Attached files
| file | filename |
|---|---|
| 8-K - 8-K - YUMANITY THERAPEUTICS, INC. | d92637d8k.htm |

Revolutionizing the Treatment of Neurodegenerative Disease January 2021 NASDAQ: YMTX Exhibit 99.1

Forward-Looking Statements This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding: future product development plans and projected timelines for the initiation and completion of preclinical and clinical trials and other activities; the potential for the results of ongoing preclinical or clinical trials and the efficacy of Yumanity’s product candidates; and future product development and regulatory strategies, including with respect to specific indications. The use of words such as, but not limited to, “believe,” “expect,” “estimate,” “project,” “intend,” “future,” “potential,” “continue,” “may,” “might,” “plan,” “will,” “should,” “seek,” “anticipate,” or “could” and other similar words or expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on Yumanity’s current beliefs, expectations and assumptions regarding the future of Yumanity’s business, future plans and strategies, clinical results and other future conditions. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. Such forward-looking statements are subject to a number of material risks and uncertainties including but not limited to those risks detailed in the definitive proxy statement/prospectus/information statement for Yumanity’s merger with Proteostasis Therapeutics filed with the Securities and Exchange Commission on November 12, 2020, as well as discussions of potential risks, uncertainties, and other important factors in Yumanity’s subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. None of Yumanity, nor its affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as result of new information, future events or otherwise, except as required by law.
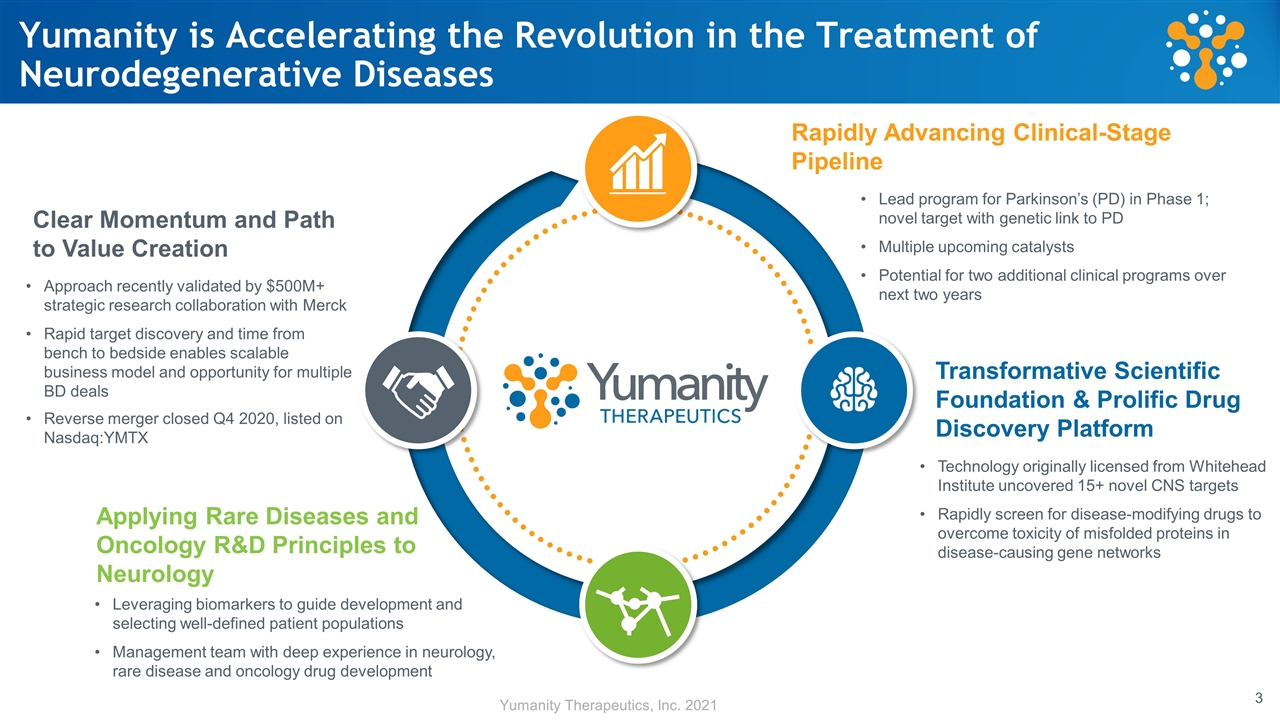
Yumanity is Accelerating the Revolution in the Treatment of Neurodegenerative Diseases Rapidly Advancing Clinical-Stage Pipeline Clear Momentum and Path to Value Creation Lead program for Parkinson’s (PD) in Phase 1; novel target with genetic link to PD Multiple upcoming catalysts Potential for two additional clinical programs over next two years Approach recently validated by $500M+ strategic research collaboration with Merck Rapid target discovery and time from bench to bedside enables scalable business model and opportunity for multiple BD deals Reverse merger closed Q4 2020, listed on Nasdaq:YMTX Transformative Scientific Foundation & Prolific Drug Discovery Platform Technology originally licensed from Whitehead Institute uncovered 15+ novel CNS targets Rapidly screen for disease-modifying drugs to overcome toxicity of misfolded proteins in disease-causing gene networks Applying Rare Diseases and Oncology R&D Principles to Neurology Leveraging biomarkers to guide development and selecting well-defined patient populations Management team with deep experience in neurology, rare disease and oncology drug development

ROBERT SCANNEVIN, PhD HEAD OF RESEARCH Previously Neuroscience Discovery, Biogen; J&J, Wyeth PhD SUNY Stony Brook, Post-doctoral Johns Hopkins School of Medicine PAULASH MOHSEN CHIEF BUSINESS OFFICER Previously Country Manager, Cubist; VP Strategy Pfizer, McKinsey MBA Harvard, MS MIT, BS Brown University RICHARD PETERS, MD, PhD CHIEF EXECUTIVE OFFICER Previously CEO of Merrimack; Global Head of Rare Diseases at Genzyme Post-grad training at MGH and HHMI Fellowship in biophysics at Harvard Proven Leadership With Established Track Record TONY COLES, MD EXECUTIVE CHAIRMAN CEO, Cerevel, Previously Chairman & CEO, Onyx Pharmaceuticals MD Duke, MPH Harvard, BS Johns Hopkins SUSAN LINDQUIST, PhD (in memoriam) FORMER DIRECTOR, WHITEHEAD INSTITUTE HHMI Investigator; Professor of Biology MIT National Medal of Science, Board of Directors J&J Distinguished Founders Experienced Management Team MICHAEL WYZGA SENIOR FINANCIAL ADVISOR Previously EVP Finance and CFO, Genzyme Corporation Chair X4 Pharmaceuticals, GenSight; Exact Sciences BRIGITTE ROBERTSON, MD CHIEF MEDICAL OFFICER Previously TA Head of Neuroscience at Shire/Takeda, GSK CEDD, Sunovion Neuropsychiatrist UCH, NIH Post-doctoral Fellowship Neuroimaging, UCSD ELLEN FOREST CHIEF HUMAN RESOURCES OFFICER Previously SVP and Head of Human Capital, Cogen Immune Medicine Previously VP and Head of Human Resources, Merrimack
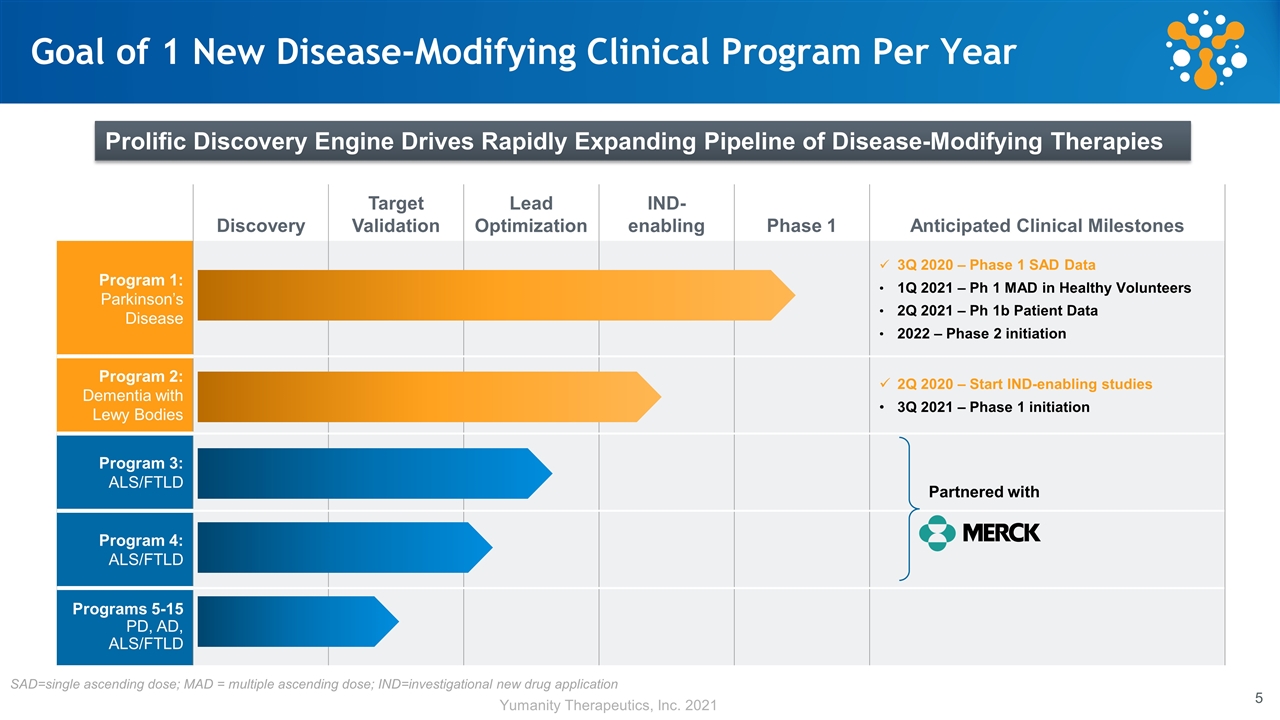
Discovery Target Validation Lead Optimization IND- enabling Phase 1 Anticipated Clinical Milestones Program 1: Parkinson’s Disease 3Q 2020 – Phase 1 SAD Data 1Q 2021 – Ph 1 MAD in Healthy Volunteers 2Q 2021 – Ph 1b Patient Data 2022 – Phase 2 initiation Program 2: Dementia with Lewy Bodies 2Q 2020 – Start IND-enabling studies 3Q 2021 – Phase 1 initiation Program 3: ALS/FTLD Program 4: ALS/FTLD Programs 5-15 PD, AD, ALS/FTLD Goal of 1 New Disease-Modifying Clinical Program Per Year Partnered with Prolific Discovery Engine Drives Rapidly Expanding Pipeline of Disease-Modifying Therapies SAD=single ascending dose; MAD = multiple ascending dose; IND=investigational new drug application

Drugging Genetic Risk Factor Networks to Modify the Course of Parkinson’s Disease
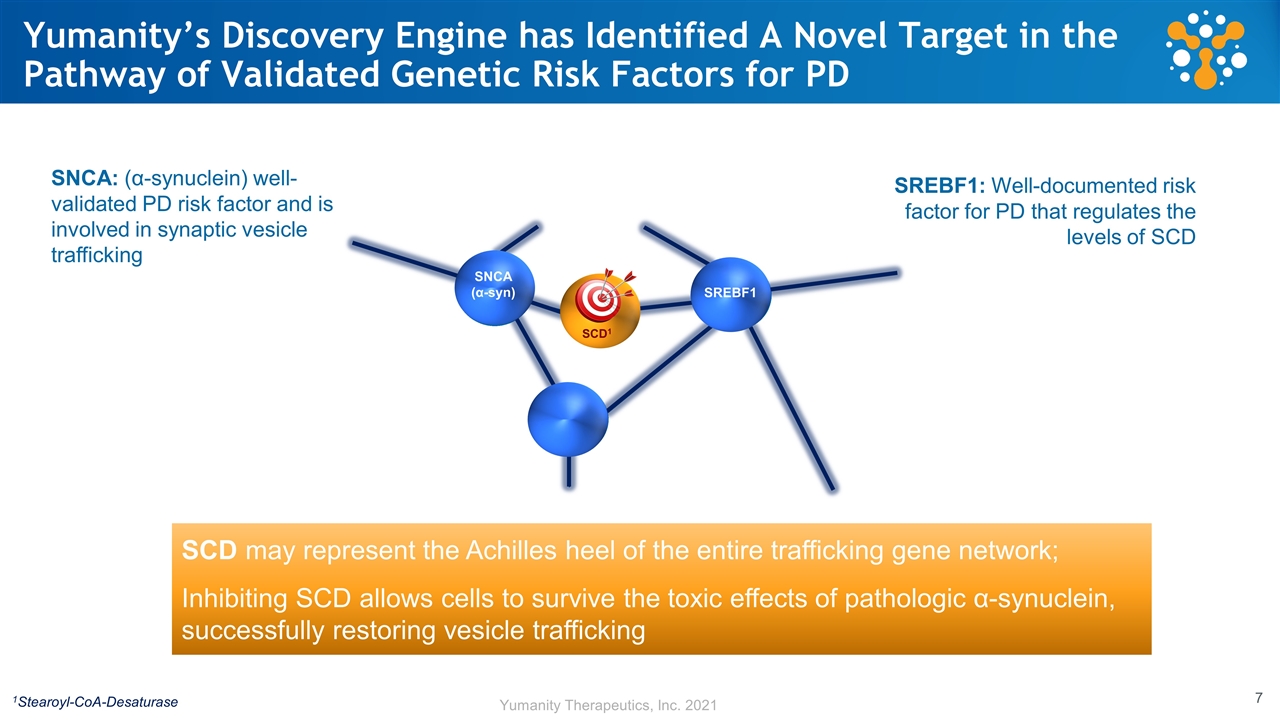
Yumanity’s Discovery Engine has Identified A Novel Target in the Pathway of Validated Genetic Risk Factors for PD SREBF1 SCD1 SNCA (α-syn) 1Stearoyl-CoA-Desaturase SNCA: (α-synuclein) well-validated PD risk factor and is involved in synaptic vesicle trafficking SREBF1: Well-documented risk factor for PD that regulates the levels of SCD SCD may represent the Achilles heel of the entire trafficking gene network; Inhibiting SCD allows cells to survive the toxic effects of pathologic α-synuclein, successfully restoring vesicle trafficking
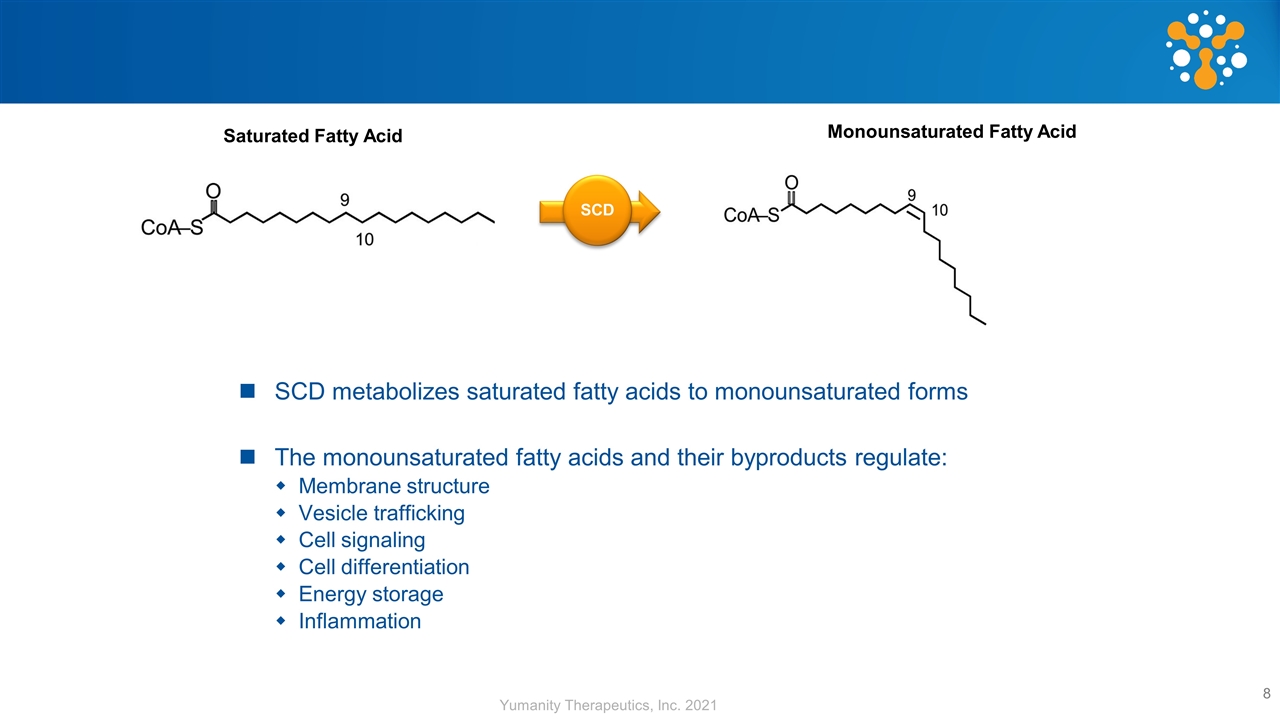
SCD metabolizes saturated fatty acids to monounsaturated forms The monounsaturated fatty acids and their byproducts regulate: Membrane structure Vesicle trafficking Cell signaling Cell differentiation Energy storage Inflammation SCD Saturated Fatty Acid Monounsaturated Fatty Acid
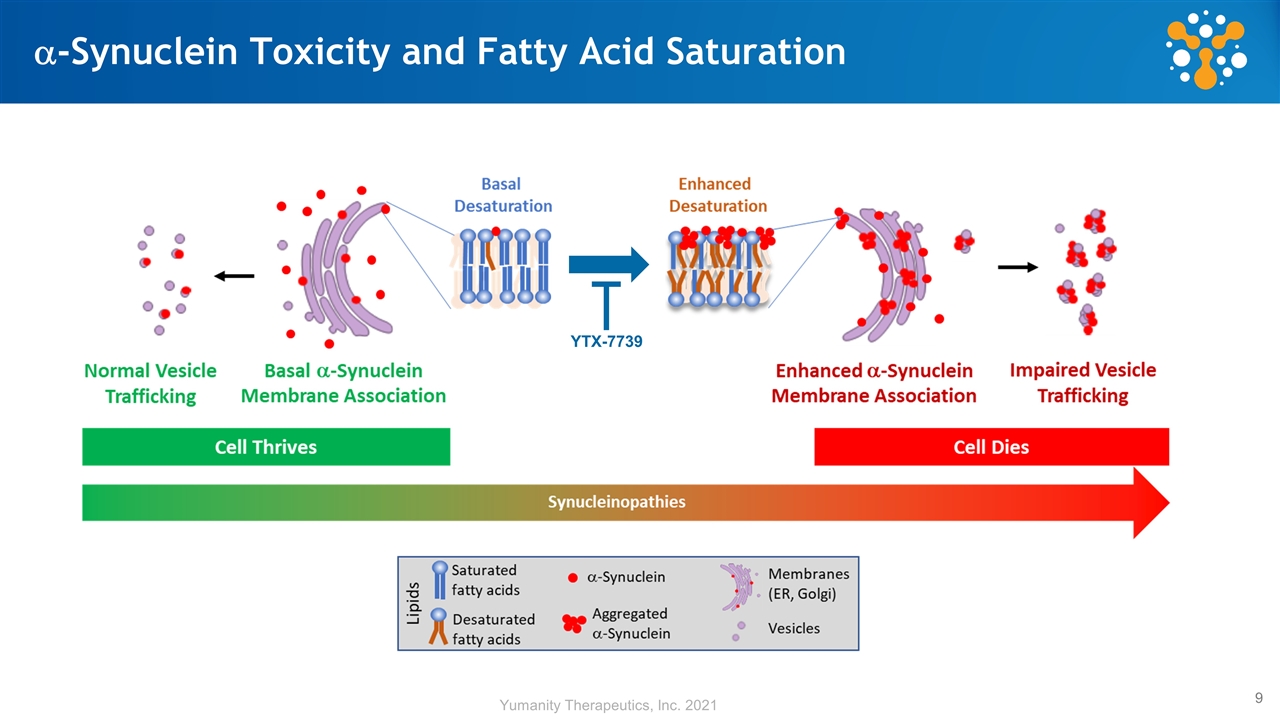
a-Synuclein Toxicity and Fatty Acid Saturation YTX-7739
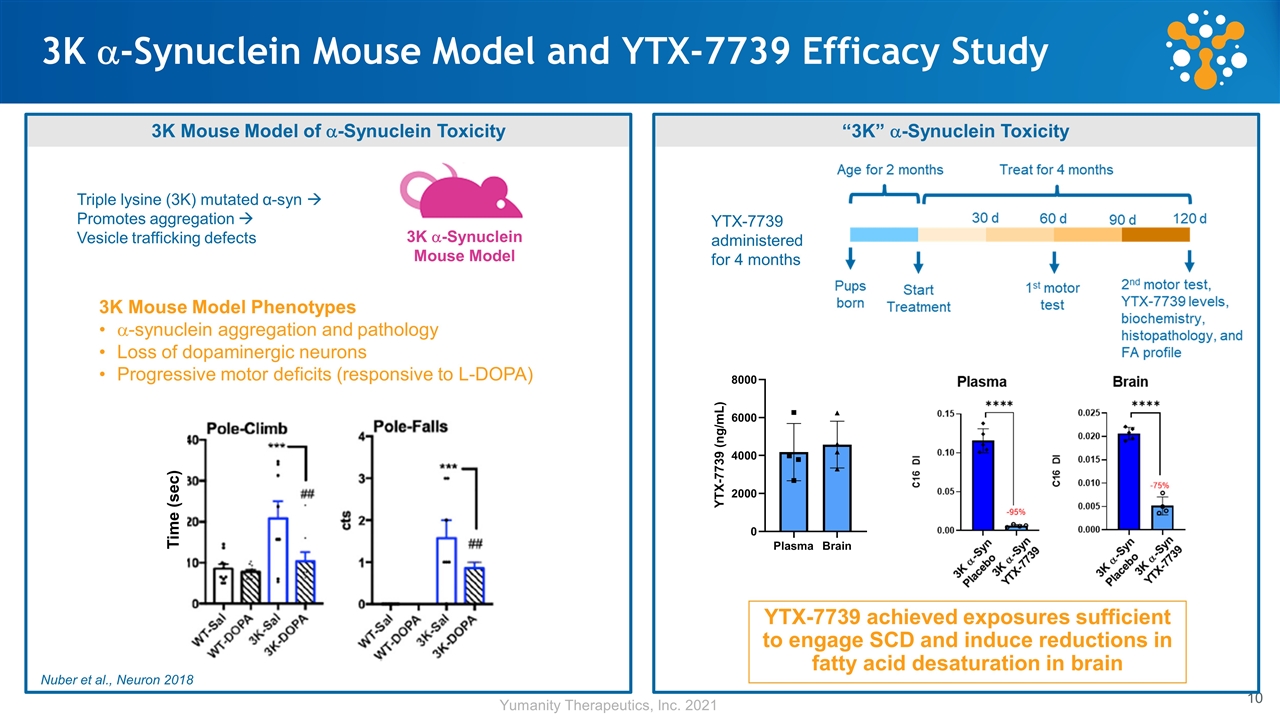
3K a-Synuclein Mouse Model and YTX-7739 Efficacy Study YTX-7739 achieved exposures sufficient to engage SCD and induce reductions in fatty acid desaturation in brain 3K Mouse Model of a-Synuclein Toxicity 3K a-Synuclein Mouse Model Nuber et al., Neuron 2018 “3K” a-Synuclein Toxicity 3K Mouse Model Phenotypes a-synuclein aggregation and pathology Loss of dopaminergic neurons Progressive motor deficits (responsive to L-DOPA) YTX-7739 administered for 4 months Time (sec) Triple lysine (3K) mutated α-syn à Promotes aggregation à Vesicle trafficking defects
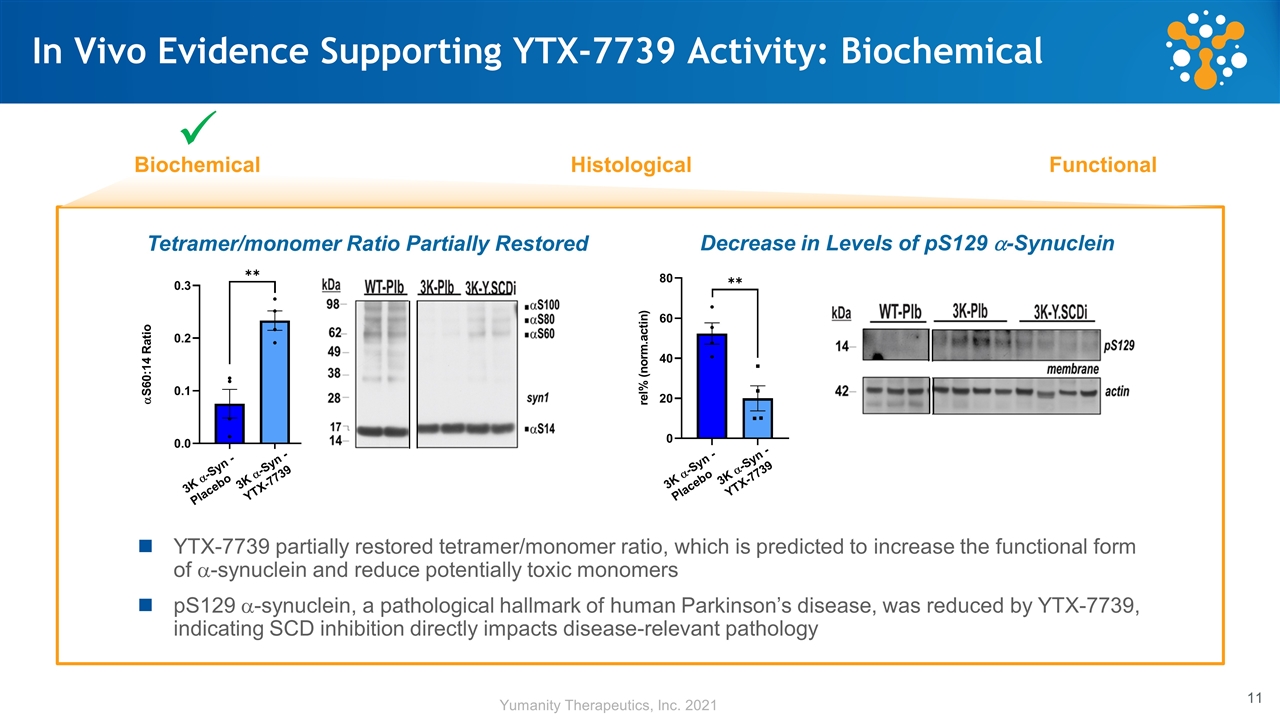
In Vivo Evidence Supporting YTX-7739 Activity: Biochemical Biochemical Histological Functional ü YTX-7739 partially restored tetramer/monomer ratio, which is predicted to increase the functional form of a-synuclein and reduce potentially toxic monomers pS129 a-synuclein, a pathological hallmark of human Parkinson’s disease, was reduced by YTX-7739, indicating SCD inhibition directly impacts disease-relevant pathology Tetramer/monomer Ratio Partially Restored 3K a-Syn - Placebo 3K a-Syn - YTX-7739 3K a-Syn - Placebo 3K a-Syn - YTX-7739 Decrease in Levels of pS129 a-Synuclein
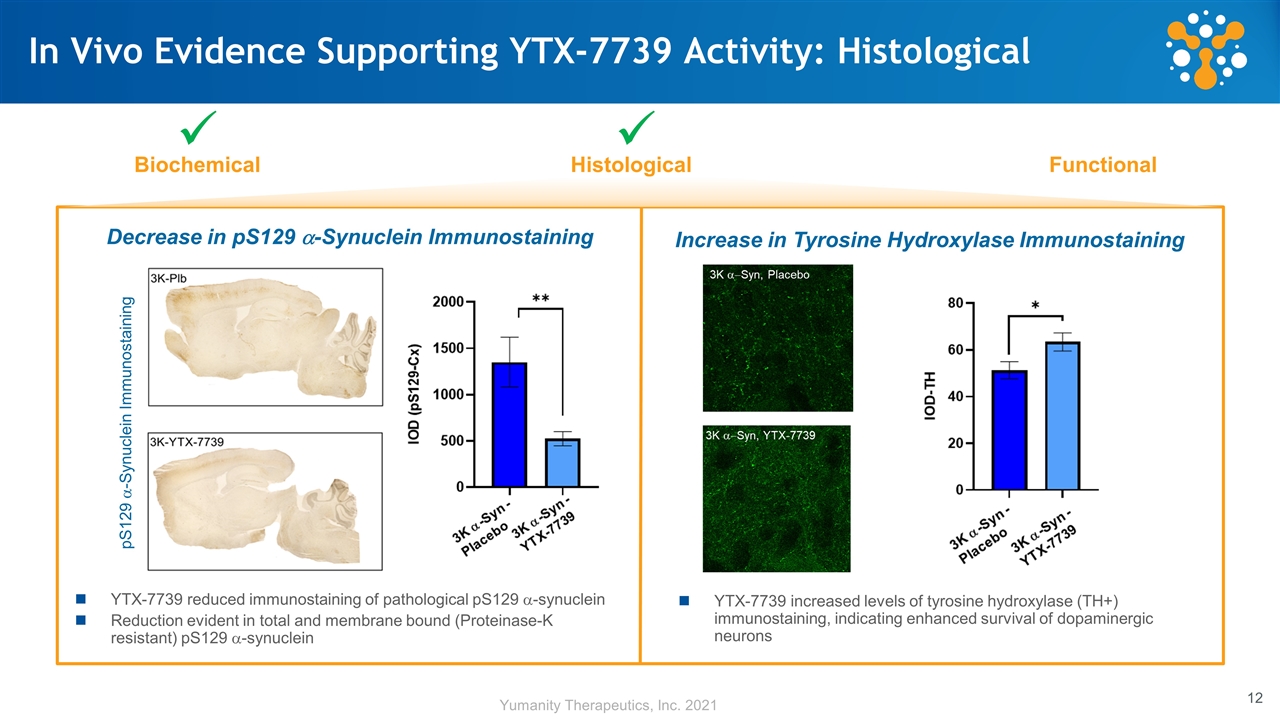
In Vivo Evidence Supporting YTX-7739 Activity: Histological Biochemical Histological Functional ü ü YTX-7739 reduced immunostaining of pathological pS129 a-synuclein Reduction evident in total and membrane bound (Proteinase-K resistant) pS129 a-synuclein Decrease in pS129 a-Synuclein Immunostaining pS129 a-Synuclein Immunostaining YTX-7739 increased levels of tyrosine hydroxylase (TH+) immunostaining, indicating enhanced survival of dopaminergic neurons Increase in Tyrosine Hydroxylase Immunostaining
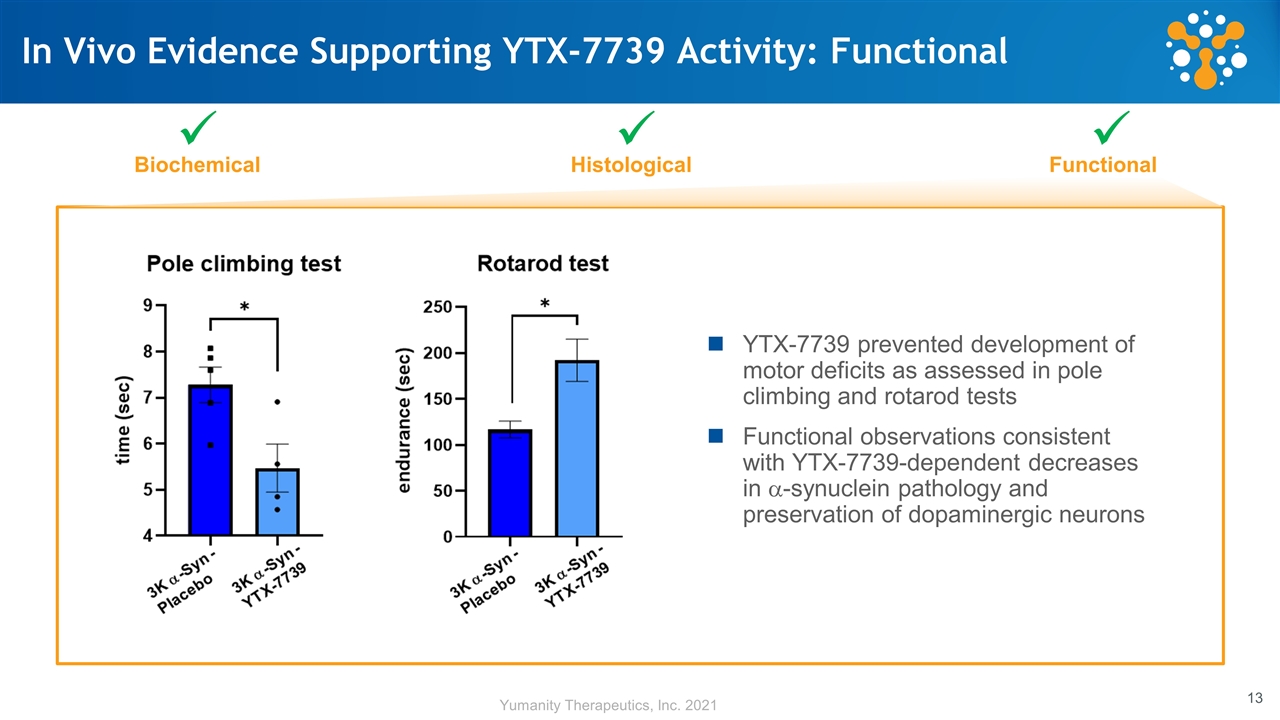
In Vivo Evidence Supporting YTX-7739 Activity: Functional Biochemical Histological Functional ü ü ü YTX-7739 prevented development of motor deficits as assessed in pole climbing and rotarod tests Functional observations consistent with YTX-7739-dependent decreases in a-synuclein pathology and preservation of dopaminergic neurons
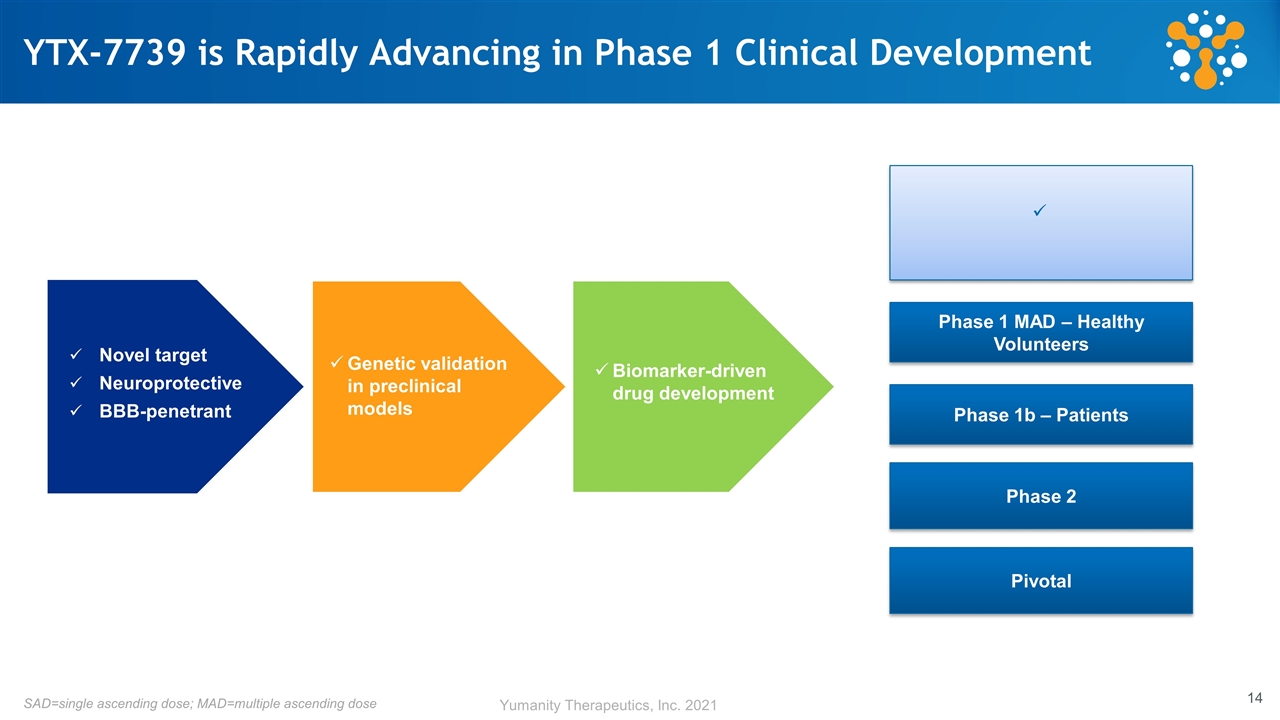
YTX-7739 is Rapidly Advancing in Phase 1 Clinical Development Novel target Neuroprotective BBB-penetrant Genetic validation in preclinical models Biomarker-driven drug development SAD=single ascending dose; MAD=multiple ascending dose Pivotal Phase 2 Phase 1b – Patients Phase 1 MAD – Healthy Volunteers
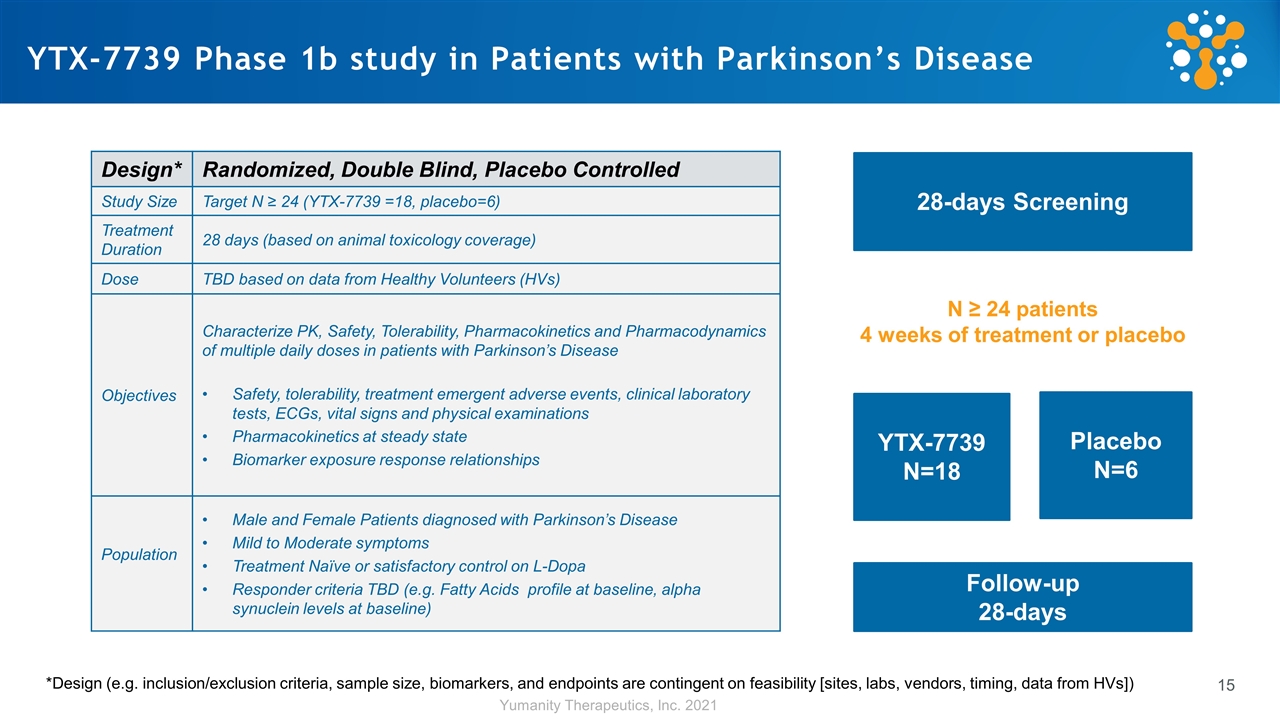
YTX-7739 Phase 1b study in Patients with Parkinson’s Disease 28-days Screening YTX-7739 N=18 Placebo N=6 *Design (e.g. inclusion/exclusion criteria, sample size, biomarkers, and endpoints are contingent on feasibility [sites, labs, vendors, timing, data from HVs]) Design* Randomized, Double Blind, Placebo Controlled Study Size Target N ≥ 24 (YTX-7739 =18, placebo=6) Treatment Duration 28 days (based on animal toxicology coverage) Dose TBD based on data from Healthy Volunteers (HVs) Objectives Characterize PK, Safety, Tolerability, Pharmacokinetics and Pharmacodynamics of multiple daily doses in patients with Parkinson’s Disease Safety, tolerability, treatment emergent adverse events, clinical laboratory tests, ECGs, vital signs and physical examinations Pharmacokinetics at steady state Biomarker exposure response relationships Population Male and Female Patients diagnosed with Parkinson’s Disease Mild to Moderate symptoms Treatment Naïve or satisfactory control on L-Dopa Responder criteria TBD (e.g. Fatty Acids profile at baseline, alpha synuclein levels at baseline) Follow-up 28-days N ≥ 24 patients 4 weeks of treatment or placebo

Prolific Drug Discovery Engine
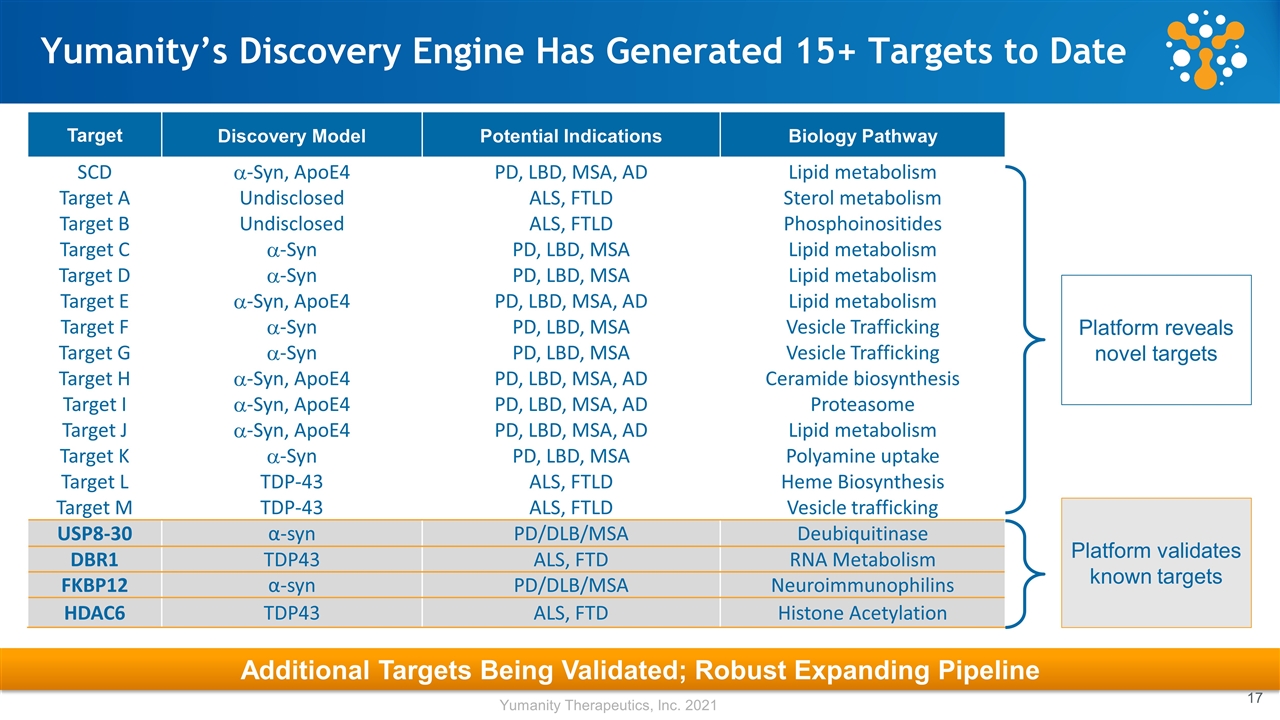
Additional Targets Being Validated; Robust Expanding Pipeline Yumanity’s Discovery Engine Has Generated 15+ Targets to Date Target Discovery Model Potential Indications Biology Pathway SCD a-Syn, ApoE4 PD, LBD, MSA, AD Lipid metabolism Target A Undisclosed ALS, FTLD Sterol metabolism Target B Undisclosed ALS, FTLD Phosphoinositides Target C a-Syn PD, LBD, MSA Lipid metabolism Target D a-Syn PD, LBD, MSA Lipid metabolism Target E a-Syn, ApoE4 PD, LBD, MSA, AD Lipid metabolism Target F a-Syn PD, LBD, MSA Vesicle Trafficking Target G a-Syn PD, LBD, MSA Vesicle Trafficking Target H a-Syn, ApoE4 PD, LBD, MSA, AD Ceramide biosynthesis Target I a-Syn, ApoE4 PD, LBD, MSA, AD Proteasome Target J a-Syn, ApoE4 PD, LBD, MSA, AD Lipid metabolism Target K a-Syn PD, LBD, MSA Polyamine uptake Target L TDP-43 ALS, FTLD Heme Biosynthesis Target M TDP-43 ALS, FTLD Vesicle trafficking USP8-30 α-syn PD/DLB/MSA Deubiquitinase DBR1 TDP43 ALS, FTD RNA Metabolism FKBP12 α-syn PD/DLB/MSA Neuroimmunophilins HDAC6 TDP43 ALS, FTD Histone Acetylation Platform validates known targets Platform reveals novel targets

Summary
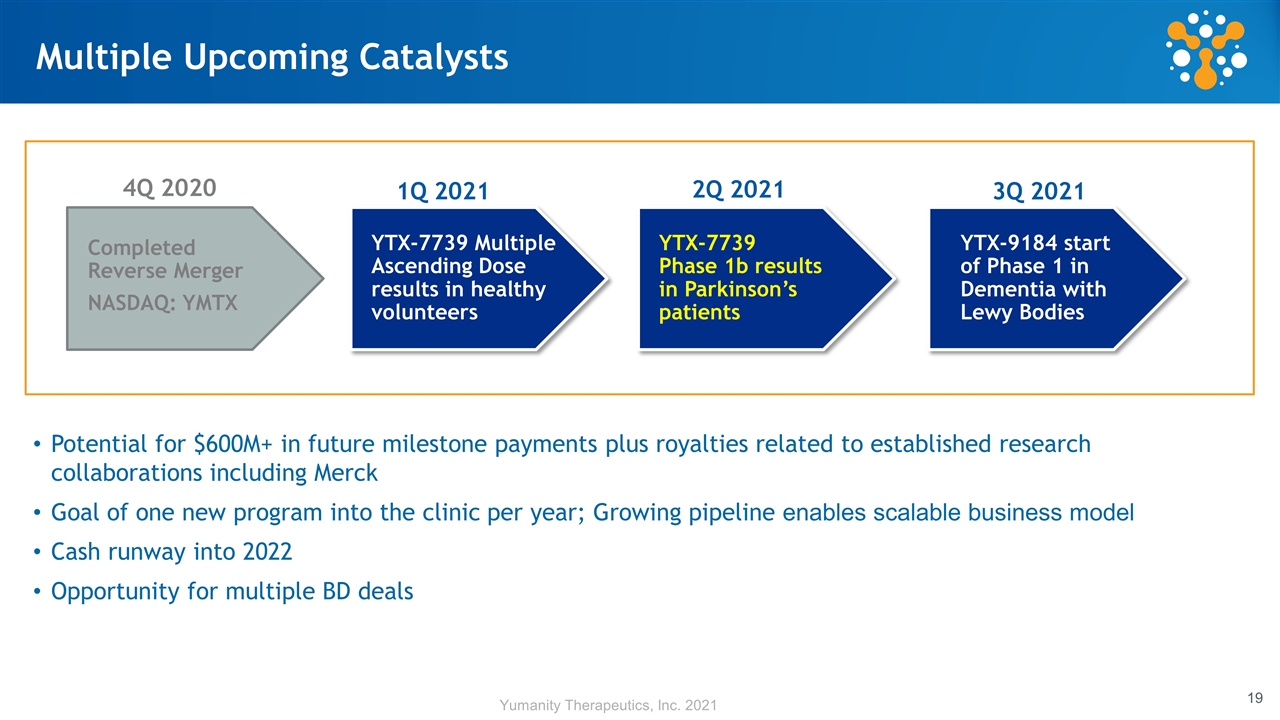
Multiple Upcoming Catalysts Completed Reverse Merger NASDAQ: YMTX YTX-7739 Multiple Ascending Dose results in healthy volunteers YTX-7739 Phase 1b results in Parkinson’s patients YTX-9184 start of Phase 1 in Dementia with Lewy Bodies 4Q 2020 1Q 2021 2Q 2021 3Q 2021 Potential for $600M+ in future milestone payments plus royalties related to established research collaborations including Merck Goal of one new program into the clinic per year; Growing pipeline enables scalable business model Cash runway into 2022 Opportunity for multiple BD deals
Yumanity is Accelerating the Revolution in the Treatment of Neurodegenerative Diseases Rapidly Advancing Clinical-Stage Pipeline Clear Momentum and Path to Value Creation Transformative Scientific Foundation & Prolific Drug Discovery Platform Applying Rare Diseases and Oncology R&D Principles to Neurology
