Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Rubius Therapeutics, Inc. | tm212708d1_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Rubius Therapeutics, Inc. | tm212708d1_ex99-1.htm |
Exhibit 99.2

JANUARY 2021 REALIZING THE POWER OF REDsv™ A NEW ERA IN CELLULAR MEDICINE 1

Forward - Looking Statements This presentation may contain forward - looking statements . Forward - looking statements are neither historical facts nor assurances of future performance . Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and other future conditions . All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters ; our current and prospective product candidates, planned clinical trials and preclinical activities, including timing related to such trials and expected results, research and development costs, current and prospective collaborations ; the estimated size of the market for our product candidates, the timing and success of our development and commercialization of our anticipated product candidates ; and the availability of alternative therapies for our target market . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise . Although we believe the expectations reflected in such forward - looking statements are reasonable, we can give no assurance that such expectations will prove to be correct . Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements . No representations or warranties (expressed or implied) are made about the accuracy of any such forward - looking statements . Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third - party sources and our own internal estimates and research . While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source . Rubius recommends that investors independently evaluate specific investments and strategies . For further information regarding these risks, uncertainties and other factors, you should read the “Risk Factors” section of our Quarterly Reports on Form 10 - Q filed for the period ended September 30 , 2020 , and Annual Report on Form 10 - K filed for the period ended December 31 , 2019 , and subsequent filings with the Securities and Exchange Commission . This presentation may contain tradenames, trademarks or servicemarks of other companies . Rubius does not intend the use or display of other parties’ tradenames, trademarks or servicemarks to imply a relationship with, or endorsement or sponsorship of, these other parties . 2

Completed dosing of 5 cohorts (n=14) in Phase 1/2 RTX - 240 solid tumor clinical trial; generating clinical data DELIVERING ON CRITICAL MILESTONES AND BUILDING THE RIGHT CULTURE Dosing patients in Phase 1 arm of RTX - 240 clinical trial in relapsed/refractory acute myeloid leukemia (AML) Screening patients in Phase 1 trial of RTX - 321 for HPV 16+ cancers; frozen drug substance with potential shelf life of up to several years Fully owned manufacturing enables execution of clinical trials; conducting cGMP runs for RTX - 240 and RTX - 321 clinical trials Presented preclinical oncology data supporting lead oncology programs at SITC, FOCIS, AACR & ASGCT Strong Execution of Key Priorities 3
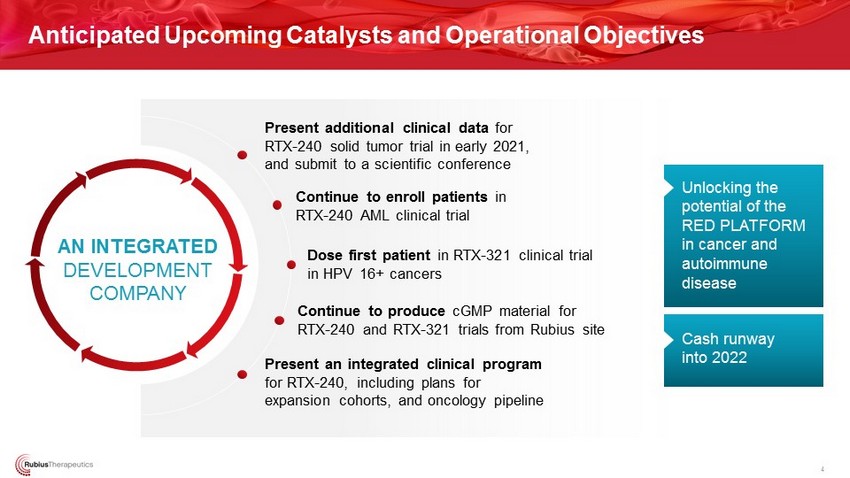
Present additional clinical data for RTX - 240 solid tumor trial in early 2021, and submit to a scientific conference Anticipated Upcoming Catalysts and Operational Objectives 4 AN INTEGRATED DEVELOPMENT COMPANY Continue to enroll patients in RTX - 240 AML clinical trial Dose first patient in RTX - 321 clinical trial in HPV 16+ cancers Continue to produce cGMP material for RTX - 240 and RTX - 321 trials from Rubius site Cash runway into 2022 Unlocking the potential of the RED PLATFORM in cancer and autoimmune disease Present an integrated clinical program for RTX - 240, including plans for expansion cohorts, and oncology pipeline

The Promise of the RED PLATFORM ® EXPANSION & DIFFERENTIATION GENETIC ENGINEERING WITH LENTIVIRUS 100 - 1000’s OF DOSES EARLY PROGENITOR CELLS ENUCLEATION & MATURATION RED CELL THERAPEUTIC SINGLE HEALTHY O - DONOR 5 FEATURES • Consistent research and manufacturing process • Only modification is lentivirus to create new product • Universal, scalable, reproducible BENEFITS • Leverages common CMC, toxicology data packages • Shorter timeline to lead candidate • Efficient cost structure

Red Cell Therapeutics™: The Future of Cellular Therapy 6 Modular platform that mimics immune biology Cellular presentation of potent immune agonists & cytokines POTENTIALLY TRANSFORMATIVE ALLOGENEIC CELLULAR THERAPIES POTENT ADVANTAGEOUS TOLERABILITY SCALABLE Biodistribution confined to vasculature Broadens therapeutic window Off - the - shelf cellular therapy from Rubius manufacturing site

Fully Owned Manufacturing and Integrated Technical Development & Operations 7 Significant potential to expand manufacturing capabilities based on future needs Providing cGMP clinical supply for RTX - 240 trial and RTX - 321 trials Small - scale production, process development, cGMP manufacturing, analytical development & quality operations Highly experienced cell therapy technical operations team with scalable process
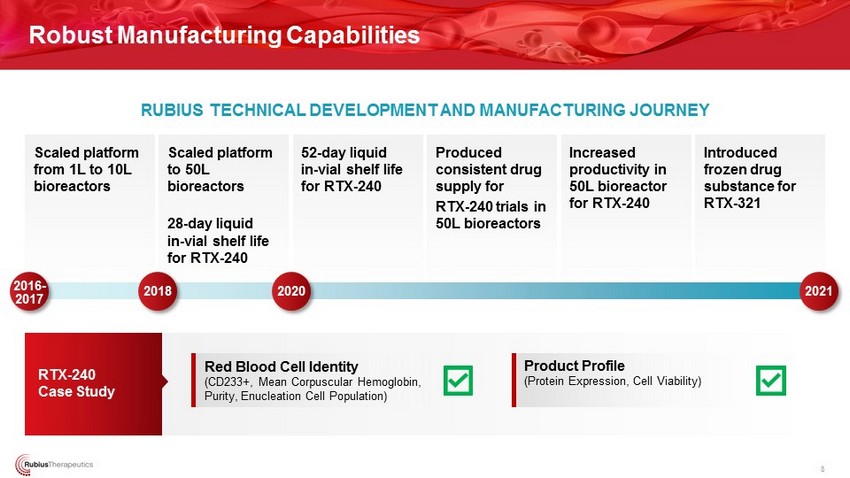
Scaled platform from 1L to 10L bioreactors Scaled platform to 50L bioreactors 28 - day liquid in - vial shelf life for RTX - 240 52 - day liquid in - vial shelf life for RTX - 240 Produced consistent drug supply for RTX - 240 trials in 50L bioreactors Increased productivity in 50L bioreactor for RTX - 240 Introduced frozen drug substance for RTX - 321 RTX - 240 Case Study Robust Manufacturing Capabilities 8 2016 - 2017 RUBIUS TECHNICAL DEVELOPMENT AND MANUFACTURING JOURNEY Product Profile (Protein Expression, Cell Viability) Red Blood Cell Identity (CD233+, Mean Corpuscular Hemoglobin, Purity, Enucleation Cell Population) 2018 2020 2021

BROAD IMMUNE SYSTEM STIMULATION Stimulate adaptive and innate immunity • Potential broad therapeutic window of agonists expressed on cell surface • Biodistribution may reduce toxicities of agonists Realize the power of immune agonists CANCER AUTOIMMUNE DISEASES ANTIGEN - SPECIFIC IMMUNE STIMULATION Drive tumor - specific responses • Drive tumor - specific responses and broad immune stimulation • Engineered red blood cells presenting all three signals* to optimally activate T cells Unlock the potential of antigen - specific immunotherapy TARGETING THE APC Induce immune tolerance • Autoantigen delivery and presentation of inhibitory molecules • Aim to create tolerogenic APCs that promote regulatory CD4+ cells Induce immune tolerance in high - potential indications RED PLATFORM ® Enables Multiple Modalities 9 Three signals: 4 - 1BBL, IL - 12, tumor - antigen peptide bound to MHC I
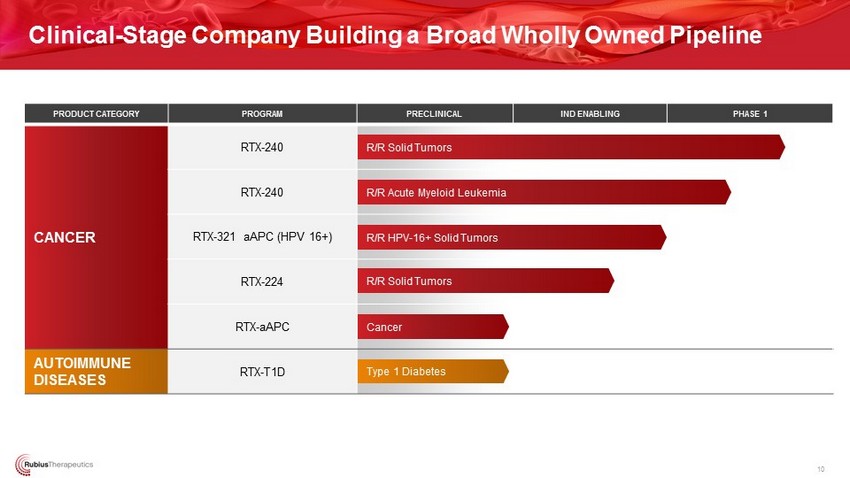
Clinical - Stage Company Building a Broad Wholly Owned Pipeline PRODUCT CATEGORY PROGRAM PRECLINICAL IND ENABLING PHASE 1 CANCER RTX - 240 RTX - 240 RTX - 321 aAPC (HPV 16+) RTX - 224 RTX - aAPC AUTOIMMUNE DISEASES RTX - T1D R/R Solid Tumors Type 1 Diabetes R/R Solid Tumors R/R Acute Myeloid Leukemia R/R HPV - 16+ Solid Tumors Cancer 10

RTX - 240: BROAD IMMUNE SYSTEM STIMULATION 11

RTX - 240: Enrolling Phase 1/2 Clinical Trial in Advanced Solid Tumors & Phase 1 Arm in Relapsed/Refractory AML 12 BROAD IMMUNE SYSTEM STIMULATION POTENTIAL BENEFITS: • Activate existing agonist pathways leading to enhanced potency • Improve anti - tumor activity • Overcome resistance to immunotherapy • Reduce toxicity given biodistribution confined to vasculature 4 - 1BBL NATURAL KILLER (NK) CELL 4 - 1BBL IL - 15TP T CELL STIMULATE ADAPTIVE AND INNATE IMMUNE CELL AGONIST PATHWAYS RTX* - 240 | (4 - 1BBL + IL - 15TP) IL - 15TP * Rubius Therapeutics Terminology: RTX – Red Cell Therapeutic product candidate; mRBC – mouse surrogate model; RCT – experimental construct

Proposed Mechanism of RTX - 240 PERIPHERAL BLOOD SPLEEN NK cell RTX RBC T cell RTX RBC T cell Red pulp White pulp NK cell T cell NK cell Tumor cell Potential for enhanced efficacy and safety by confining RTX - 240 to the vasculature 13 4 Activation Trafficking Tumor killing Expansion 1 2 3 1 2 3 4
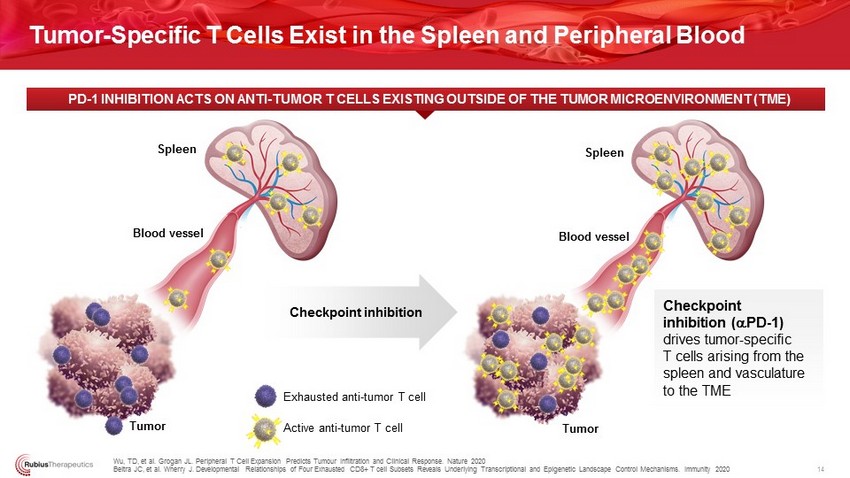
Exhausted anti - tumor T cell Active anti - tumor T cell Spleen Blood vessel Tumor Spleen Blood vessel Tumor Wu, TD, et al. Grogan JL. Peripheral T Cell Expansion Predicts Tumour Infiltration and Clinical Response. Nature 2020 Beltra JC, et al. Wherry J. Developmental Relationships of Four Exhausted CD8+ T cell Subsets Reveals Underlying Transcriptional and E pigenetic Landscape Control Mechanisms. Immunity 2020 PD - 1 INHIBITION ACTS ON ANTI - TUMOR T CELLS EXISTING OUTSIDE OF T HE TUMOR MICROENVIRONMENT (TME) 14 Tumor - Specific T Cells Exist in the Spleen and Peripheral Blood Checkpoint inhibition Checkpoint inhibition ( a PD - 1) drives tumor - specific T cells arising from the spleen and vasculature to the TME

T AND NK CELL EXPANSION IN BLOOD (In Vivo with healthy mice) 0 2×10 5 4×10 5 0 5×10 5 1×10 6 1.5×10 6 2×10 6 Number of CD8 RTX - 240 Preclinical Data Demonstrated Mechanism of Action In Vivo 15 CD8+ T Cells NK Cells 0 20 40 60 80 0 5 10 15 20 %CD8 of CD45+ %NK of CD45+ Model details: Normal mice; 4 Doses, 1x10 9 cells at days 0, 3, 7, 10; Sacrifice day 14 ~2x CD8+ T cells ~2x NK cells ~3x ~4x Number of NK Cells CT26 model details: 1x10 9 cells at days:1,5,8; PD evaluated in the tumors on Day 11 B16F10 model details: 1x10 9 cells at days:1,4,8; PD evaluated in the tumors on Day 10 CTRL mRBC - 240 1x10 9 CTRL mRBC - 240 T AND NK CELL TRAFFICKING TO TUMORS (CT26 and B16F10 tumor models)
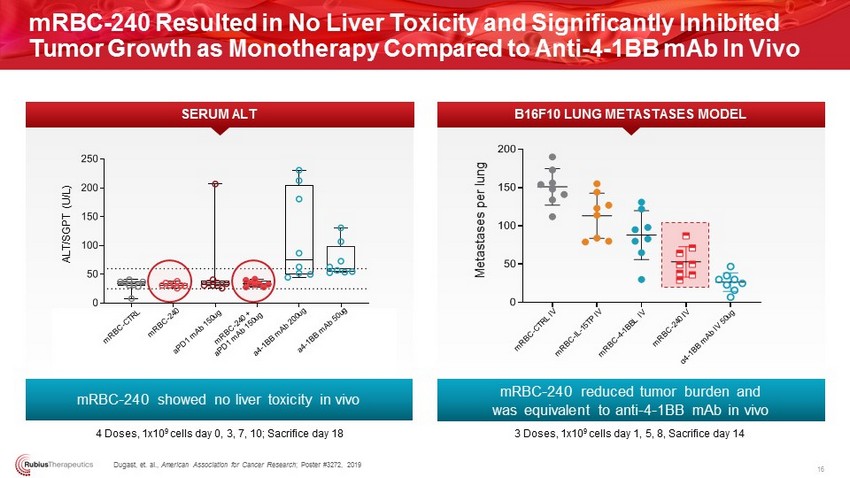
mRBC - 240 Resulted in No Liver Toxicity and Significantly Inhibited Tumor Growth as Monotherapy Compared to Anti - 4 - 1BB mAb In Vivo m R C T - C T R L m R C T - 2 1 2 1 E 9 P D 1 m A b 1 5 0 u g m R C T - 2 1 2 1 E 9 + P D 1 m A b 1 5 0 u g 4 - 1 B B m A b 2 0 0 u g 4 - 1 B B m A b 5 0 u g 0 50 100 150 200 250 4 Doses, 1x10 9 cells day 0, 3, 7, 10; Sacrifice day 18 ALT/SGPT (U/L) Dugast , et. al., American Association for Cancer Research ; Poster #3272, 2019 16 0 50 100 150 200 mRBC - 240 reduced tumor burden and was equivalent to anti - 4 - 1BB mAb in vivo B16F10 LUNG METASTASES MODEL Metastases per lung 3 Doses, 1x10 9 cells day 1, 5, 8, Sacrifice day 14 mRBC - 240 showed no liver toxicity in vivo SERUM ALT

17 RTX - 240 CLINICAL DEVELOPMENT PROGRAM & KEY TAKEAWAYS FROM INITIAL DATA

RTX - 240 Solid Tumor Clinical Trial Objectives 18 • Determine safety and tolerability, define recommended Phase 2 dose (RP2D) and interval of RTX - 240 • Assess pharmacodynamic (PD) activity of RTX - 240 measured by changes in T and NK cell number and function relative to baseline: – Activation of NK and T cells (e.g., HLA - DR expression, NKp30) – Expansion with increased absolute numbers of target cells – Tumor - trafficking via immunofluorescence* • Measure anti - tumor activity by objective response rate (ORR) • Assess pharmacokinetics (PK) of RTX - 240 18 PHASE 1 OBJECTIVES *Assays performed on a limited set of optional biopsies from participating patients PHASE 1 OBJECTIVES

RTX - 240 Solid Tumor Clinical Development Plan RTX - 240 Phase 2: Tumor - Specific Expansion Cohort #2 ENROLLING: Phase 1 Dose Escalation 5 Dose Cohorts Completed to Date (n=14) Phase 2: Tumor - Specific Expansion Cohort #1 19 • R/R or locally advanced, unresectable solid tumor for which no standard therapy exists, or for which the patient is ineligible or has declined standard therapy • Median of 3.5 prior lines of therapy (range, 1 - 10) • 10 of 14 patients had prior PD - 1/PD - L1 inhibitor therapy DEVELOPMENT PLAN 19 RELAPSED/ REFRACTORY (R/R) OR LOCALLY ADVANCED SOLID TUMORS ELIGIBILITY CRITERIA 1e8 (n=2) Q4W 1e9 (n=3) Q6W 3e9 (n=3) Q4W 1e10 (n=3) Q4W Further explore dose & interval 1e10 (n=3) Q4W* *Exploratory alternate route of administration

In the majority of patients (n=8), all of the following were observed across dose levels: ▪ Activation of NK cells ▪ Activation of T cells ▪ Expansion of NK cells ▪ Expansion of T cells Key Takeaways from Initial Data – RTX - 240 Stimulates Adaptive and Innate Immunity, Supporting Proof of Mechanism 20 No treatment - related Grade 3 - 4 adverse events and no dose - limiting toxicities observed to date (n=14) Additional clinical data to be presented in early 2021, and submitted to a scientific conference All patients showed activation of NK or T cells or both cell types (n=14)

• Determine safety and tolerability, MTD, RP2D and dosing interval of RTX - 240 • Assess PD effects of RTX - 240 as measured by changes in NK and T cell number and function relative to baseline in peripheral blood and paired bone marrow biopsies • Measure anti - tumor activity by ORR • Assess PK of RTX - 240 1e8 Q4W 1e9 Q4W 5e9 Q4W 1e10 Q4W Further explore dose and interval RTX - 240 AML Clinical Development Plan ENROLLING: Phase 1 Dose Escalation Phase 2 Expansion 21 RELAPSED/ REFRACTORY ACUTE MYELOID LEUKEMIA • R/R cytologically confirmed AML • Patients with prior stem cell transplant or transplant ineligible are included ELIGIBILITY CRITERIA KEY PHASE 1 OBJECTIVES: RTX - 240 DEVELOPMENT PLAN

RTX - 321: ANTIGEN - SPECIFIC IMMUNE STIMULATION 22

ANTIGEN PRESENTATION Rubius’ First Engineered aAPC will Target HPV+ Tumors – IND Cleared and Screening Patients for Phase 1 Clinical Trial SELECTIVE TUMOR KILLING BY EXPANDING TUMOR - SPECIFIC T CELLS BROAD IMMUNE SYSTEM STIMULATION LEADS TO EPITOPE SPREADING • Replicate immune system function to activate and expand antigen - specific T cells for a potent anti - tumor effect • Induce antigen - specific response and epitope spreading for a broad and effective anti - tumor response RTX - 321 (aAPC) | HPV 16+ Tumors Signal 2 4 - 1BBL T Cell Signal 1 HPV - 16 Antigen Signal 3 IL - 12 23 POTENTIAL BENEFITS:

0 10 20 30 40 0 2000 4000 6000 OT-1 (5E5) naïve group mRBC-OVA-4-1BBL-IL-12 previously cured (n=7) 7/7 cures 0 10 20 30 0 2000 4000 6000 OT-1 (5E5) naïve group mRBC-OVA-4-1BBL-IL-12 previously cured (n=7) 1E5 EL4 3/7 delayed 3/7 cures Re - challenge with EL4, the “parental” line of EG7.OVA. EL4 expresses other tumor antigens, but not OVA, the antigen that was presented by the aAPC Tumor volume mm 3 2E6 EG7.OVA re - challenge Days post EG7.OVA re - challenge Re - challenge with original tumor Tumor volume mm 3 Days post EL4 challenge OT - 1 (5E5) naïve group Re - challenge of Cured Mice Demonstrates Memory and Epitope Spreading Nixon, et. al., American Society of Gene & Cell Therapy ; Poster #32, 2020 24 EG7.OVA RE - CHALLENGE EL4 CHALLENGE

RTX - 321 Clinical Development Plan in Advanced HPV+ Tumors SCREENING : Phase 1 Dose Escalation Phase 2 Expansions in Specific Indications 25 PERSISTENT, RECURRENT, OR METASTATIC, UNRESECTABLE, HPV 16 - POSITIVE CANCERS • Cervical cancer, head and neck squamous cell cancer (HNSCC), or squamous cell cancer of the anal canal that is not amenable to curative therapy • HLA - A*02:01 - positive patients • Documentation of HPV 16+ tumor for patients with cervical cancer and HNSCC • Patients will have received standard platinum - or mitomycin C - based chemo and, where clinically appropriate, bevacizumab and/or PD - 1/PD - L1 therapy ELIGIBILITY CRITERIA RTX - 321 DEVELOPMENT PLAN 1e9 Q3W 5e9 Q3W 1e10 Q3W Further explore dose and interval • Determine the safety and tolerability of monotherapy RTX - 321 and RP2D PRIMARY MEASURES • Assess PD changes in immune cell populations in peripheral blood and tumor biopsies • Determine anti - tumor activity of RTX - 321 as measured by ORR, duration of response, progression free survival and overall survival SECONDARY MEASURES

EXPECTED CATALYSTS 26

Present additional clinical data for RTX - 240 solid tumor trial in early 2021, and submit to a scientific conference Anticipated Upcoming Catalysts and Operational Objectives 27 AN INTEGRATED DEVELOPMENT COMPANY Continue to enroll patients in RTX - 240 AML clinical trial Dose first patient in RTX - 321 clinical trial in HPV 16+ cancers Continue to produce cGMP material for RTX - 240 and RTX - 321 trials from Rubius site Cash runway into 2022 Unlocking the potential of the RED PLATFORM in cancer and autoimmune disease Present an integrated clinical program for RTX - 240, including plans for expansion cohorts, and oncology pipeline

REALIZING THE POWER OF RED™ A NEW ERA IN CELLULAR MEDICINE 28
