Attached files
| file | filename |
|---|---|
| 8-K - 8-K - BAXTER INTERNATIONAL INC | bax-20210111.htm |

39th Annual J.P. Morgan Healthcare Conference José (Joe) E. Almeida, Chairman & Chief Executive Officer January 11, 2021

2 Safe Harbor Statement This presentation includes forward-looking statements concerning Baxter’s preliminary financial results for fourth-quarter and full-year 2020, business development activities, capital structure, cost savings initiatives, and R&D pipeline, including results of clinical trials and planned product launches. These forward-looking statements are based on assumptions about many important factors, including the following, which could cause actual results to differ materially from those in the forward-looking statements: demand for and market acceptance of risks for new and existing products (including the impact of reduced hospital admission rates and elective surgery volumes); product development risks (including any delays in required regulatory approvals); product quality or patient safety concerns; continuity, availability and pricing of acceptable raw materials and component supply; inability to create additional production capacity in a timely manner or the occurrence of other manufacturing or supply difficulties (including as a result of a natural disaster, public health crises and epidemics/pandemics, regulatory actions or otherwise); the impact of global economic conditions (including potential trade wars) and continuing public health crises and epidemics, such as the novel strain of coronavirus (COVID-19), including related resurgences, on us and our customers and suppliers, including foreign governments in countries in which we operate; breaches or failures of the company’s information technology systems or products, including by cyberattack, unauthorized access or theft (as a result of increased remote working arrangements or otherwise); the adequacy of the company’s cash flows from operations (which may be negatively impacted by collectability concerns as a result of the COVID-19 pandemic or otherwise) and other sources of liquidity to meet its ongoing cash obligations and fund its investment program; loss of key employees or inability to identify and recruit new employees; future actions of regulatory bodies and other governmental authorities, including the FDA, the Department of Justice, the SEC, the New York Attorney General and foreign regulatory agencies, including the continued delay in lifting the warning letter at our Ahmedabad facility or proceedings related to the investigation related to foreign exchange gains and losses; the outcome of pending or future litigation, including the opioid litigation and litigation related to our internal investigation of foreign exchange gains and losses; the impacts of the material weakness identified as a result of the internal investigation and our remediation efforts, including the risk that we may experience additional material weaknesses or other deficiencies; proposed regulatory changes of the U.S. Department of Health and Human Services in kidney health policy and reimbursement, which may substantially change the U.S. end stage renal disease market and demand for our peritoneal dialysis products, necessitating significant multi-year capital expenditures, which are difficult to estimate in advance; failures with respect to compliance programs; accurate identification of and execution on business development and R&D opportunities and realization of anticipated benefits (including the acquisitions of Cheetah Medical and Seprafilm Adhesion Barrier); future actions of third parties, including payers; U.S. healthcare reform and other global austerity measures; pricing, reimbursement, taxation and rebate policies of government agencies and private payers; the impact of competitive products and pricing, including generic competition, drug reimportation and disruptive technologies; fluctuations in foreign exchange and interest rates; the ability to enforce owned or in-licensed patents or the prevention or restriction of the manufacture, sale or use of products or technology affected by patents of third parties; global, trade and tax policies; any change in laws concerning the taxation of income (including current or future tax reform), including income earned outside the United States and potential taxes associated with the Base Erosion and Anti-Abuse Tax; actions taken by tax authorities in connection with ongoing tax audits; and other risks identified in Baxter’s most recent filings on Forms 10-K and 10-Q and other SEC filings, all of which are available on Baxter’s website. Baxter does not undertake to update its forward-looking statements unless otherwise required by the federal securities laws.

Use of Non-GAAP Financial Measures 3 To supplement Baxter’s consolidated financial statements presented on a U.S. GAAP basis, the Company discloses certain non-GAAP financial measures. These non-GAAP financial measures are not in accordance with generally accepted accounting principles in the United States. An explanation of the ways in which Baxter management uses these supplemental non-GAAP measures to evaluate its business and the substantive reasons why Baxter management believes that these non-GAAP measures provide useful information to investors is included in the Company’s Form 8-K filed with the SEC on January 11, 2021. This information should be considered in addition to, and not as substitutes for, information prepared in accordance with U.S. GAAP. Baxter strongly encourages investors to review its consolidated financial statements and publicly filed reports in their entirety and cautions investors that the non-GAAP measures used by the Company may differ from similar measures used by other companies, even when similar terms are used to identify such measures. Non-GAAP financial measures used in this presentation include constant currency and operational sales growth.

4 A Global, Diversified And Market-Leading Portfolio Renal Care Medication Delivery Pharmaceuticals Clinical Nutrition Acute TherapiesAdvanced Surgery Market Leadership Across Portfolio 75M+ Patients Treated Annually Products In 100+ Countries

5 Rising To The Challenge Of COVID-19 ► Maximizing production of medically necessary products and therapies, subject to capacity constraints ► Protecting employee health and safety ► Expediting shipments to global “hot spots” ► Donating $2M+ to global relief partners
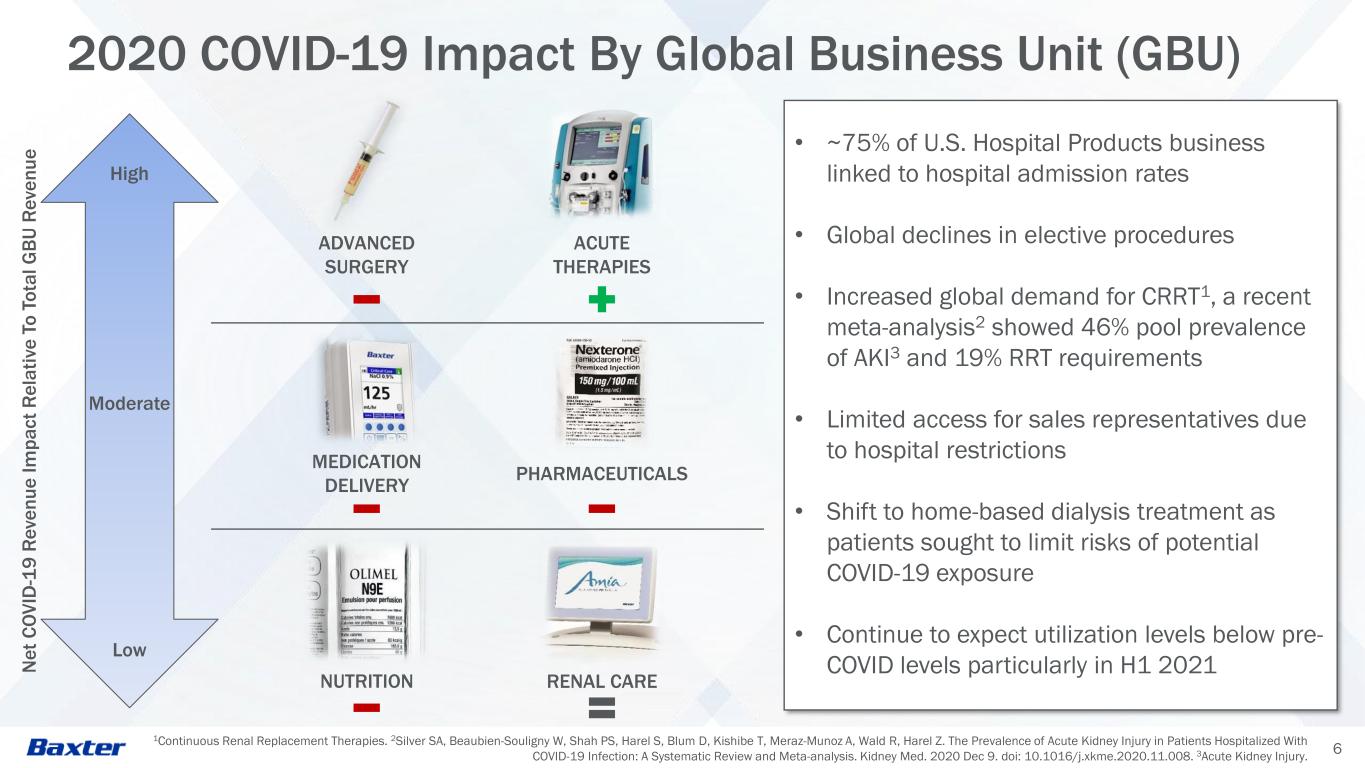
1Continuous Renal Replacement Therapies. 2Silver SA, Beaubien-Souligny W, Shah PS, Harel S, Blum D, Kishibe T, Meraz-Munoz A, Wald R, Harel Z. The Prevalence of Acute Kidney Injury in Patients Hospitalized With COVID-19 Infection: A Systematic Review and Meta-analysis. Kidney Med. 2020 Dec 9. doi: 10.1016/j.xkme.2020.11.008. 3Acute Kidney Injury. 6 2020 COVID-19 Impact By Global Business Unit (GBU) Low Moderate High • ~75% of U.S. Hospital Products business linked to hospital admission rates • Global declines in elective procedures • Increased global demand for CRRT1, a recent meta-analysis2 showed 46% pool prevalence of AKI3 and 19% RRT requirements • Limited access for sales representatives due to hospital restrictions • Shift to home-based dialysis treatment as patients sought to limit risks of potential COVID-19 exposure • Continue to expect utilization levels below pre- COVID levels particularly in H1 2021N e t C O V ID -1 9 R e v e n u e I m p a c t R e la ti v e T o T o ta l G B U R e v e n u e PHARMACEUTICALS MEDICATION DELIVERY ACUTE THERAPIES ADVANCED SURGERY RENAL CARENUTRITION

7 Strengthen our portfolio and extend our impact through transformative innovation that spans prevention to recovery Our Strategy Top Quartile Goals Industry leading performance Best place to workPatient safety and Quality Growth through innovation
8 Baxter Transformation Journey Pillars Culture & Talent Strategic Capital Deployment Financial Strength Innovation Ecosystem
9 Culture & Talent Cultivating A Best Place To Work Through Global Initiatives Ethics & Compliance Advance Racial Justice Insights & Updates Continuous Feedback Manager Effectiveness Meeting Efficiency Business Resource Groups Net Promoter Score
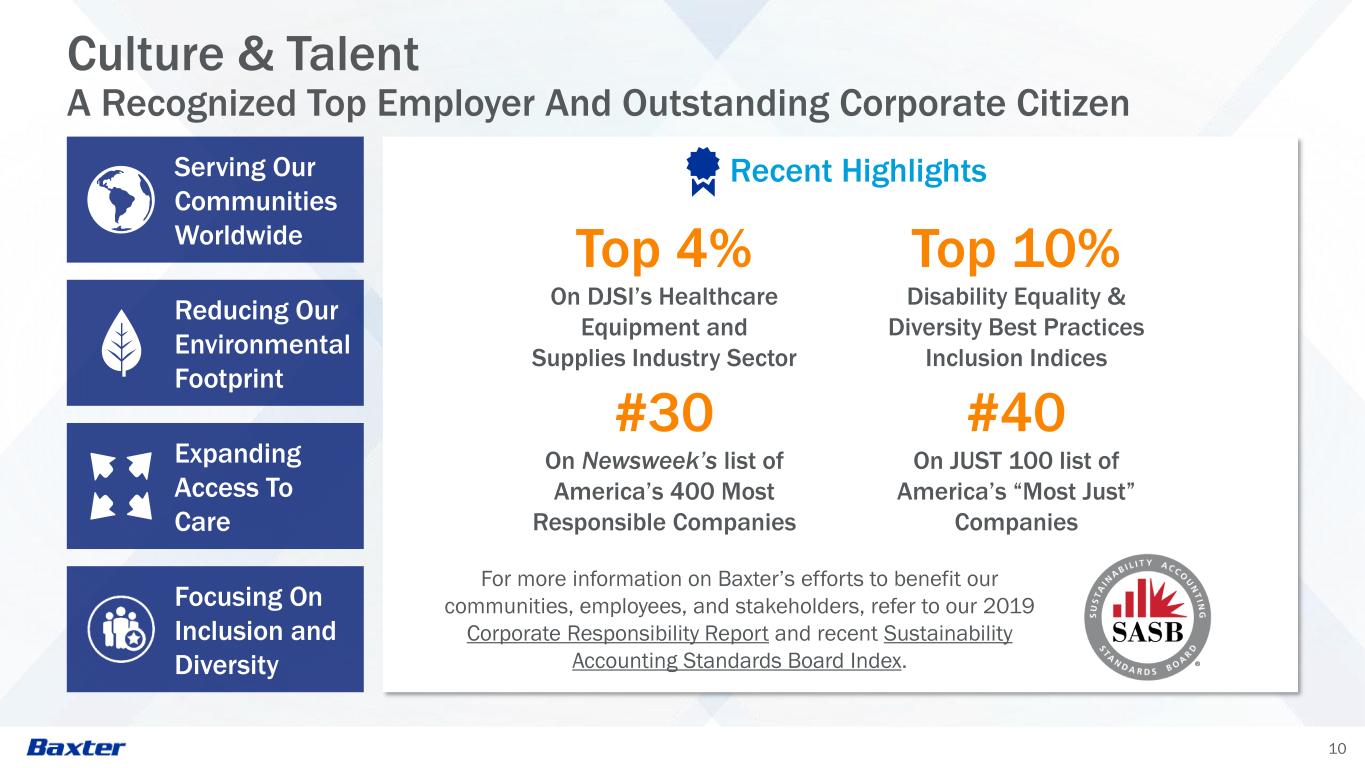
10 Culture & Talent A Recognized Top Employer And Outstanding Corporate Citizen Recent Highlights Focusing On Inclusion and Diversity Expanding Access To Care Serving Our Communities Worldwide Reducing Our Environmental Footprint For more information on Baxter’s efforts to benefit our communities, employees, and stakeholders, refer to our 2019 Corporate Responsibility Report and recent Sustainability Accounting Standards Board Index. #40 On JUST 100 list of America’s “Most Just” Companies Top 4% On DJSI’s Healthcare Equipment and Supplies Industry Sector #30 On Newsweek’s list of America’s 400 Most Responsible Companies Top 10% Disability Equality & Diversity Best Practices Inclusion Indices
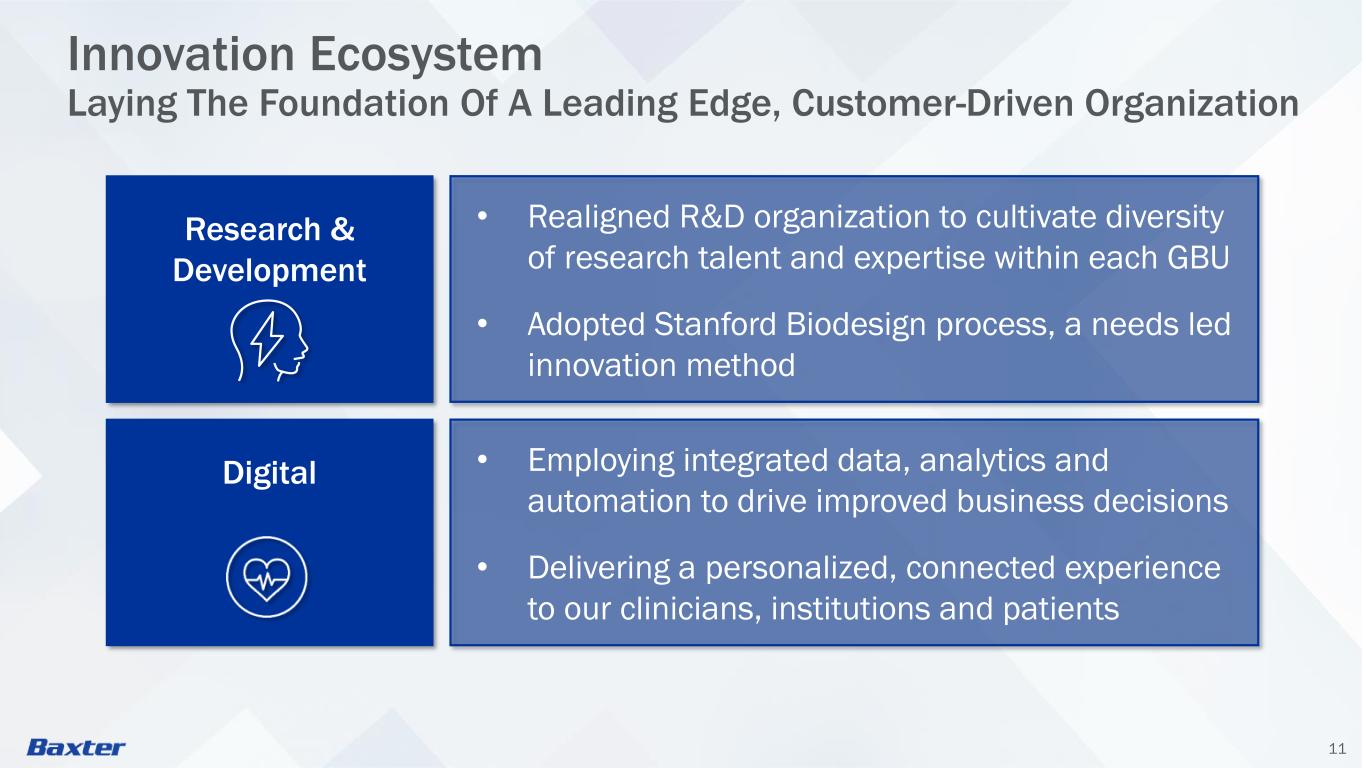
11 Innovation Ecosystem Laying The Foundation Of A Leading Edge, Customer-Driven Organization • Employing integrated data, analytics and automation to drive improved business decisions • Delivering a personalized, connected experience to our clinicians, institutions and patients Research & Development Digital • Realigned R&D organization to cultivate diversity of research talent and expertise within each GBU • Adopted Stanford Biodesign process, a needs led innovation method
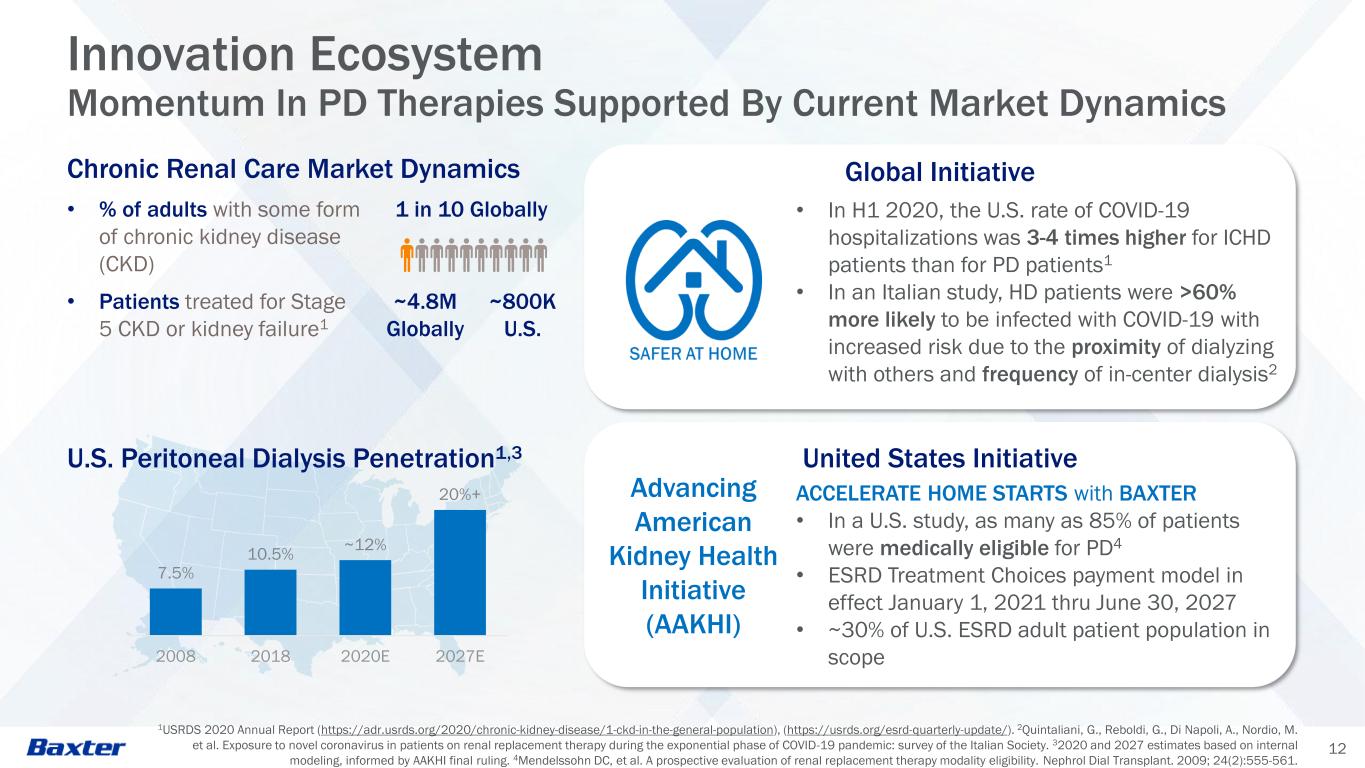
7.5% 10.5% ~12% 20%+ 2008 2018 2020E 2027E 12 Innovation Ecosystem Momentum In PD Therapies Supported By Current Market Dynamics Advancing American Kidney Health Initiative (AAKHI) • In H1 2020, the U.S. rate of COVID-19 hospitalizations was 3-4 times higher for ICHD patients than for PD patients1 • In an Italian study, HD patients were >60% more likely to be infected with COVID-19 with increased risk due to the proximity of dialyzing with others and frequency of in-center dialysis2 ACCELERATE HOME STARTS with BAXTER • In a U.S. study, as many as 85% of patients were medically eligible for PD4 • ESRD Treatment Choices payment model in effect January 1, 2021 thru June 30, 2027 • ~30% of U.S. ESRD adult patient population in scope 1USRDS 2020 Annual Report (https://adr.usrds.org/2020/chronic-kidney-disease/1-ckd-in-the-general-population), (https://usrds.org/esrd-quarterly-update/). 2Quintaliani, G., Reboldi, G., Di Napoli, A., Nordio, M. et al. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society. 32020 and 2027 estimates based on internal modeling, informed by AAKHI final ruling. 4Mendelssohn DC, et al. A prospective evaluation of renal replacement therapy modality eligibility. Nephrol Dial Transplant. 2009; 24(2):555-561. Global Initiative United States Initiative Chronic Renal Care Market Dynamics • % of adults with some form of chronic kidney disease (CKD) 1 in 10 Globally • Patients treated for Stage 5 CKD or kidney failure1 ~4.8M Globally ~800K U.S. U.S. Peritoneal Dialysis Penetration1,3
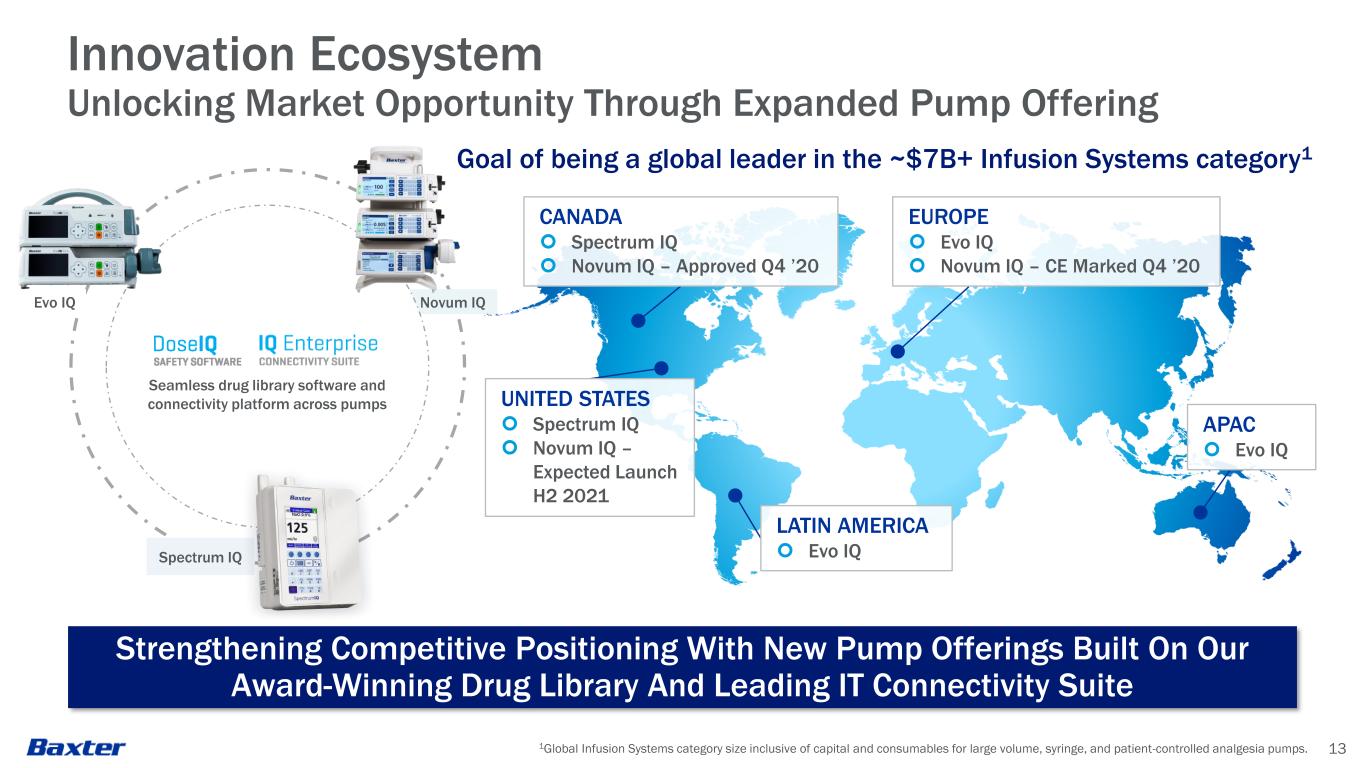
Novum IQ 1Global Infusion Systems category size inclusive of capital and consumables for large volume, syringe, and patient-controlled analgesia pumps. 13 Innovation Ecosystem Unlocking Market Opportunity Through Expanded Pump Offering Goal of being a global leader in the ~$7B+ Infusion Systems category1 EUROPE Evo IQ Novum IQ – CE Marked Q4 ’20 CANADA Spectrum IQ Novum IQ – Approved Q4 ’20 UNITED STATES Spectrum IQ Novum IQ – Expected Launch H2 2021 LATIN AMERICA Evo IQ APAC Evo IQ Strengthening Competitive Positioning With New Pump Offerings Built On Our Award-Winning Drug Library And Leading IT Connectivity Suite Spectrum IQ Evo IQ Seamless drug library software and connectivity platform across pumps
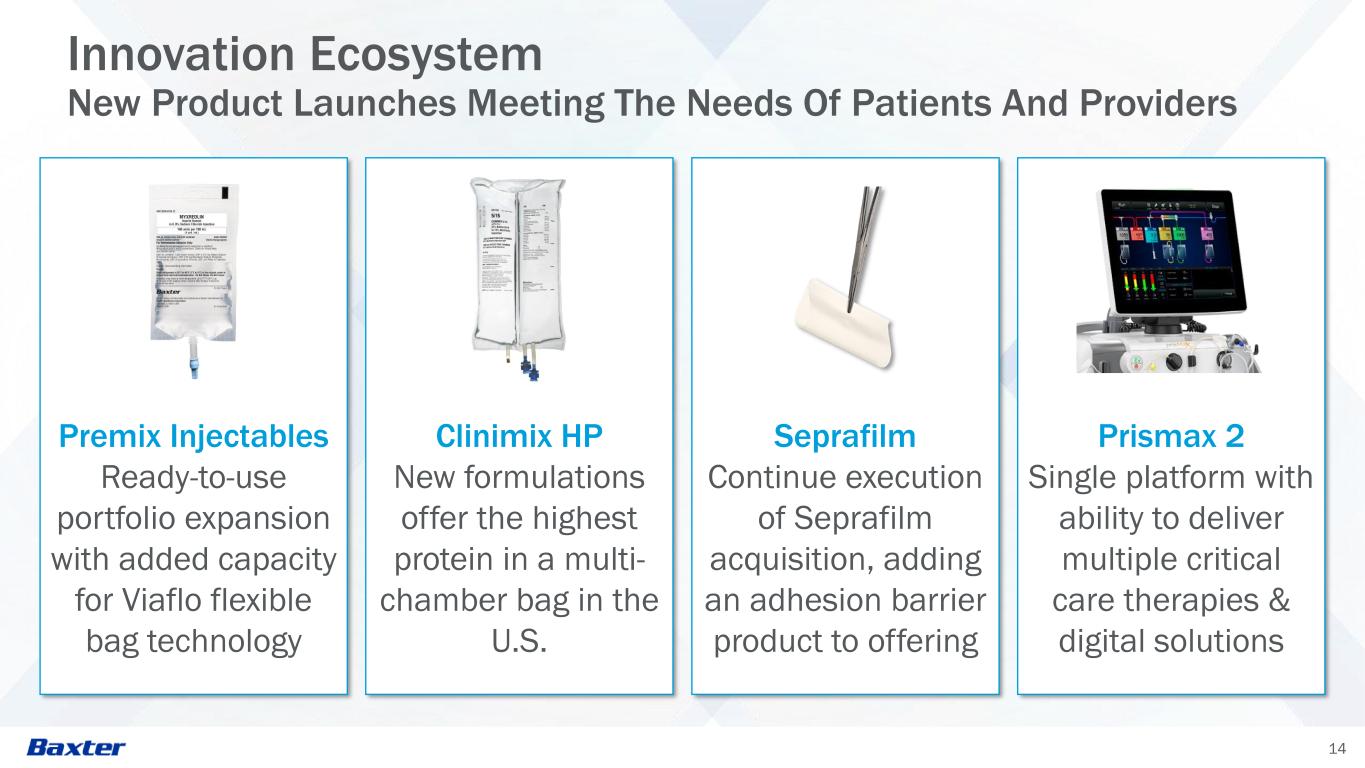
14 Innovation Ecosystem New Product Launches Meeting The Needs Of Patients And Providers Premix Injectables Ready-to-use portfolio expansion with added capacity for Viaflo flexible bag technology Clinimix HP New formulations offer the highest protein in a multi- chamber bag in the U.S. Seprafilm Continue execution of Seprafilm acquisition, adding an adhesion barrier product to offering Prismax 2 Single platform with ability to deliver multiple critical care therapies & digital solutions
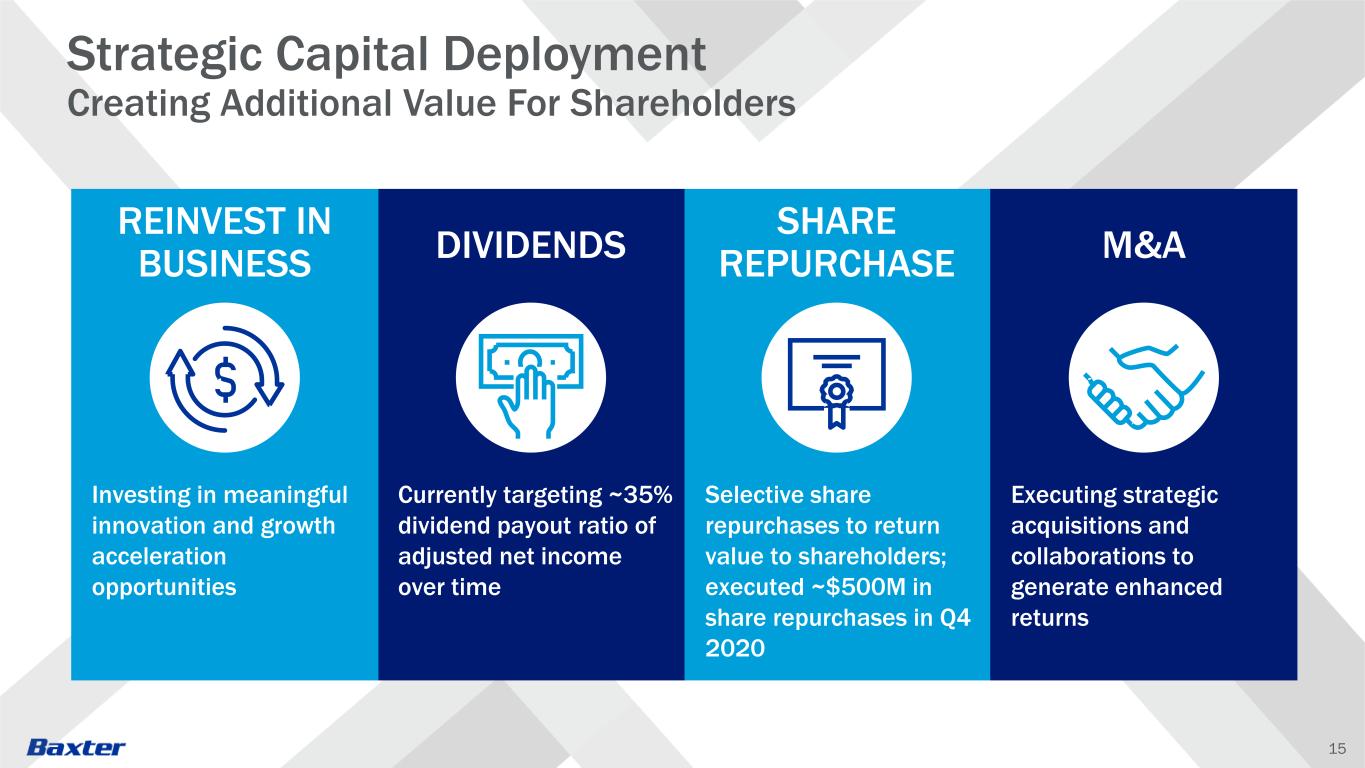
15 Investing in meaningful innovation and growth acceleration opportunities Currently targeting ~35% dividend payout ratio of adjusted net income over time Executing strategic acquisitions and collaborations to generate enhanced returns Selective share repurchases to return value to shareholders; executed ~$500M in share repurchases in Q4 2020 REINVEST IN BUSINESS DIVIDENDS M&A SHARE REPURCHASE Strategic Capital Deployment Creating Additional Value For Shareholders
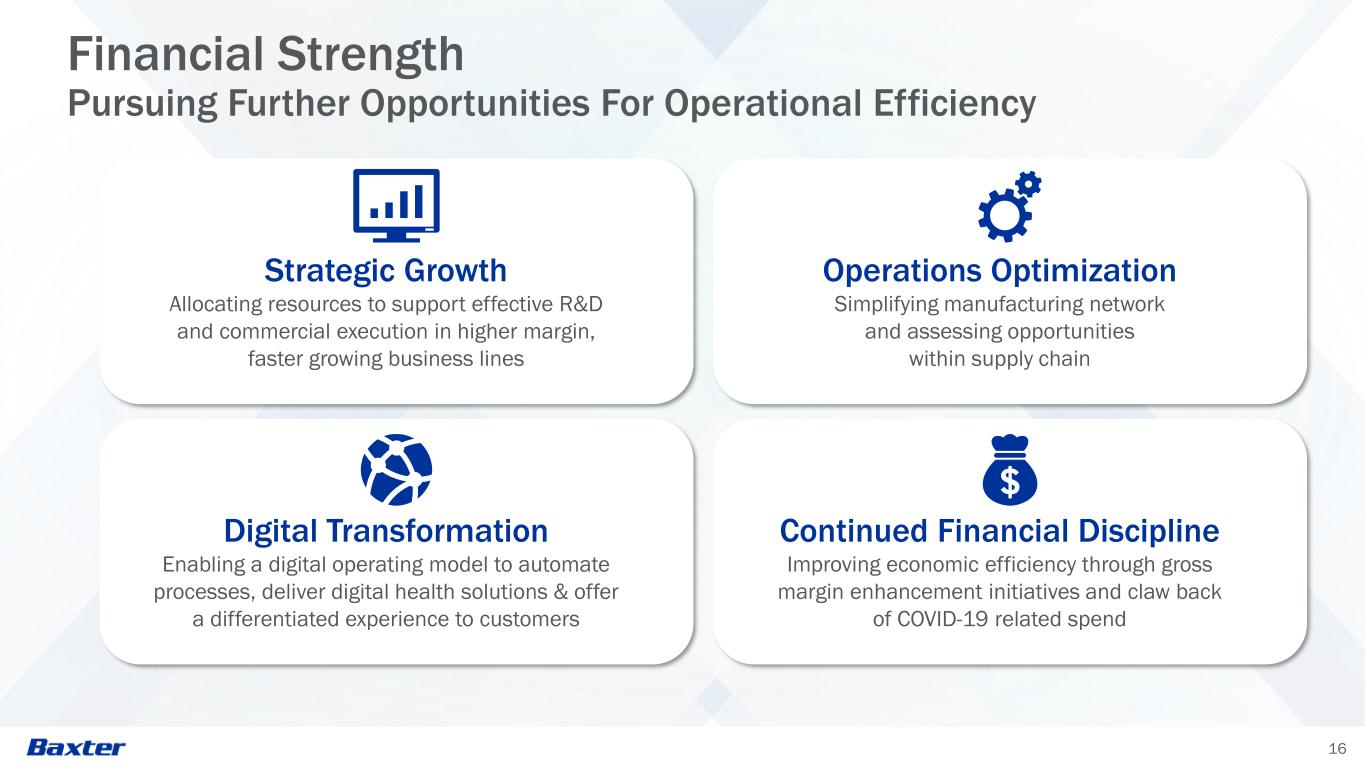
16 Financial Strength Pursuing Further Opportunities For Operational Efficiency Strategic Growth Allocating resources to support effective R&D and commercial execution in higher margin, faster growing business lines Operations Optimization Simplifying manufacturing network and assessing opportunities within supply chain Digital Transformation Enabling a digital operating model to automate processes, deliver digital health solutions & offer a differentiated experience to customers Continued Financial Discipline Improving economic efficiency through gross margin enhancement initiatives and claw back of COVID-19 related spend

1Financials referenced in this slide are preliminary and subject to change. 2Non-GAAP financial metrics referenced on this slide include constant currency and operational sales growth. Q4 2020 and FY 2020 operational sales growth excludes the impact of foreign exchange of approximately (2%) and 0%, respectively and the Seprafilm acquisition impact of approximately 1% and 1%, respectively. 17 Preliminary 2020 Financial Information1,2 Total Baxter Fourth-Quarter 2020 Revenue Full-Year 2020 Revenue $3.18B $11.67B Reported Constant Operational Reported Constant Operational +5% +3% +2% +3% +3% +2%

18 • Playing an ongoing role in pandemic response efforts • Executing on new product launches, market development and geographic expansions • Continued evaluation of strategic acquisition opportunities to unlock additional value • Committed to delivering enhanced operating margin and improved cash flow generation • Plan to host an Investor Conference in H2 2021 Continuing Momentum In 2021 And Beyond

January 11, 2021 39th Annual J.P. Morgan Healthcare Conference Appendix 19
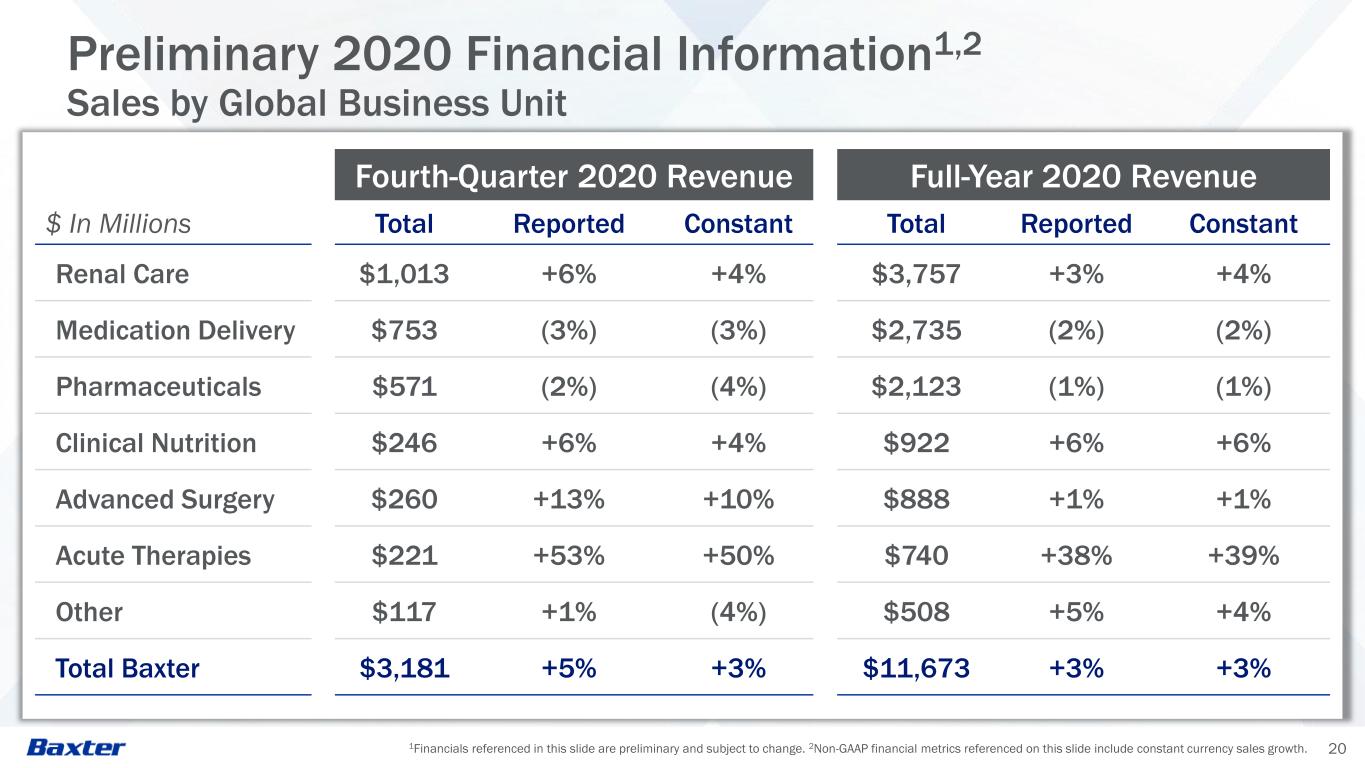
1Financials referenced in this slide are preliminary and subject to change. 2Non-GAAP financial metrics referenced on this slide include constant currency sales growth. 20 Preliminary 2020 Financial Information1,2 Sales by Global Business Unit Fourth-Quarter 2020 Revenue Full-Year 2020 Revenue $ In Millions Total Reported Constant Total Reported Constant Renal Care $1,013 +6% +4% $3,757 +3% +4% Medication Delivery $753 (3%) (3%) $2,735 (2%) (2%) Pharmaceuticals $571 (2%) (4%) $2,123 (1%) (1%) Clinical Nutrition $246 +6% +4% $922 +6% +6% Advanced Surgery $260 +13% +10% $888 +1% +1% Acute Therapies $221 +53% +50% $740 +38% +39% Other $117 +1% (4%) $508 +5% +4% Total Baxter $3,181 +5% +3% $11,673 +3% +3%
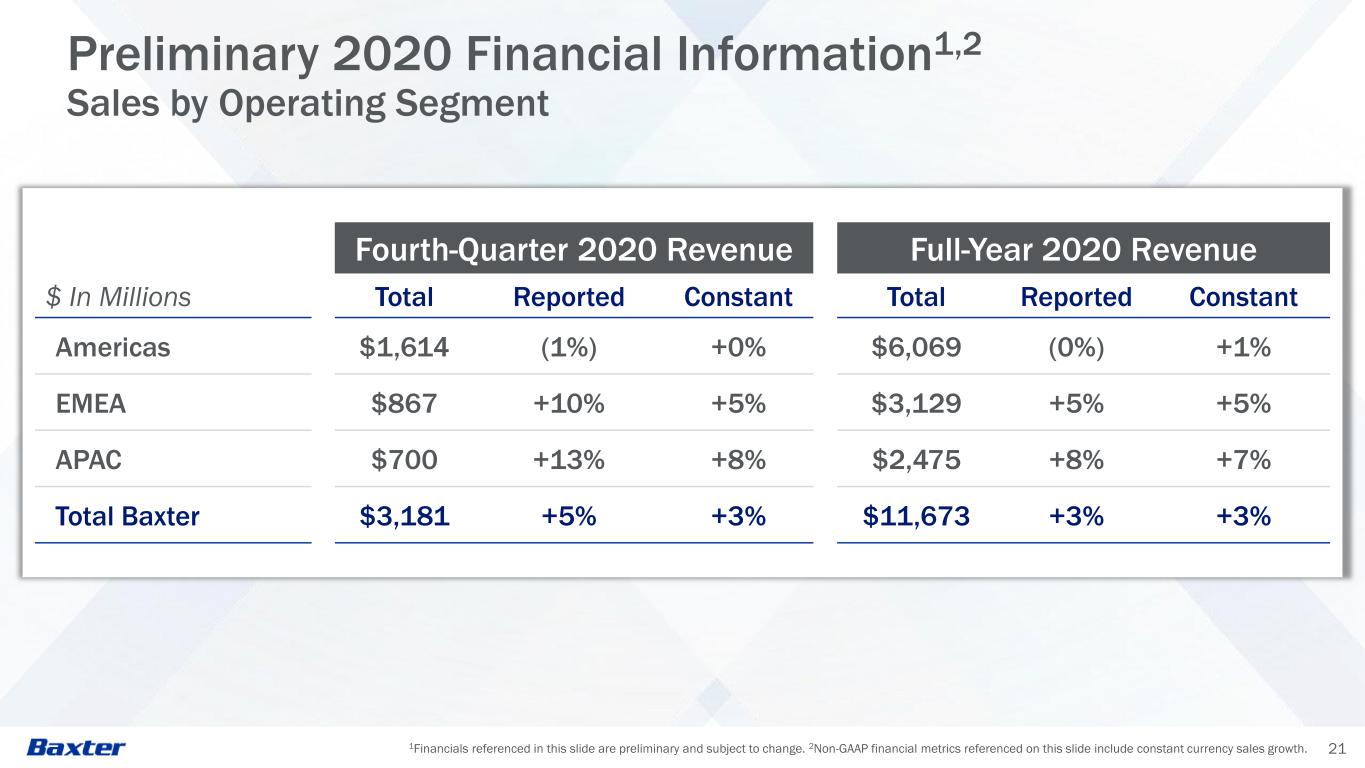
1Financials referenced in this slide are preliminary and subject to change. 2Non-GAAP financial metrics referenced on this slide include constant currency sales growth. 21 Preliminary 2020 Financial Information1,2 Sales by Operating Segment Fourth-Quarter 2020 Revenue Full-Year 2020 Revenue $ In Millions Total Reported Constant Total Reported Constant Americas $1,614 (1%) +0% $6,069 (0%) +1% EMEA $867 +10% +5% $3,129 +5% +5% APAC $700 +13% +8% $2,475 +8% +7% Total Baxter $3,181 +5% +3% $11,673 +3% +3%
