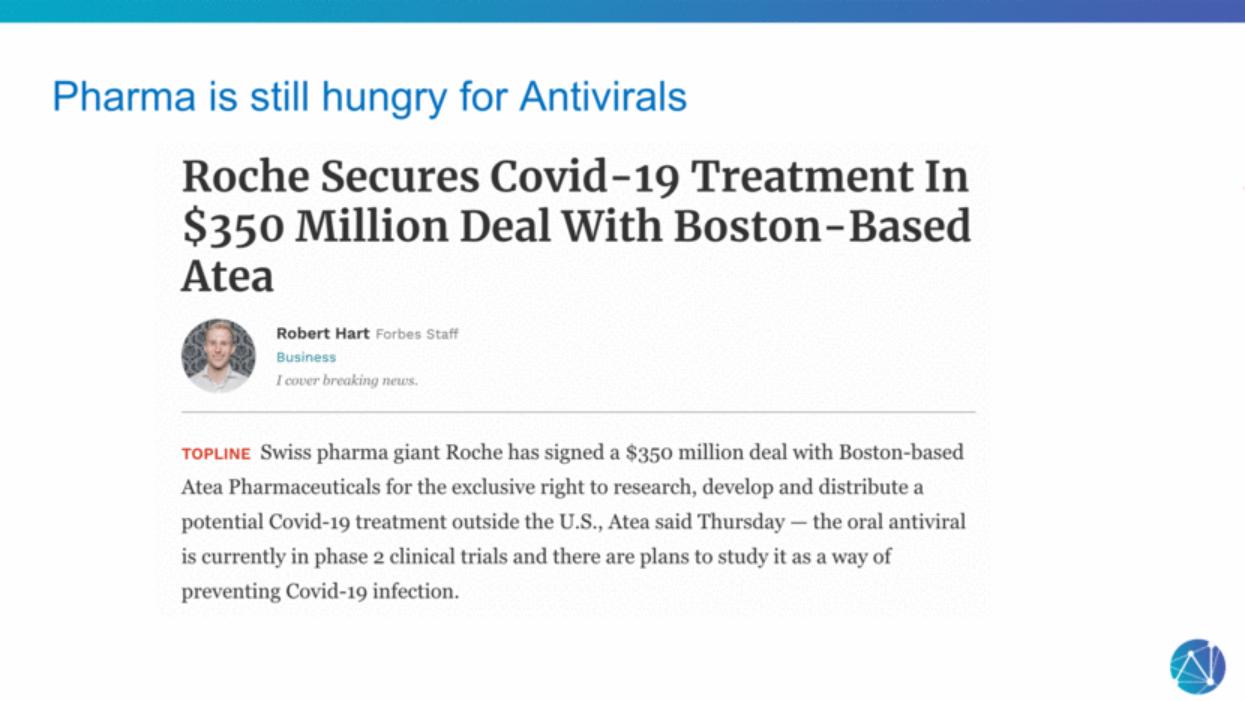Attached files
| file | filename |
|---|---|
| 8-K - 8-K - NeuroBo Pharmaceuticals, Inc. | tmb-20210108x8k.htm |
Exhibit 99.1
| Company Presentation January 8, 2021 Confidential |
| DISCLAIMERS This presentation includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Except for statements of historical fact, any information contained in this presentation may be a forward‐looking statement that reflects the Company’s current views about future events and are subject to risks, uncertainties, assumptions and changes in circumstances that may cause events or the Company’s actual activities or results to differ significantly from those expressed in any forward‐looking statement. In some cases, you can identify forward‐looking statements by terminology such as “may”, “will”, “could”, “would”, “should”, “plan”, “predict”, “potential”, “project”, “promising,” “expect,” “estimate,” “anticipate,” “intend,” “goal,” “strategy,” “believe,” and similar expressions and variations thereof. Forward‐looking statements may include statements regarding the Company’s business strategy, market size, potential growth opportunities, capital requirements and use of proceeds, clinical development activities, the timing and results of clinical trials, regulatory submissions, potential regulatory approval and commercialization of the product candidate. Although the Company believes that the expectations reflected in such forward‐looking statements are reasonable, the Company cannot guarantee future events, results, actions, levels of activity, performance or achievements. These forward‐looking statements are subject to a number of risks, uncertainties and assumptions, including those described under the heading “Risk Factors” in our Annual Report on Form 10-K for the year ending December 31, 2019 and our other filings with the SEC, including our quarterly Q and R reports on form 10-Q. These forward‐looking statements speak only as of the date of this presentation and the Company undertakes no obligation to revise or update any forward‐looking statements to reflect events or circumstances after the date hereof. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market shares and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of such products. |
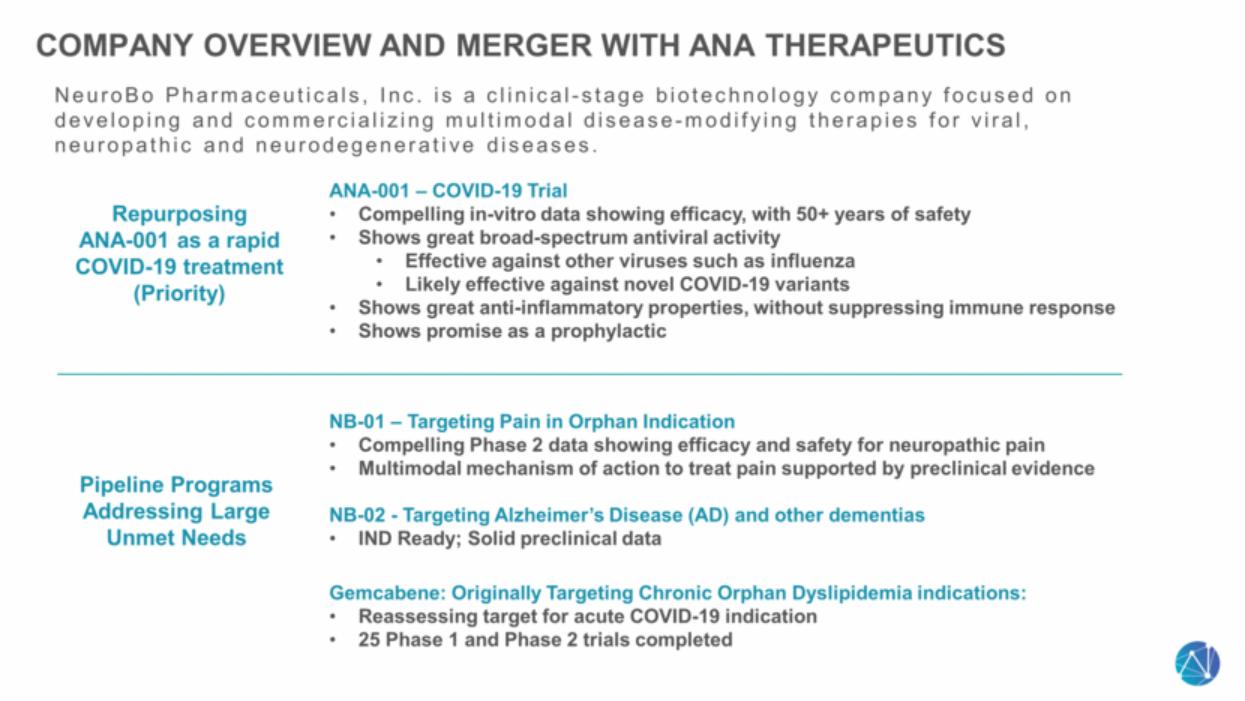
| Company Overview and Merger with ANA Therapeutics NeuroBo Pharmaceuticals, Inc. is a clinical-stage biotechnology company focused on developing and commercializing multimodal disease-modifying therapies for viral, neuropathic and neurodegenerative diseases. Repurposing ANA-001 as a rapid COVID-19 treatment (Priority) Pipeline Programs Addressing Large Unmet Needs ANA-001 – COVID-19 Trial Compelling in-vitro data showing efficacy, with 50+ years of safety Shows great broad-spectrum antiviral activity Effective against other viruses such as influenza Likely effective against novel COVID-19 variants Shows great anti-inflammatory properties, without suppressing immune response Shows promise as a prophylactic NB-01 – Targeting Pain in Orphan Indication Compelling Phase 2 data showing efficacy and safety for neuropathic pain Multimodal mechanism of action to treat pain supported by preclinical evidence NB-02 - Targeting Alzheimer’s Disease (AD) and other dementias IND Ready; Solid preclinical data Gemcabene: Originally Targeting Chronic Orphan Dyslipidemia indications: Reassessing target for acute COVID-19 indication 25 Phase 1 and Phase 2 trials completed |
| Proven Leadership Team Richard J. Kang, PhD President & CEO Akash Bakshi, MsC. Chief Operating Officer Nikki Shannon, RegN, BA VP, Clinical Operations Founder of JK BioPharma Solutions and senior management at companies including NeoImmuneTech in immuno-oncology Visiting Fellow at NIH and senior research experience in host-disease pathogen interactions Founder and CEO of ANA Therapeutics Founder and CEO of YourChoice Therapeutics Previously Assistant Director of Marketing and Technology Analysis at UC Berkeley. 26 years of drug development experience from Phase 1 to Phase 4 at Vertex (Kalydeco), Cubist/Merck, AstraZeneca, Tetraphase Leadership roles at 4 pharma companies; >55 studies including 14 Phase 3 Drug approvals: 2 NDAs, 2 MAAs EXPERT Scientific Advisory BoardS COVID-19 Roy Freeman, M.D. Expert in peripheral nerve disorders and neurodegenerative diseases Professor of Neurology, Harvard Medical School Director of the Center for Autonomic and Peripheral Nerve Disorders Neuropathic Pain Scientific Chair Warner Greene, M.D., Ph.D. Expert in virology Director of the Gladstone Institute Professor at UCSF Member of the national Academy of Medicine Gunda Georg, Ph.D. Expert in medicinal chemistry Professor and Head of the Department of Medicinal Chemistry at University of Minnesota Member of the national Academy of Medicine ALZHEIMER'S DISEASE & OTHER DEMENTIAS Christopher Davis, Ph.D. Expert in virology and clinical aspects Ex-BARDA Managed a NATO drug development program 10 years at British Intelligence as principal bioweapons anaylst |
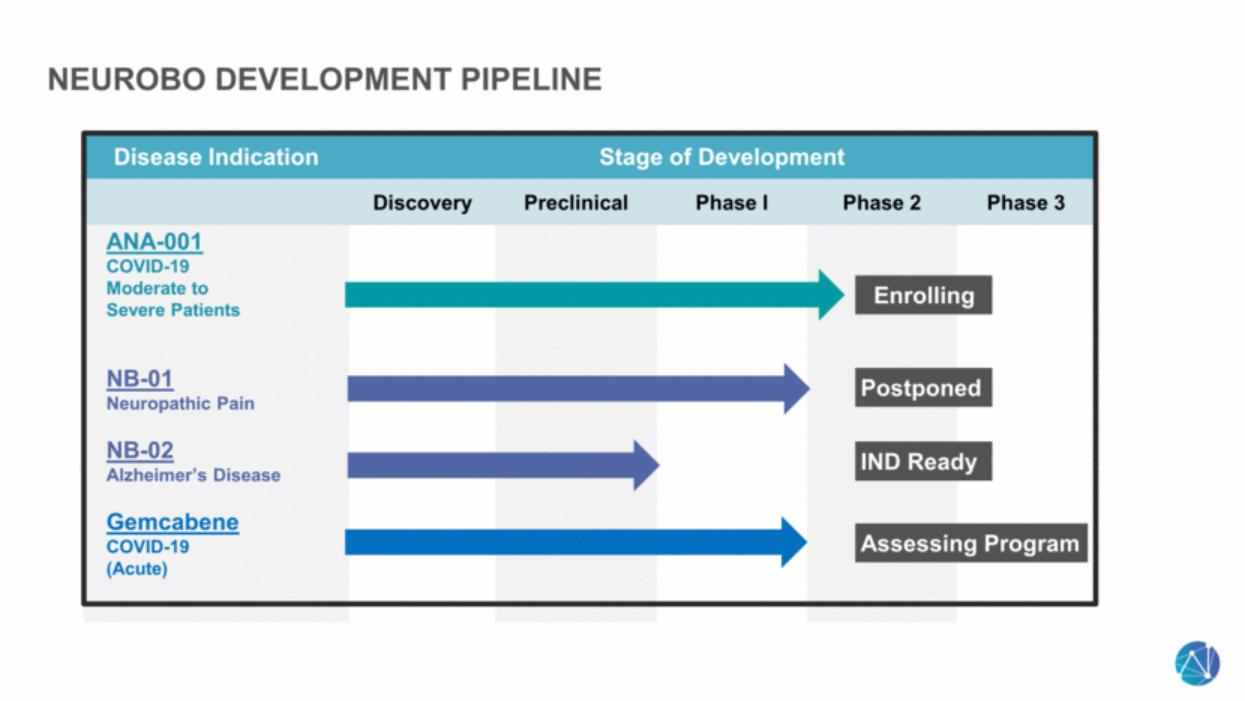
| NeuroBo Development Pipeline Disease Indication Stage of Development Discovery Preclinical Phase I Phase 2 Phase 3 ANA-001 COVID-19 Moderate to Severe Patients NB-01 Neuropathic Pain NB-02 Alzheimer’s Disease Gemcabene COVID-19 (Acute) Postponed Enrolling Assessing Program IND Ready |
| ANA-001 Targeting Covid-19 First indication: Moderate/Severe Covid-19 |
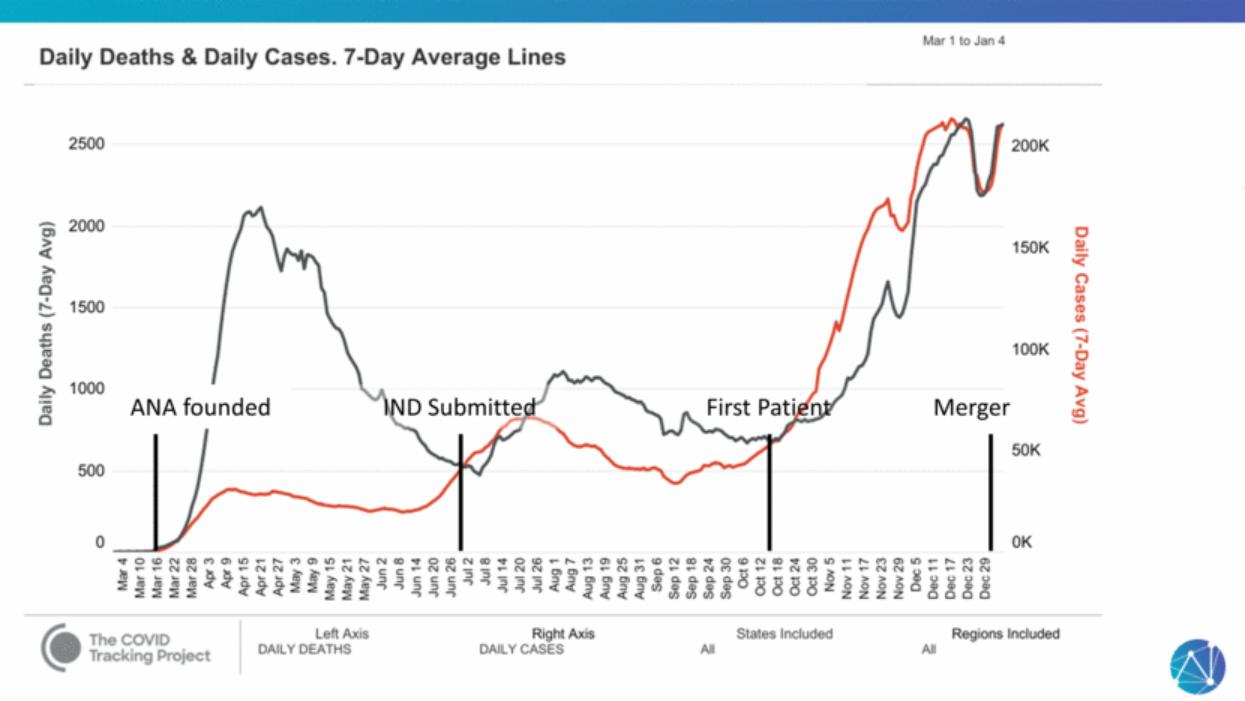
| ANA founded IND Submitted First Patient Merger |
| Background • On World Health Organization’s (WHO) list of essential medicines • Safely treated millions of patients • Currently used to treat tapeworm Niclosamide • Well-established drug: oral administration known to be safe for 50+ years • Very few, non-severe side effects • Appealing characteristics for most at risk population: elderly patients, high comorbidity, and children What is Niclosamide? |
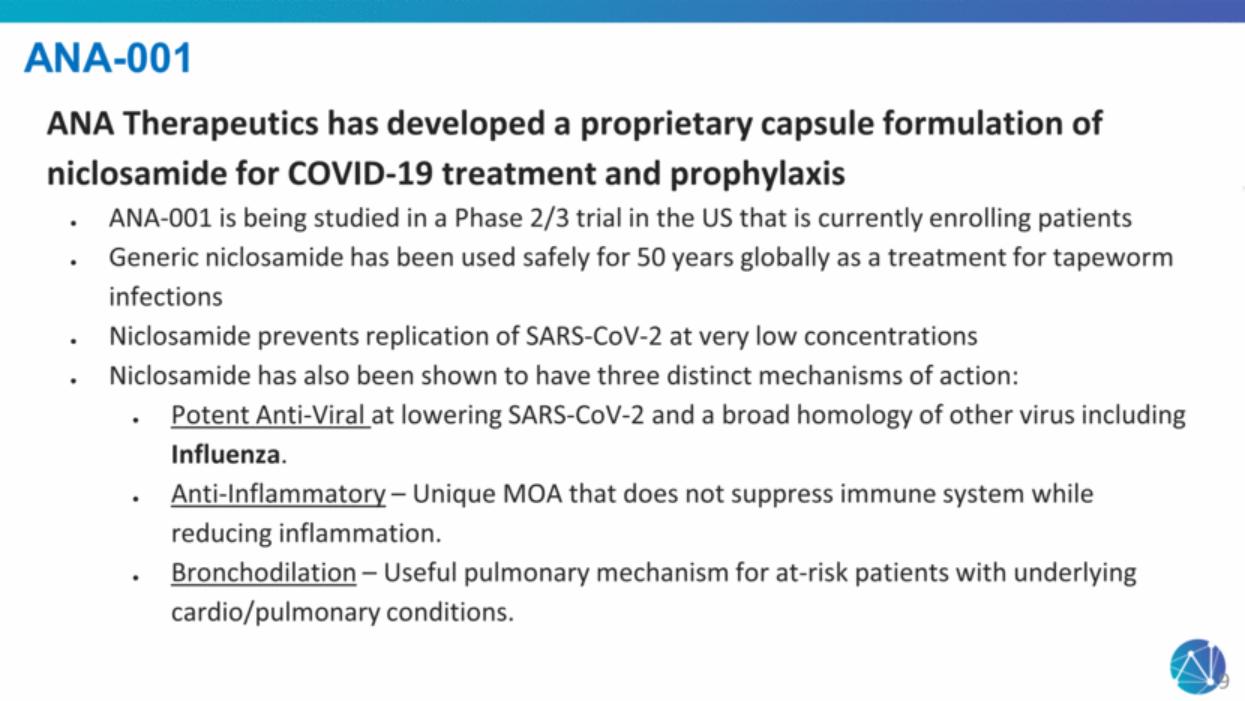
| ANA-001 ANA Therapeutics has developed a proprietary capsule formulation of niclosamide for COVID-19 treatment and prophylaxis ANA-001 is being studied in a Phase 2/3 trial in the US that is currently enrolling patients Generic niclosamide has been used safely for 50 years globally as a treatment for tapeworm infections Niclosamide prevents replication of SARS-CoV-2 at very low concentrations Niclosamide has also been shown to have three distinct mechanisms of action: Potent Anti-Viral at lowering SARS-CoV-2 and a broad homology of other virus including Influenza. Anti-Inflammatory – Unique MOA that does not suppress immune system while reducing inflammation. Bronchodilation – Useful pulmonary mechanism for at-risk patients with underlying cardio/pulmonary conditions. 9 |
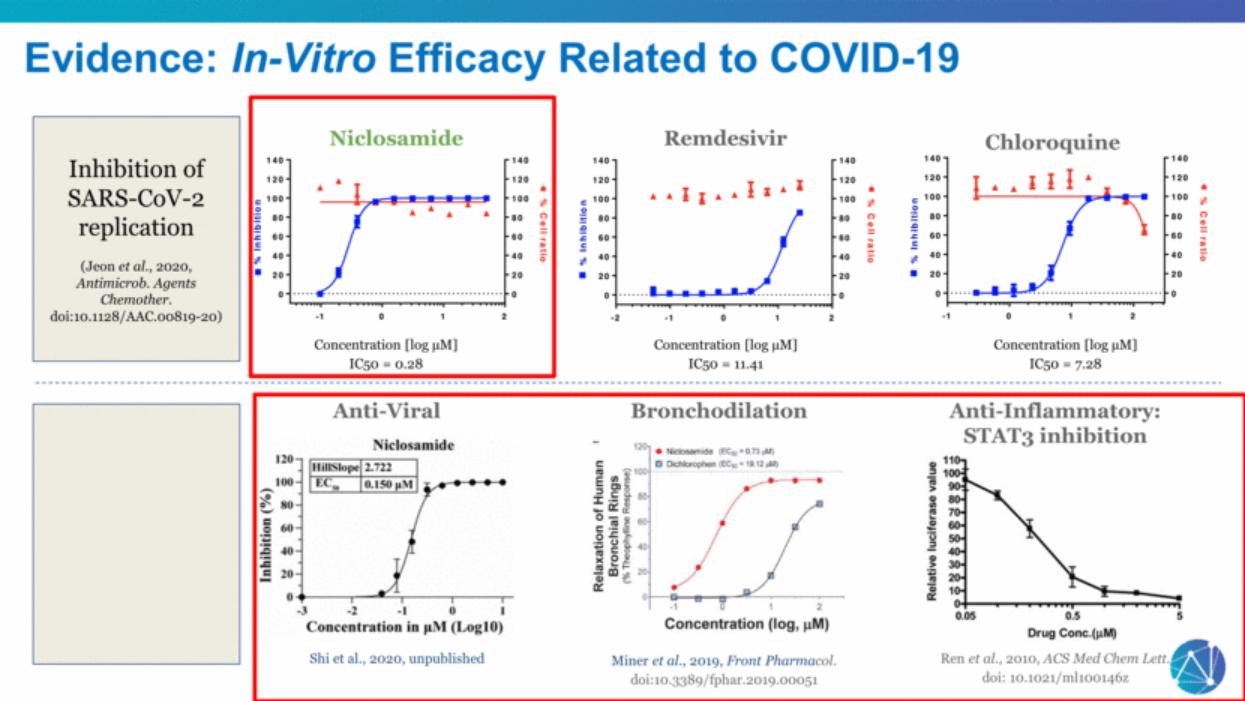
| Niclosamide Remdesivir Anti-Inflammatory: STAT3 inhibition Bronchodilation Anti-Viral Shi et al., 2020, unpublished Miner et al., 2019, Front Pharmacol. doi:10.3389/fphar.2019.00051 Ren et al., 2010, ACS Med Chem Lett. doi: 10.1021/ml100146z Concentration [log µM] IC50 = 11.41 Concentration [log µM] IC50 = 7.28 Concentration [log µM] IC50 = 0.28 Inhibition of SARS-CoV-2 replication (Jeon et al., 2020, Antimicrob. Agents Chemother. doi:10.1128/AAC.00819-20) Evidence: In-Vitro Efficacy Related to COVID-19 Chloroquine |
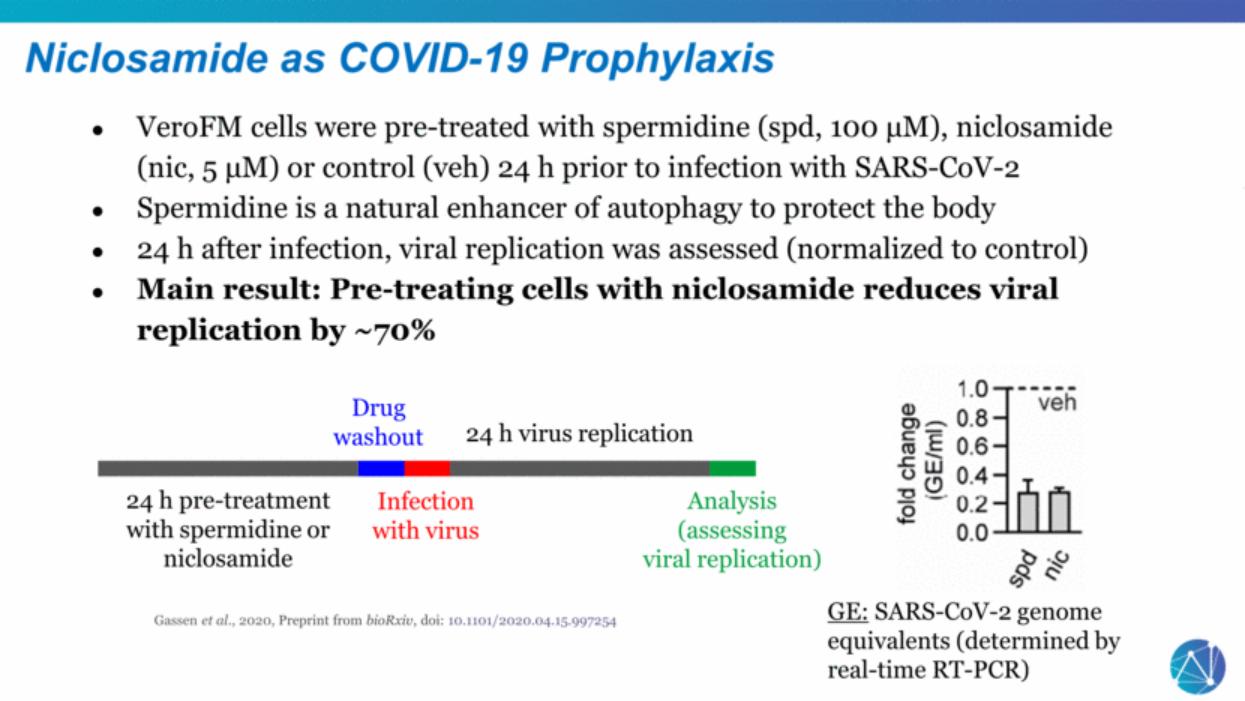
| Gassen et al., 2020, Preprint from bioRxiv, doi: 10.1101/2020.04.15.997254 Niclosamide as COVID-19 Prophylaxis VeroFM cells were pre-treated with spermidine (spd, 100 μM), niclosamide (nic, 5 μM) or control (veh) 24 h prior to infection with SARS-CoV-2 Spermidine is a natural enhancer of autophagy to protect the body 24 h after infection, viral replication was assessed (normalized to control) Main result: Pre-treating cells with niclosamide reduces viral replication by ~70% 24 h pre-treatment with spermidine or niclosamide Infection with virus 24 h virus replication Analysis (assessing viral replication) GE: SARS-CoV-2 genome equivalents (determined by real-time RT-PCR) Drug washout |
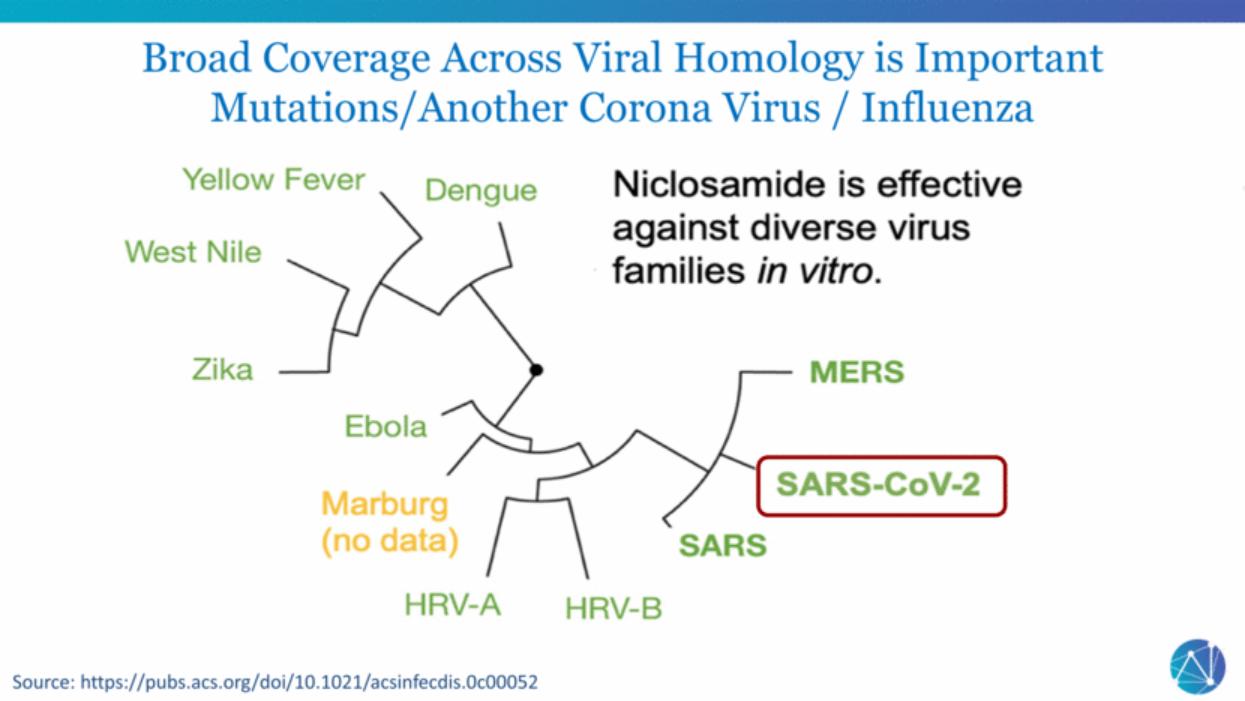
| Source: https://pubs.acs.org/doi/10.1021/acsinfecdis.0c00052 Broad Coverage Across Viral Homology is Important Mutations/Another Corona Virus / Influenza |
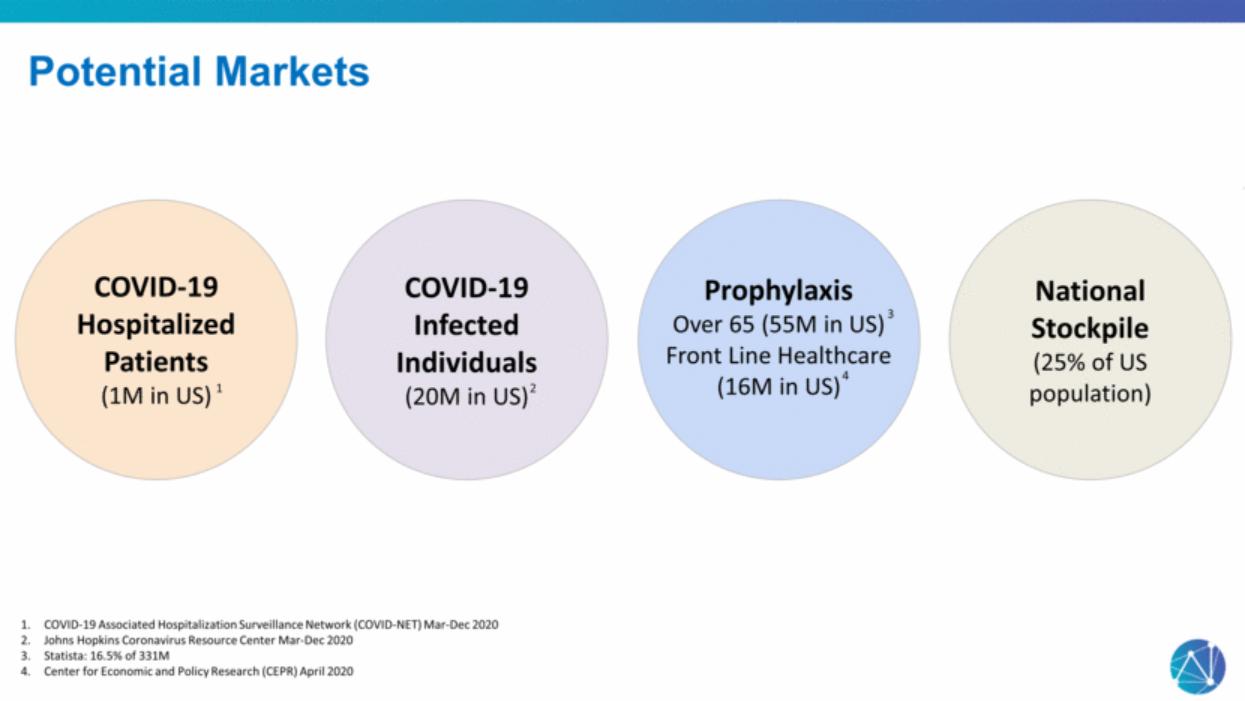
| COVID-19 Infected Individuals (20M in US) National Stockpile (25% of US population) Potential Markets COVID-19 Hospitalized Patients (1M in US) Prophylaxis Over 65 (55M in US) Front Line Healthcare (16M in US) COVID-19 Associated Hospitalization Surveillance Network (COVID-NET) Mar-Dec 2020 Johns Hopkins Coronavirus Resource Center Mar-Dec 2020 Statista: 16.5% of 331M Center for Economic and Policy Research (CEPR) April 2020 1 2 3 4 |
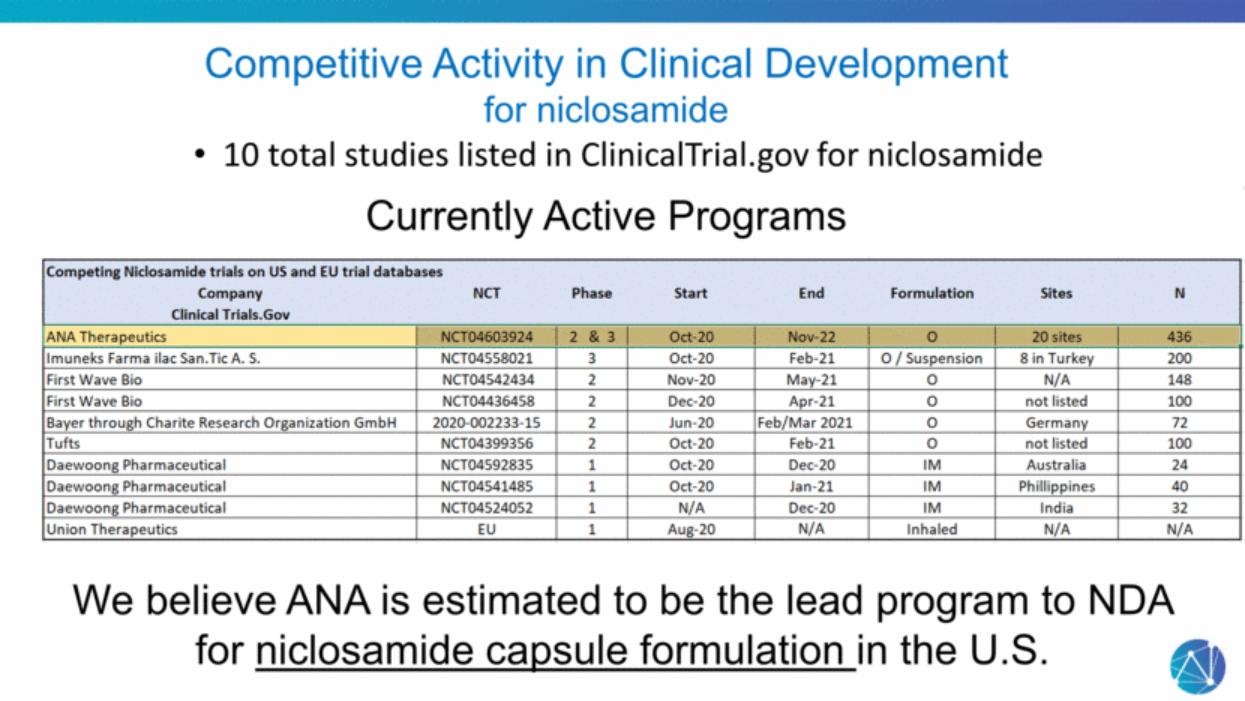
| Competitive Activity in Clinical Development for niclosamide 10 total studies listed in ClinicalTrial.gov for niclosamide Currently Active Programs We believe ANA is estimated to be the lead program to NDA for niclosamide capsule formulation in the U.S. |
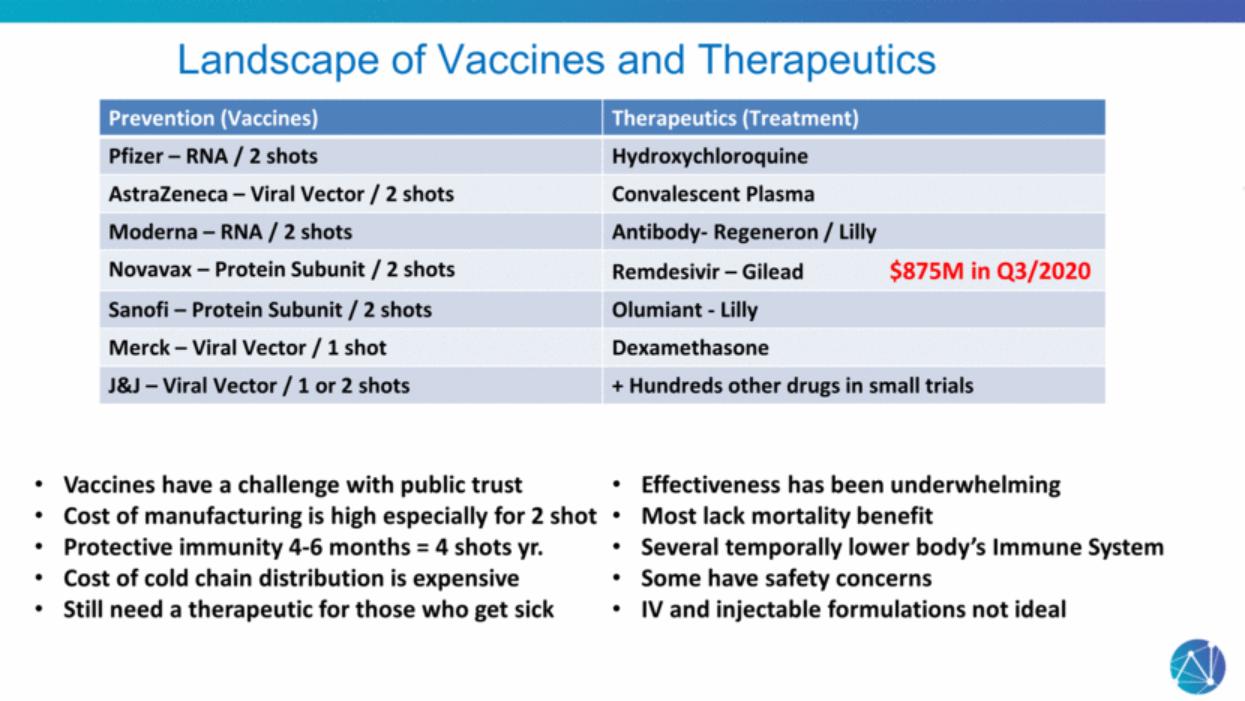
| Landscape of Vaccines and Therapeutics Prevention (Vaccines) Therapeutics (Treatment) Pfizer – RNA / 2 shots Hydroxychloroquine AstraZeneca – Viral Vector / 2 shots Convalescent Plasma Moderna – RNA / 2 shots Antibody- Regeneron / Lilly Novavax – Protein Subunit / 2 shots Remdesivir – Gilead $875M in Q3/2020 Sanofi – Protein Subunit / 2 shots Olumiant - Lilly Merck – Viral Vector / 1 shot Dexamethasone J&J – Viral Vector / 1 or 2 shots + Hundreds other drugs in small trials Vaccines have a challenge with public trust Cost of manufacturing is high especially for 2 shot Protective immunity 4-6 months = 4 shots yr. Cost of cold chain distribution is expensive Still need a therapeutic for those who get sick Effectiveness has been underwhelming Most lack mortality benefit Several temporally lower body’s Immune System Some have safety concerns IV and injectable formulations not ideal |
| Vaccines are an Important Tool in Battling COVID-19 However There are Challenges to Overcome RNA Vaccine - Ultra Cold storage (-100° F) and “cold chain” distribution scale-up Manufacturing: scale-up capacity Essential supply of vials, syringes, etc. 2 administrations necessary 28 days apart Willingness of population to get vaccinated Mutation of viral sequence may require new vaccines Unknowns: Long term efficacy Efficacy in diverse populations Safety – Side effects Long term impacts of covid infections in vaccinated individuals Can vaccinated individuals still spread COVID? |
| Pharma is still hungry for Antivirals |
| 18 ANA-001 and COVID-19 Clinical Program |
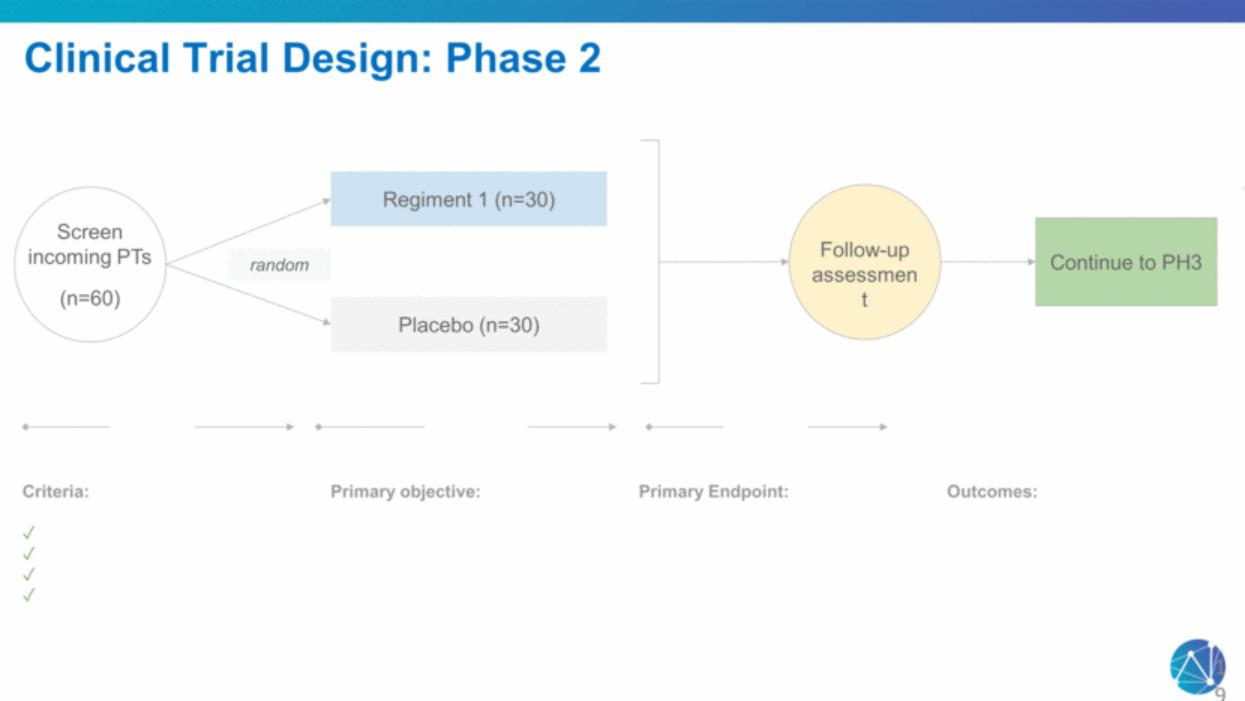
| Continue to PH3 Regiment 1 (n=30) Placebo (n=30) Criteria: ✓ ✓ ✓ ✓ random Screen incoming PTs (n=60) Primary objective: Primary Endpoint: Follow-up assessment Outcomes: Clinical Trial Design: Phase 2 19 |
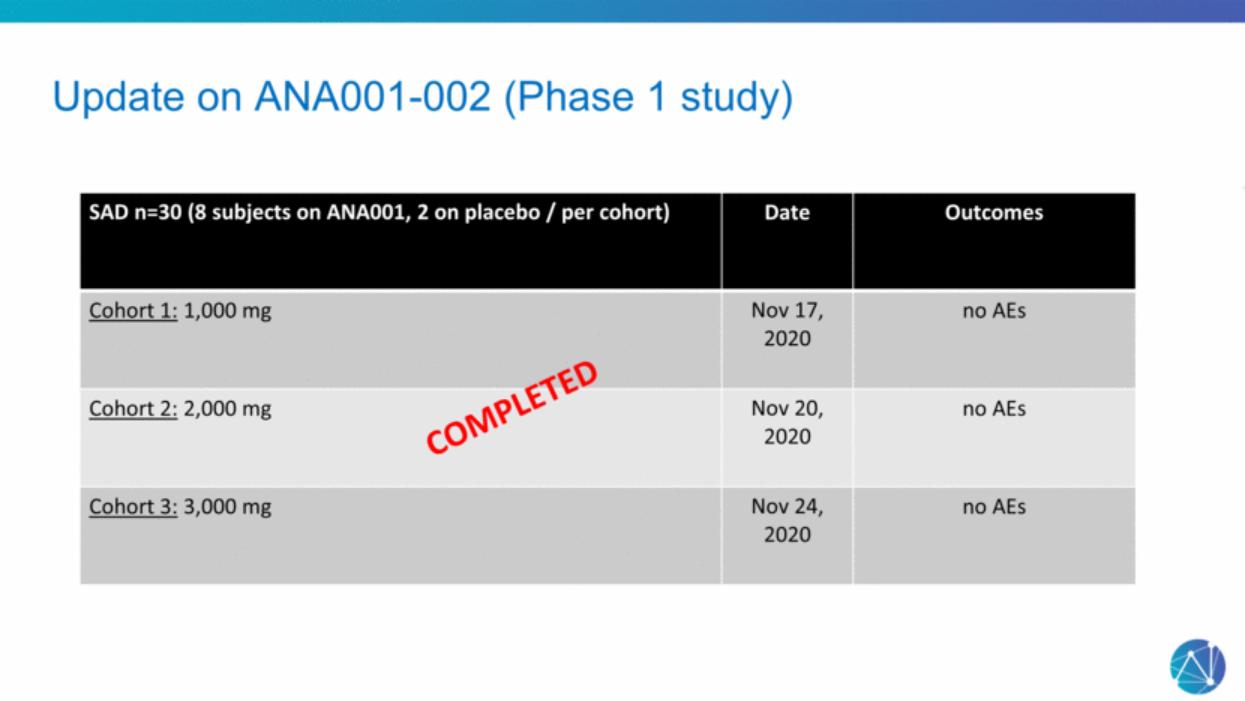
| Update on ANA001-002 (Phase 1 study) SAD n=30 (8 subjects on ANA001, 2 on placebo / per cohort) Date Outcomes Cohort 1: 1,000 mg Nov 17, 2020 no AEs Cohort 2: 2,000 mg Nov 20, 2020 no AEs Cohort 3: 3,000 mg Nov 24, 2020 no AEs COMPLETED |
| 21 ANA-001 and COVID-19 Regulatory Approval |
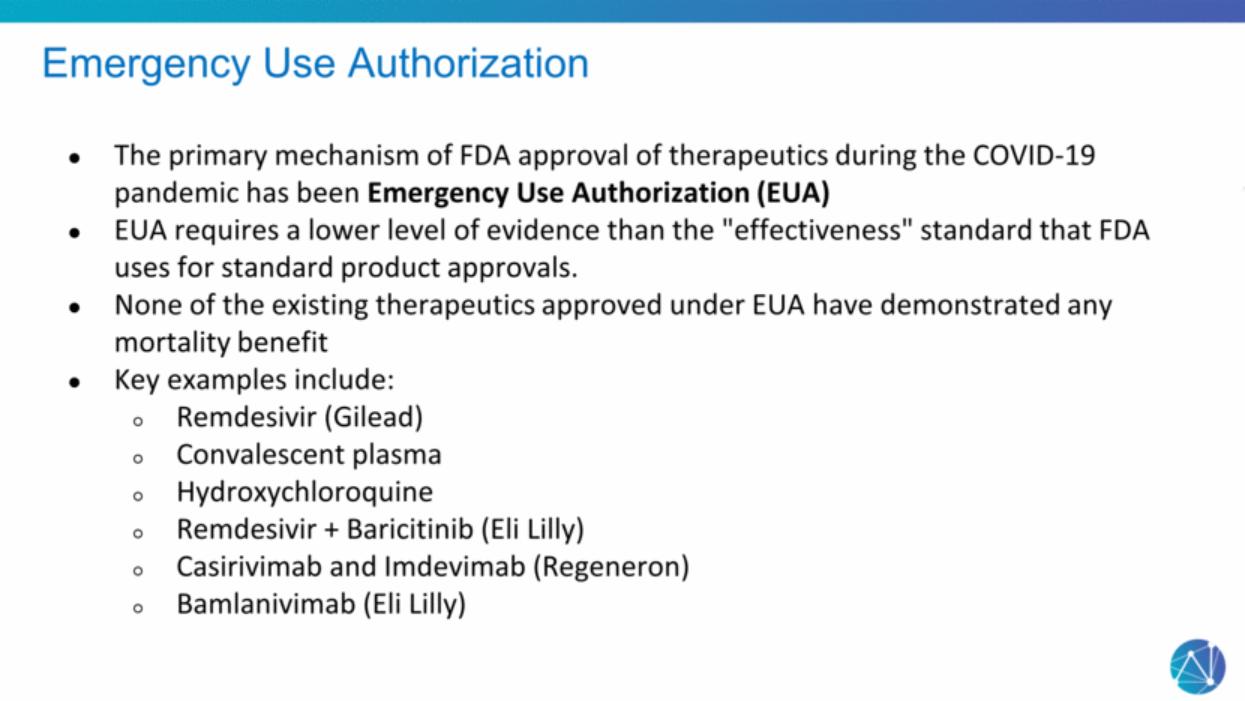
| Emergency Use Authorization The primary mechanism of FDA approval of therapeutics during the COVID-19 pandemic has been Emergency Use Authorization (EUA) EUA requires a lower level of evidence than the "effectiveness" standard that FDA uses for standard product approvals. None of the existing therapeutics approved under EUA have demonstrated any mortality benefit Key examples include: Remdesivir (Gilead) Convalescent plasma Hydroxychloroquine Remdesivir + Baricitinib (Eli Lilly) Casirivimab and Imdevimab (Regeneron) Bamlanivimab (Eli Lilly) |
| EUA Definition & Criteria What is EUA? During a public health emergency, the FDA may authorize the introduction of a drug into interstate commerce, including one which is not yet (or currently) approved under 505 of the Federal Food, Drug, and Cosmetic (FD&C) Act. Per Section 564 of the FD&C Act, EUA is appropriate in consideration of the following conditions (1) serious of life-threatening disease or condition, (2) evidence of effectiveness, (3) risk-benefit analysis, and (4) no alternatives. Each of these conditions is met in relation to the potential for ANA001 to treat COVID-19. |
| 24 ANA-001 and COVID-19 IP and Exclusivity |
| Hatch-Waxman Exclusivity and Intellectual Property NRBO is pursuing an abbreviated regulatory using A 505(b)(2) New Drug Application (NDA). This allows for referencing all the safety data from niclosamide’s original approval. A 505(b)(2) New Drug Application (NDA) provides 3 years of market exclusivity Niclosamide is not currently approved in the US, so there is unlikely to be competition Three-year exclusivity period would block the approval of any generic drugs. The three-year exclusivity period may be extended by 6 months with pediatric exclusivity NRBO will continue to supplement the provisional filing, which will include clinical data from COVID positive patients. This is a unique opportunity in biotech/pharma and expected to be particularly valuable in priority jurisdictions. |
| 26 ANA-001 and COVID-19 Clinical |
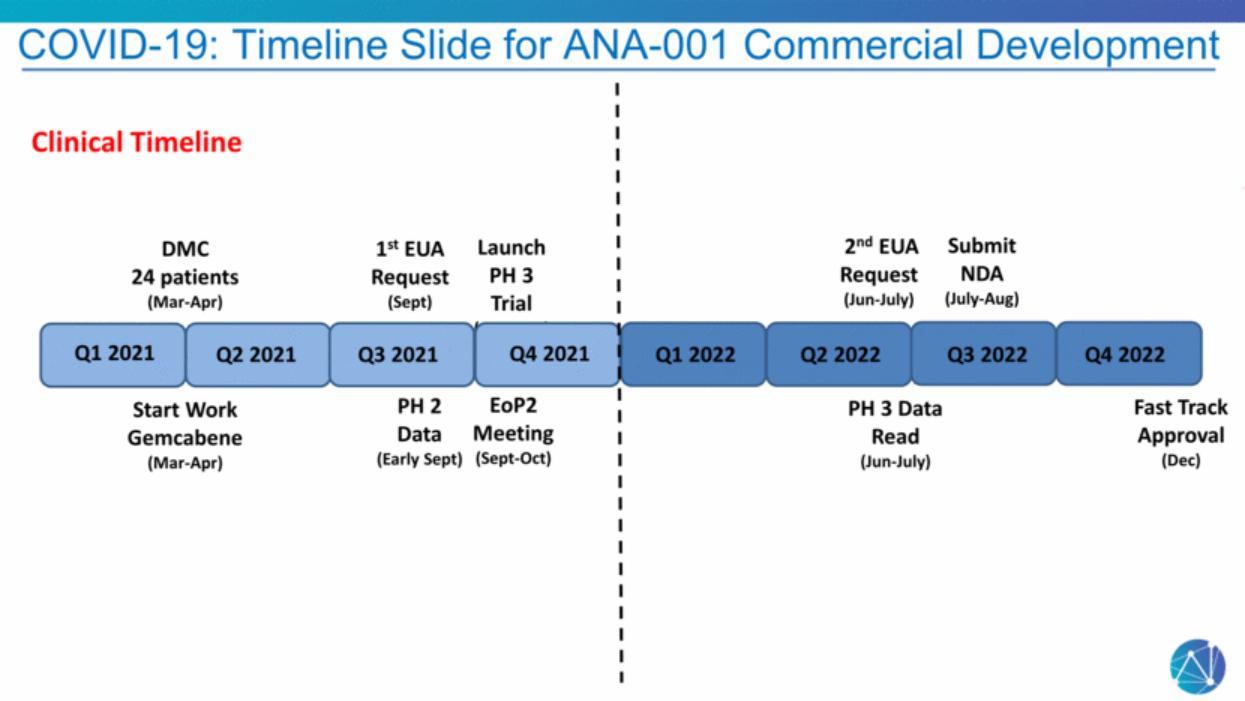
| COVID-19: Timeline Slide for ANA-001 Commercial Development PH 2 Data (Early Sept) 1st EUA Request (Sept) EoP2 Meeting (Sept-Oct) 2nd EUA Request (Jun-July) Launch PH 3 Trial (Sept-Oct) PH 3 Data Read (Jun-July) Submit NDA (July-Aug) Fast Trak Approval Fast Track Approval (Dec) Q1 2021 Q2 2021 Q3 2021 Q4 2021 Q1 2022 Q2 2022 Q3 2022 Q4 2022 Start Work Gemcabene (Mar-Apr) Clinical Timeline DMC 24 patients (Mar-Apr) |
| 28 Thank You |