Attached files
| file | filename |
|---|---|
| 8-K - 8-K - LogicBio Therapeutics, Inc. | d921256d8k.htm |
Exhibit 99.1
SUMMARY
This summary highlights selected information contained elsewhere or incorporated by reference in this prospectus supplement and the accompanying prospectus. The summary may not contain all the information that you should consider before investing in our common stock. You should read this entire prospectus supplement and the accompanying prospectus carefully, including “Risk Factors” contained in this prospectus supplement and the documents incorporated by reference herein, before making an investment decision.
Overview
We are a company dedicated to extending the reach of genetic medicine with pioneering targeted delivery platforms. Our proprietary genome editing technology platform, GeneRide, enables the site-specific integration of a therapeutic transgene without nucleases or exogenous promoters by harnessing the native process of homologous recombination. We are developing LB-001, a wholly owned genome editing program leveraging GeneRide for the treatment of methylmalonic acidemia, or MMA. In addition, we have a research collaboration with Takeda Pharmaceutical Company Limited, or Takeda, to develop LB-301, an investigational therapy leveraging GeneRide for the treatment of the rare pediatric disease Crigler-Najjar syndrome, or CN.
We are also developing a Next Generation Capsid platform for use in gene editing and gene therapy. At the American Society of Gene & Cell Therapy, or ASGCT, conference in May 2020, data was presented showing that the capsids delivered highly efficient functional transduction of human hepatocytes in a humanized mouse model. The data also showed the capsids exhibited improved manufacturability with low levels of pre-existing neutralizing antibodies in human samples. Based on this data, we believe the top-tier capsid candidates from this effort demonstrated the potential to achieve significant improvements over benchmark adeno-associated viruses, or AAVs, that are currently in clinical development. We are developing these highly potent vectors for use in our internal development candidates and potentially for business development collaborations. We plan to announce data generated from translational animal models using these capsids in early 2021.
Based on our GeneRide technology, we are developing our lead product candidate, LB-001, to treat MMA. In August 2020, we announced the clearance of an investigational new drug application, or IND, to support the initiation of a Phase 1/2 clinical trial in pediatric patients with MMA. The SUNRISE trial is a multi-center, open-label, Phase 1/2 clinical trial designed to assess the safety and tolerability of a single intravenous infusion of LB-001 in pediatric patients with MMA characterized by methylmalonyl-CoA mutase gene (MMUT) mutations. Six leading centers in the United States are expected to participate in the SUNRISE trial.
The SUNRISE Phase 1/2 clinical trial is expected to enroll eight pediatric patients with ages ranging from 6 months to 12 years, initially starting with 3 to 12 year old patients and then adding patients aged 6 months to 2 years. The SUNRISE trial will evaluate two dose cohorts of LB-001 (cohort 1 = 5 x 1013 vg/kg and cohort 2 = 1 x 1014 vg/kg). After initially starting with the lower dose in the 3 to 12 year old patient group (cohort 1, older age group, n=2), age de-escalation (cohort 1, younger age group, n=2) and dose escalation (cohort 2, older age group, n=2) are planned to occur in parallel. The decision to escalate the dose will be determined based solely on safety, whereas the decision to age de-escalate will be based on both safety and the detection of the pharmacodynamic biomarker, albumin-2A. Afterwards, based on a review of safety and/or the detection of albumin-2A, as applicable, from these two patient groups, the trial will progress to dosing additional patients in the younger age group at the higher dose (cohort 2, younger age group, n=2). The SUNRISE trial includes a six-week staggering interval between the dosing of each patient. Patients will participate in a pre-dosing observational period and will be administered a prophylactic steroid regimen. The following diagram illustrates the age de-escalation and dose escalation plan in the SUNRISE trial.
S-1
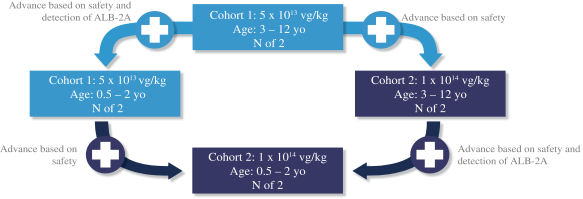
The primary endpoint of the SUNRISE trial is to assess the safety and tolerability of LB-001 at 52 weeks after a single infusion. Additional endpoints include changes in disease-related biomarkers, including serum methylmalonic acid, clinical outcomes such as growth and healthcare utilization, and the pharmacodynamic marker albumin-2A. We expect to enroll the first patient in early 2021 and provide an operational update regarding the dose escalation and age de-escalation in mid-2021. Based on the parallel age de-escalation and dose escalation plan, we expect to announce interim data from both age groups and both dose cohorts in the SUNRISE trial by the end of 2021.
At the ASGCT conference in May 2020, we released data generated in a novel modified severe MMA mouse model relying on a diet management protocol that simulated clinical management of MMA patients. In this model, animals were first stabilized under a low protein diet, before being “challenged” by switching to higher protein diet. We believe this model closely mirrors the disease progression seen in actual MMA patients by simulating both diet management and acute metabolic decompensations. A single intravenous administration of the mouse surrogate mLB-001 provided significant protection from protein challenge-induced metabolic crisis, including significant improvements in survival and body weight and a trend toward decreased circulating levels of methylmalonic acid. These findings have been replicated in both neonatal and adult animals using different doses of mLB-001, which correspond to the doses of LB-001 planned to be used in the SUNRISE trial.
In addition to the Phase 1/2 SUNRISE trial, we are also conducting a retrospective natural history study designed to evaluate disease progression in pediatric patients with MMA. We expect this study will provide us with insights into, among other matters, the course of disease progression, the impact of a liver transplant on the outcomes of MMA patients and potential endpoints such as the relevance of methylmalonic acid levels on clinical outcomes, with the goal of informing our future clinical development in MMA and our discussions with regulatory agencies as we look toward advancing our MMA program. We plan to announce preliminary findings from our retrospective natural history study in mid-2021.
Beyond LB-001, we intend to develop additional product candidates for other indications based on ongoing research and development work we perform, as well as the work of our academic partners. The criteria for selecting these proposed product candidates are initially:
| • | Genetically defined disease. As with LB-001, we expect our future product candidates to target disorders associated with genetically defined mechanisms. |
| • | High unmet need. GeneRide is designed to deliver site-specific genomic integration and limit off-target insertion of our constructs resulting in therapeutic durability to patients affected early in life by a genetic disorder. Our Next Generation Capsid platform is aimed at enhancing the delivery of gene editing approaches such as GeneRide, as well as traditional gene therapies. We plan to target underserved diseases where genetic medicines have the potential to provide lifelong benefit to patients with these two platforms. |
S-2
| • | Liver expression. Because of the modularity of our GeneRide platform in creating new product candidates in the same tissue along with the enhanced liver-tropism of our Next Generation Capsids, we will initially focus on developing therapies for indications that can be addressed by targeting the liver. We intend to evaluate the tolerability, effective targeting and expression of our therapy in our lead program in MMA, as well as our next few product candidates, before deploying additional potential therapies in other tissues. |
We expect that the initial product candidates we develop, including LB-001, will address diseases by targeting the liver, including a category of diseases known as inborn errors of metabolism, a group of genetic disorders that disrupt normal metabolic processes. We believe that achieving clinical proof of concept in an inherited liver disease such as MMA will validate our GeneRide platform technology, including its potential application to other tissues and diseases. Our approach to expanding our indication pipeline may also incorporate indications such as propionic acidemia, which like MMA is an organic acidemia, where we can leverage the experience we have gained through our research and the modularity of our platform. Furthermore, we have demonstrated proof of concept of our platform in hemophilia B and alpha-1-antitrypsin deficiency, or A1ATD, animal disease models. We expect to select future product candidates from these and other genetic diseases addressed by targeting the liver initially, and later by targeting skeletal muscle and the central nervous system, or CNS. We plan to select at least one new indication from our preclinical portfolio in 2021 and commence IND-enabling studies utilizing our modular approach and leveraging learnings from our lead programs. Depending on data and timelines, we plan to evaluate the integration of our Next Generation Capsids into our future development programs.
We have assembled a world-class team of executives, founders and advisors with years of relevant experience to enable the development of our genome editing platform and the advancement of our product candidates for patients with significant unmet medical needs. Led by Frederic (Fred) Chereau, our Chief Executive Officer, our team’s expertise spans gene therapy, homologous recombination, rare disease drug discovery and development, technical development, clinical and regulatory strategy, manufacturing strategy and operations, as well as business strategy, intellectual property, finance and commercial strategy and operations. Members of this team have been involved in developing therapies for rare diseases in both large and small biotechnology companies including Genzyme, Shire, Novartis, aTyr Pharma, Translate Bio, Genethon, Intercept Pharmaceuticals and Nightstar. Collectively, members of the team have contributed to the development of an array of approved drugs, most of which are treatments for rare diseases.
We have also established an extensive network of advisors and consultants with expertise across many critical areas of our business, from drug design, manufacturing and clinical development to regulatory approval. Our consultants and advisors possess deep experience in adeno-associated virus, or AAV, capsid development, mechanisms of DNA repair and delivery technologies, which complements our internal capabilities and supports our efforts in the development of our product candidates. Additionally, our management team is actively supported by a scientific advisory board, or SAB, and we believe that their expertise, combined with our network of consultants and advisors, is a pivotal asset for our product development efforts. We are committed to bringing much-needed therapies to children with serious genetic deficiencies and we work closely with patient foundations, such as the Organic Acidemia Association and the National Hemophilia Foundation.
S-3
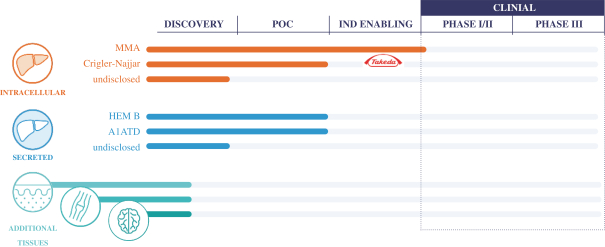
Below is a summary of our ongoing discovery, research and development programs:
Strategy
Our mission is to transform the lives of patients living with devastating genetic diseases by building the leading integrated genetic medicine company focused on developing and commercializing potentially curative therapeutics. Key elements of our strategy are to:
| • | Successfully advance LB-001 through clinical development and ultimately into commercialization. We chose a specific organic acidemia, MMA, as our initial indication to enter proof-of-concept trials in humans due to the high unmet medical need and the absence of therapeutic treatments for this disease. In August 2020, we announced the clearance of an IND to support the initiation of our SUNRISE Phase 1/2 clinical trial in pediatric patients with MMA and announced our clinical Phase 1/2 clinical design. Our goal is to develop LB-001 ourselves and, if approved, to retain global commercialization rights and commercialize through a small, targeted sales organization. |
| • | Aggressively pursue additional indications addressed by targeting the liver. For our initial animal proof-of-concept studies, we selected liver diseases with significant unmet medical need and well-validated targets with accepted disease-correlated biomarkers, and where we believe the GeneRide platform can provide unique benefits by addressing the root cause of the disease. We have established preclinical proof of concept in several indications that can be targeted through the liver using GeneRide. Our investment in developing the Next Generation Capsid platform has yielded an initial set of capsids with enhanced liver tropism, providing us with potentially differentiated delivery of our product candidates. We plan to continue our research to explore additional potential indications leveraging our modular approach, learnings from our lead program, our new capsids and our strengths in gene editing and gene therapy. |
| • | Collaborate to realize the full potential of our platforms. We plan to leverage strategic partnerships to accelerate advancement of our programs by accessing non-dilutive capital and disease-specific expertise in indications outside of our initial core focus. These indications could include other diseases addressable by targeting the liver, such as Crigler-Najjar syndrome which is being pursued using GeneRide through a research collaboration with Takeda. We also intend to seek collaborations to accelerate the development of the GeneRide platform in new tissues, such as the CNS and muscle, as well as to advance our Next Generation Capsids into gene editing and gene therapy products beyond those we plan to develop internally. |
S-4
| • | Build an exceptional team and organization. Delivering on the promise of a novel technology like GeneRide and developing differentiated, precision medicines requires an exceptional organization. We have assembled a group of leaders and scientific talent in the fields of rare diseases, genome editing and gene therapy, and expect to continue building and expanding our team, as required, to execute on our plans to develop and commercialize genetic medicines. |
| • | Maintain our scientific leadership in the field of genomic medicines. We will strive to continue optimizing all aspects of our GeneRide technology through a combination of in-house research and work by our network of academic collaborators. Additionally, we expect to continue investing in the development of our Next Generation Capsid platform that we hope will continue to enhance the utility of our GeneRide platform, and provide us with AAV assets for use in conjunction with conventional gene therapy products. We believe that our scientific leadership will provide us opportunities to expand our intellectual property portfolio. |
Impact of COVID-19
We have been actively monitoring the COVID-19 pandemic and its impact globally. Our objectives have remained the same throughout the pandemic: to support the safety of our team members and their families and to continue our research and development activities to develop genetic medicines that have the potential to durably treat rare diseases in patients with significant unmet medical need.
Since mid-March 2020, our non-laboratory employees have been working remotely in order to comply with social distancing and other applicable orders and guidelines from federal, state and local government agencies. After being limited to working in shifts on-premises through early July 2020, laboratory employees, whose work must be performed on premises, have returned to normal working schedules on-premises. We have also ceased all business travel for our employees. We plan to maintain these or similar restrictions on our business activities until we believe our employees can fully resume such business activities in accordance with federal, state and local requirements and guidelines.
Our research, development and manufacturing activities are dependent on our ability to continue our work on premises at our laboratory. We also rely on third parties located in countries that are affected by the COVID-19 pandemic, including the United States, for certain research, development and manufacturing activities. Similar to how we have restricted business activities at our premises, many of these third parties have also limited their staff from working on premises as part of their response to COVID-19. While we believe we and our third party vendors, suppliers and collaborators have largely been able to continue or resume essential business activities to a certain degree, we cannot predict the impact of the progression of the COVID-19 pandemic on future results due to a variety of factors, including the health of our and their employees, our ability to maintain operations, the ability of our third party vendors, suppliers and collaborators to continue operations, any further government and/or public actions taken in response to the pandemic and ultimately the length of the pandemic.
We plan to continue to closely monitor the COVID-19 pandemic in order to ensure the safety of our personnel and to continue advancing our research and development activities.
S-5
Corporate Information
We were incorporated in the State of Delaware in August 2014. Our principal executive offices are located at 65 Hayden Avenue, 2nd Floor, Lexington, MA, and our telephone number is (617) 245-0399. Our website address is www.logicbio.com. We do not incorporate the information on or accessible through our website into this prospectus and you should not consider any information on or that can be accessed through our website as part of this prospectus supplement or the accompanying prospectus.
S-6
