Attached files
| file | filename |
|---|---|
| 8-K - 8-K - ACCELERON PHARMA INC | xlrn-20201002.htm |

Exhibit 99.1 Phase 3 Trial Design Webinar October 2, 2020 Sotatercept is an investigational therapy that is not approved for any use in any country.

Acceleron Forward-Looking Statements THIS PRESENTATION CONTAINS FORWARD-LOOKING STATEMENTS ABOUT THE COMPANY’S STRATEGY, FUTURE PLANS AND PROSPECTS, including statements regarding the development and commercialization of sotatercept in pulmonary arterial hypertension (“PAH”) and of the Company’s other compounds, the timeline for clinical development and regulatory approval of the Company’s compounds and the expected timing for reporting of data from ongoing clinical trials. The words “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “may,” “plan,” “potential,” “project,” “should,” “target,” “will,” “would,” and similar expressions are intended to identify forward- looking statements, although not all forward-looking statements contain these identifying words. ACTUAL RESULTS COULD DIFFER MATERIALLY FROM THOSE INCLUDED IN THE FORWARD-LOOKING STATEMENTS DUE TO VARIOUS factors, risks and uncertainties, including, but not limited to, that preclinical testing of the Company's compounds and data from clinical trials may not be predictive of the results or success of ongoing or later clinical trials, that regulatory approval of the Company’s compounds in one indication or country may not be predictive of approval in another indication or country, that the development of the Company's compounds may take longer and/or cost more than planned, that the Company may be unable to successfully complete the clinical development of the Company’s compounds, that the Company may be delayed in initiating, enrolling or completing any clinical trials, that the Company's compounds may not receive regulatory approval or become commercially successful products, and that Breakthrough Therapy or Priority Medicines (PRIME) designation may not expedite the development or review of sotatercept. These and other risks and uncertainties are identified under the heading "Risk Factors" included in the Company's most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q, and other filings that the Company has made and may make with the SEC in the future. THE FORWARD-LOOKING STATEMENTS CONTAINED IN THIS PRESENTATION ARE BASED ON MANAGEMENT’S CURRENT VIEWS, PLANS, estimates, assumptions and projections with respect to future events, and the Company does not undertake and specifically disclaims any obligation to update any forward-looking statements. 2 This presentation is for investor relations purposes only – Not for product promotional purposes.

Habib Dable Chief Executive Officer This presentation is for investor relations purposes only – Not for product promotional purposes.

Sotatercept: Significant Progress in 2020 Preclinical Clinical SCIENCE TRANSLATIONAL MEDICINE RESEARCH ARTICLE Regulatory Regulatory BREAKTHROUGH THERAPY PRIME – PRIORITY MEDICINES DESIGNATION 4 This presentation is for investor relations purposes only – Not for product promotional purposes.
Our Long-Term Vision for Sotatercept in PAH BACKBONE THERAPY IN PAH Sotatercept is an investigational therapy that is not approved for any use in any country. 5 This presentation is for investor relations purposes only – Not for product promotional purposes.

Sotatercept is an investigational therapy that is not approved for any use in any country. Janethe Pena, MD, PhD VP, Medical Research, Pulmonary This presentation is for investor relations purposes only – Not for product promotional purposes.

PULSAR Trial Design Schema A Phase 2, double blind, randomized, placebo-controlled, parallel-group study. Inclusion criteria Primary treatment period Extension period (24 weeks) (18 months) • WHO Group 1 PAH Placebo + SOC Sotatercept 0.3 mg/kg + SOC • WHO Functional Class II or III (n=32) • Baseline right heart 1:1 catheterization with PVR ≥5 Wood units Sotatercept 0.7 mg/kg + SOC • Baseline 6-minute walk distance Randomization 150-550 m 3:3:4 Stratified by Sotatercept 0.3 mg/kg + SOC • Stable treatment with SOC Stratified by baseline (n=32) therapies, including mono, baseline WHOWHO FC FC double, and triple therapies Current dose + SOC – An endothelin-receptor antagonist, Trial currently in open-label a phosphodiesterase 5 inhibitor, Sotatercept 0.7 mg/kg + SOC extension phase a soluble guanylate cyclase (n=42) stimulator, and/or a prostacyclin (including IV) FC=functional class; IV=intravenous; PAH=pulmonary arterial hypertension; PVR=pulmonary vascular resistance; SOC=standard of care; WHO=World Health Organization. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 7 This presentation is for investor relations purposes only – Not for product promotional purposes.

PULSAR Baseline characteristics (N=106) PAH classification Standard-of-care PAH therapy 7% 3% 56% 9% Drug or toxin Corrected congenital Triple therapy Monotherapy induced shunt-associated 16% Heritable 58% 35% Idiopathic Double therapy 17% CTD- associated Gender WHO FC 6-minute walk distance* 87% Female 53% FC II; 47% FC III 398 ± 8.1 m Age, mean (range) Pulmonary vascular resistance* NT-proBNP* 48 (19-80) years 779 ± 33.9 dyn·s/cm5 908 ± 135.4 pg/mL Time since diagnosis, mean (range) 7.4 (0.3-26) years *Mean ± SE. FC=functional class; NT-proBNP=N-terminal-pro-B-type natriuretic peptide; PAH=pulmonary arterial hypertension; SE=standard error; WHO=World Health Organization; CTD=connective tissue disease; PAH=pulmonary arterial hypertension. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 8 This presentation is for investor relations purposes only – Not for product promotional purposes.

PULSAR Primary and Key Secondary Endpoints Primary: Pulmonary Vascular Resistance Key Secondary: 6-Minute Walk Distance Change in 6MWD from baseline to Week 24 (LS mean ± SE) Placebo-corrected LS mean difference of 25 m (SE=11.1) at Week 24 (P=0.03*) *Nominal. PVR=pulmonary vascular resistance; 6MWD=6-minute walk distance; LS=least squares; SE=standard error; SOC=standard of care. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 9 This presentation is for investor relations purposes only – Not for product promotional purposes.

PULSAR Exploratory Analyses Results were concordant across multiple exploratory endpoints at week 24 Sotatercept + SOC, All Dose n=74 Endpoint, Placebo + SOC change from baseline n=32 Absolute Change Percent Change† P Value* NT-proBNP, pg/mL +310.4 -462.4 -51% 0.0001 Right atrial pressure, mmHg +0.6 -1.4 -12% 0.03 Pulmonary arterial pressure, +0.5 -10.5 -20% 0.0001 mmHg Cardiac output, L/min +0.3 +0.1 2% 0.29 Endpoint, Placebo + SOC Sotatercept + SOC, proportion of subjects n=32 All Dose n=74 P Value* WHO FC improvement 12.5% 23% 0.20 Multi-component improvement^ 3% 38% 0.0002 † Percent change = (absolute change / mean baseline)· 100 ^ Multi-component improvement defined as meeting all three criteria: WHO FC improvement or maintenance of FC II or better, ≥30% improvement in NT-proBNP, ≥30m improvement in 6MWD * nominal 6MWD: 6-minute walk distance; FC: WHO functional class; NT-proBNP: amino-terminal brain natriuretic propeptide; SOC: standard of care. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 10 This presentation is for investor relations purposes only – Not for product promotional purposes.

PULSAR Safety Overview Sotatercept + SOC Sotatercept + SOC Placebo + SOC 0.3 mg/kg 0.7 mg/kg Events, n (%) (n=32) (n=32) (n=42) TEAEs 28 (88) 29 (91) 34 (81) Serious TEAEs 3 (9) 2 (6) 10 (24)* Serious related TEAEs 1 (3) 0 (0) 2 (5) TEAEs leading to treatment 1 (3) 2 (6) 3 (7) discontinuation • One death occurred in the study due to cardiac arrest in the sotatercept + SOC 0.7 mg/kg arm; the death was deemed unrelated to study treatment and the patient had many preexisting risk factors *These 10 patients experienced SAEs of leukopenia, neutropenia, pericardial effusion, tachycardia, chorioretinopathy, peripheral edema, pyrexia, bronchitis, influenza, respiratory tract infection, femur fracture, hypotension, device breakage, syncope, and RBC increase. RBC=red blood cell; SAE=serious adverse event; SOC=standard of care; TEAE=treatment-emergent adverse event. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 11 This presentation is for investor relations purposes only – Not for product promotional purposes.

PULSAR Conclusions Demonstrated improvement in PVR and 6MWD at week 24 in heavily pretreated patients • Subgroup analyses favored sotatercept + SOC at both dose levels and in patients receiving mono, double, or triple therapy • Concordance across multiple exploratory endpoints and analyses Generally well tolerated in patients with PAH; the safety profile was consistent with that observed in other patient populations 6MWD=6-minute walk distance; PAH=pulmonary arterial hypertension; PVR=pulmonary vascular resistance. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 12 Sotatercept is an investigational therapy that is not approved for any use in any country. This presentation is for investor relations purposes only – Not for product promotional purposes.

STELLAR Phase 3 Trial for Global Registration in PAH 13 This presentation is for investor relations purposes only – Not for product promotional purposes.

Marius Hoeper, MD* Professor, Head of the pulmonary hypertension (PH) program, and Acting Director or the intensive care unit at the Department of Respiratory Medicine, Hannover Medical School, Hannover, Germany. *Dr. Hoeper is the principal investigator of the STELLAR trial and a paid consultant to Acceleron. 14 This presentation is for investor relations purposes only – Not for product promotional purposes.

Background and Medical Center Information . Acting director of the Department of Respiratory Medicine at Hannover Medical School, Germany, in charge of the pulmonary hypertension (PH) program and senior attending physician at the intensive care unit, having published more than 350 papers in these areas. rd th th th . Task force member/chair at the 3 , 4 , 5 and 6 World Symposia on PH. Author and Section Editor of the 2009 European PH Guidelines and senior author of the 2015 European PH Guidelines. . In 2014, Professor Hoeper received the distinguished Lifetime Achievement in Pulmonary Arterial Hypertension Award from the European Respiratory Society, and in 2016, he received the Oskar award for Medicine. 15 This presentation is for investor relations purposes only – Not for product promotional purposes.
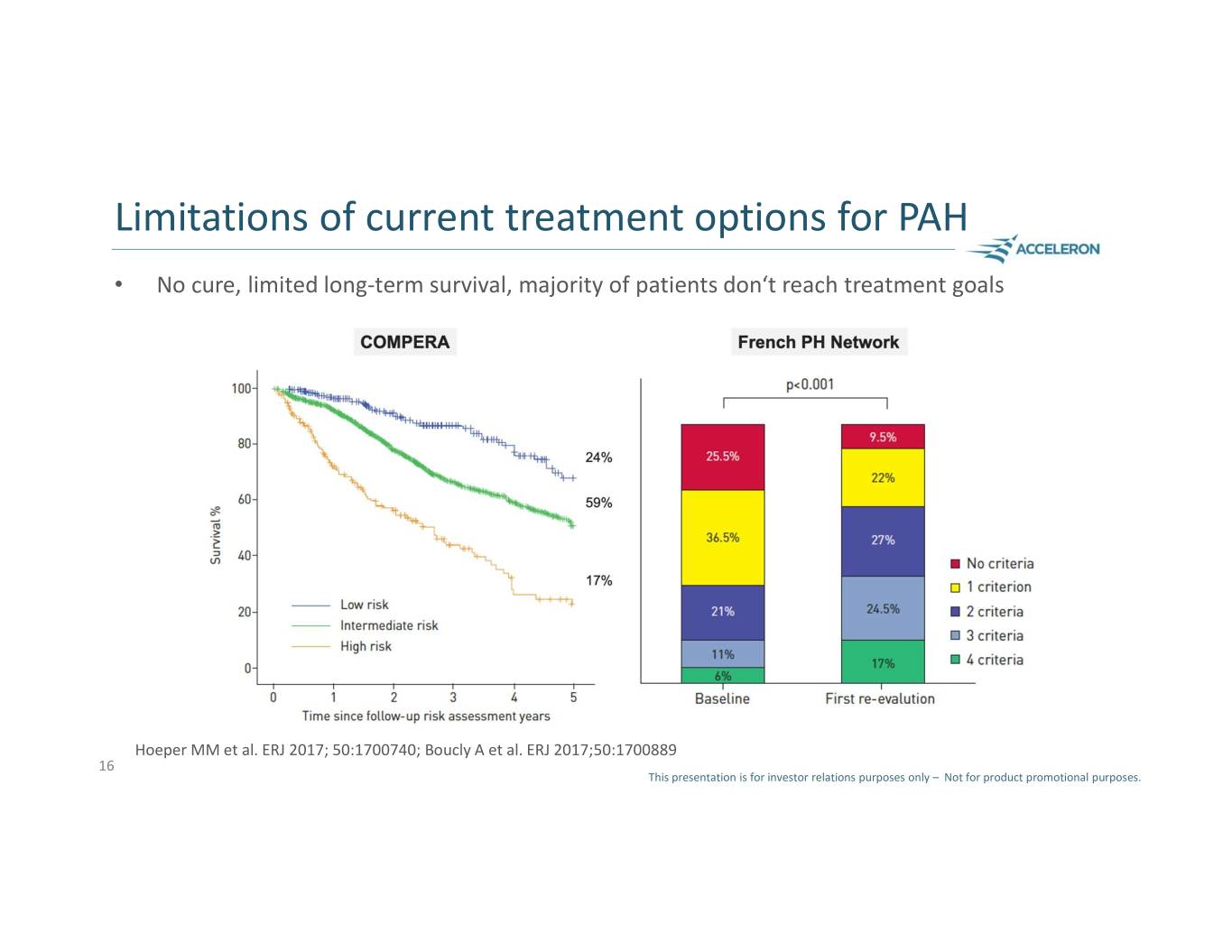
Limitations of current treatment options for PAH • No cure, limited long-term survival, majority of patients don‘t reach treatment goals Hoeper MM et al. ERJ 2017; 50:1700740; Boucly A et al. ERJ 2017;50:1700889 16 This presentation is for investor relations purposes only – Not for product promotional purposes.
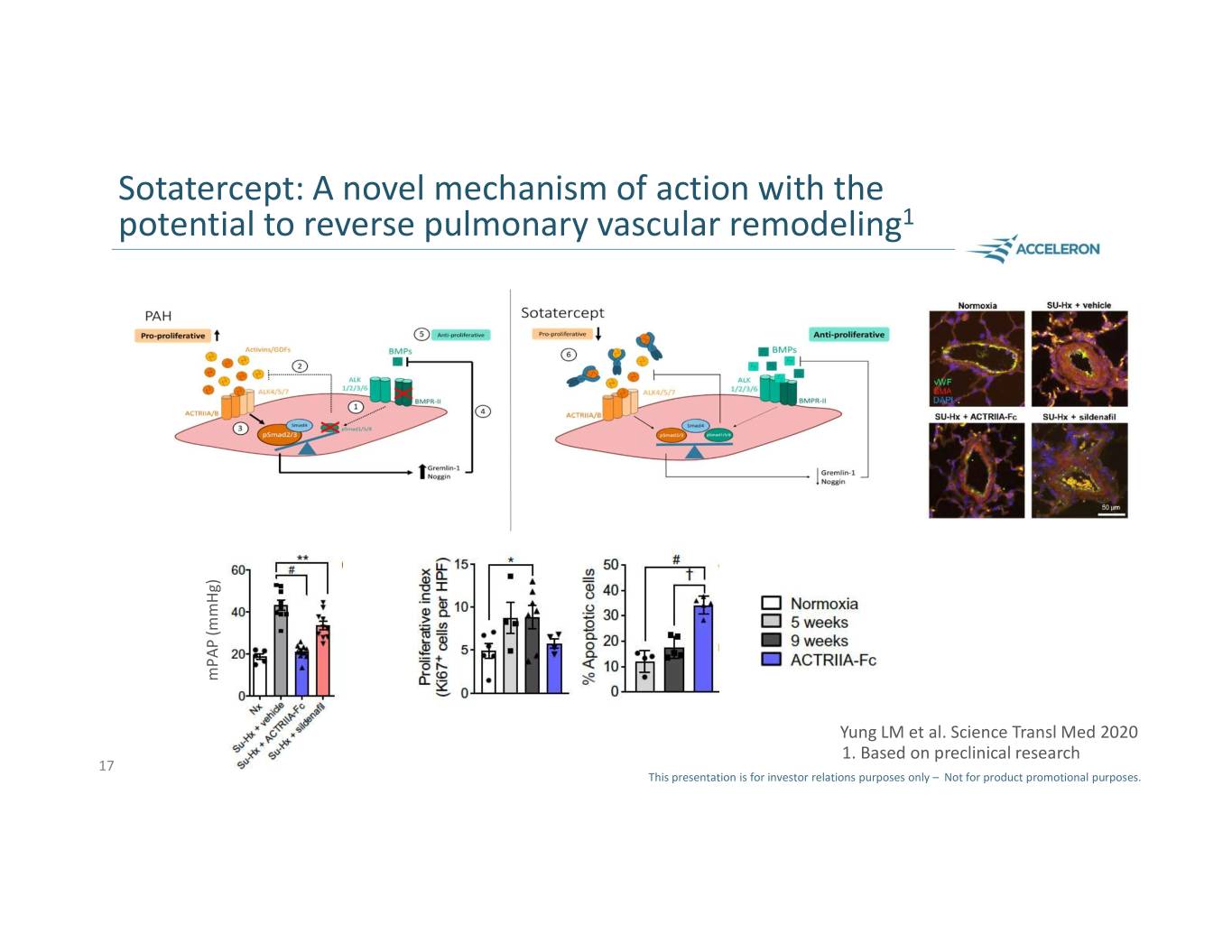
Sotatercept: A novel mechanism of action with the potential to reverse pulmonary vascular remodeling1 mPAP (mmHg) mPAP Yung LM et al. Science Transl Med 2020 1. Based on preclinical research 17 This presentation is for investor relations purposes only – Not for product promotional purposes.

Three Recommended Noninvasive Endpoints Have Been Consistently Associated With Survival Outcomes in Recent Studies Selected PAH endpoints associated with survival outcomes in recent studies WHO/NYHA BNP FC 6MWD NT-proBNP SvO2 RAP CI COMPERA1 NS n=1588* + + + + + French Registry2 NR n=603† + + + + + Giessen PH Registry3 NR NR NR NR n=685‡ + + Hannover Cohort4 n=109§ + + + + + + REVEAL 2.05 NR NR n=2529‖ + + + + *Predictive value for survival at baseline. †Univariate analysis for transplant-free survival at follow-up in subset of patients with evaluable BNP/NT-proBNP level. ‡Univariate analysis for survival. §Univariate analysis for transplant-free survival. ‖Multivariate analysis for all-cause mortality. 6MWD=6-minute walk distance; BNP=B-type natriuretic peptide; CI=cardiac index; FC=functional class; NR=not reported; NS=not significant; NT-proBNP=N-terminal-pro-B-type natriuretic peptide; NYHA=New York Heart Association; PAH=pulmonary arterial hypertension; PH=pulmonary hypertension; RAP=right atrial pressure; SvO2=mixed venous oxygen saturation; WHO=World Health Organization. 1. Hoeper MM, et al. Eur Respir J. 2017;50:1700740. 2. Boucly A, et al. Eur Respir J. 2017;50:1700889. 3. Gall H, et al. J Heart Lung Transplant. 2017;36:957-967. 4. Nickel N, et al. Eur Respir J. 2012;39:589-596. 5. Benza RL, et al. Chest. 2019;156:323-337. 18 This presentation is for investor relations purposes only – Not for product promotional purposes.

Targeting Improvement: No Single Measure Provides Sufficient Diagnostic or Prognostic Information 2015 ERS/ESC guidelines for patient assessment A multicomponent assessment is required Functional class Exercise capacity RV function (BNP/NT-proBNP (eg, 6MWD or CPET) (WHO FC I-IV) or echocardiography) 6MWD=6-minute walk distance; BNP=B-type natriuretic peptide; CPET=cardiopulmonary exercise testing; ERS=European Respiratory Society; ESC=European Society of Cardiology; FC=functional class; NT-proBNP=N-terminal-pro-B-type natriuretic peptide; RV=right ventricle; WHO=World Health Organization. Galie N, et al. Eur Respir J. 2015;46:903-975. 19 This presentation is for investor relations purposes only – Not for product promotional purposes.

A Higher Proportion of Patients Achieved the Multicomponent Improvement Endpoint With Sotatercept + SOC 60% P=0.0002* Meeting all three criteria: WHO FC: 1 40% 38% improvement or maintenance of FC II NT-proBNP: 2 ≥30% improvement Patients 20% 3 6MWD: 3% ≥30-m improvement 0% Placebo + SOC Sotatercept + SOC (n=32) all doses (n=74) *Nominal. 6MWD=6-minute walk distance; FC=functional class; NT-proBNP=N-terminal-pro-B-type natriuretic peptide; SOC=standard of care; WHO=World Health Organization. Adapted from: Badesch DB, et al. Sotatercept for the treatment of pulmonary arterial hypertension. Presented at: ATS 2020 [virtual meeting]; June 24, 2020. 20 This presentation is for investor relations purposes only – Not for product promotional purposes.

A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Compare the Efficacy and Safety of Sotatercept Versus Placebo When Added to Background Pulmonary Arterial Hypertension (PAH) Therapy for the Treatment of PAH Sotatercept is an investigational therapy that is not approved for any use in any country. 21 This presentation is for investor relations purposes only – Not for product promotional purposes.

STELLAR Phase 3 Trial Inclusion Criteria Key Inclusion criteria • Adults ≥18 years old • WHO Group 1 PAH • WHO Functional Class II or III • Baseline RHC with PVR ≥5 Wood units • Baseline 6-minute walk distance 150-500 m • Stable treatment with SOC therapies, including mono, double, and triple therapies – An endothelin-receptor antagonist, a phosphodiesterase 5 inhibitor, a soluble guanylate cyclase stimulator, and/or a prostacyclin (including IV) WHO: World Health Organization; RHC: right heart catheterization; PVR: Pulmonary vascular resistance. 22 This presentation is for investor relations purposes only – Not for product promotional purposes.

STELLAR Phase 3 Trial Design Schema Double-Blind Primary Long-term Double-Blind treatment period (24 weeks) treatment period (up to 72 weeks) Placebo Randomization + background PAH therapy (mono, double, or triple) 1:1 N = 142 Screening Stratified by (up to 4 weeks) WHO FC and Background Sotatercept 0.3 mg/kg first dose therapy to 0.7 mg/kg Q21 days + background PAH therapy (mono, double, or triple) N = 142 N=284 Primary Endpoint Analysis 23 This presentation is for investor relations purposes only – Not for product promotional purposes.
Primary Endpoint Primary endpoint Change from baseline in 6-minute walk distance (6MWD) at week 24 24 This presentation is for investor relations purposes only – Not for product promotional purposes.
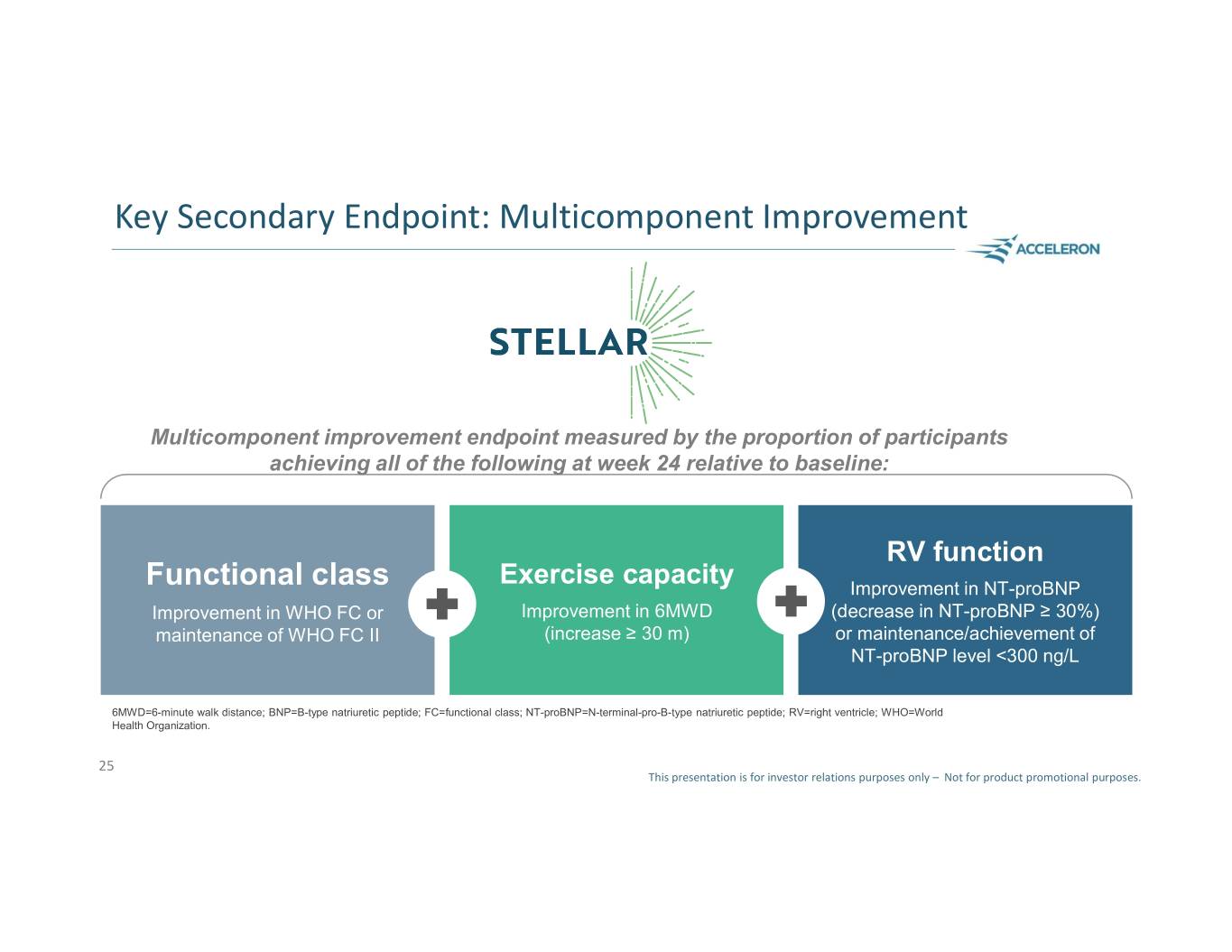
Key Secondary Endpoint: Multicomponent Improvement Multicomponent improvement endpoint measured by the proportion of participants achieving all of the following at week 24 relative to baseline: RV function Exercise capacity Functional class Improvement in NT-proBNP Improvement in WHO FC or Improvement in 6MWD (decrease in NT-proBNP ≥ 30%) maintenance of WHO FC II (increase ≥ 30 m) or maintenance/achievement of NT-proBNP level <300 ng/L 6MWD=6-minute walk distance; BNP=B-type natriuretic peptide; FC=functional class; NT-proBNP=N-terminal-pro-B-type natriuretic peptide; RV=right ventricle; WHO=World Health Organization. 25 This presentation is for investor relations purposes only – Not for product promotional purposes.
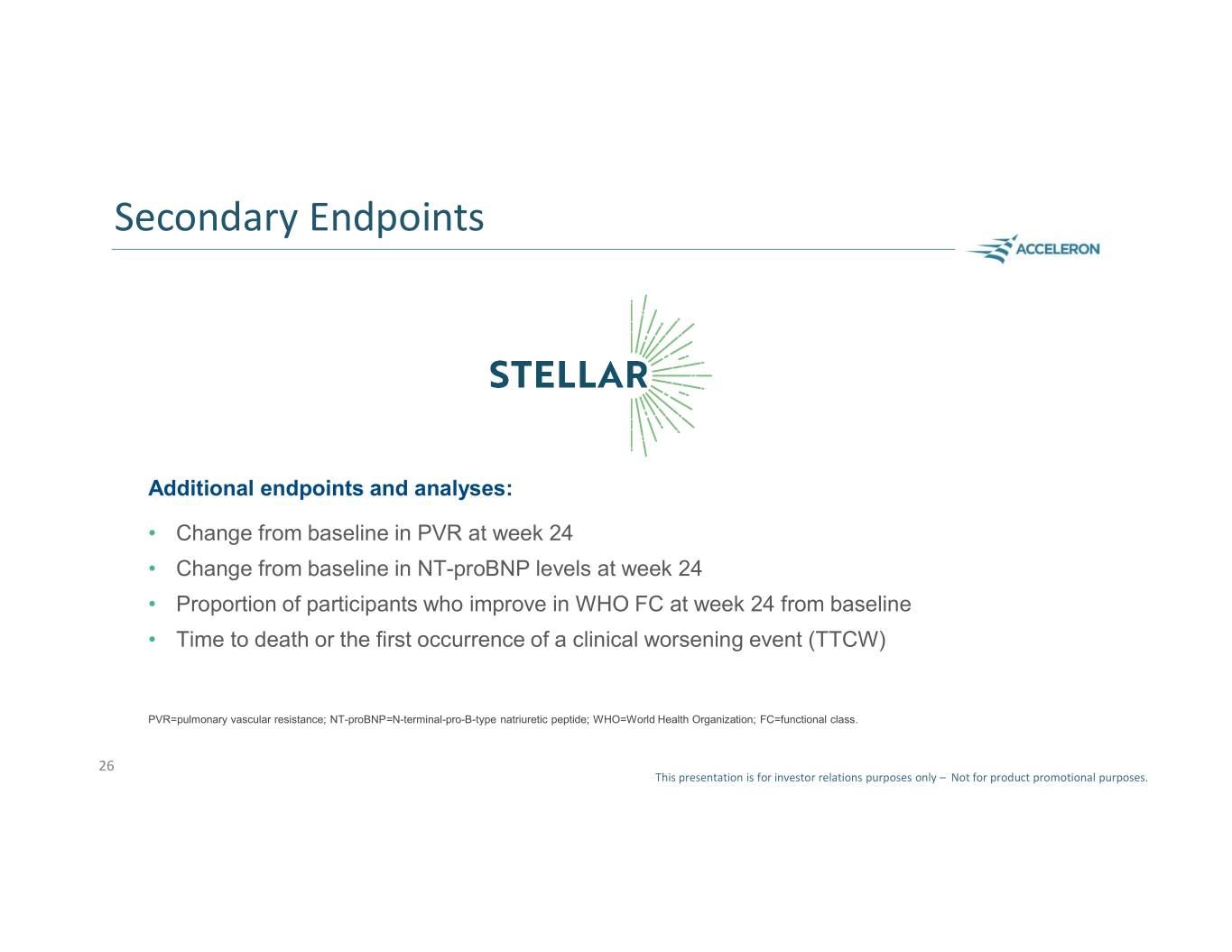
Secondary Endpoints Additional endpoints and analyses: • Change from baseline in PVR at week 24 • Change from baseline in NT-proBNP levels at week 24 • Proportion of participants who improve in WHO FC at week 24 from baseline • Time to death or the first occurrence of a clinical worsening event (TTCW) PVR=pulmonary vascular resistance; NT-proBNP=N-terminal-pro-B-type natriuretic peptide; WHO=World Health Organization; FC=functional class. 26 This presentation is for investor relations purposes only – Not for product promotional purposes.
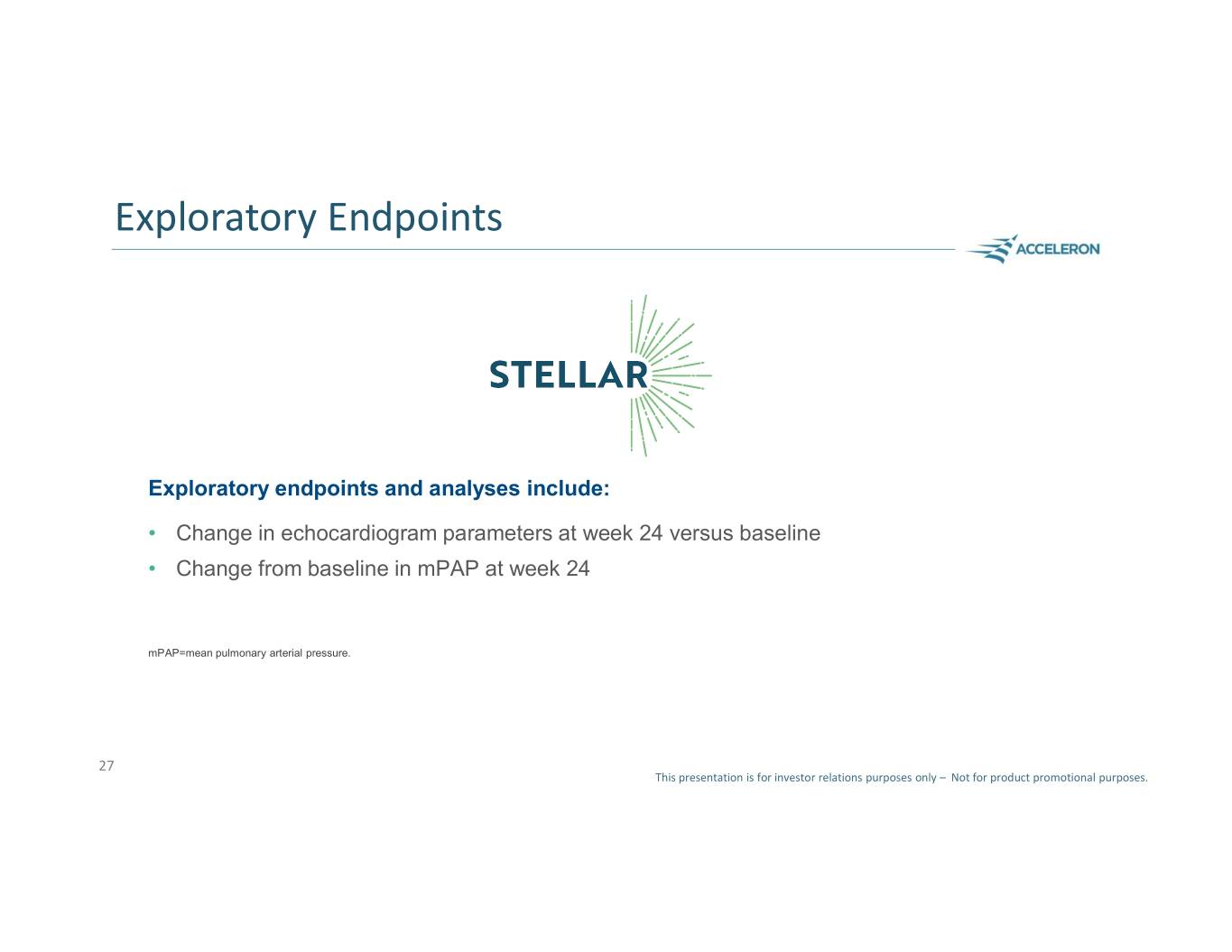
Exploratory Endpoints Exploratory endpoints and analyses include: • Change in echocardiogram parameters at week 24 versus baseline • Change from baseline in mPAP at week 24 mPAP=mean pulmonary arterial pressure. 27 This presentation is for investor relations purposes only – Not for product promotional purposes.

Summary | Jay T Backstrom, MD, MPH EVP, Head of Research & Development This presentation is for investor relations purposes only – Not for product promotional purposes.

Sotatercept Phase 3 Clinical Development Plan and Vision Registrational Label Expansion Sotatercept Vision BACKBONE THERAPY IN PAH Phase 3 Early Intervention Study Main Phase 3 Study Expect initiation before YE:2020 ZENITH Phase 3 Later Intervention Study Expect initiation in mid-2021 Sotatercept is an investigational therapy that is not approved for any use in any country. 29 This presentation is for investor relations purposes only – Not for product promotional purposes.

Priorities for Sotatercept Program in PAH . Phase 3 STELLAR trial initiation planned for YE:2020 . PULSAR open-label extension study update expected in 1H:21 . Additional results expected from the SPECTRA trial in 1H:21 . Phase 3 HYPERION (early intervention) trial and Phase 3 ZENITH (later intervention) trial initiation in expanded PAH populations planned in mid:2021 Scientific Sessions Upcoming Virtual Congress on November 13-17, 2020 . 24-week ECHO results from Phase 2 PULSAR trial expected . Preliminary interim SPECTRA results expected Sotatercept is an investigational therapy that is not approved for any use in any country. 30 This presentation is for investor relations purposes only – Not for product promotional purposes.

Question & Answer Session Habib Dable President and Chief Executive Officer Jay T Backstrom, MD, MPH EVP, Head of Research and Development Marius Hoeper, MD* Professor of Respiratory Medicine at the Hannover Medical School Janethe Pena, MD, PhD VP, Medical Research, Pulmonary Todd James SVP, Corporate Affairs and Investor Relations *Dr. Hoeper is the principal investigator of the STELLAR trial and a paid consultant to Acceleron. 31 This presentation is for investor relations purposes only – Not for product promotional purposes.
