Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PDS Biotechnology Corp | brhc10018746_8k.htm |
Exhibit 99.1

CORPORATE OVERVIEW Frank Bedu-Addo Ph.D. President & CEO JANUARY 2021

2 Forward-Looking Statements This presentation contains forward-looking statements about PDS Biotechnology Corporation (“PDSB”), and
its businesses, business prospects, strategies and plans, including but not limited to statements regarding anticipated pre-clinical and clinical drug development activities and timelines and market opportunities. All statements other than
statements of historical facts included in this presentation are forward-looking statements. The words “anticipates,” “may,” “can,” “plans,” “believes,” “estimates,” “expects,” “projects,” “intends,” “likely,” “will,” “should,” “to be,” and any
similar expressions or other words of similar meaning are intended to identify those assertions as forward-looking statements. These forward-looking statements involve substantial risks and uncertainties that could cause actual results to
differ materially from those anticipated.Factors that may cause actual results to differ materially from such forward-looking statements include those identified under the caption “Risk Factors” in the documents filed with the Securities and
Exchange Commission from time to time, including its Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. You are cautioned not to place undue reliance on these forward-looking statements, which speak
only as of the date of this presentation. Except to the extent required by applicable law or regulation, PDSB undertakes no obligation to update the forward-looking statements included in this presentation to reflect subsequent events or
circumstances.
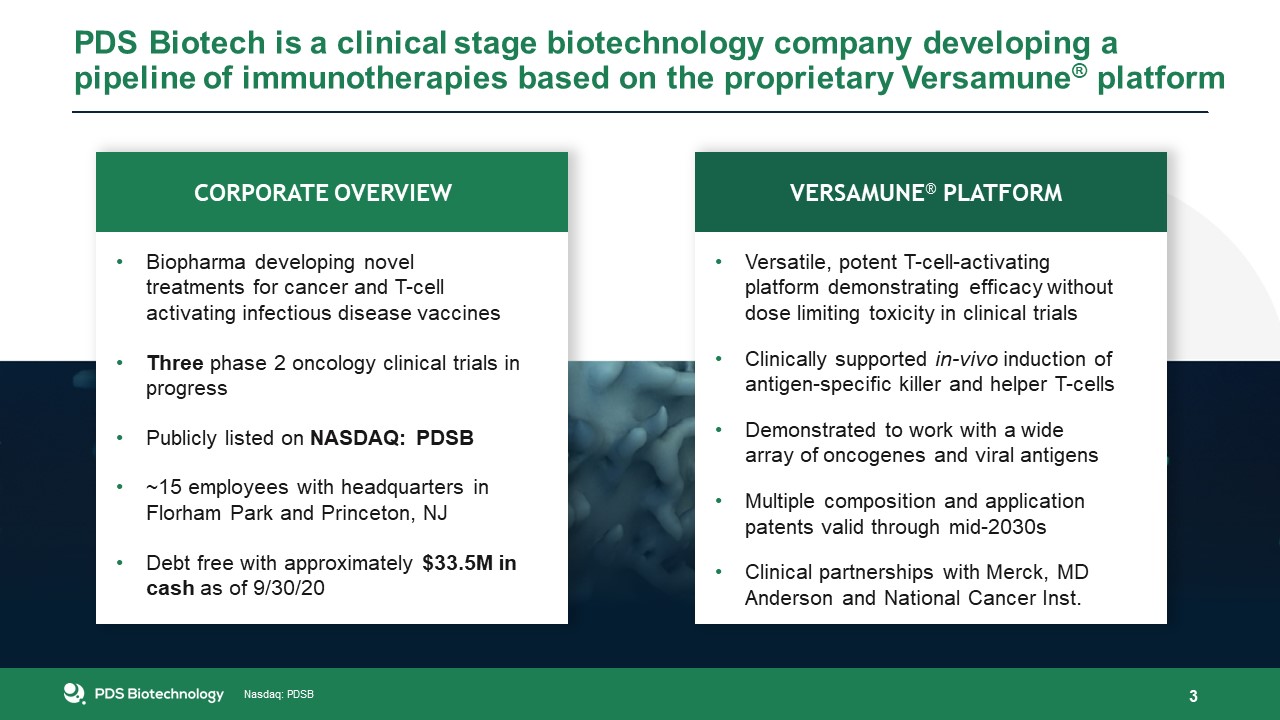
PDS Biotech is a clinical stage biotechnology company developing a pipeline of immunotherapies based on the proprietary Versamune®
platform 3 Versatile, potent T-cell-activating platform demonstrating efficacy without dose limiting toxicity in clinical trialsClinically supported in-vivo induction of antigen-specific killer and helper T-cells Demonstrated to work with a
wide array of oncogenes and viral antigensMultiple composition and application patents valid through mid-2030sClinical partnerships with Merck, MD Anderson and National Cancer Inst. Biopharma developing novel treatments for cancer and T-cell
activating infectious disease vaccinesThree phase 2 oncology clinical trials in progressPublicly listed on NASDAQ: PDSB~15 employees with headquarters in Florham Park and Princeton, NJDebt free with approximately $33.5M in cash as of
9/30/20 Pipeline Versamune® Platform Corporate Overview
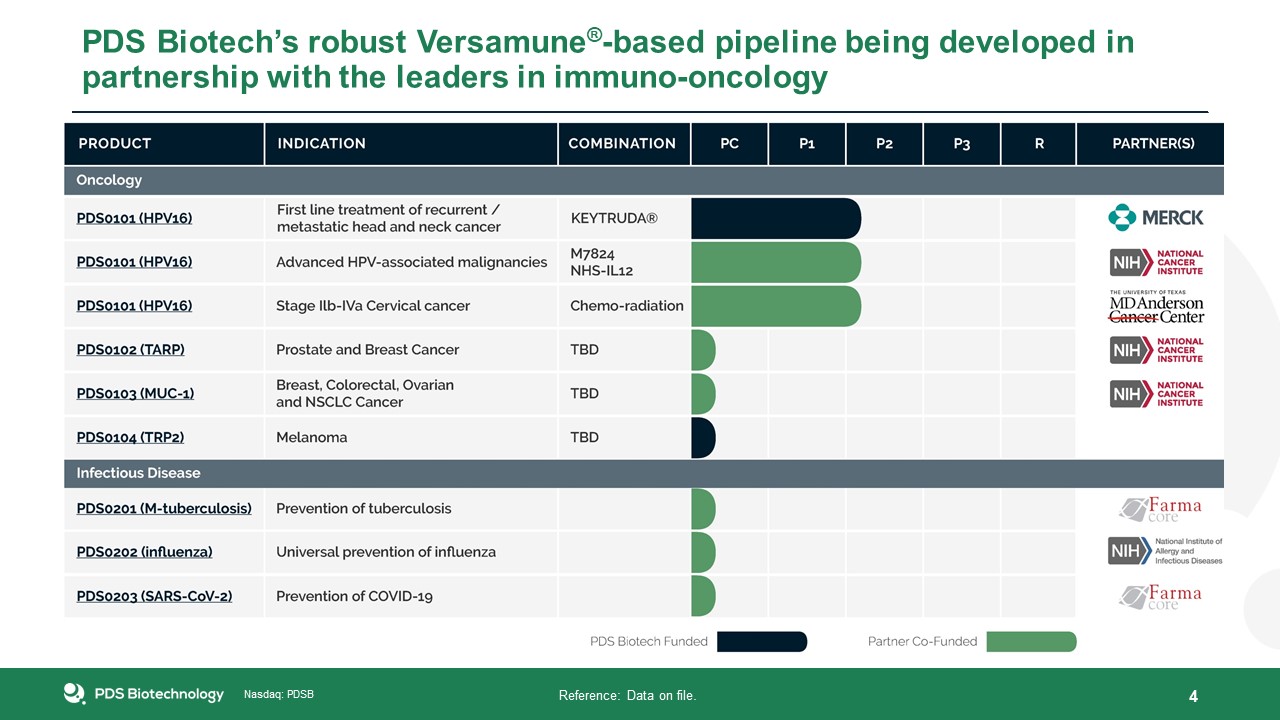
PDS Biotech’s robust Versamune®-based pipeline being developed in partnership with the leaders in immuno-oncology 4 Reference: Data
on file.

PDS Biotech executive team has demonstrated success in the development and commercialization of leading pharmaceutical
products 5 Senior executive experience with management of strategy and execution at both large pharma and biotechsNotable drug development:Abelcet® (Liposome Company/ Elan)PEG-Intron® (Schering-Plough/ Merck) Frank Bedu-Addo, PhDChief
Executive Officer Co-founder>35 years of drug development experience In-depth experience with biotech drug discovery, product development and manufacturing Gregory Conn, PhDChief Scientific Officer >30 years of translational
clinical research experienceFormer Director of Clinical Research at National Cancer Institute Center for Cancer Research (Cancer Vaccine Branch) Lauren V. Wood, MDChief Medical Officer Senior executive experience with over 20 years of
experience in high tech companiesIn-depth experience with M&A transactions, capital markets, business development and investor relations Seth Van Voorhees, PhDChief Financial Officer

Introduction to the Versamune® Platform
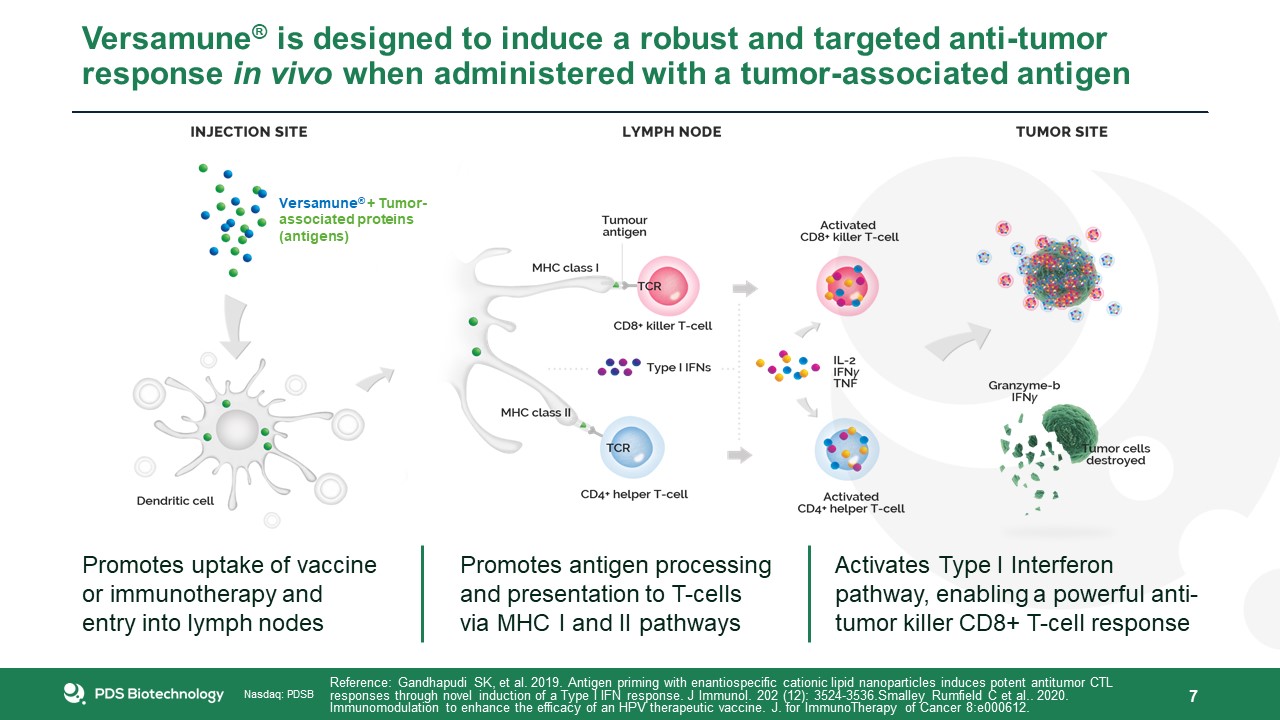
Versamune® is designed to induce a robust and targeted anti-tumor response in vivo when administered with a tumor-associated
antigen 7 Reference: Gandhapudi SK, et al. 2019. Antigen priming with enantiospecific cationic lipid nanoparticles induces potent antitumor CTL responses through novel induction of a Type I IFN response. J Immunol. 202 (12): 3524-3536.Smalley
Rumfield C et al.. 2020. Immunomodulation to enhance the efficacy of an HPV therapeutic vaccine. J. for ImmunoTherapy of Cancer 8:e000612. Promotes uptake of vaccine or immunotherapy and entry into lymph nodes Promotes antigen processing
and presentation to T-cells via MHC I and II pathways Activates Type I Interferon pathway, enabling a powerful anti-tumor killer CD8+ T-cell response Versamune® + Tumor-associated proteins (antigens)
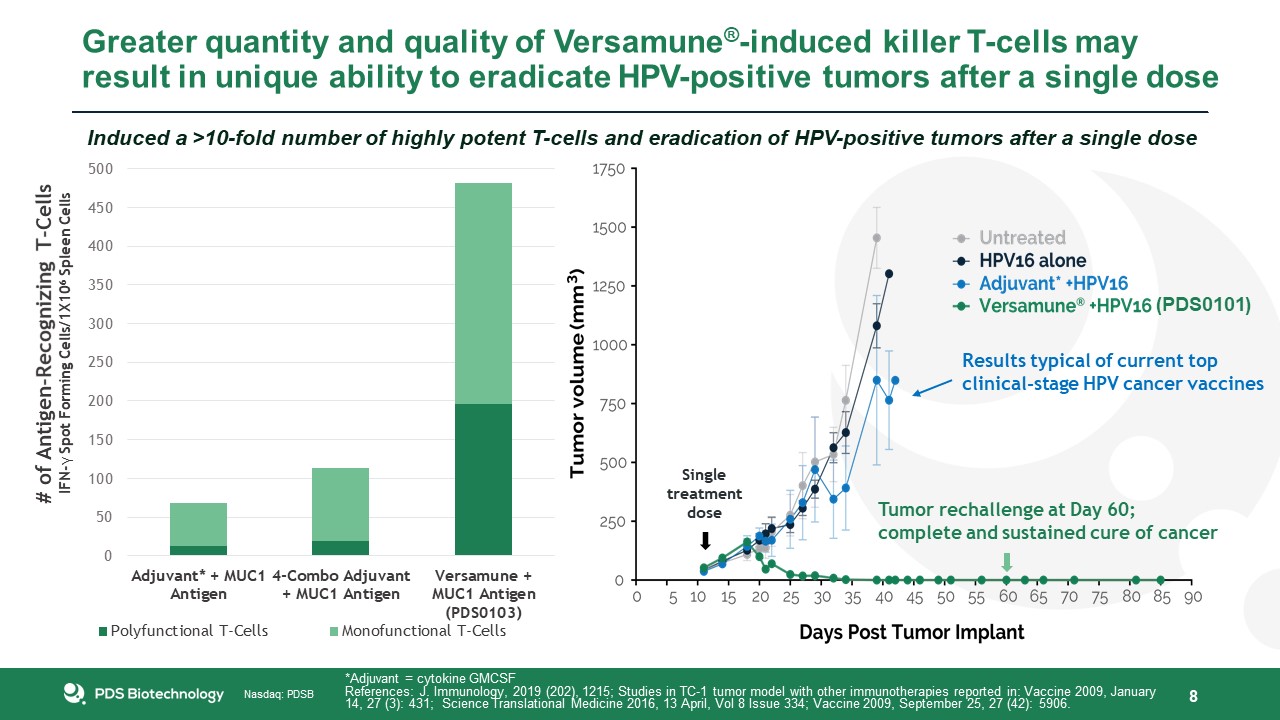
Greater quantity and quality of Versamune®-induced killer T-cells may result in unique ability to eradicate HPV-positive tumors after a
single dose 8 Induced a >10-fold number of highly potent T-cells and eradication of HPV-positive tumors after a single dose Single treatment dose Results typical of current topclinical-stage HPV cancer vaccines Tumor rechallenge at Day
60; complete and sustained cure of cancer *Adjuvant = cytokine GMCSFReferences: J. Immunology, 2019 (202), 1215; Studies in TC-1 tumor model with other immunotherapies reported in: Vaccine 2009, January 14, 27 (3): 431; Science
Translational Medicine 2016, 13 April, Vol 8 Issue 334; Vaccine 2009, September 25, 27 (42): 5906. (PDS0101)
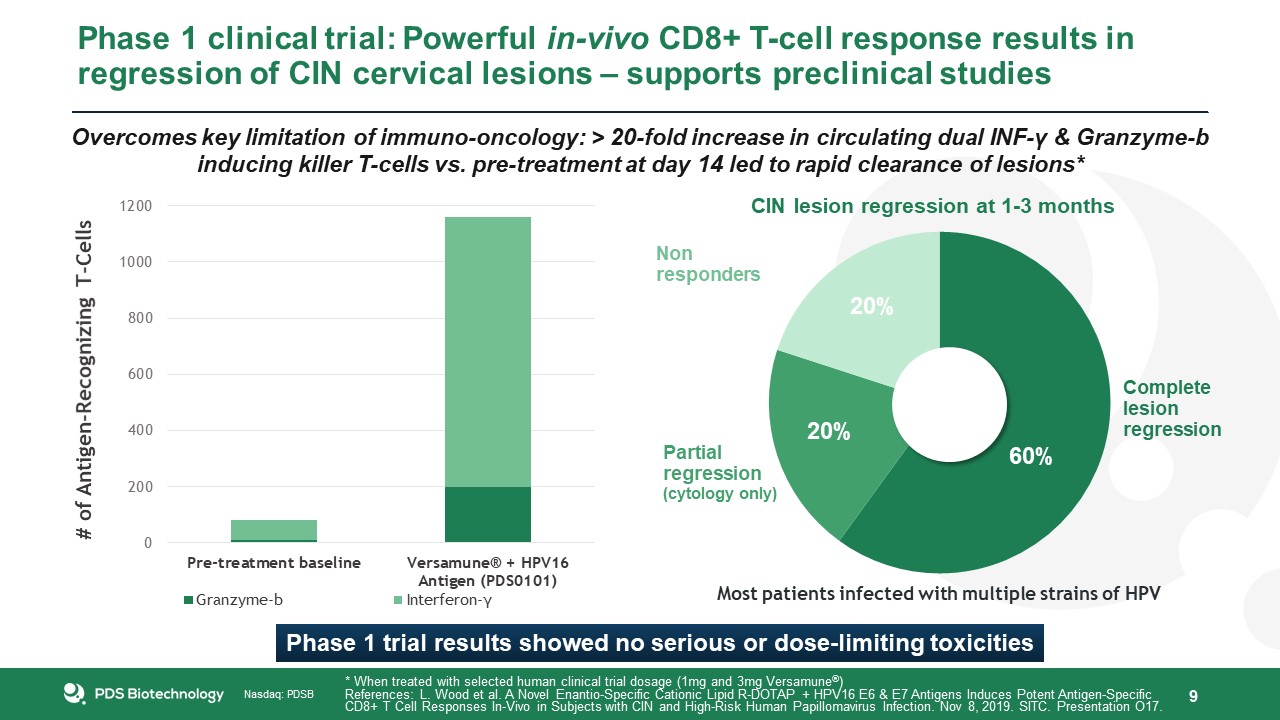
Phase 1 clinical trial: Powerful in-vivo CD8+ T-cell response results in regression of CIN cervical lesions – supports preclinical
studies 9 * When treated with selected human clinical trial dosage (1mg and 3mg Versamune®)References: L. Wood et al. A Novel Enantio-Specific Cationic Lipid R-DOTAP + HPV16 E6 & E7 Antigens Induces Potent Antigen-Specific CD8+ T Cell
Responses In-Vivo in Subjects with CIN and High-Risk Human Papillomavirus Infection. Nov 8, 2019. SITC. Presentation O17. Most patients infected with multiple strains of HPV CIN lesion regression at 1-3 months 60% 20% 20% Phase 1 trial
results showed no serious or dose-limiting toxicities Overcomes key limitation of immuno-oncology: > 20-fold increase in circulating dual INF-γ & Granzyme-b inducing killer T-cells vs. pre-treatment at day 14 led to rapid clearance of
lesions*
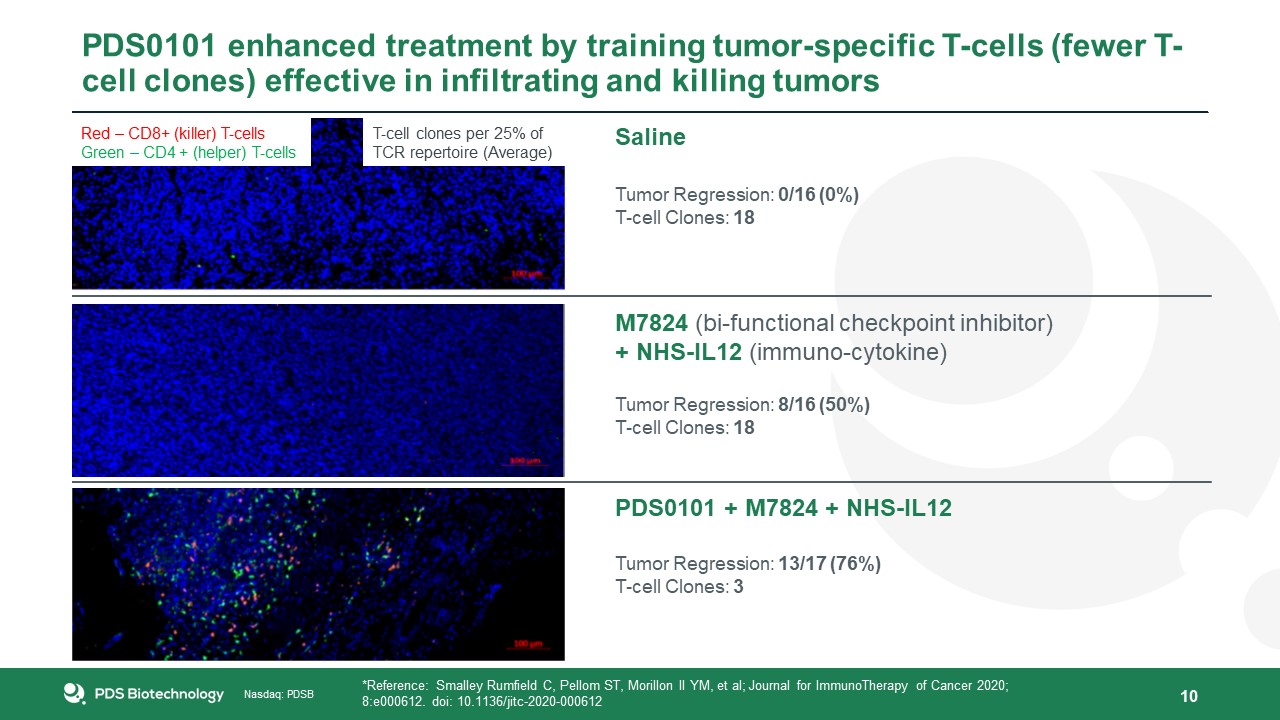
10 Saline Tumor Regression: 0/16 (0%)T-cell Clones: 18 M7824 (bi-functional checkpoint inhibitor)+ NHS-IL12 (immuno-cytokine) Tumor
Regression: 8/16 (50%)T-cell Clones: 18 PDS0101 + M7824 + NHS-IL12 Tumor Regression: 13/17 (76%)T-cell Clones: 3 *Reference: Smalley Rumfield C, Pellom ST, Morillon II YM, et al; Journal for ImmunoTherapy of Cancer 2020; 8:e000612. doi:
10.1136/jitc-2020-000612 PDS0101 enhanced treatment by training tumor-specific T-cells (fewer T-cell clones) effective in infiltrating and killing tumors Red – CD8+ (killer) T-cellsGreen – CD4 + (helper) T-cells T-cell clones per 25% of TCR
repertoire (Average)
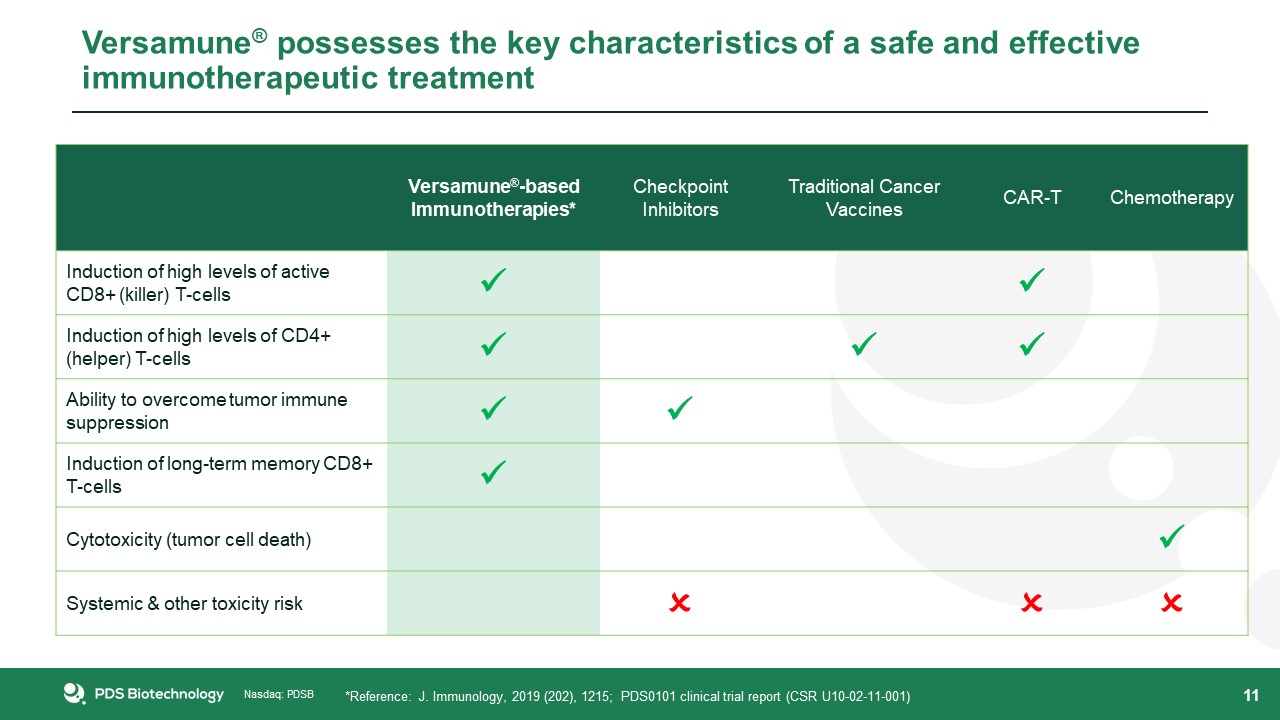
11 Versamune®-basedImmunotherapies* Checkpoint Inhibitors Traditional Cancer Vaccines CAR-T Chemotherapy Induction of high
levels of active CD8+ (killer) T-cells Induction of high levels of CD4+ (helper) T-cells Ability to overcome tumor immune suppression Induction of long-term memory CD8+ T-cells Cytotoxicity
(tumor cell death) Systemic & other toxicity risk Versamune® possesses the key characteristics of a safe and effective immunotherapeutic treatment *Reference: J. Immunology, 2019 (202), 1215; PDS0101 clinical
trial report (CSR U10-02-11-001)
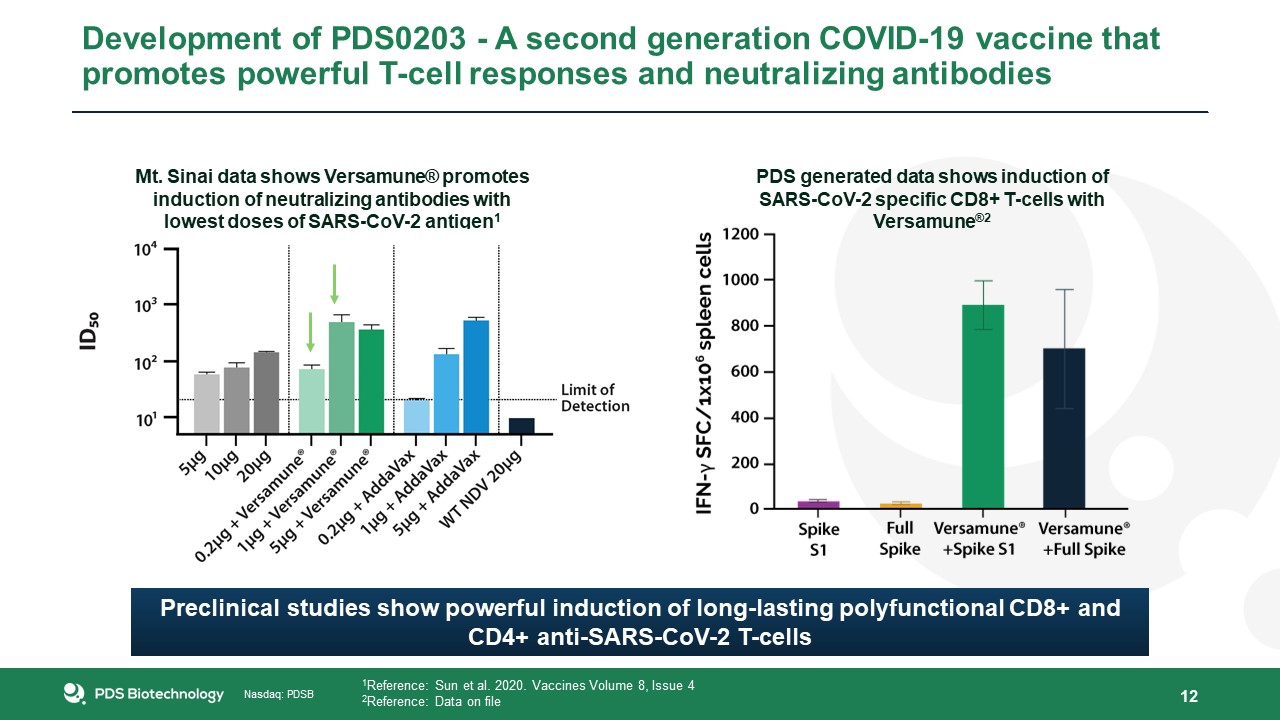
Mt. Sinai data shows Versamune® promotes induction of neutralizing antibodies with lowest doses of SARS-CoV-2 antigen1 12 1Reference:
Sun et al. 2020. Vaccines Volume 8, Issue 42Reference: Data on file PDS generated data shows induction of SARS-CoV-2 specific CD8+ T-cells with Versamune®2 Preclinical studies show powerful induction of long-lasting polyfunctional CD8+ and
CD4+ anti-SARS-CoV-2 T-cells Development of PDS0203 - A second generation COVID-19 vaccine that promotes powerful T-cell responses and neutralizing antibodies
13 Versamune® has demonstrated immunological compatibility with a wide array of tumor and pathogenic antigens Reference: Clin Cancer
Res. 2009 Sep 1;15(17):5323-37. doi: 10.1158/1078-0432.CCR-09-0737 Today, 4 tumor antigens are being utilized with the Versamune® platform, more than 75 tumor antigens have been identifiedWe are currently progressing two Versamune®-based
infectious disease vaccines, one for SARS-COVID-19, and one for universal influenzaVersamune®’s unique flexibility means it may work well with a wide range of identified tumor and pathogenic antigensPotential to continuously expand development
of Versamune®-based products through partnerships and licensing

PDS0101 Phase 2 Clinical Development
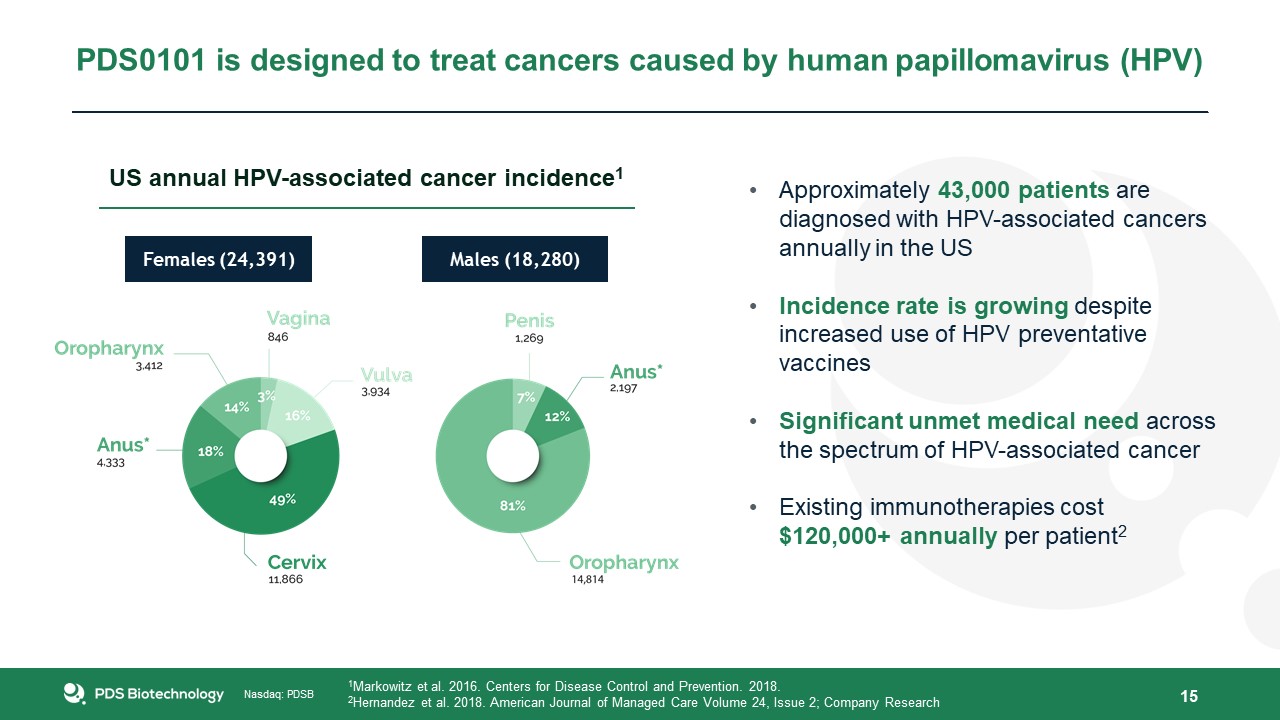
15 PDS0101 is designed to treat cancers caused by human papillomavirus (HPV) Approximately 43,000 patients are diagnosed with
HPV-associated cancers annually in the USIncidence rate is growing despite increased use of HPV preventative vaccinesSignificant unmet medical need across the spectrum of HPV-associated cancerExisting immunotherapies cost $120,000+ annually per
patient2 1Markowitz et al. 2016. Centers for Disease Control and Prevention. 2018.2Hernandez et al. 2018. American Journal of Managed Care Volume 24, Issue 2; Company Research Females (24,391) Males (18,280) US annual HPV-associated cancer
incidence1
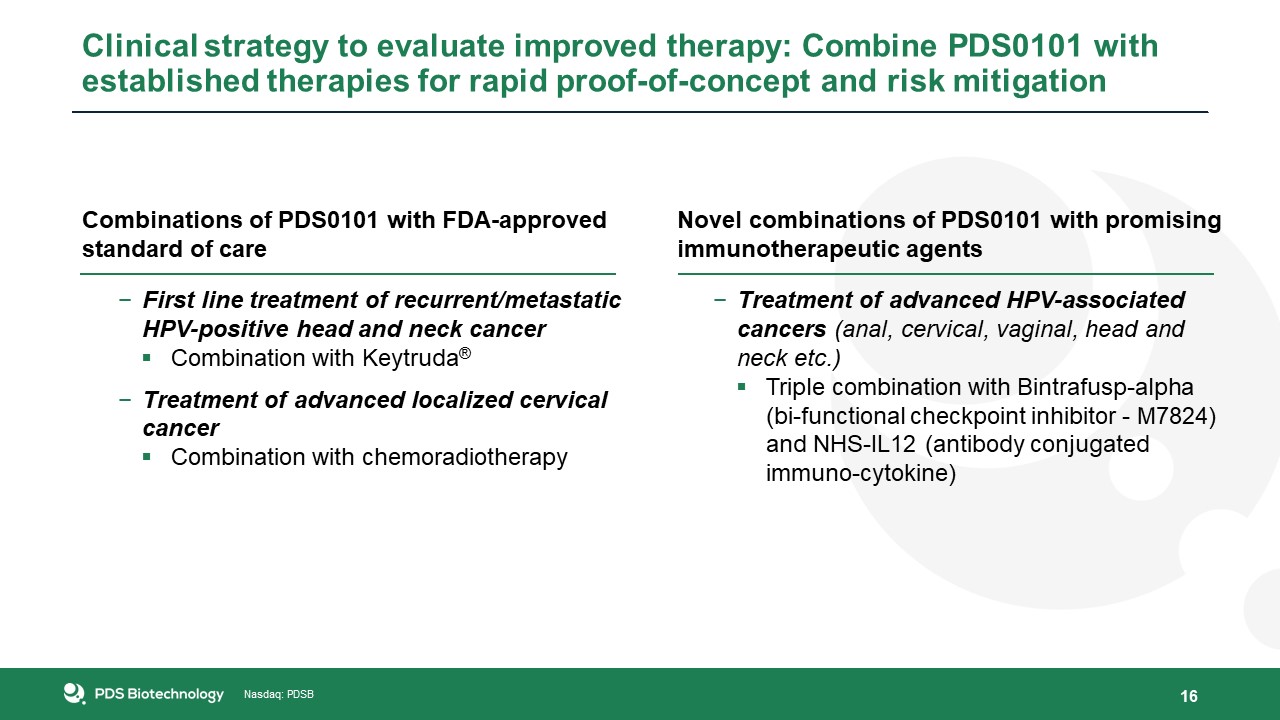
Combinations of PDS0101 with FDA-approved standard of careFirst line treatment of recurrent/metastatic HPV-positive head and neck
cancerCombination with Keytruda®Treatment of advanced localized cervical cancerCombination with chemoradiotherapy 16 Novel combinations of PDS0101 with promising immunotherapeutic agentsTreatment of advanced HPV-associated cancers (anal,
cervical, vaginal, head and neck etc.)Triple combination with Bintrafusp-alpha (bi-functional checkpoint inhibitor - M7824) and NHS-IL12 (antibody conjugated immuno-cytokine) Clinical strategy to evaluate improved therapy: Combine PDS0101 with
established therapies for rapid proof-of-concept and risk mitigation
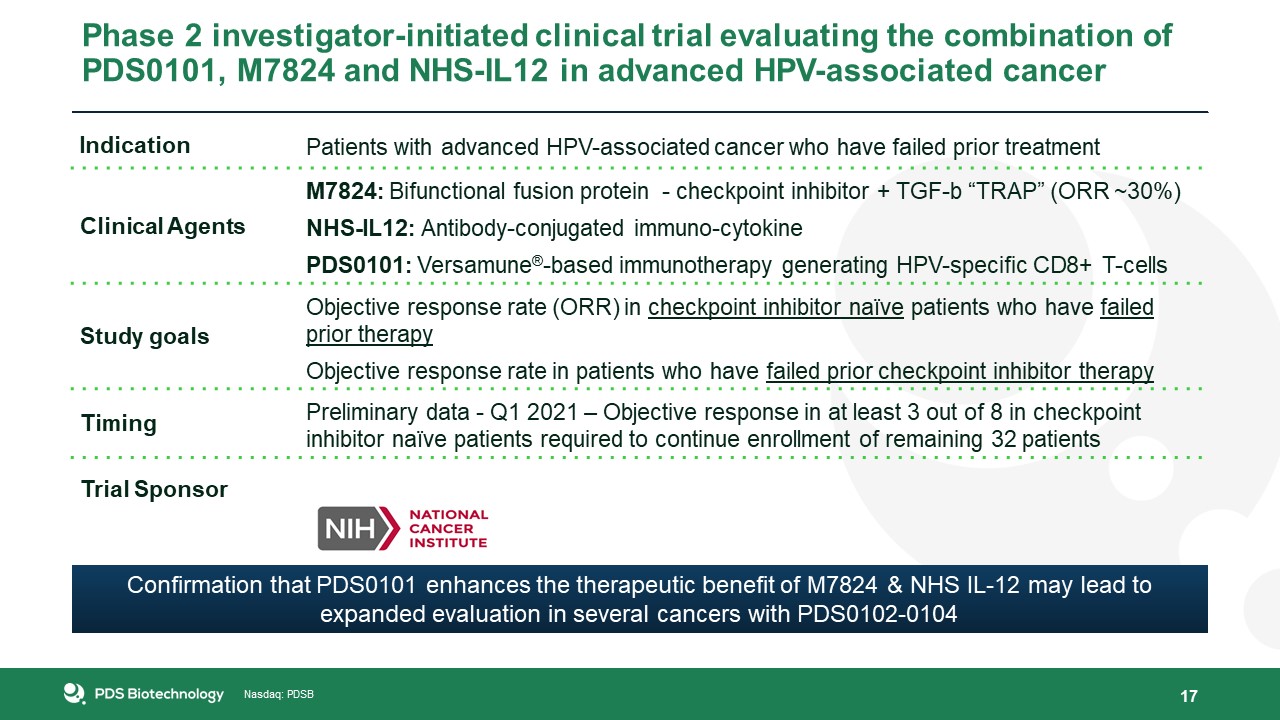
Phase 2 investigator-initiated clinical trial evaluating the combination of PDS0101, M7824 and NHS-IL12 in advanced HPV-associated
cancer 17 Indication Patients with advanced HPV-associated cancer who have failed prior treatment Clinical Agents M7824: Bifunctional fusion protein - checkpoint inhibitor + TGF-b “TRAP” (ORR ~30%)NHS-IL12: Antibody-conjugated
immuno-cytokinePDS0101: Versamune®-based immunotherapy generating HPV-specific CD8+ T-cells Study goals Objective response rate (ORR) in checkpoint inhibitor naïve patients who have failed prior therapyObjective response rate in patients who
have failed prior checkpoint inhibitor therapy Timing Preliminary data - Q1 2021 – Objective response in at least 3 out of 8 in checkpoint inhibitor naïve patients required to continue enrollment of remaining 32 patients Trial
Sponsor Confirmation that PDS0101 enhances the therapeutic benefit of M7824 & NHS IL-12 may lead to expanded evaluation in several cancers with PDS0102-0104
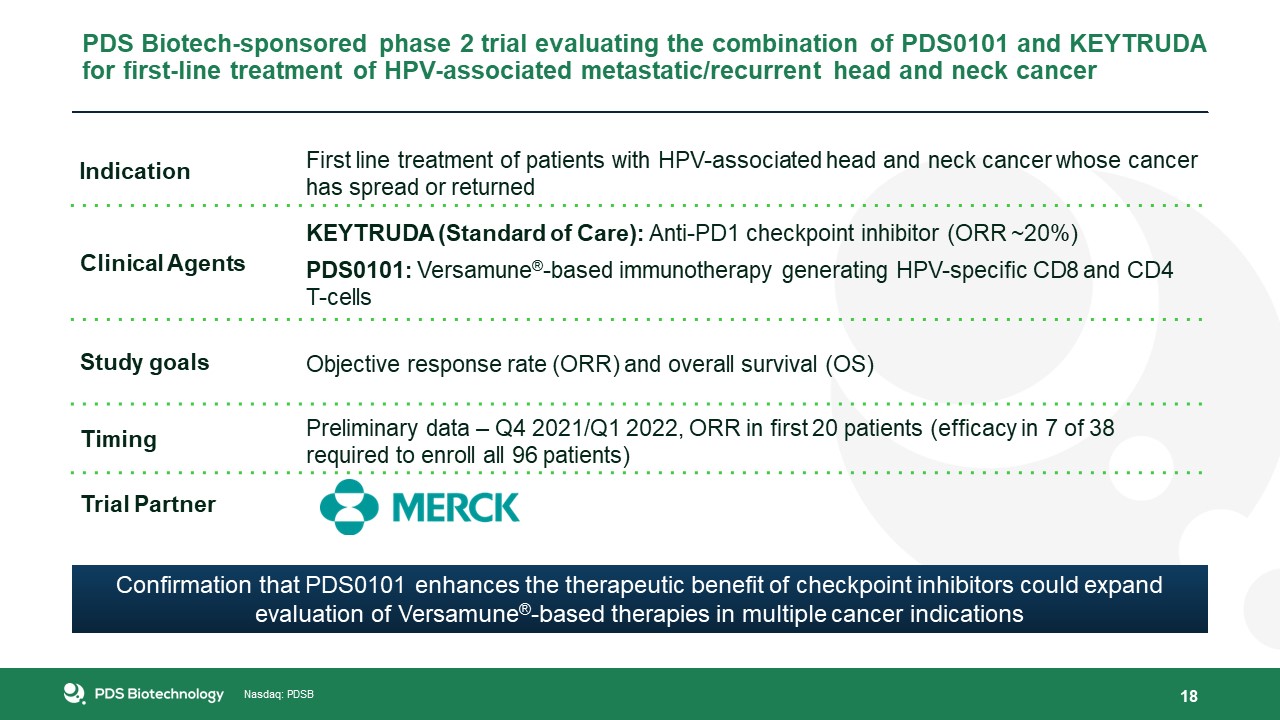
18 PDS Biotech-sponsored phase 2 trial evaluating the combination of PDS0101 and KEYTRUDA for first-line treatment of HPV-associated
metastatic/recurrent head and neck cancer Indication First line treatment of patients with HPV-associated head and neck cancer whose cancer has spread or returned Clinical Agents KEYTRUDA (Standard of Care): Anti-PD1 checkpoint inhibitor
(ORR ~20%)PDS0101: Versamune®-based immunotherapy generating HPV-specific CD8 and CD4 T-cells Study goals Objective response rate (ORR) and overall survival (OS) Timing Preliminary data – Q4 2021/Q1 2022, ORR in first 20 patients (efficacy
in 7 of 38 required to enroll all 96 patients) Trial Partner Confirmation that PDS0101 enhances the therapeutic benefit of checkpoint inhibitors could expand evaluation of Versamune®-based therapies in multiple cancer indications
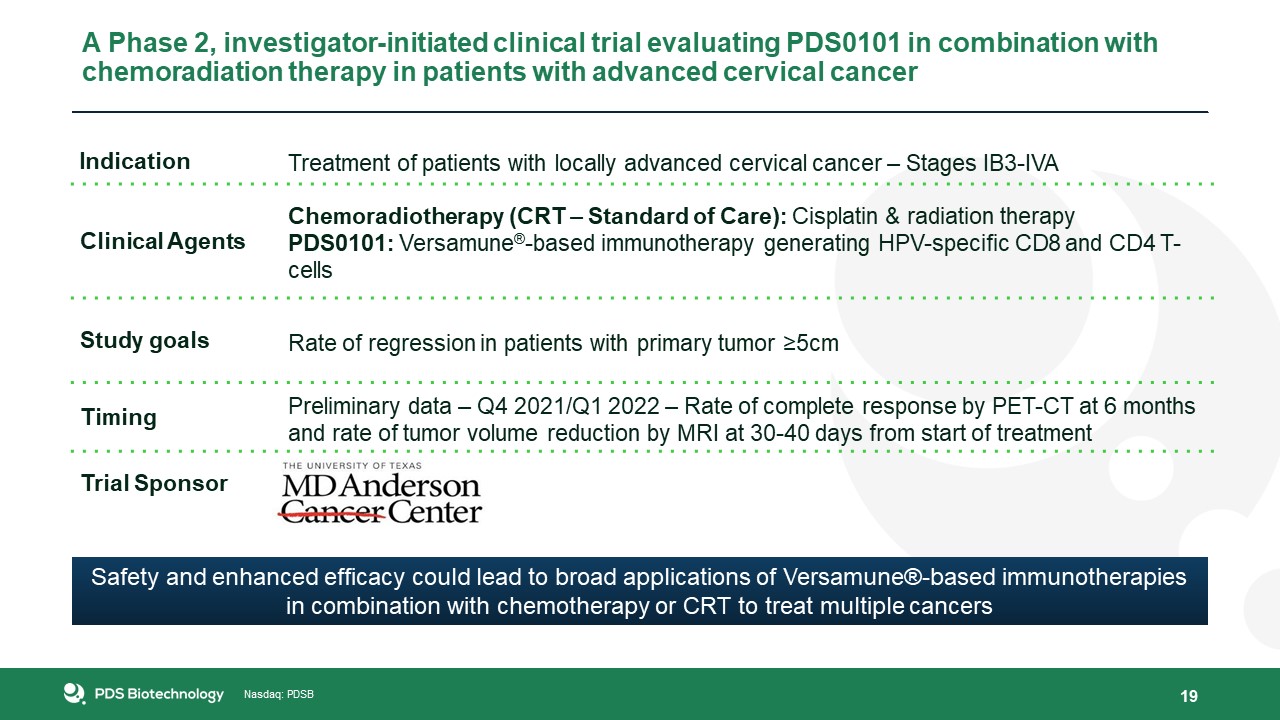
19 A Phase 2, investigator-initiated clinical trial evaluating PDS0101 in combination with chemoradiation therapy in patients with
advanced cervical cancer Indication Treatment of patients with locally advanced cervical cancer – Stages IB3-IVA Clinical Agents Chemoradiotherapy (CRT – Standard of Care): Cisplatin & radiation therapyPDS0101: Versamune®-based
immunotherapy generating HPV-specific CD8 and CD4 T-cells Study goals Rate of regression in patients with primary tumor ≥5cm Timing Preliminary data – Q4 2021/Q1 2022 – Rate of complete response by PET-CT at 6 months and rate of tumor
volume reduction by MRI at 30-40 days from start of treatment Trial Sponsor Safety and enhanced efficacy could lead to broad applications of Versamune®-based immunotherapies in combination with chemotherapy or CRT to treat multiple cancers
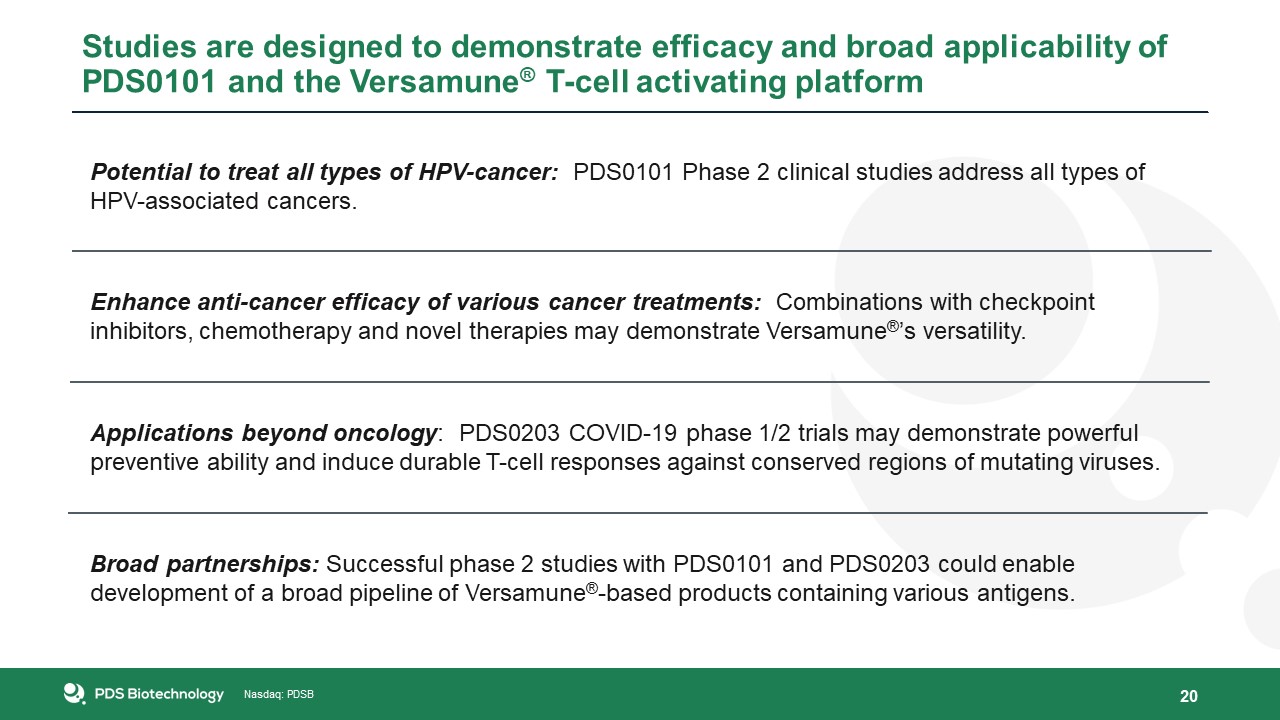
Studies are designed to demonstrate efficacy and broad applicability of PDS0101 and the Versamune® T-cell activating
platform 20 Enhance anti-cancer efficacy of various cancer treatments: Combinations with checkpoint inhibitors, chemotherapy and novel therapies may demonstrate Versamune®’s versatility. Broad partnerships: Successful phase 2 studies with
PDS0101 and PDS0203 could enable development of a broad pipeline of Versamune®-based products containing various antigens. Potential to treat all types of HPV-cancer: PDS0101 Phase 2 clinical studies address all types of HPV-associated
cancers. Applications beyond oncology: PDS0203 COVID-19 phase 1/2 trials may demonstrate powerful preventive ability and induce durable T-cell responses against conserved regions of mutating viruses.

Looking Forward
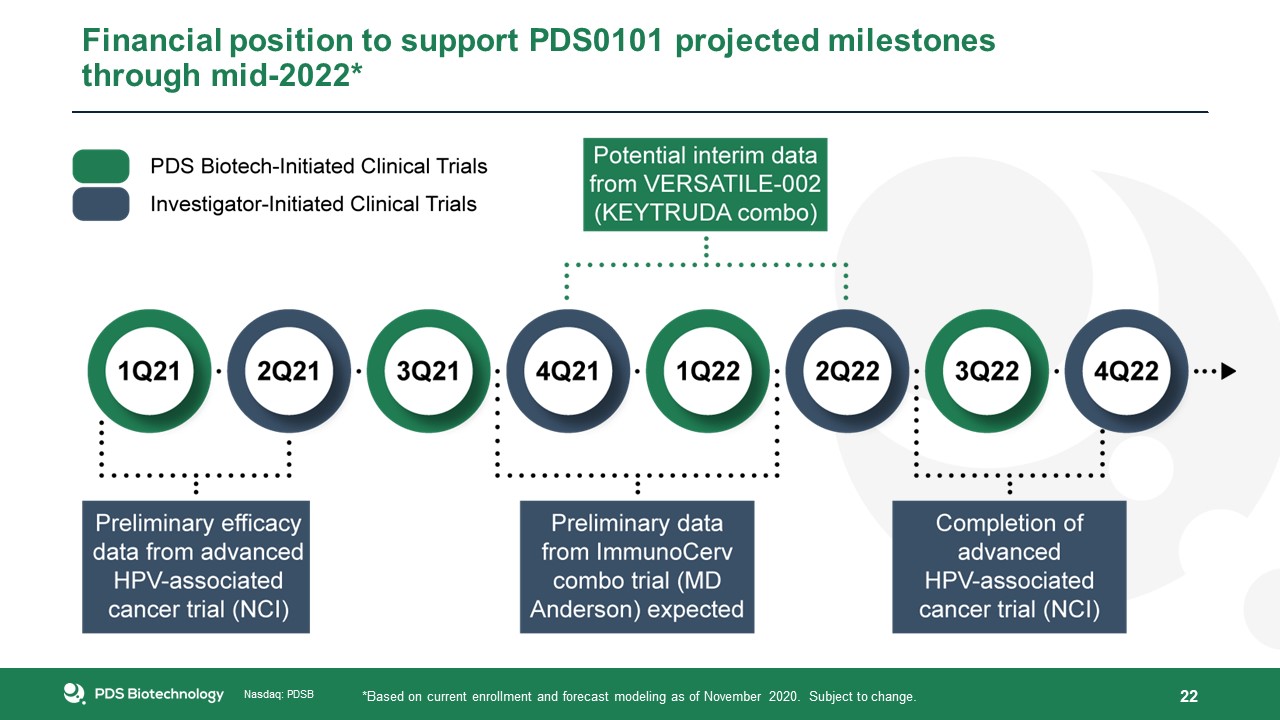
Financial position to support PDS0101 projected milestones through mid-2022* 22 *Based on current enrollment and forecast modeling as
of November 2020. Subject to change.
Positioned for accelerated development 23 Enhanced anti-cancer efficacy: Early clinical data and preclinical data suggest potentially
superior efficacy, safety and versatility of the platformNear-term milestone: PDS0101 preliminary data Q1-Q2 2021Validation of approach: All three on-going phase 2 clinical trials supported and partnered with leading and top-tier institutions
in the field of cancer and immuno-oncologyCommercialization path: Clinical studies evaluating the potential to safely enhance the clinical efficacy of FDA-approved anti-cancer products presents a potentially rapid path to commercializationRapid
adoption strategy: Evaluation of PDS0101 in combination with standard of care in multiple HPV-associated cancers Pipeline Key Advantages and Differentiators

