Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Aldeyra Therapeutics, Inc. | d109230dex992.htm |
| 8-K - 8-K - Aldeyra Therapeutics, Inc. | d109230d8k.htm |

Initial Symptom and Sign Results From Run-In Cohort of Phase 3 TRANQUILITY Trial in Dry Eye Disease DATA RELEASE January 7, 2021 Nasdaq: ALDX © Aldeyra Therapeutics, Inc. 2021 Exhibit 99.1

Disclaimers and Forward-Looking Statements This presentation and various remarks which may be made during this presentation contain forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and Section 21E of the Securities Exchange Act of 1934, as amended, including statements regarding Aldeyra’s possible or assumed future results of operations, expenses and financing needs, business strategies and plans, research and development plans or expectations, political, economic, legal, social and health risks, including the recent COVID-19 outbreak and subsequent public health measures and other responses to it, that may affect Aldeyra’s business or the global economy, the structure, timing and success of Aldeyra’s planned or pending clinical trials, expected milestones, market sizing, pricing and reimbursement, competitive position, regulatory matters, industry environment and potential growth opportunities, among other things. The results of earlier preclinical or clinical trials may not be predictive of future results. As a result of the COVID-19 pandemic, clinical site availability, staffing, and patient recruitment have been negatively affected and the timelines to complete Aldeyra’s clinical trials may be delayed. Forward-looking statements include all statements that are not historical facts and, in some cases, can be identified by terms such as “may,” “might,” “will,” “objective,” “intend,” “should,” “could,” “can,” “would,” “expect,” “believe,” “anticipate,” “project,” “target,” “design,” “estimate,” “predict,” “potential,” “plan” or similar expressions and the negatives of those terms. Forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause Aldeyra’s actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. These statements reflect Aldeyra’s current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including the development, clinical and regulatory plans or expectations for Aldeyra’s product candidates and systems-based approaches, later developments with the FDA that may be inconsistent with Aldeyra’s expectations and beliefs, including the risk that the results from smaller clinical trials or portions of clinical trials may not accurately predict results of larger scale trials or the remainder of a clinical trial, inconsistent expectations regarding FDA acceptance and review of the company’s filings and submitted data sets, and Aldeyra’s continuing review and quality control analysis of clinical data. Important factors that could cause actual results to differ materially from those reflected in Aldeyra's forward-looking statements are described in Aldeyra’s most recent Annual Report on Form 10-K and Quarterly Report on Form 10-Q, as well as Aldeyra’s subsequent filings with the Securities and Exchange Commission. All of Aldeyra's development plans and timelines may be subject to adjustment depending on funding, recruitment rate, regulatory review, preclinical and clinical results, and other factors any of which could result in changes to Aldeyra’s development plans and programs or delay the initiation, completion, or reporting of clinical trials. In addition to the risks described above and in Aldeyra's other filings with the SEC, other unknown or unpredictable factors also could affect Aldeyra's results. No forward-looking statements can be guaranteed and actual results may differ materially from such statements. The information in this presentation is provided only as of January 7, 2021, and Aldeyra undertakes no obligation to update any forward-looking statements contained in this presentation on account of new information, future events, or otherwise, except as required by law.
Initial Results From Run-In Cohort of Phase 3 TRANQUILITY Trial Support First-Line Potential of Reproxalap in Dry Eye Disease Statistical significance achieved for both sign and symptoms in dry eye chamber:* Reproxalap demonstrated statistically significant improvement over vehicle in ocular redness (p=0.03), an FDA-approvable objective sign. Reproxalap demonstrated statistically significant improvement over vehicle for the two assessed clinical symptoms: VAS Ocular Dryness (p=0.001) and Ocular Discomfort Score (p<0.0001). Acute improvements in ocular symptoms and objective sign within minutes of reproxalap administration in a dry eye chamber. 24-hour environmental results supportive of reproxalap’s rapid and broad efficacy, with consistent directional improvements over vehicle across symptoms and Schirmer’s test, and statistical significance vs. vehicle achieved in four of eight assessed outcome measurements. The main cohort of TRANQUILITY is expected to begin enrollment in February 2021, following completion of tear RASP analysis from the run-in cohort and confirmation of endpoints and patient numbers. *Based on Mixed Model Repeated Measures (MMRM p values shown above). Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Mixed effect model of repeated measures (MMRM) for change from baseline (Time 0). Source: TRANQUILITY Run-In Cohort initial results VAS = Visual Analog Scale RASP = Reactive Aldehyde Species
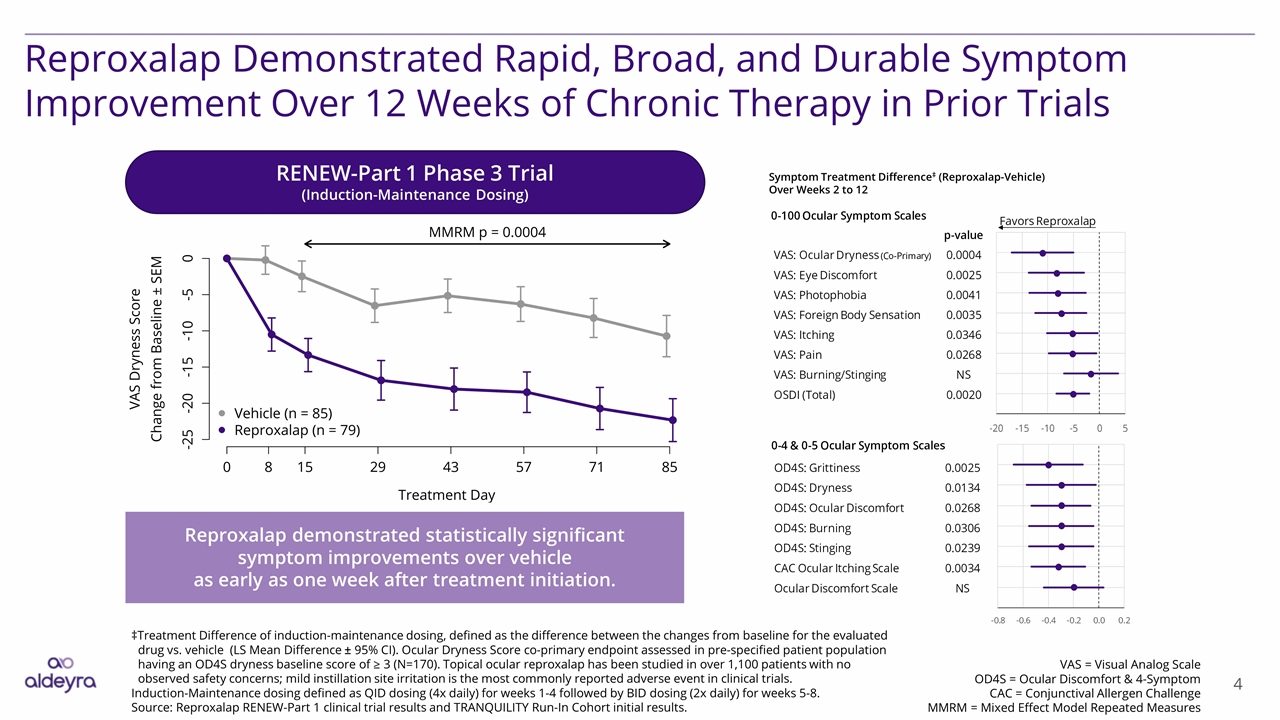
4 Reproxalap Demonstrated Rapid, Broad, and Durable Symptom Improvement Over 12 Weeks of Chronic Therapy in Prior Trials VAS = Visual Analog Scale OD4S = Ocular Discomfort & 4-Symptom CAC = Conjunctival Allergen Challenge MMRM = Mixed Effect Model Repeated Measures ‡Treatment Difference of induction-maintenance dosing, defined as the difference between the changes from baseline for the evaluated drug vs. vehicle (LS Mean Difference ± 95% CI). Ocular Dryness Score co-primary endpoint assessed in pre-specified patient population having an OD4S dryness baseline score of ≥ 3 (N=170). Topical ocular reproxalap has been studied in over 1,100 patients with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Induction-Maintenance dosing defined as QID dosing (4x daily) for weeks 1-4 followed by BID dosing (2x daily) for weeks 5-8. Source: Reproxalap RENEW-Part 1 clinical trial results and TRANQUILITY Run-In Cohort initial results. RENEW-Part 1 Phase 3 Trial (Induction-Maintenance Dosing) Reproxalap demonstrated statistically significant symptom improvements over vehicle as early as one week after treatment initiation. Symptom Treatment Difference‡ (Reproxalap-Vehicle) Over Weeks 2 to 12
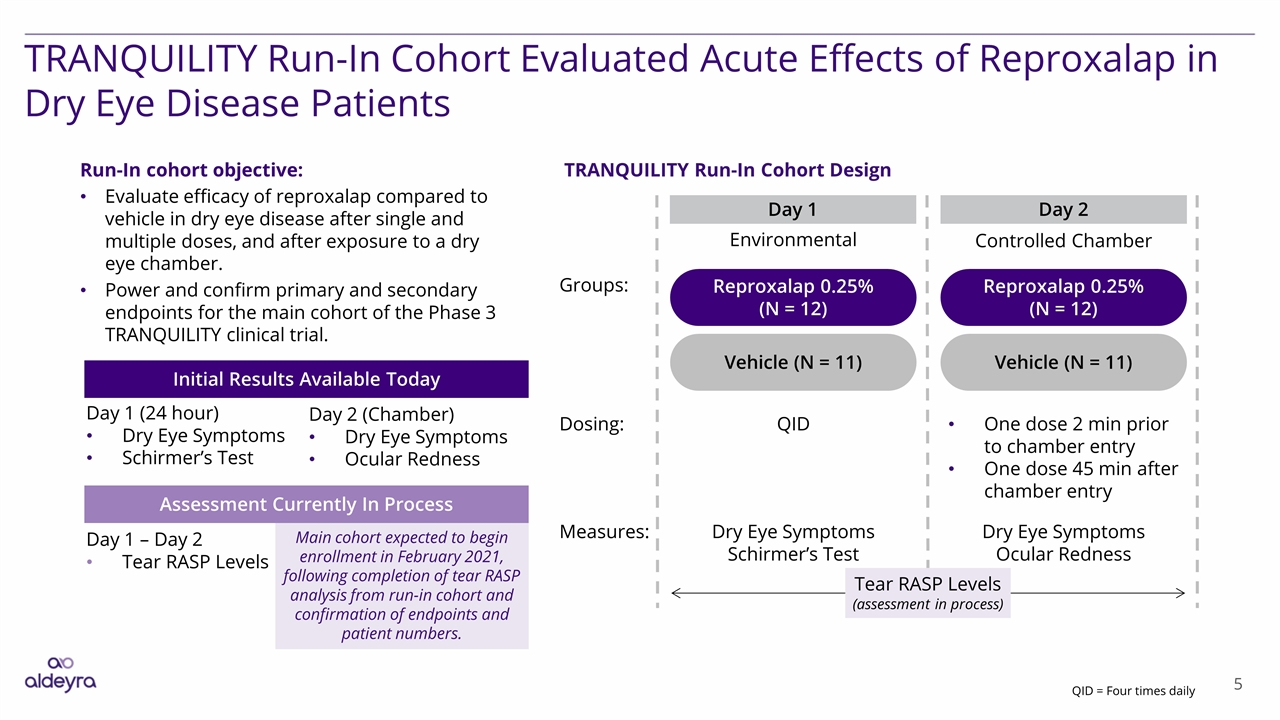
TRANQUILITY Run-In Cohort Evaluated Acute Effects of Reproxalap in Dry Eye Disease Patients Reproxalap 0.25% (N = 12) Vehicle (N = 11) Day 2 Day 1 Dosing: QID One dose 2 min prior to chamber entry One dose 45 min after chamber entry Reproxalap 0.25% (N = 12) Vehicle (N = 11) Groups: Environmental Controlled Chamber Measures: Dry Eye Symptoms Schirmer’s Test Dry Eye Symptoms Ocular Redness Initial Results Available Today Assessment Currently In Process Day 1 (24 hour) Dry Eye Symptoms Schirmer’s Test Day 1 – Day 2 Tear RASP Levels TRANQUILITY Run-In Cohort Design Run-In cohort objective: Evaluate efficacy of reproxalap compared to vehicle in dry eye disease after single and multiple doses, and after exposure to a dry eye chamber. Power and confirm primary and secondary endpoints for the main cohort of the Phase 3 TRANQUILITY clinical trial. Day 2 (Chamber) Dry Eye Symptoms Ocular Redness Main cohort expected to begin enrollment in February 2021, following completion of tear RASP analysis from run-in cohort and confirmation of endpoints and patient numbers. Tear RASP Levels (assessment in process) QID = Four times daily
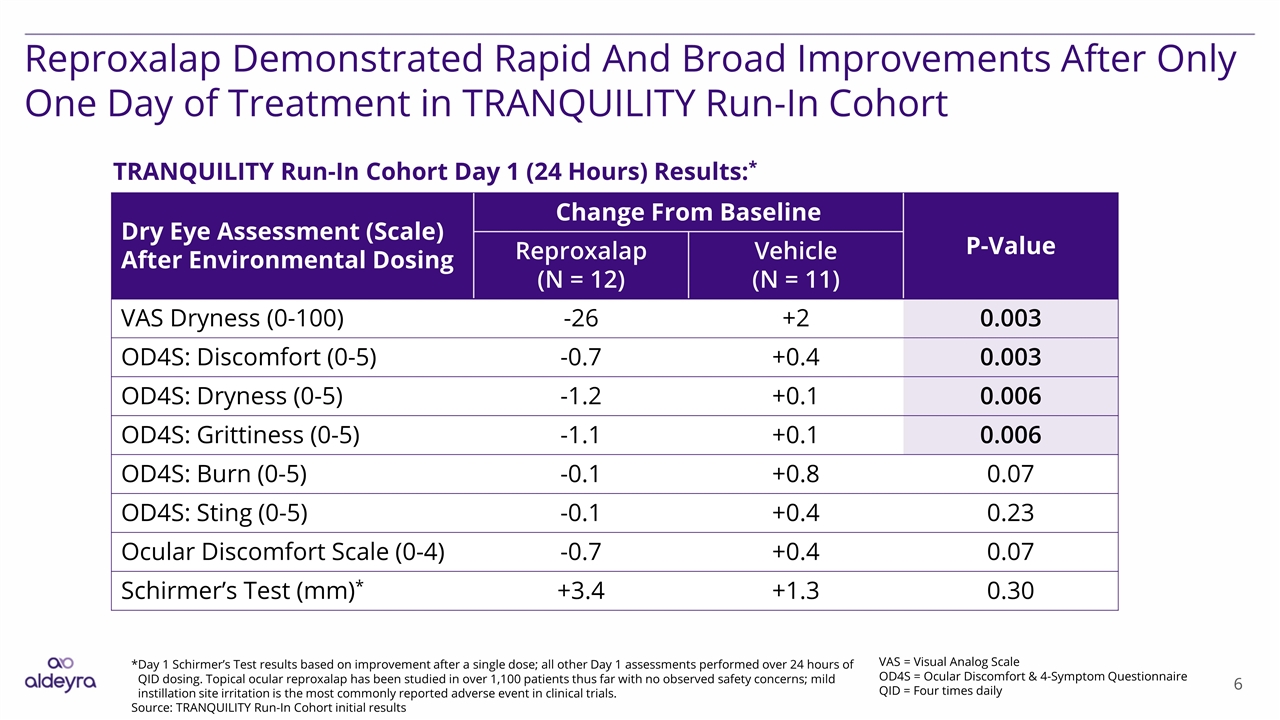
Reproxalap Demonstrated Rapid And Broad Improvements After Only One Day of Treatment in TRANQUILITY Run-In Cohort Dry Eye Assessment (Scale) After Environmental Dosing Change From Baseline P-Value Reproxalap (N = 12) Vehicle (N = 11) VAS Dryness (0-100) -26 +2 0.003 OD4S: Discomfort (0-5) -0.7 +0.4 0.003 OD4S: Dryness (0-5) -1.2 +0.1 0.006 OD4S: Grittiness (0-5) -1.1 +0.1 0.006 OD4S: Burn (0-5) -0.1 +0.8 0.07 OD4S: Sting (0-5) -0.1 +0.4 0.23 Ocular Discomfort Scale (0-4) -0.7 +0.4 0.07 Schirmer’s Test (mm)* +3.4 +1.3 0.30 VAS = Visual Analog Scale OD4S = Ocular Discomfort & 4-Symptom Questionnaire QID = Four times daily *Day 1 Schirmer’s Test results based on improvement after a single dose; all other Day 1 assessments performed over 24 hours of QID dosing. Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Source: TRANQUILITY Run-In Cohort initial results TRANQUILITY Run-In Cohort Day 1 (24 Hours) Results:*

The Dry Eye Chamber: A Demanding Real-World Drug Assessment of Dry Eye Disease Challenge-model trials utilizing a controlled chamber are an FDA accepted design for pivotal endpoints.* Dry eye chambers control relative humidity, temperature, airflow, and visual tasking in order to stress the ocular surface. Chambers simulate a “bad day” scenario in the life of a dry eye disease sufferer. Trial designs utilizing chambers are able to confirm the utility of drugs with rapid onset of action during an acute ocular surface challenge. *FDA published “Dry Eye: Developing Drugs for Treatment” draft guidance; December 2020.
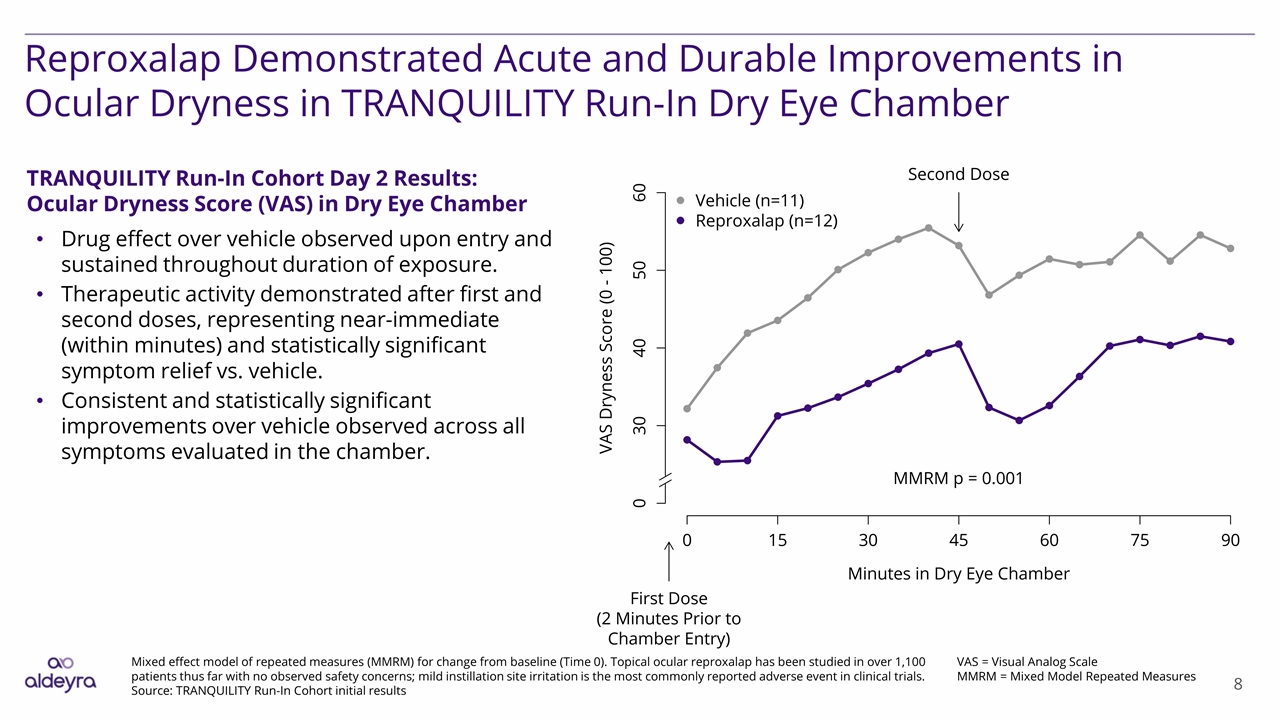
Reproxalap Demonstrated Acute and Durable Improvements in Ocular Dryness in TRANQUILITY Run-In Dry Eye Chamber VAS = Visual Analog Scale MMRM = Mixed Model Repeated Measures Mixed effect model of repeated measures (MMRM) for change from baseline (Time 0). Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Source: TRANQUILITY Run-In Cohort initial results TRANQUILITY Run-In Cohort Day 2 Results: Ocular Dryness Score (VAS) in Dry Eye Chamber Drug effect over vehicle observed upon entry and sustained throughout duration of exposure. Therapeutic activity demonstrated after first and second doses, representing near-immediate (within minutes) and statistically significant symptom relief vs. vehicle. Consistent and statistically significant improvements over vehicle observed across all symptoms evaluated in the chamber.
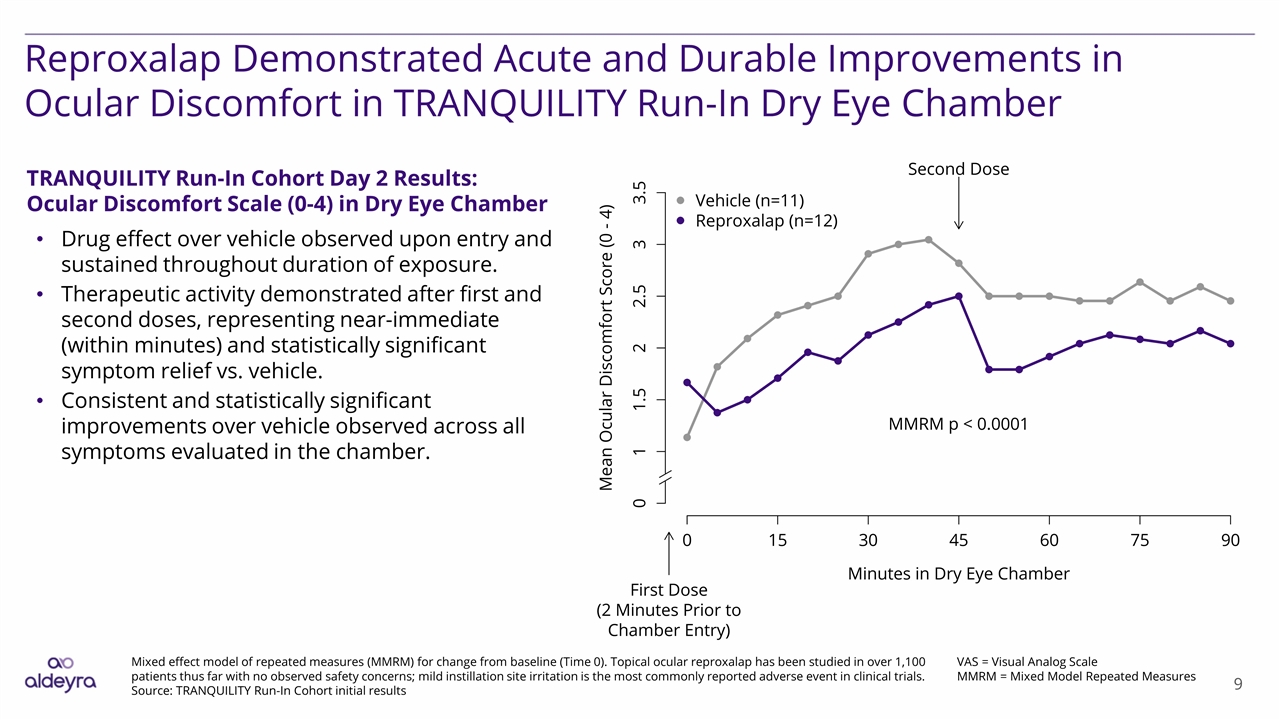
Reproxalap Demonstrated Acute and Durable Improvements in Ocular Discomfort in TRANQUILITY Run-In Dry Eye Chamber VAS = Visual Analog Scale MMRM = Mixed Model Repeated Measures Mixed effect model of repeated measures (MMRM) for change from baseline (Time 0). Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Source: TRANQUILITY Run-In Cohort initial results TRANQUILITY Run-In Cohort Day 2 Results: Ocular Discomfort Scale (0-4) in Dry Eye Chamber Drug effect over vehicle observed upon entry and sustained throughout duration of exposure. Therapeutic activity demonstrated after first and second doses, representing near-immediate (within minutes) and statistically significant symptom relief vs. vehicle. Consistent and statistically significant improvements over vehicle observed across all symptoms evaluated in the chamber.
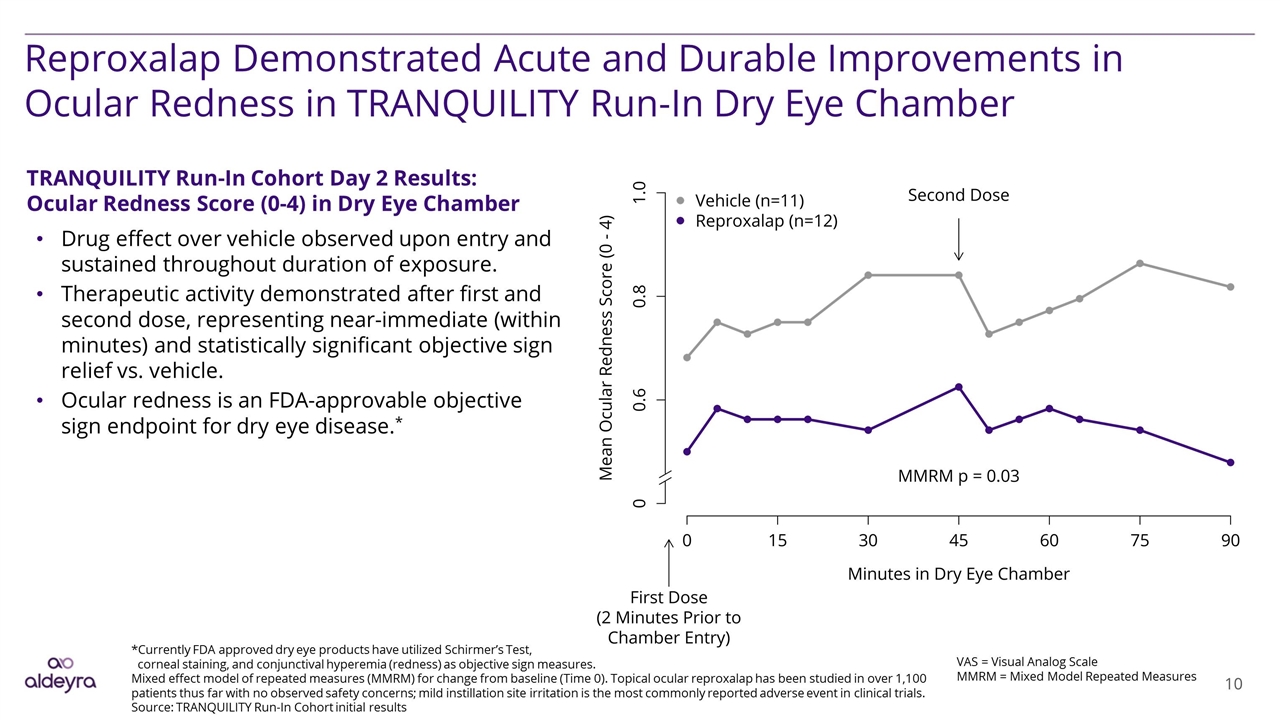
Reproxalap Demonstrated Acute and Durable Improvements in Ocular Redness in TRANQUILITY Run-In Dry Eye Chamber VAS = Visual Analog Scale MMRM = Mixed Model Repeated Measures TRANQUILITY Run-In Cohort Day 2 Results: Ocular Redness Score (0-4) in Dry Eye Chamber Drug effect over vehicle observed upon entry and sustained throughout duration of exposure. Therapeutic activity demonstrated after first and second dose, representing near-immediate (within minutes) and statistically significant objective sign relief vs. vehicle. Ocular redness is an FDA-approvable objective sign endpoint for dry eye disease.* *Currently FDA approved dry eye products have utilized Schirmer’s Test, corneal staining, and conjunctival hyperemia (redness) as objective sign measures. Mixed effect model of repeated measures (MMRM) for change from baseline (Time 0). Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Source: TRANQUILITY Run-In Cohort initial results
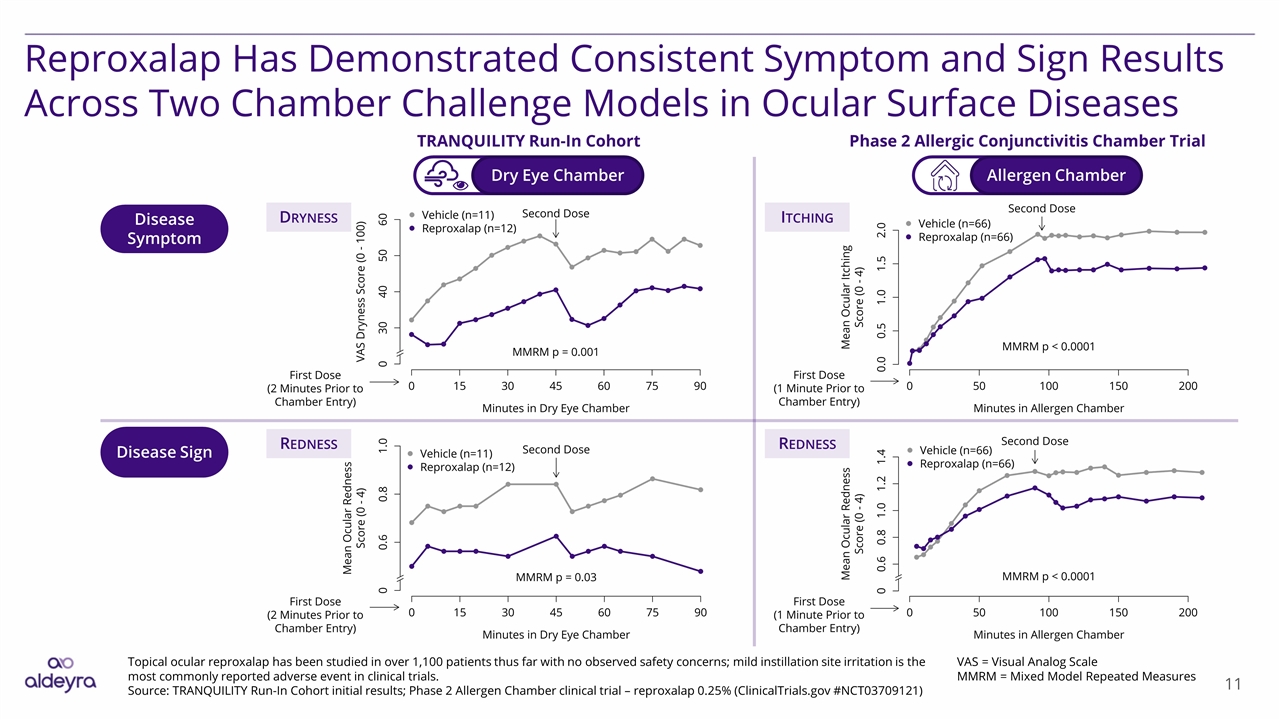
Disease Sign Reproxalap Has Demonstrated Consistent Symptom and Sign Results Across Two Chamber Challenge Models in Ocular Surface Diseases Disease Symptom Dry Eye Chamber TRANQUILITY Run-In Cohort Phase 2 Allergic Conjunctivitis Chamber Trial Allergen Chamber Topical ocular reproxalap has been studied in over 1,100 patients thus far with no observed safety concerns; mild instillation site irritation is the most commonly reported adverse event in clinical trials. Source: TRANQUILITY Run-In Cohort initial results; Phase 2 Allergen Chamber clinical trial – reproxalap 0.25% (ClinicalTrials.gov #NCT03709121) VAS = Visual Analog Scale MMRM = Mixed Model Repeated Measures Dryness Itching Redness Redness
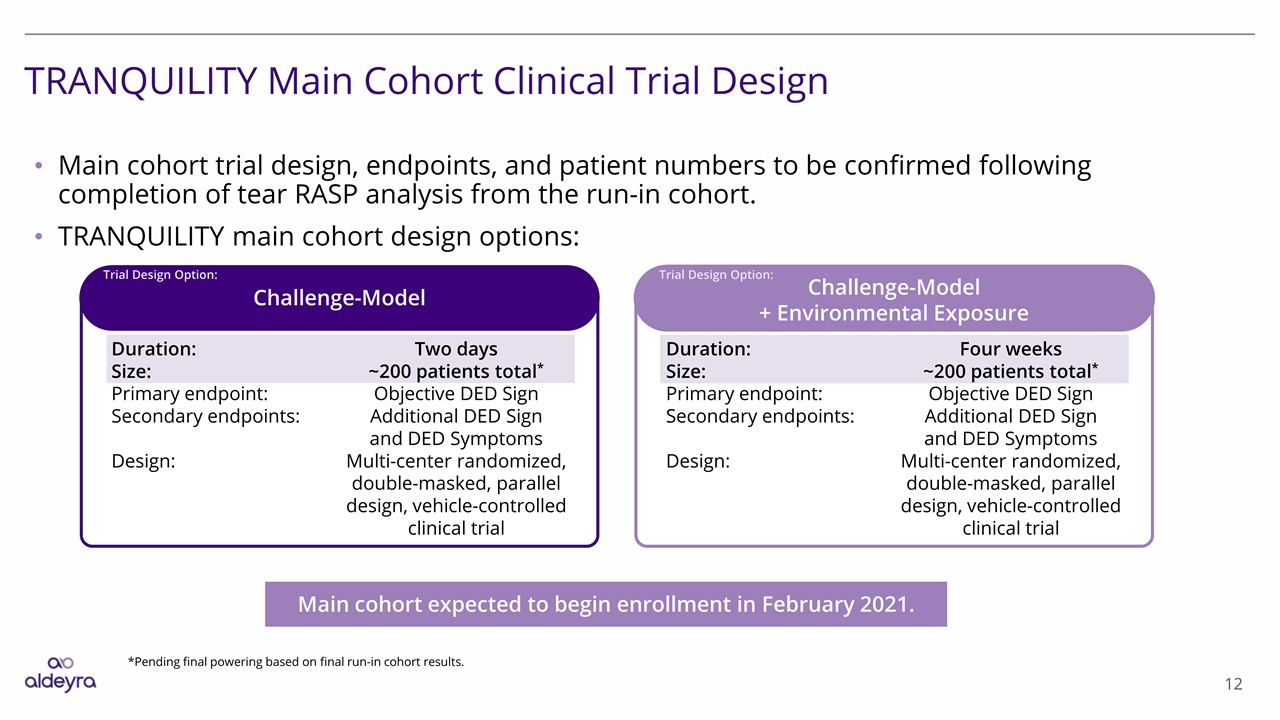
TRANQUILITY Main Cohort Clinical Trial Design Main cohort trial design, endpoints, and patient numbers to be confirmed following completion of tear RASP analysis from the run-in cohort. TRANQUILITY main cohort design options: Main cohort expected to begin enrollment in February 2021. Challenge-Model + Environmental Exposure Challenge-Model Duration: Size: Primary endpoint: Secondary endpoints: Design: Two days ~200 patients total* Objective DED Sign Additional DED Sign and DED Symptoms Multi-center randomized, double-masked, parallel design, vehicle-controlled clinical trial Duration: Size: Primary endpoint: Secondary endpoints: Design: Four weeks ~200 patients total* Objective DED Sign Additional DED Sign and DED Symptoms Multi-center randomized, double-masked, parallel design, vehicle-controlled clinical trial *Pending final powering based on final run-in cohort results. Trial Design Option: Trial Design Option:

Reproxalap allergic conjunctivitis Phase 3 INVIGORATE study top-line results H1 2021 Reproxalap dry eye disease Phase 3 TRANQUILITY-2 initiation Q1 2021 Upcoming Expected Reproxalap Development Milestones* *The timing of clinical trial initiation and completion depends, in part, on restrictions related to COVID-19, the availability of clinical research facilities and staffing, the ability to recruit patients, and regulatory feedback. Reproxalap dry eye disease Phase 3 TRANQUILITY main cohort enrollment initiation February 2021
