Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Immunome Inc. | tm2034229d1_8k.htm |
Exhibit 99.1

Developing Antibody - Based Therapeutics by Unlocking the Human Memory B Cell Response Immunome, Inc. 665 Stockton Drive, Suite 300 Exton, PA 19341 610.321.3700 www.immunome.com October 26, 2020

This presentation contains “forward - looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. The Company intends forward - looking terminology such as “believes,” “anticipates,” “plans,” “may,” “intends,” “will,” “should,” “exp ects,” and similar expressions to identify forward - looking statements. Further, forward - looking statements include, but are not limited to, statements regarding the Company’s financial position, strategy, business plans, expectations regarding the timing and achiev eme nt of its product candidate development activities and ongoing and planned preclinical studies and clinical trials, plans and expectati ons for future operations and research and development initiatives. Any statements contained herein that are not statements of historical fa cts may be deemed to be forward - looking statements. These forward - looking statements involve risks and uncertainties, including regarding t he Company’s expectation that it will incur net losses for the foreseeable future, and that it may never be profitable; its need fo r additional funding; the timing and success of preclinical studies and clinical trials it conducts; the ability to obtain and maintain re gul atory approval of its product candidates; the ability to commercialize its product candidates; its ability to compete in the marketplace; devel opm ents relating to the COVID - 19 pandemic; its ability to obtain and maintain intellectual property protection for its product candidates; and it s ability to manage growth. These or other risks or uncertainties may cause the Company’s actual results to differ materially from any for war d - looking statements. Forward - looking statements speak as of the date they are made, and the Company undertakes no obligation to update t hem. This document may contain product names, trade names, trademarks and service marks of the Company and of other organizations, of which are the properties of their respective owners. Forward Looking Statements Immunome, Inc. | Page 2

Immunome Overview Harnessing the power of the most - educated components of the human immune system • Immunome leverages its proprietary Discovery Engine, which enables unbiased interrogation of human memory B cells, to simultaneously identify potential first - in - class antibody therapeutics and novel antigen targets • Focused on oncology and infectious diseases – Two lead discovery programs: oncology (targeting IL - 38) and infectious disease (targeting SARS - CoV - 2) – Anticipate filing INDs for both programs in 2021 • Discovery Engine efficiency potentially allows 1 - 2 programs entering IND - enabling studies per year – Discovery Engine also allows for multiple partnerships; currently partnered with U.S. Department of Defense (COVID - 19) and pH Pharma (oncology antibody - drug conjugates) • Experienced management team and advisory board with deep scientific and operational expertise • Broad intellectual property estate covering all aspects of the Discovery Engine Immunome, Inc. | Page 3

Immunome, Inc. | Page 4 Immunome Approach Our approach harnesses the power of human memory B cells to discover first - in - class therapeutic monoclonal antibody candidates directed at potentially novel targets Partnerships • Contract from U.S. Department of Defense to fund COVID - 19 program, up to $13.3M • Oncology partnership (antibody - drug conjugate) with pH Pharma Infectious Diseases • Rapid identification of antibodies from convalescing patients • COVID - 19 Biosynthetic Convalescent Plasma (BCP) IND filing anticipated in 1H 2021 • Growing portfolio of 50+ novel antibody - target pairs • Lead discovery program IND filing anticipated 2H 2021 Oncology Growth strategy anticipates 1 - 2 programs entering IND enabling studies per year

The Power of Memory B Cells Memory B cells are critical players in human immune response • Memory B cells are a subset of B cells that remember specific antigens and allow for a rapid antibody response upon re - challenge 1 • Memory B cells produce the high - affinity antibodies needed to fight disease • In oncology, the presence of memory B cells in tumors is associated with favorable outcomes in response to immunotherapy 2 - 4 • Immunome immortalizes memory B cells into large, stable hybridoma* libraries; we then interrogate thousands of individual hybridoma - produced antibodies to find unique, therapeutically - relevant antibody / antigen target pairs Immunome, Inc. | Page 5 1. B Cell Localization and Migration in Health and Disease, Anja E. Hauser, Uta E. Höpken, in Molecular Biology of B Cells (Seco nd Edition), 2015 2. Helmink et al Nature 577, 549 - 555; 2020 3. Petiprezet al Nature 577, 556 - 560; 2020 4. Cabrita et al Nature 577 ,561 - 565; 2020 * Hybridomas are hybrid cells used to produce antibodies.

Immunome Discovery Engine Unbiased interrogation of memory B cells reveals potentially novel targets • Primary human memory B cells immortalized as stable hybridomas. Thousands of hybridomas per patient • High - throughput screening (20,000 antibodies / array). Deep interrogation of patient response to generate “hits” • High conversion rate from hits to targets • Potential therapeutic antibodies for use in oncology and infectious diseases Immunome, Inc. | Page 6 Antibody Screening Deep, multiplexed interrogation of patient memory B cell responses Patient Response Capture the memory B cells from cancer or infectious disease patients Antibody Validation Definitive target identification and characterization of antibody / target interactions Discovery Engine Output Unique therapeutic antibody / antigen target pairs Patient Sampling Ongoing access to new and diverse patient memory B cells to feed the engine

Immunome, Inc. | Page 7 Immunome Pipeline & Anticipated Milestones Oncology Target Product Candidate Description Discovery Next Milestone IMM - ONC - 01 IL - 38 I/O, Novel Checkpoint Anticipate IND filing 2H 2021 Anti - infectives Target Product Candidate Description Discovery Next Milestone IMM - BCP - 01 Multiple SARS - CoV - 2 proteins Biosynthetic convalescent plasma Anticipate IND filing 1H 2021

Oncology

Oncology Landscape Today Immunome, Inc. | Page 9 • Despite significant advances, the five - year survival rate for patients with advanced malignancies of the lung, liver, stomach, pancreas and other organs is less than 10% • Discovery of new targets and novel therapeutics for cancer treatment is vitally important • Immunome’s efforts are guided by patients who have effectively mounted a defense against their disease • The Immunome Discovery Engine simultaneously identifies antibodies and their antigen targets by interrogating the patient’s memory B cells in an unbiased manner

Challenges with Oncology Therapies • T cell targeted immuno - oncology approaches have redefined the way we treat cancer • Large number of patients, however, cannot be treated using T cell - targeted approaches • Tumors subvert immunity by multiple mechanisms, often simultaneously 1 • T cell compartment does not act in isolation, needing to engage other arms of the immune system Immunome, Inc. | Page 10 Limited understanding of the diversity and complexity of human tumors 1. Bonaventure et al 2019 Front Immunol doi: 10.3389/fimmu.2019.00168

Immunome’s Discovery & Output Novel “target clusters“ focus on a subset of biological functions 1. Including some commercially - validated targets such as ERBB2 2. Adv Clin Chem. 2016;74:103 - 41.DOI: 10.1016/bs.acc.2015.12.005 3. Mol Cancer. 2019 Oct 23;18(1):146. doi: 10.1186/s12943 - 019 - 1074 - 3 Immunome, Inc. | Page 11 Target Cluster 2 Target Cluster 5 Target Cluster 1 IMM20059 IMM20065 Target Cluster 3 IMM20091 IMM20130 Target Cluster 4 Target Cluster 6 13 22 22 2 10 2 DISCOVERED TO DATE 250,000 hybridomas | 1,200 hits 58 antibody / antigen pairs 1 Providing Unique Insights Into Cancer Biology Patient immune response highlights common biological processes that may have disease relevance, such as exosome control of the tumor microenvironment 2 - 3 and novel immune checkpoints that serve as functional, tumor - derived inhibitors of immunity

IMM - ONC - 01 (IL - 38) Immunome, Inc. | Page 12 Immunome Discovery Engine identified the potential utility of targeting IL - 38 • Immunome’s Discovery Engine identified the potential utility of targeting IL - 38 through interrogation of the memory B cells from a patient with head and neck cancer • Over - expression of IL - 38 is seen in certain solid tumors, including head and neck cancers 1,2 • Its over - expression appears to decrease the ability of the patient’s immune system to respond to the malignancy 1,2 IL - 38 1. Immunome analysis of the Cancer Genome Atlas (TCGA) data from Firehouse Legacy dataset 2. Takada et al PLOS ONE 2017 doi.org/10.1371/journal.pone.0181598

IL - 38 Expression in Patient Tumors Immunome, Inc. | Page 13 Inverse relationship between high IL - 38 expression and immune cell populations in tumors 1. Immunome analysis of the Cancer Genome Atlas (TCGA) data from Firehouse Legacy dataset 2. Takada et al PLOS ONE 2017 doi.org/10.1371/journal.pone.0181598 Notes: IL - 38 expressed at very high levels in some biopsies of major solid tumors. 1 Biopsy material from 400 lung cancer patients revealed association between high IL - 38 expression levels and poor patient outcomes outcomes 2 0 5 0 5 10 15 0 5 0 5 10 Prostate Adenocarcinoma Colorectal Adenocarcinoma Lung Adenocarcinoma T cells (CD3E) CD3E CD3E n = 498 R 2 = 0.005 n = 382 R 2 = 0.0003 0 5 0 5 10 15 n = 517 R 2 = 0.0001 IL - 38 expression IL - 38 expression IL - 38 expression Macrophages (CD68) CD68 CD68 0 5 0 5 10 0 5 -2 0 2 4 6 8 0 5 10 15 -2 0 2 4 6 8 n = 498 R 2 = 0.0023 n = 382 R 2 = 0.0022 n = 517 R 2 = 0.0011

IMM - ONC - 01 Immunome, Inc. | Page 14 Hit antibodies are specific for IL - 38 • IL - 38 is a lesser - known member of the IL - 1 family of cytokines • It regulates adaptive and innate immune responses by binding to receptors found on immune cell subsets (e.g., macrophages, gd T cells, dendritic cells and regulatory T cells) • Binding of two Immunome anti - IL - 38 antibodies is specific for IL - 38 with orders of magnitude less binding to related cytokine family members • Immunome antibodies were able to block the activity of IL - 38 in tissue culture experiments

IL - 38 & Inflammation Immunome, Inc. | Page 15 IL - 38 modulates global inflammatory response associated with lipopolysaccharide, LPS, stimulation Treating with anti IL - 38 antibody antagonizes the effect of IL - 38 LPS + IL38 Principal Component 1 Principal Component 2 LPS only Unstimulated Principal Component Analysis nanoString data visualized using ClustViz ( Metsalu , Tauno and Vilo , Jaak . Nucleic Acids Research , 2015) LPS + IL38 + Anti - IL38
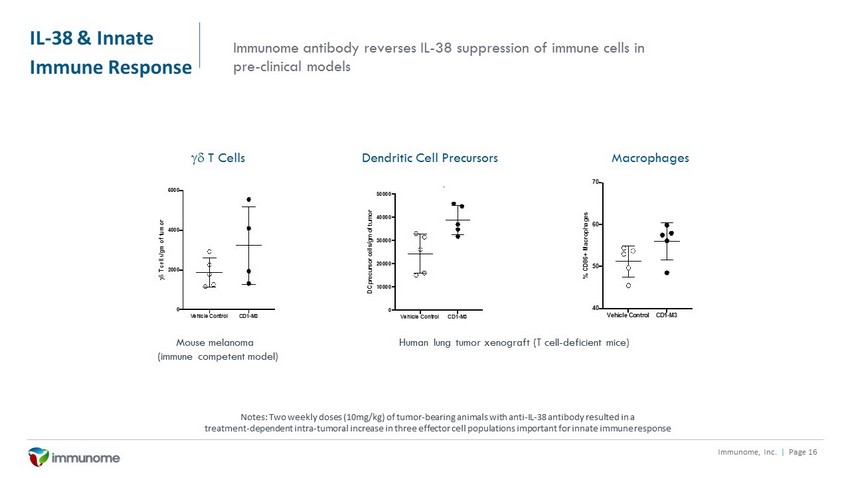
IL - 38 & Innate Immune Response Immunome, Inc. | Page 16 Vehicle Control CD1-M3 0 2000 4000 6000 g d T c e l l s / g m o f t u m o r Cells per gram of B16.F10 tumor: gd T cells Immunome antibody reverses IL - 38 suppression of immune cells in pre - clinical models Dendritic Cell Precursors Macrophages gd T Cells Mouse melanoma (immune competent model) Vehicle Control CD1-M3 0 10000 20000 30000 40000 50000 D C p r e c u r s o r c e l l s / g m o f t u m o r Cells per gram of A549 tumor: Dendritic cell precursors Vehicle Control CD1-M3 40 50 60 70 % C D 8 6 + M a c r o p h a g e s CD86+ (% of Macrophages) Human lung tumor xenograft (T cell - deficient mice) Notes: Two weekly doses (10mg/kg) of tumor - bearing animals with anti - IL - 38 antibody resulted in a treatment - dependent intra - tumoral increase in three effector cell populations important for innate immune response
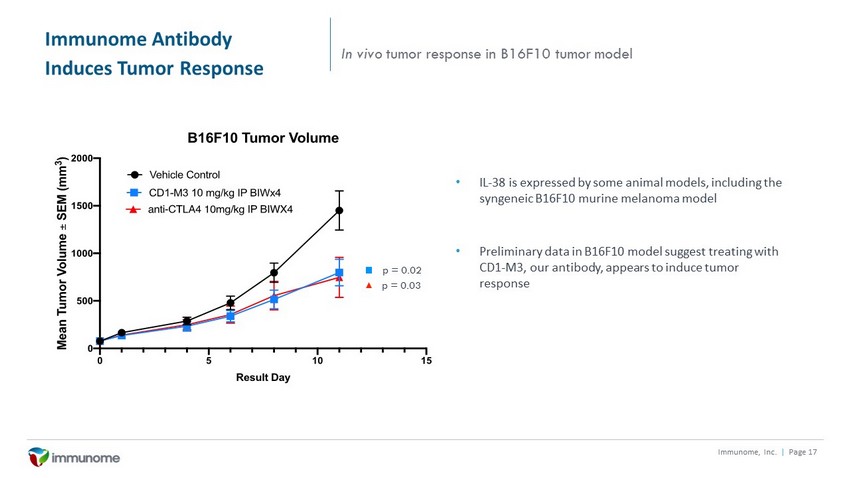
Immunome Antibody Induces Tumor Response Immunome, Inc. | Page 17 In vivo tumor response in B16F10 tumor model • IL - 38 is expressed by some animal models, including the syngeneic B16F10 murine melanoma model • Preliminary data in B16F10 model suggest treating with CD1 - M3, our antibody, appears to induce tumor response p = 0.02 p = 0.03

IMM - ONC - 01 Program Summary Immunome, Inc. | Page 18 Modulating innate immunity • IL - 38 regulates adaptive and innate immune responses by binding to receptors found on immune cell subsets • Modulation of IL - 38 levels in mouse models correlates with immune responsiveness and tumor growth • Immunome’s anti - IL - 38 antibody candidates bind and antagonize IL - 38 activity in vitro • Specifically, Immunome antibody candidate CD1 - M3 modulates intra - tumoral immune effector cell levels in vivo • Lead discovery program (IMM - ONC - 01) IND filing anticipated in 2H 2021

Infectious Diseases
Infectious Disease Application (COVID - 19) Immunome, Inc. | Page 20 Convalescent plasma treatments: while promising, challenges remain 1. Image: J Clin Invest DOI: 10.1172/JCI138003 2. Jiang et al https://doi.org/10.1101/2020.03.20.20039495 3. Bloch et al J Clin Invest https://doi.org/10.1172/JCI138745 CHALLENGES WITH CONVALESCENT PLASMA • Donor - to - donor variation; single donor will differ over the course of convalescence • Unscalable supply a single donor only treats 2 - 3 patients, which makes large - scale prophylactic treatment untenable • Must match donor to patient Treating patients with convalescent plasma CONVALESCENT PLASMA OPPORTUNITIES • Convalescing patients mount antibody responses to viral proteins in addition to Spike – Convalescent plasma has the potential to contain antibodies against several different viral proteins (e.g. S, N, Nsp5) • A broad response may induce multiple viral clearance mechanisms • National COVID - 19 Convalescent Plasma Project, among others, focus on convalescent plasma for COVID - 19 patients

COVID - 19 Biosynthetic Convalescent Plasma (BCP) Immunome, Inc. | Page 21 Single neutralizing antibody approach versus Immunome’s Biosynthetic Convalescent Plasma Neutralization Complement Fixation Phagocytosis Immunome’s BCP approach aims to leverage multiple viral clearance mechanisms to maximize the potential effectiveness of a therapeutic and/or prophylactic

Biosynthetic Convalescent Plasma Concept Collaboration with U.S. DoD awarded, up to $13.3M in funding Immunome, Inc. | Page 22 Produce antibody mixture (up to 6 Abs) using recombinant manufacture Select convalescent patients with strong anti - viral titer Biosynthetic Convalescent Plasma for potential prophylaxis and treatment Deep repertoire screening against multiple viral proteins Screen for anti - viral antibodies Collect convalescent blood and isolate memory B cells

Anti - SARS - CoV - 2 Antibodies from ”Super - Responders” More than 50% of the antibodies identified bind to SARS - CoV - 2 proteins other than Spike Immunome, Inc. | Page 23 • Immunome leverages both cell - and protein - based screening methods to identify antibodies from “super - responder” COVID - 19 patients • Screening performed for antibodies against multiple viral protein targets • To date, over 100 anti - SARS - CoV - 2 antibodies have been identified • We believe that our BCP product could elicit anti - viral effect by triggering multiple immune mechanisms and that this diversity of action may potentially decrease the likelihood of treatment resistance to any one antibody

IMM - BCP - 01 Program Summary Immunome, Inc. | Page 24 • Leverages the unbiased nature of the Immunome Discovery Engine to both capture the memory B cells of convalescent COVID - 19 patients and identify antibodies against multiple viral proteins – Patients make antibodies to multiple viral proteins, including Spike • The Immunome Discovery Engine has already identified potential antibodies against multiple viral proteins • Over 50% of them appear to bind to antigens other than Spike protein • Targeting multiple viral proteins and eliciting multiple anti - viral mechanisms may potentially decrease the likelihood of resistance to any one antibody or target • If successful, IMM - BCP - 01 could be a treatment for COVID - 19 patients, as well as a prophylactic for individuals at higher risk of contracting the SARS - CoV - 2 virus • Anticipated BCP program (IMM - BCP - 01) IND filing in 1H 2021

Immunome Summary Harnessing the power of the most - educated components of the human immune system Immunome, Inc. | Page 25 • Immunome leverages its proprietary Discovery Engine, which enables unbiased interrogation of human memory B cells, to simultaneously identify potential first - in - class antibody therapeutics and novel antigen targets • Focus on oncology and infectious diseases – Two lead discovery programs: oncology (targeting IL - 38) and infectious disease (COVID - 19) – Anticipate filing INDs for both programs in 2021 • Discovery Engine efficiency potentially allows 1 - 2 programs IND - enabling studies per year – Discovery Engine also allows for multiple partnerships; currently partnered with U.S. Department of Defense (COVID - 19) and pH Pharma (Oncology Antibody - Drug Conjugates) • Experienced management team and advisory board with deep scientific and operational expertise • Broad intellectual property estate covering all aspects of the Discovery Engine

Appendix

Management Team Michael Morin, PhD Chief Science Officer Oversaw cancer, immunology and anti - bacterial drug discovery Richard F. Fitzgerald Chief Financial Officer Former CFO of Sesen Bio, PAVmed , TechPrecision and Nucleonics, Inc. Jillian DiMuzio Senior Director, HTS & Automation Pavel Nikitin, PhD Director, Antibody Engineering Ben Harman, PhD Director, Target Identification Karen Lundgren, PhD Senior Director, Lead Development Leadership with experience in the fields of oncology, infectious disease, drug discovery and leveraging platform technologies for drug development Purnanand Sarma, PhD President & CEO Former CEO, TARIS Biomedical; VP & GM, Cephalon; VP & Managing Director, Nektar Therapeutics Immunome, Inc. | Page 27 Matthew Robinson, PhD SVP, Research & Development Sandra Stoneman, Esq. Chief Legal Officer Former Corporate Partner and Life Sciences Lead at Duane Morris Former Partner and Life Sciences Lead, Duane Morris, LLP

Michael Rapp Managing Partner, Broadband Capital Investments, LLC Richard Baron Formerly Chief Financial Officer, Zynerba Pharmaceuticals John LaMattina, PhD Formerly President, Pfizer Global Research & Development Michael Lefenfeld President and CEO, Cyanco International Co - founder of SiGNa Chemistry Purnanand Sarma, PhD President & CEO, Immunome Inc. Philip Wagenheim Managing Partner, Broadband Capital Partners, LLC Michael Widlitz, MD Formerly of clinical development & medical affairs at Pfizer Board of Directors Immunome, Inc. | Page 28

Scott Dessain, MD, PhD Chair, Founder Joseph and Ray Gordon Chair of Clinical Oncology and Research at Lankenau Institute for Medical Research George Prendergast, PhD Cancer Biology Former Editor of Cancer Research President and CEO of Lankenau Institute for Medical Research William Strohl, PhD Antibody Engineering & GMP Formerly Centocor; Biologics Lead at J&J Anthony Tolcher, MD, FRCPC Early Clinical Development NEXT Oncology, San Antonio Medical Center Louis Weiner, MD Immuno - Oncology Director, Georgetown Lombardi Comprehensive Cancer Center and Director, MedStar Georgetown Cancer Institute Scientific Advisory Board Immunome, Inc. | Page 29

Michael Diamond , MD, PhD Washington University School of Medicine The Herbert S. Gasser Professor of Medicine and Professor of Molecular Biology Pathology and Immunology Associate Director, for the Andrew M. and Jane M. Bursky Center for Human Immunology and I mmunotherapy Programs. Jeffery Henderson , MD, PhD Washington University Associate Professor of Medicine and Molecular Biology M ember, National Convalescent Plasma Project (CCPP19) Samuel Shoham , MD Johns Hopkins University School of Medicine Associate Professor of Medicine M ember, National Convalescent Plasma Project (CCPP19) Susan Weis , PhD University of Pennsylvania Perelman School of Medicine Professor and Vice Chair, Department of Microbiology Co - Director, Penn Center for Research on Coronaviruses and Other Emerging Pathogens COVID - 19 Advisory Board Immunome, Inc. | Page 30

Proprietary Screening Interrogation of patient response with depth, breadth and speed Every antibody screened against approximately 100 antigen sources To date, 1,200+ screening hits identified 20,000 antibodies per array • Proprietary high - throughput screening methodologies • Deep interrogation of patient immune response Proprietary Protein MicroArray (20,000 spots per slide) Cell - based Screening Immunome, Inc. | Page 31 Hybridoma Libraries Strength of Hit Screening hits

Target Identification & Validation High success rate in identifying potentially novel oncology targets Immunome, Inc . | Page 32 • Define targets by cross - referencing with > 80% of the predicted human proteome To date, 50+ potentially novel antibody / antigen pairs identified **Antibodies bound to single target antigen spotted in two different locations Top Five Antigens for Each Antibody Single Antigen Identified Protein MicroArray - Based Detection of Target Antigens Non - /Poly - specific

665 Stockton Drive, Suite 300 Exton, PA 19341 610.321.3700 www.immunome.com
