Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - TCR2 THERAPEUTICS INC. | d931658dex991.htm |
| 8-K - 8-K - TCR2 THERAPEUTICS INC. | d931658d8k.htm |

Exhibit 99.2 Presentation of Clinical Data from First Cohort of TC-210 Patients Trial ongoing with additional patients enrolled and treatedExhibit 99.2 Presentation of Clinical Data from First Cohort of TC-210 Patients Trial ongoing with additional patients enrolled and treated
Forward Looking Statements 2 This presentation has been prepared by TCR Therapeutics Inc. (“we,” “us,” or planned Phase 1 clinical trial of TC-110; the risk that the results from the Phase “our”) and contains forward-looking statements within the meaning of the Private 1/2 clinical trial of TC-210 will not support further development and marketing Securities Litigation Reform Act of 1995 and other federal securities laws. approval; the risk that we may be unable to gain approval of TC-210 and our Forward-looking statements are neither historical facts nor assurances of future other product candidates on a timely basis, if at all; the risk that we have over- performance. Instead, they are based on our current beliefs, expectations and estimated the potential patient population for our product candidates, if assumptions regarding the future of our business, future plans and strategies, approved; the risk that the current COVID-19 pandemic will impact our clinical our development plans, our clinical results and other future conditions. All trials and other operations; and the other risks set forth under the caption “Risk statements, other than statements of historical facts, contained in this Factors” in our most recent Annual Report on Form 10-K for the year ended presentation, including express or implied statements regarding our expectations December 31, 2019, as filed with the SEC on March 30, 2020, as updated in our for the Phase 1/2 clinical study of TC-210 and our planned Phase I clinical trial Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, as filed of TC-110, our expectations for the safety and efficacy of our product with the SEC on May 14, 2020, and in our future filings with the SEC available at candidates, including TC-210 and TC-110, compared to current T-cell therapy the SEC’s website at www.sec.gov. New risks and uncertainties may emerge approaches, and our expectations regarding the estimated patient populations from time to time, and it is not possible to predict all risks and uncertainties. You and related market opportunities in TC-210’s and TC-110’s targeted indications, should not place undue reliance on any forward‐ looking statements, which are forward-looking statements. These statements are based on management’s speak only as of the date they are made. current expectations and beliefs and are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially While we may elect to update these forward-looking statements at some point in from those discussed in such forward-looking statements. the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe Such risks and uncertainties include, among others: uncertainties inherent in the expectations reflected in such forward-looking statements are reasonable, clinical studies and in the availability and timing of data from ongoing clinical we can give no assurance that such expectations will prove to be correct. studies; whether interim results from a clinical trial will be predictive of the final Accordingly, readers are cautioned not to place undue reliance on these results of a trial; the possibility that positive results from preclinical studies and forward-looking statements. No representations or warranties (expressed or correlative studies may not necessarily be predictive of the results of our implied) are made about the accuracy of any such forward-looking statements. planned clinical trials, including the Phase 1/2 clinical trial of TC-210 and the 2Forward Looking Statements 2 This presentation has been prepared by TCR Therapeutics Inc. (“we,” “us,” or planned Phase 1 clinical trial of TC-110; the risk that the results from the Phase “our”) and contains forward-looking statements within the meaning of the Private 1/2 clinical trial of TC-210 will not support further development and marketing Securities Litigation Reform Act of 1995 and other federal securities laws. approval; the risk that we may be unable to gain approval of TC-210 and our Forward-looking statements are neither historical facts nor assurances of future other product candidates on a timely basis, if at all; the risk that we have over- performance. Instead, they are based on our current beliefs, expectations and estimated the potential patient population for our product candidates, if assumptions regarding the future of our business, future plans and strategies, approved; the risk that the current COVID-19 pandemic will impact our clinical our development plans, our clinical results and other future conditions. All trials and other operations; and the other risks set forth under the caption “Risk statements, other than statements of historical facts, contained in this Factors” in our most recent Annual Report on Form 10-K for the year ended presentation, including express or implied statements regarding our expectations December 31, 2019, as filed with the SEC on March 30, 2020, as updated in our for the Phase 1/2 clinical study of TC-210 and our planned Phase I clinical trial Quarterly Report on Form 10-Q for the quarter ended March 31, 2020, as filed of TC-110, our expectations for the safety and efficacy of our product with the SEC on May 14, 2020, and in our future filings with the SEC available at candidates, including TC-210 and TC-110, compared to current T-cell therapy the SEC’s website at www.sec.gov. New risks and uncertainties may emerge approaches, and our expectations regarding the estimated patient populations from time to time, and it is not possible to predict all risks and uncertainties. You and related market opportunities in TC-210’s and TC-110’s targeted indications, should not place undue reliance on any forward‐ looking statements, which are forward-looking statements. These statements are based on management’s speak only as of the date they are made. current expectations and beliefs and are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially While we may elect to update these forward-looking statements at some point in from those discussed in such forward-looking statements. the future, we assume no obligation to update or revise any forward-looking statements except to the extent required by applicable law. Although we believe Such risks and uncertainties include, among others: uncertainties inherent in the expectations reflected in such forward-looking statements are reasonable, clinical studies and in the availability and timing of data from ongoing clinical we can give no assurance that such expectations will prove to be correct. studies; whether interim results from a clinical trial will be predictive of the final Accordingly, readers are cautioned not to place undue reliance on these results of a trial; the possibility that positive results from preclinical studies and forward-looking statements. No representations or warranties (expressed or correlative studies may not necessarily be predictive of the results of our implied) are made about the accuracy of any such forward-looking statements. planned clinical trials, including the Phase 1/2 clinical trial of TC-210 and the 2

Introduction Garry Menzel, PhD Chief Executive Officer 3Introduction Garry Menzel, PhD Chief Executive Officer 3
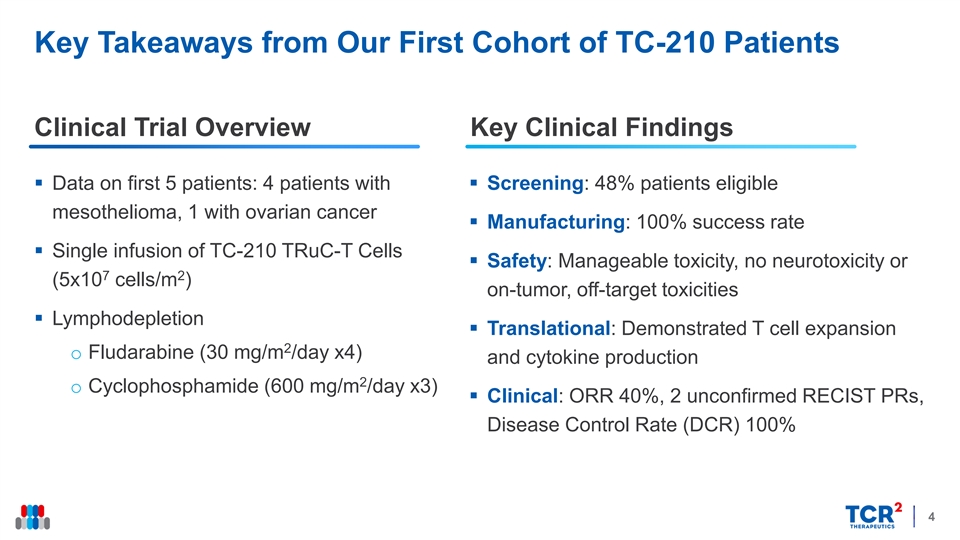
Key Takeaways from Our First Cohort of TC-210 Patients Clinical Trial Overview Key Clinical Findings § Data on first 5 patients: 4 patients with § Screening: 48% patients eligible mesothelioma, 1 with ovarian cancer § Manufacturing: 100% success rate § Single infusion of TC-210 TRuC-T Cells § Safety: Manageable toxicity, no neurotoxicity or 7 2 (5x10 cells/m ) on-tumor, off-target toxicities § Lymphodepletion § Translational: Demonstrated T cell expansion 2 o Fludarabine (30 mg/m /day x4) and cytokine production 2 o Cyclophosphamide (600 mg/m /day x3) § Clinical: ORR 40%, 2 unconfirmed RECIST PRs, Disease Control Rate (DCR) 100% 4Key Takeaways from Our First Cohort of TC-210 Patients Clinical Trial Overview Key Clinical Findings § Data on first 5 patients: 4 patients with § Screening: 48% patients eligible mesothelioma, 1 with ovarian cancer § Manufacturing: 100% success rate § Single infusion of TC-210 TRuC-T Cells § Safety: Manageable toxicity, no neurotoxicity or 7 2 (5x10 cells/m ) on-tumor, off-target toxicities § Lymphodepletion § Translational: Demonstrated T cell expansion 2 o Fludarabine (30 mg/m /day x4) and cytokine production 2 o Cyclophosphamide (600 mg/m /day x3) § Clinical: ORR 40%, 2 unconfirmed RECIST PRs, Disease Control Rate (DCR) 100% 4
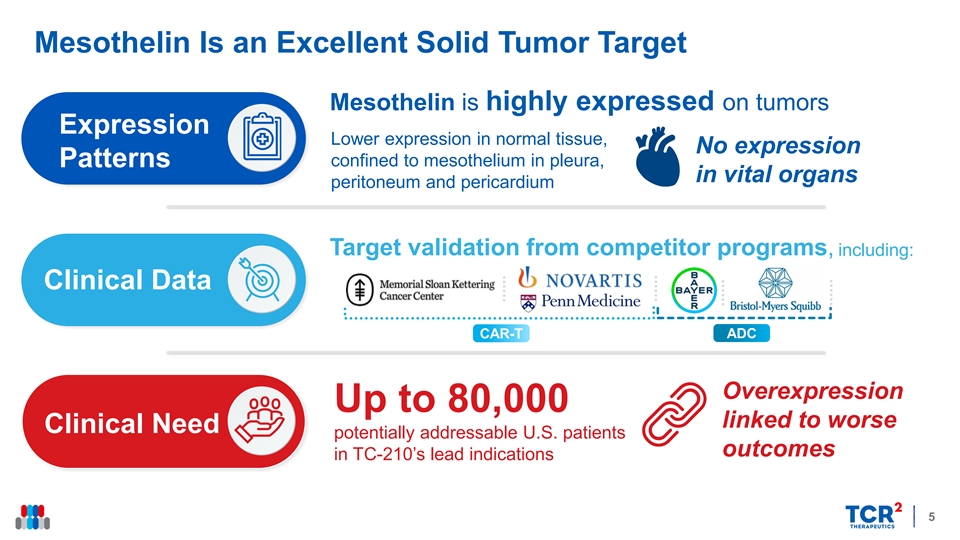
Mesothelin Is an Excellent Solid Tumor Target Mesothelin is highly expressed on tumors Expression Lower expression in normal tissue, No expression Patterns confined to mesothelium in pleura, in vital organs peritoneum and pericardium Target validation from competitor programs, including: Clinical Data ADC CAR-T Overexpression Up to 80,000 linked to worse Clinical Need potentially addressable U.S. patients outcomes in TC-210’s lead indications 5Mesothelin Is an Excellent Solid Tumor Target Mesothelin is highly expressed on tumors Expression Lower expression in normal tissue, No expression Patterns confined to mesothelium in pleura, in vital organs peritoneum and pericardium Target validation from competitor programs, including: Clinical Data ADC CAR-T Overexpression Up to 80,000 linked to worse Clinical Need potentially addressable U.S. patients outcomes in TC-210’s lead indications 5
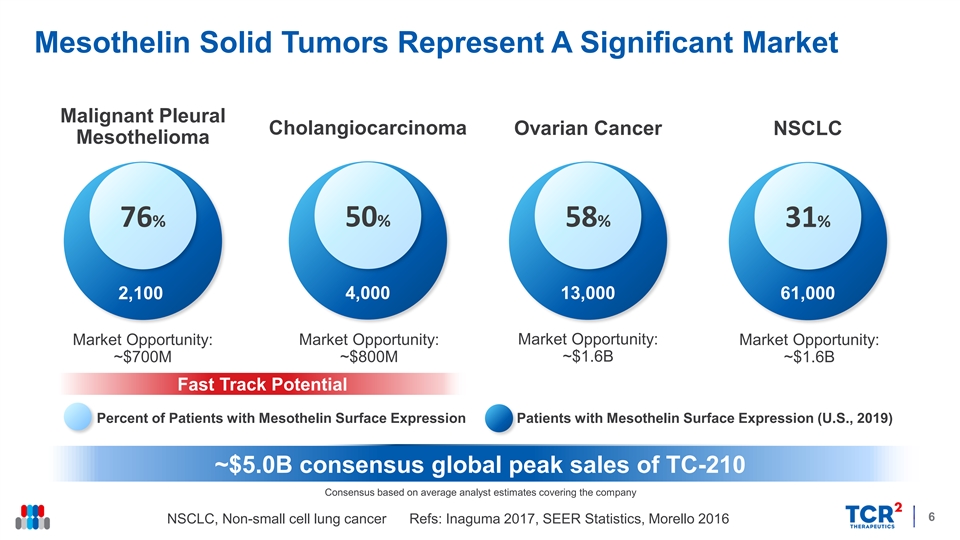
Mesothelin Solid Tumors Represent A Significant Market Malignant Pleural Cholangiocarcinoma Ovarian Cancer NSCLC Mesothelioma 76% 50% 58% 31% 2,100 4,000 13,000 61,000 Market Opportunity: Market Opportunity: Market Opportunity: Market Opportunity: ~$700M ~$800M ~$1.6B ~$1.6B Fast Track Potential Percent of Patients with Mesothelin Surface Expression Patients with Mesothelin Surface Expression (U.S., 2019) ~$5.0B consensus global peak sales of TC-210 Consensus based on average analyst estimates covering the company 6 NSCLC, Non-small cell lung cancer Refs: Inaguma 2017, SEER Statistics, Morello 2016 Mesothelin Solid Tumors Represent A Significant Market Malignant Pleural Cholangiocarcinoma Ovarian Cancer NSCLC Mesothelioma 76% 50% 58% 31% 2,100 4,000 13,000 61,000 Market Opportunity: Market Opportunity: Market Opportunity: Market Opportunity: ~$700M ~$800M ~$1.6B ~$1.6B Fast Track Potential Percent of Patients with Mesothelin Surface Expression Patients with Mesothelin Surface Expression (U.S., 2019) ~$5.0B consensus global peak sales of TC-210 Consensus based on average analyst estimates covering the company 6 NSCLC, Non-small cell lung cancer Refs: Inaguma 2017, SEER Statistics, Morello 2016

TC-210 Clinical Trial Review Alfonso Quintás-Cardama, MD Chief Medical Officer 7TC-210 Clinical Trial Review Alfonso Quintás-Cardama, MD Chief Medical Officer 7
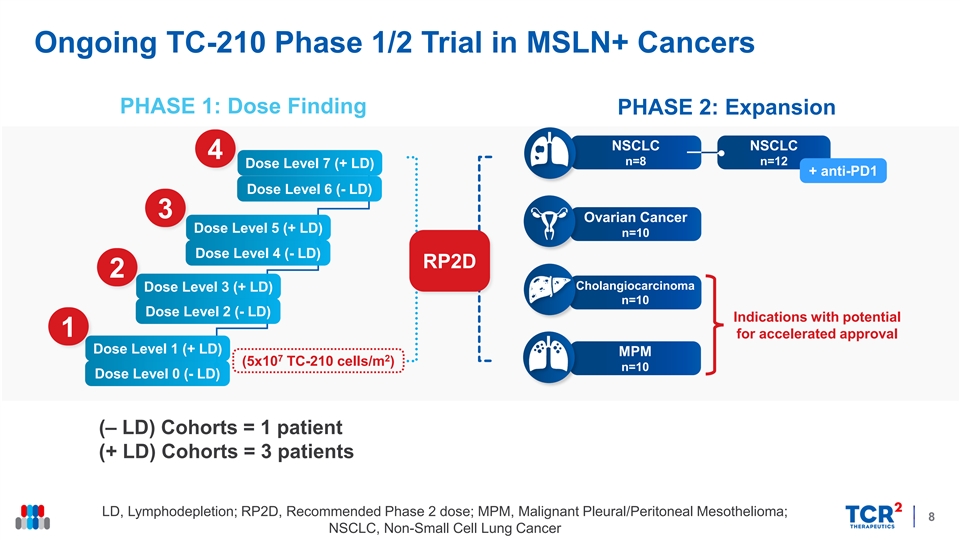
Ongoing TC-210 Phase 1/2 Trial in MSLN+ Cancers PHASE 1: Dose Finding PHASE 2: Expansion NSCLC NSCLC 4 n=8 n=12 Dose Level 7 (+ LD) + anti-PD1 Dose Level 6 (- LD) 3 Ovarian Cancer Dose Level 5 (+ LD) n=10 Dose Level 4 (- LD) RP2D 2 Cholangiocarcinoma Dose Level 3 (+ LD) n=10 Dose Level 2 (- LD) Indications with potential 1 for accelerated approval Dose Level 1 (+ LD) MPM 7 2 (5x10 TC-210 cells/m ) n=10 Dose Level 0 (- LD) (‒ LD) Cohorts = 1 patient (+ LD) Cohorts = 3 patients LD, Lymphodepletion; RP2D, Recommended Phase 2 dose; MPM, Malignant Pleural/Peritoneal Mesothelioma; 8 NSCLC, Non-Small Cell Lung CancerOngoing TC-210 Phase 1/2 Trial in MSLN+ Cancers PHASE 1: Dose Finding PHASE 2: Expansion NSCLC NSCLC 4 n=8 n=12 Dose Level 7 (+ LD) + anti-PD1 Dose Level 6 (- LD) 3 Ovarian Cancer Dose Level 5 (+ LD) n=10 Dose Level 4 (- LD) RP2D 2 Cholangiocarcinoma Dose Level 3 (+ LD) n=10 Dose Level 2 (- LD) Indications with potential 1 for accelerated approval Dose Level 1 (+ LD) MPM 7 2 (5x10 TC-210 cells/m ) n=10 Dose Level 0 (- LD) (‒ LD) Cohorts = 1 patient (+ LD) Cohorts = 3 patients LD, Lymphodepletion; RP2D, Recommended Phase 2 dose; MPM, Malignant Pleural/Peritoneal Mesothelioma; 8 NSCLC, Non-Small Cell Lung Cancer
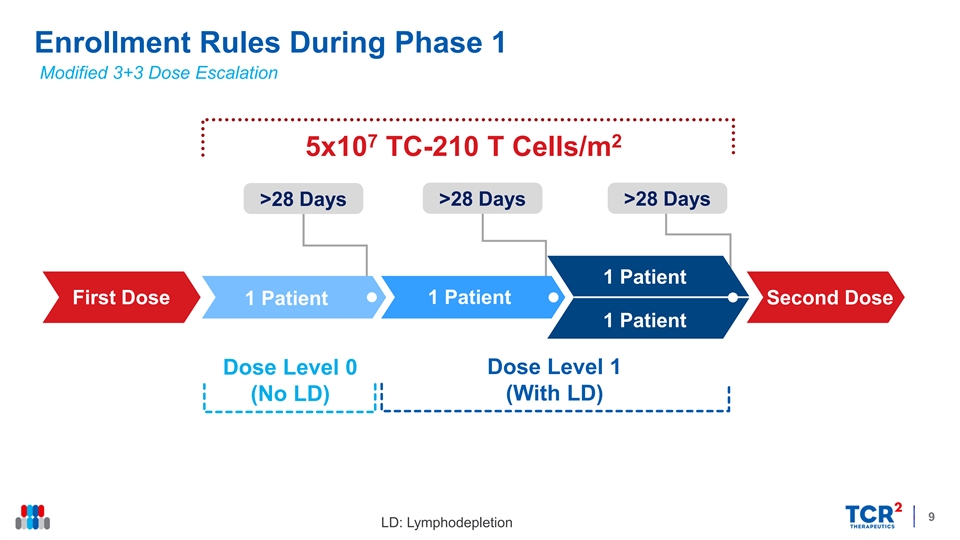
Enrollment Rules During Phase 1 Modified 3+3 Dose Escalation 7 2 5x10 TC-210 T Cells/m >28 Days >28 Days >28 Days 1 Patient First Dose 1 Patient 1 Patient Second Dose 1 Patient Dose Level 1 Dose Level 0 (No LD) (With LD) 9 LD: LymphodepletionEnrollment Rules During Phase 1 Modified 3+3 Dose Escalation 7 2 5x10 TC-210 T Cells/m >28 Days >28 Days >28 Days 1 Patient First Dose 1 Patient 1 Patient Second Dose 1 Patient Dose Level 1 Dose Level 0 (No LD) (With LD) 9 LD: Lymphodepletion
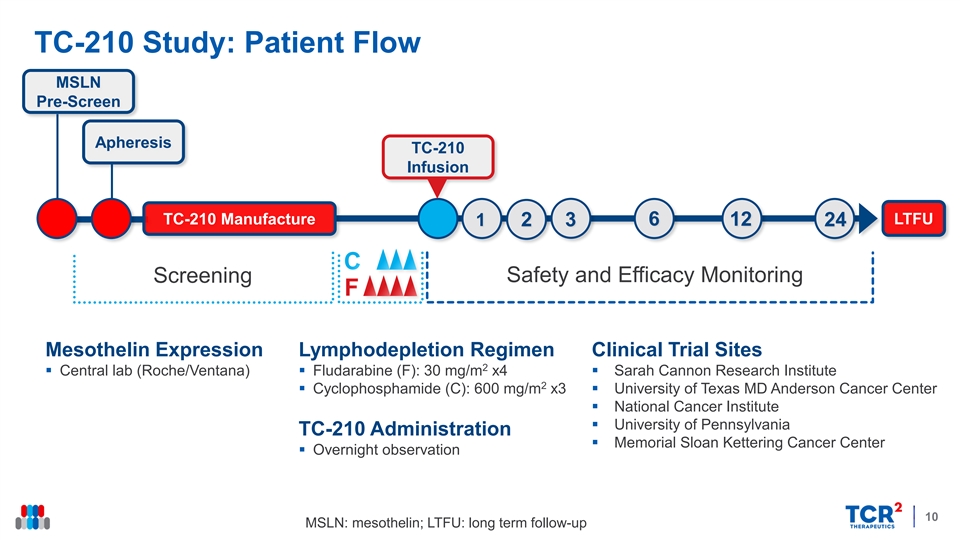
TC-210 Study: Patient Flow MSLN Pre-Screen Apheresis TC-210 Infusion LTFU TC-210 Manufacture 1 2 3 6 12 24 Safety and Efficacy Monitoring Screening Mesothelin Expression Lymphodepletion Regimen Clinical Trial Sites 2 § Central lab (Roche/Ventana)§ Fludarabine (F): 30 mg/m x4§ Sarah Cannon Research Institute 2 § Cyclophosphamide (C): 600 mg/m x3§ University of Texas MD Anderson Cancer Center § National Cancer Institute § University of Pennsylvania TC-210 Administration § Memorial Sloan Kettering Cancer Center § Overnight observation 10 MSLN: mesothelin; LTFU: long term follow-upTC-210 Study: Patient Flow MSLN Pre-Screen Apheresis TC-210 Infusion LTFU TC-210 Manufacture 1 2 3 6 12 24 Safety and Efficacy Monitoring Screening Mesothelin Expression Lymphodepletion Regimen Clinical Trial Sites 2 § Central lab (Roche/Ventana)§ Fludarabine (F): 30 mg/m x4§ Sarah Cannon Research Institute 2 § Cyclophosphamide (C): 600 mg/m x3§ University of Texas MD Anderson Cancer Center § National Cancer Institute § University of Pennsylvania TC-210 Administration § Memorial Sloan Kettering Cancer Center § Overnight observation 10 MSLN: mesothelin; LTFU: long term follow-up

TC-210 Phase 1 Dose Escalation: Objectives Primary Exploratory § Expansion, persistence, phenotype, § Safety (Establish the RP2D) functionality of TC-210 T-cells § Serum cytokine levels § Immunogenicity Secondary § ORR (CR + PR) according to RECIST v1.1 § DCR (ORR + SD) § Duration of response § Survival (PFS, OS) 11TC-210 Phase 1 Dose Escalation: Objectives Primary Exploratory § Expansion, persistence, phenotype, § Safety (Establish the RP2D) functionality of TC-210 T-cells § Serum cytokine levels § Immunogenicity Secondary § ORR (CR + PR) according to RECIST v1.1 § DCR (ORR + SD) § Duration of response § Survival (PFS, OS) 11
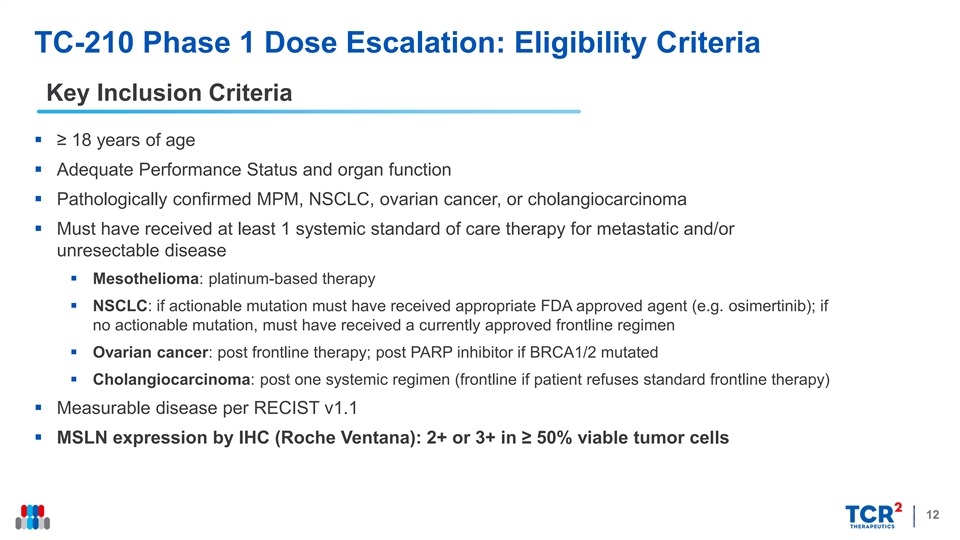
TC-210 Phase 1 Dose Escalation: Eligibility Criteria Key Inclusion Criteria § ≥ 18 years of age § Adequate Performance Status and organ function § Pathologically confirmed MPM, NSCLC, ovarian cancer, or cholangiocarcinoma § Must have received at least 1 systemic standard of care therapy for metastatic and/or unresectable disease § Mesothelioma: platinum-based therapy § NSCLC: if actionable mutation must have received appropriate FDA approved agent (e.g. osimertinib); if no actionable mutation, must have received a currently approved frontline regimen § Ovarian cancer: post frontline therapy; post PARP inhibitor if BRCA1/2 mutated § Cholangiocarcinoma: post one systemic regimen (frontline if patient refuses standard frontline therapy) § Measurable disease per RECIST v1.1 § MSLN expression by IHC (Roche Ventana): 2+ or 3+ in ≥ 50% viable tumor cells 12TC-210 Phase 1 Dose Escalation: Eligibility Criteria Key Inclusion Criteria § ≥ 18 years of age § Adequate Performance Status and organ function § Pathologically confirmed MPM, NSCLC, ovarian cancer, or cholangiocarcinoma § Must have received at least 1 systemic standard of care therapy for metastatic and/or unresectable disease § Mesothelioma: platinum-based therapy § NSCLC: if actionable mutation must have received appropriate FDA approved agent (e.g. osimertinib); if no actionable mutation, must have received a currently approved frontline regimen § Ovarian cancer: post frontline therapy; post PARP inhibitor if BRCA1/2 mutated § Cholangiocarcinoma: post one systemic regimen (frontline if patient refuses standard frontline therapy) § Measurable disease per RECIST v1.1 § MSLN expression by IHC (Roche Ventana): 2+ or 3+ in ≥ 50% viable tumor cells 12
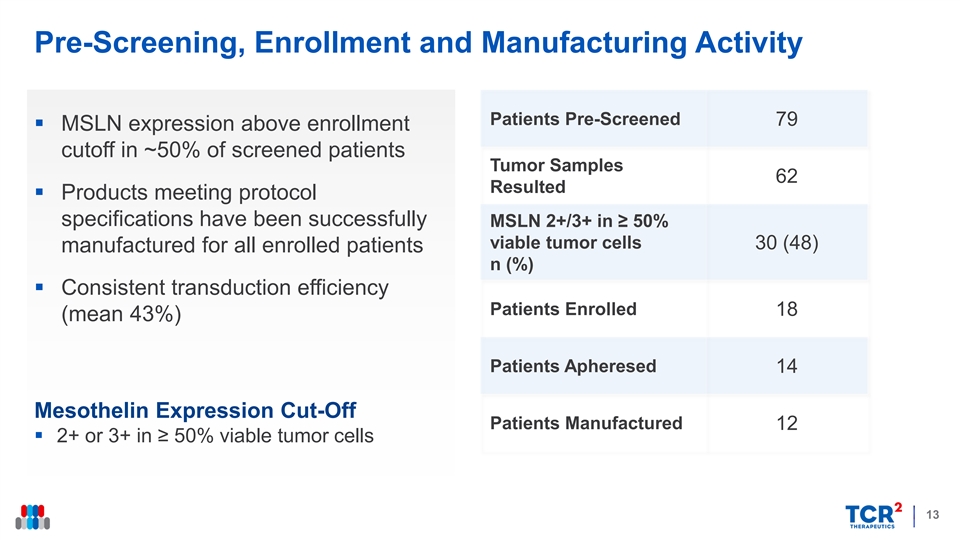
Pre-Screening, Enrollment and Manufacturing Activity Patients Pre-Screened 79 § MSLN expression above enrollment cutoff in ~50% of screened patients Tumor Samples 62 Resulted § Products meeting protocol specifications have been successfully MSLN 2+/3+ in ≥ 50% viable tumor cells 30 (48) manufactured for all enrolled patients n (%) § Consistent transduction efficiency Patients Enrolled 18 (mean 43%) Patients Apheresed 14 Mesothelin Expression Cut-Off Patients Manufactured 12 § 2+ or 3+ in ≥ 50% viable tumor cells 13Pre-Screening, Enrollment and Manufacturing Activity Patients Pre-Screened 79 § MSLN expression above enrollment cutoff in ~50% of screened patients Tumor Samples 62 Resulted § Products meeting protocol specifications have been successfully MSLN 2+/3+ in ≥ 50% viable tumor cells 30 (48) manufactured for all enrolled patients n (%) § Consistent transduction efficiency Patients Enrolled 18 (mean 43%) Patients Apheresed 14 Mesothelin Expression Cut-Off Patients Manufactured 12 § 2+ or 3+ in ≥ 50% viable tumor cells 13
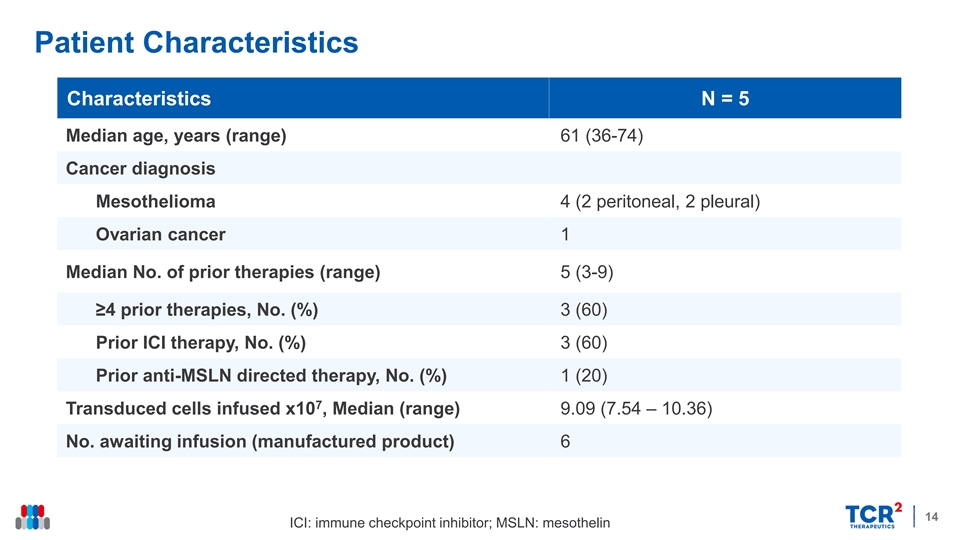
Patient Characteristics Characteristics N = 5 Median age, years (range) 61 (36-74) Cancer diagnosis Mesothelioma 4 (2 peritoneal, 2 pleural) Ovarian cancer 1 Median No. of prior therapies (range) 5 (3-9) ≥4 prior therapies, No. (%) 3 (60) Prior ICI therapy, No. (%) 3 (60) Prior anti-MSLN directed therapy, No. (%) 1 (20) 7 Transduced cells infused x10 , Median (range) 9.09 (7.54 – 10.36) No. awaiting infusion (manufactured product) 6 14 ICI: immune checkpoint inhibitor; MSLN: mesothelinPatient Characteristics Characteristics N = 5 Median age, years (range) 61 (36-74) Cancer diagnosis Mesothelioma 4 (2 peritoneal, 2 pleural) Ovarian cancer 1 Median No. of prior therapies (range) 5 (3-9) ≥4 prior therapies, No. (%) 3 (60) Prior ICI therapy, No. (%) 3 (60) Prior anti-MSLN directed therapy, No. (%) 1 (20) 7 Transduced cells infused x10 , Median (range) 9.09 (7.54 – 10.36) No. awaiting infusion (manufactured product) 6 14 ICI: immune checkpoint inhibitor; MSLN: mesothelin
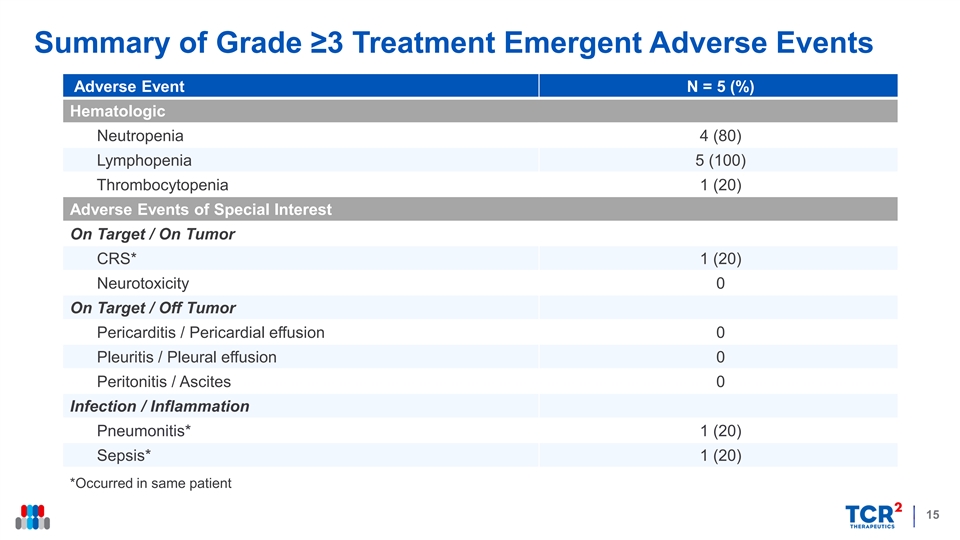
Summary of Grade ≥3 Treatment Emergent Adverse Events Adverse Event N = 5 (%) Hematologic Neutropenia 4 (80) Lymphopenia 5 (100) Thrombocytopenia 1 (20) Adverse Events of Special Interest On Target / On Tumor CRS* 1 (20) Neurotoxicity 0 On Target / Off Tumor Pericarditis / Pericardial effusion 0 Pleuritis / Pleural effusion 0 Peritonitis / Ascites 0 Infection / Inflammation Pneumonitis* 1 (20) Sepsis* 1 (20) *Occurred in same patient 15Summary of Grade ≥3 Treatment Emergent Adverse Events Adverse Event N = 5 (%) Hematologic Neutropenia 4 (80) Lymphopenia 5 (100) Thrombocytopenia 1 (20) Adverse Events of Special Interest On Target / On Tumor CRS* 1 (20) Neurotoxicity 0 On Target / Off Tumor Pericarditis / Pericardial effusion 0 Pleuritis / Pleural effusion 0 Peritonitis / Ascites 0 Infection / Inflammation Pneumonitis* 1 (20) Sepsis* 1 (20) *Occurred in same patient 15
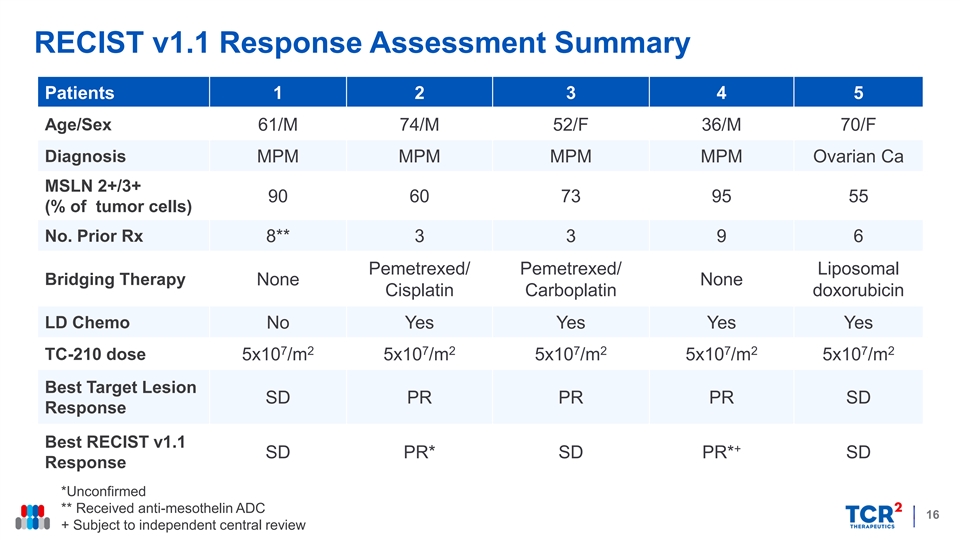
RECIST v1.1 Response Assessment Summary Patients 1 2 3 4 5 Age/Sex 61/M 74/M 52/F 36/M 70/F Diagnosis MPM MPM MPM MPM Ovarian Ca MSLN 2+/3+ 90 60 73 95 55 (% of tumor cells) No. Prior Rx 8** 3 3 9 6 Pemetrexed/ Pemetrexed/ Liposomal Bridging Therapy None None Cisplatin Carboplatin doxorubicin LD Chemo No Yes Yes Yes Yes 7 2 7 2 7 2 7 2 7 2 TC-210 dose 5x10 /m 5x10 /m 5x10 /m 5x10 /m 5x10 /m ** Best Target Lesion SD PR PR PR SD Response Best RECIST v1.1 + SD PR* SD PR* SD Response *Unconfirmed ** Received anti-mesothelin ADC 16 + Subject to independent central reviewRECIST v1.1 Response Assessment Summary Patients 1 2 3 4 5 Age/Sex 61/M 74/M 52/F 36/M 70/F Diagnosis MPM MPM MPM MPM Ovarian Ca MSLN 2+/3+ 90 60 73 95 55 (% of tumor cells) No. Prior Rx 8** 3 3 9 6 Pemetrexed/ Pemetrexed/ Liposomal Bridging Therapy None None Cisplatin Carboplatin doxorubicin LD Chemo No Yes Yes Yes Yes 7 2 7 2 7 2 7 2 7 2 TC-210 dose 5x10 /m 5x10 /m 5x10 /m 5x10 /m 5x10 /m ** Best Target Lesion SD PR PR PR SD Response Best RECIST v1.1 + SD PR* SD PR* SD Response *Unconfirmed ** Received anti-mesothelin ADC 16 + Subject to independent central review
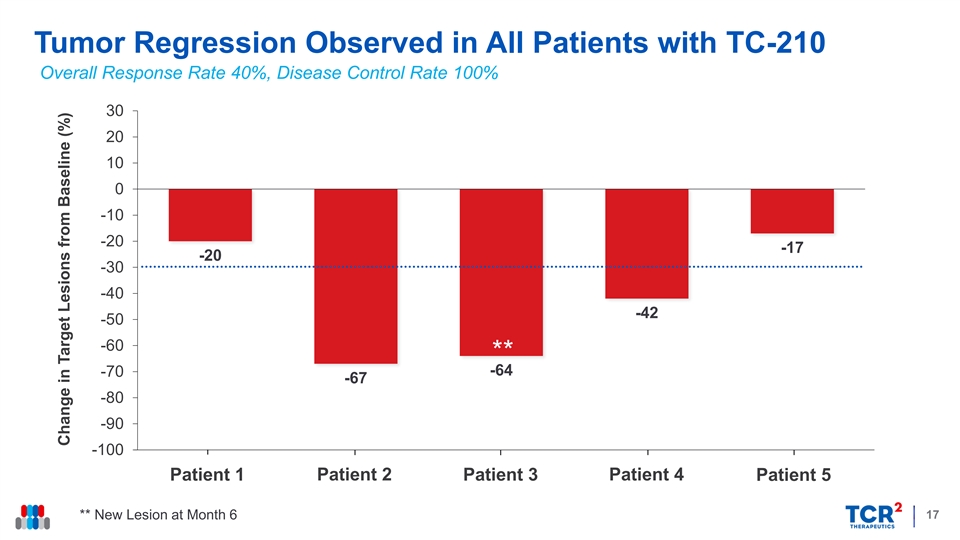
Tumor Regression Observed in All Patients with TC-210 Overall Response Rate 40%, Disease Control Rate 100% 30 20 10 0 Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 -10 -20 -17 -20 -30 -40 -42 -50 -60 ** -64 -70 -67 -80 -90 -100 Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 17 ** New Lesion at Month 6 Change in Target Lesions from Baseline (%)Tumor Regression Observed in All Patients with TC-210 Overall Response Rate 40%, Disease Control Rate 100% 30 20 10 0 Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 -10 -20 -17 -20 -30 -40 -42 -50 -60 ** -64 -70 -67 -80 -90 -100 Patient 1 Patient 2 Patient 3 Patient 4 Patient 5 17 ** New Lesion at Month 6 Change in Target Lesions from Baseline (%)
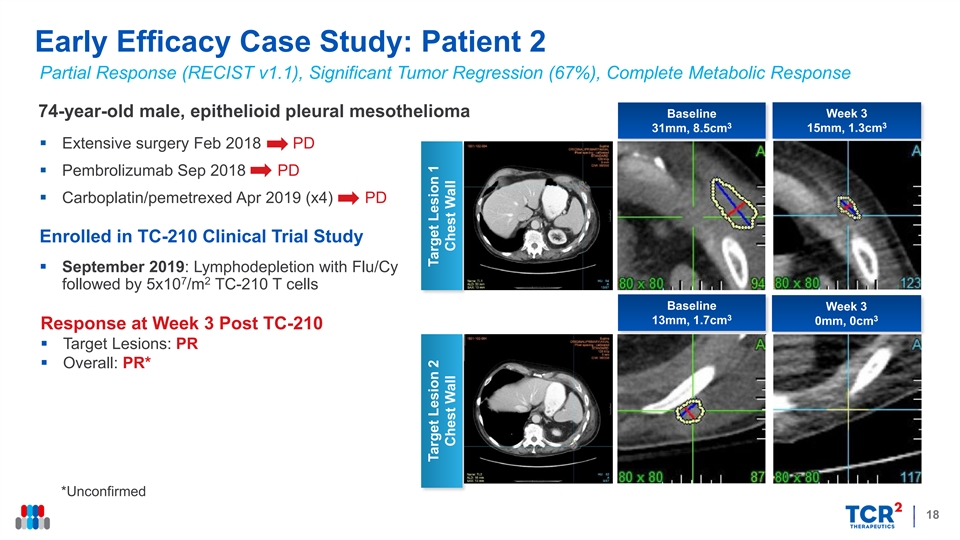
Early Efficacy Case Study: Patient 2 Partial Response (RECIST v1.1), Significant Tumor Regression (67%), Complete Metabolic Response 74-year-old male, epithelioid pleural mesothelioma Baseline Week 3 3 3 31mm, 8.5cm 15mm, 1.3cm § Extensive surgery Feb 2018 PD § Pembrolizumab Sep 2018 PD § Carboplatin/pemetrexed Apr 2019 (x4) PD Enrolled in TC-210 Clinical Trial Study § September 2019: Lymphodepletion with Flu/Cy 7 2 followed by 5x10 /m TC-210 T cells Baseline Week 3 3 3 13mm, 1.7cm 0mm, 0cm Response at Week 3 Post TC-210 § Target Lesions: PR § Overall: PR* *Unconfirmed 18 Target Lesion 2 Target Lesion 1 Chest Wall Chest WallEarly Efficacy Case Study: Patient 2 Partial Response (RECIST v1.1), Significant Tumor Regression (67%), Complete Metabolic Response 74-year-old male, epithelioid pleural mesothelioma Baseline Week 3 3 3 31mm, 8.5cm 15mm, 1.3cm § Extensive surgery Feb 2018 PD § Pembrolizumab Sep 2018 PD § Carboplatin/pemetrexed Apr 2019 (x4) PD Enrolled in TC-210 Clinical Trial Study § September 2019: Lymphodepletion with Flu/Cy 7 2 followed by 5x10 /m TC-210 T cells Baseline Week 3 3 3 13mm, 1.7cm 0mm, 0cm Response at Week 3 Post TC-210 § Target Lesions: PR § Overall: PR* *Unconfirmed 18 Target Lesion 2 Target Lesion 1 Chest Wall Chest Wall
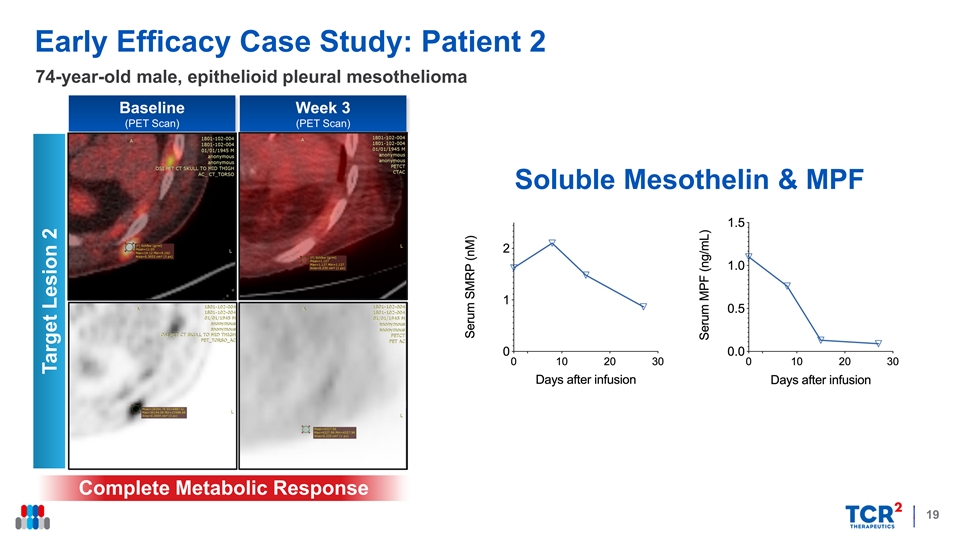
Early Efficacy Case Study: Patient 2 74-year-old male, epithelioid pleural mesothelioma Baseline Week 3 (PET Scan) (PET Scan) Soluble Mesothelin & MPF Complete Metabolic Response 19 Target Lesion 2Early Efficacy Case Study: Patient 2 74-year-old male, epithelioid pleural mesothelioma Baseline Week 3 (PET Scan) (PET Scan) Soluble Mesothelin & MPF Complete Metabolic Response 19 Target Lesion 2
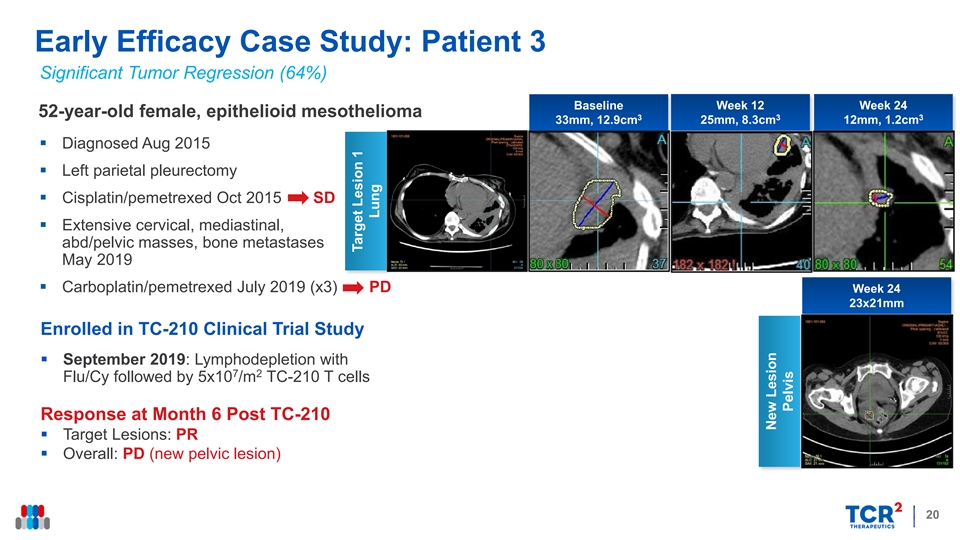
Early Efficacy Case Study: Patient 3 Significant Tumor Regression (64%) Baseline Week 12 Week 24 52-year-old female, epithelioid mesothelioma 3 3 3 33mm, 12.9cm 25mm, 8.3cm 12mm, 1.2cm § Diagnosed Aug 2015 § Left parietal pleurectomy § Cisplatin/pemetrexed Oct 2015 SD § Extensive cervical, mediastinal, abd/pelvic masses, bone metastases May 2019 § Carboplatin/pemetrexed July 2019 (x3) PD Week 24 23x21mm Enrolled in TC-210 Clinical Trial Study § September 2019: Lymphodepletion with 7 2 Flu/Cy followed by 5x10 /m TC-210 T cells Response at Month 6 Post TC-210 § Target Lesions: PR § Overall: PD (new pelvic lesion) 20 Target Lesion 1 Lung New Lesion PelvisEarly Efficacy Case Study: Patient 3 Significant Tumor Regression (64%) Baseline Week 12 Week 24 52-year-old female, epithelioid mesothelioma 3 3 3 33mm, 12.9cm 25mm, 8.3cm 12mm, 1.2cm § Diagnosed Aug 2015 § Left parietal pleurectomy § Cisplatin/pemetrexed Oct 2015 SD § Extensive cervical, mediastinal, abd/pelvic masses, bone metastases May 2019 § Carboplatin/pemetrexed July 2019 (x3) PD Week 24 23x21mm Enrolled in TC-210 Clinical Trial Study § September 2019: Lymphodepletion with 7 2 Flu/Cy followed by 5x10 /m TC-210 T cells Response at Month 6 Post TC-210 § Target Lesions: PR § Overall: PD (new pelvic lesion) 20 Target Lesion 1 Lung New Lesion Pelvis
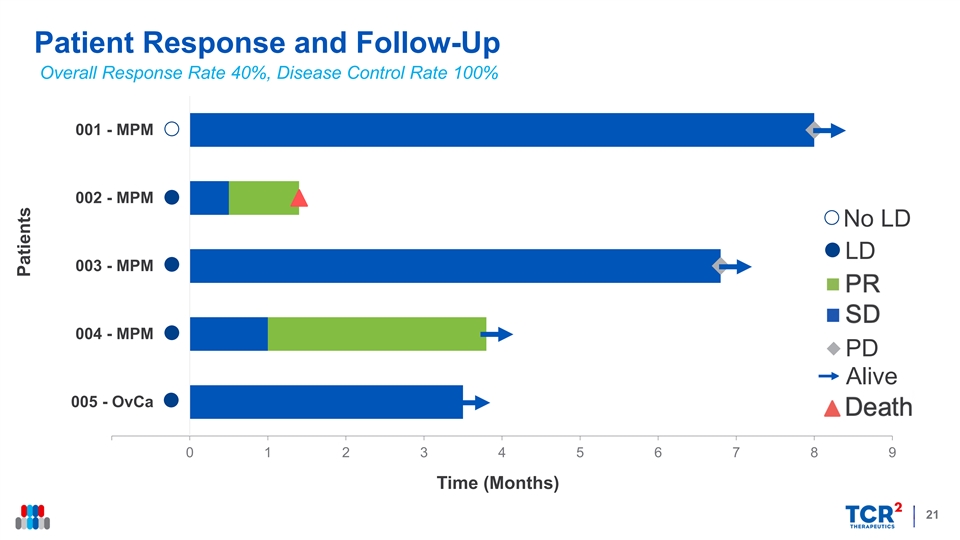
Patient Response and Follow-Up Overall Response Rate 40%, Disease Control Rate 100% 001 - MPM 002 - MPM No LD LD 003 - MPM SD PR 004 - MPM PD Alive Death 005 - OvCa 0 1 2 3 4 5 6 7 8 9 Time (Months) 21 PatientsPatient Response and Follow-Up Overall Response Rate 40%, Disease Control Rate 100% 001 - MPM 002 - MPM No LD LD 003 - MPM SD PR 004 - MPM PD Alive Death 005 - OvCa 0 1 2 3 4 5 6 7 8 9 Time (Months) 21 Patients
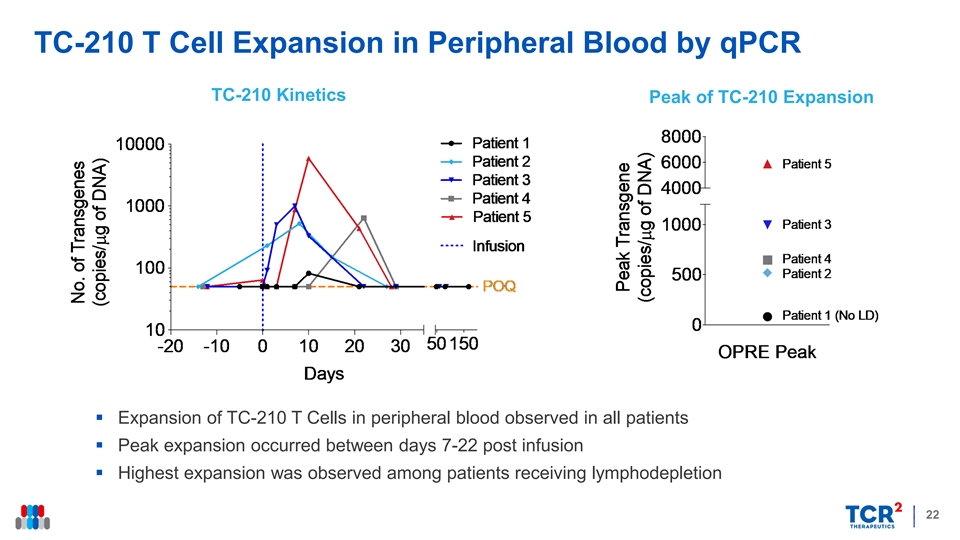
TC-210 T Cell Expansion in Peripheral Blood by qPCR TC-210 Kinetics Peak of TC-210 Expansion § Expansion of TC-210 T Cells in peripheral blood observed in all patients § Peak expansion occurred between days 7-22 post infusion § Highest expansion was observed among patients receiving lymphodepletion 22TC-210 T Cell Expansion in Peripheral Blood by qPCR TC-210 Kinetics Peak of TC-210 Expansion § Expansion of TC-210 T Cells in peripheral blood observed in all patients § Peak expansion occurred between days 7-22 post infusion § Highest expansion was observed among patients receiving lymphodepletion 22
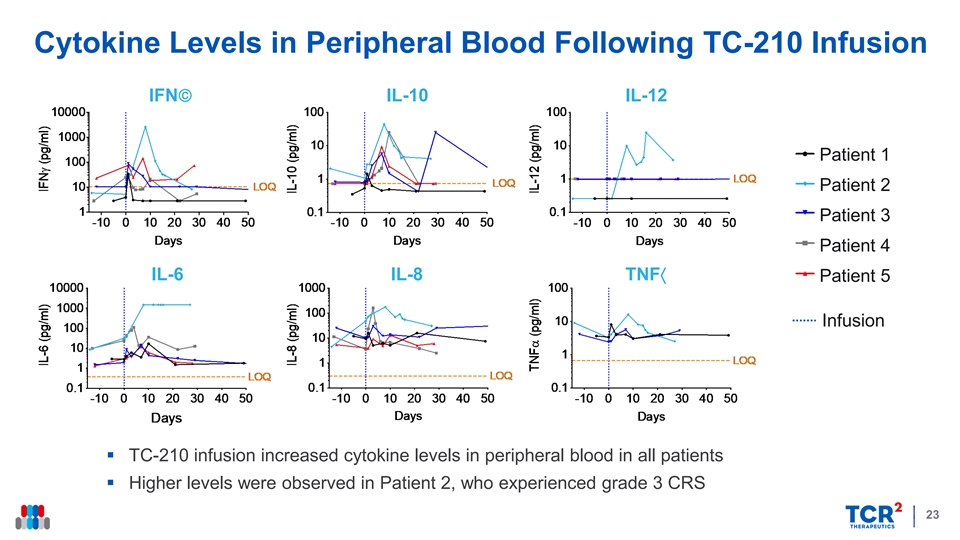
Cytokine Levels in Peripheral Blood Following TC-210 Infusion IFN IL-10 IL-12 Patient 1 Patient 2 Patient 3 Patient 4 IL-6 IL-8 TNF〈 Patient 5 Infusion § TC-210 infusion increased cytokine levels in peripheral blood in all patients § Higher levels were observed in Patient 2, who experienced grade 3 CRS 23Cytokine Levels in Peripheral Blood Following TC-210 Infusion IFN IL-10 IL-12 Patient 1 Patient 2 Patient 3 Patient 4 IL-6 IL-8 TNF〈 Patient 5 Infusion § TC-210 infusion increased cytokine levels in peripheral blood in all patients § Higher levels were observed in Patient 2, who experienced grade 3 CRS 23
TC-210: Summary and Future Direction § Encouraging data at first dose tested o Consistent clinical benefit at first TC-210 T cell dose o Manageable safety profile o High rate of patient eligibility and 100% manufacturing success § Next steps o Continue dose escalation until identification of RP2D o Initiate phase 2 portion of study, including TC-210 combination with anti-PD1 antibody § Additional data expected later in 2020 o Data on additional cohorts o Detailed translational data 24TC-210: Summary and Future Direction § Encouraging data at first dose tested o Consistent clinical benefit at first TC-210 T cell dose o Manageable safety profile o High rate of patient eligibility and 100% manufacturing success § Next steps o Continue dose escalation until identification of RP2D o Initiate phase 2 portion of study, including TC-210 combination with anti-PD1 antibody § Additional data expected later in 2020 o Data on additional cohorts o Detailed translational data 24
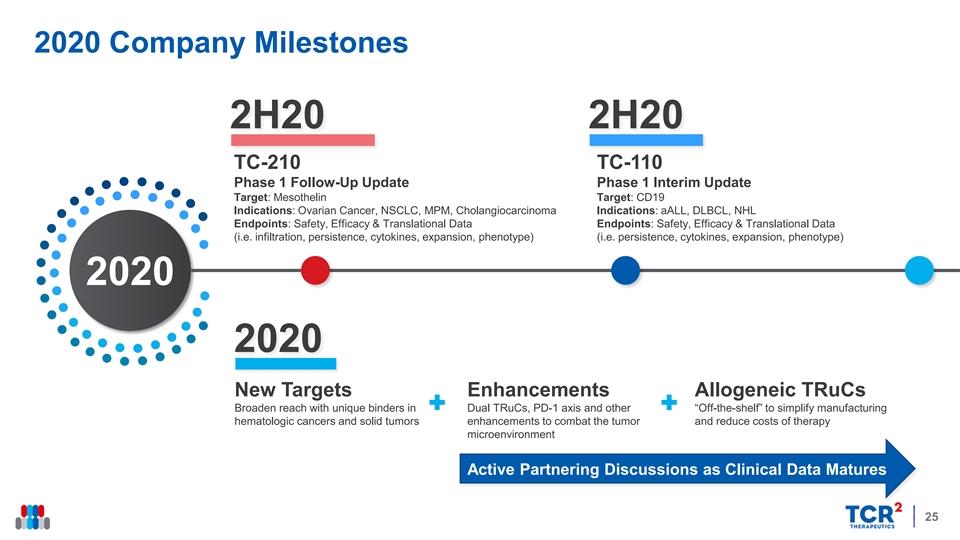
2020 Company Milestones 2H20 2H20 TC-210 TC-110 Phase 1 Follow-Up Update Phase 1 Interim Update Target: Mesothelin Target: CD19 Indications: Ovarian Cancer, NSCLC, MPM, Cholangiocarcinoma Indications: aALL, DLBCL, NHL Endpoints: Safety, Efficacy & Translational Data Endpoints: Safety, Efficacy & Translational Data (i.e. infiltration, persistence, cytokines, expansion, phenotype) (i.e. persistence, cytokines, expansion, phenotype) 2020 2020 New Targets Enhancements Allogeneic TRuCs Broaden reach with unique binders in Dual TRuCs, PD-1 axis and other “Off-the-shelf” to simplify manufacturing hematologic cancers and solid tumors enhancements to combat the tumor and reduce costs of therapy microenvironment Active Partnering Discussions as Clinical Data Matures 252020 Company Milestones 2H20 2H20 TC-210 TC-110 Phase 1 Follow-Up Update Phase 1 Interim Update Target: Mesothelin Target: CD19 Indications: Ovarian Cancer, NSCLC, MPM, Cholangiocarcinoma Indications: aALL, DLBCL, NHL Endpoints: Safety, Efficacy & Translational Data Endpoints: Safety, Efficacy & Translational Data (i.e. infiltration, persistence, cytokines, expansion, phenotype) (i.e. persistence, cytokines, expansion, phenotype) 2020 2020 New Targets Enhancements Allogeneic TRuCs Broaden reach with unique binders in Dual TRuCs, PD-1 axis and other “Off-the-shelf” to simplify manufacturing hematologic cancers and solid tumors enhancements to combat the tumor and reduce costs of therapy microenvironment Active Partnering Discussions as Clinical Data Matures 25
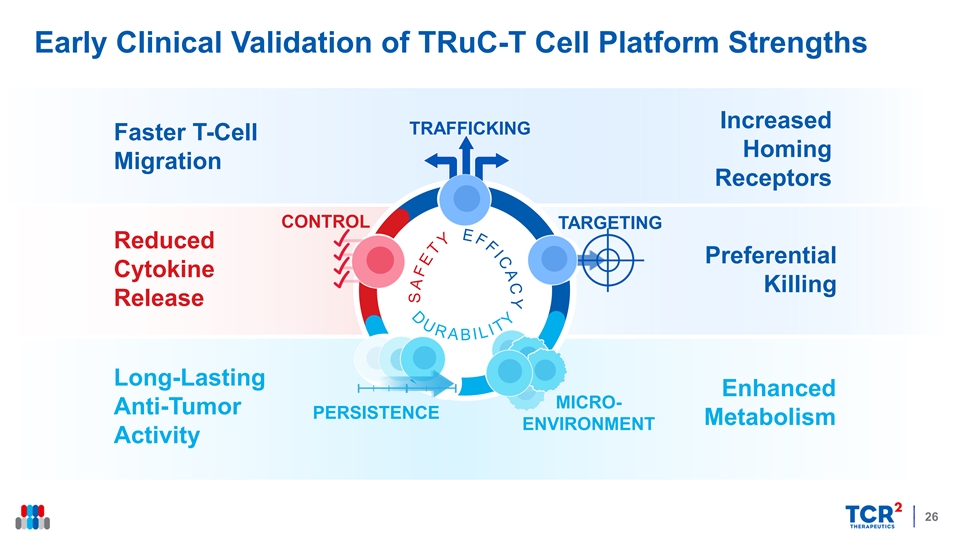
Early Clinical Validation of TRuC-T Cell Platform Strengths Increased TRAFFICKING Faster T-Cell Homing Migration Receptors CONTROL TARGETING Reduced Preferential Cytokine Killing Release Long-Lasting Enhanced MICRO- Anti-Tumor PERSISTENCE Metabolism ENVIRONMENT Activity 26Early Clinical Validation of TRuC-T Cell Platform Strengths Increased TRAFFICKING Faster T-Cell Homing Migration Receptors CONTROL TARGETING Reduced Preferential Cytokine Killing Release Long-Lasting Enhanced MICRO- Anti-Tumor PERSISTENCE Metabolism ENVIRONMENT Activity 26

Thank YouThank You
