Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Tricida, Inc. | fdatypeameetingupdate_ex.htm |
| 8-K - 8-K - Tricida, Inc. | tcda-20201025.htm |

Exhibit 99.1 Treating Metabolic Acidosis and Slowing the Progression of Chronic Kidney Disease Investor Presentation – End-of-Review Type A Meeting Update October 2020

Forward Looking Statements Any statements contained in this presentation or made during the accompanying oral presentation that are not statements of historical facts are forward-looking statements as defined under the Federal securities laws. Examples of such statements include our plans, beliefs, intentions, expectations and projections regarding, among other things: (i) the Company’s expectations with regard to its interactions and communications with the FDA; (ii) its plans and expectations as to the pathway to approval of veverimer by the FDA and the design of its ongoing clinical trials; and (iii) expectations regarding financial runway. Any such forward-looking statements are based on our current expectations and assumptions, but are subject to a number of risks and uncertainties that could cause our actual future results to differ materially from our current expectations or those implied by the forward-looking statements. The risks and uncertainties that could adversely affect our forward-looking statements include, but are not limited to: (i) the timing of the FDA’s approval of veverimer, if at all; (ii) the potential availability of the Accelerated Approval Program and the approvability of veverimer under that program; (iii) the Company’s plans and expectations with regard to its interactions with the FDA, including the potential resubmission of an NDA for veverimer; (iv) the Company’s plans and expectations for VALOR-CKD and future clinical and product development milestones; (v) the Company’s financial projections and cost estimates; (vi) risks related to COVID-19; and (vii) risks associated with the Company’s business prospects, financial results and business operations. These and other factors that may affect our future results and operations are identified and described in more detail in our filings with the Securities and Exchange Commission (the “SEC”), including the Company’s most recent Annual Report filed on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. You should not place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. Except as required by applicable law, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results, later events or circumstances or to reflect the occurrence of unanticipated events. 2

Executive Summary • Tricida has worked with FDA on accelerated approval for veverimer since 2017 • Prior to the End-of-Review Type A meeting, Tricida had 20 relevant FDA interactions all of which focused on development of veverimer based solely on the use of serum bicarbonate as the surrogate endpoint to enable accelerated approval and had not included a request for data on CKD progression from a near-term interim analysis of the VALOR-CKD trial • Tricida has developed a deep understanding of the surrogate effect of serum bicarbonate and how it reasonably likely translates to clinical benefit • In the End-of-Review Type A meeting, FDA provided feedback that Tricida believes introduces an additional requirement of data on CKD progression in addition to serum bicarbonate for accelerated approval • Based on the endpoint and patient population, Tricida does not believe it can provide early information on CKD progression without compromising the integrity of VALOR-CKD • Tricida is reorganizing the company to extend its financial runway in order to maximize its options for bringing veverimer to patients 3

20 Key FDA Interactions: No Prior Indication of a Requirement for CKD Progression Data for Accelerated Approval • Feb 2017: FDA agreement on Accelerated Approval • Aug 2019: Teleconference to discuss assessments of Program physical functioning in VALOR-CKD protocol • May 2017: FDA comments on TRCA-301 protocol to • Oct 2019: NDA Filing Letter support accelerated approval • Jan 2020: FDA comments on assessments of physical • Jul 2017: Meeting to discuss FDA’s comments on functioning in VALOR-CKD protocol TRCA-301 protocol • Jan 2020: Mid-Cycle Communication Agenda • Nov 2017: FDA response regarding proposed • Jan 2020: Mid-Cycle Meeting quantitative predictive model • Apr 2020: Late-Cycle Meeting Background Package • Mar 2018: Teleconference to discuss quantitative predictive model and VALOR-CKD study design • May 2020: Late-Cycle Meeting • May 2018: FDA response regarding quantitative • Jul 2020: FDA notification that deficiencies preclude predictive model discussion of labeling • Jun 2018: FDA response regarding draft VALOR-CKD • Jul 2020: FDA response regarding draft VALOR-CKD protocol SAP • Sep 2018: FDA comments on VALOR-CKD protocol • Aug 2020: FDA Complete Response Letter • Jun 2019: Pre-NDA Meeting • Oct 2020: FDA preliminary comments for End-of-Review Type A meeting SAP = Statistical Analysis Plan

Tricida Believes that Veverimer Is an Appropriate Candidate for Accelerated Approval • End-stage renal disease (ESRD) is a serious disease and data support the link between metabolic acidosis and progression of CKD • There is a high unmet need for an approved therapy • Serum bicarbonate is a surrogate endpoint that is reasonably likely to predict clinical benefit in patients with CKD and metabolic acidosis based on data describing the pathophysiology of metabolic acidosis, data from interventional trials and observational cohort analyses, and the availability of two validated models that consistently describe the relationship between serum bicarbonate and the renal outcome measured in VALOR-CKD • The TRCA-301 and TRCA-301E trials met all primary and secondary endpoints with high statistical significance • Tricida believes veverimer’s benefits outweigh the risk associated with use 5

Tricida has a Deep Understanding of the Surrogate Effect and How It Reasonably Likely Translates to Clinical Benefit • Tricida focused the End-of-Review Type A meeting on the topic of whether the magnitude of treatment effect observed in TRCA-301/301E was reasonably likely to predict clinical benefit and support VALOR-CKD powering – Two predictive models consistently describe the association between serum bicarbonate values and the risk of CKD progression: the new time-dependent predictive model in > 24,000 US patients with metabolic acidosis and CKD shows 8.4% lower risk for each 1 mEq/L higher serum bicarbonate value – The predictive models support Tricida’s belief that treatment effects ≥ 2 mEq/L are expected to provide clinical benefit in slowing CKD progression – The magnitude of the veverimer treatment effect in TRCA-301/301E is not best described by the between- group difference in the means because the data are not normally distributed. Between-group differences in medians are more appropriate in this case. The Week 52 median placebo-subtracted treatment effect was 3.15 mEq/L in TRCA-301E – Based on a median treatment effect of 3.15 mEq/L and the time-dependent predictive model, a hazard ratio of 0.76 is assumed for the ongoing VALOR-CKD confirmatory outcome study – We believe that VALOR-CKD is adequately designed and powered to confirm the clinical benefit of veverimer treatment. Using the 52-week median treatment effect from TRCA-301E (3.15 mEq/L), it has 87% power to show a 24% difference in primary endpoint events 6

Relevant FDA Comments in the CRL and July 30 Advice Letter • CRL indicated that FDA was seeking additional data from at least one additional adequate and well-controlled study demonstrating the efficacy of veverimer for the treatment of metabolic acidosis associated with CKD • In the CRL, the FDA expressed willingness to meet with Tricida to discuss options for approval of veverimer, including: – Completion of our ongoing confirmatory trial, VALOR-CKD, and if the trial is successful, submitting the results to support a conventional approval, or – Possibly conducting a pre-specified interim analysis of data from VALOR-CKD to support accelerated approval • In a July 30, 2020 advice letter, the FDA suggested that a group sequential design for VALOR-CKD would be more efficient than Tricida’s proposed adaptive design with unblinded sample size re-estimation 7

Tricida Now Believes FDA Will Require Data on CKD Progression for Accelerated Approval of Veverimer • During the End-of-Review Type A meeting, Tricida presented information and made proposals that the company believes addressed the issues raised by the FDA in the CRL, including: – Information to support its belief that the data from the TRCA-301 and TRCA-301E trials were sufficient to support approval under the Accelerated Approval Program, including with respect to the magnitude and durability of the treatment effect of veverimer on the surrogate endpoint of serum bicarbonate and the applicability of the findings to the US population – A proposal to conduct an interim analysis of serum bicarbonate data from VALOR-CKD in ~500 patients treated for 12 months for purposes of confirming the treatment effect of veverimer observed in TRCA-301/301E and its applicability to the U.S. population and practice of medicine – If accepted by the FDA, Tricida believes this proposal would allow resubmission of the NDA for veverimer within a matter of months • Based on feedback received during the meeting, Tricida now believes the FDA will require evidence of effect on CKD progression from a near-term interim analysis of VALOR-CKD for accelerated approval of veverimer and it is unlikely to rely solely on serum bicarbonate data – Tricida believes any requirement for early interim data on CKD progression is inconsistent with the Accelerated Approval Program – The Division did not request early interim data on CKD progression in the preliminary comments we received prior to the Type A meeting or in the CRL, nor at any time previously in the Accelerated Approval development program 8

Current Status • As of October 28, 2020, the VALOR-CKD trial has randomized 1,208 of 1,600 patients with an average treatment duration of ~11 months and has accrued 42 of the ~500 required primary endpoint events • Tricida is evaluating whether it is possible to provide the FDA with interim data on CKD progression from VALOR-CKD, however: – Tricida believes that based on the severity of kidney disease in its patient population and the double-blind, placebo controlled, time-to-event design of our outcomes trial, unblinded interim analyses of data on CKD progression are not appropriate or feasible – Tricida believes its current development program meets the requirements for accelerated approval – Tricida is committed to protecting the integrity of the VALOR-CKD trial, which was intended as the confirmatory outcomes study for approval of veverimer under the Accelerated Approval Program • Tricida plans to wait for formal meeting minutes from the FDA related to the Type A meeting prior to determining how to proceed with obtaining regulatory approval for veverimer. Tricida expects to receive the formal minutes within 30 days from the meeting 9

Metabolic Acidosis in CKD – An Unmet Need Managing Risk Clinical Practice The Goals of Treatment Factors of CKD Guidelines of Metabolic Acidosis are to: Progression KDIGO & KDOQI Good management to recommend treatment for 1 2 3 prevent nutritional decline, people with CKD whose functional decline, as well serum bicarbonate is Correct Decrease Reduce as acidosis, can help slow < 22 mEq/L. There are chronic protein catabolism down CKD progression currently no approved bone systemic caused by skeletal treatments in the US demineralization acidemia muscle proteolysis Available Limitations of Unapproved Current Supplements Supplements 4 5 Common comorbidities Sodium- & potassium- Prevent such as hypertension, Slow the containing alkali salts are heart failure, edematous protein- available to treat MA, progression however are used only in states & hyperkalemia are energy < 3% of CKD patients with absolute or relative wasting & of CKD serum bicarbonate levels contraindications to < 22 mEq/L (Dobre, 2013) treatments that ↑ sodium nutritional &/or potassium intake decline A significant unmet need exists for additional treatment options for people with CKD and metabolic acidosis. A treatment that is safe and that effectively treats metabolic acidosis without introducing unwanted ions, would address this need Bushinsky, AJKD, 2019. Dobre et al., AJKD, 2013. Kovesdy et al., Int Urol Nephrol, 2016. KDIGO CKD Work Group, Kidney Inter, Suppl 2013. Eknoyan G et al., Am J Kidney Dis., 2003. 10

Tricida Believes Veverimer’s Benefits Outweigh the Risks Associated with Use Veverimer – Potential Benefits Veverimer – Potential Risks Benefit The safety of up to 1 year of treatment with veverimer has been Serum Bicarbonate: studied in a complex population of patients with Stage 3 − 4 CKD and Rapid onset of effect metabolic acidosis: Predictable offset of effect Veverimer was well tolerated with high treatment adherence and Evidence of efficacy over 1 year of treatment an excellent safety profile Physical functioning: KDQOL-PFD: Significant improvement in score Percentage of subjects with SAEs or treatment discontinuations at week 52 from baseline in the veverimer group due to AEs was low overall, but higher in the placebo group vs. the placebo-treated group [11.42 vs. -0.71 KDQOL-PFD versus the veverimer group. points, p < 0.0001], exceeding the 3 to 5 point The only AEs reported in ≥ 5% of subjects in which the minimally important clinical difference incidence was higher (> 1% difference) for veverimer than for Chair stand test: There was a significant placebo were nontreatment limiting diarrhea and flatulence, decline in the mean time to complete the test at typically lasting a few days week 52 vs. baseline in the veverimer group vs. the placebo group [-4.28 vs. -1.42 seconds, No evidence of off-target binding p < 0.0001], exceeding the 1.7 second Negligible risk of veverimer involvement in clinically significant minimally important clinical difference drug-drug interactions Given the findings of the surrogate endpoint of serum bicarbonate, as well as the improvements observed in how subjects felt and functioned, the benefits of treatment with veverimer outweigh the risks associated with use Wesson et al., Lancet 2019; 393 1417-27. Wesson et al., Lancet 2019; 394: 396-406. 11

Organizational Update and Financial Position • Tricida is re-organizing the company to extend its financial runway in order to maximize its options for bringing veverimer to patients – Significantly reducing its headcount – Tricida will discuss its commitments with vendors and contract service providers to potentially provide additional financial flexibility • Cash and cash equivalents at September 30, 2020 were ~$375 million – Tricida has $75 million principal amount of debt with Hercules which is scheduled to be amortized from April 2021 to April 2023 – Tricida has $200 million principal amount of 3.5% Convertible Senior Notes which mature in May 2027 12

THANK YOU

Supplemental Information
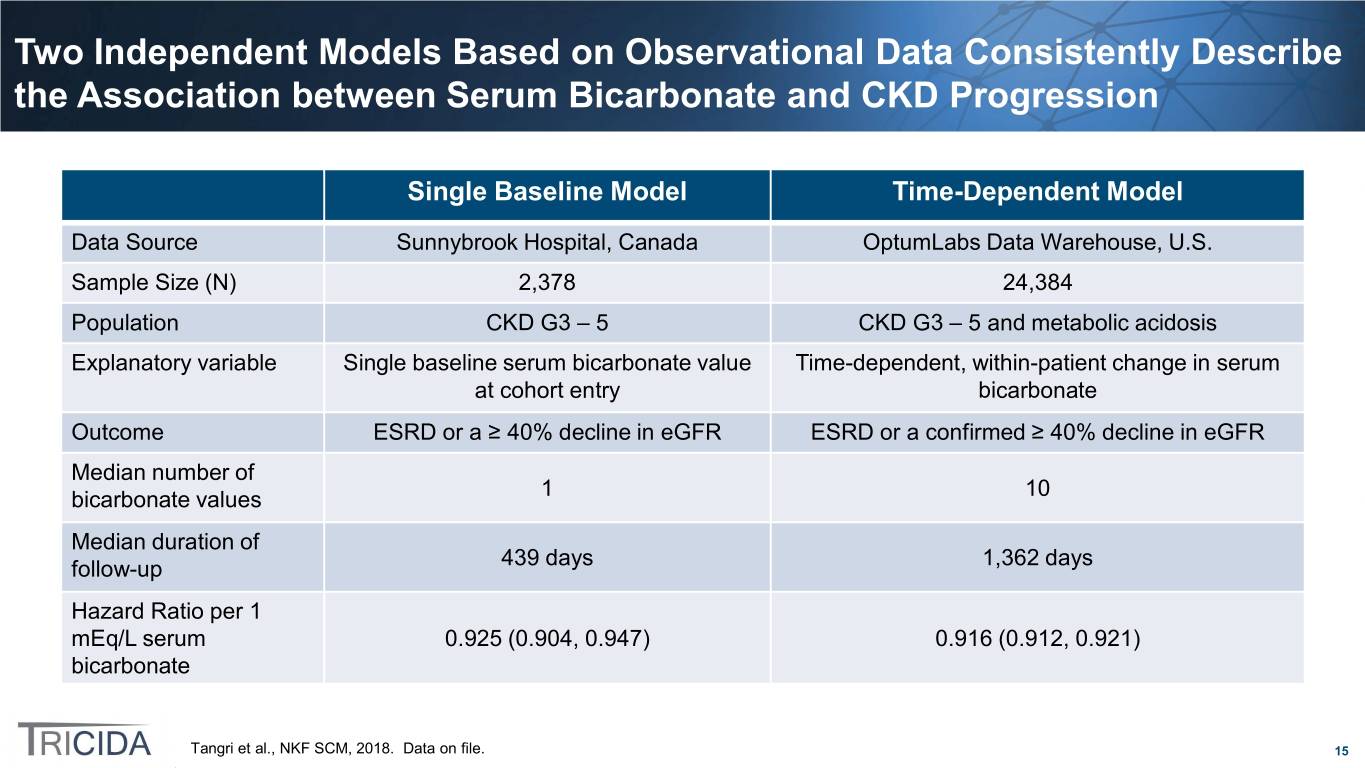
Two Independent Models Based on Observational Data Consistently Describe the Association between Serum Bicarbonate and CKD Progression Single Baseline Model Time-Dependent Model Data Source Sunnybrook Hospital, Canada OptumLabs Data Warehouse, U.S. Sample Size (N) 2,378 24,384 Population CKD G3 – 5 CKD G3 – 5 and metabolic acidosis Explanatory variable Single baseline serum bicarbonate value Time-dependent, within-patient change in serum at cohort entry bicarbonate Outcome ESRD or a ≥ 40% decline in eGFR ESRD or a confirmed ≥ 40% decline in eGFR Median number of bicarbonate values 1 10 Median duration of follow-up 439 days 1,362 days Hazard Ratio per 1 mEq/L serum 0.925 (0.904, 0.947) 0.916 (0.912, 0.921) bicarbonate Tangri et al., NKF SCM, 2018. Data on file. 15

Randomized Controlled Trials Show Clinical Benefit of Group Mean Treatment Effects of 2 – 3 mEq/L • A meta-analysis of 14 randomized controlled trials (N=1,394) found that increasing serum bicarbonate resulted in slower decline of kidney function – Average increase in bicarbonate • 3.3 mEq/L (95% CI: 2.4 – 4.3) in a random effects model • 2.3 mEq/L (95% CI: 2.1 – 2.5) in a fixed effects model • Net effect on eGFR decline = -3.28 mL/min/yr (95% CI: -4.42,-2.14; 13 trials) • Hazard ratio (HR) for ESRD = 0.32 (95% CI: 0.18, 0.56; 4 trials) Navaneethan et al, CJASN, 2019. 16

A Treatment Effect of ≥ 2 mEq/L is Reasonably Likely to Predict Clinical Benefit with a HR Consistent with Other Approved Cardio-Renal Drugs HR = Hazard Ratio. Tangri et al., NKF SCM, 2018. Data on file. Brenner et al., NEJM, 2001. Lewis et al., NEJM, 2001. Wallentin et al., NEJM, 2009. 17

Magnitude of Treatment Effect Observed in TRCA-301/301E Should be Described by the Difference in Medians • Post-hoc analysis of change from baseline in serum bicarbonate from TRCA-301/301E showed that the data were not normally distributed – Shapiro-Wilk test (p < 0.05) at Week 12 and Week 52 • Thus, the between-group difference of the medians, rather than means, are a better descriptor of the magnitude of change from baseline in serum bicarbonate (i.e., the treatment effect) and therefore we believe are more appropriate for use in estimating the power of the ongoing Median Change from Baseline in Serum Bicarbonate (mEq/L) Timepoint Veverimer Placebo Difference P-value* Week 12 4.70 1.50 3.20 <0.0001 Week 52 5.40 2.25 3.15 0.0001 *post hoc ANCOVA using ranks. Data on file. 18

Magnitude of Effect: Patients Receiving Veverimer Are Reasonably Likely to Derive Benefit Substantially more patients on veverimer than placebo have large increases in serum bicarbonate (e.g., >3, >4, >5 mEq/L) Distribution of mean changes from baseline in all bicarbonate values over the 52-week TRCA-301E study (post-hoc analysis) Proportion of Patients with Mean Increase ≥ 5 mEq/L 52.6% (60 of 114): Veverimer 8.5% (7 of 82): Placebo Proportion of Patients with Mean Increase ≥ 4 mEq/L 63.2% (72 of 114): Veverimer 15.9% (13 of 82): Placebo Proportion of Patients with Mean Increase ≥ 3 mEq/L 73.7% (84 of 114): Veverimer 26.8% (22 of 82): Placebo Data on file. 19
