Attached files
| file | filename |
|---|---|
| EX-23.2 - EXHIBIT 23.1 - YIELD10 BIOSCIENCE, INC. | ytens-1ex2320201008.htm |
| EX-5.1 - EXHIBIT 5.1 - YIELD10 BIOSCIENCE, INC. | yten-sx1exh5120201008.htm |
As filed with the Securities and Exchange Commission on October 8, 2020
Registration Statement No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM S‑1
REGISTRATION STATEMENT
under the
SECURITIES ACT OF 1933
YIELD10 BIOSCIENCE, INC.
(Exact Name of Registrant as Specified in Its Charter)
Delaware | 2870 | 04-3158289 |
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (IRS Employer Identification No.) |
19 Presidential Way
Woburn, Massachusetts 01801
(617) 583-1700
(Address, including zip code, and telephone number, including area
code, of registrant’s principal executive offices)
Dr. Oliver P. Peoples
President & Chief Executive Officer
Yield10 Bioscience, Inc.
19 Presidential Way
Woburn, Massachusetts 01801
(617) 583-1700
(Name, address, including zip code, and telephone number, including area
code, of agent for service)
_____________________________
Copies to:
Megan N. Gates, Esq.
Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C.
One Financial Center
Boston, Massachusetts 02111
(617) 542-6000
Approximate date of commencement of proposed sale to the public: From time to time after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box. ý
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | o | Accelerated filer | o | |
Non-accelerated filer | ý | Smaller reporting company | ý | |
Emerging growth company | o | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. o
_____________________________
CALCULATION OF REGISTRATION FEE
Title of each class of securities to be registered (1) | Amount to be Registered (1) | Proposed Maximum Offering Price per Share (2) | Proposed Maximum Aggregate Offering Price | Amount of Registration Fee | |||||
Common stock, par value $0.01 per share | 396,450 | $ | 7.51 | 2,977,339.50 | $ | 324.83 | |||
(1) | All of the shares of common stock offered hereby are for the account of the selling stockholders. Pursuant to Rule 416 of the Securities Act of 1933, as amended (the “Securities Act”), this registration statement also covers any additional shares of common stock which become issuable by reason of any share dividend, share split, recapitalization or any other similar transaction without receipt of consideration which results in an increase in the number of shares of common stock outstanding. |
(2) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(c) under the Securities Act based upon the average of the high and low prices for a share of the registrant’s common stock as reported on the Nasdaq Capital Market on October 5, 2020, which date is within five business days of the filing of this registration statement. |
_____________________________
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
THE INFORMATION IN THIS PROSPECTUS IS NOT COMPLETE AND MAY BE CHANGED. THE SECURITY HOLDERS IDENTIFIED IN THIS PROSPECTUS MAY NOT SELL THESE SECURITIES UNTIL THE REGISTRATION STATEMENT FILED WITH THE SECURITIES AND EXCHANGE COMMISSION IS EFFECTIVE. THIS PROSPECTUS IS NOT AN OFFER TO SELL THESE SECURITIES AND IS NOT SOLICITING AN OFFER TO BUY THESE SECURITIES IN ANY STATE WHERE THE OFFER OR SALE IS NOT PERMITTED.
SUBJECT TO COMPLETION, DATED OCTOBER 8, 2020
PRELIMINARY PROSPECTUS

YIELD10 BIOSCIENCE, INC.
396,450 SHARES OF COMMON STOCK
This prospectus relates to the proposed resale of up to 396,450 shares of Yield10 Bioscience, Inc. (the “Company”), common stock, $0.01 par value per share.
These shares will be resold from time to time by the entities or individuals listed in the section titled “Selling Security Holders” beginning on page 55, which we refer to as the selling security holders or Selling Stockholders. The shares of common stock offered under this prospectus by the selling security holders were issued pursuant to the Securities Purchase Agreement by and among the Company and the selling security holders, dated as of August 22, 2020 (the “Securities Purchase Agreement”). We are not selling any securities under this prospectus and will not receive any of the proceeds from the sale of securities by the selling security holders.
The selling security holders may sell the shares of common stock described in this prospectus in a number of different ways and at varying prices. We provide more information about how a selling security holder may sell its shares of common stock in the section titled “Plan of Distribution” on page 57. We will pay the expenses incurred in registering the securities covered by the prospectus, including legal and accounting fees.
Our common stock is traded on The Nasdaq Capital Market, or Nasdaq, under the symbol “YTEN”. On October 7, 2020, the last reported sale price of our common stock was $7.60 per share.
_______________________
AN INVESTMENT IN OUR COMMON STOCK INVOLVES RISKS. SEE THE
SECTION ENTITLED “RISK FACTORS” BEGINNING ON PAGE 8.
_______________________
Neither the Securities and Exchange Commission nor any state securities commission has
approved or disapproved of these securities or determined if this prospectus is truthful
or complete. Any representation to the contrary is a criminal offense.
_______________________
The date of this prospectus is October [●], 2020
TABLE OF CONTENTS
Prospectus Summary | |
The Offering | |
Risk Factors | |
Special Note Regarding Forward-Looking Statements | |
Use of Proceeds | |
Market for Our Common Stock | |
Dividend Policy | |
Business | |
Principal Stockholders | |
Selling Security Holders | |
Plan of Distribution | |
Description of Our Capital Stock | |
Disclosure of Commission Position on Indemnification for Securities Act Liabilities | |
Legal Matters | |
Experts | |
Where You Can Find More Information | |
Incorporation of Certain Documents by Reference | |
You should read this prospectus and any applicable prospectus supplement before making an investment in the securities of Yield10 Bioscience, Inc. See “Where You Can Find More Information” for more information. You should rely only on the information contained in this prospectus or a prospectus supplement. The Company has not authorized anyone to provide you with different information. This document may be used only in jurisdictions where offers and sales of these securities are permitted. You should assume that information contained in this prospectus, or in any prospectus supplement, is accurate only as of any date on the front cover of the applicable document. Our business, financial condition, results of operations and prospects may have changed since that date. Unless otherwise noted in this prospectus, “Yield10 Bioscience,” “Yield10,” “the Company,” “we,” “us,” “our” and similar terms refer to Yield10 Bioscience, Inc.
Smaller Reporting Company – Scaled Disclosure
Pursuant to Item 10(f) of Regulation S-K promulgated under the Securities Act of 1933, as amended (the "Securities Act"), as indicated herein, we have elected to comply with the scaled disclosure requirements applicable to “smaller reporting companies,” including providing two years of audited financial statements.
iv
PROSPECTUS SUMMARY
This summary highlights some information from this prospectus. It may not contain all the information important to making an investment decision. You should read the following summary together with the more detailed information regarding our Company and the securities being sold in this offering, including “Risk Factors” and other information incorporated by reference herein.
Business Overview
Yield10 Bioscience, Inc. is an agricultural bioscience company that uses its "Trait Factory" and the Camelina oilseed “Fast Field Testing” system to develop high value seed traits for the agriculture and food industries. Yield10 is headquartered in Woburn, Massachusetts and has an Oilseed Center of Excellence in Saskatoon, Saskatchewan, Canada. Our goal is to efficiently develop superior gene traits for the major crops including corn, soybean, canola, and other crops to enable step-change increases in crop yield of at least 10-20 percent. Our “Trait Factory” encompasses discovery of gene targets using our GRAIN (“Gene Ranking Artificial Intelligence Network”) big data mining platform, deployment of trait gene targets in the oilseed Camelina and generation of field performance data. The “Trait Factory” enables two complementary commercial opportunities with different paths to market. The first is trait licensing to the major seed companies for corn, soybean, canola and other crops. Data from our trait field testing in Camelina has enabled Yield10 to establish research license agreements with leading seed companies including the Bayer Crop Science division of Bayer AG ("Bayer"), GDM, Forage Genetics International, LLC, a division of Land O'Lakes, Inc. ("Forage Genetics") and JR Simplot Company ("Simplot"). These companies are progressing the development of Yield10 traits in soybean, forage sorghum, and potato, respectively. The second commercial opportunity is to improve the performance and value of Camelina as a platform to develop a commercial crop product business producing nutritional oils and PHA biomaterials. Using this approach, Yield10 can leverage the resources of the major seed companies to efficiently develop superior gene traits for the major crops thereby creating opportunities for licensing revenue while focusing internal resources on trait gene discovery and the commercial development of Camelina products.
Our focus in the near term is to develop a revenue generating business using Camelina to produce nutritional oils. Yield10 has discovered a series of performance gene trait leads for Camelina focused on seed yield and oil content, the two primary drivers of value. Our plan is to focus on these traits using genome editing which can be qualified as non-regulated under U.S. Department of Agriculture ("USDA") Animal and Plant Health Inspection Service ("APHIS") rules. In parallel, the Company plans to establish a program to improve the agronomics of Camelina, including the development of herbicide tolerant Camelina lines. We believe this will enable Yield10 to develop a crop oil product business with a clear path to revenue and growth. This foundation will form a strong base to produce PHA biomaterials in the longer term for use in water treatment and plastics replacement applications. Yield10 believes crop based PHA biomaterials represent a compelling new market opportunity for agriculture addressing a non-traditional market with high upside potential.
Yield10 brings a unique history and skill set, captured in our GRAIN data mining gene discovery platform, for developing advanced crop traits and increasing the concentration of specific biochemicals of commercial interest in crops. Our plan is to also use GRAIN to develop a source of revenue from funded research and development collaborations for traits, products and crops not being directly pursued internally. We are currently engaged in a range of discussions with third parties with respect to different crops, traits and products in the feed, food and pharmaceutical sectors.
Over the last four years, we have been evaluating certain of our traits in greenhouse studies and field tests conducted in the United States and Canada. We currently have four non-exclusive research license agreements in place: with the Crop Science division of Bayer, for the evaluation of our C3003 and C3004 traits in soybean; with GDM for three traits in soybean; with Forage Genetics for the evaluation of five yield traits in forage sorghum; and with Simplot for evaluation of three of our traits in potato. We have progressed our evaluation of C3003 and C3004 in field tests with Camelina and canola and are continuing our field testing in the 2020 growing season. Results to date in Camelina have demonstrated the potential of a series of traits, including C3003 and C3004 to significantly increase seed yield and genome edited traits including C3007-C3010 to increase seed oil content and filed a new patent application on a potentially breakthrough technology for producing PHA biomaterials.
1
According to a United Nations report, crop production must be increased by over 70 percent in the next 35 years to feed the growing global population, which is expected to increase from 7 billion to more than 9.6 billion by 2050. During that time period, there will be a reduction in available arable land as a result of infrastructure growth and increased pressure on scarce water resources. Consumption of meat, seafood, and dairy products is also expected to increase based on dietary changes associated with increasing wealth and living standards. This will result in increased demand for feed grains and forage crops, and that demand will need to be met with an increasing emphasis on sustainable growth metrics and climate change. Seafood production is increasingly based on aquaculture where fish diets have been increasingly moving to crop-based feed ingredients due to the limited availability and cost of processed ocean harvested fish as feed. Fish oil is the main source of omega-3 fatty acids which are essential in the human diet. Omega-3 oils have been shown to help prevent heart disease and stroke, may help control lupus, eczema, and rheumatoid arthritis, and may play protective roles in cancer and other conditions. Oils high in omega-3 fatty acids are in increasing demand as the supply of fish oil from ocean harvest is under increasing pressure. Aquaculture and other feed markets represent a growing opportunity for Camelina oil, which is high in the omega-3 fatty acid alpha linolenic acid (“ALA”).
Harvestable food production per acre and per growing season must be increased to meet this demand. At the same time, with the increasing focus on health and wellness, food safety and sustainability in developed countries, we anticipate a rise in demand for new varieties of food and food ingredients with improved nutritional properties. With crop intensification (less land available and more production needed), we expect that improved crop genetics based on new gene traits will be a key driver of increased productivity, potentially resulting in the best performing yield traits commanding disproportionate value and disrupting the seed sector. We expect farmers and growers to be the major beneficiaries of these drivers, which represent potential opportunities for increased revenue and crop diversification. Today the global food market has an estimated value of $5 trillion.
Yield10 brings unique capabilities and experience in advanced metabolic engineering and systems biology to optimize photosynthesis and carbon efficiency in crops to increase grain or biomass yield. These capabilities were developed based on sustained investment over many years when the company was named Metabolix. As Metabolix, we solved complex biological problems in industrial/synthetic biology to produce bioplastics. By 2012, we had begun work to increase photosynthesis in crops as part of those activities, which led to the creation in 2015 of the current Yield10 business focused on crop yield. In mid-2016 we sold our fermentation-based bioplastics assets to focus on our agricultural innovations and the company was rebranded as Yield10 Bioscience in January 2017.
Business Strategy
Our goal is to build a successful agricultural biotechnology company centered on demonstrating and capturing the value of our traits and technologies based on the following three potential revenue streams:
• | Licensing of our yield and performance traits for use in major row crops; |
• | Product sales revenue from products produced in the oilseed Camelina; and |
• | R&D revenue for access to our GRAIN trait gene discovery platform. |
Using our Trait Factory, we have identified and are evaluating novel yield trait genes to help address the growing global yield gap in food and feed crops. Crop yield is the key decision variable for farmers in making seed buying decisions, and as a result, is critical to the seed industry. Improvements in yield to the levels targeted by Yield10, for example 10-20 percent increases, would be expected to generate significant value to the seed and farm sectors. For example, Yield10 is targeting an approximately 10-20 percent increase in canola and soybean yields, which, if successfully deployed across North American acreage, could result in annual incremental crop value of up to $10 billion. By ultimately increasing the output of major food and feed crops and potentially reducing strains on scarce natural resources, we believe that Yield10’s technologies will also contribute to addressing global food security.
Yield10 plans to develop yield traits that enable farmers to increase their revenue, and also to license our trait innovations to the major agricultural companies so that they can be deployed in elite seed varieties. Performance traits result in increased harvest value, which is then shared based on the well-established value sharing
2
model in the seed sector. Yield10 plans to continue to focus on its core competency of advanced trait gene discovery through the Trait Factory, while also building an independent, revenue generating, specialty products business based on the Camelina oilseed.
Our C3003 yield trait is an algal gene, and we believe that it will be regulated by USDA-APHIS and other regulatory agencies in the U.S. and around the world as a biotech trait. In 2017, we signed a non-exclusive research license with the Crop Science division of Bayer AG ("Bayer") (formerly Monsanto Company), to test C3003 and the first version of C3004 in soybean. In 2019, the license was expanded to cover a new discovery and intellectual property related to a new version of C3004. Similarly, in 2018 we signed a non-exclusive research license with Forage Genetics, to test a series of traits in forage sorghum. In 2019 we signed a non-exclusive research license with Simplot for the evaluation of our traits in potato. In 2020, we signed a non-exclusive research license with GDM for the evaluation of three traits in elite soybean germplasm. We have been progressing our traits internally in canola and in corn on a fee for service basis but plan to look for partners for our traits in both crops this year. Yield10 significantly expands the development pipeline by enabling the licensees to progress our traits in major crops. Our focus is on securing a share of the upside value of our traits when we finalize the economic terms of license agreements at the point where the value of the trait is well understood.
We believe we can leverage our seed yield and oil content traits to add value to Camelina in North America in the near term. We will focus our initial development activities on the production of nutritional oils for human and aquaculture feed markets using traits that can be qualified as non-regulated by USDA-APHIS to build our commercial capabilities.
The production of PHA biomaterials in Camelina could open new markets and provide economic returns for farmers to justify large acreage adoption and enable the low-cost production of this natural biodegradable product for water treatment and plastics replacement applications. We believe crop-based production will enable an advantageous cost structure thereby eliminating one of the remaining significant barriers to entry for broad adoption of these biomaterials. By reprogramming Camelina to produce PHA in the seed, the harvested seed can then be processed to produce three products: oil, protein meal for animal feed, and PHA biomaterial. The typical costs for producing edible oils are a useful benchmark for the potential long-term cost structure for crop based PHAs. In this scenario, crop based PHAs would have a cost advantage over petroleum-based plastics.
In water treatment, the PHA biomaterial acts as a growth substrate and energy source for denitrifying bacteria which convert nitrate, a primary cause of water pollution and algal growth, to nitrogen gas which returns to the air. This end use application of PHA biomaterials is expected to be straight-forward, requiring only the production and shipment of PHA biomaterials in pellet form. Yield10 is in the early stages of developing a revenue generating business model for this opportunity.
PHA biomaterials are also useful for functionally replacing petroleum-based plastics in a wide range of packaging applications. For example, the plastics industry produces more than 350 million tons of material per year globally. This sector is facing intensive scrutiny due to increasing plastic waste pollution in the environment. As natural biomaterials, PHAs fully degrade over time in the environment yet have good processing and physical properties and can be processed like plastics to produce articles with excellent shelf life in use. When we made the transition to the Yield10 business we divested our fermentation based PHA bioplastics assets and related applications technology. However, Yield10 retained the rights to PHA production in engineered crops. Yield10 plans to eventually look for partners to produce resin-grade PHA biomaterial for supply to the plastics sector but will focus the initial launch on water treatment applications.
We are at an early stage of developing a detailed plan for the Camelina business but believe it may have considerable potential for Yield10. Completing this business plan is a key goal for 2020.
Risks Affecting Us
Our business is subject to a number of risks and uncertainties that you should understand before making an investment decision. For example, we have a history of net losses and our business may not achieve commercial success. Furthermore, our technologies are in the early stages of development and we may never commercialize a technology or product that will generate meaningful, or any, revenues. A portion of our revenue to date has been
3
from government grants. Over time, we expect our revenue to shift from being derived primarily from collaborations and government grants to royalties based on licensing of Yield10 traits and/or sales derived from niche crop products based on our technologies, but we may not be successful in achieving this transition. As of June 30, 2020, we had an accumulated deficit of $370.3 million. With the exception of 2012, we have incurred losses since our inception. We expect to have significant losses and negative cash flow for at least the next several years, as we incur additional costs and expenses for the continued development of our technology, including the ongoing expenses of research, development, commercialization and administration. The Company held unrestricted cash, cash equivalents and short-term investments of $8.5 million at June 30, 2020. Based on our cash forecasts, we believe that together with the proceeds of the registered offering and private placement completed in August 2020, we have sufficient cash, cash equivalents and short-term investments to fund operations through the end of calendar 2021. Risks are discussed more fully in the section entitled “Risk Factors” following this prospectus summary. These risks include, but are not limited to, the following:
• | We have a history of net losses and our future profitability is uncertain. |
• | We will be required to raise additional funds to finance our operations and remain a going concern; we may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us. |
• | Raising additional funds may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies. |
• | Our technologies in the area of crop science are at a very early stage of development. We may never commercialize a technology or product that will generate meaningful, or any, revenues. |
• | A portion of our revenue to date has been generated from government grants; continued availability of government grant funding is uncertain and contingent on compliance with the requirements of the grant. |
• | Our government grants may subject us to government audits, which could expose us to penalties. |
• | Our crop science product development cycle is lengthy and uncertain and will depend heavily on future collaborative partners. |
• | Even if we or our collaborators are successful in developing commercial products that incorporate our traits, such products may not achieve commercial success. |
• | If ongoing or future field trials conducted by us or our collaborators are unsuccessful, we may be unable to complete the regulatory process for, or commercialize, our products in development on a timely basis. |
• | We may not be successful using our Camelina platform to develop and commercialize niche crops to produce specialty oils and/or PHA biomaterials. |
• | Consumer and government resistance to genetically modified organisms may negatively affect the ability to commercialize crops containing our traits, as well as our public image. |
• | We may not be able to obtain or maintain the necessary regulatory approvals for our products, which could restrict our ability to sell those products in some markets. |
• | If ongoing or future field trials conducted by us or our future collaborators are unsuccessful, we may be unable to complete the regulatory process for, or commercialize, our products in development on a timely basis. |
• | Competition in the market for traits and seeds is intense and requires continuous technological development, and, if we are unable to compete effectively, our financial results will suffer. |
4
• | Our business is subject to various government regulations and if we or our collaborators are unable to timely complete the regulatory process for our products in development, our or our collaborators’ ability to market our traits could be delayed, prevented or limited. |
• | The products of third parties or the environment may be negatively affected by the unintended appearance of our trait genes, gene constructs, altered seed compositions and novel seed products. |
• | We rely on third parties to conduct, monitor, support, and oversee field trials and, in some cases, to maintain regulatory files for those products in development, and any performance issues by third parties, or our inability to engage third parties on acceptable terms, may impact our or our collaborators’ ability to complete the regulatory process for or commercialize such products. |
• | If we lose key personnel or are unable to attract and retain necessary talent, we may be unable to develop or commercialize our products under development. |
• | Patent protection for our technologies is both important and uncertain. |
• | Third parties may claim that we infringe their intellectual property, and we could suffer significant litigation or licensing expense as a result. |
• | Portions of our crop science technology are owned by or subject to retained rights of third parties. |
• | We may not be successful in obtaining necessary rights to additional technologies for the development of our products through acquisitions and in-licenses. |
• | The intellectual property landscape around genome editing technology, such as CRISPR, is highly dynamic and uncertain, and any resolution of this uncertainty could have a material adverse effect on our business. |
• | We rely in part on trade secrets to protect our technology, and our failure to obtain or maintain trade secret protection could harm our business. |
• | Trading volume in our stock is low and an active trading market for our common stock may not be available on a consistent basis to provide stockholders with adequate liquidity. Our stock price may be extremely volatile, and our stockholders could lose a significant part of their investment. |
• | We may not be able to maintain the listing of our common stock on The Nasdaq Capital Market. |
• | Provisions in our certificate of incorporation and by-laws and Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock. |
• | Concentration of ownership among our existing officers, directors and principal stockholders may prevent other stockholders from influencing significant corporate decisions and depress our stock price. |
• | Our financial condition, research and development efforts, and results of operations could be further adversely affected by the ongoing coronavirus outbreak. |
Corporate Information
We were incorporated in Massachusetts in 1992 under the name Metabolix, Inc. In September 1998, we reincorporated in Delaware. We changed our name to Yield10 Bioscience, Inc. in January 2017 to reflect our change in mission around innovations in agricultural biotechnology focused on developing disruptive technologies for step-change improvements in crop yield and niche crop products. Our corporate headquarters are located at 19 Presidential Way, Woburn, MA 01801, and our telephone number is +1 (617) 583-1700. Our website address is www.yield10bio.com. The information contained on our website or that can be accessed through our website is not
5
part of this prospectus and investors should not rely on any such information in deciding whether to purchase our securities.
6
THE OFFERING | |
Issuer | Yield10 Bioscience, Inc. |
Common stock offered by the selling security holders | 396,450 shares. |
Terms of the offering | The selling security holders and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their shares covered hereby on the Nasdaq Capital Market or any other stock exchange, market or trading facility on which the shares are traded or in private transactions. These sales may be at fixed or negotiated prices. See “Plan of Distribution.” |
Common stock to be outstanding after this offering | 3,334,048 shares |
Use of proceeds | We will not receive any of the proceeds from the sale of our common stock by the selling security holders pursuant to this prospectus. |
Nasdaq Capital Market symbol | YTEN |
Risk factors | Investing in our securities involves a high degree of risk. See “Risk Factors” on page 8 of this prospectus to read about factors that you should consider carefully before buying our securities. |
The number of shares of common stock that will be outstanding after this offering is based on 3,334,048 shares outstanding as of October 1, 2020, and excludes:
• | 326,881 shares of common stock issuable upon exercise of options to purchase our common stock outstanding as of October 1, 2020 at a weighted average exercise price of $33.52 per share; |
• | 8,500 shares of common stock issuable upon vesting of restricted stock units as of October 1, 2020; |
• | 15,450 shares of common stock reserved as of October 1, 2020 for future issuance under our 2018 Stock Option and Incentive Plan; |
• | 14,270 shares of common stock issuable upon exercise of warrants outstanding as of October 1, 2020 and issued pursuant to the Securities Purchase Agreement we entered into with certain investors on July 3, 2017 (which warrants became exercisable on January 7, 2018 at an exercise price of $201.60 per share and expire on January 7, 2024); |
• | 750 shares of common stock issuable upon exercise of immediately vested warrants outstanding as of October 1, 2020 and issued to an investor relations consultant on September 12, 2017 at an exercise price of $116.00 per share and which expire on September 11, 2024; |
• | 160,975 shares of common stock issuable upon exercise of vested Series A warrants outstanding as of October 1, 2020 pursuant to the Securities Purchase Agreement we entered into with certain investors on December 21, 2017 at an exercise price of $90.00 per share and which expire on December 21, 2022; |
• | 1,299,477 shares of common stock issuable upon exercise of Series A Warrants issued in concurrent public and private offerings in November 2019 and outstanding as of October 1, 2020 at an exercise price of $8.00 per share and which expire on May 19, 2022; and |
7
• | 1,368,227 shares of common stock issuable upon exercise of Series B Warrants issued in concurrent public and private offerings in November 2019 and outstanding as of October 1, 2020 at an exercise price of $8.00 per share and which expire on May 19, 2027. |
8
RISK FACTORS
Our business is subject to numerous risks. We caution you that the following important factors, among others, could cause our actual results to differ materially from those expressed in forward-looking statements made by us or on our behalf in filings with the SEC, press releases, communications with investors and oral statements. Any or all of our forward-looking statements contained in this prospectus and in any other public statements we make may turn out to be wrong. They can be affected by inaccurate assumptions we might make or by known or unknown risks and uncertainties. Many factors mentioned in the discussion below will be important in determining future results. Consequently, no forward-looking statement can be guaranteed. Actual future results may differ materially from those anticipated in forward-looking statements. We undertake no obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise. You are advised, however, to consult any further disclosure we make in our reports filed with the SEC.
Risks Relating to our Financial Position
We have a history of net losses and our future profitability is uncertain.
We have recorded losses every year since our inception, with the exception of 2012. As of June 30, 2020, our accumulated deficit was $370.3 million. Since 1992, we have been engaged primarily in research and development and early-stage commercial activities. Because our crop science technology is at an early stage of development, we cannot be certain that the Yield10 Bioscience business will generate sufficient revenue to become profitable. We expect to continue to have significant losses and negative cash flow for at least the next several years, as we incur additional costs and expenses for the continued development of our technology, including the ongoing expenses of research, development, commercialization and administration. The amount we spend will impact our need for capital resources as well as our ability to become profitable and this will depend, in part, on the number of new technologies that we attempt to develop. We may not achieve any or all of these goals and, thus, we cannot provide assurances that we will ever be profitable or achieve significant, or any, product revenues.
We will need to secure additional funding to finance our operations and may not be able to do so when necessary, and/or the terms of any financings may not be advantageous to us.
As of June 30, 2020, we held unrestricted cash, cash equivalents and short-term investments of $8.5 million. In March 2019, we closed on a registered direct offering of our common stock, raising $2.6 million, net of offering costs, and in November 2019, we closed on a public offering and a concurrent private placement of our securities, raising $10.2 million, net of offering costs. Through June 30, 2020, we received an additional $1.7 million from investor exercises of outstanding warrants. On August 26, 2020, we closed on concurrent public and private offerings of our common stock, receiving gross proceeds of approximately $5.7 million. We follow the guidance of Accounting Standards Codification ("ASC") Topic 205-40, Presentation of Financial Statements-Going Concern, in order to determine whether there is substantial doubt about the Company's ability to continue as a going concern for one year after the date its financial statements are issued. We believe that together with the proceeds of the registered offering and private placement completed in August 2020, we have sufficient cash and short-term investments to fund operations through the end of calendar 2021.
We continue to face significant challenges and uncertainties and, as a result, our available capital resources may be consumed more rapidly than currently expected due to any or all of the following:
• | lower than expected revenues from grants and licenses related to our technologies; |
• | changes we may make to the business that affect ongoing operating expenses; |
• | further changes we may make to our business strategy; |
• | changes in our research and development spending plans; and |
• | other items affecting our forecasted level of expenditures and use of cash resources. |
9
We will require additional capital resources to support the implementation of our business strategy and we may pursue one or more of a variety of financing options, including public or private equity financing, secured or unsecured debt financing, equity or debt bridge financing, as well as licensing or other collaborative arrangements. There can be no assurance that our financing efforts will be successful. If we are not able to secure such additional capital resources or otherwise fund our operations, we will be forced to explore strategic alternatives and/or wind down our operations and pursue options for liquidating our remaining assets, including intellectual property and equipment.
If we issue equity or debt securities to raise additional funds in the future, we may incur fees associated with such issuances, our existing stockholders may experience dilution from the issuance of new equity securities, we may incur ongoing interest expense and be required to grant a security interest in our assets in connection with any debt issuance, and the new equity or debt securities may have rights, preferences and privileges senior to those of our existing stockholders. In addition, utilization of our net operating loss and research and development credit carryforwards may be subject to significant annual limitations under Section 382 of the Internal Revenue Code of 1986, as amended (the "Code"), due to ownership changes resulting from equity financing transactions. If we raise additional funds through collaboration, licensing or other similar arrangements, it may be necessary to relinquish valuable rights to our potential products or proprietary technologies or grant licenses on terms that are not favorable to us.
We have changed our corporate strategy to focus on the crop science industry, and our technologies in this area are at a very early stage of development. We may never commercialize a technology or product that will generate meaningful, or any, revenues.
In July 2016, our Board of Directors approved a plan to implement a strategic restructuring under which Yield10 Bioscience has become our core business. As part of the restructuring, we discontinued our biopolymer operations, eliminated positions in our biopolymer operations and corporate organization, and sold certain of our biopolymer business assets.
The crop science products and technologies we are currently developing as a result of our strategic repositioning are at a very early stage of development, and the process of developing them is lengthy and uncertain. In addition, our current management has limited experience in developing technologies for the crop science industry and has never commercialized a product or technology in this industry. We may never reach a point at which our efforts result in products that allow us to achieve revenue from their license or sale.
There can be no assurance that we will be able to comply with the continued listing standards of The Nasdaq Capital Market.
We cannot assure you that we will be able to comply with the standards that we are required to meet in order to maintain a listing of our common stock on The Nasdaq Capital Market ("Nasdaq"). Nasdaq listing rules require us to maintain certain closing bid price, stockholders’ equity and other financial metric criteria in order for our common stock to continue trading on Nasdaq. For example, Nasdaq Listing Rule 5550(a)(4) requires companies to maintain a minimum of 500,000 publicly held shares. Nasdaq Listing Rule 5550(a)(2) requires listed securities to maintain a minimum bid price of $1.00 per share, and Listing Rule 5810(c)(3)(A) provides that a failure to meet the minimum bid price requirement exists if the deficiency continues for a period of 30 consecutive business days.
On June 25, 2019, we received a deficiency letter from Nasdaq which provided us a grace period of 180 calendar days, or until December 23, 2019, to regain compliance with the minimum bid price requirement. We subsequently received an additional 180 days (until June 22, 2020) to regain compliance with the requirement. On January 9, 2020, our stockholders approved an amendment to our Amended and Restated Certificate of Incorporation, as amended, authorizing a reverse stock split of our common stock. A 1-for-40 ratio for the reverse stock split was subsequently approved by our Board of Directors, and the reverse stock split took effect on January 15, 2020. As a result of the reverse stock split, every forty shares of our common stock were automatically combined and converted into one issued and outstanding share of our common stock, with no change in the par value per share. As of January 30, 2020, we had regained compliance with the minimum bid price requirement.
10
Currently, our primary source of our revenue is government grants; continued availability of government grant funding is uncertain and contingent on compliance with the requirements of the grant.
Historically, a portion of our revenue has been generated from payments to us from government entities in the form of government grants, whereby we are reimbursed for certain expenses incurred in connection with our research and development activities, subject to our compliance with the specific requirements of the applicable grant, including rigorous documentation requirements. To the extent that we do not comply with these requirements, the expenses that we incur may not be reimbursed. Any of our existing grants or new grants that we may obtain in the future may be terminated or modified.
Our ability to obtain grants or incentives from government entities in the future is subject to the availability of funds under applicable government programs and approval of our applications to participate in such programs. The application process for these grants and other incentives is highly competitive. We may not be successful in obtaining any additional grants, loans or other incentives. Recent political focus on reducing spending at the U.S. federal and state levels may continue to reduce the scope and amount of funds dedicated to crop science products, if such funds will continue to be available at all. To the extent that we are unsuccessful in being awarded any additional government grants in the future, we would lose a potential source of revenue.
Our government grants may subject us to government audits, which could expose us to penalties if we have failed to comply with the terms of the grants.
We may be subject to audits by government agencies as part of routine audits of our activities funded by our government grants. As part of an audit, these agencies may review our performance, cost structures and compliance with applicable laws, regulations and standards and the terms and conditions of the grant. If any of our costs are found to be allocated improperly, the costs may not be reimbursed, and any costs already reimbursed for such contract may have to be refunded. Accordingly, an audit could result in a material adjustment to our results of operations and financial condition. Moreover, if an audit uncovers improper or illegal activities, we may be subject to civil and criminal penalties and administrative sanctions.
Our financial condition and results of operations could be adversely affected by public health epidemics, including the ongoing coronavirus outbreak.
A novel strain of coronavirus was reported to have originated in Wuhan, Hubei Province, China in December 2019, and has been rapidly spreading across the globe, including in the United States and Canada. Any outbreak of contagious disease such as the coronavirus or other adverse public health developments could have a material and adverse effect on our business operations. Such adverse effects could include quarantines, disruptions of or restrictions on our ability and/or the ability of our collaborators’ personnel to travel or conduct normal business activities, as well as closures of our facilities or the facilities of our collaborators for an indefinite period of time (including shutdowns that may be requested or mandated by governmental authorities). Any temporary closures of facilities would likely affect our development efforts and operating results, and any disruption to the operations of our collaborators would likely impact our development efforts and operating results. The extent to which the coronavirus may impact our results will depend on future developments, which are highly uncertain and cannot be predicted, and on new information that may emerge concerning the severity of the coronavirus. However, current predictions suggest that the impact of sustained business closures and quarantines resulting from the coronavirus on the global economy will be severe, and this may have a material adverse effect on our business.
Risks Relating to our Yield10 Bioscience Crop Science Program
The crop science product development cycle is lengthy and uncertain, and our progress will depend heavily on our ability to attract third-party investment in research under license agreements and on our ability to establish future collaborative partnerships to develop and commercialize our innovations.
The technology and processes used in our crop science program and the application of our technology to enhance photosynthetic efficiency of crops are at an early stage of development. Research and development in the seed, agricultural biotechnology, and larger agriculture industries is expensive and prolonged and entails
11
considerable uncertainty. Completion of development work with respect to our products will require a significant investment of both time and money, if it can be completed at all. We expect that collaborations with established agricultural industry companies will be required to successfully develop and commercialize our innovations. Our initial development strategy is to make it attractive for established agricultural industry companies to invest financial and technical resources to introduce our traits into their elite germplasm for event selection and evaluation under research licenses. For example, in 2017 we entered into a non-exclusive research license with Monsanto, which was subsequently acquired by Bayer AG (“Bayer”), pursuant to which we granted Monsanto a non-exclusive research license to evaluate our novel C3003 and C3004 yield traits in soybean. We expanded the agreement with Bayer in 2019 to cover a new discovery and intellectual property related to C3004. In 2018, we granted a non-exclusive research license to Forage Genetics, a subsidiary of Land O’Lakes, Inc., to evaluate five of our novel yield traits in forage sorghum. The traits included in the research license include C3003 as well as four traits from our GRAIN platform, C4001, C4002, C4003 and C4029. In 2019, we granted a non-exclusive research license to J.R. Simplot Company to evaluate C3003, C3004 and C4001 in potato. In 2020, we signed a non-exclusive research license with GDM for evaluation of seed yield traits in soybean, which will provide opportunities to explore additional Yield10 commercial crop performance traits with a leading seed market participant and potentially provide access to South American acreage in Argentina and Brazil. We may not be successful in establishing or maintaining suitable relationships with established agricultural industry companies for research licenses in the future, and there can be no assurance that any such relationships will result in future collaboration agreements to develop and commercialize our innovations, with terms that are satisfactory to us or at all. In addition, industry collaborators have significant resources and development capabilities and may develop products and technologies that compete with or negatively impact the development and commercialization of our technologies.
Any potential collaborative partnerships that we may enter into in the future may not be successful, which could adversely affect our ability to develop and commercialize our innovations.
We expect that collaborations with established agricultural industry companies will be required for us to successfully develop and commercialize our innovations. The agriculture industry is highly concentrated and dominated by a small number of large companies, which could impact efforts to form the collaborations that we will need in order to complete the development of our products. To the extent that we pursue such arrangements, we will face significant competition in seeking appropriate partners. Moreover, such arrangements are complex and time-consuming to negotiate, document, implement and maintain. We may not be successful in establishing or implementing such arrangements. The terms of any partnerships, joint ventures or other collaborative arrangements that we may establish may not be favorable to us.
The success of any future collaborative partnerships is uncertain and will depend heavily on the efforts and activities of our potential partners. Such arrangements are subject to numerous risks, including the risks that:
• | our partners may have significant discretion in determining the efforts and resources that they will apply to the arrangement; |
• | our partners may not pursue the development and commercialization of our product candidates based on trial results, changes in their strategic focus, competing priorities, availability of funding, or other external factors; |
• | our partners may delay or abandon field trials, fail to conduct field trials that produce sufficient conclusory data, provide insufficient funding for field trials, or repeat or conduct new field trials; |
• | partners who have marketing, manufacturing and distribution rights with respect to a product may not commit sufficient resources to, or otherwise not perform satisfactorily in carrying out, these activities; |
• | to the extent that such arrangements provide for exclusive rights, we may be precluded from collaborating with others; |
• | our partners may not properly maintain or defend our intellectual property rights, or may use our intellectual property or proprietary information in a way that gives rise to actual or threatened |
12
litigation that could jeopardize or invalidate our intellectual property or proprietary information or expose us to potential liability;
• | disputes may arise between us and a partner that causes the delay or termination of the research, development or commercialization of our current or future products, or that results in costly litigation or arbitration that diverts management attention and resources; |
• | such arrangements may be terminated, and, if terminated, may result in a need for additional capital for our independent pursuit of matters previously covered by such arrangement; |
• | our partners may own or co-own intellectual property that results from our arrangement; and |
• | a partner’s sales and marketing activities or other operations may not be in compliance with applicable laws resulting in civil or criminal proceedings. |
Our crop science program may not be successful in developing commercial products.
We and our potential future collaborators may spend many years and dedicate significant financial and other resources developing traits that will never be commercialized. Seeds containing the traits that we develop may never become commercialized for any of the following reasons:
• | our traits may not be successfully validated in the target crops; |
• | our traits may not achieve our targeted yield improvements; |
• | we may not be able to secure sufficient funding to progress our traits through development and commercial validation; |
• | our traits may not have the desired effects sought by future collaborators for the relevant crops; |
• | development and validation of traits, particularly during field trials, may be adversely affected by environmental or other circumstances beyond our control; |
• | we or our future collaborators may be unable to obtain the requisite regulatory approvals for the seeds containing our traits, to the extent regulatory approvals are required; |
• | competitors may launch competing or more effective seed traits or seeds; |
• | a market may not exist for seeds containing our traits or such seeds may not be commercially successful; |
• | future collaborators may be unable to fully develop and commercialize products containing our seed traits or may decide, for whatever reason, not to commercialize such products; |
• | we may be unable to patent our traits in the necessary jurisdictions; and |
• | our efforts to develop niche crop products based on our Camelina platform, including specialty oils and PHB biomaterials are in the early stages and may not be successful. |
If any of these things were to occur, it could have a material adverse effect on our business and our results of operations. Research and development in the crop science industry is expensive and prolonged and entails considerable uncertainty. Because of the stringent product performance and safety criteria applied in development of crop science products, products currently under development may neither survive the development process nor ultimately receive any requisite regulatory approvals that may be needed to market such products. Even when such approvals are obtained, there can be no assurance that a new product will be commercially successful. In addition, research undertaken by competitors may lead to the launch of competing or improved products, which may affect sales of any products that we are able to develop.
13
Even if we or our future collaborators are successful in developing commercial products that incorporate our traits, such products may not achieve commercial success.
Our strategy depends upon our or our future collaborators’ ability to incorporate our traits into a wide range of crops in significant markets and geographies. Even if we or our future collaborators are able to develop commercial products that incorporate our traits, any such products may not achieve commercial success for one or more of the following reasons, among others:
• | products may fail to be effective in particular crops, geographies, or circumstances, limiting their commercialization potential; |
• | our competitors, or competitors of our collaborators, may launch competing or more effective traits or products; |
• | significant fluctuations in market prices for agricultural inputs and crops could have an adverse effect on the value of our traits; |
• | farmers are generally cautious in their adoption of new products and technologies, with conservative initial purchases and proof of product required prior to widespread deployment, and accordingly, it may take several growing seasons for farmers to adopt our or our collaborators’ products on a large scale; |
• | we may not be able to produce high-quality seeds in sufficient amounts to meet demand; and |
• | we may not be able to secure the financial or other resources needed to achieve commercial success. |
Our financial condition and results of operations could be materially and adversely affected if any of the above were to occur.
Our estimates of market opportunity and forecasts of market growth may prove to be inaccurate, and even if the markets in which we may compete in the future achieve growth, our business could fail to achieve the same growth rates as others in the industry.
Market opportunity estimates and market growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. Our estimates and forecasts relating to the size and expected growth of the global seed industry and the biotechnology seeds market, and the estimated ranges of incremental value increase that a novel, newly developed crop trait may produce, may prove to be inaccurate. Even if the markets in which we may compete in the future achieve these opportunity estimates and market growth forecasts, our business could fail to grow at similar rates, if at all.
If ongoing or future field trials conducted by us or our future collaborators are unsuccessful, we may be unable to complete the regulatory process for, or commercialize, our products in development on a timely basis.
The successful completion of multi-year, multi-site field trials is critical to the success of product development and marketing efforts for products containing our traits. If our ongoing or future field trials, or those of our future collaborators, are unsuccessful or produce inconsistent results or unanticipated adverse effects on crops, or if we or our collaborators are unable to collect reliable data, regulatory review of products in development containing our traits could be delayed or commercialization of products in development containing our traits may not be possible. In addition, more than one growing season may be required to collect sufficient data to develop or market a product containing our traits, and it may be necessary to collect data from different geographies to prove performance for customer adoption. Even in cases where field trials are successful, we cannot be certain that additional field trials conducted on a greater number of acres, or in different crops or geographies, will be successful. Generally, we or our research licensees conduct these field trials, or we pay third parties, such as farmers, consultants, contractors, and universities, to conduct field trials on our behalf. Poor trial execution or data collection, failure to follow required agronomic practices, regulatory requirements, or
14
mishandling of products in development by our collaborators or these third parties could impair the success of these field trials.
Many factors that may adversely affect the success of our field trials are beyond our control, including weather and climatic variations, such as drought or floods, severe heat or frost, hail, tornadoes and hurricanes, uncommon or unanticipated pests and diseases, or acts of protest or vandalism. For example, if there were a prolonged or permanent disruption to the electricity, climate control, or water supply operating systems in our greenhouses or laboratories, the crops in which we or our collaborators are testing our traits and the samples we or our collaborators store in freezers, both of which are essential to our research and development activities including field tests, could be severely damaged or destroyed, adversely affecting these activities and thereby our business and results of operations. Unfavorable weather conditions including drought or excessive rain, or fluctuations in temperature, which we have experienced from time to time in our field trials, can also reduce both acreages planted and incidence, or timing of, certain crop diseases or pest infestations, each of which may halt or delay our field trials. Any field test failure we may experience may not be covered by insurance and, therefore, could result in increased cost for the field trials and development of our traits, which may negatively impact our business, results of operations, and ability to secure financing. Such factors outside of our control can create substantial volatility relating to our business and results of operations.
Competition in the market for traits and seeds is intense and requires continuous technological development, and, if we are unable to compete effectively, our financial results will suffer.
We face significant competition in the markets in which we operate. The markets for traits and agricultural biotechnology products are intensely competitive and rapidly changing. In most segments of the seed and agricultural biotechnology market, the number of products available to consumers is steadily increasing as new products are introduced. At the same time, the expiration of patents covering existing products reduces the barriers to entry for competitors. We may be unable to compete successfully against our current and future competitors, which may result in price reductions, reduced margins and the inability to achieve market acceptance for any products that we or our future collaborators commercialize containing our traits. In addition, most of our competitors have substantially greater financial, marketing, sales, distribution, research and development, and technical resources than we have, and some of our potential future collaborators have more experience in research and development, regulatory matters, manufacturing, and marketing. We anticipate increased competition in the future as new companies enter the market and new technologies become available. Our technologies may be rendered obsolete or uneconomical by technological advances or entirely different approaches developed by one or more of our competitors, which will prevent or limit our ability to generate revenues from the commercialization of our traits being developed.
Our business is subject to various government regulations in the United States and Canada, the regulatory requirements for our future products in development are evolving and are subject to change, and if there are adverse changes to the current regulatory framework, our or our future collaborators’ ability to market our traits could be delayed, prevented or limited.
In the United States and Canada, where our seed traits and biotechnology-derived plant lines are developed and field tested, changes in regulatory requirements applicable to our seed traits or future products in development containing our traits could result in a substantial increase in the time and costs associated with developing and commercializing future products containing our traits, and could materially affect our ability to meet our desired development timelines or to develop and commercialize a future product containing our traits at all.
In the United States, our seed traits and any future products that are successfully developed containing our seed traits are or will be subject to USDA and FDA regulatory requirements. The USDA and FDA requirements will vary depending on the particular seed trait and the intended use of any product that will be commercialized. Our business strategy is focused on crop yield traits and we have no current plans for the development of pesticide or herbicide traits, which would be subject to regulation by the EPA.
Within USDA, the APHIS is responsible for protecting agricultural plants under the Plant Protection Act. USDA-APHIS regulates organisms and products that are known or are suspected to be plant pests or to pose a plant
15
pest risk, including those that have been altered or produced through various genetic engineering techniques. These genetically engineered plants are called “regulated articles” in the relevant USDA-APHIS regulations, which control the import, handling, interstate movement and release into the environment of regulated articles, including certain genetically engineered organisms undergoing confined experimental use or field trials. Seed traits developed using the insertion of recombinant DNA, such as our C3003 yield trait that leverages the biological functions of an algal gene, are regulated articles and are therefore subject to extensive USDA-APHIS oversight, including but not limited to permitting requirements for import, handling, interstate movement and release into the environment.
In recent years, we and others have submitted various petitions to USDA-APHIS to determine whether particular biotechnology-derived plants developed through the use of different genome editing techniques may be considered to be not regulated under the framework administered by the agency. In general, genome editing approaches to novel plant trait development have been considered not regulated by USDA-APHIS. In particular, we have submitted two petitions (also known as the “Am I Regulated?” letter) to USDA-APHIS’s Biotechnology Regulatory Services in order to confirm that the following two oil content traits are not going to be regulated by the agency under 7 CFR part 340: (i) the single trait C3008 Camelina plant line, developed using CRISPR genome editing technology for increased oil content; and (ii) the triple-edited Camelina line that combines three gene traits, C3008a, C3008b and C3009, to increase oil production. In both cases, USDA-APHIS approved our petitions and confirmed in writing that each of these novel plant lines would not be treated as a regulated article.
The USDA also announced in March 2018 that it would not require an assessment on products that used modern forms of mutagenesis if it was clear these outcomes could occur in nature. The USDA stated at that time that it did not “have any plans to regulate plants that could otherwise have been developed through traditional breeding techniques as long as they are developed without the use of a plant pest as the donor or vector and they are not themselves plant pests.” This USDA policy statement applies to genetic deletions of any size, which would include genome editing through CRISPR-Cas9 and other emerging technologies, although it remains to be seen how this policy announcement will be implemented by USDA-APHIS and what practical effect that may have on seed trait developers like us and our competitors.
There can be no guarantee that the USDA-APHIS governing regulations and policies will not change. We cannot predict whether advocacy groups will challenge existing regulations and USDA determinations, whether the USDA will alter its interpretations of existing regulations, modify existing regulations or promulgate new regulations, or whether additional laws will come into effect. If these or other developments resulted in adverse changes to the current regulatory framework, our seed traits or future products in development containing our traits could be subjected to more burdensome regulatory standards, thereby substantially increasing the time and costs associated with developing and commercializing any future products. Moreover, we cannot assure you that USDA-APHIS will analyze any of our future yield traits or products in development containing our traits in a manner consistent with its analysis of our genome edited yield traits to date. Complying with the USDA’s plant pest regulations for traits that are classified as “regulated articles,” including the permitting requirements for field testing and environmental release, is a costly, time-consuming process and could substantially delay or prevent the commercialization of any future products containing traits that we expected to be deemed non-regulated by USDA-APHIS under 7 CFR part 340.
In addition to USDA-APHIS regulation of plant breeding and planting, a biotechnology-derived plant also will be regulated by the FDA if it is intended to be used as human food or animal feed. The FDA regulates the safety of food for humans and animals, and foods derived from novel plant varieties must meet the same food safety requirements as foods derived from traditionally bred plants (also called conventional foods). Since 1992, the FDA has had in place a voluntary consultation process for developers of bioengineered food (“Biotechnology Consultations”).
Biotechnology Consultations are data-intensive and examine the new food product’s safety and nutritional profile, among other issues. Generally, the FDA has found that such food products do not pose unique health risks to humans or animals, but if a novel allergen or other distinction from the conventional food is present in the new plant variety, the agency may require specific label statements on the product to ensure that consumers are made aware of material differences between genetically engineered and conventional versions. When such a determination cannot be made, the novel plant variety may become subject to FDA premarket review and approval as a food additive.
16
As part of a broader effort to modernize its regulatory approach to all biotechnology-derived products, the FDA is currently re-evaluating its regulatory approach in light of the increasing prevalence of certain genome edited plants. In January 2017, the FDA asked for public input to help inform its thinking about human and animal foods derived from new plant varieties produced using genome editing techniques. Among other things, the FDA’s request for comments asked for data and information in response to questions about the safety of foods from genome edited plants, such as whether certain categories of genome edited plants present food safety risks different from other plants produced through traditional plant breeding. Subsequently, in October 2018, FDA leadership issued a document entitled the “Plant and Animal Biotechnology Innovation Action Plan” (“Action Plan”) that identified three key priorities for the agency in this area and stated that the FDA has reviewed the comments and other information it received in response to the January 2017 request for comments. The FDA also stated that it intended to develop guidance for industry explaining how the FDA’s existing regulatory policy for foods derived from new plant varieties applies to foods produced using genome editing. Although the expected draft guidance has not yet been released for public comment, on March 4, 2020 FDA, USDA, and EPA launched a new initiative to help consumers better understand foods created through genetic engineering, called “Feed Your Mind,” which aims to answer the most common questions that consumers have about such crops. The FDA also stated in the 2018 Action Plan that it intended to begin updating the existing procedures for voluntary Biotechnology Consultations to reflect the agency’s 25 years of experience with foods derived from biotechnology plants and to incorporate any additional issues related to genome editing of food crops. Subsequently, in February 2019, FDA completed its first consultation on a genome edited plant variety (a soybean variety modified to have increased levels of oleic acid).
We have not participated in any Biotechnology Consultations or engaged in any informal discussions with the FDA about our novel yield traits, whether those traits have been developed using genome editing or traditional genome modification using the insertion of recombinant DNA. Any delay in the regulatory consultation process, or a determination by the FDA that future product candidates containing our traits raise different safety issues than the relevant conventional crop and therefore must be approved by the agency as a new food additive through an intensive premarket safety review process, could increase the costs associated with or delay or prevent the commercialization of the future product candidate. Such delays may lead to reduced acceptance by farmers, food manufacturers or the public and an increase in competitor products that may directly compete with ours. Further, if the FDA enacts new regulations or policies with respect to genome edited plants in particular, such policies could result in additional compliance costs or delay or prevent the commercialization of any potential commercial products containing our seed traits, which could adversely affect our ability to generate revenues and to achieve profitability.
In Canada, genetically engineered crops and the food products into which they are incorporated are regulated by multiple government agencies under a federal framework for the regulation of biotechnology products that is similar to the U.S. system. First, the Canadian Food Inspection Agency ("CFIA") is the lead agency for ensuring that a new agricultural biotechnology crop will not pose new risks to Canadian plants, animals and other agricultural commodities. The CFIA’s Plant Biosafety Office ("PBO") is responsible for conducting environmental assessments of biotechnology-derived plants, referred to as “plants with novel traits” ("PNT"). Authority for the PBO includes both approving confined field trials with the PNT through permits and authorizing their “unconfined release” as a first step towards commercialization. Second, under the Food and Drugs Act and related regulations, Health Canada is responsible for reviewing a pre-market safety assessment that must be submitted by the manufacturer or importer of a “novel food,” a term of art that includes any PNT or other biotechnology-derived foods. Health Canada will evaluate the data and information about the novel food and make a determination regarding whether it is safe and nutritious before it can be sold in Canada, as well as whether any restrictions are warranted under applicable law or the product’s safety profile. Any commercialization of our yield crops in Canada is expected to be done by a third-party collaborator or other partner and complying with Health Canada’s pre-market notification requirement and safety assessment for novel foods would be the obligation of that third-party collaborator.
Our work involving the development, greenhouse testing and field testing of novel yield trait genes in crop plants requires certain government and municipal permits and we must ensure compliance with all applicable regulations including regulations relating to genetically engineered crops. With laboratories and greenhouses in both the U.S. and Canada, we are also subject to regulations governing the shipment of seeds and other plant material between our facilities in the U.S. and Canada, including USDA-APHIS permits for the import and export of plant
17
materials that could pose a risk to domestic agriculture. We also have been conducting field studies of various yield traits in Canada since 2016 under PNT permits issued by Canadian regulators.
Complying with the Canadian regulations is a costly, time-consuming process and could substantially delay or prevent the commercialization of our products. In addition, we cannot assure you that CFIA and Health Canada regulations or the agencies’ implementation of those regulations will not change or that the legislative framework in Canada for biotechnology-derived crops, whether for genome edited plants or plants modified using the insertion of recombinant DNA, will not be amended or otherwise changed in a manner that could result in additional compliance costs or delay or prevent the commercialization of any potential commercial products containing our seed traits, which could adversely affect our ability to generate revenues and to achieve profitability.
Failure to comply with applicable regulatory requirements may, among other things, result in fines, suspensions of regulatory approvals, product recalls, product seizures, operating restrictions and criminal prosecution.
If we or our future collaborators are unable to comply with and timely complete the regulatory process in the United States and Canada for our future products in development, our or our future collaborators’ ability to market our traits could be delayed, prevented or limited.
We apply for and maintain the regulatory permits in the United States and Canada necessary for our operations, particularly those covering our field trials. We anticipate that we or our future collaborators will apply for and maintain regulatory approvals, if any, necessary for the commercialization of any future products containing our seed traits. Even if we and our collaborators make timely and appropriate applications for regulatory permits for our field trials, government delays in issuing such permits can significantly affect the development timelines for our traits, particularly if the planting period for a crop growing season expires before the necessary permits are obtained.
The regulatory process is expensive and time-consuming, and the time required to complete the process is difficult to predict and depends upon numerous factors, including the substantial discretion of the regulatory authorities. We have not completed all phases of the regulatory process for any of our traits in development. Our traits could require a significantly longer time to complete the regulatory process than expected, or may never gain approval, even if we and our collaborators expend substantial time and resources seeking such approval. The time required for regulatory approval, or any delay or denial of such approval, could negatively impact our ability to generate revenues and to achieve profitability and finance our ongoing operations. In addition, changes in regulatory review policies during the development period of any of our traits, changes in, or the enactment of, additional regulations or statutes, or changes in regulatory review practices for a submitted product application may cause a delay in obtaining approval or result in the rejection of an application for regulatory approval. Regulatory approval, if obtained, may be made subject to limitations on the intended uses for which we or our collaborators may market a future product containing our traits. These limitations could adversely affect our potential revenues.
The regulatory environment for genetically engineered crops in jurisdictions outside the United States and Canada varies greatly, and some jurisdictions have more restrictive regulations that could delay, prevent or limit our or our future collaborators’ ability to market our traits.
Other jurisdictions and governmental authorities, including in South America and Asia, are increasingly taking an interest in regulating agricultural products of biotechnology. Regulatory approaches vary by jurisdiction as a result of the existing public health frameworks and phytosanitary laws, as well as other less tangible factors such as cultural and religious norms that may have an impact on individual country risk assessments and decision-making. Each jurisdiction may have its own regulatory framework, which may include restrictions and regulations on planting and growing genetically engineered plants, import of grain and other plant products, and in the consumption and labeling of feed and foods derived from such novel plants, and which may apply to future products containing our traits. We cannot predict future changes in the global regulatory landscape regarding genetically engineered plants or commercial products incorporating such novel plant varieties. The regulatory environment for such plants is greatly uncertain outside of the U.S. and Canada, and some jurisdictions have more restrictive regulations that could delay, prevent or limit our or our future collaborators’ ability to market our traits.
18
For example, regulation of all genetically engineered plants in the European Union ("EU") is far more stringent than in the U.S. and Canada. U.S. and Canadian regulators have determined that genome edited plants pose fewer risks than traditional biotechnology-derived plants subjected to modification through the insertion of recombinant DNA. In contrast, a recent EU legal ruling indicated that the existing EU regulations for genetically engineered plants modified by the insertion of recombinant DNA, which were already more stringent than corresponding U.S. and Canadian regulations, should be strictly applied to genome edited plants as well. As a result, there is a sharp distinction between how EU and U.S. and Canadian regulatory agencies oversee novel seed traits, and in particular those that are generated using the more modern techniques of genome editing.
Although we are not currently targeting EU markets for the development or commercialization of future products containing our traits, emerging oversight regimes for genetically engineered products in other jurisdictions may follow the EU approach and impose similarly strict requirements for the release of such products into the environment and their incorporation into human food or other consumer products. Such jurisdictions may also elect to regulate genetically engineered plants without distinguishing between traditional biotechnology-derived plants modified with recombinant DNA and genome edited plants. There is no guarantee that countries for which we may have or may develop future marketing plans would not take a stricter legal and regulatory approach to controlling genetically engineered plants similar to that of the EU, which could increase regulatory costs and delay, prevent or limit our or our future collaborators’ ability to market our traits in such jurisdictions.
Consumer resistance to genetically engineered crops may negatively affect the ability to commercialize future crops containing our traits, as well as our public image, and may reduce any future sales of seeds containing our yield traits.
Food and feed made from genetically engineered seeds and plants are not accepted by some consumers, and in certain countries production of certain genetically engineered crops is effectively prohibited, including throughout the EU, due to concerns over such products’ effects on food safety and the environment. Advocacy groups have engaged in publicity campaigns and filed lawsuits in various countries against companies and regulatory authorities, seeking to halt regulatory approval activities or influence public opinion against genetically engineered and/or genome edited products. Actions by consumer groups and others also may disrupt research and development or production of genetically engineered plants, seeds or food products that incorporate such novel plant varieties. The high public profile of the biotechnology industry in food and feed production, and a lack of consumer acceptance of the types of products to which we have devoted substantial development resources, could have a negative impact on the commercial success of any of products incorporating our traits that may successfully complete the development process, as to which no assurance can be given, and could materially and adversely affect our ability to obtain future collaborations and to finance our crop science program. Further, we could incur substantial liability and/or legal expenses if there are claims that genetically engineered crops damage the environment or contaminate other farm crops. This could distract our management and cause us to spend resources defending against such claims.
Government policies and regulations, particularly those affecting the agricultural sector and related industries, could adversely affect our operations and our ability to generate future revenues and to achieve profitability.
Agricultural production and trade flows are subject to government policies and regulations. Governmental policies and approvals of technologies affecting the agricultural industry, such as taxes, tariffs, duties, subsidies, incentives and import and export restrictions on agricultural commodities and commodity products can influence the planting of certain crops, the location and size of crop production, and the volume and types of imports and exports. Future government policies in the United States, Canada or in other countries could discourage farmers from using any of our products that may successfully complete the development process, as to which no assurance can be given. Similarly, these policies could discourage food processors from purchasing harvested crops containing our traits or could encourage the use of our competitors’ products, which would put us at a commercial disadvantage and could negatively impact our ability to generate any revenues and to achieve profitability.
19
The products of third parties, or the environment itself, may be negatively affected by the unintended appearance of our trait genes, novel seed compositions and novel seed products.
The potential for unintended but unavoidable trace amounts, sometimes called “adventitious presence,” of trait genes, novel seed compositions and novel seed products in conventional seed, or in the grain or products produced from conventional or organic crops, could affect acceptance by the general public or by the agricultural industry of these traits. Trace amounts of yield trait genes may unintentionally be found outside our containment area in the products of third parties, which may result in negative publicity and claims of liability brought by such third parties against us. Furthermore, in the event of an unintended dissemination of our genetically engineered materials to the environment, we could be subject to claims by multiple parties, including environmental advocacy groups, as well as governmental actions such as mandated crop destruction, product recalls or additional stewardship practices and environmental cleanup or monitoring. The occurrence of any of these events could have a material adverse effect on our business and results of operations.
Loss of or damage to our elite novel trait events and plant lines would significantly slow our product development efforts.
We have a collection of elite novel trait events and plant lines in which we are developing traits for incorporation into elite germplasm and potential seed products. Our elite novel trait events and plant lines are a key strategic asset since they form the basis for the introgression of our traits into plant breeding programs. If we suffer loss or damage to our elite novel trait events and plant lines, our research and development activities could be negatively impacted.
Our insurance coverage may be inadequate to cover all the liabilities we may incur.
We face the risk of exposure to liability claims if any products that are successfully developed containing our seed traits, as to which no assurance can be given, are defective and if any product that we develop or any product that uses our technologies or incorporates any of our traits causes injury. Although we carry insurance at levels customary for companies in our industry, such coverage may become unavailable or be inadequate to cover all liabilities we may incur. There can be no assurance that we will be able to continue to maintain such insurance, or obtain comparable insurance at a reasonable cost, if at all. If we are unable to obtain sufficient insurance coverage at an acceptable cost or otherwise, or if the amount of any claim against us exceeds the coverage under our policies, we may face significant expenses.
We rely on third parties to conduct, monitor, support, and oversee field trials and, in some cases, to maintain regulatory files for those products in development, and any performance issues by third parties, or our inability to engage third parties on acceptable terms, may impact our or our future collaborators’ ability to complete the regulatory process for or commercialize such products.
We rely on third parties to conduct, monitor, support, and oversee field trials. As a result, we have less control over the timing and cost of these trials than if we conducted these trials with our own personnel. If we are unable to maintain or enter into agreements with these third parties on acceptable terms, or if any such engagement is terminated prematurely, we may be unable to conduct and complete our trials in the manner we anticipate. In addition, there is no guarantee that these third parties will devote adequate time and resources to our studies or perform as required by our contract or in accordance with regulatory requirements, including maintenance of field trial information regarding our products in development. If any of these third parties fail to meet expected deadlines, fail to transfer to us any regulatory information in a timely manner, fail to adhere to protocols, or fail to act in accordance with regulatory requirements or our agreements with them, or if they otherwise perform in a substandard manner or in a way that compromises the quality or accuracy of their activities or the data they obtain, then field trials of our traits in development may be extended or delayed with additional costs incurred, or our data may be rejected by the applicable regulatory agencies. Ultimately, we are responsible for ensuring that each of our field trials is conducted in accordance with the applicable protocol and with legal, regulatory and scientific standards, and our reliance on third parties does not relieve us of our responsibilities. We could be subject to penalties, fines and liabilities if our third-party contractors fail to perform as required.
20
If our relationship with any of these third parties is terminated, we may be unable to enter into arrangements with alternative parties on commercially reasonable terms, or at all. Switching or adding service providers can involve substantial cost and require extensive management time and focus. Delays may occur, which can materially impact our ability to meet our desired development timelines. If we are required to seek alternative service arrangements, the resulting delays and potential inability to find a suitable replacement could materially and adversely impact our business.
In addition, there has been an increasing trend towards consolidation in the agricultural biotechnology industry. Consolidation among our competitors and third parties upon whom we rely could lead to changes in the competitive landscape, capabilities, and strategic priorities among potential service providers, which could have an adverse effect on our business and operations.
If we lose key personnel or are unable to attract and retain necessary talent, we may be unable to develop or commercialize our products under development.
We are highly dependent on our key technical and scientific personnel, who possess unique knowledge and skills related to our research and technology. If we were to lose the services of these individuals, we may be unable to readily find suitable replacements with comparable knowledge and the experience necessary to advance the research and development of our products. Because of the unique talents and experience of many of our scientific and technical staff, competition for our personnel is intense. The loss of key personnel or our inability to hire and retain personnel who have the required expertise and skills could have a material adverse effect on our research and development efforts, our business, and our ability to secure additional required financing.
Our business and operations would suffer in the event of system failures.
We utilize information technology systems and networks to process, transmit and store electronic information in connection with our business activities. As use of digital technologies has increased, cyber incidents, including deliberate attacks and attempts to gain unauthorized access to computer systems and networks, have increased in frequency and sophistication. These threats pose a risk to the security of our systems and networks and the confidentiality, availability and integrity of our data. There can be no assurance that we will be successful in preventing cyber-attacks or successful in mitigating their efforts.
Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage from such cyber-attacks, including computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such an event could cause interruption of our operations. For example, the loss of data from completed field tests for our yield traits could result in delays in our regulatory approval efforts and significantly increase our costs. To the extent that any disruption or security breach were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, we could suffer reputational harm or face litigation, or adverse regulatory action and the development of our product candidates could be delayed.
Risks Relating to Intellectual Property
Patent protection for our technologies is both important and uncertain.
Our commercial success may depend in part on our obtaining and maintaining patent protection for our technologies in the United States and other jurisdictions, as well as successfully enforcing and defending this intellectual property against third-party challenges. If we are not able to obtain or defend patent protection for our technologies, then we will not be able to exclude competitors from developing or marketing such technologies, and this could negatively impact our ability to generate sufficient revenues or profits from product sales and/or licensing to justify the cost of development of our technologies and to achieve or maintain profitability. Our currently issued patents relate to our historical business as well as two patents on our C3003 gene in-licensed from the University of Massachusetts and our C4001 U.S. patent, both of which were issued in 2019 and have expiration dates ranging from 2020 through 2035, plus any patent extensions which may be granted in the U.S. for regulatory approval delays. New outstanding patent applications owned by or licensed to us relating to crop yield
21
improvements have filing dates ranging from 2013 through 2020, including the recently filed new patent application on a breakthrough technology for producing PHA biomaterials in crops. This patent would have an expiration date in 2040 if granted, however, we may not be able to obtain sufficiently broad claims to cover the new invention.
Our patent position involves complex legal and factual questions. Accordingly, we cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. Patents may not be issued for any pending or future pending patent applications owned by or licensed to us, and claims allowed under any issued patent or future issued patent owned or licensed by us may not be valid or sufficiently broad to protect our technologies. Moreover, we may be unable to protect certain of our intellectual property in the United States or in foreign countries. Foreign jurisdictions may not afford the same protections as U.S. law, and we cannot ensure that foreign patent applications will have the same scope as the U.S. patents. There will be many countries in which we will choose not to file or maintain patents because of the costs involved. Competitors may also design around our patents or develop competing technologies.
Additionally, any issued patents owned by or licensed to us now or in the future may be challenged, invalidated, or circumvented. We could incur substantial costs to bring suits or other proceedings in which we may assert or defend our patent rights or challenge the patent rights of third parties. An unfavorable outcome of any such litigation could have a material adverse effect on our business and results of operations.
Third parties may claim that we infringe their intellectual property, and we could suffer significant litigation or licensing expense as a result.
Various U.S. and foreign issued patents and pending patent applications owned by third parties exist in areas relevant to our products and processes. We could incur substantial costs to challenge third-party patents. If third parties assert claims against us or our customers alleging infringement of their patents or other intellectual property rights, we could incur substantial costs and diversion of management resources in defending these claims, and the defense of these claims could have a material adverse effect on our business. In addition, if we are unsuccessful in defending against these claims, these third parties may be awarded substantial damages, as well as injunctive or other equitable relief against us, which could effectively block our ability to make, use, sell, distribute, or market our technologies and services based on our technologies in the United States or abroad. Alternatively, we may seek licenses to such third-party intellectual property. However, we may be unable to obtain these licenses on acceptable terms, if at all. Our failure to obtain the necessary licenses or other rights could prevent the sale, manufacture, or distribution of some of our products based on our technologies and, therefore, could have a material adverse effect on our business.
Portions of our crop science technology are owned by or subject to retained rights of third parties.
We have licensed and optioned from academic institutions certain patent rights that may be necessary or important to the development and commercialization of our crop science technology. These licenses and options may not provide exclusive rights to use such intellectual property in all fields of use in which we may wish to develop or commercialize our technology. If we fail to timely exercise our option rights and/or we are unable to negotiate license agreements for optioned patent rights on acceptable terms, the academic institutions may offer such patent rights to third parties. If we fail to comply with our obligations under these license agreements, or if we are subject to a bankruptcy or insolvency proceeding, the licensor may have the right to terminate the license. In some circumstances, we may not have the right to control the preparation, filing and prosecution of licensed patent applications or the maintenance of the licensed patents. Therefore, we cannot be certain that these patents and applications will be prosecuted, maintained and enforced in a manner consistent with the best interests of our business. Furthermore, the research resulting in certain of our licensed and optioned patent rights was funded by the U.S. government. As a result, the government may have certain rights to such patent rights and technology.
22
We may not be successful in obtaining necessary rights to additional technologies for the development of our products through acquisitions and in-licenses.
We may be unable to acquire or in-license additional technologies from third parties that we decide we need in order to develop our business. A number of more established companies may also pursue strategies to license or acquire crop science technologies that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater development and commercialization capabilities. Any failure on our part to reach an agreement for any applicable intellectual property could result in a third party acquiring the related rights and thereby harm our business.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire relevant crop science technologies on terms that would allow us to make an appropriate return on our investment.
We expect that competition for acquiring and in-licensing crop science technologies that are attractive to us may increase in the future, which may mean fewer suitable opportunities for us as well as higher acquisition or licensing costs. If we are unable to successfully obtain rights to suitable crop science technologies on reasonable terms, or at all, our business and financial condition could suffer.
Our license agreements include royalty payments that we are required to make to third parties.
We are party to license agreements that require us to remit royalty payments and other payments related to our licensed intellectual property. Under our in-license agreements, we may pay upfront fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, or timing of royalties we may owe in the future. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments.
The intellectual property landscape around genome editing technology, such as CRISPR, is highly dynamic and uncertain, and any resolution of this uncertainty could have a material adverse effect on our business.
The field of genome editing, especially in the area of CRISPR technology, is still in its infancy, and no products using this technology have reached the market. In 2018, we entered into a non-exclusive research license agreement jointly with the Broad Institute of MIT and Harvard and Pioneer, part of Corteva Agriscience™, Agriculture Division of DowDuPont Inc., for the use of CRISPR-Cas9 genome-editing technology for crops in order to demonstrate the utility of our yield trait genes in this field. The joint license covers intellectual property consisting of approximately 48 patents and patent applications on CRISPR-Cas9 technology controlled by the Broad Institute and Corteva Agriscience. Under the agreement, we have the option to renew the license on an annual basis and the right, subject to specified conditions, to convert the research license to a commercial license in the future, although there can be no assurance that we will be able to secure such commercial license on acceptable terms. CRISPR technology is uniquely suited to agricultural applications as it enables precise changes to plant DNA without the use of foreign DNA to incorporate new traits. Plants developed using CRISPR genome-editing technology have the potential to be considered not regulated by USDA-APHIS under 7 CFR part 340 for development and commercialization in the U.S., which could result in shorter developmental timelines and lower costs associated with commercialization of new traits in the U.S. as compared to regulated crops. Due to the intense research and development that is taking place by several companies, including us and our competitors, in this field, the intellectual property landscape is in flux, and it may remain uncertain for the coming years. There has been, and may continue to be, significant intellectual property related litigation and proceedings relating to this area in the future. If it is later determined that the patent rights using the CRISPR technology that we obtained under license are invalid or owned by other parties, this could have a material adverse effect on our business.
We rely in part on trade secrets to protect our technology, and our failure to obtain or maintain trade secret protection could harm our business.
We rely on trade secrets to protect some of our technology and proprietary information, especially where we believe patent protection is not appropriate or obtainable as is the case for our GRAIN trait gene discovery
23
platform. However, trade secrets are difficult to protect. Litigating a claim that a third party had illegally obtained and was using our trade secrets would be expensive and time consuming, and the outcome would be unpredictable. Moreover, if our competitors independently develop similar knowledge, methods and know-how, it will be difficult for us to enforce our rights and our business could be harmed.
Risks Relating to Owning our Common Stock
Raising additional funds may cause dilution to our existing stockholders, restrict our operations or require us to relinquish rights to our technologies.
Execution of our business plan requires additional financing. If we raise additional funds through equity offerings or offerings of equity-linked securities, including warrants or convertible debt securities, we expect that our existing stockholders will experience significant dilution, and the terms of such securities may include liquidation or other preferences that adversely affect your rights as a stockholder. Debt financing, if available, may subject us to restrictive covenants that could limit our flexibility in conducting future business activities, including covenants limiting or restricting our ability to incur additional debt, dispose of assets or make capital expenditures. We may also incur ongoing interest expense and be required to grant a security interest in our assets in connection with any debt issuance. If we raise additional funds through strategic partnerships or licensing agreements with third parties, we may have to relinquish valuable rights to our technologies or grant licenses on terms that are not favorable to us.
Trading volume in our stock can fluctuate and an active trading market for our common stock may not be available on a consistent basis to provide stockholders with adequate liquidity. Our stock price may be extremely volatile, and our stockholders could lose a significant part of their investment.
The public trading price for our common stock will be affected by a number of factors, including:
• | any change in the status of our Nasdaq listing; |
• | the need for near-term financing to continue operations; |
• | reported progress in our efforts to develop crop related technologies, relative to investor expectations; |
• | changes in earnings estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ earnings estimates; |
• | quarterly variations in our or our competitors’ results of operations; |
• | general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors; |
• | future issuances and/or sales of our securities; |
• | announcements or the absence of announcements by us, or our competitors, regarding acquisitions, new products, regulatory developments, significant contracts, commercial relationships or capital commitments; |
• | commencement of, or involvement in, litigation; |
• | any major change in our board of directors or management; |
• | changes in governmental regulations or in the status of our regulatory approvals; |
• | announcements related to patents issued to us or our competitors and to litigation involving our intellectual property; |
• | a lack of, or limited, or negative industry or security analyst coverage; |
24
• | uncertainty regarding our ability to secure additional cash resources with which to operate our business; |
• | a decision by our significant stockholders to increase or decrease their holdings in our common stock; |
• | short-selling or similar activities by third parties; and |
• | other factors described elsewhere in these risk factors. |
As a result of these factors, our stockholders may not be able to resell their shares at, or above, their purchase price. In addition, the stock prices of many technology companies have experienced wide fluctuations that have often been unrelated to the operating performance of those companies. Any negative change in the public’s perception of the prospects of industrial or agricultural biotechnology companies could depress our stock price regardless of our results of operations. These factors may have a material adverse effect on the market price and liquidity of our common stock and affect our ability to obtain required financing.
Provisions in our certificate of incorporation and by-laws and Delaware law might discourage, delay or prevent a change of control of our company or changes in our management and, therefore, depress the trading price of our common stock.
Provisions of our certificate of incorporation and by-laws and Delaware law may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which our stockholders might otherwise receive a premium for their shares of our common stock. These provisions may also prevent or frustrate attempts by our stockholders to replace or remove our management.
In addition, Section 203 of the Delaware General Corporation Law ("DGCL") prohibits a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder, which generally refers to a person which together with its affiliates owns, or within the last three years has owned, 15 percent or more of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner.
The existence of the foregoing provisions and anti-takeover measures could limit the price that investors might be willing to pay in the future for shares of our common stock. They could also deter potential acquirers of our company, thereby reducing the likelihood that our stockholders could receive a premium for their common stock in an acquisition.
Concentration of ownership among our officers, directors and principal stockholders may prevent other stockholders from influencing significant corporate decisions and depress our stock price.
Based on the number of shares outstanding as of October 1, 2020, our officers, directors and stockholders who hold at least 5% of our stock beneficially own a combined total of approximately 44.6 percent of our outstanding common stock, including shares of common stock subject to stock options and warrants that are currently exercisable or are exercisable within 60 days after October 1, 2020. If these officers, directors, and principal stockholders or a group of our principal stockholders act together, they will be able to exert a significant degree of influence over our management and affairs and control matters requiring stockholder approval, including the election of directors and approval of mergers, business combinations or other significant transactions. The interests of one or more of these stockholders may not always coincide with our interests or the interests of other stockholders. For instance, officers, directors, and principal stockholders, acting together, could cause us to enter into transactions or agreements that we would not otherwise consider. Similarly, this concentration of ownership may have the effect of delaying or preventing a change in control of our company otherwise favored by our other stockholders. As of October 1, 2020, Jack W. Schuler (and his related entities) beneficially owned approximately 39.1 percent of our common stock. To the extent that this or any other significant stockholders oppose any proposal put forth for stockholder approval by our board of directors, they control a sufficient percentage of our outstanding shares to cause such proposal to either fail or be very difficult to achieve without their support. This, in turn, could have a negative effect on the market price of our common stock. It could also prevent our stockholders
25
from realizing a premium over the market price for their shares of common stock. The concentration of ownership also may contribute to the low trading volume and volatility of our common stock.
Risks Relating to COVID-19
Our financial condition, research and development efforts, and results of operations could be further adversely affected by the ongoing coronavirus outbreak.
Any outbreak of contagious diseases, such as COVID-19, or other adverse public health developments, could have a material and adverse effect on our business operations. In response to the ongoing coronavirus pandemic, we have modified our business practices, including in response to legislation, executive orders and guidance from government entities and healthcare authorities. These directives include the temporary closing of businesses deemed “non-essential,” travel bans and restrictions, social distancing and quarantines. Since March 2020, we have limited employee, researcher and supplier access to the research facility we share with the National Research Council of Canada and our other leased facilities located in Saskatchewan, Canada. Our Canadian operations have not yet been significantly impacted by the coronavirus pandemic. Our research and development facility in Woburn were closed from March through late May 2020, and to date, we have operated our laboratories on a staggered schedule in order to help prevent the spread of the disease. To date, we have also been able to move forward with planning and operational steps required to initiate our planned 2020 field trials in Canada and the United States. It is possible, however, that current and potential future closures of our research facilities, if they continue for an extended time period, could adversely impact our anticipated time frames for completing field trials and other work we have planned to accomplish during 2020.
Additional adverse effects of the coronavirus pandemic could include quarantines, disruptions of or restrictions on our ability and/or the ability of our collaborators’ personnel to travel or conduct normal business activities, as well as additional closures of our facilities or the facilities of our collaborators for an indefinite period of time.
As COVID-19 continues to affect individuals and businesses around the globe, we will likely experience disruptions that could severely impact our business, research and field testing trials, including:
• | interruption of field testing activities due to quarantines or other limitations on travel imposed or recommended by federal or state governments, employers and others; |
• | limitations on employee resources that would otherwise be focused on the conduct of our research and field testing, including because of sickness of employees or their families or requirements imposed on employees to avoid contact with large groups of people; |
• | delays in receiving approval from regulatory authorities related to our seed traits; |
• | delays in field testing sites receiving the supplies and materials needed to conduct our trials; |
• | interruption in global shipping that may affect the transport of materials needed for our research; and |
• | limitations on government and academic grants that support our research programs. |
Additionally, our results of operations could be adversely affected to the extent that COVID-19, or any other epidemic, harms our business or the economy in general either domestically or in any other region in which we do business. The extent to which COVID-19 affects our operations will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration of the outbreak, new information that may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others, which could have an adverse effect on our business and financial condition. Current predictions suggest that the impact of sustained business closures and quarantines resulting from the coronavirus on the global economy will be severe, and this may have a material adverse effect on our business and our ability to secure funding. As we continue to actively monitor the situation, we may take further actions that affect our operations.
26
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the information incorporated by reference in this prospectus contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934 (“Exchange Act”), regarding our strategy, future, operations, future financial position, future revenues, projected costs, and plans and objectives of management. You can identify these forward-looking statements by their use of words such as “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions. You also can identify them by the fact that they do not relate strictly to historical or current facts. There are a number of important risks and uncertainties that could cause our actual results to differ materially from those indicated by forward-looking statements. For a description of these risks and uncertainties, please refer to the section entitled “Risk Factors,” any other risk factors set forth in any information incorporated by reference in this prospectus, as well as any other risk factors and cautionary statements we include or incorporate by reference into this prospectus in the future. While we may elect to update forward-looking statements wherever they appear in this prospectus or in the documents incorporated by reference in this prospectus, we do not assume, and specifically disclaim, any obligation to do so, whether as a result of new information, future events or otherwise.
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of securities by the selling security holders pursuant to this prospectus.
MARKET FOR OUR COMMON STOCK
Market Information
Our common stock currently trades under the symbol “YTEN” on The Nasdaq Capital Market.
Stockholders
As of October 1, 2020, there were approximately 35 stockholders of record.
DIVIDEND POLICY
We have never declared or paid any cash dividends on our capital stock and do not expect to pay any cash dividends for the foreseeable future. We intend to use future earnings, if any, in the operation and expansion of our business. Any future determination relating to our dividend policy will be made at the discretion of our board of directors, based on our financial condition, results of operations, contractual restrictions, capital requirements, business properties, restrictions imposed by applicable law and other factors our board of directors may deem relevant.
27
BUSINESS
Overview
Yield10 Bioscience, Inc. is an agricultural bioscience company that uses its "Trait Factory" and the Camelina oilseed “Fast Field Testing” system to develop high value seed traits for the agriculture and food industries. Yield10 is headquartered in Woburn, Massachusetts and has an Oilseed Center of Excellence in Saskatoon, Saskatchewan, Canada. Our goal is to efficiently develop superior gene traits for the major crops including corn, soybean, canola, and other crops to enable step-change increases in crop yield of at least 10-20 percent. Our “Trait Factory” encompasses discovery of gene targets using our GRAIN (“Gene Ranking Artificial Intelligence Network”) big data mining platform, deployment of trait gene targets in the oilseed Camelina and generation of field performance data. The “Trait Factory” enables two complementary commercial opportunities with different paths to market. The first is trait licensing to the major seed companies for corn, soybean, canola and other crops. Data from our trait field testing in Camelina has enabled Yield10 to establish research license agreements with leading seed companies including the Bayer Crop Science division of Bayer AG ("Bayer"), GDM Seeds (GDM), Forage Genetics International, LLC a division of Land O'Lakes, Inc. ("Forage Genetics") and JR Simplot Company ("Simplot"). These companies are progressing the development of Yield10 traits in soybean, forage sorghum, and potato. The second commercial opportunity is to improve the performance and value of Camelina as a platform to develop a commercial crop product business producing nutritional oils and PHA biomaterials. Using this approach, Yield10 can leverage the resources of the major seed companies to efficiently develop superior gene traits for the major crops thereby creating opportunities for licensing revenue while focusing internal resources on trait gene discovery and the commercial development of Camelina products.
Our focus in the near term is to develop a revenue generating business using Camelina to produce nutritional oils. Yield10 has discovered a series of performance gene trait leads for Camelina focused on seed yield and oil content, the two primary drivers of value. Our plan is to focus on these traits using genome editing which can be qualified as non-regulated under U.S. Department of Agriculture ("USDA") Animal and Plant Health Inspection Service ("APHIS") rules. In parallel, the Company plans to establish a program to improve the agronomics of Camelina, including the development of herbicide tolerant Camelina lines. We believe this will enable Yield10 to develop a crop oil product business with a clear path to revenue and growth. This foundation will form a strong base to produce PHA biomaterials in the longer term for use in water treatment and plastics replacement applications. Yield10 believes crop based PHA biomaterials represent a compelling new market opportunity for agriculture addressing a non-traditional market with high upside potential.
Yield10 brings a unique history and skill set, captured in our GRAIN data mining gene discovery platform, for developing advanced crop traits and increasing the concentration of specific biochemicals of commercial interest in crops. Our plan is to also use GRAIN to develop a source of revenue from funded research and development collaborations for traits, products and crops not being directly pursued internally. We are currently engaged in a range of discussions with third parties with respect to different crops, traits and products in the feed, food and pharmaceutical sectors.
Over the last four years, we have been evaluating certain of our traits in greenhouse studies and field tests conducted in the United States and Canada. We currently have four non-exclusive research license agreements in place: with the Crop Science division of Bayer, for the evaluation of our C3003 and C3004 traits in soybean; with GDM for three traits in soybean; with Forage Genetics for the evaluation of five yield traits in forage sorghum; and with Simplot for evaluation of three of our traits in potato. We have progressed our evaluation of C3003 and C3004 in field tests with Camelina and canola and are continuing our field testing in the 2020 growing season. Results to date in Camelina have demonstrated the potential of a series of traits, including C3003 and C3004 to significantly increase seed yield and genome edited traits including C3007-C3010 to increase seed oil content and filed a new patent application on a potentially breakthrough technology for producing PHA biomaterials.
According to a United Nations report, crop production must be increased by over 70 percent in the next 35 years to feed the growing global population, which is expected to increase from 7 billion to more than 9.6 billion by 2050. During that time period, there will be a reduction in available arable land as a result of infrastructure growth
28
and increased pressure on scarce water resources. Consumption of meat, seafood, and dairy products as well as alternative protein sources is also expected to increase based on dietary changes associated with increasing wealth and living standards. This will result in increased demand for feed grains and forage crops and that demand will need to be met with an increasing emphasis on sustainable growth metrics and climate change. Seafood production is increasingly based on aquaculture where fish diets have been increasingly moving to crop-based feed ingredients due to the limited availability and cost of processed ocean harvested fish as feed. Fish oil is the main source of omega-3 fatty acids which are essential in the human diet. Omega-3 oils have been shown to help prevent heart disease and stroke, may help control lupus, eczema, and rheumatoid arthritis, and may play protective roles in cancer and other conditions. Oils high in omega-3 fatty acids are in increasing demand as the supply of fish oil from ocean harvest is under increasing pressure. Aquaculture and other feed markets represent a growing opportunity for Camelina oil, which is high in the omega-3 fatty acid alpha linolenic acid (“ALA”).
Harvestable food production per acre and per growing season must be increased to meet this demand. At the same time, with the increasing focus on health and wellness, food safety and sustainability in developed countries, we anticipate a rise in demand for new varieties of food and food ingredients with improved nutritional properties. With crop intensification (less land available and more production needed), we expect that improved crop genetics based on new gene traits will be a key driver of increased productivity, potentially resulting in the best performing yield traits commanding disproportionate value and disrupting the seed sector. We expect farmers and growers to be the major beneficiaries of these drivers, which represent potential opportunities for increased revenue and crop diversification. Today the global food market has an estimated value of $5 trillion.
Yield10 brings unique capabilities and experience in advanced metabolic engineering and systems biology to optimize photosynthesis and carbon efficiency in crops to increase grain or biomass yield. These capabilities were developed based on sustained investment over many years when the company was named Metabolix. As Metabolix, we solved complex biological problems in the industrial/synthetic biology space to produce bioplastics. By 2012, we had begun work to increase photosynthesis in crops as part of those activities, which led to the creation in 2015 of the current Yield10 business focused on crop yield. In mid-2016 we sold our fermentation-based bioplastics assets to focus on our agricultural innovations and the company was rebranded as Yield10 Bioscience in January 2017.
Exciting new genetic engineering technologies like clustered regularly interspaced short palindromic repeats ("CRISPR") technology and other approaches to genome editing hold promise to accelerate the deployment of novel traits into commercial crops. This CRISPR method of making insertions or deletions of DNA into plants without the use of foreign DNA has been described as “precision breeding.” We signed a research license, with rights to convert to a commercial license, to CRISPR/Cas-9 technology in 2018 to support our genome editing program. We have achieved non-regulated status pursuant to 7 CFR part 340 for three genome edited traits designed to boost oil content in Camelina and for one genome edited trait to boost oil content in canola through the USDA-APHIS “Am I Regulated?” petitioning process and have petitions pending for new edited lines. Genome editing technology as well as the streamlined regulatory process supported by USDA-APHIS for certain types of plant traits may enable agricultural innovators such as Yield10 to deploy and field test new traits more quickly, potentially resulting in a shorter path to market and reduced costs as compared to the more highly regulated path required for traditional biotechnology-derived traits.
29
SUMMARY OF OUR CROP YIELD TRAITS IN DEVELOPMENT | |
R&D Area | Crops Under Evaluation |
Seed Yield Traits-Likely Regulated1 | |
C3003 | Camelina, canola, soybean, corn, potato |
C3011 | Corn, Camelina and canola |
Seed Yield Traits-Likely Not-Regulated2 | |
C3004 | Camelina, soybean, canola and corn |
Oil Enhancing Traits-Likely Not-Regulated2 | |
C3007 | Camelina and canola |
C3008a | Camelina (not-regulated4) |
Oil trait combinations - C3008a, C3008b and C3009 | Camelina (not-regulated4) |
Additional oil traits and combinations | Research in progress (target crops to be determined) |
C3014 and C3015 PHA biomaterials | Camelina in progress |
Yield Improvement Trait Discovery Platform (Traits Potentially Non-Regulated)3 | |
C4001 | Camelina, forage, sorghum and corn |
C4002 | Sorghum and corn |
C4003 | Sorghum and corn |
C4029 | Sorghum |
__________
(1) | C3003 and C3011 consist of microbial genes and are likely to be subject to regulation by UADA-APHIS. |
(2) | These traits are accessible using genome editing or other methods that do not result in the insertion of non-plant DNA. These approaches may be deemed not to be regulated by USDA-APHIS pursuant to 7CFR part 340 based on recent filings by us and other groups. |
(3) | Traits in this area were developed in our GRAIN platform and all are potentially deployable through approaches which may be not-regulated by USDA-APHIS pursuant to 7 CFR part 340. |
(4) | USDA-APHIS does not consider these lines submitted by Yield10 to be regulated pursuant to 7 CFR part 340. Commercial plant or plant lines or plant products derived from these lines may be regulated by the U.S. Food and Drug Administration ("FDA") or U.S. Environmental Protection Agency ("EPA"). |
One of the critical unmet needs in the agricultural sector is to increase the fundamental yield potential of crops to address global food security. Yield10’s Trait Factory encompasses discovery of gene targets using our GRAIN big data mining platform, genetic engineering of Camelina to modify those trait gene targets and the generation of field data with the engineered crops. Performance and molecular data from the engineered crops are then fed back into the GRAIN system to enable refinement of specific gene targets and the identification of new trait gene targets. Data from the Camelina field studies is then leveraged to form relationships with leading seed companies to progress our trait genes in major crops. Modified Camelina lines with improved performance enter the development pipeline and progress on the regulated or non-regulated path to market depending on how the plants are genetically engineered. GRAIN is a powerful new tool developed primarily to focus on Yield10 trait targets. However, we believe we may also be able to generate a revenue stream by providing access to our GRAIN platform to third parties who are interested in other trait targets and/or crops Yield10 is not pursuing.
As we continue to develop the GRAIN platform, key elements of this system have proven effective and have enabled Yield10 to produce several promising crop yield traits in our development pipeline. Yield10 has achieved and published in peer reviewed journals scientific data from growth chamber and greenhouse studies showing that significant improvements to crop yield are possible. We have achieved these results by improving fundamental crop yield through enhanced photosynthetic carbon capture and increased carbon utilization efficiency to increase seed yield. Some examples of these traits and their impact on crop yield are shown below. In order to highlight the power of our advanced metabolic engineering/systems biology approach of improving fundamental carbon conversion processes in seed we developed the C3006 trait. C3006 required a complex combination of
30
microbial genes to enhance carbon fixation from non-photosynthetic pathways in seed. This trait is based on a complex combination of 10 microbial genes which, when deployed into Camelina, more than doubled seed yield in greenhouse studies. Although the genetic complexity of C3006 creates a regulatory hurdle we believe this proof point demonstrates the value of our GRAIN platform and the potential to double Camelina seed yield. We plan to continue the development of the C3003 and C3004 traits as well as our C4000 series of yield traits in Camelina and support our licensees on their development work in corn, canola and potato.
Examples of our traits and their impact on crop yield in growth chamber and greenhouse studies | |
C3003/C3004 traits: | 23% - 65% increase in seed yield in oilseed crops (Camelina) |
C3006 advanced synthetic biology trait: | 128% increase in oilseed yield (Camelina) |
C4001, C4003 traits: | Work ongoing; 70% increase in photosynthesis, 150% increase in biomass (switchgrass) |
Yield10 has a pipeline of more than 10 novel yield traits in research and development and we expect to generate several proof points for our traits in various crops over the next two years.
We are building a portfolio of intellectual property around our crop yield technology and traits. As of June 30, 2020, we owned or held exclusive rights to 22 patents or pending patent applications worldwide related to advanced technologies for increasing yield in crops. Our portfolio of patent applications includes plant science technologies we have in-licensed globally and exclusively from the University of Massachusetts related to the yield trait gene C3003. The first U.S. patent on this trait was issued in 2019. Our portfolio of patent applications also includes advanced technologies for increasing oil content in oilseed crops in-licensed globally and exclusively from the University of Missouri in 2018 and 2019 related to the yield trait genes C3007, C3010 and C3012. Yield10 filed a patent application in 2019 for our triple edit oil content traits C3008a, C3008b and C3009. We also recently filed a new patent application on a breakthrough technology for producing PHA biomaterials in oilseeds which offers the potential for very low-cost production of a new crop product with applications in water treatment and plastics replacement.
Trait Development Process and Stages
Yield10 has effectively condensed the early phases of trait gene discovery with early development to accelerate the identification of trait leads. Novel trait gene targets, identified using our GRAIN discovery platform or in-licensed from academic institutions where proof-of-principle has been demonstrated, are deployed and evaluated in our Camelina Fast Field Testing platform. In the United States, depending on the characteristics of the trait gene, Camelina lines can be engineered using CRISPR genome editing (a non-regulated path) or traditional gene transformation technologies (a regulated path). For genome edited traits, once we have developed Camelina lines with the target trait we can file a petition with USDA-APHIS to confirm that the new Camelina lines meet the non-regulated criteria prior to field testing. For traditional traits defined as regulated at this stage of development, we can apply for regulatory approval and permits in order to carry out field testing. Following first field testing, non-regulated traits deployed in Camelina demonstrating improved yield performance in the field would then progress into the commercial development phase. This phase can be expected to last two to three years to complete activities associated with launching a new variety of Camelina. For regulated traits, the development process is considerably longer and is expected to be in the range of four to seven years based on the multiple steps required in the commercial development phase including event selection, optimization and executing steps in the regulatory approval process.
31
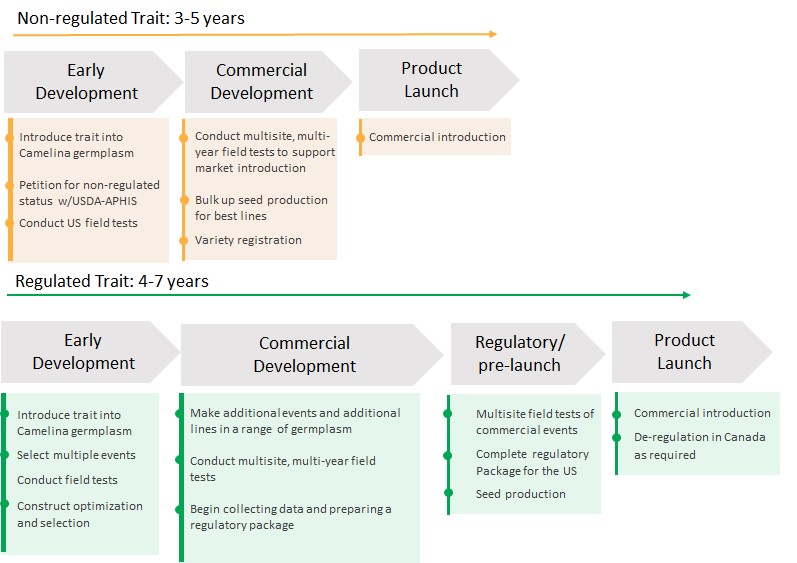
Based on using Camelina as our Fast Field Testing system, we currently have approximately 10 traits at different stages in our development pipeline. As we develop our business plan for commercial products made in Camelina based products such as oils and PHA biomaterials, we expect we will continually evaluate and prioritize our traits in development as we progress to product concepts and commercialization. A summary of our traits and their stage of development in the pipeline is presented below.
Non-Regulated Camelina Traits
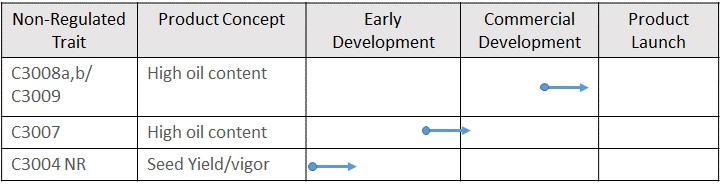
32
Regulated Camelina Traits
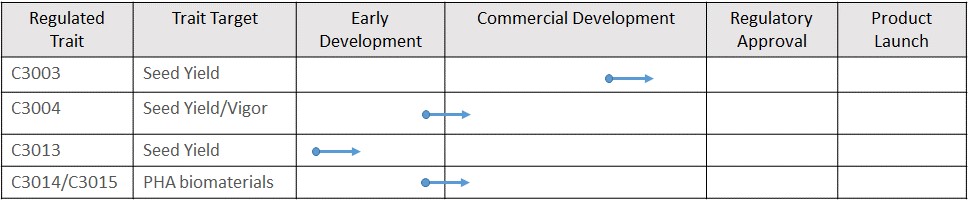
For the development of our traits in major crops such as corn, soybean, canola and others, our approach is to look for partners interested in evaluating our traits and enter into research license agreements to enable them to do so. Until such time as such licensees execute a commercial license with us, we consider our traits to be in the early development phase. Once our licensees execute a commercial license our expectation is that our licensees would place our traits in their development pipelines. We expect our licensees to use their internal capabilities and broad experience to pursue deregulation of the trait in their specific crop target, as they will want to integrate the trait with their regulatory processes, which often encompass complex trait stacking and global deregulation.
Traits Being Developed by Licensees1
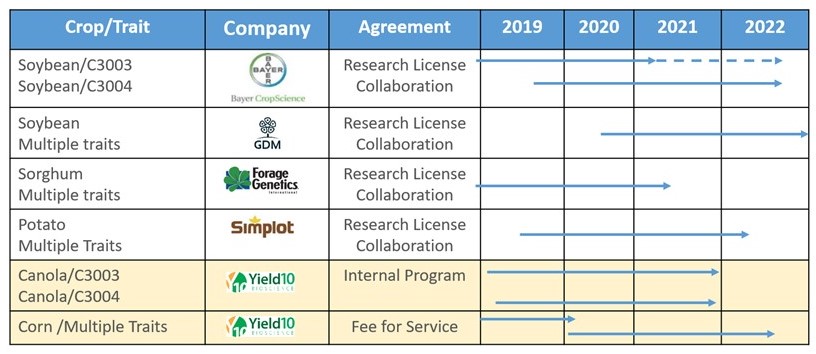
1 The time line shown in the chart reflects the duration of each partner's research license agreements.
The Unmet Need: Global Population Growth Outpacing Anticipated Global Food Supply
Yield10 is targeting a critical unmet need in agriculture based on the future disconnect between agricultural supply and the growing global population. According to a United Nations study, the global population is expected to exceed 9.6 billion people by 2050 and therefore there is a need to sustainably increase global food production including in grains, protein, seafood, dairy and edible oils to meet this demand. This will need to be achieved in the face of increased pressure on land and water resources in addition to increasingly variable weather patterns and growing environmental challenges. Solving this problem is a major global challenge requiring new crop innovation and technologies to fundamentally enhance crop productivity.
33
The Yield Gap
According to several studies described in an article published in the Public Library of Science in 2013, crop yields may no longer be increasing in different regions of the globe, and current rates of crop yield increase based on traditional plant breeding approaches are expected to fall significantly behind the levels needed to meet the demand for global food production. The researchers found that the top four global crops-maize (corn), rice, wheat and soybean-are currently witnessing average yield improvements of only between 0.9 to 1.6 percent per year, far slower than the required rates to double their production by 2050 solely from incremental yield gains. At these rates, global production of maize, rice, wheat and soybean crops may be required to increase by about 67 percent, 42 percent, 38 percent and 55 percent, respectively, by 2050, in order to meet the anticipated increase in demand for food production caused by population growth. The yield increases needed to meet the demands of the growing global population show that a significant “yield gap” exists for each of the crops evaluated in the study.
Yield10 is focused on addressing the yield gap for major crops by using our Trait Factory to optimize photosynthesis and carbon efficiency in crops to increase grain or biomass yield. We have been working in the area of increasing photosynthetic carbon capture and crop yield technologies since 2012 and we have identified several potentially promising genes for increasing yield or improving crop performance.
Health and Wellness, Food Safety and Sustainability
At the same time, with the increasing focus on health and wellness, food safety and sustainability in developed countries, we anticipate a rise in demand for new varieties of food and food ingredients with improved nutritional properties. Further, concerns about food safety have led to the concept of "seed to plate," with a focus on stringent quality control along the entire value chain. If this concept takes hold with consumers, it is likely to require identity preservation from seed to harvest and involve contract farming. This concept is currently being implemented in agricultural biotechnology, in both canola and soybean which have been modified to alter the composition of the oil produced. High oleic canola and soybean oils are being marketed as "healthier" where the value driver is the ability to make marketing claims directly to the consumer.
Camelina oil naturally contains over 30% by weight of the omega-3 fatty acid and has recently been shown in clinical studies to be more effective than fish oil for controlling LDL cholesterol indicating potential use in reducing heart disease. This oil is also finding applications in aquaculture feed due to current constraints on the availability of omega-3 fish oil from ocean harvested fish. Yield10 believes that Camelina also has considerable potential as a cover crop to reduce soil erosion and nutrient run-off from land used for row crop production. In the longer term the production of PHA biomaterials in Camelina would represent an entirely new market opportunity for farmers. This opportunity could provide economic returns for farmers to justify large acreage adoption of Camelina as a cover crop and enable the low-cost production of this product for new markets including water treatment and sustainable biodegradable plastics replacement applications.
Business Strategy
Our goal is to build a successful agricultural biotechnology company centered on demonstrating and capturing the value of our traits and technologies based on the following three potential revenue streams:
• | Licensing of our yield and performance traits for use in major row crops; |
• | Product sales revenue from products produced in the oilseed Camelina; and |
• | R&D revenue for access to our GRAIN trait gene discovery platform. |
Using our Trait Factory, we have identified and are evaluating novel yield trait genes to help address the growing global yield gap in food and feed crops. Crop yield is the key decision variable for farmers in making seed buying decisions, and as a result, is critical to the seed industry. Improvements in yield to the levels targeted by Yield10, for example 10-20 percent increases, would be expected to generate significant value to the seed and farm sectors. For example, Yield10 is targeting an approximately 10-20 percent increase in canola and soybean yields, which, if successfully deployed across North American acreage, could result in annual incremental crop value of up
34
to $10 billion. By ultimately increasing the output of major food and feed crops and potentially reducing strains on scarce natural resources, we believe that Yield10’s technologies will also contribute to addressing global food security.
Yield10 plans to develop yield traits that enable farmers to increase their revenue, and also to license our trait innovations to the major agricultural companies so that they can be deployed in elite seed varieties. Performance traits result in increased harvest value, which is then shared based on the well-established value sharing model in the seed sector. Yield10 plans to continue to focus on its core competency of advanced trait gene discovery through the Trait Factory, while also building an independent, revenue generating, specialty products business based on the Camelina oilseed.
Our C3003 yield trait is an algal gene, and we believe that it will be regulated by USDA-APHIS and other regulatory agencies in the United States and around the world as a biotech trait. In 2017, we signed a non-exclusive research license with the Crop Science division of Bayer AG ("Bayer") (formerly Monsanto Company), to test C3003 and the first version of C3004 in soybean. In 2019, the license was expanded to cover a new discovery and intellectual property related to a new version of C3004. Similarly, in 2018 we signed a non-exclusive research license with Forage Genetics, to test a series of traits in forage sorghum. In 2019 we signed a non-exclusive research license with Simplot for the evaluation of our traits in potato. In 2020, we signed a non-exclusive research license with GDM for the evaluation of three traits in soybean. We have been progressing our traits internally in canola and in corn on a fee for service basis but plan to look for partners for our traits in both crops this year. Yield10 significantly expands the development pipeline by enabling the licensees to progress our traits in major crops. Our focus is on securing a share of the upside value of our traits when we finalize the economic terms of license agreements at the point where the value of the trait is well understood.
We believe we can leverage our seed yield and oil content traits to add value to Camelina in North America in the near term. We will focus our initial development activities on the production of nutritional oils for human and aquaculture feed markets using traits that can be qualified as non-regulated by USDA-APHIS to build our commercial capabilities.
The production of PHA biomaterials in Camelina could open new markets and provide economic returns for farmers to justify large acreage adoption and enable the low-cost production of this natural biodegradable product for water treatment and plastics replacement applications. We believe crop-based production will enable an advantageous cost structure thereby eliminating one of the remaining significant barriers to entry for broad adoption of these biomaterials. By reprogramming Camelina to produce PHA in the seed, the harvested seed can then be processed to produce three products: oil, protein meal for animal feed, and PHA biomaterial. The typical costs for producing edible oils are a useful benchmark for the potential long-term cost structure for crop based PHAs. In this scenario, crop based PHAs would have a cost advantage over petroleum-based plastics.
In water treatment, the PHA biomaterial acts as a growth substrate and energy source for denitrifying bacteria which convert nitrate, a primary cause of water pollution and algal growth, to nitrogen gas which returns to the air. This application is technically straight-forward, requiring only the production and shipment of PHA biomaterials in pellet form. Yield10 is in the early stages of developing a revenue generating business model for this opportunity.
PHA biomaterials are also useful for functionally replacing petroleum-based plastics in a wide range of packaging applications. For example, the plastics industry produces more than 350 million tons of material per year globally. This sector is facing intensive scrutiny due to increasing plastic waste pollution in the environment. As natural biomaterials, PHAs fully degrade over time in the environment yet have good processing and physical properties and can be processed like plastics to produce articles with excellent shelf life in use. When we made the transition to the Yield10 business we divested our fermentation based PHA bioplastics assets and related applications technology. However, Yield10 retained the rights to PHA production in engineered crops. Yield10 plans to eventually look for partners to produce resin-grade PHA biomaterial for supply to the plastics sector but will focus the initial launch on water treatment applications.
We are at an early stage of developing a detailed plan for the Camelina business but believe it may have considerable potential for Yield10. Completing this business plan is a key goal for 2020.
35
Our History
We have a significant track record and expertise in the metabolic engineering of microbes and have made significant progress translating this capability to plants.
As part of the legacy biopolymers and biobased chemicals business of our predecessor company, Metabolix had supported a crop science research program to produce PHA biomaterials in crops as a potential low-cost production system. Historically, these efforts were focused on producing the simplest member of the PHA family, known as PHB, which is a microbial carbon storage biopolymer, in high concentration in the seeds of oilseed crops or in the leaves of biomass crops such as switchgrass. The PHB biomaterial is useful as a natural water treatment product and as a replacement for petroleum-based plastics.
As we made progress on producing PHB in plants, we learned that basic carbon supply from photosynthesis was a bottleneck. To address this carbon shortfall, in 2012 we began developing new metabolic engineering and bioinformatics approaches to enhancing basic crop photosynthetic carbon capture. Discoveries from these two approaches became the foundation of our GRAIN crop trait discovery platform. We also began building intellectual property on novel yield trait gene technologies discovered in these programs and realized that our experience in re-engineering the flow of carbon in microorganisms could be applied to building better plants with higher yield potential. Improving the yield potential of major crops is an essential step to increase seed and/or biomass yield and, therefore, food production.
Our Approach
Our GRAIN platform provides us with a unique approach for discovering novel yield trait genes.
We have integrated advanced metabolic flux modeling capabilities with transcriptome network analysis to form the foundation of our GRAIN big data mining gene discovery platform. This discovery platform is the core of our Trait Factory. In the case of crops, the levers to increase seed yield are the metabolic infrastructure through which carbon flows from photosynthesis to seed production and the gene regulators or transcription factors which control the various pathways. Over the last 20 years, the agricultural sector has generated vast numbers of data points. During this same period, there have been very few new crop traits produced. GRAIN efficiently mines big data sets and prioritizes actionable gene targets to improve crop productivity. We have employed this approach to discover a range of potential yield trait genes.
We developed the Camelina Fast Field Test model system to characterize, evaluate and de-risk novel yield trait genes.
One of the challenges the agricultural industry has faced over the years is translating early crop science discovery into value generating traits. In part this is because results from greenhouse studies in model plants have not translated well into field results in major crops. Translating success with non-plant genes in major crops has been successful for traits such as insect resistance and herbicide tolerance and the current biotechnology seed sector, which accounted for 457 million acres of crops worldwide in 2016, is based on using microbial genes in plants. The long timelines to progress early discoveries successfully into major crops and generate field data adds to the challenge.
For these reasons, Yield10 has put in place a process we call “Fast Field Testing” based on our Camelina oilseed platform. Camelina is an industrial oilseed well-suited to field trials, and we believe it is a promising new crop for farmers. It is also very fast to modify, develop genetically stable seed and scale up seed for field planting. Ideally, we hope to be able to progress from trait identification to field planting in about 12 months. Results from our field studies in Camelina can then be used to generate partner interest in progressing our traits in corn, soybean, canola and other crops through research license agreements.
36
We are focused on identifying and developing technologies that will enable us to produce step-change improvements to crop yield and value.
Yield10 is targeting a critical unmet need in agriculture based on the anticipated disconnect between agricultural supply and the growing global population. Food production must be increased by over 70 percent in the next 35 years to feed the growing global population, which is expected to increase from 7 billion to more than 9.6 billion by 2050. Global climate change is also resulting in regional shifts to historical growing conditions. Given the projection for population growth, recent studies show a “yield gap” for major food and feed crops that cannot be addressed by incremental improvements to yield brought about by traditional plant breeding and existing biotech traits. Current biotech traits deployed in crops by the seed industry are based primarily on using microbial-sourced genes to impart yield protection through herbicide, pest, disease and even drought resistance, whereas Yield10 is focused on increasing fundamental crop yield through enhanced carbon capture and utilization. The demand for edible vegetable oils and healthier edible oils is also increasing.
Yield10 is using the Trait Factory to optimize photosynthesis and carbon efficiency in crops to increase grain or biomass yield targeting step-change increases in the range of 10-20 percent in crop yield.
We have assembled a pipeline of crop yield traits for development that are applicable to major commercial crops and established agreements with major seed companies.
Our unique approach to crop yield trait discovery utilizing our GRAIN platform, which integrates advanced metabolic engineering concepts to address critical bottlenecks in carbon metabolism, has enabled us to discover a series of yield genes with potential use for producing step-change improvements in crop yield. Through our research and early development efforts we have identified and begun characterizing our C3000 and C4000 series of traits. To initially characterize the potential yield trait genes, we test our yield trait candidates using our Camelina platform. As a yield trait innovator, our objective is to identify novel yield traits that act at a fundamental level in crop metabolism to provide the potential for broad deployment of our traits across multiple crop types. Following our work with these trait genes in Camelina our approach is to enter into license agreements or form collaborations with major agricultural companies so they can incorporate our novel yield traits into their seed products.
We believe our business model will allow us to capture value for our yield trait discoveries and provide a path to commercialization for important new yield traits for major crops.
Yield10 is working to advance our own developments as well as form business alliances to progress our traits through development, launch and commercialization. Key to our strategy is to retain, where practical, control of timelines and maximize, where possible, the opportunity for value creation and optionality around future value realization strategies. We are focused on identifying and signing additional research and development collaborations to accelerate commercial development of our promising yield traits. Based on this strategy Yield10 can focus internal resources on trait gene discovery and developing an independent Camelina based products business.
We have signed non-exclusive research licenses for our novel yield traits with agriculture industry leaders.
In 2017 we granted a non-exclusive global research license to Bayer to evaluate our novel yield traits C3003 and C3004 in soybean. The license was expanded in 2019 to include a new discovery and intellectual property for C3004. Bayer is a leader in the development and commercialization of biotech-derived soybean seed. In 2018, we granted a research license with a similar structure to Forage Genetics, a leader in forage crops used for animal feed, to evaluate five traits in forage sorghum. In 2019 we granted a research license with a similar structure to Simplot, a leader in potato. In 2020, we signed a non-exclusive research license with GDM for the evaluation of three traits in elite soybean germplasm.
These licenses are intended to provide market leaders in their respective crops with an attractive opportunity to test our traits and develop data at their own expense. At any time during the term, they have the option to negotiate a broader agreement with us. At the same time, we have the right to sign licenses with other companies for these traits. This structure allows us the flexibility to expand the testing of our traits with investment by other companies and to potentially enter negotiations for development and commercial licenses when the value of
37
our traits is better understood. In 2019, we continued to explore additional opportunities to expand the testing of our traits through similar arrangements with other companies and as part of our evolving strategy we now plan to look for partners in canola and corn.
We have identified promising potential traits which can be modified using genome editing. We believe that such targets may be subject to less regulatory complexity in the U.S. during development and along the path to commercialization and may provide opportunities for licensing.
Genome editing techniques, including CRISPR, which involve making small targeted changes to the DNA of a target organism, have been of interest to the agricultural biotechnology industry because this approach is believed to have the potential to significantly reduce development costs and regulatory timelines for crop trait development and market introduction. In 2018, we signed a non-exclusive research license for CRISPR/Cas-9 technology with the Broad Institute of MIT and Harvard and Pioneer, part of Corteva Agriscience.
USDA-APHIS has streamlined the regulatory path for genome edited plant lines that do not contain any remaining foreign DNA (i.e., DNA sequences not from the plant being engineered) from the procedure used to edit the plant. These plant lines may not be subject to certain USDA-APHIS crop regulations in the U.S. See the “Regulatory Requirements” section below. This significantly decreased the timeline and cost of developing and bringing some new traits to commercialization in the U.S. The GRAIN platform is particularly well suited to the challenge of identifying new gene targets for genome editing that can generate economic value. This has opened the potential for Yield10 to exploit a second tier of novel traits addressable with genome editing.
From its internal GRAIN discovery platforms and those in-licensed through academic collaborations, Yield10 has identified gene targets suitable for deployment in crops through genome editing. We have deployed genome editing technology based on our C3008a trait in Camelina as well as our triple edited-line based on our C3008a, C3008b and C3009 traits in Camelina, which were deemed non-regulated by USDA-APHIS in 2017 and 2018, respectively. In 2020, we filed petitions to confirm non-regulated status for a number of genome edited C3007 Camelina lines and C3007 canola lines. These C3007 plant lines were deemed non-regulated by USDA-APHIS in 2020. C3007 is a trait in-licensed from the University of Missouri for increasing oil content. We also believe that our C3004 yield trait can be deployed in Camelina through a non-regulated process. Plants that are not regulated by USDA-APHIS may still be subject to regulation by the FDA or the EPA depending on certain characteristics and the plant’s intended uses. We believe our GRAIN platform for identifying genome editing targets as well as improved crops we could develop using this approach may enable us to form collaborations or enter into license arrangements with a broader set of potential commercial partners in order to bring these genome edited traits forward into development in the near-term.
We believe Camelina has high potential as a commercial crop for producing nutritional oils and PHA biomaterials in North America.
Based on our 10-year investment in the Camelina platform and trait proof points achieved to date, we believe Yield10 has established the foundation for a crop product business producing nutritional oils and PHA biomaterials. Camelina or Camelina sativa is an oilseed crop currently in limited cultivation in North America and Europe. Camelina oil has historically been used in food and production is increasing because of its natural omega-3 fatty acid content. Results from a randomized controlled study published in 2018 in the journal Molecular Nutrition and Food Research have shown that Camelina sativa oil, but not fatty fish or lean fish, improved serum lipid profile in subjects with impaired glucose metabolism. Camelina protein meal left over following oil extraction by cold crushing has been approved by regulatory authorities for use in animal feed applications in the U.S. and Canada. In the cold crushing process to extract oil, some of the omega oil remains in the meal, making it attractive for use as chicken feed because it increases the omega-3 content of eggs.
We believe that our Camelina development capabilities, together with our yield and oil content trait improvements, will enable an attractive Camelina products business focused on nutritional oils in the near-term. In the longer term, the potential for production of PHA biomaterials in Camelina could provide economic returns for farmers to justify very large acreage adoption and enable the low-cost production of this product. PHA biomaterials with the right cost structure have applications in very large markets not currently served by agriculture including
38
water treatment and plastics replacement applications. We believe crop-based production will enable broad-based global adoption of these materials.
Our Oilseed Operation based in Canada provides us with unique capabilities in the development of oilseed crops.
We established our oilseeds subsidiary in Canada in 2010 to produce robust oilseed germplasm with engineered value-added traits for commercial crop production in western North America. Our oilseeds team is based in Saskatoon, Saskatchewan, with laboratories in the National Research Council (NRC) - Saskatoon facility and commercial greenhouse and laboratory facilities at nearby Innovation Place. Our team has developed and implemented technology to improve and accelerate engineering and trait evaluation of Camelina and canola. The team also plays a key role in designing and conducting greenhouse and field tests required to effectively evaluate novel yield traits.
We have a network of commercial and science advisors to provide us with insight and opportunities to advance our industry alliances, crop research and development, and key intellectual property.
Yield10 has pursued academic collaborations that have led to the discovery of novel yield trait genes. Researcher Danny Schnell, Ph.D. discovered the C3003 trait in an ARPA-e (a division of the U.S. Department of Energy ("DOE") funded collaborative project at the University of Massachusetts in which Yield10 was a partner. In 2015, Prof. Schnell moved to Michigan State University where he is Chairperson, Department of Plant Biology and remains a collaborator on C3003. In 2018, Yield10 announced signing a global license agreement with the University of Missouri for advanced technology to boost oil content in oilseed crops, including C3007 and C3010, which are based on the discovery of a key regulatory mechanism controlling oil production in oilseed crops which can be used to increase oil content. Jay J. Thelen, Ph.D., Professor of Biochemistry at the University of Missouri, who discovered this mechanism, joined our Scientific Advisory Board in 2018.
We plan to seek U.S. and Canadian government grants to support our research and development goals.
Yield10 has been awarded grants over the last several years supporting research on strategies to improve the efficiency of photosynthesis, increase seed oil content, identify novel yield traits and test these novel traits in Camelina. This work is valuable because traits developed in Camelina have the potential to be developed and deployed in other oilseed crops. For example, in 2017, we were selected as a sub-awardee on a new DOE grant led by Michigan State University to conduct research aimed at boosting oilseed yield in Camelina. We plan to continue to pursue government grants to defray research costs associated with our research and development activities.
We are operating with a lean organizational footprint which is evaluating our novel yield traits in greenhouse and field tests while maintaining efficient use of cash resources.
As of June 30, 2020, we had 25 full-time employees, with the majority directly involved with our research and development activities. We believe that our organizational capabilities are aligned with our research priorities and are complemented by our use of third-party infrastructure and certain service providers. With this approach we can leverage third-party infrastructure and capability without having to spend the time and capital needed to recreate them in-house. This is allowing us to focus our limited resources on deploying our core strengths against our key development goals. We expect to grow our research and development operations over time commensurate with building value in our business and advancing our traits through development while at the same time tightly managing overhead costs.
Our “GRAIN” Technology Platform
In the last two decades there has been a dramatic expansion of new genetic engineering and systems biology tools: genomics data, metabolic engineering, high-throughput analytical tools, including whole organism gene expression analysis and metabolomics, and powerful genome editing technologies. As a result, the seed sector has tested thousands of single genes and generated billion if not trillions of data points yet step change improvements in crop yield have remained elusive. Yield10 is bringing new approaches and innovation based on
39
over 30 years of experience in advanced synthetic biology and metabolic systems modelling to improve inherent yield of major food and feed crops.
At a fundamental level, increasing crop yield is a complex two-step carbon optimization problem. Harvested seed is mostly carbon fixed from carbon dioxide in the air by photosynthesis with oxygen coming from water in the soil and smaller amounts of nitrogen and phosphate both of which are applied as fertilizer. Based on our experience optimizing carbon flow in living systems, we know that increasing seed yield will likely require multiple trait genes to increase carbon fixation by photosynthesis at the front-end and direct the increased fixed carbon to the seed.
Plant growth is based on a series of chemical reactions and these can be modeled to determine the best ways to optimize the yield of the targeted product. We have integrated advanced metabolic flux modeling capabilities with transcriptome network analysis to form the foundation of the GRAIN gene discovery platform. GRAIN is a powerful new data mining tool which the company has protected as a trade secret. Yield10 has used GRAIN to identify a pipeline of traits it is developing in Camelina to determine performance and then through a series of license arrangements with major seed companies in other crops. We also believe our integrated GRAIN platform can be used to successfully identify new targets for improving crop yield and are working to leverage the platform in the near-term to secure research and development funding from industry partners.
Traits in Development
Based on our early innovations, the development of the fully integrated GRAIN trait gene discovery platform and the execution of our in-licensing strategy, Yield10 has established a strong pipeline of traits in development. By using our Camelina platform as a Fast Field Testing platform, to generate initial trait performance data, our traits are furthest along in Camelina. This has formed the foundation of our strategy to develop a Camelina based specialty products business. Data from Camelina field studies have also helped us to establish research license agreements with four seed companies (Bayer, Forage Genetics, Simplot, GDM) to enable them to progress our traits in major crops. Yield10 is progressing proprietary traits in corn through a service agreement. In 2019 Yield10 continued to progress the development of our C3000 series of seed yield and oil content traits as well as some of our C4000 series traits in the oilseed Camelina. We progressed several of these traits into major crops including corn, soybean, forage sorghum and potato through third party relationships and in canola using internal resources. In 2019, Yield10 announced the filing of a new patent application on a technology breakthrough for producing high levels of a PHA biomaterial, a product with very large market potential in oilseeds.
Novel Yield Trait Gene C3003
C3003 is an algal gene, in-licensed from the University of Massachusetts. We believe based on GRAIN modelling that C3003 reduces the well-understood yield losses that occur through photorespiration, a side reaction of photosynthesis in C3 crops based on early positive results. C3 photosynthesis, the simplest type of plant photosynthetic system, exists in most agricultural crops used for human consumption, including canola, soybean, rice, wheat and potato. Yield10 is progressing the introduction of the C3003 trait gene as well as improvements to the C3003 trait in Camelina, canola, and corn.
Canola is an important North American oilseed crop harvested for its oil. We are targeting step-changes of 10-20% in the evaluation and development of novel traits to increase seed yield in canola. In our small scale field tests of canola in 2018, we achieved seed yield improvements in some events at the low end of this range (11%), and based on these results, we continued to progress C3003 into the preliminary commercial development phase in canola in 2019. Key activities in 2019 included field testing of the C3003 Canola lines from the 2018 trial and the development of additional commercial quality C3003 canola events. The 2019 growing season was particularly challenging with wide swings in weather patterns and as a result we were unable to generate statistically significant performance data. However, based on earlier results and our increasing understanding of the underlying biological mechanism, we remain committed to progressing the development of C3003. In 2019, we produced 15 additional high quality C3003 canola lines and are continuing the field testing and development programs with this trait in Camelina and canola in 2020.
40
Under a research license, Bayer is working with C3003 in its soybean program as a strategy to improve seed yield. We anticipate that Bayer will generate field test data with C3003 pursuant to the research license. The Bayer license was expanded in 2019 to include a new discovery relating to C3004 that will enable them to begin deploying and testing this trait in their soybean program. In 2020, we signed a research license with GDM for three traits in soybean which will allow GDM to deploy our traits in their elite soybean varieties.
Novel Yield Trait Gene C3004
While the role of C3004 is currently not well understood and we continue to investigate the role of the gene in plant metabolism, we believe that it may affect carbon partitioning (the flow of carbon from green photosynthetic tissue to seed development) in plants. Our ongoing research will continue to investigate the activity of C3004 alone and in combination with C3003 to produce increases in seed yield in crops.
We began our investigation of C3004 by preparing genetic constructs to increase the expression of the C3004 gene in Camelina. Stable C3004 Camelina lines were developed and we performed yield studies in a greenhouse and a controlled environment growth chamber. In these studies, increased expression of C3004 in Camelina resulted in a significant increase in plant growth and vigor, increased branching and seed yield, and in some cases increased individual seed weight. In 2019 we continued the development of additional C3004 Camelina lines, conducted greenhouse studies and our first field tests.
Our 2019 greenhouse studies included additional C3004 Camelina lines with different Camelina genetic backgrounds. We again observed increased vigor, branching and increases in seed yield consistent with our 2018 observations. In our 2019 field tests, photosynthetic measurements were taken during the growing season on C3004 Camelina lines at similar developmental stages. Five lines tested showed statistically significant increases in several important photosynthetic parameters for plants, including CO2 fixation, electron transport rate, and the conversion of light energy to chemical energy (effective quantum yield). While field conditions throughout Western Canada were harsh, including severe drought, there were indications that the C3004 plants produced more seed than wildtype Camelina; however, substantial variability among the test plots under these severe conditions confounded the collection of statistically significant seed yield data from the study. In 2020, Yield10 is field testing C3004 Camelina lines at an expanded number of sites to collect agronomic and seed yield data; we are also field testing C3004 in canola for the first time. We currently have research license agreements in place with seed companies to evaluate the Camelina C3004 gene in soybean, corn and potato.
The version of the C3004 trait we have tested so far in our Camelina studies was genetically engineered using a traditional transgenic approach; however, we believe that it may be possible to develop versions of C3004 Camelina that are non-regulated under current USDA-APHIS rules and expect this will form a key part of our Camelina commercial development effort.
Oil Enhancing Traits: C3007, C3008, C3009, C3010 and C3012
Edible oils or vegetable oils are derived from fruits and vegetables, such as palm, soybean, rapeseed (canola) and sunflower. These oils are used in frying, baking, other types of cooking and in food preparation and flavoring such as salad dressings and bread dips. Edible oils are of increasing importance among health-conscious consumers as key functional ingredients which may reduce the risk of cardiovascular disorders along with potentially lowering the possibility of certain kinds of breast cancer. Based on these drivers the global edible oil market is anticipated to witness a substantial growth in demand for unrefined, unprocessed, healthy, and organic oil.
This is leading to the development and commercialization of modified soybean and canola oils with higher levels of healthier unsaturated fatty acids. Health benefits of omega-3 fatty acids from fish oil are creating additional interest in plant-based omega-3 vegetable oils as a nutritional constituent in the food industry on account of their exceptional anti-inflammatory and other health attributes. Camelina and flax naturally produce omega-3 oils and are seeing increased commercial interest and we believe that Yield10 is well positioned to become the leader in Camelina oil production.
Yield10 is progressing a series of traits targeted at increasing the oil content in Camelina where the oil is the main value driver. Based on the results we obtain with Camelina we may be able to license these traits to seed
41
companies for use in other oilseed crops including canola and soybean. Yield10 is building significant capabilities and intellectual property around key oil biosynthesis pathways in plants based on technologies for increasing oil content in seeds. In cases where the edible oil is the primary economic value driver for the crop, increasing oil content is a valuable trait. Improving the oil content and yield of Camelina seed would make this an attractive crop for producing omega-3 nutritional oils. Based on our study of metabolic pathways in oilseed crops, we believe there is an opportunity to apply genome editing to significantly increase oil content in oilseed crops including canola, soybean, sunflower and Camelina. We began the technical work in Camelina in 2016 with our C3008a, C3008b and C3009 traits which regulate the production and degradation of oils in oilseed crops. In 2017 and 2018, we received confirmation from USDA-APHIS’s Biotechnology Regulatory Services (BRS) that two types of our genome-edited Camelina plant lines developed using CRISPR/Cas-9 genome editing technology for increased oil content were not considered to be regulated under 7 CFR part 340, clearing the way for field testing in the U.S. The first type is based on the inactivation of an enzyme expected to decrease turnover of oil in mature seeds and reduce free fatty acids in oil, a trait we have designated as C3008a. The other type is based on the inactivation of three enzymes to both enhance the production of oil and decrease turnover of oil in mature seeds and is designated as our triple edit, or C3008a, C3008b and C3009 trait containing line. We completed our first field trial with these edited Camelina lines in the U.S. during the 2019 growing season. Some of the Camelina lines with edits to the three genes produced an increase in oil content in individual seeds as well as an increase in seed oil content as a percentage of seed weight as compared to control plants. The best performing line produced an average 11.8 percent increase in oil per individual seed, an 8.7 percent increase in individual seed weight, and a 4.7 percent increase in seed oil content as a percentage of overall seed weight. No significant change in oil composition was observed. Yield10 is conducting additional field tests with the best Camelina line (E3902) in the 2020 growing season and is scaling up pure seed production in anticipation of potential commercial use.
In 2018, we signed an exclusive global license agreement with the University of Missouri for advanced oilseed technology including the C3007 and C3010 gene traits, promising targets focused on the central role of Acetyl-CoA Carboxylase ("ACCase") a key metabolic control point for oil production. In 2019, we signed an additional exclusive global license with University of Missouri for another ACCase related gene target we named C3012. We have produced genome edited versions of C3007 in Camelina and canola. We filed petitions with USDA-APHIS to confirm that the agency does not consider the Camelina and canola lines to be regulated under 7 CFR part 340. We received confirmation of non-regulated status from USDA-APHIS in 2020. We are testing the C3007 Camelina genome edited lines in the field during the 2020 growing season and plan to test the C3007 canola genome edited lines in the 2021 growing season.
PHA Traits: C3014 and C3015
While we continue the discovery of novel yield traits for licensing to seed companies and focus on deploying our non-regulated traits to improve the performance and value of Camelina to produce nutritional oils in the near-term, we believe there may be significant market opportunity for producing PHA biomaterials in Camelina in the future. PHA biomaterials (PHAs) are natural microbial high molecular weight polymeric storage polymers. These polymers are natural polyesters and can be recovered from the microbes which produce them and processed using standard plastics processing equipment into a range of product forms. PHAs have applications in a wide range of markets including animal nutrition, wastewater treatment and the replacement of petroleum plastics. Commercialization of PHAs based on fermentation technologies continues to receive considerable media and investment interest even although this approach has proven challenging due to the high capital and operating costs. Feedstock and energy costs dominate, the net result being PHA products with limited market adoption. We believe direct production of PHAs in crops can lead to low production costs and open large market opportunities. Seeds are natural, stable storage sites for large amounts of oil and proteins deposited by plants to nourish seedlings following seed germination in the field. The stability of seeds at ambient temperatures allows them to be readily harvested, transported and stored prior to processing and makes them the ideal site in a plant for producing PHA biomaterials. In 2019, Yield10 filed a U.S. Patent application for new technology potentially enabling low-cost production of PHA biomaterials in the seeds of Camelina. The new Yield10 patent application describes a discovery around maintaining the viability and vigor of Camelina seed programed to produce high levels of the PHA biomaterial PHB. By introducing the three genes encoding the pathway for producing PHA from the plant metabolite acetyl-CoA we have demonstrated the production of up to 10% PHB in seeds of Camelina with good seedling viability in growth
42
chambers. We believe crop-based production will enable an advantaged cost structure thereby eliminating a barrier to entry for broad adoption of these materials. The key concept was to introduce PHB as a new component of the seed composition and by processing the PHB producing seed, to produce oil, polymer, and protein rich seed meal. The combination of all three products improves the overall value proposition and we believe that in time this will result in PHA biomaterial costs in line with canola and soybean oils. Yield10 plans to develop and commercialize Camelina seed based PHA biomaterials for water treatment applications and look for commercial partnering opportunities for plastics replacements markets. We currently have two new PHA biomaterial traits, C3014 and C3015, in our development pipeline and plan to carry out the first field tests in 2020 with these traits, pending regulatory approval. In the planned tests, we will also bulk up C3014 and C3015 Camelina seed for planting in the 2021 growing season so we can begin to scale production and produce crop based PHA samples for testing and demonstration purposes.
C4000 Series Traits
We used our GRAIN platform to study global transcription factors and identify novel yield traits in the C4000 series. These traits may be powerful regulators of plant growth and represent a potentially valuable resource for identifying genome editing traits for crops. We have recently shown that traits from the C4000 series can significantly increase photosynthetic efficiency, above ground biomass, and below ground biomass production in our switchgrass plants engineered to overexpress the transcription factors. We reported these results for our novel C4001 and C4003 traits in 2018 in the journal Plant Science. Switchgrass plants expressing C4001 resulted in a total increase in biomass of 75-100 percent in leaves and stems as compared to controls. Expression of C4003 in switchgrass resulted in a total increase in biomass of 100-160 percent in leaves and stems as compared to control plants. Increasing biomass yield is important for forage crops such as sorghum, silage corn, and alfalfa.
We are progressing the development of certain of our C4000 series of traits in Camelina and corn. Depending on the field performance of the C4000 series Camelina lines, Yield10 plans to integrate them into a commercial Camelina seed business. Recognizing our limited internal capabilities and resources in corn, the Company plans to seek partners interested in progressing these traits in corn under a license agreement like the one in place with Bayer for soybean. Forage Genetics began work with certain of our C4000 series traits through a research license signed in 2018 to assess the potential of our traits to increase biomass in forage sorghum. Simplot is testing the C4001 trait in potato. We also began early development work in late 2018 to assess certain C3000 and C4000 series traits in corn through a third-party agricultural company.
We expect evaluation of C4000 series traits in these target crops will continue to advance in 2020. Traits in this series and the proof points we expect to generate may provide us with an opportunity to selectively partner with others for the development of these traits in major commercial food, feed, and forage crops.
Target Crop: The Oilseed Camelina sativa
As we continued to make progress on the development of our novel yield traits for major crops through research license agreements with major seed companies, we have been working to develop Camelina based business opportunities for Yield10. Our vision is to use our proprietary non-regulated gene traits to improve Camelina seed oil content and yield in the near term to produce nutritional oils. In the longer term, we believe optimizing the production of PHA biomaterials in Camelina will enable large acre production as a cash cover crop. Some estimates from USDA indicate a potential of up to 30 million acres in the upper corn belt of the U.S., which would make Camelina the third largest crop in the U.S. Ideally, cash cover crops should improve the sustainability of food production by reducing nutrient pollution of our waterways and provide additional sources of revenue for farmers. A new product like PHA biomaterials may have the potential to create value-added bioproducts markets in water treatment and plastics replacement. Production of PHA biomaterials in cover crops may also provide environmental benefits in that it would reduce fertilizer run-off from farming on the front end, and produce a natural biodegradable product that can be used to reduce nitrate pollution from aquaculture and septic systems on the back end.
Camelina is an attractive choice of crop for the following reasons:
43
• | There is a growing demand for crops that diversify the crop landscape, have lower environmental footprints and have the potential to produce high value secondary products, opening new opportunities for farmers. |
• | Camelina oil is rich in an omega-3 fatty acid (ALA) which is creating demand for the oil as a substitute for fish oil in aquaculture. |
• | Camelina is readily segregated from the major row crops and readily engineered using genetic engineering tools, making it an ideal platform for producing novel products. |
• | Camelina, as an underdeveloped crop has high technology upside potential to improve agronomics, seed yield and seed value. |
Camelina (Camelina sativa) is currently in limited cultivation in North America and Europe and was grown extensively in Europe in medieval times for food and fuel. Interest in biofuels resulted in additional investment beginning in the mid-2000s, as a result of which several beneficial Camelina attributes have been shown. Camelina is amenable to production practices used for canola, grows on marginal lands, has enhanced drought and cold tolerance, displays early maturation and requires fewer inputs than other oilseeds. The fast growth cycle is particularly attractive in areas in the Northwest U.S. and into Canada with shorter growing seasons. It is naturally resistant to diseases that impact canola and it performs well across Canada and parts of the U.S.
Camelina oil, like flax seed oil, is rich in omega-3 fatty acids which are essential in the human diet. There are three main types of omega-3 fatty acids, two of which EPA (Eicosapentaenoic acid) and DHA (docosahexaenoic acid), come mainly from fish. The third ALA (alpha-linolenic acid), the most common omega fatty acid in the Western diet, can come from fish or plant sources including nuts, flaxseed and Camelina. Omega-3 oils have been shown to help prevent heart disease and stroke, may help control lupus, eczema, and rheumatoid arthritis, and may play protective roles in cancer and other conditions. Recent clinical studies have shown that Camelina sativa oil, but not fatty fish, or lean fish improved serum lipid profile in subjects with impaired glucose metabolism in a randomized controlled study published in the journal Molecular Nutrition and Food Research, U. Schwab, et. al. (2018). In addition, seafood production is increasingly based on aquaculture, where using ocean harvested fish as feed is not sustainable and cannot meet the growing global demand. Aquaculture feed is now increasingly based on crop-based feed ingredients such as soy protein meal and soybean oil due to the availability and lower cost, However, fish oil, the primary source of the essential omega-3 fatty acids is the most difficult and expensive feed ingredient to replace. The high content of the omega-3 ALA makes it a preferred oil for use in aquaculture feed as compared to soybean oil. This is creating a growing market for Camelina oil.
Harvested Camelina seed is typically cold crushed to produce oil and an oil containing protein meal. In the United States an application for GRAS ("Generally Regarded as Safe" under FDA guidelines) status for Camelina oil was filed with FDA in and received a favorable response letter from the FDA in 2018. The Canadian Food Inspection Agency (CFIA) has approved the use of mechanically extracted Camelina as a feed ingredient for farmed salmon and trout, a policy change likely to benefit the aquaculture industry in Canada, where there is high demand for omega-3 oils. Camelina protein meal left over following oil extraction by cold crushing has also been approved for use in animal feed applications in the U.S. and Canada. The residual oil in the meal provides additional value as animal feed.
When Yield10 was launched, we started working on Camelina as the basis for our “Fast Field Testing” system. Prior to becoming Yield10, our oilseeds team had worked with Camelina for several years to develop it as a production platform for PHA biomaterials. We currently use Camelina to develop the initial field performance data for our gene trait targets identified using our GRAIN gene discovery tool because it is possible to go from trait gene concept to engineered stable seed suitable for field work. Camelina has a small seed size and is readily segregated from commodity crops including canola, soybean and corn and for these reasons we consider it highly unlikely that engineered Camelina seed will contaminate commodity crops slated for export markets.
These commodities are exported to geographies with strict regulations regarding the use and import of farm products which have been genetically engineered. The importance of avoiding contamination of commodity crops with new GMO varieties has resulted in companies delaying commercial release until any new trait is globally
44
deregulated. This has resulted in a large increase in both the costs and the timelines to commercialize new GMO varieties of commodity crops. The approval processes in the U.S. and Canada, although different in each country, are more straightforward to navigate. The use of new tools such as genome editing is also receiving favorable treatment in the U.S. and some traits developed using this approach can be defined as non-regulated under USDA-APHIS rules. Yield10 has developed non-regulated Camelina lines with both single and three gene edits, taken them through the USDA-APHIS process and has executed field trials with them. Camelina has also been engineered by Yield10 to produce PHA biomaterials and by third parties to produce modified oils including fish oil replacements. We expect these crops will be considered regulated under the USDA-APHIS rules but also recognize the potential upside from the ongoing process to modernize these rules, taking into consideration the track record of safety and learning from the last 25 years.
Camelina is a relatively underdeveloped crop with no barriers to entry for companies like Yield10 to develop a Camelina based seed and products business. Due to the limited market opportunities for Camelina seed mainly focused on biofuels, this crop has not been subjected to intensive breeding efforts or the use of input traits like herbicide tolerance and disease resistance. We believe the growing interest in omega-3 oils will change this and because Yield10 has been using Camelina as a trait development tool we have in hand several proof points of the upside potential using our proprietary yield and oil content traits. We have demonstrated the potential to more than double Camelina seed yield with our complex C3006 trait. Our single gene C3003 and C3004 yield traits have also been shown to increase Camelina seed yield and because C3004 is a Camelina gene it has the potential to be re-engineered in Camelina to develop non-regulated Camelina lines with higher seed yield. In addition to the seed yield traits, we have progressed genome edited oil content traits which are showing promise for increased seed oil content, the primary economic value driver for Camelina seed. Further, we are developing doubled haploid winter and spring varieties of Camelina as foundation genetics for deploying our performance and compositional traits.
Target Crops for Trait Licensing
Our research and early development work with our C3000 and C4000 series traits in Camelina and other crops suggests that our technology may be applicable to a wide range of crops harvested for food and animal feed uses. We believe that if novel yield traits could be successfully developed and commercialized in any of these crops, farmers would be able to improve the productivity of their land to meet rising demand for food and feed, thereby creating significant economic value.
In considering our strategy to develop our technologies we segregate our trait genes into two classes: trait genes based on using non-plant genes to add new functionality to crops which are by definition genetically modified, or GM, due to the insertion of foreign recombinant DNA; and trait genes that we may be able to deploy in lines that are not considered regulated by USDA-APHIS, which encompass our trait genes that are based exclusively on plant genes. We see the opportunity to deploy our trait technology in a broader set of food and feed crops, many of which are not currently GM. We plan to pursue our GM trait genes in crops which are currently GM and where the economics can sustain the cost and timelines for deregulation. We are aware of the current USDA-APHIS GM crop regulation review and the reality that GM likely will remain an issue for some NGO groups regardless of the science. For our GM yield trait genes, we are targeting seed yield increases on the order of 10 to 20 percent over the current elite seed lines, increases which reflect the order of magnitude step-changes necessary to address global food security.
The crops we are targeting for development are described below.
Canola (Brassica napus) is a cultivar of rapeseed which produces a higher value edible oil favored by consumers because it has a healthier fatty acid profile than corn or soybean oil. The canola crop was developed in Canada where it is primarily grown today with additional acreage grown in the U.S. Currently the vast majority of the canola grown in North America contains two seed enhancement technologies, herbicide tolerance and hybrid seed. Both Roundup Ready (Monsanto, now Bayer) and Liberty-Link (Bayer) varieties of canola are grown and were introduced to the market in the 1990s. Approximately 24.7 million acres were planted in Canada and the U.S. in the 2018 growing season. The Canola Council of Canada has set yield goals of 52 bushels/acre for 26 million metric tons of production to meet global market demand for canola by 2025. Yield10 is targeting a 10-20 percent or greater increase in canola seed yield. With a 2017 harvest of 939 million bushels of canola (Statistics Canada) and
45
assuming an average farm gate price of $10.00 per bushel, a 20 percent yield increase in canola represents a total potential added annual value of $1.9 billion that could be shared among the companies in the canola value chain.
Soybean or Glycine max is an oilseed crop used for food, food ingredients, food additives and animal feed. The soybean can be harvested for oil used in food and industrial applications, and soybean meal is a significant source of protein for use mostly in animal feed but also for direct human consumption. Fermented soy foods include soy sauce and tempeh, and non-fermented food uses include soy milk and tofu. Soybeans are widely cultivated in North and South America, where a majority of the seed planted is genetically modified. An estimated 94.4 million acres of soybean will be planted in the U.S. and Canada in the 2018/2019 growing season. According to the USDA, the U.S., Brazil and Argentina together represent approximately 80 percent of global soybean production. Yield10 is targeting a 20 percent or greater increase in soybean seed yield. Assuming a 2018/2019 U.S. harvest of 4.5 billion bushels (USDA) and an average farm gate price of $10.00 per bushel, a 20 percent yield increase in soybean represents a total potential added annual value of $8.8 billion that could be shared among the companies in the soybean value chain.
Corn is a crop grown globally and used for animal feed and for producing starch which can be used as a raw material for producing food ingredients and food additives, as well as for use in the production of paper, packaging materials and other items. GM maize was grown for the first time in the U.S. and Canada in 1997. Currently, about 80 percent of maize/corn production in the U.S. is genetically modified. It was estimated that more than 83 million acres of corn were planted in North America in the 2018 growing season. The traits commonly used in today’s corn cultivars provide insect resistance and herbicide tolerance. In many GM seeds sold today, these traits are stacked (“stacked” refers to the practice of adding multiple traits to an elite plant line). Europe has limited production of GM corn, where Spain is a leading producer. In this case, the most widely used GM trait (Bt) protects against the corn borer insect. Special protocols must be followed in Europe to avoid mixing of GM corn with conventional corn. Corn has the more efficient C4 photosynthesis system and Yield10 is targeting a 10 percent yield increase in corn. With a projected 2018/2019 U.S. harvest of 14.4 billion bushels and an average per bushel price of $3.50, a 10 percent yield increase in corn represents a total potential added annual value of $5.1 billion that could be shared among the companies in the corn value chain.
Potato is the most important non-cereal staple food crop for humans after wheat and rice. In the United States the potato harvest acreage is around 1 million acres, the harvest value however is around $6 billion, and the frozen French fry sector has a value of around $20 billion. Yield10 has no in-house R&D activities specific to potato but has executed a research license agreement with Simplot to enable the evaluation of three of our traits in potato.
Forage Sorghum. Forage crops are grown expressly for biomass used for feeding livestock. Typical forage crops include both annual and perennial crops such as various grasses, silage corn, alfalfa and sorghum. Biotechnology traits have been previously introduced into silage corn and alfalfa. Other forage crops could be amenable to gene editing strategies to increase biomass yield per acre. We believe that our technology and traits that increase biomass may have application to forage crops. Yield10 has no in-house R&D activities specific to forage sorghum but has executed a research license agreement with Forage Genetics to enable them to evaluate five of our traits in this crop.
Regulatory Requirements
Since the first successful commercialization of a biotechnology-derived agricultural crop in the 1990s, many new crop varieties have been developed and made available to farmers in the U.S. and worldwide. U.S. farmers have rapidly adopted many of these new biotechnology-derived varieties. In 2016, 92 percent of the corn, 93 percent of the cotton and 94 percent of the soybeans planted in the U.S. were varieties produced through traditional forms of genetic engineering. A significant percentage of the production of other crops planted and harvested in the U.S., such as alfalfa, papaya and sugar beet, are also biotechnology-derived.
Biotechnology-derived or genetically engineered crops are subject to a significant amount of regulation in the U.S. and around the world. Field tests and field trials of such crops need to ensure that traits in development do not escape or mix with native plants, and crops that may be used as human food or animal feed must meet certain
46
safety standards, but government regulations, regulatory systems and the politics that influence them vary significantly among jurisdictions.
For purposes of this discussion, the term “GE” includes both biotechnology-derived or genetically engineered plants that are modified by the insertion of recombinant DNA (“Traditional Genome Modification”) and biotechnology-derived or genetically engineered plants that are modified through the application of more modern techniques of genome editing. We have seed traits that fall within each of these two generalized categories of GE plants, as summarized above under the subheading “Traits in Development.”
United States Regulation
The U.S. government agencies primarily responsible for overseeing the products of modern agricultural biotechnology are the USDA, the FDA and the EPA. Depending on its characteristics, a product may be subject to the jurisdiction of one or more of these agencies under the federal government’s 1986 Coordinated Framework for the Regulation of Biotechnology, as updated. Regulatory officials from the three agencies regularly communicate and exchange information to ensure that any safety or regulatory issues that may arise are appropriately resolved within the scope of authority afforded to each agency under their respective statutes. Other environmental laws or regulations also may be implicated, depending on the specific product and its potential applications or intended uses. EPA’s principal oversight role is for biotechnology-derived products that are intended for use as pesticides or herbicides, under the authorities granted to the agency under the Federal Insecticide, Fungicide, and Rodenticide Act and the Toxic Substances Control Act. Our business strategy for major grain crops is to develop yield and performance traits for licensing to the major seed companies. We have no current plans for the development of pesticide or herbicide GE traits that would be subject to the procedures and requirements of the EPA under these statutes.
Our seed traits and any future products that are successfully developed containing our seed traits, however, are or will be subject to USDA and FDA regulatory requirements. Those requirements will vary depending on the particular seed trait and the type and intended use of any product that will be commercialized. Future products that we plan to produce and sell, for example for use in water treatment may potentially have EPA regulatory requirements, and the regulations relating to manufacturing and consumer protection will need to be addressed.
Within USDA, APHIS is responsible for protecting agricultural plants from pests, diseases and noxious weeds. Under the Plant Protection Act ("PPA"), USDA-APHIS has regulatory oversight over products of modern biotechnology that could pose such a risk to domestic agriculture and native plants. Accordingly, USDA-APHIS regulates organisms and products that are known or are suspected to be plant pests or to pose a plant pest risk, including those that have been altered or produced through various genetic engineering techniques. These GE plants are called “regulated articles” in the relevant USDA-APHIS regulations, which are codified at 7 CFR part 340. The PPA and the implementing regulations in 7 CFR part 340 empower USDA-APHIS to regulate the import, handling, interstate movement and release into the environment of regulated articles, including certain GE organisms undergoing confined experimental use or field trials. Regulated articles are reviewed to ensure that, under the proposed conditions of use, they do not present a plant pest risk by ensuring appropriate handling, confinement and disposal.
Seed traits developed using Traditional Genome Modification, such as our C3003 yield trait that leverages the biological functions of an algal gene, are regulated under 7 CFR part 340. Regulated articles are subject to extensive USDA-APHIS oversight, including but not limited to permitting requirements for import, handling, interstate movement and release into the environment.
If, however, USDA-APHIS determines that a GE plant is unlikely to present a greater plant pest risk than its unmodified counterpart, the newly developed crop will no longer be subject to the permitting and other regulatory processes that are overseen by the agency (i.e., it will no longer be treated as a potential plant pest). Such a determination by the USDA-APHIS is called "not regulated" under the 7 CFR part 340 regulatory framework. The regulations establish detailed procedures for how a developer of a new GE plant may petition USDA-APHIS to determine if modified plant lines are not regulated under the 7 CFR part 340 framework, which is an official agency
47
finding that the particular article is unlikely to pose a plant pest risk and therefore no longer needs to be regulated under 7 CFR part 340 and the PPA.
USDA-APHIS conducts a comprehensive science-based review of the petition to assess, among other things, plant pest risk, environmental considerations pursuant to the National Environmental Policy Act, and any potential impacts on endangered species. The duration of the petition process varies based on a number of factors, including the agency’s familiarity with similar GE products, the type and scope of the environmental review conducted, and the number and types of public comments received. If, upon the completion of the review, USDA-APHIS approves the petition and the product is no longer deemed a “regulated article,” the developer may commercialize the product, subject to any conditions set forth in the USDA-APHIS written decision issued in response to the petition for determination of non-regulated status.
As previously described, our seed traits developed using Traditional Genome Modification are regulated under 7 CFR part 340 and are subject to USDA-APHIS permitting requirements. In recent years, however, we and others have submitted various petitions to USDA-APHIS to determine whether particular GE plants developed through the use of different genome editing techniques meet the not regulated status under the 7 CFR part 340 framework administered by the agency. In general, lines developed using genome editing approaches have been deemed not to be regulated by USDA-APHIS under 7 CFR part 340. The USDA also announced in March 2018 that it would not require an assessment on products that use modern forms of mutagenesis if it is clear these outcomes could occur in nature. The USDA stated at that time that it did not “have any plans to regulate plants that could otherwise have been developed through traditional breeding techniques as long as they are developed without the use of a plant pest as the donor or vector and they are not themselves plant pests.” This USDA policy statement applies to genetic deletions of any size, which would include genome editing through CRISPR-Cas9 and other emerging technologies, although it remains to be seen how this policy announcement will be implemented by USDA-APHIS and what practical effect that may have on seed trait developers like us and our competitors.
Historically, changes to the U.S. regulatory paradigm for agricultural biotechnology have been infrequent, are typically preceded by notice, and are most often subject to public comment, but there can be no guarantee that the USDA-APHIS governing regulations and policies will not change.
We have submitted four petitions under 7 CFR part 340 for a determination of the regulatory status (also known as the “Am I Regulated?” letter) to USDA-APHIS’s Biotechnology Regulatory Services in order to confirm that the following four traits designed to increase oil content using CRISPR genome editing technology are not going to be regulated by the agency: (i) the single trait C3008 Camelina plant line, ; (ii) the triple-edited Camelina line that combines three gene traits, C3008a, C3008b and C3009, ; (3) the single trait C3007 Camelina plant line, and (4) the single trait C3007 canola plant line. . In all cases, USDA-APHIS's Biotechnology Regulatory Services approved our petitions and confirmed that each of these novel plant lines would not be treated as a regulated article.
To our knowledge, our triple-edited Camelina line which was determined to not be regulated under 7 CFR part 340 in September 2018, is the first CRISPR-edited triple-trait plant determined by the agency to be not to be regulated. Given our business strategy to develop certain multi-trait genome edited plant lines, this achievement should facilitate our ability to put more of our novel yield traits through the petitioning process and the agency’s scientifically driven decision-making process, with the expected end result of having lines containing more of our traits treated as not to be regulated under 7 CFR part 340 (as compared to our seed traits developed using Traditional Genome Modification, which are regulated articles). We expect to continue to make appropriate use of USDA-APHIS procedures to clarify the regulatory status of our new GE seed traits as they are developed.
Also, we tested the C3008 single-trait Camelina line in a 2018 field evaluation that took place in the United States following a notification in 2017 that the line would not be regulated under 7 CFR part 340.
Separate from the plant breeding and planting issues and USDA-APHIS regulation under 7 CFR part 340, a GE plant also will be regulated by the FDA if it is intended to be used as human food or animal feed. The FDA regulates the safety of food for humans and animals, and foods derived from GE plants must meet the same food safety requirements as foods derived from traditionally bred plants (also called conventional foods).
48
Since 1992, the FDA has had in place a voluntary consultation process for developers of bioengineered food (“Biotechnology Consultations”). Final agency decisions and other information from these Biotechnology Consultations are made publicly available by the FDA. Biotechnology Consultations are data-intensive and examine the new food product’s safety and nutritional profile, among other issues. Generally, the FDA has found that such food products do not pose unique health risks to humans or animals, but if a novel allergen or other distinction from the conventional food is present in the new plant variety, the agency may require specific label statements on the product to ensure that consumers are made aware of material differences between GE and conventional versions. The FDA primarily derives its regulatory power from the Federal Food, Drug, and Cosmetic Act, which has been amended over time by several subsequent laws. Among other oversight and inspection responsibilities, the FDA regulates ingredients, packaging, and labeling of foods, including nutrition and health claims and the nutrition facts panel. Foods are typically not subject to premarket review and approval requirements, with limited exceptions.
As part of a broader effort to modernize its regulatory approach to all biotechnology-derived products, the FDA is currently re-evaluating its regulatory approach in light of the increasing prevalence of certain genome edited plants. In January 2017, the FDA asked for public input to help inform its thinking about human and animal foods derived from new plant varieties produced using genome editing techniques. Among other things, the FDA’s request for comments asked for data and information in response to questions about the safety of foods from genome edited plants, such as whether certain categories of genome edited plants present food safety risks different from other plants produced through traditional plant breeding.
In October 2018, FDA leadership issued a document entitled the “Plant and Animal Biotechnology Innovation Action Plan” (the “Action Plan”) that identified three key priorities for the agency in this area: 1) advancing human and animal health by promoting product innovation and applying modern, efficient and risk-based regulatory pathways; 2) strengthening public outreach and communication regarding the FDA’s approach to innovative plant and animal biotechnology; and 3) increasing engagement with domestic and international partners on biotechnology issues. The Action Plan also stated that the FDA has reviewed the comments and other information it received in response to the January 2017 request for comments, and that it intends to develop guidance for the industry explaining how the FDA’s existing regulatory policy for foods derived from new plant varieties applies to foods produced using genome editing. The FDA also stated in the Action Plan that it intends to begin updating the existing procedures for voluntary Biotechnology Consultations to reflect the agency’s 25 years of experience with foods derived from biotechnology plants and to incorporate any additional issues related to genome editing of food crops. Such procedural updates are expected to be developed and implemented over the next two years.
Canadian Regulation
In Canada, GE crops and the food products into which they are incorporated are regulated by multiple government agencies under a federal framework for the regulation of biotechnology products that is similar to the U.S. system. First, the CFIA is the lead agency for ensuring that a new agricultural biotechnology crop will not pose new risks to Canadian plants, animals and other agricultural commodities. The Plant Biosafety Office ("PBO") is responsible for conducting environmental assessments of biotechnology-derived plants, referred to as "plants with novel traits" ("PNT"). Authority for the PBO includes both approving confined field trials with the PNT through permits and authorizing their “unconfined release” as a first step towards commercialization. PNTs are defined in the Canadian Seeds Regulations as (i) plants into which a trait or traits have been intentionally introduced, and (ii) where the trait is new in Canada and has the potential to impact the environment. The CFIA also has in place a remutation policy, whereby plants containing the same mutation as a previously authorized plant of the same species are included in the authorization of the original PNT and are therefore subject to the same conditions.
Under the Food and Drugs Act and related regulations, Health Canada is responsible for reviewing a pre-market safety assessment that must be submitted by the manufacturer or importer of a “novel food,” a term of art that includes any PNT or other or biotechnology-derived foods. The safety assessment should provide assurances that the novel food is safe when prepared or consumed according to its intended use before it enters the Canadian market and food system. A multi-disciplinary team of experts from Health Canada will evaluate the data and information about the novel food and make a determination regarding whether it is safe and nutritious before it can be sold in Canada, as well as whether any restrictions are warranted under applicable law or the product’s safety profile. Health Canada’s final decision documents regarding the safety of these novel foods are made available to
49
the public by the government. As in the United States, approval of a PNT or a novel food product does not take into account the method with which such product was produced. Rather, Health Canada employs a product-based (as opposed to a process-based) approach to its regulatory oversight of such emerging foods and food ingredients.
As the lead agency for public health and safety, Health Canada also works in conjunction with the CFIA on food labeling oversight when it has identified a potential health or safety issues with a food that could be mitigated through labeling or other disclosures. For example, if the biotechnology-derived food contains a new allergen that is otherwise not present in the conventional version of the food, then specific label statements will be required to alert consumers to that important health information. However, the CFIA has primary oversight over non-health issues related to food labeling, packaging, and advertising. Accordingly, the CFIA is the lead agency for ensuring that food labeling, and advertising meet the legal requirements of the Food and Drugs Act, and that labeling representations do not create a potential risk of fraud or consumer confusion and are compliant with Canada’s voluntary disclosure standard for GE food ingredients.
Environment Canada is also available to serve as a regulatory “safety net” if a novel product does not naturally fall within the jurisdiction of the CFIA, Health Canada, or the Pest Management Regulatory Agency that oversees pesticide products.
Our work involving the development, greenhouse testing and field testing of novel yield trait genes in crop plants requires certain government and municipal permits and we must ensure compliance with all applicable regulations including regulations relating to GE crops. With laboratories and greenhouses in both the U.S. and Canada, we are also subject to regulations governing the shipment of seeds and other plant material (including GE seeds and GE plant material) between our facilities in the U.S. and Canada, including USDA-APHIS and CFIA permits for the import and phytosanitary certificates for the export of plant materials that could pose a risk to domestic agriculture.
Having deployed our own research and development operations in Saskatoon, Canada in 2010, we have been conducting field studies of various yield traits in that country since 2016 under PNT permits issued by Canadian regulators. During 2018, we conducted field studies of C3003 in canola, Camelina and soybean at field sites in Canada.
Finally, as one of Canada’s major field crops, canola in particular is subject to variety registration, which is a regulatory requirement of the Seeds Act and is also administered by the CFIA. Any future sales of our seed traits or products in Canada would be done by a third-party collaborator or other partner, and that third party would be responsible for complying with registration requirements for the canola varieties, if applicable.
Regulation in Other Jurisdictions
Other jurisdictions and governmental authorities, including in South America and Asia, are increasingly taking an interest in regulating agricultural products of biotechnology. Regulatory approaches vary by jurisdiction, the existing public health framework and phytosanitary laws in the country, and other less tangible factors such as cultural and religious norms that may have an impact on individual country risk assessments and decision-making. We cannot predict future changes in the global regulatory landscape regarding GE plants subjected to Traditional Genome Modification or GE plants subjected to genome editing.
Further, although U.S. and Canadian regulatory authorities have taken similar approaches to overseeing both traditional biotechnology-derived plants and genome edited plants under their national plant health and biosafety laws, regulation of all GE plants in the EU is significantly more stringent than in North America. U.S. and Canadian regulators have also determined that genome edited GE plants pose fewer risks that those subjected to Traditional Genome Modification, while a recent EU legal ruling indicates that the existing European regulations for GE plants modified by the insertion of recombinant DNA should be strictly applied to genome edited plants as well. There is thus a sharp distinction between how European and North American regulatory agencies oversee novel seed traits, including those that are generated using the more modern techniques of genome editing. It is possible that emerging oversight regimes for GE products in other jurisdictions could follow the EU approach and impose similar strict requirements for the release of such products into the environment and their incorporation into human food or other consumer products.
50
Regulation of biotechnology-derived products in the EU is primarily based on Directive 2001/18/EC (the “2001 EC Directive”). The 2001 EC Directive defines “genetically modified organisms” ("GMOs") broadly as “organism[s], with the exception of human beings, in which the genetic material has been altered in a way that does not occur naturally by mating and/or natural recombination.” In July 2018, the Court of Justice of the European Union (CJEU) issued an important ruling clarifying that the 2001 EC Directive and its pre-market authorization and associated risk assessment requirements required for such “GMOs” should also apply in full to organisms developed using more modern “directed” mutagenesis techniques.
The July 2018 CJEU decision is being interpreted to cover all modern genome editing tools such as CRISPR-Cas9, TALEN and oligonucleotide-directed mutagenesis. This recent clarification by the CJEU regarding the scope of EU regulations suggests that novel seed trait developers who are seeking to bring genome edited seed traits to commercial markets in the EU will face hurdles comparable to what has historically been required in Europe for introducing and commercializing Traditional Genome Modification traits.
Although we are not currently targeting European markets for the development or commercialization of our products, the EU approach to regulating GE plants without regard to the scientific distinctions between Traditional Genome Modification and directed genome editing could be adopted by emerging oversight regimes for GE products in other jurisdictions. There is no guarantee that countries for which we may have or may develop future marketing plans would not take a stricter legal and regulatory approach to controlling GE plants similar to that of the EU.
License Agreement with the University of Massachusetts
Pursuant to a license agreement with the University of Massachusetts ("UMASS") dated as of June 30, 2015, we have an exclusive, worldwide license under certain patents and patent applications, including issued patents covering our yield trait gene C3003, relating to the manufacture of plants with enhanced photosynthesis. The agreement provides an exclusive, worldwide license to make, have made, use, offer for sale, sell, have sold and import any transgenic plant seed or plant grown therefrom or transgenic plant material developed for sale to a farmer or grower for planting in the field, which transgenic plant seed or plant grown therefrom or transgenic plant material is covered by, embodies or is derived from (in whole or in part) one or more issued or pending claims of the licensed patents or patent applications.
Pursuant to the UMASS license agreement, we are required to use diligent efforts to develop licensed products throughout the field of use and to introduce licensed products into the commercial market. In that regard, we are obligated to fulfill certain development and regulatory milestones relating to C3003, including completion of multi-site field demonstrations of a crop species in which C3003 has been introduced, and filing for regulatory approval of a crop species in which C3003 has been introduced within a specified period. Our failure to achieve any milestone provided for under the agreement would give UMASS the right to terminate the agreement, following a notice period, unless we are able to reach agreement with UMASS as to a potential adjustment to the applicable milestone.
We are obligated to pay UMASS milestone payments relating to any regulatory filings and approvals covered by the agreement, royalties on any sales of licensed products following regulatory approval, as well as a percentage of any sublicense income related to the licensed products.
We may terminate the agreement at any time upon 90 days prior written notice to UMASS. Either party may terminate for material breach immediately upon written notice for a breach that is not cured within 60 days after receiving written notice of the breach. In addition, UMASS may terminate this agreement with respect to certain patent rights immediately upon written notice in the event we contest the validity or enforceability of such patent rights.
License Agreement with the University of Missouri
Pursuant to a license agreement with the University of Missouri (“UM”) dated as of May 17, 2018, we have an exclusive, worldwide license to two novel gene technologies to boost oil content in crops. Both technologies are based on significant new discoveries around the function and regulation of ACCase, a key rate-limiting enzyme
51
involved in oil production. The first technology, named C3007, is a gene for a negative controller that inhibits the enzyme activity of ACCase. The second technology, named C3010, is a gene which, if over-expressed, results in increased activity of ACCase. The UM license was expanded during May 2019 to include an exclusive worldwide license to a third gene in the ACCase complex, that we have designated C3012, that may complement the activity of C3007 to boost oil content in crops.
Pursuant to the UM license agreement, we are required to use diligent efforts to develop licensed products throughout the licensed field and to introduce licensed products into the commercial market. In that regard, we are obligated to fulfill certain research, development and regulatory milestones relating to C3007, C3010 and C3012, including completion of multi-site field demonstrations of a crop species in which C3007, C3010 and C3012 have been introduced, and filing for regulatory approval of a crop species in which C3007, C3010 and C3012 have been introduced within a specified period. Our failure to achieve any milestone provided for under the license agreement would give UM the right to terminate the license agreement or render it nonexclusive, unless we are able to reach agreement with UM as to the potential adjustment of the applicable milestone.
We are obligated to pay UM a license execution payment, milestone payments relating to any regulatory filings and approvals covered by the license agreement, royalties on any sales of licensed products following regulatory approval, as well as a percentage of any sublicense royalties related to the licensed products.
We may terminate the license agreement at any time upon 90 days’ prior written notice to UM. Either party may terminate the license agreement upon written notice for a breach that is not cured within 30 days after receiving written notice of the breach. In addition, UM may terminate the license agreement with respect to certain patent rights immediately upon written notice in the event we contest the validity or enforceability of such patent rights.
Agricultural Industry Landscape
Following advances in biotechnology in the 1970s through early 1990s, the first genetically modified ("GM") crops were commercially introduced in the U.S. in the years 1994 and 1995. Today, the U.S. leads the world in the adoption of GM crops in terms of crop value and acreage planted. GM crops have had both their supporters and their detractors over the years. Consumer sentiment including concerns about the safety of GM crops have limited the introduction and adoption of GM crops in Europe. However, recent studies by the National Academy of Science continue to support the 20-year history of safe use of GM crops.
The International Service for the Acquisition of Agri-Biotech Applications (ISAAA), an industry research group, reported that 457 million acres worldwide were planted with GM crops in 2016, the most recent year for which data is available. The planting of GM crops is centered in the Americas with North America at approximately 45 percent of the acres and South America at approximately 43 percent. China and India follow with approximately 8 percent and the balance of the total worldwide GM crop acreage in 2016 was planted in the EU and the rest of world. The primary GM crops in the U.S. are corn, soybean, cotton and sugar beet. In Canada, the oilseed crop canola is the primary GM crop. Cotton is the primary GM crop grown in India and China.
In contrast to the Americas, the EU has been resistant to the adoption of GM crops and has relied heavily on plant breeding programs for capturing crop yield improvements over the last 20 years. In 2016, Spain was the largest producer of GM crops in Europe, based on cultivation of GM corn representing approximately 20 percent of the country’s crop that year. Certain GM crops have been approved for cultivation in some European countries, while other countries have imposed outright bans on cultivation of GM crops.
According to the market research firm, Research and Markets, the total global seed business was estimated at $68 billion in 2017 and is projected to grow to more than $100 billion by 2022. According to an ISAAA report, the global GM seed business represented a $17.2 billion market in 2017 and biotech crops were grown on approximately 469 million acres that year. The traits being commercialized today by the agricultural industry mainly address crop protection, which involves preventing crop damage by weeds, insects and other pests that lower expected crop yield. As technology has advanced, “trait stacking,” or the practice of adding multiple traits to an elite plant line, has become commonplace as a strategy to protect yield. As the industry has developed, the practice of inter-licensing traits between research and development driven seed companies has led to a proliferation of branded seed products on the market today.
52
The GM seed business is dominated by large multinational companies and their subsidiaries including BASF Corporation, Bayer, DuPont de Nemours, Inc., Syngenta AG and AgReliant Genetics, LLC. These companies have significant resources, experience and track records of successfully developing, testing and commercializing high performing seed lines as well as new traits for GM crops. They offer farmers conventional and biotechnology seeds as well as crop protection chemicals, biologicals, fertilizers and other products and technologies aimed at supporting the on-farm efficiency of managing crops in the field as well as managing the overall cost of crop production to successful harvest. Many of these companies were recently involved in consolidation of the sector with the merger of DuPont de Nemours, Inc. and Dow Chemical Company, the acquisition of Syngenta AG by the China National Chemical Corporation, and the acquisition of The Monsanto Company by Bayer in 2018.
Privately owned, U.S. retail seed companies play a key role in the industry by developing, marketing and selling high performing seed to U.S. farmers. These companies include Beck’s Hybrids and Stine Seed. These companies have capabilities in both biotechnology and plant breeding. They source traits from the multinational companies and input these traits into elite plant germplasm to produce seeds optimized for a variety of soil, climate and field conditions. Both companies offer a broad arrange of GM corn and soybean products to their customers.
Recent advances in biotechnology including gene editing have led to the formation of companies focusing on yield trait discovery, biologicals for pest control, agbiome strategies and precision agriculture. There are startups, privately held and publicly traded companies involved in this space. Such companies include AgBiome LLC, Arcadia Biosciences, Inc., Benson Hill Biosystems, Inc., BioCeres S.A., Calyxt, Inc., Cibus Ltd., Evogene Ltd., Inari Agriculture, Inc., Indigo Agriculture, Inc., Kaiima Bio-Agritech Ltd., Marrone Bio Innovation, Inc., and Pairwise Plants LLC, many of which have greater resources and experience than we have.
Intellectual Property
Our continued success depends in large part on our proprietary technology. As of June 30, 2020, we owned or held exclusive rights to 22 patents and pending patent applications worldwide related to advanced technologies for increasing yield in crops. Our portfolio of patent applications includes plant science technologies we have in-licensed globally and exclusively from the University of Massachusetts related to the yield trait gene C3003 and other advanced technologies based on advanced metabolic engineering methods to improve carbon capture and selectively control carbon partitioning in plants. Our portfolio of patent applications also includes advanced technologies for oilseed crops that we in-licensed globally and exclusively from the University of Missouri in 2018 and 2019 related to the yield trait genes C3007, C3010 and C3012.
We continue to seek, develop and evaluate new technologies and related intellectual property that might enhance our Company's business strategy, industry position or deployment options.
Employees
As of June 30, 2020, we had 25 full-time employees. Of those employees, 21 were in research and development. Among our staff, 11 hold Ph.D.’s and 12 hold masters’ or bachelors’ degrees in their respective disciplines. Our technical staff has expertise in the following areas: plant genetics, plant biology, microbial genetics, bioinformatics, metabolic engineering and systems biology. Our headquarters are located in Massachusetts, and we maintain a research and development facility, including greenhouse facilities, in Saskatoon, Canada. None of our employees are subject to a collective bargaining agreement. We consider our relationship with our employees to be good.
Corporate History and Investor Information
In 1992, our Company was incorporated in Massachusetts under the name Metabolix, Inc. In September 1998, we reincorporated in Delaware and in January 2017 we changed our name to Yield10 Bioscience, Inc. to reflect our change in mission around innovations in agricultural biotechnology focused on developing disruptive technologies for step-change improvements in crop yield. Financial and other information about our Company is available on our website at www.yield10bio.com.
53
We make available on our website, free of charge, copies of our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after filing such material electronically or otherwise furnishing it to the Securities and Exchange Commission (the "SEC").
In addition, the SEC maintains an internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC. Our filings with the SEC may be accessed through the SEC's website at http://www.sec.gov.
PRINCIPAL STOCKHOLDERS
The following table sets forth certain information with respect to the beneficial ownership of our common stock as of October 1, 2020 for (a) our named executive officers, (b) our directors, (c) our executive officers and directors as a group, and (d) each stockholder known to us to beneficially own more than five percent of our common stock. Beneficial ownership is determined in accordance with the rules of the SEC and includes voting or investment power with respect to the securities. We deem shares that may be acquired by an individual or group within 60 days following October 1, 2020, pursuant to the exercise of options or warrants to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. Except as otherwise indicated, we believe that the stockholders named in the table have sole voting and investment power with respect to all shares shown to be beneficially owned by them based on information provided to us by these stockholders. Percentage ownership is based on a total of 3,334,048 shares of our common stock issued and outstanding on October 1, 2020. Unless otherwise noted below, the address of each person listed on the table is c/o Yield10 Bioscience, Inc., 19 Presidential Way, Woburn, MA 01801.
Beneficial Owner | Shares of Common Stock(1) | Options Exercisable Within 60 Days(2) | Warrants Exercisable Within 60 Days (2) | Total Shares Beneficially Owned | Percentage of Outstanding Shares(3) | ||||||||||
5% Stockholders: | |||||||||||||||
Jack W. Schuler(4) 28161 North Keith Drive Lake Forest, IL 60045 | 793,636 | — | 840,429 | 1,634,065 | 39.1 | % | |||||||||
Lind Global Macro Fund, LP (5) 444 Madison Avenue, Floor 41 New York, NY 10022 | 170,593 | — | — | 170,593 | 5.1 | % | |||||||||
Directors, Nominees and Named Executive Officers: | |||||||||||||||
Lynne. H. Brum(8) | 4,311 | 6,042 | — | 10,353 | * | ||||||||||
Oliver P. Peoples, Ph.D.(6) | 7,219 | 22,503 | — | 29,722 | * | ||||||||||
Kristi D. Snell, Ph.D.(9) | 4,780 | 11,607 | — | 16,387 | * | ||||||||||
Sherri M. Brown, Ph.D. | — | 2,688 | — | 2,688 | * | ||||||||||
Richard W. Hamilton, Ph.D. | 76 | 1,410 | — | 1,486 | * | ||||||||||
Anthony J. Sinskey, Sc.D.(7) | 309 | 6,240 | — | 6,549 | * | ||||||||||
Robert L. Van Nostrand | 282 | 5,127 | — | 5,409 | * | ||||||||||
All directors and executive officers as a group (8 persons)(10) | 21,264 | 61,671 | — | 82,935 | 2.4 | % | |||||||||
_______________________________________________________________________________
* | less than 1%. |
(1) | Beneficial ownership, as such term is used herein, is determined in accordance with Rule 13d-3(d)(1) promulgated under the Securities Exchange Act of 1934, as amended, and includes voting and/or investment power with respect to shares of Common Stock. Unless otherwise indicated, the named person possesses sole voting and investment power with respect to the shares. |
54
(2) | Consists of shares of Common Stock subject to stock options and warrants held by the person that are currently vested or will vest within 60 days after October 1, 2020. |
(3) | Percentages of ownership are based upon 3,334,048 shares of Common Stock issued and outstanding as of October 1, 2020. Shares of Common Stock that may be acquired pursuant to options and warrants that are vested and exercisable within 60 days after October 1, are deemed outstanding for computing the percentage ownership of the person holding such options, but are not deemed outstanding for the percentage ownership of any other person. |
(4) | The reported securities consist of 759,616 shares of Common Stock and 840,429 shares of Common Stock underlying the warrants owned by the JWS Living Trust, 33,999 shares of Common Stock owned by the Schuler Family Foundation, and 21 shares of Common Stock owned by the Renate Schuler Trust. Mr. Schuler has sole voting and investment power over the shares issued to the JWS Living Trust, the Schuler Family Foundation and Renate Schuler Trust. Beneficial ownership information for Mr. Schuler has been derived from his historical SEC filings. |
(5) | Beneficial ownership information for the Lind Global Macro Fund, LP, has been derived from historical SEC filings. |
(6) | Includes 3,766 shares held for Dr. Peoples in the Company's 401(k) plan. |
(7) | Includes 20 shares owned by Dr. Sinskey's spouse and 4 shares owned by a trust over which Dr. Sinskey may be deemed to share voting and investment power. Dr. Sinskey disclaims beneficial ownership of such shares. |
(8) | Includes 2,712 shares held for Ms. Brum in the Company's 401(k) plan. |
(9) | Includes 3,255 shares held for Dr. Snell in the Company's 401(k) plan. |
(10) | Includes Charles B. Haaser, who is an executive officer but not a named executive officer. |
55
SELLING SECURITY HOLDERS
The shares of Common Stock being offered by the selling security holders consist of 396,450 shares of Common Stock previously issued to the selling security holders in a private placement offering that occurred during August 2020. We are registering the shares of Common Stock in order to permit the selling security holders to offer the shares for resale from time to time. Other than as described below, the selling security holders have not had any material relationship with us within the past three years.
The selling security holders might not sell any or all of the shares covered by this prospectus or may sell or dispose of some or all of the shares other than pursuant to this prospectus. Because the selling security holders may not sell or otherwise dispose of some or all of the shares covered by this prospectus and because there are currently no agreements, arrangements or understandings with respect to the sale or other disposition of any of the shares, we cannot estimate the number of the shares that will be held by the selling security holders after completion of the offering.
The table below lists the selling security holders and other information regarding the beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended, and the rules and regulations thereunder) of the shares of Common Stock held by each of the selling security holders. The table is prepared based on information supplied to us by the selling security holders. The second column lists the number of shares of Common Stock beneficially owned by the selling security holders, based on their respective ownership of shares as of October 1, 2020. The third column lists the shares of Common Stock being offered by this prospectus by the selling security holders. The fourth column assumes the sale of all of the shares offered by the selling security holders pursuant to this prospectus.
Prior to the Offering | After the Offering | |||||||||
Selling Security Holder (1) | Number of Shares of Common Stock Beneficially Owned (2) | Percent of Common Stock Outstanding (3) | Number of Shares of Common Stock Being Registered for Resale | Number of Shares of Common Stock Beneficially Owned (4) | Percent of Common Stock Outstanding (3)(4) | |||||
Jack W. Schuler Living Trust (5) | 1,600,045 | 38.3 | % | 186,450 | 1,413,595 | 35.4 | % | |||
Tino Hans Schuler Trust (6) | 380,702 | 10.8 | % | 60,000 | 320,702 | 9.2 | % | |||
Tanya Eva Schuler Trust (7) | 380,703 | 10.8 | % | 60,000 | 320,703 | 9.2 | % | |||
Therese Heidi Schuler Trust (8) | 341,325 | 9.7 | % | 60,000 | 281,325 | 8.2 | % | |||
Schuler Grandchildren LLC (9) | 77,601 | 2.3 | % | 30,000 | 47,601 | 1.4 | % | |||
Total Shares of Common Stock | 2,780,376 | 58.1 | % | 396,450 | 2,383,926 | 54.3 | % | |||
(1) | This table and the information in the notes below are based upon information supplied by the selling security holders, including reports and amendments thereto filed with the SEC on Schedule 13D. |
(2) | The number of shares of common stock beneficially owned includes shares of common stock underlying warrants that are convertible or exercisable within 60 days of October 1, 2020. |
(3) | Percentage ownership is based on a denominator equal to the sum of (i) 3,334,048 shares of common stock outstanding as of October 1, 2020 and (ii) the number of shares of common stock underlying warrants that are convertible or exercisable within 60 days of October 1, 2020 that are beneficially owned by the applicable selling stockholder. |
(4) | Assumes that all shares of common stock being registered under the registration statement of which this prospectus forms a part are sold in this offering, and that none of the selling stockholders acquire additional shares of our common stock after the date of this prospectus and prior to completion of this offering. |
(5) | Jack W. Schuler is the trustee of the Jack W. Schuler Living Trust, an Illinois trust, and has sole voting and investment control over the shares being offered. Mr. Schuler may be deemed to be the beneficial owner of all shares of common stock held by the Jack W. Schuler Living Trust. |
56
(6) | Tino Schuler is trustee of the Tino Hans Schuler Trust, an Illinois trust, and has voting and investment control over the shares being offered. |
(7) | Tanya Sharman is trustee of the Tanya Eva Schuler Trust, an Illinois trust, and has voting and investment control over the shares being offered. |
(8) | George Schuler is trustee of the Therese Heidi Schuler Trust, an Illinois trust, and has voting and investment control over the shares being offered. |
(9) | George Schuler is the sole manager of Schuler Grandchildren LLC, an Illinois limited liability company, and has sole voting and investment control over the shares being offered. Mr. Schuler disclaims beneficial ownership over the shares held by Schuler Grandchildren LLC. |
Relationships with Selling Security Holders
The transactions described below involve entities affiliated with Jack W. Schuler within the past three years. All share amounts and share prices below have been adjusted to reflect the 1-for-40 reverse stock split that took effect on January 15, 2020.
August 2020 Offerings
On August 22, 2020, we entered into the Securities Purchase Agreement with Jack W. Schuler, our largest shareholder, and entities related to him to sell 396,450 shares of our Common Stock for gross proceeds of approximately $1.7 million. The closing of the private placement occurred on August 26, 2020. The issuance and sale of the securities in the private placement was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
Concurrently with the private placement, on August 22, 2020, we entered into an underwriting agreement with Maxim Group LLC, pursuant to which we sold, in a registered public offering, 835,000 shares of Common Stock at a public offering price of $4.25 per share. In addition to their participation in the private placement, Mr. Schuler and entities affiliated with him purchased an aggregate of 178,791 shares of our Common Stock in the registered public offering. The closing of the registered offering occurred on August 26, 2020.
November 2019 Offerings
On November 14, 2019, we entered into a securities purchase agreement with entities affiliated with Mr. Schuler pursuant to which Mr. Schuler and his affiliated entities purchased, in an unregistered private offering, 5,750 Units, priced at $1,000 per unit, with each unit receiving one share of our Series B Convertible Preferred Stock, contingently convertible into 125 shares of common stock at an exercise of $8.00, Series A Warrants to purchase 125 shares of our common stock, exercisable at a price of $8.00 for a two and one-half year period, and Series B Warrants to purchase 125 shares of our common stock, exercisable at a price of $8.00 per share for a seven and one-half year period. As of the November 19, 2019 closing date of the offering, we did not have sufficient authorized and available shares of common stock to permit conversion of the Series B Convertible Preferred Stock or to permit exercise of the Series A Warrants and Series B Warrants. Upon our filing of a Charter Amendment to effect a 1-for-40 reverse stock split on January 15, 2020, sufficient shares of our common stock became available and the Series B Convertible Preferred Stock held by the Schuler entities automatically converted to 718,750 shares of common stock and their Series A Warrants and Series B Warrants became eligible for exercise.
March 2019 Offering
On March 14, 2019, we entered into a securities purchase agreement with certain investors, including entities affiliated with Mr. Schuler, pursuant to which Mr. Schuler and his affiliated entities purchased, in a registered public offering, an aggregate of 24,598 shares of our common stock, at an offering price of $48.40 per share for gross proceeds from all investors participating in the offering of approximately $2.9 million (the “March 2019 Offering”).
57
December 2017 Offering
On December 19, 2017, we entered into an underwriting agreement with Ladenburg pursuant to which we agreed to issue and sell, in a registered public offering, Class A Units, each consisting of one share of our common stock, a warrant to purchase one share of our common stock, exercisable at a price of $90.00 for a five year period (a “2017 Series A Warrant”), and a warrant to purchase 0.5 of one share of our common stock, exercisable at a price of $90.00 per share for a nine month period (a “2017 Series B Warrant”), with each Class A Unit to be offered to the public at an offering price of $90.00 per Class A Unit and (b) Class B Units, each consisting of one share of Series A Preferred Stock, a 2017 Series A Warrant to purchase 11 shares of Common Stock and a 2017 Series B Warrant to purchase 6 shares of Common Stock, with each Class B Unit to be offered to the public at an offering price of $1,000 per Class B Unit (the “December 2017 Offering”). Entities affiliated with Mr. Schuler purchased 64,335 Class A Units and none of the Class B Units in the December 2017 Offering.
PLAN OF DISTRIBUTION
Each selling stockholder of the shares of the securities and any of their pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on the Nasdaq Capital Market or any other stock exchange, market or trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. A selling stockholder may use any one or more of the following methods when selling securities:
• | ordinary brokerage transactions and transactions in which the broker‑dealer solicits purchasers; |
• | block trades in which the broker‑dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
• | purchases by a broker‑dealer as principal and resale by the broker‑dealer for its account; |
• | an exchange distribution in accordance with the rules of the applicable exchange; |
• | privately negotiated transactions; |
• | settlement of short sales; |
• | in transactions through broker‑dealers that agree with the Selling Stockholders to sell a specified number of such securities at a stipulated price per security; |
• | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
• | a combination of any such methods of sale; or |
• | any other method permitted pursuant to applicable law. |
The Selling Stockholders may also sell securities under Rule 144 or any other exemption from registration under the Securities Act, if available, rather than under this prospectus.
Broker‑dealers engaged by the Selling Stockholders may arrange for other brokers‑dealers to participate in sales. Broker‑dealers may receive commissions or discounts from the Selling Stockholders (or, if any broker‑dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this Prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
In connection with the sale of the securities or interests therein, the Selling Stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the securities in the course of hedging the positions they assume. The Selling Stockholders may also sell securities
58
short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in turn may sell these securities. The Selling Stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Stockholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling Stockholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities.
The Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the securities. The Company has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities under the Securities Act.
We agreed to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Stockholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for the Company to be in compliance with the current public information under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Stockholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common stock by the Selling Stockholders or any other person. We will make copies of this prospectus available to the Selling Stockholders and have informed them of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
DESCRIPTION OF OUR CAPITAL STOCK
General
The following summary of our capital stock is based on certain provisions of our amended and restated certificate of incorporation, as amended, and amended and restated by-laws and on the applicable provisions of the DGCL. This summary does not purport to be complete and is qualified in its entirety by reference to the applicable provisions in our amended and restated certificate of incorporation, as amended, and amended and restated by-laws and the DGCL. For a complete description you should refer to our amended and restated certificate of incorporation, as amended, and our amended and restated by-laws, copies of which have been incorporated by reference herein, and to the applicable provisions of the DGCL.
Our authorized capital stock consists of 65,000,000 shares, with a par value of $0.01 per share, of which:
• | 60,000,000 shares are designated as common stock; and |
• | 5,000,000 shares are designated as preferred stock. Previously issued shares of Series A Convertible Preferred Stock and Series B Convertible Preferred Stock have been fully converted to common stock and are no longer outstanding. |
59
Common Stock
The holders of our Common Stock are entitled to one vote per share on all matters submitted to a vote of our stockholders and do not have cumulative voting rights. Subject to preferences that may be applicable to any preferred stock outstanding at the time, the holders of outstanding shares of Common Stock are entitled to receive ratably any dividends declared by our board of directors out of assets legally available. Upon our liquidation, dissolution or winding up, holders of our Common Stock are entitled to share ratably in all assets remaining after payment of liabilities and the liquidation preference of any then outstanding shares of preferred stock. Holders of Common Stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to our Common Stock.
Preferred Stock
Our amended and restated certificate of incorporation, as amended, provides for a class of its authorized stock known as preferred stock, consisting of 5,000,000 shares, $0.01 par value per share, issuable from time to time in one or more series. Our board of directors may designate the rights, preferences, privileges and restrictions of the preferred stock, including dividend rights, conversion rights, voting rights, terms of redemption, liquidation preference, sinking fund terms and the number of shares constituting any series or the designation of any series.
Warrants
As of October 1, 2020, we had warrants outstanding to purchase 2,843,699 shares of our common stock.
Anti-Takeover Provisions
Certain provisions of the DGCL and our amended and restated certificate of incorporation, as amended, and amended and restated by-laws may have the effect of delaying, deferring or discouraging another party from acquiring control of our company. These provisions, which are summarized below, may discourage certain types of coercive takeover practices and inadequate takeover bids and encourage anyone seeking to acquire control of our company to first negotiate with our board of directors. These provisions might also have the effect of preventing changes in our management and could make it more difficult to accomplish transactions that stockholders might otherwise deem to be in their best interests. However, we believe that the advantages gained by protecting our ability to negotiate with any unsolicited and potentially unfriendly acquirer outweigh the disadvantages of discouraging such proposals, because, among other reasons, the negotiation of such proposals could result in improving their terms.
Amended and Restated Certificate of Incorporation and Bylaw Provisions
Our amended and restated certificate of incorporation, as amended, and amended and restated by-laws include a number of provisions that may have the effect of delaying, deferring or discouraging another party from acquiring control of our company or preventing changes in our management, including the following:
• | Issuance of Undesignated Preferred Stock. Our board of directors has the authority, without further action by the stockholders, to issue up to 5,000,000 shares of undesignated preferred stock with rights, preferences and privileges designated from time to time by our board of directors without further action by stockholders. These rights, preferences and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences and sinking fund terms, any or all of which may be greater than the rights of common stock. |
• | Size of the Board of Directors and Filling Vacancies. The number of directors constituting our board of directors may be set only by resolution adopted by a majority vote of our entire board of directors. Any vacancy on our board of directors, however occurring, including a vacancy resulting from an increase in the size of the board of directors, may only be filled by the affirmative vote of a majority of our directors then in office, even if less than a quorum. |
60
• | Classified Board. Our board of directors is divided into three classes of directors, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. |
• | No Cumulative Voting. Our amended and restated certificate of incorporation, as amended, and amended and restated by-laws do not permit cumulative voting in the election of directors. Cumulative voting allows a stockholder to vote a portion, or all of its shares for one or more candidates. The absence of cumulative voting makes it more difficult for a minority stockholder to gain a seat. |
• | Removal of Directors. Directors can only be removed by our stockholders for cause and removal of a director will require a 75% stockholder vote. |
• | No Written Consent of Stockholders. All stockholder actions are required to be taken by a vote of the stockholders at an annual or special meeting. Stockholders may not take action by written consent in lieu of a meeting. The inability of stockholders to take action by written consent means that a stockholder would need to wait until the next annual or special meeting to bring business before the stockholders for a vote. |
• | Special Meetings of Stockholders. Special meetings of our stockholders may be called only by our board of directors acting pursuant to a resolution approved by the affirmative vote of a majority of the directors then in office. Only those matters set forth in the notice of the special meeting may be considered or acted upon at a special meeting of our stockholders. |
• | Advance Notice Requirements for Stockholder Proposals and Director Nominations. Our amended and restated by-laws provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. These procedures provide that notice must be given in writing not later than the close of business on the 90th day nor earlier than the close of business on the 120th day prior to the first anniversary of the preceding year’s annual meeting. These procedures may have the effect of precluding the conduct of certain business at a meeting if the proper procedures are not followed or may discourage or deter a potential acquirer from conducting a solicitation of proxies to elect its own slate of directors or otherwise attempt to obtain control of us. |
• | Amendment to Amended and Restated Certificate of Incorporation and By-laws. Any amendment, repeal or modification of certain provisions of our amended and restated certificate of incorporation or amended and restated by-laws requires a 75% stockholder vote. Provisions requiring such supermajority vote include, among other things, any amendment, repeal or modification of the provisions relating to the classification of our board of directors, the requirement that stockholder actions be effected at a duly called annual or special meeting of our stockholders and the designated parties entitled to call a special meeting of our stockholders. |
Section 203 of the DGCL
We are subject to Section 203 of the DGCL. In general, Section 203 of the DGCL prohibits a publicly held Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a three-year period following the time that this stockholder becomes an interested stockholder, unless it satisfies one of the following conditions:
• | the transaction is approved by the board of directors prior to the time that the interested stockholder became an interested stockholder; |
• | upon consummation of the transaction which resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced; or |
In general, Section 203 defines “business combination” to include the following:
61
• | at or subsequent to such time that the stockholder became an interested stockholder, the business combination was approved by the board of directors and authorized at an annual or special meeting of stockholders by at least two-thirds of the outstanding voting stock which is not owned by the interested stockholder. |
• | any merger or consolidation involving the corporation and the interested stockholder; |
• | any sale, lease, exchange, mortgage, pledge, transfer or other disposition of the assets of the corporation with an aggregate market value of 10% or more of either the aggregate market value of all assets of the corporation on a consolidated basis or the aggregate market value of all of the outstanding stock of the corporation involving the interested stockholder; |
• | subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation to the interested stockholder; |
• | any transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series of the corporation beneficially owned by the interested stockholder; or |
• | the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits by or through the corporation. |
In general, Section 203 defines an “interested stockholder” as an entity or person who, together with the stockholder’s affiliates and associates (as defined in Section 203), beneficially owns, or within three years prior to the time of determination of interested stockholder status did own, 15% or more of the outstanding voting stock of the corporation.
Treatment of Options Upon Change of Control
In general, under the terms of our Stock Option and Incentive Plans and our executive employment agreements, in the event of certain change in control transactions, if the successor corporation does not assume our outstanding options or issue replacement awards, or if an option holder’s employment is involuntarily terminated in connection with such change in control, the vesting of the options outstanding under such plans will accelerate.
Transfer Agent and Registrar
The transfer agent and registrar for our Common Stock is American Stock Transfer & Trust Company, LLC. The transfer agent’s telephone number is (718) 921-8200.
Stock Exchange Listing
Our Common Stock is listed on The Nasdaq Capital Market under the symbol “YTEN”.
62
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been informed that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
LEGAL MATTERS
Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., Boston, Massachusetts, will pass upon the validity of the issuance of the securities offered by this prospectus.
EXPERTS
The consolidated financial statements of Yield10 Bioscience, Inc. as of December 31, 2019 and 2018 and for each of the years in the two-year period ended December 31, 2019, incorporated in this Preliminary Prospectus by reference from the Yield10 Bioscience, Inc. Annual Report on Form 10-K for the year ended December 31, 2019 have been audited by RSM US LLP, an independent registered public accounting firm, as stated in their report thereon, incorporated herein by reference, and have been incorporated in this Preliminary Prospectus and Registration Statement in reliance upon such reports and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and other periodic reports, proxy statements and other information with the SEC. You can read our SEC filings over the Internet at the SEC’s website at www.sec.gov.
Our Internet address is www.yield10bio.com. There we make available free of charge, on or through the investor relations section of our website, annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed pursuant to Section 13(a) or 15(d) of the Exchange Act as soon as reasonably practicable after we electronically file such material with the SEC. The information found on our website is not part of this prospectus supplement or the accompanying prospectus.
The SEC maintains a website at http://www.sec.gov that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC.
INCORPORATION OF CERTAIN DOCUMENTS BY REFERENCE
The SEC allows us to “incorporate by reference” much of the information we file with them, which means that we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus is considered to be part of this prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus is continually updated and those future filings may modify or supersede some of the information included or incorporated in this prospectus. You should refer to the registration statement, including the exhibits, for further information about us and the securities we may offer pursuant to this prospectus. Statements in this prospectus regarding the provisions of certain documents filed with, or incorporated by reference in, the registration statement are not necessarily complete and each statement is qualified in all respects by that reference. We incorporate by reference into this prospectus the documents listed below and any future filings made by us with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (1) after the date of this prospectus and prior to the time that all of the securities offered by this prospectus are sold or the earlier termination of the offering, and (2) after the date of the initial registration statement of which this prospectus forms a part and prior to the effectiveness of the registration statement (except in each case in which the information contained in such documents is “furnished” and not “filed”). The documents we are incorporating by reference as of their respective dates of filing are:
63
• | Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on March 24, 2020; |
• | Definitive Proxy Statement on Schedule 14A for the annual meeting of our stockholders held on May 19, 2020, filed with the SEC on March 25, 2020; |
• | Current Reports on Form 10-Q for the quarters ended March 31, 2020 and June 30, 2020; |
• | Current Reports on Form 8-K filed on January 9, 2020, January 15, 2020, January 31, 2020, February 13, 2020, March 19, 2020, May 20, 2020, June 9, 2020, August 11, 2020, and August 25, 2020; and |
• | The description of our common stock contained in Item 1 of our Registration Statement on Form 8-A filed with the SEC on November 6, 2006, including any amendments or reports filed for the purpose of updating the description. |
The SEC file number for each of the documents listed above is 001-33133. We will provide, without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request of such person, a copy of any or all of the documents incorporated herein by reference other than exhibits, unless such exhibits are specifically incorporated by reference into such documents or this document. Requests for such documents should be addressed in writing or by telephone to:
Investor Relations
Yield10 Bioscience, Inc.
19 Presidential Way
Woburn, Massachusetts 01801
(617) 583-1700
64
PART II INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth the costs and expenses, payable by the Company in connection with the registration and sale of the common stock being registered. All amounts are estimates except the SEC registration fee.
Amount to be paid ($) | |||
SEC registration fee | $ | 324.83 | |
Legal fees and expenses | 40,000.00 | ||
Accounting fees and expenses | 10,000.00 | ||
Other | 1,000.00 | ||
Total | $ | 51,324.83 | |
Item 14. Indemnification of Directors and Officers.
Pursuant to Section 145 of the Delaware General Corporation Law (the “DGCL”), our amended and restated bylaws provide that each director or officer of Yield10 Bioscience, who was or is made a party or is threatened to be made a party to or is involved in any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he or she, or a person of whom he or she is the legal representative, is or was a director or officer of Yield10 Bioscience, or is or was serving at the request of Yield10 Bioscience as a director, officer, employee or agent of another corporation or of a partnership, joint venture, trust or other enterprise, including service with respect to employee benefit plans, shall be indemnified and held harmless by Yield10 Bioscience to the fullest extent authorized by the DGCL.
Pursuant to Section 102(b)(7) of the DGCL, Article 7 of our amended and restated certificate of incorporation, as amended, eliminates the liability of a director to us or our stockholders for monetary damages for such a breach of fiduciary duty as a director, except for liabilities arising:
• | from any breach of the director’s duty of loyalty to us or our stockholders; |
• | from acts or omissions not in good faith or which involve intentional misconduct or a knowing violation of law; |
• | under Section 174 of the Delaware General Corporation Law; and |
• | from any transaction from which the director derived an improper personal benefit. |
We carry insurance policies insuring our directors and officers against certain liabilities that they may incur in their capacity as directors and officers. In addition, we have entered into indemnification agreements with our directors and officers.
The foregoing discussion of our certificate of incorporation, bylaws and Delaware law is not intended to be exhaustive and is qualified in its entirety by such certificate of incorporation, bylaws or law.
Item 15. Recent Sales of Unregistered Securities
On January 9, 2020, the Company issued 3,715 shares of common stock to participants in its Yield10 Bioscience, Inc. 401(k) Plan as quarterly matching contributions. On April 10, 2020, the Company issued 10,114 shares of common stock to participants in the Yield10 Bioscience, Inc. 401(k) Plan as a matching contribution. On July 2, 2020, the Company issued 3,689 shares of common stock to participants in the Yield10 Bioscience, Inc. 401(k) Plan as a matching contribution. The issuance of these securities is exempt from registration pursuant to Section 3(a)(2) of the Securities Act as exempted securities. On August 22, 2020, the Company entered into a
65
Securities Purchase Agreement with Jack W. Schuler, its largest shareholder, and entities related to him to sell 396,450 shares of its Common Stock for gross proceeds of approximately $1.7 million. The closing of the private placement occurred on August 26, 2020. The issuance and sale of the securities in the private placement was exempt from registration pursuant to Section 4(a)(2) of the Securities Act.
Item 16. Exhibits and Financial Statement Schedules.
(a) The exhibits listed below are filed as part of or incorporated by reference into this Registration Statement on Form S-1. Where certain exhibits are incorporated by reference from a previous filing, the exhibit numbers and previous filings are identified in parentheses.
Exhibit No. | Identification of Exhibit | |
Amended and Restated Certificate of Incorporation, as amended, of the Registrant, (incorporated by reference herein to the exhibits to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 (File No. 001-33133)). | ||
Certificate of Amendment to the Amended and Restated Certificate of Incorporation of the Registrant (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on January 15, 2020 (File No. 001-33133)). | ||
Certificate of Designation of Preferences, Rights and Limitations with respect to the Series A Preferred Stock (incorporated by reference herein to the exhibits to the Company’s Report on Form 8-K filed on November 20, 2019 (File No. 001-33133)). | ||
Certificate of Designation of Preferences, Rights and Limitations with respect to the Series B Preferred Stock (incorporated by reference herein to the exhibits to the Company’s Report on Form 8-K filed on November 20, 2019 (File No. 001-33133)). | ||
Amended and Restated By-laws of the Registrant (incorporated by reference herein to the exhibits to the Company’s Report on Form 8-K filed on January 6, 2017 (File No. 001-33133)). | ||
Specimen Stock Certificate for shares of the Registrant's Common Stock (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed on September 21, 2006 (File No. 333-135760)). | ||
Form of Investor Warrant to Purchase Common Stock (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on July 5, 2017 (File No. 001-33133)). | ||
Form of Series A Common Warrant to purchase shares of Common Stock (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed December 15, 2017 (File No. 333-221283)). | ||
Form of Common Stock Purchase Warrant (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on November 20, 2019 (File No. 001-33133)). | ||
* | Opinion of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. | |
@ | 2006 Stock Option and Incentive Plan (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed on October 20, 2006 (File No. 333-135760)). | |
@ | 2006 Stock Option and Incentive Plan, Form of Incentive Stock Option Agreement (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed on October 20, 2006 (File No. 333-135760)). | |
@ | 2006 Stock Option and Incentive Plan, Form of Non-Qualified Stock Option Agreement (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed on October 20, 2006 (File No. 333-135760)). | |
@ | 2006 Stock Option and Incentive Plan, Form of Director Non-Qualified Stock Option Agreement (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed on October 20, 2006 (File No. 333-135760)). | |
@ | 2014 Stock Option and Incentive Plan, Revised and Restated (incorporated by reference herein to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2015 (File No. 001-33133)). | |
66
@ | 2014 Stock Option and Incentive Plan, Form of Incentive Stock Option Award (incorporated by reference herein to the exhibits to the Company's 2014 Annual Report on Form 10-K filed on March 25, 2015 (File No. 001-33133)). | |
@ | 2014 Stock Option and Incentive Plan, Form of Non-Qualified Stock Option Award (incorporated by reference herein to the exhibits to the Company's 2014 Annual Report on Form 10-K filed on March 25, 2015 (File No. 001-33133)). | |
@ | 2014 Stock Option and Incentive Plan, Form of Restricted Stock Unit Award (incorporated by reference herein to the exhibits to the Company's 2014 Annual Report on Form 10-K filed on March 25, 2015 (File No. 001-33133)). | |
@ | 2018 Stock Option and Incentive Plan (incorporated by reference herein to the exhibits to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 (File No. 001-33133)). | |
@ | 2018 Stock Option and Incentive Plan, Form of Stock Option Agreement (incorporated by reference herein to the exhibits to the Company's Annual Report on Form 10-K filed on March 28, 2019 (File No. 001-33133)). | |
@ | 2018 Stock Option and Incentive Plan, Form of Restricted Stock Unit Agreement (incorporated by reference herein to the exhibits to the Company's Annual Report on Form 10-K filed on March 25, 2020 (File No. 001-33133)). | |
@ | Employment Agreement between the Company and Oliver P. Peoples dated March 28, 2017 (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | |
@ | Employment Agreement between the Company and Charles B. Haaser dated March 28, 2017 (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | |
@ | Employment Agreement between the Company and Lynne H. Brum dated March 28, 2017 (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | |
@ | Employment Agreement between the Company and Kristi Snell dated March 28, 2017 (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | |
@ | Noncompetition, Confidentiality and Inventions Agreement between the Company and each of Oliver Peoples, Charles Haaser, Lynne H. Brum and Kristi Snell, dated March 28, 2017 (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | |
@ | Form of Indemnification Agreement between the Registrant and its Directors and Officers (incorporated by reference herein to the exhibits to the Company's Registration Statement on Form S-1/A filed on October 20, 2006 (File No. 333-135760)). | |
Standstill Agreement dated June 19, 2015 between the Company and Jack W. Schuler, Renate Schuler and the Schuler Family Foundation (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on June 17, 2015 (File No. 001-33133)). | ||
Lease Agreement between the Company and ARE MA Region No. 20, LLC dated January 20, 2016 for the premises located at 19 Presidential Way, Woburn, MA (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on January 26, 2016 (File No. 001-33133)). | ||
+ | Exclusive License Agreement, dated as of June 30, 2015, between the Company and the University of Massachusetts (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | |
Sublease between CJ Research Center LLC and the Company, dated as of September 16, 2016 (incorporated by reference herein to the exhibits to the Company's 2016 Annual Report on Form 10-K filed on March 30, 2017 (File No. 001-33133)). | ||
Form of Securities Purchase Agreement, dated July 3, 2017, between the Company and the Purchasers named therein (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on July 5, 2017 (File No. 333-33133)). | ||
67
+ | Exclusive License Agreement, dated May 17, 2018, between the Company and the University of Missouri (incorporated by reference herein to the exhibits to the Company's Quarterly Report on Form 10-Q for the quarter ended June 30, 2018 (File No. 001-33133)). | |
Form of Securities Purchase Agreement, dated as of March 14, 2019, by and among the Company and the purchasers named therein (incorporated by reference herein to the exhibits on Form 8-K filed on March 15, 2019 (File No. 001-33133)). | ||
Securities Purchase Agreement, dated November 14, 2019, by and between Yield10 Bioscience, Inc. and the Investors listed on Schedule I thereto (incorporated by reference herein to the exhibits to the Company’s Report on Form 8-K filed on November 20, 2019 (File No. 001-33133)). | ||
Securities Purchase Agreement, dated August 22, 2020, by and between Yield10 Bioscience, Inc. and the Investors listed on Schedule I thereto (incorporated by reference herein to the exhibits to the Company's Report on Form 8-K filed on August 25, 2020 (File No. 001-33133)) | ||
Yield10 Bioscience, Inc. Code of Business Conduct and Ethics (incorporated by reference herein to the exhibits to the Company's 2018 Annual Report on Form 10-K filed on March 28, 2019) | ||
Subsidiaries of the Registrant (incorporated by reference herein to the exhibits to the Company's 2018 Annual Report on Form 10-K filed on March 28, 2019) (File No. 001-33133)). | ||
* | Consent of RSM US LLP, an independent registered public accounting firm. | |
23.3 | Consent of Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C. (included in Exhibit 5.1). | |
24.1 | Power of Attorney (included in the signature pages to the Registration Statement). | |
101.INS | * | XBRL Instance Document. |
101.SCH | * | XBRL Taxonomy Extension Schema. |
101.CAL | * | XBRL Taxonomy Extension Calculation Linkbase. |
101.DEF | * | XBRL Taxonomy Extension Definition Linkbase. |
101.LAB | * | XBRL Taxonomy Extension Label Linkbase. |
101.PRE | * | XBRL Taxonomy Extension Presentation Linkbase. |
* | Filed herewith. |
@ | Indicates a management contract or any compensatory plan, contract or arrangement. |
+ | Confidential treatment has been requested for certain portions of this document. |
68
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by Section 10(a)(3) of the Securities Act;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement.
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act to any purchaser: each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(5) Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this Registration Statement on Form S-1 to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Woburn, Massachusetts, on October 8, 2020.
69
YIELD10 BIOSCIENCE, INC. | |||
By | /s/ Oliver P. Peoples | ||
Oliver P. Peoples President and Chief Executive Officer | |||
POWER OF ATTORNEY
We, the undersigned officers and directors of Yield10 Bioscience, Inc., hereby severally constitute and appoint Oliver P. Peoples, Charles B. Haaser, and Lynne H. Brum, and each of them singly, our true and lawful attorneys, with full power to them, and to each of them singly, to sign for us and in our names in the capacities indicated below, the registration statement on Form S-1 filed herewith, and any and all pre-effective and post-effective amendments to said registration statement, and any registration statement filed pursuant to Rule 462(b) under the Securities Act of 1933, as amended, in connection with the registration under the Securities Act of 1933, as amended, of equity securities of the Company, and to file or cause to be filed the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as each of us might or could do in person, and hereby ratifying and confirming all that said attorneys, and each of them, or their substitute or substitutes, shall do or cause to be done by virtue of this Power of Attorney.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement on Form S-1 has been signed below by the following persons in the capacities and on the dates indicated.
Signatures | Title | Date |
/s/ Oliver P. Peoples Oliver P. Peoples | Director, President and Chief Executive Officer (Principal Executive Officer) | October 8, 2020 |
/s/ Charles B. Haaser Charles B. Haaser | Chief Accounting Officer (Principal Financial Officer and Principal Accounting Officer) | October 8, 2020 |
/s/ Sherri M. Brown Sherri M. Brown | Director | October 8, 2020 |
/s/ Richard W. Hamilton Richard W. Hamilton | Director | October 8, 2020 |
/s/ Anthony J. Sinskey Anthony J. Sinskey | Director | October 8, 2020 |
/s/ Robert L. Van Nostrand Robert L. Van Nostrand | Director | October 8, 2020 |
70
