Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Inmune Bio, Inc. | ea128926-8k_inmunebio.htm |
Exhibit 99.1

www.roth.com Member: FINRA / SIPC 1 of 15 COVID - 19 Review October 2020

www.roth.com Member: FINRA / SIPC 2 of 15 Forward Looking Statements

www.roth.com Member: FINRA / SIPC 3 of 15 Speaker: RJ Tesi MD CEO/CMO Dr . Tesi has been President, Chief Executive Officer and acting Chief Medical Officer since the formation of the Company in September 2015 . Dr . Tesi’s career in medicine and biopaharma has focused on strategies to manipulate the immune system . First, as an academic liver/pancreas/kidney transplant sugeon , he focused on controlling the immune systems attempt to reject life saving organs . While managing immunosuppression on a daily basis, he was introduced to the tricky balance between enough immunosuppression to prevent rejection and too much immunosuppression resulting in life threatening viral infections . In biopharma, his focus has been on controlling the immune response as it relates to disease including autoimmune disease, cancer, neurodegenerative, metabolic and infectious diseases . Dr . Tesi is one of the three founders of INmune Bio, a company focused on reprograming the innate immune system to treat disease . Before his roles at INmune Bio, Dr . Tesi has held senior leadership roles in private and public companies . Dr . Tesi received his medical degree from Washington University in St . Louis and held board certification is general surgery and transplant surgery during his academic career .

www.roth.com Member: FINRA / SIPC 4 of 15 INmune Bio Company Overview Product Platforms • Founded September 2015 • NASDAQ listed (INMB) • Simple capital structure • Multiple clinical programs • Strong IP portfolio • Identifiable near - term catalysts . • DN - TNF platform for treatment dysregulated innate immunity • XPro1595 for Alzheimer’s disease • XPro1595 for Treatment Resistant Depression • Quellor for treatment of COVID19 cytokine storm • INB03 for MUC4 expressing cancers • LIVNate for NASH • INKmune to eliminate minimal residual disease • Treatment of high - risk MDS • Eliminate MRD in CaOva Dark programs are in or soon to be in clinic

www.roth.com Member: FINRA / SIPC 5 of 15 Company Overview Development Platforms • Founded Sept 2015 • NASDAQ: INMB • Simple cap structure • Innate immune system focused • DN - TNF for CNS, immuno - oncology, COVID19 and metabolic diseases • INKmune – an NK cell focused platform targeting minimal residual disease

www.roth.com Member: FINRA / SIPC 6 of 15 Modifying the Catastrophic Complications of COVID19 by targeting the Master Cytokine with Quellor A Phase II trial

What brings patients to the hospital with C19? • Reality #1: At time of admission viral titer is decreasing • Reality #2: The dysregulated innate immune response to the virus causes Cytokine Storm • Problem: Cytokine Storm is making patients sick • Hypothesis: Blunting the effects of Cytokine Storm will alter the arch of the disease • Solution: Target the Master Cytokine of the Cytokine Storm with Quellor Quellor non - confidential October 2020 7
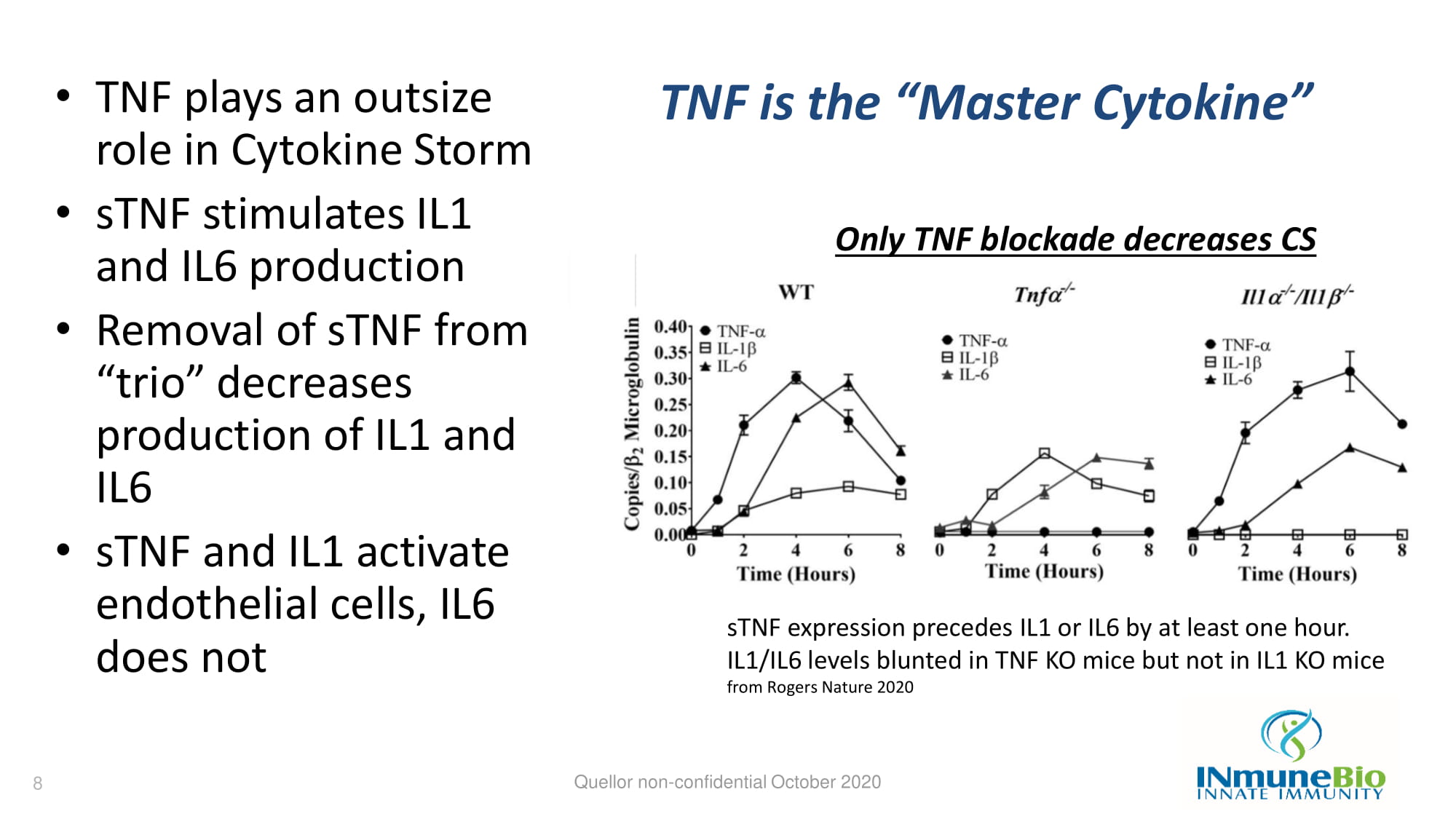
TNF is the “Master Cytokine” • TNF plays an outsize role in Cytokine Storm • sTNF stimulates IL1 and IL6 production • Removal of sTNF from “trio” decreases production of IL1 and IL6 • sTNF and IL1 activate endothelial cells, IL6 does not Quellor non - confidential October 2020 sTNF expression precedes IL1 or IL6 by at least one hour. IL1/IL6 levels blunted in TNF KO mice but not in IL1 KO mice from Rogers Nature 2020 Only TNF blockade decreases CS 8

The ‘3’ pathologies of TNF in COVID - 19 1. Immune cell activation - activated cells produce cytokines 2. Endothelial cell activation – Up - regulation of ICAM/VCAM for transmigration of immune cells from blood vessel to tissue – Up - regulation of Tissue Factor to cause coagulopathy 3. Promotes infection of cells – TNF up - regulates Neurophilin - 1 - both Neurophilin - 1 and ACE2 receptor binding required for COVID19 entry to cell Quellor non - confidential October 2020

Quellor MOA see Steed et al., Science , 301, 2003 Receptor binding, Receptor binding, "small" domain (subunit A) Subunit A Receptor binding, (subunit A) Mutation Y87H (subunit C) Mutation R31C (for 10kD pegylation) "large" domain Subunit C Subunit B Mutation A145R Nonglycosylated small homotrimeric protein expressed in E.coli 10 Quellor non - confidential October 2020 Quellor neutralizes sTNF without effecting tmTNF

Phase I experience - AD • Phase I oncology – ACTRN12618000675 224 – Safe and ↓CRP, CCL2, and IL6 • Phase I AD – NCT03943264 – On - going – reported preliminary data on 6 patients 13 July 2020 Quellor non - confidential October 2020 White Mater Free Water is a biomarker of neuroinflammation Dose response in white matter free water safe mask Decreased neuroinflammation in arcuate fasciculus – a language white matter tract 11
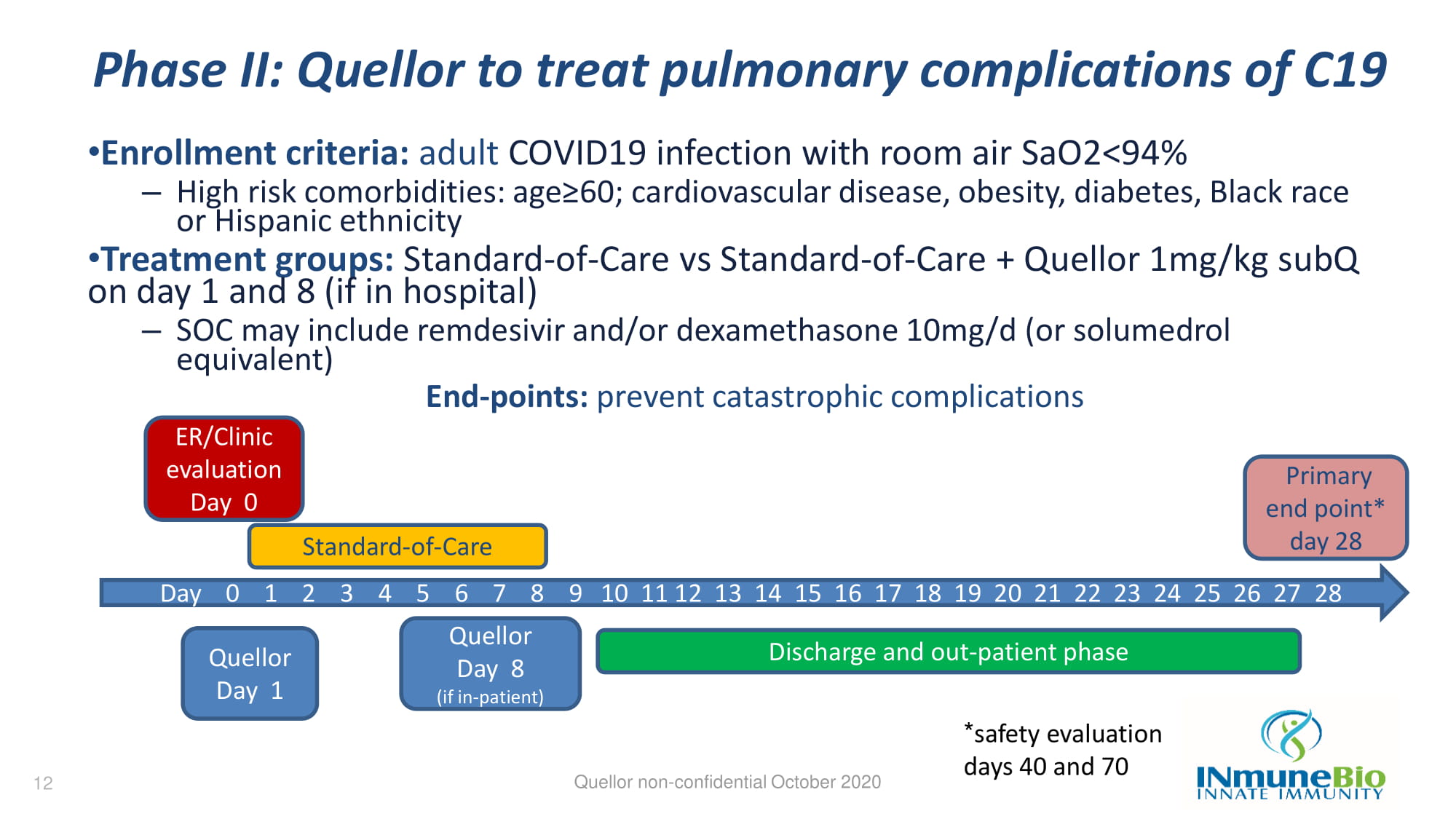
Phase II: Quellor to treat pulmonary complications of C19 • Enrollment criteria: adult COVID19 infection with room air SaO2<94% – High risk comorbidities: age≥60; cardiovascular disease, obesity, diabetes, Black race or Hispanic ethnicity • Treatment groups: Standard - of - Care vs Standard - of - Care + Quellor 1mg/kg subQ on day 1 and 8 (if in hospital) – SOC may include remdesivir and/or dexamethasone 10mg/d (or solumedrol equivalent) End - points: prevent catastrophic complications Quellor non - confidential October 2020 Day 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 ER/Clinic evaluation Day 0 Quellor Day 8 (if in - patient) Quellor Day 1 Standard - of - Care Discharge and out - patient phase Primary end point* day 28 12 * safety evaluation days 40 and 70

www.roth.com Member: FINRA / SIPC 13 of 15
