Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Evofem Biosciences, Inc. | evfm_x11x18x2020xstifelpre.htm |

Revolutionizing Women’s Sexual and Reproductive Health Saundra Pelletier, CEO Stifel Virtual Healthcare Conference November 18, 2020 NASDAQ: EVFM For investor discussions only. 1 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.

Forward-Looking Statements This presentation contains forward looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws. In some cases, you can identify forward looking statements by terms such as “may,” ”will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “strategy,” “objective,” “designed,” “suggest,” “currently,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from those, express or implied, in these forward-looking statements. Factors that may cause differences between current expectations and actual results include, but are not limited to, the following: the rate and degree of market acceptance of Phexxi® (lactic acid, citric acid and potassium bitartrate) vaginal gel o ® o Evofem’s ability to successfully commercialize Phexxi and its ability to develop sales and marketing capabilities o Evofem’s ability to maintain and protect its intellectual property o Evofem’s ability to rely on existing cash reserves to fund its current development plans and operations and to raise additional capital when needed Evofem’s reliance on third-party providers, such as third-party manufacturers and clinical research organizations o ® o the presence or absence of any adverse events or side effects relating to the use of Phexxi and EVO100 o the outcome or success of Evofem’s clinical trials including EVOGUARD o Evofem’s ability to retain members of its management and other key personnel o general risks to the economy represented by spread of the COVID-19 virus o Evofem’s ability to obtain the necessary regulatory approvals for its product candidates and the timing of such approvals, and, o any other risk factors detailed in Evofem’s filings from time to time with the US Securities and Exchange Commission including, without limitation, the 10-K filed on March 12, 2020, 8-K filed on June 2, 2020 and subsequent filings. The forward looking statements in this presentation represent Evofem’s views only as of the date of this presentation, November 18, 2020, and Evofem expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Evofem’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based for any reason, except as required by law, even as new information becomes available or other events occur in the future. All forward- looking statements in this presentation are qualified in their entirety by this cautionary statement. For investor discussions only. 2 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
Evofem Biosciences 101 • Commercial-stage biopharmaceutical company • Mission: to improve women’s lives by developing and commercializing innovative prescription products to address critical unmet needs in women’s sexual and reproductive health • Launched Phexxi® in September 2020 o First-and-only FDA-approved hormone-free, on-demand, woman-controlled contraceptive vaginal gel 1 o 21 million US women at risk for pregnancy but NOT using hormonal contraception o “Best in Class” commercial team with more than 60 product launches • Phase 3 investigational candidate for prevention of chlamydia and prevention of gonorrhea in women o Tremendous unmet need – all sexually active women at risk o Top-line data expected mid 2022 1. Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief. 2018; 327: 1-14. For investor discussions only. 3 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
Phexxi® is Off to a Strong Start Encouraging October Data • Monthly TRx and NRx growing • Number of unique Phexxi prescribers more than doubled versus September o Geographically diverse o Over 800 unique HCPs prescribed Phexxi from launch through October • Over 1/3 of Phexxi prescribers have written more than one Phexxi prescription o Top 10 prescribers wrote 8 or more scripts each o Highest prescribing HCP has written 70 Phexxi scripts As of 30 October 2020 For investor discussions only. 4 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.

Phexxi® is Off to a Strong Start Building Awareness • 650,000 visits to consumer site1 • Strong engagement driving conversions on HCP-Phexxi.com 2 o 31k unique visitors, 53k page views • Successfully harnessing social media that resonates with our key audience segments 1 o ~250M social media impressions since launch 1 o Phexxi influencers have 3.6M Instagram followers • 38 earned media pieces since launch 1. As of 15 November 2020 2. As of 27 October 2020 For investor discussions only. 5 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
Phexxi® Telehealth Platform is Exceeding Expectations 41% of women who qualified for Phexxi through our Phexxi Concierge Service have requested a Phexxi prescription Populus Media: “Phexxi is our most successful launch to date” Source: Populus AWS – 15 Nov 2020 For investor discussions only. 6 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
Phexxi® Concierge Recruitment Platform with Nurse Support Driving Significant Leads Harnessing AI to generate high-quality leads • 83Bar metrics projected generation of 200 leads/month • Consistently generating over 200 leads/week o 4x to 5x higher than expectations • ~75% of women who engaged have spoken with a Phexxi support nurse and requested to see an HCP for a Phexxi prescription Source: 83Bar Metrics - 15 November 2020 For investor discussions only. 7 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
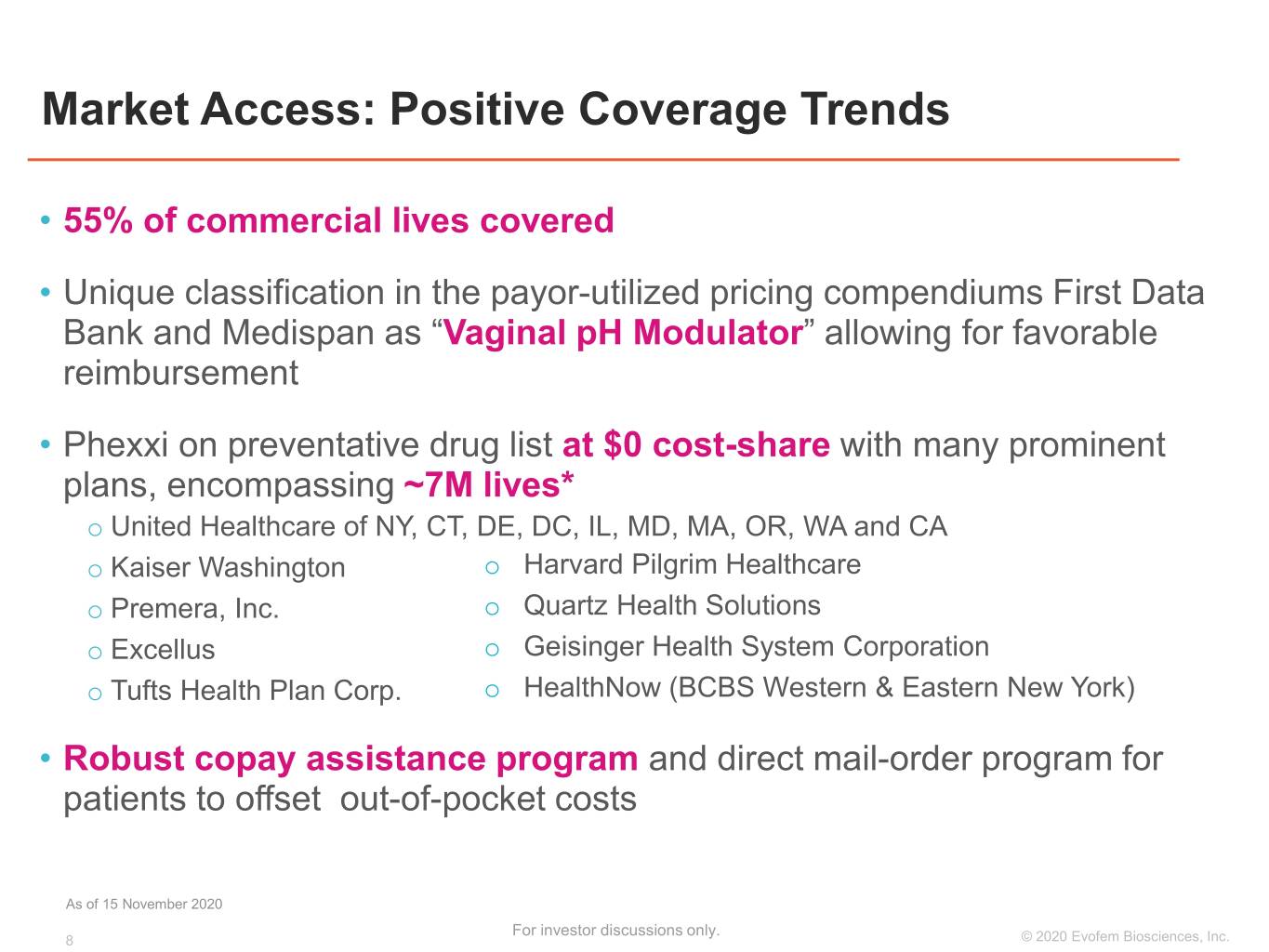
Market Access: Positive Coverage Trends • 55% of commercial lives covered • Unique classification in the payor-utilized pricing compendiums First Data Bank and Medispan as “Vaginal pH Modulator” allowing for favorable reimbursement • Phexxi on preventative drug list at $0 cost-share with many prominent plans, encompassing ~7M lives* o United Healthcare of NY, CT, DE, DC, IL, MD, MA, OR, WA and CA o Kaiser Washington o Harvard Pilgrim Healthcare o Premera, Inc. o Quartz Health Solutions o Excellus o Geisinger Health System Corporation o Tufts Health Plan Corp. o HealthNow (BCBS Western & Eastern New York) • Robust copay assistance program and direct mail-order program for patients to offset out-of-pocket costs As of 15 November 2020 For investor discussions only. 8 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
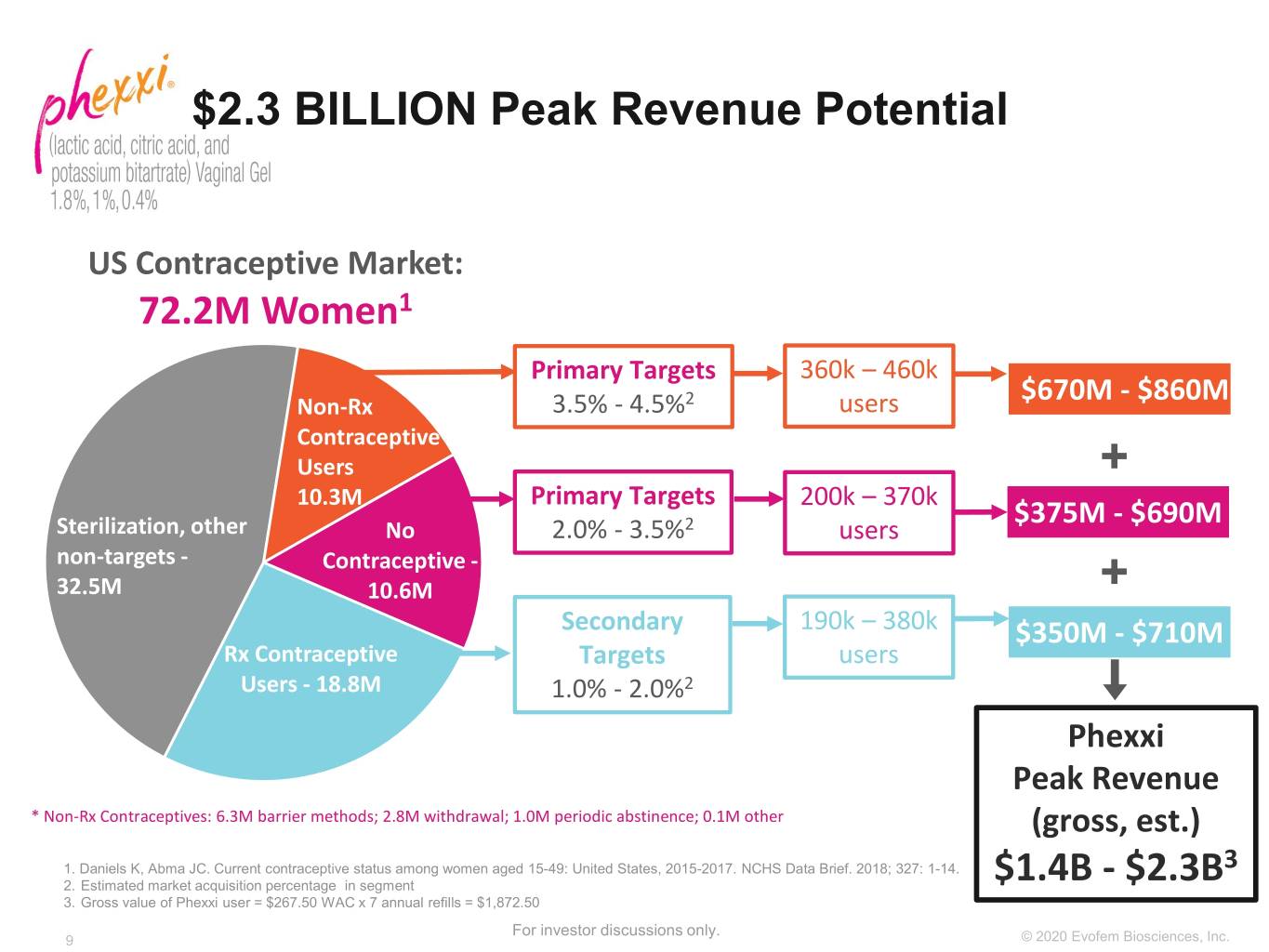
$2.3 BILLION Peak Revenue Potential US Contraceptive Market: 72.2M Women1 Primary Targets 360k – 460k Non-Rx 3.5% - 4.5%2 users $670M - $860M Contraceptive Users + 10.3M Primary Targets 200k – 370k $375M - $690M Sterilization, other No 2.0% - 3.5%2 users non-targets - Contraceptive - 32.5M 10.6M + Secondary 190k – 380k $350M - $710M Rx Contraceptive Targets users Users - 18.8M 1.0% - 2.0%2 Phexxi Peak Revenue * Non-Rx Contraceptives: 6.3M barrier methods; 2.8M withdrawal; 1.0M periodic abstinence; 0.1M other (gross, est.) 1. Daniels K, Abma JC. Current contraceptive status among women aged 15-49: United States, 2015-2017. NCHS Data Brief. 2018; 327: 1-14. 3 2. Estimated market acquisition percentage in segment $1.4B - $2.3B 3. Gross value of Phexxi user = $267.50 WAC x 7 annual refills = $1,872.50 For investor discussions only. 9 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
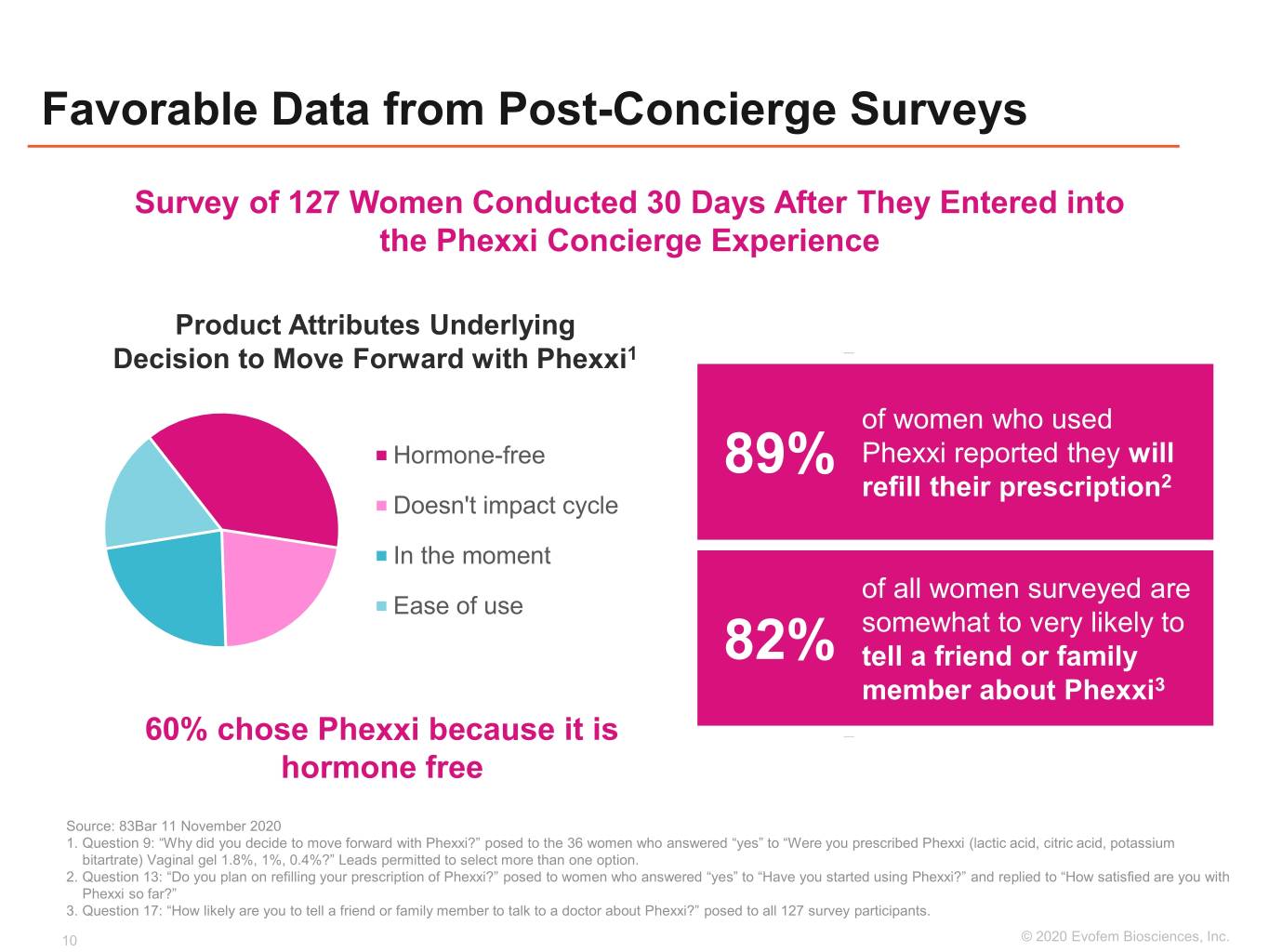
Favorable Data from Post-Concierge Surveys Survey of 127 Women Conducted 30 Days After They Entered into the Phexxi Concierge Experience Product Attributes Underlying Decision to Move Forward with Phexxi1 of women who used Hormone-free Phexxi reported they will 89% refill their prescription2 Doesn't impact cycle In the moment of all women surveyed are Ease of use somewhat to very likely to 82% tell a friend or family member about Phexxi3 60% chose Phexxi because it is hormone free Source: 83Bar 11 November 2020 1. Question 9: “Why did you decide to move forward with Phexxi?” posed to the 36 women who answered “yes” to “Were you prescribed Phexxi (lactic acid, citric acid, potassium bitartrate) Vaginal gel 1.8%, 1%, 0.4%?” Leads permitted to select more than one option. 2. Question 13: “Do you plan on refilling your prescription of Phexxi?” posed to women who answered “yes” to “Have you started using Phexxi?” and replied to “How satisfied are you with Phexxi so far?” 3. Question 17: “How likely are you to tell a friend or family member to talk to a doctor about Phexxi?” posed to all 127 survey participants. 10 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.

® 11 ©©2019 2020 EvofemEvofem Biosciences,Biosciences, Inc.Inc.
