Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ACCELERON PHARMA INC | xlrn-20210111.htm |

J . P . M O R G A N 3 9 T H A N N U A L H E A L T H C A R E C O N F E R E N C E J A N U A R Y 1 1 , 2 0 2 0 Exhibit 99.1

Acceleron Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS ABOUT THE COMPANY’S STRATEGY, FUTURE PLANS AND PROSPECTS, including statements regarding the development and commercialization of the Company's compounds, the timeline for clinical development and regulatory approval of the Company’s compounds and the expected timing for reporting of data from ongoing clinical trials, the Company’s future cash position, and the potential of REBLOZYL® (luspatercept-aamt) as a therapeutic drug. The words “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. ACTUAL RESULTS COULD DIFFER MATERIALLY FROM THOSE INCLUDED IN THE FORWARD-LOOKING STATEMENTS DUE TO VARIOUS factors, risks and uncertainties, including, but not limited to, that preclinical testing of the Company’s compounds and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that regulatory approval of the Company’s compounds in one indication or country may not be predictive of approval in another indication or country, that the development of the Company's compounds may take longer and/or cost more than planned or accelerate faster than currently expected, that the Company or its collaboration partner, Bristol Myers Squibb (“BMS”), may be unable to successfully complete the clinical development of the Company’s compounds, that the Company or BMS may be delayed in initiating, enrolling or completing any clinical trials, and that the Company's compounds may not receive regulatory approval or become commercially successful products. These and other risks and uncertainties are identified under the heading "Risk Factors" included in the Company's most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q, and other filings that the Company has made and may make with the SEC in the future. THE FORWARD-LOOKING STATEMENTS CONTAINED IN THIS PRESENTATION ARE BASED ON MANAGEMENT’S CURRENT VIEWS, PLANS, estimates, assumptions and projections with respect to future events, and the Company does not undertake and specifically disclaims any obligation to update any forward-looking statements. This presentation is for investor relations purposes only – Not for product promotional purposes. 2

M I S S I O N & T H E R A P E U T I C A R E A F O C U S OUR MISSION is to transform the lives of patients with serious and rare diseases 3 HEMATOLOGY PULMONARY This presentation is for investor relations purposes only – Not for product promotional purposes.
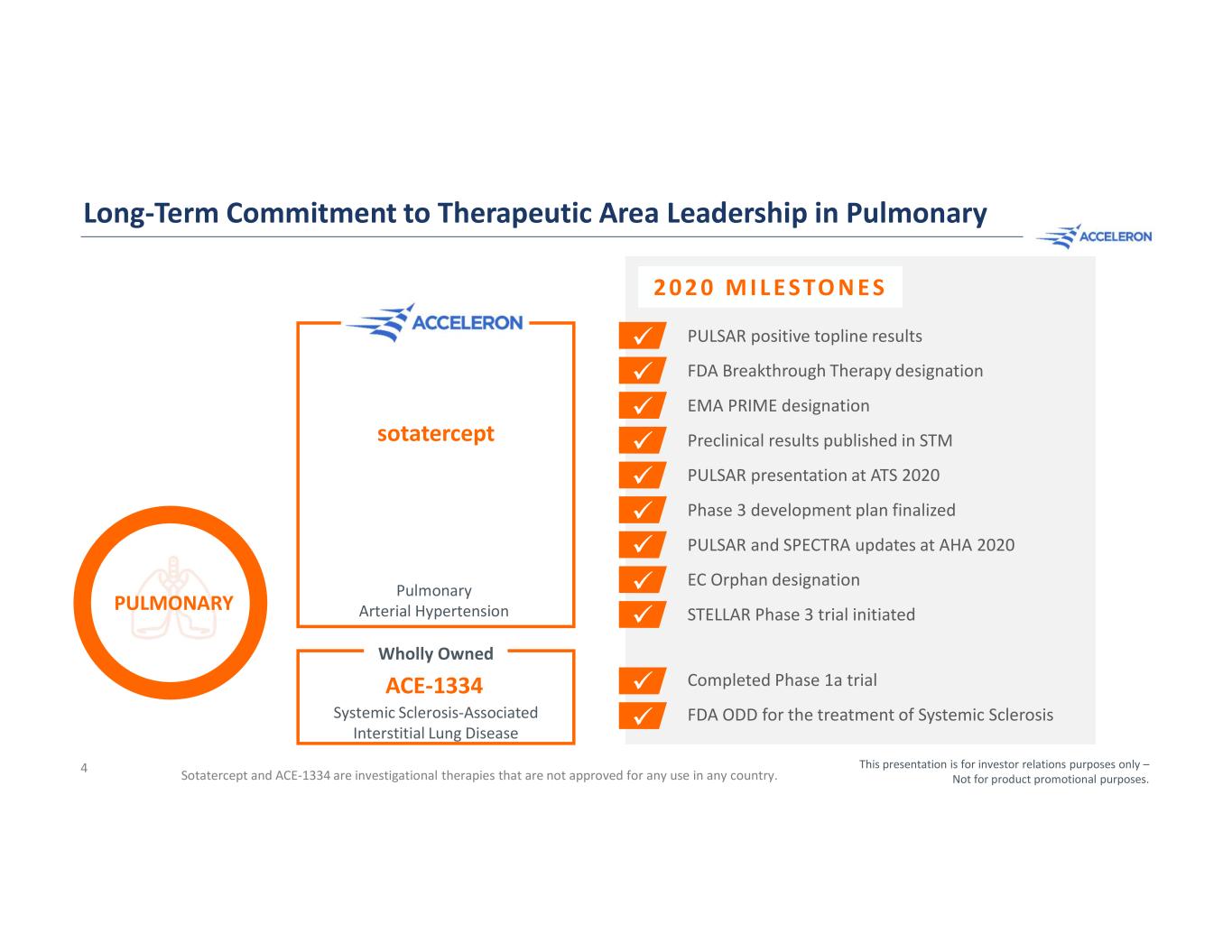
Long-Term Commitment to Therapeutic Area Leadership in Pulmonary 4 sotatercept Pulmonary Arterial Hypertension PULSAR positive topline results FDA Breakthrough Therapy designation EMA PRIME designation Preclinical results published in STM PULSAR presentation at ATS 2020 Phase 3 development plan finalized PULSAR and SPECTRA updates at AHA 2020 EC Orphan designation STELLAR Phase 3 trial initiated ACE-1334 Systemic Sclerosis-Associated Interstitial Lung Disease Wholly Owned Sotatercept and ACE-1334 are investigational therapies that are not approved for any use in any country. This presentation is for investor relations purposes only – Not for product promotional purposes. PULMONARY Completed Phase 1a trial FDA ODD for the treatment of Systemic Sclerosis 2 0 2 0 M I L E S TO N E S
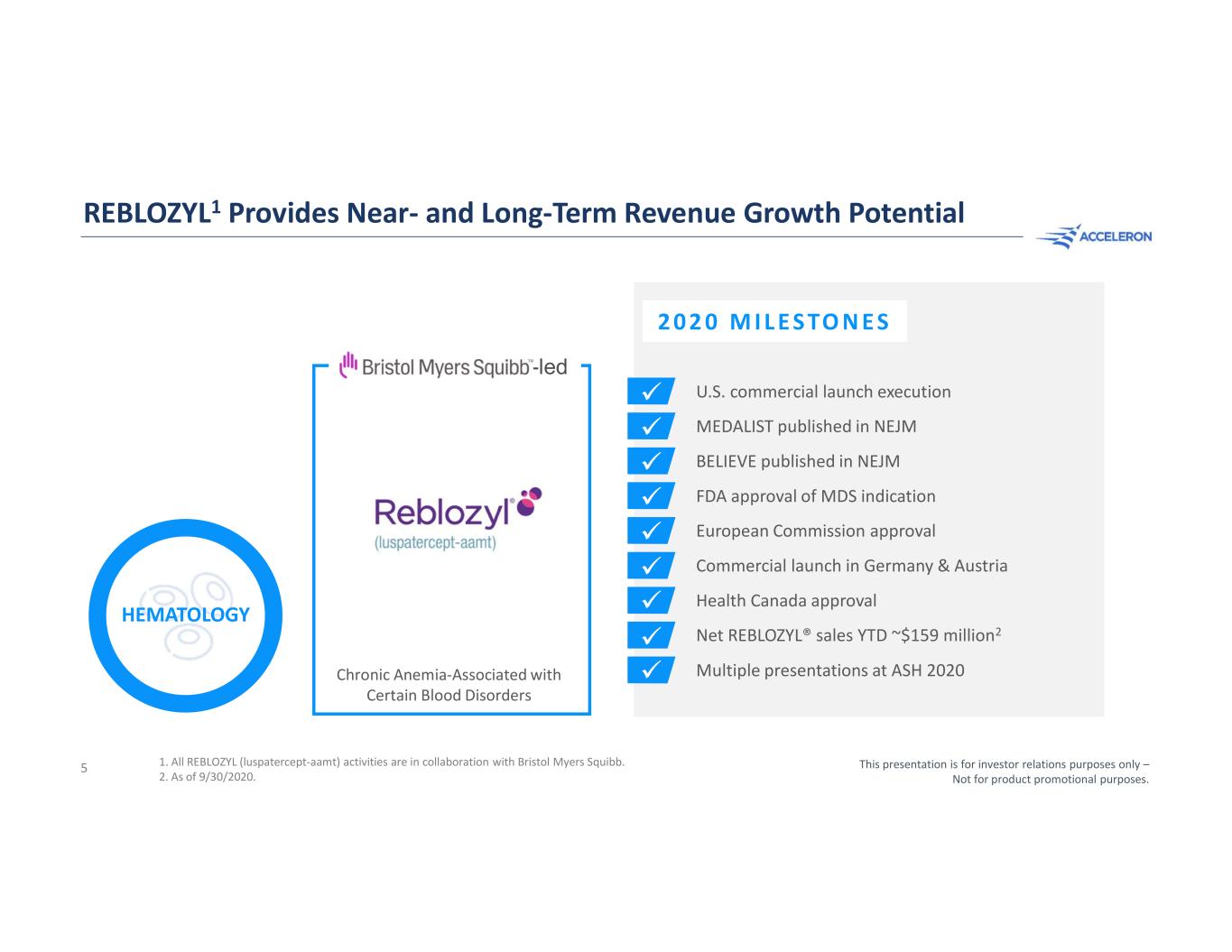
REBLOZYL1 Provides Near- and Long-Term Revenue Growth Potential 5 U.S. commercial launch execution MEDALIST published in NEJM BELIEVE published in NEJM FDA approval of MDS indication European Commission approval Commercial launch in Germany & Austria Health Canada approval Net REBLOZYL® sales YTD ~$159 million2 Multiple presentations at ASH 2020 This presentation is for investor relations purposes only – Not for product promotional purposes. 2 0 2 0 M I L E S TO N E S HEMATOLOGY 1. All REBLOZYL (luspatercept-aamt) activities are in collaboration with Bristol Myers Squibb. 2. As of 9/30/2020. Chronic Anemia-Associated with Certain Blood Disorders -led
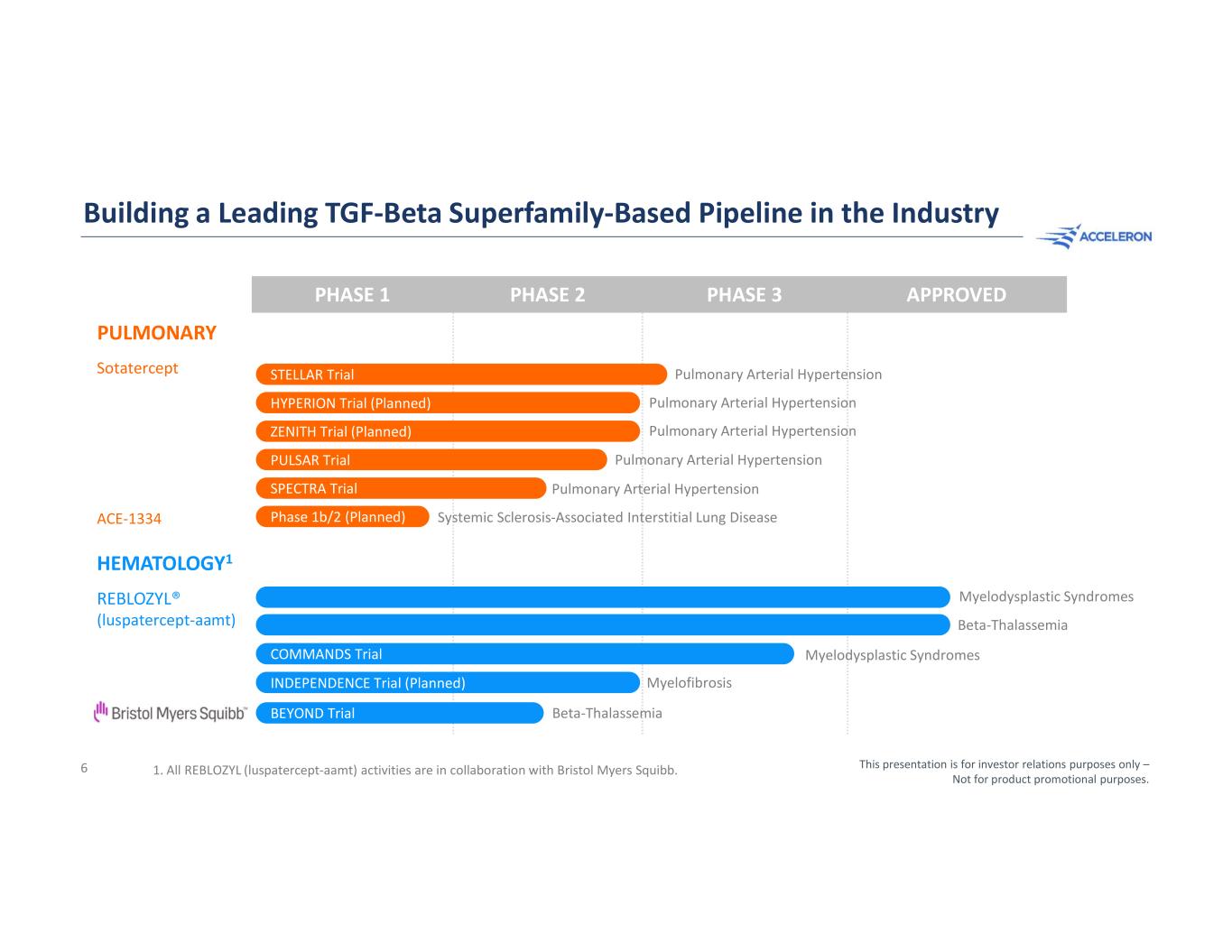
Building a Leading TGF-Beta Superfamily-Based Pipeline in the Industry PHASE 1 PHASE 2 PHASE 3 APPROVED PULMONARY Sotatercept ACE-1334 HEMATOLOGY1 REBLOZYL® (luspatercept-aamt) 1. All REBLOZYL (luspatercept-aamt) activities are in collaboration with Bristol Myers Squibb. Pulmonary Arterial Hypertension Pulmonary Arterial Hypertension Systemic Sclerosis-Associated Interstitial Lung Disease Pulmonary Arterial Hypertension Pulmonary Arterial Hypertension Pulmonary Arterial Hypertension PULSAR Trial STELLAR Trial SPECTRA Trial HYPERION Trial (Planned) ZENITH Trial (Planned) Phase 1b/2 (Planned) Beta-Thalassemia Myelodysplastic Syndromes Myelodysplastic Syndromes Myelofibrosis COMMANDS Trial INDEPENDENCE Trial (Planned) This presentation is for investor relations purposes only – Not for product promotional purposes. 6 Beta-ThalassemiaBEYOND Trial

Pulmonary Disease 7 This presentation is for investor relations purposes only – Not for product promotional purposes.

Pulmonary Arterial Hypertension (PAH) Disease Background Common Symptoms ~70K U.S./E.U. PAH Patients Shortness of Breath Chest Pain Dizziness and/or Fainting Exertion Fatigue This presentation is for investor relations purposes only – Not for product promotional purposes. 8

PAH: A Disease of Vascular Remodeling This presentation is for investor relations purposes only – Not for product promotional purposes. 9 Normal Elevated pressure in the pulmonary circulation can lead to heart failure Arterial Hypertension Smooth muscle cell hypertrophy Endothelial cell proliferation Intimal fibrosis P UL MO NA RY A RT ERI ES

Current Therapies Are Not Enough DRUG APPROVALS 14 Median Survival Rate YEARS~5-7 Multiple Pathways Primarily Promote Vasodilation This presentation is for investor relations purposes only – Not for product promotional purposes. 10

Sotatercept: Significant Progress in 2020 BREAKTHROUGH THERAPY DESIGNATION PRIME – PRIORITY MEDICINES SCIENCE TRANSLATIONAL MEDICINE RESEARCH ARTICLE P R E C L I N I C A L R EG U L ATO R Y C L I N I C A L November 13-17, 2020 A virtual experience This presentation is for investor relations purposes only – Not for product promotional purposes. 11

Positive Phase 2 Results Establish Proof-of-Concept in PAH This presentation is for investor relations purposes only – Not for product promotional purposes. 12 Demonstrated improvement in PVR and 6MWD at week 24 Concordance across multiple exploratory endpoints and analyses and in patients receiving mono, double, or triple therapy Presentation of PULSAR echocardiogram results at week 24 Sotatercept associated with measures of improved right ventricular function Preliminary SPECTRA hemodynamic data consistent with PULSAR ― Reduction in PVR and mPAP Improvement in exercise tolerance and capacity Sotatercept is an investigational therapy that is not approved for any use in any country. Sotatercept was generally well tolerated in patients with PAH; the safety profile was consistent with that observed in other patient populations Cardiopulmonary Best Abstract Breaking News Session

L A B E L E X PA N S I O NR E G I S T R AT I O N A L S O TAT E R C E P T V I S I O N Main Phase 3 Study Phase 3 Early Intervention Study Phase 3 WHO Functional Class IV Study ZENITH BACKBONE THERAPY IN PAH Sotatercept is an investigational therapy that is not approved for any use in any country. Sotatercept Phase 3 Clinical Development Plan and Vision This presentation is for investor relations purposes only – Not for product promotional purposes. 13 Now open for enrollment Planned

Advancing ACE-1334 in Systemic Sclerosis with Interstitial Lung Disease (SSc-ILD) 1. Anti-fibrotic activity in multiple preclinical models of fibrosis ACE-1334 is an investigational therapy that is not approved for any use in any country. This presentation is for investor relations purposes only – Not for product promotional purposes. 14 COMMON SYMPTOMS: Dyspnea on exertion Cough Fatigue Chest pain D I S EA S E A R EA S Sc - I LD KEY ATTRIBUTES: Acceleron-discovered Wholly owned TGF-beta ligand trap Anti-fibrotic activity1 PROGRAM HIGHLIGHTS: Phase 1 trial completed FDA Fast Track designation FDA Orphan Drug designation Phase 1b/2 trial planned P R O D U C T C A N D I DAT E ACE- 1 3 3 4 KEY IDENTIFIERS: High unmet medical need Median survival of 5 to 8 years TGF-beta driven fibrosis ~50K patients in U.S./E.U.

This presentation is for investor relations purposes only – Not for product promotional purposes. 15 REBLOZYL® (luspatercept-aamt) is US FDA approved for the treatment of anemia in adult patients with beta thalassemia who require regular red blood cell transfusions and for the treatment of anemia failing an ESA and requiring 2 or more RBC units over 8 weeks in adult patients with very low- to intermediate-risk MDS-RS or with MDS/MPN-RS-T. REBLOZYL is EMA approved for the treatment of adult patients with transfusion-dependent anemia associated with beta thalassemia and for the treatment of adult patients with transfusion-dependent anemia due to very low-, low- and intermediate-risk MDS with ring sideroblasts, who had an unsatisfactory response or are ineligible for erythropoietin-based therapy. REBLOZYL is approved by Health Canada for the treatment of adult patients with red blood cell (RBC) transfusion-dependent anemia associated with beta(β)-thalassemia. REBLOZYL is not approved for any other use in the US, EU and Canada or for any use in any other country. 1. All REBLOZYL activities are in collaboration with Bristol Myers Squibb. Hematology1
Chronic Anemia Due to Rare Blood Disorders Bone marrow failure disorder Bone marrow failure isorder CHRONIC ANEMIA L O W E R - R I S K M D S Bone marrow failure disorder Inherited hemoglobin disorderB E T A - T H A L A S S E M I A Bone marrow failure disorder Fib tic bone marr w diseaseM Y E L O F I B R O S I S This presentation is for investor relations purposes only – Not for product promotional purposes. 16

REBLOZYL1 Regulatory Approvals 17 North America Co-Promote, with Bristol Myers Squibb Responsible for ROW U.S. FDA approves REBLOZYL for beta- thalassemia indication U.S. FDA approves REBLOZYL for MDS indication European Commission approves REBLOZYL for MDS and beta-thalassemia indications Health Canada approves REBLOZYL for beta- thalassemia indication Co-promote Bristol Myers Squibb N O V E M B E R 2 0 1 9 A P R I L 2 0 2 0 J U N E 2 0 2 0 S E P T E M B E R 2 0 2 0 1. All REBLOZYL (luspatercept-aamt) activities are in collaboration with Bristol Myers Squibb. This presentation is for investor relations purposes only – Not for product promotional purposes.

R E B L O Z Y L 1 C O M M E R C I A L L A U N C H Q3 2020 Highlights2 • Net REBLOZYL sales of ~$159M YTD • ~$19.3M in royalty revenue from net REBLOZYL sales of ~$96M in Q3 • E.U. launch in Austria and Germany underway Launch Commentary • A significant proportion of early uptake driven by pent-up demand • Moving forward, we expect REBLOZYL demand to be driven primarily by patients earlier in their LR MDS (RS+) journey This presentation is for investor relations purposes only – Not for product promotional purposes. 18 1. All REBLOZYL (luspatercept-aamt) activities are in collaboration with Bristol Myers Squibb. 2. As of 9/30/2020.

Building a Multi-Billion Dollar Anemia Brand A C C E L E R O N ’ S E S T I M AT E D A N N U A L P E A K S A L E S P O T E N T I A L Future Clinical Development Expansion REBLOZYL® (luspatercept-aamt) is not approved to treat patients with treatment naïve myelodysplastic syndromes, myelofibrosis, pediatric beta-thalassemia, or non-transfusion-dependent beta-thalassemia in any country. All REBLOZYL activities are in collaboration with Bristol Myers Squibb. $4 Billion+ This presentation is for investor relations purposes only – Not for product promotional purposes. 19 Lower-Risk MDS, First-line O N G O I N G P H A S E 3 T R I A L MF in combination with JAK2 inhibitor P L A N N E D P H A S E 3 T R I A L Beta-Thalassemia (NTD) O N G O I N G P H A S E 2 T R I A L
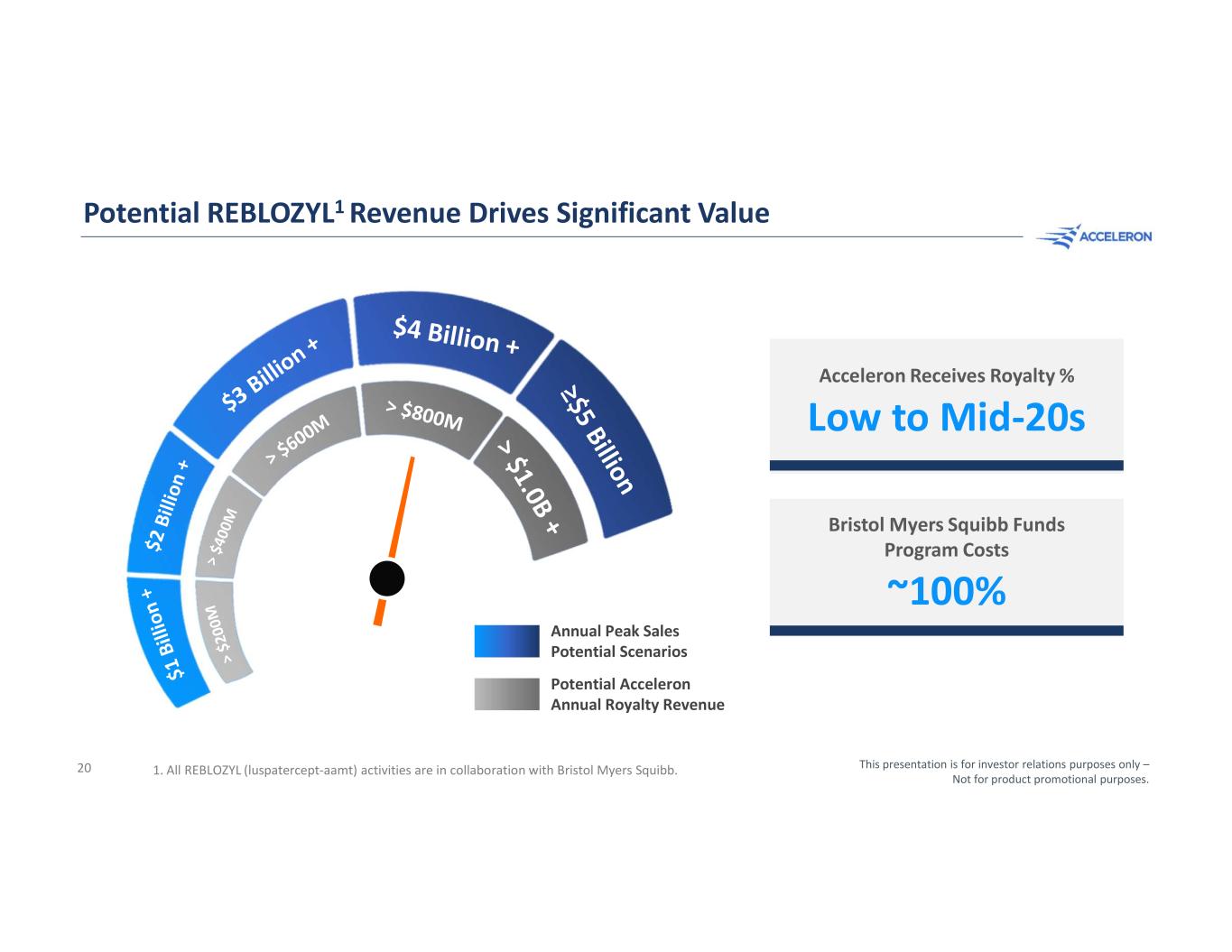
Potential REBLOZYL1 Revenue Drives Significant Value Acceleron Receives Royalty % Bristol Myers Squibb Funds Program Costs Low to Mid-20s ~100% Annual Peak Sales Potential Scenarios Potential Acceleron Annual Royalty Revenue 1. All REBLOZYL (luspatercept-aamt) activities are in collaboration with Bristol Myers Squibb. This presentation is for investor relations purposes only – Not for product promotional purposes. 20

Upcoming and Ongoing Corporate Priorities PULMONARY Sotatercept – STELLAR Phase 3 trial execution – PULSAR open-label extension study update expected in 1H 2021 – Additional results from SPECTRA Phase 2 trial expected in 1H 2021 – HYPERION and ZENITH Phase 3 trial initiations in mid:2021 – Evaluate development of sotatercept in broader PH indications ACE-1334 – Initiate Phase 1b/2 trial in SSc-ILD expected in 2021 HEMATOLOGY REBLOZYL1 – U.S., E.U., and Canada commercial launch – BEYOND trial results presentation expected in 1H 2021; COMMANDS trial topline results expected in 2022+; INDEPENDENCE trial start expected in Q1 2021 – Potential clinical development expansion into other disease states CORPORATE PRIORITIES R&D day planned for the middle of 2021 1. All REBLOZYL (luspatercept-aamt) activities are in collaboration with Bristol Myers Squibb. This presentation is for investor relations purposes only – Not for product promotional purposes. 21

A P P E N D I X

STELLAR Phase 3 Trial Design Schema Randomization 1:1 Stratified by WHO FC and Background therapy Double-Blind Primary treatment period (24 weeks) Placebo + background PAH therapy (mono, double, or triple) N = 142 Sotatercept 0.3 mg/kg first dose to 0.7 mg/kg Q21 days + background PAH therapy (mono, double, or triple) N = 142 Long-term Double-Blind treatment period (up to 72 weeks) N=284 Primary Endpoint Analysis WHO: World Health Organization; RHC: Right heart catheterization; PVR: Pulmonary vascular resistance. K E Y I N C L U S I O N C R I T E R I A Adults ≥18 years old WHO Group 1 PAH WHO Functional Class II or III Baseline RHC with PVR ≥5 Wood units Baseline 6-minute walk distance 150-500 m Stable treatment with SOC therapies, including mono, double, and triple therapies – An endothelin-receptor antagonist, a phosphodiesterase 5 inhibitor, a soluble guanylate cyclase stimulator, and/or a prostacyclin (including IV) This presentation is for investor relations purposes only – Not for product promotional purposes. 23 NOW OPEN FOR ENROLLMENT
