Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - VYNE Therapeutics Inc. | tm2030482d1_ex99-1.htm |
| 8-K - FORM 8-K - VYNE Therapeutics Inc. | tm2030482-1_8k.htm |
Exhibit 99.2

Investor Presentation September 2020

Forward Looking Statements This presentation includes forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 , including, but not limited to statements regarding the development and commercialization of VYNE’s products and product candidates and other statements regarding the future expectations, plans and prospects of VYNE . All statements in this presentation which are not historical facts are forward - looking statements . Any forward - looking statements are based on VYNE’s current knowledge and its present beliefs and expectations regarding possible future events and are subject to risks, uncertainties and assumptions that could cause actual results to differ materially and adversely from those set forth or implied by such forward - looking statements . These risks and uncertainties include, but are not limited to, the outcome and cost of clinical trials for current and future product candidates ; determination by the FDA that results from VYNE’s clinical trials are not sufficient to support registration or marketing approval of product candidates ; adverse events associated with the commercialization of AMZEEQ ® and ZILXI™ ; the outcome of pricing, coverage and reimbursement negotiations with third party payors for AMZEEQ ® , ZILXI™ or any other products or product candidates that VYNE may commercialize in the future ; whether, and to what extent, third party payors impose additional requirements before approving AMZEEQ ® or ZILXI™ prescription reimbursement ; the eligible patient base and commercial potential of AMZEEQ ® , ZILXI™ or any of VYNE’s other product or product candidates ; risks that VYNE’s intellectual property rights, such as patents, may fail to provide adequate protection, may be challenged and one or more claims may be revoked or interpreted narrowly or will not be infringed ; risks that any of VYNE’s patents may be held to be narrowed, invalid or unenforceable or one or more of VYNE’s patent applications may not be granted and potential competitors may also seek to design around VYNE’s granted patents or patent applications ; additional competition in the acne and dermatology markets ; inability to raise additional capital on favorable terms or at all ; VYNE’s ability to recruit and retain key employees ; and VYNE’s ability to stay in compliance with applicable laws, rules and regulations . For a discussion of other risks and uncertainties, and other important factors, any of which could cause VYNE’s actual results to differ from those contained in the forward - looking statements, see the sections titled “Risk Factors” in VYNE’s most recent quarterly report on Form 10 Q as well as discussions of potential risks, uncertainties, and other important factors in VYNE’s subsequent filings with the U . S . Securities and Exchange Commission . Although VYNE believes these forward - looking statements are reasonable, they speak only as of the date of this presentation and VYNE undertakes no obligation to update publicly such forward - looking statements to reflect subsequent events or circumstances, except as otherwise required by law . Given these risks and uncertainties, you should not rely upon forward - looking statements as predictions of future events . The trademarks included herein are the property of the owners thereof and are used for reference purposes only . This presentation concerns product candidates that are under clinical investigation . None of such product candidates have been approved for marketing by the FDA or the EMA, and such product candidates are currently limited to investigational use, and no representation is made as to their safety or effectiveness for the purposes for which they are being investigated . 2

VYNE Investment Highlights x Commercial stage company focused on large and growing markets in dermatology x Two commercial products: AMZEEQ ® and ZILXI TM employ proprietary differentiated MST TM technology x Strong intellectual property with latest patent into 2037 and barriers to genericization inherent among topical medications x Addition of FCD105 expands potential product offering and enhances company position as a scaled leader in dermatology x Portfolio synergies allow for leverageable commercial infrastructure x Experienced management team demonstrating execution capabilities with AMZEEQ ® ramp x Well capitalized with $100M in cash as of June 30 th and ~168M shares outstanding x Broad investor base with a healthy mix of institutional and retail investors 3

Proprietary Technology Innovative and Proprietary Molecule Stabilizing Technology (MST™) 1. Hazot Y, et al. J Anal Pharm Res. 2017;4(5):00117. 2. Amzeeq (minocycline) topical foam, 4% [package insert]. Bridgewater, NJ; Foamix Pharmaceuticals Inc. • Stabilizes hydrophobic molecules • Surfactant & irritant free formulation designed to maintain barrier function, improve tolerability and compliance 1 • Low mechanical sheer designed to enhance spreadability • Delivers unstable drugs that have been difficult to formulate topically 4 • Targets delivery of minocycline directly into the pilosebaceous unit • Enabled the first topical minocycline 2 • Intellectual property/patents out to 2037

Products/Pipeline Advancing a leading dermatology portfolio Dermatology assets positioned for growth and value creation Safety and efficacy of these investigational products have not been established. There is no guarantee that pipeline products will receive FDA approval or become commercially available. 5 Product Preclinical Phase 1 Phase 2 Phase 3 Approved / Marketed Key Milestones FCD105 Foam • NDA approval and launch of first topical minocycline • NDA approval and launch anticipated Q4 2020 • Phase 2 topline results Q2 2020 • Anticipate FPI Phase 3 Trial 1H 2021

Commercial Strategy for AMZEEQ ® and ZILXI™ 6 • Opportunity for ownable, unique product positioning rooted in novel delivery (MST TM ) and first - to - market topical minocycline Brand Positioning and Messaging • Untapped opportunity in consumer space • Target gen Z (age 9 - 24) consumers digitally for AMZEEQ • Target millennials (age 30 - 50) via traditional media for ZILXI Consumer Activation • Use prescribing behavior as focused guide for HCP targeting • Synergistic cross - brand footprint for efficient sales activity Smart Deployment • Ensure broad access, manage corporate gross - to - net and limit patient out - of - pocket burden Comprehensive Access

The minocycline you know and trust. The tolerability of a gentle foam. The innovation you’ve been waiting for. INDICATIONS AND USAGE AMZEEQ ® (minocycline) topical foam, 4% is indicated for the treatment of inflammatory lesions of non - nodular moderate to severe acne vulgaris in adults and pediatric patients 9 years of age and older. Limitations of Use: This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug - resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, AMZEEQ should be used only as indicated. IMPORTANT SAFETY INFORMATION Contraindications: Persons who have shown hypersensitivity to any of the tetracyclines or any other ingredient in AMZEEQ. Please see Important Safety Information on slide 26 7 The touch of minocycline

AMZEEQ ® Opportunity Large, Growing Acne Market 1. Symphony Health Solutions METYS, data ending DEC’19 - weighted 2. Symphony Health Solutions, MAT ending April ’19 3. AAD. Acne Stats and Facts. https://www.aad.org/media/stats - numbers . Accessed June 1, 2020 4. Symphony Health Solutions IDV Vantage, 2/19 8 $5.1B in sales in 2019 1 >2M active, diagnosed acne patients per year under HCP care 2 Consistently 40 - 50M acne sufferers 3 (of which 10M are moderate - to - severe) Prevalence data yield 60% of acne patients are managed by 5K HCPs 4 22M TRxs in 2019 growing YOY ~5% 1

AMZEEQ ® Market Research Feedback In market research, physicians and health care providers have consistently expected Amzeeq to disrupt the acne market and displace a broad range of products. 1. Source: Data on File, Foamix Pharmaceuticals, 2019. 9 “It’s quite unique in that it’s a minocycline topical; I don’t like a lot of the minocycline oral [products], but minocycline topical – I can certainly get excited about.” US Dermatologist(s), Core Visual Aid Market Research, September 2019 “This is new and exciting. Not your grandfather’s acne medication!” - KOL MD, Amzeeq Advisory Board May 2019 “No one has done it (topical tetracycline) like this before. It’s a big step – pretty powerful.” US Dermatologist(s), Creative Market Research, June 2019

AMZEEQ ® Prescription Volume Performance • Rapid uptake of Amzeeq reflected in strong Rx performance in Q1 pre COVID - 19 • Implemented virtual sales and speaker efforts during state and local stay - at - home orders to stabilize business • As statewide and county restrictions have lifted , direct selling efforts have resumed resulting in monthly Rx growth surpassing pre - COVID levels Symphony METYS – LTD as of 1/10/20 to present 10 COVID - 19 Shutdown 3,035 8,120 8,819 5,504 7,050 9,040 10,337 11,394 0 2,000 4,000 6,000 8,000 10,000 12,000 2020-1 2020-2 2020-3 2020-4 2020-5 2020-6 2020-7 2020-8 Amzeeq TRxs

AMZEEQ ® Target Penetration and Productivity Launch - to - date (LTD), 62% of platinum targets have prescribed at least 1 Rx and on average prescribed 25 Rx’s 11 45% 62% 49% 0% 10% 20% 30% 40% 50% 60% 70% Gold Platinum Total Target Penetration LTD* 14 25 17 0 5 10 15 20 25 30 Gold Platinum Total Prescriber Productivity by Segment (Prescriptions LTD) Source: Symphony Vantage Prescriber Insights – LTD as of 1/10/20 to present; Prescriber Segment 3Q Target List, AMZEEQ ® Weekly TRx by Segment LTD as of 8/14/20 * Percent of target segment that has prescribed since launch, as of August 28 th , 2020
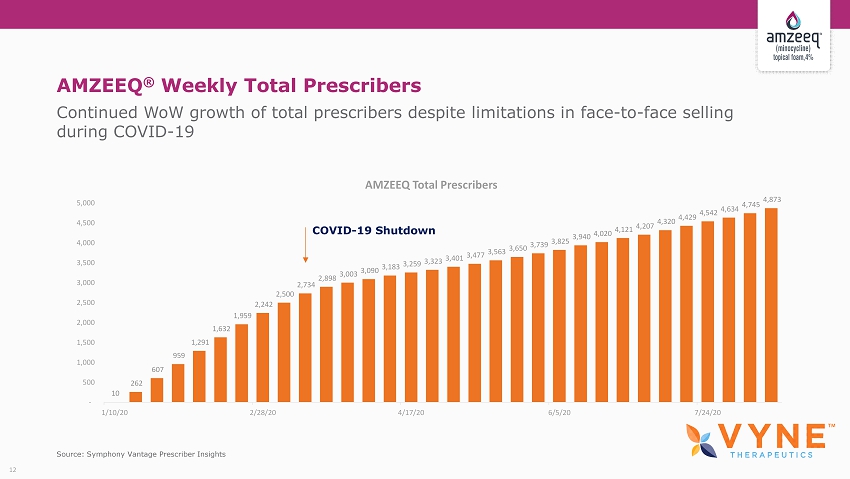
AMZEEQ ® Weekly Total Prescribers Continued WoW growth of total prescribers despite limitations in face - to - face selling during COVID - 19 Source: Symphony Vantage Prescriber Insights 12 10 262 607 959 1,291 1,632 1,959 2,242 2,500 2,734 2,898 3,003 3,090 3,183 3,259 3,323 3,401 3,477 3,563 3,650 3,739 3,825 3,940 4,020 4,121 4,207 4,320 4,429 4,542 4,634 4,745 4,873 - 500 1,000 1,500 2,000 2,500 3,000 3,500 4,000 4,500 5,000 1/10/20 2/28/20 4/17/20 6/5/20 7/24/20 AMZEEQ Total Prescribers COVID - 19 Shutdown

AMZEEQ ® Market Access ~63% of Covered Lives with Additional Contract Talks Ongoing * Includes Express Scripts, EnvisionRx, OptumRx Source: 2020 Managed Markets Insight & Technology, LLC. 13 • Approximately 63% of commercial lives with covered or better status* • Ongoing discussions with payors are productive and in process. Covered 63% In Process 37% Covered vs. Non - Covered

14 Please see Important Safety Information on slide 27 ZILXI is indicated for the treatment of inflammatory lesions of rosacea in adults. Limitations of Use: This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug - resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ZILXI should be used only as indicated. Indication

ZILXI™ (FMX103) Clinical Results: Highlights 1. Co - primary endpoint: Investigator's Global Assessment (IGA) Treatment Success at W eek 12 [Score Clear (0) or Almost Clear (1) ]; Significantly greater number of subjects receiving FMX103 achieved IGA treatment success defined as an IGA score of 0 (clear) or 1 (almost clear) a nd at least a 2 - grade improvement at Week 12 when compared to vehicle treatment, (50.6% vs.41.0%; p<0.001; integrated summary of efficacy), respect iv ely. 2. ZILXI Prescribing Information, June 2020. 3. Study FX2016 - 13, data on file, Foamix Pharmaceuticals. 4. Assessment of facial local tolerability using a 5 - point severity scale in studies FX2016 - 11 and FX2016 - 12 double - blind studies, integrated summary of safety. 15 Baseline IGA 3 “Moderate” Week 12 IGA 1 “Almost Clear” Efficacy: • 51% of patients treated with ZILXI were assessed to be clear or almost clear at week 12 1,2 • 83% reduction in lesion count and 82% achieved IGA treatment success after 52 weeks of therapy 3 Safety: • Cutaneous TEAEs occurred in <1% of subjects in the ZILXI treatment groups2 • ~45% of patients’ erythema (redness) was clear or almost clear at week 12 in facial local tolerability assessments 2,4

ZILXI Opportunity 1. Symphony Health Solutions METYS, data ending DEC’19 - weighted 2. Kantar Media, Jan. 2015 thru Dec. 2018 3. FMX103 Demand Study, Consumer Arm June 2019 4. Symphony Health unprojected patient claims data Period of Analysis – Jan 2015 – Dec 2018 16 Large, Unsatisfied Rosacea Market 4.4M TRxs in 2019 1 >$1B in sales in 2019 1 98% of competitor promotional spend on HCPs 2 73% of patients indicate that they are likely to seek a better solution than their current treatment 3 70% switch or discontinue Rosacea therapy after first diagnosis. 4 Over

ZILXI Market Research: Intent to Prescribe • 76% of physicians are likely to prescribe ZILXI based on blinded product profile and 8.5 out of 10 are likely to use after hearing the complete ZILXI promotional story. Source: Ipsos HCP ATU September 2020 Topline Report Q630. How likely would you be to prescribe Product X for patients with moderate - to - severe papulopustular rosacea? 17 Physicians Likely to Prescribe 2% 4% 19% 49% 27% Definitely would prescribe Probably would prescribe Might or might not prescribe Probably would not prescribe Definitely would not prescribe 8.5 0 2 4 6 8 10 Likehood to Use Likelihood to Use After Hearing Complete ZILXI Story Source: Zilxi Core Visual Aid Testing, July 2020

Portfolio Synergies Complimentary Patient Types and Call Points 18 COMMON STRATEGIES Similar HCP Targets Focused Positioning Predictive Analytics Consumer Outreach Broad Market Access ACNE PATIENTS, AGE 13 - 24: bacteria and in f la mm a t i o n ROSACEA PATIENTS, AGE 30 - 60: inflammatory lesions

Layering ZILXI™ Targeting with AMZEEQ ® Sales Efforts 19 Zilxi™ targets are already being called on 87% Amzeeq Only 40% Zilxi Only 6% Both 54% ~6700 Unique Targets of diagnosed patient volume captured within target universe 75% High volume prescribers captured without field footprint alternative ~85%

FCD105 Combines the bacteriostatic and anti - inflammatory properties of minocycline with the comedolytic, antiproliferative and anti - inflammatory properties of adapalene 3% minocycline, 0.3% adapalene combination foam formulation being investigated for the treatment of moderate - to - severe acne vulgaris in patients 12 years of age and older. 20 • 3 - week dermal toxicity complete • 12 - week dermal toxicity started April 2019 • Phase 2 started September 2019 • Phase 2 completed June 2020 Development Milestones :

FCD105 Commercial Opportunity - Adapalene is one of the most widely prescribed retinoids available for acne therapy. - Adapalene and its combination products represent a 1.7M annual Rx opportunity, with branded adapalene products representing ~15% of total acne branded market. Source: Symphony Health Solutions METYS, data ending 5/15/20, acne weighted market 21 $630M Adapalene $ Volume 2019 1.7M Adapalene TRx Volume 2019

FCD105 Study Design (Study FX2016 - 40*) *A Prospective, Multicenter, Randomized, Double - Blind, Vehicle - Controlled Phase 2 Study to Evaluate the Safety and Efficacy of a Combination of Minocycline and Adapalene Topical Foam Formulation for the Treatment of Acne Vulgaris (Study FX2016 - 40) 22 • Subjects aged 12 years or older. • 20 - 50 inflammatory lesions. • 25 - 100 non - inflammatory lesions. • Score of 3 “Moderate” or 4 “Severe” on 5 point IGA scale. • No more than 2 active nodules on the face. Key Inclusion Criteria: Subjects randomized 5:4:4:3 to either combination, monads or vehicle respectively; Planned N=400 from ~35 US sites FCD105 Foam, N=125 3% Minocycline Foam, N=100 0.3% Adapalene Foam, N=100 Vehicle Foam, N=75 Baseline Week 4 Week 8 Week 12 (End of Treatment) Week 16 (Safety Follow - up) . Co - Primary Efficacy Endpoints: • Absolute change from Baseline in inflammatory and non - inflammatory lesion counts at Week 12. • Proportion of subjects (%) with IGA score of 0/1 (“Clear” or “Minimal”) at Week 12. Safety Assessments: • Treatment Emergent Adverse Events. • Local skin tolerability Assessments (burning/stinging, itching, dryness, scaling, erythema and hyperpigmentation). • Vital signs. • Physical Examination. Secondary Efficacy Endpoints: • Percent change from Baseline in the inflammatory and non - inflammatory lesion counts at Weeks 12, 8 and 4. • IGA Treatment Success of IGA score of 0 or 1 and at least a 2 - grade improvement (decrease) at Weeks 8 and 4. • FCD105 vs. 0.3% adapalene foam, and FCD105 vs. 3% minocycline foam for all co - primary endpoints at Week 12. IGA: Investigator’s Global Assessment

Co - Primary Efficacy Endpoints at Week 12: Absolute Change in Inflammatory & Non - inflammatory Lesion Counts; IGA0/1 Treatment Success (ITT population) 1: Cochran - Mantel - Haenszel test stratified by analysis center. P - value is for the null hypothesis that the Risk Ratio equals 1. 2: P - value obtained from an ANCOVA model with treatment as a main effect, baseline (non)inflammatory lesion count as a covariate, a nd analysis center as a blocking factor. 23 • FCD105 treatment group was statistically superior to Vehicle and 0.3% Adapalene treatment groups at Week 12 for IGA Treatment Su ccess (IGA0/1). • FCD105 treatment group was statistically superior to Vehicle and 0.3% Adapalene treatment groups at Week 12 for reduction in inf lammatory lesions. • FCD105 treatment group was statistically superior to 0.3% Adapalene and 3% Minocycline treatment groups at Week 12 for reduction in non - inflammatory lesions.

Safety: Local Skin Tolerance Assessments (LSTA, Safety Population) Summary of LSTA at Week 12 (Severity Score of 0 “None” or 1 “Mild”) 1 1: Observed cases. 24 • 93% of subjects or greater who received FCD105 treatment had either “None” or “Mild” local skin tolerance scores at Week 12. • In general, subjects receiving 0.3% adapalene experienced lower overall tolerability scores compared to the other treatment g rou ps. • Treatment emergent adverse events were few in type and frequency; most were mild in severity and no serious adverse events were reported. • Subject discontinuations due to a TEAE were low.

Summary • Continued momentum demonstrated as company executes against business plan o AMZEEQ script trends have recovered and are growing on a week/week basis o Prescriber trends are robust and recent formulary addition strengthens coverage o FDA approval of ZILXI in rosacea complements our existing commercial infrastructure • FCD105 showed highly statistically significant improvement in disease burden versus vehicle foam for absolute change in inflammatory lesion count and IGA treatment success (IGA0/1) at Week 12. Numerical superiority was demonstrated between FCD105 and Vehicle for absolute change in non - inflammatory lesion counts at Week 12. o Numerical superiority of FCD105 over both 3% minocycline foam and 0.3% adapalene foam was observed across all endpoints with majority of comparisons being statistically significant • Treatment emergent adverse events were few in type and frequency; most were mild in severity and no serious adverse events were reported, and subject discontinuations due to a TEAE were low. • ≥93% of subjects receiving FCD105 had scores of 0 “none” or 1 “mild” for local skin tolerability assessments at Week 12. • Data is suggestive of a potential best - in - class treatment of moderate - to - severe acne vulgaris. 25

AMZEEQ ® (minocycline) topical foam, 4% Indication and Important Safety Information 26 INDICATIONS AND USAGE AMZEEQ™ (minocycline) topical foam, 4% is indicated for the treatment of inflammatory lesions of non - nodular moderate to severe acne vulgaris in adults and pediatric patients 9 years of age and older. Limitations of Use : This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug - resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, AMZEEQ should be used only as indicated. IMPORTANT SAFETY INFORMATION Contraindications: Persons who have shown hypersensitivity to any of the tetracyclines or any other ingredient in AMZEEQ. Warnings and Precautions Flammability: The propellant in AMZEEQ is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. AMZEEQ is a topical foam. While systemic absorption of AMZEEQ is low, and serious adverse reactions were not seen in clinical studies, the following adverse reactions associated with oral minocycline should be considered: • Teratogenic effects, inhibition of bone growth & permanent tooth discoloration: Use during the second and third trimesters of pregnancy, infancy and childhood up to the age of 8 years may cause permanent discoloration of the teeth (yellow - gray - brown) and reversible inhibition of bone growth. • Clostridium difficile associated diarrhea (CDAD): If CDAD occurs, discontinue AMZEEQ. • Hepatotoxicity & metabolic effects: If renal impairment exists or if liver injury suspected, discontinue AMZEEQ. • Central nervous system effects: Patients experiencing light - headedness, dizziness or vertigo should be cautioned about driving vehicles or operating heavy machinery. • Intracranial hypertension: Clinical manifestations include headache, blurred vision, diplopia, and vision loss. Discontinue AMZEEQ immediately if symptoms occur. • Autoimmune syndromes: Symptoms may be manifested by fever, rash, arthralgia, and malaise. Discontinue AMZEEQ immediately if symptoms occur. • Photosensitivity: Patients should minimize or avoid exposure to natural or artificial sunlight while using AMZEEQ. Advise patients to discontinue treatment with AMZEEQ at the first evidence of sunburn. • Hypersensitivity reactions: Discontinue AMZEEQ immediately if symptoms of anaphylaxis, serious skin reactions, erythema multiforme, and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome occur. • Tissue hyperpigmentation: Discoloration of organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (teeth, mucosa, alveolar bone), sclerae and heart valves. • Superinfection: Overgrowth of non - susceptible organisms, including fungi. If superinfection occurs, discontinue AMZEEQ and institute appropriate therapy. Adverse Reactions: The most common adverse reaction reported during clinical trials of AMZEEQ was headache. Please visit www.amzeeq.com for full Prescribing Information. To report side effects of prescription drugs to the FDA, visit http://www.fda.gov/medwatchor call 1 - 800 - FDA - 1088.

ZILXI™ (minocycline) topical foam, 1.5% Indication and Important Safety Information 27 INDICATION ZILXI TM (minocycline) topical foam, 1.5% is a tetracycline - class drug indicated for the treatment of inflammatory lesions of rosacea in adults. Limitations of Use: This formulation of minocycline has not been evaluated in the treatment of infections. To reduce the development of drug - resistant bacteria as well as to maintain the effectiveness of other antibacterial drugs, ZILXI should be used only as indicated. IMPORTANT SAFETY INFORMATION Contraindications: Persons who have shown hypersensitivity to any of the tetracyclines or any other ingredient in ZILXI. Warnings and Precautions Flammability: The propellant in ZILXI is flammable. Instruct the patient to avoid fire, flame, and smoking during and immediately following application. ZILXI is a topical foam. While systemic absorption of ZILXI is low, and serious adverse reactions were not seen in clinical studies, the following adverse reactions associated with oral minocycline should be considered: • Teratogenic effects, inhibition of bone growth & permanent tooth discoloration: Use during the second and third trimesters of pregnancy, infancy and childhood up to the age of 8 years may cause permanent discoloration of the teeth (yellow - gray - brown) and reversible inhibition of bone growth. • Clostridioides difficile associated diarrhea (CDAD): If CDAD occurs, discontinue ZILXI. • Hepatotoxicity & metabolic effects: If renal impairment exists or if liver injury suspected, discontinue ZILXI. • Central nervous system effects: Patients experiencing light - headedness, dizziness or vertigo should be cautioned about driving vehicles or operating heavy machinery. • Intracranial hypertension: Clinical manifestations include headache, blurred vision, diplopia, and vision loss. Discontinue ZILXI immediately if symptoms occur. • Autoimmune syndromes: Symptoms may be manifested by fever, rash, arthralgia, and malaise. Discontinue ZILXI immediately if symptoms occur. • Photosensitivity: Patients should minimize or avoid exposure to natural or artificial sunlight while using ZILXI. Advise patients to discontinue treatment with ZILXI at the first evidence of sunburn. • Hypersensitivity reactions: Discontinue ZILXI immediately if symptoms of anaphylaxis, serious skin reactions, erythema multiforme, and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome occur. • Tissue hyperpigmentation: Discoloration of organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (teeth, mucosa, alveolar bone), sclerae and heart valves. • Superinfection: Overgrowth of non - susceptible organisms, including fungi. If superinfection occurs, discontinue ZILXI and institute appropriate therapy. Adverse Reactions: The most common adverse reaction reported during clinical trials of ZILXI was diarrhea. Please visit www.zilxi.com for full prescribing information.

Investor Presentation September 2020
