Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Turning Point Therapeutics, Inc. | d139828dex992.htm |
| 8-K - FORM 8-K - Turning Point Therapeutics, Inc. | d139828d8k.htm |

TRIDENT-1 Phase 2 Study of Repotrectinib: Early Interim Data Update August 19, 2020 Exhibit 99.1

Statements in this Presentation that are not statements of historical fact are forward-looking statements. Such forward-looking statements include, without limitation, statements regarding our research and clinical development activities, plans and projected timelines, business strategy and plans, regulatory matters, objectives of management for future operations, market size and opportunity, our ability to complete certain milestones and our expectations regarding the relative benefits of our drug candidates versus competitive therapies. Words such as “believe,” “can”, “continue,” “anticipate,” “could,” “estimate,” “plan,” “predict,” “expect,” “intend,” “will,” “may,” “goal,” “upcoming,” “near term”, “milestone”, “potential,” “target” or the negative of these terms or similar expressions are intended to identify forward-looking statements, though not all forward-looking statements necessarily contain these identifying words. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Risks that contribute to the uncertain nature of the forward-looking statements include, without limitation: our preclinical studies and clinical trials may not be successful; regulatory authorities, including the U.S. Food and Drug Administration (FDA) may not agree with our interpretation of the data from clinical trials of our drug candidates; we may decide, or regulatory authorities may require us, to conduct additional clinical trials or to modify our ongoing clinical trials; we may experience delays in the commencement, enrollment, completion or analysis of clinical testing for our drug candidates, or significant issues regarding the adequacy of our clinical trial designs or the execution of our clinical trials may arise, which could result in increased costs and delays, or limit our ability to obtain regulatory approval; our drug candidates may not receive regulatory approval or be successfully commercialized; unexpected adverse side effects or inadequate therapeutic efficacy of our drug candidates could delay or prevent regulatory approval or commercialization; the COVID-19 pandemic may disrupt our business and that of third parties on which we depend, including delaying or otherwise disrupting our research and development activities; and we may not be able to obtain additional financing. These forward-looking statements should not be taken as forecasts or promises nor should they be taken as implying any assurance or guarantee that the assumptions on which such forward-looking statements have been made are correct or exhaustive. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date this Presentation is given. Other risks and uncertainties affecting us are described more fully in our filings with the Securities and Exchange Commission. We undertake no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made. This Presentation discusses drug candidates that are under clinical study and which have not yet been approved for marketing by the U.S. Food and Drug Administration. No representation is made as to the safety or effectiveness of these drug candidates for the use for which such drug candidates are being studied. Forward-Looking Statements
Our Vision Drives our Strategy Pipeline Expansion Clinical Excellence Leadership in Precision Oncology Potential for Best-in-Class Products Addressing Patients with High Unmet Need in TKI-Naïve and Resistance Settings Deep Oncology Development Expertise Rapid Clinical Proof-of-Concept Data Therapeutic Area Focus with Indication Expansion with Synergistic Combinations Mine Existing Macrocyclic Scaffolds New Discovery against Novel or Validated Targets TKI-Pretreated TKI-Naive NSCLC
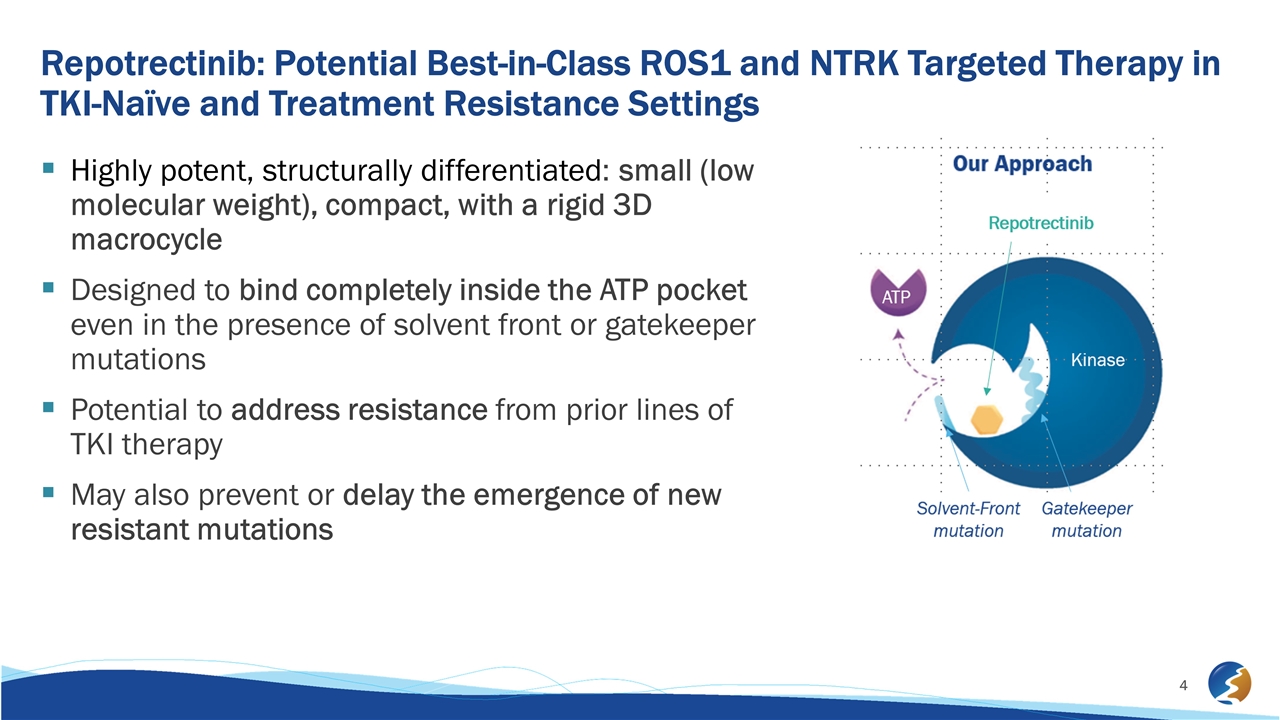
Repotrectinib: Potential Best-in-Class ROS1 and NTRK Targeted Therapy in TKI-Naïve and Treatment Resistance Settings Highly potent, structurally differentiated: small (low molecular weight), compact, with a rigid 3D macrocycle Designed to bind completely inside the ATP pocket even in the presence of solvent front or gatekeeper mutations Potential to address resistance from prior lines of TKI therapy May also prevent or delay the emergence of new resistant mutations
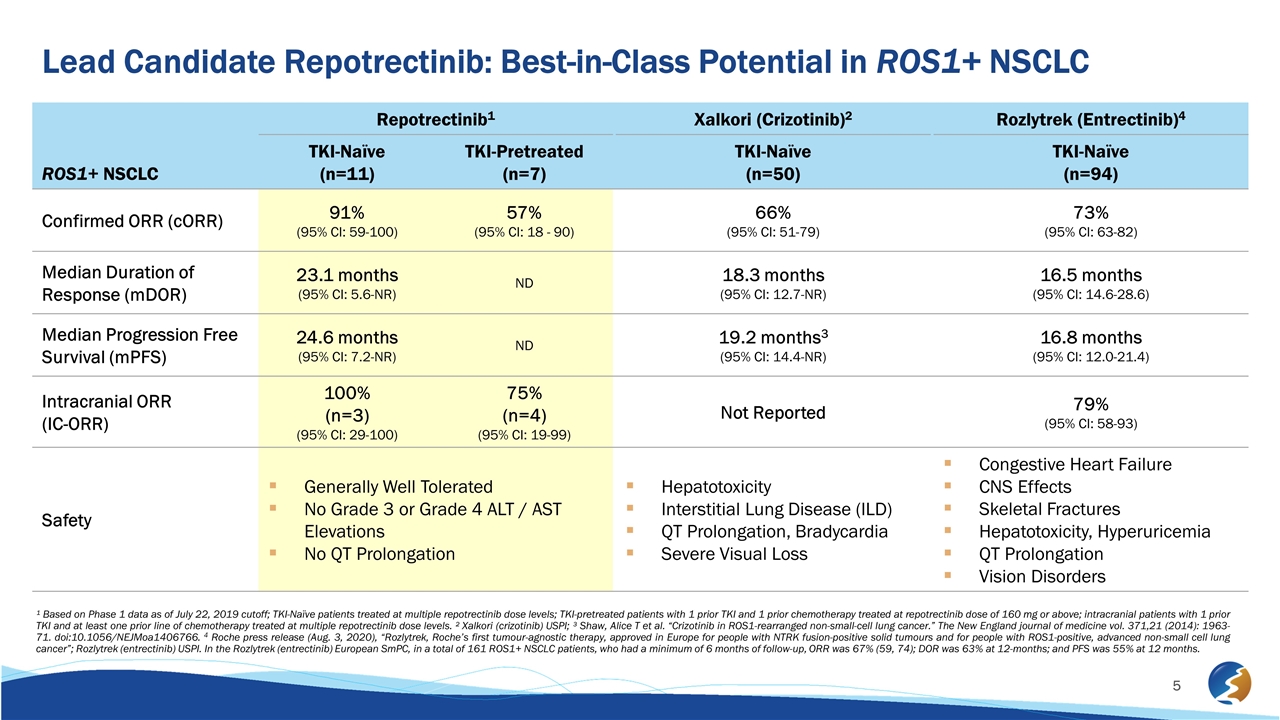
¹ Based on Phase 1 data as of July 22, 2019 cutoff; TKI-Naïve patients treated at multiple repotrectinib dose levels; TKI-pretreated patients with 1 prior TKI and 1 prior chemotherapy treated at repotrectinib dose of 160 mg or above; intracranial patients with 1 prior TKI and at least one prior line of chemotherapy treated at multiple repotrectinib dose levels. ² Xalkori (crizotinib) USPI; ³ Shaw, Alice T et al. “Crizotinib in ROS1-rearranged non-small-cell lung cancer.” The New England journal of medicine vol. 371,21 (2014): 1963-71. doi:10.1056/NEJMoa1406766. 4 Roche press release (Aug. 3, 2020), “Rozlytrek, Roche’s first tumour-agnostic therapy, approved in Europe for people with NTRK fusion-positive solid tumours and for people with ROS1-positive, advanced non-small cell lung cancer”; Rozlytrek (entrectinib) USPI. In the Rozlytrek (entrectinib) European SmPC, in a total of 161 ROS1+ NSCLC patients, who had a minimum of 6 months of follow-up, ORR was 67% (59, 74); DOR was 63% at 12-months; and PFS was 55% at 12 months. ROS1+ NSCLC Repotrectinib1 Xalkori (Crizotinib)2 Rozlytrek (Entrectinib)4 TKI-Naïve (n=11) TKI-Pretreated (n=7) TKI-Naïve (n=50) TKI-Naïve (n=94) Confirmed ORR (cORR) 91% (95% CI: 59-100) 57% (95% CI: 18 - 90) 66% (95% CI: 51-79) 73% (95% CI: 63-82) Median Duration of Response (mDOR) 23.1 months (95% CI: 5.6-NR) ND 18.3 months (95% CI: 12.7-NR) 16.5 months (95% CI: 14.6-28.6) Median Progression Free Survival (mPFS) 24.6 months (95% CI: 7.2-NR) ND 19.2 months3 (95% CI: 14.4-NR) 16.8 months (95% CI: 12.0-21.4) Intracranial ORR (IC-ORR) 100% (n=3) (95% CI: 29-100) 75% (n=4) (95% CI: 19-99) Not Reported 79% (95% CI: 58-93) Safety Generally Well Tolerated No Grade 3 or Grade 4 ALT / AST Elevations No QT Prolongation Hepatotoxicity Interstitial Lung Disease (ILD) QT Prolongation, Bradycardia Severe Visual Loss Congestive Heart Failure CNS Effects Skeletal Fractures Hepatotoxicity, Hyperuricemia QT Prolongation Vision Disorders Lead Candidate Repotrectinib: Best-in-Class Potential in ROS1+ NSCLC
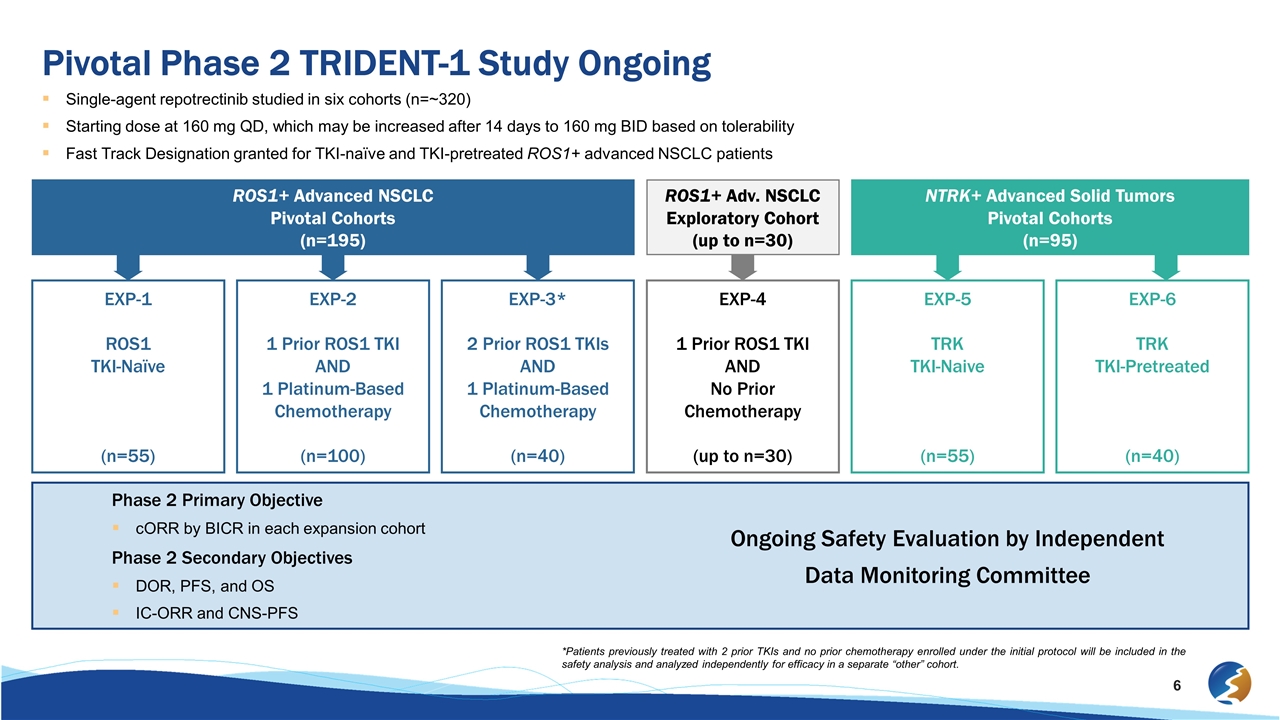
Pivotal Phase 2 TRIDENT-1 Study Ongoing Single-agent repotrectinib studied in six cohorts (n=~320) Starting dose at 160 mg QD, which may be increased after 14 days to 160 mg BID based on tolerability Fast Track Designation granted for TKI-naïve and TKI-pretreated ROS1+ advanced NSCLC patients *Patients previously treated with 2 prior TKIs and no prior chemotherapy enrolled under the initial protocol will be included in the safety analysis and analyzed independently for efficacy in a separate “other” cohort. ROS1+ Advanced NSCLC Pivotal Cohorts (n=195) ROS1+ Adv. NSCLC Exploratory Cohort (up to n=30) NTRK+ Advanced Solid Tumors Pivotal Cohorts (n=95) EXP-1 ROS1 TKI-Naïve (n=55) EXP-2 1 Prior ROS1 TKI AND 1 Platinum-Based Chemotherapy (n=100) EXP-3* 2 Prior ROS1 TKIs AND 1 Platinum-Based Chemotherapy (n=40) EXP-4 1 Prior ROS1 TKI AND No Prior Chemotherapy (up to n=30) EXP-5 TRK TKI-Naive (n=55) EXP-6 TRK TKI-Pretreated (n=40) Phase 2 Primary Objective cORR by BICR in each expansion cohort Phase 2 Secondary Objectives DOR, PFS, and OS IC-ORR and CNS-PFS Ongoing Safety Evaluation by Independent Data Monitoring Committee
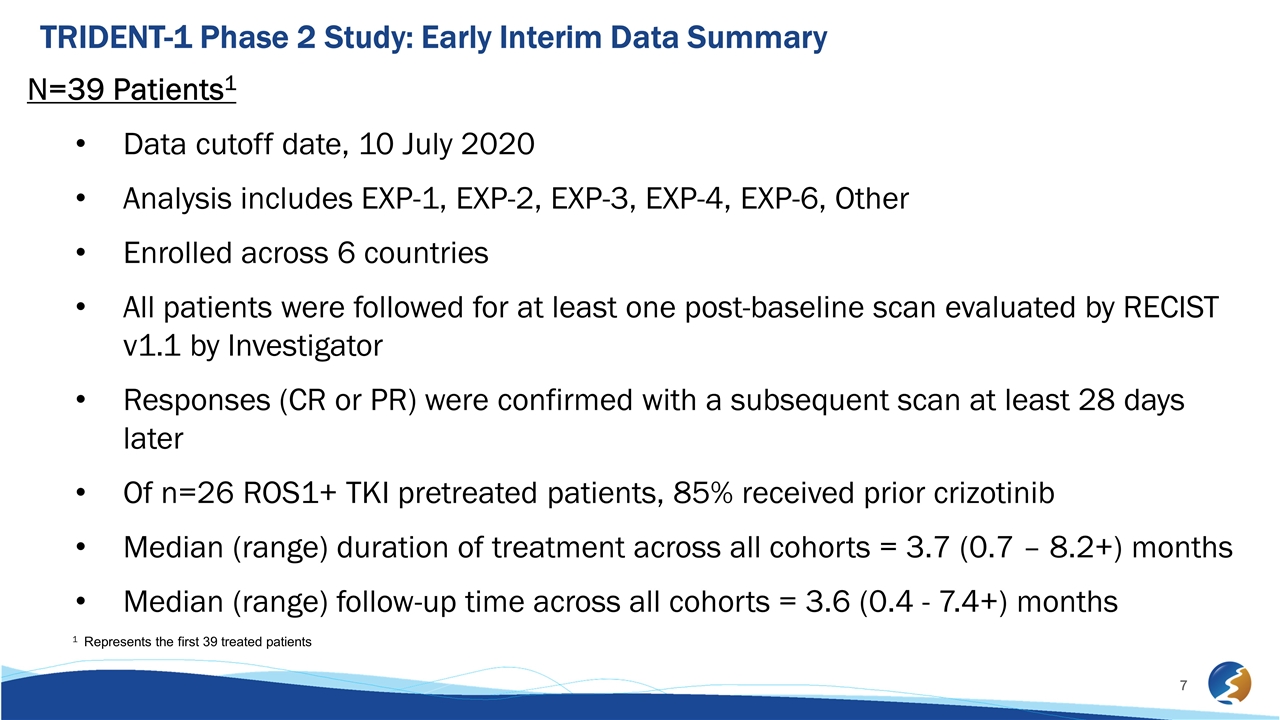
TRIDENT-1 Phase 2 Study: Early Interim Data Summary N=39 Patients1 Data cutoff date, 10 July 2020 Analysis includes EXP-1, EXP-2, EXP-3, EXP-4, EXP-6, Other Enrolled across 6 countries All patients were followed for at least one post-baseline scan evaluated by RECIST v1.1 by Investigator Responses (CR or PR) were confirmed with a subsequent scan at least 28 days later Of n=26 ROS1+ TKI pretreated patients, 85% received prior crizotinib Median (range) duration of treatment across all cohorts = 3.7 (0.7 – 8.2+) months Median (range) follow-up time across all cohorts = 3.6 (0.4 - 7.4+) months 1 Represents the first 39 treated patients
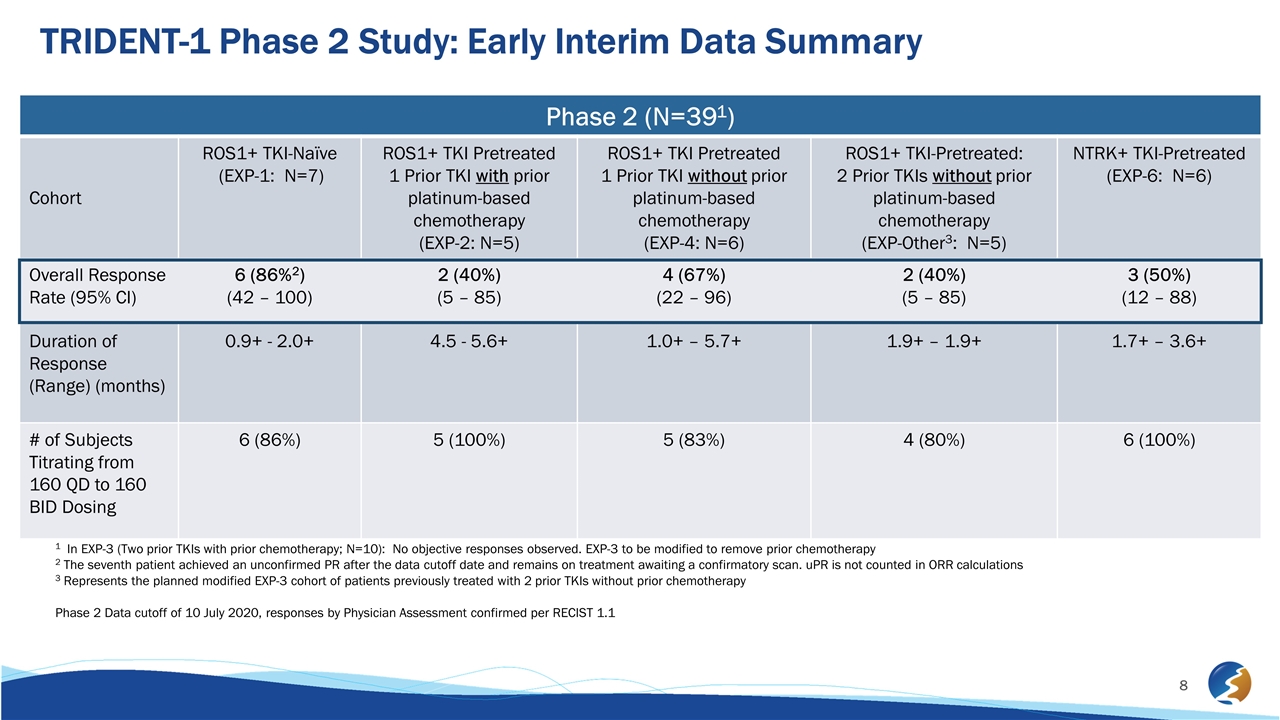
TRIDENT-1 Phase 2 Study: Early Interim Data Summary Phase 2 (N=391) Cohort ROS1+ TKI-Naïve (EXP-1: N=7) ROS1+ TKI Pretreated 1 Prior TKI with prior platinum-based chemotherapy (EXP-2: N=5) ROS1+ TKI Pretreated 1 Prior TKI without prior platinum-based chemotherapy (EXP-4: N=6) ROS1+ TKI-Pretreated: 2 Prior TKIs without prior platinum-based chemotherapy (EXP-Other3: N=5) NTRK+ TKI-Pretreated (EXP-6: N=6) Overall Response Rate (95% CI) 6 (86%2) (42 – 100) 2 (40%) (5 – 85) 4 (67%) (22 – 96) 2 (40%) (5 – 85) 3 (50%) (12 – 88) Duration of Response (Range) (months) 0.9+ - 2.0+ 4.5 - 5.6+ 1.0+ – 5.7+ 1.9+ – 1.9+ 1.7+ – 3.6+ # of Subjects Titrating from 160 QD to 160 BID Dosing 6 (86%) 5 (100%) 5 (83%) 4 (80%) 6 (100%) 1 In EXP-3 (Two prior TKIs with prior chemotherapy; N=10): No objective responses observed. EXP-3 to be modified to remove prior chemotherapy 2 The seventh patient achieved an unconfirmed PR after the data cutoff date and remains on treatment awaiting a confirmatory scan. uPR is not counted in ORR calculations 3 Represents the planned modified EXP-3 cohort of patients previously treated with 2 prior TKIs without prior chemotherapy Phase 2 Data cutoff of 10 July 2020, responses by Physician Assessment confirmed per RECIST 1.1
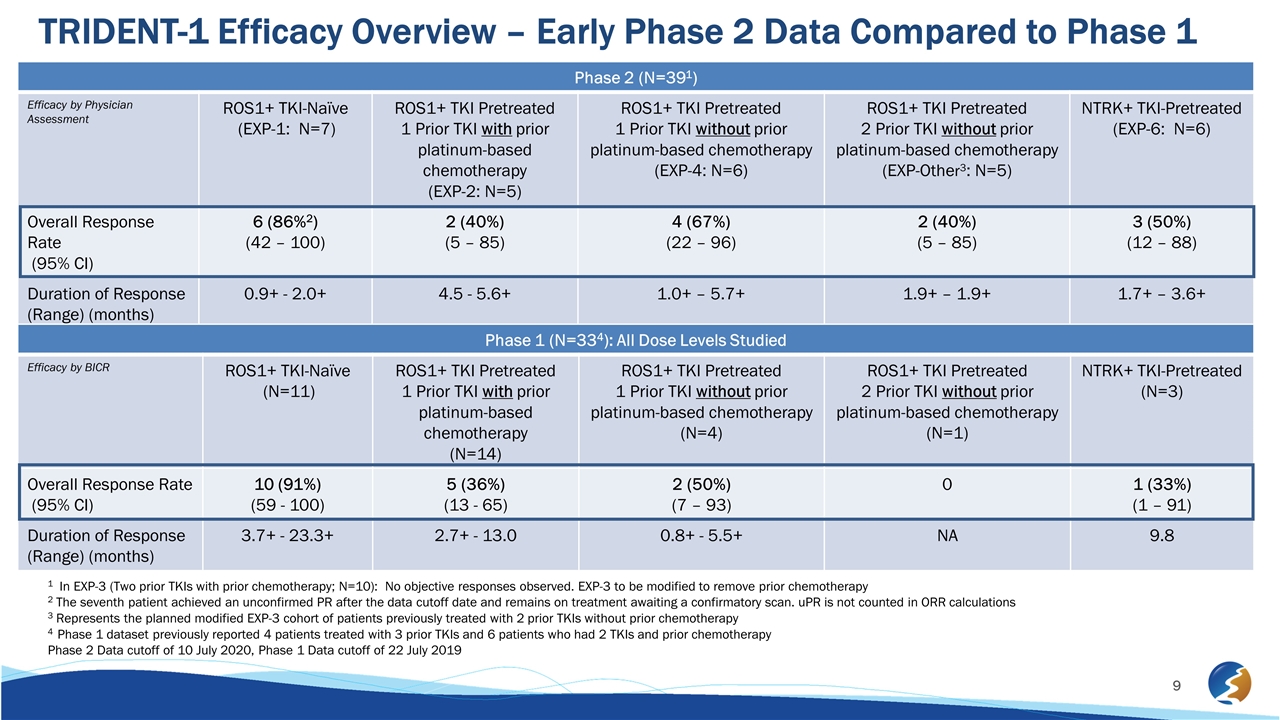
TRIDENT-1 Efficacy Overview – Early Phase 2 Data Compared to Phase 1 Phase 2 (N=391) Efficacy by Physician Assessment ROS1+ TKI-Naïve (EXP-1: N=7) ROS1+ TKI Pretreated 1 Prior TKI with prior platinum-based chemotherapy (EXP-2: N=5) ROS1+ TKI Pretreated 1 Prior TKI without prior platinum-based chemotherapy (EXP-4: N=6) ROS1+ TKI Pretreated 2 Prior TKI without prior platinum-based chemotherapy (EXP-Other3: N=5) NTRK+ TKI-Pretreated (EXP-6: N=6) Overall Response Rate (95% CI) 6 (86%2) (42 – 100) 2 (40%) (5 – 85) 4 (67%) (22 – 96) 2 (40%) (5 – 85) 3 (50%) (12 – 88) Duration of Response (Range) (months) 0.9+ - 2.0+ 4.5 - 5.6+ 1.0+ – 5.7+ 1.9+ – 1.9+ 1.7+ – 3.6+ Phase 1 (N=334): All Dose Levels Studied Efficacy by BICR ROS1+ TKI-Naïve (N=11) ROS1+ TKI Pretreated 1 Prior TKI with prior platinum-based chemotherapy (N=14) ROS1+ TKI Pretreated 1 Prior TKI without prior platinum-based chemotherapy (N=4) ROS1+ TKI Pretreated 2 Prior TKI without prior platinum-based chemotherapy (N=1) NTRK+ TKI-Pretreated (N=3) Overall Response Rate (95% CI) 10 (91%) (59 - 100) 5 (36%) (13 - 65) 2 (50%) (7 – 93) 0 1 (33%) (1 – 91) Duration of Response (Range) (months) 3.7+ - 23.3+ 2.7+ - 13.0 0.8+ - 5.5+ NA 9.8 1 In EXP-3 (Two prior TKIs with prior chemotherapy; N=10): No objective responses observed. EXP-3 to be modified to remove prior chemotherapy 2 The seventh patient achieved an unconfirmed PR after the data cutoff date and remains on treatment awaiting a confirmatory scan. uPR is not counted in ORR calculations 3 Represents the planned modified EXP-3 cohort of patients previously treated with 2 prior TKIs without prior chemotherapy 4 Phase 1 dataset previously reported 4 patients treated with 3 prior TKIs and 6 patients who had 2 TKIs and prior chemotherapy Phase 2 Data cutoff of 10 July 2020, Phase 1 Data cutoff of 22 July 2019
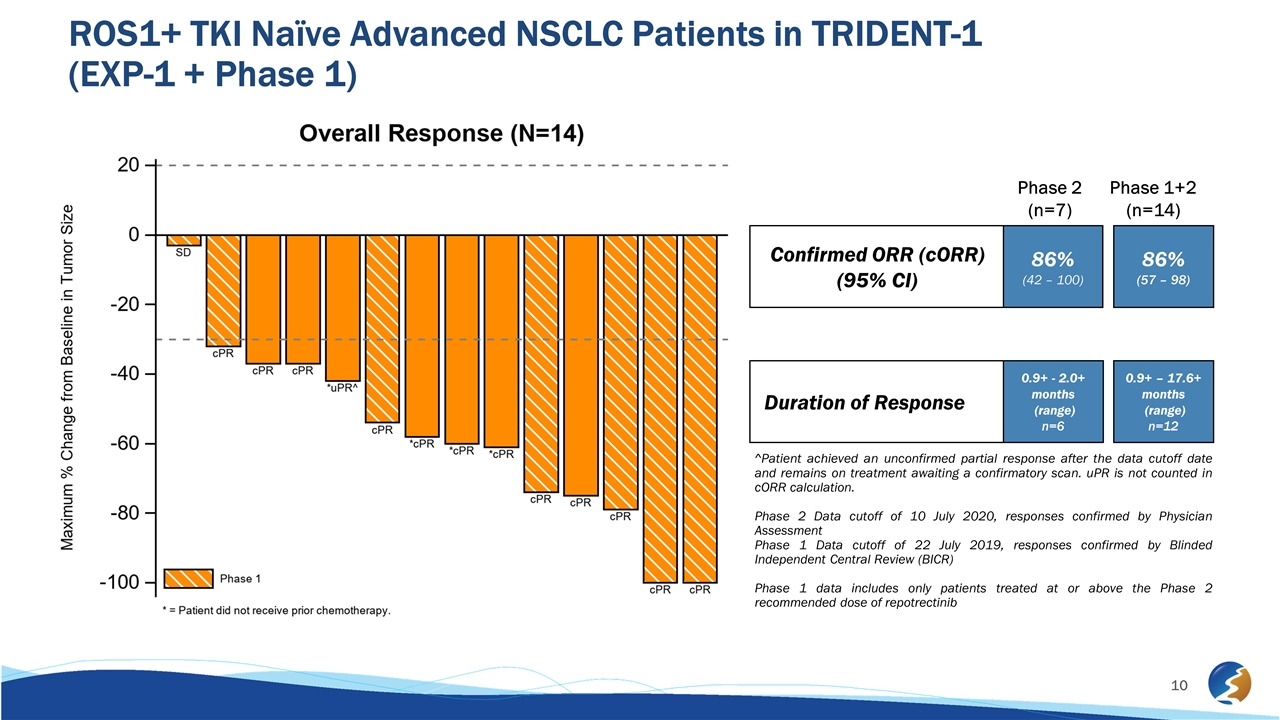
ROS1+ TKI Naïve Advanced NSCLC Patients in TRIDENT-1 (EXP-1 + Phase 1) ^Patient achieved an unconfirmed partial response after the data cutoff date and remains on treatment awaiting a confirmatory scan. uPR is not counted in cORR calculation. Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib Confirmed ORR (cORR) (95% CI) Duration of Response 0.9+ - 2.0+ months (range) n=6 0.9+ – 17.6+ months (range) n=12 86% (42 – 100) 86% (57 – 98) Phase 2 (n=7) Phase 1+2 (n=14)
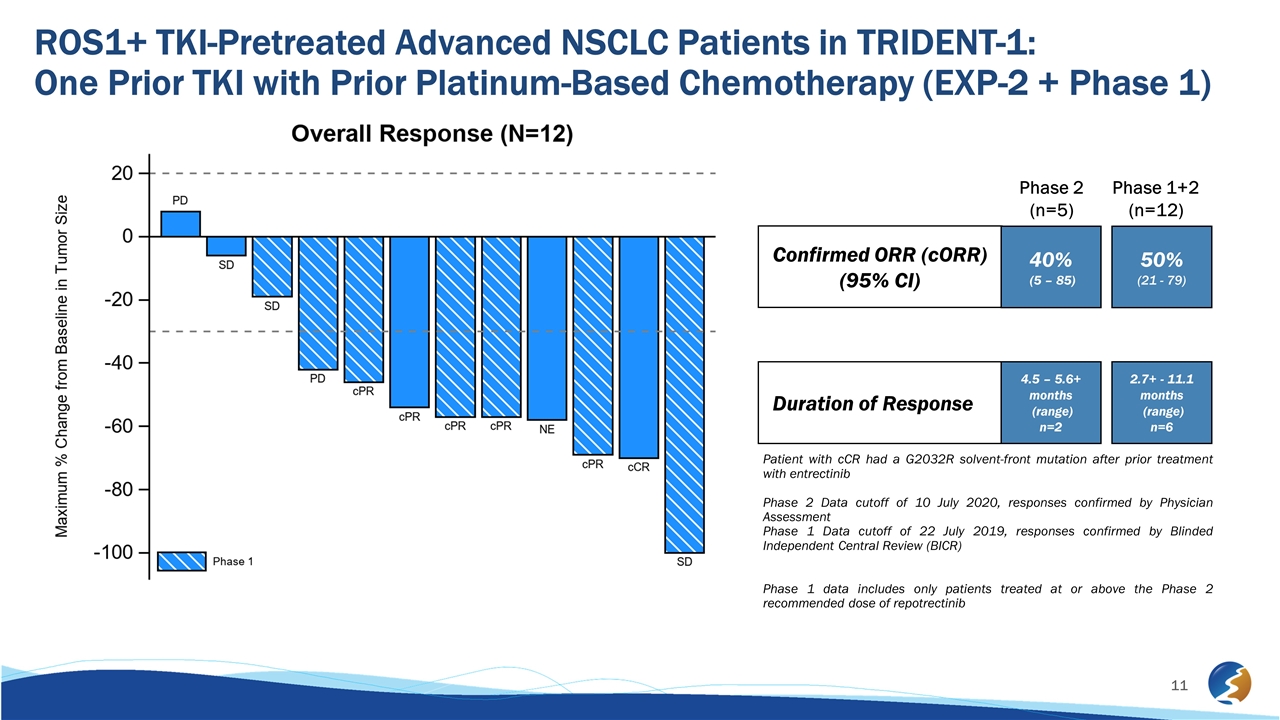
ROS1+ TKI-Pretreated Advanced NSCLC Patients in TRIDENT-1: One Prior TKI with Prior Platinum-Based Chemotherapy (EXP-2 + Phase 1) Patient with cCR had a G2032R solvent-front mutation after prior treatment with entrectinib Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib Confirmed ORR (cORR) (95% CI) Duration of Response 40% (5 – 85) 4.5 – 5.6+ months (range) n=2 50% (21 - 79) 2.7+ - 11.1 months (range) n=6 Phase 2 (n=5) Phase 1+2 (n=12)
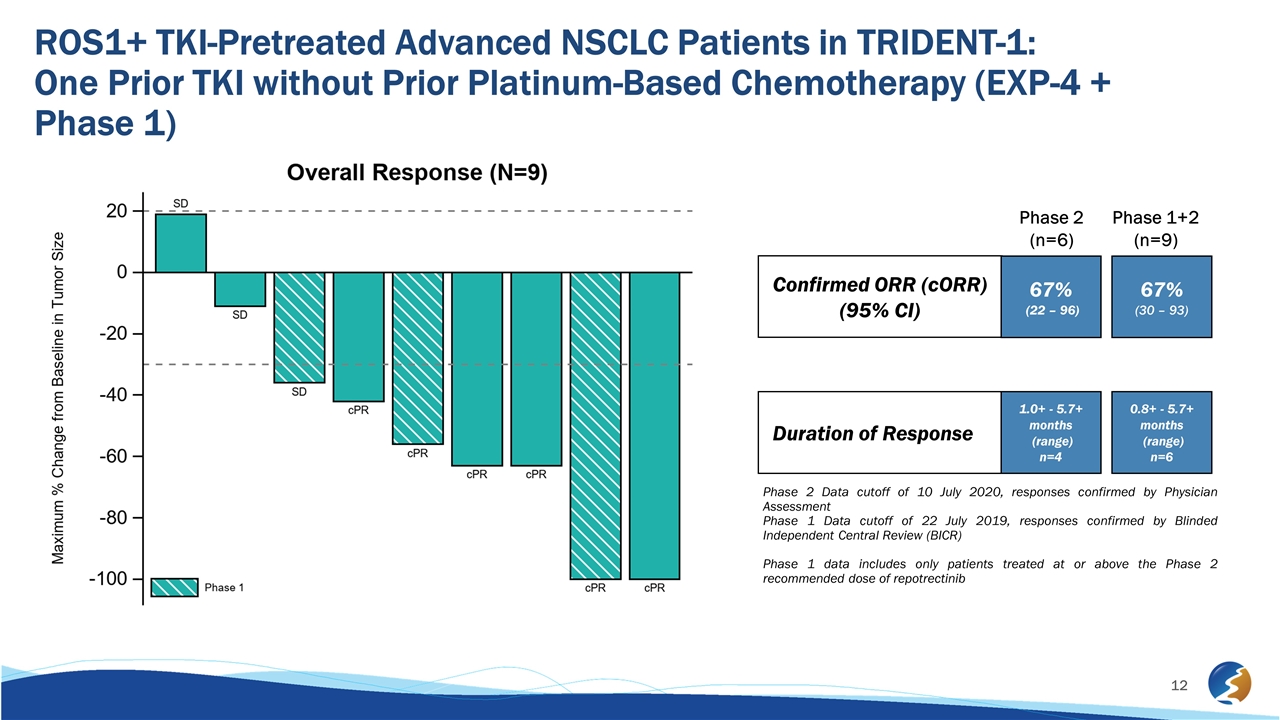
ROS1+ TKI-Pretreated Advanced NSCLC Patients in TRIDENT-1: One Prior TKI without Prior Platinum-Based Chemotherapy (EXP-4 + Phase 1) Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib Confirmed ORR (cORR) (95% CI) Duration of Response 67% (22 – 96) 1.0+ - 5.7+ months (range) n=4 67% (30 – 93) 0.8+ - 5.7+ months (range) n=6 Phase 2 (n=6) Phase 1+2 (n=9)
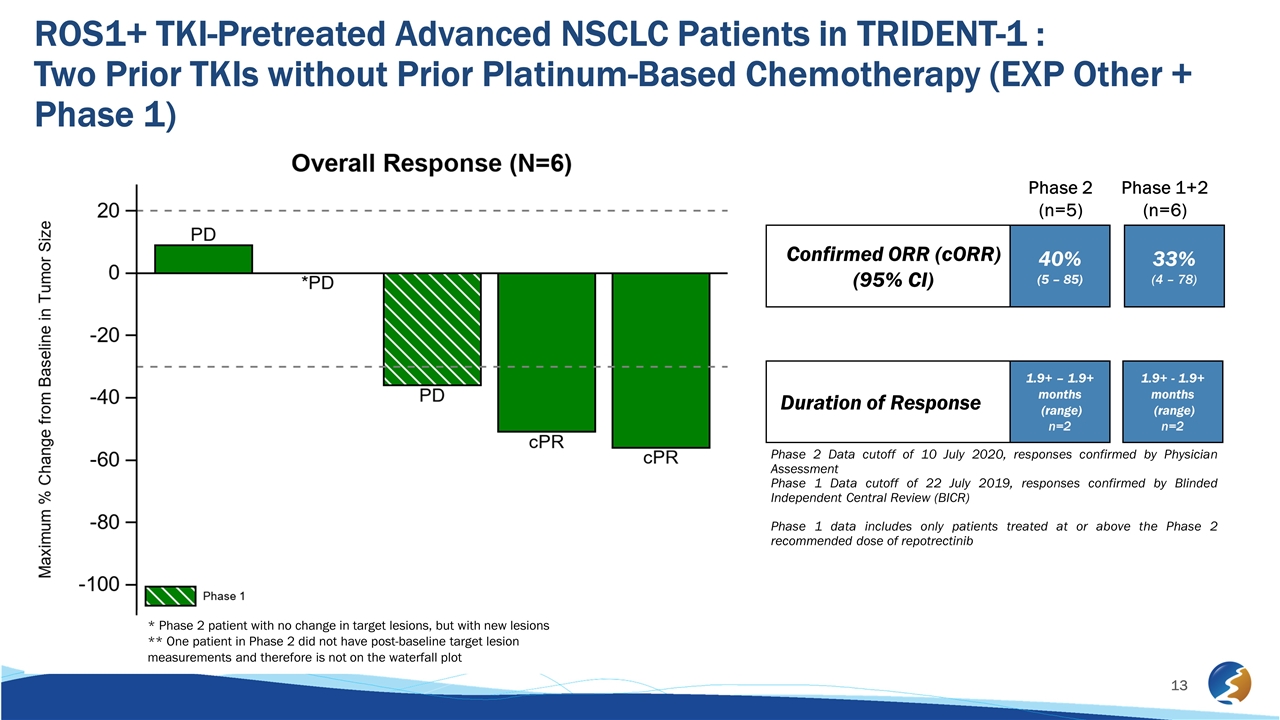
ROS1+ TKI-Pretreated Advanced NSCLC Patients in TRIDENT-1 : Two Prior TKIs without Prior Platinum-Based Chemotherapy (EXP Other + Phase 1) Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib Confirmed ORR (cORR) (95% CI) Duration of Response 40% (5 – 85) 1.9+ – 1.9+ months (range) n=2 33% (4 – 78) 1.9+ - 1.9+ months (range) n=2 Phase 2 (n=5) Phase 1+2 (n=6) * Phase 2 patient with no change in target lesions, but with new lesions ** One patient in Phase 2 did not have post-baseline target lesion measurements and therefore is not on the waterfall plot
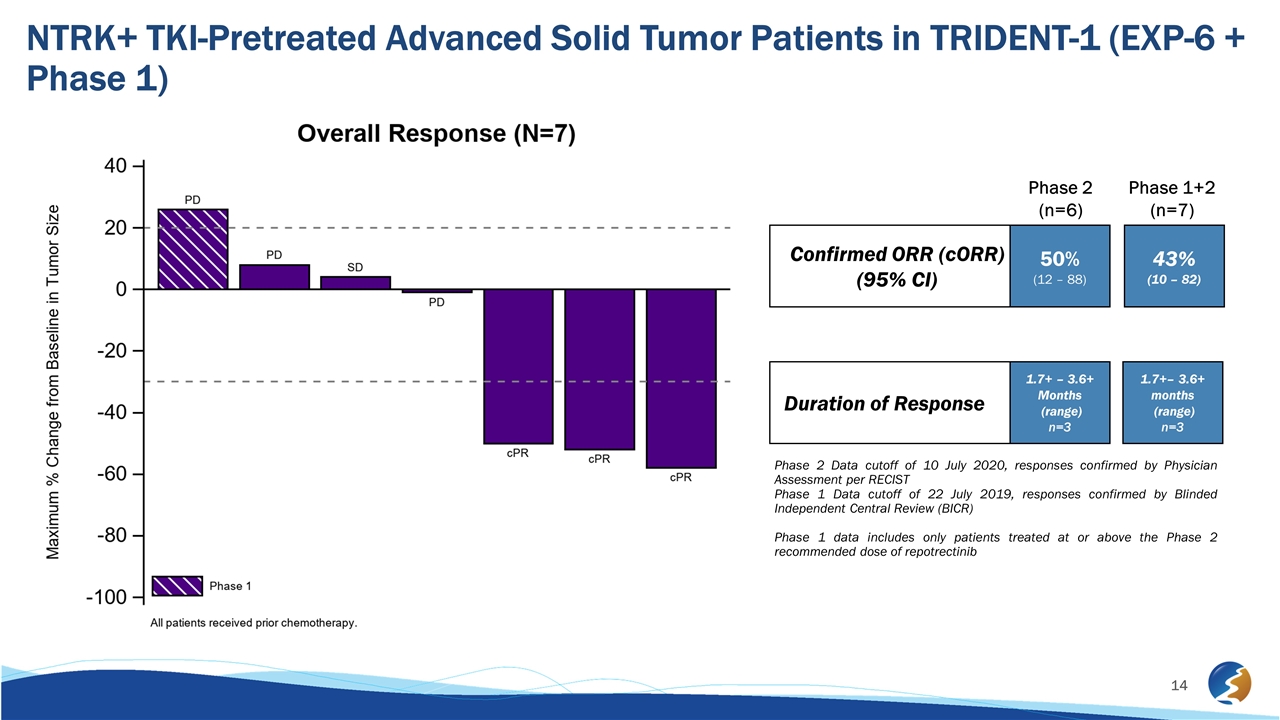
NTRK+ TKI-Pretreated Advanced Solid Tumor Patients in TRIDENT-1 (EXP-6 + Phase 1) Confirmed ORR (cORR) (95% CI) Duration of Response Phase 2 Data cutoff of 10 July 2020, responses confirmed by Physician Assessment per RECIST Phase 1 Data cutoff of 22 July 2019, responses confirmed by Blinded Independent Central Review (BICR) Phase 1 data includes only patients treated at or above the Phase 2 recommended dose of repotrectinib 50% (12 – 88) 1.7+ – 3.6+ Months (range) n=3 43% (10 – 82) 1.7+– 3.6+ months (range) n=3 Phase 2 (n=6) Phase 1+2 (n=7)
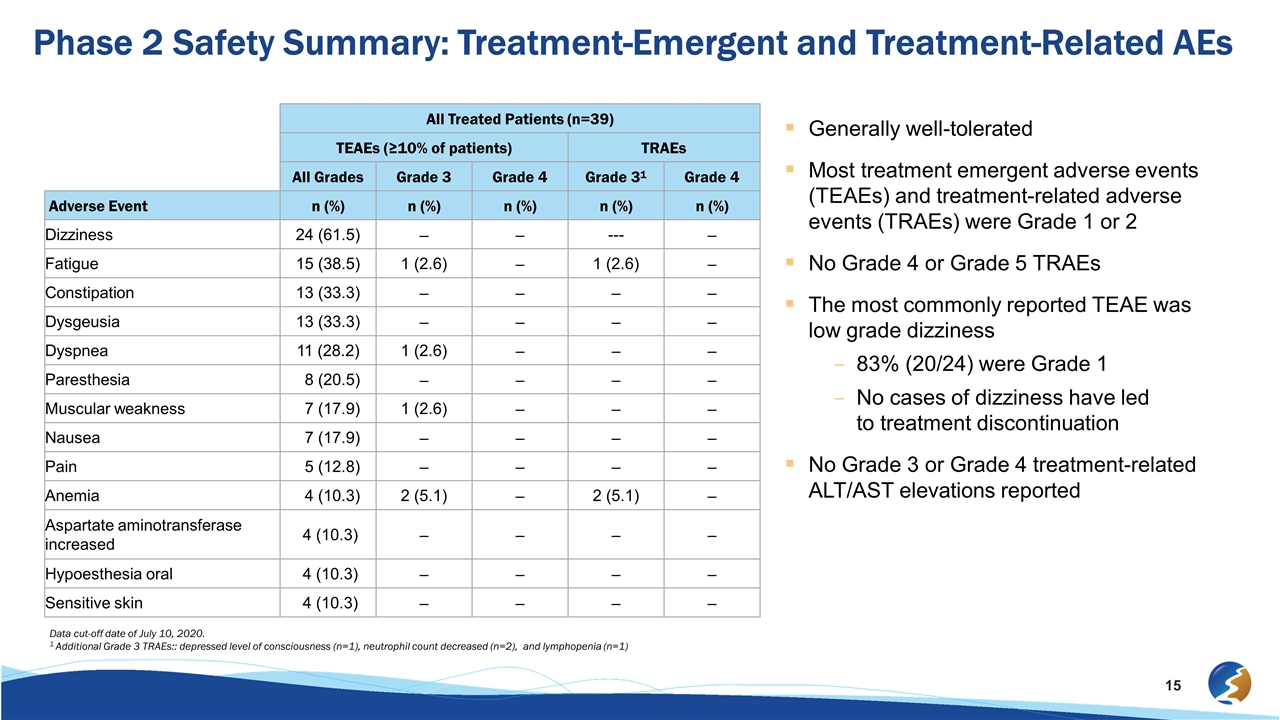
All Treated Patients (n=39) TEAEs (≥10% of patients) TRAEs All Grades Grade 3 Grade 4 Grade 31 Grade 4 Adverse Event n (%) n (%) n (%) n (%) n (%) Dizziness 24 (61.5) – – --- – Fatigue 15 (38.5) 1 (2.6) – 1 (2.6) – Constipation 13 (33.3) – – – – Dysgeusia 13 (33.3) – – – – Dyspnea 11 (28.2) 1 (2.6) – – – Paresthesia 8 (20.5) – – – – Muscular weakness 7 (17.9) 1 (2.6) – – – Nausea 7 (17.9) – – – – Pain 5 (12.8) – – – – Anemia 4 (10.3) 2 (5.1) – 2 (5.1) – Aspartate aminotransferase increased 4 (10.3) – – – – Hypoesthesia oral 4 (10.3) – – – – Sensitive skin 4 (10.3) – – – – Generally well-tolerated Most treatment emergent adverse events (TEAEs) and treatment-related adverse events (TRAEs) were Grade 1 or 2 No Grade 4 or Grade 5 TRAEs The most commonly reported TEAE was low grade dizziness 83% (20/24) were Grade 1 No cases of dizziness have led to treatment discontinuation No Grade 3 or Grade 4 treatment-related ALT/AST elevations reported Phase 2 Safety Summary: Treatment-Emergent and Treatment-Related AEs Data cut-off date of July 10, 2020. 1 Additional Grade 3 TRAEs:: depressed level of consciousness (n=1), neutrophil count decreased (n=2), and lymphopenia (n=1)
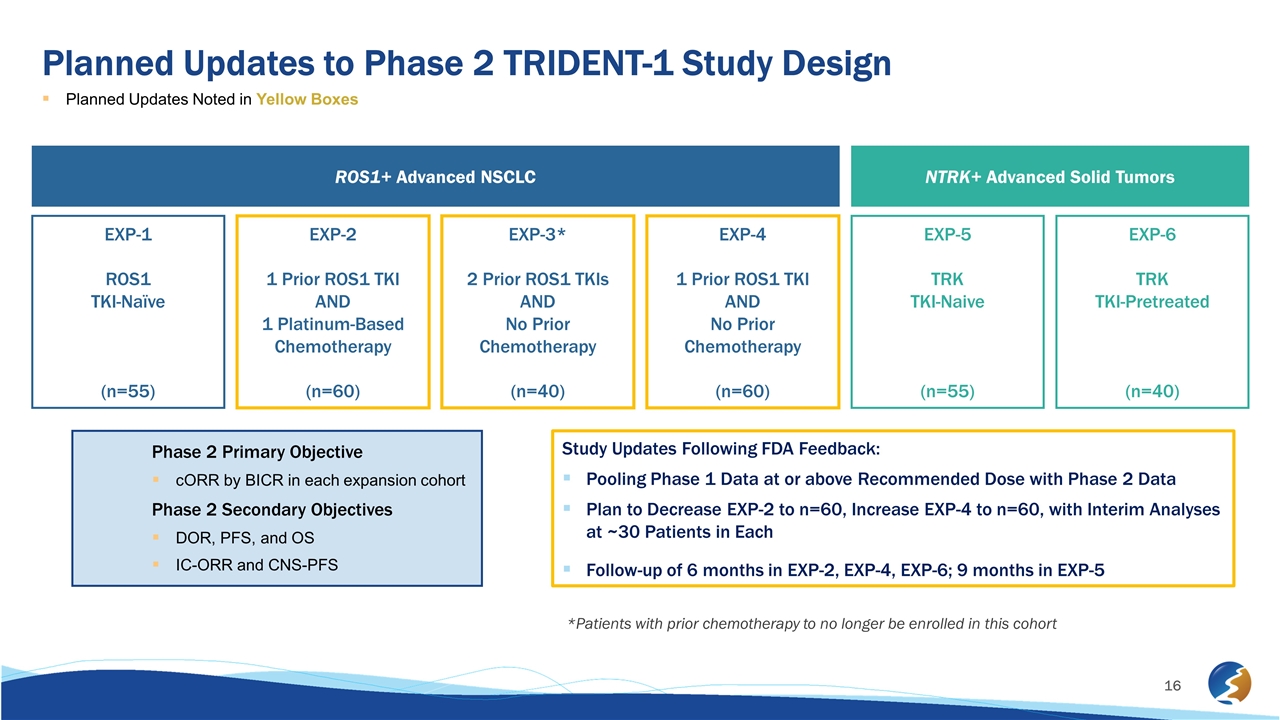
Planned Updates to Phase 2 TRIDENT-1 Study Design Planned Updates Noted in Yellow Boxes ROS1+ Advanced NSCLC NTRK+ Advanced Solid Tumors EXP-1 ROS1 TKI-Naïve (n=55) EXP-2 1 Prior ROS1 TKI AND 1 Platinum-Based Chemotherapy (n=60) EXP-3* 2 Prior ROS1 TKIs AND No Prior Chemotherapy (n=40) EXP-4 1 Prior ROS1 TKI AND No Prior Chemotherapy (n=60) EXP-5 TRK TKI-Naive (n=55) EXP-6 TRK TKI-Pretreated (n=40) Phase 2 Primary Objective cORR by BICR in each expansion cohort Phase 2 Secondary Objectives DOR, PFS, and OS IC-ORR and CNS-PFS Study Updates Following FDA Feedback: Pooling Phase 1 Data at or above Recommended Dose with Phase 2 Data Plan to Decrease EXP-2 to n=60, Increase EXP-4 to n=60, with Interim Analyses at ~30 Patients in Each Follow-up of 6 months in EXP-2, EXP-4, EXP-6; 9 months in EXP-5 *Patients with prior chemotherapy to no longer be enrolled in this cohort

TRIDENT-1 Phase 2 Data Update – Key Takeaways Early, interim data in 39 patients from ongoing Phase 2 study reaffirm repotrectinib as potentially best-in-class therapy Wider global population: Patients enrolled across 6 countries as compared to 2 countries in the Phase 1 portion Dose-titration approach: showing 90% titrate up from daily to twice daily without new safety concerns Generally consistent adverse event profile to prior update in Phase 1 portion of TRIDENT-1 Recent feedback from FDA may provide faster path to potential approval Goal to achieve full site activation in early 2021 and provide update on overall study timeline
