Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Larimar Therapeutics, Inc. | d917825dex991.htm |
| 8-K - FORM 8-K - Larimar Therapeutics, Inc. | d917825d8k.htm |

Corporate Presentation August 2020 (8.11.20 update) Exhibit 99.2

Forward Looking Statements This presentation contains forward-looking statements that are based on the Company’s beliefs and assumptions and on information currently available to management. All statements contained in this presentation other than statements of historical fact are forward-looking statements, including but not limited to statements regarding the potential benefits of the merger, the use of proceeds of the private placement, Company’s ability to develop and commercialize CTI-1601 and other planned product candidates, Company’s planned research and development efforts, and other matters regarding Company’s business strategies, use of capital, results of operations and financial position, and plans and objectives for future operations. In some cases, you can identify forward-looking statements by the words “may,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements involve risks, uncertainties and other factors that may cause actual results, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. These risks, uncertainties and other factors include, among others, the success, cost and timing of the Company’s product development activities, studies and clinical trials; the ongoing impact of the COVID-19 pandemic on the Company’s clinical trial timelines, ability to raise additional capital and general economic conditions; the Company’s ability to optimize and scale CTI-1601’s manufacturing process; the Company’s ability to obtain regulatory approval for CTI-1601 and future product candidates; the Company’s ability to develop sales and marketing capabilities, whether alone or with potential future collaborators, and successfully commercialize any approved product candidates; the Company’s ability to raise the necessary capital to conduct its product development activities; and other risks described in the filings made by the Company with the Securities and Exchange Commission (SEC), including but not limited to the Form 8-K/A filed on June 26, 2020, and the Company’s subsequent periodic reports, including the annual report on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K, filed with or furnished to the Securities and Exchange Commission and available at www.sec.gov. These forward-looking statements are based on a combination of facts and factors currently known by the Company and its projections of the future, about which it cannot be certain. As a result, the forward-looking statements may not prove to be accurate. These forward-looking statements are based on information currently available to us, and we assume no obligation to update any forward-looking statements, except as required by law.
Larimar Therapeutics Introduction Created by reverse merger of Zafgen and Chondrial Therapeutics Traded under ticker “LRMR” Merger accompanied by $80 million private placement – World-class life science investor shareholder base Financing led by Cowen Healthcare Investments with Acuta, Janus, Logos, OrbiMed, RA Capital and Vivo Continued shareholder support of Deerfield Management and Atlas Ventures Company has ~ $116 million in cash from merger and financing Leadership team bolstered Joseph Truitt – Board chair Nancy Ruiz, MD, FACP, FIDSA – CMO Michael Celano - CFO

Investment Highlights Clinical-stage biotech with novel protein replacement therapy platform to address untreated, serious and complex rare diseases Lead candidate, CTI-1601, in ongoing Phase 1 clinical development for treatment of Friedreich’s ataxia (FA) To our knowledge, frataxin (FXN) protein replacement therapy is only protein replacement therapy in clinical development Nonclinical studies demonstrated promising results in several models of FA, including heart, brain and muscle function, and overall survival Multiple FDA designations: Orphan Drug, Rare Pediatric Disease, Fast Track; Recent EMA Committee for Orphan Medicinal Products positive opinion for Orphan Drug designation Topline Phase 1 data expected in 1H 2021 Experienced leadership team Extensive IP with 12 years market exclusivity expected if approved; patents pending around efficacy biomarkers Strong balance sheet with ~ $114M in cash and investments at 6/30/20; based on current estimates of funding needs, cash expected to last for ~ 2 years into first half of 2022
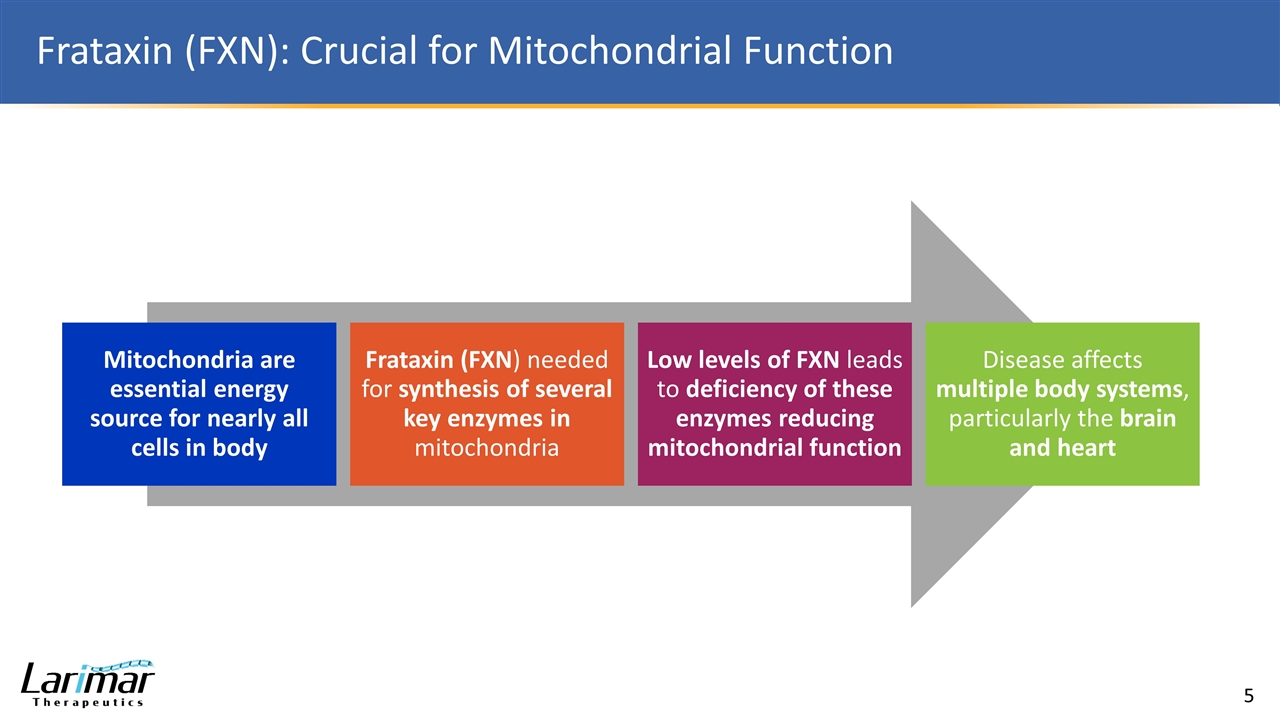
Frataxin (FXN): Crucial for Mitochondrial Function Mitochondria are essential energy source for nearly all cells in body Frataxin (FXN ) needed for synthesis of several key enzymes in mitochondria Low levels of FXN leads to deficiency of these enzymes reducing mitochondrial function Disease affects multiple body systems , particularly the brain and heart

Friedreich’s ataxia (FA): Rare and Progressive Disease Rare disease caused by genetic defect resulting in abnormally low levels of FXN Affects ~5,000 patients in U.S. and ~20,000 patients in EU Onset: Age of onset correlated with severity and speed of progression >70% of patients present before age 14 Initial symptoms may include unsteady posture, frequent falling and progressive difficulty in walking By the time symptoms occur, heart damage has already occurred Progression of disease: Symptoms worsen and patients are eventually confined to wheelchair Life expectancy of 30-50 years, early death usually caused by heart disease Treatment limited to symptom management; currently no approved therapies
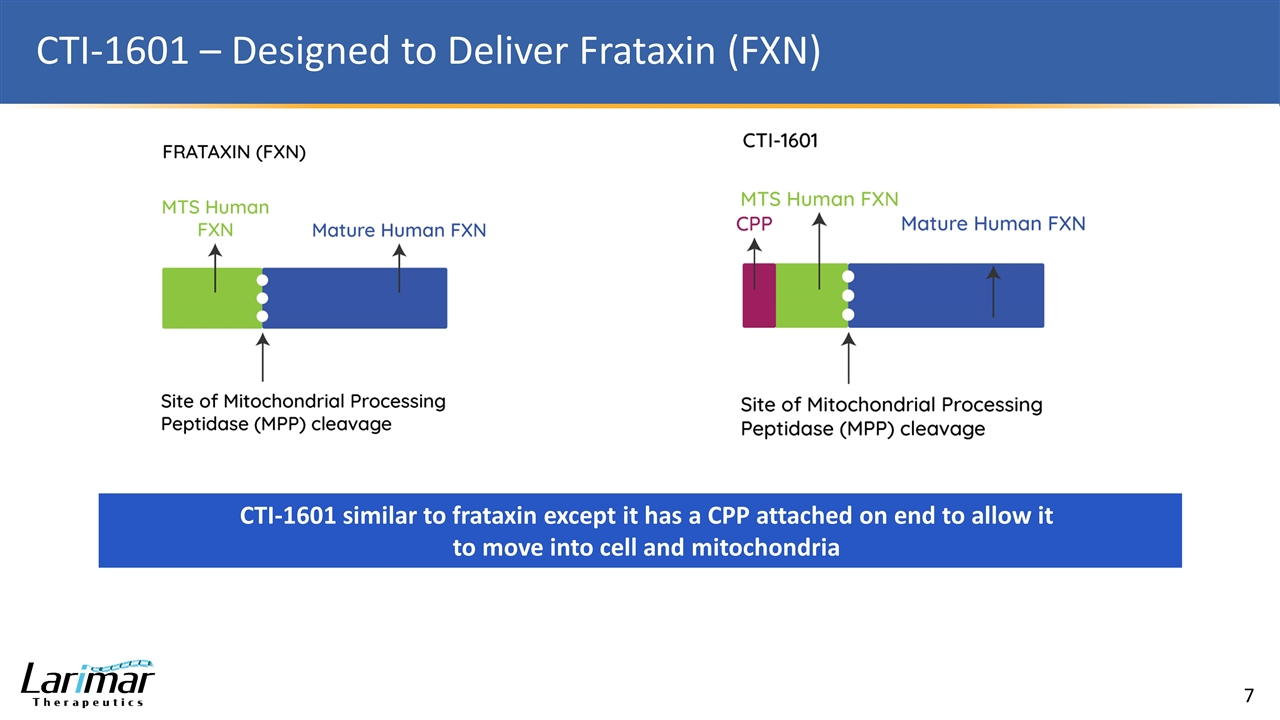
CTI-1601 – Designed to Deliver Frataxin (FXN) CTI-1601 similar to frataxin except it has a CPP attached on end to allow it to move into cell and mitochondria
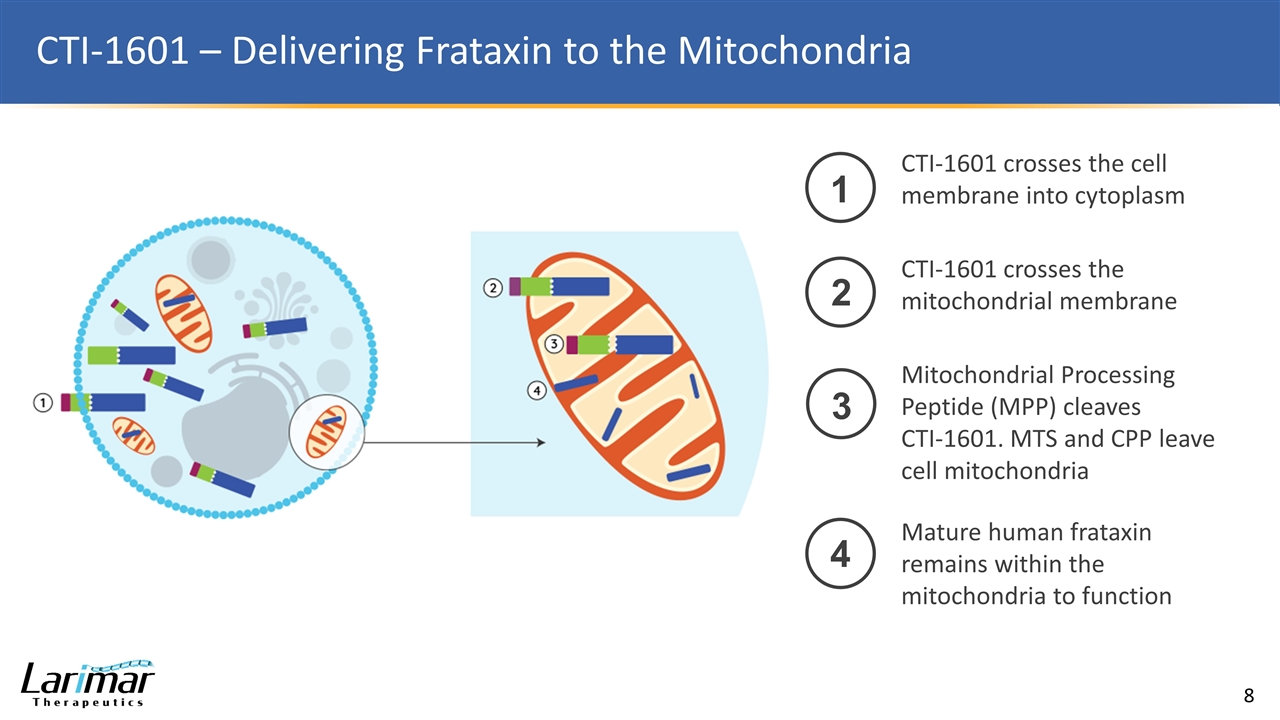
CTI-1601 – Delivering Frataxin to the Mitochondria 1 CTI-1601 crosses the cell membrane into cytoplasm 2 CTI-1601 crosses the mitochondrial membrane 3 Mitochondrial Processing Peptide (MPP) cleaves CTI-1601. MTS and CPP leave cell mitochondria 4 Mature human frataxin remains within the mitochondria to function
CTI-1601 – POC Achieved Through Multiple Non-Clinical Studies Nonclinical efficacy and PD data support of continued development to potentially replace FXN in patients with FA Extended survival in a well-characterized nonclinical model of FA Prevented ataxic gait in another nonclinical model of FA Demonstrated capability of delivering sufficient amounts of FXN to mitochondria Prevented left ventricle dilation and maintained function Safe and well tolerated in multiple species
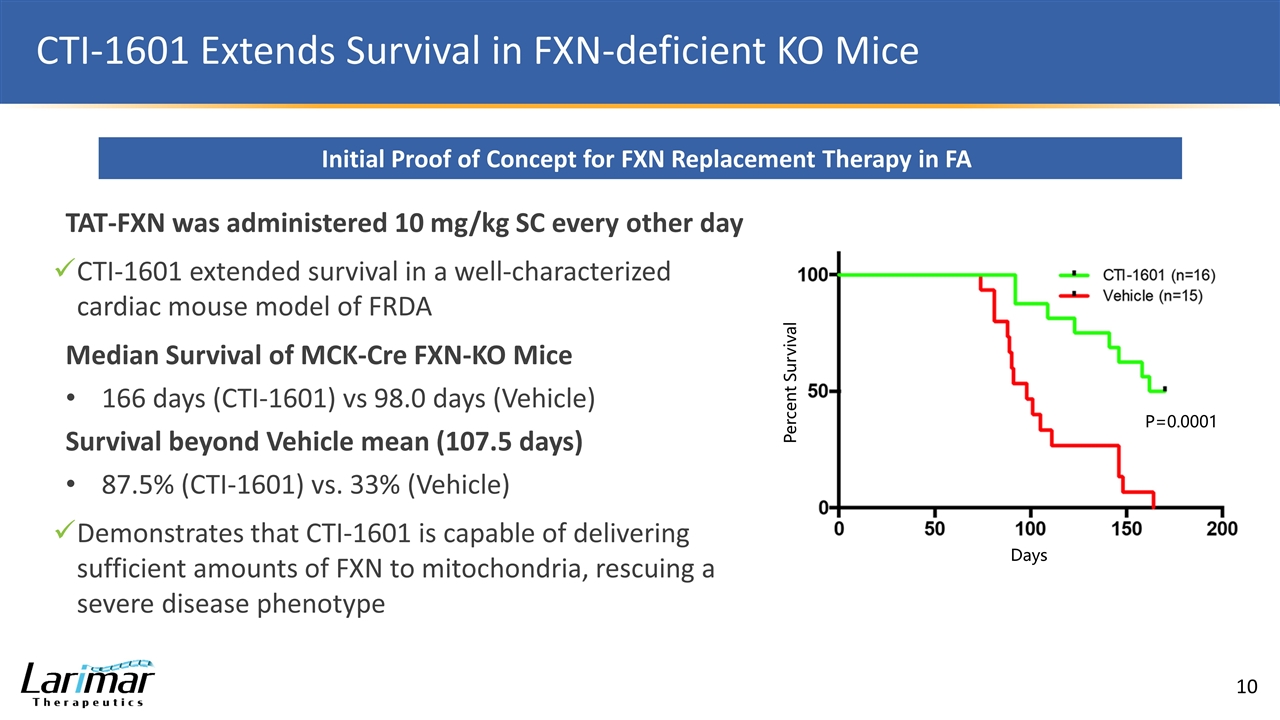
CTI-1601 Extends Survival in FXN-deficient KO Mice Initial Proof of Concept for FXN Replacement Therapy in FA TAT-FXN was administered 10 mg/kg SC every other day CTI-1601 extended survival in a well-characterized cardiac mouse model of FRDA Median Survival of MCK-Cre FXN-KO Mice 166 days (CTI-1601) vs 98.0 days (Vehicle) Survival beyond Vehicle mean (107.5 days) 87.5% (CTI-1601) vs. 33% (Vehicle) Demonstrates that CTI-1601 is capable of delivering sufficient amounts of FXN to mitochondria, rescuing a severe disease phenotype Days Percent Survival P=0.0001
CTI-1601 Prevents Neurological Phenotype and Ataxic Gait in KO mice In-Vivo Efficacy Data in Neurologic KO Mouse Model Pvalb-Cre FXN-KO mouse Single dose level: 10 mg/kg CTI-1601 or Vehicle given intraperitoneally three times per week hFXN replacement with CTI-1601 prevents development of ataxic gait Treated mice survive longer than untreated mice Human frataxin present in brain, dorsal root ganglia and spinal cord
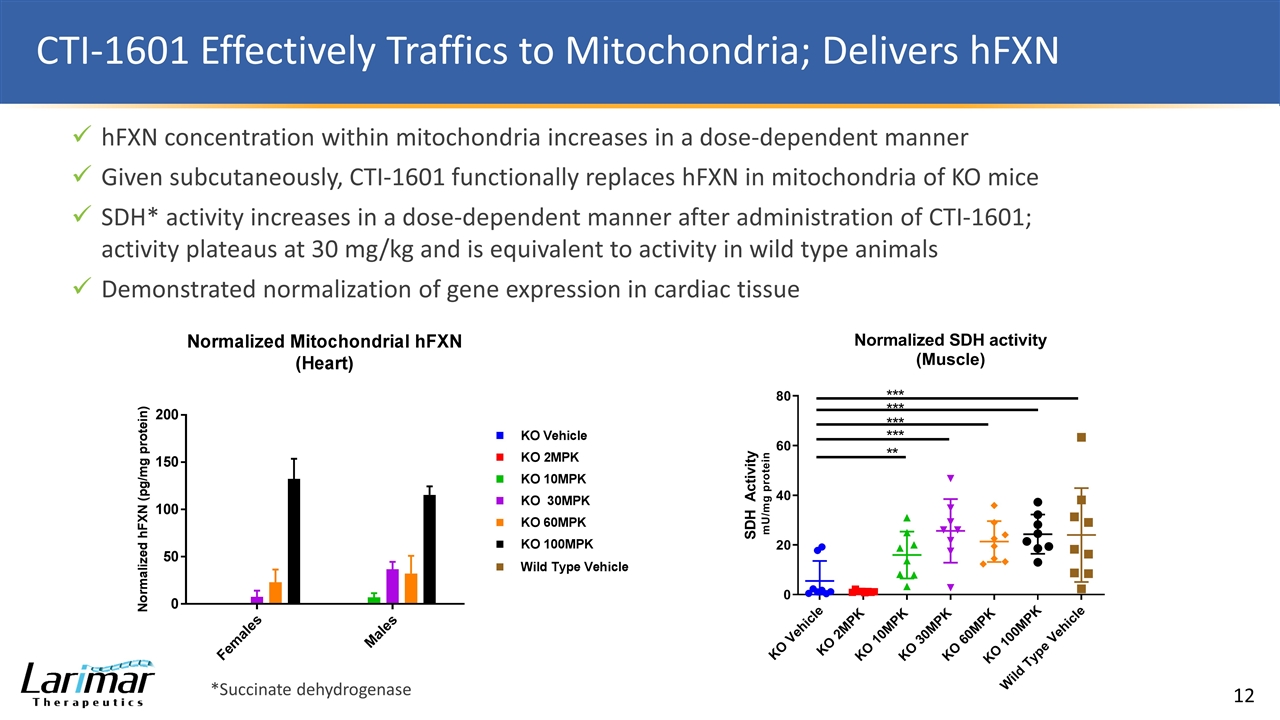
CTI-1601 Effectively Traffics to Mitochondria; Delivers hFXN hFXN concentration within mitochondria increases in a dose-dependent manner Given subcutaneously, CTI-1601 functionally replaces hFXN in mitochondria of KO mice SDH* activity increases in a dose-dependent manner after administration of CTI-1601; activity plateaus at 30 mg/kg and is equivalent to activity in wild type animals Demonstrated normalization of gene expression in cardiac tissue *Succinate dehydrogenase
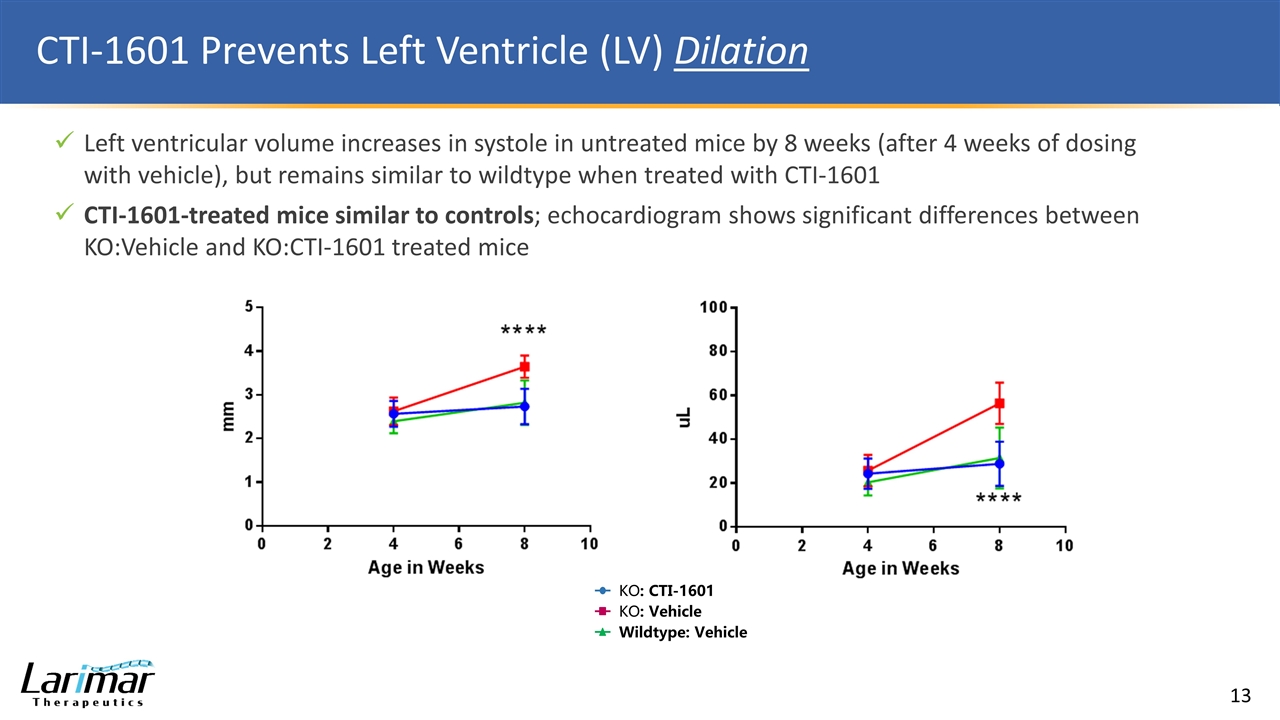
CTI-1601 Prevents Left Ventricle (LV) Dilation Left ventricular volume increases in systole in untreated mice by 8 weeks (after 4 weeks of dosing with vehicle), but remains similar to wildtype when treated with CTI-1601 CTI-1601-treated mice similar to controls; echocardiogram shows significant differences between KO:Vehicle and KO:CTI-1601 treated mice KO: CTI-1601 KO: Vehicle Wildtype: Vehicle
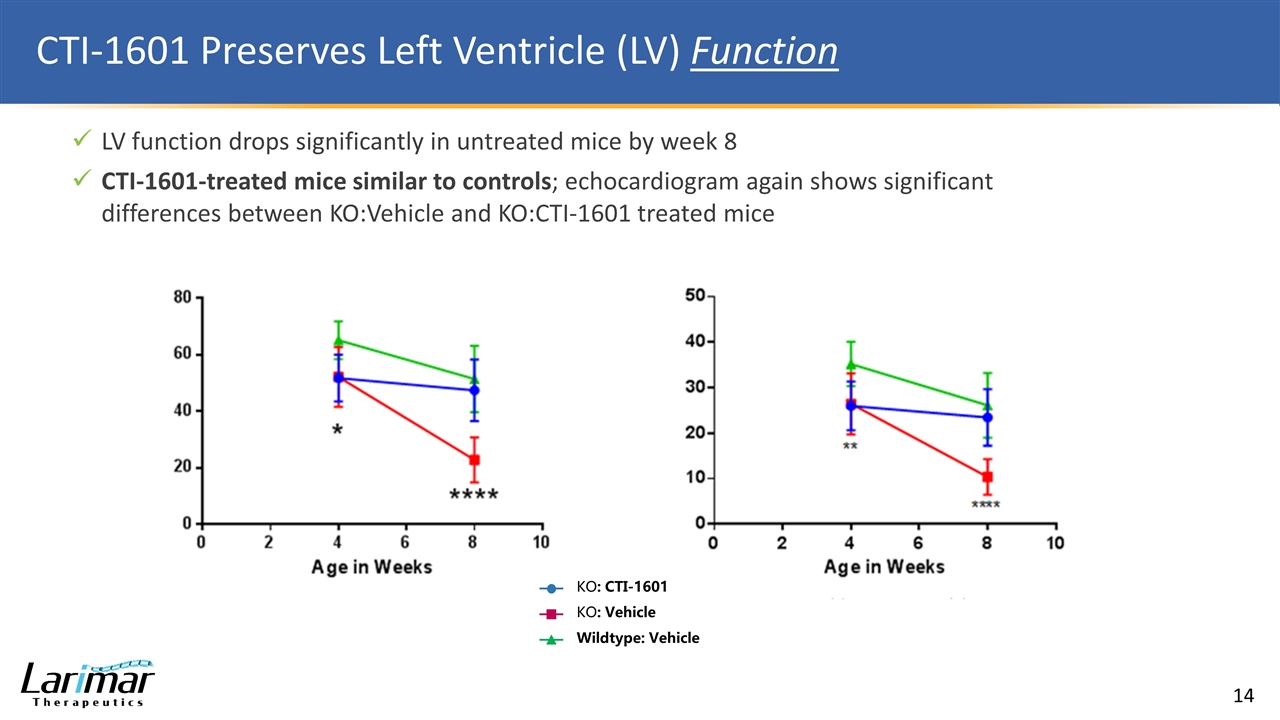
CTI-1601 Preserves Left Ventricle (LV) Function LV function drops significantly in untreated mice by week 8 CTI-1601-treated mice similar to controls; echocardiogram again shows significant differences between KO:Vehicle and KO:CTI-1601 treated mice KO: CTI-1601 KO: Vehicle Wildtype: Vehicle
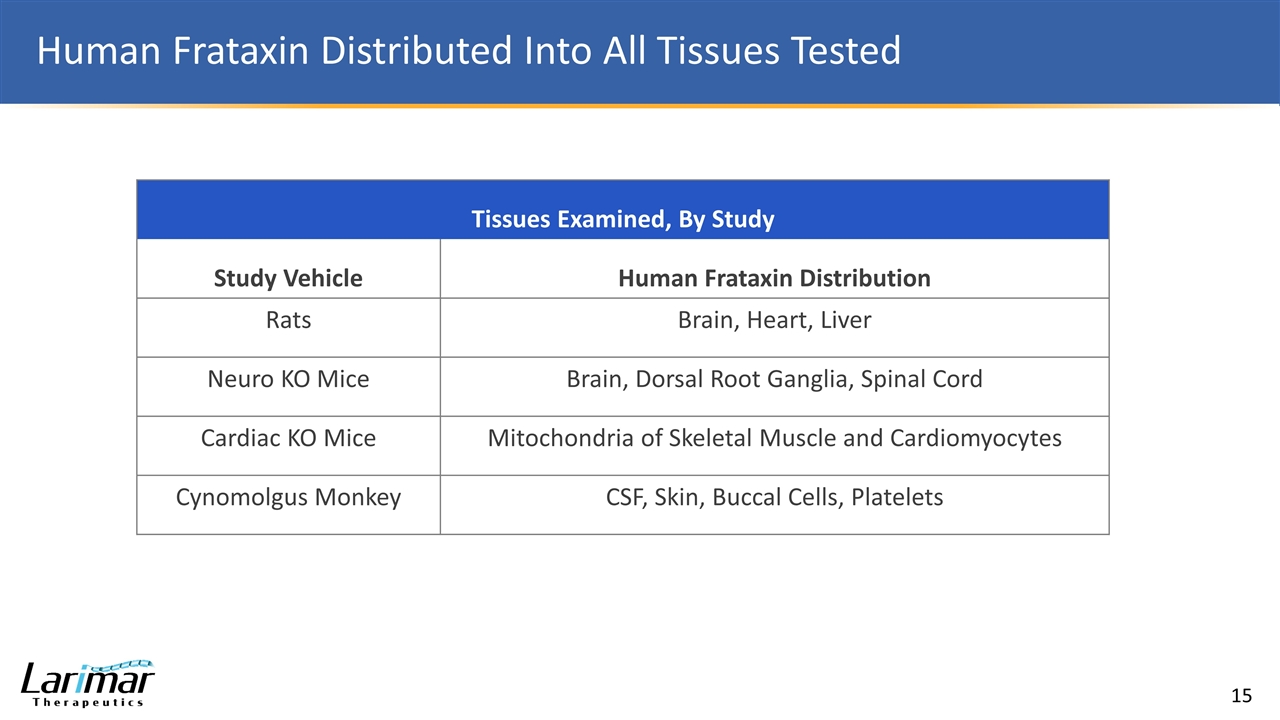
Human Frataxin Distributed Into All Tissues Tested Tissues Examined, By Study Study Vehicle Human Frataxin Distribution Rats Brain, Heart, Liver Neuro KO Mice Brain, Dorsal Root Ganglia, Spinal Cord Cardiac KO Mice Mitochondria of Skeletal Muscle and Cardiomyocytes Cynomolgus Monkey CSF, Skin, Buccal Cells, Platelets
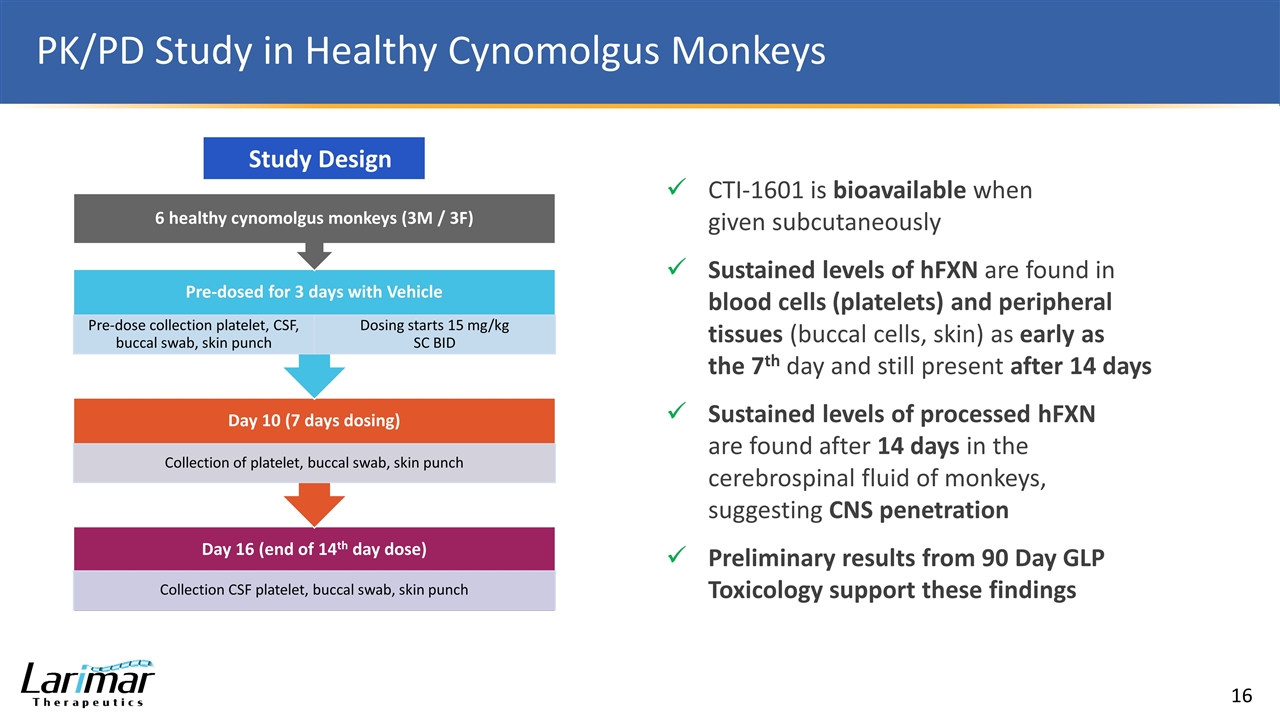
PK/PD Study in Healthy Cynomolgus Monkeys Study Design CTI-1601 is bioavailable when given subcutaneously Sustained levels of hFXN are found in blood cells (platelets) and peripheral tissues (buccal cells, skin) as early as the 7th day and still present after 14 days Sustained levels of processed hFXN are found after 14 days in the cerebrospinal fluid of monkeys, suggesting CNS penetration Preliminary results from 90 Day GLP Toxicology support these findings 6 healthy cynomolgus monkeys (3M / 3F) Pre-dosed for 3 days with Vehicle Pre-dose collection platelet, CSF, buccal swab, skin punch Dosing starts 15 mg/kg SC BID Day 10 (7 days dosing) Collection of platelet, buccal swab, skin punch Day 16 (end of 14 th day dose) Collection CSF platelet, buccal swab, skin punch
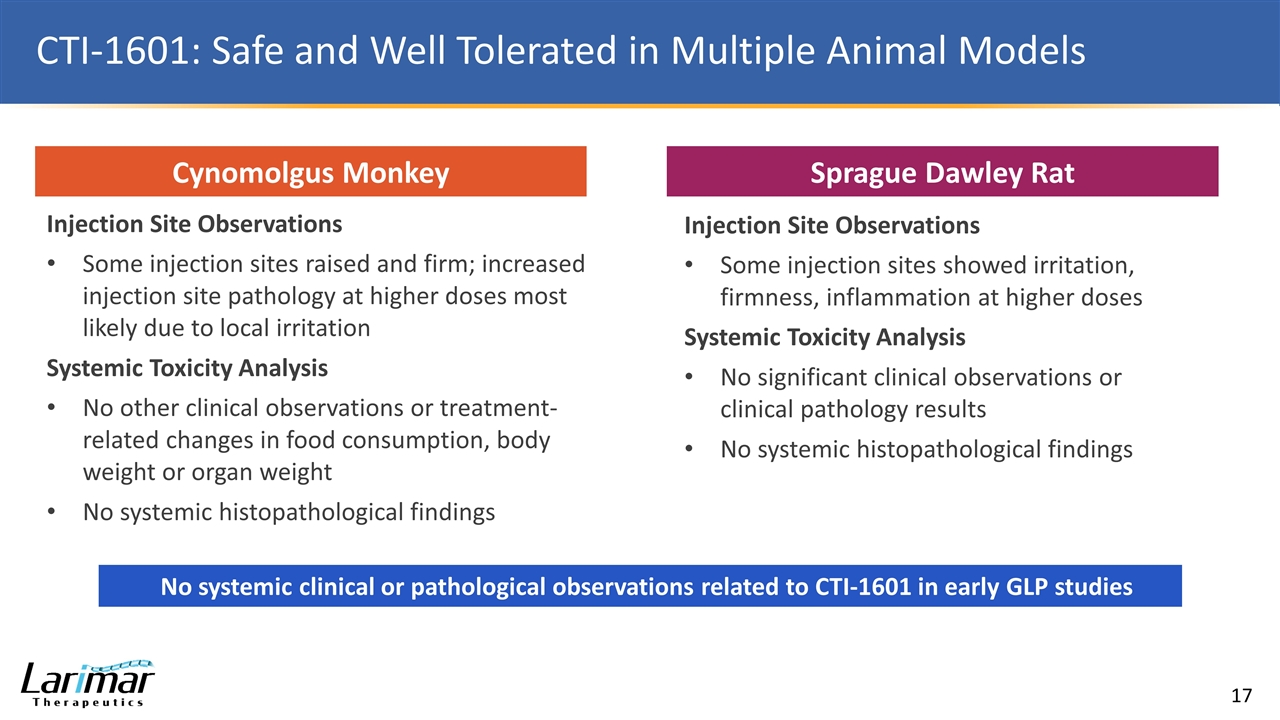
CTI-1601: Safe and Well Tolerated in Multiple Animal Models Cynomolgus Monkey Sprague Dawley Rat Injection Site Observations Some injection sites raised and firm; increased injection site pathology at higher doses most likely due to local irritation Systemic Toxicity Analysis No other clinical observations or treatment-related changes in food consumption, body weight or organ weight No systemic histopathological findings Injection Site Observations Some injection sites showed irritation, firmness, inflammation at higher doses Systemic Toxicity Analysis No significant clinical observations or clinical pathology results No systemic histopathological findings No systemic clinical or pathological observations related to CTI-1601 in early GLP studies

CTI-1601: Ongoing Phase 1 Clinical Program in FA Patients Dosing regimen: Single Ascending Doses given SC (SAD); Multiple Ascending Doses given SC (MAD) Patient dosing began December 2019 Three cohorts dosed- SAD Phase 1 Number of subjects: SAD: approximately 32-34 subjects (currently 4 cohorts planned) MAD: 24-30 subjects (currently 3 cohorts planned) Outcome measures: Primary: Safety and tolerability Secondary: PK; PD (hFXN, gene expression in buccal swab and blood); multiple exploratory Sufficient drug supply for Phase 1 clinical program (5 GMP batches) Topline results from Phase 1 clinical program expected in 1H 2021
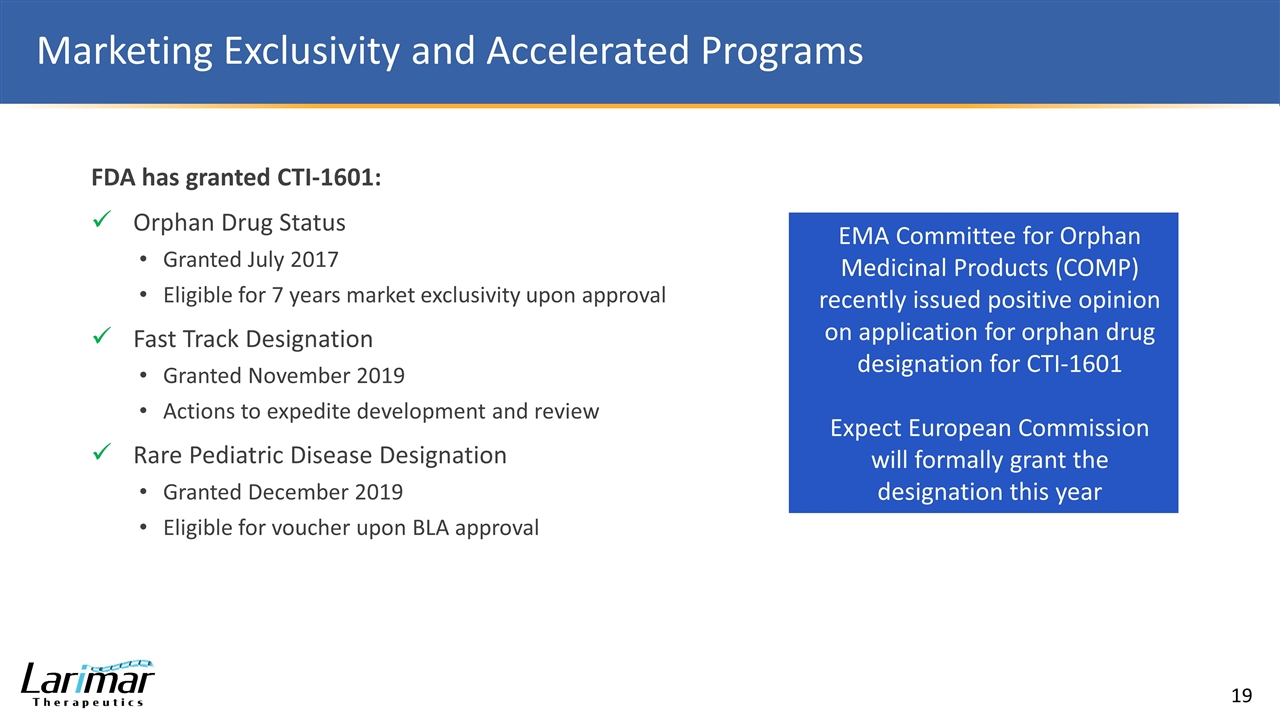
Marketing Exclusivity and Accelerated Programs EMA Committee for Orphan Medicinal Products (COMP) recently issued positive opinion on application for orphan drug designation for CTI-1601 Expect European Commission will formally grant the designation this year FDA has granted CTI-1601: Orphan Drug Status Granted July 2017 Eligible for 7 years market exclusivity upon approval Fast Track Designation Granted November 2019 Actions to expedite development and review Rare Pediatric Disease Designation Granted December 2019 Eligible for voucher upon BLA approval
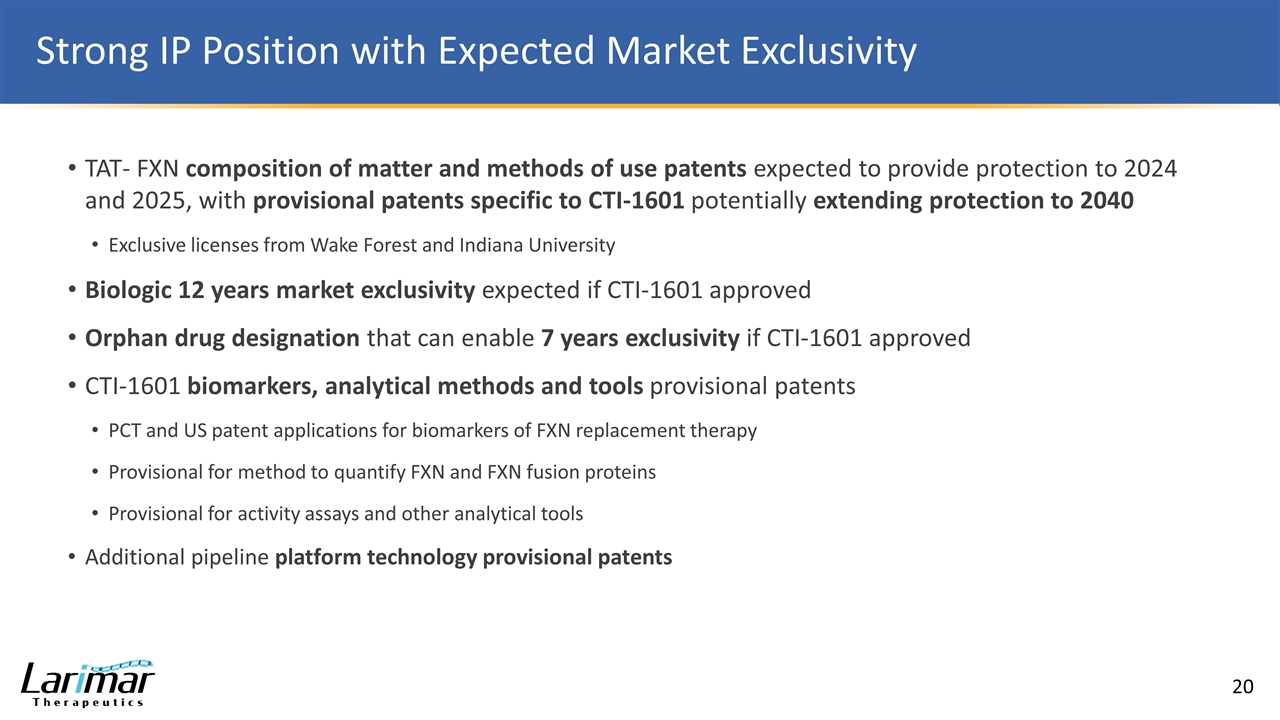
Strong IP Position with Expected Market Exclusivity TAT- FXN composition of matter and methods of use patents expected to provide protection to 2024 and 2025, with provisional patents specific to CTI-1601 potentially extending protection to 2040 Exclusive licenses from Wake Forest and Indiana University Biologic 12 years market exclusivity expected if CTI-1601 approved Orphan drug designation that can enable 7 years exclusivity if CTI-1601 approved CTI-1601 biomarkers, analytical methods and tools provisional patents PCT and US patent applications for biomarkers of FXN replacement therapy Provisional for method to quantify FXN and FXN fusion proteins Provisional for activity assays and other analytical tools Additional pipeline platform technology provisional patents

Strong Relationship with FARA Company has strong relationship with Friedreich’s Ataxia Research Alliance (FARA) National, non-profit organization dedicated to the pursuit of scientific research leading to treatments and a cure for FA FARA provides industry with several key items Assists with patient recruitment and education Access to Global Patient Registry with demographic and clinical information on more than 1,000 FA patients Sponsored a Patient-Focused Drug Development Meeting in 2017 resulting in a publication called The Voice of the Patient

Summary Novel protein replacement therapy platform CTI-1601 in Phase 1 clinical development for treatment of Friedreich’s ataxia (FA) Topline Phase 1 data expected in 1H 2021 Experienced leadership team Extensive IP Strong balance sheet of ~ $114M in cash and investments as of 6/30/20

