Attached files
| file | filename |
|---|---|
| 8-K - 8-K - IDEAYA Biosciences, Inc. | idya-8k_20210111.htm |
IDEAYA Biosciences Improving Lives Through Transformative Precision Medicines JPM Healthcare Conference January 2021 Exhibit 99.1 NASDAQ: IDYA

Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future financial performance of IDEAYA Biosciences, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any expectations regarding the Company’s target discovery platform or new target validation efforts as creating opportunities for research and development initiatives; any projections of financial information, market opportunities, cash runway or profitability; any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of management for development programs or future operations; any statements about the timing of preclinical research, clinical development, regulatory filings, manufacturing or release of data; any statements of expectation or belief regarding future events, potential markets or market size, technology developments, or receipt of cash milestones, option exercise fees or royalties; and any statements of assumptions underlying any of the items mentioned. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company's control. These and other important factors may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. The forward-looking statements in this presentation are made only as of the date hereof. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see the Company's periodic filings with the Securities and Exchange Commission (the "SEC"), including its Annual Report on Form 10-K for the year ended December 31, 2019, its Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, and any current and periodic reports filed thereafter. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation concerns anticipated products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA). It is currently limited by Federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. Safe Harbor Statement 2
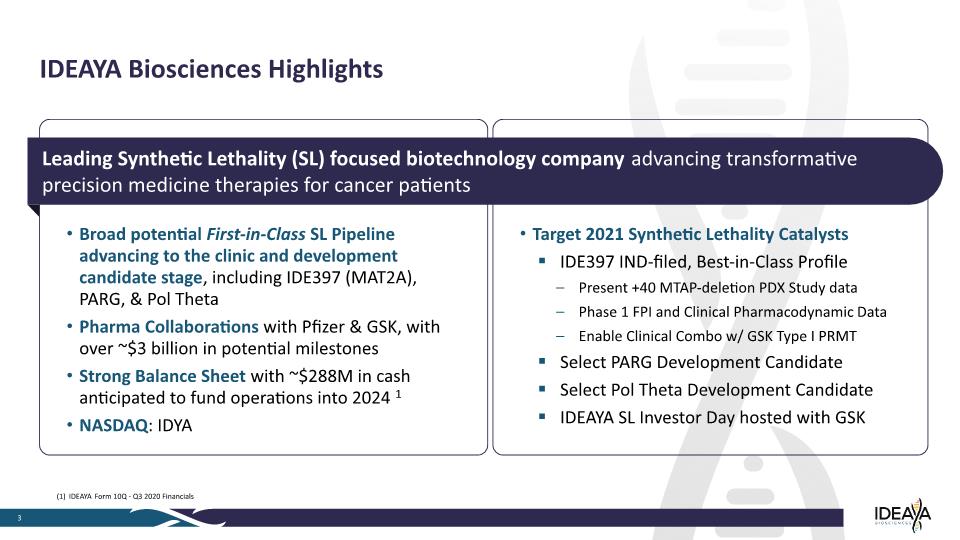
Target 2021 Synthetic Lethality Catalysts IDE397 IND-filed, Best-in-Class Profile Present +40 MTAP-deletion PDX Study data Phase 1 FPI and Clinical Pharmacodynamic Data Enable Clinical Combo w/ GSK Type I PRMT Select PARG Development Candidate Select Pol Theta Development Candidate IDEAYA SL Investor Day hosted with GSK 3 IDEAYA Biosciences Highlights Broad potential First-in-Class SL Pipeline advancing to the clinic and development candidate stage, including IDE397 (MAT2A), PARG, & Pol Theta Pharma Collaborations with Pfizer & GSK, with over ~$3 billion in potential milestones Strong Balance Sheet with ~$288M in cash anticipated to fund operations into 2024 1 NASDAQ: IDYA Leading Synthetic Lethality (SL) focused biotechnology company advancing transformative precision medicine therapies for cancer patients (1) IDEAYA Form 10Q - Q3 2020 Financials
Synthetic lethality occurs when the simultaneous perturbation of two genes results in cell death Synthetic lethal interactions with tumor-specific mutations (biomarker) may be exploited to develop anticancer therapies Large-scale screening for synthetic lethal targets has progressed through advances in molecular biology (e.g., RNA interference, CRISPR-Cas9 editing) Synthetic Lethality 4 The Next Frontier in Precision Medicine Oncology Synthetic Lethality provides a powerful approach to discover novel precision medicine therapies with patient biomarkers, including MTAP-deletion (~15% of solid tumors), BRCA/HRD (Breast, Prostate, Ovarian), and high-MSI (15% GI Cancers) Reference: Charles Boone Nature Reviews Genetics, Vol. 18, 2017, Hieter, et al Nature REVIEWS GENETICS Mitochondria Ribosome & translation RNA processing Chromatin & transcription Nuclear-cytoplasmic transport Nuclear migration & protein degradation Mitosis & chr. segregation Cell polarity & morphogenesis Protein folding & glycosylation Cell wall biosynthesis Secretion & vesicle transport Metabolism & amino acid biosynthesis Peroxisome
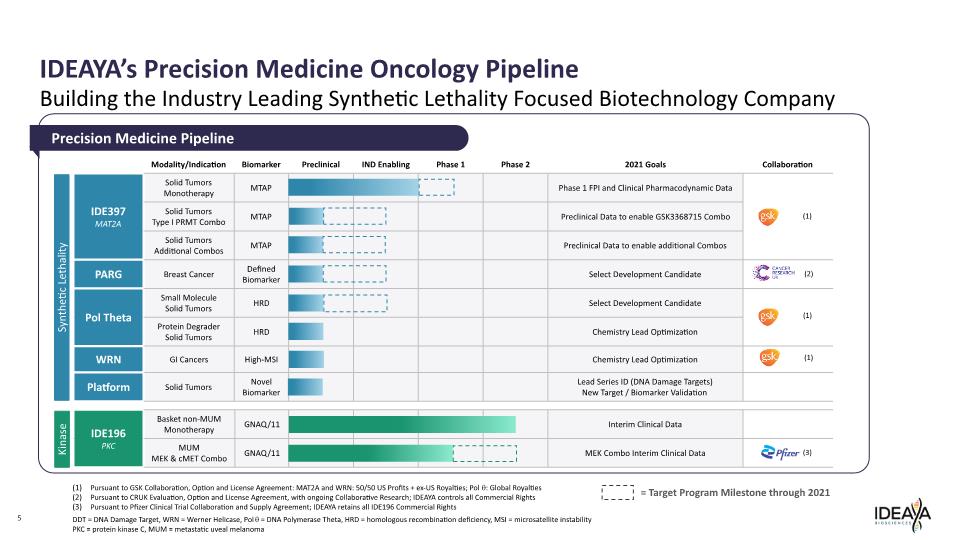
IDEAYA’s Precision Medicine Oncology Pipeline 5 Building the Industry Leading Synthetic Lethality Focused Biotechnology Company Precision Medicine Pipeline Pursuant to GSK Collaboration, Option and License Agreement: MAT2A and WRN: 50/50 US Profits + ex-US Royalties; Polq: Global Royalties Pursuant to CRUK Evaluation, Option and License Agreement, with ongoing Collaborative Research; IDEAYA controls all Commercial Rights Pursuant to Pfizer Clinical Trial Collaboration and Supply Agreement; IDEAYA retains all IDE196 Commercial Rights DDT = DNA Damage Target, WRN = Werner Helicase, Polq = DNA Polymerase Theta, HRD = homologous recombination deficiency, MSI = microsatellite instability PKC = protein kinase C, MUM = metastatic uveal melanoma (1) (1) (1) (2) (3) Synthetic Lethality Kinase Target Program Milestone through 2021 Modality/Indication Biomarker Preclinical IND Enabling Phase 1 Phase 2021 Goals Collaboration IDE397 MAT2A PARG Pol Theta WRN Platform IDE196 PKC Solid Tumors Monotherapy Solid Tumors Type I PRMT Combo Solid Tumors Additional Combos Breast Cancer Small Molecule Solid Tumors Protein Degrader Solid Tumors GI Cancers Solid Tumors Basket non-MUM Monotherapy MUM MEK & cMET Combo MTAP MTAP MTAP Defined Biomarker HRD HRD High-MSI Novel Biomarker GNAQ/11 GNAQ/11 Phase 1 FPI and Clinical Pharmacodynamic Data Preclinical Data to enable GSK3368715 Combo Preclinical Data to enable additional Combos Select Development Candidate Select Development Candidate Chemistry Lead Optimization Chemistry Lead Optimization Lead Series ID (DNA Damage Targets) New Target / Biomarker Validation Interim Clinical Data MEK Combo Interim Clinical Data (1) (2) (1) (1) Pfizer (3) gsk CANCER RESEARCH UK gsk gsk

IDEAYA Team and Scientific Advisory Board 6 Proven Team & Scientific Thought Leaders in Precision Medicine Oncology IDEAYA Executives & R&D Leadership IDEAYA Scientific Advisory Board Yujiro Hata, M.B.A. President, Chief Executive Officer, Director Paul Stone, J.D. SVP, Chief Financial Officer Michael Dillon, Ph.D. SVP, Chief Scientific Officer Head of Research Mark Lackner, Ph.D. SVP, Head of Biology & Translational Sciences Mick O’Quigley, M.B.A. VP, Development Operations Paul Barsanti, Ph.D. VP, Head of Drug Discovery Jason Throne, J.D. SVP, General Counsel Alan D’Andrea, M.D. IDEAYA SAB Chair Harvard Medical School, Professor Director, Center of DNA Damage and Repair, Dana Farber William Sellers, M.D. Broad Institute, Dana Farber, and Harvard, Professor Novartis, Former Head Oncology Research, SL Project Drive initiative Frank McCormick, Ph.D. UCSF, Professor and former Director, Helen Diller Cancer Center Former President AACR; Founder and CSO, Onyx Trey Ideker, Ph.D. UCSD, Professor, Co-Director Cancer Genomes & Networks Program, Research in Dual-CRISPR and SL interaction maps Jeffrey Hager, Ph.D. Former Chief Technology Officer, IDEAYA Elizabeth Swisher, M.D. University of Washington, Professor; Co-Leader, Breast and Ovarian Cancer Research Program, Seattle Cancer Care Alliance Principal Investigator on multiple PARP inhibitor trials Brian Daniels, M.D. Bristol Myers Squibb, Former SVP Global Development & Medical Affairs
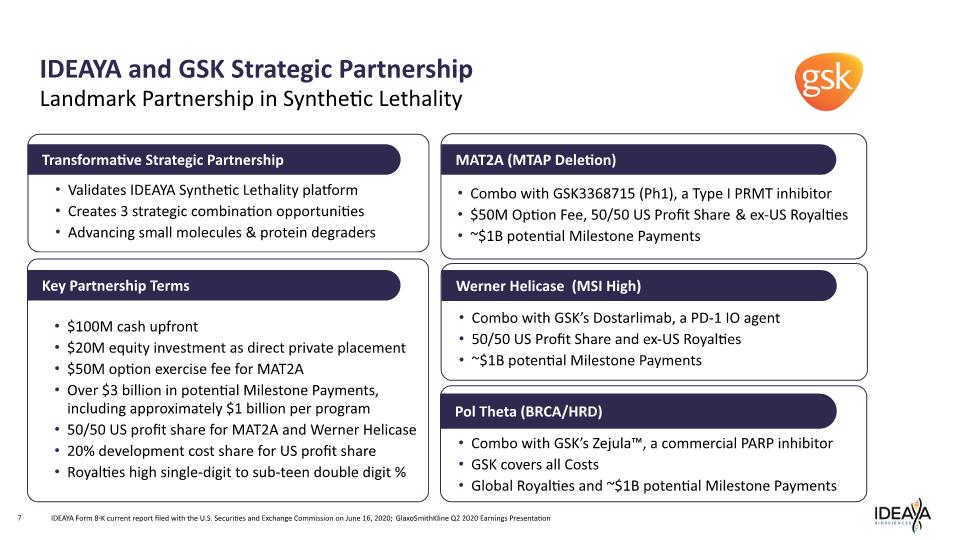
IDEAYA and GSK Strategic Partnership 7 Landmark Partnership in Synthetic Lethality Validates IDEAYA Synthetic Lethality platform Creates 3 strategic combination opportunities Advancing small molecules & protein degraders Transformative Strategic Partnership $100M cash upfront $20M equity investment as direct private placement $50M option exercise fee for MAT2A Over $3 billion in potential Milestone Payments, including approximately $1 billion per program 50/50 US profit share for MAT2A and Werner Helicase 20% development cost share for US profit share Royalties high single-digit to sub-teen double digit % Key Partnership Terms Pol Theta (BRCA/HRD) Werner Helicase (MSI High) MAT2A (MTAP Deletion) Combo with GSK’s Zejula™, a commercial PARP inhibitor GSK covers all Costs Global Royalties and ~$1B potential Milestone Payments Combo with GSK3368715 (Ph1), a Type I PRMT inhibitor $50M Option Fee, 50/50 US Profit Share & ex-US Royalties ~$1B potential Milestone Payments IDEAYA Form 8-K current report filed with the U.S. Securities and Exchange Commission on June 16, 2020; GlaxoSmithKline Q2 2020 Earnings Presentation Combo with GSK’s Dostarlimab, a PD-1 IO agent 50/50 US Profit Share and ex-US Royalties ~$1B potential Milestone Payments
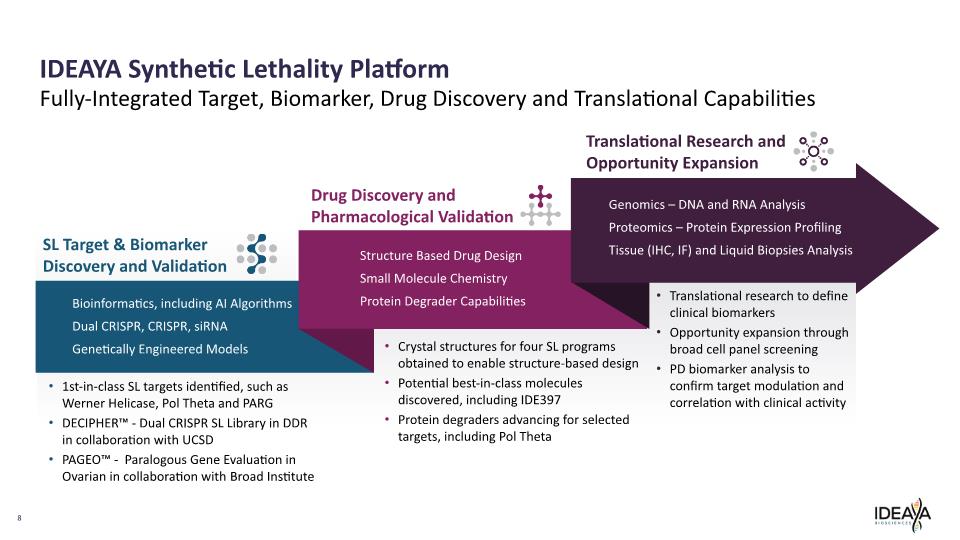
8 SL Target & Biomarker Discovery and Validation Bioinformatics, including AI Algorithms Dual CRISPR, CRISPR, siRNA Genetically Engineered Models Drug Discovery and Pharmacological Validation Structure Based Drug Design Small Molecule Chemistry Protein Degrader Capabilities 1st-in-class SL targets identified, such as Werner Helicase, Pol Theta and PARG DECIPHER™ - Dual CRISPR SL Library in DDR in collaboration with UCSD PAGEO™ - Paralogous Gene Evaluation in Ovarian in collaboration with Broad Institute Crystal structures for four SL programs obtained to enable structure-based design Potential best-in-class molecules discovered, including IDE397 Protein degraders advancing for selected targets, including Pol Theta Translational Research and Opportunity Expansion Genomics – DNA and RNA Analysis Proteomics – Protein Expression Profiling Tissue (IHC, IF) and Liquid Biopsies Analysis IDEAYA Synthetic Lethality Platform Translational research to define clinical biomarkers Opportunity expansion through broad cell panel screening PD biomarker analysis to confirm target modulation and correlation with clinical activity Fully-Integrated Target, Biomarker, Drug Discovery and Translational Capabilities
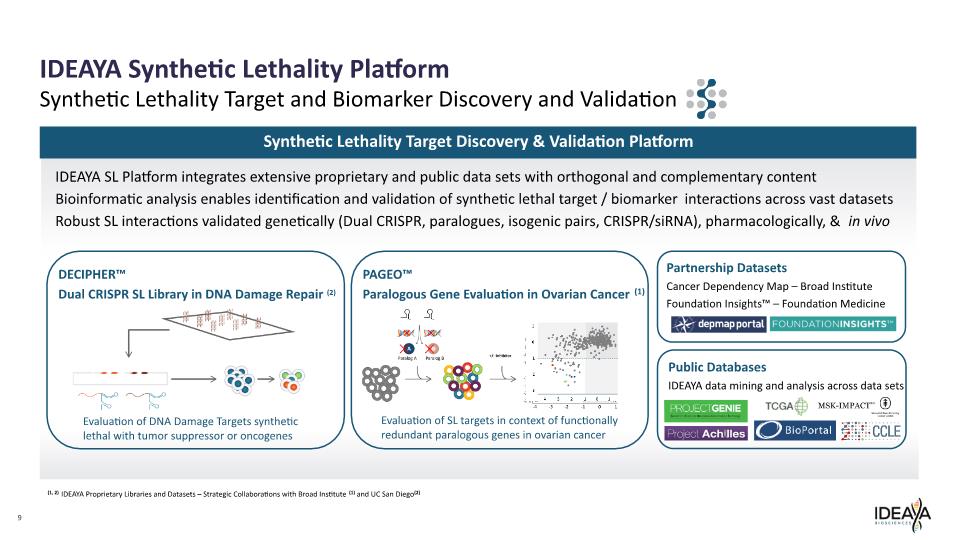
Partnership Datasets Cancer Dependency Map – Broad Institute Foundation Insights™ – Foundation Medicine 9 IDEAYA Synthetic Lethality Platform Synthetic Lethality Target and Biomarker Discovery and Validation DECIPHER™ Dual CRISPR SL Library in DNA Damage Repair (2) PAGEO™ Paralogous Gene Evaluation in Ovarian Cancer (1) Public Databases IDEAYA data mining and analysis across data sets Evaluation of SL targets in context of functionally redundant paralogous genes in ovarian cancer Evaluation of DNA Damage Targets synthetic lethal with tumor suppressor or oncogenes Synthetic Lethality Target Discovery & Validation Platform IDEAYA SL Platform integrates extensive proprietary and public data sets with orthogonal and complementary content Bioinformatic analysis enables identification and validation of synthetic lethal target / biomarker interactions across vast datasets Robust SL interactions validated genetically (Dual CRISPR, paralogues, isogenic pairs, CRISPR/siRNA), pharmacologically, & in vivo (1, 2) IDEAYA Proprietary Libraries and Datasets – Strategic Collaborations with Broad Institute(1) and UC San Diego(2)
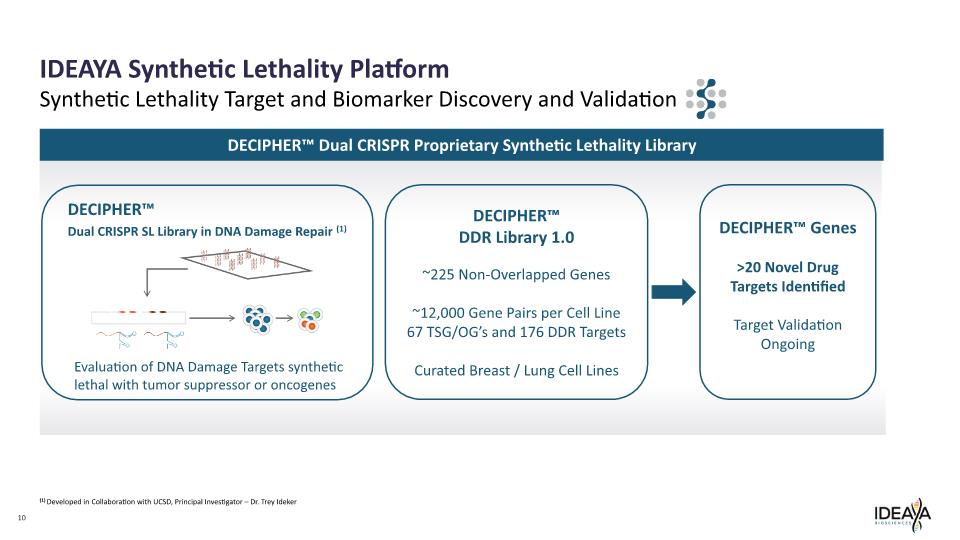
10 IDEAYA Synthetic Lethality Platform Synthetic Lethality Target and Biomarker Discovery and Validation DECIPHER™ Dual CRISPR SL Library in DNA Damage Repair (1) Evaluation of DNA Damage Targets synthetic lethal with tumor suppressor or oncogenes DECIPHER™ Dual CRISPR Proprietary Synthetic Lethality Library (1) Developed in Collaboration with UCSD, Principal Investigator – Dr. Trey Ideker DECIPHER™ DDR Library 1.0 ~225 Non-Overlapped Genes ~12,000 Gene Pairs per Cell Line 67 TSG/OG’s and 176 DDR Targets Curated Breast / Lung Cell Lines DECIPHER™ Genes >20 Novel Drug Targets Identified Target Validation Ongoing
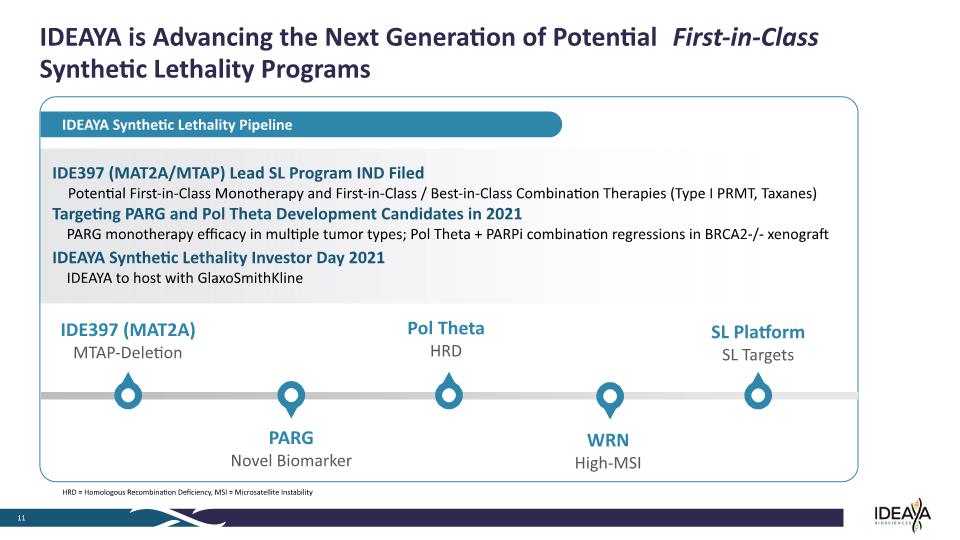
IDE397 (MAT2A/MTAP) Lead SL Program IND Filed Potential First-in-Class Monotherapy and First-in-Class / Best-in-Class Combination Therapies (Type I PRMT, Taxanes) Targeting PARG and Pol Theta Development Candidates in 2021 PARG monotherapy efficacy in multiple tumor types; Pol Theta + PARPi combination regressions in BRCA2-/- xenograft IDEAYA Synthetic Lethality Investor Day 2021 IDEAYA to host with GlaxoSmithKline IDEAYA is Advancing the Next Generation of Potential First-in-Class Synthetic Lethality Programs 11 IDEAYA Synthetic Lethality Pipeline IDE397 (MAT2A) MTAP-Deletion WRN High-MSI SL Platform SL Targets Pol Theta HRD PARG Novel Biomarker HRD = Homologous Recombination Deficiency, MSI = Microsatellite Instability
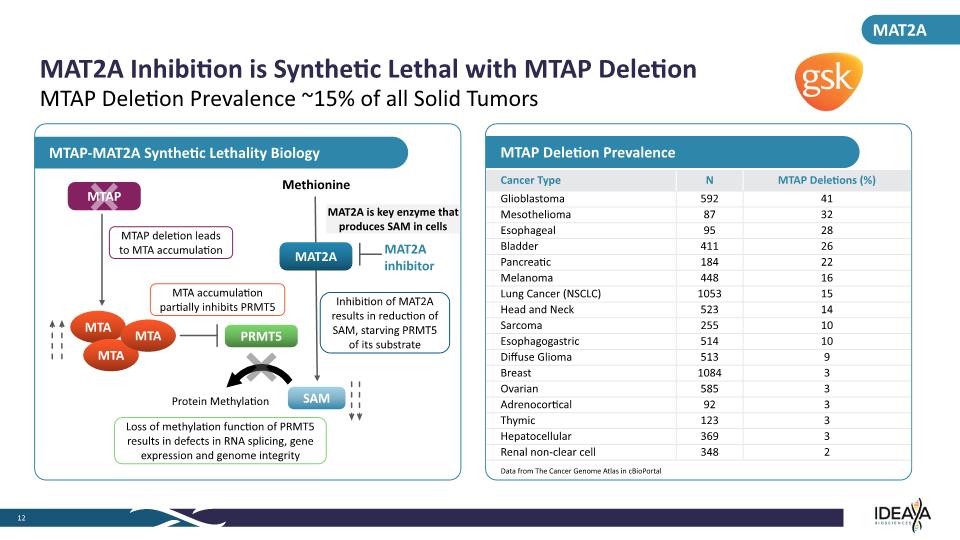
MTAP Deletion Prevalence MTAP-MAT2A Synthetic Lethality Biology MAT2A Inhibition is Synthetic Lethal with MTAP Deletion 12 MTAP Deletion Prevalence ~15% of all Solid Tumors Data from The Cancer Genome Atlas in cBioPortal MAT2A MAT2A inhibitor MAT2A Methionine MAT2A is key enzyme that produces SAM in cells SAM PRMT5 MTAP Protein Methylation MTAP deletion leads to MTA accumulation MTA accumulation partially inhibits PRMT5 Loss of methylation function of PRMT5 results in defects in RNA splicing, gene expression and genome integrity Inhibition of MAT2A results in reduction of SAM, starving PRMT5 of its substrate Cancer Type N MTAP Deletions (%) Glioblastoma 592 41 Mesothelioma 87 32 Esophageal 95 28 Bladder 411 26 Pancreatic 184 22 Melanoma 448 16 Lung Cancer (NSCLC) 1053 15 Head and Neck 523 14 Sarcoma 255 10 Esophagogastric 514 10 Diffuse Glioma 513 9 Breast 1084 3 Ovarian 585 3 Adrenocortical 92 3 Thymic 123 3 Hepatocellular 369 3 Renal non-clear cell 348 2
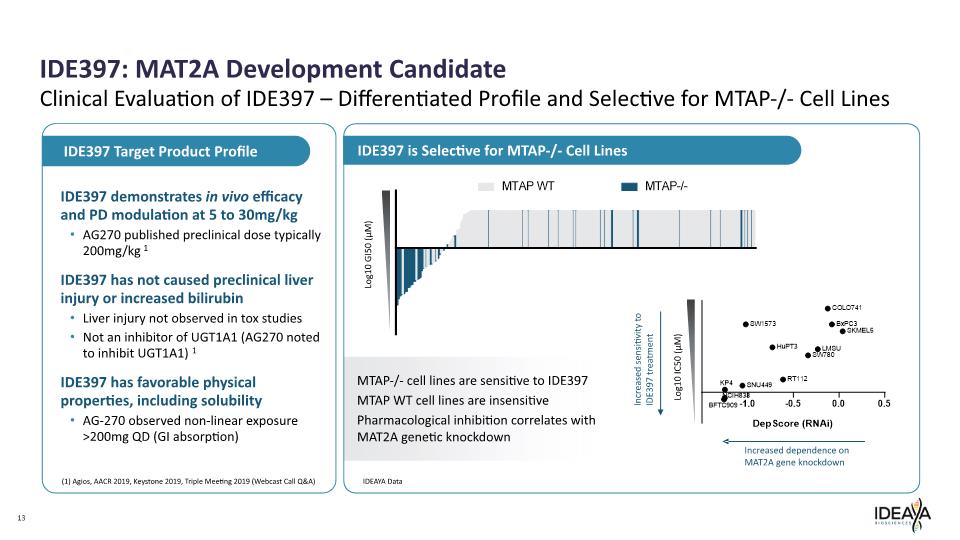
IDE397: MAT2A Development Candidate 13 Clinical Evaluation of IDE397 – Differentiated Profile and Selective for MTAP-/- Cell Lines IDE397 demonstrates in vivo efficacy and PD modulation at 5 to 30mg/kg AG270 published preclinical dose typically 200mg/kg 1 IDE397 has not caused preclinical liver injury or increased bilirubin Liver injury not observed in tox studies Not an inhibitor of UGT1A1 (AG270 noted to inhibit UGT1A1) 1 IDE397 has favorable physical properties, including solubility AG-270 observed non-linear exposure >200mg QD (GI absorption) IDE397 Target Product Profile IDE397 is Selective for MTAP-/- Cell Lines IDEAYA Data MTAP-/- cell lines are sensitive to IDE397 MTAP WT cell lines are insensitive Pharmacological inhibition correlates with MAT2A genetic knockdown Log10 GI50 (µM) Log10 IC50 (µM) (1) Agios, AACR 2019, Keystone 2019, Triple Meeting 2019 (Webcast Call Q&A) Increased sensitivity to IDE397 treatment Increased dependence on MAT2A gene knockdown MTAP WT MTAP-/- SW1573 COLO741 BxPC3 SKMEL5 HuPT3 LMSU SW780 KP4 SNU449 RT 112 NCIH 833 BFTC909 -1.0 -0.5 0.0 0.5
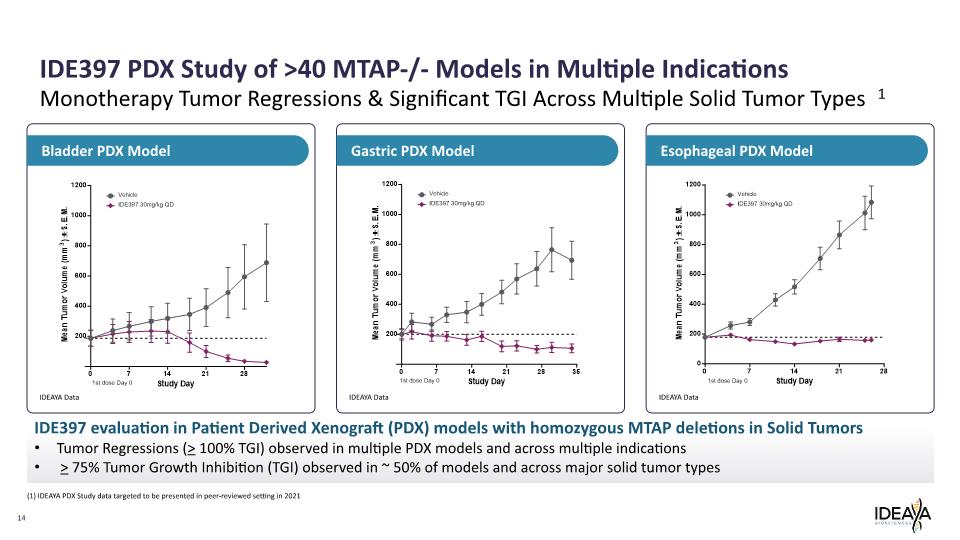
14 Monotherapy Tumor Regressions & Significant TGI Across Multiple Solid Tumor Types 1 Esophageal PDX Model Gastric PDX Model Bladder PDX Model IDE397 PDX Study of >40 MTAP-/- Models in Multiple Indications IDE397 evaluation in Patient Derived Xenograft (PDX) models with homozygous MTAP deletions in Solid Tumors Tumor Regressions (> 100% TGI) observed in multiple PDX models and across multiple indications > 75% Tumor Growth Inhibition (TGI) observed in ~ 50% of models and across major solid tumor types IDEAYA Data IDEAYA Data IDEAYA Data (1) IDEAYA PDX Study data targeted to be presented in peer-reviewed setting in 2021 Mean Tumor Volume (mm3) ± S.E.M. 1200 1000 800 600 400 200 0 7 14 21 28 1st dose Day 0 Study Day Vehicle IDE397 30mg/kg QD Mean Tumor Volume (mm3) ± S.E.M. 1200 1000 800 600 400 200 0 7 14 21 28 35 1st dose Day 0 Study Day Vehicle IDE397 30mg/kg QD Mean Tumor Volume (mm3) ± S.E.M. 1200 1000 800 600 400 200 0 7 14 21 28 1st dose Day 0 Study Day Vehicle IDE397 30mg/kg QD
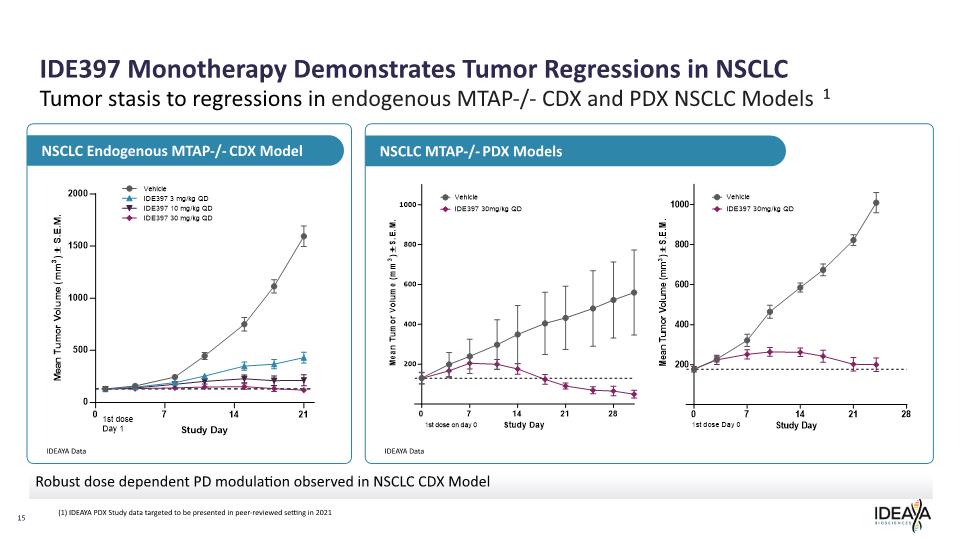
Robust dose dependent PD modulation observed in NSCLC CDX Model 15 NSCLC MTAP-/- PDX Models NSCLC Endogenous MTAP-/- CDX Model Tumor stasis to regressions in endogenous MTAP-/- CDX and PDX NSCLC Models 1 IDE397 Monotherapy Demonstrates Tumor Regressions in NSCLC (1) IDEAYA PDX Study data targeted to be presented in peer-reviewed setting in 2021 IDEAYA Data IDEAYA Data Mean Tumor Volume (mm3) ± S.E.M.2000 1500 1000 500 0 0 7 14 21 1st dose Day 1 Study Day Vehicle IDE397 3 mg/kg QD IDE397 10 mg/kg QD IDE397 30 mg/kg QD Mean Tumor Volume (mm3) ± S.E.M. 1000 800 600 400 200 0 7 14 21 28 1st dose Day 0 Study Day Vehicle IDE397 30mg/kg QD Tumor Volume (mm3) ± S.E.M. 1000 800 600 400 200 0 7 14 21 28 1st dose Day 0 Study Day Vehicle IDE397 30mg/kg QD
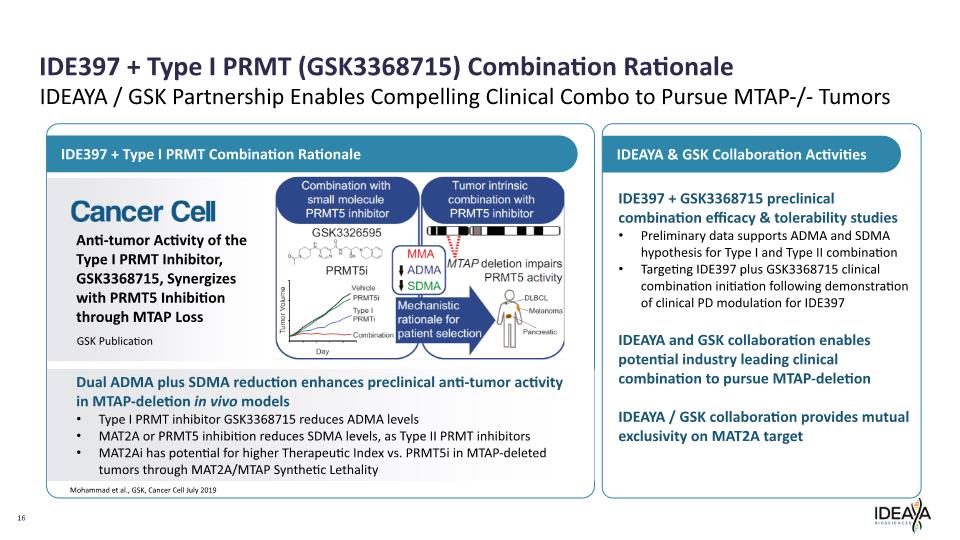
IDE397 + Type I PRMT (GSK3368715) Combination Rationale 16 Mohammad et al., GSK, Cancer Cell July 2019 Anti-tumor Activity of the Type I PRMT Inhibitor, GSK3368715, Synergizes with PRMT5 Inhibition through MTAP Loss Dual ADMA plus SDMA reduction enhances preclinical anti-tumor activity in MTAP-deletion in vivo models Type I PRMT inhibitor GSK3368715 reduces ADMA levels MAT2A or PRMT5 inhibition reduces SDMA levels, as Type II PRMT inhibitors MAT2Ai has potential for higher Therapeutic Index vs. PRMT5i in MTAP-deleted tumors through MAT2A/MTAP Synthetic Lethality IDE397 + Type I PRMT Combination Rationale IDEAYA & GSK Collaboration Activities IDE397 + GSK3368715 preclinical combination efficacy & tolerability studies Preliminary data supports ADMA and SDMA hypothesis for Type I and Type II combination Targeting IDE397 plus GSK3368715 clinical combination initiation following demonstration of clinical PD modulation for IDE397 IDEAYA and GSK collaboration enables potential industry leading clinical combination to pursue MTAP-deletion IDEAYA / GSK collaboration provides mutual exclusivity on MAT2A target IDEAYA / GSK Partnership Enables Compelling Clinical Combo to Pursue MTAP-/- Tumors GSK Publication Cancer Cell Combination with small molecule PRMT5 inhibitor GSK3326595 PRMT5i Vehicle PRMT5i Type I PRMTi Combination Day Tumor Volume MMA ADMA SDMA Mechanistic rationale patient selection Tumor intrinsic combination with PRMT5 inhibitor MTAP deletion impairs PRMT5 activity DLBCL Melanoma Pancreatic
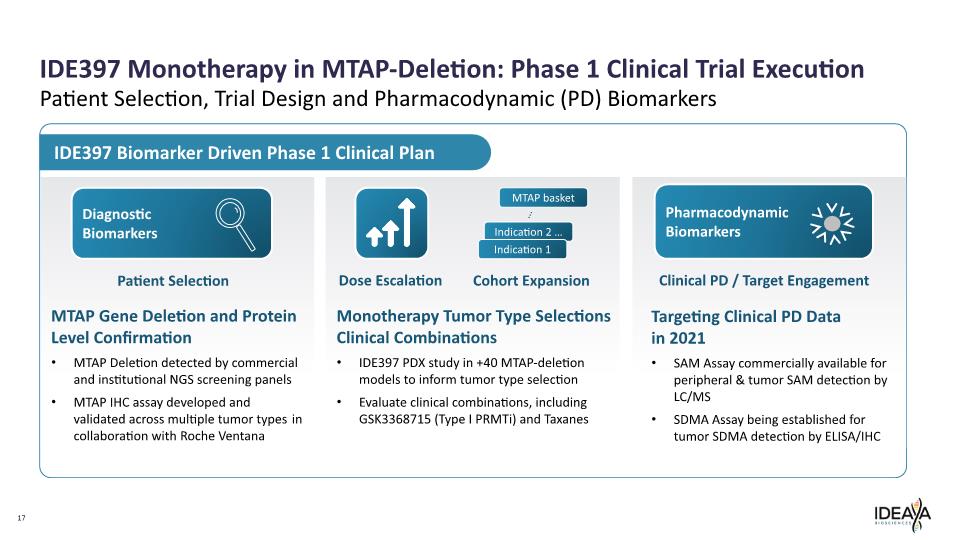
IDE397 Monotherapy in MTAP-Deletion: Phase 1 Clinical Trial Execution 17 Patient Selection, Trial Design and Pharmacodynamic (PD) Biomarkers MTAP Gene Deletion and Protein Level Confirmation MTAP Deletion detected by commercial and institutional NGS screening panels MTAP IHC assay developed and validated across multiple tumor types in collaboration with Roche Ventana Diagnostic Biomarkers Patient Selection IDE397 Biomarker Driven Phase 1 Clinical Plan Dose Escalation Cohort Expansion Monotherapy Tumor Type Selections Clinical Combinations IDE397 PDX study in +40 MTAP-deletion models to inform tumor type selection Evaluate clinical combinations, including GSK3368715 (Type I PRMTi) and Taxanes Pharmacodynamic Biomarkers Clinical PD / Target Engagement Targeting Clinical PD Data in 2021 SAM Assay commercially available for peripheral & tumor SAM detection by LC/MS SDMA Assay being established for tumor SDMA detection by ELISA/IHC Indication 2 … MTAP basket Indication 1 …
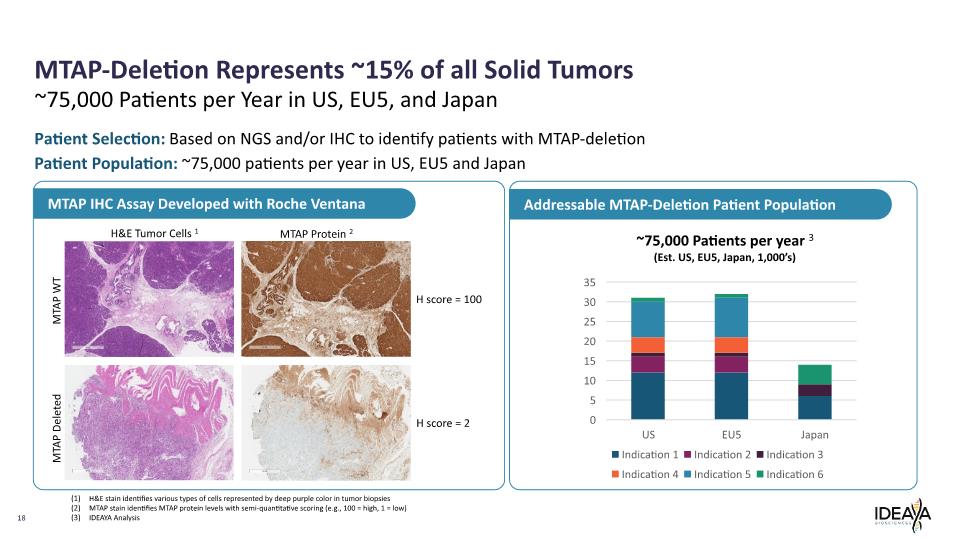
18 ~75,000 Patients per Year in US, EU5, and Japan H&E Tumor Cells 1 MTAP Protein 2 H score = 100 H score = 2 MTAP WT MTAP Deleted Patient Selection: Based on NGS and/or IHC to identify patients with MTAP-deletion Patient Population: ~75,000 patients per year in US, EU5 and Japan H&E stain identifies various types of cells represented by deep purple color in tumor biopsies MTAP stain identifies MTAP protein levels with semi-quantitative scoring (e.g., 100 = high, 1 = low) IDEAYA Analysis MTAP-Deletion Represents ~15% of all Solid Tumors MTAP IHC Assay Developed with Roche Ventana Addressable MTAP-Deletion Patient Population ~75,000 Patients per year 3 (Est. US, EU5, Japan, 1,000’s) (1) (2) (3) 35 30 25 20 15 10 5 0 US EU5 Japan Indication 1 Indication 2 Indication 3 Indication 4 Indication 5 Indication 6
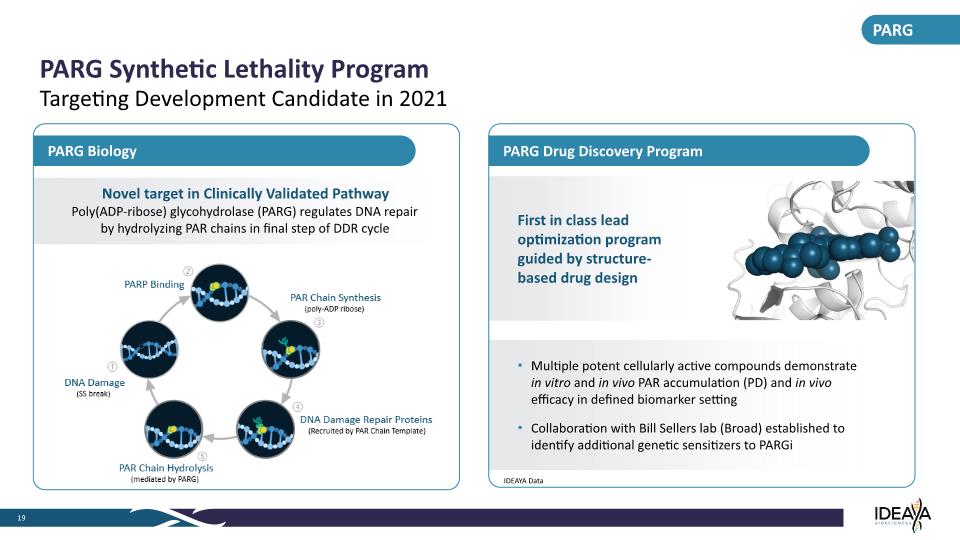
PARG Synthetic Lethality Program 19 Targeting Development Candidate in 2021 PARG Biology PARG Novel target in Clinically Validated Pathway Poly(ADP-ribose) glycohydrolase (PARG) regulates DNA repair by hydrolyzing PAR chains in final step of DDR cycle Multiple potent cellularly active compounds demonstrate in vitro and in vivo PAR accumulation (PD) and in vivo efficacy in defined biomarker setting Collaboration with Bill Sellers lab (Broad) established to identify additional genetic sensitizers to PARGi First in class lead optimization program guided by structure-based drug design PARG Drug Discovery Program IDEAYA Data 1 DNA Damage (SS break) 2 PARP Binding 3 PAR Chain Synthesis (poly-ADP ribose) 4 DNA Damage Repair Proteins (Recruited by PAR Chain Template) 5 PAR Chain Hydrolysis (mediated by PARG)
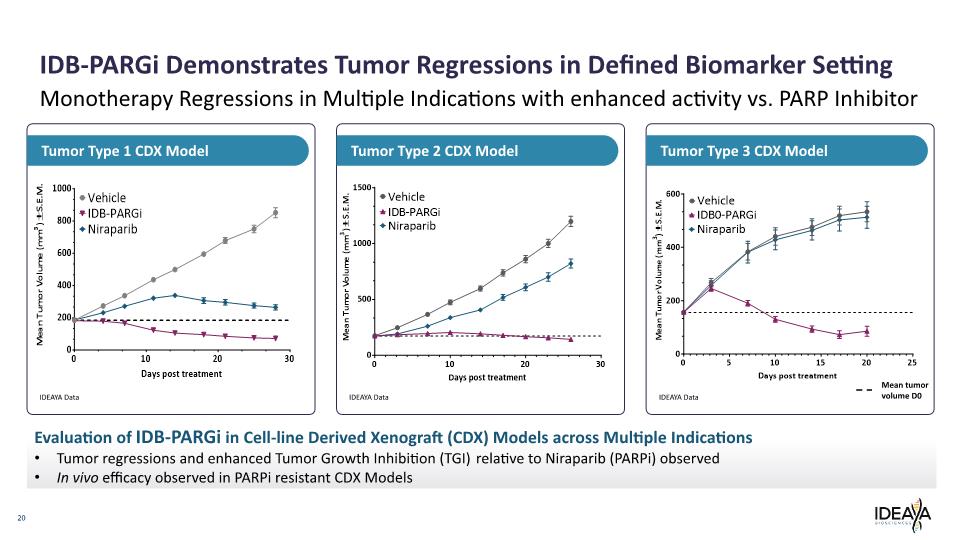
20 Monotherapy Regressions in Multiple Indications with enhanced activity vs. PARP Inhibitor Tumor Type 3 CDX Model Tumor Type 2 CDX Model Tumor Type 1 CDX Model IDB-PARGi Demonstrates Tumor Regressions in Defined Biomarker Setting Evaluation of IDB-PARGi in Cell-line Derived Xenograft (CDX) Models across Multiple Indications Tumor regressions and enhanced Tumor Growth Inhibition (TGI) relative to Niraparib (PARPi) observed In vivo efficacy observed in PARPi resistant CDX Models IDEAYA Data IDEAYA Data IDEAYA Data Mean tumor volume D0 Mean Tumor Volume (mm3) ± S.E.M. 1000 800 600 400 200 0 0 10 20 30 Days post treatment Vehicle IDB-PARGi Niraparib Mean Tumor Volume (mm3) ± S.E.M. 1500 1000 500 0 0 10 20 30 Days post treatment Vehicle IDB-PARGi Niraparib Mean Tumor Volume (mm3) ± S.E.M. 600 400 200 0 0 5 10 15 20 25 Vehicle IDB-PARGi Niraparib
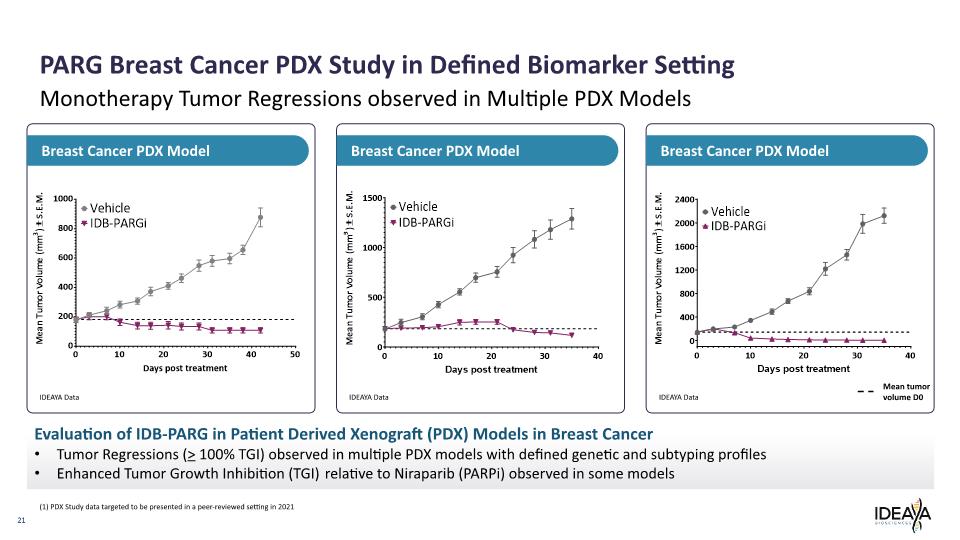
21 Monotherapy Tumor Regressions observed in Multiple PDX Models Breast Cancer PDX Model Breast Cancer PDX Model Breast Cancer PDX Model PARG Breast Cancer PDX Study in Defined Biomarker Setting Evaluation of IDB-PARG in Patient Derived Xenograft (PDX) Models in Breast Cancer Tumor Regressions (> 100% TGI) observed in multiple PDX models with defined genetic and subtyping profiles Enhanced Tumor Growth Inhibition (TGI) relative to Niraparib (PARPi) observed in some models IDEAYA Data IDEAYA Data IDEAYA Data (1) PDX Study data targeted to be presented in a peer-reviewed setting in 2021 Mean tumor volume D0 Mean Tumor Volume (mm3) ± S.E.M. 1000 800 600 400 200 0 0 10 20 30 40 50 Days post treatment Vehicle IDB-PARGi Mean Tumor Volume (mm3) ± S.E.M. 1500 1000 500 0 0 10 20 30 40 Days post treatment Vehicle IDB-PARGi Mean Tumor Volume (mm3) ± S.E.M. 2400 2000 1600 1200 800 400 0 0 10 20 30 40 Days post treatment Vehicle IDB-PARGi
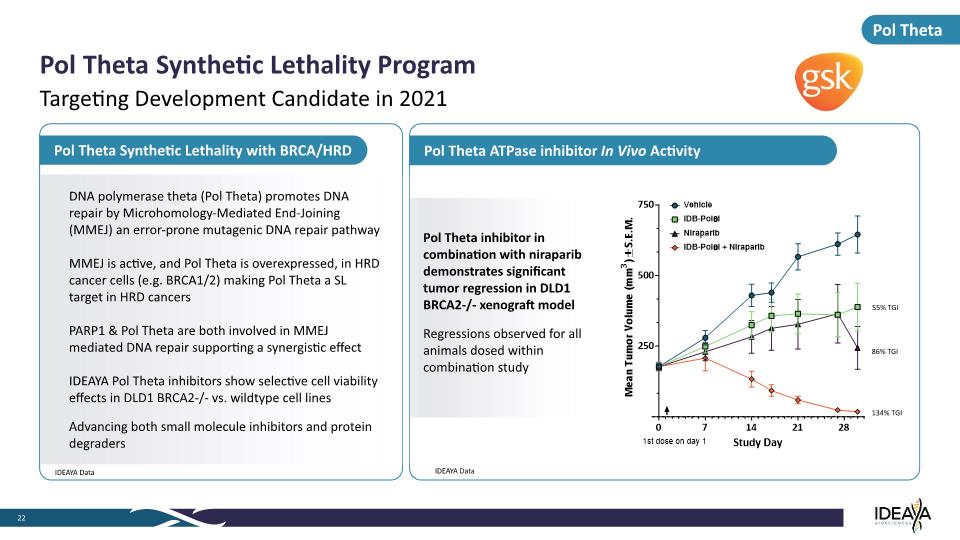
Pol Theta ATPase inhibitor In Vivo Activity Pol Theta Synthetic Lethality with BRCA/HRD Pol Theta Pol Theta Synthetic Lethality Program 22 Targeting Development Candidate in 2021 IDEAYA Data DNA polymerase theta (Pol Theta) promotes DNA repair by Microhomology-Mediated End-Joining (MMEJ) an error-prone mutagenic DNA repair pathway MMEJ is active, and Pol Theta is overexpressed, in HRD cancer cells (e.g. BRCA1/2) making Pol Theta a SL target in HRD cancers PARP1 & Pol Theta are both involved in MMEJ mediated DNA repair supporting a synergistic effect IDEAYA Pol Theta inhibitors show selective cell viability effects in DLD1 BRCA2-/- vs. wildtype cell lines Advancing both small molecule inhibitors and protein degraders IDEAYA Data Pol Theta inhibitor in combination with niraparib demonstrates significant tumor regression in DLD1 BRCA2-/- xenograft model Regressions observed for all animals dosed within combination study 134% TGI 86% TGI 55% TGI Mean Tumor Volume (mm3) ± S.E.M. 750 500 250 0 7 14 21 28 1st dose on day 1 Study Day Vehicle IDB-Pol0i Niraparib IDB-Pol0i + Niraparib
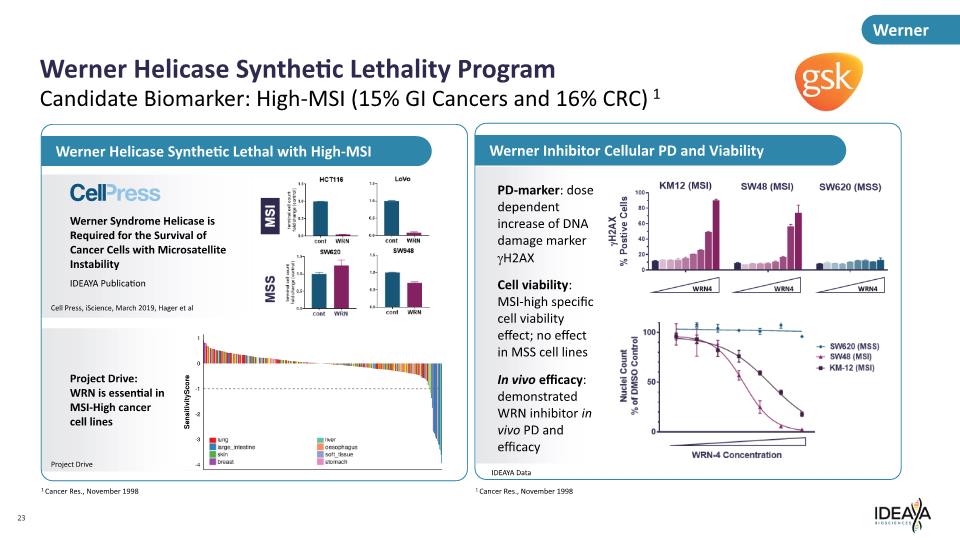
Werner Inhibitor Cellular PD and Viability Werner Helicase Synthetic Lethality Program 23 Candidate Biomarker: High-MSI (15% GI Cancers and 16% CRC) 1 PD-marker: dose dependent increase of DNA damage marker gH2AX Cell viability: MSI-high specific cell viability effect; no effect in MSS cell lines In vivo efficacy: demonstrated WRN inhibitor in vivo PD and efficacy IDEAYA Data 1 Cancer Res., November 1998 Werner Project Drive: WRN is essential in MSI-High cancer cell lines Werner Syndrome Helicase is Required for the Survival of Cancer Cells with Microsatellite Instability IDEAYA Publication Werner Helicase Synthetic Lethal with High-MSI Project Drive Cell Press, iScience, March 2019, Hager et al 1 Cancer Res., November 1998 Cell press MSI MSS terminal cell count told change(control) HCT116 1.5 1.0 0.0 cont WRN LoVo 1.5 1.0 0.5 0.0 cont WRN terminal cell count told change(control) SW620 1.5 1.0 0.5 0.0 cont WRN SW948 1.5 1.0 0.5 0.0 cont WRN lung large intestine skin breast liver oesophagus soft tissue stomach KM12 (MSI) SW48 (MSI) SW620 (MSS) H2AX % Positive Cells 100 80 60 40 20 0 Nuclei Count % of DMSO Control 100 50 0 WRN-4 Concentration SW620 (MSS)SW48(MSI) KM-12 (MSI)
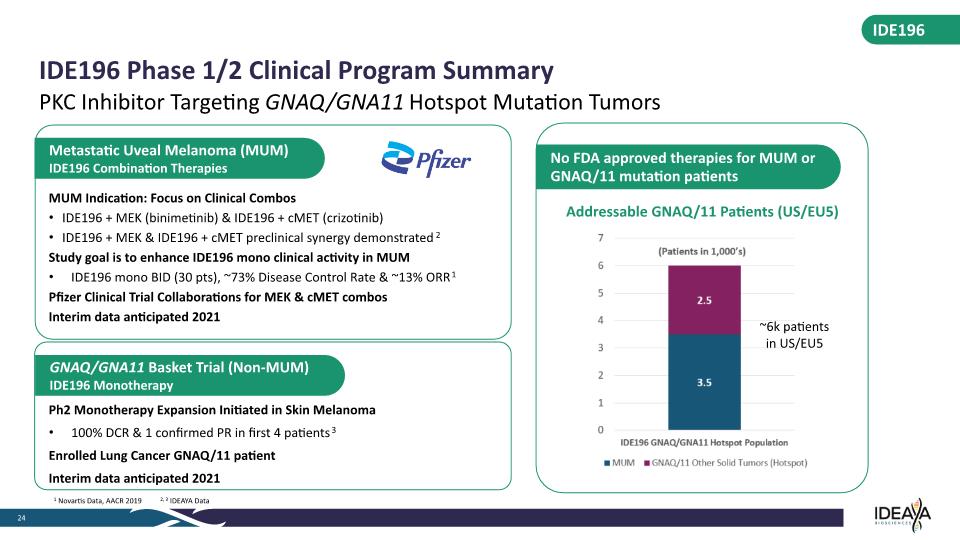
MUM Indication: Focus on Clinical Combos IDE196 + MEK (binimetinib) & IDE196 + cMET (crizotinib) IDE196 + MEK & IDE196 + cMET preclinical synergy demonstrated2 Study goal is to enhance IDE196 mono clinical activity in MUM IDE196 mono BID (30 pts), ~73% Disease Control Rate & ~13% ORR1 Pfizer Clinical Trial Collaborations for MEK & cMET combos Interim data anticipated 2021 Metastatic Uveal Melanoma (MUM) IDE196 Combination Therapies IDE196 IDE196 Phase 1/2 Clinical Program Summary 24 PKC Inhibitor Targeting GNAQ/GNA11 Hotspot Mutation Tumors GNAQ/GNA11 Basket Trial (Non-MUM) IDE196 Monotherapy 1 Novartis Data, AACR 2019 2, 3 IDEAYA Data Ph2 Monotherapy Expansion Initiated in Skin Melanoma 100% DCR & 1 confirmed PR in first 4 patients3 Enrolled Lung Cancer GNAQ/11 patient Interim data anticipated 2021 Addressable GNAQ/11 Patients (US/EU5) ~6k patients in US/EU5 No FDA approved therapies for MUM or GNAQ/11 mutation patients (Patients in 1,000’s) 7 6 5 4 3 2 1 0 2.5 3.5 IDE196 GNAQ/GNA11 Hotspot Population MUM GNAQ/11 Other Solid Tumors (Hotspot)
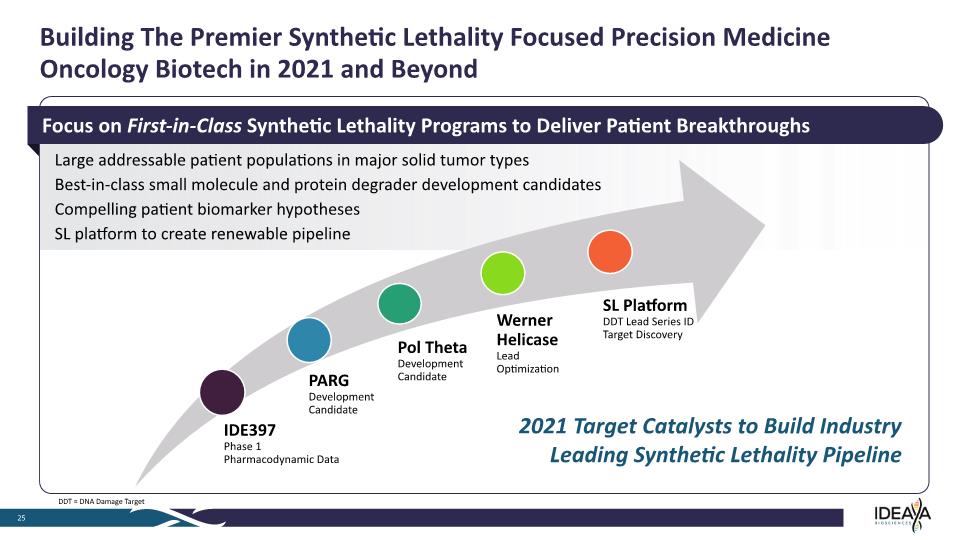
Building The Premier Synthetic Lethality Focused Precision Medicine Oncology Biotech in 2021 and Beyond 25 Large addressable patient populations in major solid tumor types Best-in-class small molecule and protein degrader development candidates Compelling patient biomarker hypotheses SL platform to create renewable pipeline 2021 Target Catalysts to Build Industry Leading Synthetic Lethality Pipeline Focus on First-in-Class Synthetic Lethality Programs to Deliver Patient Breakthroughs DDT = DNA Damage Target IDE397 Phase 1 Pharmacodynamic Data PARG Development Candidate Pol Theta Development Candidate Werner Helicase Lead Optimization SL Platform DDT Lead Series ID Target Discovery
