Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Intra-Cellular Therapies, Inc. | d831321dex991.htm |
| 8-K - 8-K - Intra-Cellular Therapies, Inc. | d831321d8k.htm |
Exhibit 99.2
Overview
We are a biopharmaceutical company focused on the discovery, clinical development and commercialization of innovative, small molecule drugs that address underserved medical needs primarily in neuropsychiatric and neurological disorders by targeting intracellular signaling mechanisms within the central nervous system, or CNS. In December 2019 CAPLYTATM (lumateperone) was approved by the U.S. Food and Drug Administration, or FDA, for the treatment of schizophrenia in adults (42 mg/day) and we initiated the commercial launch of CAPLYTA in late March of 2020. In support of our commercialization efforts, we hired a national sales force consisting of approximately 240 sales representatives. As used in this prospectus supplement, “CAPLYTA” refers to lumateperone approved by the FDA for the treatment of schizophrenia in adults, and “lumateperone” refers to, where applicable, CAPLYTA as well as lumateperone for the treatment of indications beyond schizophrenia.
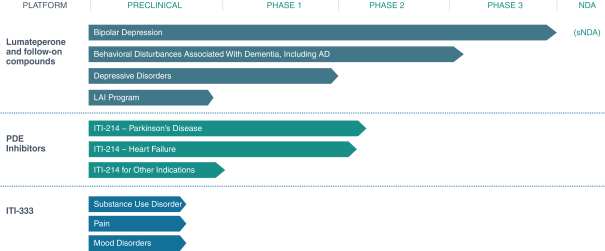
Lumateperone is also in Phase 3 clinical development as a novel treatment for bipolar depression. Our lumateperone bipolar depression Phase 3 clinical program consists of three monotherapy studies and one adjunctive study. In September 2020, we announced positive topline results from our third Phase 3 clinical trial, Study 402, conducted globally, evaluating lumateperone as adjunctive therapy to lithium or valproate in the treatment of major depressive episodes associated with Bipolar I or Bipolar II disorder. In Study 402, once daily lumateperone 42 mg met the primary endpoint for improvement in depression as measured by change from baseline versus placebo on the Montgomery-Åsberg Depression Rating Scale, or MADRS, total score (p=0.0206; effect size = 0.27). Lumateperone 42 mg also met the key secondary endpoint, the Clinical Global Impression Scale for Bipolar for Severity of Illness, or CGI-BP-S, Depression Score (p=0.0082; effect size = 0.31). The lower lumateperone dose, 28 mg, showed a trend for a dose-related improvement in symptoms of depression but the results did not reach statistical significance. Previously, in July 2019, we announced topline results from our first monotherapy study, Study 401, conducted in the U.S., and our second monotherapy study, Study 404, conducted globally, evaluating lumateperone as monotherapy in the treatment of major depressive episodes associated with Bipolar I or Bipolar II disorder. In Study 404, lumateperone 42 mg met the primary endpoint for improvement in depression as measured by change from baseline versus placebo on the MADRS total score (p<0.0001; effect size = 0.56). These benefits were statistically significant in both Bipolar I and Bipolar II patients. Study 404 also met its key secondary endpoint, CGI-BP-S Total Score (p<0.001; effect size = 0.46). Study 401 tested two doses of lumateperone, 42 mg and 28 mg along with placebo. In this trial, neither dose of lumateperone met the primary endpoint of statistical separation from placebo as measured by change from baseline on the MADRS total score. There was a high placebo response in this trial. Lumateperone was generally well-tolerated in all three bipolar depression studies, with a favorable safety profile. The rates of discontinuation due to treatment emergent adverse events for both doses of lumateperone were low. In addition, while our Phase 3 bipolar depression trials were powered for the overall patient population and not powered for subpopulation analyses, statistically significant benefit versus placebo was seen in the subgroup of patients with Bipolar I and Bipolar II disorder in Study 404 and in patients with Bipolar I disorder in Study 402, but the Bipolar II subgroup was not statistically significant in Study 402. Based on Study 402 and Study 404, in late 2020 or early 2021 we expect to submit a supplemental new drug application, or sNDA, to the FDA for potential regulatory approval of lumateperone for the treatment of bipolar depression in patients with Bipolar I or II disorder as monotherapy and adjunctive therapy.
1
We are also pursuing clinical development of lumateperone for the treatment of additional CNS diseases and disorders. We believe lumateperone may have utility for treating agitation, aggression and sleep disturbances in diseases that include dementia, Alzheimer’s disease, or AD, Huntington’s disease and autism spectrum disorders. At a dose of 42 mg, lumateperone has been shown effective in treating the symptoms associated with schizophrenia, and we believe this dose may merit further investigation for the treatment of bipolar disorder, depressive disorders and other neuropsychiatric diseases.
Within the lumateperone portfolio, we are also developing a long-acting injectable formulation to provide more treatment options to patients suffering from mental illness. We have completed the preclinical development of a long-acting injectable formulation and plan to initiate a Phase 1 clinical trial by the end of 2020. Given the encouraging tolerability data to date with oral lumateperone, we believe that a long-acting injectable option, in particular, may lend itself to being an important formulation choice for patients.
We may investigate the use of lumateperone, either on our own or with a partner, as a treatment for agitation, aggression and sleep disturbances in additional diseases that include autism spectrum disorders, depressive disorder, intermittent explosive disorder, non-motor symptoms and motor complications associated with Parkinson’s disease, and post-traumatic stress disorder. We hold exclusive, worldwide commercialization rights to lumateperone and a family of compounds from Bristol-Myers Squibb Company pursuant to an exclusive license.
We have a second major program called ITI-002 that has yielded a portfolio of compounds that selectively inhibit the enzyme phosphodiesterase type 1, or PDE1. PDE1 enzymes are highly active in multiple disease states and our PDE1 inhibitors are designed to reestablish normal function in these disease states. Abnormal PDE1 activity is associated with cellular proliferation and activation of inflammatory cells. Our PDE1 inhibitors ameliorate both of these effects in animal models. We intend to pursue the development of our phosphodiesterase, or PDE, program, for the treatment of several CNS and non-CNS conditions with a focus on diseases where excessive PDE1 activity has been demonstrated and increased inflammation is an important contributor to disease pathogenesis. Our potential disease targets include heart failure, immune system regulation, neurodegenerative diseases, and other non-CNS disorders. ITI-214 is our lead compound in this program. We believe ITI-214 is the first compound in its class to successfully advance into Phase 1 clinical trials. Following the favorable safety and tolerability results in our Phase 1 program, we initiated our development program for ITI-214 for Parkinson’s disease and commenced patient enrollment in the third quarter of 2017 in a Phase 1/2 clinical trial of ITI-214 in patients with Parkinson’s disease to evaluate safety and tolerability in this patient population, as well as motor and non-motor exploratory endpoints. In the fourth quarter of 2018, we announced that the Phase 1/2 clinical trial of ITI-214 has been completed and topline results demonstrated ITI-214 was generally well-tolerated with a favorable safety profile and clinical signs consistent with improvements in motor symptoms and dyskinesias. In addition, in the second quarter of 2020, we announced topline results from Study ITI-214-104, a Phase 1/2 translational study of single ascending doses of ITI-214 in patients with chronic systolic heart failure with reduced ejection fraction. In this study, ITI-214 improved cardiac output by increasing heart contractility and decreasing vascular resistance. Agents that both increase heart contractility (inotropism) and decrease vascular resistance (vasodilation) are called inodilators. Inodilators in current clinical use are associated with the development of arrhythmias, which are abnormal heart rhythms that when serious can impair heart function and lead to mortality. ITI-214, which acts through a novel mechanism of action, was not associated with arrhythmias in this study and was generally well tolerated in all patients.
Our pipeline also includes programs that are focused on advancing drugs for symptomatic and disease modifying treatments for schizophrenia, Parkinson’s disease, AD and other neuropsychiatric and neurodegenerative disorders. We have an ongoing early stage clinical program evaluating a new molecule as a potential treatment for behavioral disturbances in patients with dementia. In addition, ITI-333 is being evaluated as a potential treatment for substance use disorders, pain and psychiatric comorbidities including depression and anxiety. There is a pressing need to develop new drugs to treat opioid addiction and safe, effective, non-addictive treatments to manage pain. We expect to initiate early phase clinical studies with ITI-333 by the end of 2020.
2
We have assembled a management team with significant industry experience to lead the discovery, development and potential commercialization of our product candidates. We complement our management team with a group of scientific and clinical advisors that includes recognized experts in the fields of schizophrenia and other CNS disorders.
Our therapeutic pipeline
Our strategy
Our goal is to discover, develop and commercialize novel small molecule therapeutics for the treatment of CNS diseases and other diseases in order to improve the lives of people suffering from such illnesses. Using our key understanding of intracellular signaling, we seek to accomplish our goal, using our in-house expert drug discovery and clinical development teams, in two ways:
| • | we seek to have the capability to develop first-in-class medications with novel mechanisms that have the potential to treat CNS diseases and other diseases for which there are no previously marketed drugs; and |
| • | we seek to develop drugs that either can differentiate themselves in competitive markets by addressing aspects of CNS diseases and other diseases which are not adequately treated by currently marketed drugs or can be effective with fewer side effects. |
The key elements of our strategy are to:
| • | continue to commercialize CAPLYTA, which has been approved by the FDA for the treatment of schizophrenia in adults, in the United States; |
| • | commercialize lumateperone for the treatment of bipolar depression, if approved by the FDA; |
| • | complete the development of lumateperone for additional neuropsychiatric indications, such as bipolar disorder, behavioral disturbances in dementia, including AD, residual symptoms in schizophrenia and major depressive disorder, or MDD; |
| • | expand the commercial potential of lumateperone by investigating its usefulness in additional neurological areas, such as autism spectrum disorder, and in additional neuropsychiatric indications, such as sleep disorders associated with neuropsychiatric and neurological disorders; |
3
| • | continue to develop PDE inhibitor compounds, such as ITI-214, for the treatment of CNS and other disorders; and |
| • | advance earlier stage product candidates in our pipeline, such as ITI-333, for substance use disorders, pain and psychiatric comorbidities including depression and anxiety. |
4
