castrate-Resistant + pembrolizumab Initiated 3Q 2020 Prostate Cancer + paclitaxel- Triple-Negative Initiated 4Q 2020 Safety 4Q 2021 carboplatin Breast Cancer Anti-TIGIT mAb Fc�� R-Engaging Presentation of initial Dose Finding, PK/PD Solid Tumors Initiated 1Q 2020 results 2Q 2021 + IMID Multiple Myeloma Initiation mid-2021 Mid 2022 EOS-448 + pembrolizumab Solid Tumors Initiation mid-2021 Mid 2022 + Inupadenant Solid Tumors Initiation mid-2021 Mid 2022 Preclinical Pipeline Candidate Oncology Adenosine pathway inhibitor selection 2021 4

Inupadenant Potentially Best-in-Class Adenosine Receptor Antagonist Phase 1/2 Program with Early Single Agent Activity 5Inupadenant Potentially Best-in-Class Adenosine Receptor Antagonist Phase 1/2 Program with Early Single Agent Activity 5
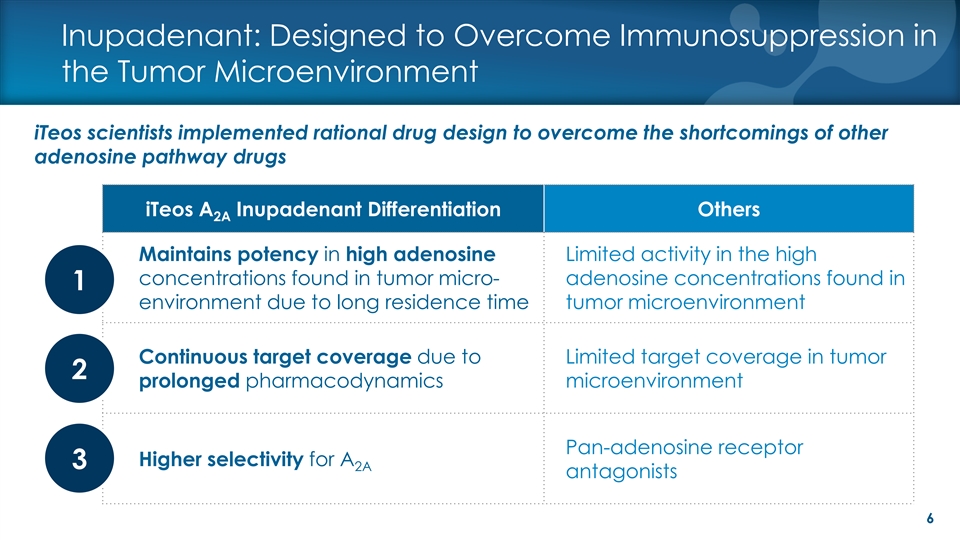
Inupadenant: Designed to Overcome Immunosuppression in the Tumor Microenvironment iTeos scientists implemented rational drug design to overcome the shortcomings of other adenosine pathway drugs iTeos A Inupadenant Differentiation Others 2A Maintains potency in high adenosine Limited activity in the high concentrations found in tumor micro- adenosine concentrations found in 1 environment due to long residence time tumor microenvironment Continuous target coverage due to Limited target coverage in tumor 2 prolonged pharmacodynamics microenvironment Pan-adenosine receptor Higher selectivity for A 3 2A antagonists 6Inupadenant: Designed to Overcome Immunosuppression in the Tumor Microenvironment iTeos scientists implemented rational drug design to overcome the shortcomings of other adenosine pathway drugs iTeos A Inupadenant Differentiation Others 2A Maintains potency in high adenosine Limited activity in the high concentrations found in tumor micro- adenosine concentrations found in 1 environment due to long residence time tumor microenvironment Continuous target coverage due to Limited target coverage in tumor 2 prolonged pharmacodynamics microenvironment Pan-adenosine receptor Higher selectivity for A 3 2A antagonists 6
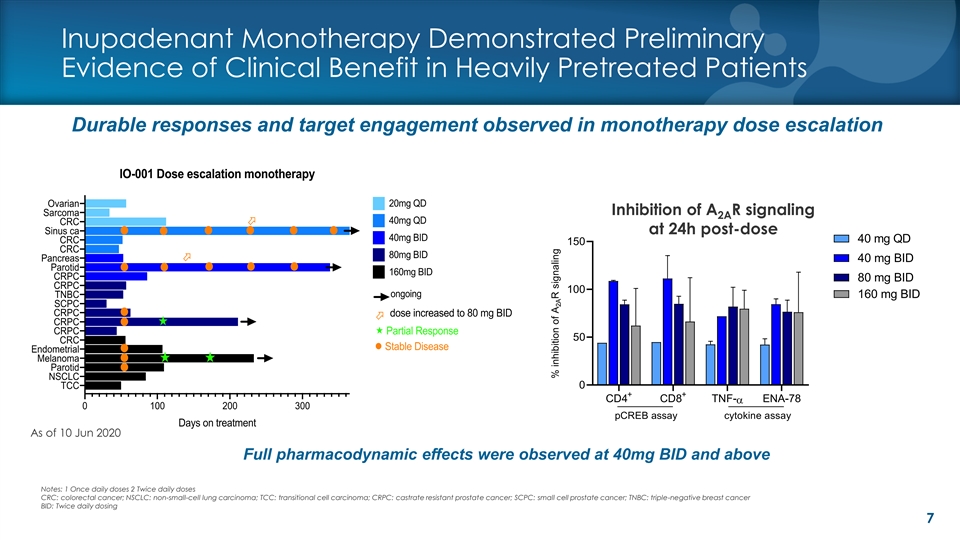
Inupadenant Monotherapy Demonstrated Preliminary Evidence of Clinical Benefit in Heavily Pretreated Patients Durable responses and target engagement observed in monotherapy dose escalation Inhibition of A R signaling 2A Test at 24h post-dose at 24h post-dose 40 mg QD 150 40 mg BID 80 mg BID 100 160 mg BID 50 0 + + CD4 CD8 TNF- ENA-78 a pCREB assay cytokine assay As of 10 Jun 2020 Full pharmacodynamic effects were observed at 40mg BID and above Notes: 1 Once daily doses 2 Twice daily doses CRC: colorectal cancer; NSCLC: non-small-cell lung carcinoma; TCC: transitional cell carcinoma; CRPC: castrate resistant prostate cancer; SCPC: small cell prostate cancer; TNBC: triple-negative breast cancer BID: Twice daily dosing 7 % inhibition of A R signaling 2AInupadenant Monotherapy Demonstrated Preliminary Evidence of Clinical Benefit in Heavily Pretreated Patients Durable responses and target engagement observed in monotherapy dose escalation Inhibition of A R signaling 2A Test at 24h post-dose at 24h post-dose 40 mg QD 150 40 mg BID 80 mg BID 100 160 mg BID 50 0 + + CD4 CD8 TNF- ENA-78 a pCREB assay cytokine assay As of 10 Jun 2020 Full pharmacodynamic effects were observed at 40mg BID and above Notes: 1 Once daily doses 2 Twice daily doses CRC: colorectal cancer; NSCLC: non-small-cell lung carcinoma; TCC: transitional cell carcinoma; CRPC: castrate resistant prostate cancer; SCPC: small cell prostate cancer; TNBC: triple-negative breast cancer BID: Twice daily dosing 7 % inhibition of A R signaling 2A

Inupadenant Treatment Results: Confirmed PRs with Substantial Tumor Reduction CHECKPOINT INHIBITOR-REFRACTORY METASTATIC MELANOMA: HEAVILY PRE-TREATED mCRPC: è 44% tumor reductionè 49% tumor reduction è Patient reported decreased pain & improved mobilityè Patient reported decreased bone pain è Single-agent activity observed è Single-agent activity observed Prior Treatments: Inupadenant Treatment History: Prior Treatments: Inupadenant Treatment History: Heavily pre-treated with Stable disease at 7 weeks Heavily pre-treated with 5 Stable disease at 8 weeks multiple CPIs• 26% tumor reduction previous rounds of therapy PR at 16 weeks • 2 previous cycles of • Prior treatments include PR at 16 weeks• 40% tumor reduction pembro antiandrogen therapy • 44% tumor reduction Confirmed PR at 30 weeks • 1 previous cycle of ipi and 2 lines of • 49% tumor reduction chemotherapy Confirmed PR at 24 weeks Baseline Week 16 Baseline Follow-up 1 Follow-up 2 Follow-up 3 10/25/2019 01/02/2020 02/27/2020 05/25/2020 Target Lesions Posterior R arm Posterior R arm TO1 Lymph node axillary right Lymph node axillary right TO2 Lymph node para-aortic right Lymph node para-aortic Anterior R arm Anterior R arm right TO3 Adrenal gland right Adrenal gland right 8Inupadenant Treatment Results: Confirmed PRs with Substantial Tumor Reduction CHECKPOINT INHIBITOR-REFRACTORY METASTATIC MELANOMA: HEAVILY PRE-TREATED mCRPC: è 44% tumor reductionè 49% tumor reduction è Patient reported decreased pain & improved mobilityè Patient reported decreased bone pain è Single-agent activity observed è Single-agent activity observed Prior Treatments: Inupadenant Treatment History: Prior Treatments: Inupadenant Treatment History: Heavily pre-treated with Stable disease at 7 weeks Heavily pre-treated with 5 Stable disease at 8 weeks multiple CPIs• 26% tumor reduction previous rounds of therapy PR at 16 weeks • 2 previous cycles of • Prior treatments include PR at 16 weeks• 40% tumor reduction pembro antiandrogen therapy • 44% tumor reduction Confirmed PR at 30 weeks • 1 previous cycle of ipi and 2 lines of • 49% tumor reduction chemotherapy Confirmed PR at 24 weeks Baseline Week 16 Baseline Follow-up 1 Follow-up 2 Follow-up 3 10/25/2019 01/02/2020 02/27/2020 05/25/2020 Target Lesions Posterior R arm Posterior R arm TO1 Lymph node axillary right Lymph node axillary right TO2 Lymph node para-aortic right Lymph node para-aortic Anterior R arm Anterior R arm right TO3 Adrenal gland right Adrenal gland right 8

Inupadenant Phase 1/2 Clinical Plan: Rapidly Expanding in Several Tumor Types in Multiple Combinations Single Safety and PK/PD Signal-Seeking Agent Expansion Cohorts 2-Stage Expansions Inupadenant Single-agent Generation of additional data to Melanoma, CRPC, analyze the MoA and the specific CRPC (n= up to 27) Endometrial, NSCLC, (n=24) CRPC population for inupadenant Dose Escalation w/matched tumor biopsies (Completed) Large market potential - Prostate tissue CRPC (n= up to 48) Advanced solid contains a non-canonical source of Inupadenant + Pembro adenosine production tumor patients Solid Tumors (n=10) (n=21) Anti-PD-1-Resistant Potential for Proof of Concept in PD-1 melanoma (n= up to 33) resistant patients Biomarker-rich study w/matched tumor Chemotherapy leads to biopsies immunogenic cell death and Inupadenant + Chemo 1L TNBC (n= up to 38) promotes necrosis and hypoxia that TNBC (n=6) lead to adenosine production - CD73 expression is associated with a poor prognosis and reduced anti- Initial results reported at Initial results of expansion tumor immunity AACR 2020 cohorts in 2Q21 TNBC: Triple Negative Breast Cancer CRPC: Castration Resistant Prostate Cancer 9 NSCLC: Non-small Cell Lung CancerInupadenant Phase 1/2 Clinical Plan: Rapidly Expanding in Several Tumor Types in Multiple Combinations Single Safety and PK/PD Signal-Seeking Agent Expansion Cohorts 2-Stage Expansions Inupadenant Single-agent Generation of additional data to Melanoma, CRPC, analyze the MoA and the specific CRPC (n= up to 27) Endometrial, NSCLC, (n=24) CRPC population for inupadenant Dose Escalation w/matched tumor biopsies (Completed) Large market potential - Prostate tissue CRPC (n= up to 48) Advanced solid contains a non-canonical source of Inupadenant + Pembro adenosine production tumor patients Solid Tumors (n=10) (n=21) Anti-PD-1-Resistant Potential for Proof of Concept in PD-1 melanoma (n= up to 33) resistant patients Biomarker-rich study w/matched tumor Chemotherapy leads to biopsies immunogenic cell death and Inupadenant + Chemo 1L TNBC (n= up to 38) promotes necrosis and hypoxia that TNBC (n=6) lead to adenosine production - CD73 expression is associated with a poor prognosis and reduced anti- Initial results reported at Initial results of expansion tumor immunity AACR 2020 cohorts in 2Q21 TNBC: Triple Negative Breast Cancer CRPC: Castration Resistant Prostate Cancer 9 NSCLC: Non-small Cell Lung Cancer

EOS-448 Fc�� R-engaging Anti-TIGIT Antibody Currently in Dose Escalation Phase 1/2 Trial 10EOS-448 Fc�� R-engaging Anti-TIGIT Antibody Currently in Dose Escalation Phase 1/2 Trial 10

EOS-448 is Designed to Enhance Anti-Tumor Immune Response Through T Cell Activation & FcgR Engagement Multiple programs have demonstrated that IgG1 antibodies are well tolerated at effective doses 3 Mechanisms of Action: Inhibition of TIGIT triggering activation Depletion of immunosuppressive Reverse activation of myeloid cells LOW HIGH of TIGIT T cells and NK cells Treg and exhausted TIGIT T cells via FcγR engagement Killing of tumor cells by Depletion of Treg and Further activation of anti-tumoral T and NK cells: exhausted T cells: response: CD112 FCgR EOS-448 Dendritic NK cell cell TIGIT- TIGIT- Tumor NK or T expressing expressing EOS-448 EOS-448 cell cell cell cell CD226 Macro- Macro- phage CD155 phage Effector T cells or NK cells with low FCgR levels of TIGIT Treg or exhausted T cell expressing high levels of TIGIT IgG1 isotype ✓✓✓ Silent isotype ✕ ✓✕ 11EOS-448 is Designed to Enhance Anti-Tumor Immune Response Through T Cell Activation & FcgR Engagement Multiple programs have demonstrated that IgG1 antibodies are well tolerated at effective doses 3 Mechanisms of Action: Inhibition of TIGIT triggering activation Depletion of immunosuppressive Reverse activation of myeloid cells LOW HIGH of TIGIT T cells and NK cells Treg and exhausted TIGIT T cells via FcγR engagement Killing of tumor cells by Depletion of Treg and Further activation of anti-tumoral T and NK cells: exhausted T cells: response: CD112 FCgR EOS-448 Dendritic NK cell cell TIGIT- TIGIT- Tumor NK or T expressing expressing EOS-448 EOS-448 cell cell cell cell CD226 Macro- Macro- phage CD155 phage Effector T cells or NK cells with low FCgR levels of TIGIT Treg or exhausted T cell expressing high levels of TIGIT IgG1 isotype ✓✓✓ Silent isotype ✕ ✓✕ 11

EOS-448’s Ability to Block TIGIT is Associated with Superior Immune Activation EOS-448 is associated with enhanced IL-2 EOS-448 blocks binding of TIGIT to CD155 mediated gene expression EOS884448 EOS-448 4000 Mereo Mereo Genentech 3000 Genentech BMS Merck 2000 Merck BMS Arcus 1000 Arcus 0 -6 -4 -2 0 2 4 Log inhibitor concentration (nM) Log Inhibitor concentration (nM) Differentiated ability to block TIGIT binding Evidence of differentiated potency Mereo = 313M32 from US2016/0376365 A1; Genentech = 4.1D3 from WO2017/053748 A2; BMS = 22G2 from US2016/0176963 A1; Merck = Clone 31C6 from WO2016/028656vA1; Arcus = TIG1 from WO2017/152088 A1 12 Mean Fluorescence Intensity Mean Fluorescence Intensity Mean Fluorescence Intensity CD155 binding to TIGIT CD155 binding to Jurkat hTIGIT CD155 binding to TIGITEOS-448’s Ability to Block TIGIT is Associated with Superior Immune Activation EOS-448 is associated with enhanced IL-2 EOS-448 blocks binding of TIGIT to CD155 mediated gene expression EOS884448 EOS-448 4000 Mereo Mereo Genentech 3000 Genentech BMS Merck 2000 Merck BMS Arcus 1000 Arcus 0 -6 -4 -2 0 2 4 Log inhibitor concentration (nM) Log Inhibitor concentration (nM) Differentiated ability to block TIGIT binding Evidence of differentiated potency Mereo = 313M32 from US2016/0376365 A1; Genentech = 4.1D3 from WO2017/053748 A2; BMS = 22G2 from US2016/0176963 A1; Merck = Clone 31C6 from WO2016/028656vA1; Arcus = TIG1 from WO2017/152088 A1 12 Mean Fluorescence Intensity Mean Fluorescence Intensity Mean Fluorescence Intensity CD155 binding to TIGIT CD155 binding to Jurkat hTIGIT CD155 binding to TIGIT
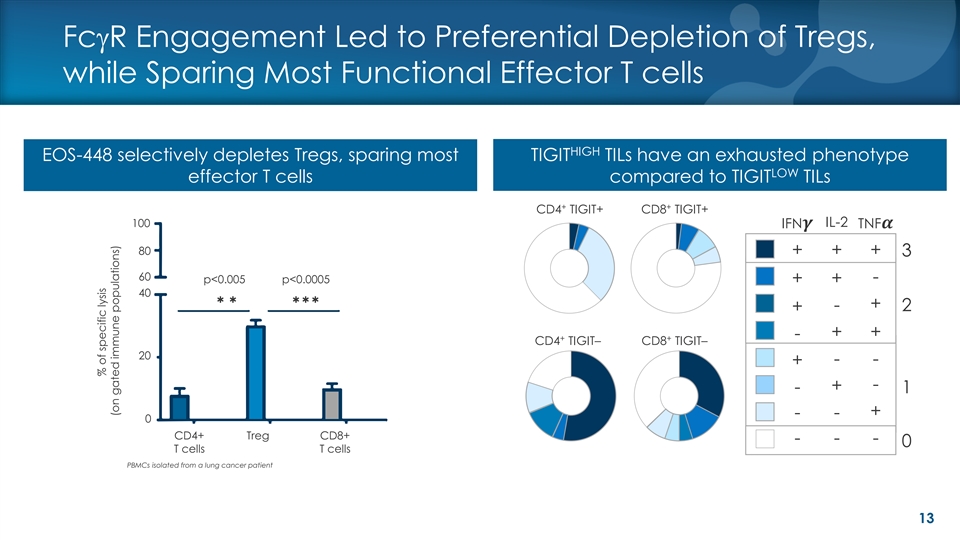
FcgR Engagement Led to Preferential Depletion of Tregs, while Sparing Most Functional Effector T cells HIGH EOS-448 selectively depletes Tregs, sparing most TIGIT TILs have an exhausted phenotype LOW effector T cells compared to TIGIT TILs + + CD4 TIGIT+ CD8 TIGIT+ 100 IL-2 IFN�� TNF�� Fill 80 + + + le 3 ve l 1 60 p<0.005 p<0.0005 - + + 40 ÞÞÞÞÞ + - 2 + + + - + + CD4 TIGIT– CD8 TIGIT– 20 - - + - + - 1 + - - 0 CD4+ Treg CD8+ - - - 0 T cells T cells PBMCs isolated from a lung cancer patient 13 % of specific lysis (on gated immune populations)FcgR Engagement Led to Preferential Depletion of Tregs, while Sparing Most Functional Effector T cells HIGH EOS-448 selectively depletes Tregs, sparing most TIGIT TILs have an exhausted phenotype LOW effector T cells compared to TIGIT TILs + + CD4 TIGIT+ CD8 TIGIT+ 100 IL-2 IFN�� TNF�� Fill 80 + + + le 3 ve l 1 60 p<0.005 p<0.0005 - + + 40 ÞÞÞÞÞ + - 2 + + + - + + CD4 TIGIT– CD8 TIGIT– 20 - - + - + - 1 + - - 0 CD4+ Treg CD8+ - - - 0 T cells T cells PBMCs isolated from a lung cancer patient 13 % of specific lysis (on gated immune populations)

EOS-448 Initial Clinical Plan: Biologically Driven with a Focus on Addressing Unmet Medical Needs Single Combination Agent POC Trials Rationale • Strong biological rationale • TIGIT upregulated on CD8+ T cells during progression EOS-448 + IMID • In vivo model suggests that TIGIT expression in post-transplant Multiple Myeloma Dose Escalation setting is associated with exhausted T cells and shows benefit of (Ongoing) IMID combination Advanced solid • High TIGIT expression observed in Tumor-infiltrating lymphocytes – EOS-448 + tumor patients frequently co-expressed with PD-1 pembrolizumab • Strong external validation by successful Ph II trials of αTIGIT/PD(L)-1 (n=30) Solid Tumors combo in NSCLC Biomarker-rich study w/matched tumor • Complementary mechanisms of immunosuppression biopsies EOS-448 + Inupadenant • Targeting multiple immune cells in the tumor micro-environment Solid Tumors • Additive benefit observed in animal models Anticipate reporting Anticipate commencement in 1H2021 in mid-2021 TNBC: Triple Negative Breast Cancer TNBC: Triple Negative Breast Cancer CRPC: Castration Resistant Prostate Cancer CRPC: Castration Resistant Prostate Cancer 14 NSCLC: Non-small Cell Lung Cancer NSCLC: Non-small Cell Lung CancerEOS-448 Initial Clinical Plan: Biologically Driven with a Focus on Addressing Unmet Medical Needs Single Combination Agent POC Trials Rationale • Strong biological rationale • TIGIT upregulated on CD8+ T cells during progression EOS-448 + IMID • In vivo model suggests that TIGIT expression in post-transplant Multiple Myeloma Dose Escalation setting is associated with exhausted T cells and shows benefit of (Ongoing) IMID combination Advanced solid • High TIGIT expression observed in Tumor-infiltrating lymphocytes – EOS-448 + tumor patients frequently co-expressed with PD-1 pembrolizumab • Strong external validation by successful Ph II trials of αTIGIT/PD(L)-1 (n=30) Solid Tumors combo in NSCLC Biomarker-rich study w/matched tumor • Complementary mechanisms of immunosuppression biopsies EOS-448 + Inupadenant • Targeting multiple immune cells in the tumor micro-environment Solid Tumors • Additive benefit observed in animal models Anticipate reporting Anticipate commencement in 1H2021 in mid-2021 TNBC: Triple Negative Breast Cancer TNBC: Triple Negative Breast Cancer CRPC: Castration Resistant Prostate Cancer CRPC: Castration Resistant Prostate Cancer 14 NSCLC: Non-small Cell Lung Cancer NSCLC: Non-small Cell Lung Cancer

iTeos has Built the Foundation to Support Transformative Acceleration in 2021 Company well capitalized to fund aggressive growth in preclinical and clinical operations Significant data updates on both clinical programs in Q2 2021 Continue to progress Inupadenant ongoing monotherapy and combination studies in multiple solid tumor types. Advance EOS-448 into combination studies in both solid and liquid tumor types rd Select lead for 3 internally-discovered IO program to advance into clinical trials and continue to advance discovery engine 15iTeos has Built the Foundation to Support Transformative Acceleration in 2021 Company well capitalized to fund aggressive growth in preclinical and clinical operations Significant data updates on both clinical programs in Q2 2021 Continue to progress Inupadenant ongoing monotherapy and combination studies in multiple solid tumor types. Advance EOS-448 into combination studies in both solid and liquid tumor types rd Select lead for 3 internally-discovered IO program to advance into clinical trials and continue to advance discovery engine 15

Pioneering Novel IO Therapies Focused on Key Mechanisms of Immunosuppression JANUARY 2021Pioneering Novel IO Therapies Focused on Key Mechanisms of Immunosuppression JANUARY 2021
