Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Tricida, Inc. | tcda-20210111.htm |

Treating Metabolic Acidosis and Slowing the Progression of Chronic Kidney Disease Investor Presentation January 2021 Exhibit 99.1

2 Forward Looking Statements Any statements contained in this presentation or made during the accompanying oral presentation that are not statements of historical facts are forward-looking statements as defined under the Federal securities laws. Examples of such statements include our plans, beliefs, intentions, expectations and projections regarding, among other things: (i) the approvability of veverimer by the FDA; (ii) our future clinical trial and product development milestones; (iii) the market opportunity, competition and rate of adoption for veverimer; (iv) the availability of coverage and reimbursement for veverimer; and (v) our financial projections and cost estimates. Any such forward-looking statements are based on our current expectations and assumptions, but are subject to a number of risks and uncertainties that could cause our actual future results to differ materially from our current expectations or those implied by the forward-looking statements. In addition, this presentation contains industry and market data prepared by third parties or by us. We have not independently verified this third-party data, and our data is based on our estimates and assumptions, which are subject to uncertainty and risk. As a result, you should not place undue reliance on this industry and market data. The risks and uncertainties that could adversely affect our forward-looking statements and the industry and market data include, but are not limited to: (i) the timing of the FDA’s approval of veverimer, if at all; (ii) the potential availability of the Accelerated Approval Program and the approvability of veverimer under that program; (iii) the Company’s plans and expectations with regard to its interactions with the FDA, including the potential resubmission of an NDA for veverimer; (iv) the Company’s plans and expectations for VALOR-CKD and future clinical and product development milestones; (iv) the enforceability of our patent estate; (v) risks related to COVID-19; (vi) the Company’s financial projections and cost estimates; (vii) risks associated with the Company’s business prospects, financial results and business operations; and (viii) risks related to our capital requirements and ability to raise sufficient funds for our operations. These and other factors that may affect our future results and operations are identified and described in more detail in our filings with the Securities and Exchange Commission (the “SEC”), including the Company’s most recent Annual Report filed on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. You should not place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. Except as required by applicable law, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results, later events or circumstances or to reflect the occurrence of unanticipated events.
3*Veverimer is not yet approved by the FDA First and ONLY FDA-Approved Therapy Significant unmet medical need to treat chronic metabolic acidosis Improve How Patients Feel and Function Enhance physical functioning and physical functioning-related quality of life Disease Modifying Slow CKD progression through the treatment of chronic metabolic acidosis which affects ~3 M patients with CKD in the US Our Goals for Veverimer* An Investigational Drug Candidate for the Treatment of Metabolic Acidosis in Patients with CKD

4 August 22, 2020 Complete Response Letter (CRL) Veverimer Is Being Developed to Treat Metabolic Acidosis and Potentially Slow CKD Progression 4Q 2018 Initiated a confirmatory outcomes trial based on accelerated approval guidelines Substantive discussions with the FDA to submit the veverimer NDA through the Accelerated Approval Program Completed pivotal Phase 3 efficacy trial Completed Phase 3 long-term safety and durability of effect trial Pivotal Phase 3 trial results published Phase 3 long-term safety and durability of effect trial results published NDA on file with review granted under the Accelerated Approval Program / PDUFA goal date of August 22, 2020 2019 2020 4Q End of Review Type A Meeting A Formal Dispute Resolution Request (FDRR) has been filed with the Office of New Drugs (OND) 2021 Anticipate interim analysis for early stopping for efficacy after accrual of 150 endpoint events in VALOR-CKD 2Q 1Q 3Q 3Q 3Q 4Q Interim analysis for early stopping for efficacy after accrual of 250 endpoint events in VALOR-CKD anticipated in 2022 Completion of study and final analyses after accrual of 511 endpoint events in VALOR-CKD anticipated in 2024 2022 and Beyond Awaiting response from FDA on FDRR and clarity on the path forward for resubmitting the NDA 1Q
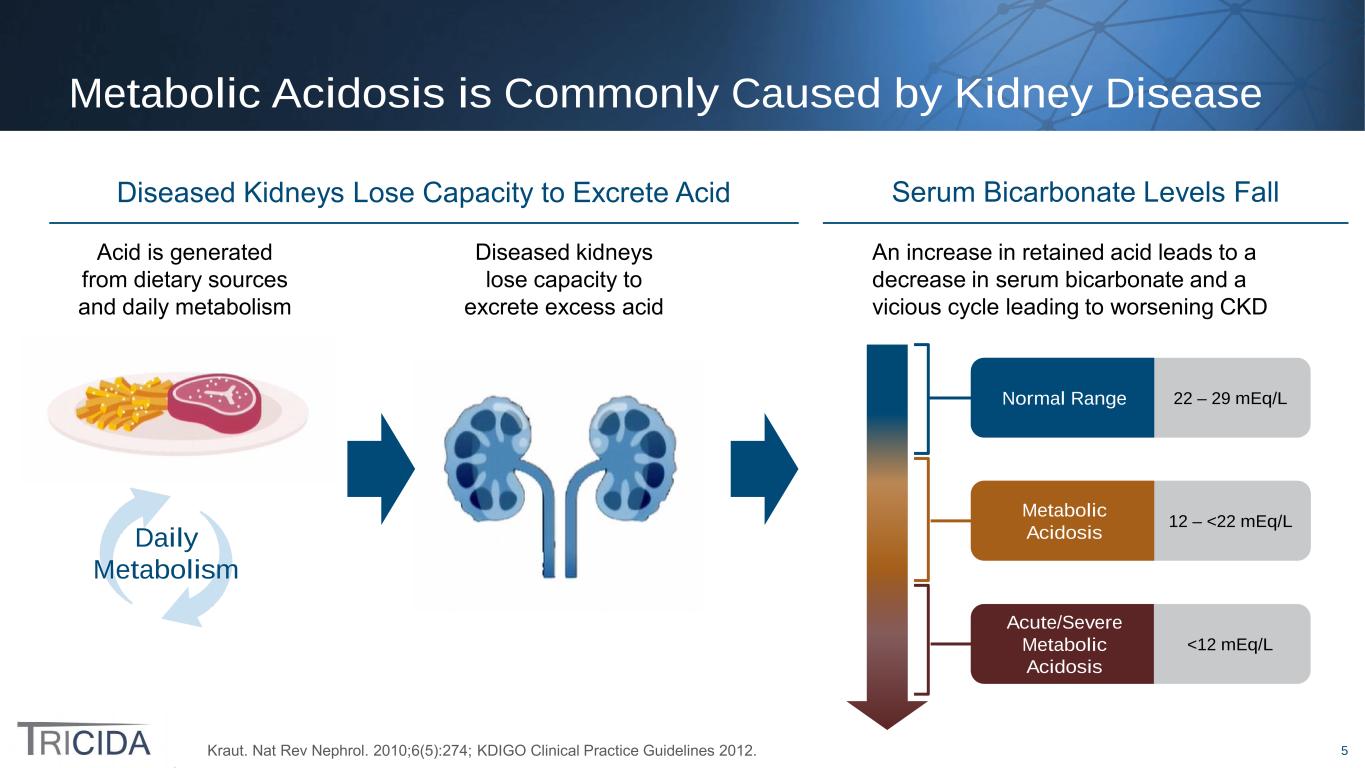
5 Daily Metabolism Metabolic Acidosis is Commonly Caused by Kidney Disease Kraut. Nat Rev Nephrol. 2010;6(5):274; KDIGO Clinical Practice Guidelines 2012. Serum Bicarbonate Levels FallDiseased Kidneys Lose Capacity to Excrete Acid Acid is generated from dietary sources and daily metabolism Diseased kidneys lose capacity to excrete excess acid An increase in retained acid leads to a decrease in serum bicarbonate and a vicious cycle leading to worsening CKD Normal Range Acute/Severe Metabolic Acidosis <12 mEq/L Metabolic Acidosis 12 – <22 mEq/L 22 – 29 mEq/L

6 Metabolic Acidosis Is a Serious Condition Harmful Needs to Be Treated 0% 20% 40% 60% 80% 100% Dobre 2013 Tangri 2020 TRCA-301 Tangri 2019 < 3% < 10% 15% < 6% % o f P at ie nt s R ec ei vi ng O ra l A lk al i T he ra pyThe Majority of Patients with Metabolic Acidosis are NOT treated No FDA Approved Treatments 3,000,000 Patients with CKD are afflicted with Metabolic AcidosisCommon Accelerates CKD Progression Impacts Bone and Muscle Health
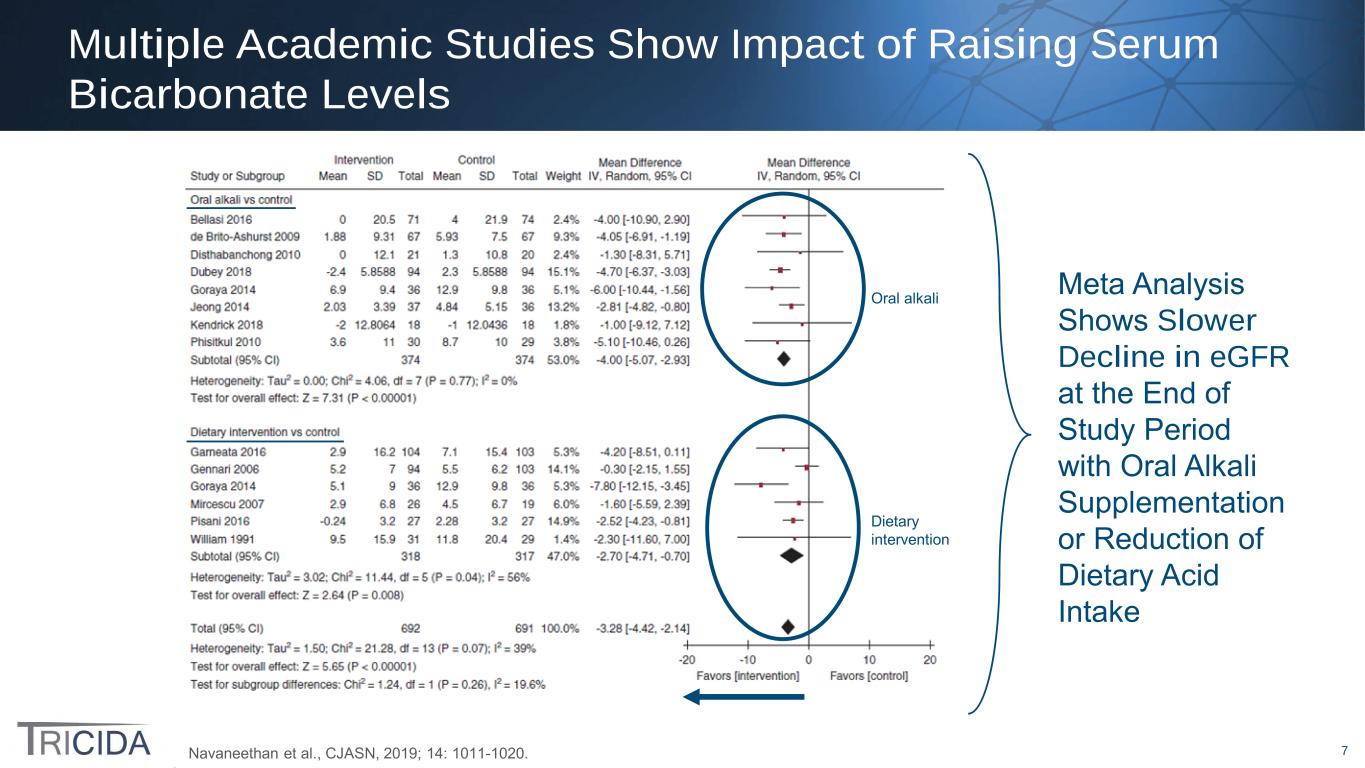
7 Oral alkali Dietary intervention Multiple Academic Studies Show Impact of Raising Serum Bicarbonate Levels Navaneethan et al., CJASN, 2019; 14: 1011-1020. Meta Analysis Shows Slower Decline in eGFR at the End of Study Period with Oral Alkali Supplementation or Reduction of Dietary Acid Intake
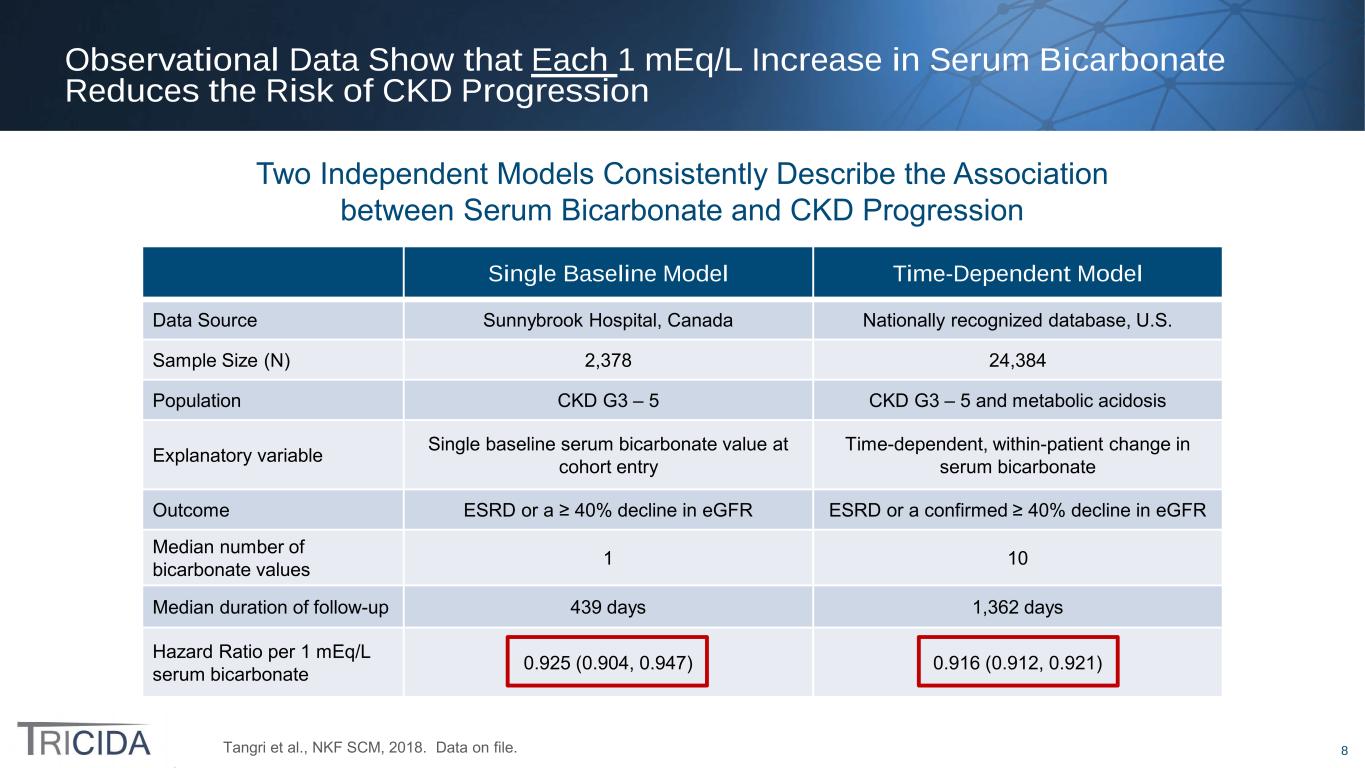
8 Observational Data Show that Each 1 mEq/L Increase in Serum Bicarbonate Reduces the Risk of CKD Progression Tangri et al., NKF SCM, 2018. Data on file. Single Baseline Model Time-Dependent Model Data Source Sunnybrook Hospital, Canada Nationally recognized database, U.S. Sample Size (N) 2,378 24,384 Population CKD G3 – 5 CKD G3 – 5 and metabolic acidosis Explanatory variable Single baseline serum bicarbonate value at cohort entry Time-dependent, within-patient change in serum bicarbonate Outcome ESRD or a ≥ 40% decline in eGFR ESRD or a confirmed ≥ 40% decline in eGFR Median number of bicarbonate values 1 10 Median duration of follow-up 439 days 1,362 days Hazard Ratio per 1 mEq/L serum bicarbonate 0.925 (0.904, 0.947) 0.916 (0.912, 0.921) Two Independent Models Consistently Describe the Association between Serum Bicarbonate and CKD Progression

Veverimer: Designed to Bind and Remove Acid in the GI Tract to Raise Serum Bicarbonate to Treat Metabolic Acidosis and Slow CKD Progression

10GI: Gastrointestinal. HCl: Hydrochloric acid. *Veverimer’s maximum theoretical HCl binding capacity is approximately 10 mEq/gram. Excreted, resulting in removal of HCl* Binds protons (H+) and then selectively binds chloride (Cl–) in the GI tract Excretion in Feces O ra l I ng es tio n A ci d B in di ng in G I T ra ct NH2 NH2 NH2 NH2 veverimer NH3+ NH3+ NH3+ NH3+ Cl-Cl- Cl- Cl- veverimer Cl- H+ NH3+ NH3+ NH3+ NH3+ veverimer Non-absorbed polymer veverimerCitrate Chloride Fatty Acids Bile Acids Phosphate GI Anions Ranked by Size The high degree of crosslinking in veverimer provides a size exclusion mechanism that leads to high selectivity for binding chloride (the smallest anion) over larger anions Veverimer: Designed to Bind and Remove HCl with High Capacity and Selectivity High Binding Capacity High Binding Selectivity

11 Veverimer: Ease of Administration* Once-a-day dosing Orally administered as a suspension in water 3 g, 6 g or 9 g dose sizes No observed drug-drug interactions** 2 oz. Water *Targeted profile if approved ** Data on file

Veverimer: Clinical Development Program and Regulatory Path

13 Veverimer Clinical Program QD = Once daily. Wesson DE et al., Lancet 2019; 393: 1417-27. Wesson DE et al., Lancet 2019; 394: 396-406. Phase 3 Clinical Trials: TRCA-301 / TRCA-301E VALOR-CKD Outcomes Trial Veverimer QD (n~800) Placebo QD (n~800)Tr ea tm en t ~ 4 Y ea rs End of study: 511 primary endpoint events 1,600 subjects with serum bicarbonate of 12 – 20 mEq/L, eGFR of 20 – 40 ml/min/1.73m2 Screening Time-to-Event, Double-Blind, Randomized, Placebo-Controlled Treatment (Primary Endpoint: Time to confirmed ≥40% decline in eGFR, ESRD, or renal death) Subjects not eligible: Safety Follow-up 2- weeks Veverimer QD 6 g QD (n=124) Placebo QD (n=93)Tr ea tm en t 12 W ee ks Veverimer QD (n=114) Placebo QD (n=82)T re at m en t 40 W ee ks Safety follow-up for adverse event collection and for assessment of offset of effects TRCA-301 Treatment (12 Weeks, 4:3 Randomization) TRCA-301E: 40-Week Extension Trial (196 Subjects were eligible to participate. Same regimen as TRCA-301) 217 subjects with serum bicarbonate of 12 – 20 mEq/L, eGFR of 20 – 40 ml/min/1.73m2 Screening

14 Baseline Disease Characteristics of Subjects Enrolled in TRCA-301 Veverimer N=124 Placebo N=93 Baseline eGFR, mean 29 mL/min/1.73m2 28 mL/min/1.73m2 Baseline serum bicarbonate, mean 17 mEq/L 17 mEq/L Selected Medical History CKD 100% 100% Hypertension 97% 97% Diabetes 61% 70% Left ventricular hypertrophy 47% 41% Congestive heart failure 29% 33% Medication Use Veverimer N=124 Placebo N=93 ACE inhibitors / ARBs 67% 82% Diuretics 60% 63% Calcium channel blockers 56% 60% Anti-diabetic drugs 53% 56% Beta blockers 46% 53% Lipid modifying agents 44% 53% Anti-thrombotic agents 38% 48% Drugs for acid related disorders 10% 6% Sodium bicarbonate 10% 8% Wesson DE et al., Lancet 2019; 393: 1417-27.

15 TRCA-301 Veverimer-Treated Subjects Experienced a Mean Increase from Baseline in Serum Bicarbonate of 4.5 mEq/L Veverimer: Summary of TRCA-301/301E Clinical Trial Results BL: Baseline. W: Week. Error bars: Standard error of the mean. DD50: Death, dialysis or ≥50% reduction in eGFR. Wesson DE et al., Lancet 2019; 393:1417-27. Wesson DE et al., Lancet 2019; 394: 396-406. 17.3 18.1 18.4 18.6 18.6 19.0 18.5 18.9 17.3 20.2 20.8 21.6 21.6 22.0 21.7 21.7 17 18 19 20 21 22 BL Week 1 Week 2 Week 4 Week 6 Week 8 Week 10 Week 12Placebo Veverimer 4.5 mEq/L (P< 0.0001) TRCA-301 / TRCA-301E Prespecified Time-to-Event Analyses (52 Weeks) 4% DD50 incidence rate 12% DD50 incidence rate 65% Reduction in the Annualized Event Rate for Veverimer-Treated Subjects Time since randomisation (weeks) TRCA-301 / TRCA-301E Repeated Chair Stand Test 00 0 0 12 40 52 -1.4 -4.3 Im proved physical function 11.4 -0.7 Im proved physical function TRCA-301 / TRCA-301E KDQOL Physical Function Domain
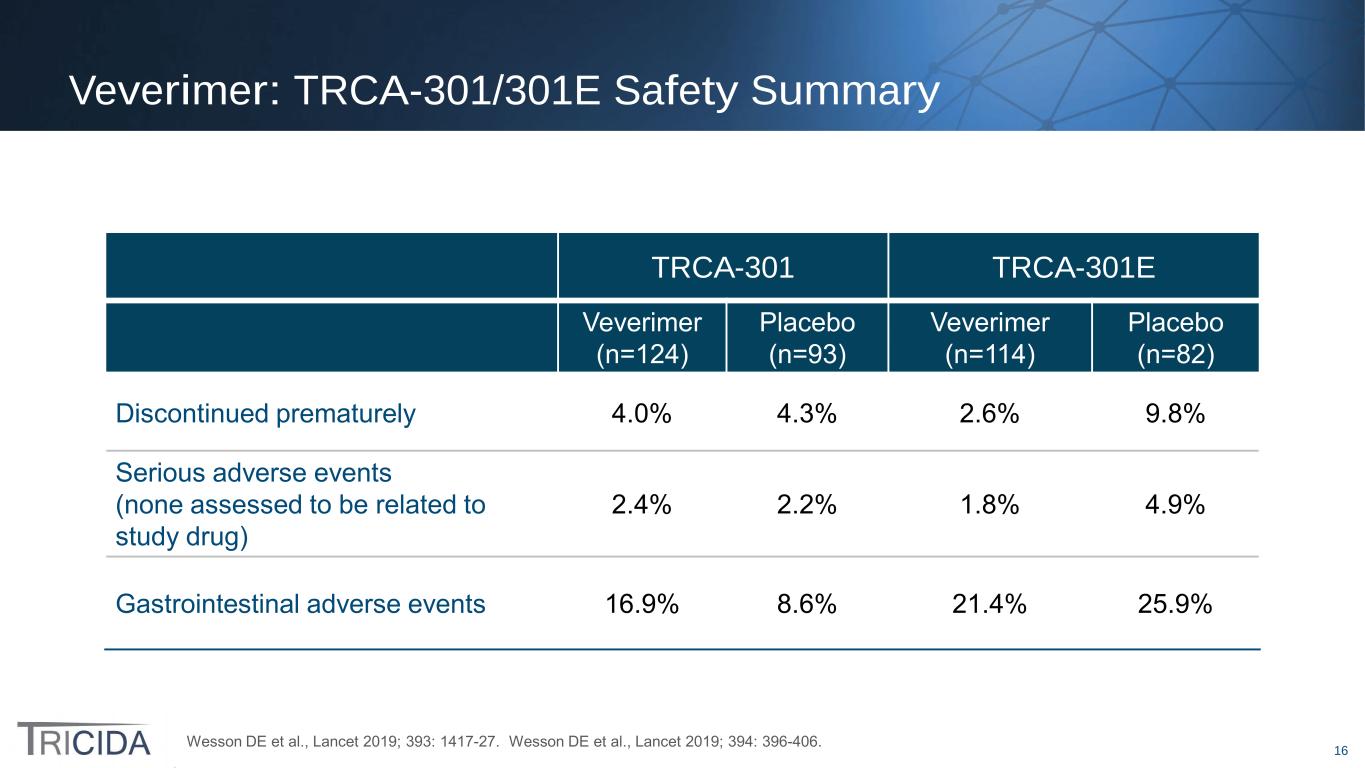
16 Veverimer: TRCA-301/301E Safety Summary TRCA-301 TRCA-301E Veverimer (n=124) Placebo (n=93) Veverimer (n=114) Placebo (n=82) Discontinued prematurely 4.0% 4.3% 2.6% 9.8% Serious adverse events (none assessed to be related to study drug) 2.4% 2.2% 1.8% 4.9% Gastrointestinal adverse events 16.9% 8.6% 21.4% 25.9% Wesson DE et al., Lancet 2019; 393: 1417-27. Wesson DE et al., Lancet 2019; 394: 396-406.

17 Veverimer: TRCA-301 and TRCA-301E Trial Results Published in The Lancet TRCA-301 Results TRCA-301E Results Wesson DE et al., Lancet 2019; 393: 1417-27. Wesson DE et al., Lancet 2019; 394: 396-406.

VALOR-CKD Confirmatory Outcomes Trial

19 VALOR-CKD Confirmatory Outcomes Trial The trial terminates when the independent blinded Clinical Endpoint Adjudication Committee (CEAC) has positively adjudicated 511 subjects with primary efficacy endpoint events • Two interim analyses are planned for early stopping for efficacy Time-to-event trial to evaluate the efficacy and safety of veverimer in delaying CKD progression Primary endpoint: Progression of renal disease, defined by time to first occurrence of any event in the composite renal endpoint consisting of renal death, ESRD or ≥ 40% reduction in eGFR (DD40) As of January 8, 2021, the VALOR-CKD trial has randomized 1,378 of 1,600 subjects (86%) with an average treatment duration of ~1 year and has accrued 57 of the 511 required subjects (11%) with positively adjudicated primary endpoint events (renal death, ESRD, and/or ≥ 40% reduction in eGFR) • Trial recruitment outside of North America and Western Europe was closed in late 2020 and it is projected to be completed by the end of 2022
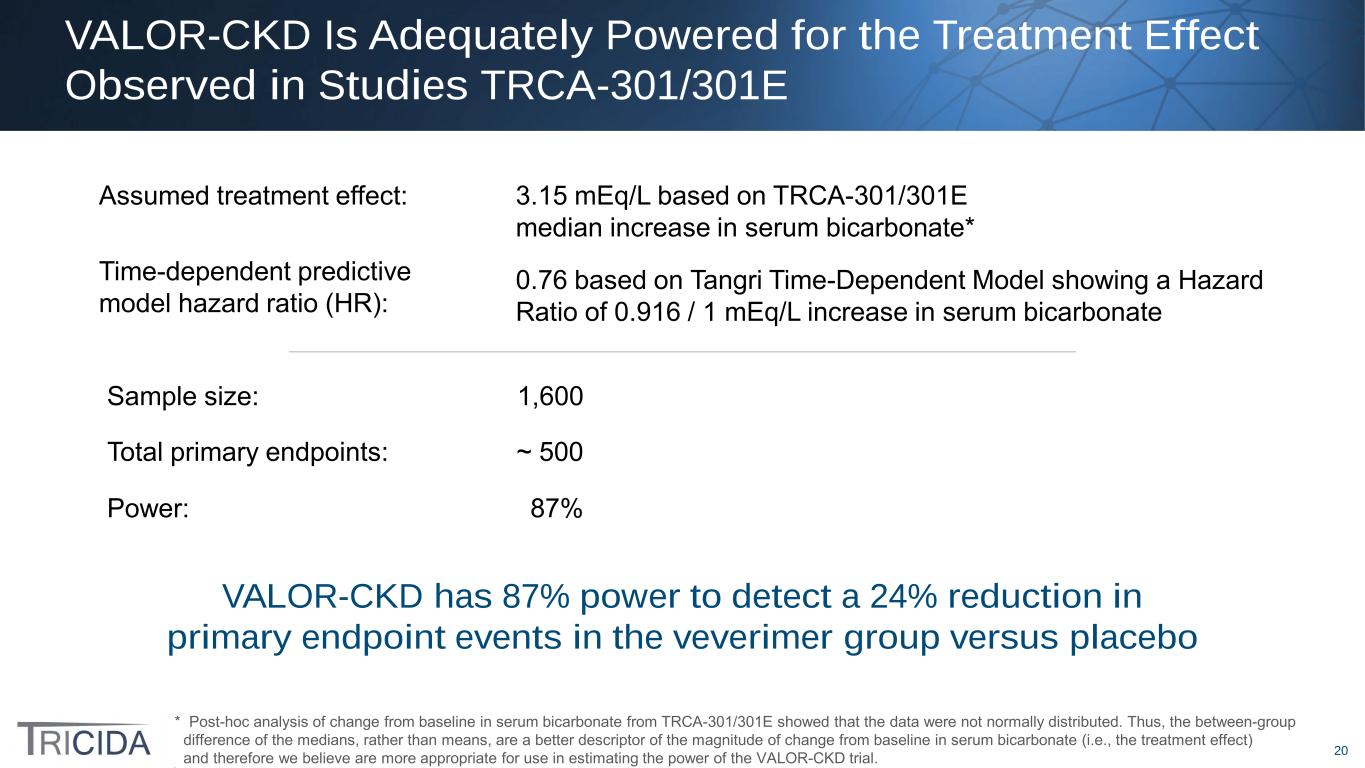
20 VALOR-CKD Is Adequately Powered for the Treatment Effect Observed in Studies TRCA-301/301E * Post-hoc analysis of change from baseline in serum bicarbonate from TRCA-301/301E showed that the data were not normally distributed. Thus, the between-group difference of the medians, rather than means, are a better descriptor of the magnitude of change from baseline in serum bicarbonate (i.e., the treatment effect) and therefore we believe are more appropriate for use in estimating the power of the VALOR-CKD trial. Assumed treatment effect: 3.15 mEq/L based on TRCA-301/301E median increase in serum bicarbonate* Time-dependent predictive model hazard ratio (HR): 0.76 based on Tangri Time-Dependent Model showing a Hazard Ratio of 0.916 / 1 mEq/L increase in serum bicarbonate Sample size: 1,600 Total primary endpoints: ~ 500 Power: 87% VALOR-CKD has 87% power to detect a 24% reduction in primary endpoint events in the veverimer group versus placebo

21 Interim Analyses Are Planned for Early Stopping for Efficacy • Interim alpha spending approach: O'Brien-Fleming alpha spending function – Nominal one-sided alpha required for significance at • Interim 1: 0.00004; observed HR will need to be < 0.50 • Interim 2: 0.00151; observed HR will need to be < 0.67 • Final: 0.0245; observed HR will need to be < 0.83 True Hazard Ratio Probability of stopping at interim 1 (%) Probability of stopping at interim 2 (%) Cumulative probability of stopping by interim 2 (%) Probability of stopping at final analysis (%) Cumulative probability of stopping by final analysis (%) 0.76 1 21 22 65 87 0.70 4 40 44 54 98 • Interim analysis 1: 150 events • Interim analysis 2: 250 events • Final analysis: 511 events Tricida will use a group sequential design for VALOR-CKD, as recommended by the FDA in a July 30, 2020 advice letter. In the letter, the FDA suggested that a group sequential design for VALOR-CKD would be more efficient than Tricida’s proposed adaptive design with unblinded sample size re-estimation.

22 *57 Events VALOR-CKD Primary Endpoint Event Tracking Interim analysis 1: Anticipated 2H 2021 Interim analysis 2: Anticipated mid-2022 Final analysis: Anticipated 2024 VALOR-CKD anticipated event rates based on tracking against event rates observed in previous CKD trials * Data on event rates among 2197 control group patients in 29 CKD trials. Required baseline eGFR of 20 to 40 mL/min/1.73m2 Source: Inker et al. JASN 30:1735-1745, 2019

FDA Regulatory Status

24FDA Guidance for Industry—Expedited Programs for Serious Conditions—Drugs and Biologics. Treats a Serious, Life-Threatening Condition Increasing blood bicarbonate may slow progression to ESRD for patients with CKD and metabolic acidosis Meaningful Advantage Over Standard of Care There are no FDA-approved therapies for the treatment of chronic metabolic acidosis Surrogate Endpoint Reasonably Likely to Predict Clinical Benefit Increasing blood bicarbonate has been linked to the slowing of CKD progression Accelerated Approval Program Requirements We Believe that Veverimer Meets the Requirements for Approval through the Accelerated Approval Program* Benefits of Treating Metabolic Acidosis in Patients with CKD Veverimer Development Program Designed to Meet Accelerated Approval Requirements TRCA-301 / TRCA301E Statistically significant increase in serum bicarbonate versus placebo and an excellent safety profile VALOR-CKD Outcomes Trial Ongoing time-to-event trial designed to evaluate the efficacy and safety of veverimer in delaying CKD progression * We received a CRL on our August 2020 PDUFA date. In December, we submitted a Formal Dispute Resolution Request (FDRR) to the FDA to seek clarity on the path forward for resubmitting our NDA under the Accelerated Approval Program.

25 Recent FDA Interactions • We received a Complete Response Letter (CRL) in August 2020 indicating that the FDA was seeking additional data from at least one additional adequate and well-controlled study demonstrating the efficacy of veverimer for the treatment of metabolic acidosis associated with CKD • In the CRL, the FDA expressed willingness to meet with Tricida to discuss options for approval of veverimer, including: – Completion of our ongoing confirmatory trial, VALOR-CKD, and if the trial is successful, submitting the results to support a traditional approval, or – Possibly conducting a pre-specified interim analysis of data from VALOR-CKD to support accelerated approval • During the End-of-Review Type A meeting, Tricida presented information and made proposals that the company believes addressed the issues raised by the FDA in the CRL • FDA End-of-Review Type A meeting minutes are consistent with October 30, 2020 disclosures. We still believe that the FDA will require evidence of effect on CKD progression from a near-term interim analysis of data on CKD progression from the ongoing VALOR-CKD study for accelerated approval of veverimer and it is unlikely to rely solely on serum bicarbonate data.

26 A Formal Dispute Resolution Request (FDRR) Has Been Submitted that Focuses on the Narrow Issue of Requisite Magnitude of Benefit • Addressing the issues in the CRL is not a worthwhile endeavor unless Tricida can reach agreement with the FDA that the already-demonstrated magnitude of serum bicarbonate change is reasonably likely to predict clinical benefit and therefore should serve as the basis for accelerated approval • FDRR requests that the Office of New Drugs (OND) find that the magnitude of serum bicarbonate change seen in the TRCA-301 and TRCA-301E trials is reasonably likely to predict clinical benefit in the treatment of metabolic acidosis associated with CKD. Data provided in the FDRR supports the following: – Veverimer’s effects on serum bicarbonate are in line with clinical practice targets for the treatment of metabolic acidosis in patients with CKD – Veverimer’s effects on daily activities, as measured by patient reported outcomes, and on physical function, as measured by the repeated chair stand test, provide additional benefit – A predictive model, developed in close consultation with the Division, provides evidence that the magnitude of veverimer-induced change in serum bicarbonate is reasonably likely to predict clinical benefit in renal outcomes – Veverimer’s excellent safety profile in a complex population of patients with Stage 3 and 4 CKD with multiple accompanying comorbidities

27 • FDRR was submitted to the FDA in December 2020 and confirmation of acceptance has been confirmed • Timing and next steps will be dependent upon the Office of New Drug’s (OND’s) decision. Potential scenarios include: – If the FDRR is granted,* the Division would be directed to have label discussions with Tricida in order to allow for approval of veverimer under the Accelerated Approval Program – Even if the FDRR is denied, OND could provide an alternative path forward for resubmission of Tricida’s NDA under Accelerated Approval and Tricida would then work with the Division of Cardiology and Nephrology to fulfill any additional requests based on the recommendation of the OND • Tricida intends for the VALOR-CKD trial to either: – Serve as the confirmatory trial for accelerated approval OR – Form the basis for traditional approval of veverimer * According to the FDA, during the time period from 2003 to 2014, “[o]f the 140 unique appeals accepted and reviewed [131 of which were in OND], CDER granted 23 (16%) appeals and denied 117 (84%).” We Have Multiple Potential Paths to FDA Approval for Veverimer

Veverimer Market Opportunity

29 3,000,000 Patients with CKD and metabolic acidosis 1,100,000 Diagnosed patients ~600,000 Nephrologist- treated patients Concentrated Patient Population Allows Focus of Commercial Efforts on the Nephrology Community Focus on nephrologist-treated patients provides initial opportunity for veverimer Expansion beyond nephrologists provides additional opportunity Increased awareness drives higher metabolic acidosis diagnosis and treatment Targeting ~600,000 Nephrology-Treated Patients Concentrated Market Allows for Targeting ~5K Nephrologists 0% 20% 40% 60% 80% 100% C um ul at iv e % P ot en tia l Cumulative % of nephrologists with highest number of pre- dialysis patients with Stages 3-5 CKD 80% of Potential Target Patient Population ~5,000 Nephrologists Symphony Health Solutions IDV® Data on file. NHANES 1999-2004 reports prevalence of CKD Stages 3 and 4 for the US adult population ages 20 and older. CKD Stage 3 and 4 prevalence was calculated using NHANES prevalence and 2016 US Census data. Stage 3a (70%) and 3b (30%) were approximated using NCCD-CDC Surveillance System. MA prevalence by Stage 3a, 3b, and 4 reported in Inker LA et al., J Am Soc Nephrol 22:2322-31, 2011. Symphony Health Solutions Claims Data

30 Analysis of Major Payer Database Shows Metabolic Acidosis Increases the Cost of Health Care Metabolic Acidosis Has a Significant Impact on Health Care Costs Optum® EMR, De-identified electronic medical records 2007–2017. Reaven N, Association of Managed Care Pharmacy; Oct 31, 2019 and ASN Kidney Week Nov 7, 2019 ~$40K of added costs/year CKD stage 3 to 5 non-dialysis patients with metabolic acidosis costs ~$40K more than CKD patients with normal serum bicarbonate Impact of metabolic acidosis comparing all-cause cost in an acidotic vs. non-acidotic population Each 1 mEq/L increase in serum bicarbonate was associated with a 7% decrease in all cause monthly healthcare costs (p<0.0001) Impact of increase in serum bicarbonate as a continuous variable in combined acidotic and non-acidotic population

Intellectual Property, Financial Position and Upcoming Milestones

32 • Six already issued Orange Book eligible patents provide patent protection until 2034 • Several pending patent applications United States • Three issued EPO patents providing patent protection until 2034 and a fourth issued patent providing protection until 2035 • Several pending EPO patent applications Europe Rest of World • Issued patents provide protection in numerous countries/regions outside the US and Europe* • Corresponding pending patent applications in other commercially significant countries New Veverimer Composition of Matter Patent Will Extend Patent Coverage to 2038 in the United States Patent term does not include any extension related to Hatch-Waxman in the United States, or a Supplementary Protection Certificate in Europe. * Patent protection expected to provide protection for veverimer until at least 2034 in Australia, China, Hong Kong, India, Israel, Japan, and certain other markets and at least 2035 in Europe, Mexico and Russia. A recently allowed Orange Book eligible patent, upon issuance, will extend patent coverage to 2038

33 Financial Position • ~ $332 million cash, cash equivalents and investments as of December 31, 2020* • Debt includes: • $200 million of 3.50% Convertible Senior Notes due 2027 • $75 million currently drawn under a debt facility with Hercules (final maturity of April 2023) • ~ 50 million total shares outstanding as of December 31, 2020* * Unaudited. Anticipate reporting full Q4 2020 financial results in late February 2021

34*Veverimer is not yet approved by the FDA Key Veverimer Milestones* Obtain clarity on the path forward for resubmitting the veverimer NDA through the Accelerated Approval Program Conduct interim analysis 1 for potential early stopping of VALOR-CKD for efficacy Anticipated completion of trial without early stopping for efficacy Conduct interim analysis 2 for potential early stopping of VALOR-CKD for efficacy 1Q’21 ~3Q’21 ~2Q’22 ~1Q’24

THANK YOU
