Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - HOOKIPA Pharma Inc. | tm2037251d1_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - HOOKIPA Pharma Inc. | tm2037251d1_ex99-1.htm |
Exhibit 99.2

HB - 101 CMV Vaccine Phase 2 Trial in Kidney Transplantation Preliminary Results of Interim Analysis November 30, 2020

2 HOOKIPA Pharma Confidential Disclaimer This presentation and other related material may contain a number of “forward looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 as amended, including statements regarding HOOKIPA’s expectation about any or all of the following (i) the success, cost, results and timing of HOOKIPA’s product development activities and clinical trials ; (ii) the timing, scope or likelihood of regulatory filings and approvals, including timing of Investigational New Drug Application and Biological Licensing Application filings for HOOKIPA’s current and future product candidates, and final U . S . Food and Drug Administration, European Medicines Agency or other foreign regulatory authority approval of HOOKIPA’s current and future product candidates ; (iii) HOOKIPA’s ability to develop and advance its current product candidates and programs into, and successfully complete, clinical studies ; (iv) HOOKIPA’s manufacturing, commercialization and marketing capabilities and strategy ; (v) the potential benefits of and HOOKIPA’s ability to maintain its collaboration with Gilead Sciences, Inc . and establish or maintain future collaborations or strategic relationships or obtain additional funding ; (vi) risks relating to business interruptions resulting from the coronavirus (COVID - 19 ) disease outbreak or similar public health crises and other matters that could affect the sufficiency of existing cash to fund operations and HOOKIPA’s ability to achieve the milestones under the agreement with Gilead and (vii) the rate and degree of market acceptance and clinical utility of HOOKIPA’s current and future product candidates Forward looking statements can be identified by terms such as “ believes,”“ expects,”“ plans,”“ potential,”“ or similar expressions and the negative of those terms HOOKIPA has based these forward looking statements largely on its current expectations and projections about future events and financial trends that it believes may affect its business, financial condition and results of operations . Although HOOKIPA believes that such statements are based on reasonable assumptions, forward looking statements are neither promises nor guarantees and they are necessarily subject to a high degree of uncertainty and risk . Because forward looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond HOOKIPA’s control, you should not rely on these forward looking statements as predictions of future events . These risks and uncertainties include, among others outcomes of HOOKIPA’s planned clinical trials and studies may not be favorable that one or more of HOOKIPA’s product candidate programs will not proceed as planned for technical, scientific or commercial reasons availability and timing of results from preclinical studies and clinical trials uncertainty about regulatory approval to conduct clinical trials or to market a products uncertainties regarding intellection property protection and those risk and uncertainties described under the heading “Risk Factors” in HOOKIPA’s Annual Report on Form 10 - K filed with the U . S . Securities and Exchange Commission on March 19 , 2020 , and HOOKIPA’s Quarterly Reports on Form 10 - Q filed with the U . S . Securities and Exchange Commission on May 14 , 2020 , August 13 , 2020 , and November 12 , 2020 and in any other subsequent filings made by HOOKIPA with the U . S . Securities and Exchange Commission, which are available at www . sec . gov . Existing and prospective investors are cautioned not to place undue reliance on these forward looking statements, which speak only as of the date they are made . HOOKIPA disclaims any obligation or undertaking to update or revise any forward looking statements contained in this presentation, other than to the extent required by law .

3 HOOKIPA Pharma Confidential H B - 101 P h a s e 2 I n t e r i m An a l y s i s Data Overview • HB - 101 is a C ytomegalovirus (CMV) vaccine based on H O O K I P A ’s non - replicating technology • This interim analysis of the ongoing Phase 2 study of HB - 101 supports dose - finding for the 3 - dose schedule • The 3 - dose schedule of HB - 101 has demonstrated: o Good tolerability profile o Promising immunogenicity o Encouraging interim efficacy in decreasing rates of viremia, antiviral use, and CMV disease • While from a small number of patients, early efficacy data show the potential of HB - 101 to help address the unmet need in CMV

4 HOOKIPA Pharma Confidential Cytomegalovirus (CMV) in Solid Organ Transplant Recipients Kotton CN et al. The Third International Consensus Guidelines on Management of Cytomegalovirus in Solid - organ Transplantation. T ransplantation. 2019;102:900 - 931; Ljungman P et al. Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials. Clinical Infectious D ise ase. 2017;64(1):87 - 91; Global Observatory on Donation and Transplantation. 2019; Azevedo LS et al. Cytomegalovirus infection in transplant recipients. Clinics. 2015;70(70):515 - 523. CMV viremia CMV syndrome CMV disease CMV detection in body fluid Fever, malaise, leukopenia, and/or thrombocytopenia End organ disease, pneumonia, hepatitis, rejection Donor - - + + Recipient - + + - Majority of kidney donors are deceased; living donor transplants offer the ideal opportunity to assess post - transplant efficacy relatively quickly CMV Risk to Organ Recipient TWO TREATMENT APPROACHES Pre - emptive antivirals vs. Prophylactic antivirals CMV can cause severe complications in solid organ transplant recipients

5 HOOKIPA Pharma Confidential • Uses our proprietary non - replicating technology, with two arenaviral vectors (LCMV) • Bi - valent vaccine incorporating 2 CMV antigens: o Glycoprotein B (“gB”) fusion protein, a B cell antigen o Phosphoprotein 65 kDa (“pp65”), a T cell antigen • Vaccine stimulates both arms of the adaptive immune system: o Antibodies against gB fusion protein o T cells against pp65 T cell antigen • Intra - muscular delivery HB - 101 Product Details HOOKIPA’s HB - 101: Arenavirus - based Prophylactic Vaccine to Prevent CMV Infections or Reactivations in Immuno - Compromised Populations

6 HOOKIPA Pharma Confidential Patients Eligible for a Kidney Transplant from a Live Donor Randomized to HB - 101 or Placebo Pre - transplant Stratified by Post - transplant Treatment Intent Measure decrease of post - transplant viremia in the absence and presence of antivirals Endpoints • Primary: immunogenicity & safety • Secondary: CMV viremia, antiviral use, CMV disease, transplant rejection ARM 2: CMV - Recipients HB - 101 2 - 3 doses pre - transplant HB - 101 2 - 3 doses pre - transplant Placebo Placebo 3 - 6 months prophylactic antiviral therapy Pre - emptive antiviral therapy ARM 3: CMV+ Recipients HB - 101 2 - 3 doses pre - transplant Pre - emptive or prophylactic SoC 1 ARM 1: CMV - Recipients Pre - transplant Post - transplant HB - 101 Ongoing Phase 2 Clinical Trial: Prophylactic CMV Vaccine in Kidney Transplant Patients Trial Objectives 1 SoC: Standard of care.
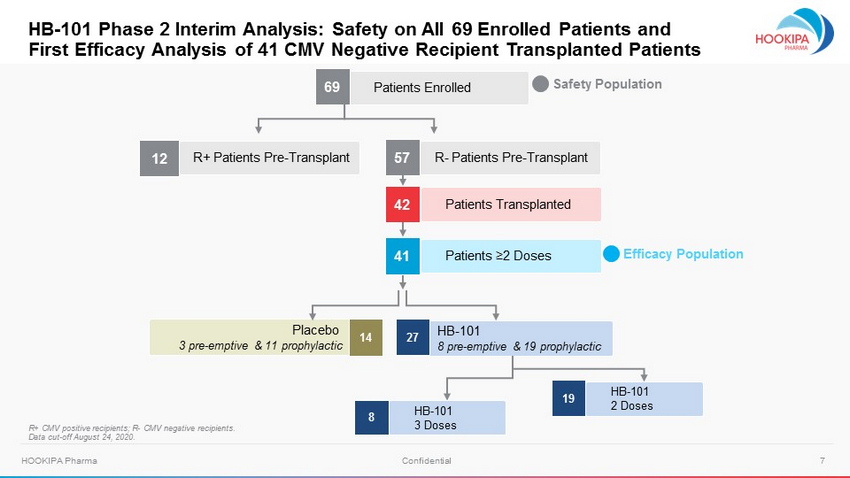
7 HOOKIPA Pharma Confidential 57 Patients Transplanted 42 Safety Population Efficacy Population Placebo 3 pre - emptive & 11 prophylactic 14 HB - 101 8 pre - emptive & 19 prophylactic 27 HB - 101 2 Doses 19 HB - 101 3 Doses 8 Patients ≥2 Doses 41 Patients Enrolled 69 R+ Patients Pre - Transplant 12 R+ CMV positive recipients; R - CMV negative recipients. Data cut - off August 24, 2020. HB - 101 Phase 2 Interim Analysis: Safety on All 69 Enrolled Patients and First Efficacy Analysis of 41 CMV Negative Recipient Transplanted Patients R - Patients Pre - Transplant

8 HOOKIPA Pharma Confidential Safety Population : • 69 patients prior to kidney transplantation Most common mild AEs : • Influenza - like illness (N=2) • Injection site pain (N=2) Human Leukocyte Antigen (HLA) Sensitization : • HLA - sensitization is a known complication in renal dialysis patients awaiting transplantation, occurring at a rate of 4 % 2 • HLA sensitization requires identification of a new donor organ ; it can be managed clinically via risk stratification based on recipient’s HLA profile • 2 cases were classified as both severe and serious AEs ; 1 additional case was not considered an AE Pre - Transplant AEs Related to Study Medication N (%) Grade 1 – Mild 8 (11.6%) Grade 2 – Moderate 2 (2.9%) Grade 3 – Severe 2 (2.9%)* Grade 4 – Life - Threatening 0 (0.0%) Death 0 (0.0%) Serious (SAE) 2 (2.9%)* Discontinued Study Medication Due to AE 0 (0.0%) HB - 101 Phase 2 Interim Analysis Preliminary Safety 1 *Recipient HLA - sensitization 1 Data cut - off August 24, 2020. 2 N ephrol Dial Transplant (2013) 28: 2908 - 2918.

9 HOOKIPA Pharma Confidential 33 R - patients measured on the day of transplant • 21 patients received vaccine • 12 patients received placebo Seroconversion Rates CMV Antibody Levels Rate s of CMV - neutralizing antibodies development: • 100% in 3 dose group (N=6) • 20% in 2 dose group (N=15) 3 doses of HB - 101 more immunogenic than 2 doses Antibody level induced by 3 doses of HB - 101 was significantly superior to that induced by: • 2 doses of HB - 101 ( p =0.03) • Placebo ( p =0.0095) Assessment of antibody responses was completed for a subset of the 41 - patient efficacy group at the time of cut - off. Data cut - of f August 24, 2020. H B - 101 P h a s e 2 I n t e r i m An a l y s i s of CMV - Neutralizing Antibody Responses: 3 Doses Induce 100% Seroconversion at Levels Superior to 2 Doses or Placebo
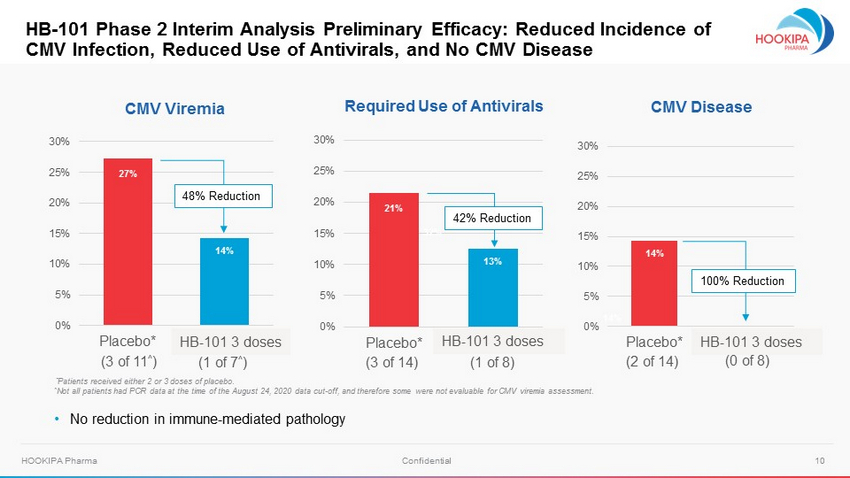
10 HOOKIPA Pharma Confidential 0% 5% 10% 15% 20% 25% 30% Placebo* 3 doses HB - 101 Phase 2 Interim Analysis Preliminary Efficacy: Reduced Incidence of CMV Infection, Reduced Use of Antivirals, and No CMV Disease CMV Viremia Required Use of Antivirals CMV Disease 0% 5% 10% 15% 20% 25% 30% Placebo* 3 doses 0% 5% 10% 15% 20% 25% 30% Placebo* 3 doses (3 of 11 ^ ) 27% 21% 32% 13% 14% 14% 14% (1 of 7 ^ ) 48% Reduction (3 of 14) (1 of 8) 42% Reduction (2 of 14) (0 of 8) 100% Reduction • No reduction in immune - mediated pathology HB - 101 3 doses HB - 101 3 doses HB - 101 3 doses * Patients received either 2 or 3 doses of placebo. ^ Not all patients had PCR data at the time of the August 24, 2020 data cut - off, and therefore some were not evaluable for CMV vir emia assessment.

11 HOOKIPA Pharma Confidential Based on P h a s e 2 I n t e r i m An a l y s i s , HB - 101 Appears to be Well Tolerated, and in Patients Receiving Three Doses, Immunogenic and Efficacious (Preliminary Data) Safety: • HB - 101 appears to be well tolerated with a low incidence of adverse events (mostly mild to moderate) • Three cases of HLA - sensitization were reported, two as serious adverse events Immunogenicity: • Patients who received three doses of HB - 101 had 100% response rate for CMV - neutralizing antibodies (N=6) • CMV - neutralizing antibody levels induced by the three doses of HB - 101 are statistically superior to those seen with placebo ( p =0.0095) • Patients who received three doses of HB - 101 had 100% response rate for CMV - specific cellular (T cell) responses (N=3, as reported in June 2020 interim analysis) Efficacy: • Reduced viremia in patients who received three doses of HB - 101 compared to placebo by 48% • Reduced antiviral use required in patients who received three doses of HB - 101 compared to placebo by 42% • No CMV disease in eight patients who received three doses of HB - 101

12 HOOKIPA Pharma Confidential HB - 101 Phase 2 Dataset Continues to Build and Inform Path to Registration • Interim Phase 2 safety and immunogenicity data have been reviewed with investigators: o Encouraged to complete the pre - transplant three dose vaccination protocol (whenever possible in the context of these live donor transplants) and thereby maximize immunogenicity at transplantation and potential patients benefits . • Continuing accrual, as permitted by COVID - 19 considerations at our participating sites • Phase 2 data will enable us to explore a path to a Phase 3 registration study in a real world, all - comers population, wherein most recipients are on a transplant waiting list and a three - dose vaccination schedule is easily managed • Next Phase 2 data update will be provided in H2 2021

13 HOOKIPA Pharma Confidential 2021 Outlook: Catalysts & Upcoming Immuno - Oncology Clinical Data • From non - replicating to single - vector replicating to dual vector replicating technology o HB - 101 is an immunogenic, single - vector non - replicating technology o HB - 201 is expected to be more immunogenic, single - vector replicating technology o HB - 202/HB - 201 is expected to be most immunogenic, alternating two - vector replicating technology • Based on published preclinical data, we hope to demonstrate o Favorable safety across the non - replicating and replicating platforms o Increasing immunogenicity and efficacy for single - vector replicating as compared to non - replicating o Increasing immunogenicity and efficacy for alternating two - vector replicating therapy as compared to single - vector replicating therapy • Initial clinical data release from the HB - 201 Phase 1/2 study is planned for early 2021 • Funded to reach beyond major value inflections: $82m cash (30 September 2020) Add’l HB - 101 efficacy data ( H 2 2021) HB - 202/201 data ( m i d 2021) Initial HB - 201 data ( e a r l y 2021) Q1/21 Q3/21 Q2/21 Q4/21

