Attached files
| file | filename |
|---|---|
| 8-K - Guardion Health Sciences, Inc. | form8-k.htm |
Exhibit 99.1

Guardion Health Sciences Reports Results of Operations
for Quarter Ended June 30, 2020
Asian Nutraceutical Sales Drive Second Quarter Revenues
SAN DIEGO, California – August 12, 2020 – Guardion Health Sciences, Inc. (“Guardion” or the “Company”) (NASDAQ: GHSI), ), a specialty health sciences company that develops medical foods and medical devices in the ocular health space and nutraceutical products that the Company believes provide supportive health benefits to consumers, reported financial results for the three months and six months ended June 30, 2020.
Fiscal 2020 Financial Highlights (Unaudited) (all dollar amounts rounded to the nearest ‘000)
Three Months Ended June 30, 2020
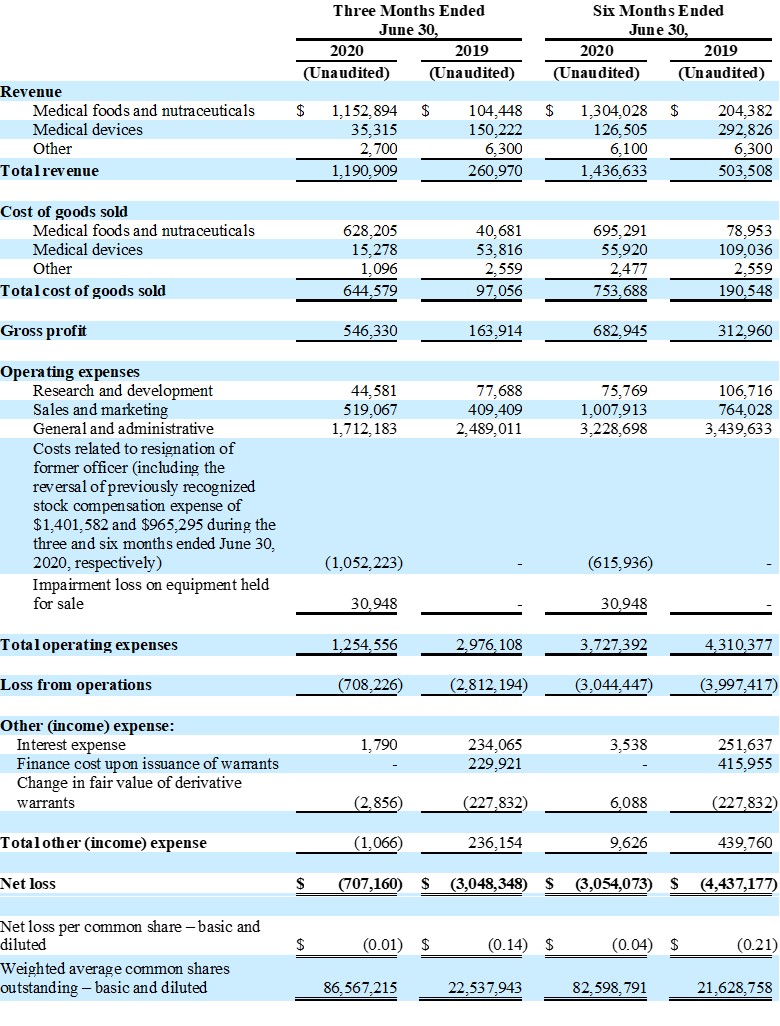
| ● | Total revenue was $1,191,000 for the three months ended June 30, 2020, as compared to total revenue of $261,000 for the three months ended June 30, 2019, an increase of 356%, due to shipment of a large nutraceutical initial test order to Malaysian distributor | |
| ● | Completed shipment and cash collection of $890,000 for nutraceutical initial test order to Malaysian distributor | |
| ● | Medical foods sales up 26% year over year | |
| ● | Medical devices sales were down 75% year over year, primarily as a result of medical facility closures due to COVID-19 “Stay at Home” orders | |
| ● | Review and rationalization of product lines, resulting in the decision to wind-down the Trans-Cranial Doppler business unit at June 30, 2020 | |
| ● | Net loss for the three months ended June 30, 2020 was ($707,000) or ($0.01) per share, as compared to a net loss of ($3,048,000) or ($0.14) per share for the three months ended June 30, 2019 | |
| ● | Warrant exercises netted $999,000 in cash during the three months ended June 30, 2020 | |
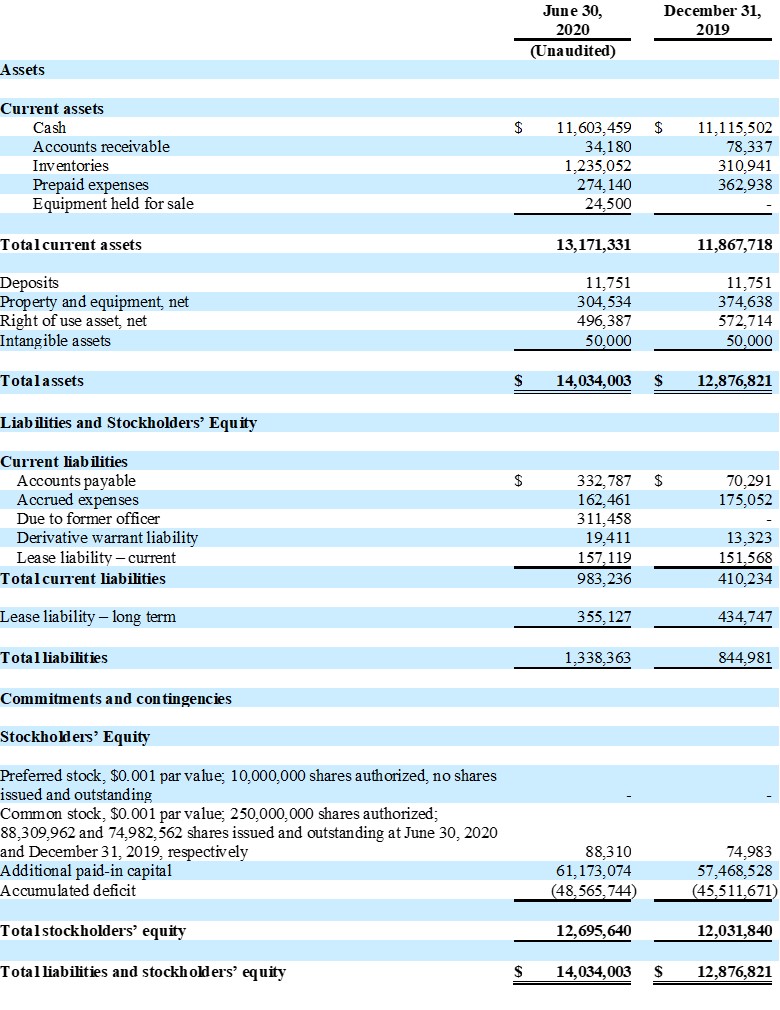
| ● | Ending cash balance at June 30, 2020 was $11,600,000 | |
| ● | Recently published research shows an absorption rate three to four times higher, and thus similarly increased bioavailability, of Lumega-Z ® versus AREDS2 gel caps |
Six Months Ended June 30, 2020
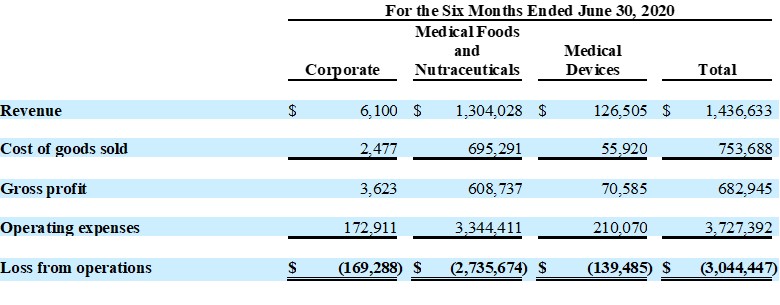
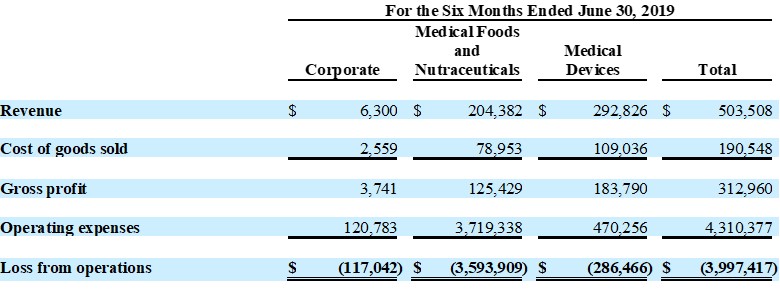
| ● | Total revenue was $1,437,000 for the six months ended June 30, 2020, as compared to total revenue of $504,000 for the six months ended June 30, 2019, an increase of 185%, due to shipment of a large nutraceutical initial test order to Malaysian distributor | |
| ● | Medical foods sales were up 33% year over year | |
| ● | Medical devices sales were down 57% as a result of medical facility closures due to COVID-19 “Stay at Home” orders | |
| ● | Net loss for the six months ended June 30, 2020 was ($3,054,000) or ($0.04) per share, as compared to a net loss of ($4,437,000) or ($0.21) per share for the six months ended June 30, 2019 | |
| ● | Warrant exercises netted $4,549,000 in cash during the six months ended June 30, 2020 |
| Page 1 of 8 |
Management Commentary
Robert N. Weingarten, Guardion’s recently appointed Chairman of the Board of Directors, commented, “The last few months have seen major changes in the executive leadership of Guardion Health Sciences, Inc., changes that the board believes are necessary to focus the Company on effective deployment of its capital and maximization of shareholder value. These ongoing changes, some of which will take some time to develop and implement, include new research studies, development and roll-out of an e-commerce platform, enhanced trade promotion, and other programs and strategies that the Company expects will generate value over the long term, are being made to pave the way for a better and brighter future for Guardion and its shareholders.”
Mr. Weingarten added, “The Board of Directors is working closely with Dr. David Evans, Ph.D., MBA, Guardion’s Chief Science Officer and Interim President and Interim Chief Executive Officer, and with Andrew Schmidt, who was recently appointed as Chief Financial Officer of the Company, to finalize plans to take advantage of the market and commercialization opportunities for the Company’s proprietary products and technologies. The Company is focusing on expanding its product lines with unique formulas under a comprehensive branding strategy that is expected, over time, to result in a sustainable improvement in the operating performance of our business units.”
Dr. David Evans, Ph.D., MBA, Guardion’s Chief Science Officer and Interim President and Interim Chief Executive Officer, commented, “I am encouraged by our strong second quarter revenues and the market opportunities that such sales in Asia indicate. We are engaged in a wide-ranging series of discussions with existing and potential new distributors to identify additional business opportunities, both domestically and internationally. The new leadership team and I are focused on implementing initiatives across our core product lines to continue this positive momentum through the rest of the year and beyond, particularly in light of signs that many doctors’ offices are reopening and patients are beginning to return for eye health care.”
Dr. Evans concluded, “From a scientific perspective, we are pleased with the newly published peer-review research demonstrating the superiority of our medical foods over the competition, as well as the commencement of new research studies to collect and publish data showing the excellent efficacy of our medical food products, which we believe will further validate the technology-based health benefits of our products.”
Andrew Schmidt, Guardion’s newly-appointed Chief Financial Officer, commented, “My financial team and I are focused on supporting the Company’s efforts to improve revenue growth and operational performance in the near-term. I am looking forward to working with Dr. Evans and the rest of the Guardion management team to expand the distribution of our proprietary products and technologies through different channels, including by building an on-line commercial presence, as well as expanding international distribution.”
Three Months Ended June 30, 2020 Financial Results
Total revenue for the second quarter of fiscal 2020 increased 356% to $1,191,000, as compared to $261,000 for the second fiscal quarter of 2019. This increase was due to a large initial test order of a nutraceutical product placed by a Malaysian distributor and increased sales of medical food product lines, partially offset by a decrease in medical device sales which were affected by the impact of COVID-19 closures.
Operating expenses for the second quarter of fiscal 2020 of $1,255,000 were significantly lower than operating expenses for the second quarter of fiscal 2019 of $2,976,000, primarily due to a one-time gain of $1,402,000 related to a reversal of stock-based compensation as a result of the resignation of the Company’s former President and Chief Executive Officer in June 2020.
| Page 2 of 8 |
Operating loss for the second quarter of fiscal 2020 was ($708,000), a decrease of $2,104,000 from the operating loss of ($2,812,000) for the second quarter of fiscal 2019. Net loss for the second quarter of fiscal 2020 was ($707,000), or ($0.01) per share, as compared with a net loss of ($3,048,000), or ($0.14) per share, for the second quarter of fiscal 2019.
Six Months Ended June 30, 2020 Financial Results
Total revenue for the six months ended June 30, 2020 increased 185% to $1,437,000, as compared to $504,000 for the six months ended 2019. This increase was due to a large initial test order of a nutraceutical product placed by a Malaysian distributor and increased sales of medical food product lines, partially offset by a decrease in medical device sales which were affected by the impact of COVID-19 closures.
Operating expense for the six months ended June 30, 2020 of $3,727,000 was lower than operating expense for the for the six months ended June 30, 2019 of $4,310,000, primarily due to a reduction of $965,000 in stock compensation expense, related to a reversal of stock-based compensation as a result of the resignation of the Company’s former President and Chief Executive Officer in June 2020.
Operating loss the six months ended June 30, 2020 was ($3,044,000), a decrease of $953,000 from the operating loss of ($3,997,000) for the six months ended June 30, 2019. Net loss for the six months ended June 30, 2020 was ($3,054,000), or ($0.04) per share, as compared with a net loss of ($4,437,000), or ($0.21) per share, for the six months ended June 30, 2019.
Earnings Conference Call
Guardion will conduct a conference call and webcast today at 4:30 p.m. Eastern Time (1:30pm Pacific Time) to discuss its fiscal 2020 second quarter results. To access the live call, dial 800-353-6461 (United States and Canada) or +1 334-323-0501 (International) and give the participant passcode 7353998. In addition, a live and archived webcast of the conference call will be accessible on the Investor Relations section of the Company’s website at www.guardionhealth.com. In addition, a phone replay will be available approximately two hours following the end of the call and will remain available for one week. To access the call replay dial-in information, please click here.
About Guardion Health Sciences, Inc.
Guardion is a specialty health sciences company that develops (i) medical foods and medical devices for the ocular health marketplace, and (ii) nutraceutical products that the Company believes provide supportive health benefits to consumers. Information and risk factors with respect to Guardion and its business, including its ability to successfully develop and commercialize its proprietary products and technologies, may be obtained in the Company’s filings with the SEC at www.sec.gov.
About VectorVision®
VectorVision® specializes in the standardization of contrast sensitivity, glare sensitivity, low contrast acuity, and ETDRS acuity vision testing. Its patented standardization system provides the practitioner or researcher the ability to delineate very small changes in visual capability, either as compared to the population or from visit to visit. VectorVision’s® patented technology is considered the standard of care for clinical trials. VectorVision® is a wholly owned subsidiary of Guardion.
| Page 3 of 8 |
About NutriGuard™
NutriGuard™ formulates high-quality nutraceuticals which are designed to supplement consumers’ diets. NutriGuard uses industry standards to establish the safety and efficacy of the products it develops and markets, maintains that commitment through prudent manufacturing and quality assurance programs, and only uses manufacturers who comply with FDA current Good Manufacturing Practices (cGMP) requirements. Guardion plans to increase NutriGuard’s existing customer base and build on its product platform by making NutriGuard products available to patients directly and through recommendations by their physicians.
Forward-Looking Statement Disclaimer
With the exception of the historical information contained in this news release, the matters described herein may contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Statements preceded by, followed by or that otherwise include the words “believes,” “expects,” “anticipates,” “intends,” “projects,” “estimates,” “plans” and similar expressions or future or conditional verbs such as “will,” “should,” “would,” “may” and “could” are generally forward-looking in nature and not historical facts, although not all forward-looking statements include the foregoing. These statements involve unknown risks and uncertainties that may individually or materially impact the matters discussed herein for a variety of reasons that are outside the control of the Company, including, but not limited to, the Company’s ability to raise sufficient financing to implement its business plan, the impact of the COVID-19 pandemic on the Company’s business, operations and the economy in general, and the Company’s ability to successfully develop and commercialize its proprietary products and technologies. Readers are cautioned not to place undue reliance on these forward- looking statements, as actual results could differ materially from those described in the forward-looking statements contained herein. Readers are urged to read the risk factors set forth in the Company’s filings with the SEC, which are available at the SEC’s website (www.sec.gov). The Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Investor Relations
MKR Investor Relations, Inc.
Todd Kehrli
ghsi@mkr-group.com
| Page 4 of 8 |
Guardion Health Sciences, Inc.
Condensed Consolidated Balance Sheets
| Page 5 of 8 |
Guardion Health Sciences, Inc.
Condensed Consolidated Statements of Operations
| Page 6 of 8 |
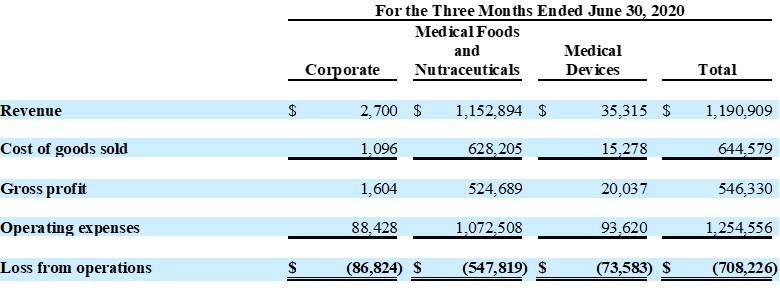
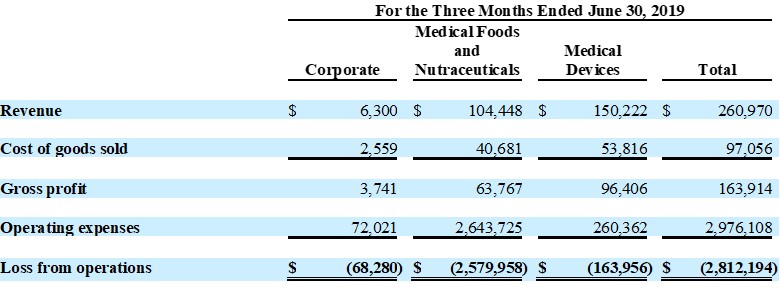
Guardion Health Sciences, Inc.
Unaudited Segment Reporting Details
| Page 7 of 8 |
| Page 8 of 8 |
