Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Orchard Therapeutics plc | ortx-ex991_6.htm |
| 8-K - 8-K - Orchard Therapeutics plc | ortx-8k_20210111.htm |

2021 JP Morgan Presentation Bobby Gaspar, M.D., Ph.D. Chief executive officer January 13, 2021 Exhibit 99.2

Certain information set forth in this presentation and in statements made orally during this presentation contains “forward-looking statements”. Except for statements of historical fact, information contained herein constitutes forward-looking statements and may include, but is not limited to, the Company’s expectations regarding: (I) the safety and efficacy of Libmeldy and its product candidates; (II) the expected development of the Company’s business and product candidates; (III) the timing of regulatory submissions for approval of its product candidates; (IV) the timing of interactions with regulators and regulatory submissions related to ongoing and new clinical trials for its product candidates; (V) the timing of announcement of preclinical and clinical data for its product candidates and the likelihood that such data will be positive and support further development and regulatory approval of these product candidates; (VI) the timing and likelihood of approval of such product candidates by the applicable regulatory authorities; (VII) the adequacy of the Company’s supply chain and ability to commercialize Libmeldy, including the ability to secure adequate pricing and reimbursement to support continued development and commercialization of Libmeldy; (VIII) execution of the Company’s vision and growth strategy, including with respect to global growth; (IX) the size and value of potential markets for the Company’s product candidates; and (X) projected financial performance and financial condition, including the sufficiency of the Company’s cash and cash equivalents to fund operations in future periods and future liquidity, working capital and capital requirements. The words “may,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts," “potential,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Forward-looking statements are provided to allow investors the opportunity to understand management’s beliefs and opinions in respect of the future so that they may use such beliefs and opinions as one factor in evaluating an investment. These statements are neither promises nor guarantees of future performance. Such forward-looking statements necessarily involve known and unknown risks and uncertainties, which include, without limitation, the severity of the impact of the COVID-19 pandemic on the Company’s business, including on preclinical and clinical development and commercial programs, which may cause actual performance and financial results in future periods to differ materially from any projections of future performance or results expressed or implied by such forward-looking statements. You are cautioned not to place undue reliance on forward-looking statements. These statements are subject to a variety of risks and uncertainties, many of which are beyond the Company’s control, which could cause actual results to differ materially from those contemplated in these forward-looking statements. For additional disclosure regarding these and other risks faced by the Company, see the disclosure contained in the Company’s public filings with the U.S. Securities and Exchange Commission (the “SEC”), including in the Company’s quarterly report on Form 10-Q filed with the SEC on November 3, 2020, as well as subsequent filings and reports filed with the SEC. These forward-looking statements speak only as of the date of this presentation. The Company undertakes no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise, except as may be required by law. Forward Looking Statements 2

Curing the incurable The potential of HSC gene therapy
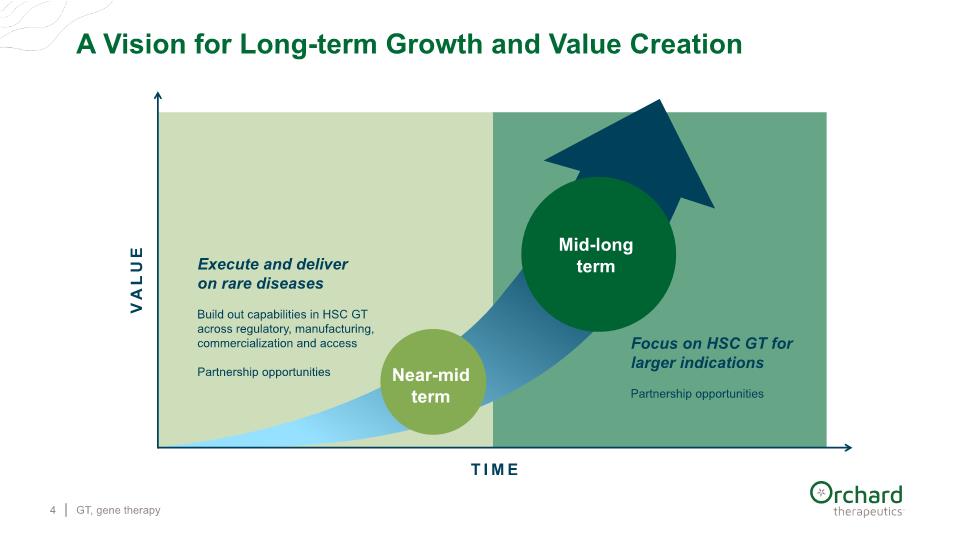
A Vision for Long-term Growth and Value Creation GT, gene therapy 4 TIME VALUE Execute and deliver on rare diseases Build out capabilities in HSC GT across regulatory, manufacturing, commercialization and access Partnership opportunities Focus on HSC GT for larger indications Partnership opportunities Mid-long term Near-mid term
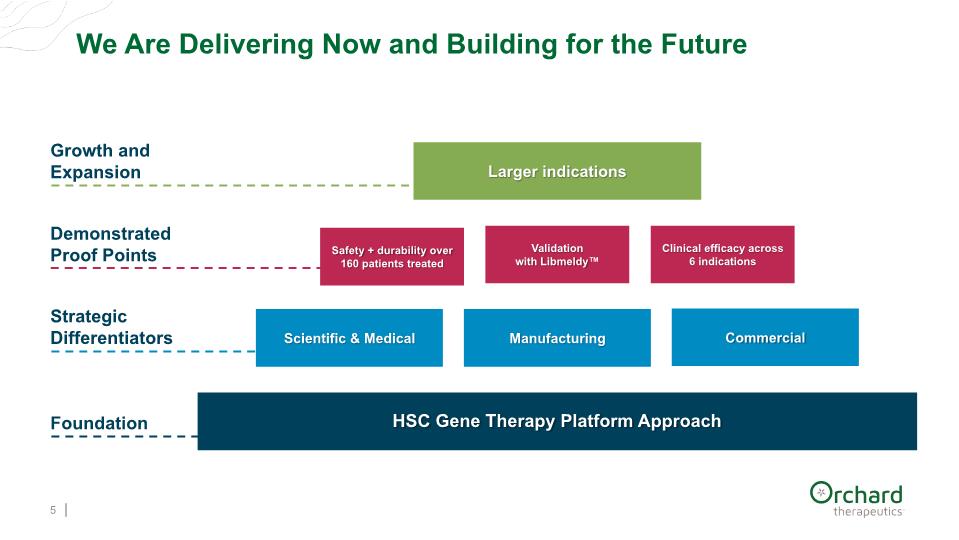
We Are Delivering Now and Building for the Future 5 Foundation Strategic Differentiators Demonstrated Proof Points Growth and Expansion HSC Gene Therapy Platform Approach Scientific & Medical Safety + durability over 160 patients treated Larger indications Clinical efficacy across 6 indications Manufacturing Commercial Validation with Libmeldy™
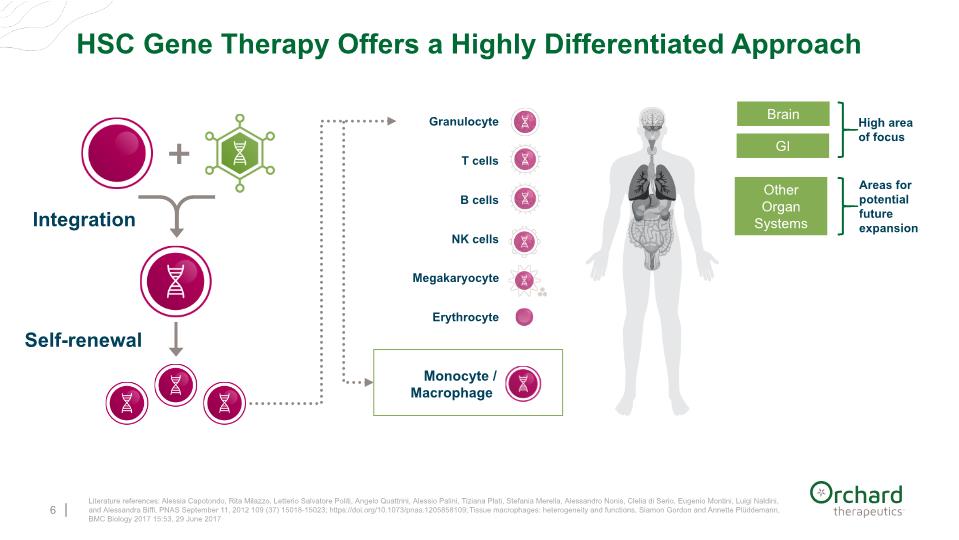
HSC Gene Therapy Offers a Highly Differentiated Approach 6 Monocyte / Macrophage T cells B cells NK cells Megakaryocyte Erythrocyte Granulocyte Literature references: Alessia Capotondo, Rita Milazzo, Letterio Salvatore Politi, Angelo Quattrini, Alessio Palini, Tiziana Plati, Stefania Merella, Alessandro Nonis, Clelia di Serio, Eugenio Montini, Luigi Naldini, and Alessandra Biffi, PNAS September 11, 2012 109 (37) 15018-15023; https://doi.org/10.1073/pnas.1205858109; Tissue macrophages: heterogeneity and functions, Siamon Gordon and Annette Plüddemann, BMC Biology 2017 15:53, 29 June 2017 Integration Self-renewal
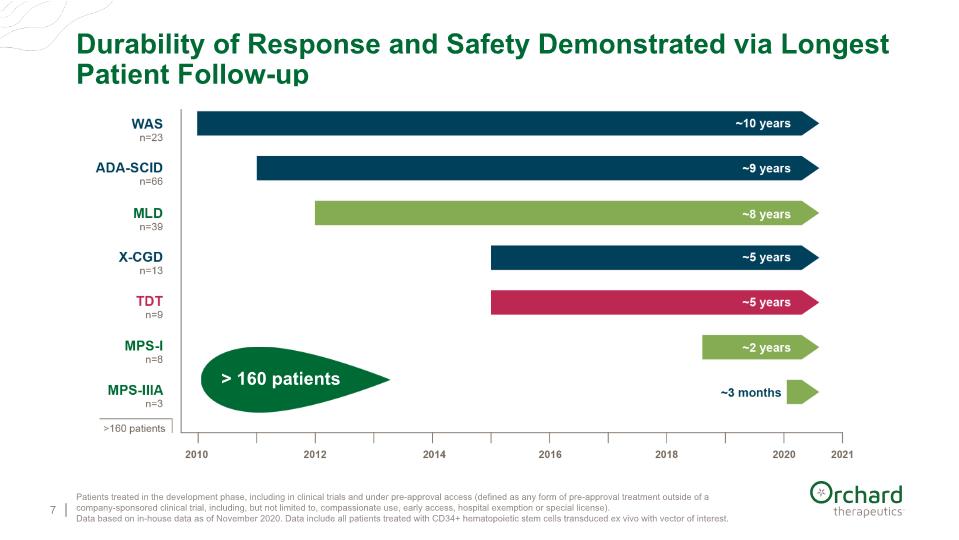
Durability of Response and Safety Demonstrated via Longest Patient Follow-up 7 Patients treated in the development phase, including in clinical trials and under pre-approval access (defined as any form of pre-approval treatment outside of a company-sponsored clinical trial, including, but not limited to, compassionate use, early access, hospital exemption or special license). Data based on in-house data as of November 2020. Data include all patients treated with CD34+ hematopoietic stem cells transduced ex vivo with vector of interest.
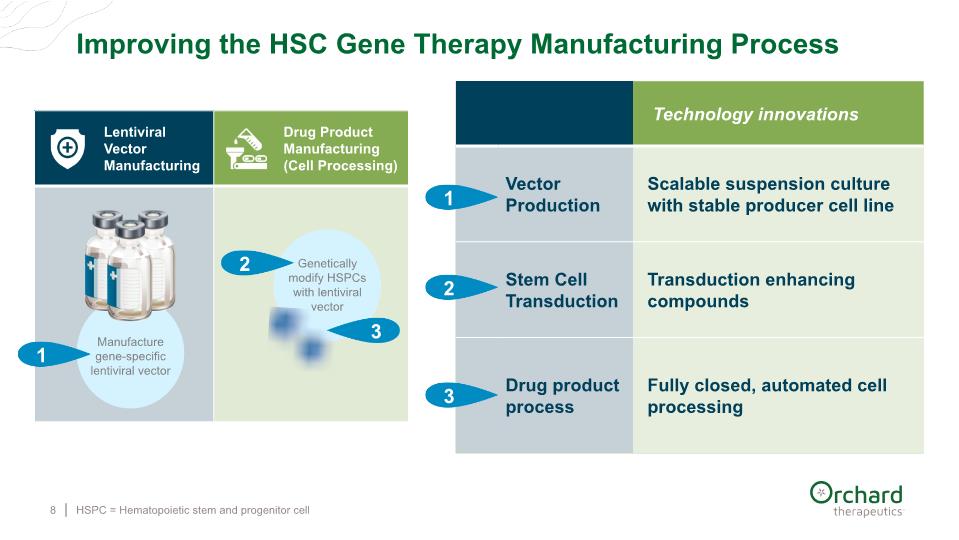
Improving the HSC Gene Therapy Manufacturing Process HSPC = Hematopoietic stem and progenitor cell 8
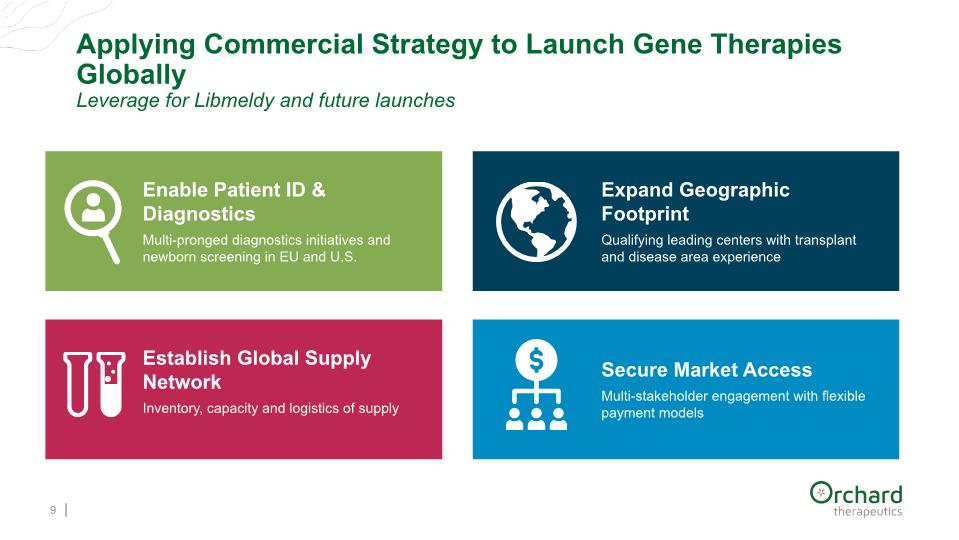
Applying Commercial Strategy to Launch Gene Therapies Globally Leverage for Libmeldy and future launches 9 Enable Patient ID & Diagnostics Multi-pronged diagnostics initiatives and newborn screening in EU and U.S. Expand Geographic Footprint Qualifying leading centers with transplant and disease area experience Establish Global Supply Network Inventory, capacity and logistics of supply Secure Market Access Multi-stakeholder engagement with flexible payment models

HSC Gene Therapy: Meeting the Need in Severe Neurodegenerative Disorders 10
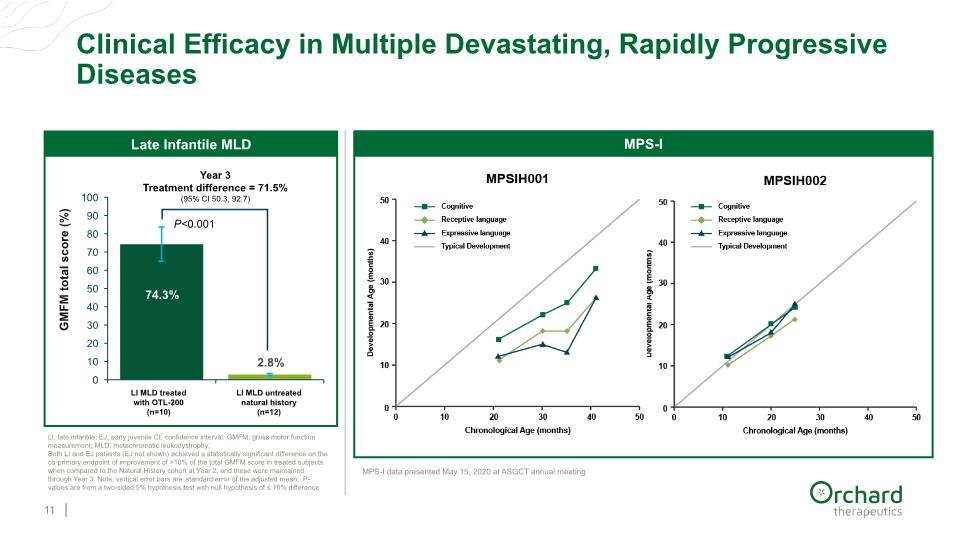
Clinical Efficacy in Multiple Devastating, Rapidly Progressive Diseases 11 LI, late infantile; EJ, early juvenile CI, confidence interval; GMFM, gross motor function measurement; MLD, metachromatic leukodystrophy; Both LI and EJ patients (EJ not shown) achieved a statistically significant difference on the co-primary endpoint of improvement of >10% of the total GMFM score in treated subjects when compared to the Natural History cohort at Year 2, and these were maintained through Year 3. Note: vertical error bars are standard error of the adjusted mean; P-values are from a two-sided 5% hypothesis test with null hypothesis of ≤ 10% difference MPS-I MPS-I MPS-I data presented May 15, 2020 at ASGCT annual meeting
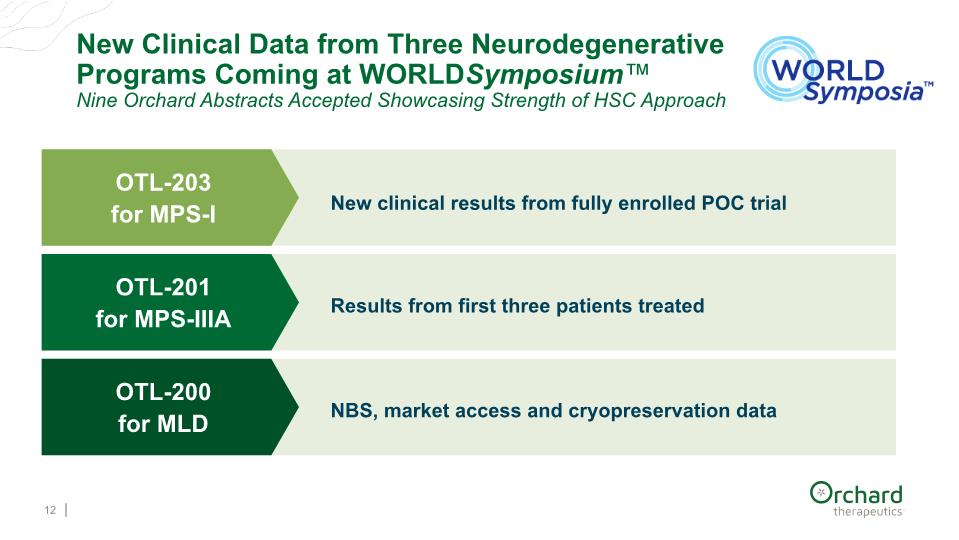
12 New Clinical Data from Three Neurodegenerative Programs Coming at WORLDSymposium™ Nine Orchard Abstracts Accepted Showcasing Strength of HSC Approach
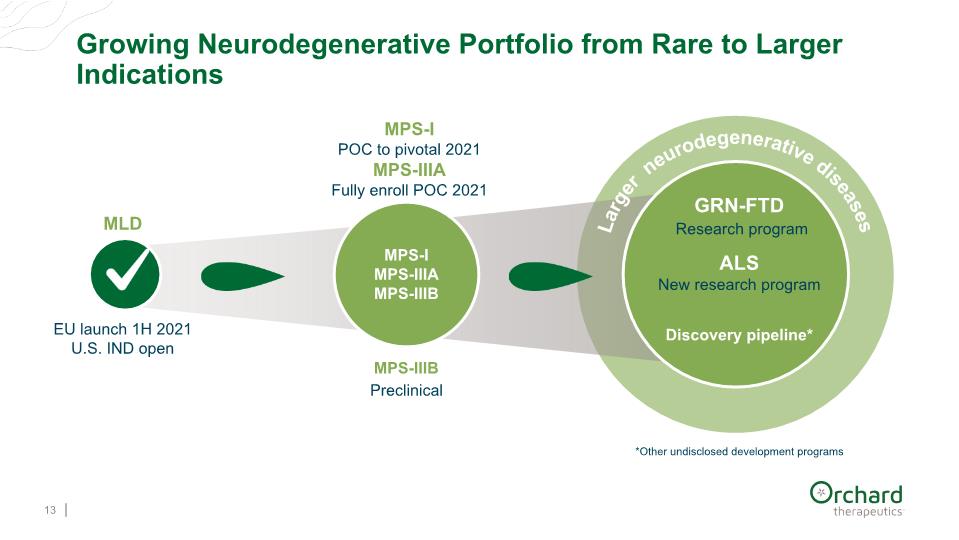
Growing Neurodegenerative Portfolio from Rare to Larger Indications 13 GRN-FTD Research program ALS New research program Discovery pipeline* MPS-I MPS-IIIA MPS-IIIB MPS-IIIB Preclinical MPS-I POC to pivotal 2021 MPS-IIIA Fully enroll POC 2021 MLD Larger neurodegenerative diseases *Other undisclosed development programs EU launch 1H 2021 U.S. IND open
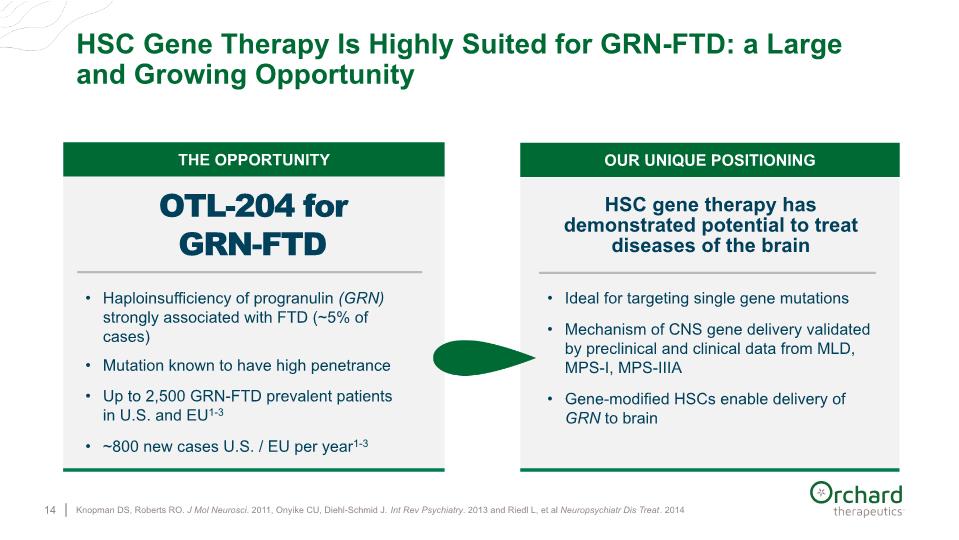
Knopman DS, Roberts RO. J Mol Neurosci. 2011, Onyike CU, Diehl-Schmid J. Int Rev Psychiatry. 2013 and Riedl L, et al Neuropsychiatr Dis Treat. 2014 HSC Gene Therapy Is Highly Suited for GRN-FTD: a Large and Growing Opportunity 14

HSC Gene Therapy: Advancing the Treatment Landscape in Immunological Disorders 15
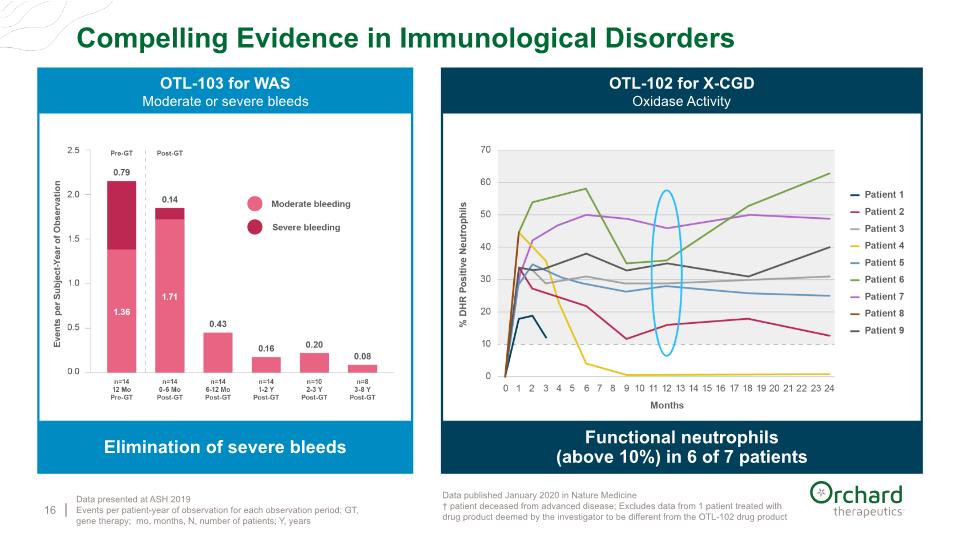
16 Elimination of severe bleeds OTL-103 for WAS Moderate or severe bleeds Data published January 2020 in Nature Medicine † patient deceased from advanced disease; Excludes data from 1 patient treated with drug product deemed by the investigator to be different from the OTL-102 drug product Functional neutrophils (above 10%) in 6 of 7 patients OTL-102 for X-CGD Oxidase Activity † † Compelling Evidence in Immunological Disorders Data presented at ASH 2019 Events per patient-year of observation for each observation period; GT, gene therapy; mo, months, N, number of patients; Y, years
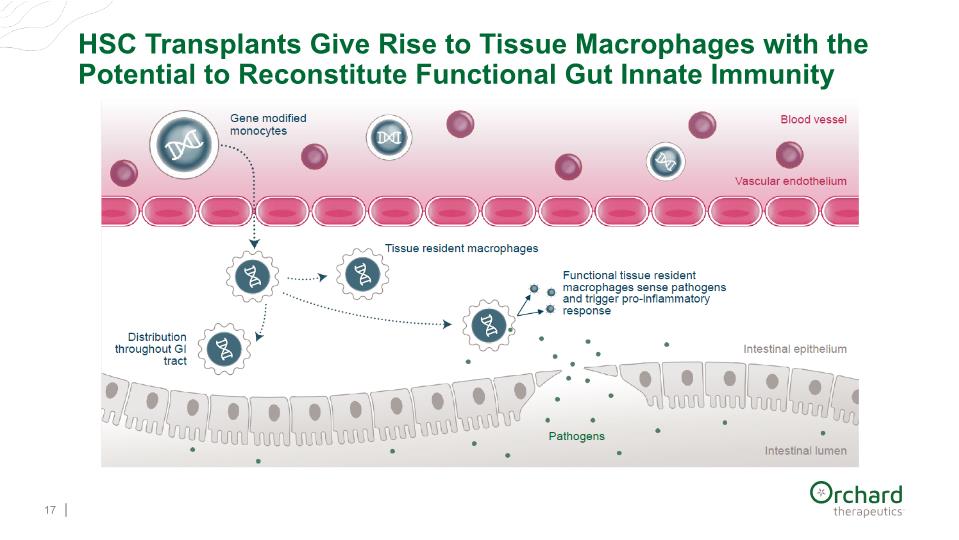
HSC Transplants Give Rise to Tissue Macrophages with the Potential to Reconstitute Functional Gut Innate Immunity 17
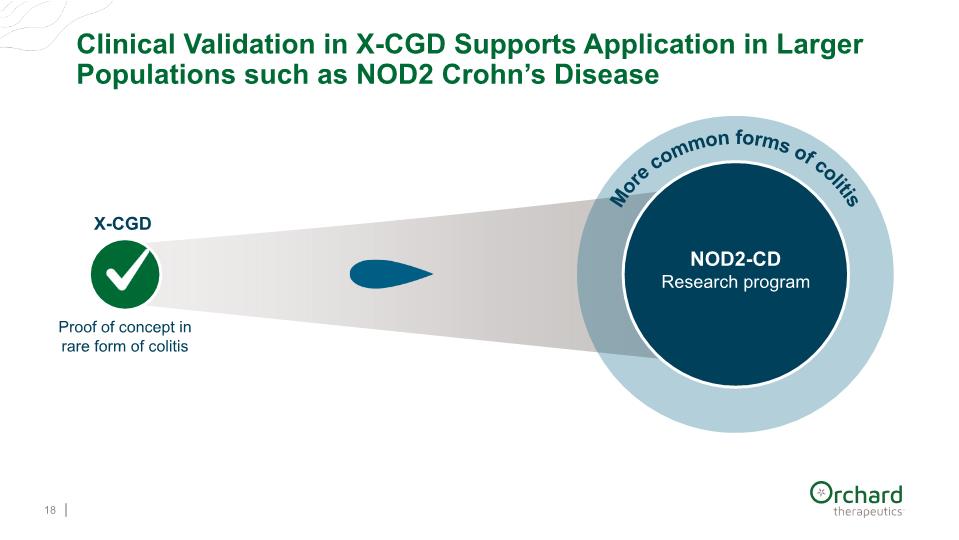
Clinical Validation in X-CGD Supports Application in Larger Populations such as NOD2 Crohn’s Disease 18 Proof of concept in rare form of colitis NOD2-CD Research program X-CGD More common forms of colitis
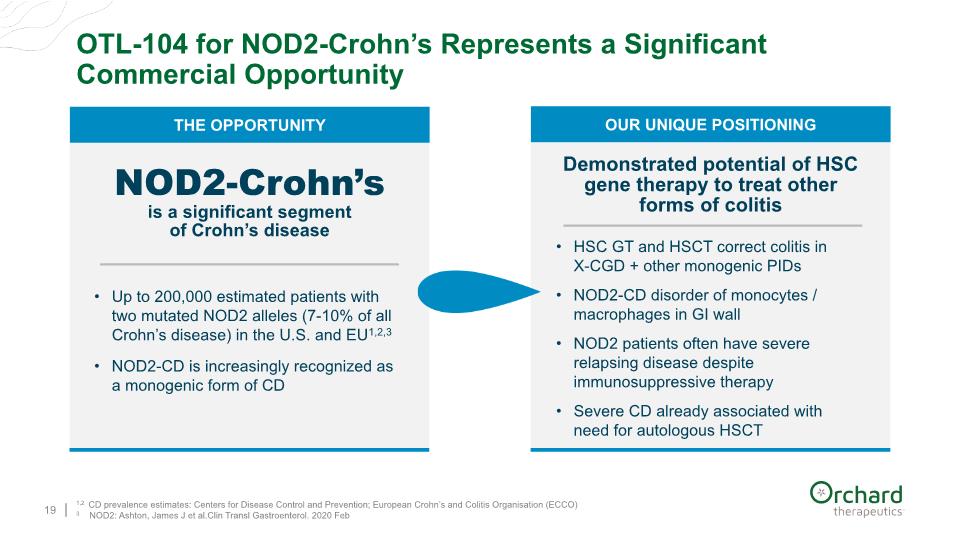
OTL-104 for NOD2-Crohn’s Represents a Significant Commercial Opportunity 19 1,2 CD prevalence estimates: Centers for Disease Control and Prevention; European Crohn’s and Colitis Organisation (ECCO) 3 NOD2: Ashton, James J et al.Clin Transl Gastroenterol. 2020 Feb

Operations and Upcoming Milestones 20
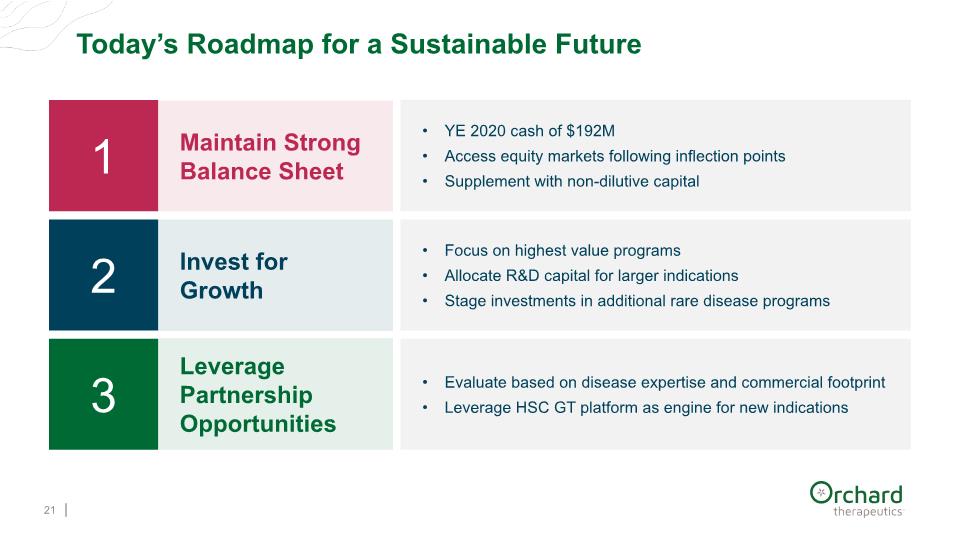
Today’s Roadmap for a Sustainable Future 21 1 YE 2020 cash of $192M Access equity markets following inflection points Supplement with non-dilutive capital 2 Focus on highest value programs Allocate R&D capital for larger indications Stage investments in additional rare disease programs 3 Invest for Growth Maintain Strong Balance Sheet Leverage Partnership Opportunities Evaluate based on disease expertise and commercial footprint Leverage HSC GT platform as engine for new indications
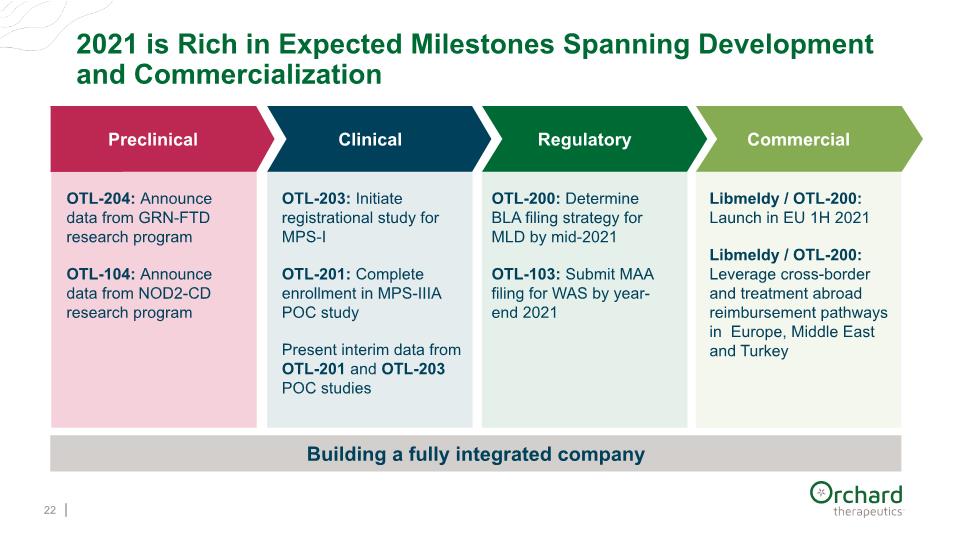
2021 is Rich in Expected Milestones Spanning Development and Commercialization 22 Building a fully integrated company Clinical Regulatory Commercial OTL-204: Announce data from GRN-FTD research program OTL-104: Announce data from NOD2-CD research program OTL-203: Initiate registrational study for MPS-I OTL-201: Complete enrollment in MPS-IIIA POC study Present interim data from OTL-201 and OTL-203 POC studies OTL-200: Determine BLA filing strategy for MLD by mid-2021 OTL-103: Submit MAA filing for WAS by year-end 2021 Libmeldy / OTL-200: Launch in EU 1H 2021 Libmeldy / OTL-200: Leverage cross-border and treatment abroad reimbursement pathways in Europe, Middle East and Turkey
$192M in cash as of YE 2020 and runway into the first half of 2022 Compelling Fundamentals Driving Near and Long-term Growth 23 1x treatment – HSC gene therapy approach offers curative potential Strong clinical track record – over 160 patients treated Clinical validation in rare diseases increases confidence for larger indications now approved for early-onset MLD in the EU
