Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Arcutis Biotherapeutics, Inc. | arqt-20200929.htm |

Exhibit 99.1 Seborrheic Dermatitis September 2020

Legal Disclaimers This presentation and the accompanying oral presentation contain “forward-looking” statements that are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include all statements other than statements of historical fact contained in this presentation, including information concerning our current and future financial performance, business plans and objectives, current and future clinical and preclinical development activities, timing and success of our ongoing and planned clinical trials and related data, the timing of announcements, updates and results of our clinical trials and related data, our ability to obtain and maintain regulatory approval, the potential therapeutic benefits and economic value of our product candidates, competitive position, industry environment and potential market opportunities. Forward-looking statements are subject to known and unknown risks, uncertainties, assumptions and other factors including, but not limited to, those related to the success, cost and timing of our product candidate development activities and ongoing and planned clinical trials; our plans to develop and commercialize targeted therapeutics, including our lead product candidates ARQ-151 and ARQ-154; the progress of patient enrollment and dosing in our clinical trials; the ability of our product candidates to achieve applicable endpoints in the clinical trials; the safety profile of our product candidates; the potential for data from our clinical trials to support a marketing application, as well as the timing of these events; our ability to obtain funding for our operations, development and commercialization of our product candidates; the timing of and our ability to obtain and maintain regulatory approvals; the rate and degree of market acceptance and clinical utility of our product candidates; the size and growth potential of the markets for our product candidates, and our ability to serve those markets; our commercialization, marketing and manufacturing capabilities and strategy; future agreements with third parties in connection with the commercialization of our product candidates; our expectations regarding our ability to obtain and maintain intellectual property protection; our dependence on third party manufacturers; the success of competing therapies that are or may become available; our ability to attract and retain key scientific or management personnel; our ability to identify additional product candidates with significant commercial potential consistent with our commercial objectives; and our estimates regarding expenses, future revenue, capital requirements and needs for additional financing. Moreover, we operate in a very competitive and rapidly changing environment, and new risks may emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed herein may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Further information on these and other factors that could affect these forward-looking statements is contained in our our Form 10-Q filed with U.S. Securities and Exchange Commission (SEC) on August 11, 2020, and other reports filed with the SEC from time to time. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances described in the forward-looking statements will be achieved or occur. We undertake no obligation to publicly update any forward- looking statements, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, projections, assumptions and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk. 2 Copyright ARCUTIS 2020

Seborrheic Dermatitis Phase 2 Data Call 3 Copyright ARCUTIS 2020

Frank Watanabe President & CEO 4 Copyright ARCUTIS 2020

Seborrheic Dermatitis (Seb Derm) • Common, chronic inflammatory skin disease • Affects 10M people in the U.S. • Appears as itchy red patches covered by greasy, flaking scales on the scalp, face & chest 5 Copyright ARCUTIS 2020

Negative Impact on Quality of Life (QoL) Seb derm can have a significant, negative influence on QoL Women Express Particular Psychological Distress Self-Consciousness Oily skin and flakiness in visible areas Limits clothing choices (no black), hairstyle causes psychological distress (due to Rx shampoos), and make-up ! Significant QoL Impact Perception of Poor Hygiene QoL is Key Driver for Rx QoL impacted by all Patients are perceived as High patient QoL burden symptoms: erythema, flaking, “dirty,” causes negative impact motivates dermatologists to oily skin, and pruritus1 on self-esteem treat seb derm Szepietowski JC, Reich A, Wesołowska-Szepietowska E, Baran E. National quality of life in dermatology group. 2009 6 Copyright ARCUTIS 2020

Efficacy Benchmarks Total Severity Score Clearance Rate 67% 70% 70% 61% 60% 57% 60% 56% 50% 50% 42% 38% 40% 40% 30% 30% 4 Weeks 4 20% 20% Responders at Responders 10% 10% 0% 0% Clobetasol Propionate Ketoconazole (Nizoral ) Ciclopirox 1% Placebo Ketoconazole 2% Placebo 0.05% (Spray Clobex) 2% Shampoo (Loprox) Foam n= 326 n= 183 n= 1,162 • Total severity score (TSS) ≥2 defined as sum of erythema, loose • Responders equals none or • IGA score of 0 or 1 at 4 weeks desquamation, and adherent desquamation at 4 weeks1 slight (0-1 scores) at 4 weeks2 equals treatment success3 • Moderate-to-severe scalp SD (IGA of 3 or 4 on a 5-point scale) • Placebo rate: 42% • TEAEs: 5% • Predominantly mild subjects • TEAEs: 14% References: 1. 2011 (Ortonne, JP – Galderma funded) 2. 2004 (Abeck, D) 3. 2007 (Elewski, BE) 7 Copyright ARCUTIS 2020

Patrick Burnett, M.D., Ph.D., FAAD Chief Medical Officer 8 Copyright ARCUTIS 2020

Seb Derm Contributing Factors Genetic factors Overall health Particular yeast Emotional or Climate that lives on all physical stress, such (usually worse in human skin as lack of sleep cold weather) 9 Copyright ARCUTIS 2020

Limitations of Current Seb Derm Treatments Topical AntiEasy-Fungals to Diagnose No single product appropriate for both scalp and face/body • Often used as first-line therapy • Many patients use 3-5 products • Often ineffective for long-term remission • Time management challenge and Topical steroids complexity • Increased risk of glaucoma and cataracts • Reduces patient compliance • No chronic high-potency steroid use • Increases time / expense (multiple co-pays) beyond 2-4 weeks Rx shampoos • Skin atrophy concerns since skin on face and scalp is thin • Usage usually 2x/week for up to 4 weeks • Texture of vehicle can mess up hair styles Non-steroidals and dry out hair • Perceived lack of efficacy and/or • Perceived unpleasant smell tolerability 10 Copyright ARCUTIS 2020

Topical Roflumilast Foam Roflumilast foam offers a highly differentiated clinical profile Investigated for use as a once More potent (25- to 300-fold) daily, non-steroidal, anti- than the two other FDA- inflammatory topical formulation approved PDE4 inhibitors Selective, highly potent “Leave on” foam formulation Oral roflumilast anti-inflammatory PDE4 allows for use on all body approved by FDA for inhibitor parts, including hair-bearing COPD in 2011 areas 11 Copyright ARCUTIS 2020

Phase 2 Study of Roflumilast Foam in Seb Derm Endpoints 2:1 ARQ-154 foam Primary • IGA success at week 8 Eligibility 0.3% QD Secondary • Diagnosis of at least moderate • Overall assessment of erythema seb derm • Overall assessment of scaling • Aged ≥18 y • WI-NRS • ≤20% BSA Randomize Vehicle Exploratory QD • Scalpdex • DLQI N=226 • BSA Safety and tolerability 8 weeks dosing aIGA success was defined as IGA score of 0 or 1 (clear or almost clear) with at least a two-grade improvement from baseline. BSA, body surface area; DLQI, dermatology life quality index; IGA, investigator global assessment; QD, once daily; WI-NRS, worst itch numeric rating scale. NCT04091646. https://clinicaltrials.gov/ct2/show/NCT04091646. Accessed July 20, 2020. 12 Copyright ARCUTIS 2020
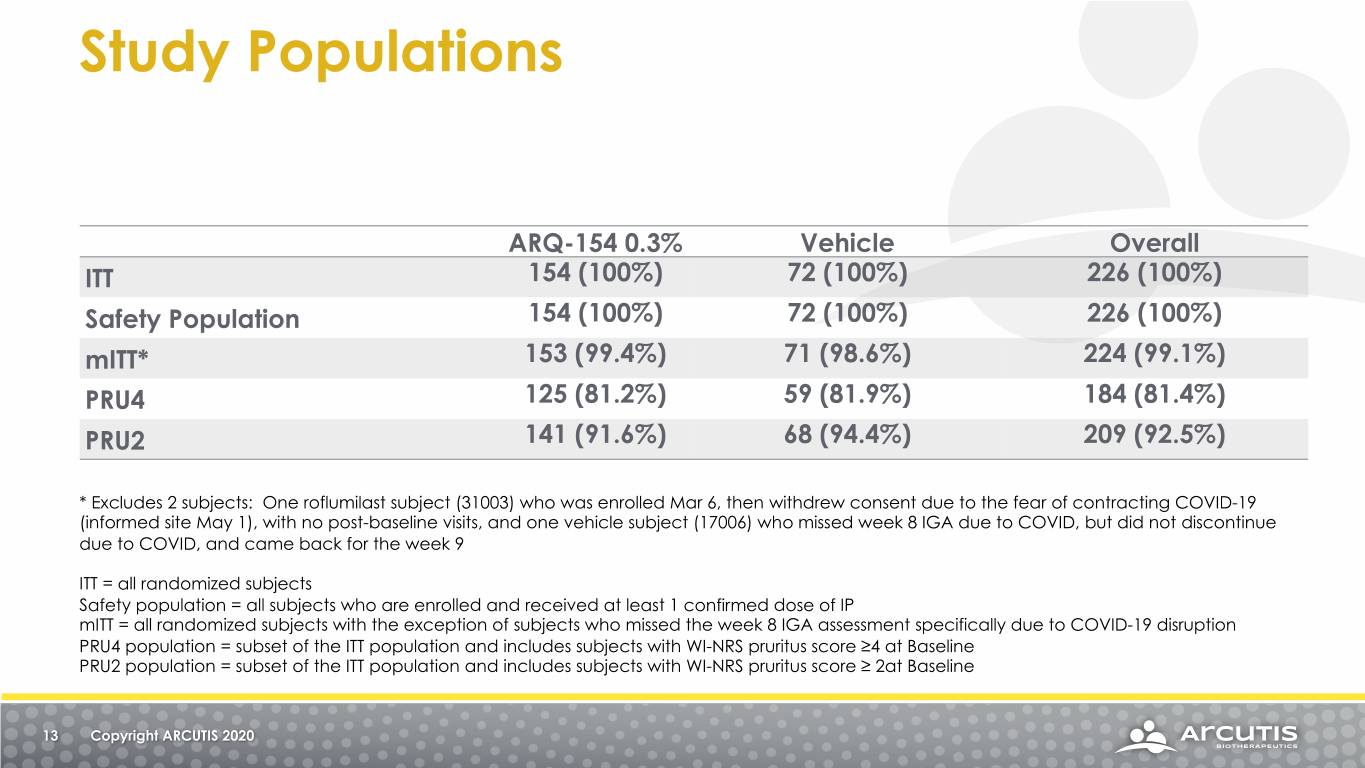
Study Populations ARQ-154 0.3% Vehicle Overall ITT 154 (100%) 72 (100%) 226 (100%) Safety Population 154 (100%) 72 (100%) 226 (100%) mITT* 153 (99.4%) 71 (98.6%) 224 (99.1%) PRU4 125 (81.2%) 59 (81.9%) 184 (81.4%) PRU2 141 (91.6%) 68 (94.4%) 209 (92.5%) * Excludes 2 subjects: One roflumilast subject (31003) who was enrolled Mar 6, then withdrew consent due to the fear of contracting COVID-19 (informed site May 1), with no post-baseline visits, and one vehicle subject (17006) who missed week 8 IGA due to COVID, but did not discontinue due to COVID, and came back for the week 9 ITT = all randomized subjects Safety population = all subjects who are enrolled and received at least 1 confirmed dose of IP mITT = all randomized subjects with the exception of subjects who missed the week 8 IGA assessment specifically due to COVID-19 disruption PRU4 population = subset of the ITT population and includes subjects with WI-NRS pruritus score ≥4 at Baseline PRU2 population = subset of the ITT population and includes subjects with WI-NRS pruritus score ≥ 2at Baseline 13 Copyright ARCUTIS 2020

Subject Disposition ARQ-154 0.3% Vehicle Overall (N=154) (N=72) (N=226) Completed 141 (91.6%) 67 (93.1%) 208 (92.0%) Prematurely discontinued 13 (8.4%) 5 (6.9%) 18 (8.0%) Reason for discontinuation Withdrawal by subject 4 (2.6%) 1 (1.4%) 5 (2.2%) Sponsor decision 0 0 0 PI Decision 0 0 0 Non-compliance 0 0 0 Protocol violation 0 1 (1.4%) 1 (0.4%) Lost to follow-up 6 (3.9%) 2 (2.8%) 8 (3.5%) Adverse event 2 (1.3%) 1 (1.4%) 3 (1.3%) Death 0 0 0 Pregnancy 0 0 0 Other 1 (0.6%) 0 1 (0.4%) 14 Copyright ARCUTIS 2020

Demographics (Safety Population) ARQ-154 0.3% Vehicle Overall (N=154) (N=72) (N=226) Age, mean (yrs) 45.3 44.2 44.9 Gender Male 76 (49.4%) 40 (55.6%) 116 (51.3%) Female 78 (50.6%) 32 (44.4%) 110 (48.7%) Ethnicity Hispanic or Latino 29 (18.8%) 16 (22.2%) 45 (19.9%) Not Hispanic or Latino 125 (81.2%) 56 (77.8%) 181 (80.1%) Race American-Indian or Alaskan Native 1 (0.6%) 0 1 (0.4%) Asian 7 (4.5%) 1 (1.4%) 8 (3.5%) Black or African-American 17 (11.0%) 6 (8.3%) 23 (10.2%) Native Hawaiian or Other Pacific Islander 0 0 0 White 123 (79.9%) 62 (86.1%) 185 (81.9%) Other 1 (0.6%) 2 (2.8%) 3 (1.3%) More than one race 5 (3.2%) 1 (1.4%) 6 (2.7%) 15 Copyright ARCUTIS 2020

Baseline Characteristics (Safety Population) ARQ-154 0.3% Vehicle Overall (N=154) (N=72) (N=226) BSA, mean (%) 3.3 3.0 3.2 Baseline IGA (0-4) 3 – Moderate 141 (91.6%) 69 (95.8%) 210 (92.9%) 4 – Severe 13 (8.4%) 3 (4.2%) 16 (7.1%) Baseline Erythema (0-3) 2 – Moderate 135 (87.7%) 66 (91.7%) 201 (88.9%) 3 – Severe 19 (12.3%) 6 (8.3%) 25 (11.1%) Baseline Scaling (0-3) 2 – Moderate 130 (84.4%) 58 (80.6%) 188 (83.2%) 3 – Severe 24 (15.6%) 14 (19.4%) 38 (16.8%) WINRS Mean 5.8 (2.66) 5.7 (2.33) 5.8 (2.56) Median 6.0 6.0 6.0 >4 125 (81.2%) 59 (81.9%) 184 (81.4%) Facial involvement 100 (64.9%) 36 (50.0%) 136 (60.2%) 16 Copyright ARCUTIS 2020

IGA Success at Each Visit (mITT) 74% of Patients Achieved IGA Success 100% 90% p < 0.0001 80% 70% p = 0.0002 60% 50% p = 0.0033 40% 30% Percentage of Patients of Percentage 20% 10% 0% Week 2 Week 4 Week 8 0.3% ARQ-154 Vehicle IGA Success = Clear or Almost Clear with at least a 2-grade improvement from baseline 17 Copyright ARCUTIS 2020 17

WI-NRS 4-pt Response (PRU4 Population) 65% of Patients Achieved a WI-NRS 4-pt Response 100% 90% 80% p = 0.0007 70% p = 0.0009 p = 0.0007 60% 50% 40% 30% Percentage of Patients of Percentage 20% 10% 0% Week 2 Week 4 Week 8 0.3% ARQ-154 Vehicle 18 Copyright ARCUTIS 2020 18

Low Rates of Adverse Events (Safety Population) ARQ-154 0.3% Vehicle Overall (N=154) (N=72) (N=226) Subjects with any TEAE 37 (24.0%) 13 (18.1%) 50 (22.1%) Subjects with any Tx-Related TEAE 3 (1.9%) 3 (4.2%) 6 (2.7%) Subjects with any SAE 0 0 0 Subjects who discontinued Study 2 (1.3%) 2 (2.8%) 4 (1.8%) Drug due to AE Subjects who discontinued Study 2 (1.3%) 1 (1.4%) 3 (1.3%) due to AE 19 Copyright ARCUTIS 2020

Most Common TEAE’s by Preferred Term > 2% in any group ARQ-154 0.3% Vehicle Overall Preferred Term (N=154) (N=72) (N=226) Contact Dermatitis 3 (2%) 2 (3%) 5 (2%) Insomnia 3 (2%) 1 (1%) 4 (2%) Nasopharyngitis 3 (2%) 0 (0%) 3 (1%) 20 Copyright ARCUTIS 2020

Dr. Matthew Zirwas, M.D. Founder of the Bexley Dermatology Research Clinic and Investigator in the Trial 21 Copyright ARCUTIS 2020

Ken Lock Chief Commercial Officer 22 Copyright ARCUTIS 2020

Seb Derm Prevalence Additional opportunities to drive value in Seb Derm: Seb derm patients • Market growth due to educational 10.0M efforts and promotional investment Treated • U.S. patients treated by other 3.2M patients specialties (e.g., PCPs) Rx treated • Ex-US markets topically 2.7M Rx treated topically in 1.8M derm setting Unresponsive to 1st-line treatment 530K 23 Copyright ARCUTIS 2020

In Derm Offices the Volume and Severity Is In-line with Psoriasis average number of seborrheic dermatitis 55 patients seen in a Severity of Seborrheic Dermatitis patients typical month 41% 42% 17% Mild Moderate Severe Symptoms Experienced in Each Area From qualitative 36% 33% research and pilot 24% interviews, most of 6% the combinations HCPs are seeing are Face only Scalp only Body only (not face Combination of any Face + Scalp or scalp) of the above areas Arcutis Quantitative Seb Derm Research August 2020, n=100 Dermatology HCPs 24 Copyright ARCUTIS 2020

FDA Approved Seb Derm Treatment Options Regimens Side Effects Approx List Price LOCOID Solution Burning, itching, irritation, dryness, folliculitis Hydrocortisone Butyrate 0.1% 2-3x/ daily (these reactions are listed in an $65 Approved 1982 approximate decreasing order of occurrence) LOPROX Shampoo 2x/ week for 4 weeks with 1% application site reaction Ciclopirox 1% $55 a min of 3 days between 1% increased itching Approved 1997 applications (n=626) 4% application site burning XOLEGEL Gel 1x/ day (the most common treatment- $970 Ketoconazole 2% for 2 weeks Approved 2006 related adverse reaction) EXTINA Foam Burning: Ketoconazole 2% 2x/ day 10% Extina $785 Approved 2007 10% vehicle * Data from USPIs of Select Products 25

Most Patients Require 2 or More Products Mild Moderate Severe % Patients getting 2 or more Treatments 43% 75% 88% 47% 2 treatments in combination 54% 3 or more 34% 41% treatments in 21% combination 9% Arcutis Quantitative Seb Derm Research August 2020, n=100 Dermatology HCPs 26 CONFIDENTIAL - Do Not Duplicate

TRx Trends for Approved Therapies 10,000,000 2,000,000 • There are >9M on-label TRx 9,000,000 1,800,000 on an annual basis for FDA 8,000,000 1,600,000 TRx Approved therapies 7,000,000 1,400,000 • Other off-label products 6,000,000 1,200,000 are used (e.g. TCSs, TCIs) 5,000,000 1,000,000 Ciclopirox / . • Ketoconazole is 4,000,000 800,000 Ketoconazole TRx Ketoconazole dominant therapy and 3,000,000 600,000 Hydrocort utilization is growing 2,000,000 400,000 1,000,000 200,000 MAT Jun MAT Jun MAT Jun MAT Jun MAT Jun MAT Jun 2015 2016 2017 2018 2019 2020 Ketoconazole Hydrocortisone (0.1%) Ciclopirox Source: IQVIA June 2020 Data 27 Copyright ARCUTIS 2020

Payor Sentiment Top National Pharmacy Benefit Managers % and Health Plans representing over ~70 80 million formulary lives were surveyed • Seborrheic dermatitis is considered a lower payer management priority compared to Surveyed currently conditions like psoriasis and atopic dermatitis view seborrheic dermatitis as a • Review of current medical policies of top medical condition National PBMs and Health Plans demonstrate that warrants Rx coverage and benefit exclusions are rare prescription therapy • Payers expressed minimal budget impact and superior efficacy were the most likely ways for a brand product to avoid management in predominantly generic/OTC categories Source: Arcutis Payer market research (August 2020, n=25) 28 Copyright ARCUTIS 2020

High Interest in Roflumilast Foam Dermatologist Likelihood to Prescribe Roflumilast Foam % 2% % % % 87 11 27 60 Very or Somewhat Likely Very Likely Extremely Likely Extremely likely to Rx It sounds like an attractive option Very, very excited as it is a foam and thus can be Provides another possible option that a PDE inhibitor used on the scalp and face. I also for these difficult-to-treat cases.(…) would come to like that it does not have alcohol The most important symptoms for market especially in a which may sting the skin. It’s great most patients is the itching. foam vehicle and a that it is not a topical steroid and non-steroidal!” the time frames listed for improvement are reasonable.” Arcutis Quantitative Seb Derm Research August 2020, n=100 Dermatology HCPs 29 Copyright ARCUTIS 2020

Most Compelling Aspects of Roflumilast Foam Compelling Product Profile Statements (top 2 – very/extremely compelling) Used for long-term management of seb derm 90% Safe to use on all parts of the body: face, scalp, and body 90% Highly favorable tolerability profile (no stinging/burning) 90% Convenient for patients (single product for face, scalp, body) 83% Use once-daily 81% 70% IGA success at week 4, continued thru week 8 (primary endpoint) 81% Efficacy superior to antifungals 77% Arcutis Quantitative Seb Derm Research August 2020, n=100 Dermatology HCPs 30 CONFIDENTIAL - Do Not Duplicate

Pricing of Current Foam Therapies Ranges from ~$365 - $1100 WAC as of September 2020 $1,200 $1,000 $800 $600 $1,113 $992 $400 $785 $829 $829 $200 $366 $0 Finacea 15% Foam Extina 2% Foam Lexette 0.05% Foam Sorilux 0.005% Foam Verdeso 0.05% Foam Enstilar 0.005% Foam (Rosacea) (Seb D) (PsO) (PsO) (AD) (PsO) Source: ProspectRx, September 2020 31 Copyright ARCUTIS 2020

Seb Derm Competitive Pipeline Development timeline for Seb Derm therapies ARQ-154 Phase IIb trial initiated in seborrheic dermatitis patients 2018 2019 2020 2021 2022 2023 2024 2025 2026 2027 ARQ-154 Omiganan Gel Topline Phase IIb Phase II trial initiated results • 1.75% BID Gel • Facial SD Only • Mild to Moderate Pts • Antifungal MOA Source: Clintrials.gov Sept 2020 Seborrheic Dermatitis Trials ARCUTIS CONFIDENTIAL – DO NOT DUPLICATE

~5 Million Patients Currently Treated Topically by Dermatologists in US US Patient Populations (Millions) Psoriasis Atopic Dermatitis Seborrheic Dermatitis Prevalence 8.6 19.2 10.0 Rx treated 3.5 6.3 2.7 Topically treated 2.5 5.4 2.7 Rx treated in Derm Setting 2.8 1.2 1.8 Rx treated (Topically) in 2.0 1.0 1.8 Derm Setting Additional opportunities to unlock value of our molecules: • U.S. patients treated by other specialties (e.g., PCPs or pediatricians) • Ex-US markets 33 Copyright ARCUTIS 2020

If Approved, Roflumilast Foam: Novel Mechanism Convenience • Will be first treatment in Easy to Diagnose • Will be an easy-to-use, once decades to offer a novel daily, single treatment option for mechanism of action for the both scalp and face/body treatment of seb derm Suitability “Best in Class” • Will be suitable for use in hair- • Has potential to be a “best in bearing areas (unlike creams), class” treatment for patients as well as face and around the with seb derm eyes (unlike steroids) 34 Copyright ARCUTIS 2020

The Potential of Roflumilast Foam Current Treatments Roflumilast Foam No single product works for scalp, Roflumilast can be used on all body face and body areas, including hair-bearing Most patients need an arsenal of Once-a-day roflumilast offers the products to manage disease convenience of a single product Steroids not meant to be used Has shown efficacy and is well chronically tolerated – suitable for long-term use Shampoos can be drying Dries quickly, is unscented and contains no drying ethanol 35 Copyright ARCUTIS 2020

Thank You 36 Copyright ARCUTIS 2020
