Attached files
| file | filename |
|---|---|
| EX-23.1 - PetVivo Holdings, Inc. | ex23-1.htm |
| EX-21.1 - PetVivo Holdings, Inc. | ex21-1.htm |
| EX-14.1 - PetVivo Holdings, Inc. | ex14-1.htm |
| EX-10.4 - PetVivo Holdings, Inc. | ex10-4.htm |
| EX-3.1.5 - PetVivo Holdings, Inc. | ex3-1_5.htm |
As filed with the Securities and Exchange Commission on October 13, 2020
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
PetVivo Holdings, Inc.
(Exact name of registrant as specified in its charter)
| Nevada | 3841 | 99-0363559 | ||
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S.
Employer Identification No.) |
| 5251
Edina Industrial Blvd. Edina, MN 55439 (952) 405-6216 |
| (Address, Including Zip Code, and Telephone Number, Area Code, of Principal Executive Offices) |
| Copies to: | ||
Laura M. Holm, Esq. Patrick Pazderka, Esq. Fox & Rothschild, LLP Campbell Mithun Tower 222 S. Ninth St., Suite 2000 Minneapolis MN 55402-3338 |
M. Ali Panjwani, Esq. Pryor Cashman LLP 7 Times Square New York, NY 10036-6569 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, check the following box. [ ]
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is a large accelerated file, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | [ ] | Accelerated filer | [ ] |
Non-accelerated filer (Do not check if a smaller reporting company) |
[ ] | Smaller reporting company | [X] |
| Emerging Growth Company | [X] | ||
If an emerging growth company, indicate by check mark if the registrant has elected to not use the extended transition period for complying with any new or revised financial accounting standards provided to Section 7(a)(2)(B) of the Securities Act. [ ]
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1) | Amount of Registration Fee | ||||||
| Units(2) | $ | 11,500,000 | $ | 1,245.65 | ||||
| Common stock, par value $0.001 per share, included in the units | — | (4) | — | (4) | ||||
| Warrants to purchase common stock, par value $0.001 per share, included in the units | — | (4) | — | (4) | ||||
| Common stock, par value $0.001 per share, underlying the warrants included in the units(3) | $ | 12,650,000 | $ | 1,380.12 | ||||
| Underwriter’s warrant to purchase common stock | $ | — | (5) | $ | — | (5) | ||
| Common stock issuable upon exercise of Underwriter’s warrants to purchase common stock (6) | $ | 625,000 | $ | 68.19 | ||||
| TOTAL | $ | 24,775,000 | $ | 2,693.96 | ||||
| (1) | Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(o) of the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | Each unit consists of one share of common stock, par value $0.001 per share, and one warrant to purchase one share of common stock, par value $0.001 per share. Includes shares of common stock and/or warrants to purchase shares of common stock, which may be issued upon exercise of a 45-day option granted to the underwriters to cover over-allotments, if any. |
| (3) | The warrants are exercisable at a per share exercise price equal to ___% of the public offering price per share of common stock. The proposed maximum aggregate public offering price of the shares of common stock issuable upon exercise of the warrants was calculated to be $12,650,000 (which is 110% of $11,500,000 since each investor will receive a warrant to purchase one share of common stock for each share of common stock purchased in this offering). Pursuant to Rule 416, the registrant is also registering an indeterminate number of additional shares of common stock that are issuable by reason of the anti-dilution provisions of the warrants. |
| (4) | Included in the price of the units. No fee required pursuant to Rule 457(g) under the Securities Act. |
| (5) | No fee required pursuant to Rule 457(g) under the Securities Act. |
| (6) | The underwriter’s warrants are exercisable at a per share exercise price equal to 125% of the public offering price per share. As estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act, the proposed maximum aggregate offering price of the representative’s warrants is $625,000 which is equal to 125% of $500,000 (5% of $10,000,000 shares of common stock sold in the offering). Pursuant to Rule 416, the registrant is also registering an indeterminate number of additional shares of common stock that are issuable by reason of the anti-dilution provisions of the underwriter’s warrants. |
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Commission acting pursuant to said Section 8(a) may determine.
The information in this Prospectus is not complete and may be changed. We may not sell the securities until the registration statement filed with the Securities and Exchange Commission becomes effective. This Prospectus is not an offer to sell the securities and we are not soliciting offers in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED OCTOBER __, 2020 |
|
_____ Units |
|

PetVivo Holding, Inc.
This is a firm commitment offering of units (the “Units”) of PetVivo Holding, Inc., a Nevada corporation. Each Unit consists of one share of our common stock and one warrant to purchase one share of our common stock at an exercise price per share of $ ( % of the public offering price of one Unit in this offering). The warrants will expire on the five-year anniversary of the initial exercise date. The units will have no stand-alone rights and will not be issued or certificated as stand-alone securities. Purchasers will receive only shares of common stock and warrants. The shares of common stock and warrants may be transferred separately, immediately upon issuance. The offering also includes the shares of common stock issuable from time to time upon exercise of the warrants.
Our common stock is currently quoted on the OTCQB tier of the OTC Market Group, Inc. under the symbol “PETV.” The last reported sale price of our common stock on October 12, 2020 was $0.65 per share. At present, there is a very limited market for our common stock. Prior to this offering, there has been no public market for the warrants. We have applied to list our common stock and warrants on [ ] (“Exchange”) under the symbols “PETV” and “PETVW,” respectively. There is no assurance that our listing application will be approved by the Exchange. If our common stock and warrants are not listed on the Exchange, we will not consummate this offering.
We are an “emerging growth company” under applicable federal securities laws and are subject to reduced public company reporting requirements.
Investing in our common stock involves a high degree of risks. See “Risk Factors” beginning on page 7.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved these securities or determined whether this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Unit | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions(1) | $ | $ | ||||||
| Proceeds to us, before expenses | $ | $ | ||||||
(1) Underwriting discounts and commissions do not include a non-accountable expense allowance equal to 1.0% of the public offering price payable to the underwriters. We refer you to “Underwriting” beginning on page 61 for additional information regarding underwriters’ compensation.
We have granted a 45 day option to the representative of the underwriters to purchase up to _____ additional shares of common stock and/or warrants to purchase __ shares of common stock solely to cover over-allotments, if any.
The underwriters expect to deliver our securities on or about , 2020.
ThinkEquity
a division of Fordham Financial Management, Inc.
The date of this prospectus is ___________, 2020

TABLE OF CONTENTS
You may only rely on the information contained in this prospectus. We have not authorized anyone to provide you with different information. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities other than the common stock and warrants offered by this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any common stock or warrant in any circumstances in which such offer or solicitation is unlawful. Neither the delivery of this prospectus nor any sale made in connection with this prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus or that the information incorporated by reference to this prospectus is correct as of any time after its date.
This summary highlights information contained elsewhere in this prospectus and does not contain all of the information you should consider before investing in our common stock and warrants. You should read this entire prospectus carefully, especially the risks of investing in our common stock and warrant discussed under “RISK FACTORS” and the financial statements and notes to those financial statements before making an investment decision. PetVivo Holdings, Inc. is referred to in this prospectus as “PetVivo,” the “Company,” “we” or “us.”
The Company
PetVivo is a veterinary biotech and biomedical device company headquartered in suburban Minneapolis, Minnesota that is primarily engaged in the business of commercializing and licensing products in the veterinary market to treat companion animals such as dogs and horses. Most of our technology was developed for human biomedical applications, and we intend to leverage the investments already expended in their development to commercialize treatments for pets in a capital and time-efficient way.
Many of the Company’s products are derived from proprietary biomaterials that simulate a body’s cellular tissue by virtue of their reliance upon natural protein compositions which incorporate such “tissue building blocks” as collagen, elastin and heparin. Since these are naturally-occurring in the body, we believe they have an enhanced biocompatibility with living tissues compared to synthetic biomaterials such as those based upon alpha-hydroxy polymers (PLA, PLGA and the like) and other “natural” biomaterials that may lack the multiple proteins incorporated into our biomaterials. These proprietary protein-based biomaterials appear to mimic the body’s tissue thus allowing integration and tissue repair in long-term implantation in certain applications
Our initial product, Kush™, is a veterinary device designed to help reinforce articular cartilage tissue for the treatment and prevention of osteoarthritis and other joint related afflictions in companion animals. Kush™ uses an intra-articular injection of non-dissolving, cartilage like patented particles that are slippery, wet-permeable, durable and resilient to enhance the force cushioning function of the synovial fluid. The particles mimic natural cartilage in composition, structure and hydration. Multiple joints can be treated simultaneously. Our particles are comprised of collagen, elastin and heparin, the same components found in natural cartilage. These particles show an effectiveness to augment the cartilage and enhance the functionality of the joint (e.g. provide cushion or shock-absorbing features to the joint and to provide joint lubricity).
Osteoarthritis, the most common inflammatory joint disease in both dogs and horses, is a chronic, progressive, degenerative joint disease that is caused by a loss of synovial fluid and/or the deterioration of joint cartilage. Osteoarthritis affects 1 out of 4 dogs and over half of all horses. It is estimated that there are over 20 million dogs with osteoarthritis in the United States and the European Union with a market size of $2.6 billion and over 1 million lame horses in the US for a $600 million United States and the European market. See “Johnston, Spencer, Osteoarthritis. Joint Anatomy, Physiology, and Pathobiology,” The Veterinary Clinics of North America, 1997:699-72.”
Despite the market size, veterinary clinics and hospitals have very few treatments and/or drugs for use in treating osteoarthritis in dogs, horses and other pets. As there is no cure for osteoarthritis, the various treatment methods are focused on managing the related symptoms of pain and inflammation. The current treatment for osteoarthritis in dogs generally consists of the use of nonsteroidal anti-inflammatory drugs (or “NSAIDs”) which present the potential for side effects relating to gastrointestinal, kidney and liver damage. In contrast, the Company’s treatment using Kush™, to our knowledge, has not elicited any adverse side effects in dogs. Remarkably, Kush™-treated dogs have shown an increase in activity even after they no longer are receiving pain medication. Other treatments for osteoarthritis include steroid and/or hyaluronic acid injections, which are often slow acting and/or short lasting.
We believe Kush™ is superior to existing treatments to safely improve joint function in animals for several reasons:
| ● | Kush™ addresses the underlying problem which relate to bones being too close and a lack of synovial fluid. Kush™ provides a biocompatible lubricious cushion to the joint, which establishes a barrier between the bones, thereby protecting the remaining cartilage and bone. | |
| ● | Kush™ is easily administered with the standard intra-articular injection technique. Multiple joints can be treated simultaneously. |
| 1 |
| ● | Case studies indicate many canines have long-lasting multi-month improvement in lameness after having been treated with Kush™ | |
| ● | After receiving a Kush™ injection, many canines are able to discontinue the use of NSAID’s, eliminating the negative side effects | |
| ● | Kush™ is an effective and economical solution for treating osteoarthritis. A single injection of Kush™ is approximately $400 per joint and typically lasts for at least 12 months. |
Historically, drug sales represent up to 30% of revenues at a typical veterinary practice (Veterinary Practice News). Revenues and margins at veterinary practices are being eroded because online, big-box and traditional pharmacies have recently started filling veterinary prescriptions. Veterinary practices are looking for ways to replace lost prescription revenues with safe and effective products. Kush™ is veterinarian-administered and should expand practice revenues and margins. We believe that the increased revenues and margins provided by Kush™ will accelerate its adoption rate and propel it forward as the standard of care for canine and equine lameness related to or due to synovial joint issues.
Kush™ is classified as an animal device under the United States Food and Drug Administration (“FDA”) rules and pre-market approval is not required by the FDA. Kush™ has completed a safety and efficacy study in rabbits in 2007. Since that time, more than 100 horses and dogs have been successfully treated with Kush™. We are in the process of preparing a protocol to conduct a pilot study with a major university within the United States or another academic institute that has an internationally recognized faculty. We anticipate this study will be a 12-month study that will be primarily used to expand our distribution outlets since the large international and national distributors generally require a third-party university study to include a product in their catalog of products.
We plan to commercialize Kush™ and other products developed by us in the United States through the use of in-house marketing personnel who will oversee the efforts of independent distributors we engage on a regional or national basis. We plan to support our distributors with the use of social media and other methods to educate and inform key opinion leaders and decision makers at the top distributors and high prescriber veterinarians for companion animals of the availability and benefits of Kush™. We have developed a commercialization strategy, which provides for our (i) initial launch in key regional markets (Colorado, Minnesota and Texas) by internal sales representatives in the third and fourth quarter of 2020, (ii) development of alliances with 3 to 4 of the top national distributors in the first and second quarter of 2021 and (iii) development of a full national roll-out strategy to deliver our products to customers in the third quarter of 2021.
We have established an ISO 7 certified clean room manufacturing facility located in our Minneapolis facility using a patented and scalable self-assembly production process, which reduces the infrastructure requirements and manufacturing risks to deliver a consistent, high-quality product while being responsive to volume requirements. While we are not currently manufacturing commercial quantities, we have built out an ISO 7 certified facility that will be able to handle projected production in units for at least the next five years.
In addition to Kush™, we have engaged a strategic partner, Emerald Organic Products, Inc. (“Emerald”), through an exclusive license agreement, dated July 31, 2019, to allow Emerald to bring our mucoadhesive drug delivery technology to the human nutraceutical market for the delivery of various active nutraceuticals including cannabidiol (“CBD”), caffeine, and citicoline. Since such products tout up to a 10-times increase in bioavailability of active agents, we believe that we have an advantage over other delivery methods in the CBD and nutritional supplement markets. We have agreed that the license to Emerald will include use of PetVivo’s proprietary technology in the formulation, manufacture and sale of Emerald’s nutritional supplements including its hemp-based CBD wellness products.
We also have a pipeline which includes 17 therapeutic devices for both veterinary and human clinical applications. Some such devices may be regulated by the FDA or other equivalent regulatory agencies, including but not limited to the Center for Veterinary Medicine (“CVM”). We anticipate growing our product pipeline through the acquisition or in-licensing of additional proprietary products from human medical device companies specifically for use in pets. In addition to commercializing our own products in strategic market sectors and in view of the Company’s vast proprietary product pipeline, the Company may establish strategic out-licensing partnerships to provide secondary revenues.
| 2 |
Recent Developments
On June 15, 2020, we issued and sold to an institutional investor a note in the principal amount of $352,941 and warrants to purchase 557,143 shares of our common stock for gross proceeds of $300,000 (representing an original issue discount of 15%). The note matures on March 15, 2021. We have the right to redeem all or a portion of the note upon ten days prior written notice, during which time the holder of the note may convert the principal amount and all accrued and unpaid interest thereon into common stock as further discussed below.
We have the option to issue and sell to the investor, and which the investor is required to purchase, an additional note and warrant, for aggregate gross proceeds of $300,000, provided, however, that the investor will not be required to purchase such additional securities if we are in default under the purchase agreement, the outstanding note or if certain other customary closing conditions are not met. The second tranche closing may not occur later than December 31, 2020.
Business Strategy
Our mission is to provide safe and effective products that address unmet medical needs in the animal markets and further develop our unique intellectual property. Key elements of our business strategy include:
| ● | Launching Kush™ in key regional markets (CO, MN and TX) in the 3rd and 4th quarters of fiscal 2021; | |
| ● | Complete a clinical study with a major university to prove the safety and efficacy of Kush™ for use in dogs; | |
| ● | Secure partnerships with 3 or 4 large national distributors to sell Kush™ in the United States to effectuate our national distribution plan; | |
| ● | Complete the build-out of our manufacturing facility in Minneapolis and develop strategic partnerships with other vendors for package and delivery of Kush™; | |
| ● | Advance research on equine lameness due to navicular disease and/or digital cushion impairment; | |
| ● | Continue our partnership with Emerald for the distribution of our proprietary mucoadhesive drug delivery technology platform in CBD wellness products; | |
| ● | Leverage our current product pipeline in additional animal species and/or humans; and | |
| ● | Develop strategic out-licensing partnerships to provide secondary revenues. |
Risks Related to Our Business
Our business, and our ability to execute our business strategy, is subject to a number of risks as more fully described in the section titled “Risk Factors.” These risks include, among others, the following:
| ● | We have a limited operating history, have not yet generated any material revenues, expect to continue to incur significant research and development and other expenses, and may never become profitable. | |
| ● | Our independent registered public accounting firm has expressed substantial doubt about our ability to continue as a going concern. | |
| ● | We have never generated any material revenue from operations and may need to raise additional capital to achieve our goals. | |
| ● | We are substantially dependent on the success of our lead product, Kush™, and cannot be certain that this product will be successfully commercialized by us. | |
| ● | We have a limited marketing and sales organization, and if our current marketing and sales personnel are insufficient or inadequate to support the current introduction of Kush™, we may not be able to sell this product in the quantities needed to become commercially successful. | |
| ● | Our business will depend significantly on the sufficiency and effectiveness of our marketing and product promotional programs and incentives. | |
| ● | Our lead product, Kush™, will face significant competition in our industry, and our failure to compete effectively may prevent us from achieving any significant market penetration. |
| 3 |
Implications of Being an Emerging Growth Company
As a company with less than $1.07 billion in revenues during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting requirements that are otherwise applicable generally to public companies. These provisions include:
| ● | A requirement to have only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations; | |
| ● | An exemption from compliance with the auditor attestation requirement on the effectiveness of our internal controls over financial reporting; | |
| ● | An exemption from compliance with any requirement that the Public Company Accounting Oversight Board may adopt regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | Reduced disclosure about our executive compensation arrangements; and | |
| ● | Exemptions from the requirements to obtain a non-binding advisory vote on executive compensation or a shareholder approval of any golden parachute arrangements. |
Under the JOBS Act, we will remain an “emerging growth company” until the earliest of: (a) the last day of the fiscal year during which we have total annual gross revenue of $1.07 billion or more; (b) the last day of the fiscal year following the fifth anniversary of the effective date of the registration statement of which this prospectus forms a part; (c) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; or (d) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended, or the Exchange Act (we will qualify as a large accelerated filer as of the first day of the first fiscal year after we have (i) more than $700 million in outstanding common equity held by our non-affiliates and (ii) been public for at least 12 months; the value of our outstanding common equity will be measured each year on the last day of our second fiscal quarter).
We may choose to take advantage of some of the available benefits under the JOBS Act, and have taken advantage of some reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different from the information contained in prospectuses from other United States public companies.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This provision allows an emerging growth company to delay the adoption of some accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
Corporate History and Structure
We were incorporated as Pharmascan Corp. in the State of Nevada on March 31, 2009. On September 21, 2010, we filed a Certificate of Amendment to our Articles of Incorporation and changed our name to Technologies Scan Corp. On April 1, 2014, we filed a Certificate of Amendment to our Articles of Incorporation and changed our name to PetVivo Holdings, Inc. On March 11, 2014, our Board of Directors authorized the execution of a securities exchange agreement dated March 11, 2014 (the “Securities Exchange Agreement”) with PetVivo Inc., a Minnesota corporation (“PetVivo”). PetVivo was founded in 2013 by John Lai and John Dolan and engaged in the business of acquiring, in-licensing and adapting human biomedical technology and products for commercial sale in the veterinary market.
In accordance with the terms and provisions of the Securities Exchange Agreement, we acquired all of the issued and outstanding shares of stock of PetVivo and it became our wholly-owned subsidiary. John Lai and John Dolan were controlling shareholders of PetVivo Holdings, Inc at the time of the securities exchange. In August of 2013, in exchange for 1,305,000 shares of the Company’s common stock, PetVivo entered into an exclusive worldwide license for the commercialization of a patented biomaterials technology for the veterinary treatment of animals having orthopedic joint afflictions (“Technology”). The Technology was developed by Gel-Del Technologies Inc., a Minnesota corporation (“Gel-Del”). Gel-Del was a biomaterials development and manufacturing company focused on human and companion animal applications of its biomaterials technology; our initial product, Kush™, is derived from the licensed Technology.
| 4 |
Thereafter, a wholly-owned subsidiary of ours (which was incorporated in Minnesota expressly for this transaction) completed a triangular merger (the “Merger”) with Gel-Del. Pursuant to the Merger, Gel-Del was the surviving entity and concurrently became our wholly-owned subsidiary, resulting in our obtaining full ownership of Gel-Del. Our primary reason to effect the Merger was to obtain 100% ownership and control of Gel-Del and its patented bioscience technology, including ownership of Cosmeta, a subsidiary of Gel-Del. The effective date for the Merger was April 10, 2017 when the Merger was filed officially with the Secretary of State of Minnesota.
Our principal executive office is located at 5251 Edina Industrial Blvd., Minneapolis, MN 55439 and our telephone number is (952) 405-6216. Our website is www.petvivo.com. Information contained in, or that can be accessed through, our website is not incorporated by reference into this prospectus, and you should not consider information on our website to be part of this prospectus. Our design logo and our other registered and common law trade names, trademarks and service marks are the property of PetVivo, Inc.
Exchange Listing and Reverse Stock Split
We have filed an application to list our common stock and warrants on the Exchange under the symbols “PETV” and “PETVW,” respectively. No assurance can be given that our application will be approved. If our application to the Exchange is not approved or we otherwise determine that we will not be able to secure the listing of the common stock and warrants on the Exchange, we will not complete the offering.
We held a meeting of our shareholders on September 22, 2020 for the purpose of having our shareholders approve a reverse stock split of the outstanding shares of our common stock in the range from one-for-two (1-for-2) to one-for-sixteen (1-for-16), which ratio is to be selected by the board of directors. The board of directors anticipates setting the ratio of the reverse stock split, and the reverse stock split becoming effective following approval by both the board of directors and FINRA of the reverse stock split, prior to the effective date of the registration statement (of which this prospectus forms a part). The reverse stock split is intended to allow us to meet the minimum share price requirement of the Exchange.
Except as otherwise indicated and except in our financial statements and the notes thereto, all references to our common stock, share data, per share data and related information has been adjusted to depict an assumed reverse stock split ratio of 1-for-[•] (“Reverse Stock Split”) until final determination by the board of directors as if it was effective and as if it had occurred at the beginning of the earliest period presented. The Reverse Stock Split, when effective, will combine each shares of our outstanding common stock into one share of common stock, without any change in the par value per share, and the Reverse Stock Split correspondingly will adjust, among other things, the exercise rate of our warrants and options into our common stock. No fractional shares will be issued in connection with the Reverse Stock Split, and any fractional shares resulting from the Reverse Stock Split will be rounded up to the nearest whole share.
| 5 |
The Offering
| Securities offered | Units, each Unit consisting of one share of our common stock and one warrant to purchase one share of our common stock. Each warrant will have an exercise price of $____ per share ( % of the assumed public offering price of one Unit), is exercisable immediately and will expire five (5) years from the date of issuance. The Units will not be certificated or issued in stand-alone form. The shares of our common stock and the warrants comprising the Units are immediately separable upon issuance and will be issued separately in this offering. |
| Common stock offered by us | ____ shares of common stock |
| Warrants offered by us | Warrants to purchase _____ shares of common stock |
| Over-allotment option | We have granted the underwriter an option for a period of 45 days to purchase up to an additional _________ shares and/or warrants, in any combination, to cover over-allotments, if any. |
|
Common stock outstanding(1)(2): Before offering After offering |
24,876,189 shares _________ shares |
| Public offering price | $___ per Unit |
| Use of proceeds | We expect the net proceeds to us from the offering will be approximately $_______ after deduction of the underwriting discount and estimated offering expenses. If the underwriter fully exercises its over-allotment option, we estimate we would receive an additional $_______ of net proceeds. Such proceeds will be used as working capital to fund our efforts to produce, market and distribute our Kush™ product and to pay off outstanding debt. |
| Proposed Exchange Trading Symbols | Our common stock is currently traded on the OTCQB over the counter market under the symbol “PETV.” We have applied to list our common stock and warrants on the Exchange under the symbols “PETV” and “PETVW,” respectively. The listing of our common stock and warrants on the Exchange is a condition of consummating this offering. |
| Description of the Warrants | The exercise price of the warrants is $[●] per share ( % of the assumed public offering price of one Unit). Each warrant is exercisable for one share of common stock, subject to adjustment in the event of stock dividends, stock splits, stock combinations, reclassifications, reorganizations or similar events affecting our common stock as described herein. A holder may not exercise any portion of a warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of the outstanding common stock after exercise, as such percentage ownership is determined in accordance with the terms of the warrants, except that upon notice from the holder to us, the holder may waive such limitation up to a percentage, not in excess of 9.99%. Each warrant will be exercisable immediately upon issuance and will expire five years after the initial issuance date. The terms of the warrants will be governed by a Warrant Agreement, dated as of the effective date of this offering, between us and [●], as the warrant agent (the “Warrant Agent”). This prospectus also relates to the offering of the shares of common stock issuable upon exercise of the warrants. For more information regarding the warrants, you should carefully read the section titled “Description of Securities—Warrants and Options” in this prospectus. |
| Risk factors | Investing in our securities involves a high degree of risk. You should carefully review and consider the “RISK FACTORS” section of this prospectus for a discussion of factors to consider before deciding to invest in our Units. |
| 6 |
(1) Does not include shares of our common stock underlying the Underwriters’ over-allotment option.
(2)The number of shares of our common stock to be outstanding after this offering is based on 24,876,189 shares of our common stock outstanding as of September 30, 2020 and includes the automatic conversion of convertible notes in the amount of $___ into __ shares of our common stock upon completion of our listing on the Exchange. It does not include:
| ● | 5,763,610 shares of our common stock issuable upon the exercise of outstanding warrants with a weighted-average exercise price of $0.52 per share; | |
| ● | 4,000,000 shares reserved for issuance our PetVivo 2020 Equity Incentive Plan; | |
| ● | Warrants to purchase up to [●] shares of our common stock issued as part of the Units; and | |
| ● | Warrants to purchase up to [●] shares of our common stock issuable to the Underwriter in connection with this offering. |
Unless otherwise indicated, all information in this prospectus reflects or assumes the following: (i) no exercise or termination of any options or warrants outstanding as of September 30, 2020 and (ii) no exercise by the underwriters of their overallotment option.
Statements contained in this prospectus that are not factual or purely historical are considered to be “forward-looking statements” within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended. (the “Exchange Act”). These forward-looking statements include, but are not limited to: any statements or projections regarding future revenues, earnings, or other financial items; any statements of the strategies, plans and objectives of our management for future operations; any statement regarding proposed development of new products or technology; any statements regarding anticipated marketing or production operations; any statements regarding our future capital needs, any statements regarding the value or use of current or future patents or patent applications or other intellectual property; any statements regarding anticipated regulatory requirements; any statements regarding future economic conditions; and any statements of belief or assumptions. Forward-looking statements may include the words “may,” “will,” “estimate,” “intend,” “continue,” “believe,” “expect,” or “anticipate” or any other similar words. These statements represent our expectations, beliefs, anticipations, commitments, intentions and strategies regarding the future and include, but are not limited to, the risks and uncertainties outlined and set forth in the following sections of this prospectus entitled “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” Readers of this prospectus are cautioned that actual results could differ materially from the anticipated results or other expectations that are expressed in our forward-looking statements. Our forward-looking statements included in this prospectus speak only as of the date of the prospectus, and we undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Given these many risks and uncertainties, readers of this prospectus are cautioned not to place undue reliance on our forward-looking statements.
An investment in our common stock and warrants involves a high degree of risk. You should carefully consider the following described risks together with all other information included in this prospectus before making an investment decision with regard to this offering. If one or more of the following risks occurs, our business, financial condition, and results of operations could be materially harmed, which most likely would result in a decline in the trading price of our common stock and warrants and investors losing part or even all of their investment.
| 7 |
Risks Relating to Our Financial Condition
We have incurred substantial losses to date and could continue to incur such losses.
We have incurred substantial losses since commencing our current business. For fiscal quarter ended June 30, 2020, we lost approximately $0.81 million without obtaining any commercial revenues and had an accumulated deficit of approximately $55.40 million. For our fiscal years ended March 31, 2020 and 2019, we lost approximately $2.1 million and $4.76 million, respectively, without obtaining any commercial revenues. In order to achieve and sustain future revenues, we must succeed in our current efforts to commercialize Kush™ for treatment of dogs and horses suffering from osteoarthritis. That will require us to produce our products effectively in commercial quantities, establish adequate sales and marketing systems, conduct clinical trials and tests which show the safety and efficacy of Kush™ in dogs and horses and gain significant support from veterinarians in the use of our products. We expect to continue to incur losses until such time, if ever, as we succeed in significantly increasing our revenues and cash flow beyond what is necessary to fund our ongoing operations and pay our obligations as they become due. We may never generate revenues sufficient to become profitable or to sustain profitability.
Our auditors have expressed doubt about our ability to continue as a going concern.
The report of our independent registered accounting firm that audited our March 31, 2020 and 2019 financial statements included an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern. Our ability to continue as a going concern is contingent primarily upon our continuing to raise sufficient working capital to support our operations until attaining profitability, which may never happen.
If we are unable to obtain sufficient funding, we may have to reduce materially or even discontinue our business.
As of September 30, 2020, we had cash or cash equivalents of $169,493. Accordingly, our ability to commercialize our Kush™ products is dependent on our receipt of the net proceeds from this offering. We anticipate that our cash on hand with the net proceeds from this offering will be adequate to satisfy operational and capital requirements for the next 21 months. If we are unable to realize substantial revenues in the near future, we will need to seek additional financing beyond this 21-month period to continue our operations. We also most likely will require additional financing to develop additional new products or to expand into foreign markets.
Along with establishing effective production, marketing, sales and distribution of our Kush™ products, we believe that our future capital requirements depend upon the timing and costs of many factors with some of them beyond our control, including our ability to establish an adequate base of veterinarian clinics using our products, costs in obtaining patents and any required regulatory approvals for future products, costs of any future target animal studies, costs related to new product development, costs of finished product inventory, expenses to attract and retain skilled personnel as needed, increased costs related to being a listed public company, and the costs of any future acquisitions of existing companies or IP technologies. There is no assurance that future additional capital will be available to us as needed, or if available upon terms acceptable to us.
Risks Relating to Our Business
We have no operating history upon which to base an evaluation of our prospects.
We have had no material commercial operations, since our primary efforts and resources have been directed toward acquiring our technology to produce and sell proprietary products for the animal market. Our lack of an operating history makes an evaluation of our business and prospects very difficult. Our prospects must be considered speculative, especially considering the risks, expenses and difficulties frequently encountered in the establishment of an early-stage company. Our ability to operate our business successfully remains unknown and untested. If we cannot commercialize our products effectively, or are significantly delayed or limited in doing so, our business and operations will be harmed substantially, and we may even need to cease operations.
| 8 |
We are substantially dependent upon the success of Kush™ and any failure of Kush™ to achieve market acceptance would harm us significantly.
Our recent efforts and financial resources have primarily been directed toward commercialization of the Kush™ products for the treatment of dogs and horses suffering from osteoarthritis. Accordingly, our prospects rely heavily on the successful launch and follow-up marketing of this products. In addition to establishing effective production, marketing, sales, distribution and training for the use of Kush™, we believe its successful commercialization will depend on other material factors including our ability to educate and convince veterinarians and pet owners about the benefits, safety and effectiveness of our Kush™ products, the occurrence and severity of any side effects to pets from use of our products, maintaining regulatory compliance and effective quality control for our products, our ability to maintain and enforce our patents and other intellectual property rights, any increased manufacturing costs from third-party contractors or suppliers, and the availability, cost and effectiveness of treatments offered by competitors.
If we fail to attract and retain qualified management and key scientific personnel, we may be unable to successfully commercialize our current products or develop new products effectively.
Our success will significantly be dependent upon our current management and key scientific technicians, and also on our ability to attract, retain and motivate future management and employees. We are highly dependent upon our current management and technology personnel, and the loss of the services of any of them could delay or prevent the successful commercialization or development of current or future products. Competition to obtain qualified personnel in the animal health field is intense due to the limited number of individuals possessing the skills and experience required by our industry. We may not be able to attract or retain qualified personnel as needed on acceptable terms, or at all, which would harm our business and operations.
Our operations will rely on third parties to produce our raw materials to produce our products.
We will rely on independent third parties to produce the raw materials (e.g. collagen, elastin and heparin) that we use to produce our Kush™ products. As such, we will be dependent upon their services and will not be in a position to control their operations as we might if we directly produced these raw materials. While we believe the raw materials used to manufacture our Kush products are readily available and can be obtained from multiple reliable sources on a timely basis, circumstances outside our control may impair our ability to have an adequate supply of raw materials to produce our Kush products.
If we experience the rapid commercial growth we anticipate, we may not be able to manage such growth effectively.
We contemplate rapid growth for our business as we bring our Kush™ products to market and anticipate that will place significant new demands on our management and our operational and financial resources. Our organizational structure will become more complex as we add additional personnel, and we would likely require more financial and staff resources to support and continue our growth. If we are unable to manage our growth effectively, our business, financial condition and results of operations may be materially harmed.
We have a limited marketing and sales organization, and if our current marketing and sales personnel are insufficient or inadequate to support the current introduction of our Kush products, we may not be able to sell these products in quantities to become commercially successful.
We have a limited marketing and sales organization, and we have minimal prior experience in the marketing, sale and distribution of pet care products. There are significant risks involved in our building and managing an effective sales organization, including our ability to hire, adequately train, maintain and motivate qualified individuals, generate sufficient sales leads and other contacts, and establish effective product distribution channels. Any failure or substantial delay in the development of our internal sales, marketing and distribution capabilities would adversely impact our business and financial condition.
| 9 |
Our business will depend significantly on the sufficiency and effectiveness of our marketing and product promotional programs and incentives.
Due to the highly competitive nature of our industry, we must effectively and efficiently promote and market our products through internet, television and print advertising, social media, and through trade promotions and other incentives to sustain and improve our competitive position in our market. Moreover, from time to time we may have to change our marketing strategies and spending allocations based on responses from our veterinarian customers and pet owners. If our marketing, advertising and trade promotions are not successful to create and sustain consistent revenue growth or fail to respond to marketing strategy changes in our industry, our business, financial condition and results of operations may be adversely affected.
Any damage to our reputation or our brand may materially harm our business.
Developing, maintaining and expanding our reputation and brand with veterinarians, pet owners and others will be critical to our success. Our brand may suffer if our marketing plans or product initiatives are unsuccessful. The importance of our brand and demand for our products may decrease if competitors offer products with benefits similar to or as effective as our products and at lower costs to consumers. Although we maintain procedures to ensure the quality, safety and integrity of our products and their production processes, we may be unable to detect or prevent product and/or ingredient quality issues such as contamination or deviations from our established procedures. If any of our products cause injury to animals, we may incur material expenses for product recalls, and may be subject to product liability claims, which could damage our reputation and brand substantially.
We may not be able to manage our manufacturing and supply chain effectively, which would harm our results of operations.
We must accurately forecast demand for our products in order to have adequate product inventory available to fill customer orders timely. Our forecasts will be based on multiple assumptions that may cause our estimates to be inaccurate, and thus affect our ability to ensure adequate manufacturing capability to satisfy product demand. Any material delay in our ability to obtain timely product inventories from our manufacturing facility and our ingredient suppliers could prevent us from satisfying increased consumer demand for our products, resulting in material harm to our brand and business. In addition, we will need to continuously monitor our inventory and product mix against forecasted demand to avoid having inadequate product inventory or having too much product inventory on hand. If we are unable to manage our supply chain effectively, our operating costs may increase materially.
Failure to protect our intellectual property could harm our competitive position or cause us to incur significant expenses and personnel resources to enforce our rights.
Our success will depend significantly upon our ability to protect our intellectual property (“IP”) rights, including patents, trademarks, trade secrets, and process know-how, which valuable assets support our brand and the perception of our products. We rely on patent, trademark, trade secret and other intellectual property laws, as well as non-disclosure and confidentiality agreements to protect our intellectual property. Our non-disclosure and confidentiality agreements may not always effectively prevent disclosure of our proprietary IP rights, and may not provide an adequate remedy in the event of an unauthorized disclosure of such information, which could harm our competitive position. We also may need to engage in costly litigation to enforce or protect our patent or other proprietary IP rights, or to determine the validity and scope of proprietary rights of others. Any such litigation could require us to expend significant financial resources and also divert the efforts and attention of our management and other personnel from our ongoing business operations. If we fail to protect our intellectual property, our business, brand, financial condition and results of operations may be materially harmed.
We may be subject to intellectual property infringement claims, which could result in substantial damages and diversion of the efforts and attention of our management.
We must respect prevailing third-party intellectual property, and the procedures and steps we take to prevent our misappropriation, infringement or other violation of the intellectual property of others may not be successful. If third parties assert infringement claims against us, our suppliers, or veterinarians using our products and technology, we could be required to expend substantial financial and personnel resources to respond to and litigate or settle any such third-party claims. Although we believe our patents, manufacturing processes and products do not infringe in any material respect on the intellectual property rights of other parties, we could be found to infringe on such proprietary rights of others. Any claims that our products, processes or technology infringe on third-party rights, regardless of their merit or resolution could be very costly to us and also materially divert the efforts and attention of our management and technical personnel. Any adverse outcome to us from one or more such claims against us could, among other things, require us to pay substantial damages, to cease the sale of our products, to discontinue our use of any infringing processes or technology, to expend substantial resources to develop non-infringing products or technology, or to license technology from the infringed party. If one or more of such adverse outcomes occur, our ability to compete could be affected significantly and our business, financial condition and results of operations could be harmed substantially.
| 10 |
We may be unable to obtain required regulatory approvals for future products timely or at all, and the denial or substantial delay of any such approval could delay materially or even prevent our efforts to commercialize new products, which could adversely impact our ability to generate future revenues.
Based on our determination that our Kush™ products constitute a device for the treatment of animals rather than being a pharmaceutical product, we believe we are not required to obtain regulatory approval to produce and market them for their current intended uses. However, we have not received confirmation from any regulatory authority that our determination is correct. The production, marketing and sale of any future products for the treatment of animals based on our proprietary technology may be require us to obtain regulatory approval from the Center for Veterinarian Medicine (“CVM”), a branch of the FDA, and/or the USDA, and also certain state regulatory authorities. Any substantial delay or inability to obtain required regulatory approvals for any new products developed by us could substantially delay or even prevent their commercialization, which would materially adversely impact our business and prospects.
Moreover, at such future time that we commence business internationally, our products will need to obtain regulatory approval for labeling, marketing and sale in foreign countries from authorities such as the European Commission (“EU”) or the European Medicine Agency (“EMA”). Any substantial delay or inability to obtain any necessary foreign regulatory approvals for our products could harm our business and prospects materially.
Our products will face significant competition in our industry, and our failure to compete effectively may prevent us from achieving any significant market penetration.
The development and commercialization of animal care products is highly competitive, including significant competition from major pharmaceutical, biotechnology, and specialty animal health medical companies. Our competitors include Zoetis, Inc.; Merck Animal Health, the animal health division of Merck & Co., Inc.; Merial, the animal health division of Sanofi, S.A.; Elanco, the animal health division of Eli Lilly and Company; Bayer Animal Health, the animal health division of Bayer AG; Novartis Animal Health, the animal health division of Novartis AG; Boehringer Ingelheim Animal Health; Virbac Group; Ceva Animal Health; Vetaquinol; and Dechra Pharmaceuticals PLC. There also are several smaller stage animal health companies which have recently emerged in our industry and are developing therapeutics products that may compete with our products, including Kindred Bio, Aratana Therapeutics, Next Vet, and VetDC.
Since we are an early-stage company with limited operations and financing, virtually all our competitors have substantially more financial, technical and personnel resources than us. Most of them also have established brands and substantial experience in the development, production, regulation and commercialization of animal health care products. Regarding our development of any new products or technology, we also compete with academic institutions, governmental agencies and private organizations that conduct research in the field of animal health medicines. We expect that competition in our industry is based on several factors including primarily product reliability and effectiveness, product pricing, product branding, adequate patent and other IP protection, safety of use, and product availability.
Although for the foreseeable future, our efforts and financial resources will continue to focus on successfully commercializing our Kush™ products, our future business strategy plan includes the identification of additional animal care products we may license, acquire or develop, and then commercializing such products into a branded product portfolio along with our Kush™ products. Even if we successfully license, acquire or develop such animal care products from our proprietary technology, or acquire any such new products, we may still fail to commercialize them successfully for various reasons, including competitors offering alternative products which are more effective than ours, our discovery of third-party IP rights already covering the products, harmful side effects caused to animals by the products, inability to produce products in commercial quantities at an acceptable cost, or the products not being accepted by veterinarians and pet owners as being safe or effective. If we fail to successfully obtain and commercialize future new animal care products, our business and prospects may be harmed substantially.
| 11 |
We will rely on third-parties to conduct studies of our current and new products, and if these third parties do not successfully perform their contracted commitments effectively or substantially fail to meet expected study deadlines, we could be delayed significantly or even prevented from obtaining regulatory approval for and/or effectively commercializing our future products.
We intend to engage one or more educational institutions with a veterinary medical curriculum to conduct studies of Kush™ and other products to be introduced by us. We expect to have limited control over the timing and resources that such third parties will devote to the studies. Although we must rely on the third parties to conduct our studies, we remain responsible for ensuring any of our studies are conducted in compliance with protocols, regulations and standards set by industry regulatory authorities and commonly referred to as current good clinical practices (“cGCPs”) and good laboratory practices (“GLPs”). These required clinical and laboratory practices include many items regarding the conducting, monitoring, recording and reporting the results of target animal studies to ensure that the data and results of these studies are objective and scientifically credible and accurate.
A failure of one or more key information technology systems, networks or processes may harm our ability to conduct our business effectively.
The effective operation of our business and operations will depend significantly on our information technology and computer systems. We will rely on these systems to effectively manage our sales and marketing, accounting and financial, and legal and compliance functions, new product development efforts, research and development data, communications, supply chain and product distribution, order entry and fulfillment, and other business processes. Any material failure of our information technology systems to perform satisfactorily, or their damage or interruption from circumstances beyond our control such as power outages or natural disasters, could disrupt our business materially and result in transaction errors, processing inefficiencies, and even the loss of sales and customers., causing our business and results of operations to suffer materially.
Natural disasters and other events beyond our control could materially adversely affect us.
Natural disasters or other catastrophic events may cause damage or disruption to our operations, international commerce and the global economy, and thus could have a strong negative effect on us. Our business operations are subject to interruption by natural disasters, fire, power shortages, pandemics (including the ongoing Coronavirus (COVID-19) epidemic) and other events beyond our control. Although we maintain crisis management and disaster response plans, such events could make it difficult or impossible for us to deliver our services to our customers, and could decrease demand for our services.
Risks Related to this Offering, our Common Stock and Warrants
Ownership and control of our Company is concentrated in our management.
As of the date of this Prospectus, our officers and directors beneficially own or control approximately 58% of our outstanding shares of common stock. Following this Offering, our directors and directors will own approximately __% of our common stock, which concentrated ownership and control by our management could adversely affect the status and perception of our common stock and/or warrants. In addition, any material sales of common stock of our management, or even the perception that such sales will occur, could cause a material decline in the trading price of our common stock and/or warrants.
Due to this ownership concentration, our management has the ability to control all matters requiring stockholder approval including the election of all directors, the approval of mergers or acquisitions, and other significant corporate transactions. Any person acquiring our common stock most likely will have no effective voice in the management of our company. This ownership concentration also could delay or prevent a change of control of the Company, which could deprive our stockholders from receiving a premium for their common shares.
| 12 |
There has been no consistent active trading market for our common stock, and public trading or our common stock and warrants may continue to be inactive and fluctuate substantially.
There has never been a consistent active trading market for our common stock. Our common stock currently trades over-the-counter on the QB tier of OTC Markets Group, Inc. under the symbol “PETV.” We plan on listing our warrants on the Exchange under the symbol “___.” There is no assurance that the trading market for our common stock and warrants will become more active or liquid. Furthermore, there can be no assurance any broker will be interested in trading our stock or warrants. Therefore, it may be difficult to sell your shares of common stock or warrants if you desire or need to sell them. Our underwriters are not obligated to make a market in our securities, and even if they make a market, they can discontinue market making at any time without notice. Neither we nor the underwriters can provide any assurance that an active and liquid trading market in our securities will develop or, if developed, that such market will continue.
Moreover, the trading price of our common stock has fluctuated substantially over the past few years, and there remains a significant risk that our common stock price may continue to fluctuate substantially in the future in response to various factors, including any material variations in our periodic operating results, departures or additions of management or other key personnel, announcements of acquisitions, mergers, or new technology or patents, new product developments, significant litigation matters, gain or loss of significant customers, significant capital transactions, substantial sales of our common stock in our trading market, and general and specific market and economic conditions.
The market price of our common stock and warrants is likely to be highly volatile because of several factors, including a limited public float.
The market price of our common stock has been volatile in the past and the market price of our common stock and our warrants is likely to be highly volatile in the future. You may not be able to resell shares of our common stock or our warrants following periods of volatility because of the market’s adverse reaction to volatility.
Other factors that could cause such volatility may include, among other things:
| ● | actual or anticipated fluctuations in our operating results; | |
| ● | the absence of securities analysts covering us and distributing research and recommendations about us; | |
| ● | we may have a low trading volume for a number of reasons, including that a large portion of our stock is closely held; | |
|
● | overall stock market fluctuations; |
| ● | announcements concerning our business or those of our competitors; | |
| ● | actual or perceived limitations on our ability to raise capital when we require it, and to raise such capital on favorable terms; | |
| ● | conditions or trends in the industry; | |
| ● | litigation; | |
| ● | changes in market valuations of other similar companies; | |
| ● | future sales of common stock; | |
| ● | departure of key personnel or failure to hire key personnel; and | |
| ● | general market conditions. |
| 13 |
Any of these factors could have a significant and adverse impact on the market price of our common stock and/or warrants. In addition, the stock market in general has at times experienced extreme volatility and rapid decline that has often been unrelated or disproportionate to the operating performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock and/or warrants, regardless of our actual operating performance.
Our common stock has in the past been a “penny stock” under SEC rules, and our warrants may be subject to the “penny stock” rules in the future. It may be more difficult to resell securities classified as “penny stock.”
In the past (including immediately prior to this offering), our common stock was a “penny stock” under applicable Securities and Exchange Commission (“SEC”) rules (generally defined as non-exchange traded stock with a per-share price below $5.00). While our common stock (and trading warrants) will not be considered “penny stocks” following this offering since they will be listed on the Exchange, if we are unable to maintain that listing and our common stock and warrants are no longer listed on the Exchange, unless we maintain a per-share price above $5.00, our common stock and warrants will become “penny stocks.” These rules impose additional sales practice requirements on broker-dealers that recommend the purchase or sale of penny stocks to persons other than those who qualify as “established customers” or “accredited investors.” For example, broker-dealers must determine the appropriateness for non-qualifying persons of investments in penny stocks. Broker-dealers must also provide, prior to a transaction in a penny stock not otherwise exempt from the rules, a standardized risk disclosure document that provides information about penny stocks and the risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, disclose the compensation of the broker-dealer and its salesperson in the transaction, furnish monthly account statements showing the market value of each penny stock held in the customer’s account, provide a special written determination that the penny stock is a suitable investment for the purchaser, and receive the purchaser’s written agreement to the transaction.
Legal remedies available to an investor in “penny stocks” may include the following:
| ● | If a “penny stock” is sold to the investor in violation of the requirements listed above, or other federal or states securities laws, the investor may be able to cancel the purchase and receive a refund of the investment. | |
| ● | If a “penny stock” is sold to the investor in a fraudulent manner, the investor may be able to sue the persons and firms that committed the fraud for damages. |
These requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security that becomes subject to the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our securities, which could severely limit the market price and liquidity of our securities. These requirements may restrict the ability of broker-dealers to sell our common stock or our warrants and may affect your ability to resell our common stock and our warrants.
Many brokerage firms will discourage or refrain from recommending investments in penny stocks. Most institutional investors will not invest in penny stocks. In addition, many individual investors will not invest in penny stocks due, among other reasons, to the increased financial risk generally associated with these investments. For these reasons, penny stocks may have a limited market and, consequently, limited liquidity. We can give no assurance at what time, if ever, our common stock or our warrants will not be classified as a “penny stock” in the future.
| 14 |
If we fail to maintain effective internal control over financial reporting, the price of our securities may be adversely affected.
Our internal controls over financial reporting have weaknesses and conditions that require correction or remediation. For the year ended March 31, 2020 and the quarter ended June 30, 2020, we identified three material weakness in our assessment of the effectiveness of disclosure controls and procedures. We have (i) deficiencies in the segregation of duties, (ii) deficiencies in the staffing of our financial accounting department and (iii) limited checks and balances in processing cash and other transactions. We are committed to improving our financial reporting processes and plan on increasing the size of our accounting staff at the appropriate time for our business and its size to ameliorate our concern that we do not effectively segregate certain accounting duties or have adequate staffing, which we believe would resolve these material weakness in disclosure controls and procedures. However, there can be no assurances as to the timing of any such action or that we will be able to do so. Any failure by us to implement the changes necessary to maintain an effective system of internal controls could harm our operating results materially and cause investors and financial analysts to lose confidence in our reported financial information. Any such loss of confidence in the investment community would have a negative effect on the trading and price of our common stock and warrants.
We are required to comply with certain provisions of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (the “Sarbanes-Oxley Act”) and if we fail to continue to comply, our business could be harmed, and the price of our securities could decline.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act require an annual assessment of internal controls over financial reporting, and for certain issuers, an attestation of this assessment by the issuer’s independent registered public accounting firm. The standards that must be met for management to assess the internal controls over financial reporting as effective are evolving and complex, and require significant documentation, testing, and possible remediation to meet the detailed standards. We expect to incur significant expenses and to devote resources to Section 404 compliance on an ongoing basis. It is difficult for us to predict how long it will take or costly it will be to complete the assessment of the effectiveness of our internal controls over financial reporting for each year and to remediate any deficiencies in our internal control over financial reporting. As a result, we may not be able to complete the assessment and remediation process on a timely basis. In the event that our Chief Executive Officer or Chief Financial Officer determines that our internal controls over financial reporting are not effective as defined under Section 404, we cannot predict how regulators will react or how the market prices of our securities will be affected; however, we believe that there is a risk that investor confidence and the market value of our securities may be negatively affected.
Our management has broad discretion over the use of proceeds received by us from this offering and may apply these proceeds in ways that do not improve our operating results or increase the value of your investment.
Our management will retain broad discretion as to the use and allocation of the net proceeds received by us in this offering. Accordingly, our investors will not have any opportunity to evaluate the economic, financial and other relevant information considered by us regarding the application of the net proceeds. Management could apply the proceeds in ways that you would not approve of, and which do not improve our business or increase the value of your investment.
You will experience immediate and substantial dilution in the value of any Units that you purchase from us in this offering.
The price per common share in this public offering is substantially higher than the net tangible book value of each outstanding share of our common stock, and accordingly purchasers of Units in this offering will experience immediate and substantial dilution. See “Dilution.”
Assuming we obtain an Exchange listing, we will incur material increased costs and become subject to additional regulations and requirements.
As a newly Exchange-listed public company, we will incur material additional legal, accounting and other expenses including recruiting and retaining qualified independent directors, payment of annual Exchange fees, and satisfying Exchange standards for companies listed with it. If we are unable to satisfy our obligations and responsibilities as an Exchange-listed company, we could be subject to delisting of our common stock and warrants from the Exchange and other regulatory action, and potentially even civil litigation.
We do not anticipate paying any dividends on our common stock for the foreseeable future.
We have not paid any dividends on our common stock to date, and we do not anticipate paying any such dividends in the foreseeable future. We anticipate that any earnings experienced by us will be retained to finance the implementation of our operational business plan and expected future growth.
| 15 |
The elimination of monetary liability against our directors and executive officers under Nevada law and the existence of indemnification rights held by them granted by our bylaws could result in substantial expenditures by us.
Our Articles of Incorporation eliminate the personal liability of our directors and officers to the Company and its stockholders for damages for breach of fiduciary duty to the maximum extent permissible under Nevada law. In addition, our Bylaws provide that we are obligated to indemnify our directors or officers to the fullest extent authorized by Nevada law for costs or damages incurred by them involving legal proceedings brought against them relating to their positions with the Company. These indemnification obligations could result in our incurring substantial expenditures to cover the cost of settlement or damage awards against our directors or officers.
Our Articles of Incorporation, Bylaws, and Nevada law may have anti-takeover effects that could discourage, delay or prevent a change in control, which may cause our stock price to decline.
Our Articles of Incorporation, Bylaws, and Nevada law could make it more difficult for a third party to acquire us, even if closing such a transaction would be beneficial to our stockholders. We are authorized to issue up to 1,000,000 shares of preferred stock. This preferred stock may be issued in one or more series, the terms of which may be determined at the time of issuance by our board of directors without further action by stockholders. The terms of any series of preferred stock may include voting rights (including the right to vote as a series on particular matters), preferences as to dividend, liquidation, conversion and redemption rights and sinking fund provisions. None of our preferred stock will be outstanding at the closing of this offering. The issuance of any preferred stock could materially adversely affect the rights of the holders of our common stock, and therefore, reduce the value of our common stock. In particular, specific rights granted to future holders of preferred stock could be used to restrict our ability to merge with, or sell our assets to, a third party and thereby preserve control by the present management.
Provisions of our Articles of Incorporation, Bylaws and Nevada law also could have the effect of discouraging potential acquisition proposals or making a tender offer or delaying or preventing a change in control, including changes a stockholder might consider favorable. Such provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management. In particular, our certificate of incorporation and by-laws and Delaware law, as applicable, among other things:
| ● | provide the board of directors with the ability to alter the by-laws without stockholder approval; | |
| ● | establishing advance notice requirements for nominations for election to the board of directors or for proposing matters that can be acted upon at stockholder meetings; and | |
| ● | provide that vacancies on the board of directors may be filled by a majority of directors in office, although less than a quorum. |
Warrants are speculative in nature.
The warrants offered in this offering do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to acquire shares of our common stock at a fixed price for a limited period of time. Specifically, commencing on the date of issuance, holders of the warrants may exercise their right to acquire the common stock and pay an exercise price of $[●] per share (100)% of the assumed public offering price of a Unit), prior to five (5) years from the date of issuance, after which date any unexercised warrants will expire and have no further value. In addition, there is no established trading market for the warrants and we do not expect a market to develop.
Holders of the warrants will have no rights as a common stockholder until they acquire our common stock.
Until holders of the warrants acquire shares of our common stock upon exercise of the warrants, the holders will have no rights with respect to shares of our common stock issuable upon exercise of the warrants. Upon exercise of the warrants, the holder will be entitled to exercise the rights of a common stockholder as to the security exercised only as to matters for which the record date occurs after the exercise.
| 16 |
There is no established market for the warrants to purchase shares of our common stock being offered in this offering.
There is no established trading market for the warrants and we do not expect a market to develop. Although we have applied to list the warrants on the Exchange there can be no assurance that there will be an active trading market for the warrants. Without an active trading market, the liquidity of the warrants will be limited.
Provisions of the warrants offered by this prospectus could discourage an acquisition of us by a third party.
In addition to the discussion of the provisions of our Articles of Incorporation and our Bylaws, certain provisions of the warrants offered by this prospectus could make it more difficult or expensive for a third party to acquire us. The warrants prohibit us from engaging in certain transactions constituting “fundamental transactions” unless, among other things, the surviving entity assumes our obligations under the warrants. These and other provisions of the warrants offered by this prospectus could prevent or deter a third party from acquiring us even where the acquisition could be beneficial to you.
This prospectus contains estimates, projections and other information concerning our industry, our business and the markets for our drug candidates, including data regarding the estimated size of such markets and the incidence of certain medical conditions. We obtained the industry, market and similar data set forth in this prospectus from our internal estimates and research and from academic and industry research, publications, surveys and studies conducted by third parties, including governmental agencies. In some cases, we do not expressly refer to the sources from which this data is derived. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. While we believe our internal research is reliable, such research has not been verified by any third party.
Based on the public offering price of $ per Unit, we estimate that the net proceeds to us from this public offering will be approximately $ after deducting the underwriting discounts and commissions and other estimated offering expenses. Based on our current projections, we expect such net proceeds will satisfy our operational and working capital needs for the next twelve months. We intend to use these net proceeds as follows:
| Marketing support and expenses, including sales personnel and representatives | $ | 3,000,000 | ||
| Research & Development | 130,000 | |||
| Debt Repayment | 440,000 | |||
| Note Payable – Related Party | 130,000 | |||
| Raw materials, supplies and direct labor - Kush | 1,300,000 | |||
| Purchase of equipment | 40,000 | |||
| Working Capital | 3,960,000 | |||
| Total net proceeds | $ | 9,000,000 |
We currently intend to use the net proceeds from this offering for general corporate purposes including marketing of our Kush™ products, debt repayment, research and development of our current product pipeline, purchase of manufacturing equipment and working capital. Our total planned debt repayment of $440,000 consists of (i) $360,000 in a Convertible Note due to RedDiamond Partners, LLC and maturing on March 15, 2021, consisting of approximately $353,000 in principal and $7,000 in interest, which accrues interest at a rate of 12.5%, is automatically converted into common stock at $0.72 per share if an uplisting to the Exchange occurs and the price per share in the uplisting is at least $0.864; (ii) $40,000 in accrued expenses relating to a past employee’s accrued salary; (iii) $40,000 in a note payable that accrues interest payable on a monthly basis at a rate of 6.5% interest and matures on May 3, 2026. We also plan to repay $130,000 in a notes payable to a related party, as follows: (i) payment in full of the remaining balance, on the Amendment (“Amendment”) to the Promissory Note dated September 1, 2020, which is $60,000 and (ii) a partial payment of $70,000 on the promissory note dated September 1, 2020 (“Note”) in the principal amount of $195,000 due to the related party. The Amendment bears interest at the rate of 3% per annum, has accrued interest due of $400 and has a maturity date of August 31, 2022. The Note bears interest at the rate of 8% per annum, has accrued interest due of $6,000, and a maturity date of June 30, 2022. Management plans to use net proceeds from this offering to repay debt including but not limited to: a bridge note used for working capital purposes, a note payable to a related party and accrued interest entered into for working capital purposes, accounts payable relating to general operating expenses and accrued expenses and accrued expenses – related party, both due to general operating expenses. See “Risk Factors” for a discussion of certain risks that may affect our intended use of the net proceeds from this offering.
| 17 |
We anticipate expending approximately $800,000 to fund independent studies for the use of our products in dogs and horses as part of the $3,000,000 for marketing support. We have had discussions with several universities that have veterinary studies in their curriculum regarding conducting such studies for us. We also anticipate expending approximately $130,000 for research and development relating to advancing products within our muco-adhesion technology included within our product pipeline.
These anticipated expenditures represent our current estimates and intentions based upon our present plans and assumptions regarding our anticipated future revenues and expenses. As of the date of this prospectus, we cannot predict accurately all of the particular uses for these net proceeds, and accordingly our management will have broad discretion regarding our use of net proceeds from this offering. Moreover, the amounts and timing of our actual expenditures will depend upon numerous factors, including the future results of our marketing and other commercialization efforts for our initial Kush System products. Pending our use of proceeds as above, we expect to invest them in investment-grade, short-term interest-bearing deposit certificates or similar securities.
MARKET FOR OUR STOCK AND RELATED STOCKHOLDER MATTERS
Market Information
During the fiscal years ended March 31, 2019 and 2020, our common stock was listed for quotation on the OTC Pink Sheets and OTCQB markets under the symbol “PETV.” The following table sets forth the high and low bid prices relating to our common stock on a quarterly basis for the periods indicated as quoted by the OTC Pink Sheets and OTCQB markets. These quotations reflect inter-dealer prices without retail mark-up, mark-down, or commissions. There can be no assurance that an active public market for our common stock will develop or be sustained.
| High Bid | Low Bid | |||||||
| Fiscal Year 2021 | ||||||||
Second Quarter Ended September 30, 2020 | 0.95 | 0.40 | ||||||
First Quarter Ended June 30, 2020 | 0.39 | 0.14 | ||||||
Fiscal Year 2020: | ||||||||
| Fourth Quarter Ended March 31, 2020 | 0.55 | 0.12 | ||||||
| Third Quarter Ended December 31, 2019 | 0.92 | 0.37 | ||||||
| Second Quarter Ended September 30, 2019 | 0.41 | 0.22 | ||||||
| First Quarter Ended June 30, 2019 | 0.41 | 0.10 | ||||||
| Fiscal Year 2019: | ||||||||
| Fourth Quarter Ended March 31, 2019 | 0.83 | 0.32 | ||||||
| Third Quarter Ended December 31, 2018 | 0.71 | 0.22 | ||||||
| Second Quarter Ended September 30, 2018 | 1.11 | 0.66 | ||||||
| First Quarter Ended June 30, 2018 | 1.81 | 1.02 | ||||||
Number of Stockholders
At September 30, 2020, there were approximately 287 stockholders of record. The number of stockholders of record does not include certain beneficial owners of our common stock, whose shares are held in the names of various dealers, clearing agencies, banks, brokers and other fiduciaries.
Dividends
We have never declared or paid any cash dividends on our common stock and do not anticipate paying any cash dividends on our common stock at any time in the foreseeable future. We currently intend to retain all available funds and any future earnings for use in the operation of our business and do not anticipate paying any dividends on our common stock in the foreseeable future. Any future determination to declare dividends will be made at the discretion of our Board and will depend on, among other factors, our financial condition, operating results, capital requirements, general business conditions, the terms of any future credit agreements and other factors that our Board may deem relevant.
| 18 |
Warrants and Stock Options
As of September 30, 2020, we have options and warrants outstanding for the purchase of 5,763,610 shares of our common stock. This does not include the underwriters’ warrants and warrants offered by us in this offering as part of the Units.
Restricted Stock Units
As of September 30, 2020, we have not granted any restricted stock units to any employees, consultants or other third parties.
DETERMINATION OF OFFERING PRICE
The offering price for the Units offered by this prospectus will be based upon negotiations between the Company and the Underwriters as described in “UNDERWRITING.”
The following table is illustrative only, and our capitalization following the completion of the offering will be adjusted based on the actual offering price the Units and other terms of our offering determined on such pricing. You should read the following table in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of Operations and our financial statements and notes appearing elsewhere in this prospectus.
The table sets forth our cash and cash equivalents and our capitalization, as of June 30, 2020:
| ● | on an actual basis; | |
| ● | on a pro forma basis to give effect to the issuance of ______ Units in this offering after deducting the underwriter discount and our estimated offering expenses; | |
| ● | on a pro forma as adjusted basis to give effect to the sale of ________ Units in this offering at a price of $_.__ per Unit, after deducting underwriting discounts and estimated offering expenses payable by us. |
| 19 |
As of June 30, 2020 (unaudited) | ||||||||||||
| Actual | Pro forma | Pro
forma as adjusted | ||||||||||
| Cash and Cash Equivalents | $ | 128,564 | $ | |||||||||
| Notes due related parties | 62,445 | |||||||||||
| Stockholders’ (Deficit) Equity: | ||||||||||||
| Preferred stock, $0.001 par value, 20,000,000 authorized, none issued or outstanding | -0- | -0- | -0- | |||||||||
| Common stock, $0.001 par value, 225,000,000 shares authorized, 23,111,857 shares issued and outstanding actual; _________ shares issued and outstanding pro forma and _________ shares issued and outstanding pro forma as adjusted | 23,111 | |||||||||||
| Additional paid-in capital | 53,804,107 | |||||||||||
| Accumulated deficit | (55,402,653 | ) | ||||||||||
| Total stockholders’ deficit | (1,575,435 | ) | $ | |||||||||
| Total Capitalization | $ | (2,959,861 | ) | |||||||||
The number of shares of common stock to be outstanding after this offering is based on 24,876,189 shares outstanding as of September 30, 2020, ________ shares issued between October 1, 2020 through and immediately prior to the date of this prospectus and _________ Units to be issued in this Offering; and does not include _________ shares reserved for exercise of outstanding warrants, underwriters warrants for ________ shares and up to _________ Units for the over-allotment option.
If you invest in the Units offered pursuant to this prospectus, your interest will be diluted to the extent of the difference between the public offering price you pay and the adjusted net tangible book value per common share of our stock. Net tangible book value as of June 30, 2020 was ($0.05) per common share of our common stock. Net tangible book value per share is the difference between our total assets less our intangible assets and total liabilities, divided by the number of shares of common stock outstanding.
Dilution per share represents the difference between the amount per share paid by new investors who purchase Units in this offering and the adjusted net tangible book value per share of our common stock immediately after completion of this offering as of June 30, 2020, after giving effect to the sale by us of ________ Units at the public offering price of $__ per Unit.
| Public offering price per share (attributing no value to the warrants) | $ | |||||||||||
| Net tangible book value per share as of June 30, 2020 | $ | (0.05 | ) | |||||||||
| Increase in net tangible book value per share attributable to this offering | ( | ) | ||||||||||
| As adjusted net tangible book value per share after this offering | ||||||||||||
| Dilution in net tangible book value per share to investors in this offering |
The number of shares of common stock to be outstanding after this offering is based on 24,876,189 shares outstanding as of September 30, 2020, ________ shares issued between October 1, 2020 through and immediately prior to the date of this prospectus and _________ Units to be issued in this Offering; and does not include _________ shares reserved for exercise of outstanding warrants, underwriters warrants for ________ shares and up to _________ Units for the over-allotment option.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read in conjunction with our financial statements and related notes that appear elsewhere in this prospectus. In addition to historical consolidated financial information, the following discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results could differ materially from those discussed in the forward looking statements. Factors that could cause or contribute to those differences include those discussed below and elsewhere in this prospectus, particularly in “RISK FACTORS.” We caution the reader not to place undue reliance on these forward-looking statements, which reflect management’s analysis only as of the date of this prospectus.
| 20 |
General Overview
We are a smaller reporting company and have not generated any material revenues to date and have incurred substantial losses in connection with our limited operations. We need substantial capital to pursue our current plans to bring our first products to market. The first of such products is a proprietary gel-like protein-based biomedical material for injection into the afflicted body parts of animals suffering from osteoarthritis or other impairments to be marketed under the trade name Kush™. It will provide to veterinarians an innovative treatment for dogs and horses suffering from osteoarthritis.
Going Concern
The independent auditor’s report accompanying our March 31, 2020 financial statements contains an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern. The financial statements have been prepared “assuming that we will continue as a going concern,” which contemplates that we will realize our assets and satisfy our liabilities and commitments in the ordinary course of business. We have suffered recurring losses from operations, have a substantial working capital deficit and are currently in default of the payment terms of certain note agreements. These factors raise substantial doubt about our ability to continue as a going concern.
RESULTS OF OPERATION
For Three Months Ended June 30, 2020 | For Three Months Ended June 30, 2019 | For Fiscal Year Ended March 31, 2020 | For Fiscal Year Ended March 31, 2019 | |||||||||||||
| Revenues | $ | 2,006 | - | $ | 3,588 | $ | 0 | |||||||||
| Total Cost of Sales | 0 | 0 | 19,710 | 77,936 | ||||||||||||
| Total Operating Expenses | 440,074 | 493,721 | 2,000,010 | 4,594,872 | ||||||||||||
| Total Other Income (Expense) | (371,940 | ) | (7,166 | ) | (66,602 | ) | (84,950 | ) | ||||||||
| Net Loss | (814,008 | ) | (500,887 | ) | (2,082,734 | ) | (4,757,758 | ) | ||||||||
| Net loss per share - basic and diluted | $ | (.04 | ) | $ | (.03 | ) | $ | (.10 | ) | $ | (.26 | ) | ||||
For Fiscal Year Ended March 31, 2020 (“fiscal 2020”) Compared to Fiscal Year Ended March 31, 2019 (“fiscal 2019”)
Total Revenues. For fiscal 2020, we earned $3,588 in revenue compared to earning no revenue during fiscal 2019. Our sales in fiscal 2020 represented sample product sales, with no commercial sales in fiscal 2019.
Total Cost of Sales. For fiscal 2020, we incurred $19,710 in expenses compared to $77,936 in fiscal 2019. Cost of sales during fiscal 2020 was made up of costs during the year and subsequent reserve for obsolete inventory. Cost of sales during fiscal 2019 was made up of a reserve for obsolete inventory taken due to an analysis that included the product status, shelf life, and lack of material sales.
Operating Expenses. Operating expenses for fiscal 2020 were $2,000,010 compared to $4,594,872 for fiscal 2019, a decrease of $2,594,862. Operating expenses consisted of: (i) research and development expenses, (ii) sales and marketing, (iii) general and administrative expenses and (iv) intangible impairment expenses. General and administrative expenses generally include corporate overhead, financial and administrative contracted services, marketing, and consulting costs.
Sales and marketing expenses increased significantly to $171,509 in fiscal 2020 compared to $38,348 in fiscal 2019; this increase was due to increased sales and marketing expenses relating to promoting Kush™ to veterinarians.
| 21 |
Research and development expenses (“R&D”) decreased to $12,672 in fiscal 2020 compared to $200,982 in fiscal 2019. We completed most of our R&D for Kush™ in fiscal 2019 and therefore did not incur significant R&D expense in fiscal 2020. Sales and marketing expenses decreased to $38,348 in fiscal 2020 compared to $171,509 in fiscal 2019. General administrative (“G&A”) expenses were $1,784,557 in fiscal 2020 compared to $4,251,742 in fiscal 2019. In fiscal 2019, we had significant G&A expenses attributable to a charge in the amount of $861,592 attributable to common stock issued to our CEO to replace shares he surrendered pursuant to an escrow agreement, and $584,501 in common stock issued to our CEO to replace shares he gave up to secure funding for the Company in 2015. Our intangible impairment expense was $31,272 in fiscal 2020 compared to $103,800 in fiscal 2019.
Operating Loss. As a result of the foregoing, our operating loss for fiscal 2020 was $2,016,132 compared to an operating loss of $4,672,808 for fiscal 2019.
Other Income (Expense). Other expense for fiscal 2020 was $66,602 compared to other expense of $84,950 for fiscal 2019 in fiscal 2019, our only expense was interest expense of $84,950. In fiscal 2020, interest expense decreased significantly to $32,180 due to the retirement of certain outstanding debt. In addition, we have other expense in fiscal 2020 consisting of (i) a $47,710 gain on settlement, (ii) a loss on sale of assets of ($389) and (iii) a loss on debt extinguishment of ($81,738).
Net Loss before Taxes and Net Loss. Our net loss before taxes for fiscal year ended March 31, 2020 was ($2,082,734) or ($0.10) per share as compared to ($4,757,758) or ($0.26) per share for fiscal year ended March 31, 2019. Net loss generally decreased primarily due to the following differences in fiscal year ended March 31, 2019: increased expense related to sales and marketing of $133,161, a decrease in expense related to research and development of $188,310, a decrease in intangible impairment of $72,528, and a decrease in general and administrative expenses of $2,467,185. The weighted average number of shares outstanding during fiscal 2020 was 21,222,359 compared to 18,451,797 for fiscal 2019.
Results of Operations for the Three Months Ended June 30, 2020 (“first quarter 2020”) and June 30, 2019 (“first quarter 2019”)
Total Revenues. In the first quarter of 2020, revenue was $2,006 which consisted of Kush™ product sales to veterinary clinics, compared to no revenues in the first quarter 2019.
Total Cost of Sales. We did not incur any costs of sales in the first quarter of 2020 or first quarter of 2019 because we were not manufacturing any new Kush™ products.
Gross Profit. Our gross profit was $2,006 for the first quarter of 2020, compared to $0 in the first quarter of 2019.
Operating Expenses. Operating expenses for the first quarter of 2020 were $444,074, compared to $493,721 for the first quarter of 2019. The decrease of $49,647 was due primarily to a decrease in depreciation and amortization of $92,285 since several of our patents have become fully-amortized. The decrease in depreciation and amortization was partially offset by an increase in sales and marketing expense of $43,886 due to various stock-based agreements being relieved from prepaids to expense.
The majority of our operating expenses in both of these quarters was general and administrative expenses. During the three-month period ended June 30, 2020, operating expenses were primarily made up of $51,805 (compared to $144,090 for the comparable quarter in 2019) in depreciation and amortization and $183,244 (compared to $190,801 for the comparable quarter in 2019) in stock-based compensation.
Other Expense. Other Expense for the three months ended June 30, 2020 of $371,940 is primarily due to derivative expense in the amount of $324,200 related to the setup of a derivative liability as described in Note 8 to these financial statements. Other Expense for the three months ended June 30, 2019 of $7,166 was primarily made up of interest accrued on outstanding notes payable, notes payable – related party, and convertible notes payable in the amount of $7,616, which was partially offset by a $450 gain on sale of asset that was fully depreciated at the time of the sale.
Net Loss before Taxes and Net Loss – Our net loss for the three months ended June 30, 2020 was $814,008, or $0.04 per share, compared to $500,887, or $0.03 per share for the three months ended June 30, 2019. The increased loss of $313,121 was attributable primarily to derivative expense of $342,200 recognized during the three months ended June 30, 2020. The weighted average number of shares outstanding during the three-month periods ended June 30, 2020 and June 30, 2019 were 22,938,231 and 19,867,200, respectively.
| 22 |
LIQUIDITY AND CAPITAL RESOURCES
Our financial position and future prospects depend significantly on our access to financing to fund our operations during our commercialization stage. Much of our current cost structure is based on costs related to personnel and facilities, and not subject to material variability. In order to fund our operations and working capital needs, we historically have utilized loans from accredited investors and others, equity sales of common stock to accredited investors and others with whom we have pre-existing relationships, and substantial issuances of stock-based compensation to satisfy outstanding debt and pay for development, management, financial, professional and other services.
As of June 30, 2020, our current assets were $268,434 and our current liabilities were $2,013,558, which resulted in a working capital deficiency of $1,745,124. As of June 30, 2020, we had approximately $128,564 in cash, $2,000 in accounts receivable, $1,500 in investment – equity securities, and $136,370 in prepaid expenses. In comparison, our current liabilities as of that date were $2,013,558, consisting of $780,835 of accounts payable and accrued expenses, $239,372 of accrued expenses – related party, $548,200 in derivative liability, $301,446 in convertible notes and accrued interest payable, net of debt discount, $25,197 in convertible notes and accrued interest – related party, $15,947 in PPP loan – short term, $15,325 in notes payable, $62,445 in notes payable and accrued interest – related party, and $24,791 in operating lease liability – short term.
We currently have little cash to support our operations and projected commercial growth. We will need to raise substantial additional capital through private or public offerings of our equity or debt securities, or a combination thereof, and we may have to use a material portion of any capital raised to repay past due debt obligations. To the extent any capital raised is insufficient to both satisfy operational working capital needs and meet any required debt payments, we will most likely need to either extend, refinance or convert to equity our outstanding indebtedness.
If we complete this offering, we believe the net proceeds will be sufficient to fund our operational working capital for at least the next 12 months. In the event we cannot complete this offering or obtain any such financing when needed on terms acceptable to us, if at all, our business would suffer substantially.
Liquidity represents the ability of a company to generate sufficient cash to provide for its immediate cash needs, which our continued losses have made it difficult for us to accomplish. Over the past several years, we have continued to incur substantial losses without any source of material revenues or liquid assets, which has caused a serious and harmful effect to our liquidity and a substantial strain on our ongoing business operations.
We have not generated any operating cash flows since we are a development stage company which has not yet realized any significant commercial revenues.
Net Cash Used in Operating Activities
We used $235,331 of net cash in operating activities for the three months ended June 30, 2020 compared to $173,797 of net cash for the three months ended June 30, 2019. This increase in cash used in operating activities was primarily attributable to a decrease in the amount of depreciation and amortization recognized of $92,285.
We used $496,598 and $736,445 of net cash in operating activities in fiscal 2020 and fiscal 2019, respectively. Net cash flows used in operating activities during fiscal 2020 consisted primarily of a net loss of $2,082,734, which was offset by: (i) $863,012 of stock-based compensation and (ii) $559,544 in depreciation and amortization expenses. Net cash used in operating activities during fiscal 2019 consisted primarily of a net loss of $4,757,758, which was offset by: (i) $1,642,869 in in stock based compensation, (ii) $1,446,093 for shares issued to our CEO to replace shares given up in escrow and (iii) $646,921 in depreciation and amortization. The stock-based compensation expenses, including warrant and common stock issuances, helped us to operate the business with minimal amounts of cash on hand at any given time during the year.
| 23 |
Net Cash Used in Investing Activities
We used $45,890 of net cash in investing activities in the three months ended June 30, 2020, consisting of $5,136 of costs capitalized to intangible assets, $482 in sale of equipment, and $41,236 of costs capitalized to property and equipment. This is compared to $21,260 of net cash used in investing activities in the three months ended June 30, 2019, which was primarily due to $19,760 of costs capitalized to intangible assets.
We used $65,196 and $103,807 of net cash in investing activities in fiscal 2020 and 2019, respectively. Our use in fiscal 2020 consisting primarily of the purchase of equipment for $32,791 and an increase of patents and trademarks for $43,386, which were offset by proceeds from the sale of equipment in the amount of $12,481.
Net Cash Provided by Financing Activities
During the three months ended June 30, 2020, we were provided with net cash of $399,203 from financing activities consisting of $322,500 from entering into various convertible notes payable, $38,665 from the proceeds of a loan we received pursuant to the Paycheck Protection Program offered by the U.S. Small Business Administration pursuant to Title I of the Coronavirus Aid, Relief and Economic Security Act, and $52,000 in stock and warrants sale proceeds, which was partially offset by a repayment of convertible notes in the amount of $13,962. In comparison, during the three months ended June 30, 2019, we were provided by financing activities with net cash of $254,238 consisting of $280,000 in proceeds from entering into convertible notes payable, which was partially offset by $25,762 in repayments of various notes, notes payable to related parties, and convertible notes payable.
In fiscal 2020, our net cash flows provided from financing activities was $565,907 consisting of: (i) $339,000 in common stock and warrants issued for cash; (ii) $15,000 in proceeds from notes payable, which was partially offset by $19,565 in repayments and (iii) $280,000 proceeds from convertible notes payable, which was partially offset by $18,537 in repayments.
In fiscal 2019, our net cash flows provided from financing activities was $609,377, consisting of (i) $399,865 in common stock and warrants issued for cash, (ii) $215,000 in proceeds for notes payable, which was partially offset by $50,842 in repayments and (iii) $70,000 in notes payable from related parties, which was offset by $25,006 in repayments.
Inventory
Inventories are stated at cost, subject to the lower of cost or net realizable value. Cost includes materials, labor, and manufacturing overhead related to the purchase and production of inventories. Net realizable value is the estimated selling price less estimated costs of completion, disposal, and transportation. We regularly review inventory quantities on hand through an inventory count.
At June 30, 2020, the Company’s inventory has a carrying value of $-0- and is broken down into $43,744 of finished goods inventory, which is fully offset by a $43,744 reserve for obsolete inventory.
At March 31, 2020, the Company’s inventory had a carrying value of $-0- and was broken down into $50,357 of finished goods inventory, which was fully offset by a $50,357 reserve for obsolete inventory.
MATERIAL COMMITMENTS
Accrued Salary
We are indebted to certain related parties with respect to accrued salaries. At June 30, 2020, we are obligated for unpaid officer salaries and amounts payable to related parties of $239,372. This amount is included in accrued expenses – related party.
| 24 |
Notes and Convertible Notes Payable
As of June 30, 2020, we were obligated for the following notes:
| Notes Payable | Convertible Notes Payable | |||||||||
| 1. | Third Parties – Principal | $ | 15,000 | $ | 632,941 | |||||
| Third Parties – Unamortized Debt Discount | - | (333,333 | ) | |||||||
| Third Parties – Interest | 325 | 1,838 | ||||||||
| Third Parties – Total | 15,325 | 301,446 | ||||||||
| 2. | Related Parties – Principal | 59,642 | 25,000 | |||||||
| Related Parties – Interest | 2,803 | 197 | ||||||||
| Related Parties – Total | 62,445 | 25,197 | ||||||||
| Total | $ | 77,770 | $ | 326,643 | ||||||
As of September 30, 2020, an aggregate of $25,197 of the principal and interest on a convertible note payable to a related party was converted into equity.
OFF-BALANCE SHEET ARRANGEMENTS
We do not have during the periods presented, and we do not currently have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
GOING CONCERN
The independent auditors’ report accompanying our March 31, 2020 financial statements contains an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern. The financial statements have been prepared assuming that we will continue as a going concern, which contemplates that we will realize our assets and satisfy our liabilities and commitments in the ordinary course of business. We have suffered recurring losses from operations and have a working capital deficit. These factors raise substantial doubt about our ability to continue as a going concern.
Critical AccountING Policies
We prepare our consolidated financial statements in accordance with generally accepted accounting standards in the United States of America. Our significant accounting policies are described in Note 3 to our consolidated financial statements attached hereto. We believe the following critical accounting policies involve the most significant judgments and estimates used in the preparation of the consolidated financial statements.
RECENTLY ISSUED ACCOUNTING STANDARDS
The following describes the recently issued accounting standards used in reporting our financial condition and results of operations. In some cases, accounting standards allow more than one alternative accounting method for reporting. In those cases, our reported results of operations would be different should we employ an alternative accounting method.
The FASB issued ASC 606 as guidance on the recognition of revenue from contracts with customers in May 2014 with amendments in 2015 and 2016. Revenue recognition will depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance also requires disclosures regarding the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. The guidance permits two methods of adoption: retrospectively to each prior reporting period presented, or retrospectively with the cumulative effect of initially applying the guidance recognized at the date of initial application (the cumulative catch-up transition method). The company adopted the guidance on April 1, 2018 and applied the cumulative catch-up transition method. There was no transition adjustment upon adoption of the new standard.
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. Topic 842 affects any entity that enters into a lease, with some specified scope exemptions. The guidance in this ASU supersedes Topic 840, Leases. The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from leases. A lessee should recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use (“ROU”) asset representing its right to use the underlying asset for the lease term. The Company adopted Topic 842 on April 1, 2019 and resulted in a right of use asset and liability of $154,917.
| 25 |
All newly issued accounting pronouncements but not yet effective have been deemed either immaterial or not applicable.
JOBS Act
On April 5, 2012, the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, was enacted. Section 107 of the JOBS Act provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. In other words, an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies.
We have chosen to opt out of the extended transition periods available to emerging growth companies under the JOBS Act for complying with new or revised accounting standards. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition periods for complying with new or revised accounting standards is irrevocable.
We are in the process of evaluating the benefits of relying on other exemptions and reduced reporting requirements provided by the JOBS Act. Subject to certain conditions set forth in the JOBS Act, as an “emerging growth company,” we intend to rely on certain of these exemptions, including without limitation, (i) providing an auditor’s attestation report on our system of internal controls over financial reporting pursuant to Section 404(b) of the Sarbanes-Oxley Act and (ii) complying with any requirement that may be adopted by the PCAOB regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, known as the auditor discussion and analysis. We will remain an “emerging growth company” until the earliest of (i) the last day of the fiscal year in which we have total annual gross revenues of $1.0 billion or more; (ii) the last day of our fiscal year following the fifth anniversary of the date of the completion of this offering; (iii) the date on which we have issued more than $1 billion in nonconvertible debt during the previous three years; or (iv) the date on which we are deemed to be a large accelerated filer under the rules of the SEC.
PetVivo is a veterinary biotech and biomedical device company headquartered in suburban Minneapolis, Minnesota that is primarily engaged in the business of commercializing and licensing products in the veterinary market to treat companion animals such as dogs and horses. Most of our technology was developed for human biomedical applications, and we intend to leverage the investments already expended in their development to commercialize treatments for pets in a capital and time-efficient way.
Many of the Company’s products are derived from proprietary biomaterials that simulate a body’s cellular tissue by virtue of their reliance upon natural protein compositions which incorporate such “tissue building blocks” as collagen, elastin and heparin. Since these are naturally-occurring in the body, we believe they have an enhanced biocompatibility with living tissues compared to synthetic biomaterials such as those based upon alpha-hydroxy polymers (PLA, PLGA and the like) and other “natural” biomaterials that may lack the multiple proteins incorporated into our biomaterials. These proprietary protein-based biomaterials appear to mimic the body’s tissue thus allowing integration and tissue repair in long-term implantation in certain applications.
Our initial product, Kush™, is a veterinary device designed to help reinforce articular cartilage tissue for the treatment and prevention of osteoarthritis and other joint related afflictions in companion animals. Kush™ uses an intra-articular injection of non-dissolving, cartilage-like, patented particles that are slippery, wet-permeable, durable and resilient to enhance the force cushioning function of the synovial fluid. The particles mimic natural cartilage in composition, structure and hydration. Multiple joints can be treated simultaneously. Our particles are comprised of collagen, elastin and heparin, the same components found in natural cartilage. These particles show an effectiveness to augment the cartilage and enhance the functionality of the joint (e.g. provide cushion or shock-absorbing features to the joint and to provide joint lubricity).
| 26 |
Osteoarthritis, the most common inflammatory joint disease in both dogs and horses, is a chronic, progressive, degenerative joint disease that is caused by a loss of synovial fluid and/or the deterioration of joint cartilage. Osteoarthritis affects 1 out of 4 dogs and over half of all horses. It is estimated that there are over 20 million dogs with osteoarthritis in the United States and the European Union with a market size of $2.6 billion and over 1 million lame horses in the US for a $600 million United States and the European market. See Johnston, Spencer A. “Osteoarthritis. Joint anatomy, physiology, and pathobiology.” The Veterinary Clinics of North (1997):699-723.
Despite the market size, veterinary clinics and hospitals have very few treatments and/or drugs for use in treating osteoarthritis in dogs, horses and other pets. As there is no cure for osteoarthritis, the various treatment methods are focused on managing the related symptoms of pain and inflammation. The current treatment for osteoarthritis in dogs generally consists of the use of nonsteroidal anti-inflammatory drugs (or “NSAIDs”) which present the potential for side effects relating to gastrointestinal, kidney and liver damage. In contrast, the Company’s treatment using Kush™, to our knowledge, has not elicited any adverse side effects in dogs. Remarkably, Kush™-treated dogs have shown an increase in activity even after they no longer are receiving pain medication. Other treatments for osteoarthritis include steroid and/or hyaluronic acid injections, which are often slow acting and/or short lasting.
We believe Kush™ is superior to existing treatments to safely improve joint function in animals for several reasons:
| ● | Kush™ addresses the underlying problem which relate to bones being too close and a lack of synovial fluid. | |
| ● | Kush™ provides a biocompatible lubricious cushion to the joint, which establishes a barrier between the bones, thereby protecting the remaining cartilage and bone. | |
| ● | Kush™ is easily administered with the standard intra-articular injection technique. Multiple joints can be treated simultaneously. | |
| ● | Case studies indicate many canines have long-lasting, multi-month improvement in lameness after having been treated with Kush™. | |
| ● | After receiving a Kush™ injection, many canines are able to discontinue the use of NSAID’s, eliminating the negative side effects that comes from their use. | |
| ● | Kush™ is an effective and economical solution for treating osteoarthritis. A single injection of Kush™ is approximately $400 to the end consumer and typically lasts up to a year or longer. |
Historically, drug sales represent up to 30% of revenues at a typical veterinary practice (Veterinary Practice News). Revenues and margins at veterinary practices are being eroded because online, big-box and traditional pharmacies have recently started filling veterinary prescriptions. Veterinary practices are looking for ways to replace lost prescription revenues with safe and effective products. Kush™ is veterinarian-administered and should expand practice revenues and margins. We believe that the increased revenues and margins provided by Kush™ will accelerate its adoption rate and propel it forward as the standard of care for canine and equine lameness related to or due to synovial joint issues
Kush™ is classified as an animal device under the FDA rules and pre-market approval is not required by the FDA. Kush™ has completed a safety and efficacy study in rabbits in 2007. Since that time, more than 100 horses and dogs have been successfully treated with Kush™. We are in the process of preparing a protocol to conduct a pilot study with a major university or another academic institute. We anticipate this study will be a 12-month study that will be primarily used to expand our distribution outlets since the large international and national distributors generally require a third-party university study to include a product in their catalog of products.
We plan to commercialize Kush™ and other products developed by us in the United States through the use of in-house marketing personnel who will oversee the efforts of independent distributors we engage on a regional or national basis. We plan to support our distributors with use of social media and other methods to educate and inform to key opinion leaders and decision makers at the top distributors and high prescriber veterinarians for companion animals of the availability and benefits of KushTM . We have developed a commercialization strategy, which provides for our (i) initial launch in key regional markets (Colorado, Minnesota and Texas) by internal sales representatives, (ii) development of alliances with 3 to 4 of the top national distributors and (iii) development of a full national roll-out strategy to deliver our products to customers commencing in July of 2021.
We have established an ISO 7 certified clean room manufacturing facility located in our Minneapolis facility using a patented and scalable self-assembly production process, which reduces the infrastructure requirements and manufacturing risks to deliver a consistent, high-quality product while being responsive to volume requirements. While we are not currently manufacturing commercial quantities, we have built out an ISO 7 certified facility that will be able to handle projected production in units for at least the next five years.
In addition to Kush™, we have engaged a strategic partner, Emerald Organic Products, Inc. (“Emerald”), through an exclusive license agreement dated July 31, 2019 to allow Emerald to bring our mucoadhesive drug delivery technology to the human nutraceutical market for the delivery of various active nutraceuticals including cannabidiol (“CBD”), caffeine, and citicoline. Since such products tout up to a 10-times increase in bioavailability of active agents, we believe that we have an advantage over other delivery methods in the CBD and nutritional supplement markets. We have agreed that the license to Emerald will include use PetVivo’s proprietary technology in the formulation, manufacture and sale of Emerald’s nutritional supplements including its hemp-based CBD wellness products.
We also have a pipeline which includes 17 therapeutic devices for both veterinary and human clinical applications. Some such devices may be regulated by the FDA or other equivalent regulatory agencies, including but not limited to the CVM. We anticipate growing our product pipeline through the acquisition or in-licensing of additional proprietary products from human medical device companies specifically for use in pets. In addition to commercializing our own products in strategic market sectors and in view of the Company’s vast proprietary product pipeline, the Company may establish strategic out-licensing partnerships to provide secondary revenues.
| 27 |
Product Pipeline

Possible Future Use In Humans
Dr. David Masters’ and Gel-Del’s efforts in the development of the gel-like substance used in Kush™, was intended for use in humans. Such efforts included pursuit of premarket approval for use of the material for injection as a dermal filler for cosmetic wrinkle treatment. Details regarding the FDA clinical trial may be found at the FDA’s website for clinical trails https://www.clinicaltrials.gov/ct2/show/NCT00414544?spons=Gel+Del&rank=1. In 2006, the technology was licensed for such purposes on an exclusive basis to a California-based marketer of facial rejuvenation products; however, the arrangement was terminated after approximately eighteen months largely due to economic conditions in 2007.
While we currently have no immediate plans to pursue marketing Kush™ for use in treatment of humans or to license it to third parties for such marketing, we believe there are significant opportunities for such use in the future. We have no intention to devote proceeds from this offering or current efforts in the near term towards such opportunities in treatment of humans.
| 28 |
Dermal Filler
Our biomaterials are constructed from purified water, protein, and carbohydrate, tailored to simulate different body tissues that biologically integrate (bio-integration). Our biomaterials can be manufactured and used as a dermal filler for wrinkle treatment by injection. These formed, gel particles fill, integrate and rejuvenate dermal skin tissue to remove the wrinkle. This product was taken through an FDA clinical trial under the name CosmetaLife®, the results of which can be found at www.clinicaltrials.gov (NCT00414544).
Cardiovascular Device
Our blood-compatible biomaterial, which allows blood contact and bio-integrative processes to occur without clotting, platelet attachment, or thrombogenesis, is used to repair cardiovascular tissue. VasoGraft®, a blood vessel graft made from VasoCover™ material, is designed to mimic natural blood vessel tissue in almost every respect, including the components used.
Drug Delivery
Unique fabrication techniques allow us to homogeneously distribute drug in milligram to nanogram amounts, resulting in optimum performance and manufacturing capabilities for a variety of delivery methods, such as coatings, injectables, implantables or transmucosal delivery. The first planned transmucosal product has been optimized and tested with peptide drugs with better efficacy than oral dosing via swallowing.
Orthopedic Devices
Another of our materials can be used in a variety of shapes for orthopedic and dental applications. The first products, OrthoGelic™ and OrthoMetic™, will be aimed at difficult-to-heal, non-union broken bones, by using particles to fill the empty space. The orthopedic biomaterial, made to mimic the structural components of bone, can allow integration and healing in the break and exclude non-bone tissue infiltration.
Overview of the Animal Health Market
Over the last several decades, we believe the animal health market and industry has been a strong component in the overall U.S. economy and is more resistant to economic cycles. The veterinary sector is as an attractive area to participate in the growth of the broader healthcare industry without reimbursement risk. According to the American Pet Products Association (“APPA”) 2019-2020 National Pet Owners Survey, in 2019, U.S. pet spending was $95.7 billion, with veterinary care and products accounting for $29.3 billion of these sales, followed by supplies, live animals and OTC medicines at $19.2 billion and other services at $10.3 billion. The APAA projects that pet spending will increase to $99 billion, a 3.5% increase in 2020.
Management expects growth in the companion animal market to continue for several years. Factors influence the growth in demand for veterinarian care and pet therapeutics include: (i) increased pet ownership globally, (ii) pets living longer, and (iii) increased pet spending as pets are viewed as members of the family. The APPA projects that the global veterinary medicine market is expected to grow from $22.9 billion in 2019 to $29.7 billion by 2027, registered a compound annual growth rate of 4.6% from 2020 to 2027.1
According to the APPA, U.S. pet ownership reached record levels in 2019. Specifically, 68% of all U.S. households (approximately 84.9 million households) owned a pet in 2019. The APPA also reported that there were 63.4 million dogs, 42.7 million cats and 1.6 million horses held by U.S. households in 2019. According to the APPA, pet ownership increased at a 1.58% annual compound growth rate in the United States while the human population in the United States increased at a 0.83% compound annual growth rate over the last 15 years.
Pets are living longer, with the average lifespan for dogs increasing by half a year to 11 years between 2002 and 2012 according to a study by Banfield Pet Hospital. With pets living longer, consumers are spending more disposable income to give their “family member” a healthier, more comfortable life. Most veterinary expenditures are paid out of pocket. Even in times of recession, pet owners are less sensitive to the overall price of pet care than to other aspects of their lifestyle. As new innovations emerge, pet owners now have a greater ability to extend the life of their pet by treating chronic diseases and ailments associated with old age.
1 Allied Market Research, July 2020 report titled: “Veterinarian Medicine Market by Product (Drugs, Vaccines, and Medicated Feed Additives), Routes of Administration (Oral Route, Parenteral Route and Topical Route), Animal Type (Companion Animals and Livestock Animals), and Distribution Channel (Veterinary Hospitals Pharmacies, and Retail Veterinary Pharmacies): Global Opportunity Analysis and Industry Forecast, 2020-2027 – July 2020.
| 29 |
Osteoarthritis Market Size
Osteoarthritis, the most common inflammatory joint disease in both dogs and horses, is a progressive condition that is caused by a deterioration of joint cartilage. Over time, the joint cartilage deterioration creates joint stiffness from mechanical stress resulting in inflammation, pain and loss of range of motion, which may be referred to as lameness. Osteoarthritis joint stiffness and lameness worsens with time from gradual cartilage degeneration and an ongoing loss of protective cushion and lubricity (i.e., loss of slippery padding).
Canine Osteoarthritis
The prevalence of companion animal osteoarthritis is estimated through a variety of methods. In looking at the incidence of osteoarthritis in dogs, Spencer Johnston’s article “Osteoarthritis. Joint anatomy, physiology, and pathobiology” is often cited, which reports that 20% of all dogs over the age of one year suffer from osteoarthritis. Using the following simple methodology and management’s estimate that 20% of the total dog population is under the age of one, we estimate that 14.4 million dogs in the U.S. suffer from osteoarthritis.
89.7 million dogs in the U.S. x 80% = 71.8 million total dogs x 20% with osteoarthritis = 14.4 million dogs with osteoarthritis in the U.S.
Craig-Hallum’s July 22, 2013 institutional research report on Aratana Therapeutics estimates the U.S. dog osteoarthritis market at 16.6 million dogs. William Blair & Company, L.L.C. released a July 25, 2013 Equity Research report by Aratana Therapeutics that concluded that roughly 10% of dogs and cats suffer from osteoarthritis (89.7 million dogs x 10% = 9 million dogs with OA). Stifel issued a report on Aratana Therapeutics dated July 22, 2013 that estimated the osteoarthritis market to be 55% of dogs over the age of 10. This equates to a US market in 2014 of 7.1 million dogs with osteoarthritis.
Horse Osteoarthritis (Lameness)
Equine osteoarthritis is the most common cause of lameness in horses. The annual average costs for diagnosis and treatment of equine lameness is $3,000 per horse, with downtime & homecare costs being much higher (Oke and McIlwraith, 2010). “The USDA National Economic Cost of Equine Lameness… in the United States” published by 1978 places the annual incidence of lameness at 8.5 to 13.7 lameness events per 100 horses.
As noted previously, the APPA reported the total number of horses owned by U.S. households was 7.6 million in 2018. A 2007 publication by Emily Kilby “The Demographics of the U.S. Equine Population” concludes the U.S. horse population to be 9.5 million in 2006 with racehorses being 9% of that population or 846,000 horses. The article “The Occurrence and Causes of Lameness and Laminitis in the U.S. Horse Population” estimates that 17% of racehorses and 5.4% of the rest of the horse population go lame annually. Based on the above assumptions we calculate that there are approximately 500,000 new lame horses each year.
Treatments for Osteoarthritis
Despite the market size, veterinary clinics and hospitals have very few treatments and/or drugs for use in treating osteoarthritis, one of the most common inflammatory joint diseases in both dogs and horses. As there is no cure for osteoarthritis, the various treatment methods are focused on managing the related symptoms of pain and inflammation. Veterinarians recommend several treatments depending on the severity of the disease, including a combination of rest, weight loss, physical rehabilitation, and a regimen of pain and NSAIDs. Non-steroidal anti-inflammatory drugs (NSAIDs and hyaluronic (“HA”) injections in the joints) are used to alleviate the pain and inflammation caused by osteoarthritis.
NSAID’s are administered once or twice per day in a pill form. Many NSAID’s, including Rimadyl and Elanco’s Galliprant, present the potential for gastrointestinal, kidney and liver side effects in the animal and do not treat the underlying condition. Use of NSAID’s in dogs may have serious side effects in dogs that limit their long-term use and may require ongoing monitoring by veterinarians. Furthermore, NSAID’s do not halt or slow joint degeneration. In addition, the owner may need to pay for toxicity monitoring. We estimate the annual cost of treatment with NSAIDs to be from $350 to $700 (exclusive of toxicity monitoring which may be implemented).
| 30 |
Another palliative treatment for osteoarthritis is the injection of HA into the joint, which avoids risks of the side effects associated with NSAIDs. However, the HA injections are often slow-acting and/or short lasting. The duration of the treatment may be limited to thirty days or less. Generally, an animal will receive HA injections once a month at the vet. We estimate the cost to be from $150 to $250 per HA injection. On an annual basis, the cost is $1,800 to $3,000.
Joint replacement is a more extreme treatment and is limited to larger or specialty surgical veterinary practices. While both administration of Kush™ and HA injections may involve sedation of the animal, joint replacement involves invasive surgery and rehabilitation. As such there are risks of surgical complications and higher cost, which we estimate to be between $2,000 and $4,000.
There are other treatments which may have a more regenerative effect, including stem cell and platelet rich plasma therapy. These treatments require painful tissue harvesting and are time-sensitive, time-consuming and expensive. Furthermore, the long-term effects of these treatments are not well known at this time.
Use of Kush™ to Treat Osteoarthritis
Overview
Kush™ is veterinary device designed to help reinforce articular cartilage tissue for the treatment and prevention of osteoarthritis and other joint related afflictions in companion animals. Kush™ uses an intra-articular injection of non-dissolving, cartilage like patented particles to enhance the force cushioning function of the synovial fluid. No special training is required for the administration of Kush™ to treat osteoarthritis, degenerative disk disease or hip dysplasia. The veterinarian simply injects the material into the synovial joint space using standard intra-articular injection techniques. Many veterinarians, especially in canines, will choose to sedate the animal in connection with the injection. Multiple joints can be treated simultaneously. There are no special post-injection treatment requirements
The particles in Kush™ are comprised of collagen, elastin and heparin, the same components found in natural cartilage. These particles show an effectiveness to augment the cartilage and enhance the functionality of the joint (e.g. provide cushion or shock-absorbing features to the joint and to provide joint lubricity) based on experiences reported by our customers.
Once injected, the extent of bone-on-bone contact is lessened immediately, and results are typically seen within days. In-vivo studies indicate that Kush™ can easily be combined with synovial fluid in a rabbit knee to form a joint cushion, buffering the adjacent bones and cartilage; in this rabbit study it was shown that no damage was caused to the cartilage from replacing the synovial fluid. The particles show an effectiveness to augment the cartilage and enhance the functionality of the joint (e.g. provide cushion or shock-absorbing features to the joint and to provide joint lubricity).
Kush is the Superior Solution
We believe Kush™ is superior to existing treatments to safely improve joint function in companion animals for several reasons.
Address the Underlying Cause. Kush™ addresses the underlying cause of the pain and discomfort (not enough synovial fluid) with no known side effects. Kush provides a biocompatible lubricious cushion to the join, which establishes a barrier between the bones, thereby protecting the remaining cartilage and bone. Upon injection into the afflicted joint the spongy Kush™ gel-like substance provides long-lasting lubrication and cushioning which mimic the composition and protective function of the synovial membrane and cartilage (i.e., providing both a slippery cushion and healing scaffolding). Essentially, the injected particles function in a way that may protect the joint as an artificial cartilage. NSAID’s and HA mask the symptoms and do not treat the underlying condition.
Easy Delivery. Kush™ is easily administered with the standard intra-articular injection technique. Multiple joints can be treated simultaneously Veterinarians do not need any special training to make a Kush™ injection. There are no special post treatment requirements. In contrast, animals who receive NSAID’s must receive pills every day and may need to undergo toxicity monitoring. HA injections require follow up treatments every month.
| 31 |
No Known Side Effects. We are not aware of any adverse side effects when treating dogs or horses with Kush™. When dogs are treated with NSAID’s, there are many possible side effects including gastric, kidney or liver problems.
Superior Outcomes. Veterinarians have used Kush™ to treat osteoarthritis and lameness successfully in both dogs and horses for many years beginning in 2014. Case studies indicate long-lasting multi-month improvement in lameness. Furthermore, many animals have multi-year post treatment records.
Economic Solution. Kush is an effective and economical solution for treating OA. The cost for one treatment is $400 to $600 per joint. The cost for other treatments is generally higher, with less effective results.
Composition
Kush’s proprietary biomaterials simulate a body’s cellular tissue by virtue of their reliance upon natural protein compositions which incorporate such “tissue building blocks” as collagen, elastin and heparin. Since these are naturally-occurring in the body, we believe they have an enhanced biocompatibility with living tissues compared to synthetic biomaterials such as those based upon alpha-hydroxy polymers (PLA, PLGA and the like) and other “natural” biomaterials that may lack the multiple proteins incorporated into our biomaterials. These proprietary protein-based biomaterials appear to mimic the body’s tissue thus allowing integration and tissue repair in long-term implantation in certain applications.
Mechanism of Action
The Kush™ components self-assemble to form an insoluble matrix which is slippery, wet permeable, durable and resilient. The components mimic natural cartilage in composition, structure and hydration. These biocompatible particles are lubricious, “cushioning” and reside in joint synovial space. These spongy augmentation particles mimic the protective function of cartilage – providing both a slippery cushion and a protective barrier between bones. Kush™ augments the synovial membrane function, without pharmacologic, chemical or metabolic action.
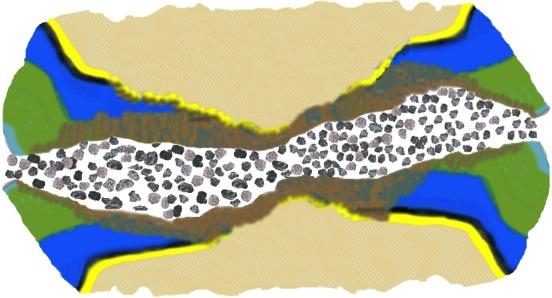
Figure 1 - Kush Particles mimic cartilage
Use of Kush™ to Treat Lameness due to Navicular Disease and/or Digital Cushion Impairment
We believe Kush™ to be superior to existing treatments to safely treat lameness due to navicular disease and/or other deficiencies in the digital cushion of hoofed animals. Navicular syndrome, often called navicular disease, is a syndrome of lameness problems in horses. It most commonly describes an inflammation or degeneration of the navicular bone and its surrounding tissues, usually on the front feet. It can lead to significant and even disabling lameness. The digital cushion is a major component of the circulation and shock dissipation apparatus of the animal’s hoof. It is an elastic, wedge-shaped mass composed of fibrous fatty tissue located in the animal’s hoof that has varying consistencies depending on the animal’s health.
The structure of the hoof differs from species to species; however, the basic anatomy is very similar between species. Cattle, sheep, goats and pigs are cloven-footed animals while a horse has a single hoof at the end of each of its legs. The exterior of the hoof is a hard surface, structurally similar to the human fingernail, but functionally like human skin with the exterior being formed by the tissue directly beneath it. Underneath the hoof is a slightly softer area called the sole. Within the hoof are located bones that serve to shape the hoof and provide a supporting structure. The digital cushion is located above the sole and separated from it by the corium (which acts much like the quick of a human fingernail).
| 32 |
Horses may experience compression of the navicular bone, loss of cartilage and/or a reduction and/or compression of the digital cushion due to excessive walking, trotting or running over a short period of time and/or normal work over a longer period of time. Such use can cause the occurrence of Navicular Disease and/or other lameness that may be attributed to compression of the navicular bone, cartilage degeneration and/or a reduction in digital cushion thickness. Kush™ may be used to treat such afflictions by simply injecting Kush into the digital cushion to increase its thickness.
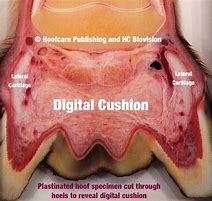 |
The images to the left and right are of cross sections of hoofs and display the location of the digital cushion. |
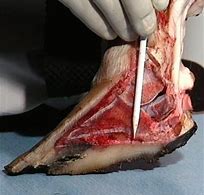 |
Kush™ Manufacturing and Product Information
The Company manufactures Kush™ in a two-phased approach. In Phase One, the sterile Kush™ particles are produced by the Company. The Company uses a proprietary coacervation and particle production process. In Phase Two, the Company’s personnel or strategic partner will perform aseptic syringe filling, packaging, analytics and quality assurance of Kush™ particles for final sale and distribution.
Once produced, Kush™ is packaged in sterile syringes containing approximately 2 cc of the product and delivered to veterinary clinics and/or veterinarians in protective boxes. Kush™ is generally stored under room temperature (approximately 60° to 80° F) and has been shown to be stable in a 25-month stability study.
The Company is currently in the process of completing the build-out of its manufacturing facility located in suburban Minneapolis, Minnesota. The manufacturing facility is ISO 7 and ISO 8 certified with ISO 5 environments totaling approximately 500 square feet for the production of the Company’s Kush™ product. The Company anticipates completion of our facility and initial production of the Kush™ product in the third quarter of 2020 pending successful funding. The initial batch runs will be for 500 syringes and are planned to increase to runs of approximately 5,000 syringes in 12 months. The Company believes that this facility will handle capacity of approximately 500,000 units per year. Additionally, the Company may engage strategic partners to perform syringe filling, packaging, analytics and quality assurance prior to final sale and distribution.
Sales and Marketing
We are currently focusing on marketing Kush™ to veterinarians for use in the treatment of osteoarthritis in dogs and horses. We plan to commercialize Kush™ for use in the United States through use of in-house marketing personnel who will oversee the efforts of independent distributors we engage on a regional or national basis.
Most U.S. veterinarians buy a majority of their equipment and supplies from a preferred distributor. More than 75% of veterinarians name Covetrus, Patterson, MWI Animal Health (a division of AmerisourceBergen), Midwest Veterinary Supply, Inc. or Victor Medical Company as their preferred distributor. We believe, these top tier distributors sell a substantial majority by revenue, of the products sold to companion animal veterinarians in the U.S. Covetrus, Patterson and MWI are recognized by manufacturers, distributors and veterinarians as the pre-eminent national companion animal veterinary supply distributors in the US. There are no other distributors that provide equivalent levels of service to manufacturers and regularly visit veterinarians in as wide a geographic area as Covetrus, Patterson or MWI. Midwest and Victor are large, regional distributors, also with strong reputations for high-quality service.
| 33 |
We have developed a commercialization strategy, which provides for our (i) initial launch in key regional markets (Colorado, Minnesota and Texas) in the third and fourth quarter of fiscal 2020 (ii) developing alliances with 3 to 4 of the top national distributors in the first and second quarter of 2021 and (iii) a developing a full national roll-out to deliver our products to customers in the third quarter of 2021.
Our product distribution will leverage the existing supply chain, veterinary clinic and clinician relationships already established by these large distributors. We intend to support and supplement this distribution channel with regional business development & training representatives. Our business development staff will provide product training to distribution representatives, veterinarians and other veterinary staff. In addition, we will exhibit at key veterinary conferences in addition to supporting ongoing case studies. All of these sales, distribution, marketing and education efforts will also be supported by both veterinarian and pet owner product education and treatment awareness campaigns that will be conducted utilizing a variety of advertising and social media tools. The unique nature and the anticipated benefits provided by our initial Kush™ products are expected to generate significant consumer response.
Particle Devices

Orthopedic Joint Treatments
Kush is a treatment for joint pain, which is made of injected, protein-based, gel-like particles. In vivo studies indicate that the gel particle device can easily be combined with synovial fluid in a rabbit knee to form a joint cushion, buffering the adjacent bones/cartilage where no damage was caused to the cartilage from replacing the synovial fluid. The particles show an effectiveness to repair, reconstitute or remodel the tissue, cartilage, ligaments and/or bone and/or enhance the functionality of the joint (e.g. repair deteriorated components present in the joint to provide cushion or shock-absorbing features to the joint and to provide joint lubricity).
| 34 |
| AppTec Laboratories accomplished a gel-particle rabbit study in which six New Zealand white rabbits were injected in both stifle joints (knees) to fill but not extend the synovial space (~0.5 cc GDP/site). Rabbits were tested every other day for abnormal clinical signs including range of motion and joint observations until sacrifice. Behavioral testing revealed no abnormal scores for range of motion, withdrawal response, or joint observations (all animals were 100% normal). At one week and at four weeks the animals were sacrificed. AppTec pathologists evaluated knee joint histology. The reported cartilage surfaces of the femoral and tibia condyles and the menisci were grossly and histologically 100% normal for all animals and test sites. | 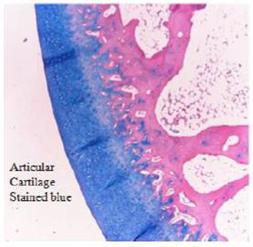 |
Clinical Studies
The particle devices for joint injections have been extensively studied for a broad range of applications including the treatment of wrinkles as dermal filler. Described below is an overview of the pre-clinical and clinical studies completed for CosmetaLife, which is the name used for the particle device when it was used as a dermal filler.
Particle Integration after 12 Weeks
 |
Particle Integration after 12 Weeks
The image at left shows collagen in blue, fibroblasts in red and CosmetaLife in gray. Note the typical cellularization and integration of collagen within the CosmetaLife matrix perimeter. Also notice the fibroblasts (collagen producers) are integrated throughout the injection site. Microvascularization, indicated by arrowheads, is also present in several locations. There is little to no sign of inflammation.
Trichrome Stain - 20x Objective |
CosmetaLife Particles
CosmetaLife is an easy-to-inject, water-protein-based dermal filler that not only fills nasolabial wrinkle depressions but also helps rejuvenate the dermal tissues, counteracting damage that causes wrinkles. The dermal cells are attracted to the CosmetaLife gel-particles, attach to them, and then slowly replace them with natural dermal material (extracellular matrix). The natural biological replacement process of CosmetaLife to collagen is estimated to take 6 to 12 months. CosmetaLife clinical trial on nasolabial folds supports this estimate.
CosmetaLife injections allow the body to create more natural dermal structure in and around every particle. Enhancing the natural process of dermal tissue construction with CosmetaLife allows for long-term dermal contouring, corrections, and rejuvenation with little to no adverse side effects noted in clinical trials.
| 35 |
Particle Device Clinical Studies
 |
The Company has conducted several biocompatibility animal studies. In the implantation study, no abnormal clinical signs were noted for any of the rabbits. The results of the sensitization study in guinea pigs showed a sensitization response equivalent to the negative controls.
The results of the histological report on the rabbit skin biopsies clearly demonstrate structural integration of the particles into the host tissues by week 12. Evaluators observed the particle material integration with normal tissue, remodeled and/or new collagen, and fibroblasts throughout the injected particles, mild to no inflammation, and new collagen-matrix production. |
An FDA/IDE approved pivotal human clinical trial began with CosmetaLife late in 2006. The clinical trial was a randomized, double-blind, parallel assignment, multi-center comparison of the safety and efficacy of CosmetaLife versus Restylane® (Control) for the correction of nasolabial folds. One hundred seventy-one patients were skin tested and 145 were treated at six trial sites. The number of study exits after treatment totaled four subjects. This clinical trial was reported and published at www.clinicaltrials.gov (NCT00414544).
The feedback from physician investigators has been positive with respect to CosmetaLife injection qualities, cosmetic appearance, and its feel to the touch. During the first three to four months of the study, CosmetaLife showed no decrease in efficacy, as compared to Restylane that showed an 11 percent decrease in efficacy. The FDA/IDE approved human clinical trial for the CosmetaLife product through twelve months was found to be the same as compared to control hyaluronic acid product, Restylane (for each interval the consensus of the blinded subjects tested preferred CosmetaLife or showed no preference at 3, 6, 9 and 12 months).
 |
CosmetaLife particles, shown in figure to the left, were photographed from a light microscope under high magnification and immersed in a saline solution to help disperse them for better viewing. These particles are approximately 100 microns in size (0.1 mm in diameter). |
We use existing, scalable processes to reduce the infrastructure requirements and manufacturing risks to deliver a consistent, high-quality product while being responsive to volume requirements.
Particles Safety Study
Patients injected with CosmetaLife were found to have no or mild inflammatory, irritation, or immunogenic responses. These results suggest the particles are biocompatible because it closely matches the skin structure, composition, and moisture content. The no-to-low immunogenic responses are attributed to the tight cross-linking of the CosmetaLife matrix, which prevents immunogenic progenitor cells from producing antibodies to the matrix.
| 36 |
 |
In the clinical trial, the incidence of possible reaction to a skin test was 2.55 percent, with only one subject showing a reaction to a second test or 0.6%, (1 out of 171). We also have a study report by AppTec, Inc., our Contract Research Organization, that indicates CosmetaLife did not produce an antibody response during the clinical trial further supporting our belief that it is safe to use.
CosmetaLife is composed of materials that approximately meet the Generally Regarded As Safe (“GRAS”) requirements of the FDA. CosmetaLife contains materials from certified bovine and porcine tissue sources that do not harbor prion disease or BSE. Additionally, steps in the manufacturing process have been validated for deactivating viruses. |
Extrusion force testing and the Clinical Trial usage both demonstrate the consistent and easy injection of CosmetaLife. Twenty-five month stability testing shows that CosmetaLife is stable at room temperature conditions. Moreover, CosmetaLife has been shown to be stable at 40 °C (104 °F) conditions for at least 3 months.
Muco-Adhesion Technology Overview
 |
Our muco-adhesion technology is an active agent delivery platform that is based on an eroding mucoadhesive protein matrix, is well patented and uses only materials that satisfy the FD’s GRAS requirements. In wafer form, it sticks very strongly and comfortably to buccal mucosal tissue, which covers the roof of mouth, cheeks and gums. Other active agent delivery applications include particle and pill forms for nasal and oral routes. The fabrication steps are reproducible and economical, including an aqueous active agent solution and protein mixture to form a dried protein matrix from which mucoadhesive particles are made at any exact active agent dose (milligrams to nanograms), and then pill pressed into wafers. |
Wafer eroding characteristics are quite adjustable (up to 15 minutes before the wafer dissolves), allowing drug to release and absorb into mucosal tissue and the blood stream, thereby avoiding alimentary canal deactivation and first pass liver elimination. Since our muco-adhesion particles contain exact doses of homogenously and reversibly bound active agent as protein-encased molecules, their nasal applications work well by controlling active agent delivery and irritation in the sinus passages, allowing for active agent penetration into the brain via olfactory bulb transport paths. Alternatively, our buccal wafers instantly attach to buccal mucosa tissue and the roof of the mouth, soften from mucus wetting absorption and dissolve at the mucosal surface to release active agent into the mucus membrane. The protein matrix tastes good and can include substances to mask active agent taste and enhance active agent tissue absorption.
In clinical tests, our wafers were used for buccal delivery of the peptide drug, desmopressin, which were reportedly about 10 times more effective in inhibiting urination urge than a swallowed pill. Moreover, these wafers can be very effective in the veterinary market, especially because of their strong non-friable ability to bind mucosal tissue so strongly that while it dissolves away completely it cannot be removed by the animal’s tongue.
Since this novel delivery technology allows for very low doses, reduces active agent irritation, enhances bioavailability, and can use alimentary canal sensitive active agents, it expands the ability to develop active agents (e.g., highly potent actives, neuropeptides, others).
| 37 |
Exclusive License Agreement
On July 31, 2019, the Company entered into an exclusive license agreement with Emerald Organic Products, Inc. (“Emerald”; OTCPink: EMOR) whereby we licensed our muco-adhesion technology for use of cannabidiol (“CBD”), caffeine, and citicoline in the human nutraceutical market. Emerald touts a strong management team with a pedigree in commercializing products within the human nutraceutical market. It is our belief that Emerald is a great partner for us because of their expertise in commercializing similar product to our muco-adhesion technologies and their relationships established within distribution channels we believe can generate significant licensing revenues to us if utilized effectively.
CBD Market
The CBD Market is projected to reach $20 billion with a compound annual growth rate of 49% by 20242. We believe our muco-adhesion technology is positioned uniquely to capture a significant portion of this market as it is projected to continually grow over the next several years. However, a major problem in the CBD industry is that CBD has exceptionally poor bioavailability, as low as 3%, because like other cannabinoids, it is not readily soluble in water and it faces extensive first-pass metabolism in the liver before it reaches circulation. An efficacious dose of CBD oil is upward of 5 milligrams per kilogram of body weight taken via tincture, which is s approximately a 500 milligram dose for a 220-pound person. In addition, as currently marketed, a typical 30-day supply of tinctured CBD is 500 milligrams at a price of approximately $60. This price becomes uneconomical for end users as they are typically spending $60 or more on a product that needs to be over 15-times stronger for an average 160-pound person to become efficacious. Since our product touts up to a 10-times increase in bioavailability of active agents, we believe that we have an advantage over other delivery methods in the CBD market and will become the premier CBD product in the market.
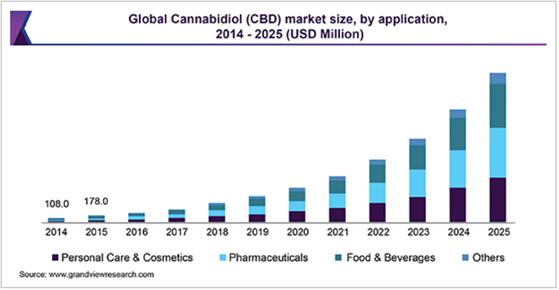
Muco-Adhesive Device Manufacturing
Most other biomaterials have difficulty incorporating low quantities of drugs homogeneously because all prior techniques require dry powder mixing. For example, one microgram of active drug in a 100 mg pill requires a mixing ratio of one gram of powder into 100,000 grams of powder, which cannot be reliably accomplished homogeneously. However, unique fabrication techniques will allow our muco-adhesion material to homogeneously distribute any drug in small or large quantities, resulting in what we believe will be optimum performance from an active-delivery standpoint and little to no risk of inhomogeneous dosages.
2 BDS Analytics and Arcview Market Research press release dated May 9, 2019 at https://bdsa.com/u-s-cbd-market-anticipated-to-reach-20-billion-in-sales-by-2024/#:~:text=BDS%20Analytics’%20predicts%20US%20sales,annual%20growth%20rate%20of%2049%25.&text=CBD%20consumers%20are%20an%20average,to%20be%20employed%20full%20time
| 38 |
Our muco-adhesion technology uses only safe materials (FDA-GRAS). The fabrication steps are reproducible and economical, including an aqueous drug solution and protein mixture that we use to form a dried protein matrix from which mucoadhesive particles are made at any exact drug dose (milligrams to nanograms), and then pill pressed into wafers.
Competition
The development and commercialization of new animal health medicines and treatments is highly competitive, and we expect considerable competition from major pharmaceutical, biotechnology and specialty animal health medicines companies. As a result, there are, and likely will continue to be, extensive research and substantial financial resources invested in the discovery and development of new animal health medicines.
Our potential competitors include large animal health companies, such as Zoetis, Inc. Merck Animal Health (the animal health division of Merck & Co., Inc.), Merial (the animal health division of Sanofi S.A.), Elanco (the animal health division of Eli Lilly and Company), Bayer Animal Health (the animal health division of Bayer AG), NAH (the animal health division of Novartis AG), Boehringer Ingelheim Animal Health (the animal health division of Boehringer Ingelheim GmbH), Virbac Group, Ceva Animal Health, Vetoquinol and Dechra Pharmaceuticals PLC. We are also aware of several smaller, early-stage animal health companies, such as Kindred Bio, Aratana Therapeutics Inc., Parnell, Veterinary Sciences Inc., Luitpold Pharmaceuticals, NextVet and VetDC that are developing products for use in the pet therapeutics market.
The current standard for treatment of osteoarthritis is use of NSAIDs. NSAIDs may cause gastral tract bleeding and kidney damage. Another pitfall of NSAIDs treatment is the lack of pet-owner compliance when administering the treatment. Treatment of osteoarthritis using the KushTM has no known negative side effects, bypasses pet-owner compliance issues, and has shown increased duration of lameness improvement.
Intellectual Property
Our intellectual property portfolio is comprised of patents, patent applications, trademarks and trade secrets. We have one U.S. trademark application pending (for Kush™), nine issued United States Patents with an additional two U.S. patent applications currently being examined. In addition to our U.S. patent portfolio, we also have one EU trademark, nine non-U.S. patents issued or granted and one non-U.S. patent application currently pending in key markets around the world, including Australia (1), Canada (4), Germany (1), Europe (1), France (2) and Great Britain (1).
We believe we have developed a broad and deep patent portfolio around our biomaterials and manufacturing processes in addition to the application of these biomaterials for use as medical devices and pharmaceutical delivery devices. The Company relies on trade secret law to secure and protect other technological know-how and also possesses one trademark protected pursuant to trademark common law.
| 39 |
United States Patents:
| 9,107,937 – | Wound Treatments with Crosslinked Protein Amorphous Biomaterials |
| 8,871,267 – | Protein Matrix Materials, Devices and Methods of Making and Using Thereof |
| 8,623,393 – | Biomatrix Structural Containment and Fixation Systems and Methods of Use Thereof |
| 8,529,939 – | Mucoadhesive Drug Delivery Devices and Methods of Making and Using Thereof |
| 8,465,537 – | Encapsulated or Coated Stent Systems |
| 8,153,591 – | Protein Biomaterials and Biocoacervates and Methods of Making and Using Thereof |
| 9,999,705 – | Protein Biomaterials and Bioacervates and Methods of Making and Using Thereof |
| 10,016,534 – | Protein Biomaterial and Biocoacervate Vessel Graft System and Methods of Making and Using Thereof |
| 10,744,236 – | Protein Biomaterial and Biocoacervate Vessel Graft System and Methods of Making and Using Thereof |
To maximize the strength and value of our patent portfolio many of the claims use the transitional term “comprising”, which is synonymous with “including,” This use of transitional language is inclusive or open-ended and does not exclude additional, unrecited elements or method steps. Our patents also include method claims covering many of the applications and uses of the biomaterials as medical devices and drug delivery systems. With 10 issued or allowed United States Patents that contain over 300 claims, our intellectual property portfolio strongly protects our proprietary technology, including the composition of raw elements used to produce our formulations, the fabricated biomaterials and their application in end products, thereby making our material and devices much more attractive to industry partners.
We protect our products and technologies through a combination of patents, and proprietary know-how. Our goal is to obtain, maintain and enforce patent protection for our products, formulations, processes, methods and other proprietary technologies, preserve our trade secrets, and operate without infringing on the proprietary rights of other parties, both in the United States and in other countries. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current products and any future products in development. We also strenuously protect our proprietary information and proprietary technology through a combination of contractual arrangements, trade secrets and patents, both in the United States and abroad. However, even patent protection may not always afford us with complete protection against competitors who seek to circumvent our patents.
We depend upon the skills, knowledge and experience of our scientific and technical personnel, as well as that of our advisors, consultants and other contractors. To help protect our proprietary know-how which may not be patentable, or for which patents may be difficult to obtain or enforce, we rely on trade secret protection and confidentiality agreements. We generally require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit disclosure of confidential information and, where applicable, require disclosure and assignment of ownership to us any of the ideas, developments, discoveries and inventions important to our business.
Government Regulation – Medical Device Veterinary Use
The FDA provides a general exception to the requirement of pre-market approval for the use of medical devices in veterinary medicine. Our Kush™ product has already been classified and referred to as a medical device in past correspondence issued by the FDA. Accordingly, we believe the veterinary application of this medical device, Kush™, falls under this FDA exception, which allows us to commercialize our Kush products without obtaining prior regulatory approval.
| 40 |
Government Regulation – Veterinary and/or Human Use
A number of the formulations and medical devices that we manufacture for veterinary applications, and plan to manufacture for human applications, are subject to regulation by numerous regulatory bodies, including the FDA and comparable international regulatory agencies. These agencies require manufacturers of medical devices to comply with applicable laws and regulations governing the development, testing, manufacturing, labeling, marketing and distribution of medical devices. Medical devices are generally subject to varying levels of regulatory control, the most comprehensive of which requires that a clinical evaluation program be conducted before a device receives approval for commercial distribution.
In the EU, medical devices are required to comply with the Medical Devices Directive and obtain CE Mark certification in order to market medical devices. The CE Mark certification, granted following approval from an independent Notified Body, is an international symbol of adherence to quality assurance standards and compliance with applicable European Medical Devices Directives. Distributors of medical devices may also be required to comply with other foreign regulations such as Ministry of Health Labor and Welfare approval in Japan. The time required to obtain these foreign approvals to market our products may be longer or shorter than that required in the U.S., and requirements for those approvals may differ from those required by the FDA. In Europe, our devices are classified as Class IIa or IIb, and will need to conform to the Medical Devices Directive.
In the U.S., specific permission from the FDA to distribute a new device is usually required (that is, other than in the case of very low risk devices), and we expect that some form of marketing authorization will be necessary for our devices. Marketing authorization is generally sought and obtained in one of two ways. The first process requires that a pre-market notification (510(k) Submission) be made to the FDA to demonstrate that the device is as safe and effective as, or “substantially equivalent” to, a legally-marketed device that is not subject to pre-market approval (“PMA”). A legally-marketed device is a device that (i) was legally marketed prior to May 28, 1976, (ii) has been reclassified from Class III to Class II or I, or (iii) has been found to be substantially equivalent to another legally-marketed device following a 510(k) Submission. The legally-marketed device to which equivalence is drawn is known as the “predicate” device. Applicants must submit descriptive data and, when necessary, performance data to establish that the device is substantially equivalent to a predicate device. In some instances, data from human clinical studies must also be submitted in support of a 510(k) Submission. If so, these data must be collected in a manner that conforms with specific requirements in accordance with federal regulations including the Investigational Device Exemption (IDE) and human subjects protections or “Good Clinical Practice” regulations. After the 510(k) application is submitted, the applicant cannot market the device unless FDA issues “510(k) clearance” deeming the device substantially equivalent. After an applicant has obtained clearance, the changes to existing devices covered by a 510(k) Submission which do not significantly affect safety or effectiveness can generally be made without additional 510(k) Submissions, but evaluation of whether a new 510(k) is needed is a complex regulatory issue, and changes must be evaluated on an ongoing basis to determine whether a proposed change triggers the need for a new 510(k), or even PMA. The 510(k) clearance pathway is not available for all devices: whether it is a suitable path to market depends on several factors, including regulatory classifications, the intended use of the device, and technical and risk-related issues for the device.
The second, more rigorous, process requires that an application for PMA be made to the FDA to demonstrate that the device is safe and effective for its intended use as manufactured. This approval process applies to most Class III devices. A PMA submission includes data regarding design, materials, bench and animal testing, and human clinical data for the medical device. Again, clinical trials are subject to extensive FDA regulation. Following completion of clinical trials and submission of a PMA, the FDA will authorize commercial distribution if it determines there is reasonable assurance that the medical device is safe and effective for its intended purpose. This determination is based on the benefit outweighing the risk for the population intended to be treated with the device. This process is much more detailed, time-consuming, and expensive than the 510(k) process. Also, FDA may impose a variety of conditions on the approval of a PMA.
Both before and after a device for the U.S. market is commercially released, we would have ongoing responsibilities under FDA regulations. The FDA reviews design and manufacturing practices, labeling and record keeping, and manufacturers’ required reports of adverse experiences and other information to identify potential problems with marketed medical devices. We would also be subject to periodic inspection by the FDA for compliance with the FDA’s quality system regulations, which govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, and servicing of all finished medical devices intended for human use. In addition, the FDA and other U.S. regulatory bodies (including the Federal Trade Commission, the Office of the Inspector General of the Department of Health and Human Services, the Department of Justice (DOJ), and various state Attorneys General) monitor the manner in which we promote and advertise our products. Although physicians are permitted to use their medical judgment to employ medical devices for indications other than those cleared or approved by the FDA, we are prohibited from promoting products for such “off-label” uses and can only market our products for cleared or approved uses. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could require us to notify health professionals and others that the devices present unreasonable risks of substantial harm to the public health, order a recall, repair, replacement, or refund of such devices, detain or seize adulterated or misbranded medical devices, or ban such medical devices. The FDA may also impose operating restrictions, enjoin and/or restrain certain conduct resulting in violations of applicable law pertaining to medical devices, including a hold on approving new devices until issues are resolved to its satisfaction, and assess civil or criminal penalties against our officers, employees, or us. The FDA may also recommend prosecution to the DOJ. Conduct giving rise to civil or criminal penalties may also form the basis for private civil litigation by third-party payers or other persons allegedly harmed by our conduct.
| 41 |
The delivery of our devices in the U.S. market would be subject to regulation by the U.S. Department of Health and Human Services and comparable state agencies responsible for reimbursement and regulation of health care items and services. U.S. laws and regulations are imposed primarily in connection with the Medicare and Medicaid programs, as well as the government’s interest in regulating the quality and cost of health care.
Federal health care laws apply when we or customers submit claims for items or services that are reimbursed under Medicare, Medicaid, or other federally-funded health care programs. The principal federal laws include: (1) the False Claims Act which prohibits the submission of false or otherwise improper claims for payment to a federally-funded health care program; (2) the Anti-Kickback Statute which prohibits offers to pay or receive remuneration of any kind for the purpose of inducing or rewarding referrals of items or services reimbursable by a Federal health care program; (3) the Stark law which prohibits physicians from referring Medicare or Medicaid patients to a provider that bills these programs for the provision of certain designated health services if the physician (or a member of the physician’s immediate family) has a financial relationship with that provider; and (4) health care fraud statutes that prohibit false statements and improper claims to any third-party payer. There are often similar state false claims, anti-kickback, and anti-self-referral and insurance laws that apply to state-funded Medicaid and other health care programs and private third-party payers. In addition, the U.S. Foreign Corrupt Practices Act can be used to prosecute companies in the U.S. for arrangements with physicians, or other parties outside the U.S. if the physician or party is a government official of another country and the arrangement violates the law of that country.
The laws applicable to us are subject to change, and subject to evolving interpretations. If a governmental authority were to conclude that we are not in compliance with applicable laws and regulations, we and our officers and employees could be subject to severe criminal and civil penalties including substantial fines and damages, and exclusion from participation as a supplier of product to beneficiaries covered by Medicare or Medicaid.
The process of obtaining clearance to market products is costly and time-consuming in virtually all of the major markets in which we expect to sell products and may delay the marketing and sale of our products. Countries around the world have recently adopted more stringent regulatory requirements, which are expected to add to the delays and uncertainties associated with new product releases, as well as the clinical and regulatory costs of supporting those releases. No assurance can be given that any of our other medical devices will be approved on a timely basis, if at all. In addition, regulations regarding the development, manufacture and sale of medical devices are subject to future change. We cannot predict what impact, if any, those changes might have on our business. Failure to comply with regulatory requirements could have a material adverse effect on our business, financial condition and results of operations.
Exported devices are subject to the regulatory requirements of each country to which the device is exported. Some countries do not have medical device regulations, but in most foreign countries medical devices are regulated. Frequently, medical device companies may choose to seek and obtain regulatory approval of a device in a foreign country prior to application in the U.S. given the differing regulatory requirements. However, this does not ensure approval of a device in the U.S.
Research and Development
The Company is currently pursuing advancements in the composition, methods of manufacture and use for its proprietary biomaterials. It is anticipated that within the next twelve months the Company will pursue additional third-party studies related to the use of Kush™ for the treatment of osteoarthritis in canine and equine patients. The Company also anticipates that resources will be expended to advance and improve the manufacturing systems for Kush™ that will increase product volume and overall efficiency. Finally, the Company anticipates that research and testing will be conducted in the next eighteen months involving the existing Kush™ formulation and other variations to identify and determine the next commercial product(s) that may be administered to the digital cushion of horses for the treatment of navicular disease.
| 42 |
We intend to use a portion of the net proceeds from the offering to expand and scale our manufacturing capabilities to fill larger quantity orders should they be placed. We anticipate expending approximately $800,000 to engage independent studies of the use of our products in dogs and horses. We have had discussions with several universities that have veterinary studies in their curriculum regarding conducting such studies for us.
Employees and Services Performed by our Independent Contractors
We currently have two employees, consisting of our CEO and CFO, and have contracted with two independent contractors, who serve as our General Counsel and Director of Science and Technology. Our CEO is a full-time employee and our CFO is currently a part-time employee. With the proceeds received from this offering, we have identified and plan to add additional staff including a Director of Manufacturing, a Director of Quality Assurance and Studies, an Office Administrator, a Business Development Director and two manufacturing technicians.
We also engage outside consultants to assist with research and development, clinical development and regulatory matters, business development, operations and other functions from time to time.
Insurance
We currently maintain a “life science” commercial insurance policy with coverage in the amount of $1 million for our products and operations. The policy has been designed for those engaged in the life science business. We may face claims in excess of the limits of such insurance. As well, claims made against us may fall outside of our coverage. The policy is a “claims made” policy. Thus, our coverage must be maintained at the time a claim is made for us to be entitled to seek coverage from the issuer of the policy for such claims.
Corporate History and Structure
In 1999, Dr. David Masters, a biochemist, formed Gel-Del to pursue the development of biomaterials that could be used in connection with implantable therapeutic medical devices and treatments. Gel-Del’s efforts were principally funded by grants received from various federal governmental agencies. In connection with those efforts, Dr. Masters obtained United States Patents for methods of production and use of biomaterials that were assigned to Gel-Del. In August 2013, Gel-Del entered into a licensing agreement with PetVivo, Inc., a Minnesota Corporation (“PetVivo MN”) which had then been recently formed by John Lai and John Dolan to pursue the development and/or licensing of biomedical technology for application in the veterinary medicine markets.
The Company was incorporated in 2009 in Nevada under a former name and pursued a business unrelated to our current operations. In March 2014 the Company entered into a share exchange agreement with PetVivo MN and the five shareholders of PetVivo MN, whereby we acquired all of the common stock of PetVivo MN, resulting in PetVivo MN becoming our wholly-owned subsidiary, in exchange for the issuance to the shareholders of PetVivo MN of 4,159,692 shares of our common stock, as adjusted for a reverse stock split soon after this merger. In 2014 we changed our name to PetVivo Holdings, Inc., and our common stock’s trading symbol was changed to “PETV”.
In November 2014, the Boards of Directors of the Company and Gel-Del approved the terms of an agreement providing for a merger of the Company and Gel-Del. The merger was subject to approval by shareholders of both companies. The Gel-Del shareholders approved the merger in March 2015 and our shareholders approved it in April 2015; however, the required documents to effect the merger were not filed with the offices of the Secretary of State for Nevada or Minnesota. As we deemed ourselves to then be in control of Gel-Del, we combined its operations with ours and began to include its financial operating results in our financial statements as a “variable interest entity” where an investor has a controlling interest in a company, that is not based on ownership of a controlling equity interest.
Primarily due to an elongated delay to close the merger and a substantial decline in the prices for our shares, we found it necessary to adjust the terms of the original agreement between Gel-Del and the Company to complete the merger. In order provide compensation for such a delay and decline in our share price, in early 2017, we agreed to provide to the Gel-Del shareholders a larger number of our shares than was required in the initial merger agreement. The prior agreement was terminated and we entered into a new agreement among the Company, a newly established subsidiary of the Company and Gel-Del providing for Gel-Del to merge into that subsidiary with our issuing 4,905,000 shares upon the merger, then representing approximately 30% of our then outstanding shares. The shares were issued on the effective date for the Merger, which was April 10, 2017.
| 43 |
We rent our Edina, Minnesota office in suburban Minneapolis under the provisions of a long-term lease. Our executive, administrative and operating offices are located at 5251 Edina Industrial Blvd., Edina, Minnesota 55439. We believe that our facility is adequate for our needs and that additional suitable space will be available on acceptable terms as required.
From time to time, the Company is involved in various legal claims and actions arising in the ordinary course of business. While from time to time, claims are asserted that may make demands for sums of money, we do not believe that the resolution of any of these others matters, either individually or in the aggregate, will materially affect our financial position, cash flows or the results of our operations.
Board of Directors and Executive Officers
Our executive officers are appointed by and serve at the discretion of our board of directors. The following table includes the names, ages and positions held by our executive officers and directors as of September 30, 2020:
| Name | Age | Management and/or Director Positions | ||
| John Lai | 57 | Chief Executive Officer, President and Director | ||
| John Carruth | 31 | Chief Financial Officer | ||
| John F. Dolan | 55 | General Counsel, Secretary and Director | ||
| Gregory Cash | 63 | Chairman | ||
| David Deming | 60 | Director | ||
| Joseph Jasper | 56 | Director | ||
| Scott Johnson | 56 | Director | ||
| James Martin | 81 | Director | ||
| David B. Masters, Ph.D. | 62 | Director | ||
| Randall A. Meyer | 56 | Director | ||
| Robert Rudelius | 65 | Director |
Each of our directors will be elected at our annual meeting of stockholders and hold office until the next annual meeting of stockholders, or until a successor is elected and qualified. If any director resigns, dies or is otherwise unable to serve out the director’s term, or if the Board increases the number of directors, the Board may fill the vacancy by the vote of a majority of the directors then in office. A director elected to fill a vacancy shall serve for the unexpired term of such director’s predecessor.
John Lai. Mr. Lai has been a director and senior executive officer since March 2014, serving in various capacities that include Principal Financial Officer, President and CEO; and serving as our Chief Executive Officer from March 2014 to May 2017 and June 2019 to present. From March 2012 to April 2016, Mr. Lai also was Chief Executive Officer and a director of Blue Earth Resources, Inc., a small public company which acquired and managed working interests in producing oil and gas leases in Louisiana. Mr. Lai has over thirty years of senior executive and operational management and financial experience while holding key executive positions with several public companies in various industries. In 1992, Mr. Lai founded, and until December 2012 was the principal owner and President of Genesis Capital Group, Inc., which provided significant consulting services to many public and private companies in powersports, technology and other industries, while advising its clients in corporate development, mergers and acquisitions, and private and public capital-raising through equity offerings. Mr. Lai’s role as a co-founder of the company and his many years of experience as a chief executive officer of many public or private companies are material factors regarding his qualifications to serve on our Board of Directors.
| 44 |
John Carruth. Mr. Carruth joined our Company as our Controller in April 2018, was promoted to serve as our Acting Chief Financial Officer in December 2018 and has served as our Chief Financial Officer since July 2019. He has spent several years in the accounting field with continually-progressing responsibilities. His areas of expertise include internal controls over financial reporting, SEC reporting, and GAAP compliance. He holds three degrees in accounting, including a Master of Accountancy degree from the University of Minnesota. Since April of 2020 he has provided services to Granicus, LLC as an Accounting Manager specializing in accounting systems administration and acquisitions for approximately 5 hours per week. He has previously been employed at Merrill Corporation, where he worked on SEC reporting from May 2015 until September 2015, at Prime Therapeutics from September 2015 to March 2016, where he worked on special projects; and most recently, from March 2016 until April 2018 at Supervalu focusing on GAAP compliance and emerging and special projects.
John F. Dolan. Mr. Dolan has been a director since March 2014, and he served as our Chief Financial Officer from March 2014 to November 2017. Since March 2013, Mr. Dolan also has served as corporate and intellectual property counsel for KILO, Inc. and TerraCOH, Inc., both alternative energy companies. Mr. Dolan has also served as general counsel for Traust IP Finance, LLC since June, 2019. From June 2000 to July 2012, Mr. Dolan was a shareholder in the intellectual property group of the Minneapolis law firm of Fredrikson & Byron, where he specialized in securing and protecting domestic and foreign patent and other IP rights for various clients including biomaterials technology and products. During the past five years, Mr. Dolan also has provided consulting services to several early stage companies on all aspects of IP asset protection as well as new technology and corporate development. His extensive career in the intellectual property field includes serving as a patent examiner with the U.S. Patent and Trademark Office. Mr. Dolan’s role as a cofounder of the Company and his extensive experience in intellectual property, mergers and acquisitions, private equity, corporate governance and general corporate law are material factors which demonstrate his qualifications to serve on our Board of Directors.
Gregory Cash. Mr. Cash has served as a director of the Company since July 2019. He has more than 35 years senior management and/or key sales and marketing executive experience in the life sciences industry, including being Chief Executive Officer or Division President of publicly traded and privately held cardiovascular medical device companies. Since 2011, he has been the Chief Executive Officer and principal owner of Argent International LLC, Minneapolis, MN, a consulting firm he founded to provide management, marketing and financial consulting services to start-up and established companies in the life sciences industry. Prior to founding Argent, Mr. Cash served for over thirty years in senior executive management or marketing roles with leading medical device companies, including five years with Boston Scientific Corporation and over fourteen years with Medtronic, Incorporated. His many industry achievements also feature extensive and high-level overseas experience including being Chief Executive Officer or a senior marketing executive of both start-up and established international medical device companies in European countries including The United Kingdom, France and Italy, as well as serving for several years as the Marketing Manager in Asia for all Medtronic product lines. Mr. Cash’s many years of experience as an executive in the medical device industry are material factors regarding his qualification to serve on our Board of Directors.
James Martin. Mr. Martin has served as a director of the Company since July 2019. He is a retired Certified Public Accountant (“CPA”) and attorney whose career included his responsibility as Partner in Charge of KPMG’s tax practice for its Newport Beach, California office. In that role he provided and oversaw the rendition of tax services for numerous clients in varied industries including those for which KPMG provided a certified audit. He retains his AICPA membership and holds Accounting and Law Degrees from the University of Washington and, on a Fellowship, received a Master of Laws Degree from New York University. Mr. Martin’s extensive accounting expertise is a material factor which demonstrate his qualifications to serve on our Board of Directors.
Scott Johnson. Mr. Johnson has served as a director of the Company since July 2019. He is a licensed professional engineer with over 30 years experience providing life science engineering leadership, risk management, production engineering, quality control, auditing, and FDA compliance for numerous manufacturers. Since 2012 he has been the Chief Executive Officer and principal owner of Stratego, Inc., a life sciences consulting corporation he founded. Significant engagements of Stratego include risk management services for defibrillator products at Philips Healthcare, risk management and quality audit services for combination products at Hospira, a subsidiary of Pfizer and Baxter, quality remediation management for implantable medical devices at St. Jude Medical, a product regulatory roadmap for Varuna Biomedical and engineering PMA submissions content at Zimmer Biomet –Biologics. Mr. Johnson’s lengthy past employment and consulting include five years of employment with SciMed Life Systems, five years systems engineering, testing and compliance for PumpWorks, and being FDA compliance project manager at Boston Scientific. His engineering projects for the production of medical devices include substantial domestic and foreign facility experience. Mr. Johnson’s many years of experience as an executive in the life science industries and expertise with medical product design and regulatory issues are material factors which demonstrate his qualifications to serve on our Board of Directors.
| 45 |
David B. Masters, Ph.D. Dr. Masters has been a director since April 2015, and he served as our Chief Technical Officer from April 2015 to December 2017. Dr. Masters is the founder of and served as Chief Executive Officer and Chief Technology Officer of Gel-Del Technologies, Inc., from 1999 to December 2017, while for Gel-Del he developed and obtained significant patents for the proprietary biomaterial technology and product applications acquired by us from Gel-Del. Dr. Masters is recognized internationally as a leading expert in biomaterials and local drug delivery, and over the past twenty years he has developed and obtained patents for many novel biomaterials and drug delivery products, including implantable medical devices for neurologic, vascular, orthopedic, urologic and dermal applications. Dr. Masters’ former academic career included teaching courses and doing significant research at Harvard Medical School and The Mayo Clinic. He received a B.A. Degree in Biochemistry, a Master’s Degree in Chemistry, and a Ph.D. in Behavioral and Neural Sciences from Rutgers University. Dr. Master’s role as the founder of Gel-Del and his long professional career in developing and obtaining patents for many biomaterials and drug delivery products are material factors regarding his qualifications to serve on our Board of Directors.
Randall A. Meyer. Mr. Meyer has been a director since April 2015, and he served as our Chief Operating Officer from April 2015 to November 2017. From January 2009 to April 2015, Mr. Meyer served as Chief Operating Officer of Gel-Del Technologies, Inc. while being in charge of all operational and marketing activities of Gel-Del. Prior to joining Gel-Del, Mr. Meyer’s substantial medical device industry management experience included being Chief Operating Officer of Softscope Medical Technologies, Inc. and being Chief Executive Officer of Tactile Systems Technology, Inc. Mr. Meyer’s role as the senior operational officer of Gel-Del for many years and his long experience as an executive officer of several companies in the medical device industry are material factors regarding his qualifications to serve on our Board of Directors.
David Deming. Mr. Deming has been a director of the Company since September 2017. Mr. Deming has over 35 years of institutional investment management experience with pensions, endowments, family offices and high net worth investors in roles. He is currently serving as the Chief Investment Officer of Business Brokers Investment Company, a main street business brokerage firm, which he joined in January 2020. He served for over 19 years as the Director of Business Development at Arbor Capital Management, LLC from January 1997 until October 2016. Subsequent thereto, he served as the Director of Marketing and Investor Relations at BCCM Advisors, an alternative investment platform, from August 2018 to March 2020 and as the Director of Business Development, Chief Compliance Officer and Partner at Asymmetric Capital Management from October 2016 until August 2018. Prior thereto, he held positions with brokerage and trading firms, including, Merrill Lynch, Paine Webber and Leuthold Weeden Capital Management and was a floor trader at the Chicago Board of Trade. Mr. Deming’s extensive experience in the finance industry is a material factor which demonstrates his qualifications to serve on our Board of Directors.
Robert Rudelius. Mr. Rudelius has been a director of the Company since August 2018. Currently, he is the Chief Executive Officer and Managing Director of Noble Ventures, LLC, a company he founded in 2001 that provides advisory and consulting services to early and mid-stage companies in the information technology, communications, medical technology and social e-commerce industries. He is also the co-founder, President & CEO of MedicaMetrix, Inc., a company that is building a commercialization engine that will launch a stream of medical devices aimed at delivering transformative healthcare solutions for unmet medical needs. From April 1999 through May 2001, when it was acquired by StarNet L.P., Mr. Rudelius was the founder and CEO of Media DVX, Inc., a start-up business that provided a satellite-based, IP-multicasting alternative to transmitting television commercials via analog videotapes to television stations, networks and cable television operators throughout North America. From April 1998 to April 1999, Mr. Rudelius was the President and Chief Operating Officer of Control Data Systems, Inc., during which time Mr. Rudelius reorganized and re-positioned the software company as a professional technology services company, resulting in the successful sale of the company to British Telecom. From October 1995 through April 1998, Mr. Rudelius was the founding Managing Partner of AT&T Solutions, Inc., a subsidiary of AT&T Inc. (NYSE: T) and headed the Media, Entertainment & Communications industry practice. From January 1990 through September 1995, Mr. Rudelius was a partner in McKinsey & Company’s information, technology and systems practice, during which time he headed the practice in Japan and the United Kingdom. Mr. Rudelius began his career at Arthur Andersen & Co. where he was a leader in the firm’s financial accounting systems consulting practice. Mr. Rudelius served as a member of the Axogen, Inc. (NASDAQ: AXGN) Board of Directors for ten years from September 2010 through September 30, 2020, where he served on the audit committee and as a member of the compensation committee. Mr. Rudelius has an M.B.A. from the Kellogg School of Management at Northwestern University and a B.S. in mathematics and economics from Gustavus Adolphus College in St. Peter, Minnesota. Mr. Rudelius’ qualifications to serve on our Board of Directors include his extensive executive leadership and financial experience, particularly in connection with rapid growth technology businesses, and his experience as a director of publicly traded companies.
| 46 |
Joseph Jasper. Mr. Jasper has been a director of the Company since August 20, 2018. He is a CFA who since 2005 has been Chief Executive Officer of Vermillion Capital Management, an institutional investment firm. From 2002 to 2005, Mr. Jasper was Managing Director and Director of Fixed Income Strategy and Marketing for Piper Jaffray Company. Prior to 2002, he spent 20 years managing, structuring and selling fixed income and equity securities at several leading investment banking firms, including U.S. Bancorp Libra and UBS PaineWebber. Mr. Jasper also serves as Vice Chairman of the Board of Directors of MicroNet, Inc. and as a director of GroundCloud, Inc. both privately-held companies. He has previously served as a director or principal advisor to many operating and venture-stage companies across a broad range of industries. Mr. Jasper received a MBA degree from the University of St. Thomas, where he also has served as its Adjunct Professor of Finance. Mr. Jasper’s extensive financing and accounting expertise are material factors which demonstrate his qualifications to serve on our Board of Directors
Family Relationships
There are no family relationships between any of our executive officers and directors.
Board of Directors
Each of our directors will be elected at our annual meeting of stockholders and hold office until the next annual meeting of stockholders, or until a successor is elected and qualified. If any director resigns, dies or is otherwise unable to serve out the director’s term, or if the Board increases the number of directors, the Board may fill the vacancy by the vote of a majority of the directors then in office. A director elected to fill a vacancy shall serve for the unexpired term of such director’s predecessor.
Director Independence
We have applied to have our common stock and warrants listed on the Exchange, subject to notice of issuance under the trading symbols “PETV” and “PETVW,” respectively. The Exchange Listing Rules require that independent directors compose a majority of a listed company’s board of directors within one year of listing. In addition, the Exchange Listing Rules require that, subject to specified exceptions, each member of a listed company’s audit, compensation, and nominating and corporate governance committees be independent and that audit committee members also satisfy independence criteria set forth in Rule 10A-3 under the Exchange Act of 1934. Under the Exchange Listing Rules, a director will only qualify as an “independent director” if, in the opinion of our board of directors, that person does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In order to be considered independent for purposes of Rule 10A-3 under the Exchange Act, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the board of directors, or any other board committee: (1) accept, directly or indirectly, any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
In addition members of the compensation committee must satisfy additional independence requirements set forth in the Exchange Listing Rules. In order to be considered independent for purposes of the Exchange Listing Rules, a member of a compensation committee of a listed company may not, other than in his or her capacity as a member of the compensation committee, the board of directors, or any other board committee, accept, directly or indirectly any consulting, advisory, or other compensatory fee from the listed company or any of its subsidiaries. Additionally, the board of directors of the listed company must consider whether the compensation committee member is an affiliated person of the listed company or any of its subsidiaries and if so, must determine whether such affiliation would impair the director’s judgment as a member of the compensation committee.
| 47 |
In July 2020, our board of directors undertook a review of its composition, the composition of its committees and the independence of each director. Based upon information requested from and provided by each director concerning his background, employment and affiliations, including family relationships, our board of directors determined that Messrs. Cash, Deming, Johnson, Jasper and Martin do not have any relationships that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each is considered an “independent” director as that term is defined under the applicable SEC rules and the Exchange Listing Rules. In making those determinations, our board of directors considered the current and prior relationships that each non-employee director has with our Company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director.
Committees of the Board of Directors
We have an Audit Committee, Compensation Committee, and Nominating Committee. Our Audit Committee consists of three independent directors who are David Deming, James Martin and Joseph Jasper, with Mr. Martin considered as an “audit committee financial expert” within the meaning of Regulation S-K of the SEC. Our Compensation Committee consists of three independent directors who are David Deming, Scott Johnson and Robert Rudelius. Our Nominating Committee consists of two independent directors who are Joseph Jasper and Robert Rudelius.
Code of Ethics
We have adopted a Code of Ethics which applies to our board of directors, executive officers and other employees. Our Code of Ethics outlines the broad principles of ethical business conduct we have adopted, including subject areas such as confidentiality, conflicts of interest, corporate opportunities, public disclosure reporting, protection of company assets, and compliance with applicable laws. A copy of our Code of Ethics is available without charge to any person by written request to us at our principal offices at 5251 Edina Industrial Blvd., Edina, MN 55439.
| 48 |
EXECUTIVE AND DIRECTOR COMPENSATION
The following is a discussion and analysis of the compensation arrangements for our named executive officers, or NEOs. For fiscal 2020 and 2019, our named executive officers were John Lai, our Chief Executive Officer and President, John Carruth, our Chief Financial Officer, John F. Dolan, our General Counsel and Secretary.
Summary Compensation Table
The following table sets forth information regarding the compensation paid to or earned by our named executive officers for the fiscal years ended March 31, 2019 and 2020.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock Awards ($)(1) | All
Other Compensation ($) | Total ($) | ||||||||||||||||||
| John Lai, CEO and President(2) | 2020 | 4,797 | 0 | 350,684 | 0 | 355,481 | ||||||||||||||||||
| 2019 | 60,000 | (3) | 0 | 0 | 0 | 60,000 | ||||||||||||||||||
| John Carruth, CFO (4) | 2020 | 100,000 | 0 | 249,978 | 0 | 349,978 | ||||||||||||||||||
| 2019 | 72,775 | 10,000 | 148,247 | 0 | 231,022 | |||||||||||||||||||
| John F. Dolan, General Counsel and Secretary(5) | 2020 | 2,283 | (6) | 0 | 126,797 | 0 | 129,080 | |||||||||||||||||
| 2019 | 0 | 0 | 102,778 | 0 | 102,788 | |||||||||||||||||||
| (1) | The value in this column reflects the aggregate grant date fair value of the stock option award as computed in accordance with ASC Topic 718. Information regarding the valuation assumptions used in the calculations are included in “Note 15 – Stockholders’ deficit” to our audited consolidated financial statements included in this prospectus. | |
| (2) | Mr. Lai was appointed to serve as the Company’s CEO in June 2019. | |
| (3) | Half of Mr. Lai’s salary ($30,000) was accrued in fiscal 2019. | |
| (4) | Mr. Carruth served as our Acting Chief Financial Officer from December 2018 until July 2019 and as our Chief Financial Officer since July 2019. | |
| (5) | Mr. Dolan has served as our General Counsel since November 2019. He is an independent contractor. | |
| (6) | In lieu of receiving cash, Mr. Dolan received his consulting fee with a grant of warrants awarded to him in fiscal 2020. |
In February of 2020, the Company issued John Lai 61,396 shares of common stock pursuant to his cashless conversion of an outstanding warrant for 168,750 shares of common stock with a strike price of $.33/share.
| 49 |
Outstanding Equity Awards at Fiscal Year-End
The table below provides information regarding unexercised option and warrants awards held by each of our named executive officers as of our fiscal year-end, March 31, 2020. All share amounts and exercise prices reflect the effect of the reverse split of our common stock effected on ______________.
| Option Awards | |||||||||||||||||
| Name | Grant Date | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price ($) | Option Expiration Date | ||||||||||||
| John Lai | 3/31/2020 | 98,093 | (1) | - | $ | 0.32 | 3/31/2025 | ||||||||||
| 6/02/2017 | 168,750 | (2) | - | $ | 0.33 | 10/31/2020 | |||||||||||
| 6/02/2017 | 168,750 | (2) | - | $ | 0.33 | 12/30/2020 | |||||||||||
| 6/02/2017 | 168,750 | (2) | - | $ | 0.33 | 6/30/2021 | |||||||||||
| 12/31/2019 | 79,397 | (1) | - | $ | 0.49 | 12/31/2024 | |||||||||||
| 10/31/2019 | 60,000 | (3) | 480,000 | (3) | $ | 0.56 | 10/31/2024 | ||||||||||
| John Carruth | 4/30/2018 | 101,250 | (4) | 33,750 | $ | 0.33 | 12/11/2023 | ||||||||||
| 4/30/2018 | 72,000 | (5) | - | $ | 0.33 | 4/30/2023 | |||||||||||
| 10/31/2019 | 60,000 | (4) | 390,000 | (4) | $ | 0.56 | 10/30/2024 | ||||||||||
| John F. Dolan | 1/5/2019 | 168,750 | (1) | - | $ | 0.30 | 1/15/2029 | ||||||||||
| 3/31/2020 | 35,314 | (1) | - | $ | 0.32 | 3/31/2025 | |||||||||||
| 12/31/2019 | 15,880 | (1) | - | $ | 0.49 | 12/31/2024 | |||||||||||
| 10/31/2019 | 40,500 | (5) | 180,000 | (5) | $ | 0.56 | 10/31/2024 | ||||||||||
| (1) | These warrants were granted to Mr. Lai and Mr. Dolan in lieu of compensation and vested immediately on their grant dates. | |
| (2) | These warrants were granted to Mr. Lai for serving as our President and vested semi-annually over a 2 year period from their grant date and were fully vested as of March 31, 2020. | |
| (3) | Mr. Lai was granted a warrant to purchase up to 540,000 shares of our common stock at an exercise price of $0.56 per share pursuant to his employment agreement. The warrants have a five-year term and 360,000 warrants vest quarterly over a three-year term and 180,000 warrants vest based on certain performance conditions; 90,000 of which vest if the Company completes a successful listing on the Exchange and sustaining a stock price of at least $4.00 for the thirty consecutive days of trading. | |
| (4) | Mr. Carruth was granted a warrant to purchase 135,000 shares of our common stock at an exercise price of $0.33 per share for acting CFO services. The warrants have a five-year term and vest at the rate of 1/8 per quarter (16,875) beginning on December 31, 2018. | |
| (5) | Mr. Carruth was granted a warrant to purchase 72,000 shares of our common stock at an exercise price of $0.56 per share for serving as the Controller. These warrants have a five-year term and vested at the rate of 1/8 per quarter (9,000) beginning on June 30, 2018. All warrants are fully vested as of March 31, 2020. | |
| (6) | Mr. Carruth was granted a warrant to purchase up to 360,000 shares of our common stock at an exercise price of $0.56 per share pursuant to his employment agreement. These warrants have a five-year term and vest quarterly over a three-year term. He also received a warrant to purchase up to 90,000 shares of the Company’s common stock at an exercise price of $0.56 per share that vest upon certain milestones, which includes vesting of 45,000 shares upon the Company’s filing and approval of an S-1 registration statement by the SEC and (ii) 45,000 shares on the date of completion of a successful uplisting to the Exchange or similar national exchange. | |
| (7) | Mr. Dolan received a warrant to purchase 220,500 shares of the Company’s common stock at an exercise price of $0.56 per share for his services as an independent contractor, of which 40,500 vested immediately, 90,000 warrants vest upon achieving certain performance milestones and 90,000 vest in equal installments of 7,500 warrants each quarter over the three years ended September 30, 2022. |
2020 Equity Incentive Plan
The PetVivo Holdings, Inc. 2020 Equity Incentive Plan (“Plan”) was adopted by the Board of Directors on July 10, 2020 and approved by our stockholders on September 22, 2020 The Plan permits the following types of awards: stock options, stock appreciation rights, restricted stock awards, restricted stock units, deferred stock units, performance awards, non-employee director awards and other types of awards. The Company reserved 4,000,000 shares of common stock for issuance under the 2020 Plan. As of today’s date, the Company has not granted any stock awards under the 2020 Plan. The Plan will terminate on July 10, 2030.
| 50 |
Executive and Consultant Employment and Consulting Agreements
John Lai
On October 1, 2019, we entered into employment agreement with John Lai to serve as our Chief Executive Officer for a term of three years to expire on September 30, 2022. This agreement may be terminated by the Company without cause at any time upon 10 days’ notice or for Cause (as defined in Mr. Lai’s employment agreement). Mr. Lai’s annual base salary is a minimum of $100,000 or such higher annual salary, if approved by the Board. The Board may elect to pay Mr. Lai’s salary in cash or warrants to purchase shares of the Company’s common stock at 125% of the cash value calculated using the volume weight average price of the Company’s common stock in the last week of the quarter that the base salary is accrued but that conversion rate shall never be less than $0.35 per share. Mr. Lai is eligible to receive discretionary bonuses, as determined by the Board and is eligible for all employee benefits provided to executives of similar tenure.
On October 31, 2019, Mr. Lai was granted a warrant to purchase up to 540,000 shares of our common stock at an exercise price of $.56 per share. The warrants have a five-year term and 360,000 warrants vest quarterly over a three-year term and 180,000 warrants vest based on certain performance conditions; 90,000 of which vest if the Company completes a successful listing on the Exchange and sustaining a stock price of at least $4.00 for the thirty consecutive days of trading. Mr. Lai’s employment agreement contains customary confidentiality and non-competition provisions which survive for a period of one year after his employment with the Company is terminated.
During fiscal 2020, Mr. Lai received an aggregate grant of 177,490 warrants exercisable at prices ranging from $0.32 to $0.49 per share in lieu of his salary. These warrants expire five years after their respective grant date. In June 2020, he received a grant of 90,000 warrants exercisable at a price of $0.35 per share that will vest if the Company completes a successful listing on the Exchange and sustaining a stock price of at least $4.00 for the thirty consecutive days of trading. In June 2020, My Lai also received a warrant for 29,762 shares of common stock, vested immediately, at a price of $0.40 per share in lieu of his salary.
John Carruth
On October 1, 2019, we entered into an employment agreement with John Carruth to serve as our Chief Financial Officer for a term of three years, to expire on September 30, 2022. This agreement may be terminated by the Company at any time upon 10 days’ notice or for Cause (as defined in Mr. Carruth’s employment agreement). Under this agreement, Mr. Carruth’s annual base salary is a minimum of $100,000 or such higher annual salary, approved by the Board. The Board may elect to pay Mr. Carruth’s salary in cash or warrants to purchase shares of the Company’s common stock at exercise price of $0.35 per share. Mr. Carruth is eligible to receive discretionary bonuses, as determined by the Board and is eligible for all employee benefits provided to executives of similar tenure.
On October 1, 2019, Mr. Carruth was granted a warrant to purchase up to 360,000 shares of our common stock at an exercise price of $0.56 per share. These warrants have a five-year term and vest quarterly over a three-year term. He also received a warrant to purchase up to 90,000 shares of the Company’s common stock at an exercise price of $0.56 per share that vest upon certain milestones, which includes vesting of 45,000 shares upon the Company’s filing and approval of an S-1 registration statement by the SEC and (ii) 45,000 shares on the date of completion of a successful uplisting to the Exchange or similar national exchange. Mr. Carruth’s employment agreement contains customary confidentiality and non-competition provisions which survive for a period of one year after his employment with the Company is terminated.
We amended our employment agreement with Mr. Carruth pursuant to an amendment (“Amendment”) dated June 15, 2020, with an effective date of April 14, 2020 to provide that Mr. Carruth’s employment would be part-time and to reduce his salary to a maximum of $33,000 per year. All other terms of the employment agreement remain the same.
John Dolan
We entered into an independent contractor agreement with John Dolan, our General Counsel and Secretary on November 20, 2019. This agreement has a term of three years and is set to expire on September 30, 2022. Mr. Dolan will receive compensation at the rate of $3,000 per month for the performance of general counsel services, which shall be provided for approximately 25% of his normal monthly time allocated to such services. The Company may pay the monthly compensation at its discretion in cash or warrants for common at 125% of the cash value calculated by using the volume weighted average price of the Company’s common stock in the last week of the quarter that compensation is accrued.
In connection with this agreement, Mr. Dolan received a warrant to purchase 220,500 shares of the Company’s common stock at an exercise price of $0.56 per share, of which 40,500 vested immediately, 90,000 warrants vest upon achieving certain performance milestones and 90,000 vest in equal installments of 7,500 warrants each quarter over the three years ended September 30, 2022. All of these 220,500 warrants expire on October 31, 2024.
| 51 |
In fiscal 2020, the Company did not pay any cash consulting fees to Mr. Dolan and paid Mr. Dolan’s consulting fee with the grant of an aggregate of 51,194 warrants at exercise prices of $0.32 to $0.49 per share. These warrants expire between December 31, 2024 and March 31, 2025.
Potential Payments Upon Termination of Employment or Change-in-Control
Termination and Change-in-Control Agreements or Arrangements
We do not have any contracts, agreements, or arrangements with any of our named executive officers providing for additional benefits or payments in connection with a termination of employment, change in job responsibility, or change-in-control. Upon termination of employment for any reason, all unvested restricted stock units expire.
Change in Control Provisions of the 2020 Plan
Subject to the terms of the applicable award agreement or an individual agreement between the Company and a participant, upon a change in control, the Board may, in its discretion, determine whether some or all outstanding options shall become exercisable in full or in part, whether the restriction period and performance period applicable to some or all outstanding restricted stock awards and restricted stock units, shall lapse in full or in part, and whether the performance measures applicable to some or all outstanding awards shall be deemed to be satisfied. The Board may further require that shares of stock of the Company resulting from such a change in control, thereof, be substituted for some or all of our shares of common stock subject to an outstanding award and that any outstanding awards, in whole or in part, be surrendered to us by the holder, to be immediately cancelled by us, in exchange for a cash payment, shares of capital stock of the corporation resulting from or succeeding us, or a combination of both cash and such shares of stock.
Director Compensation
Directors who are also executive officers do not receive any compensation regarding their role as a director. Currently, our policy for non-executive director compensation is as follows: i) independent directors receive a grant of 100,000 warrants to purchase shares of our common stock at the exercise price on the date of grant for a term of with a 5-year term for each 2 years of service, ii) directors who serve on committees are compensated in cash or warrants, at the Company’s discretion, at various pre-set amounts for different levels of service ranging from $1,500 to $5,000 per year per director. We also have a clawback provision which provides that if a director quits or is terminated for service prior to the end of the fiscal year, all warrant issued to that director are automatically cancelled.
The following table provides information on compensation paid to our current independent directors for their services as members of our board of directors during our fiscal year ended March 31, 2020:
| Name of director | Fees paid in cash ($) | Stock awards ($) | Warrant awards ($)(1) | All other compensation ($) | Total ($) | |||||||||||||||
| Gregory Cash(1) | — | — | 44,269 | — | 44,269 | |||||||||||||||
| David Deming | — | — | 25,667 | — | 25,667 | |||||||||||||||
| Joseph Jasper | — | — | 2,201 | — | 2,201 | |||||||||||||||
| Scott M. Johnson(1) | — | — | 42,892 | — | 42,892 | |||||||||||||||
| Robert Rudelius | — | — | 3,578 | — | 3,578 | |||||||||||||||
| (1) | These directors were elected as directors effective as of June 23, 2019. |
Each of our three newly elected directors, Messrs. Johnson, Cash and Martin, were issued warrants with five-year terms to purchase 90,000 shares of our common stock in consideration for their agreements to serve as a director, which warrants are fully vested, exercisable at $0.33 per share, and include a cashless exercise provision, and each of these three directors also received similar warrants to serve as a director for a second year with the exception that the second year warrants vest at a rate of 11,250 shares quarterly provided they continue to serve as a director.
| 52 |
Each of the independent directors, in consideration of their service as members of our Board of Directors, has been issued warrants for the purchase of 90,000 shares of our common stock. The dollar value in the table above for warrant awards to independent directors for the fiscal year ended March 31, 2020 reflects valuations using the Black-Scholes model of grants that occurred during the fiscal year ended March 31, 2020. The amounts disclosed were determined in accordance with ASC Topic 718.
Securities authorized for issuance under equity compensation plans.
The following table sets forth securities authorized for issuance under any equity compensation plan approved by our stockholders as well as any equity compensation plans not approved by our stockholders as of September 30, 2020
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted average exercise price of outstanding options, warrants and rights | Number of Securities remaining available for future issuance under equity compensation plans (excluding securities reflected in table) | |||||||||
| Plans approved by shareholders(1) | 0 | - | 4,000,000 | |||||||||
| Plans not approved by shareholders(2) | 4,739,097 | $ | 0.46 | |||||||||
| (1) | PetVivo Holdings, Inc. 2020 Equity Incentive Plan. | |
| (2) | Represents warrants granted to officers, directors, employees, financial advisors, consultants, investors, and other service providers pursuant to individual contracts, investments, awards or arrangements for compensatory purposes. |
As of September 30, 2020 (the “Record Date”), we had 24,876,189 shares of our common stock issued and outstanding. The following table sets forth, as of the Record Date, information concerning the beneficial ownership of shares of our common stock held by our directors, our named executive officers, our directors and executive officers as a group, and each person known by us to be a beneficial owner of more than 5% of our outstanding common stock. Unless otherwise indicated, the business address of each of our directors, executive officers and beneficial owners of more than 5% of our outstanding common stock is c/o PetVivo Holdings, Inc., 5251 Edina Industrial Blvd., Edina, MN 55439. Each person has sole voting and investment power with respect to the shares of our common stock, except as otherwise indicated. Beneficial ownership consists of a direct interest in the shares of common stock, except as otherwise indicated.
As used in this section, the term “beneficial ownership” with respect to a security is defined by Rule 13d-3 under the Securities Exchange Act of 1934, as amended, as consisting of sole or shared voting power (including the power to vote or direct the vote) and/or sole or shared investment power (including the power to dispose of or direct the disposition of) with respect to the security through any contract, arrangement, understanding, relationship or otherwise, subject to community property laws where applicable. Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission which provide that shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or become exercisable within 60 days of the date of the table are deemed beneficially owned by their holders. Subject to community property laws, where applicable, the persons or entities named in the table above have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
| 53 |
| Name
of Beneficial Owners, Officers and Directors | Amount
and Nature of Beneficial Owner | Percent
of Class | ||||
| John Lai | 3,827,096 shares (1) | 15.01 | % | |||
| John Carruth | 431,867
shares (2) | 1.71 | % | |||
| John F. Dolan | 2,195,386
shares (3) | 8.72 | % | |||
| David B. Masters | 4,563,125 shares (4) | 18.26 | % | |||
| Randall A. Meyer | 2,221,281 shares (5) | 8.91 | % | |||
| Scott Johnson | 722,853 shares (6) | 2.89 | % | |||
| Gregory Cash | 137,800 shares (7) | 0.55 | % | |||
| David Deming | 286,655 shares (8) | 1.14 | % | |||
| James Martin | 313,776
shares (9) | 1.26 | % | |||
| Joseph Jasper | 229,734
shares (10) Director | 0.92 | % | |||
| Robert Rudelius | 720,278
shares (11) | 2.87 | % | |||
| All
directors and named executive (officers as a group 11 persons) | 15,649,851 shares (12) | 57.68 | % | |||
| 5% Stockholders | ||||||
| Stanley Cruden | 2,283,287
shares (13) | 9.18 | % | |||
| (1) | Amount consists of 3,200,848 shares owned directly by Mr. Lai and warrants to purchase 626,250 shares that are vested or will vest within 60 days of the Record Date. |
| (2) | Amount consists of 104,867 shares owned directly by Mr. Carruth and warrants to purchase 327,000 shares that are vested or will vest within 60 days of the Record Date. |
| (3) | Amount consists of 1,904,942 shares held directly by Mr. Dolan and warrants to purchase 290,444 shares that are vested or will vest within 60 days of the Record Date. |
| (4) | Amount consists of 4,456,328 shares held directly by Dr. Masters and warrants to purchase 106,797 shares that are vested or will vest within 60 days of the Record Date. |
| (5) | Amount consists of 2,167,593 shares that are owned directly by Mr. Meyer and includes warrants to purchase 53,688 shares that are vested or will vest within 60 days of the Record Date. |
| (6) | Amount consists of 554,973 shares held by Mr. Johnson directly, 66,380 held in IRA accounts and warrants to purchase 101,500 shares that are vested or will vest within 60 days of the Record Date. |
| (7) | Amount consists of 63,159 shares held by Mr. Cash directly and warrants to purchase 74,641 shares that are vested or will vest within 60 days of the Record Date. |
| (8) | Amount consists of 43,748 shares held by Mr. Deming directly, 13,500 shares held by Mr. Deming with his spouse and warrants to purchase 229,407 shares that are vested or will vest within 60 days of the Record Date. |
| (9) | Amount consists of 77,209 shares held by Mr. Martin directly, 156,930 shares held in his two IRA accounts, 9,155 shares held by Martinmoore Holdings, LLP, a company controlled by Mr. Martin who exercises sole voting and dispositive power over the shares, 450 shares held by Mr. Martin’s wife and warrants to purchase 70,032 shares that are vested or will vest within 60 days of the Record Date. |
| (10) | Amount includes 36,835 shares held directly by Mr. Jasper and warrants to purchase 192,899 shares that are vested or will vest within 60 days of the Record Date. |
| (11) | Amount includes 152,010 shares held by Mr. Rudelius directly, 301,352 shares held in his IRA, 84,001 shares held by Noble Ventures, LLC, a company controlled by Mr. Rudelius and warrants to purchase 182,915 shares that are vested or will vest within 60 days of the Record Date. |
| (12) | Amount includes warrants owned by all of our named executive officers and directors, as a group, to purchase an aggregate of 2,255,573 shares that are vested or will vest within 60 days of the Record Date. |
| (13) | As reported in Mr. Cruden’s Amendment No. 1 to his Schedule 13G filed with the SEC on October 2, 2019. |
| 54 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
The following is a summary of the transactions since the beginning of the 2019 fiscal year between the Company and its executive officers, directors, nominees for directors, principal shareholders and related parties involves amounts in excess of $120,000 or that the Company has chosen to voluntarily disclose.
David Masters
Effective September 1, 2020, the Company entered into two debt settlement agreements with David B. Masters, a director of the Company, pursuant to (i) an Amendment to Promissory Note (“Amendment”) which amended certain outstanding promissory notes dated September 5, 2013, February 11, 2014 and August 14, 2014 (collectively, the “Outstanding Notes”) issued by Gel-Del, the Company’s wholly-owned subsidiary, with an aggregate amount owed of $65,700 and (ii) a Promissory Note (“Note”) having a principal amount of $195,000, which represents accrued salary owed to Dr. Masters. The Amendment extends, for up to an additional two years and under the same terms as originally entered into, the Outstanding Notes. The Company also entered into a Settlement and General Release (“Settlement Agreement”) with Dr. Masters that provides for the settlement and general release of any and all past claims, demands, damages, judgements, causes of action and liabilities that Dr. Masters may have had, may currently have or may acquire against the Company and its subsidiaries, including, but not limited to any claims related to (a) the ownership, operation, business, or financial condition of Company or its business, (b) any promissory note, loan, contract, agreement or other arrangement, whether verbal or written, including all unpaid interest charges, late fees, penalties or any other charges thereon, entered into or established between Dr. Masters and his affiliates and the Company on or prior to the September 1, 2020 or (c) the employment of Dr. Masters by the Company (except for claims directly relating to the breach of the Amendment, the Note or the Consulting Agreement).
As of September 30, 2020, the Company owes David Masters an aggregate amount of $254,010.
John Lai
In May 2018, our Board of Directors approved and the Company issued John Lai 803,385 shares of our common stock, which includes 324,723 to replace shares he had surrendered to a lender in 2016 to obtain past significant financing, and 478,662 shares to restore escrowed shares subject to an escrowed agreement, provided that Mr. Lai still satisfies certain financing terms contained in the escrow agreement. In February of 2020, the Company issued John Lai 61,396 shares of common stock pursuant to his cashless conversion of an outstanding warrant for 168,750 shares of common stock with a strike price of $0.33 per share.
John Lai, John Dolan and Randy Meyer
On September 11, 2019, the Company issued an aggregate of 1,439,851 shares of common stock to John Lai, John Dolan and Randy Meyer, a former employee and current director of the Company, an aggregate amount of $455,965 in exchange for their forgiveness of an aggregate amount of $455,965 in accrued salary and release of all claims against the Company for unpaid compensation.
Related Party Transaction Policy and Approval
We are adopting a written policy that any transaction between the Company and executive officers, directors, principal shareholders or their affiliates takes place on an arm’s length basis and requires the approval of a majority of our independent directors, as defined in the Exchange Listing Rules.
| 55 |
Our authorized capital stock consists of 225,000,000 shares of common stock and 20,000,000 shares of preferred stock. As of September 30, 2020, there were 24,876,189 shares of our common stock issued and outstanding and no shares of our preferred stock outstanding.
Common Stock
Holders of our common stock are entitled to one vote for each share on all matters submitted to a stockholder vote, and also are entitled to share ratably in all dividends declared by our board of directors from legally available funds. Holders of our common stock do not have any cumulative voting rights. In the event of our liquidation, dissolution or winding up, subject to preferences of any outstanding preferred stock, holders of our common stock will participate ratably in all assets that remain after payment of liabilities. Holders of our common stock have no conversion, redemption, preemptive or other subscription rights.
Preferred Stock
Without further stockholder approval, our board of directors may establish and issue one or more series or classes of preferred stock fixing the relative rights, priorities, preferences, qualifications, limitations and restrictions for each such series or class. Different series of preferred stock may differ with respect to voting rights, dividend rates, conversion rights, redemption provisions, amounts payable on liquidation, sinking fund provisions and other material matters. Such preferred stock may rank senior to our common stock for the payment of dividends and the distribution of assets on liquidation and the terms may include those which limits the payment of dividends on our common stock while such preferred stock is outstanding.
Warrants and Options
We currently have outstanding stock purchase warrants to purchase an aggregate of 5,763,610 shares of our common stock at exercise prices ranging from $0.30 to $3.89 per share with a weighted average price of $0.51 per share and having expiration dates ranging from October 2020 to January 2029. We currently have no outstanding stock options.
Overview. The following summary of certain terms and provisions of the warrants offered hereby is not complete and is subject to, and qualified in its entirety by, the provisions of the warrant agent agreement between us the Warrant Agent, and the form of warrant, both of which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the warrant agent agreement, including the annexes thereto, and form of warrant.
The warrants issued in this offering entitle the registered holder to purchase one share of our common stock at a price equal to $[●] per share (based on an assumed offering price of $[●] per Unit), subject to adjustment as discussed below, immediately following the issuance of such warrant and terminating at [●] p.m., New York City time, five years after the closing of this offering. As described below, we have applied to list the warrants on the Exchange under the symbol “PETVW.”
The exercise price and number of shares of common stock issuable upon exercise of the warrants may be adjusted in certain circumstances, including in the event of a stock dividend or recapitalization, reorganization, merger or consolidation. However, the warrants will not be adjusted for issuances of common stock at prices below its exercise price.
Exercisability. The warrants are exercisable at any time after their original issuance and at any time up to the date that is five (5) years after their original issuance. The warrants may be exercised upon surrender of the warrant certificate on or prior to the expiration date at the offices of the Warrant Agent, with the exercise form on the reverse side of the warrant certificate completed and executed as indicated, accompanied by full payment of the exercise price, by bank check payable to us, for the number of warrants being exercised. Under the terms of the Warrant Agreement, we must use our reasonable best efforts to maintain the effectiveness of the registration statement and current prospectus relating to common stock issuable upon exercise of the warrants until the expiration of the warrants. If we fail to maintain the effectiveness of the registration statement and current prospectus relating to the common stock issuable upon exercise of the warrants, the holders of the warrants shall have the right to (i) rescind their election to exercise the warrants or (ii) exercise the warrants via a cashless exercise feature provided for in the warrants, until such time as there is an effective registration statement and current prospectus.
Exercise Limitation. A holder may not exercise any portion of a warrant to the extent that the holder, together with its affiliates and any other person or entity acting as a group, would own more than 4.99% of the outstanding common stock after exercise, as such percentage ownership is determined in accordance with the terms of the warrant, except that upon prior notice from the holder to us, the holder may waive such limitation up to a percentage not in excess of 9.99%.
| 56 |
Exercise Price. The exercise price per whole share of common stock purchasable upon exercise of the warrants is $[●] per share (based on an assumed public offering price of $[●] per Unit) or 100% of public offering price of the common stock. The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications or similar events affecting our common stock and also upon any distributions of assets, including cash, stock or other property to our stockholders.
Fractional Shares. No fractional shares of common stock will be issued upon exercise of the warrants. If, upon exercise of the warrant, a holder would be entitled to receive a fractional interest in a share, we will, upon exercise, pay a cash adjustment in respect of such fraction in an amount equal to such fraction multiplied by the exercise price.
Transferability. Subject to applicable laws, the warrants may be offered for sale, sold, transferred or assigned without our consent.
Exchange Listing. We have applied to list our warrants on the Exchange under the symbol “PETVW.” No assurance can be given that our listing application will be approved.
Warrant Agent; Global Certificate. The warrants will be issued in registered form under a warrant agent agreement between the Warrant Agent and us. The warrants shall initially be represented only by one or more global warrants deposited with the Warrant Agent, as custodian on behalf of The Depository Trust Company (DTC) and registered in the name of Cede & Co., a nominee of DTC, or as otherwise directed by DTC.
Fundamental Transactions. In the event of a fundamental transaction, as described in the warrants and generally including any reorganization, recapitalization or reclassification of our common stock, the sale, transfer or other disposition of all or substantially all of our properties or assets, our consolidation or merger with or into another person, the acquisition of more than 50% of our outstanding common stock, or any person or group becoming the beneficial owner of 50% of the voting power represented by our outstanding common stock, the holders of the warrants will be entitled to receive the kind and amount of securities, cash or other property that the holders would have received had they exercised the warrants immediately prior to such fundamental transaction.
Rights as a Stockholder. The warrant holders do not have the rights or privileges of holders of common stock or any voting rights until they exercise their warrants and receive shares of common stock. After the issuance of shares of common stock upon exercise of the warrants, each holder will be entitled to one vote for each share held of record on all matters to be voted on by stockholders.
Governing Law. The warrants and the warrant agent agreement are governed by New York law.
Anti-takeover Effects of Nevada Law
The following paragraphs summarize certain provisions of Nevada law and our amended and restated articles of incorporation, as amended, and bylaws. The summary does not purport to be complete and is subject to and qualified in its entirety by reference to Nevada law and to our amended and restated articles of incorporation, as amended, and bylaws, as amended, copies of which are on file with the SEC as exhibits to reports previously filed by us. See “Where You Can Find More Information.”
General
Certain provisions of our articles of incorporation, as amended, and bylaws and Nevada law could make our acquisition by a third party, a change in our incumbent management, or a similar change in control more difficult, including:
| ● | an acquisition of us by means of a tender or exchange offer; |
| ● | an acquisition of us by means of a proxy contest or otherwise; or |
| ● | the removal of a majority or all of our incumbent officers and directors. |
| 57 |
These provisions, which are summarized below, are likely to discourage certain types of coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that these provisions help to protect our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us, and that this benefit outweighs the potential disadvantages of discouraging such a proposal because our ability to negotiate with the proponent could result in an improvement of the terms of the proposal. The existence of these provisions which are described below could limit the price that investors might otherwise pay in the future for our securities.
Articles of Incorporation and Bylaws
Authorized But Unissued Capital Stock. We have shares of common stock and preferred stock available for future issuance without stockholder approval, subject to any limitations imposed by the listing standards of any securities exchange on which our stock may be listed. We may utilize these additional shares for a variety of corporate purposes, including for future public offerings to raise additional capital or facilitate corporate acquisitions or for payment as a dividend on our capital stock. The existence of unissued and unreserved common stock and preferred stock may enable our board of directors to issue shares to persons friendly to current management or to issue preferred stock with terms that could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a controlling interest in our company by means of a merger, tender offer, proxy contest, or otherwise. In addition, if we issue preferred stock, the issuance could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation.
Blank Check Preferred Stock. Our board of directors, without stockholder approval, has the authority under our amended and restated articles of incorporation, as amended, to issue preferred stock with rights superior to the rights of the holders of common stock. As a result, preferred stock could be issued quickly and easily, could impair the rights of holders of common stock, and could be issued with terms calculated to delay or prevent a change in control or make removal of management more difficult.
Election of Directors. Our bylaws provide that a majority of directors then in office may fill any vacancy occurring on our board of directors, even though less than a quorum may then be in office. These provisions may discourage a third party from voting to remove incumbent directors and simultaneously gaining control of our board of directors by filling the vacancies created by that removal with its own nominees.
Business Combinations
The “business combination” provisions of Sections 78.411 to 78.444, inclusive, of the Nevada Revised Statutes, or NRS, generally prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder for a period of two years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the board of directors prior to the date the interested stockholder obtained such status or the combination is approved by the board of directors and thereafter is approved at a meeting of the stockholders by the affirmative vote of stockholders representing at least 60% of the outstanding voting power held by disinterested stockholders, and extends beyond the expiration of the two-year period, unless:
| ● | the combination was approved by the board of directors prior to the person becoming an interested stockholder or the transaction by which the person first became an interested stockholder was approved by the board of directors before the person became an interested stockholder or the combination is later approved by a majority of the voting power held by disinterested stockholders, or | |
● |
if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the two years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher. |
| 58 |
A “combination” is generally defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer, or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, (c) 10% or more of the earning power or net income of the corporation, and (d) certain other transactions with an interested stockholder or an affiliate or associate of an interested stockholder.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within two years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
Control Share Acquisitions
The “control share” provisions of Sections 78.378 to 78.3793, inclusive, of the NRS apply to “issuing corporations” that are Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents, and that conduct business directly or indirectly in Nevada. The control share statute prohibits an acquirer, under certain circumstances, from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Generally, once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control shares” and such control shares are deprived of the right to vote until disinterested stockholders restore the right. These provisions also provide that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures established for dissenters’ rights.
A corporation may elect to not be governed by, or “opt out” of, the control share provisions by making an election in its articles of incorporation or bylaws, provided that the opt-out election must be in place on the 10th day following the date an acquiring person has acquired a controlling interest, that is, crossing any of the three thresholds described above. We have not opted out of the control share statutes, and will be subject to these statutes if we are an “issuing corporation” as defined in such statutes.
The effect of the Nevada control share statutes is that the acquiring person, and those acting in association with the acquiring person, will obtain only such voting rights in the control shares as are conferred by a resolution of the stockholders at an annual or special meeting. The Nevada control share law, if applicable, could have the effect of discouraging takeovers of our company.
Limitations of Liability and Indemnification of Officers and Directors
Our Articles of Incorporation and Bylaws limit the liability of directors to the fullest extent permitted by Nevada law. In addition, our Articles of Incorporation and Bylaws provide that we will indemnify our directors and officers to the fullest extent permitted by law.
Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities arising under the Securities Act may be permitted for directors, officers, or controlling persons pursuant to the provisions described in the preceding paragraph, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Transfer Agent
The transfer agent and registrar for our common stock is Equity Stock Transfer, LLC, 237 W. 37th Street, Suite 601, New York, NY 10018.
| 59 |
SHARES ELIGIBLE FOR FUTURE SALE
Future sales of substantial amounts of our common stock in the public market, including shares issued upon the exercise of outstanding options or warrants, or upon debt conversion, or the anticipation of these sales, could adversely affect market prices prevailing from time to time and could impair our ability to raise capital through sales of equity securities.
Upon completion of this offering we estimate that we will have ____________ outstanding shares of our common stock, calculated as of September 30, 2020, assuming no further exercise of outstanding warrants, and no sale of shares reserved for the underwriter for over-allotment allocation, if any.
Sale of Restricted Securities
The shares of our common stock and warrants sold pursuant to this offering will be registered under the Securities Act or 1933, as amended, and therefore freely transferable, except for our affiliates. Our affiliates will be deemed to own “control” securities that are not registered for resale under the registration statement covering this prospectus. Individuals who may be considered our affiliates after the offering include individuals who control, are controlled by or are under common control with us, as those terms generally are interpreted for federal securities law purposes. These individuals may include some or all of our directors and executive officers. Individuals who are our affiliates are not permitted to resell their shares of our common stock unless such shares are separately registered under an effective registration statement under the Securities Act of 1933, as amended, or an exemption from the registration requirements of the Securities Act of 1933, as amended, is available, such as Rule 144.
Rule 144
In general, under Rule 144 as currently in effect, a person (or persons whose shares are aggregated), including an affiliate, who beneficially owns “restricted securities” (i.e. securities that are not registered by an effective registration statement) of a “reporting company” may not sell these securities until the person has beneficially owned them for at least six months. Thereafter, affiliates may not sell within any three-month period a number of shares in excess of the greater of: (i) 1% of the then outstanding shares of Common Stock as shown by the most recent report or statement published by the issuer; and (ii) the average weekly reported trading volume in such securities during the four preceding calendar weeks.
Sales under Rule 144 by our affiliates will also be subject to restrictions relating to manner of sale, notice and the availability of current public information about us and may be affected only through unsolicited brokers’ transactions.
Persons not deemed to be affiliates who have beneficially owned “restricted securities” for at least six months but for less than one year may sell these securities, provided that current public information about the Company is “available,” which means that, on the date of sale, we have been subject to the reporting requirements of the Exchange Act for at least 90 days and are current in our Exchange Act filings. After beneficially owning “restricted securities” for one year, our non-affiliates may engage in unlimited re-sales of such securities.
Shares received by our affiliates in the distribution or upon exercise of stock options or upon vesting of other equity-linked awards may be “controlled securities” rather than “restricted securities.” “Controlled securities” are subject to the same volume limitations as “restricted securities” but are not subject to holding period requirements.
| 60 |
We have entered into an underwriting agreement with ThinkEquity with respect to the Units subject to this offering. Subject to certain conditions, we have agreed to sell such underwriter such Units at a price which is ____% less than the public offering price for the Units. Such underwriter is offering the Units subject to its acceptance of the Units from us and subject to prior sale. The underwriting agreement provides that the obligation of such underwriter to pay for and accept delivery of the Units offered by this prospectus is subject to the approval of certain legal matters by its legal counsel and certain other conditions. Such underwriter is obligated to take and pay for all of the Units if any of the Units are taken. Such underwriter is not, however, required to take or pay for Units covered by the over-allotment option described below.
Advisory Services
As of May 18, 2020, we engaged ThinkEquity to act as our financial advisor with respect to our conducting an offering of our securities. Under the terms of such engagement we agreed to pay to ThinkEquity an engagement retainer fee of $10,000 and to reimburse ThinkEquity for travel expenses and other out-of-pocket expenditures. As of September 30, 2020, we were obligated to pay ThinkEquity $0 for such services and also for reimbursement of expenses of which $0 remains payable.
Over-Allotment Option
We have granted to the underwriter an option, exercisable for 45 days from the date of this prospectus to purchase up to (i) ___ additional shares of common stock and/or (ii) additional warrants to purchase shares of common stock at a price of $ per warrant (___% of the common stock and warrants such underwriter has agreed to take and pay for), in any combination, to cover over-allotments, if any, of the Units offered by this prospectus.
Discount, Commissions and Expenses
ThinkEquity has advised us that it will offer the common stock and warrants to the public at the price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $__ per Unit. Such underwriter may allow, and certain dealers may reallow, a discount from the concession not in excess of $ __ per Unit to certain brokers and dealers. After this offering, the public offering price, concession and reallowance to dealers may be changed by such underwriter. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The Units offered by such underwriter are subject to receipt and acceptance by it and is subject to such underwriter’s right to reject any order in whole or in part. The underwriter has informed us that it does not intend to confirm sales to any accounts over which it exercises discretionary authority.
We have agreed to sell the Units to ThinkEquity for $_ per Unit, which is ___% less than the public offering price for the Units. We have also agreed reimburse such underwriter for certain out-of-pocket expenses, including “road show” expenses, out of pocket due diligence expense and fees of such underwriter’s counsel (not to exceed $200,000 in the aggregate).
Underwriters Warrants
We have agreed to issue to the underwriters warrants to purchase up to a total of [●] shares of common stock (5% of the shares of common stock sold in this offering). The warrants are exercisable at [●] per share (125% of the public offering price) commencing on a date which is six (6) months from the effective date of the offering under this prospectus and expiring on a date which is no more than five (5) years from the effective date of the offering in compliance with FINRA Rule 5110(f)(2)(G). The warrants have been deemed compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The underwriters (or their permitted assignees under the Rule) will not sell, transfer, assign, pledge, or hypothecate these warrants or the securities underlying these warrants, nor will it engage in any hedging, short sale, derivative, put, or call transaction that would result in the effective economic disposition of the warrants or the underlying securities for a period of 180 days from effectiveness. In addition, the warrants provide for registration rights upon request, in certain cases. We will bear all fees and expenses attendant to registering the securities issuable on exercise of the warrants other than underwriting commissions incurred and payable by the holders. The exercise price and number of shares issuable upon exercise of the warrants may be adjusted in certain circumstances including in the event of a stock dividend, extraordinary cash dividend or our recapitalization, reorganization, merger or consolidation.
| 61 |
Right of First Refusal
We have also granted the underwriter a right of first refusal to act as sole investment banker, sole book-runner and/or sole placement agent for any future public or private equity or debt offering, including equity-linked offerings, by us for the eighteen month period following the closing of the offering.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933, as amended, or the Securities Act, and liabilities arising from breaches of representations and warranties contained in the underwriting agreement, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
Lock-up Agreements
We, our officers, directors and certain of our stockholders have agreed, subject to limited exceptions, for a period of six months following the date of this prospectus, not to offer, sell, contract to sell, pledge, grant any option to purchase, make any short sale or otherwise dispose of, directly or indirectly any shares of our common stock or any securities convertible into or exchangeable such common stock either owned as of the date of this prospectus or thereafter acquired without the prior written consent of ThinkEquity. Such underwriter may, in its sole discretion and at any time or from time to time before the termination of the lock-up period, without notice, release all or any portion of the securities subject to lock-up agreements.
Price Stabilization, Short Positions and Penalty Bids
In connection with the offering ThinkEquity may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions and penalty bids in accordance with Regulation M under the Exchange Act:
| ● | Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. | |
| ● | Over-allotment involves sales by such underwriter of shares in excess of the number such underwriter is obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of securities over-allotted by the underwriter is not greater than the number of securities that it may purchase in the over-allotment option. In a naked short position, the number of securities involved is greater than the number of securities in the over-allotment option. The underwriter may close out any covered short position by either exercising their over-allotment option and/or purchasing securities in the open market. | |
| ● | Syndicate covering transactions involve purchases of securities in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of securities to close out the short position, the underwriter will consider, among other things, the price of securities available for purchase in the open market as compared to the price at which it may purchase securities through the over-allotment option. If the underwriter sells more securities than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying securities in the open market. A naked short position is more likely to be created if the underwriter is concerned that there could be downward pressure on the price of the securities in the open market after pricing that could adversely affect investors who purchase in the offering. | |
| ● | Penalty bids permit the underwriter to reclaim a selling concession from a syndicate member when the securities originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
| 62 |
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our common stock and warrants or preventing or retarding a decline in the market price of the common stock or warrants. As a result, the price of our common stock and warrants may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriter make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our common stock. In addition, neither we nor the underwriter may make any representations that the underwriter will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
Passive Market Making
In connection with this offering, the underwriter and any selling group members may engage in passive market making transactions in our common stock and warrants on the Exchange in accordance with Rule 103 of Regulation M under the Securities Exchange Act of 1934, as amended, during a period before the commencement of offers or sales of our common stock and warrants and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. If all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded.
Electronic Distribution
This prospectus in electronic format may be made available on websites or through other online services maintained by ThinkEquity. Other than this prospectus in electronic format, the information on any selling group member’s website and any information contained in any other website maintained by such underwriter, selling group member or their affiliates is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or such underwriter in its capacity as underwriter, and should not be relied upon by investors.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to this offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
| 63 |
European Economic Area - Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| ● | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; | |
| ● | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); | |
| ● | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or | |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
| 64 |
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), or ISA, nor have such securities been registered for sale in Israel. The securities may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with this offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Societ-$$-Aga e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
| 65 |
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company.
| 66 |
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriter is not required to comply with the disclosure requirements of NI33-105 regarding underwriter conflicts of interest in connection with this offering.
Certain legal matters in connection with the offering and the validity of the common stock offered by this Prospectus was passed upon by Fox and Rothschild, LLP, Minneapolis, Minnesota. Certain legal matters in connection with the offering was passed upon for the underwriter by Pryor Cashman LLP, New York, New York.
The consolidated financial statements as of March 31, 2020 and 2019 and for the years then ended, included in this Prospectus, have been audited by Assurance Dimensions Inc., an independent registered public accounting firm, as stated in their report (which expresses an unqualified opinion and includes an explanatory paragraph relating to the substantial doubt about the Company’s ability to continue as a going concern) appearing herein, and elsewhere in the registration statement, and have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We are required to comply with the information and periodic reporting requirements of the Securities Exchange Act of 1934, and, in accordance with the requirements of such Act, will file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the regional offices, public reference facilities and internet site of the SEC referred to below.
We filed with the SEC a registration statement on Form S-1 under the Securities Act for the Units to be sold in this offering. This prospectus does not contain all of the information in the registration statement and the exhibits and schedules that were filed with the registration statement. For further information with respect to the Units and us, we refer you to the registration statement and the exhibits and schedules that were filed with the registration statement. Statements made in this prospectus regarding the contents of any contract, agreement or other document that is filed as an exhibit to the registration statement are not necessarily complete, and we refer you to the full text of the contract or other document filed as an exhibit to the registration statement.
| 67 |
A copy of the registration statement and the exhibits and schedules that were filed with the registration statement may be inspected without charge at the public reference facilities maintained by the SEC, 100 F Street, Washington, DC 20549. Copies of all or any part of the registration statement may be obtained from the SEC upon payment of the prescribed fee. Information regarding the operation of the public reference rooms may be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements and other information regarding registrants that file electronically with the SEC. The address of the site is https://www.sec.gov.
You can find more information about us on our website, which is located at https://www.petvivo.com .
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Under the Nevada Corporation Code and our Articles of Incorporation, as amended, our directors will have no personal liability to us or our stockholders for monetary damages incurred as the result of the breach or alleged breach by a director of his “duty of care.” This provision does not apply to the directors’ (i) acts or omissions that involve intentional misconduct or a knowing and culpable violation of law, (ii) acts or omissions that a director believes to be contrary to the best interests of the corporation or its stockholders or that involve the absence of good faith on the part of the director, (iii) approval of any transaction from which a director derives an improper personal benefit, (iv) acts or omissions that show a reckless disregard for the director’s duty to the corporation or its stockholders in circumstances in which the director was aware, or should have been aware, in the ordinary course of performing a director’s duties, of a risk of serious injury to the corporation or its stockholders, (v) acts or omissions that constituted an unexcused pattern of inattention that amounts to an abdication of the director’s duty to the corporation or its stockholders, or (vi) approval of an unlawful dividend, distribution, stock repurchase or redemption. This provision would generally absolve directors of personal liability for negligence in the performance of duties, including gross negligence.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
| 68 |
INDEX TO FINANCIAL STATEMENTS
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors
PetVivo Holdings, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of PetVivo Holdings, Inc. (the Company) as of March 31, 2020 and 2019, the related consolidated statements of operations, changes in stockholders’ deficit and cash flows for each of the years in the two-year period ended March 31, 2020 and 2019 and the related notes (collectively referred to as the consolidated financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of March 31, 2020 and 2019 and the results of its operations and its cash flows for the years ended March 31, 2020 and 2019 in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 12 to the financial statements, the Company had a net loss and cash used from operations of approximately $2,082,734 and $496,589, respectively, for the year ended of March 31, 2020 and a working capital deficit of approximately $950,700 as of March 31, 2020. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regards to these matters are also described in Note 12. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the auditing standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Very truly yours,
| /s/ Assurance Dimensions | |
We have served as the Company’s auditor since 2019 |
|
Margate, Florida |
|
June 29, 2020 |
| F-2 |
CONSOLIDATED BALANCE SHEETS
| March 31, 2020 | March 31, 2019 | |||||||
| Assets: | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 888 | $ | 6,460 | ||||
| Accounts receivable | 1,000 | - | ||||||
| Equity sale proceeds receivable | 52,000 | - | ||||||
| Restricted cash | 9,694 | - | ||||||
| Inventory, net | - | 12,495 | ||||||
| Employee advance | - | 2,500 | ||||||
| Prepaids | 132,023 | 34,327 | ||||||
| Investments – equity securities | 1,500 | - | ||||||
| Total Current Assets | 197,105 | 55,782 | ||||||
| Property and Equipment: | ||||||||
| Property & equipment | 221,493 | 149,802 | ||||||
| Less: accumulated depreciation | (111,586 | ) | (112,453 | ) | ||||
| Total Fixed Assets | 109,907 | 37,349 | ||||||
| Other Assets: | ||||||||
| Trademark and patents-net | 58,611 | 589,817 | ||||||
| Operating lease right-of-use asset | 148,693 | - | ||||||
| Security deposit | 8,201 | 8,201 | ||||||
| Total Other Assets | 215,505 | 598,018 | ||||||
| Total Assets | $ | 522,517 | $ | 691,149 | ||||
| Liabilities and Stockholders’ Equity (Deficit) | ||||||||
| Current Liabilities | ||||||||
| Accounts payable & accrued expenses | $ | 794,057 | $ | 854,990 | ||||
| Accrued expenses - related party | 252,607 | 576,393 | ||||||
| Operating lease liability – short term | 24,791 | - | ||||||
| Notes payable and accrued interest | 15,095 | 18,831 | ||||||
| Notes payable and accrued interest - related party | 61,255 | 85,752 | ||||||
| Total Current Liabilities | 1,147,805 | 1,535,966 | ||||||
| Other Liabilities | ||||||||
| Convertible notes and accrued interest payable | 286,981 | - | ||||||
| Operating lease liability (net of current) | 123,901 | - | ||||||
| Total Other Liabilities | 410,882 | - | ||||||
| Total Liabilities | $ | 1,558,687 | $ | 1,535,966 | ||||
| Commitments and Contingencies (Note 14) | ||||||||
| Stockholders’ Equity (Deficit): | ||||||||
| Preferred stock, par value $0.001, 20,000,000 shares authorized, issued 0 and 0 shares outstanding at March 31, 2020 and March 31, 2019 | ||||||||
| Common stock, par value $0.001, 225,000,000 shares authorized, issued 22,911,857 and 19,867,200 shares outstanding at March 31, 2020 and March 31, 2019 | 22,911 | 22,074 | ||||||
| Common stock to be issued | 52,000 | 86,333 | ||||||
| Additional Paid-In Capital | 53,477,565 | 51,552,688 | ||||||
| Accumulated Deficit | (54,588,646 | ) | (52,505,912 | ) | ||||
| Total Stockholders’ Deficit | (1,036,170 | ) | (844,817 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 522,517 | $ | 691,149 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended | Year Ended | |||||||
| March 31, 2020 | March 31, 2019 | |||||||
| Revenues | $ | 3,588 | $ | - | ||||
| Inventory Write-Down | 19,710 | 77,936 | ||||||
| Total Cost of Sales | 19,710 | 77,936 | ||||||
| Gross Profit | (16,122 | ) | (77,936 | ) | ||||
| Operating Expenses: | ||||||||
| Research and Development | 12,672 | 200,982 | ||||||
| Intangible Impairment | 31,272 | 103,800 | ||||||
| Sales and Marketing | 171,509 | 38,348 | ||||||
| General and Administration: | ||||||||
| Depreciation and Amortization | 559,544 | 646,921 | ||||||
| Other General and Administrative | 1,225,013 | 3,604,821 | ||||||
| Total General and Administration | 1,784,557 | 4,251,742 | ||||||
| Total Operating Expenses | 2,000,010 | 4,594,872 | ||||||
| Operating Loss | (2,016,132 | ) | (4,672,808 | ) | ||||
| Other Income (Expense) | ||||||||
| Gain on Settlements | 47,710 | - | ||||||
| Loss on Sale of Assets | (389 | ) | - | |||||
| Loss on Extinguishment of Debt | (81,738 | ) | - | |||||
| Interest Expense | (32,185 | ) | (84,950 | ) | ||||
| Total Other Income (Expense) | (66,602 | ) | (84,950 | ) | ||||
| Net Loss before taxes | $ | (2,082,734 | ) | $ | (4,757,758 | ) | ||
| Income Tax Provision | - | - | ||||||
| Net Loss | (2,082,734 | ) | (4,757,758 | ) | ||||
| Net Loss Per Share – Basic And Diluted | $ | (0.10 | ) | $ | (0.26 | ) | ||
| Weighted Average Common Shares Outstanding - Basic And Diluted | 21,222,359 | 18,451,797 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY (DEFICIT)
| Additional | Common | |||||||||||||||||||||||
| Common Stock | Paid-in | Accumulated | Stock to be | |||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Issued | Total | |||||||||||||||||||
| Balance March 31, 2018 | 16,451,167 | $ | 18,279 | $ | 47,257,557 | $ | (47,748,154 | ) | $ | 608,966 | $ | 136,648 | ||||||||||||
| Common stock issued | 904,758 | 1,005 | 607,961 | - | (608,966 | ) | - | |||||||||||||||||
| Common stock returned | - | - | (177,600 | ) | - | - | (177,600 | ) | ||||||||||||||||
| Common stock sold | 999,923 | 1,111 | 398,754 | - | - | 399,865 | ||||||||||||||||||
| Stock-based compensation | 24,384 | 27 | 1,556,509 | - | 86,333 | 1,642,869 | ||||||||||||||||||
| Stock granted for debt conversion | 763,921 | 849 | 386,863 | - | - | 387,712 | ||||||||||||||||||
| Common stock issued to replace shares to officer | 723,047 | 803 | 1,445,290 | - | - | 1,446,093 | ||||||||||||||||||
| Common stock issued by officer | - | - | 77,354 | - | - | 77,354 | ||||||||||||||||||
| Net loss | - | - | - | (4,757,758 | ) | - | (4,757,758 | ) | ||||||||||||||||
| Balance March 31, 2019 | 19,867,200 | $ | 22,074 | $ | 51,552,688 | $ | (52,505,912 | ) | $ | 86,333 | $ | (844,817 | ) | |||||||||||
| Adjustment for 9-for-10 reverse stock split | 254 | (2,206 | ) | 2,206 | - | - | - | |||||||||||||||||
| Common stock issued | 77,700 | 78 | 86,255 | - | (86,333 | ) | - | |||||||||||||||||
| Common stock sold | 1,006,000 | 1,006 | 303,385 | - | 34,709 | 339,100 | ||||||||||||||||||
| Warrants sold | - | - | 34,609 | - | 17,291 | 51,900 | ||||||||||||||||||
| Warrant conversions | 124,537 | 124 | (124 | ) | - | - | - | |||||||||||||||||
| Stock-based compensation | 540,300 | 540 | 962,138 | - | - | 962,678 | ||||||||||||||||||
| Stock granted for settlement | 1,295,866 | 1,295 | 536,408 | - | - | 537,703 | ||||||||||||||||||
| Net loss | - | - | - | (2,082,734 | ) | - | (2,082,734 | ) | ||||||||||||||||
| Balance March 31, 2020 | 22,911,857 | $ | 22,911 | $ | 53,477,565 | $ | (54,588,646 | ) | $ | 52,000 | $ | (1,036,170 | ) | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
Shares retroactively restated for 9-for-10 reverse stock split in November of 2019
| F-5 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the year ended | For the year ended | |||||||
| March 31, 2020 | March 31, 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net Loss For The Year | $ | (2,082,734 | ) | $ | (4,757,758 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: | ||||||||
| Stock-based compensation | 863,012 | 1,642,869 | ||||||
| Depreciation and amortization | 559,544 | 646,921 | ||||||
| Loss on debt extinguishment | 81,738 | - | ||||||
| Intangible impairment | 31,272 | 103,800 | ||||||
| Loss on sale of assets | 389 | - | ||||||
| Gain on settlement | (47,710 | ) | - | |||||
| Common stock issued to replace shares to officer | - | 1,446,093 | ||||||
| Common stock issued by officer on behalf of PetVivo | - | 77,354 | ||||||
| Beneficial conversion feature | - | 66,248 | ||||||
| Write-off of accounts receivable | - | 163 | ||||||
| Common stock returned | - | (177,600 | ) | |||||
| Changes in Operating Assets and Liabilities | ||||||||
| Decrease in inventory | 12,495 | 13,059 | ||||||
| Increase in prepaid expenses and employee advances | 4,470 | (14,379 | ) | |||||
| Increase in receivables | (1,000 | ) | - | |||||
| Interest accrued on convertible notes payable | 25,518 | - | ||||||
| Interest accrued on notes payable - related party | 5,504 | 9,201 | ||||||
| Interest accrued on notes payable | 820 | 8,593 | ||||||
| Increase (Decrease) in accounts payable and accrued expense | (4,232 | ) | 182,482 | |||||
| Increase (Decrease) in accrued expenses - related party | 54,325 | 16,509 | ||||||
| Net Cash Used In Operating Activities | (496,589 | ) | (736,445 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Decrease in security deposit | - | 1,999 | ||||||
| Increase in investments - equity securities | (1,500 | ) | - | |||||
| Proceeds from sale of equipment | 12,481 | - | ||||||
| Purchase of equipment | (32,791 | ) | (27,119 | ) | ||||
| Increase in patents and trademarks | (43,386 | ) | (78,687 | ) | ||||
| Net Cash Used in Investing Activities | (65,196 | ) | (103,807 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from stock and warrants sale | 339,000 | 399,865 | ||||||
| Proceeds from convertible notes | 280,000 | - | ||||||
| Proceeds from notes | 15,000 | 215,000 | ||||||
| Proceeds from notes - related parties | - | 70,000 | ||||||
| Repayments of convertible notes | (18,537 | ) | - | |||||
| Repayments of notes payable | (19,556 | ) | (50,482 | ) | ||||
| Repayments of notes payable - related party | (30,000 | ) | (25,006 | ) | ||||
| Net Cash Provided by Financing Activities | 565,907 | 609,377 | ||||||
| Net Increase (Decrease) in Cash and Restricted Cash | 4,122 | (230,875 | ) | |||||
| Cash and Restricted Cash at Beginning of Year | 6,460 | 237,335 | ||||||
| Cash and Restricted Cash at End of Year | $ | 10,582 | $ | 6,460 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash Paid During The Year For: | ||||||||
| Interest | $ | 23,805 | $ | 20,181 | ||||
| Income taxes paid | $ | - | $ | - | ||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH INVESTING AND FINANCING ACTIVITIES: | ||||||||
| Notes Payable interest converted into common stock | $ | - | $ | 4,280 | ||||
| Notes Payable interest converted into common stock - related parties | $ | - | $ | 1,722 | ||||
| Proceeds not received at balance sheet date pursuant to Stock and Warrant sales | $ | 52,000 | $ | - | ||||
| Leasehold improvements included in accounts payable | $ | 67,361 | $ | - | ||||
| Warrants converted | $ | 124 | $ | - | ||||
| Prepaid stock-based compensation for services | $ | 99,664 | $ | - | ||||
| Stock granted for debt conversions | $ | - | $ | 387,712 | ||||
| Stock granted pursuant to settlements | $ | 537,703 | $ | - | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
Notes to Consolidated Financial Statements
March 31, 2020
NOTE 1 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND ORGANIZATION
(A) Organization and Description
The Company is in the business of licensing and commercializing our proprietary medical devices and biomaterials for the treatment of afflictions and diseases in animals, initially for dogs and horses. The Company’s operations are conducted from its headquarter facilities in suburban Minneapolis, Minnesota.
(B) Basis of Presentation
PetVivo Holdings, Inc. (the “Company”) was incorporated in Nevada under a former name in 2009 and entered its current business in 2014 through a stock exchange reverse merger with PetVivo, Inc., a Minnesota corporation. This merger resulted in Minnesota PetVivo becoming a wholly-owned subsidiary of the Company. In April 2017, the Company acquired another Minnesota corporation, Gel-Del Technologies, Inc., through a statutory merger, which is also a wholly-owned subsidiary of the Company.
In November 2019, the Company effected a 9-for-10 reverse split of our authorized and outstanding shares of common stock. Pursuant to this reverse stock split, each ten (10) shares of PetVivo’s outstanding common stock, $.001 par value per share, was combined and converted into nine (9) post-split outstanding shares of common stock, $.001 par value per share; 24,974,518 pre-reverse-split shares of common stock were combined during the 9-for-10 reverse split into 22,477,320 shares of post-reverse-split shares of common stock with 254 shares being issued for fractional shares through the date of the balance sheet. Accordingly, all references to number of shares of common stock and per share data have been adjusted retroactively where applicable to account for this reverse split.
(C) Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its two wholly-owned Minnesota corporations, Gel-Del Technologies, Inc. and PetVivo, Inc. All intercompany accounts have been eliminated upon consolidation.
(D) Use of Estimates
In preparing financial statements in conformity with generally accepted accounting principles, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and revenues and expenses during the reported period. Actual results could differ from those estimates. Significant estimates include estimated useful lives and potential impairment of property and equipment, estimate of fair value of share-based payments and derivative instruments and recorded debt discount, valuation of deferred tax assets and valuation of in-kind contribution of services and interest.
(E) Cash and Cash Equivalents
The Company considers all highly-liquid, temporary cash investments with an original maturity of three months or less to be cash equivalents. At March 31, 2020, and March 31, 2019 the Company had no cash equivalents.
(F) Concentration-Risk
The Company maintains its cash with various financial institutions, which at times may exceed federally insured limits. As of March 31, 2020, the Company did not have any cash balances in excess of the federally insured limits.
| F-7 |
(H) Property & Equipment
Property and equipment are recorded at cost. Expenditures for major additions and betterments are capitalized. Maintenance and repairs are charged to operations as incurred. Depreciation is computed by the straight-line method (after taking into account their respective estimated residual values) over the assets estimated useful life of (3) years for equipment, (5) years for automobile, and (7) years for furniture and fixtures.
(I) Patents and Trademarks
The Company capitalizes direct costs for the maintenance and advancement of their patents and trademarks and amortizes these costs over the lesser of a useful life of 60 months or the life of the patent. We evaluate the recoverability of intangible assets periodically by taking into account events or circumstances that may warrant revised estimates of useful lives or that indicate the asset may be impaired.
(J) Loss Per Share
Basic loss per share is computed by dividing net loss by weighted average number of shares of common stock outstanding during each period. Diluted loss per share is computed by dividing net loss by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during the period.
The Company has 4,901,119 warrants outstanding as of March 31, 2020, with varying exercise prices ranging from $3.89 to $.30/share. The weighted average exercise price for these warrants is $.53/share. These warrants are excluded from the weighted average number of shares because they are considered antidilutive.
The Company had 3,818,919 warrants outstanding as of March 31, 2019 with varying exercise prices ranging from $3.89 to $.33/share. The weighted average exercise price for these warrants was $.55/share. These warrants are excluded from the weighted average number of shares because they are considered antidilutive.
The Company uses the guidance in Accounting Standards Codification # 260 (“ASC 260”) to determine if-converted loss per share. ASC 260 states that convertible securities should be considered exercised at the later date of the first day of the reporting period’s quarter or the inception date of the debt instrument. Also, the if-converted method shall not be applied for the purposes of computing diluted EPS if the effect would be antidilutive.
At March 31, 2020, the Company had $280,000 in convertible notes and $6,981 in accrued interest outstanding, these notes mature in our fiscal quarter ended June 30, 2021; see Note 9 to these financial statements for more information on these convertible notes. If converted, the $286,981 in outstanding principal and accrued interest would convert into 397,359 shares of common stock at a rate of $.72 per share.
(K) Revenue Recognition
The Company will recognize revenue on arrangements in accordance with FASB ASC No. 606, “Revenue From Contracts With Customers”. Revenue is recognized upon transfer of control of promised products or services to customers in an amount that reflects the consideration we expect to receive in exchange for those products or services. The Company adopted the guidance on April 1, 2018 using the cumulative catch-up transition method. This change in accounting did not have any material effect on the Company’s financial statements.
(L) Research and Development
The Company expenses research and development costs as incurred.
(M) Fair Value of Financial Instruments
The Company applies the accounting guidance under FASB ASC 820-10, “Fair Value Measurements”, as well as certain related FASB staff positions. This guidance defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact business and considers assumptions that marketplace participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance.
| F-8 |
The guidance also establishes a fair value hierarchy for measurements of fair value as follows:
| ● | Level 1 - quoted market prices in active markets for identical assets or liabilities. | |
| ● | Level 2 - inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | |
| ● | Level 3 - unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company’s financial instruments consist of accounts receivable, accounts payable, accrued expenses, accrued expenses – related parties, notes payable and accrued interest, and notes payable and accrued interest - related party, and others. The carrying amount of the Company’s financial instruments approximates their fair value as of March 31, 2020 and March 31, 2019, due to the short-term nature of these instruments and the Company’s borrowing rate of interest.
In instances where the determination of the fair value measurement is based on inputs from different levels of the fair value hierarchy, the level in the fair value hierarchy within which the entire fair value measurement falls is based on the lowest level input that is significant to the fair value measurement in its entirety. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment and considers factors specific to the asset or liability. The valuation of the Company’s notes recorded at fair value is determined using Level 3 inputs, which consider (i) time value, (ii) current market and (iii) contractual prices.
The Company had no assets and liabilities measured at fair value on a recurring basis at March 31, 2020 and March 31, 2019.
(N) Stock-Based Compensation - Non-Employees
Equity Instruments Issued to Parties Other Than Employees for Acquiring Goods or Services
The Company accounts for equity instruments issued to parties other than employees for acquiring goods or services under guidance of Sub-topic 505-50 of the FASB Accounting Standards Codification (“Sub-topic 505-50”).
Pursuant to ASC Section 505-50-30, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If the Company is a newly formed corporation or shares of the Company are thinly traded the use of share prices established in the Company’s most recent private placement memorandum (“PPM”), or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
The fair value of share options and similar instruments is estimated on the date of grant using a Black-Scholes option-pricing valuation model. The ranges of assumptions for inputs are as follows:
| ● | Expected term of share options and similar instruments: Pursuant to Paragraph 718-10-50-2(f)(2)(i) of the FASB Accounting Standards Codification the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and holder’s expected exercise behavior into the fair value (or calculated value) of the instruments. The Company uses historical data to estimate holder’s expected exercise behavior. If the Company is a newly formed corporation or shares of the Company are thinly traded the contractual term of the share options and similar instruments is used as the expected term of share options and similar instruments as the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term. |
| F-9 |
| ● | Expected volatility of the entity’s shares and the method used to estimate it. Pursuant to ASC Paragraph 718-10-50-2(f)(2)(ii) a thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index. The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market. | |
| ● | Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected term of the share options and similar instruments. | |
| ● | Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the expected term of the share options and similar instruments. |
Pursuant to Paragraphs 505-50-25-8 and 505-50-25-9, an entity may grant fully vested, non-forfeitable equity instruments that are exercisable by the grantee only after a specified period of time if the terms of the agreement provide for earlier exercisability if the grantee achieves specified performance conditions. Any measured cost of the transaction shall be recognized in the same period(s) and in the same manner as if the entity had paid cash for the goods or services or used cash rebates as a sales discount instead of paying with, or using, the equity instruments. A recognized asset, expense, or sales discount shall not be reversed if a share option and similar instrument that the counterparty has the right to exercise expires unexercised.
(O) Income Taxes
The Company accounts for income taxes under Accounting Standards Codification (ASC) Topic 740. Deferred tax assets and liabilities are determined based upon differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
As required by ASC Topic 450, the Company recognizes the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority. At the adoption date, the Company applied ASC Topic 740 to all tax positions for which the statute of limitations remained open. As a result of the implementation of ASC Topic 740, the Company did not recognize any change in the liability for unrecognized tax benefits.
The Company is not currently under examination by any federal or state jurisdiction.
The Company’s policy is to record tax-related interest and penalties as a component of operating expenses.
(P) Inventory
Inventories are recorded in accordance with ASC 330 and are stated at the lower of cost or net realizable value. We account for inventories using the first in first out (FIFO) methodology and capitalize costs on a project basis as they occur. The current marketed shelf life of our Kush inventory is 3 years. However, management reserves the right to review and adjust this as necessary.
| F-10 |
(Q ) Recent Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. Topic 842 affects any entity that enters into a lease, with some specified scope exemptions. The guidance in this ASU supersedes Topic 840, Leases. The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from leases. A lessee should recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use (“ROU”) asset representing its right to use the underlying asset for the lease term. The Company adopted Topic 842 on April 1, 2019 and resulted in a right of use asset and liability of $154,917.
In January 2017, the FASB issued ASU No. 2017-04, “Intangibles and Other (Topic 350): Simplifying the Test for Goodwill Impairment”, which eliminates the requirement to calculate the implied fair value of goodwill, but rather requires an entity to record an impairment charge based on the excess of a reporting unit’s carrying value over its fair value. This amendment is effective for annual or interim goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted. We do not expect the adoption of this ASU to have a material effect on our consolidated financial statements.
In July 2018, the FASB issued ASU 2018-07, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting, an accounting standard update to improve non-employee share-based payment accounting. The accounting standard update more closely aligns the accounting for employee and non-employee share-based payments. The accounting standards update is effective as of the beginning of 2019 with early adoption permitted. We have elected to adopt this standard as of April 1, 2018, the beginning of our 2019 fiscal year, with the main reason for adoption being comparability between both employee and non-employee share-based payments. The adoption of this standard did not have any material effect on the Company’s financial statements or any component of stockholder’s equity.
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurements (Topic 820): Disclosure Framework Changes to the Disclosure Requirements for Fair Value Measurement. The amendments in this update modify the disclosure requirements on fair value measurements in Topic 820. The ASU is effective for the Registrants for fiscal years beginning after December 15, 2019, and interim periods therein. Early adoption is permitted. The Company is currently assessing the impact of this standard on their Financial Statements.
All other newly issued accounting pronouncements but not yet effective have been deemed either immaterial or not applicable.
NOTE 2 – INVENTORY
As of March 31, 2020 and March 31, 2019, respectively, the Company had approximately $50,000 and $78,000 of finished goods inventory; however, reserves of equal amounts for each respective period were taken because of the substantial doubt in the Company’s ability to utilize this inventory to obtain material sales, primarily due to (among other things) the fact the Company has not obtained controlled study data detailing the safe and effective use of Kush™ in dogs and horses.
As of March 31, 2019, all of the Company’s finished goods inventory were in quarantine due to a contamination issue. During the year ended March 31, 2020, the Company released some inventory for sale and sample to the public.
Total Inventory is broken out as follows:
| March 31, 2020 | March 31, 2019 | |||||||
| Finished Goods | $ | 50,357 | $ | 77,936 | ||||
| Reserve for Obsolete Inventory | (50,357 | ) | (77,936 | ) | ||||
| Work in Process | -0- | -0- | ||||||
| Manufacturing Supplies | -0- | 3,127 | ||||||
| Raw Materials | -0- | 9,368 | ||||||
| Total Inventory | $ | -0- | $ | 12,495 | ||||
NOTE 3 – INVESTMENTS – EQUITY SECURITIES
On June 28, 2019, the Company entered into a purchase agreement with a third-party to purchase 1,500,000 shares of Emerald Organic Products, Inc. (OTC Pink: “EMOR”) common stock for consideration of $1,500 in cash. The Company applied guidance from ASU No. 2016-01 Financial Instruments – Overall – Recognition and Measurement of Financial Assets and Financial Liabilities and ASC 820 to arrive at a fair value at March 31, 2020, of $1,500. The Company took into account many factors when determining the stock’s fair value including, but not limited to, the nature and duration of the restriction on the stock, the extent to which potential buyers would be limited by the restriction, and qualitative and quantitative factors specific to both the instrument and the issuer.
| F-11 |
NOTE 4 – PROPERTY AND EQUIPMENT
During fiscal years 2020 and 2019, depreciation expense was $16,224 and $8,342, respectively. During the year ended March 31, 2020, we recorded a loss on sale of assets in the amount of $389. The $389 loss on sale of assets was made up of the sale of assets with carrying books values totaling $12,870 sold for purchase prices totaling $12,481.
During the fiscal year ended March 31, 2020, the Company also built onto its Edina manufacturing facility to include: (i) proper HVAC equipment valued at $64,000; (ii) proper cleanroom structural environment and walls valued at $13,657; and (iii) proper electrical capabilities valued at $8,947.
The Company anticipates incurring approximately an additional $40,000 in facilities build-out expenses to obtain an operational manufacturing facility.
The components of property and equipment were as follows:
| As of March 31 | ||||||||
| 2020 | 2019 | |||||||
| Leasehold improvements | $ | 98,706 | $ | 4,602 | ||||
| Furniture and office equipment | 10,130 | 10,130 | ||||||
| Production equipment | 87,473 | 108,882 | ||||||
| R&D equipment | 25,184 | 26,188 | ||||||
| Total, at cost | 221,493 | 149,802 | ||||||
| Accumulated depreciation | (111,586 | ) | (112,453 | ) | ||||
| Total, net | $ | 109,907 | $ | 37,349 | ||||
NOTE 5 – INTANGIBLE ASSETS
The components of intangible assets, all of which are finite-lived, were as follows:
| As of March 31 | ||||||||
| 2020 | 2019 | |||||||
| Patents | $ | 3,822,542 | $ | 3,820,374 | ||||
| Trademarks | 25,023 | 22,829 | ||||||
| Total, at cost | 3,847,565 | 3,843,203 | ||||||
| Accumulated Amortization | (3,788,954 | ) | (3,253,386 | ) | ||||
| Total, net | $ | 58,611 | $ | 589,817 | ||||
During fiscal years 2020 and 2019, amortization expense was $543,320 and $638,579, respectively. The Company performed intangible impairment analyses throughout the year ended March 31, 2020 and concluded that approximately $31,000 (2019: $104,000) in patents needed to be impaired. We conducted these analyses pursuant to ASC 350 and ASC 360.
NOTE 6 – PREPAID EXPENSES
As of March 31, 2020, the Company had approximately $132,023 in prepaid expenses recorded on our balance sheet, respectively, as follows: i) approximately $100,000 in marketing services; ii) approximately $10,000 in annual OTC listing license; and iii) approximately $6,000 in insurance costs.
As of March 31, 2019, the Company had approximately $34,327 in prepaid expenses recorded on our balance sheet, respectively, as follows: i) approximately $10,000 in annual OTC listing license; ii) $2,000 in SEC filing service services; iii) $7,000 in insurance costs; and iv) $10,000 in legal services.
| F-12 |
NOTE 7 - RELATED PARTY NOTES PAYABLE
At March 31, 2020, the Company is obligated for a related party note payable and accrued interest in the total amount of $61,255 (2019: $85,752); the maturity date of this note is April 30, 2020. As of the date of this filing we are in default on this note. The related party note payable terms are accrual of interest at eight percent annually with payments of $3,100 per month, which are applied to interest first, then principal. The terms also include a stipulation that if the Company receives additional financing during any 24-month period from the date of the note in the amount greater than $3,500,000, the Company will immediately pay the officer the principal amount of the note along with all interest due.
During the year ended March 31, 2019, the Company entered into bridge note agreements with related parties totaling $70,000 in principal. Upon entering into these bridge note agreements, the note-holders were issued one warrant for every $2.00 in principal loaned to the Company. These warrants were exercisable at $1.11 for a term of three years and vested immediately. Pursuant to ASC 470 the relative fair value of the warrants attributable to a discount on the debt was $15,677. The note terms dictate 12% simple interest, compounding daily based on a 365-day year, paid out 6 months from the date of the note along with the principal amount loaned to the Company; these notes were to mature in calendar Q1 of 2019. The entire $70,000 in principal and $1,722 in accrued interest was converted into 215,166 shares of common stock at a rate of $.33 per share pursuant to bridge note conversion agreements in December of 2018.
An additional $13,333 in equity issuance expense was recognized due to a beneficial conversion feature whereby $20,000 of the $70,000 in principal was converted at $.33 per share when the stock price on the date of the conversion agreement was $.55 per share.
Also, pursuant to the bridge note conversion agreements, for every $2.00 in outstanding balance converted into equity the note-holder received one warrant exercisable at $.33 per share through December 31, 2018; 32,275 of these warrants were issued. The entire balance remaining in debt discount of $15,677 was charged to interest expense upon conversion of these notes.
NOTE 8 – NOTES PAYABLE
At March 31, 2020, the Company is obligated for one note payable and accrued interest in the total amounts of $15,000 and $95, respectively. The note terms dictate 6.5% per annum on the unpaid outstanding principal per year from the date funds were first advanced, which was February 25, 2020. This note originated from a lease amendment whereby we extended our lease at our Edina facility for two years (see Note 14); if certain criteria are met, the Company is able to receive an additional $27,500 in loan proceeds pursuant to this promissory note agreement.
At March 31, 2019, the Company was obligated for one note payable and accrued interest in the total amounts of $18,831 and $-0-, respectively. The note terms dictate 12% simple interest, compounding daily based on a 365-day year, paid out 6 months from the date of the note and the issuance of a detachable warrant for purchase of half of the principal amount in shares exercisable at $1.11 per share for a 3-year term. All debt discount associated with the warrants issued in conjunction with this note was charged to interest expense as of the maturity date of the note in February of 2019. Upon maturity of the note we entered into a note amendment whereby instead of paying out the entire outstanding balance of principal and interest, we were to pay an initial installment of $5,000 and then monthly payments of $3,000 until the amended maturity date of September 30, 2019, at which time the entire outstanding balance was paid.
During the year ended March 31, 2019 the Company entered into bridge note agreements with several bridge note holders in the principal amount of $215,000. There were 96,750 detachable warrants issued in conjunction with bridge notes entered into in the year ending March 31, 2019. Pursuant to ASC 470 the relative fair value of the warrants attributable to a discount on the debt is $49,880; this amount was amortized to interest expense on a straight-line basis over the term of the loans.
During the year ended March 31, 2019 and pursuant to bridge note conversion agreements, $150,000 in principal and $4,280 in accrued interest was converted into 462,838 shares of common stock at a rate of $.33 per share. Pursuant to the conversion of the notes, each note-holder who converted their note(s) received a warrant for purchase of half of the outstanding balance in shares exercisable at $.33 per share through December 31, 2018; 69,426 of these warrants were issued. An additional $33,822 in equity issuance expense was recognized due to a beneficial conversion feature whereby $50,734 in principal and interest was converted at $.33 per share when the stock price on the date of the conversion agreement was $.55 per share. During the year ended March 31, 2019, $46,169 in principal was repaid, and $4,313 in accrued interest was paid out. Each of the warrants issued pursuant to conversion of these notes, if exercised, qualified for 1 additional share of common stock transferred from a founder of the Company for every 3 shares received through exercising of these warrants; 30,016 shares were transferred to these note-holders by a founder. During the year ended March 31, 2019 the entire total of $49,880 in debt discount has been relieved to interest expense due to amortization and the conversions.
| F-13 |
NOTE 9 – CONVERTIBLE NOTES PAYABLE
At March 31, 2020, the Company is obligated for several convertible notes payable in the total amount of $286,981 made up of $280,000 in principal and $6,981 in interest. The Company entered into these convertible notes during the quarter ended June 30, 2019. All of these convertible notes mature during the quarter ended June 30, 2021, two years from their inception dates. These convertible notes accrue interest at a rate of 10%. Accrued interest is due and payable each calendar quarter in cash; during the years ended March 31, 2020 and 2019, the Company paid out $18,536 and $-0-, respectively, in accrued interest to these convertible note holders. These convertible notes automatically convert into shares of common stock at a rate of $.72 per share at the earlier of the maturity date or an uplist to a national securities exchange (e.g. Exchange or New York Stock Exchange) provided that the Company’s stock price is at least $.87 at the time of the uplist. The convertible note holders have the right to convert their outstanding principal and interest into shares of the Company’s common stock at any time during their note’s term at $.72 per share. No note holders have converted their notes through the date of this report. As of March 31, 2020, these convertible notes did not include a beneficial conversion feature.
NOTE 10 – ACCOUNTS PAYABLE AND ACCRUED EXPENSES
At March 31, 2020, the Company is obligated to pay $794,057 (2019: $854,990) in accounts payable and accrued expenses. Of the total, $556,653 (2019: $524,273) is made up of accounts payable, while the $237,404 (2019: $330,717) in accrued expenses is made up of past employee’s accrued salaries and related payroll taxes payable. The Company has not paid the related payroll taxes, consisting primarily of Social Security and Medicare taxes. As a result, the Company has established an accrued liability for the unpaid salaries, along with related taxes of approximately $22,026 (2019: $58,124) at March 31, 2020 and 2019, respectively.
NOTE 11 – ACCRUED EXPENSES – RELATED PARTY
At March 31, 2020, the Company was obligated to pay $252,607 in accrued expenses due to related parties. Of the total, $38,954 was made up of accounts payable, while $213,653 was made up of accrued salaries.
At March 31, 2019, the Company is obligated to pay $576,393 in accrued expenses due to related parties. Of the total, $89,186 is made up of accounts payable, while $487,207 is made up of accrued salaries and payroll taxes payable.
NOTE 12 - GOING CONCERN
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern.
The Company incurred net losses of $2,082,734 for the year ended March 31, 2020 and had net cash used in operating activities of $496,589 for the same period. Additionally, the Company has an accumulated deficit of $54,588,646, working capital of ($950,700), and a stockholders’ deficit of $1,036,170, at March 31, 2020. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of at least twelve months after the date of issuance on these financial statements. In view of these matters, the Company’s ability to continue as a going concern is dependent upon the Company’s ability to achieve a level of profitability and/or to obtain adequate financing through the issuance of debt or equity in order to finance its operations.
Management intends to raise additional funds either through a private placement or public offering of its equity securities. Management believes that the actions presently being taken to further implement its business plan will enable the Company to continue as a going concern. While the Company believes in its viability to raise additional funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and raise additional funds.
COVID-19 has had an impact on the global economy, which directly or indirectly may have an impact on our ability to continue as a going concern.
These financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
| F-14 |
NOTE 13 – COMMON STOCK AND WARRANTS
During the fiscal year ended March 31, 2020, the Company had several equity transactions as follows:
Common Stock
On November 22nd, 2019, the Company approved and declared a reverse stock split of all its outstanding common stock at a ratio of 9-for-10 shares. Pursuant to this reverse stock split, each ten (10) shares of PetVivo’s outstanding common stock, $.001 par value per share, was combined and converted into nine (9) post-split outstanding shares of common stock, $.001 par value per share. This reverse stock split affected all PetVivo shareholders uniformly and accordingly will not alter any shareholder’s percentage interest or ownership of PetVivo equity. Through the date of this filing, 254 shares of common stock have been issued due to rounding up of fractional shares.
During fiscal year ended March 31, 2020 and to date, the Company issued an aggregate of 3,044,657 shares of unregistered common stock which included the following:
i) 348,000 shares to John Lai (CEO, President & Director) pursuant to a Settlement Agreement whereby Mr. Lai agreed to release the Company of all claims through the date of the agreement, September 11, 2019, including accrued compensation he had earned in the amount of $116,000 and hold the shares for a period of at least 3 years;
ii) 575,806 shares to Randall Meyer (Director) pursuant to a Settlement Agreement whereby Mr. Meyer agreed to release the Company of all claims through the date of the agreement, September 11, 2019, including accrued compensation he had earned in the amount of $191,936 and hold the shares for a period of at least 3 years;
iii) 204,000 shares to John Dolan (Secretary & Director) pursuant to a Settlement Agreement whereby Mr. Dolan agreed to release the Company of all claims through the date of the agreement, September 11, 2019, including accrued compensation he had earned in the amount of $68,000 and hold the shares for a period of at least 3 years; and
iv) 168,060 shares to a former employee pursuant to a Settlement Agreement dated August 29, 2019, whereby this individual agreed to release the Company of all claims, including compensation earned in the amount of $80,029; and
v) 108,000 shares to a service provider for $120,000 worth of services provided during the one-year period ended July 13, 2019 and valued at $1.11/share over that period on a pro-rata basis; and
vi) 360,000 shares to one shareholder that the Company sold in exchange for $100,000, which equates to a price per share of $.28/share; and
vii) 270,000 shares to one service provider valued at $102,000 whereby this service provider agreed to provide video production, investor relations, and promotional services in exchange for 270,000 shares of common stock. The scope of services includes but is not limited to coordinating the airing of 96 commercials nationally on Bloomberg T.V. network and producing 12, monthly, 10-minute interviews; and
viii) 486,000 shares to various accredited investors in exchange for $135,000 in cash, which equates to a price per share of $.28/share; and
ix) 90,000 shares to service providers for $55,000 of investor relations and marketing services performed by Barry Kaplan Associates during the six-month period ending in April 2020; and
x) 160,000 units in exchange for $104,000, which equates to $.65/unit, whereby a unit is made up of one share of common stock and ½ warrant share wherein the common stock was recorded at its relative fair value of $69,391 and the warrants are described below in this Form 10-K’s Note 13’s “Warrants” subsection; and
xi) 150,000 shares of common stock to a service provider, Launchpad IR, at $.42/share for total consideration of $70,500, for investor relations services.
xii) 63,141 shares of common stock to a former Director of the Company pursuant to a cashless conversion feature within the former Director’s warrant for 168,750 shares, equating to a conversion rate of .37:1.00; and
xiii) 61,396 shares of common stock to a John Lai, the CEO of the Company, pursuant to a cashless conversion feature within his warrant for 168,750 shares, equating to a conversion rate of .36:1.00.
| F-15 |
The transactions outlined directly above and enumerated i) through iii) yielded a reduction of $375,936 in Accrued Expenses – Related Party that was owed and payable to them arising from services they provided in the past. The settlement of $80,029 explained in number iv above for a former employee’s accrued salary was accounted for as a reduction of Accounts Payable and Accrued Expenses. A loss on extinguishment of debt was recorded in the amount of $81,738 related to the transactions numbered i) through iv).
On October 31, 2019, the Company’s Board of Directors also approved a compensation plan for John Lai that included his retention of 600,000 escrowed shares.
After the balance sheet date of March 31, 2020, the Company sold and agreed to issue 80,000 units in exchange for $52,000, which equates to $.65/unit, whereby a unit is made up of one share of common stock and ½ warrant share wherein the common stock was recorded at its relative fair value of $34,709 and the 40,000 warrants are valued at $17,291 and are exercisable for 3 years from the date of the grant at $1.00/share. The $52,000 was recorded as a receivable at March 31, 2020 pursuant to ASC 310-10-S99-2, which permits the Company to record such a note as an asset if the note is collected prior to issuance of the financial statements; as outlined in Note 17, we received the funds pursuant to this sale prior to the issuance of this Annual Report on Form 10-K.
Warrants
During the year ended March 31, 2020, the Company granted 360,000 warrants to management team members that vest upon achieving certain performance conditions (milestones). These 360,000 warrants were valued using the Black Scholes valuation model at $199,982. On a quarterly basis, the Company evaluates the probability of these certain milestones being reached and recognizes expense relating to these warrants based on that probability and other criteria. As of March 31, 2020, these milestones were not met and were not probable to occur and as a result the Company recognized $-0- in expense related to these 360,000 warrants that may or may not vest pursuant to their respective milestones.
During the year ended March 31, 2020, the Company granted warrants to purchase a total of 1,905,700 shares of common stock valued using the Black-Scholes model including:
i) warrants for 270,000 shares, valued at $119,954, to three new Directors, Messrs. Scott Johnson, Gregory Cash, and James Martin, with 135,000 vested immediately and 135,000 vesting quarterly between August 2020 and May 2021, and exercisable over a five-year term at $.33/share; and
ii) warrants for 220,500 shares, valued at $122,489, to John Dolan, whereby 40,500 were granted as a bonus and were vested immediately on the October 31, 2019 grant date, 90,000 that vest upon a performance-based milestone, and 90,000 that vest quarterly over three years starting on October 1, 2019; and whereby all of these warrants are exercisable for a five-year term at $.56/share; and
iii) warrants for 540,000 shares, valued at $299,973, to John Lai, whereby 180,000 vest upon performance-based milestones and 360,000 vest quarterly over three years starting on October 1, 2019; and whereby all of these warrants are exercisable for a five-year term at $.56/share; and
iv) warrants for 450,000 shares, valued at $249,997, to John Carruth, whereby 90,000 vest upon performance-based milestones and 360,000 vest quarterly over three years starting on October 1, 2019; and whereby all of these warrants are exercisable for a five-year term at $.56/share; and
v) warrants for 41,250 shares, valued at $22,915, to David Deming, whereby they vest monthly during the eleven-month period ending August 31, 2020, have a strike price of $.49 and a five-year term; and
vi) warrants for 79,397 shares, valued at $38,744, to John Lai, whereby they vested on December 31, 2019, have a strike price of $.50 and a five-year term; and
vii) warrants for 15,880 shares, valued at $7,749, to John Dolan, whereby they vested on December 31, 2019, have a strike price of $.50 and a five-year term; and
viii) warrants for 80,000 shares, issued as a detachable warrant in purchased units with a relative fair value of $34,609, whereby an accredited investor purchased 160,000 units for $104,000 at a rate of $.65/unit and a unit equates to one share of common stock and one-half warrant, and furthermore where the warrants are exercisable for a term of 3 years, have a strike price of $1.00/share and are vested immediately; and
| F-16 |
ix) warrants to several directors for service to the Company, issued and vested on December 31, 2019, with a strike price of $.49/share, and exercisable for a five-year term as follows:
| a) | To Gregory Cash, 7,059 warrants, valued at $3,445; and | |
| b) | To Robert Rudelius, 5,735 warrants, valued at $2,799; and | |
| c) | To Scott Johnson, 4,852 warrants, valued at $2,368; and | |
| d) | To Randall Meyer, 4,852 warrants, valued at $2,368; and | |
| e) | To David Deming, 4,412 warrants, valued at $2,153; and | |
| f) | To James Martin, 4,412 warrants, valued at $2,153; and | |
| g) | To Joseph Jasper, 3,528 warrants, valued at $1,722; and | |
| h) | To David Masters, 2,647 warrants, valued at $1,292. |
x) warrants for 98,093 shares, valued at $11,967, to John Lai, whereby they vested on March 31, 2020, have a strike price of $.32 and a five-year term; and
xi) warrants for 35,314 shares, valued at $4,308, to John Dolan, whereby they vested on March 31, 2020, have a strike price of $.32 and a five-year term; and
xii) warrants to several directors for service to the Company, issued and vested on March 31, 2020, with a strike price of $.32/share, and exercisable for a five-year term as follows:
| a) | To Gregory Cash, 6,867 warrants, valued at $838; and | |
| b) | To Robert Rudelius, 6,376 warrants, valued at $778; and | |
| c) | To Scott Johnson, 4,415 warrants, valued at $539; and | |
| d) | To Randall Meyer, 4,415 warrants, valued at $539; and | |
| e) | To David Deming, 4,905 warrants, valued at $598; and | |
| f) | To James Martin, 4,905 warrants, valued at $598; and | |
| g) | To Joseph Jasper, 3,924 warrants, valued at $479; and | |
| h) | To David Masters, 1,962 warrants, valued at $239. |
During the year ended March 31, 2020, the Company cancelled warrants to purchase a total of 396,000 shares of common stock including:
i) warrants for 270,000 shares, valued at $300,770 using the Black-Scholes model, $117,144 in expense of which had yet to be taken at the time of cancellation were cancelled pursuant to the terms of such warrants dictating cancellation upon the two-month anniversary of a cease of service; and
ii) warrants for 54,000 shares that were never originally valued, were to be vested upon billing from service providers, and were cancelled because those services were never received; and
iii) warrants for 36,000 shares, valued at $68,000 using the Black-Scholes model, $17,000 in expense of which had yet to be taken at the time of cancellation were cancelled pursuant to the holder’s service agreement’s term lapsing and requisite clauses contained therein; and
iv) warrants for 36,000 shares, valued at $68,000 using the Black-Scholes model, $-0- in expense of which had yet to be taken at the time of cancellation were cancelled pursuant to the holder’s service agreement’s term lapsing and requisite clauses contained therein.
During the year ended March 31, 2020, the Company had warrants to purchase a total of 90,000 shares of common stock expire including:
i) warrants for 90,000 shares, valued at $49,996 using the Black-Scholes model, $49,996 in expense of which had yet to be taken at the time of expiration, held by John Lai, but had not vested pursuant to the performance milestones included in the same.
During the year ended March 31, 2020, the Company had warrants to purchase a total of 337,500 shares of common stock converted on a cashless basis including:
i) warrants for 168,750 shares, valued at $56,223 using the Black-Scholes model, $-0- in expense of which had yet to be taken at the time of conversion, held and converted by John Lai into 61,396 shares of common stock at a conversion rate of .36:1.00; and
| F-17 |
ii) warrants for 168,750 shares, valued at $102,807 using the Black-Scholes model, $-0- in expense of which had yet to be taken at the time of conversion were converted into 63,141 shares of common stock at a conversion rate of .37:1.00 by a former director of the Company.
During the fiscal year ended March 31, 2019 the Company had several equity transactions as follows:
Common Stock Issued
The Company issued a total of 904,759 shares of common stock (adjusted for the stock split that occurred during fiscal year 2020) during the fiscal year ended March 31, 2019 pursuant to agreements entered into in previous years as follows:
| i) | 382,759 shares pursuant to conversions of $181,966 in debt; $66,230 was converted into 85,153 shares at $.78 per share and $115,736 was converted into 297,606 shares at $.39 per share; | |
| ii) | 279,000 shares pursuant to subscription agreements for $310,000 in cash; | |
| iii) | 54,000 shares pursuant to a warrant exercise agreement for $60,000 in cash; | |
| iv) | 9,000 shares valued at $1.67 per share to a service provider for management consulting services rendered in the amount of $15,000; | |
| v) | 180,000 shares valued based on the stock price on the date of the issuance on June 7, 2017 at $.23 per share for total consideration of $42,000 to the Company’s former CEO, Wesley Hayne, for serving in that capacity. |
Common Stock Returned
During the fiscal year ended March 31, 2019, upon the departure of the former CEO Wesley Hayne, 540,000 shares held in escrow were returned to John Lai and a reduction of expense and corresponding reduction of additional paid in capital was recorded in the amount of ($177,600), which was based on the $.33 share price at the time of original valuation.
Common Stock Sold
During the fiscal year ended March 31, 2019, the Company
| i) | issued 299,507 shares of common stock to several accredited investors in consideration of $166,393 in cash pursuant to warrant exercises; | |
| ii) | issued 700,415 shares of common stock to several accredited investors in consideration of $233,472 in cash pursuant to discounted warrant exercise agreements whereby the company offered all warrant holders the option to exercise their warrants at $.33 per share and they would receive 1 share for every 3 shares received pursuant to the discounted warrant exercise agreement from John Lai, the President of the Company. |
Stock-Based Compensation Granted
During the fiscal year ended March 31, 2019, the Company issued 24,384 shares of common stock to two service providers as follows:
| i) | 1,884 shares of common stock valued at $2,700 for website services; | |
| ii) | 22,500 shares of common stock valued at $24,750 for marketing services. |
Also, stock-based compensation expense was recognized pursuant to several warrants’ vesting periods in the amount of $1,449,348 as follows:
| i) | $99,882 in expense pursuant to vesting of warrants granted to service providers; | |
| ii) | $258,031 in expense pursuant to vesting of warrants granted to advisors; | |
| iii) | $780,181 in expense pursuant to vesting of warrants granted to directors; | |
| iv) | $161,750 in expense pursuant to vesting of warrants granted to employees; | |
| v) | $149,505 in expense pursuant to vesting of warrants granted to officers. |
There were also several warrants granted in conjunction with bridge notes that were entered into during the fiscal year ended March 31, 2019 that led to recognition of $14,181 in stock-based compensation expense. Also, warrants granted in conjunction with these bridge notes led to the setup and subsequent amortization of a debt discount to interest expense in the amount of $65,557 with the offset recorded in additional paid in capital.
| F-18 |
Finally, pursuant to a manufacturing and production agreement with CytoMedical Design Group (“CMDG”) the Company had granted but not issued CMDG 77,700 shares of common stock valued at $86,333 which had been recorded to general and administrative expense with an offset to stock to be issued during the fiscal year ended March 31, 2019.
Stock Granted for Debt Conversion
During the fiscal year ended March 31, 2019, the Company issued 85,916 shares of common stock to a third party to convert their accounts payable in the amount of $95,462. We also issued 678,006 shares of common stock pursuant to conversions of bridge notes with principal and accrued interest in the total amount of $226,002; some of these conversions took place on a date when the stock price was publicly-quoted at a price higher than that of the conversion price, which led to expense recognized due to these beneficial conversion features with an offset to additional paid in capital in the amount of $66,248.
Common Stock Issued to Replace Shares to Officer
During the fiscal year ended March 31, 2019, the Company issued 723,047 shares of common stock valued at $1,446,093 to John Lai, the Company’s President, to replace shares he had previously given up as follows:
| i) | 292,251 shares of common stock valued at $584,501; these shares were issued to replace 292,251 shares given to a third party by John Lai in order to secure funding in 2015; this transaction is included in Common stock issued to replace shares to officer on the statement of equity; | |
| ii) | 430,796 shares of common stock valued at $861,592; these shares were issued to virtually restore 450,000 shares of common stock John lost to escrow pursuant to its terms. |
Common Stock Issued by Officer
During the fiscal year ended March 31, 2019, the Company recognized $77,354 in stock-based compensation expense with an offset to additional paid in capital pursuant to stock transfer agreements whereby John Lai transferred 1 share for every 3 shares warrant holders received pursuant to their discounted warrant exercises entered into in December of 2018 during our discounted warrant exercise offering; this is explained more in the below section titled Warrant Grants.
Warrant Grants
During the fiscal year ended March 31, 2019, the Company granted warrants to purchase a total of 1,782,478 shares of common stock including:
i) warrants for 72,000 shares to two advisory board members for service, vested semi-annually over two years, and exercisable over a five-year term at $1.11/share and valued at $70,434;
ii) warrants for 207,000 shares to John Carruth, the Company’s Acting CFO at the time, in consideration of his employment, vested quarterly over two years, with a strike price of $.33 per share and exercisable over a five-year term and valued at $69,072;
iii) warrants for 27,000 shares to a lawyer for general legal counsel, fully-vested and exercisable over a five-year term at $1.11/share valued at $52,818;
iv) warrants for 54,000 shares to various information technology service providers for IT services, vested as billed, exercisable over a five-year term, which are valued as earned and have not yet been earned;
v) warrants for 270,000 shares to three new Directors in consideration of their service, vested quarterly over two years, and exercisable over a five-year term at $1.11/share and valued at $259,920;
vi) warrants for 128,250 shares to several note holders pursuant to their bridge note agreements, vested immediately, and exercisable over a three-year term at $1.11/share and valued at $85,218;
vii) warrants for 101,728 shares to several note holders pursuant to their conversion of notes into equity, vested immediately, exercisable through December 31, 2018 at $.33/share and valued at $11,170;
| F-19 |
viii) warrants for 922,501 shares to several board members, valued at $561,910, vested immediately, for a term of ten years with a strike price of $.30/share and a one-time protection against a reverse split whereby the strike price will not be adjusted upon combination of outstanding shares of stock, as follows:
| i) Sheryll Grisewood | 168,750 | |
| ii) David Merrill | 168,750 | |
| iii) John Dolan | 168,750 | |
| iv) David Deming | 84,375 | |
| v) Peter Vezmar | 84,375 | |
| vi) Joseph Jasper | 84,375 | |
| vii) Robert Rudelius | 78,750 | |
| viii) David Masters | 42,188 | |
| ix) Randall Meyer | 42,188 |
Also, during the year ended March 31, 2019, the Company reduced the strike price of 528,750 warrants for members of the board of directors to $.33 per share. They also reduced the strike price of 72,000 warrants to $.33 per share issued to John Carruth, the Acting CFO at the time. Pursuant to ASC 718-20-35-3 the Company did not realize any additional expense associated with these reductions in strike price, as the change in fair value of these instruments was not in excess of the original instrument.
During the fiscal year ended March 31, 2019, the Company cancelled previous grants of warrants to purchase 90,000 shares of common stock including:
i) warrants for 54,000 shares from a service provider due to the termination of a contract pursuant to its terms that were valued at $102,000;
ii) warrants for 36,000 shares from a former advisory board member due to the termination of a contract that were valued at $68,000.
During December 2018 the Company offered its warrant-holders the option to exercise their warrants at a discounted rate of $.33 per share if exercised within 15 days of the offer date. Pursuant to this discounted warrant exercise agreement (“DWEA”), warrant-holders were entitled to 1 share issued by way of stock transfer from a founder of the Company for every 3 shares received pursuant to the DWEA. Several warrant-holders entered into such agreements whereby they received 610,369 shares of newly-issued common stock and 203,456 shares of common stock from John Lai, a founder of the Company, in exchange for $203,456 in cash.
During December 2018, the Company offered its note-holders the option to convert their notes and receive 1 warrant for every $2.00 in outstanding balance of principal and interest converted. There were 101,729 of these warrants issued; 11,680 expired on December 31, 2018 and the remaining 90,049 were exercised in exchange for $30,016 in cash. Pursuant to these exercised warrants, each warrant-holder received 1 share of common stock from a founder, John Lai; the total number of shares transferred by John Lai to these warrant-holders was 30,016 shares, which were valued at $11,759.
A summary of warrant activity for fiscal years ending March 31, 2019 and 2020 is as follows:
| Number of Warrants | Weighted- Average Exercise Price | Warrants Exercisable | Weighted- Average Exercisable Price | |||||||||||||
| Outstanding, March 31, 2018 | 3,138,046 | .66 | 2,190,241 | .63 | ||||||||||||
| Granted | 1,782,478 | .46 | ||||||||||||||
| Exercised | (999,925 | ) | .40 | |||||||||||||
| Expired | (11,680 | ) | .33 | |||||||||||||
| Cancelled | (90,000 | ) | 1.11 | |||||||||||||
| Outstanding, March 31, 2019 | 3,818,919 | .55 | 3,035,035 | .54 | ||||||||||||
| Granted | 1,905,700 | .52 | ||||||||||||||
| Cashless Conversions | (337,500 | ) | .32 | |||||||||||||
| Expired | (90,000 | ) | .56 | |||||||||||||
| Cancelled | (396,000 | ) | .58 | |||||||||||||
| Outstanding, March 31, 2020 | 4,901,119 | .55 | 4,072,369 | .53 | ||||||||||||
| F-20 |
At March 31, 2020, the range of warrant prices for shares under warrants and the weighted-average remaining contractual life is as follows:
| Warrants Outstanding | Warrants Exercisable | ||||||||||||||||||||
| Range
of Warrant Exercise Price | Number of Warrants | Weighted- Average Exercise Price | Weighted- (Years) | Number of Warrants | Weighted- Average Exercise Price | ||||||||||||||||
| .30-.50 | 2,299,701 | .38 | 5.48 | 2,434,701 | .33 | ||||||||||||||||
| .51-1.00 | 2,105,739 | .57 | 2.92 | 1,141,989 | .59 | ||||||||||||||||
| 1.01-3.50 | 495,679 | 1.42 | 2.36 | 495,679 | 1.42 | ||||||||||||||||
| Total | 4,901,119 | .53 | 4.06 | 4,072,369 | .53 | ||||||||||||||||
The Company granted warrants during the fiscal years ended March 31, 2020 and 2019 based on the following ranges:
| Fiscal Year Ended March 31, | ||||||||
| 2020 | 2019 | |||||||
| Stock price on valuation date | $ | .12 - $.56 | $ | .27 - $2.00 | ||||
| Exercise price | $ | .32 - $.56 | $ | .33 - $1.67 | ||||
| Term (years) | .003 - 10 | .003 - 10 | ||||||
| Weighted-average volatility* | 348 | % | 238 | % | ||||
| Risk-free rate | 1.5% - 2.4 | % | 1.7% - 2.4 | % | ||||
*Weighted-average volatility disclosed as opposed to a range
The fair value of each warrant award is estimated on the date of grant using a Black-Scholes valuation model that uses the assumptions noted in the table above. Because the Black-Scholes valuation model incorporates ranges of assumptions for inputs, those ranges are disclosed in the table above. Implied volatilities are based on historical volatility of the Company’s stock. No reserve is taken for warrants granted that we estimate will not vest as there is not enough historical data to come to a reasonable estimation of the same. The risk-free rate for periods within the contractual lives of the warrants is based on the 13-week U.S. Treasury bill rates in effect at the time of grants.
For the years ended March 31, 2020 and 2019, the total stock-based compensation on all instruments was $962,678 and $1,642,869, respectively. It is expected that the Company will recognize expense after March 31, 2020 related to warrants issued, outstanding, and valued using the Black Scholes pricing model as of March 31, 2020 in the amount of approximately $500,000. Additionally, the Company has approximately $150,000 of expense to recognize for warrants with potential future milestones.
| F-21 |
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
June 30, 2020 (Unaudited) | March 31, 2020 | |||||||
| Assets: | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 128,564 | $ | 888 | ||||
| Restricted cash | - | 9,694 | ||||||
| Accounts receivable | 2,000 | 1,000 | ||||||
| Equity sale proceeds receivable | - | 52,000 | ||||||
| Investments - equity securities | 1,500 | 1,500 | ||||||
| Prepaid expenses | 136,370 | 132,023 | ||||||
| Total Current Assets | 268,434 | 197,105 | ||||||
| Property and Equipment: | ||||||||
| Property and equipment | 262,730 | 221,493 | ||||||
| Less: accumulated depreciation | (118,075 | ) | (111,586 | ) | ||||
| Total Property and Equipment, net | 144,655 | 109,907 | ||||||
| Other Assets: | ||||||||
| Operating lease right-of-use | 142,854 | 148,693 | ||||||
| Trademark and patents, net | 14,825 | 58,611 | ||||||
| Security deposit | 8,201 | 8,201 | ||||||
| Total Other Assets | 165,880 | 215,505 | ||||||
| Total Assets | $ | 578,969 | $ | 522,517 | ||||
| Liabilities and Stockholders’ Equity (Deficit) | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 780,835 | $ | 794,057 | ||||
| Accrued expenses - related party | 239,372 | 252,607 | ||||||
| Derivative liability | 548,200 | - | ||||||
| Convertible notes and accrued interest payable, net of debt discount | 301,446 | 286,981 | ||||||
| Convertible notes and accrued interest payable - related party | 25,197 | - | ||||||
| PPP loan – short term | 15,947 | - | ||||||
| Notes payable and accrued interest | 15,325 | 15,095 | ||||||
| Notes payable and accrued interest - related party | 62,445 | 61,255 | ||||||
| Operating lease liability – short term | 24,791 | 24,791 | ||||||
| Total Current Liabilities | 2,013,558 | 1,434,786 | ||||||
| Other Liabilities: | ||||||||
| PPP loan (net of current) | 22,783 | - | ||||||
| Operating lease liability (net of current) | 118,063 | 123,901 | ||||||
| Total Other Liabilities | 140,846 | 123,901 | ||||||
| Total Liabilities | $ | 2,154,404 | $ | 1,558,687 | ||||
| Commitments and Contingencies | ||||||||
| Stockholders’ Equity (Deficit): | ||||||||
| Preferred stock, par value $0.001, 20,000,000 shares authorized 0 and 0 shares outstanding at June 30, 2020 and March 31, 2020, respectively | ||||||||
| Common stock, par value $0.001, 225,000,000 shares authorized 23,111,857 and 22,911,857 shares outstanding at June 30, 2020 and March 31, 2020, respectively | 23,111 | 22,912 | ||||||
| Common stock to be issued | - | 52,000 | ||||||
| Additional Paid-In Capital | 53,804,107 | 53,477,563 | ||||||
| Accumulated Deficit | (55,402,653 | ) | (54,588,645 | ) | ||||
| Total Stockholders’ Deficit | (1,575,435 | ) | (1,036,170 | ) | ||||
| Total Liabilities and Stockholders’ Deficit | $ | 578,969 | $ | 522,517 | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-22 |
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
(UNAUDITED)
| Three Months Ended | ||||||||
| June 30, | June 30, | |||||||
| 2020 | 2019 | |||||||
| Revenues | $ | 2,006 | $ | - | ||||
| Cost of Sales | - | - | ||||||
| Gross Profit | 2,006 | - | ||||||
| Operating Expenses: | ||||||||
| Sales and Marketing | 46,682 | 2,796 | ||||||
| Intangible impairment | 3,606 | - | ||||||
| General and Administrative: | ||||||||
| Depreciation and Amortization | 51,805 | 144,090 | ||||||
| Other General and Administrative | 341,981 | 346,835 | ||||||
| Total General and Administration | 393,786 | 490,925 | ||||||
| Total Operating Expenses | 444,074 | 493,721 | ||||||
| Operating Loss | $ | (442,068 | ) | $ | (493,721 | ) | ||
| Other Income (Expense) | ||||||||
| Gain on Sale of Asset | 482 | 450 | ||||||
| Derivative expense | (342,200 | ) | - | |||||
| Interest Expense | (30,222 | ) | (7,616 | ) | ||||
| Total Other Expense | (371,940 | ) | (7,166 | ) | ||||
| Net Loss before taxes | $ | (814,008 | ) | $ | (500,887 | ) | ||
| Income Tax Provision | - | - | ||||||
| Net Loss | (814,008 | ) | (500,887 | ) | ||||
| Net Loss Per Share: | ||||||||
| Basic and Diluted | $ | (0.04 | ) | $ | (0.03 | ) | ||
| Weighted Average Common Shares Outstanding: | ||||||||
| Basic and Diluted | 22,938,231 | 19,867,200 | ||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
Shares retroactively restated for 9-for-10 reverse stock split in November of 2019
| F-23 |
CONDENSED CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS’ EQUITY
(UNAUDITED)
Three Months Ended June 30, 2020
| Common Stock | Additional Paid-in | Accumulated | Common Stock to | |||||||||||||||||||||
| Shares | Amount | Capital | Deficit | be Issued | Total | |||||||||||||||||||
| Balance at March 31, 2020 | 22,911,857 | $ | 22,911 | $ | 53,477,563 | $ | (54,588,645 | ) | $ | 52,000 | $ | (1,036,171 | ) | |||||||||||
| Common stock and warrants sold | 80,000 | 80 | 51,920 | - | (52,000 | ) | - | |||||||||||||||||
| Warrants issued in conjunction with convertible debt | - | - | 91,500 | - | - | 91,500 | ||||||||||||||||||
| Stock-based compensation | 120,000 | 120 | 183,124 | - | - | 183,244 | ||||||||||||||||||
| Net loss | - | - | - | (814,008 | ) | - | (814,008 | ) | ||||||||||||||||
| Balance at June 30, 2020 | 23,111,857 | $ | 23,111 | $ | 53,804,107 | $ | (55,402,653 | ) | $ | - | $ | (1,575,435 | ) | |||||||||||
Three Months Ended June 30, 2019
| Common Stock | Additional Paid-in | Accumulated | Common Stock to | |||||||||||||||||||||
| Shares | Amount | Capital | Deficit | be Issued | Total | |||||||||||||||||||
| Balance at March 31, 2019 | 19,867,200 | $ | 22,074 | $ | 51,552,688 | $ | (52,505,912 | ) | $ | 86,333 | $ | (844,817 | ) | |||||||||||
| Stock-based compensation | - | - | 157,134 | - | 33,667 | 190,801 | ||||||||||||||||||
| Net loss | - | - | - | (500,887 | ) | - | (500,887 | ) | ||||||||||||||||
| Balance at June 30, 2019 | 19,867,200 | $ | 22,074 | $ | 51,709,822 | $ | (53,006,799 | ) | $ | 120,000 | $ | (1,154,903 | ) | |||||||||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
Shares retroactively restated for 9-for-10 reverse stock split in November of 2019
| F-24 |
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| For the Three Months Ended | ||||||||
| June 30, 2020 | June 30, 2019 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net Loss For The Period | $ | (814,008 | ) | $ | (500,887 | ) | ||
| Adjustments to Reconcile Net Loss to Net Cash Used in Operating Activities: | ||||||||
| Derivative expense | 342,200 | - | ||||||
| Stock-based compensation | 183,244 | 190,801 | ||||||
| Depreciation and amortization | 51,805 | 144,090 | ||||||
| Amortization of debt discount | 19,608 | - | ||||||
| Intangible impairment | 3,606 | - | ||||||
| Gain on sale of asset | (482 | ) | (450 | ) | ||||
| Changes in Operating Assets and Liabilities | ||||||||
| Increase in prepaid expenses | (4,347 | ) | (11,157 | ) | ||||
| Increase in accounts receivable | (1,000 | ) | - | |||||
| Interest accrued on convertible notes payable | 8,819 | 5,362 | ||||||
| Interest accrued on notes payable - related party | 1,387 | 1,590 | ||||||
| Interest accrued on notes payable | 295 | 471 | ||||||
| Decrease in accounts payable and accrued expense | (13,224 | ) | (5,322 | ) | ||||
| Increase (decrease) in accrued expenses - related party | (13,234 | ) | 1,705 | |||||
| Net Cash Used In Operating Activities | (235,331 | ) | (173,797 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Increase in investments - equity securities receivable | - | (1,500 | ) | |||||
| Sale of equipment | 482 | - | ||||||
| Purchase of equipment | (41,236 | ) | - | |||||
| Increase in patents and trademarks | (5,136 | ) | (19,760 | ) | ||||
| Net Cash Used in Investing Activities | (45,890 | ) | (21,260 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from stock and warrants sale | 52,000 | - | ||||||
| Proceeds from PPP loan | 38,665 | - | ||||||
| Proceeds from convertible notes | 322,500 | 280,000 | ||||||
| Repayments of convertible notes | (13,962 | ) | (5,362 | ) | ||||
| Repayments of notes payable | - | (9,000 | ) | |||||
| Repayments of notes payable - related party | - | (11,400 | ) | |||||
| Decrease in notes payable and accrued interest | - | - | ||||||
| Net Cash Provided by Financing Activities | 399,203 | 254,238 | ||||||
| Net Increase in Cash | 117,982 | 59,181 | ||||||
| Cash and Restricted Cash at Beginning of Period | 10,582 | 6,460 | ||||||
| Cash at End of Period | $ | 128,564 | $ | 65,641 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash Paid During The Period For: | ||||||||
| Interest | $ | 13,962 | $ | 7,676 | ||||
| SUPPLEMENTAL DISCLOSURE ON NON-CASH FINANCING AND INVESTING ACTIVITIES | ||||||||
| Derivative treated as debt discount | $ | 352,941 | $ | - | ||||
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements.
| F-25 |
Notes to Financial Statements
June 30, 2020
(Unaudited)
NOTE 1 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES AND ORGANIZATION
(A) Organization and Description
The Company is in the business of licensing and commercializing our proprietary medical devices and biomaterials for the treatment of afflictions and diseases in animals, initially for dogs and horses. The Company’s operations are conducted from its headquarter facilities in suburban Minneapolis, Minnesota.
(B) Basis of Presentation
PetVivo Holdings, Inc. (the “Company”) was incorporated in Nevada under a former name in 2009 and entered its current business in 2014 through a stock exchange reverse merger with PetVivo, Inc., a Minnesota corporation. This merger resulted in Minnesota PetVivo becoming a wholly-owned subsidiary of the Company. In April 2017, the Company acquired another Minnesota corporation, Gel-Del Technologies, Inc., through a statutory merger, which is also a wholly-owned subsidiary of the Company.
In November 2019, the Company effected a 9-for-10 reverse split of our authorized and outstanding shares of common stock. Pursuant to this reverse stock split, each ten (10) shares of PetVivo’s outstanding common stock, $.001 par value per share, was combined and converted into nine (9) post-split outstanding shares of common stock, $.001 par value per share; 24,974,518 pre-reverse-split shares of common stock were combined during the 9-for-10 reverse split into 22,477,320 shares of post-reverse-split shares of common stock with 254 shares being issued for fractional shares through the date of the balance sheet. Accordingly, all references to number of shares of common stock and per share data have been adjusted retroactively where applicable to account for this reverse split.
The accompanying condensed consolidated financial statements are unaudited. These unaudited interim financial statements have been prepared in accordance with accounting principles generally accepted in the United States (“GAAP”) and pursuant to the rules and regulations of the SEC. Certain information and note disclosures, which are included in annual financial statements, have been omitted pursuant to these rules and regulations. We believe the disclosures made in these interim unaudited financial statements are adequate to make the information not misleading.
Although these interim financial statements at June 30, 2020 and for the three months ended June 30, 2020 and 2019 are unaudited, in the opinion of our management, such statements include all adjustments (consisting of normal recurring entries) necessary to present fairly our financial position, results of operations and cash flows for the periods presented. The results for the three months ended June 30, 2020 are not necessarily indicative of the results to be expected for the year ended March 31, 2021 or for any future period.
These unaudited interim financial statements should be read and considered in conjunction with our audited financial statements and the notes thereto for the year ended March 31, 2020, included in our annual report on Form 10-K filed with the SEC on June 29, 2020.
(C) Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its two wholly-owned Minnesota corporations. All intercompany accounts have been eliminated upon consolidation.
(D) Use of Estimates
In preparing financial statements in conformity with generally accepted accounting principles, management is required to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and revenues and expenses during the reported period. Actual results could differ from those estimates. Significant estimates include estimated useful lives and potential impairment of property and equipment, estimate of fair value of share-based payments and derivative instruments and recorded debt discount, valuation of deferred tax assets and valuation of in-kind contribution of services and interest.
| F-26 |
(E) Cash and Cash Equivalents
The Company considers all highly-liquid, temporary cash investments with an original maturity of three months or less to be cash equivalents. At June 30, 2020, the Company had $128,564 in cash and no cash equivalents. At March 31, 2020, the Company had $10,582 in cash and restricted cash and no cash equivalents.
(F) Concentration-Risk
The Company maintains its cash with various financial institutions, which at times may exceed limits insured by the Federal Deposit Insurance Corporation (FDIC). At June 30, 2020, cash did not exceed the FDIC uninsured balances and management believes the Company is not exposed to any significant credit risk on cash.
(G) Property & Equipment
Property and equipment are recorded at cost less accumulated depreciation. Expenditures for major additions and betterments are capitalized. Maintenance and repairs are charged to operations as incurred. Depreciation is computed by the straight-line method (after taking into account their respective estimated residual values) over the asset’s estimated useful life of (3) years for equipment, (5) years for automobile, and (7) years for furniture and fixtures.
(H) Patents and Trademarks
The Company capitalizes direct costs for the maintenance and advancement of their patents and trademarks and amortizes these costs over the lesser of a useful life of 60 months or the life of the patent. We evaluate the recoverability of intangible assets periodically by taking into account events or circumstances that may warrant revised estimates of useful lives or that indicate the asset may be impaired.
(I) Loss Per Share
Basic loss per share is computed by dividing net loss by weighted average number of shares of common stock outstanding during each period. Diluted loss per share is computed by dividing net loss by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during the period.
The Company has 5,728,610 warrants outstanding as of June 30, 2020 with varying exercise prices ranging from $3.89 to $.30/share. The weighted average exercise price for these warrants is $.51/share. These warrants are excluded from the weighted average number of shares because they are considered anti-dilutive.
The Company had 3,933,663 warrants outstanding as of June 30, 2019, with varying exercise prices ranging from $3.89 to $.30/share. The weighted average exercise price for these warrants was $.53/share. These warrants are excluded from the weighted average number of shares because they are considered antidilutive.
The Company uses the guidance in ASC 260 to determine if-converted loss per share. ASC 260 states that convertible securities should be considered exercised at the later date of the first day of the reporting period’s quarter or the inception date of the debt instrument. Also, the if-converted method shall not be applied for the purposes of computing diluted EPS if the effect would be antidilutive.
At June 30, 2020 and 2019, the Company had $280,000 in convertible notes outstanding, these notes mature in our fiscal quarter ended June 30, 2021. If converted, the $280,000 in outstanding principal and accrued interest would convert into 387,693 shares of common stock at a rate of $.72 per share. Also at June 30, 2020, the Company had a 15% OID convertible note outstanding in the principal amount of $352,941 ($-0- at June 30, 2019) that had accrued $1,838 ($-0- at June 30, 2019) in accrued interest to yield an outstanding balance of $354,779 that is convertible into shares at a rate of $.28/share into 1,267,068 shares of common stock on or after December 15, 2020. See Note 7 to these financial statements for more information on the convertible notes discussed in this paragraph.
At June 30, 2020, the Company had $25,197 ($-0- at June 30, 2019) in convertible notes outstanding held by related parties, these notes mature August 14, 2021. If converted, the $25,197 in outstanding principal and accrued interest would convert into 99,279 shares of common stock at a rate of $.2538 per share. See Note 9 to these financial statements for more information on the convertible notes due to related parties discussed in this paragraph.
| F-27 |
(J) Revenue Recognition
The Company recognizes revenue on arrangements in accordance with FASB ASC No. 606, “Revenue From Contracts With Customers.” Revenue is recognized upon transfer of control of promised products or services to customers in an amount that reflects the consideration we expect to receive in exchange for those products or services. The Company adopted the guidance on April 1, 2018 using the cumulative catch-up transition method. This change in accounting did not have any material effect on the Company’s financial statements.
(K) Research and Development
The Company expenses research and development costs as incurred.
(L) Fair Value of Financial Instruments
The Company applies the accounting guidance under FASB ASC 820-10, “Fair Value Measurements,” as well as certain related FASB staff positions. This guidance defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact business and considers assumptions that marketplace participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance.
The guidance also establishes a fair value hierarchy for measurements of fair value as follows:
| ● | Level 1 - quoted market prices in active markets for identical assets or liabilities. | |
| ● | Level 2 - inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. | |
| ● | Level 3 - unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. |
The Company’s financial instruments consist of investments – equity securities receivable, notes payable and accrued interest, notes payable and accrued interest - related party, and convertible notes payable. The carrying amount of the Company’s financial instruments approximates their fair value as of June 30, 2020 and March 31, 2020, due to the short-term nature of these instruments and the Company’s borrowing rate of interest.
In instances where the determination of the fair value measurement is based on inputs from different levels of the fair value hierarchy, the level in the fair value hierarchy within which the entire fair value measurement falls is based on the lowest level input that is significant to the fair value measurement in its entirety. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires judgment, and considers factors specific to the asset or liability. The valuation of the Company’s notes recorded at fair value is determined using Level 3 inputs, which consider (i) time value, (ii) current market and (iii) contractual prices.
The Company measured its investments – equity securities receivable at fair value at June 30, 2020, and March 31, 2020, see Note 4 to the financial statements included in this Form 10-Q.
| F-28 |
The Company accounts for derivative instruments in accordance with Accounting Standards Codification 815, Derivatives and Hedging (“ASC 815”), which establishes accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other contracts, and for hedging activities. ASC 815 requires that an entity recognize all derivatives as either assets or liabilities in the balance sheet and measure those instruments at fair value.
If certain conditions are met, a derivative may be specifically designated as a hedge, the objective of which is to match the timing of gain or loss recognition on the hedging derivative with the recognition of (i) the changes in the fair value of the hedged asset or liability that are attributable to the hedged risk or (ii) the earnings effect of the hedged forecasted transaction. For a derivative not designated as a hedging instrument, the gain or loss is recognized in income in the period of change.
(M) Stock-Based Compensation - Non-Employees
Equity Instruments Issued to Parties Other Than Employees for Acquiring Goods or Services
The Company accounts for equity instruments issued to parties other than employees for acquiring goods or services under guidance of Sub-topic 505-50 of the FASB Accounting Standards Codification (“Sub-topic 505-50”).
Pursuant to ASC Section 505-50-30, all transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. The measurement date used to determine the fair value of the equity instrument issued is the earlier of the date on which the performance is complete or the date on which it is probable that performance will occur. If the Company is a newly formed corporation or shares of the Company are thinly traded the use of share prices established in the Company’s most recent private placement memorandum (“PPM”), or weekly or monthly price observations would generally be more appropriate than the use of daily price observations as such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market.
The fair value of share options and similar instruments is estimated on the date of grant using a Black-Scholes option-pricing valuation model. The ranges of assumptions for inputs are as follows:
| ● | Expected term of share options and similar instruments: Pursuant to Paragraph 718-10-50-2(f)(2)(i) of the FASB Accounting Standards Codification the expected term of share options and similar instruments represents the period of time the options and similar instruments are expected to be outstanding taking into consideration of the contractual term of the instruments and holder’s expected exercise behavior into the fair value (or calculated value) of the instruments. The Company uses historical data to estimate holder’s expected exercise behavior. If the Company is a newly formed corporation or shares of the Company are thinly traded the contractual term of the share options and similar instruments is used as the expected term of share options and similar instruments as the Company does not have sufficient historical exercise data to provide a reasonable basis upon which to estimate expected term. | |
| ● | Expected volatility of the entity’s shares and the method used to estimate it. Pursuant to ASC Paragraph 718-10-50-2(f)(2)(ii) a thinly-traded or nonpublic entity that uses the calculated value method shall disclose the reasons why it is not practicable for the Company to estimate the expected volatility of its share price, the appropriate industry sector index that it has selected, the reasons for selecting that particular index, and how it has calculated historical volatility using that index. The Company uses the average historical volatility of the comparable companies over the expected contractual life of the share options or similar instruments as its expected volatility. If shares of a company are thinly traded the use of weekly or monthly price observations would generally be more appropriate than the use of daily price observations as the volatility calculation using daily observations for such shares could be artificially inflated due to a larger spread between the bid and asked quotes and lack of consistent trading in the market. | |
| ● | Expected annual rate of quarterly dividends. An entity that uses a method that employs different dividend rates during the contractual term shall disclose the range of expected dividends used and the weighted-average expected dividends. The expected dividend yield is based on the Company’s current dividend yield as the best estimate of projected dividend yield for periods within the expected term of the share options and similar instruments. |
| F-29 |
| ● | Risk-free rate(s). An entity that uses a method that employs different risk-free rates shall disclose the range of risk-free rates used. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant for periods within the expected term of the share options and similar instruments. |
Pursuant to Paragraphs 505-50-25-8 and 505-50-25-9, an entity may grant fully vested, non-forfeitable equity instruments that are exercisable by the grantee only after a specified period of time if the terms of the agreement provide for earlier exercisability if the grantee achieves specified performance conditions. Any measured cost of the transaction shall be recognized in the same period(s) and in the same manner as if the entity had paid cash for the goods or services or used cash rebates as a sales discount instead of paying with, or using, the equity instruments. A recognized asset, expense, or sales discount shall not be reversed if a share option and similar instrument that the counterparty has the right to exercise expires unexercised.
(N) Income Taxes
The Company accounts for income taxes under Accounting Standards Codification (ASC) Topic 740. Deferred tax assets and liabilities are determined based upon differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. A valuation allowance is provided when it is more likely than not that some portion or all of a deferred tax asset will not be realized.
The Company accounts for income taxes under Accounting Standards Codification (ASC) Topic 740.
As required by ASC Topic 740, the Company recognizes the financial statement benefit of a tax position only after determining that the relevant tax authority would more likely than not sustain the position following an audit. For tax positions meeting the more-likely-than-not threshold, the amount recognized in the financial statements is the largest benefit that has a greater than 50 percent likelihood of being realized upon ultimate settlement with the relevant tax authority. The Company applied ASC Topic 740 to all tax positions for which the statute of limitations remained open. As a result of the implementation of ASC Topic 740, the Company did not recognize any change in the liability for unrecognized tax benefits.
The Company is not currently under examination by any federal or state jurisdiction.
The Company’s policy is to record tax-related interest and penalties as a component of operating expenses.
(O) Inventory
Inventories are recorded in accordance with ASC 330 and are stated at the lower of cost or net realizable value. We account for inventories using the first in first out (FIFO) methodology and capitalize costs on a project basis as they occur. The current marketed shelf life of our Kush inventory is 3 years. However, management reserves the right to review and adjust this as appropriate.
(P) Recently Issued and Adopted Accounting Pronouncements
In February 2016, the FASB issued ASU No. 2016-02, Leases (Topic 842) to increase transparency and comparability among organizations by recognizing lease assets and lease liabilities on the balance sheet and disclosing key information about leasing arrangements. Topic 842 affects any entity that enters into a lease, with some specified scope exemptions. The guidance in this ASU supersedes Topic 840, Leases. The core principle of Topic 842 is that a lessee should recognize the assets and liabilities that arise from leases. A lessee should recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use (“ROU”) asset representing its right to use the underlying asset for the lease term. The Company adopted Topic 842 on April 1, 2019 and resulted in a right of use asset and liability of $154,917.
In January 2017, the FASB issued ASU No. 2017-04, “Intangibles and Other (Topic 350): Simplifying the Test for Goodwill Impairment”, which eliminates the requirement to calculate the implied fair value of goodwill, but rather requires an entity to record an impairment charge based on the excess of a reporting unit’s carrying value over its fair value. This amendment is effective for annual or interim goodwill impairment tests in fiscal years beginning after December 15, 2019. We adopted this ASU on April 1, 2020 and it did not have a material effect on our consolidated financial statements.
| F-30 |
In August 2018, the FASB issued ASU No. 2018-13, Fair Value Measurements (Topic 820): Disclosure Framework Changes to the Disclosure Requirements for Fair Value Measurement. The amendments in this update modify the disclosure requirements on fair value measurements in Topic 820. The ASU is effective for the Registrants for fiscal years beginning after December 15, 2019, and interim periods therein. The Company adopted this ASU on April 1, 2020 and it did not have a material impact on the financial statements.
All other newly issued accounting pronouncements but not yet effective have been deemed either immaterial or not applicable.
NOTE 2 - GOING CONCERN
The accompanying financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern.
The Company incurred net losses of $814,008 for the three-month period ended June 30, 2020 and had net cash used in operating activities of $235,331 for the same period. Additionally, the Company has an accumulated deficit of $55,402,653, working capital deficit of $1,745,124, and a stockholders’ deficit of $1,575,435 at June 30, 2020. These conditions raise substantial doubt about the Company’s ability to continue as a going concern for a period of at least twelve months after the date of issuance on these financial statements. In view of these matters, the Company’s ability to continue as a going concern is dependent upon the Company’s ability to achieve a level of profitability and/or to obtain adequate financing through the issuance of debt or equity in order to finance its operations.
Management intends to raise additional funds either through a private placement or public offering of its equity securities. Management believes that the actions presently being taken to further implement its business plan will enable the Company to continue as a going concern. While the Company believes in its viability to raise additional funds, there can be no assurances to that effect. The ability of the Company to continue as a going concern is dependent upon the Company’s ability to further implement its business plan and raise additional funds.
COVID-19 has had an impact on the global economy, which directly or indirectly may have an impact on our ability to continue as a going concern.
These financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
NOTE 3 – LEASE AND COMMITMENTS
Rent expense for the three-month periods ended June 30, 2020 and June 30, 2019 were $13,568 and $14,546, respectively.
The Company entered into an eighty-four-month lease for 3,577 square feet of newly constructed office, laboratory and warehouse space located in Edina, Minnesota on May 3, 2017. The Company resided in the facility starting in November of 2017. The base rent is $2,078 per month and the Company is responsible for its proportional share of common space expenses, property taxes, and building insurance. This lease is terminable by the landlord if damage causes the property to no longer be utilized as an integrated whole and by the Company if damage causes the facility to be unusable for a period of 45 days. The Company entered into a lease amendment whereby the lease term was extended through November of 2026 in exchange for a loan of $42,500 and a grant of $7,500 for lease improvements. Through the balance sheet date, the Company has received $15,000 in loan proceeds and expects to receive the remaining loan amount of $27,500 and grant amount of $7,500 if certain criteria are met relating to the build out of our Edina facility; some of these criteria are not contingent upon the Company’s performance.
The following is a maturity analysis of the annual undiscounted cash flows of the operating lease liabilities as of June 30, 2020:
| Year Ended March 31, | |||||
| 2021 | $ | 18,702 | |||
| 2022 | 24,936 | ||||
| 2023 | 24,936 | ||||
| 2024 | 24,936 | ||||
| 2025 | 24,936 | ||||
| Thereafter | 29,092 | ||||
| $ | 147,538 | ||||
| Less: Amount representing interest | (4,684 | ) | |||
| Present value of lease liabilities | $ | 142,854 | |||
| F-31 |
In compliance with ASC 842 the Company adopted new guidance in relation to lease accounting on April 1, 2019 whereby we recognized operating lease right-to-use assets and corresponding and equal operating lease liabilities for the lease of our facility in Edina, MN. As of June 30, 2020, planned future base rent lease payments total $147,538, which has been discounted to $142,854 using the 52-week treasury bill coupon equivalent discount rate of 2.18% and a present value model. As of June 30, 2020, the Company only had one operating lease so that the remaining lease term and weighted average discount rate are approximately 6 years and 2.18%, respectively.
| June 30, 2020 | ||||
| Present value of future base rent lease payments | $ | 142,854 | ||
| Base rent payments included in prepaid expenses | - | |||
| Present value of future base rent lease payments – net | $ | 142,854 | ||
As of June 30, 2020, the present value of future base rent lease payments – net is classified between current and non-current assets and liabilities as follows:
| June 30, 2020 | ||||
| Operating lease right-of-use asset | $ | 142,854 | ||
| Total operating lease assets | 142,854 | |||
| Operating lease current liability | 24,791 | |||
| Operating lease other liability | 118,063 | |||
| Total operating lease liabilities | $ | 142,854 | ||
Pursuant to a lease wherein our subsidiary, Gel-Del Technologies, Inc., was the lessee until the lease’s termination in 2017, the Company owes approximately $330,000 to the lessor as of June 30, 2020; this amount is included in accounts payable.
NOTE 4 – INVESTMENTS – EQUITY SECURITIES
On June 28, 2019, the Company entered into a purchase agreement with a third-party to purchase 1,500,000 shares of Emerald Organic Products, Inc. (OTC Pink: “EMOR”) common stock for consideration of $1,500. The Company applied guidance from ASU No. 2016-01 Financial Instruments – Overall – Recognition and Measurement of Financial Assets and Financial Liabilities and ASC 820 to arrive at a fair value at June 30, 2019 of $1,500. The Company took into account many factors when determining the stock’s fair value including, but not limited to, the nature and duration of the restriction on the stock, the extent to which potential buyers would be limited by the restriction, and qualitative and quantitative factors specific to both the instrument and the issuer.
NOTE 5 – PROPERTY AND EQUIPMENT
The components of property and equipment were as follows:
| June 30, 2020 | March 31, 2020 | |||||||
| Leasehold improvements | $ | 139,943 | $ | 98,706 | ||||
| Furniture and office equipment | 10,130 | 10,130 | ||||||
| Production equipment | 87,473 | 87,473 | ||||||
| R&D equipment | 25,184 | 25,184 | ||||||
| Total, at cost | 262,730 | 221,493 | ||||||
| Accumulated depreciation | (118,075 | ) | (111,586 | ) | ||||
| Total, net | $ | 144,655 | $ | 109,907 | ||||
During the three months ended June 30, 2020 and 2019, depreciation expense was $6,489 and $6,981, respectively.
| F-32 |
NOTE 6 – INTANGIBLE ASSETS
The components of intangible assets, all of which are finite-lived, were as follows:
| June 30, 2020 | March 31, 2020 | |||||||
| Patents | $ | 3,824,272 | $ | 3,822,542 | ||||
| Trademarks | 25,023 | 25,023 | ||||||
| Total, at cost | 3,849,295 | 3,847,565 | ||||||
| Accumulated Amortization | (3,834,470 | ) | (3,788,954 | ) | ||||
| Total, net | $ | 14,825 | $ | 58,611 | ||||
During the three-month periods ended June 30, 2020 and 2019, amortization expense was $45,316 and $137,109, respectively.
NOTE 7 – CONVERTIBLE NOTES AND NOTES PAYABLE
At June 30, 2020, the Company is obligated for $77,770 in notes payable and $326,643 in convertible notes payable.
At March 31, 2020, the Company is obligated for $66,350 in notes payable and $286,981 in convertible notes payable.
| June 30, 2020 | March 31, 2020 | |||||||||||||||||
| Notes Payable | Convertible Notes Payable | Notes Payable | Convertible Notes Payable | |||||||||||||||
| 1. | Third Parties – Principal | $ | 15,000 | $ | 632,941 | $ | 15,000 | $ | 280,000 | |||||||||
| Third Parties – Unamortized Debt Discount | - | (333,333 | ) | - | - | |||||||||||||
| Third Parties – Interest | 325 | 1,838 | 95 | 6,981 | ||||||||||||||
| Third Parties – Total | 15,325 | 301,446 | 15,095 | 286,981 | ||||||||||||||
| 2. | Related Parties – Principal | 59,642 | 25,000 | 59,642 | - | |||||||||||||
| Related Parties – Interest | 2,803 | 197 | 1,613 | - | ||||||||||||||
| Related Parties – Total | 62,445 | 25,197 | 61,255 | - | ||||||||||||||
| Total | $ | 77,770 | $ | 326,643 | $ | 76,350 | $ | 286,981 | ||||||||||
At June 30, 2020, the Company is obligated for one convertible note payable held by RedDiamond Partners, LLC (“RDCN”); the Company entered into the RDCN on June 15, 2020, whereby the note is convertible on or after January 15, 2021 and before maturity on March 15, 2021 at a rate of $.28/share. The RDCN was issued in the principal amount of $352,941 with $52,941 being made up of a 15% Original Issue Discount (“OID”) and included a conversion feature because there are exercise contingencies triggered by the occurrence of an Event of Default, which includes events that are outside the control of the Company (i.e. not based solely on the market for the Company’s stock or the Company’s own operations). Additionally, the RDCN accrues interest at a rate of 12.5% per annum, calculated on a 360-day-per-year-basis. At June 30, 2020, the Company owed $354,779 in outstanding balance whereby $352,941 was made up of principal and $1,838 was made up of accrued interest. This RDCN was issued alongside a warrant for purchase of 557,143 shares of Company common stock (“RDCN Warrants”) with a relative value of $91,500; see Note 13 for more information on these RDCN Warrants. The outstanding principal balance of the RDCN was reduced to $-0- by various discounts on the debt totaling ($352,941) as follows: i) the RDCN Warrants generated a discount on the debt of ($91,500) based on the relative value of the same; ii) $2,500 in investor legal costs was treated as a discount on the debt of ($2,500) since this was paid by the Company; iii) $52,941 of OID was treated as a discount on the debt of ($52,941); iv) a discount of ($206,000) was taken due to the conversion option being treated as a derivative. As of June 30, 2020, the Company had ($333,333) ($-0- at March 31, 2020) in unamortized debt discount remaining. In evaluating the various instruments and their components within this transaction (including issuance of the RDCN and RDCN Warrants) for treatment as a derivative and the respective accounting treatment of the same, the Company referenced ASC 470 and ASC 815 in conjunction with interpretive guidance. Subsequently, at June 30, 2020, the Company amortized a pro-rata portion of the discount on the debt on a straight-line basis to interest expense in the amount of $19,608. In conjunction with the RDCN and RDCN Warrants issuances, the Company also paid $30,000 and issued 75,000 warrants (“Think Warrants”) valued at $31,500 using the Black-Scholes model to Think Equity for soliciting the RedDiamond Partners, LLC transaction; see Note 13 for more information on these warrants. The total issuance costs paid to Think Equity of $61,500 of cash and warrants, which the Company recorded the relative value (as noted in Note 13) of $52,399 to expense since no further discount was available to be taken on the debt.
| F-33 |
At June 30, 2020 and March 31, 2020, the Company is/was obligated for several convertible notes payable held by accredited investors (“Accredited Investor Convertible Notes” or “AICN” or “AICNs”). At June 30, 2020 the total outstanding balance of these AICNs of $280,000 was made up of $280,000 in principal and $-0- in accrued interest. At March 31, 2020, the total outstanding balance of these AICNs of $286,981 was made up of $280,000 in principal and $6,981 in accrued interest. The Company entered into these AICNs during the quarter ended June 30, 2019. All of these AICNs mature during the quarter ended June 30, 2021, two years from their inception dates. These AICNs accrue interest at a rate of 10% annually. Accrued interest is due and payable each calendar quarter in cash; during the three-month periods ended June 30, 2020 and 2019, the Company paid out $13,962 and $5,362, respectively, in accrued interest to these AICN note holders. These AICNs automatically convert into shares of common stock at a rate of $.72 per share at the earlier of the maturity date or an uplist to a national securities exchange (e.g. Exchange or New York Stock Exchange) provided that the Company’s stock price is at least $.87 at the time of the uplist. The AICN note holders have the right to convert their outstanding principal and interest into shares of the Company’s common stock at any time during their note’s term at $.72 per share. No AICN note holders have converted their notes through the date of this report. As of June 30, 2020 and March 31, 2020, these AICNs did not include a beneficial conversion feature.
NOTE 8 – DERIVATIVE LIABILITY
The Company evaluates its convertible instruments, options, warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for under ASC Topic 815, “Derivatives and Hedging.” The result of this accounting treatment is that the fair value of the derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operation as other income (expense). Upon conversion or exercise of a derivative instruments, the instrument is marked to fair value at the conversion date then that fair value is reclassified to equity. Equity instruments that are initially classified as equity that become subject to reclassification under ASC Topic 815 are reclassified to liabilities at the fair value of the instrument on the reclassification date.
The Company used the following assumptions for determining the fair value of the conversion feature in the RDCN referenced in Note 7 to these financial statements, under the binomial pricing model with Monte Carlo simulations at June 15, 2020 and June 30, 2020, the issuance and balance sheet dates, respectively:
| June 15, 2020 | June 30, 2020 | |||||||
| Stock price on valuation date | $ | .42 | $ | .44 | ||||
| Conversion price | $ | .28 | $ | .28 | ||||
| Days to maturity | 273 | 258 | ||||||
| Weighted-average volatility* | 367 | % | 367 | % | ||||
| Risk-free rate | .18 | % | .18 | % | ||||
The initial valuation of $526,800 at June 15, 2020, generated a discount on the debt of $206,000, which net the convertible note liability to $-0- and forced a recognition of derivative expense of $320,800 and a corresponding offset to derivative liability of $526,800. At June 30, 2020, the Company revalued the derivative liability at $548,200 and recognized $21,400 to derivative expense and derivative liability.
| F-34 |
The Company recorded derivative liability transactions during the three-month period ended June 30, 2020 as follows:
| 3 Months Ended June 30, 2020 | ||||
| Convertible note embedded derivative liability | ||||
| Balance at March 31, 2020 | $ | -0- | ||
| Initial recognition of derivative liability | 526,800 | |||
| Change in fair value | 21,400 | |||
| Balance at June 30, 2020 | $ | 548,200 | ||
NOTE 9 – CONVERTIBLE NOTES AND NOTES PAYABLE – RELATED PARTY
At June 30, 2020 and March 31, 2020, the Company was obligated for a related party note payable and accrued interest in the total amount of $62,445 and $61,255, respectively; the maturity date of this note was April 30, 2020 and the Company is in default on this note as of the date of this filing. The related party note payable terms are accrual of interest at 8% annually with payments of $3,100 per month, which are applied to interest first, then principal. The Company is currently in negotiations to resolve this note that is in default and payable to Dr. David Masters.
At June 30, 2020, the Company was obligated for related party convertible notes payable principal and accrued interest in the total amount of $25,000 and $197, respectively, recorded to the Convertible notes and accrued interest payable - related party account. At March 31, 2020, the Company was not obligated for any related party convertible notes payable or accrued interest. These notes were entered into on May 14, 2020, accrue interest at 6% per annum, are convertible at $.2538 per share, and mature on August 14, 2020.
NOTE 10 – ACCOUNTS PAYABLE AND ACCRUED EXPENSES
At June 30, 2020 and March 31, 2020, the Company is obligated to pay $780,835 and $794,057, respectively, in accounts payable and accrued expenses. Of the total at June 30, 2020 of $780,835, $543,430 is made up of accounts payable, while the $237,404 in accrued expenses is made up of past employee’s accrued salaries and related payroll taxes payable. Of the total at March 31, 2020 of $794,057, $556,653 is made up of accounts payable, while the $237,404 in accrued expenses is made up of past employee’s accrued salaries and related payroll taxes payable. The potential payroll taxes owed are not due until the accrued compensation has been paid. Since the Company has not paid these accrued wages, the Company has appropriately left the potential payroll taxes associated with these accrued wages unpaid. The Company has established an accrued liability for the potential taxes of approximately $22,025 and $22,025 at June 30, 2020 and March 31, 2020, respectively.
NOTE 11 – PPP LOAN
On May 1, 2020, the Company received $38,665 in loan proceeds pursuant to the Paycheck Protection Program enacted by the 2020 US Federal government Coronavirus Aid, Relief, and Economic Security Act. At June 30, 2020, the Company was obligated for the outstanding balance of $38,730, made up of $38,665 in principal and $65 in accrued interest. The principal and accrued interest may be forgivable and the Company has applied for forgiveness. The loan accrues interest at a rate of 1% per annum and matures on May 1, 2022; if not forgiven prior to December 1, 2020, the Company is required to pay monthly installments toward principal and interest until the note is paid in full.
NOTE 12 – ACCRUED EXPENSES – RELATED PARTY
At June 30, 2020, the Company is obligated to pay $239,372 in accrued expenses due to related parties. Of the total, $24,882 is made up of accounts payable, while $214,490 is made up of accrued salaries and potential payroll taxes payable.
At March 31, 2020, the Company was obligated to pay $252,607 in accrued expenses due to related parties. Of the total, $17,502 was made up of accounts payable, while $235,105 is made up of accrued salaries and potential payroll taxes payable.
| F-35 |
NOTE 13 - COMMON STOCK AND WARRANTS
Common Stock
During the three-month period ended June 30, 2020 the Company issued 200,000 shares of common stock as follows:
i) 120,000 shares valued using the Black-Scholes model at $40,680 and recorded in Stock-based compensation to a service provider for video marketing services over a 6-month term;
ii) 80,000 shares with a relative value of $34,709 pursuant to a purchase of 80,000 units whereby a unit is made up of 1 share of common stock and ½ warrant. The value of $34,709 along with the relative value of the warrants associated with this transaction of $17,291 ($52,000 total) was recorded during the quarter ended March 31, 2020 to Common Stock Subscribed and moved to Additional Paid in Capital and Capital Stock upon receipt of funds and issuance of shares of common stock during the quarter ended June 30, 2020.
During the three-month period ended June 30, 2019 the Company did not issue any stock.
Warrants
During the three-month period ended June 30, 2020, the Company granted warrants to purchase a total of 827,491 shares of common stock, which is included in the vested stock based compensation of approximately $184,000, including:
i) warrants for 40,000 shares, valued at $17,291 using the Black-Scholes model, to one investor, whereby the value was recorded during the quarter ended March 31, 2020 to Common Stock Subscribed and moved to Additional Paid in Capital upon receipt of funds and issuance of warrants during the quarter ended June 30, 2020 and further whereas the warrants vested immediately upon issuance and are exercisable at $1.00 per share for 3 years from the grant date of April 6, 2020;
ii) warrants for 90,000 shares, valued at $28,964 using the Black-Scholes model, to John Lai, whereby the value was recorded to Stock-based compensation and the warrants vest upon the Company raising $10,000,000 or more through an S-1 offering as long as that occurs prior to October 31, 2020; if these warrants vest they will be exercisable for a period of 5-years at $.35 per share;
iii) warrants for 557,143 shares (RDCN Warrants), valued at $234,000 using the Black-Scholes model, to RedDiamond Partners, LLC, whereby the relative value of $91,500 was recorded to Warrants issued in conjunction with convertible debt on the statement of equity; the warrants have a cashless conversion feature, are exercisable at $.35 per share for a term of five years from the date of the grant of June 15, 2020 when the Company entered into a securities purchase agreement and issued a convertible note as outlined in Note 7;
iv) warrants for 75,000 shares (Think Warrants), valued at $31,500 using the Black-Scholes model, whereby the relative value of the warrants was recorded to Stock-based compensation, whereas the warrants are exercisable for 5 years from the date of the grant of June 15, 2020 at an exercise price of $.35 per share. These were issued to Think Equity as a placement fee for soliciting the RedDiamond transaction as described in roman numeral iii above and Note 7 to these financial statements;
v) warrants for 27,237 shares, valued at $11,984 using the Black-Scholes model, whereby the value of the warrants was recorded to Stock-based compensation, whereas the warrants are exercisable for 5 years from the date of the grant at $.40 per share to various directors of the Company for services on various committees;
vi) warrants for 29,762 shares, valued at $13,095 using the Black-Scholes model, whereby the value of the warrants was recorded to Stock-based compensation on the statement of equity, whereas the warrants are exercisable for 5 years from the date of the grant at $.40 per share to John Lai;
vii) warrants for 8,349 shares, valued at $3,674 using the Black-Scholes model, whereby the value of the warrants was recorded to Stock-based compensation on the statement of equity, whereas the warrants are exercisable for 5 years from the date of the grant at $.40 per share to a service provider for various consulting services.
| F-36 |
During the three-month period ended June 30, 2019, the Company granted warrants to purchase a total of 270,000 shares of common stock including:
i) warrants for 270,000 shares, valued at $119,954 using the Black-Scholes model, to three Directors, Messrs. Scott Johnson, Gregory Cash, and James Martin, with 135,000 vested immediately and 135,000 vesting quarterly between August 2020 and May 2021, and exercisable over a five-year term at $.33 per share.
A summary of warrant activity for the year ending March 31, 2020 and three-month period ending June 30, 2020 is as follows:
| Number
of Warrants | Weighted- Average Exercise Price | Warrants Exercisable | Weighted- Average Exercisable Price | |||||||||||||
| Outstanding, March 31, 2019 | 3,818,919 | .55 | 3,035,035 | .54 | ||||||||||||
| Granted | 1,905,700 | .52 | ||||||||||||||
| Cashless conversions | (337,500 | ) | .32 | |||||||||||||
| Expired | (90,000 | ) | .56 | |||||||||||||
| Canceled | (396,000 | ) | .58 | |||||||||||||
| Outstanding, March 31, 2020 | 4,901,119 | .53 | 4,072,369 | .53 | ||||||||||||
| Issued in conjunction with convertible debt | 632,143 | .35 | ||||||||||||||
| Sold | 40,000 | 1.00 | ||||||||||||||
| Granted | 155,348 | .37 | ||||||||||||||
| Outstanding, June 30, 2020 | 5,728,610 | .51 | 4,939,235 | .51 | ||||||||||||
At June 30, 2020, the range of warrant prices for shares under warrants and the weighted-average remaining contractual life is as follows:
| Warrants Outstanding | Warrants Exercisable | ||||||||||||||||||||
Range of Warrant Exercise Price | Number of Warrants | Weighted- Average Exercise Price | Weighted- Average Remaining Contractual Life (Years) | Number of Warrants | Weighted- Average Exercise Price | ||||||||||||||||
| .30-.50 | 3,087,192 | .37 | 4.71 | 3,182,817 | .33 | ||||||||||||||||
| .51-1.00 | 2,145,739 | .58 | 2.67 | 1,260,739 | .60 | ||||||||||||||||
| 1.01-3.89 | 495,679 | 1.42 | 2.11 | 495,679 | 1.42 | ||||||||||||||||
| Total | 5,728,610 | .51 | 3.72 | 4,939,235 | .51 | ||||||||||||||||
For the three-month periods ended June 30, 2020 and 2019, the total stock-based compensation on all instruments was $183,244 and $157,134, respectively. It is expected that the Company will recognize expense after June 30, 2020 related to warrants issued, outstanding, and valued using the Black Scholes pricing model as of June 30, 2020 in the amount of approximately $431,000.
| F-37 |
NOTE 14 – SUBSEQUENT EVENTS
Prior to the issuance date of this Form 10-Q and subsequent to the balance sheet date, the Company entered into an agreement to issue 10,000 shares of common stock to a service provider for legal services valued at $4,400; these shares remain unissued as of the date of filing of this Form 10-Q with the SEC.
Prior to the issuance date of this Form 10-Q and subsequent to the balance sheet date, the Company issued 40,000 shares of common stock to a service provider for investor relations services valued at $17,600.
Prior to the issuance date of this Form 10-Q and subsequent to the balance sheet date, the Company issued 61,027 shares of common stock pursuant to a warrant conversion whereby the warrant holder converted 168,750 warrants valued at $102,807 using the Black-Scholes pricing model into 61,027 shares of common stock.
On July 1, 2020, the Company issued 15,000 warrants to two directors of the Company, valued at $6,600, vesting in equal amounts over the two-month period ending August 31, 2020 at $.40 per share for a term of 5 years from the date of the grant.
On August 14, 2020, three directors, Messrs. Johnson, Martin, and Cash, who held convertible notes as detailed in Note 9, converted $25,382 in outstanding principal and interest into 100,008 shares of common stock.
| F-38 |
Units

PetVivo Holdings, Inc.
PROSPECTUS
ThinkEquity
a division of Fordham Financial Management, Inc.
, 2020
Through and including , 2020 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
| 69 |
PART II. INFORMATION NOT REQUIRED IN PROSPECTUS
ITEM 13. OTHER EXPENSES OF ISSUANCE AND DISTRIBUTION
We have expended, or will expend fees in relation to this registration statement as detailed below:
| Expenditure Item | Amount | |||
| Attorney Fees (estimated) | $ | * | ||
| Audit Fees (estimated) | $ | * | ||
| Transfer Agent Fees | $ | 5,000 | ||
| SEC Registration and Blue Sky Registration fees (estimated) | $ | * | ||
| Exchange Registration Fee | $ | [•] | ||
| Printing Costs and Miscellaneous Expenses (estimated) | $ | * | ||
Total |
$ | * | ||
*To be provided by amendment.
ITEM 14. INDEMNIFICATION OF DIRECTORS AND OFFICER
We are a Nevada corporation and generally governed by the Nevada Private Corporations Code, Title 78 of the Nevada Revised Statutes, or NRS.
Section 78.138 of the NRS provides that, unless the corporation’s articles of incorporation provide otherwise, a director or officer will not be individually liable unless it is proven that (i) the director’s or officer’s acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud, or a knowing violation of the law.
Section 78.7502 of the NRS permits a company to indemnify its directors and officers against expenses, judgments, fines, and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending, or completed action, suit, or proceeding, if the officer or director (i) is not liable pursuant to Section 78.138 of the NRS, or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful. Section 78.7502 of the NRS also precludes indemnification by the corporation if the officer or director has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court determines that in view of all the circumstances, the person is fairly and reasonably entitled to indemnity for such expenses and requires a corporation to indemnify its officers and directors if they have been successful on the merits or otherwise in defense of any claim, issue, or matter resulting from their service as a director or officer.
Section 78.751 of the NRS permits a Nevada corporation to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit, or proceeding as they are incurred and in advance of final disposition thereof, upon determination by the stockholders, the disinterested board members, or by independent legal counsel. Section 78.751 of the NRS provides that the articles of incorporation, the bylaws or an agreement may require a corporation to advance expenses as incurred upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the corporation if so provided in the corporation’s articles of incorporation, bylaws, or other agreement. Section 78.751 of the NRS further permits the corporation to grant its directors and officers additional rights of indemnification under its articles of incorporation, bylaws, or other agreement.
| 70 |
Section 78.752 of the NRS provides that a Nevada corporation may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee, or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee, or agent of another company, partnership, joint venture, trust, or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee, or agent, or arising out of his status as such, whether or not the corporation has the authority to indemnify him against such liability and expenses. We have obtained primary and excess insurance policies insuring our directors and officers and our subsidiaries against certain liabilities they may incur in their capacity as directors and officers. Under such policies, the insurer, on our behalf, may also pay amounts for which we have granted indemnification to the directors or officers.
The foregoing discussion of indemnification merely summarizes certain aspects of indemnification provisions and is limited by reference to the above discussed sections of the Nevada Private Corporations Code.
Indemnification for Securities Act Liabilities
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, or the Securities Act, may be permitted for directors, officers, or controlling persons pursuant to the provisions described in the preceding paragraph, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable
ITEM 15. RECENT SALES OF UNREGISTERED SECURITIES
During the three years preceding the filing of this registration statement, we issued and sold the following securities that were not registered under the Securities Act:
2017
In April 2017, the Company issued an aggregate of 60,000 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to two accredited investors at a price of $0.35 for total proceeds of $21,000.
In May 2017, the Company issued an aggregate of 14,300 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to an accredited investor at a price of $0.35 for total proceeds of $5,005.
In July 2017, the Company issued an aggregate of 60,000 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to two accredited investors at a price of $0.35 for total proceeds of $21,000.
In August 2017, the Company issued an aggregate of 57,143 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to an accredited investor at a price of $0.35 for total proceeds of $20,000.
In September 2017, the Company issued an aggregate of 510,714 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to eight accredited investors at a price of $0.35 for total proceeds of $178,750.
In September 2017, the Company issued 4,905,000 shares of its common stock on a pro rata basis to shareholders of Gel-Del Technologies, Inc. to complete the acquisition of all of Gel-Del’s outstanding capital stock, for aggregate consideration of $2,160,000 (which assumed a price of $0.44 per share).
In October 2017, the Company issued an aggregate of 727,844 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to sixteen accredited investors at a price of $0.35 for total proceeds of $254,745.
| 71 |
In November 2017, the Company issued an aggregate of 45,000 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to three accredited investors at a price of $0.35 for total proceeds of $15,750.
In November and December 2017, the Company issued an aggregate of 384,559 shares of its common stock pursuant to convertible note conversions by two accredited investors in the aggregate amount of $181,967.
In December 2017, the Company issued an aggregate of 25,000 units, consisting of 1 share of common stock and 1 warrant for purchase of 1 share of common stock at a price of $1.00 per unit, to an accredited investor at a price of $0.35 for total proceeds of $8,750.
On December 29, 2017, the Company issued 250,000 shares of its common stock to an accredited investor at a price of $1.00 per share in a private offering.
2018
In February 2018, the Company engaged a new patent law firm and granted the law firm warrants to purchase 18,000 shares of its common stock at an exercise price of $1.11 per share with a three year term.
On April 20, 2018, the Company issued 2,093 shares of its common stock to a service provider for website consulting services valued at $2,700.
On April 23, 2018, the Company issued 85,916 shares of common stock pursuant to an assignment of debt held in accounts payable to an accredited investor in an aggregate amount of $95,462.
On April 30, 2018, the Company granted warrants to purchase for 72,000 shares to John Carruth, the Company’s Controller, in consideration for his employment, vesting quarterly over two years, and exercisable over a five-year term at an exercise price of $1.11 share.
In May 2018, the Company issued 723,047 shares of its common stock to John Lai, the Company’s President, including 430,796 shares to replace shares he placed into escrow in 2017 valued at $861,592 and 292,251 shares valued at $584,500, to replace shares given up to obtain financing in 2015.
In May 2018, the Company granted warrants to purchase: (i) 72,000 shares of the Company’s common stock to two advisory board members for their service on the Company’s advisory board, vesting semi-annually over two years, and exercisable over a five-year term at an exercise price of $1.11 per share; (ii) 27,000 shares to a lawyer for general legal counsel, fully-vested and exercisable over a five-year term at an exercise price of $1.11 per share and (iii) warrants to purchase 60,000 shares of the Company’s common stock to various information technology service providers for information technology services, to vest pursuant to the provider’s invoices, exercisable over a five-year term.
In June and July 2018, the Company issued 166,393 shares to six accredited investors for cash upon exercise of their warrants at an exercise price of $0.50 per share.
On July 15, 2018, the Company issued 22,500 shares a service provider for marketing services valued at $24,750.
On October 2, 2018, the Company issued 22,500 shares of common stock to an accredited investor for cash upon exercise of its warrants at an exercise price of at $0.56 per share.
In December 2018, the Company issued 778,240 shares of its common stock to 15 investors upon exercise of their warrants at an exercise price of $0.30 per share, for aggregate gross proceeds of $233,472.
In December 2018, the Company issued 753,338 shares of its common stock to 7 noteholders pursuant to their note conversion agreements whereby principal and interest in an aggregate amount of $226,001 were converted into common stock at a rate of $0.30 per share.
| 72 |
2019
On March 31, 2019, the Company returned to treasury an aggregate of 540,000 shares of its common stock pursuant to the termination of an escrow agreement between the Company and Wesley Hayne, its former Chief Executive Officer.
In June 2019, the Company granted an aggregate of 270,000 warrants to three of its directors for two years of Board service. 135,000 warrants vested immediately, while 135,000 warrants vest in equal tranches of 33,750 each quarter during the second year of Board service. All 270,000 warrants have an exercise price of $0.33 per share and expire five years after the date of issuance.
On July 13, 2019, the Company issued 108,000 shares to a service provider for services provided during the one-year period ended July 13, 2019, valued at $1.11 per share over such period on a pro-rata basis.
On September 11, 2019, the Company issued an aggregate of 1,295,866 shares of common stock to 2 executives and 1 former employee and current director for settlement of an aggregate of $455,965 in accrued salary and release of all claims against the Company for unpaid compensation.
On September 13, 2019, the Company sold 360,000 shares of common stock at a price of $0.28 per share to one shareholder for gross proceeds of $100,000 in a private offering.
On September 18, 2019, the Company issued 270,000 shares of its common stock to a service provider for services to be provided during the one-year period ended December 31, 2020, which included video production, investor relations and promotional services. The period of service was scheduled to begin on September 18, 2019, but the parties have delayed the start date until the first video to be produced by such service provider airs on Bloomberg Television Network.
On October 18, 2019, the Company issued 90,000 shares of its common stock to a service provider for marketing services to be provided during the six-month period ending April 18, 2020.
In October 2019, the Company sold 486,000 shares of its common stock to four accredited and/or sophisticated investors at a price of $0.28 per shares, with aggregate offering proceeds of $135,000.
On December 9, 2019, the Company issued 150,000 shares of its common stock, valued at $0.47 per share, to a service provider for investor relations services.
On December 31, 2019, the Company sold and agreed to issue 160,000 units consisting of one share of common stock and one warrant to purchase one half of one share of the Company’s common stock in exchange for $104,000, which equates to $0.65 per unit, to an accredited investor. The exercise price of the warrants is $1.00 per share and the warrants expire on December 31, 2022.
2020
On January 10, 2020, the Company issued 63,141 shares of common stock to a former Director of the Company pursuant to a cashless conversion feature within the former Director’s warrant for 168,750 shares, equating to a conversion rate of 0.37:1.00.
On January 31, 2020, the Company issued 61,396 shares of common stock to a John Lai, the CEO of the Company, pursuant to a cashless conversion feature within his warrant for 168,750 shares, equating to a conversion rate of 0.36:1.00.
On February 25, 2020, the Company sold and agreed to issue 80,000 units consisting of one share of common stock and one warrant to purchase one half of one share of the Company’s common stock, in exchange for $52,000, which equates to $0.65 per unit, to an accredited investor. The exercise price of the warrants is $1.00 per share and the warrants expire on April 6, 2023.
On June 8, 2020, the Company granted warrants to purchase for 90,000 shares of the Company’s common stock to John Lai, which will vest upon the Company raising $10,000,000 or more in a registered public offering on Form S-1 offering, as long as such offering occurs prior to October 31, 2020. If the warrants vest at all, they will be exercisable until June 8, 2025 at an exercise price of $0.35 per share.
| 73 |
On June 15, 2020, the Company issued and sold to Red Diamond Partners LLC, an accredited investor, a convertible note (“Note”) in the principal amount of $352,941 and warrants to purchase 557,143 shares of the Company’s common stock for gross proceeds of $300,000 (representing an original issue discount of 15% on the convertible note) (the “Financing”). The note matures on January 15, 2021. The Note bears interest at the rate of 12.5% per annum and is convertible into shares of the Company’s common stock at a conversion price equal to $0.28 per share or upon the occurrence or continuance of an event of default, if lower, at a conversion price equal to 70% of the variable averaged weight price of the Company’s common stock during the 15 consecutive trading days prior to the conversion date. The warrants are exercisable to purchase shares of common stock for a purchase price of $0.35 per share, subject to adjustment, at any time on or prior to June 15, 2025, and may be exercised on a cashless basis if the shares of common stock underlying the warrants are not then registered under the Securities Act.
In connection with the Financing transaction, the Company entered into an Engagement Agreement (the “Engagement Agreement”) with ThinkEquity, a division of Fordham Financial Management, Inc. (“ThinkEquity”), a FINRA registered broker dealer, pursuant to which we paid ThinkEquity (a) a cash fee of $30,000, which is equal to 10% of the gross proceeds received by the Company in the Financing and (b) warrants to purchase up to 10% of the aggregate number of shares of common stock underlying the purchase price paid for the Notes, which, in the case of the initial closing, equals 75,000 shares of common stock, at an exercise price of $0.35 (the “Placement Agent Warrants”).
The Placement Agent Warrants are exercisable, in whole or in part, commencing on June 15, 2020 and have an exercise period of five years. In the event that there is not an effective registration statement permitting for the resale of the shares underlying the Placement Agent Warrants, the Placement Agent Warrants shall be exercisable on a cashless basis. There are significant restrictions pursuant to FINRA Rule 5110 against transferring the Placement Agent’s Warrants and the shares issuable upon exercise of the Placement Agent Warrants during the 180 days after the closing date of such transaction.
On June 30, 2020, Company granted warrants to purchase 27,237 shares of its common stock to seven directors for their services on various committees of the Board. The exercise price of the warrants is $0.40 per share and the warrants are exercisable for 5 years from the date of the grant.
On July 1, 2020, the Company issued 10,000 shares of common stock to a service provider for legal services valued at $4,400.
On July 1, 2020, the Company granted 15,000 warrants to two directors of the Company, at a price of $0.40 per share, vesting in equal amounts over the two-month period ending August 31, 2020 for a term of 5 years from the date of the grant.
On August 14, 2020, three directors, Messrs Johnson, Martin, and Cash, who held convertible notes converted $25,382 in outstanding principal and interest into 100,008 shares of common stock.
Exemption from Registration
All of the issuances described herein were exempt from registration under the Securities Act in reliance on Section 4(a)(2) of the Securities Act, as a transaction by an issuer not involving a public offering. The purchasers of securities in each of these transactions represented their intention to acquire the securities for investment only and not with a view to offer or sell, in connection with any distribution of the securities, and appropriate legends were affixed to the share certificates and instruments issued in such transactions.
| 74 |
Item 16. Exhibits and Financial Statements
| 75 |
Item 17. Undertakings
a) The undersigned registrant hereby undertakes:
(a)(1) The undersigned registrant hereby undertakes to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: (i) To include any Prospectus required by Section 10(a)(3) of the Securities Act of 1933; (ii) To reflect in the Prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of Prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and (iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
(2) The undersigned registrant hereby undertakes that, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) The undersigned registrant hereby undertakes to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(h) Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and will be governed by the final adjudication of such issue.
| 76 |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the Registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Edina, Minnesota on October 13, 2020.
| PETVIVO HOLDINGS INC. | ||
| By: | /s/ John Lai | |
| Name: | John Lai | |
| Title: | President and Chief Executive Officer | |
| 77 |
KNOW ALL MEN BY THESE PRESENTS, that the undersigned officers and directors of PetVivo Holdings Inc., a Nevada corporation (the “Corporation”), hereby constitute and appoint John Lai the true and lawful agent and attorney-in-fact of the undersigned with full power and authority in said agent and attorney-in-fact to sign for the undersigned and in their respective names as an officer/director of the Corporation, any and all amendments (including post-effective amendments) to this registration statement on Form S-1 (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act) and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, and with full power of substitution, hereby ratifying and confirming all that said attorney-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Capacity | Date | ||
| /s/ John Lai | President and Chief Executive Officer and Director | October 13, 2020 | ||
| John Lai | (Principal Executive Officer) | |||
| /s/ John Carruth | Chief Financial Officer | October 13, 2020 | ||
| John Carruth | (Principal Financial Officer and Principal Accounting Officer) |
|||
| /s/ John Dolan | General Counsel and Director | October 13, 2020 | ||
| John Dolan | ||||
| /s/ Gregory D. Cash | Chairman | October 13, 2020 | ||
| Gregory D. Cash | ||||
| /s/ David Deming | Director | October 13, 2020 | ||
| David Deming | ||||
| /s/ Joseph Jasper | Director | October 13, 2020 | ||
| Joseph Jasper | ||||
| /s/ Scott Johnson | Director | October 13, 2020 | ||
| Scott Johnson | ||||
| /s/ David A. Masters, Ph.D. | Director | October 13, 2020 | ||
| David A. Masters, Ph.D. | ||||
| /s/ James Martin | Director | October 13, 2020 | ||
| James Martin | ||||
| /s/ Randall A. Myer | Director | October 13, 2020 | ||
| Randall A. Myer | ||||
| /s/ Robert Rudelius | Director | October 13, 2020 | ||
| Robert Rudelius |
| 78 |
