Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ARVINAS, INC. | d98837d8k.htm |
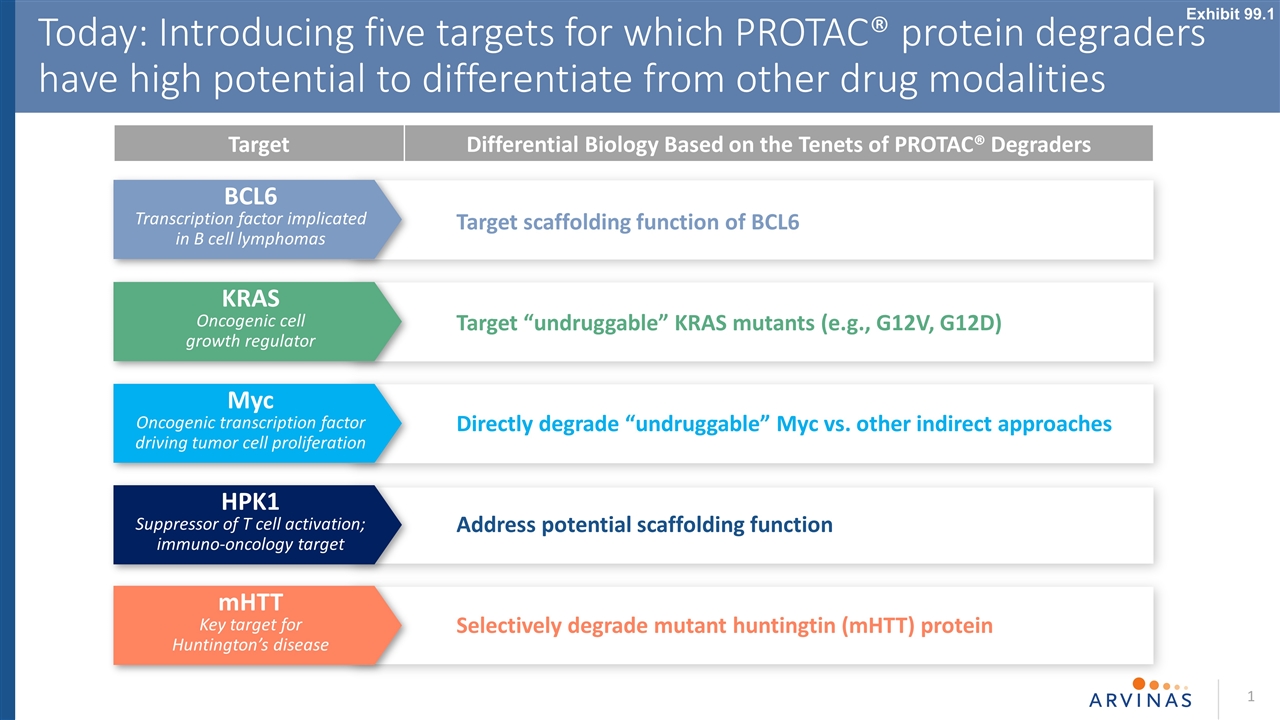
1 Today: Introducing five targets for which PROTAC® protein degraders have high potential to differentiate from other drug modalities KRAS Oncogenic cell growth regulator BCL6 Transcription factor implicated in B cell lymphomas Myc Oncogenic transcription factor driving tumor cell proliferation HPK1 Suppressor of T cell activation; immuno-oncology target mHTT Key target for Huntington’s disease Target Differential Biology Based on the Tenets of PROTAC® Degraders Target scaffolding function of BCL6 Target “undruggable” KRAS mutants (e.g., G12V, G12D) Directly degrade “undruggable” Myc vs. other indirect approaches Address potential scaffolding function Selectively degrade mutant huntingtin (mHTT) protein Exhibit 99.1
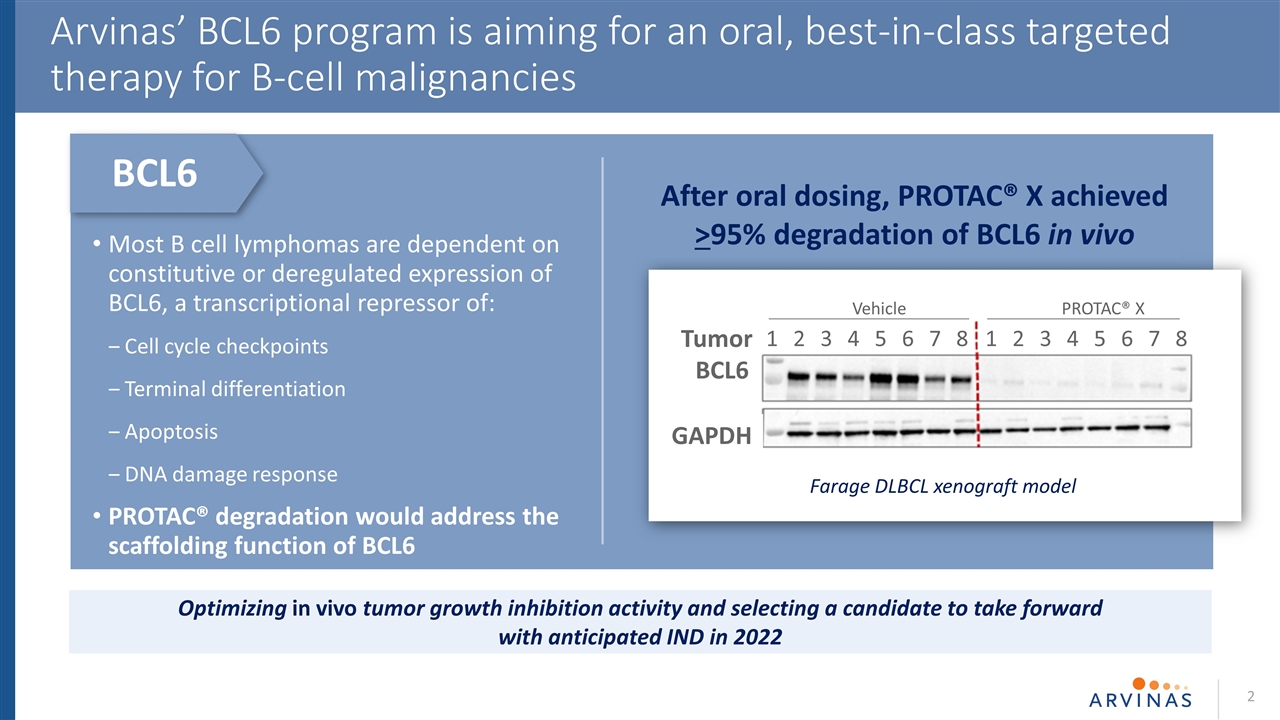
Most B cell lymphomas are dependent on constitutive or deregulated expression of BCL6, a transcriptional repressor of: Cell cycle checkpoints Terminal differentiation Apoptosis DNA damage response PROTAC® degradation would address the scaffolding function of BCL6 2 Arvinas’ BCL6 program is aiming for an oral, best-in-class targeted therapy for B-cell malignancies Optimizing in vivo tumor growth inhibition activity and selecting a candidate to take forward with anticipated IND in 2022 After oral dosing, PROTAC® X achieved >95% degradation of BCL6 in vivo Farage DLBCL xenograft model BCL6 Vehicle PROTAC® X 1 2 3 4 5 6 7 8 1 2 3 4 5 6 7 8 Tumor BCL6 GAPDH
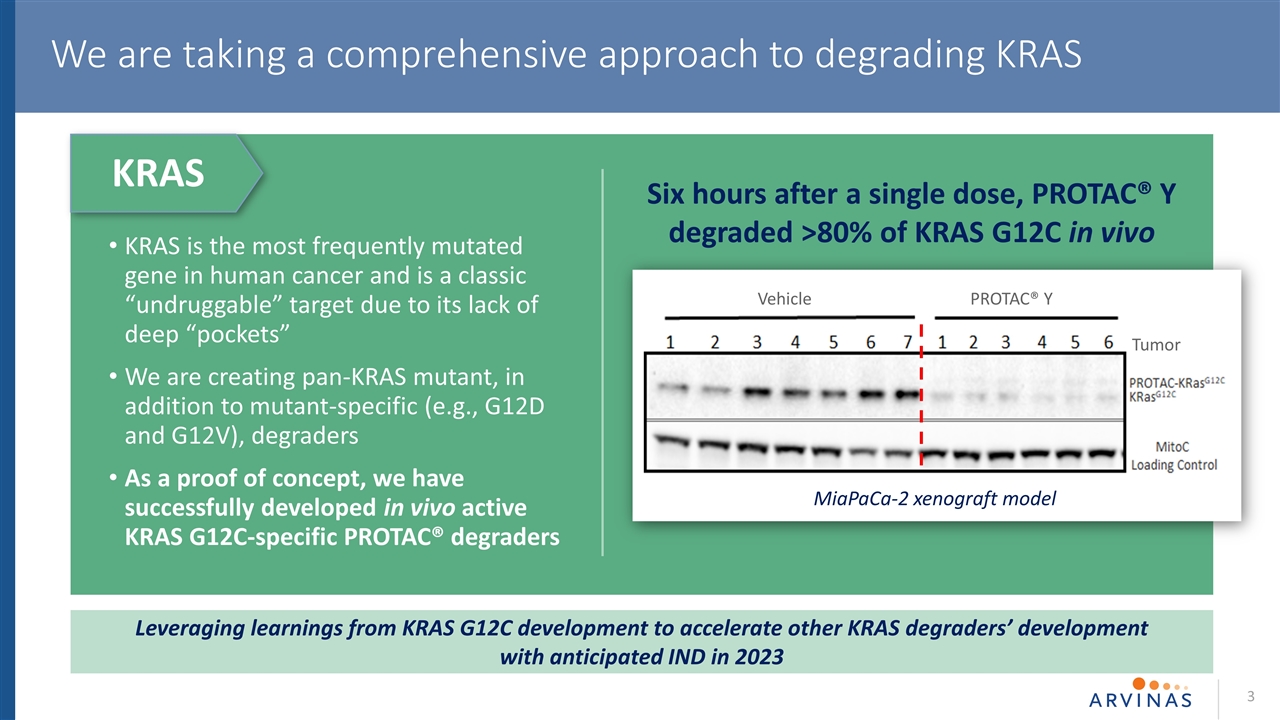
3 We are taking a comprehensive approach to degrading KRAS KRAS is the most frequently mutated gene in human cancer and is a classic “undruggable” target due to its lack of deep “pockets” We are creating pan-KRAS mutant, in addition to mutant-specific (e.g., G12D and G12V), degraders As a proof of concept, we have successfully developed in vivo active KRAS G12C-specific PROTAC® degraders MiaPaCa-2 xenograft model Vehicle PROTAC® Y Leveraging learnings from KRAS G12C development to accelerate other KRAS degraders’ development with anticipated IND in 2023 Six hours after a single dose, PROTAC® Y degraded >80% of KRAS G12C in vivo KRAS Tumor
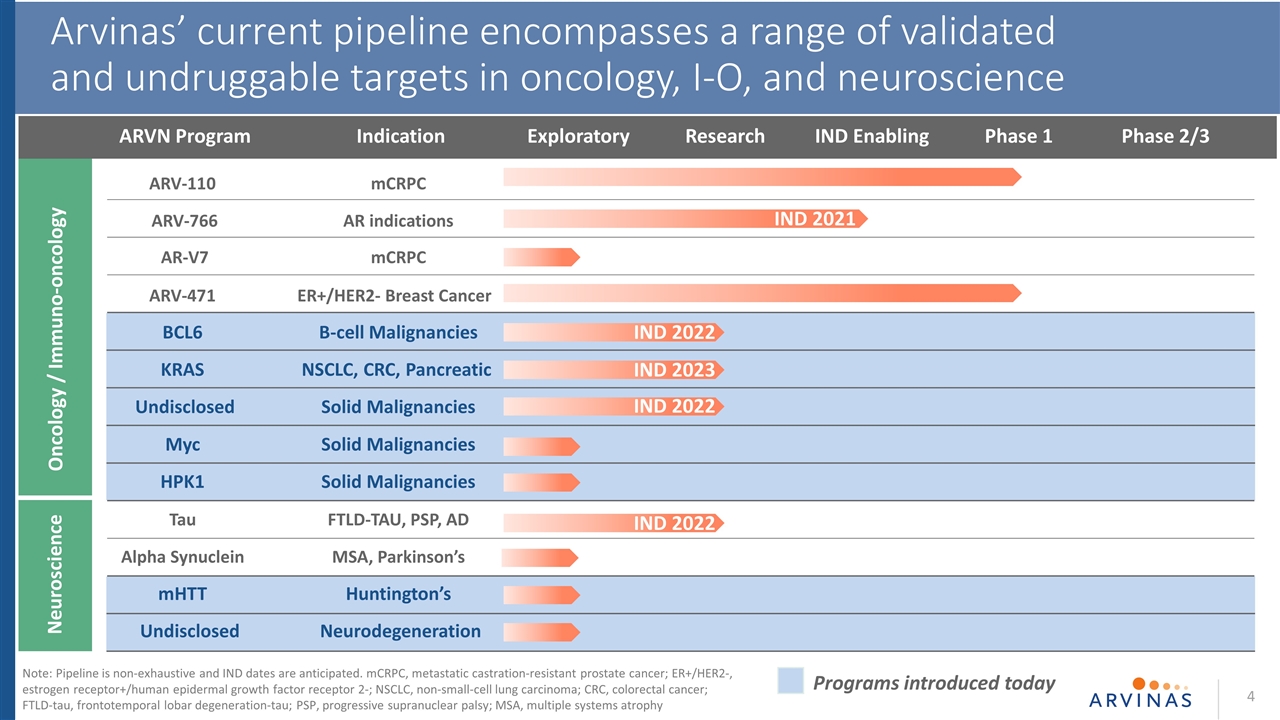
Neuroscience Oncology / Immuno-oncology ARVN Program Research IND Enabling Phase 1 Phase 2/3 Exploratory 4 Arvinas’ current pipeline encompasses a range of validated and undruggable targets in oncology, I-O, and neuroscience Indication IND 2021 IND 2022 IND 2023 IND 2022 Programs introduced today IND 2022 ARV-110mCRPC ARV-766AR indications AR-V7mCRPC ARV-471ER+/HER2- Breast Cancer BCL6B-cell Malignancies KRASNSCLC, CRC, Pancreatic UndisclosedSolid Malignancies MycSolid Malignancies HPK1 Solid Malignancies TauFTLD-TAU, PSP, AD Alpha Synuclein MSA, Parkinson’s mHTTHuntington’s Undisclosed Neurodegeneration Note: Pipeline is non-exhaustive and IND dates are anticipated. mCRPC, metastatic castration-resistant prostate cancer; ER+/HER2-, estrogen receptor+/human epidermal growth factor receptor 2-; NSCLC, non-small-cell lung carcinoma; CRC, colorectal cancer; FTLD-tau, frontotemporal lobar degeneration-tau; PSP, progressive supranuclear palsy; MSA, multiple systems atrophy
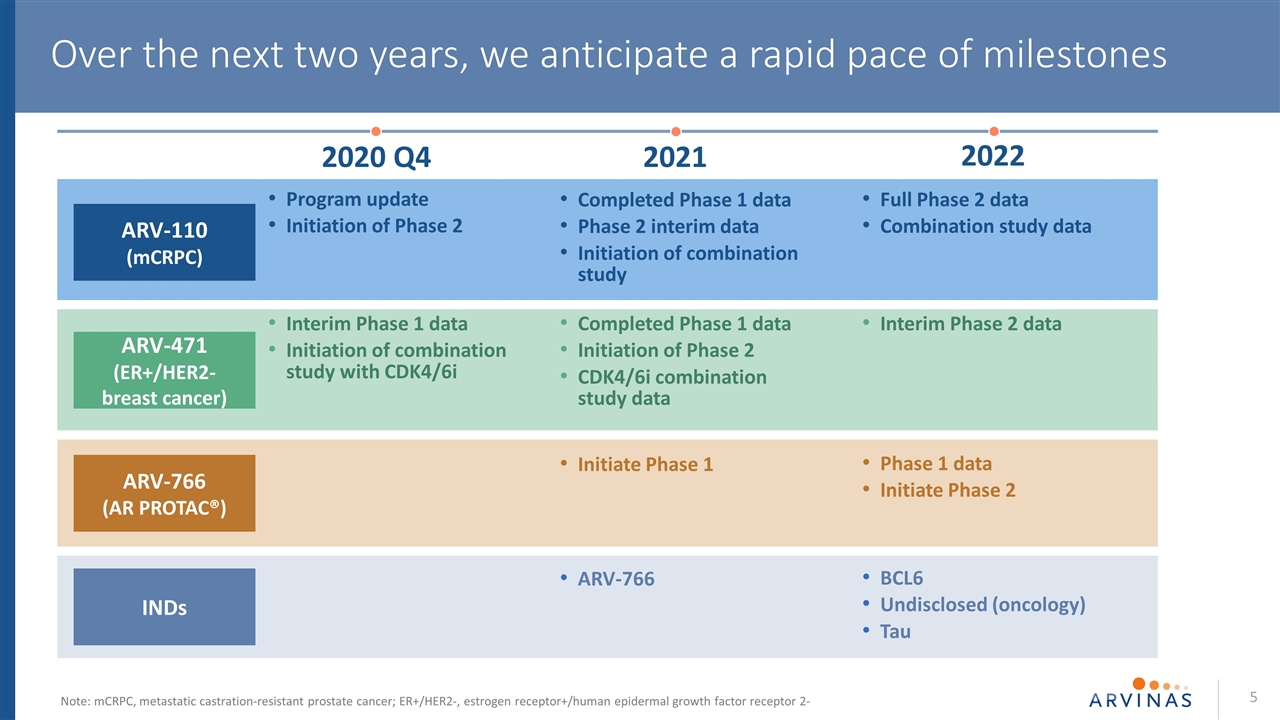
Program update Initiation of Phase 2 Completed Phase 1 data Phase 2 interim data Initiation of combination study Full Phase 2 data Combination study data ARV-110 (mCRPC) Interim Phase 1 data Initiation of combination study with CDK4/6i Completed Phase 1 data Initiation of Phase 2 CDK4/6i combination study data Interim Phase 2 data ARV-471 (ER+/HER2- breast cancer) ARV-766 BCL6 Undisclosed (oncology) Tau INDs Initiate Phase 1 Phase 1 data Initiate Phase 2 ARV-766 (AR PROTAC®) Over the next two years, we anticipate a rapid pace of milestones 2021 2020 Q4 2022 Note: mCRPC, metastatic castration-resistant prostate cancer; ER+/HER2-, estrogen receptor+/human epidermal growth factor receptor 2-

Arvinas 2024 Vision: Ascending to new heights in bringing the benefits of PROTAC® degraders to patients Built Arvinas’ Foundation as a Pioneer in Protein Degradation Proved the Concept of Our PROTAC® Discovery Engine 2013-2018 2019-2020 2024 Vision Integrated biotech poised for launch First PROTAC® degraders proven to benefit patients in registrational studies Sustainably nominating ≥1 clinical candidate per year Our PROTAC® Discovery Engine delivering candidates with tissue- and disease-specific degradation Completing build-out of the resources and capabilities to bring PROTAC® therapeutics to market
