Attached files
| file | filename |
|---|---|
| 8-K - 8-K - JPM DECK - ARENA PHARMACEUTICALS INC | arna-8k_20210111.htm |

JANUARY 2021 • NASDAQ: ARNA Exhibit 99.1

Forward Looking Statements This presentation includes forward-looking statements that involve a number of risks and uncertainties, including statements about catalysts, value, our investigative stage drug candidates, including with respect to their potential (including to become first or best-in-class), safety, efficacy, indications, significance of data, development plans, differentiation, the market and unmet needs, expected growth in market size, broad portfolio expansion over the next 5-7 years, commercialization, expected data readouts, initiation and progress of clinical trials and regulatory approval; our expected pipeline growth and ability to succeed long-term and create long-term value; our focus, goals, strategy, plans, timelines and guidance; our partnered programs; ; and other statements that are not historical facts, including statements that may include words such as “can,” “may,” “will,” “intend,” “plan,” “expect,” “believe,” “potential,” “opportunity,” “up to,” “long-term” or other similar words. For such statements, we claim the protection of the Private Securities Litigation Reform Act of 1995. Actual events or results may differ materially from expectations, and you are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the time they were made. Factors that could cause actual results to differ materially from such statements include, without limitation: Drug development programs are expensive, time consuming, uncertain and susceptible to change, interruption, delay or termination; the timing and outcome of research, development and regulatory review and feedback is uncertain; we expect to need additional funds to advance all of our programs, and you and others may not agree with the manner in which we allocate our resources; topline data may not accurately reflect the complete results of a particular study or trial; results of clinical trials and other studies are subject to different interpretations and may not be predictive of future results; new data may be unexpected or unfavorable; our drug candidates may not advance in development or be approved for marketing; clinical trials and other studies may not proceed at the time or in the manner expected or at all; enrolling patients in our ongoing and intended clinical trials is competitive and challenging; the duration and severity of the COVID-19 pandemic, including but not limited to the impact on Arena's clinical operations, the operations of Arena's suppliers, partners, collaborators, licensees, and capital markets, which in each case remains uncertain; clinical and nonclinical data is voluminous and detailed, and regulatory agencies may interpret or weigh the importance of data differently and reach different conclusions than Arena or others, request additional information, have additional recommendations or change their guidance or requirements; data and information related to our programs may not meet regulatory requirements or otherwise be sufficient for further development, regulatory review, partnering or approval at all or on our projected timeline; other risks related to developing, seeking regulatory approval of and commercializing drugs, including regulatory, manufacturing, supply and marketing issues and drug availability; Arena's and third parties' intellectual property rights; competition; reimbursement and pricing decisions; risks related to relying on partners and other third parties; and satisfactory resolution of litigation or other disagreements. Additional factors that could cause actual results to differ materially from those stated or implied by our forward-looking statements are disclosed in our Securities and Exchange Commission (SEC) filings, including under the heading “Risk Factors” of our Quarterly Report on Form 10-Q for the quarter ended September 30, 2020. We disclaim any intent or obligation to update these forward-looking statements, other than as may be required under applicable law 2
TRANSFORMING INTO A GLOBAL BUSINESs 3
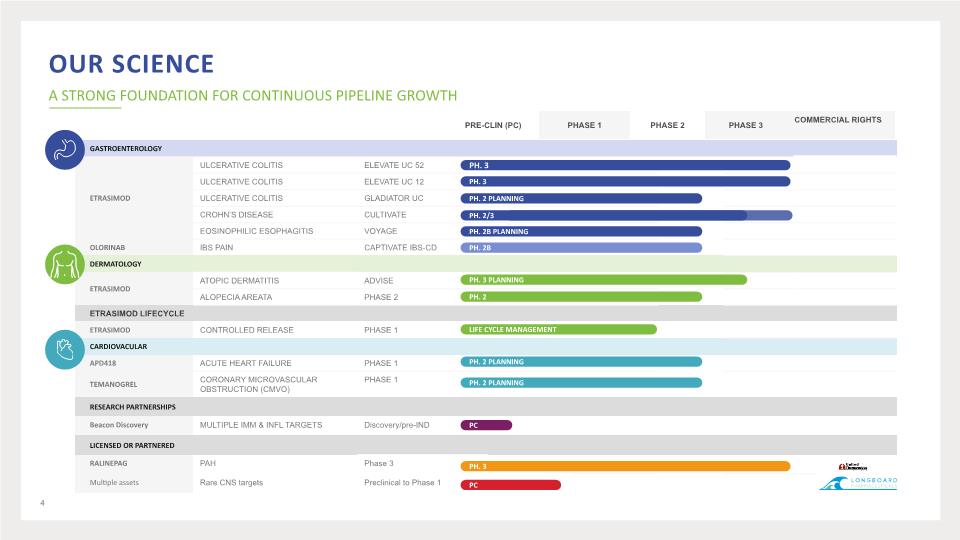
Our Science A STRONG FOUNDATION FOR CONTINUOUS PIPELINE GROWTH LIFE CYCLE MANAGEMENT PC 4
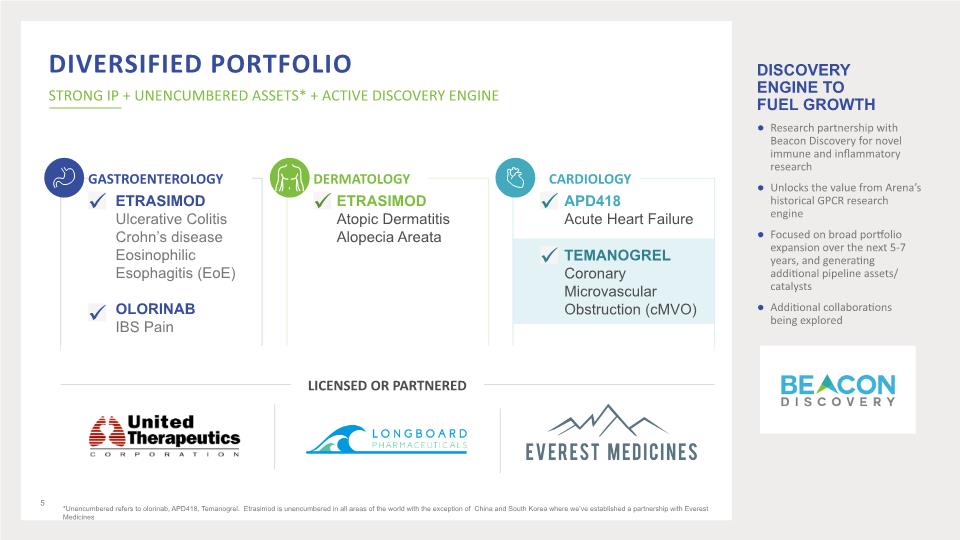
Diversified Portfolio STRONG IP + UNENCUMBERED ASSETS* + ACTIVE DISCOVERY ENGINE *Unencumbered refers to olorinab, APD418, Temanogrel. Etrasimod is unencumbered in all areas of the world with the exception of China and South Korea where we’ve established a partnership with Everest Medicines 5
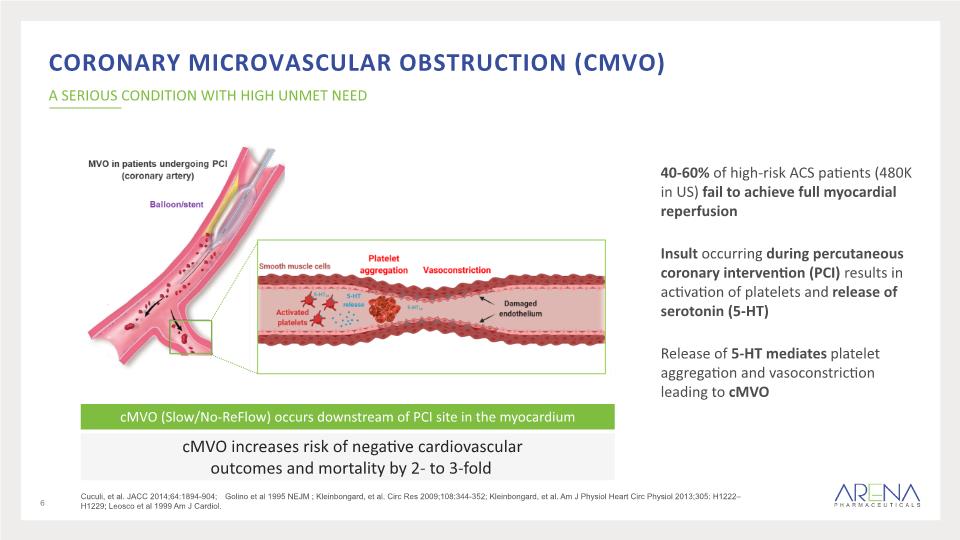
40-60% of high-risk ACS patients (480K in US) fail to achieve full myocardial reperfusion Insult occurring during percutaneous coronary intervention (PCI) results in activation of platelets and release of serotonin (5-HT) Release of 5-HT mediates platelet aggregation and vasoconstriction leading to cMVO Coronary Microvascular Obstruction (cMVO) A SERIOUS CONDITION WITH HIGH UNMET NEED cMVO increases risk of negative cardiovascular outcomes and mortality by 2- to 3-fold cMVO (Slow/No-ReFlow) occurs downstream of PCI site in the myocardium 6 Cuculi, et al. JACC 2014;64:1894-904; Golino et al 1995 NEJM ; Kleinbongard, et al. Circ Res 2009;108:344-352; Kleinbongard, et al. Am J Physiol Heart Circ Physiol 2013;305: H1222–H1229; Leosco et al 1999 Am J Cardiol.
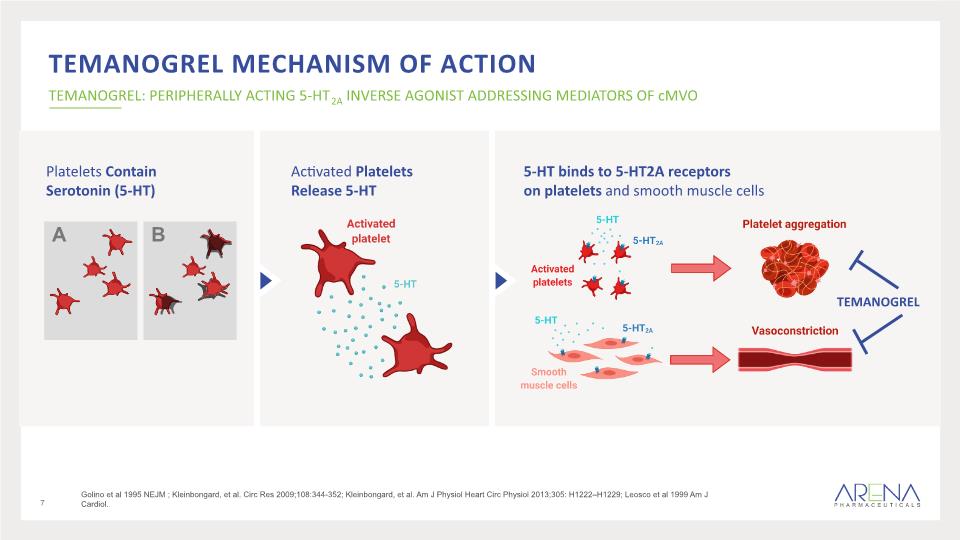
temanogrel Mechanism of Action TEMANOGREL: PERIPHERALLY ACTING 5-HT2A INVERSE AGONIST ADDRESSING MEDIATORS OF cMVO Platelets Contain Serotonin (5-HT) 7 Golino et al 1995 NEJM ; Kleinbongard, et al. Circ Res 2009;108:344-352; Kleinbongard, et al. Am J Physiol Heart Circ Physiol 2013;305: H1222–H1229; Leosco et al 1999 Am J Cardiol.
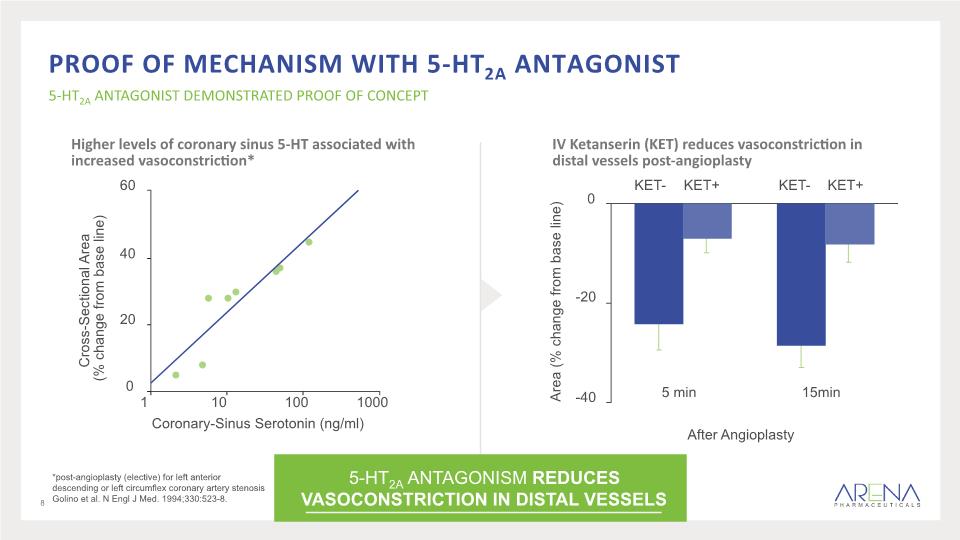
Higher levels of coronary sinus 5-HT associated with increased vasoconstriction* IV Ketanserin (KET) reduces vasoconstriction in distal vessels post-angioplasty *post-angioplasty (elective) for left anterior descending or left circumflex coronary artery stenosis Golino et al. N Engl J Med. 1994;330:523-8. Proof of Mechanism With 5-HT2A Antagonist 5-HT2A ANTAGONIST DEMONSTRATED PROOF OF CONCEPT 8
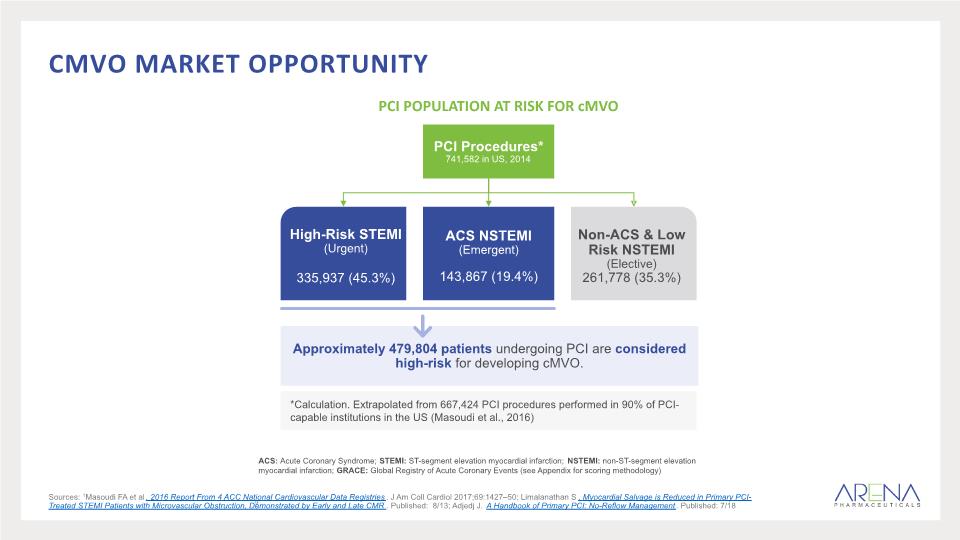
cmvo market opportunity 9 Sources: 1Masoudi FA et al. 2016 Report From 4 ACC National Cardiovascular Data Registries. J Am Coll Cardiol 2017;69:1427–50; Limalanathan S. Myocardial Salvage is Reduced in Primary PCI-Treated STEMI Patients with Microvascular Obstruction, Demonstrated by Early and Late CMR. Published: 8/13; Adjedj J. A Handbook of Primary PCI: No-Reflow Management. Published: 7/18 ACS: Acute Coronary Syndrome; STEMI: ST-segment elevation myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction; GRACE: Global Registry of Acute Coronary Events (see Appendix for scoring methodology) PCI POPULATION AT RISK FOR cMVO

Temanogrel Development Program CAPITALIZING ON PROGRESS AND FAST-TRACK DESIGNATION IV Formulation: 1 completed Phase 1 study Oral Formulation: 4 completed Phase 1 studies Phase 2a: In Elective NSTEMI PCI Single IV bolus - dose selection for Phase 2 (791-202) study Single + repeated doses (with and without DAPT) Initial study during PCI in Elective and Non-Urgent NSTEMI – Q2 2021 Readout expected H2 2022 FDA Fast-Track Designation Granted July 2019 10
Diversified Portfolio STRONG IP + UNENCUMBERED ASSETS* + ACTIVE DISCOVERY ENGINE 11 *Unencumbered refers to olorinab, APD418, Temanogrel. Etrasimod is unencumbered in all areas of the world with the exception of China and South Korea where we’ve established a partnership with Everest Medicines
Etrasimod Launch Readiness MARKET INSIGHTS Generate key market insights to inform clinical and payer evidence CLINICAL VALUE Enhanced formulation, executing peri-approval studies SCIENTIFIC EXCHANGE Data generation, medical communications, MSLs, advisory boards ENSURE ACCESS Harness AI to create predictable value-based program SCALE FOR SUCCESS Build unique customer engagement model 12
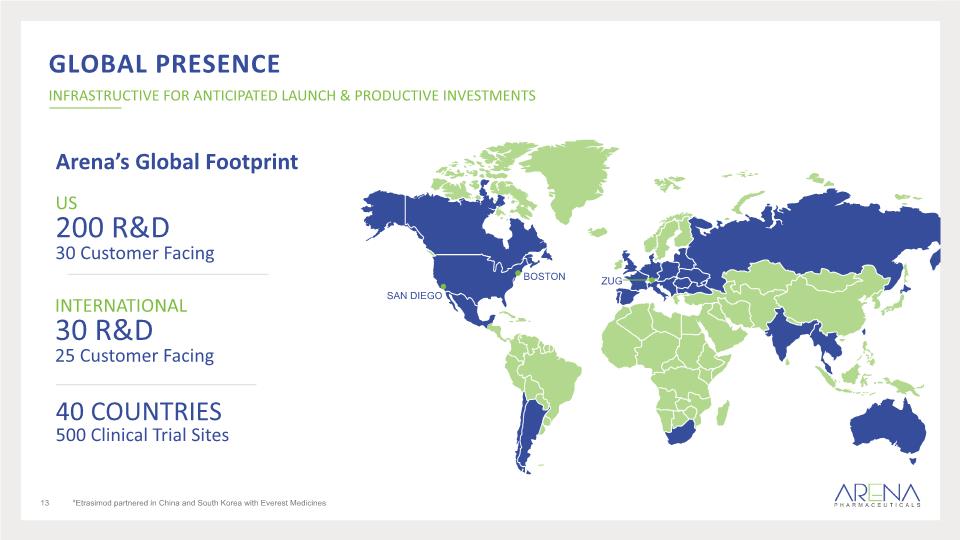
GLOBAL PRESENCE Arena’s Global Footprint *Etrasimod partnered in China and South Korea with Everest Medicines INTERNATIONAL 30 R&D 25 Customer Facing 40 COUNTRIES 500 Clinical Trial Sites US 200 R&D 30 Customer Facing SAN DIEGO BOSTON ZUG INFRASTRUCTIVE FOR ANTICIPATED LAUNCH & PRODUCTIVE INVESTMENTS 13
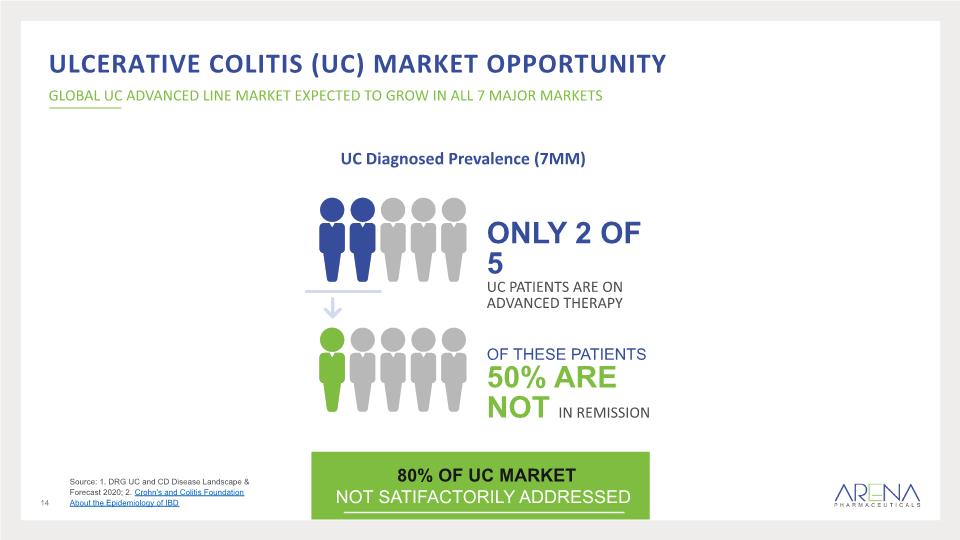
Ulcerative colitis (uc) Market Opportunity GLOBAL UC ADVANCED LINE MARKET EXPECTED TO GROW IN ALL 7 MAJOR MARKETS Source: 1. DRG UC and CD Disease Landscape & Forecast 2020; 2. Crohn's and Colitis Foundation About the Epidemiology of IBD UC Diagnosed Prevalence (7MM) ONLY 2 OF 5 UC PATIENTS ARE ON ADVANCED THERAPY OF THESE PATIENTS 50% ARE NOT IN REMISSION 14 80% OF UC MARKET NOT SATIFACTORILY ADDRESSED
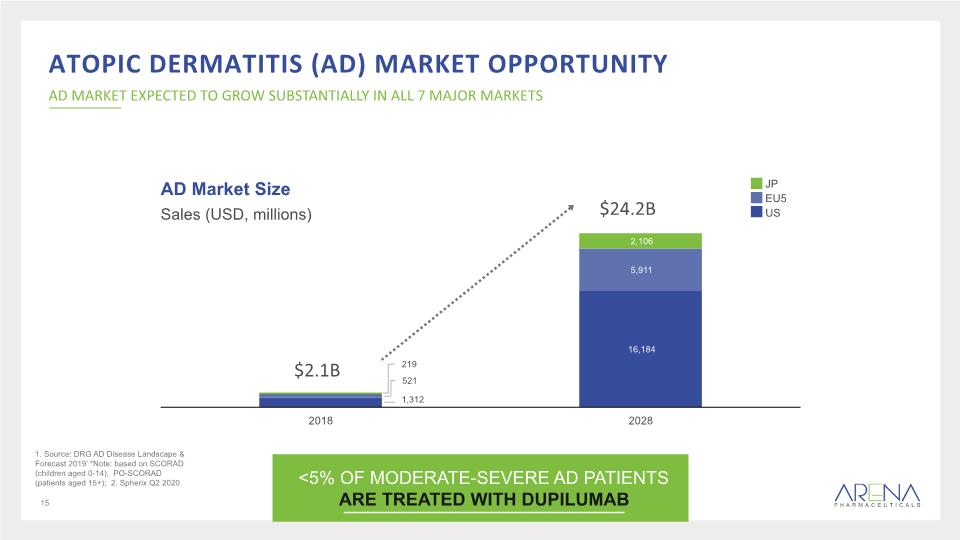
Atopic dermatitis (AD) Market Opportunity AD MARKET EXPECTED TO GROW SUBSTANTIALLY IN ALL 7 MAJOR MARKETS 15 <5% OF MODERATE-SEVERE AD PATIENTS ARE TREATED WITH DUPILUMAB AD Market Size Sales (USD, millions) $2.1B $24.2B 1. Source: DRG AD Disease Landscape & Forecast 2019’ *Note: based on SCORAD (children aged 0-14), PO-SCORAD (patients aged 15+); 2. Spherix Q2 2020
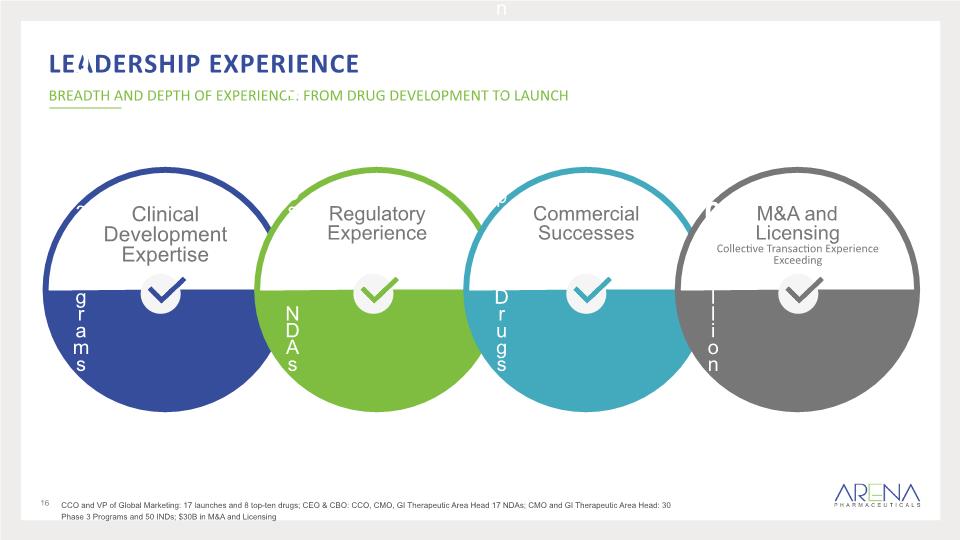
Clinical Development Expertise Leadership experience BREADTH AND DEPTH OF EXPERIENCE: FROM DRUG DEVELOPMENT TO LAUNCH 30 Phase 3 Programs Regulatory Experience 50 INDs 17 NDAs Commercial Successes 17 Launches 8 Top-Ten Drugs M&A and Licensing Collective Transaction Experience Exceeding $30 Billion 16 CCO and VP of Global Marketing: 17 launches and 8 top-ten drugs; CEO & CBO: CCO, CMO, GI Therapeutic Area Head 17 NDAs; CMO and GI Therapeutic Area Head: 30 Phase 3 Programs and 50 INDs; $30B in M&A and Licensing

High-Performance Culture BUILDING A SUSTAINABLE BUSINESS OUR COMMITMENT TO SOCIAL RESPONSIBILITY 17
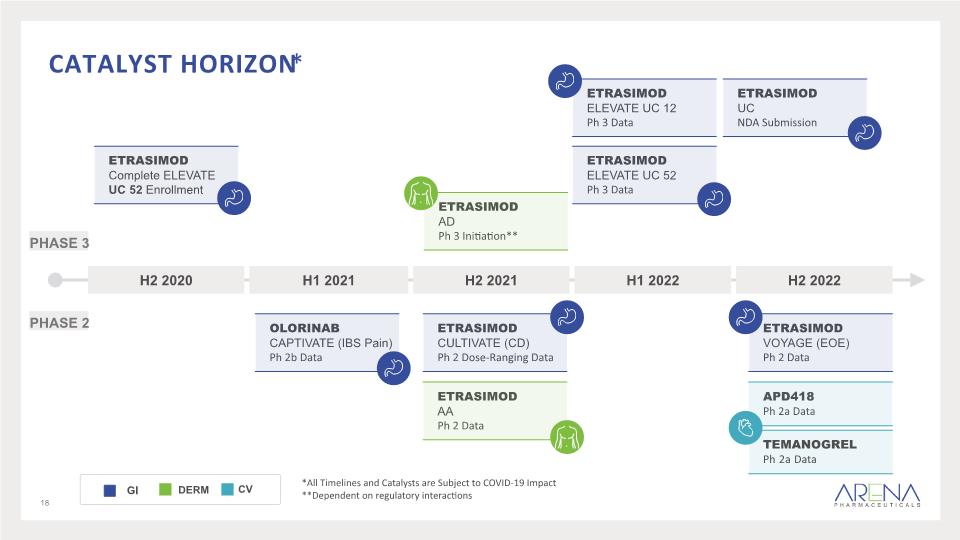
Catalyst Horizon* 18 *All Timelines and Catalysts are Subject to COVID-19 Impact **Dependent on regulatory interactions PHASE 3 PHASE 2 Etrasimod AA Ph 2 Data Etrasimod ELEVATE UC 52 Ph 3 Data APD418 Ph 2a Data Etrasimod CULTIVATE (CD) Ph 2 Dose-Ranging Data Olorinab CAPTIVATE (IBS Pain) Ph 2b Data Etrasimod Complete ELEVATE UC 52 Enrollment Etrasimod ELEVATE UC 12 Ph 3 Data Etrasimod UC NDA Submission Etrasimod VOYAGE (EOE) Ph 2 Data Etrasimod AD Ph 3 Initiation** Temanogrel Ph 2a Data

Arena by the numbers 4 CLINICAL STAGE ASSETS* 8 DISEASE AREAS 2 PH 3 5 PH 2 1 IND EXPECTED EACH YEAR THROUGH [2022] 7 DATA READOUTS: 2 PRECLINICAL ASSETS 3 THERAPEUTIC AREAS *Etrasimod, olorinab, APD418, temanogrel

TOWARD a bold future Pipeline Growth • Commercialization Excellence Leadership & Culture • Long-Term Value 20

JANUARY 2021 • NASDAQ: ARNA
