Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Gemini Therapeutics, Inc. /DE | ea128273-8k_fsdevlopment.htm |
| EX-99.1 - PRESS RELEASE, DATED OCTOBER 15, 2020 - Gemini Therapeutics, Inc. /DE | ea128273ex99-1_fsdevelop.htm |
Exhibit 99.2

© Gemini Therapeutics, 2020 Confidential © Gemini Therapeutics, 2020 October 2020
© Gemini Therapeutics, 2020 Confidential Disclaimer This presentation is being furnished solely for the purpose of considering a potential business combination involving Gemini The rapeutics, Inc (“Gemini” or the “Company”), as contemplated in the definitive merger agreement entered into by Gemini and FS. Development Corp. By accepting this presentation, the recipient ac kno wledges and agrees that all of the information contained herein is confidential, that the recipient will distribute, disclose and use such information only for such purpose and that the rec ipi ent shall not distribute, disclose or use such information in any way detrimental to the Company. Important Information About the Merger and Where to Find It A full description of the terms of the business combination will be provided in a registration statement on Form S - 4 to be filed with the Securities and Exchange Commission (the “SEC”) by FS Development Corp. that will include a prospectus with respect to the securities of the combined company upon the closing of t he business combination, to be issued in connection with the business combination, and a proxy statement with respect to the shareholder meeting of FS Development Corp. to vote on the business co mbi nation . FS Development Corp. urges its investors, shareholders and other interested persons to read, when available, the preliminary proxy statement/ prospectus as well as oth er documents filed with the SEC because these documents will contain important information about FS Development Corp., Gemini and the business combination. After the registration statement is declared effective, the definitive proxy statement/prospectus to be included in the registration statement will be mailed to shareholders of FS Development Corp . a s of a record date to be established for voting on the proposed business combination. Once available, shareholders will also be able to obtain a copy of the S - 4, including the proxy statement/ prospectus, and other documents filed with the SEC without charge, by directing a request to: FS Development Corp., Attn: Secretary, 600 Montgomery Street, Suite 4500, San Francisco, Califor nia 94111. The preliminary and definitive proxy statement/prospectus to be included in the registration statement, once available, can also be obtained, without charge, at t he SEC’s website ( www.sec.gov ). Participants in the Solicitation FS Development Corp. and Gemini and their respective directors and executive officers may be considered participants in the s oli citation of proxies with respect to the proposed business combination described in this presentation under the rules of the SEC. Information about the directors and executive officers of FS Development Corp. is set forth in FS Development Corp.’s final prospectus filed with the SEC pursuant to Rule 424(b) of the Securities Act of 1933, as amended (the “Securities Act”) on Aug ust 13, 2020, and is available free of charge at the SEC’s website at www.sec.gov or by directing a request to: FS Development Corp., Attn: Secretary, 600 Montgomery Street, Suite 4500, San Francisco, Calif or nia 94111. Information regarding the persons who may, under the rules of the SEC, be deemed participants in the solicitation of the FS Development Corp. shareholders in conne cti on with the proposed business combination will be set forth in the registration statement containing the proxy statement/prospectus for the proposed business combination when it is filed with the SEC. These documents can be obtained free of charge from the sources indicated above. Trademarks This presentation may contain trademarks, service marks, trade names and copyrights of other companies, which are the propert y o f their respective owners. Solely for convenience, some of the trademarks, service marks, trade names and copyrights referred to in this presentation may be listed without the TM, SM, © or ® symbols, but FS Development Corp. and Gemini will assert, to the fullest extent under applicable law, the rights of the applicable owners, if any, to these trademarks, service marks, trade n ame s and copyrights. 2

© Gemini Therapeutics, 2020 Confidential Disclaimer (Cont.) Forward - Looking Statements This presentation contains forward - looking statements that are based on beliefs and assumptions and on information currently ava ilable. In some cases, you can identify forward - looking statements by the following words: “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “beli eve ,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward - looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievements to be materially different from the information express ed or implied by these forward - looking statements. Although we believe that we have a reasonable basis for each forward - looking statement contained in this presentation, we caution you that these sta tements are based on a combination of facts and factors currently known by us and our projections of the future, about which we cannot be certain. Forward - looking statements in this presentation include, but are not limited to, statements regarding the proposed business combination, including the timing and structure of the business combination, the proceeds of the business combinatio n, the initial market capitalization of the combined company and the benefits of the business combination, as well as statements about the potential attributes and benefits of Gemini’s product c and idates and the format and timing of Gemini’s product development activities and clinical trials. We cannot assure you that the forward - looking statements in this presentation will prove to be a ccurate. These forward - looking statements are subject to a number of significant risks and uncertainties that could cause actual results to differ materially from expected results, including, am ong others, the ability to complete the business combination due to the failure to obtain approval from FS Development Corp.’s shareholders or satisfy other closing conditions in the merger agreeme nt, the occurrence of any event that could give rise to the termination of the merger agreement, the ability to recognize the anticipated benefits of the business combination, the outcome of any le gal proceedings that may be instituted against FS Development Corp. or Gemini following announcement of the proposed business combination and related transactions, the impact of COVID - 19 on Gemini ’s business and/or the ability of the parties to complete the business combination, the ability to obtain or maintain the listing of FS Development Corp.’s common stock on Nasdaq followin g t he proposed business combination, costs related to the proposed business combination, changes in applicable laws or regulations, the possibility that FS Development Corp. or Gemini may be a dve rsely affected by other economic, business, and/or competitive factors, and other risks and uncertainties, including those to be included under the header “Risk Factors” in the registratio n s tatement on Form S - 4 to be filed by FS Development Corp. with the SEC and those included under the header “Risk Factors” in the final prospectus of FS Development Corp. related to its initial pu blic offering. Most of these factors are outside of FS Development Corp.’s and Gemini’s control and are difficult to predict. Furthermore, if the forward - looking statements prove to be inaccurate , the inaccuracy may be material. In light of the significant uncertainties in these forward - looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame, or at all. The forward - looking statements in this presentation represent our views as of the date o f this presentation. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward - looking statements at some poin t in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward - looking statements as representing ou r views as of any date subsequent to the date of this presentation. Non - Solicitation This presentation is not a proxy statement or solicitation of a proxy, consent or authorization with respect to any securitie s o r in respect of the proposed business combination and shall not constitute an offer to sell or a solicitation of an offer to buy any securities nor shall there be any sale of securities in any state or jurisdiction in which such offer, solicitation, or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. No offer of secu rit ies shall be made except by means of a prospectus meeting the requirements of the Securities Act of 1933, as amended. 3

© Gemini Therapeutics, 2020 Confidential 4 © Gemini Therapeutics, 2020 Precision Medicine for Genetically Defined Dry AMD Correcting Factor H in Patients with Genetically Reduced Function
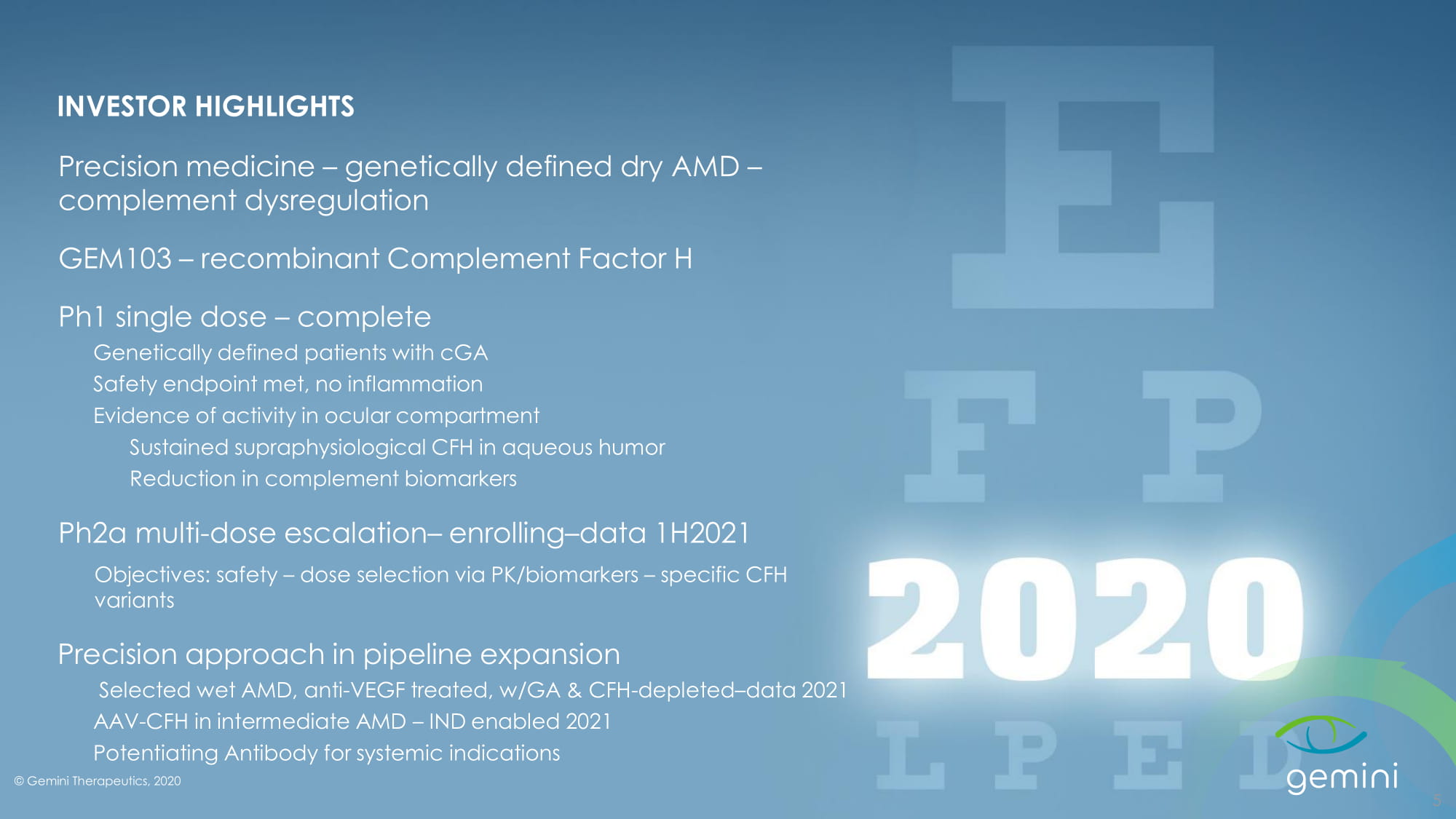
© Gemini Therapeutics, 2020 Confidential INVESTOR HIGHLIGHTS Precision medicine – genetically defined dry AMD – complement dysregulation GEM103 – recombinant Complement Factor H Ph1 single dose – complete Genetically defined patients with cGA Safety endpoint met, no inflammation Evidence of activity in ocular compartment Sustained supraphysiological CFH in aqueous humor Reduction in complement biomarkers Ph2a multi - dose escalation – enrolling – data 1H2021 Objectives: safety – dose selection via PK/biomarkers – specific CFH variants Precision approach in pipeline expansion Selected wet AMD, anti - VEGF treated, w/GA & CFH - depleted – data 2021 AAV - CFH in intermediate AMD – IND enabled 2021 Potentiating Antibody for systemic indications 5 © Gemini Therapeutics, 2020

© Gemini Therapeutics, 2020 Confidential Led by experienced management and backed by tier 1 investor syndicate Jason Meyenburg , MBA CEO, Orchard, Vtesse , Alexion Scott Lauder, PhD CTO, Merrimack, EMD Serono Walter Strapps , PhD VP Gene Therapy Intellia , Merck, Sirna 6 Suresh Katti , PhD VP Research, Alexion, Optherion , Bayer Marc Uknis , MD, FACS CMO, CSL - Behring, ViroPharma , Achillion Gregg Beloff , JD, MBA Interim CFO Leadership Team Investors Board of Directors Jason Meyenburg CEO Steve Squinto , PhD Chairman, OrbiMed Jason Rhodes Atlas Venture Carl Gordon, PhD OrbiMed Jean George Lightstone Ventures Phil Reilly, MD, JD Independent Hannah Chang, MD, PhD Wu Capital David Lubner Independent Tuyen Ong, MD Biogen
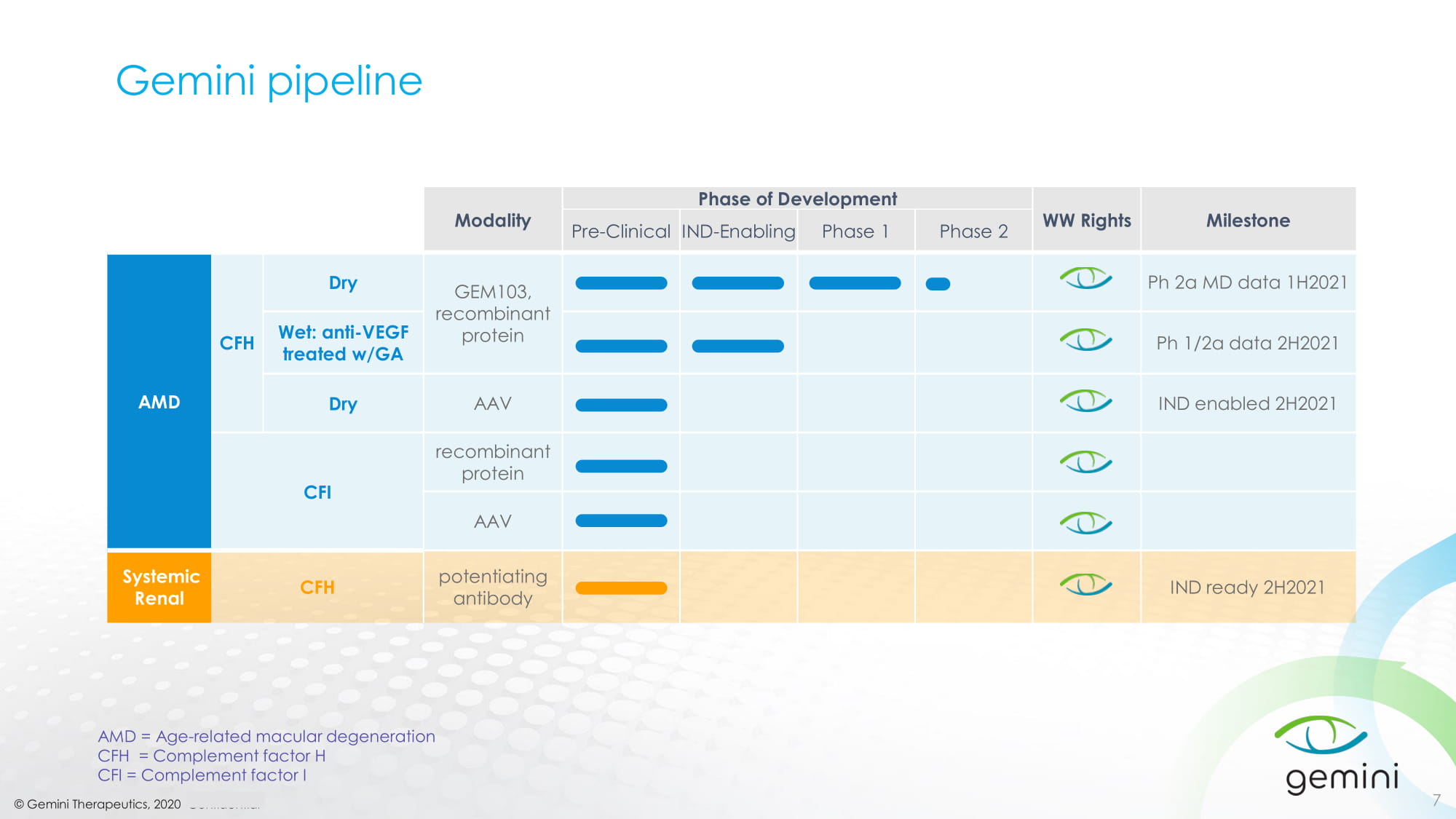
© Gemini Therapeutics, 2020 Confidential Gemini pipeline 7 Modality Phase of Development WW Rights Milestone Pre - Clinical IND - Enabling Phase 1 Phase 2 AMD CFH Dry GEM103, recombinant protein Ph 2a MD data 1H2021 Wet: anti - VEGF treated w/GA Ph 1/2a data 2H2021 Dry AAV IND enabled 2H2021 CFI recombinant protein AAV Systemic Renal CFH potentiating antibody IND ready 2H2021 AMD = Age - related macular degeneration CFH = Complement factor H CFI = Complement factor I

© Gemini Therapeutics, 2020 Confidential Dry AMD represents ~90% of all AMD cases and leads to vision loss due to geographic atrophy 8 some conversion Normal Retina Early AMD (drusen) Intermediate dry AMD ( drusen ) Advanced AMD (geographic atrophy) GA lesion size progresses with no treatment anti - VEGF therapy is mainstay 1 Source: SEVEN - UP Study 90% 10% Wet AMD
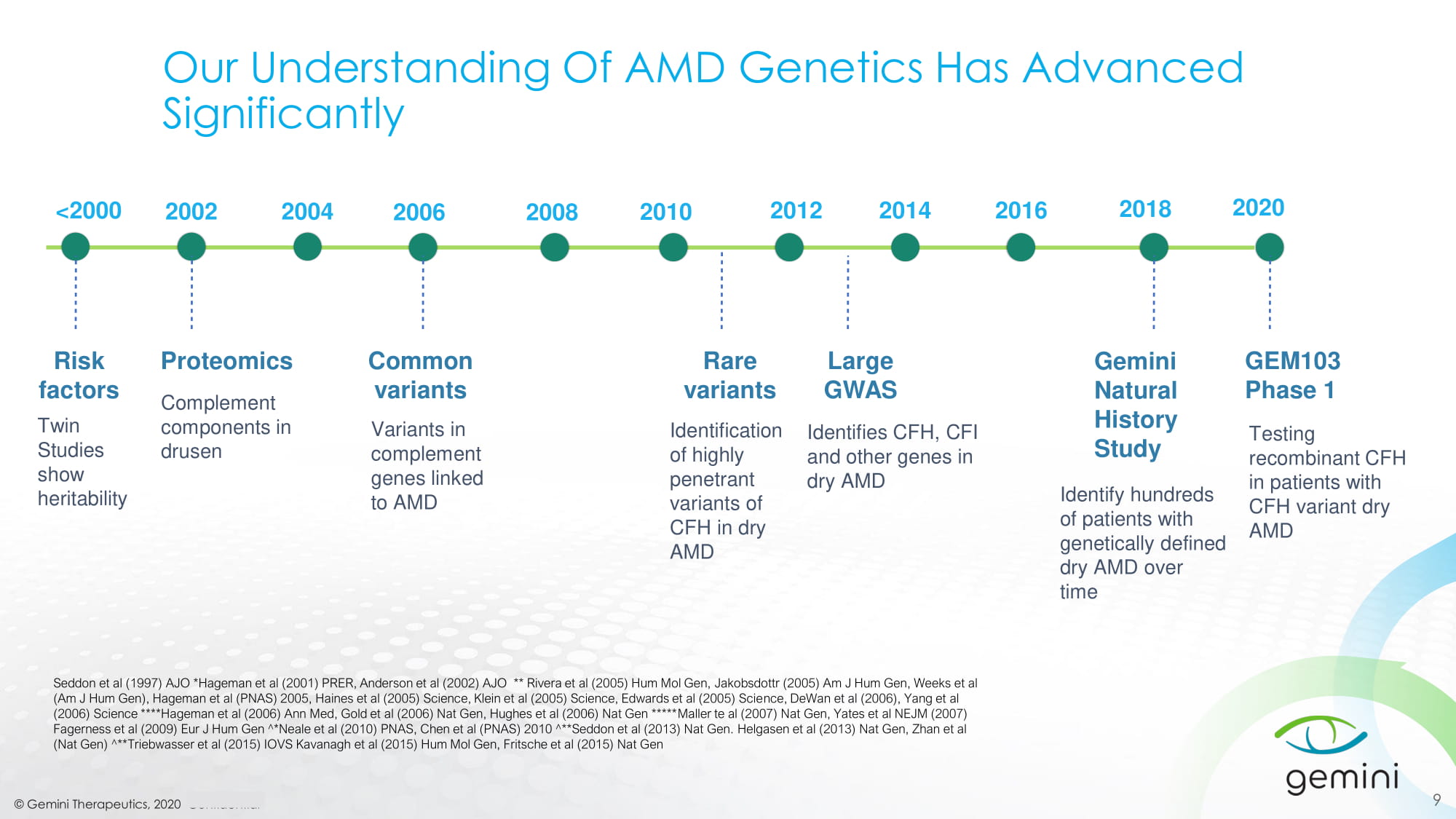
© Gemini Therapeutics, 2020 Confidential 9 <2000 2014 Gemini Natural History Study Identify hundreds of patients with genetically defined dry AMD over time Risk factors Twin Studies show heritability Proteomics Complement components in drusen 2002 Seddon et al (1997) AJO *Hageman et al (2001) PRER, Anderson et al (2002) AJO ** Rivera et al (2005) Hum Mol Gen, Jakobsdottr (2005) Am J Hum Gen, Weeks et al (Am J Hum Gen), Hageman et al (PNAS) 2005, Haines et al (2005) Science, Klein et al (2005) Science, Edwards et al (2005) Scie nce , DeWan et al (2006), Yang et al (2006) Science ****Hageman et al (2006) Ann Med, Gold et al (2006) Nat Gen, Hughes et al (2006) Nat Gen ***** Maller te al (2007) Nat Gen, Yates et al NEJM (2007) Fagerness et al (2009) Eur J Hum Gen ^*Neale et al (2010) PNAS, Chen et al (PNAS) 2010 ^**Seddon et al (2013) Nat Gen. Helgasen et al (2013) Nat Gen, Zhan et al (Nat Gen) ^** Triebwasser et al (2015) IOVS Kavanagh et al (2015) Hum Mol Gen, Fritsche et al (2015) Nat Gen Common variants Rare variants Large GWAS Identifies CFH, CFI and other genes in dry AMD 2004 2006 2008 2010 2012 2016 2018 Our Understanding Of AMD Genetics Has Advanced Significantly Variants in complement genes linked to AMD Identification of highly penetrant variants of CFH in dry AMD 2020 GEM103 Phase 1 Testing recombinant CFH in patients with CFH variant dry AMD
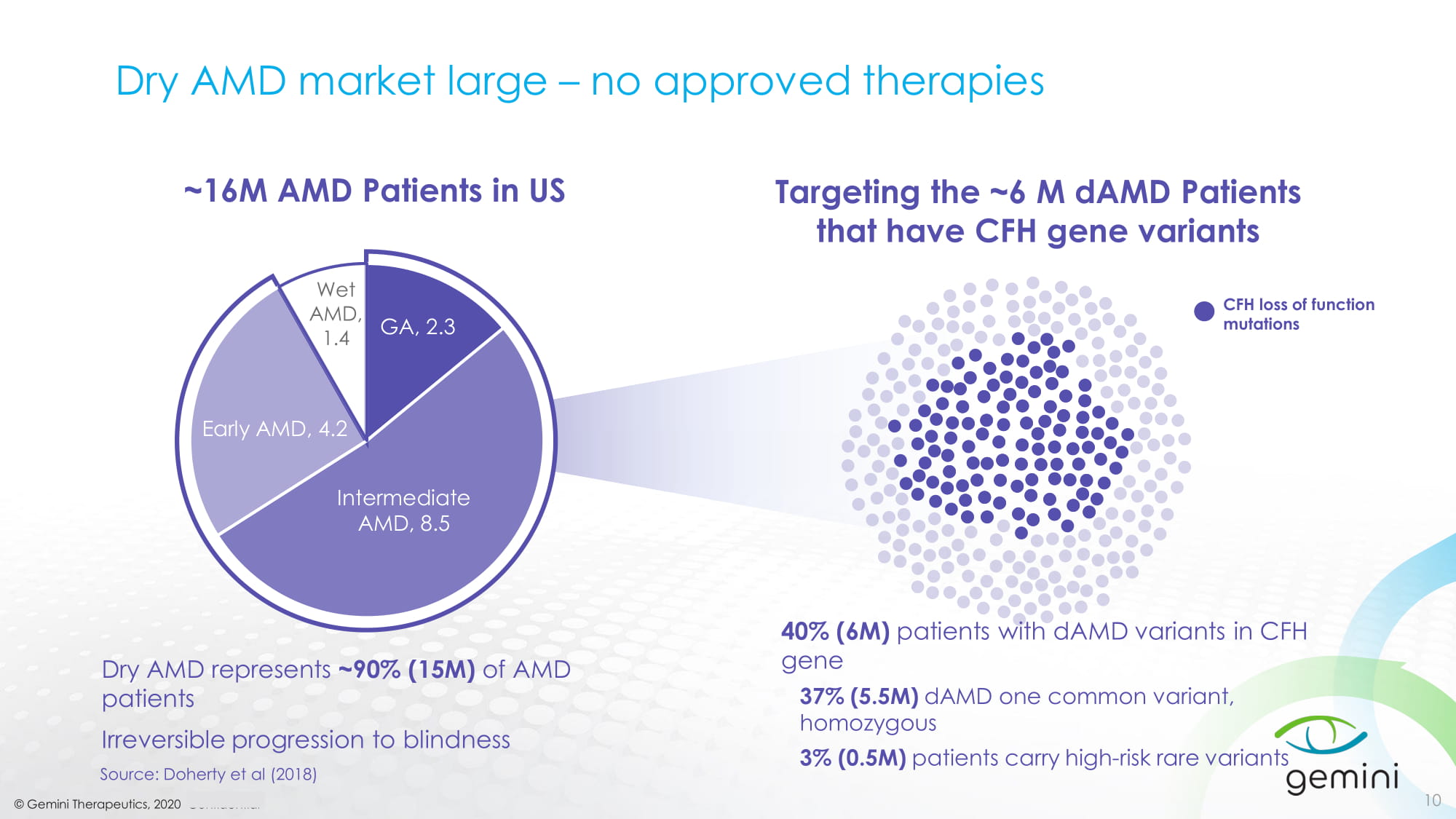
© Gemini Therapeutics, 2020 Confidential 10 Source: Doherty et al (2018) Dry AMD represents ~90% (15M) of AMD patients Irreversible progression to blindness 40% (6M) patients with dAMD variants in CFH gene 37% (5.5M) dAMD one common variant, homozygous 3% (0.5M) patients carry high - risk rare variants CFH loss of function mutations GA, 2.3 Early AMD, 4.2 Intermediate AMD, 8.5 Wet AMD, 1.4 ~16M AMD Patients in US Targeting the ~6 M dAMD Patients that have CFH gene variants Dry AMD market large – no approved therapies
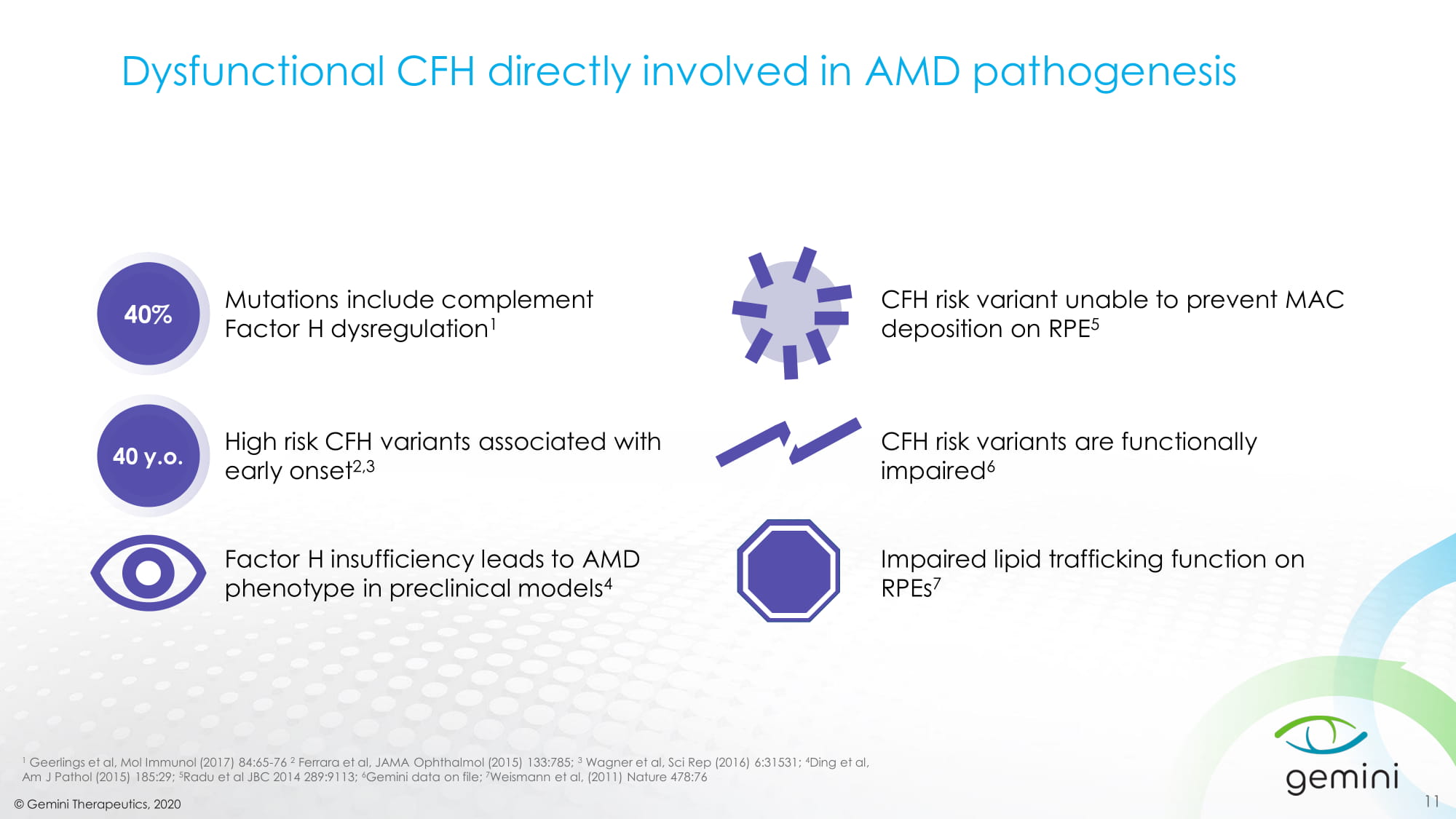
© Gemini Therapeutics, 2020 Dysfunctional CFH directly involved in AMD pathogenesis 1 Geerlings et al, Mol Immunol (2017) 84:65 - 76 2 Ferrara et al, JAMA Ophthalmol (2015) 133:785; 3 Wagner et al, Sci Rep (2016) 6:31531; 4 Ding et al , Am J Path ol (2015) 185:29; 5 Radu et al JBC 2014 289:9113; 6 Gemini data on file; 7 Weismann et al, (2011) Nature 478:76 40% Mutations include complement Factor H dysregulation 1 40 y.o . High risk CFH variants associated with early onset 2,3 Factor H insufficiency leads to AMD phenotype in preclinical models 4 CFH risk variant unable to prevent MAC deposition on RPE 5 CFH risk variants are functionally impaired 6 Impaired lipid trafficking function on RPEs 7 11

© Gemini Therapeutics, 2020 12 CFH – endogenous complement regulator and… Selectively binds & protects self - tissues Prevents damage from terminal complement pathway mediators Efficient, inhibitor of complement pathways Critical to maintain retinal health Factor H critical regulatory complement component necessary for retinal health
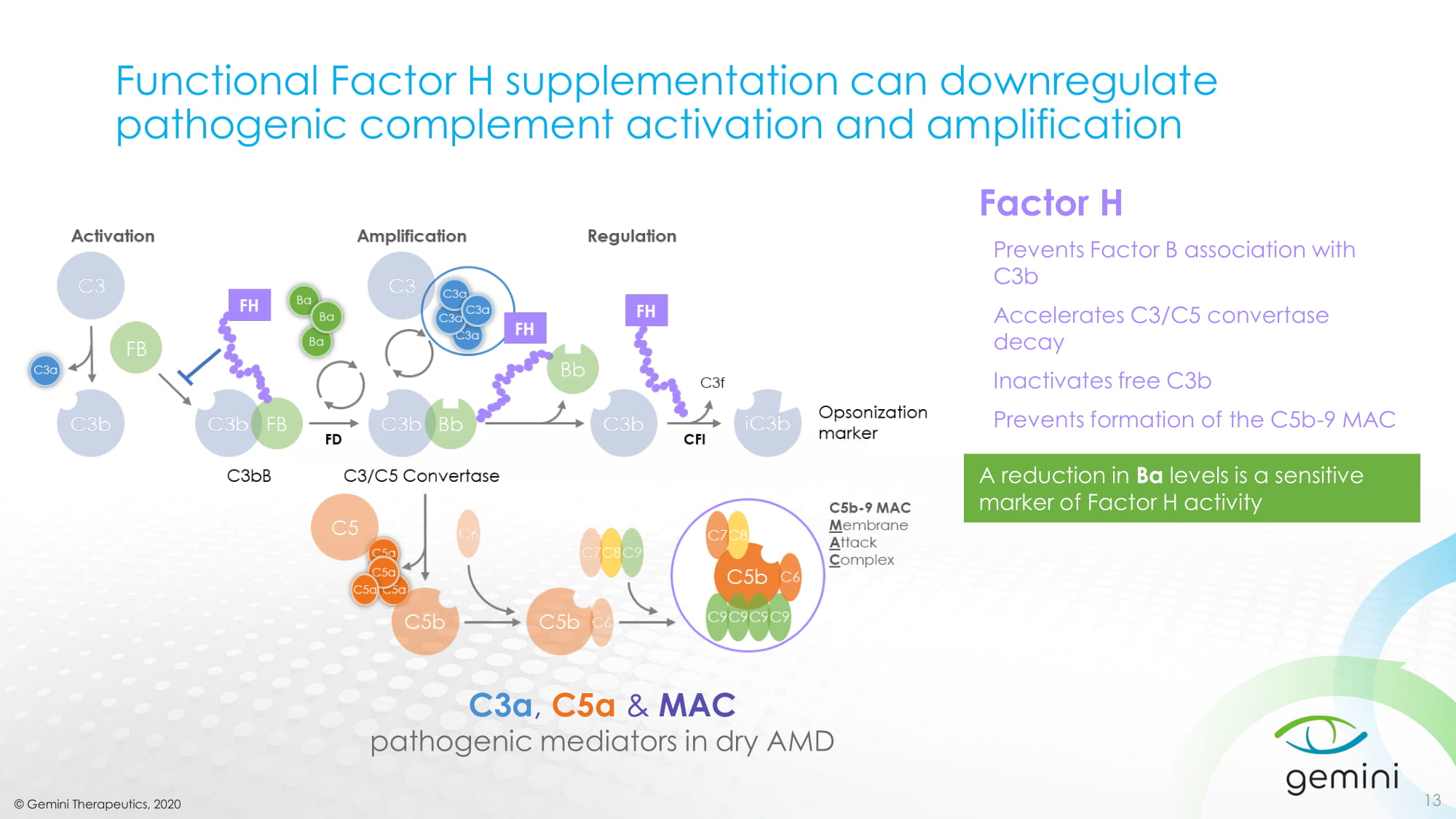
© Gemini Therapeutics, 2020 Functional Factor H supplementation can downregulate pathogenic complement activation and amplification C3a , C5a & MAC pathogenic mediators in dry AMD 13 A reduction in Ba levels is a sensitive marker of Factor H activity Factor H Prevents Factor B association with C3b Accelerates C3/C5 convertase decay Inactivates free C3b Prevents formation of the C5b - 9 MAC
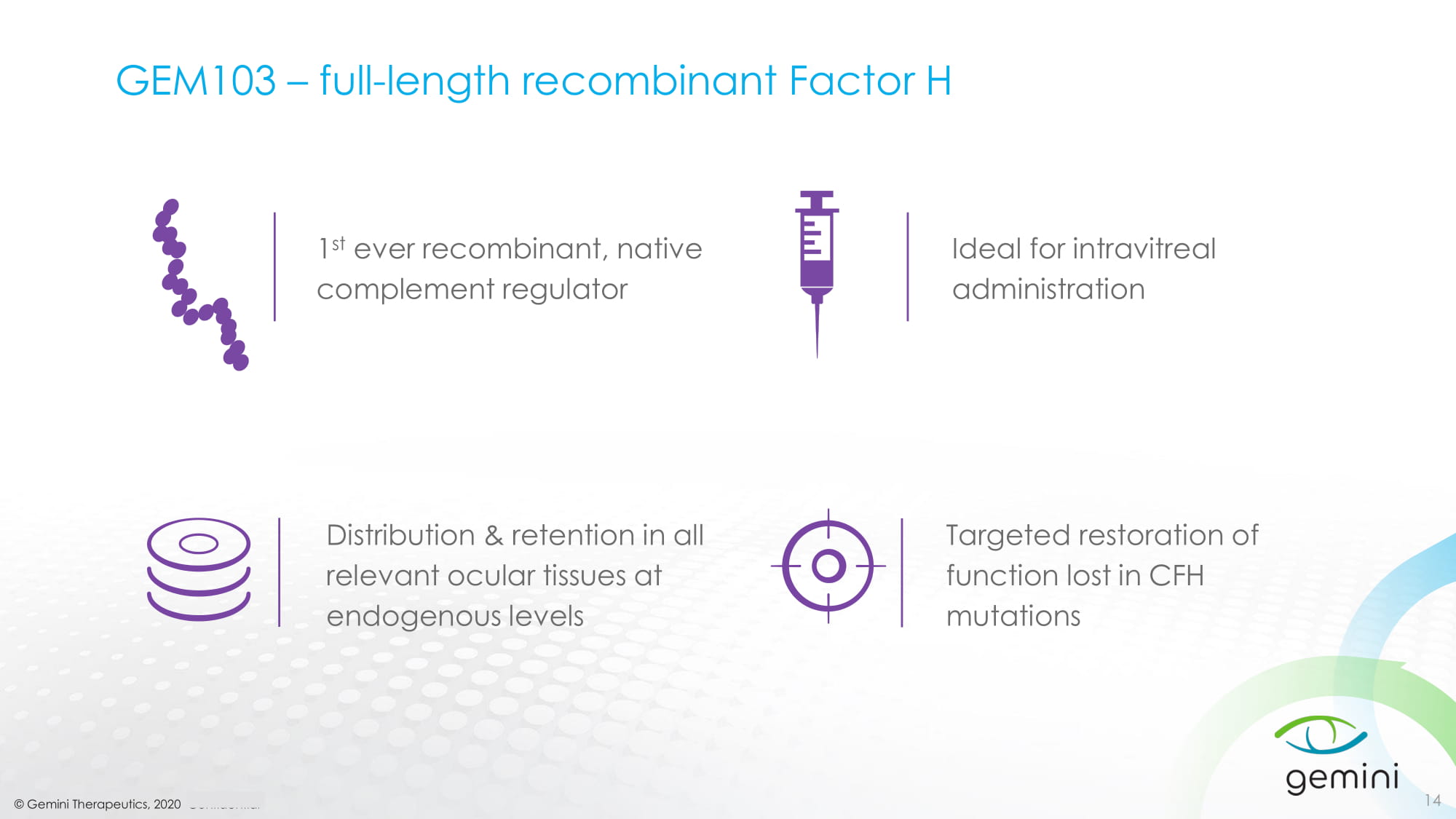
© Gemini Therapeutics, 2020 Confidential GEM103 – full - length recombinant Factor H 14 Ideal for intravitreal administration Targeted restoration of function lost in CFH mutations Distribution & retention in all relevant ocular tissues at endogenous levels 1 st ever recombinant, native complement regulator

© Gemini Therapeutics, 2020 Confidential GEM103 restores physiologic complement activity…without unintended consequences of current inhibitory approaches 15 Preserves Beneficial Phagocytosis and Retinal Debris Clearance More Efficient Inhibition [test article] anti - C3 mAb GEM103 0 50 100 P e r c e n t p h a g o c y t o s i s GEM103 C3 inhib. P < 0.05 Phagocytosis and retinal debris clearance inhibited

© Gemini Therapeutics, 2020 Confidential After IVT administration GEM103 present at high levels in RPE 16 0 7 14 21 28 35 42 49 0.1 1 10 100 1000 10000 100000 Days post Administration A b s o l u t e G E M 1 0 3 L e v e l s 285x181x RPE VH AH NHP Biodistribution (I - 125) Aqueous Humor CFH levels underestimates CFH levels on retina (RPE)
© Gemini Therapeutics, 2020 Confidential GEM103 Recombinant Human CFH - Precision Ophthalmology for Dry AMD 17
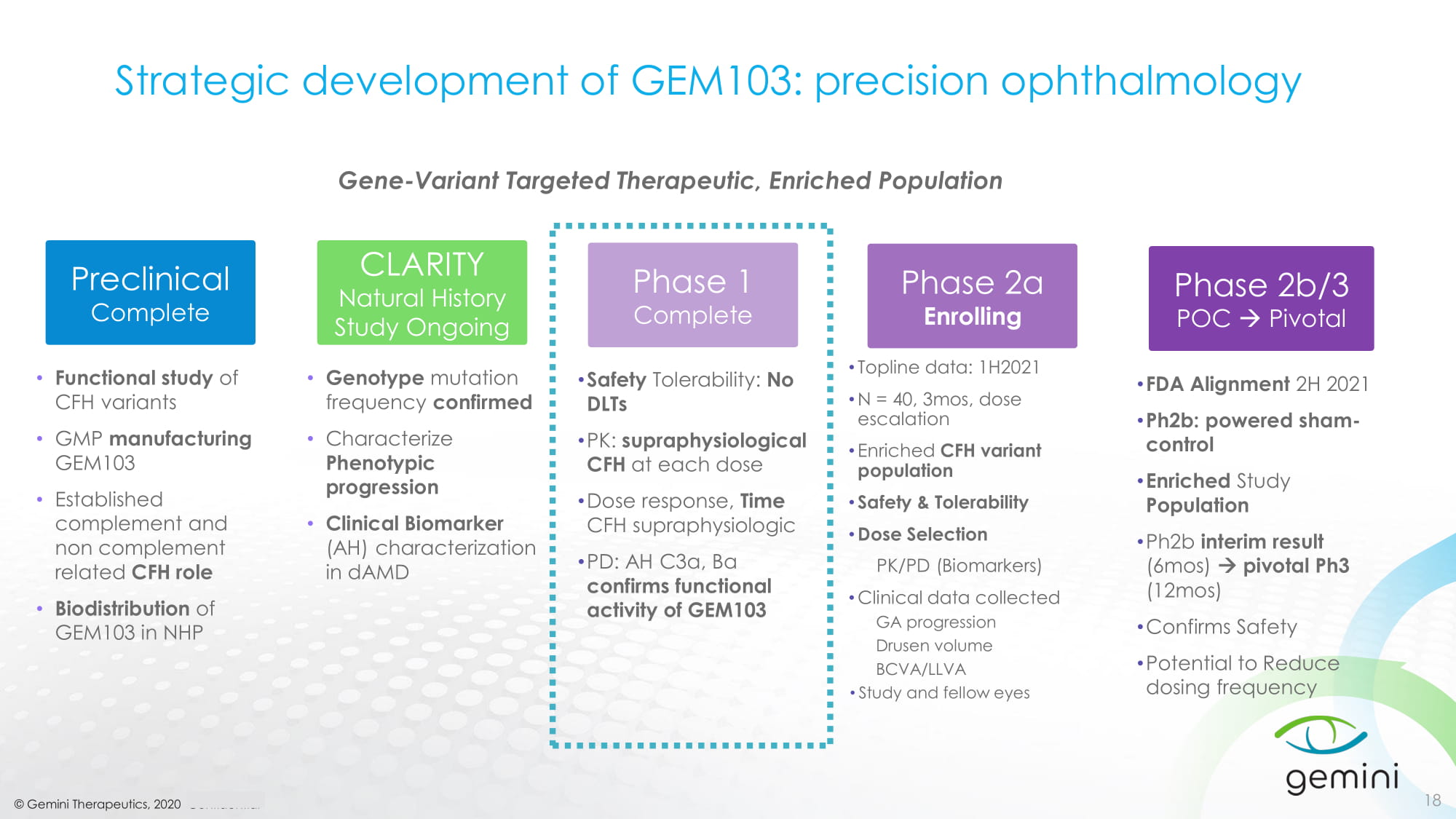
© Gemini Therapeutics, 2020 Confidential Strategic development of GEM103: precision ophthalmology 18 • Functional study of CFH variants • GMP manufacturing GEM103 • Established complement and non complement related CFH role • Biodistribution of GEM103 in NHP Preclinical Complete • Genotype mutation frequency confirmed • Characterize Phenotypic progression • Clinical Biomarker (AH) characterization in dAMD CLARITY Natural History Study Ongoing • Safety Tolerability: No DLTs • PK: supraphysiological CFH at each dose • Dose response, Time CFH supraphysiologic • PD: AH C3a, Ba confirms functional activity of GEM103 Phase 1 Complete • Topline data: 1H2021 • N = 40, 3mos, dose escalation • Enriched CFH variant population • Safety & Tolerability • Dose Selection PK/PD (Biomarkers) • Clinical data collected GA progression Drusen volume BCVA/LLVA • Study and fellow eyes Phase 2a Enrolling • FDA Alignment 2H 2021 • Ph2b: powered sham - control • Enriched Study Population • Ph2b interim result (6mos) pivotal Ph3 (12mos) • Confirms Safety • Potential to Reduce dosing frequency Phase 2b/3 POC Pivotal Gene - Variant Targeted Therapeutic, Enriched Population
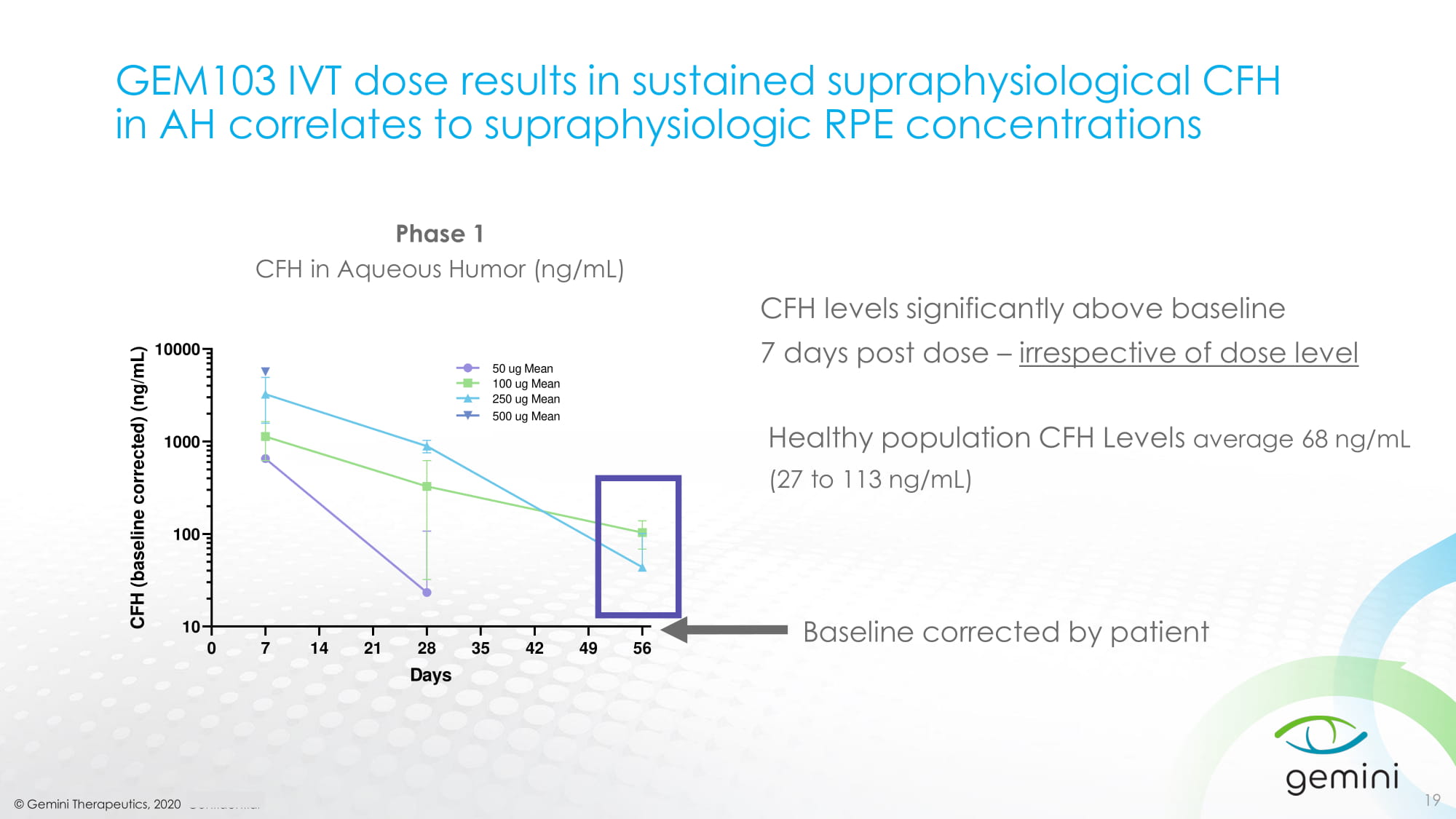
© Gemini Therapeutics, 2020 Confidential GEM103 IVT dose results in sustained supraphysiological CFH in AH correlates to supraphysiologic RPE concentrations 19 Phase 1 CFH in Aqueous Humor (ng/mL) 0 7 14 21 28 35 42 49 56 10 100 1000 10000 CFH in Aqueous Humor (ng/mL) Days C F H ( b a s e l i n e c o r r e c t e d ) ( n g / m L ) 250 ug Mean 100 ug Mean 50 ug Mean 500 ug Mean Healthy population CFH Levels average 68 ng/mL (27 to 113 ng/mL) CFH levels significantly above baseline 7 days post dose – irrespective of dose level Baseline corrected by patient

© Gemini Therapeutics, 2020 Confidential Decrease in Ba after single GEM103 dose confirms functionality 20 Baseline Ba levels elevated • Ba, 8 - 42 ng/mL 7 14 21 28 35 42 49 56 -30 -20 -10 0 10 Ba in Aqueous Humor (ng/mL) Days B a B a s e l i n e C o r r e c t e d ( n g / m L ) 50 ug Subject 1 50 ug Subject 3 100 ug Subject 1 100 ug Subject 2 100 ug Subject 3 250 ug Subject 1 250 ug Subject 2 250 ug Subject 3 500 ug Subject 1 Healthy population Ba, 7.8 ng/mL (6 - 11 ng/mL)

© Gemini Therapeutics, 2020 Confidential GEM103 achieves safety endpoint in central GA patients • Substantial baseline disease • Central GA, BCVA 27 - 43, 70 - 95yo In presence of persistent supraphysiological CFH (GEM103) • No dose - limiting toxicity (DLT) – no adverse drug reactions • No ocular inflammation • No CNV • Visual acuity maintained • Independent safety review committee confirmation • All patients in 3 cohorts, 50 - 250 µg single dose IVT • 500 µg single dose IVT: no DLTs 21
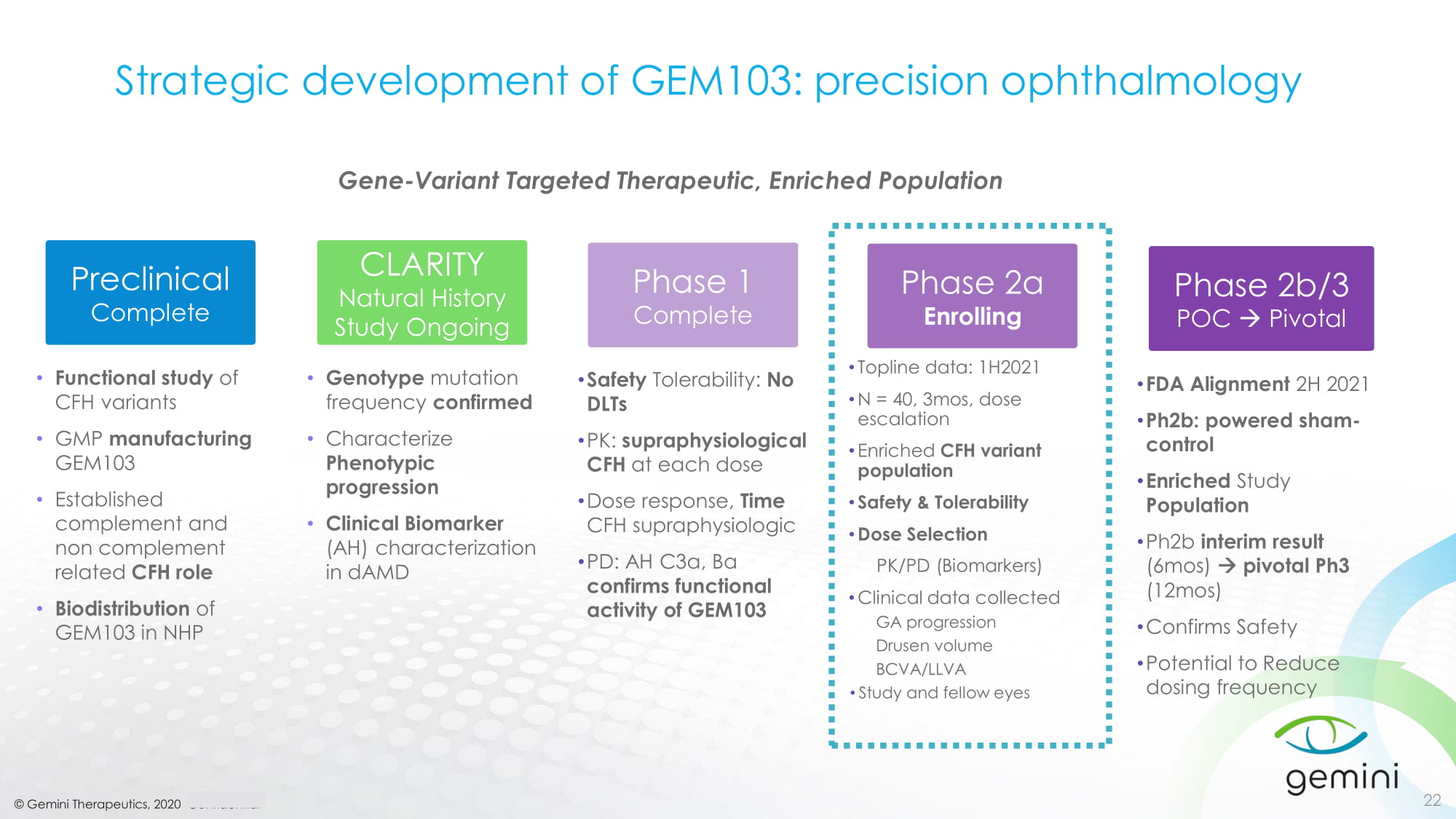
© Gemini Therapeutics, 2020 Confidential Strategic development of GEM103: precision ophthalmology 22 • Functional study of CFH variants • GMP manufacturing GEM103 • Established complement and non complement related CFH role • Biodistribution of GEM103 in NHP Preclinical Complete • Genotype mutation frequency confirmed • Characterize Phenotypic progression • Clinical Biomarker (AH) characterization in dAMD CLARITY Natural History Study Ongoing • Safety Tolerability: No DLTs • PK: supraphysiological CFH at each dose • Dose response, Time CFH supraphysiologic • PD: AH C3a, Ba confirms functional activity of GEM103 Phase 1 Complete • Topline data: 1H2021 • N = 40, 3mos, dose escalation • Enriched CFH variant population • Safety & Tolerability • Dose Selection PK/PD (Biomarkers) • Clinical data collected GA progression Drusen volume BCVA/LLVA • Study and fellow eyes Phase 2a Enrolling • FDA Alignment 2H 2021 • Ph2b: powered sham - control • Enriched Study Population • Ph2b interim result (6mos) pivotal Ph3 (12mos) • Confirms Safety • Potential to Reduce dosing frequency Phase 2b/3 POC Pivotal Gene - Variant Targeted Therapeutic, Enriched Population

© Gemini Therapeutics, 2020 Confidential Ph2a open - label dose escalation in enriched CFH variant GA population to confirm PK and complement PD effect 23 Phase 2a, Open - Label Dose Escalation Study Minimum 3mos exposure at MTD N = 40 Population: 402H homozygous (N=30), rare variants (N=10) 250 μg N = 10, q30d for 12wks 500 μg Expand to N = 40 q30d for 12wks 500μg N = 40 q30d for 52wks, interim analysis 6 &12mos exposure Escalate based on Safety GEM103 Exposure Pts 1 - 10, 3X 250μg, 3X 500μg over 6mos Pts 11 - 40, 3X 500μg over 3mos Open - Label Extension Cumulative ≥ 12mos exposure at MTD Topline data: 1H2021 Safety & Tolerability Dose Selection: PK/PD (Biomarkers) Clinical data collected: GA progression, BCVA/LLVA (study and fellow eye) Alignment with FDA on Ph2b/3

© Gemini Therapeutics, 2020 Therapeutic Landscape 24
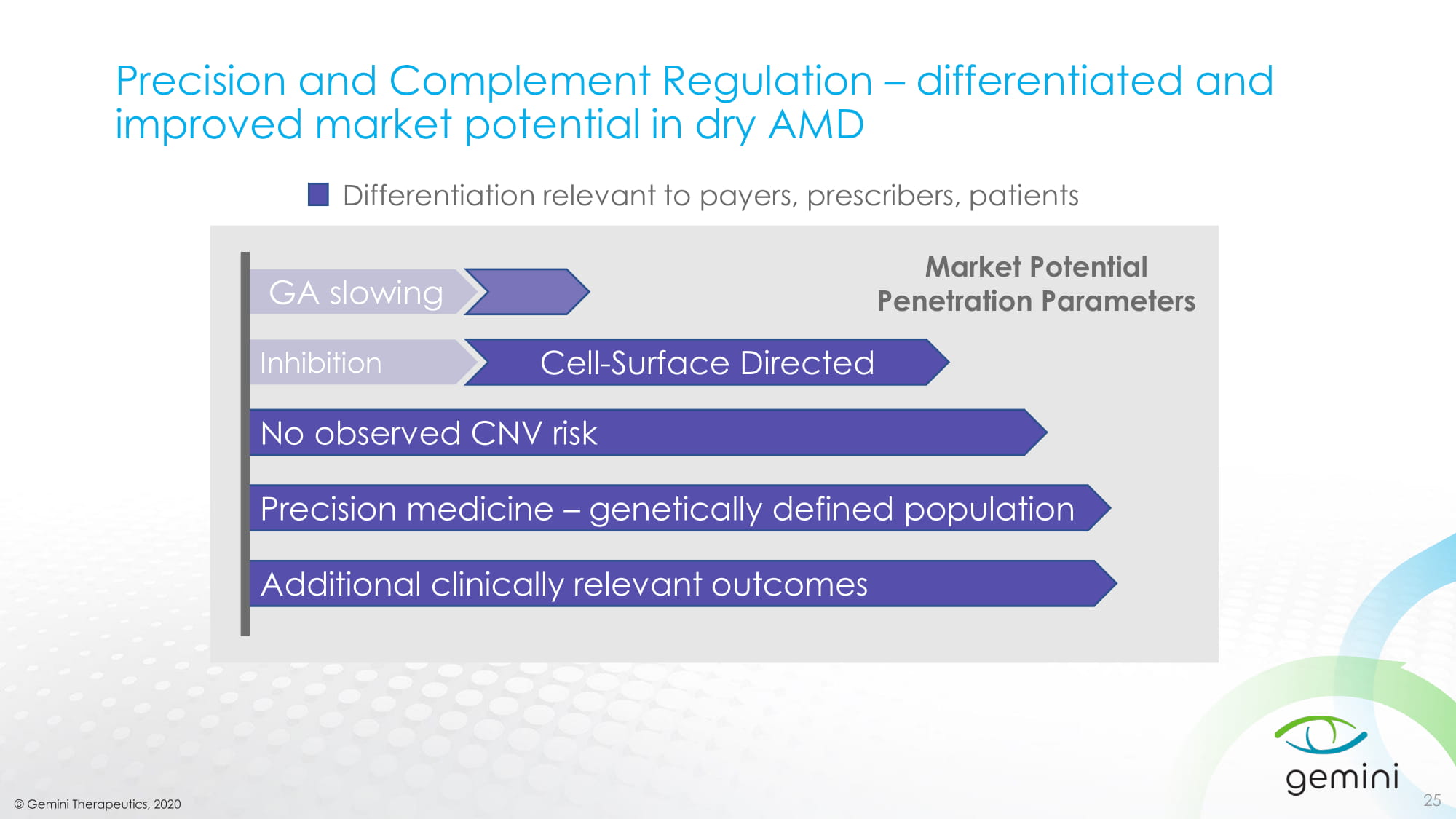
© Gemini Therapeutics, 2020 Precision and Complement Regulation – differentiated and improved market potential in dry AMD 25 GA slowing No observed CNV risk Inhibition Cell - Surface Directed Additional clinically relevant outcomes Precision medicine – genetically defined population Market Potential Penetration Parameters Differentiation relevant to payers, prescribers, patients

© Gemini Therapeutics, 2020 Confidential Highlights and Milestones 26

© Gemini Therapeutics, 2020 Confidential $200 mln funds pipeline through 2022 and the completion of anticipated GEM103 dry AMD pivotal studies in 2023 27 AMD GEM103 Dry Wet: anti - VEGF treated AAV - CFH CFI SYSTEMIC RENAL CFH Potentiating Antibody Ph 1 2H 2020 2021 2022 Define construct Phase 2a Process feasibility & tox Define construct Phase 1/2a IND Enabling Preclinical Phase 2b/3 Pivotal IND – ready Late - stage development

© Gemini Therapeutics, 2020 Confidential 28 Transaction Overview ▪ Gemini and FS Development Corp. (FSDC) have entered into a definitive merger agreement ▪ Expected post transaction equity value of approximately $465 million ▪ Expected to be completed by January 2021 Transaction Summary ▪ PIPE investors include lead investor Foresite Capital, as well as Fidelity Management & Research Company LLC, Wellington Management, Boxer Capital of Tavistock Group, Alyeska Investment Group, L.P., Suvretta Capital Management, CVF, DAFNA Capital, and Acorn Bioventures ▪ Existing Gemini shareholders, including Orbimed Healthcare Fund Management, Atlas Venture, Lightstone Ventures and Wu Capital Premier Healthcare Investor Base ▪ At the time of closing, expected to have approximately $200 million in cash and cash equivalents ▪ Funding expected to generate multiple data readouts across its pipeline ▪ Expected to provide cash runway into 2023 Use of Proceeds ▪ Combined company to be led by Jason Meyenburg ▪ Anticipated directors*: Jason Meyenburg, Jim Tananbaum Key Management and Board * BOD will include 5 members of Gemini’s current BOD

© Gemini Therapeutics, 2020 Confidential Terms of Transaction 29 (1) Assuming no redemptions from FSDC shareholders Shares % Ownership FSDC Sponsor (Foresite) 5.0 11% Sponsor Shares 3.5 7% PIPE Shares 1.5 3% Public Shareholders (1) (excl. FSDC Sponsor) 12.1 26% Current Gemini Shareholders 21.5 46% PIPE Investors (excl. FSDC Sponsor) 8.0 17% Total 46.5 100% Pro Forma Shares Outstanding (1) 46.5 Implied Share Price $10.00 Pro Forma Equity Value $465.4 Less: Pro Forma Cash ($199.8) Plus: Pro Forma Debt - Pro Forma Valuation (1) $265.6 Equity Issued to Gemini Shareholders $215.0 Estimated Transaction Fees & Expenses $16.0 Remaining Cash (Balance Sheet) (1) $199.8 Uses $430.8 Cash Held in Trust (1) $120.8 Gemini Shareholder Equity Rollover $215.0 PIPE Proceeds $95.0 Sources $430.8 Shares and $ in millions (other than share price) Pro Forma Valuation Sources of Funds Uses of Funds Pro Forma Ownership (1)

© Gemini Therapeutics, 2020 Confidential INVESTOR HIGHLIGHTS Precision medicine – genetically defined dry AMD – complement dysregulation GEM103 – recombinant Complement Factor H Ph1 single dose – complete Genetically defined patients with cGA Safety endpoint met, no inflammation Evidence of activity in ocular compartment Sustained supraphysiological CFH in aqueous humor Reduction in complement biomarkers Ph2a multi - dose escalation – enrolling – data 1H2021 Objectives: safety – dose selection via PK/biomarkers – specific CFH variants Precision approach in pipeline expansion Selected wet AMD, anti - VEGF treated, w/GA & CFH - depleted – data 2021 AAV - CFH in intermediate AMD – IND enabled 2021 Potentiating Antibody for systemic indications 30 © Gemini Therapeutics, 2020

© Gemini Therapeutics, 2020 © Gemini Therapeutics, 2020 31
