Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Passage BIO, Inc. | tm212291d1_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Passage BIO, Inc. | tm212291d1_ex99-1.htm |
Exhibit 99.2

39th Annual J.P. Morgan Healthcare Conference Bruce Goldsmith, PhD | CEO January 11, 2021 NASDAQ GS: PASG Fulfilling the Promise of Gene Therapies for Central Nervous System Disorders

Forward - Looking Statement This presentation includes “forward - looking statements” within the meaning of, and made pursuant to the safe harbor provisions o f, the Private Securities Litigation Reform Act of 1995, including, but not limited to: our expectation about timing and execution of antici pat ed milestones, including our planned IND submissions and initiation of clinical trials; our expectations about our collaborators’ and partne rs’ ability to execute key initiatives; estimates regarding our cash forecasts; the expected impact of the COVID - 19 pandemic on our operations; and the abi lity of our lead product candidates to treat the underlying causes of their respective target monogenic CNS disorders. These forward - looking stat ements may be accompanied by such words as “aim,” “anticipate,” “believe,” “could,” “estimate,” “expect,” “forecast,” “goal,” “intend,” “ma y,” “might,” “plan,” “potential,” “possible,” “will,” “would,” and other words and terms of similar meaning. These statements involve risks and un cer tainties that could cause actual results to differ materially from those reflected in such statements, including: our ability to develop, obtain reg ulatory approval for and commercialize PBGM01, PBFT02, PBKR03 and future product candidates; the timing and results of preclinical studies and clinica l t rials; the risk that positive results in a preclinical study or clinical trial may not be replicated in subsequent trials or success in early st age clinical trials may not be predictive of results in later stage clinical trials; risks associated with clinical trials, including our ability to adeq uat ely manage clinical activities, unexpected concerns that may arise from additional data or analysis obtained during clinical trials, regulatory authorities m ay require additional information or further studies, or may fail to approve or may delay approval of our drug candidates; the occurrence of advers e s afety events; failure to protect and enforce our intellectual property, and other proprietary rights; failure to successfully execute or realize th e a nticipated benefits of our strategic and growth initiatives; risks relating to technology failures or breaches; our dependence on collaborators and othe r t hird parties for the development of product candidates and other aspects of our business, which are outside of our full control; risks associated wit h current and potential delays, work stoppages, or supply chain disruptions caused by the coronavirus pandemic; risks associated with curre nt and potential future healthcare reforms; risks relating to attracting and retaining key personnel; failure to comply with legal and regulat ory requirements; risks relating to access to capital and credit markets; and the other risks and uncertainties that are described in the Risk Factor s s ection of our most recent filings with the U.S. Securities and Exchange Commission. These statements are based on our current beliefs and expect ati ons and speak only as of the date of this presentation. We do not undertake any obligation to publicly update any forward - looking statements e xcept as required by law. By attending or receiving this presentation you acknowledge that you are cautioned not to place undue reliance on the se forward - looking statements, which speak only as of the date such statements are made; you will be solely responsible for your own assessment of the market and our market position; and that you will conduct your own analysis and be solely responsible for forming your own view of the p ote ntial future performance of Passage Bio. 2

~7,000 Rare diseases OUR VISION: To fulfill the promise of gene therapy by developing groundbreaking therapies that transform the lives of patients with rare monogenic CNS diseases 70% of rare genetic diseases produce abnormalities of the CNS 1 790+ are rare monogenic CNS diseases with few treatment options 2 Passage Bio is currently targeting rare CNS disorders that affect: BABIES ADULTS 1. Lee, C., Singleton, K., Wallin, M., Faundez, V. (2020). Rare Genetic Diseases: Nature’s Experiments on Human Development . iScience 2020 May 22;23(5): 101123 2. M arket research conducted by Health Advances Approved disease - modifying treatment options for our targeted rare diseases 80% of rare diseases have a genetic component 1 who suffer from severe clinical manifestations and severely shortened survival 3

Patients with rare CNS disorders need therapeutic options.

The Passage Bio Advantage Corporate model designed for success 5 PENN GTP PARTNERSHIP Led by renowned innovator James M. Wilson, MD, PhD Access to cutting - edge research and development Access to next - generation technologies BROAD AND ROBUST PIPELINE 17 total program license options 3 clinical - stage therapies expected in 2021 4 research - stage pipeline candidates 10 additional new pipeline license options INTEGRATED MANUFACTURING SUPPLY CHAIN Flexible, scalable Dedicated CGMP suite Internal CMC analytics and assay infrastructure State - of - the - art technology WELL - POSITIONED CORPORATE FOUNDATION Robust financial position Leaders in pediatric and adult neurodegenerative disease therapy development Deep experience in gene therapy manufacturing

Passage Bio’s Differentiated Path for Clinical Success 17 program license options from GTP focused on rare monogenic CNS disorders 6 GTP expertise and insights into research and development Optimization of delivery approaches Disease - specific existing and novel capsid selection Integration of next - generation research advances . Patient need Optimal capsid, transgene and promoter Preclinical safety and efficacy data Availability of measurable, predictive biomarkers Avoids need to cross blood brain barrier Better CNS biodistribution Lower dose compared to systemic delivery Reduced immune response RIGOROUS PRODUCT CANDIDATE SELECTION DIRECT DELIVERY TO CNS PIPELINE WITH HIGHER PROBABILITY OF TECHNICAL AND REGULATORY SUCCESS

PROGRAM 1 / INDICATION GENE DISCOVERY CANDIDATE SELECTION IND - ENABLING PHASE 1/2 PIVOTAL PEDIATRIC PB GM01 2 Infantile GM1 Gangliosidosis GLB1 PB KR03 Krabbe GALC PBML04 Metachromatic Leukodystrophy ARSA PBCM06 Charcot - Marie - Tooth Type 2A MFN2 ADULT PB FT02 Frontotemporal Dementia - GRN GRN PBAL05 Amyotrophic Lateral Sclerosis C9orf72 Undisclosed CNS Undisclosed A Broad and Robust Pipeline with Global Rights Multiple potential value creating catalysts in 2021 7 1 10 additional new pipeline license options 2 Program includes ongoing natural history study of infantile and juvenile GM1 gangliosidosis patients

PBGM01 Infantile GM1 Gangliosidosis

GM1 Gangliosidosis A D evastating Pediatric Disease 9 FATAL, PEDIATRIC NEUROLOGICAL LYSOSOMAL STORAGE DISORDER caused by GLB1 gene mutations characterized by destruction of neurons in the brain and spinal cord. Characterized by rapidly progressive neurological decline resulting in reduced muscle tone, progressive CNS dysfunction, deafness, blindness, rigidity and skeletal dysplasia. RARE AND UNDERSERVED populations with incidence of up to ~1 per 100,000 live births worldwide. No disease - modifying therapies are presently available.

PBGM01 A Potential Transformative Therapy For a Rare, Underserved Disorder ▪ Next - generation, proprietary AAVhu68 capsid delivers functional GLB1 gene encoding β - gal to the brain and peripheral tissues ▪ Meaningful transduction of both central nervous system and critical peripheral organs in preclinical models ▪ Received regulatory clearances from FDA and MHRA, for global Phase 1/2 clinical trial program ▪ Received Orphan Drug and Rare Pediatric Disease designations by FDA and Orphan Drug designation by EC for treatment in GM1 ▪ First patient enrollment planned for 1Q21 in Phase 1/2 trial ▪ Initial 30 - day safety and biomarker data planned for mid - 2021 10 Source: NIH, CHOP, American Journal of Neuroradiology

11 PBGM01 Supportive Preclinical Findings Improvement in biomarker and pathophysiology in knock - out mouse model p<0.05, p<0.01, NS=not significant. GLB1 +/ - + PBS GLB1 - / - + PBS GLB1 - / - + AAV 10,000 10 1 10.1 CSF 100 1,000 ß - gal activity ( nmol /mL/h) GLB1 +/ - + PBS GLB1 - / - + PBS GLB1 - / - + AAV GLB1 +/ - + PBS GLB1 - / - + PBS GLB1 - / - + AAV 10,000 1,000 100 10 NS Liver GLB1 +/ - + PBS GLB1 - / - + PBS GLB1 - / - + AAV NS Increased Biomarker Activity Histological Confirmation GLB1 +/ - + vehicle GLB1 - / - + vehicle GLB1 - / - + PBGM01 ▪ Dose - dependent reduction of brain lysosomal storage lesions as measured by LAMP1 positive cells ▪ Stable, dose - dependent increases in β - gal enzyme activity in brain, cerebrospinal fluid, serum, and critical peripheral tissues

Dose - Related Effect on Survival GLB1 - / - (KO) GLB1 +/ - (HET) Dose 4 (highest) Dose 3 Dose 2 Dose 1 (lowest) Vehicle Vehicle Percent Survival 0 Day 100 200 300 0 50 100 Preservation of Neurological Function 0 5 10 15 20 Total score ( + SEM) Days 240 180 120 60 0 Neurological Examinations GLB1 – / – (KO) GLB1 +/ – (HET) Dose 4 (highest) Dose 3 Dose 2 Dose 1 (lowest) Vehicle Vehicle 12 PBGM01 Supportive Preclinical Findings Improvement in neurological function and survival in knock - out mouse model ▪ Treated mice demonstrated increased survival at all doses tested ▪ Top 2 doses achieved 100% survival to study endpoint ▪ Dose related improvements in neurological function demonstrated ▪ Top two doses similar to GLB +/ - vehicle
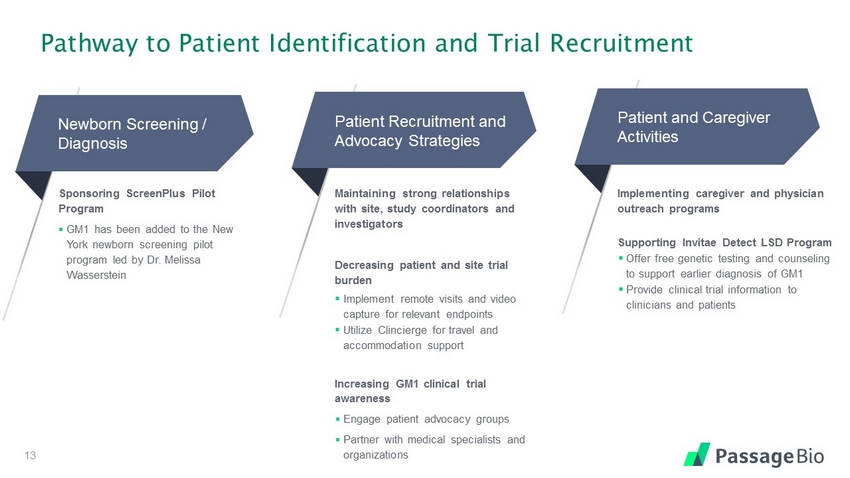
Pathway to Patient Identification and Trial Recruitment 13 Newborn Screening / Diagnosis Patient Recruitment and Advocacy Strategies Patient and Caregiver Activities Sponsoring ScreenPlus Pilot Program ▪ GM1 has been added to the New York newborn screening pilot program led by Dr. Melissa Wasserstein Implementing caregiver and physician outreach programs Supporting Invitae Detect LSD Program ▪ Offer free genetic testing and counseling to support earlier diagnosis of GM1 ▪ Provide clinical trial information to clinicians and patients Maintaining strong relationships with site, study coordinators and investigators Decreasing patient and site trial burden ▪ Implement remote visits and video capture for relevant endpoints ▪ Utilize Clincierge for travel and accommodation support Increasing GM1 clinical trial awareness ▪ Engage patient advocacy groups ▪ Partner with medical specialists and organizations

Adapted from Regier DS, et al. 2016 GLB - 1 Disorders, In Adam MP, et al. GeneReviews. Goal of the Imagine - 1 Trial in Early and Late Infantile GM1 Elevate β - gal activity to preserve neurological function and improve developmental potential and survival CLINICAL TRIAL OBJECTIVES 1. Assess the safety and tolerability of a single intra - cisterna magna dose of PBGM01 2. Demonstrate treatment - related increase in β – Gal enzyme activity in CSF and serum 3. Demonstrate resultant normalization of disease biomarkers and pathophysiology 4. Demonstrate improvement in clinical outcomes through developmental milestone assessment GM1 Gangliosidosis is a Continuum Disease Severity Residual Enzyme Activity Imagine - 1 Trial will include Type I (Early Infantile) and Type IIa (Late Infantile) patients Negligible to 5% ~ 1 - 5% ~ 3 – 10% Type I (Early Infantile) • Onset <6 months • Skeletal dysplasia • Developmental regression • Hypotonia • Seizures • Survival: <2 years Type IIa (Late - Infantile) • Onset 6 - 24 months • Developmental plateau, followed by regression • Impaired ambulation • Decreased cognition • Seizures • Survival: 5 to 10 years Type II (Juvenile) • Onset 2 - 5 years • Impaired ambulation • Dysarthria • Variable skeletal disease • Decreased cognition • Survival into 2nd decade 14

Imagine - 1 Global Phase 1/2 Trial with PBGM01 First patient enrollment expected in 1Q21 15 Trial Design Phase 1/2, multi - center, open - label, single - arm, 2+2 dose escalation and confirmatory study Separate cohorts of pediatric subjects with Late Onset Infantile GM1 in cohorts 1 and 2 and Early Onset Infantile GM1 in cohort 3 and 4 Intervention Single ICM dose of AAVhu68.hGLB1 First Read Out 30 - day safety and biomarker data of cohort 1 to be reported mid - 2021 Duration Two years, with rollover into a separate long - term follow - up study Primary Endpoints Cohorts 1 - 4: Safety and tolerability Expansion Cohorts: Efficacy, safety and tolerability COHORT 4 Early Infantile n = 2 H IGH D OSE L OW D O S E Expansion Cohort Early Infantile n = 6 Expansion Cohort Late Infantile n = 6 COHORT 2 Late Infantile n = 2 COHORT 3 Early Infantile n = 2 COHORT 1 Late Infantile n = 2 DSMB review

PBKR03 Krabbe Disease

Krabbe Lysosomal Storage Disease PBKR03 — A potential transformative therapy for a rare, underserved disorder 17 Source: NIH, CHOP, American Journal of Neuroradiology, Third Party Research Severe form of disease ▪ Infantile Krabbe disease is the most severe form, accounting for 60% to 70% of diagnoses ▪ Disease progression is highly predictable and includes loss of acquired milestones, staring episodes, peripheral neuropathy, seizures, blindness and deafness ▪ Rapid progression with mortality by ~2 years Rare and underserved ▪ Estimated worldwide incidence of Krabbe is 2.6:100,000 births ▪ Currently no approved disease - modifying therapies PBKR03 — Our solution ▪ Next - generation, proprietary AAVhu68 capsid delivers a functional GALC gene encoding galactosylceramidase (GALC) to the brain and peripheral tissues ▪ In preclinical models, observed meaningful transduction of both CNS and peripheral nerves ▪ PBKR03 - treated Krabbe dogs improved central and peripheral myelination, reduced neuroinflammation and increased survival rates with full phenotypic recovery ▪ Received Orphan Drug and Rare Pediatric Disease designations by FDA for treatment in Krabbe disease ▪ Filed Investigational New Drug application ▪ Clinical trial initiation in 1H21 Infantile Krabbe PBKR03

PBKR03 Disease Model Data – Twitcher Mouse and Dog Models Meaningful brain and peripheral transduction with improved pathophysiology and function 18 GALC Increase in Brain and Periphery Twitcher Mouse Model: • Increased GALC activity in brain, liver and serum • AAV treated twitcher mouse showed clinical scores comparable to wt mice GALC Activity (FU/50 µg) 2 0,000 15,000 10,000 0 5,000 +/+ t wi / twi t wi / twi PBS GTP - 206 Brain PBKR03 GALC Activity (FU/50 µg) 2, 000 1,500 0 500 +/+ t wi / twi t wi / twi PBS GTP - 206 1,000 Liver PBKR03 GALC and Psychosine Normalization 200 240 280 320 WT (untreated) AAV-treated (6M post- treatment) FU / 10 µl CSF CSF GALC Activity (6M post - treatment ) CSF Psychosine Levels (70 days post - treatment) ng/ml Canine Model: • Increased GALC activity in CSF • Decreased psychosine levels in CSF 0 0.5 1 1.5 2 Vehicle-treated AAV-treated

Naturally occurring Krabbe Dog model study showed substantial benefit with AAV treatment, including full phenotypic recovery and increased survival 0 20 40 60 80 K948 K930 K938 K939 K937 K933 Weeks PBKR03 Patient Enrollment Expected in 1H 2021 Supported by extensive preclinical studies 19 TRIAL DESIGN A Phase 1/2 Dose Escalation Study of a Single ICM Dose of PBKR03 in in pediatric subjects with infantile Krabbe disease ENDPOINTS Primary: Safety and tolerability Secondary: CSF and serum GALC levels; disease biomarkers; Clinical outcome measure Phase 1/2 Study Summary Survival Extended by AAV Treatment Euthanized due to disease progression Euthanized as planned per protocol Euthanized for seizure (AAV3) and weight loss (AAV4) Myelination Improvement in CNS and PNS Brain Peripheral Nerves Animal ID Average Severity Score (Grade 1 - 4) Vehicle AAV WT Vehicle AAV

PBFT02 Frontotemporal Dementia - GRN 20

FTD - GRN Frontotemporal Dementia Caused by a Progranulin Deficiency PBFT02 – A potential transformative therapy for a rare, underserved disorder 21 Devasting form of dementia ▪ FTD is one of the more common causes of early - onset dementia ▪ Approximately 5 - 10% of FTD is caused by a GRN gene mutation, causing progranulin (PGRN) deficiency ▪ Progresses to immobility and severe behavioral changes and loss of speech and expression ▪ Rapid progression, with average survival of 8 years, after onset of symptoms Rare and underserved ▪ Estimated prevalence of F TD - GR N in the United States is ~3,000 to 6,000 patients ▪ Currently no approved disease - modifying therapies PBFT02 — Our solution ▪ Proprietary AAV1 capsid - based product candidate ▪ Designed to deliver to the brain a functional GRN gene encoding PGRN ▪ Meaningful transduction in NHP studies showing high transduction of ependymal cells that line brain ventricles ▪ Increases in CSF PGRN concentrations in NHP studies to >50 - fold normal human CSF PGRN concentrations ▪ Received Orphan Drug designation by FDA ▪ Filed Investigational New Drug application ▪ Clinical trial initiation 1H21 FTD - GRN PBFT02

PBFT02 Biomarker Improvement Across Brain Regions Decreases markers of lysosomal function and microgliosis *** * PBFT02 high dose vs vehicle in GRN - / - mice; post baseline data is 90 days post - dose * = p< 0.05*** = p<0.001 0% 20% 40% 60% 80% 100% 120% Baseline Vehicle PBFT02 0 0.2 0.4 0.6 0.8 1 1.2 Baseline Vehicle PBFT02 Lipofuscin Accumulation Stopped in Thalamus Hexosaminidase Activity Normalization in Cortex Reduced CD68 in Hippocampus • Lipofuscin accumulation is implicated in various neurodegenerative conditions • Treatment stopped lipofuscin accumulation across brain regions • Hexosaminidase activity is upregulated in setting of lysosomal dysfunction • Treatment reduced enzyme activity at the highest dose • CD68 is a lysosomal protein expressed by macrophages and activated microglia with increases reflecting higher inflammation • Treatment reduced CD68 levels across brain regions 22

PBFT02 Patient Enrollment Expected in 1H 2021 Supported by extensive NHP preclinical studies utilizing ICM delivery 23 Phase 1/2 study summary PBFT02 Showed D ose - Related Increase in CSF PGRN at 14d PD 0 2 4 6 8 10 12 High Dose Medium Dose Low Dose Vehicle Human PGRN (ng/ml) Production of Human PGRN in CSF CSF PGRN (ng/mL) 0 10 2 0 30 4 0 CSF 0.1 1 10 100 LLOQ Normal Day RA2981 RA2982 RA3027 RA3153 RA3151 RA3170 RA3155 RA3160 AAVhu68 AAVhu68 (v2) AAV1 AAV5 RA2981 RA2982 RA3027 RA3153 RA3151 RA3170 RA3155 RA3160 AAVhu68 AAVhu68 (v2) AAV1 AAV5 RA2981 RA2982 RA3027 RA3153 RA3151 RA3170 RA3155 RA3160 AAVhu68 AAVhu68 (v2) AAV1 AAV5 AAVhu68 AAVhu68 (v2) AAV5 AAV1 Normal LLOQ AAVhu68 AAV1 Ependyma 1 – 2% transduction 48% transduction AAV1 Vector Expressing GFP 48% transduction of the ependymal cells in the animals treated with AAV1 Trial Design A Phase 1/2 Dose Escalation Study of a Single ICM Dose of PBFT02 in Subjects with Frontotemporal Dementia and Heterozygous Mu tat ions in the Granulin Precursor GRN Gene Primary Endpoints Primary: Safety and tolerability Secondary: CSF progranulin levels; disease biomarkers (e.g., NfL, MRI); Clinical outcome measure

State - of - the - Art Manufacturing

Manufacturing That is Dedicated, Flexible, and Scalable 25 CMC Infrastructure Capability Investment in CMC laboratory with state - of - the - art analytical capabilities, clinical assay development and validation, biomarker assay validation, and clinical product testing End - to - End Supply Chain Establishing manufacturing and global distribution from clinical development through initial commercialization Dedicated Manufacturing Suite Partnership with gene therapy manufacturing leader Catalent facilitates flexible and scalable capacity Qualified CGMP suite dedicated to Passage Bio is completed and manufacturing initiated

Corporate Foundation 26 SOURCE: www.brandywinerealty.com

Financial Summary 27 Strong cash balance of $335M at September 30, 2020 Cash on hand to fund operations into 2023 Runway to potentially deliver meaningful catalysts over multiple programs

Anticipated Upcoming Milestones 28 Phase 1/2 trial for PBGM01 in patients with infantile GM1 1Q21 INITIATE Phase 1/2 trial for PBFT02 in patients with FTD - GRN in 1H21 INITIATE Advance preclinical programs for MLD, ALS CMT2A ADVANCE Evaluate and expand pipeline by pursuing new licenses in partnership with Penn’s GTP BUILD Phase 1/2 trial for PBKR03 in patients with Krabbe disease in 1H21 INITIATE PBGM01 safety and biomarker data mid - 2021 REPORT
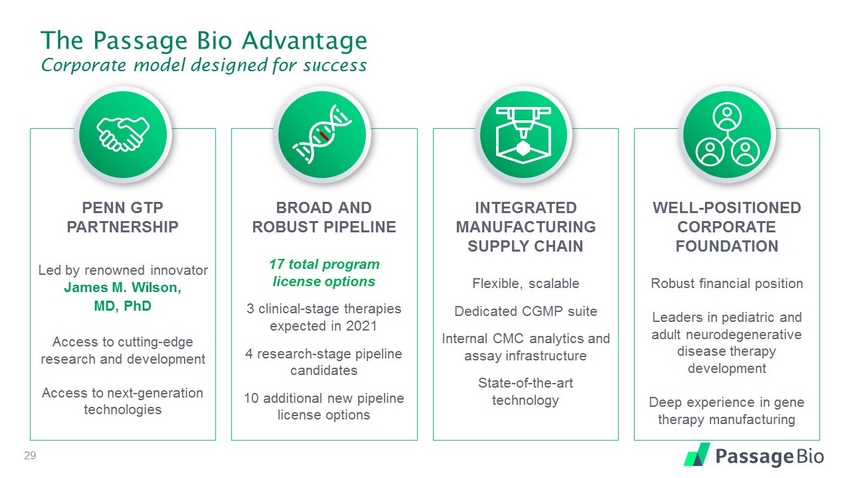
The Passage Bio Advantage Corporate model designed for success 29 PENN GTP PARTNERSHIP Led by renowned innovator James M. Wilson, MD, PhD Access to cutting - edge research and development Access to next - generation technologies BROAD AND ROBUST PIPELINE 17 total program license options 3 clinical - stage therapies expected in 2021 4 research - stage pipeline candidates 10 additional new pipeline license options INTEGRATED MANUFACTURING SUPPLY CHAIN Flexible, scalable Dedicated CGMP suite Internal CMC analytics and assay infrastructure State - of - the - art technology WELL - POSITIONED CORPORATE FOUNDATION Robust financial position Leaders in pediatric and adult neurodegenerative disease therapy development Deep experience in gene therapy manufacturing

www.passagebio.com NASDAQ GS: PASG THANK YOU 30

Appendix

Demonstrated Leadership Deep experience in rare disease, CNS disorders and gene therapy 32 LEADERSHIP TEAM BOARD OF DIRECTORS Tachi Yamada, M.D. (Chair) Frazier Athena Countouriotis, M.D. Turning Point Bruce Goldsmith, Ph.D. Passage Bio Patrick Heron Frazier Saqib Islam, J.D. SpringWorks Sandip Kapadia Intercept Liam Ratcliffe, M.D., Ph.D. Access Industries Stephen Squinto, Ph.D. OrbiMed Tom Woiwode, Ph.D. Versant Rich Morris Chief Financial Officer Chip Cale General Counsel Alex Fotopoulos Chief Technology Officer Bruce Goldsmith, Ph.D. Chief Executive Officer Jill M. Quigley Chief Operating Officer Gary Romano, M.D., Ph.D. Chief Medical Officer Stephen Squinto, Ph.D. Acting Head of R&D

Cutting - Edge Gene Therapy R&D Collaboration 17 program license options for Passage Bio focused on rare monogenic CNS disorders 33 Founded by renowned innovator and pioneer James M. Wilson, M.D., Ph.D. Chief Scientific Advisor, Passage Bio Director, Gene Therapy Program Rose H. Weiss Professor and Director, Orphan Disease Center Professor of Medicine and Pediatrics, Department of Medicine Cutting - edge research, expertise, next - generation technologies ~300 full - time employees Research contributed to successful clinical programs for approved gene therapies Glybera ® , Luxturna ® and Zolgensma ® Supporting natural history studies and early patient identification World - class Gene Therapy Program
