Attached files
| file | filename |
|---|---|
| 8-K - 8-K - REATA PHARMACEUTICALS INC | reta-8k_20210111.htm |

Investor Presentation JANUARY 2021 Exhibit 99.1
Forward-Looking Statements This presentation contains certain “forward-looking” statements that are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical or present facts, are forward-looking statements, including statements regarding our future financial condition, future revenues, projected costs, prospects, business strategy, and plans and objectives of management for future operations, including our plans to submit for regulatory filings. In some cases, you can identify forward-looking statements by terminology such as “believe,” “will,” “may,” “might,” “estimate,” “continue,” “anticipate,” “intend,” “target,” “project,” “model,” “should,” “would,” “plan,” “expect,” “predict,” “could,” “seek,” “goal,” “potential,” or the negative of these terms or other similar terms or expressions that concern our expectations, strategy, plans, or intentions. These statements are based on our intentions, beliefs, projections, outlook, analyses, or current expectations using currently available information, and are not guarantees of future performance, and involve certain risks and uncertainties. Although we believe that the expectations reflected in these forward-looking statements are reasonable, we cannot assure you that our expectations will prove to be correct. Therefore, actual outcomes and results could materially differ from what is expressed, implied, or forecasted in these statements. Any differences could be caused by a number of factors including but not limited to: our expectations regarding the timing, costs, conduct, and outcome of our clinical trials, including statements regarding the timing of the initiation and availability of data from such trials; the timing and likelihood of regulatory filings and approvals for our product candidates; whether regulatory authorities determine that additional trials or data are necessary in order to obtain approval; our ability to obtain funding for our operations, including funding necessary to complete further development and commercialization of our product candidates; our plans to research, develop, and commercialize our product candidates; the commercialization of our product candidates, if approved; the rate and degree of market acceptance of our product candidates; our expectations regarding the potential market size and the size of the patient populations for our product candidates, if approved for commercial use, and the potential market opportunities for commercializing our product candidates; the success of competing therapies that are or may become available; our expectations regarding our ability to obtain and maintain intellectual property protection for our product candidates; the ability to license additional intellectual property relating to our product candidates and to comply with our existing license agreements; our ability to maintain and establish relationships with third parties, such as contract research organizations, suppliers, and distributors; our ability to maintain and establish collaborators with development, regulatory, and commercialization expertise; our ability to attract and retain key scientific or management personnel; our ability to grow our organization and increase the size of our facilities to meet our anticipated growth; the accuracy of our estimates regarding expenses, future revenue, capital requirements, and needs for additional financing; our expectations related to the use of our available cash; our ability to develop, acquire, and advance product candidates into, and successfully complete, clinical trials; the initiation, timing, progress, and results of future preclinical studies and developments and projections relating to our competitors and our industry. Additional factors that could cause actual results to differ materially from our expectations can be found in our Securities and Exchange Commission filings. Moreover, we operate in a very competitive and rapidly changing environment. New risk factors emerge from time to time, and it is not possible for our management to predict all risk factors, nor can we assess the effects of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in, or implied by, any forward-looking statements. All forward-looking statements included in this presentation are expressly qualified in their entirety by these cautionary statements. The forward-looking statements speak only as of the date made and, other than as required by law, we undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise. Bardoxolone methyl, omaveloxolone, and RTA 901 are investigational drugs, and their safety and efficacy have not been established by any agency. 2
Reata at a Glance CKD1 Pipeline Positive Phase 3 data for bardoxolone in Alport syndrome NDA filing planned for 1Q21 Pipeline in rare forms of CKD including the ongoing Phase 3 FALCON study in ADPKD Neurology Pipeline Plan to initiate 2nd pivotal study of omaveloxolone in FA in 2H21, following discussion with FDA Plan to study omaveloxolone in additional neurological diseases Plan to initiate Phase 2 study for RTA 901 in DPNP in 4Q21 Global Opportunity Few or no effective therapies approved for lead indications Worldwide commercial rights to all pipeline assets2 Robust IP protection for bardoxolone, omaveloxolone, and RTA 901 1Definitions in slide 53; 2Ex-Asia for bardoxolone 3
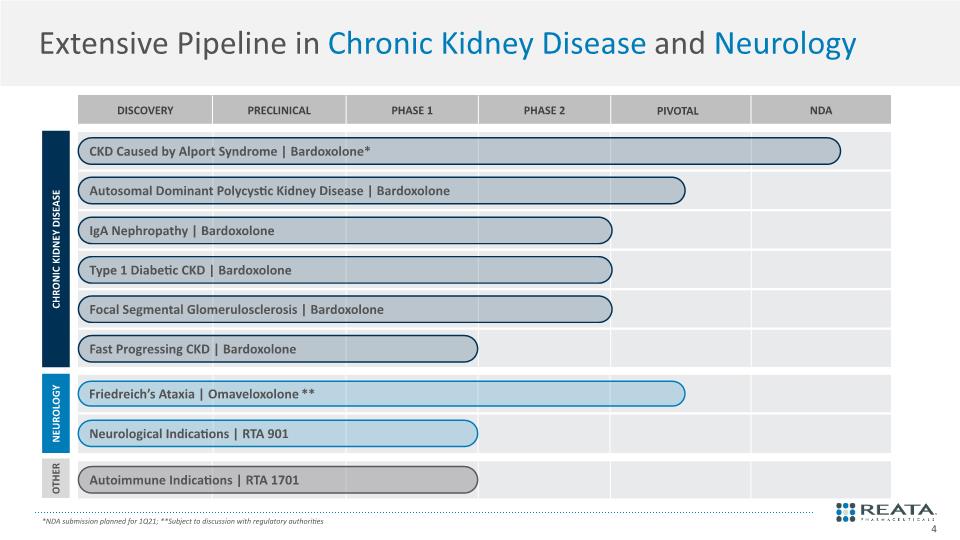
Extensive Pipeline in Chronic Kidney Disease and Neurology Autoimmune Indications | RTA 1701 CKD Caused by Alport Syndrome | Bardoxolone* IgA Nephropathy | Bardoxolone Type 1 Diabetic CKD | Bardoxolone Focal Segmental Glomerulosclerosis | Bardoxolone Autosomal Dominant Polycystic Kidney Disease | Bardoxolone Friedreich’s Ataxia | Omaveloxolone** Neurological Indications | RTA 901 CHRONIC KIDNEY DISEASE NEUROLOGY OTHER *NDA submission planned for 1Q21; **Subject to discussion with regulatory authorities Fast Progressing CKD | Bardoxolone 4

Key 2021 Goals CKD Caused by ADPKD | Bardoxolone Continued enrollment in Phase 3 FALCON study Submit NDA in 1Q21 If designated as priority review, PDUFA expected in 4Q21 Submit MAA in 4Q21 CKD Caused by Alport Syndrome | Bardoxolone Plan to initiate Phase 2 study in 1Q21 Data expected 2H21 Fast Progressing CKD | Bardoxolone Plan to initiate second pivotal study in 2H21 following discussion with FDA Friedreich’s Ataxia | Omaveloxolone Plan to initiate additional Phase 1 studies in 2Q21 Plan to initiate Phase 2 study in 4Q21 DPNP | RTA 901 CHRONIC KIDNEY DISEASE NEUROLOGY 5

Chronic Kidney Disease Pipeline
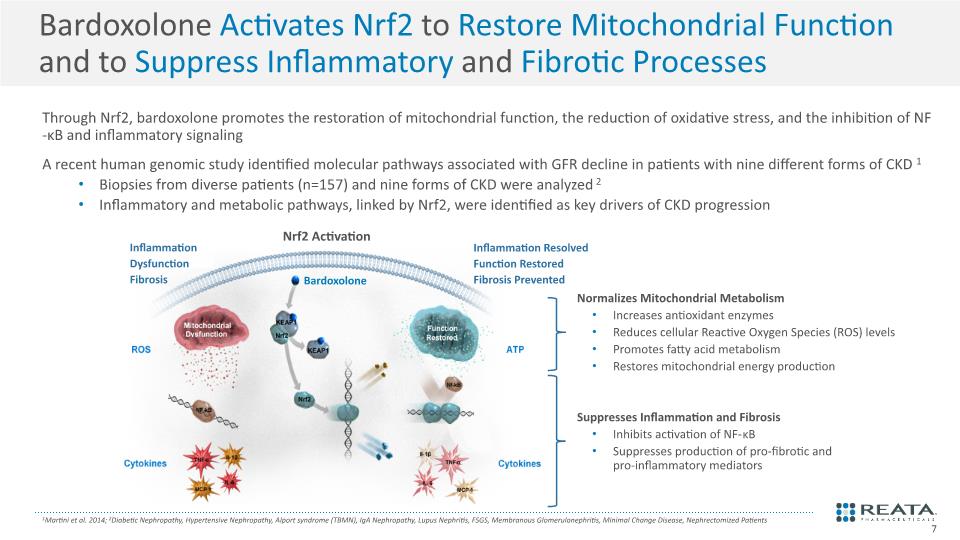
Bardoxolone Activates Nrf2 to Restore Mitochondrial Function and to Suppress Inflammatory and Fibrotic Processes Through Nrf2, bardoxolone promotes the restoration of mitochondrial function, the reduction of oxidative stress, and the inhibition of NF-κB and inflammatory signaling A recent human genomic study identified molecular pathways associated with GFR decline in patients with nine different forms of CKD1 Biopsies from diverse patients (n=157) and nine forms of CKD were analyzed2 Inflammatory and metabolic pathways, linked by Nrf2, were identified as key drivers of CKD progression 1Martini et al. 2014; 2Diabetic Nephropathy, Hypertensive Nephropathy, Alport syndrome (TBMN), IgA Nephropathy, Lupus Nephritis, FSGS, Membranous Glomerulonephritis, Minimal Change Disease, Nephrectomized Patients 7
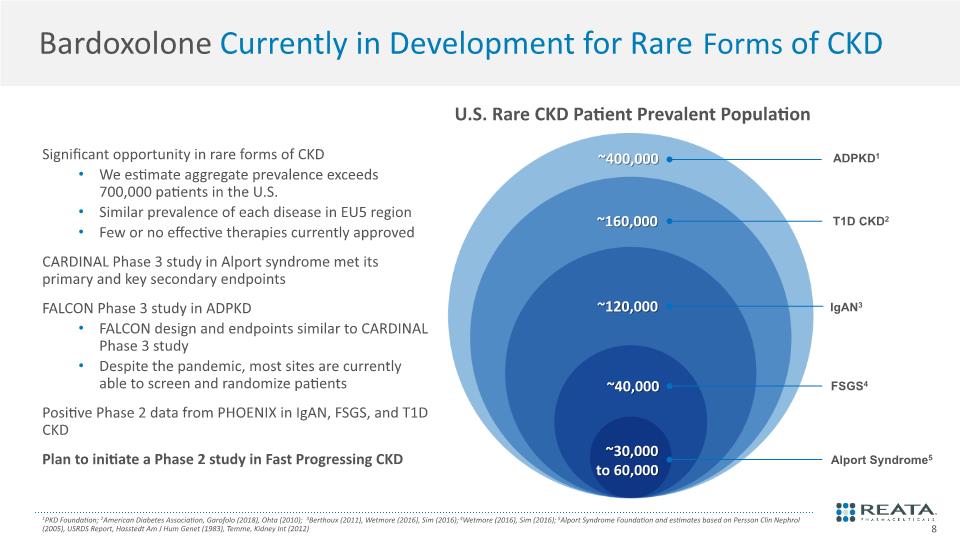
Bardoxolone Currently in Development for Rare Forms of CKD Significant opportunity in rare forms of CKD We estimate aggregate prevalence exceeds 700,000 patients in the U.S. Similar prevalence of each disease in EU5 region Few or no effective therapies currently approved CARDINAL Phase 3 study in Alport syndrome met its primary and key secondary endpoints FALCON Phase 3 study in ADPKD FALCON design and endpoints similar to CARDINAL Phase 3 study Despite the pandemic, most sites are currently able to screen and randomize patients Positive Phase 2 data from PHOENIX in IgAN, FSGS, and T1D CKD Plan to initiate a Phase 2 study in Fast Progressing CKD 1PKD Foundation; 2American Diabetes Association, Garofolo (2018), Ohta (2010); 3Berthoux (2011), Wetmore (2016), Sim (2016); 4Wetmore (2016), Sim (2016); 5Alport Syndrome Foundation and estimates based on Persson Clin Nephrol (2005), USRDS Report, Hasstedt Am J Hum Genet (1983), Temme, Kidney Int (2012) U.S. Rare CKD Patient Prevalent Population FSGS4 Alport Syndrome5 ~120,000 ~30,000 to 60,000 ~160,000 IgAN3 T1D CKD2 ADPKD1 ~40,000 ~400,000 8
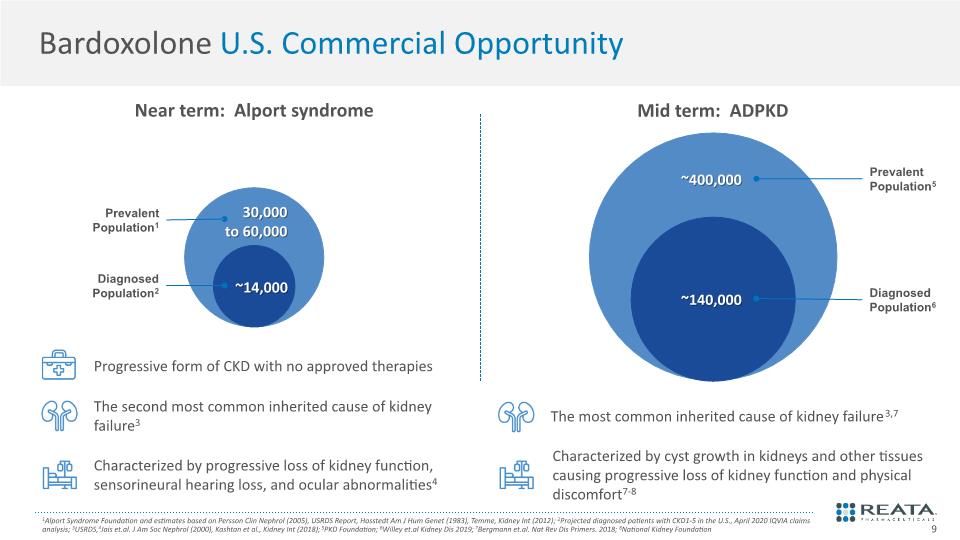
Bardoxolone U.S. Commercial Opportunity 9 1Alport Syndrome Foundation and estimates based on Persson Clin Nephrol (2005), USRDS Report, Hasstedt Am J Hum Genet (1983), Temme, Kidney Int (2012); 2Projected diagnosed patients with CKD1-5 in the U.S., April 2020 IQVIA claims analysis; 3USRDS,4Jais et.al. J Am Soc Nephrol (2000), Kashtan et al., Kidney Int (2018); 5PKD Foundation; 6Willey et.al Kidney Dis 2019; 7Bergmann et.al. Nat Rev Dis Primers. 2018; 8National Kidney Foundation Mid term: ADPKD Near term: Alport syndrome

Bardoxolone in Alport Syndrome
1Kruegel et al., Nat Rev Nephrol (2013), Gomez et al., JCI (2015); 2Groopman et al. N Engl J Med. 2019; 3Kashtan et al., Kidney Int (2018); 4Jais et al., JASN (2000); 5NKF-ASF-FDA Voice of the Patient Externally Led Patient-Focused Drug Development Meeting on Alport Syndrome, August 3, 2018; 6CARDINAL Phase 3 historical eGFR decline rate Alport Syndrome Background 11
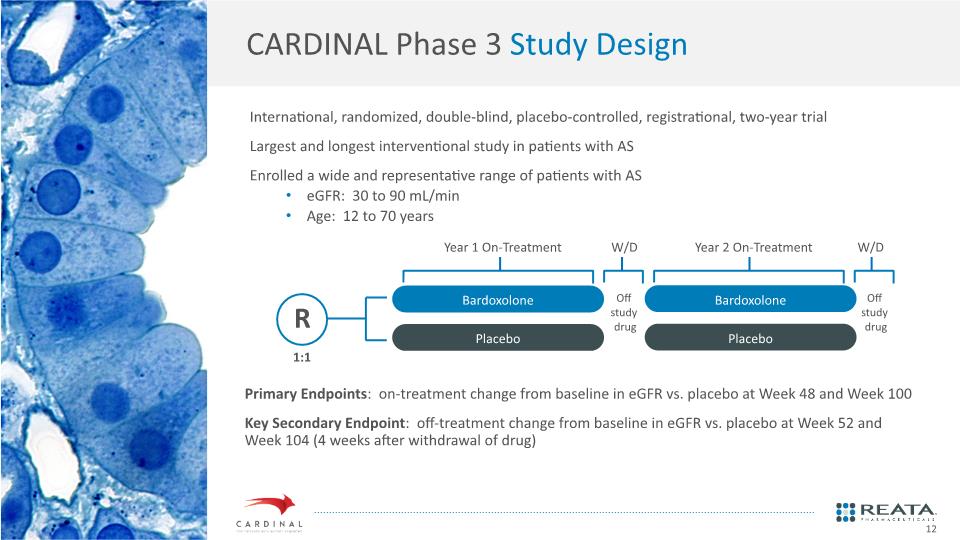
CARDINAL Phase 3 Study Design Primary Endpoints: on-treatment change from baseline in eGFR vs. placebo at Week 48 and Week 100 Key Secondary Endpoint: off-treatment change from baseline in eGFR vs. placebo at Week 52 and Week 104 (4 weeks after withdrawal of drug) International, randomized, double-blind, placebo-controlled, registrational, two-year trial Largest and longest interventional study in patients with AS Enrolled a wide and representative range of patients with AS eGFR: 30 to 90 mL/min Age: 12 to 70 years 12
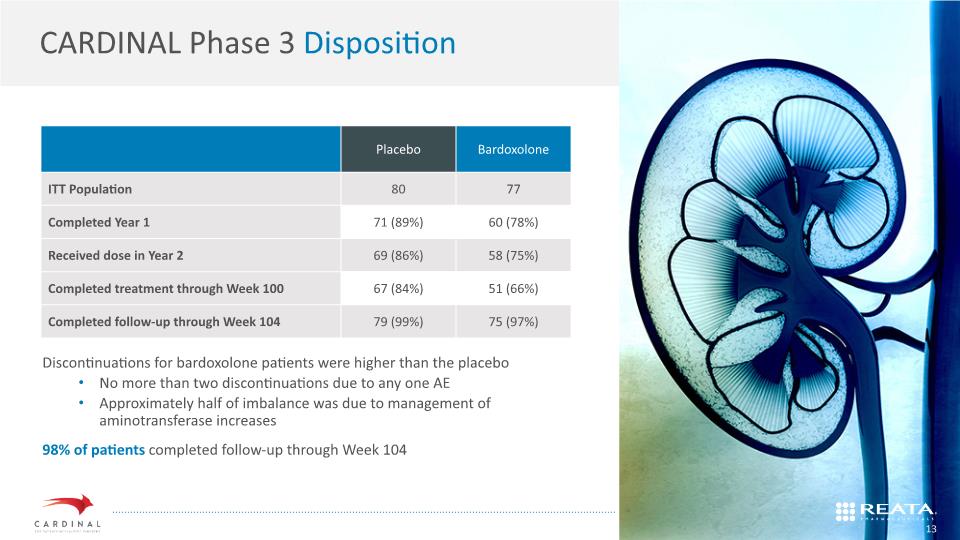
CARDINAL Phase 3 Disposition KEYWORD Discontinuations for bardoxolone patients were higher than the placebo No more than two discontinuations due to any one AE Approximately half of imbalance was due to management of aminotransferase increases 98% of patients completed follow-up through Week 104 13
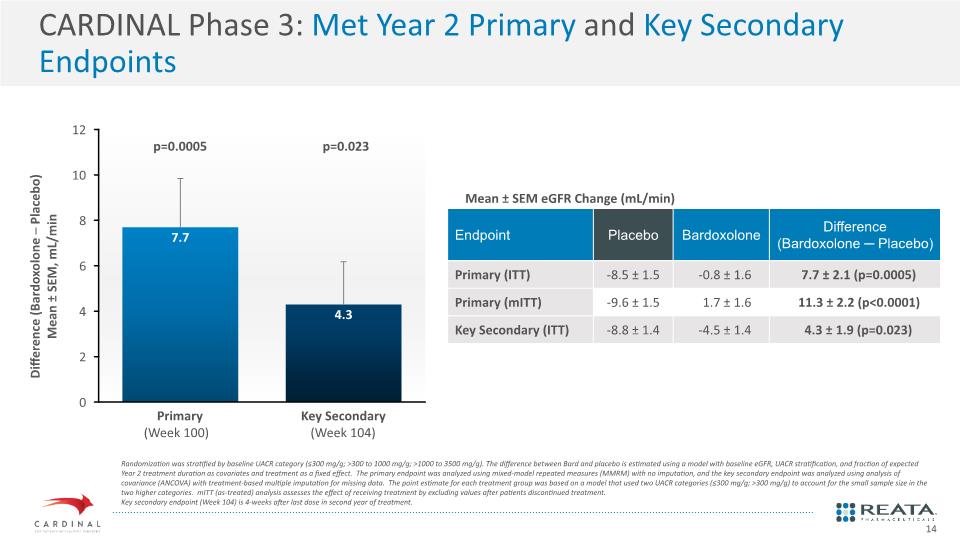
CARDINAL Phase 3: Met Year 2 Primary and Key Secondary Endpoints Mean ± SEM eGFR Change (mL/min) Randomization was stratified by baseline UACR category (≤300 mg/g; >300 to 1000 mg/g; >1000 to 3500 mg/g). The difference between Bard and placebo is estimated using a model with baseline eGFR, UACR stratification, and fraction of expected Year 2 treatment duration as covariates and treatment as a fixed effect. The primary endpoint was analyzed using mixed-model repeated measures (MMRM) with no imputation, and the key secondary endpoint was analyzed using analysis of covariance (ANCOVA) with treatment-based multiple imputation for missing data. The point estimate for each treatment group was based on a model that used two UACR categories (≤300 mg/g; >300 mg/g) to account for the small sample size in the two higher categories. mITT (as-treated) analysis assesses the effect of receiving treatment by excluding values after patients discontinued treatment. Key secondary endpoint (Week 104) is 4-weeks after last dose in second year of treatment. 14
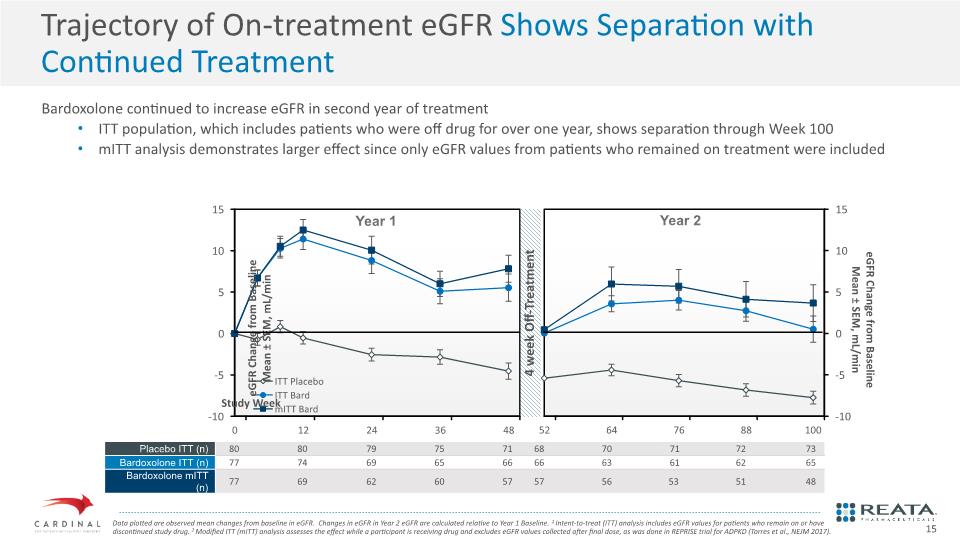
Bardoxolone continued to increase eGFR in second year of treatment ITT population, which includes patients who were off drug for over one year, shows separation through Week 100 mITT analysis demonstrates larger effect since only eGFR values from patients who remained on treatment were included Trajectory of On-treatment eGFR Shows Separation with Continued Treatment Data plotted are observed mean changes from baseline in eGFR. Changes in eGFR in Year 2 eGFR are calculated relative to Year 1 Baseline. 1 Intent-to-treat (ITT) analysis includes eGFR values for patients who remain on or have discontinued study drug. 2 Modified ITT (mITT) analysis assesses the effect while a participant is receiving drug and excludes eGFR values collected after final dose, as was done in REPRISE trial for ADPKD (Torres et al., NEJM 2017). 15
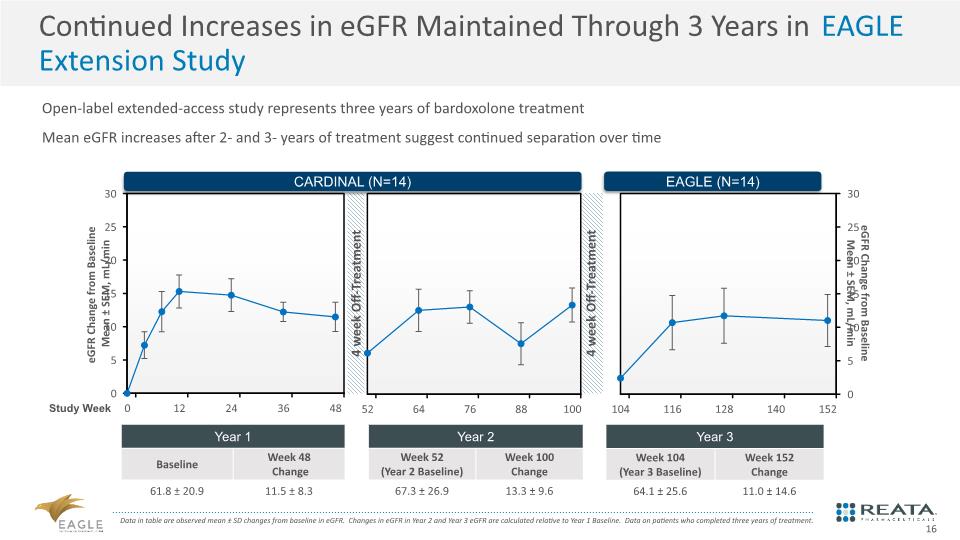
Open-label extended-access study represents three years of bardoxolone treatment Mean eGFR increases after 2- and 3- years of treatment suggest continued separation over time Continued Increases in eGFR Maintained Through 3 Years in EAGLE Extension Study Data in table are observed mean ± SD changes from baseline in eGFR. Changes in eGFR in Year 2 and Year 3 eGFR are calculated relative to Year 1 Baseline. Data on patients who completed three years of treatment. 16
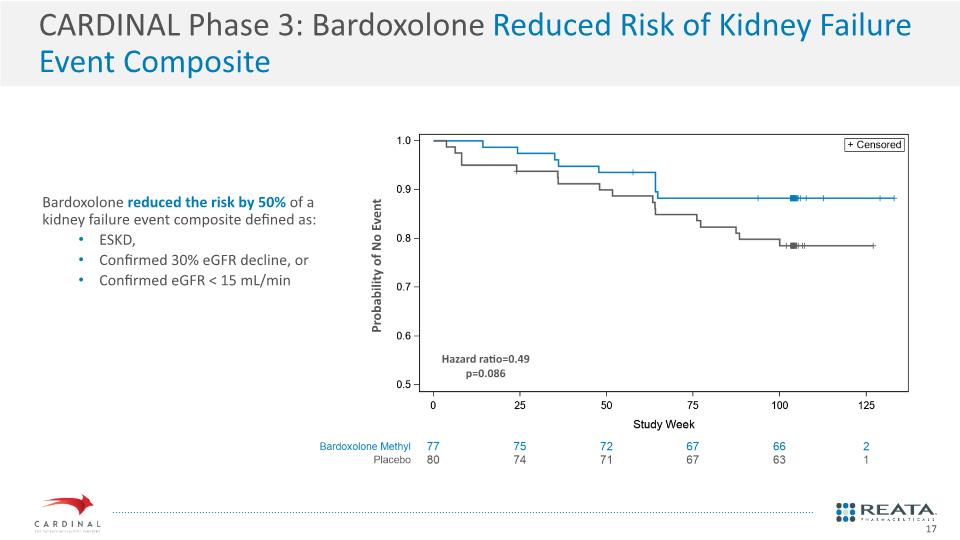
CARDINAL Phase 3: Bardoxolone Reduced Risk of Kidney Failure Event Composite Hazard ratio=0.49 p=0.086 Probability of No Event Bardoxolone reduced the risk by 50% of a kidney failure event composite defined as: ESKD, Confirmed 30% eGFR decline, or Confirmed eGFR < 15 mL/min 17
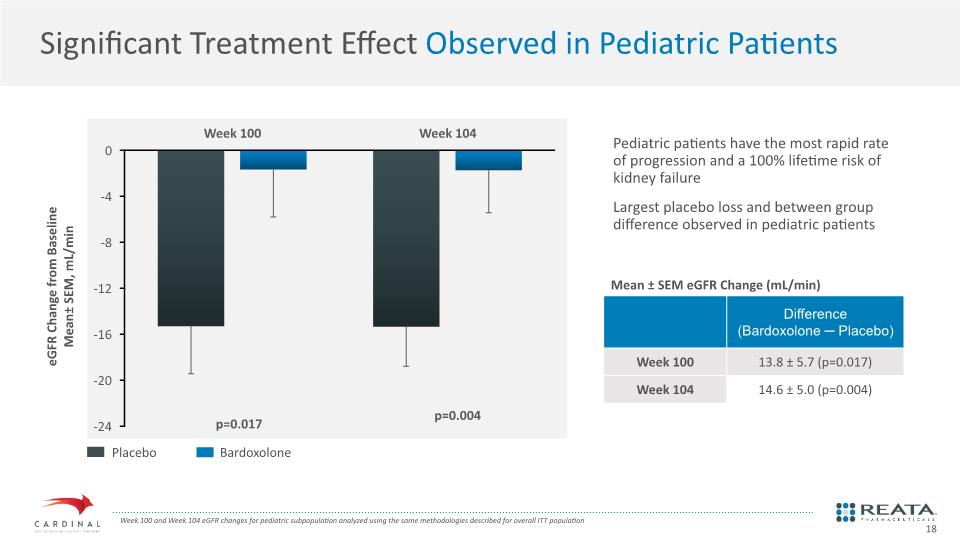
Significant Treatment Effect Observed in Pediatric Patients eGFR Change from Baseline Mean± SEM, mL/min Placebo Bardoxolone Week 100 and Week 104 eGFR changes for pediatric subpopulation analyzed using the same methodologies described for overall ITT population p=0.004 p=0.017 Mean ± SEM eGFR Change (mL/min) Pediatric patients have the most rapid rate of progression and a 100% lifetime risk of kidney failure Largest placebo loss and between group difference observed in pediatric patients 18
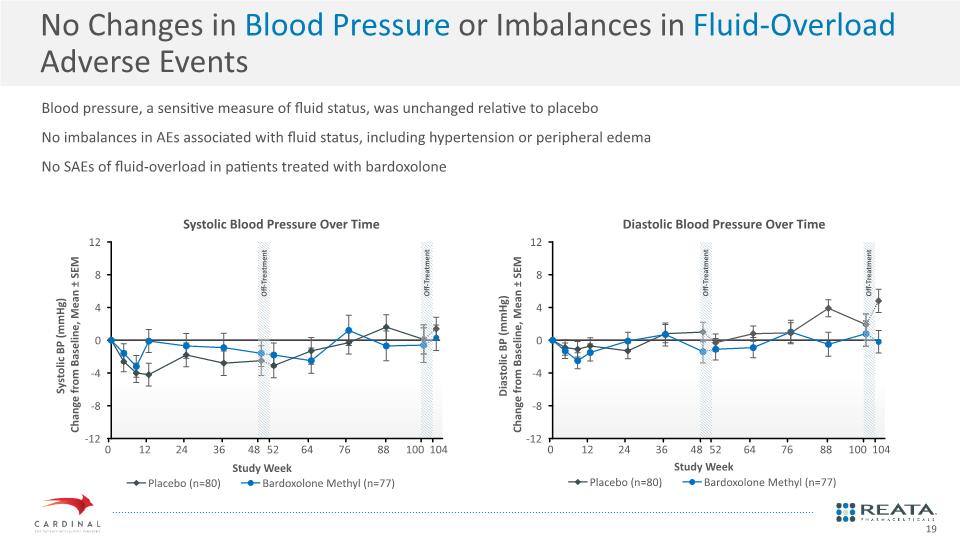
No Changes in Blood Pressure or Imbalances in Fluid-Overload Adverse Events Blood pressure, a sensitive measure of fluid status, was unchanged relative to placebo No imbalances in AEs associated with fluid status, including hypertension or peripheral edema No SAEs of fluid-overload in patients treated with bardoxolone Systolic Blood Pressure Over Time Diastolic Blood Pressure Over Time 19
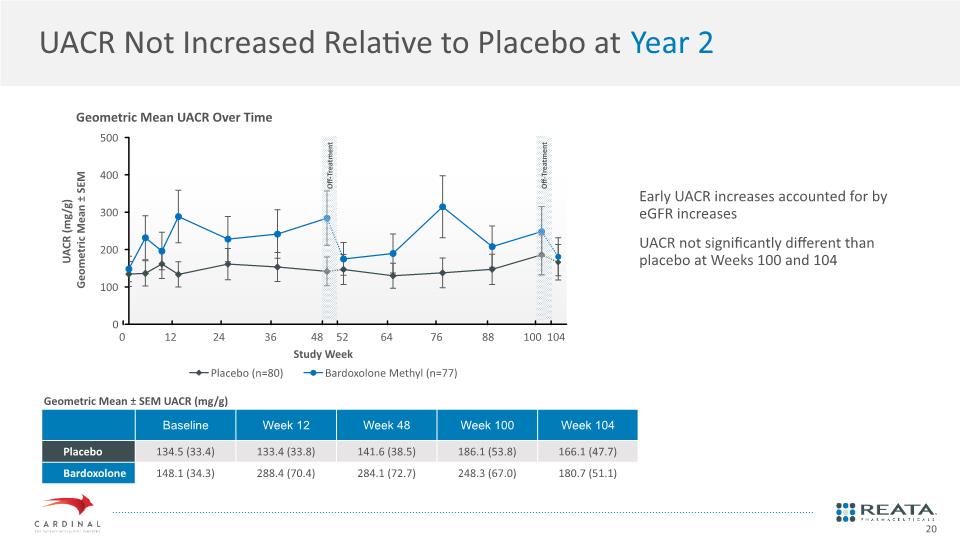
UACR Not Increased Relative to Placebo at Year 2 Early UACR increases accounted for by eGFR increases UACR not significantly different than placebo at Weeks 100 and 104 Geometric Mean UACR Over Time Geometric Mean ± SEM UACR (mg/g) 20
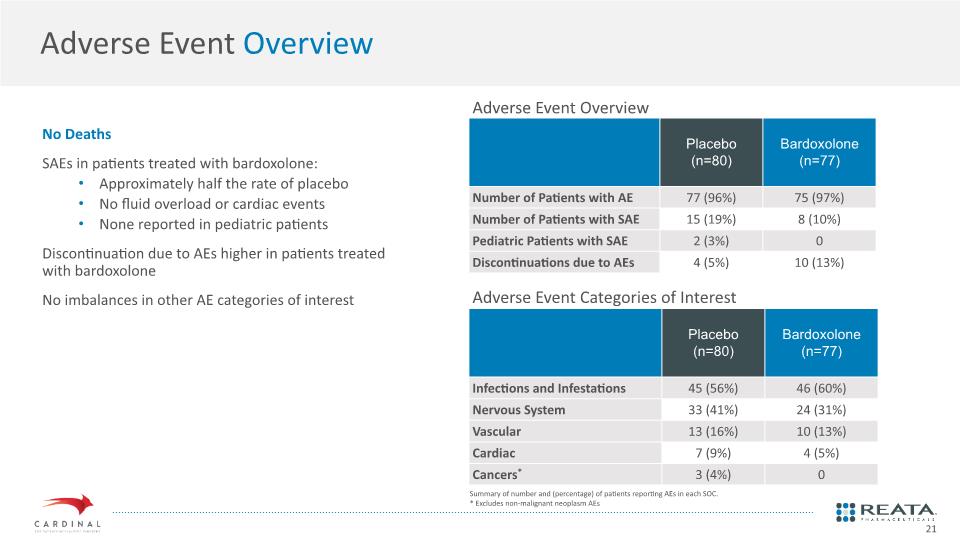
Adverse Event Overview No Deaths SAEs in patients treated with bardoxolone: Approximately half the rate of placebo No fluid overload or cardiac events None reported in pediatric patients Discontinuation due to AEs higher in patients treated with bardoxolone No imbalances in other AE categories of interest Adverse Event Overview Adverse Event Categories of Interest Summary of number and (percentage) of patients reporting AEs in each SOC. * Excludes non-malignant neoplasm AEs 21
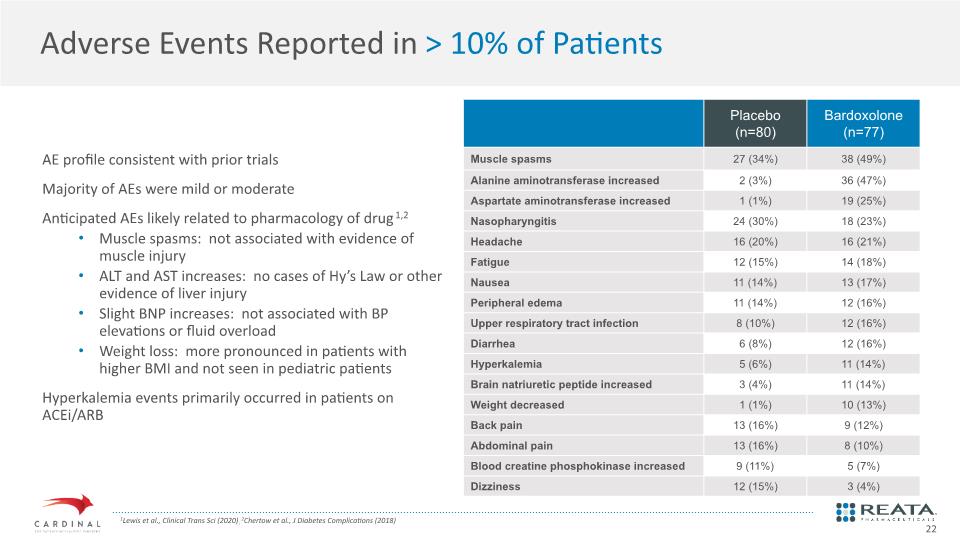
Adverse Events Reported in > 10% of Patients AE profile consistent with prior trials Majority of AEs were mild or moderate Anticipated AEs likely related to pharmacology of drug1,2 Muscle spasms: not associated with evidence of muscle injury ALT and AST increases: no cases of Hy’s Law or other evidence of liver injury Slight BNP increases: not associated with BP elevations or fluid overload Weight loss: more pronounced in patients with higher BMI and not seen in pediatric patients Hyperkalemia events primarily occurred in patients on ACEi/ARB 1Lewis et al., Clinical Trans Sci (2020); 2Chertow et al., J Diabetes Complications (2018) 22
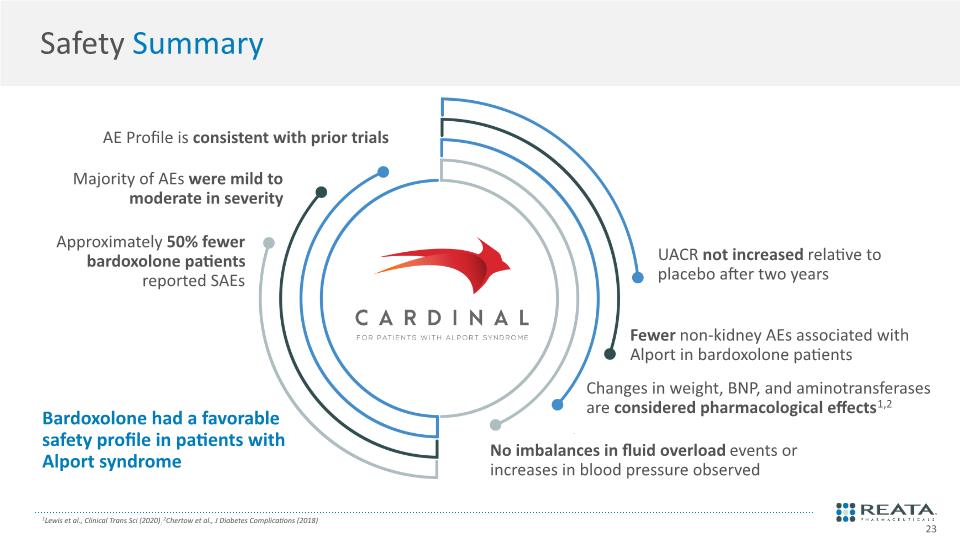
Safety Summary AE Profile is consistent with prior trials Majority of AEs were mild to moderate in severity Approximately 50% fewer bardoxolone patients reported SAEs UACR not increased relative to placebo after two years Fewer non-kidney AEs associated with Alport in bardoxolone patients Changes in weight, BNP, and aminotransferases are considered pharmacological effects1,2 No imbalances in fluid overload events or increases in blood pressure observed Bardoxolone had a favorable safety profile in patients with Alport syndrome 1Lewis et al., Clinical Trans Sci (2020); 2Chertow et al., J Diabetes Complications (2018) 23
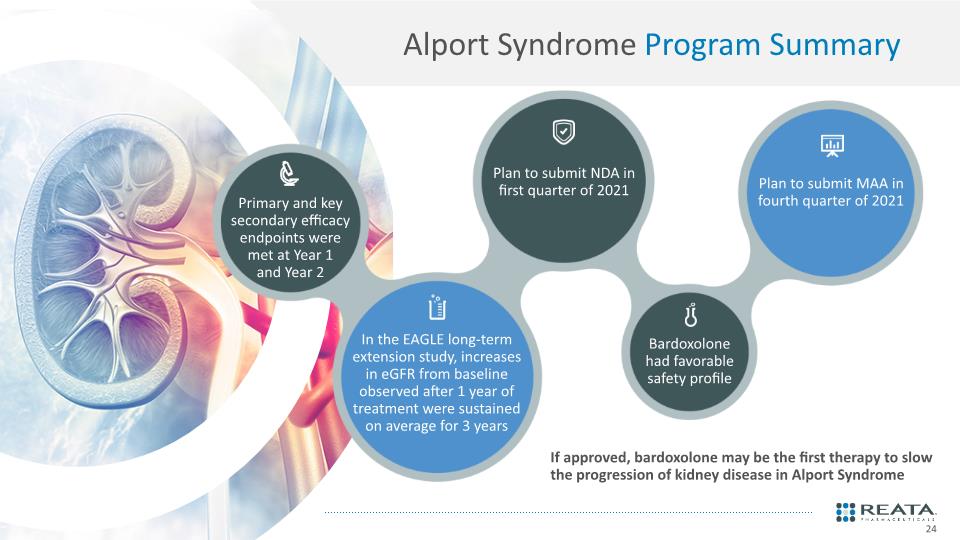
Primary and key secondary efficacy endpoints were met at Year 1 and Year 2 Plan to submit NDA in first quarter of 2021 In the EAGLE long-term extension study, increases in eGFR from baseline observed after 1 year of treatment were sustained on average for 3 years Bardoxolone had favorable safety profile Plan to submit MAA in fourth quarter of 2021 If approved, bardoxolone may be the first therapy to slow the progression of kidney disease in Alport Syndrome Alport Syndrome Program Summary 24
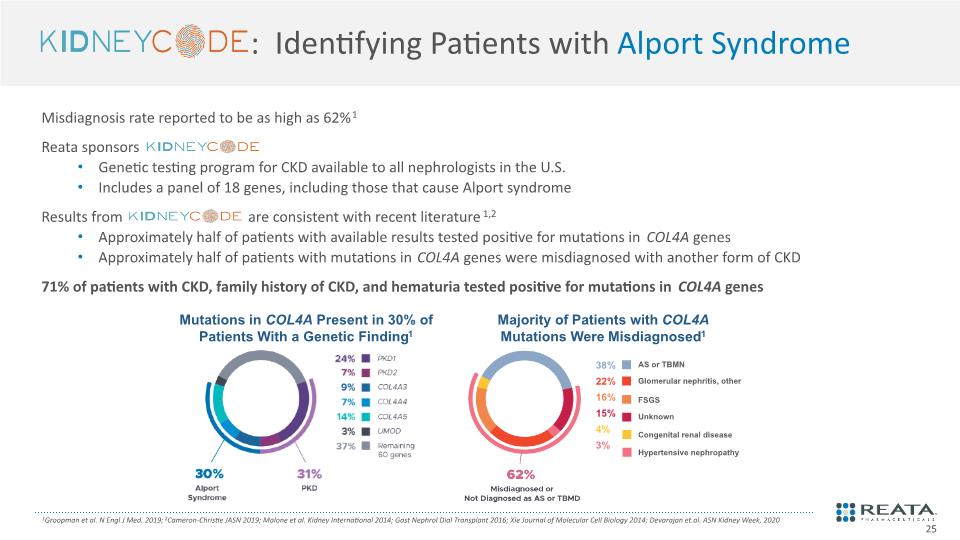
: Identifying Patients with Alport Syndrome 1Groopman et al. N Engl J Med. 2019; 2Cameron-Christie JASN 2019; Malone et al. Kidney International 2014; Gast Nephrol Dial Transplant 2016; Xie Journal of Molecular Cell Biology 2014; Devarajan et.al. ASN Kidney Week, 2020 Misdiagnosis rate reported to be as high as 62%1 Reata sponsors Genetic testing program for CKD available to all nephrologists in the U.S. Includes a panel of 18 genes, including those that cause Alport syndrome Results from are consistent with recent literature1,2 Approximately half of patients with available results tested positive for mutations in COL4A genes Approximately half of patients with mutations in COL4A genes were misdiagnosed with another form of CKD 71% of patients with CKD, family history of CKD, and hematuria tested positive for mutations in COL4A genes 25
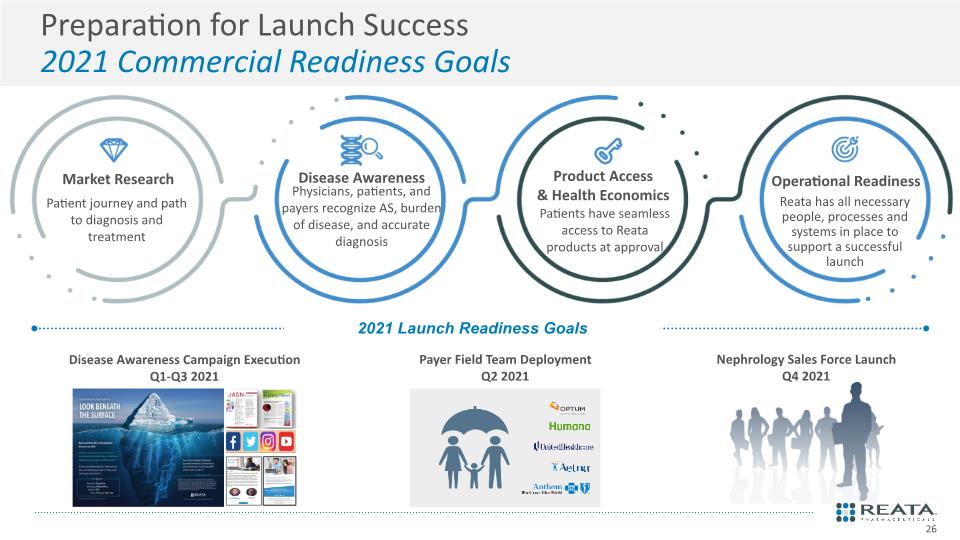
Disease Awareness Market Research Product Access & Health Economics Operational Readiness Reata has all necessary people, processes and systems in place to support a successful launch Preparation for Launch Success 2021 Commercial Readiness Goals Physicians, patients, and payers recognize AS, burden of disease, and accurate diagnosis Patients have seamless access to Reata products at approval Patient journey and path to diagnosis and treatment 26

Bardoxolone in ADPKD
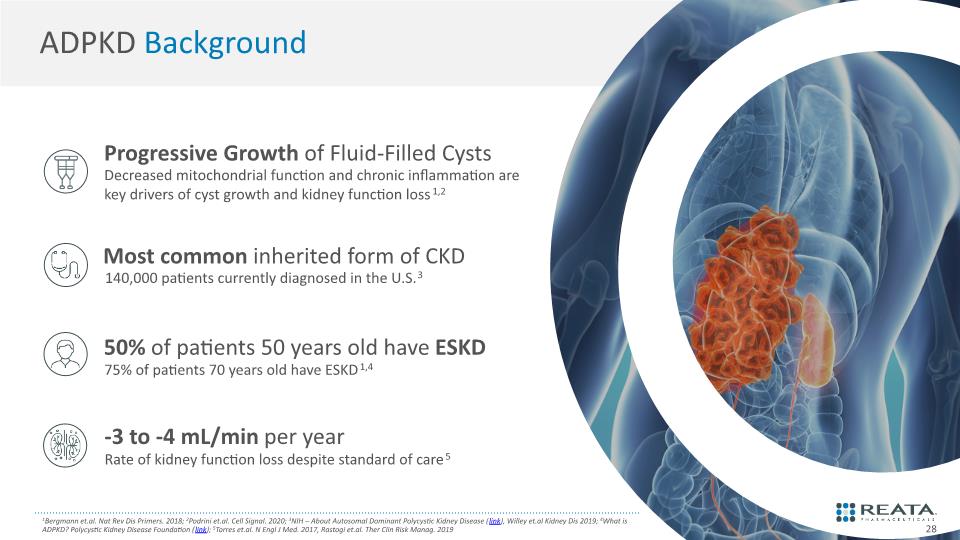
1Bergmann et.al. Nat Rev Dis Primers. 2018; 2Podrini et.al. Cell Signal. 2020; 3NIH – About Autosomal Dominant Polycystic Kidney Disease (link), Willey et.al Kidney Dis 2019; 4What is ADPKD? Polycystic Kidney Disease Foundation (link); 5Torres et.al. N Engl J Med. 2017, Rastogi et.al. Ther Clin Risk Manag. 2019 ADPKD Background 28

FALCON Phase 3 Study of Bardoxolone in ADPKD Phase 3 similar in design to CARDINAL with two-year treatment duration Planning to enroll approximately 300 patients across approximately 100 sites in the U.S., Europe, Australia, and Japan eGFR 30-90 mL/min Age 18-70 years old Primary Endpoints: off-treatment change from baseline in eGFR at Week 52 (4 weeks after withdrawal of drug at Week 48) Key Secondary Endpoint: off-treatment change from baseline in eGFR at Week 104 (4 weeks after withdrawal of drug at Week 100) Despite the pandemic, most sites are currently able to screen and randomize patients First patients have reached the second year of the trial 29
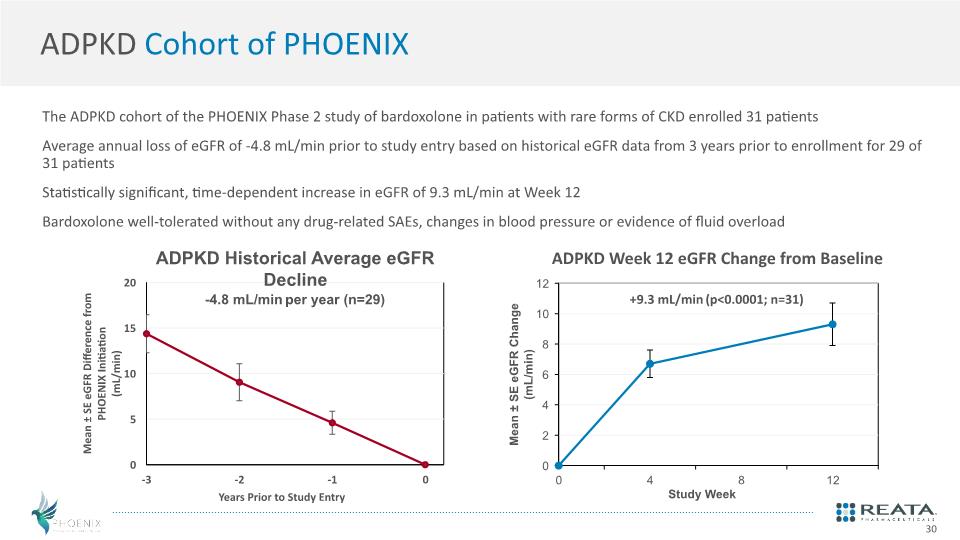
ADPKD Cohort of PHOENIX The ADPKD cohort of the PHOENIX Phase 2 study of bardoxolone in patients with rare forms of CKD enrolled 31 patients Average annual loss of eGFR of -4.8 mL/min prior to study entry based on historical eGFR data from 3 years prior to enrollment for 29 of 31 patients Statistically significant, time-dependent increase in eGFR of 9.3 mL/min at Week 12 Bardoxolone well-tolerated without any drug-related SAEs, changes in blood pressure or evidence of fluid overload 30


Omaveloxolone in Friedreich’s ataxia
1Cook and Giunti, Br. Med. Bull. 2017; 2Patel et. al. Ann. Clin. Transl. Neurol. 2016 Friedreich’s Ataxia Background 33

MOXIe Part 2 Study Design International, double-blind, placebo-controlled, randomized, registrational trial (n=103) Enrolled a wide and representative range of patients with FA Baseline mFARS: 20 to 80 Age: 16 to 40 years Patients randomized 1:1 to 150 mg omaveloxolone or placebo 48 week treatment duration Primary endpoint: change from baseline in mFARS at Week 48 Secondary endpoints: PGIC, CGIC, 9-HPT, 25FWT, frequency of falls, peak work, FA-ADL 34
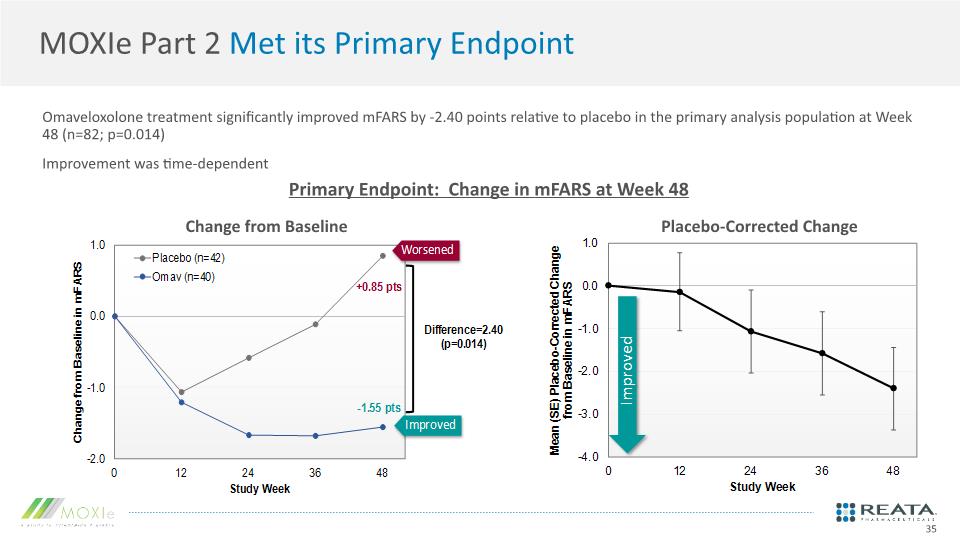
MOXIe Part 2 Met its Primary Endpoint Omaveloxolone treatment significantly improved mFARS by -2.40 points relative to placebo in the primary analysis population at Week 48 (n=82; p=0.014) Improvement was time-dependent 35
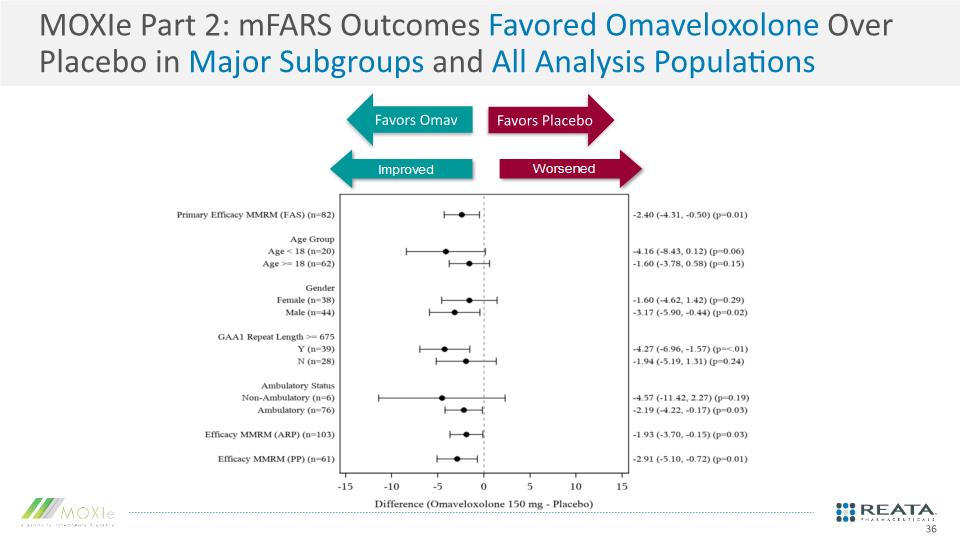
MOXIe Part 2: mFARS Outcomes Favored Omaveloxolone Over Placebo in Major Subgroups and All Analysis Populations Favors Placebo Favors Omav 36
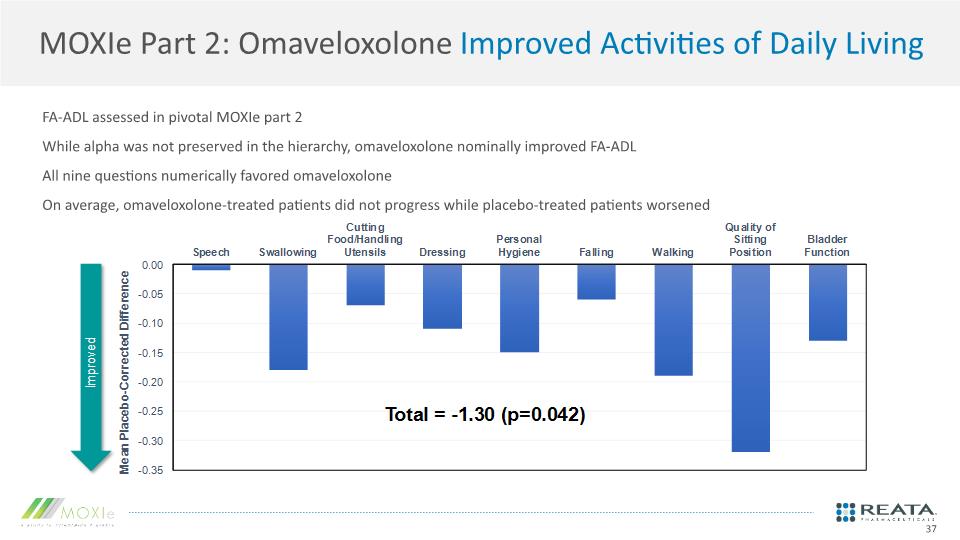
MOXIe Part 2: Omaveloxolone Improved Activities of Daily Living FA-ADL assessed in pivotal MOXIe part 2 While alpha was not preserved in the hierarchy, omaveloxolone nominally improved FA-ADL All nine questions numerically favored omaveloxolone On average, omaveloxolone-treated patients did not progress while placebo-treated patients worsened 37
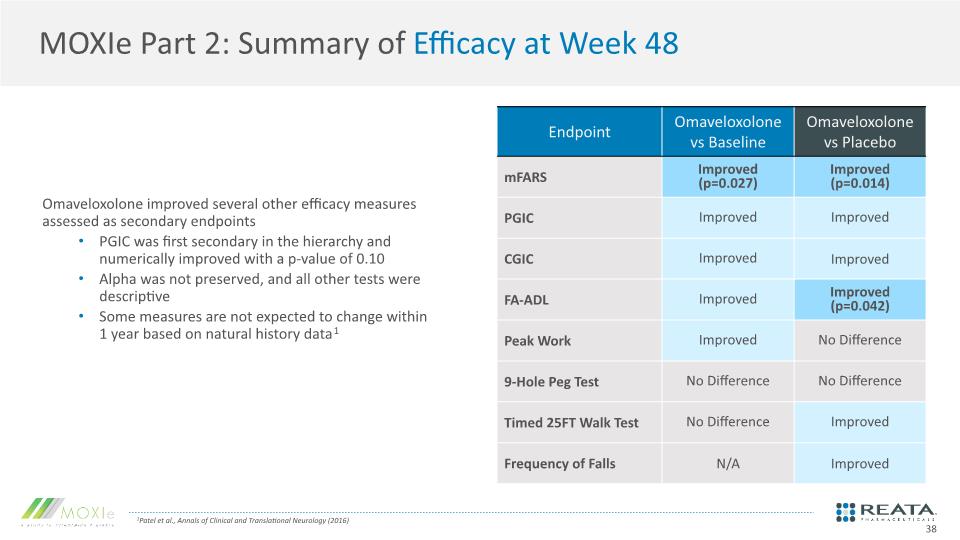
MOXIe Part 2: Summary of Efficacy at Week 48 Omaveloxolone improved several other efficacy measures assessed as secondary endpoints PGIC was first secondary in the hierarchy and numerically improved with a p-value of 0.10 Alpha was not preserved, and all other tests were descriptive Some measures are not expected to change within 1 year based on natural history data1 1Patel et al., Annals of Clinical and Translational Neurology (2016) 38
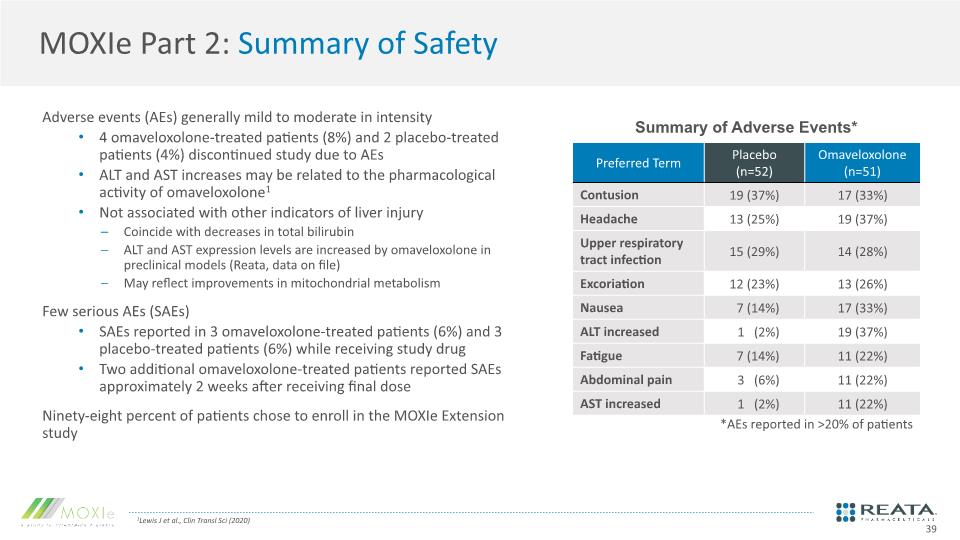
MOXIe Part 2: Summary of Safety 1Lewis J et al., Clin Transl Sci (2020) Adverse events (AEs) generally mild to moderate in intensity 4 omaveloxolone-treated patients (8%) and 2 placebo-treated patients (4%) discontinued study due to AEs ALT and AST increases may be related to the pharmacological activity of omaveloxolone1 Not associated with other indicators of liver injury Coincide with decreases in total bilirubin ALT and AST expression levels are increased by omaveloxolone in preclinical models (Reata, data on file) May reflect improvements in mitochondrial metabolism Few serious AEs (SAEs) SAEs reported in 3 omaveloxolone-treated patients (6%) and 3 placebo-treated patients (6%) while receiving study drug Two additional omaveloxolone-treated patients reported SAEs approximately 2 weeks after receiving final dose Ninety-eight percent of patients chose to enroll in the MOXIe Extension study Summary of Adverse Events* 39

Baseline-Controlled Study: Objective and Design Baseline-controlled study (cross-over study) assessed the strength and certainty of the positive primary endpoint findings in MOXIe Part 2 Treatment-naïve patients from MOXIe Part 1 and Part 2 served as their own controls to assess changes in mFARS in MOXIe Extension Primary efficacy endpoint: paired difference in annualized mFARS slope for treatment period vs pre-treatment period Primary analysis population: Part 1 patients and Part 2 placebo patients without the pes cavus foot deformity who had an mFARS assessment at Week 48 of MOXIe Extension (n=34) Multiple sensitivity analyses conducted to assess robustness of results Baseline-controlled study maintained operational and analytical rigor mFARS assessments conducted in a similar and rigorous manner throughout MOXIe Part 1, Part 2, and Extension Investigators and patients remained blinded to prior treatment assignments 40
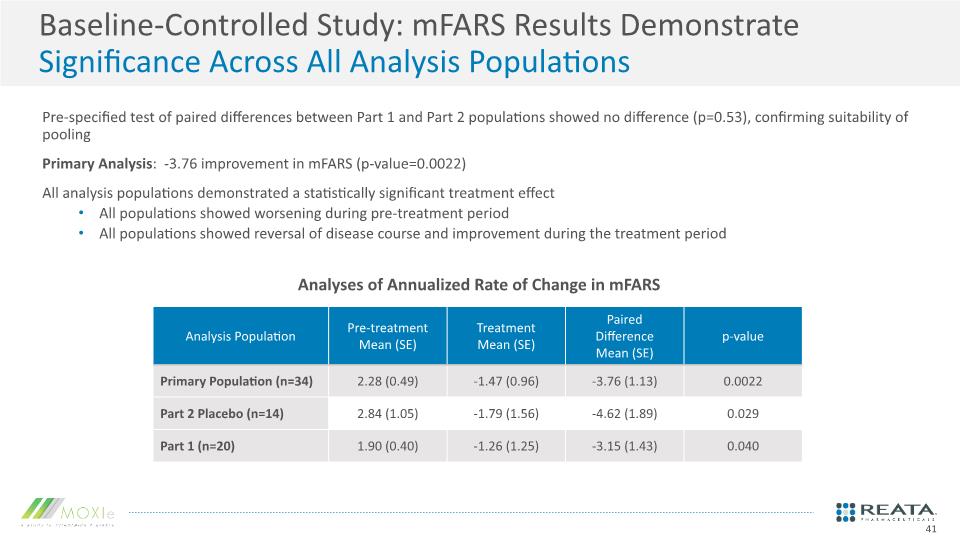
Baseline-Controlled Study: mFARS Results Demonstrate Significance Across All Analysis Populations Pre-specified test of paired differences between Part 1 and Part 2 populations showed no difference (p=0.53), confirming suitability of pooling Primary Analysis: -3.76 improvement in mFARS (p-value=0.0022) All analysis populations demonstrated a statistically significant treatment effect All populations showed worsening during pre-treatment period All populations showed reversal of disease course and improvement during the treatment period Analyses of Annualized Rate of Change in mFARS 41

Omaveloxolone Regulatory Update FDA has provided guidance that it is not concerned about the reliability of the mFARS primary endpoint results in the MOXIe Part 2 study, but is not convinced that the results are sufficient to support a single study approval Results of the baseline-controlled study analyses were recently provided to FDA and after an internal review FDA concluded that they do not believe the results strengthen the results of Part 2 of the MOXIe study FDA proposed additional exploratory analyses but stated that the potential for these analyses to sufficiently strengthen the study results was questionable due to the small number of patients available for analysis We plan to submit the analyses to FDA and to request a meeting with FDA to discuss the development program We plan to initiate a second pivotal study of omaveloxolone in FA in 2H21, following discussion with FDA 42
cycle Friedreich’s ataxia is a rare, severe disease with no approved therapies MOXIe Part 2 demonstrated a time-dependent PBO-corrected improvement in mFARS. At week 48, it showed a PBO-corrected 2.40 point mean improvement Baseline-Controlled Study met its primary endpoint of paired difference of 3.76 points in annualized mFARS slope Omaveloxolone was generally reported to be well-tolerated Plan to initiate 2nd pivotal study of omaveloxolone in FA in 2H21, following discussion with FDA Friedreich’s Ataxia Program Summary 43
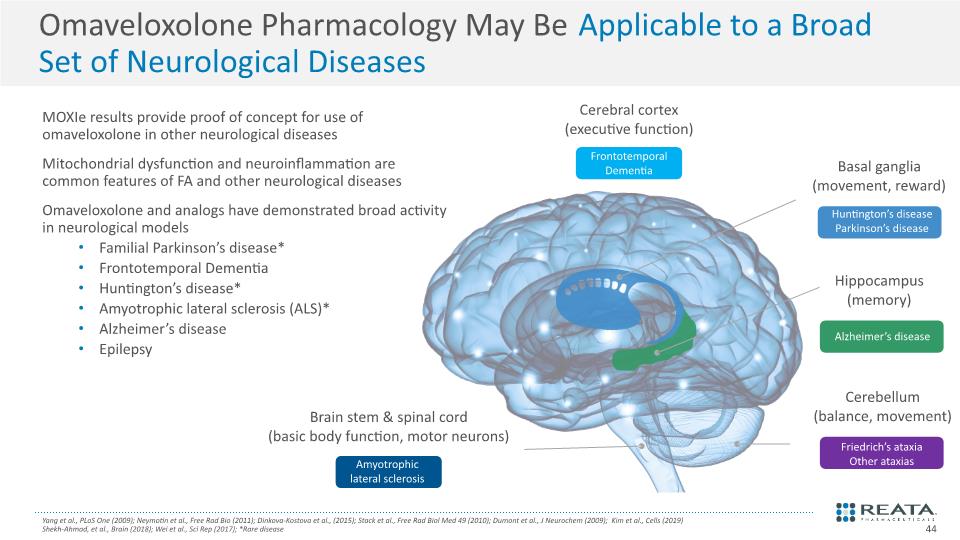
Omaveloxolone Pharmacology May Be Applicable to a Broad Set of Neurological Diseases Yang et al., PLoS One (2009); Neymotin et al., Free Rad Bio (2011); Dinkova-Kostova et al., (2015); Stack et al., Free Rad Biol Med 49 (2010); Dumont et al., J Neurochem (2009); Kim et al., Cells (2019) Shekh-Ahmad, et al., Brain (2018); Wei et al., Sci Rep (2017); *Rare disease MOXIe results provide proof of concept for use of omaveloxolone in other neurological diseases Mitochondrial dysfunction and neuroinflammation are common features of FA and other neurological diseases Omaveloxolone and analogs have demonstrated broad activity in neurological models Familial Parkinson’s disease* Frontotemporal Dementia Huntington’s disease* Amyotrophic lateral sclerosis (ALS)* Alzheimer’s disease Epilepsy 44

RTA 901 Hsp90 Modulator for Diabetic Neuropathy
RTA 901 in Diabetic Peripheral Neuropathic Pain Overview of diabetic peripheral neuropathic pain (DPNP) in the U.S. Overall prevalence of roughly 4 million patients with moderate or severe DPNP1-3 Estimated 2 million adult patients diagnosed with DPNP seek treatment annually2 Currently approved medicines provide room for improvement1,4 Roughly half of patients do not achieve a meaningful reduction in pain Tolerability issues such as somnolence and dizziness contribute to discontinuation Phase 1 SAD/MAD study of RTA 901 in healthy adult volunteers completed Oral, once-daily administration resulted in exposures approximately 10-fold larger than required for efficacy in preclinical models of DPNP No safety or tolerability concerns identified Development path ahead Additional Phase 1 clinical pharmacology studies expected to begin in the second quarter of 2021 Launch of a randomized, placebo-controlled Phase 2 study in DPNP expected in the fourth quarter 2021 1American Diabetes Association; 2GlobalData Jan 2018 GDHC164PIDR PharmaPoint: Painful Diabetic Neuropathy Global Drug Forecast and Market Analysis to 2026; 3Yang et.al. Front Endocrinol (Lausanne). 2020, Hicks et.al. Curr Diab Rep. 2019; 4FDA product labels for Lyrica, Lyrica CR, Cymbalta, Nucynta ER, and Qutenza, Tesfaye et.al. Pain (2013) 46
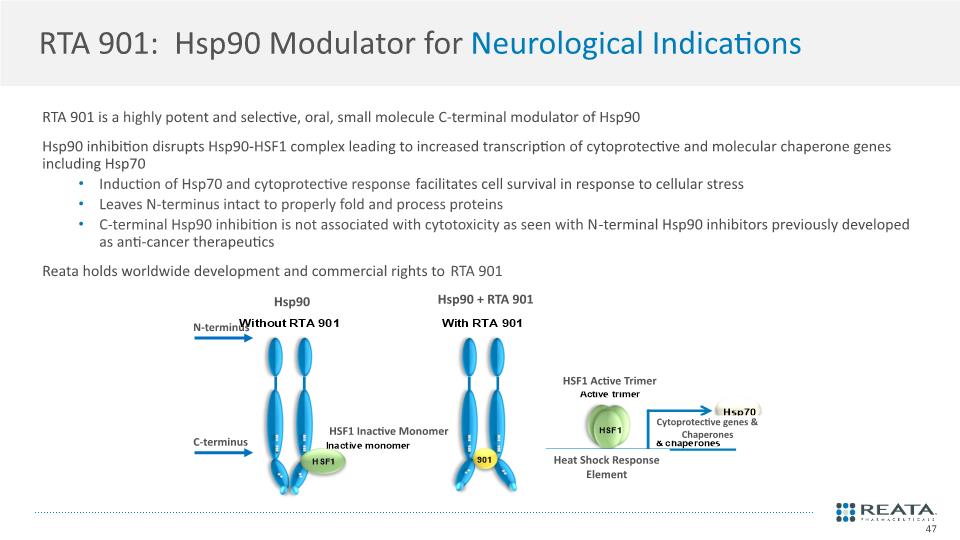
RTA 901: Hsp90 Modulator for Neurological Indications RTA 901 is a highly potent and selective, oral, small molecule C-terminal modulator of Hsp90 Hsp90 inhibition disrupts Hsp90-HSF1 complex leading to increased transcription of cytoprotective and molecular chaperone genes including Hsp70 Induction of Hsp70 and cytoprotective response facilitates cell survival in response to cellular stress Leaves N-terminus intact to properly fold and process proteins C-terminal Hsp90 inhibition is not associated with cytotoxicity as seen with N-terminal Hsp90 inhibitors previously developed as anti-cancer therapeutics Reata holds worldwide development and commercial rights to RTA 901 47
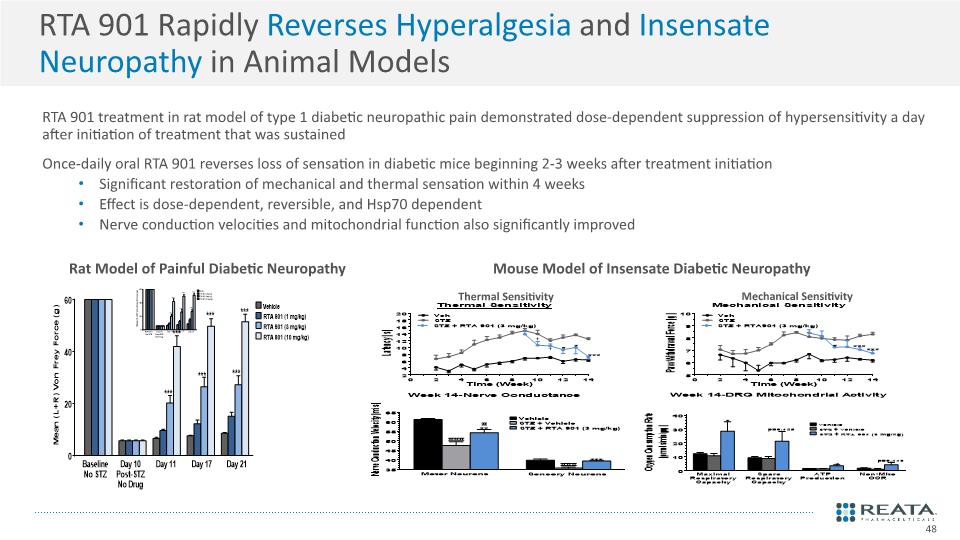
RTA 901 Rapidly Reverses Hyperalgesia and Insensate Neuropathy in Animal Models RTA 901 treatment in rat model of type 1 diabetic neuropathic pain demonstrated dose-dependent suppression of hypersensitivity a day after initiation of treatment that was sustained Once-daily oral RTA 901 reverses loss of sensation in diabetic mice beginning 2-3 weeks after treatment initiation Significant restoration of mechanical and thermal sensation within 4 weeks Effect is dose-dependent, reversible, and Hsp70 dependent Nerve conduction velocities and mitochondrial function also significantly improved Mouse Model of Insensate Diabetic Neuropathy Mechanical Sensitivity Thermal Sensitivity 48
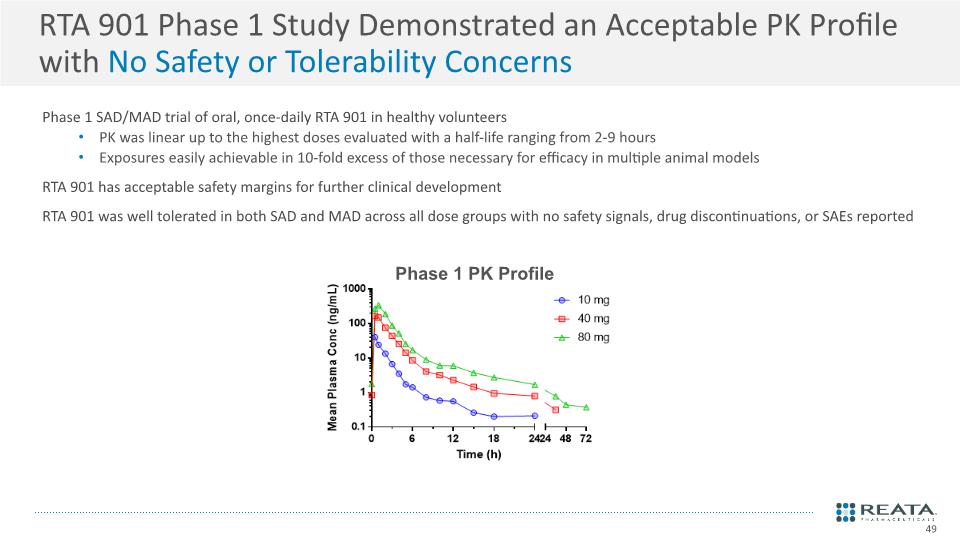
RTA 901 Phase 1 Study Demonstrated an Acceptable PK Profile with No Safety or Tolerability Concerns Phase 1 SAD/MAD trial of oral, once-daily RTA 901 in healthy volunteers PK was linear up to the highest doses evaluated with a half-life ranging from 2-9 hours Exposures easily achievable in 10-fold excess of those necessary for efficacy in multiple animal models RTA 901 has acceptable safety margins for further clinical development RTA 901 was well tolerated in both SAD and MAD across all dose groups with no safety signals, drug discontinuations, or SAEs reported 49

Operations, Financials, Intellectual Property
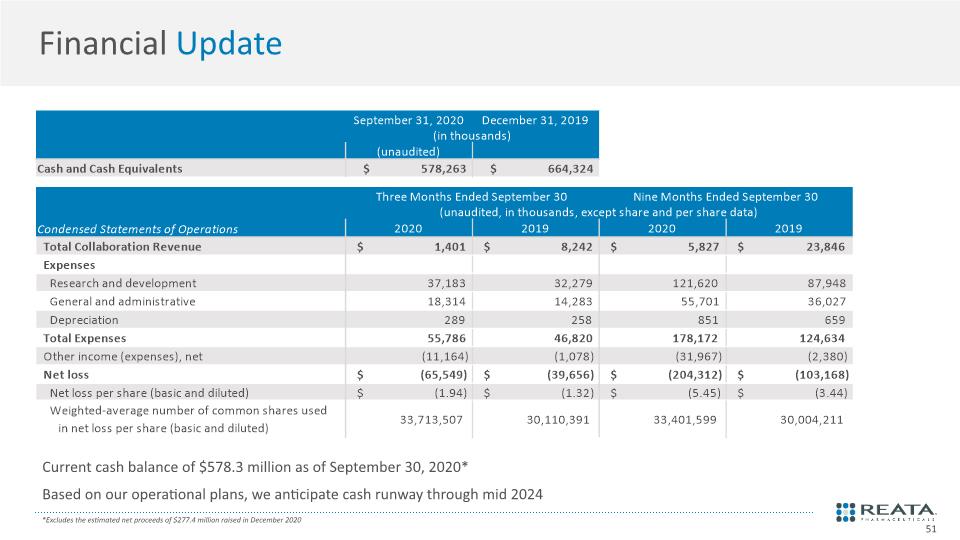
Financial Update Current cash balance of $578.3 million as of September 30, 2020* Based on our operational plans, we anticipate cash runway through mid 2024 *Excludes the estimated net proceeds of $277.4 million raised in December 2020 51

Reata Patent Portfolio Morphic form patent (US 8,088,824) claims commercial (amorphous) forms of bardoxolone Claims granted in U.S., EU, Japan, Canada, China, Mexico, Eurasia, and 9 other territories, with applications pending in 8 countries Anticipated protection to 2034 in U.S. and 2033 in rest of world with term extensions BARDOXOLONE OMAVELOXOLONE RTA 901 Composition of matter patent (US 8,993,640) claims omaveloxolone and several solid forms Granted in U.S., Europe, Japan, China, Eurasia, 15 other territories, and pending in 12 other countries Could be extended as late as 2038 depending on approval date Genus patent (US 8,124,799) claims granted in 17 foreign territories, and pending in 1 more Original composition of matter patent will expire February 2033 unless extended Second composition of matter patent will provide protection through at least Feb 2039 52
List of Abbreviations 53
