Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Ra Medical Systems, Inc. | rmed-8k_20210105.htm |

Ra Medical Systems, Inc. NYSE American: RMED Corporate Presentation dated January 5, 2021 Exhibit 99.1

Disclaimer Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future financial performance of Ra Medical Systems, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any expectations regarding investment returns; any projections of financial information; any statements about historical results that may suggest trends for our business; any statements of the plans, strategies, and objectives of management for future operations; any statements of expectation or belief regarding future events, potential markets, market size, market opportunities, or technology developments; any statements regarding sales and expansion strategies; any statements regarding our intention to seek additional indications for our products; and any statements of assumptions underlying any of the items mentioned. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company’s control. These and other important factors may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. For a list and description of the risk and uncertainties inherent in the forward-looking statements, see the Company’s Annual Report on Form 10-K for the year ended December 31, 2019 and in its other filings with the Securities and Exchange Commission. The forward-looking statements in this presentation are made only as of the date hereof. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation also contains estimates, projections and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry and our business. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. We have not independently verified the accuracy and completeness of the information obtained by third parties included in this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the solutions and services of the company or this proposed offering.
Focused on developing Pharos growth strategy to build on ~$5-6 million annual revenue rate Highlights * IDE trial for atherectomy indication in process

DABRA—Excimer laser that utilizes disposable catheters for crossing total chronic occlusions (CTOs) and ablating a channel in occlusive peripheral vascular disease. DABRA is used as a tool to treat peripheral artery disease (PAD), a form of peripheral vascular disease. Photoablation to disintegrate plaque in the artery Designed to track the patient’s true lumen Established safety profile, effective, easy-to-use, and competitively priced No serious device-related adverse events reported in our 2017 pivotal study or in our post-market surveillance Regulatory clearances in US and Europe PHAROS—Dermatology, same laser platform as DABRA US FDA 510(k) clearance and Europe CE Mark for psoriasis, vitiligo, atopic dermatitis and leukoderma Ra Medical Technology
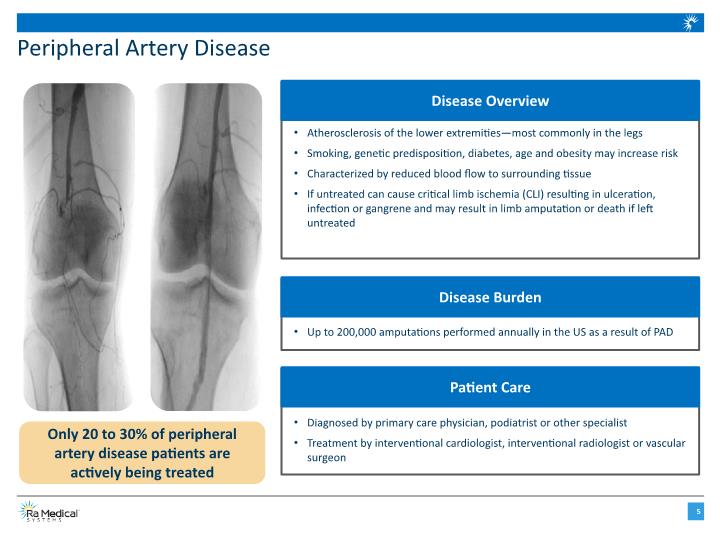
Peripheral Artery Disease Only 20 to 30% of peripheral artery disease patients are actively being treated
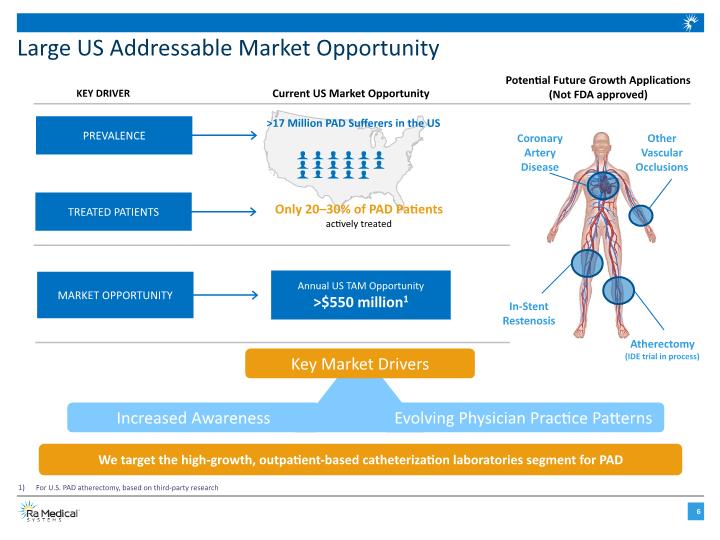
Large US Addressable Market Opportunity PREVALENCE TREATED PATIENTS MARKET OPPORTUNITY Current US Market Opportunity KEY DRIVER >17 Million PAD Sufferers in the US Only 20–30% of PAD Patients actively treated Annual US TAM Opportunity >$550 million1 For U.S. PAD atherectomy, based on third-party research Key Market Drivers Increased Awareness Evolving Physician Practice Patterns We target the high-growth, outpatient-based catheterization laboratories segment for PAD Potential Future Growth Applications (Not FDA approved) Other Vascular Occlusions Coronary Artery Disease In-Stent Restenosis Atherectomy (IDE trial in process)
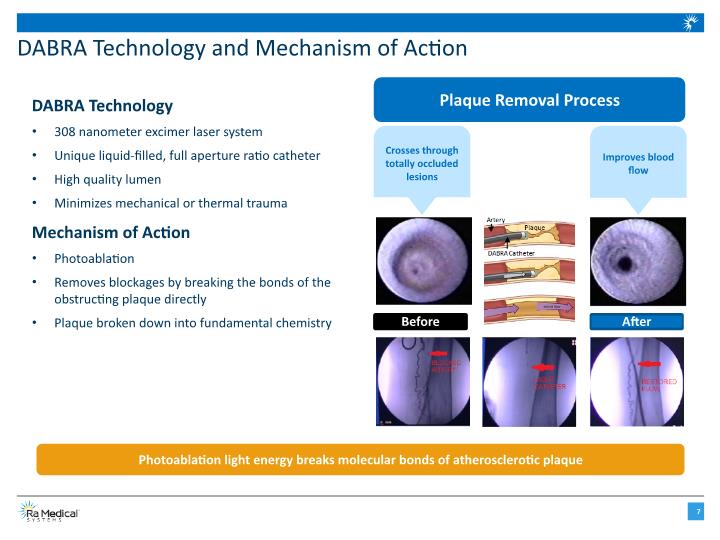
DABRA Technology and Mechanism of Action DABRA Technology 308 nanometer excimer laser system Unique liquid-filled, full aperture ratio catheter High quality lumen Minimizes mechanical or thermal trauma Mechanism of Action Photoablation Removes blockages by breaking the bonds of the obstructing plaque directly Plaque broken down into fundamental chemistry Photoablation light energy breaks molecular bonds of atherosclerotic plaque Plaque Removal Process Crosses through totally occluded lesions Improves blood flow

DABRA Advantages
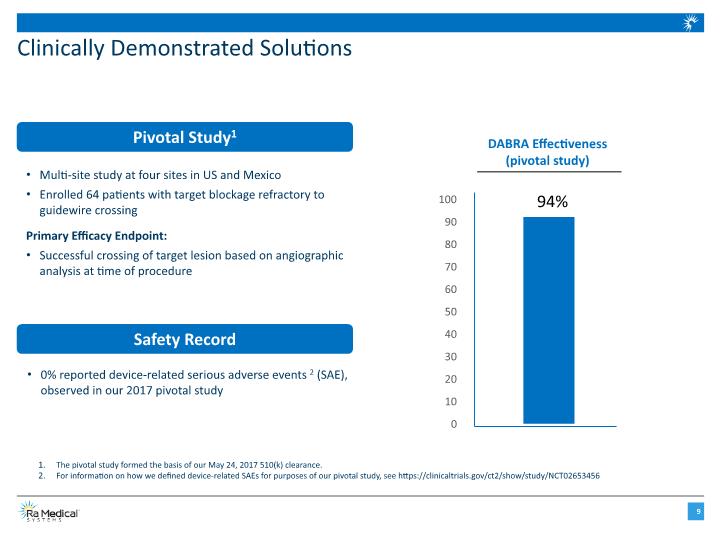
Clinically Demonstrated Solutions Safety Record Pivotal Study1 Multi-site study at four sites in US and Mexico Enrolled 64 patients with target blockage refractory to guidewire crossing Primary Efficacy Endpoint: Successful crossing of target lesion based on angiographic analysis at time of procedure 0% reported device-related serious adverse events2 (SAE), observed in our 2017 pivotal study DABRA Effectiveness (pivotal study) 94% The pivotal study formed the basis of our May 24, 2017 510(k) clearance. For information on how we defined device-related SAEs for purposes of our pivotal study, see https://clinicaltrials.gov/ct2/show/study/NCT02653456
DABRA Will Target Office-Based Labs We believe our solution expands provider economics

Engineering Efforts Focused on Three Initiatives to Improve DABRA’s Performance Extend shelf life 6 months minimum with target 12 months or greater Improve deliverability Develop a catheter with an enhanced outer-jacket to allow physicians to better access difficult anatomy Develop guidewire compatible platform Project outsourced to an experienced engineering firm to develop a version of the DABRA catheter that is compatible with standard interventional guidewires

An FDA-Approved IDE Atherectomy Indication Study is Underway Primary safety endpoint: The incidence of 30-day Major Adverse Events (MAEs) as adjudicated by the Clinical Events Committee (CEC) : All-cause mortality, Unplanned major target limb amputation (at or above the ankle), and/or Clinically driven target limb revascularization (CD-TLR). The incidence of MAEs at 30-Days is < 20% Status: 5 sites cleared to enroll, 20 subjects treated as of December 31, 2020 Study size: Up to 10 sites, 100 patients Primary efficacy endpoint: Mean reduction in percent diameter stenosis in each subject’s primary lesion as measured by angiography following treatment with the DABRA Laser System and before any other treatment. The mean difference in percent diameter stenosis, post-procedure, is > 20%

Pharos Excimer Laser
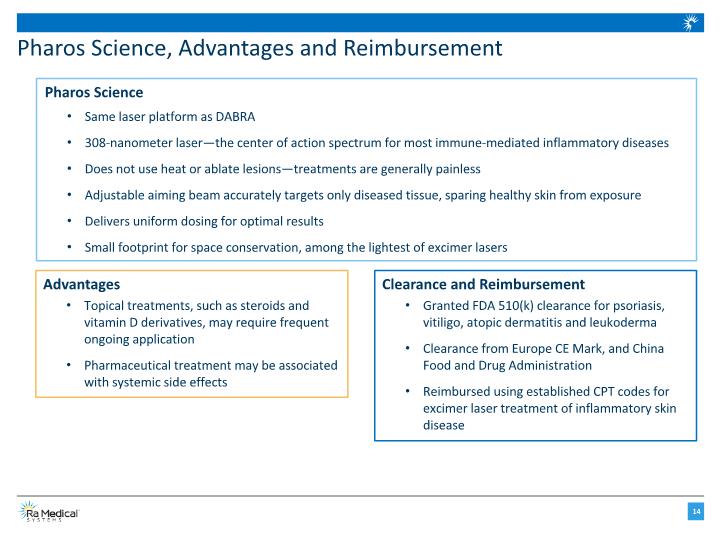
Pharos Science, Advantages and Reimbursement Pharos Science Same laser platform as DABRA 308-nanometer laser—the center of action spectrum for most immune-mediated inflammatory diseases Does not use heat or ablate lesions—treatments are generally painless Adjustable aiming beam accurately targets only diseased tissue, sparing healthy skin from exposure Delivers uniform dosing for optimal results Small footprint for space conservation, among the lightest of excimer lasers Advantages Topical treatments, such as steroids and vitamin D derivatives, may require frequent ongoing application Pharmaceutical treatment may be associated with systemic side effects Clearance and Reimbursement Granted FDA 510(k) clearance for psoriasis, vitiligo, atopic dermatitis and leukoderma Clearance from Europe CE Mark, and China Food and Drug Administration Reimbursed using established CPT codes for excimer laser treatment of inflammatory skin disease

Fully Operational Manufacturing Facility Carlsbad, CA Sizable capacity for laser and catheter production 41,000 sq. ft. Carlsbad, CA with three controlled environments manufacturing facility fully staffed and operational Existing facility expected to be capable of manufacturing > 400 lasers/year and 140,000 catheters/year Fully capitalized with all equipment owned ISO13485 certified, FDA and CA state inspected Laser Assembly Controlled Environments
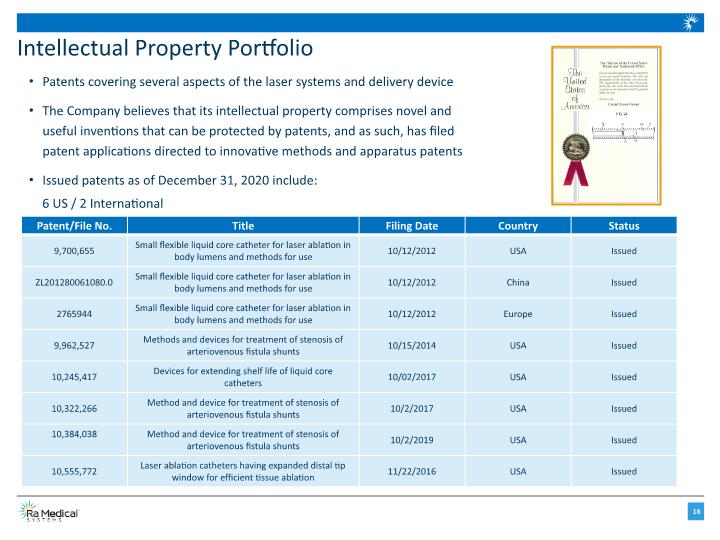
Intellectual Property Portfolio Patents covering several aspects of the laser systems and delivery device The Company believes that its intellectual property comprises novel and useful inventions that can be protected by patents, and as such, has filed patent applications directed to innovative methods and apparatus patents Issued patents as of December 31, 2020 include: 6 US / 2 International
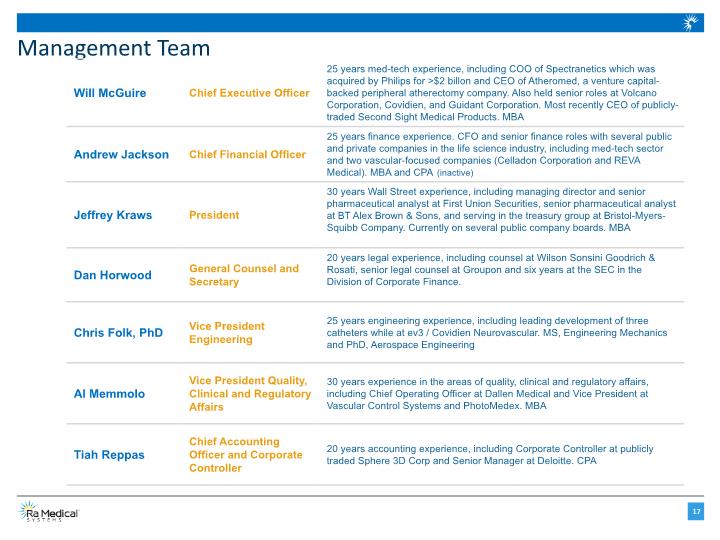
Management Team
Financial Overview
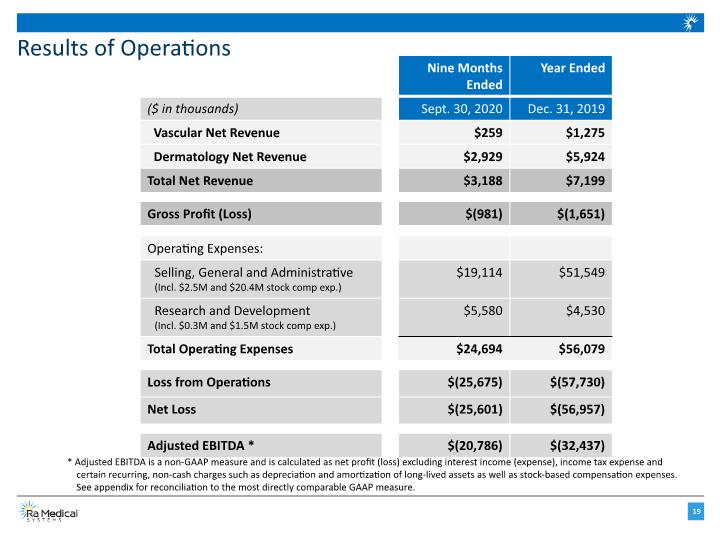
Results of Operations * Adjusted EBITDA is a non-GAAP measure and is calculated as net profit (loss) excluding interest income (expense), income tax expense and certain recurring, non-cash charges such as depreciation and amortization of long-lived assets as well as stock-based compensation expenses. See appendix for reconciliation to the most directly comparable GAAP measure.
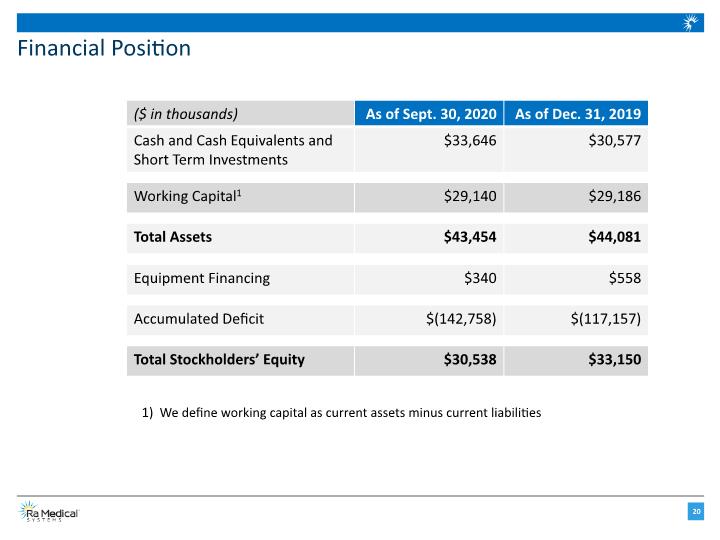
Financial Position We define working capital as current assets minus current liabilities
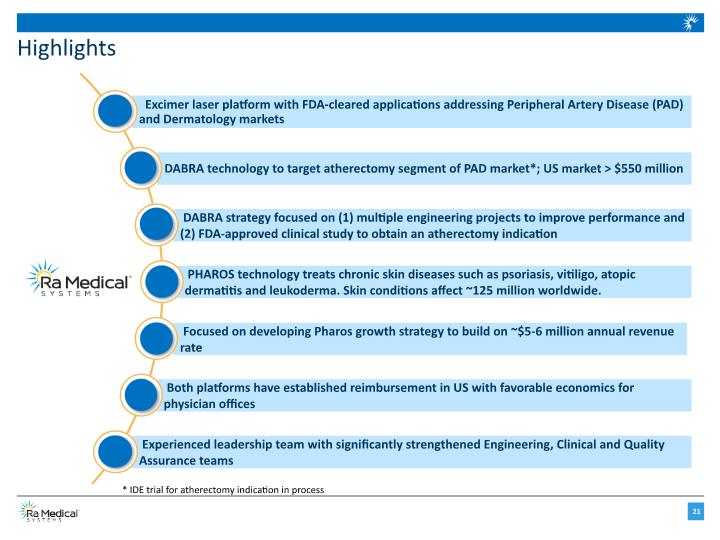
Focused on developing Pharos growth strategy to build on ~$5-6 million annual revenue rate Highlights * IDE trial for atherectomy indication in process
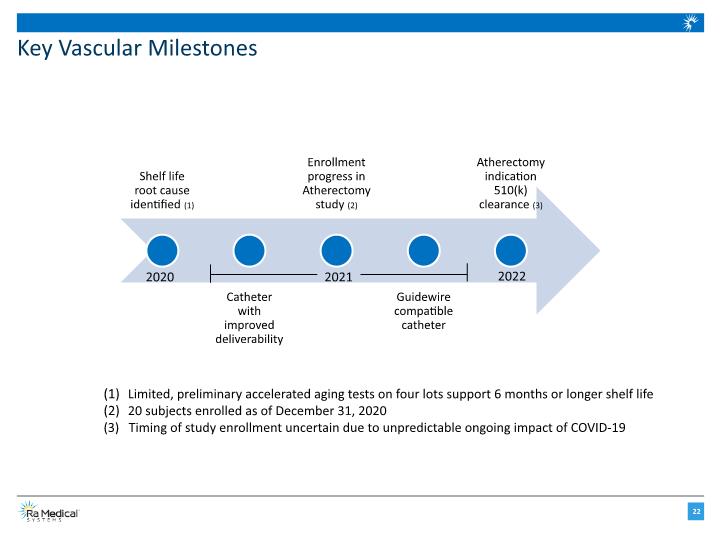
Key Vascular Milestones 2020 2021 2022 Limited, preliminary accelerated aging tests on four lots support 6 months or longer shelf life 20 subjects enrolled as of December 31, 2020 (3) Timing of study enrollment uncertain due to unpredictable ongoing impact of COVID-19

Appendix
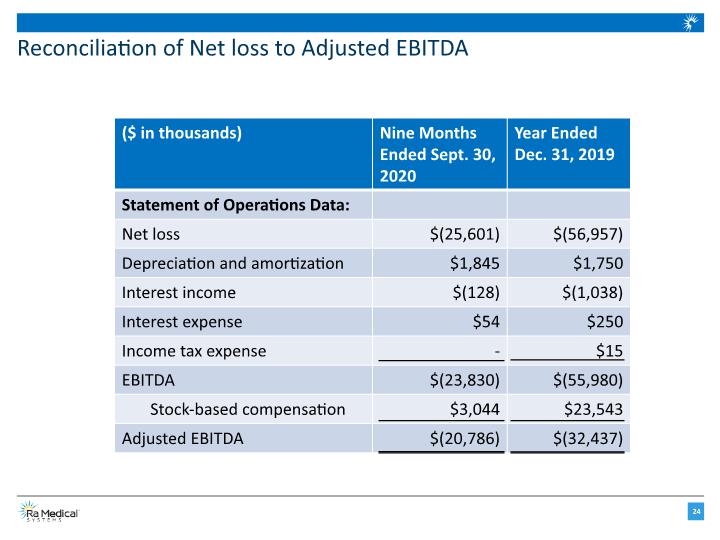
Reconciliation of Net loss to Adjusted EBITDA

Board of Directors
