Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Jounce Therapeutics, Inc. | jnce-20210111.htm |

Jounce Therapeutics A Next Gen Immunotherapy Company January 2021

Legal Disclaimer Various statements concerning our future expectations, plans and prospects, including without limitation, our expectations regarding the timing, progress and results of discovery programs, preclinical studies and clinical trials for our product candidates and any future product candidates, the potential benefits of any of these product candidates and the timing or likelihood of regulatory filings, our analyses and plans for data disclosures, our cash position, and our plans and expectations in light of the COVID-19 pandemic and its impacts on global healthcare systems and our business may constitute forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws and are subject to substantial risks, uncertainties and assumptions. You should not place reliance on these forward looking statements, which often include words such as “anticipate,” “can,” “estimate,” “expect,” “explore,” “goal,” “initiate,” “intend,” “in development,” “may,” “on track,” “plan,” “position,” “potential,” “predict,” “predictive,” “target,” “tracking,” “up to,” or similar terms, variations of such terms or the negative of those terms. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee such outcomes. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, risks that the COVID-19 pandemic may disrupt our business and/or the global healthcare system more severely than we have anticipated, which may have the effect of further delaying our ability to initiate, enroll and complete our ongoing clinical trials, delaying our timelines for conducting analyses of our clinical trial data, preparing regulatory submissions or disclosing data, or delaying or interrupting the work of our third-party partners, our ability to successfully demonstrate the efficacy and safety of our product candidates and future product candidates, the preclinical and clinical results for our product candidates, which may not support further development and marketing approval, the potential advantages of our product candidates, the development plans of our product candidates, the management of our supply chain for the delivery of drug product and materials for use in our clinical trials and research and development activities, the use of our product candidates in combination with other therapies and our ability to obtain such therapies, actions of regulatory agencies, which may affect the initiation, timing and progress of preclinical studies and clinical trials of our product candidates, our anticipated milestones, our ability to obtain, maintain and protect its intellectual property, our ability to enforce its patents against infringers and defend its patent portfolio against challenges from third parties, the market opportunity for our product candidates, timing, cost or other aspects of a potential commercial launch of our product candidates and potential future sales of our current product candidates or any other potential products if any are approved for marketing, competition from others developing products for similar uses, our ability to manage operating expenses, our ability to establish or maintain collaborations, our dependence on third parties for development, manufacture, marketing, sales and distribution of product candidates and unexpected expenditures, as well as those risks more fully discussed in the section entitled “Risk Factors” in Jounce’s most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission as well as discussions of potential risks, uncertainties, and other important factors in our subsequent filings with the Securities and Exchange Commission. All such statements speak only as of the date made, and we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. 2 | © 2021 Jounce Therapeutics, Inc.

The Jounce Difference 3 | © 2021 Jounce Therapeutics, Inc. Translational Science Platform – Proven Value Generation Platform Pipeline Corporate Profile Near-Term Milestones • Management team with proven track record in IO, oncology R&D and business development • Founded by IO pioneers with Nobel, Coley and Lasker Prize winners • Cash runway into 2023 • Highly productive Translational Science Platform • Robust discovery across different immune cell types • Translational analysis from clinical trial samples - pharmacodynamic & predictive biomarkers • Potential FIC development programs: ICOS, LILRB2, CCR8 • POC studies underway on our two lead programs (INNATE & SELECT) • PD-1i as combination Immuno-oncology backbone • JTX-1811 (CCR8): IND on track for 1H21 filing • JTX-8064 (LILRB2 / ILT4): Establish RP2D and safety in 2H21 • Vopratelimab & JTX-4014: Ph 2 data expected late 2021

The Core of Our Approach: Translational Science Platform Translational Drug Discovery • Sustainable engine • Multiple immune cell types • New IND every 12 – 18 months Reverse Translational Analysis • Patients with clinical outcomes • PD* and predictive biomarkers • Patients more likely to respond 4 | © 2021 Jounce Therapeutics, Inc. *PD= Pharmacodynamic

IO Sensitive & IO Resistant Tumors: Significant But Different Opportunities Green – T cells Green – T cells Red – Macrophages PD-(L)1i* Sensitive PD-(L)1i Resistant IO Market Growth *PD-(L)1i = PD-1 inhibitor or PD-L1 inhibitor **GlobalData Drug Sales and Consensus Forecast 2020 (GlobalData Inc.) Current PD-(L)1i Market $27B** Most Patients Don’t Benefit Growing Market Requiring New Mechanisms • PD-(L)1i naïve patients • Durable clinical benefit in a minority of patients • Opportunities in biomarker selection of patients / new mechanisms • PD-(L)1i naïve or experienced patients • Primary or secondary PD-(L)1i resistance • Chemo remains SOC; little IO impact • Opportunities in new mechanisms that may restore IO responsiveness Few Immune Cells 5 | © 2021 Jounce Therapeutics, Inc.

Not Disclosed Myeloid Not Disclosed Stroma Not Disclosed PD-(L)1i resistance mechanisms Jounce Immunotherapy Pipeline Program Target Biology Discovery Preclinical IND Enabling Clinical Phase 1 Phase 2 Phase 3 Vopratelimab ICOS CD4 T cell focused JTX-4014 PD-1 CD8 T cell focused JTX-8064 LILRB2 (ILT4) Suppressive macrophage JTX-1811 CCR8 T-regulatory Development Programs Discovery Programs Jounce Wholly Owned 6 | © 2021 Jounce Therapeutics, Inc. SELECT (PD-(L)1i naïve patients) SELECT & INNATE INNATE (PD-(L)1i experienced & naïve patients)

Recent News by Program 7 | © 2021 Jounce Therapeutics, Inc. POC Studies Underway JTX-8064 INNATE Study – IND accepted Nov 2020 – Sites open and patient screening began Dec 2020 Vopra SELECT Study – Biomarker selected patients began enrolling Oct 2020 JTX-4014: PD-1i Used in INNATE & SELECT Discovery Engine Continues to Create Value – JTX-1811: Gilead out-license – Multiple potential FIC discovery programs

Lead Macrophage Program: JTX-8064

JTX-8064: Potential Macrophage Checkpoint Inhibitor • Novel, highly selective & potent IgG4 antibody to LILRB2 (ILT4) • Designed to reprogram tumor macrophages (M2 to M1) • Potential to reverse PD-(L)1i resistance JTX-8064 Antibody • IND acceptance Nov 2020; Sites open and patient screening began Dec 2020 • PD-(L)1i resistant and sensitive tumors • Supportive clinical data on the target presented at ESMO 2020* • Updates anticipated at multiple scientific meetings in 2021 • INNATE Trial: RP2D, safety and opening of expansion cohorts expected in 2021 Program Highlights 9 | © 2021 Jounce Therapeutics, Inc. *Siu et al. ESMO 2020 (Merck’s LILRB2 / ILT4 Program)
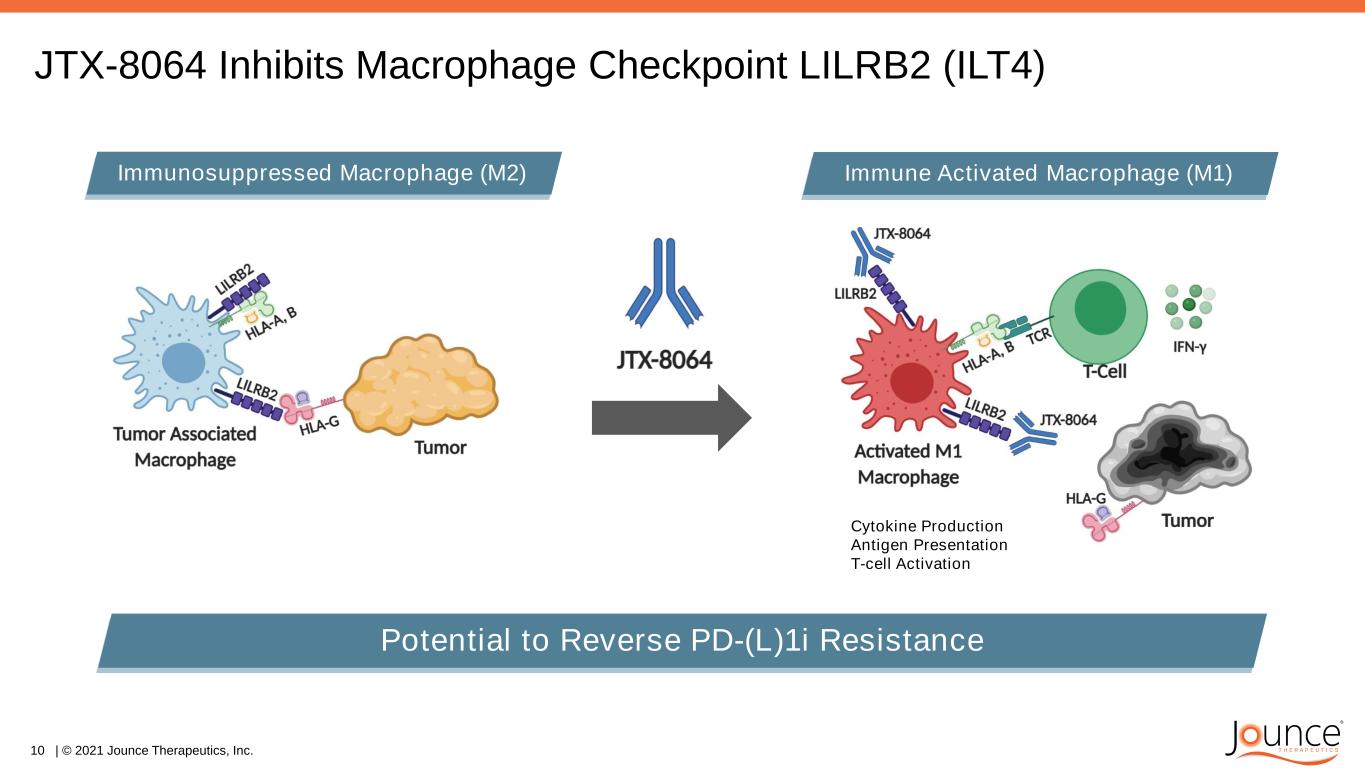
JTX-8064 Inhibits Macrophage Checkpoint LILRB2 (ILT4) 10 | © 2021 Jounce Therapeutics, Inc. Potential to Reverse PD-(L)1i Resistance Immunosuppressed Macrophage (M2) Immune Activated Macrophage (M1) Cytokine Production Antigen Presentation T-cell Activation

INNATE: JTX-8064 Mono and PD-1i Combo Dose Escalation and Expansion 11 | © 2021 Jounce Therapeutics, Inc. • Designed to rapidly demonstrate POC • Indications selected based on: – Biology, RNA signatures and differentiated development opportunities – Unmet medical need: PD-(L)1i sensitive and resistant tumors, PD-(L)1i experienced and naïve patients • Assessment of pharmacodynamic and potential predictive biomarkers Monotherapy Solid Tumors 1H 2021 Stage 1: Dose Escalation All Comers 2H 2021 Stage 3: Expansion Cohorts Indication Specific PD-1 Combination Solid Tumors 2H 2021 Stage 2: Dose Escalation All Comers 2H 2021 Stage 4: Expansion Cohorts Indication Specific

• Potential to move to front line therapy in IO responsive tumor type • High unmet need due to minimal to no response to PD-(L)1i • Potential for rapid registration 12 | © 2021 Jounce Therapeutics, Inc. Opportunities Across Major IO Segments INNATE: Indication Selection & Unmet Need PD-(L)1i Naïve Patients No Approved PD-(L)1 • High unmet need as PD-(L)1i approvals increase in early line therapy • Preliminary data on MK-4830 promising in primary resistance • Potential for rapid registration of JTX-8064 +/- JTX-4014 PD-(L)1i Experienced Patients PD-(L)1i Naïve Patients Where PD-(L)1s Approved

Vopratelimab & JTX-4014
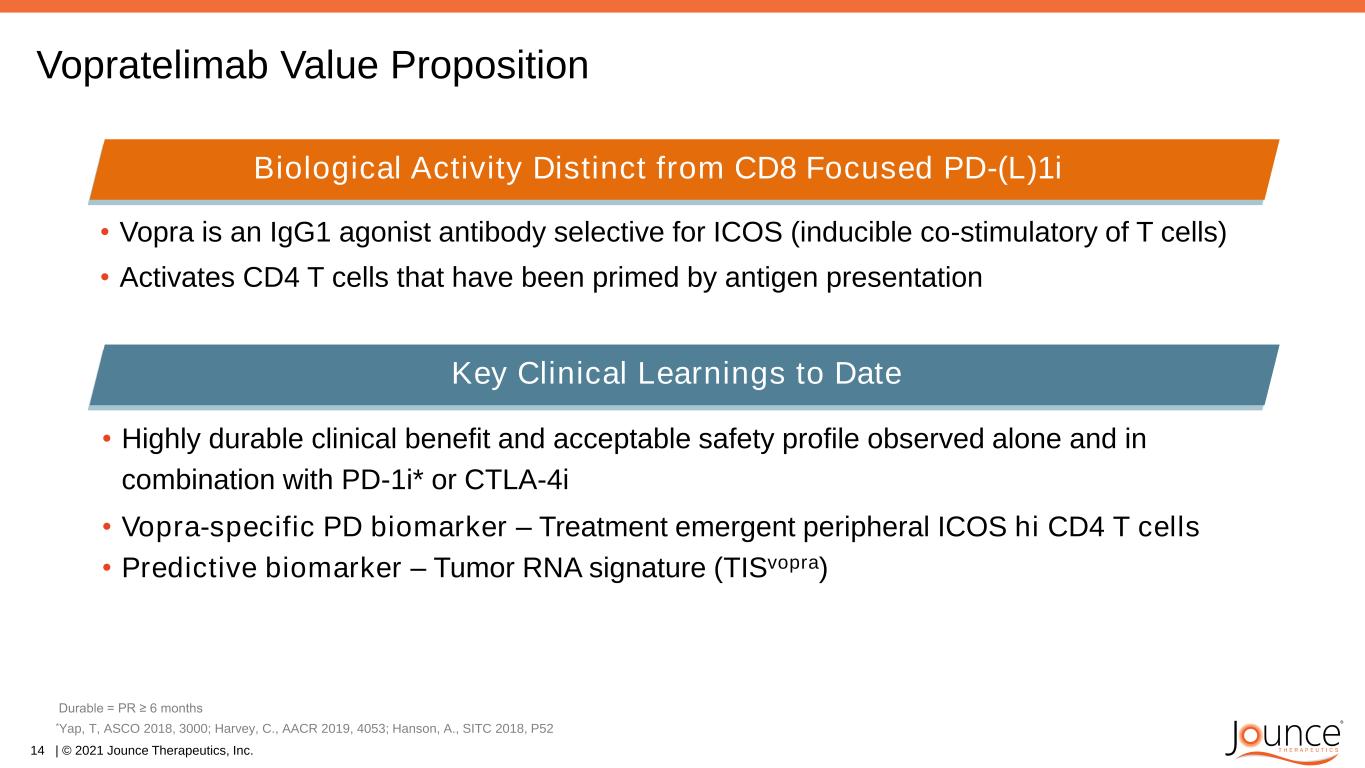
Vopratelimab Value Proposition • Highly durable clinical benefit and acceptable safety profile observed alone and in combination with PD-1i* or CTLA-4i • Vopra-specific PD biomarker – Treatment emergent peripheral ICOS hi CD4 T cells • Predictive biomarker – Tumor RNA signature (TISvopra) 14 | © 2021 Jounce Therapeutics, Inc. Biological activity beyond CD8 focused PD-1 inhibitors *Yap, T, ASCO 2018, 3000; Harvey, C., AACR 2019, 4053; Hanson, A., SITC 2018, P52 Biological activity distinct from CD8 focused PD-(L)1i* Key Learnings from ICONIC**Key Clinical Learnings to Date Durable = PR ≥ 6 months • Vopra is an IgG1 agonist antibody selective for ICOS (inducible co-stimulatory of T cells) • Activates CD4 T cells that have been primed by antigen presentation Biological Activity Distinct from CD8 Focused PD-(L)1i

TISvopra Predicts for ICOS hi CD4 T Cells and Potential Clinical Benefit ICONIC Overall Survival by ICOS hi CD4 Status* Treatment emergent ICOS hi CD4 T cells associated with improved survival TISvopra predicts for on treatment emergence of ICOS hi CD4 T cells and OS ICONIC Overall Survival by TISvopra Status 15 | © 2021 Jounce Therapeutics, Inc. ICOS hi ICOS lo As of 23-JUL-2020, n = 44 Below TISvopra Cut-Off Median OS = 6.2 mo Above TISvopra Cut-Off Median OS = 16.9 mo Above Biomarker Cut-Off Below Biomarker Cut-Off As of 23-JUL-2020, n = 89 Pharmacodynamic Biomarker: Predictive Biomarker: *Subset of 44 patients with evaluable blood samples and tumor measurements; based on investigator assessment
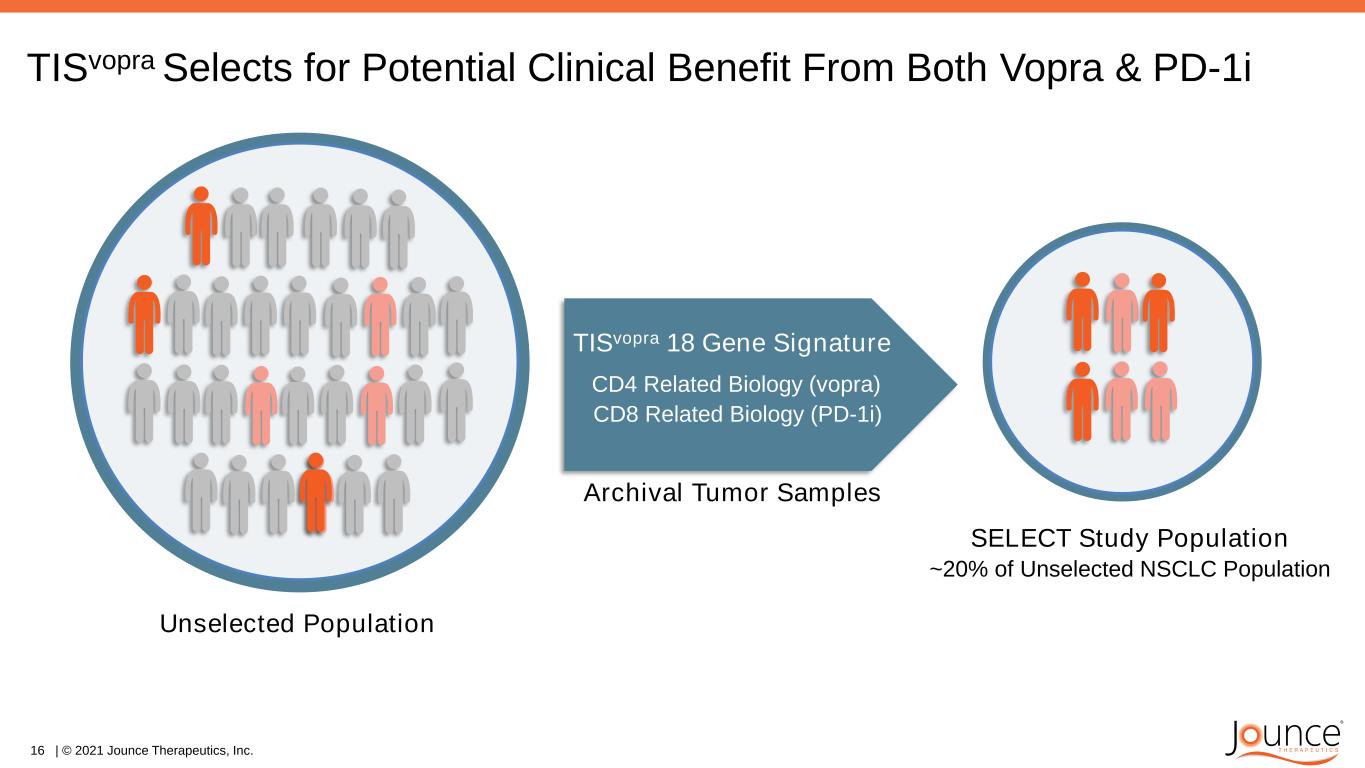
TISvopra Selects for Potential Clinical Benefit From Both Vopra & PD-1i SELECT Study Population ~20% of Unselected NSCLC Population Unselected Population TISvopra 18 Gene Signature CD8 Related Biology (PD-1i) CD4 Related Biology (vopra) Archival Tumor Samples 16 | © 2021 Jounce Therapeutics, Inc.

• IO naïve 2L NSCLC in ~75 patients selected with TISvopra • Powered to demonstrate statistical superiority of vopra + JTX-4014 versus JTX-4014 • Enrollment initiated October 2020, clinical data expected in late 2021 SELECT: Phase 2 Predictive Biomarker Study Clinical Study of Two Wholly Owned Antibodies with New Predictive Biomarker Establish combo POC in TISvopra selected patients Vopra + JTX-4014 (PD-1i) JTX-4014 (PD-1i) vs 17 | © 2021 Jounce Therapeutics, Inc. Phase 2 SELECT Randomized POC Study Study Design Study Hypothesis

Data cut-off 30-OCT-2020 JTX-4014: PD-1 Inhibitor in Two POC Combination Studies Phase 1 Study Results* • 3/18 (16.7%) confirmed responses • No deaths or dose limiting toxicities Opportunities for JTX-4014 • Biomarker selected IO naïve patients (SELECT) • PD-(L)1i naïve & experienced patients (INNATE) Well-Characterized PD-1 Antibody, Fully Human IgG4 * Lymph node 80% reduction to 5mm, qualifies as CR by RECIST criteria. 80mg q3w 240mg q3w 800mg q3w 800mg q6w 400mg q3w 1200mg q3w C h a n g e f ro m B a s e li n e % Months Since Treatment Initiation Target Lesion Change from Baseline Over Time *Papadopoulos, K., Cancer Immunology, Immunotherapy 2020 18 | © 2021 Jounce Therapeutics, Inc.

Discovery

Creating Value Through Discovery: Translational Science Platform Novel & Sustainable Discovery • Right IO for right patients • Novel targets beyond T cells • Biomarker driven approach • New IND every 12 – 18 months Value Creation to Date • 3 wholly owned clinical programs • $360M in upfront business development payments • Potential for milestones & royalties 20 | © 2021 Jounce Therapeutics, Inc.

JTX-1811: Selective Depletion of Tumor Infiltrating T Regulatory Cells • Highly selective IgG1 monoclonal antibody to CCR8 • CCR8 is a chemokine receptor enriched on tumor infiltrating T-reg cells (T-regs) – T-regs cells are immunosuppressive in the TME • First in class mechanism designed for targeted & enhanced depletion of T-regs JTX-1811 Antibody • First T-reg program from Jounce’s Translational Science Platform • IND filing expected 1H21 Program Highlights 21 | © 2021 Jounce Therapeutics, Inc.

Gilead Licensed JTX-1811: Continued Value Generation 22 | © 2021 Jounce Therapeutics, Inc. • JTX-1811: Exclusively licensed to Gilead in October 2020 • $85M upfront license fee • $35M equity investment – ~14% ownership, 5.5M shares • Up to $685M in potential milestone payments – Up to $510M in development and regulatory – Up to $175M in commercial – Royalties high single digit to mid-teens on WW sales • Jounce to progress JTX-1811 to IND clearance • Clinical development and commercialization by Gilead License Terms

Milestones & Financials
2021 Milestones ▪ JTX-8064 (INNATE): ▪ Establish safety and RP2D ▪ Open tumor specific expansion cohorts ▪ Vopratelimab (SELECT): ▪ Complete trial enrollment (75 patients) ▪ Report preliminary efficacy data ▪ JTX-1811: ▪ IND acceptance ▪ Continue to advance multiple new targets through discovery pipeline with a goal of an IND every 12 – 18 months 24 | © 2021 Jounce Therapeutics, Inc.
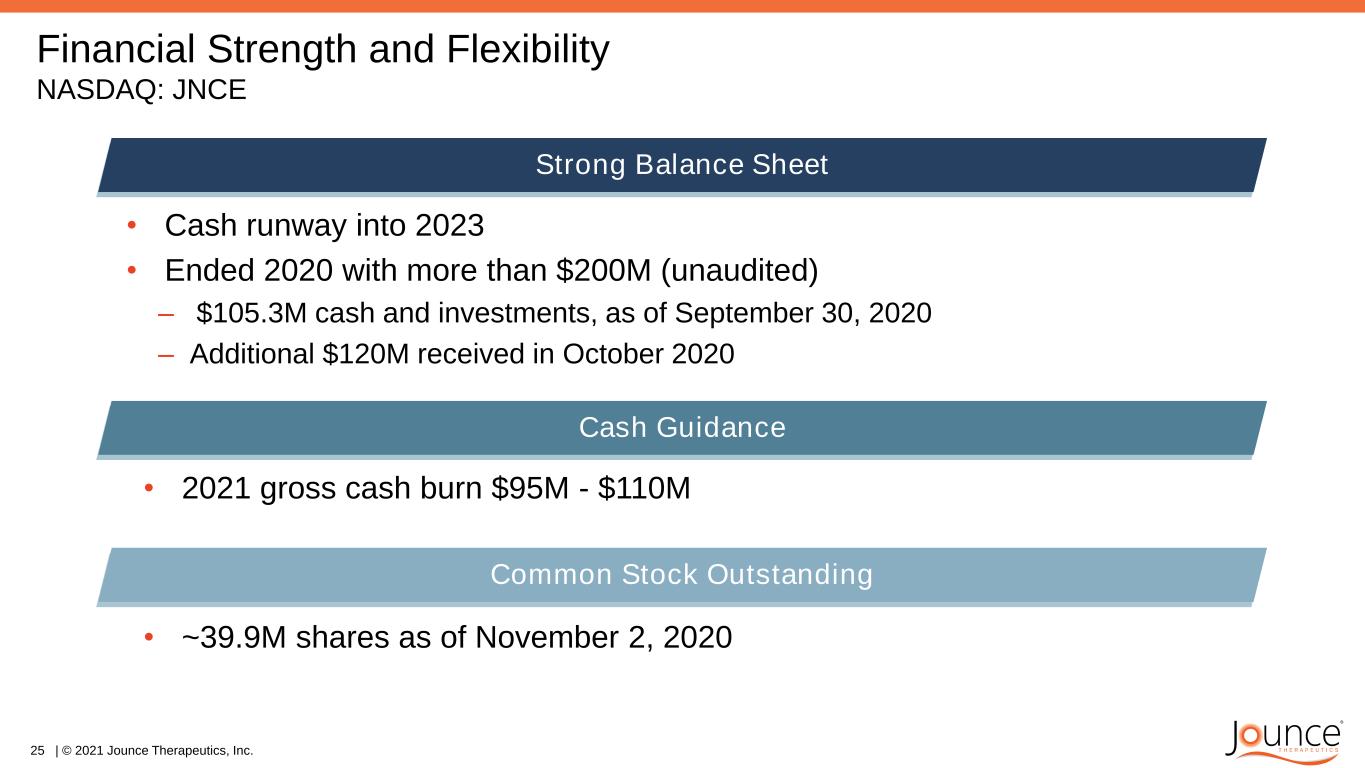
Financial Strength and Flexibility NASDAQ: JNCE • Cash runway into 2023 • Ended 2020 with more than $200M (unaudited) – $105.3M cash and investments, as of September 30, 2020 – Additional $120M received in October 2020 25 | © 2021 Jounce Therapeutics, Inc. • ~39.9M shares as of November 2, 2020 Strong Balance Sheet Cash Guidance • 2021 gross cash burn $95M - $110M Common Stock Outstanding

Jounce Therapeutics A Next Gen Immunotherapy Company
