Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Entasis Therapeutics Holdings Inc. | tm2025497d1_8k.htm |
Exhibit 99.1

Non - Confidential Targeted Solutions for Antibacterial Resistance Precision antibiotics focused against critical pathogens July 2020

Non - Confidential Disclaimer This presentation contains forward - looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as ‘‘anticipate,’’ ‘‘believe,’’ ‘‘continue,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘target,’’ ‘‘should,’’ ‘‘would,’’ and similar expressions are intended to identify forward - looking statements, although not all forward - looking statements contain these identifying words. All statements, other than statements of historical facts, contained in this presentation are forward - looking statements, including statements regarding the plans of Entasis Therapeutics Holdings Inc. (the “Company”) to develop and commercialize its product candidates, the Company's ongoing and planned clinical trials, the timing and availability of data from its clinical trials, advancement of ETX0462 into a phase 1 clinical trial, the efficacy and safety data from its ongoing phase 3 trials that, if positive, will be sufficient to support the submission of a new drug application (NDA) to the US Food and Drug Administration (FDA), its ability to obtain grants or other government funding to develop product candidates, its ability to take advantage of benefits offered by current and pending legislation related to the development of products addressing antimicrobial resistance, the timing of its planned regulatory filings, the timing of and ability to obtain and maintain regulatory approval for its product candidates, the clinical utility of its product candidates and the potential advantages compared to other treatments, its commercialization, marketing and distribution capabilities and strategy, its ability to establish and maintain arrangements for the manufacture of its product candidates, its ability to establish and maintain collaborations and to recognize the potential benefits of such collaborations, its estimates regarding the market opportunities for its product candidates, its intellectual property position and duration of its patent rights and its estimates regarding future expenses, capital requirements and needs for additional financing. Forward - looking statements are based on the Company’s current expectations and are subject to inherent uncertainties, risks and assumptions that are difficult to predict. Factors that could cause actual results to differ include, but are not limited to, unexpected safety or efficacy data observed during preclinical or clinical trials, clinical trial site activation or enrollment rates that are lower than expected, changes in expected or existing competition, changes in the regulatory environment, failure of the Company’s collaborators to support or advance collaborations or product candidates, unexpected litigation or other disputes and the coronavirus pandemic. Many of these factors are beyond the Company’s control. These and other risks and uncertainties are discussed in the Company’s filings with the U.S. Securities and Exchange Commission, including the “Risk Factors” sections contained therein. Forward - looking statements contained in this presentation are made as of the date of this presentation, and except as required by law, the Company assumes no obligation to update any forward - looking statements contained herein to reflect any change in expectations, even as new information becomes available. T his presentation contains estimates and other statistical data made by independent parties and by the Company relating to market size and other data about the Company's industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such data and estimates. In addition, projections, assumptions and estimates of the Company's future performance and the future performance of the markets in which the Company operates are necessarily subject to a high degree of uncertainty and risk. 1

Non - Confidential Accomplished Management Team MANAGEMENT TEAM WITH EXTENSIVE ANTI - INFECTIVE EXPERIENCE Manos Perros, Ph.D. President and Chief Executive Officer Michael Gutch, Ph.D. Chief Financial Officer and Chief Business Officer Ruben Tommasi, Ph.D. Chief Scientific Officer John Mueller, Ph.D. Chief Development Officer David Altarac, M.D. Chief Medical Officer Eric Kimble Chief Commercial Officer Elizabeth Keiley, J.D. General Counsel • Spun out from AZ with 2 early stage programs in 2015 • Today, advancing 4 novel antibacterial programs • ~50 FTEs Boston BioHub, Waltham, MA 2

Non - Confidential Company Highlights Abbreviations: CRE, Carbapenem - resistant Enterobacterales; MDR, Multidrug resistant ; UTIs, Urinary tract infections 3 Leader in targeted antibiotic development ► Multidrug - resistant, pathogen - targeted focus from discovery through commercialization Innovative and robust pipeline ► Two Phase 3 clinical trials against highest - priority pathogens 1. Sulbactam - durlobactam (SUL - DUR) against Acinetobacter 2. Zoliflodacin against N. gonorrhoeae ► Two additional programs in early - stage development 3. ETX0282CPDP against MDR UTIs, including CRE 4. ETX0462 against Pseudomonas aeruginosa Clear commercial opportunities ► Potential best - in - class and first - to - market product candidates with significant revenue potential

Non - Confidential Pathogen Focus We focus on the resistance mechanisms of these multidrug resistant pathogens. Source: Centers for Disease Control and Prevention, Antibiotic Resistance Threats in the United States, 2019 4 CDC “ Urgent ” Threats CDC “ Serious ” Threat
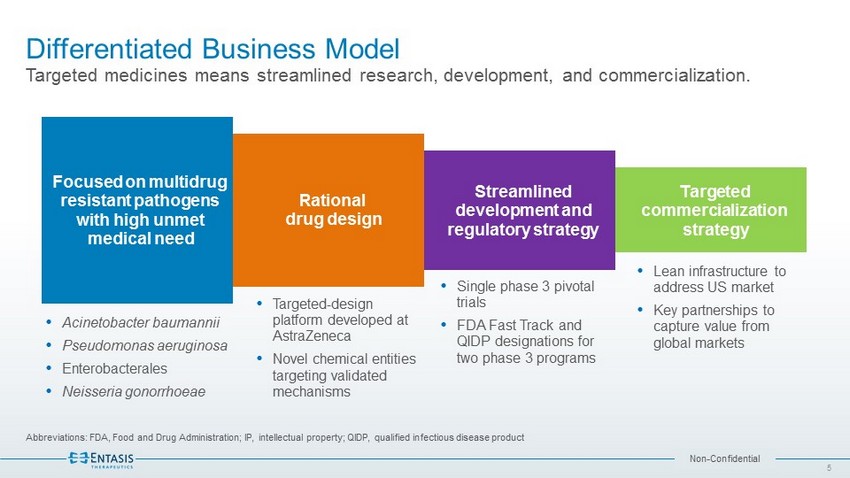
Non - Confidential Differentiated Business Model Targeted medicines means streamlined research, development, and commercialization. Abbreviations: FDA, Food and Drug Administration; IP, intellectual property; QIDP, qualified infectious disease product 5 Focused on multidrug resistant pathogens with high unmet medical need Rational drug design Streamlined development and regulatory strategy Targeted commercialization strategy Targeted - design platform developed at AstraZeneca Novel chemical entities targeting validated mechanisms Acinetobacter baumannii Pseudomonas aeruginosa Enterobacterales Neisseria gonorrhoeae Single phase 3 pivotal trials FDA Fast Track and QIDP designations for two phase 3 programs Lean infrastructure to address US market Key partnerships to capture value from global markets

Non - Confidential Robust Pipeline of Novel Antibiotics Entasis has fully - integrated research and development in our Waltham site. Abbreviations: CRE, carbapenem - resistant Enterobacterales; ESBL, extended spectrum β - lactamases; IV, intravenous; NBP, non β - lac tam penicillin binding protein inhibitor. a Zai Lab has licensed Asia - Pacific rights to sulbactam - durlobactam b Global Antibiotic Research and Development Partnership (GARDP) will fully fund the phase 3 development program and has commer cia l rights in low - income and specified middle - income countries. Entasis has retained commercial rights in all major markets in North America, Europe, and Asia - Pacific. 6 PRODUCT CANDIDATE/ INDICATION PRE - CLINICAL PHASE 1 PHASE 2 PHASE 3 PROGRAM STATUS COMMERCIAL RIGHTS PARTNERSHIPS/ GRANT FUNDING Sulbactam - durlobactam (IV) Carbapenem - resistant Acinetobacter infections Phase 3 trial actively enrolling Worldwide excluding Asia - Pacific a Zoliflodacin (Oral) Uncomplicated gonorrhea Phase 3 trial actively enrolling All developed countries b ETX0282CPDP (Oral) Complicated urinary tract infections (UTIs) ( Enterobacterales including CRE and ESBL - producing) Post - phase 1 formulation work ongoing Worldwide ETX0462 ( IV) Multidrug - resistant Pseudomonas Clinical candidate selection Worldwide NBP - 2 ( IV) Gram - negative infections Lead optimization Worldwide

Non - Confidential Sulbactam - durlobactam (SUL - DUR; formerly ETX2514SUL) For Acinetobacter Infections Partnered with 7

Non - Confidential Carbapenem - Resistant Acinetobacter : Overview Global carbapenem - resistant Acinetobacter rates exceed 50%. Abbreviations: CRAB, carbapenem - resistant Acinetobacter baumannii; EU5, European Union Five (France, Germany, Italy, Spain, United Kingdom); Sources: 1. Data on file. Decision Resources. 2. Chung DR, et al; Asian Network for Surveillance of Resistant Pathogens Study Gr oup. Am J Respir Crit Care Med . 2011;184:1409 - 1417. 3. Du, et al. American Journal of Infection Control 00 (2019) 1 - 6. 4. Poirel L, et al. Antimicrob Agents Chemother . 2010;54:24 - 38. 5. Karageorgopoulos DE, Falagas ME. Lancet Infect Dis . 2008;8:751 - 762. 6. Raible KM, et al. Ann Clin Microbiol Antimicrob . 2017;16:75. 7. Data on file. Entasis Therapeutics. IHMA (2014 - 2017). 8. CARSS (China Antimicrobial Resistance Surveillance system), 2017 Annual Report. 8 ► Estimated annual CRAB incidence 1,7,8 ► Limited therapeutic options Polymyxin - based polypharmacy Mortality rate: ~50% 2,3 ► Resistance to β - lactams mediated by Classes A, C, and D β - lactamases 4 - 6 20,000 - 40,000 (United States) 45,000 - 60,000 (Europe and Middle East) Global Percentages of Carbapenem Resistance in A. baumannii 7 *Local surveillance studies >150,000 (China)

Non - Confidential Durlobactam: Overview Durlobactam is a molecule with best - in - class Class A,C & D β - lactamase coverage. Abbreviations: IV, intravenous; SUL - DUR, sulbactam - durlobactam. 9 ► A novel IV broad - spectrum β - lactamase inhibitor Differentiator is its broad Class D β - lactamase coverage , essential for the treatment of carbapenem - resistant Acinetobacter infections ► Extensive preclinical and clinical studies demonstrate antibacterial activity and favorable safety profile Extensive pharmacokinetic and pharmacodynamic modeling employed to project efficacious SUL - DUR dosing regimen Well tolerated in a phase 2 and three phase 1 clinical trials, including at doses that are well in excess of phase 3 clinical trial dose ► Has the potential to restore the antibiotic activity of sulbactam against multidrug - resistant Acinetobacter ► Phase 3 clinical trial ongoing

Non - Confidential SUL - DUR: Pre - Clinical Data Compelling preclinical data differentiates SUL - DUR from existing therapies, including colistin. Abbreviations: CFU, colony - forming unit; MIC, minimal inhibitory concentration; SUL - DUR, sulbactam - durlobactam 10 a Extensively drug - resistant (XDR) Acinetobacter baumannii ARC3486 (OXA - 72, OXA - 66, TEM - 1, AmpC) in neutropenic mice; MIC (sulbactam) ≥32 mg/L, MIC(SUL - DUR) = 0.5 mg/L. b Durlobactam, sulbactam, and colistin were dosed subcutaneously. Colistin was injected to maximum tolerated dose. 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0.06 0.12 0.25 0.5 1 2 4 8 16 32 % of Strain Inhibited In Vitro Activity Against 5,567 Acinetobacter Strains SUL-DUR Colistin Sulbactam Amikacin Impenem Meropenem 90% Inhibition 0 2 4 6 8 10 Pretreatment Vehicle 2.5/0.625 5/1.25 10/2.5 20/5 30/7.5 40/10 80/20 Colistin 40 mg/kg Bacterial Load (Log CFU/g) In Vivo Activity Against XDR Acinetobacter Infection in Mouse Lung Model a,b 1 - Log Reduction Sulbactam - durlobactam (mg/kg)

Non - Confidential SUL - DUR: Clinical Data Summary We have conducted extensive clinical trials to optimize SUL - DUR heading into phase 3. Abbreviations: cUTI , complex urinary tract infection; FDA, Food and Drug Administration; PK, pharmacokinetics; QIDP, qualified infectious diseas e p roduct; SUL - DUR, sulbactam - durlobactam. 11 Phase 1 (n=188) Successfully demonstrated safety & dose proportional PK No dose - limiting toxicities up to 8 grams in single dose No drug - drug interactions Predicted therapeutic levels achieved in urine, plasma, and lung Renal dosing study completed to inform phase 3 dosing Phase 2 (n=80) Additional safety in 53 cUTI patients receiving SUL - DUR PK was consistent with PK observed in phase 1 Successful eradication of imipenem - nonsensitive strains (n=3) Phase 3 Phase 3 ongoing Received Fast Track and QIDP designation by the US FDA
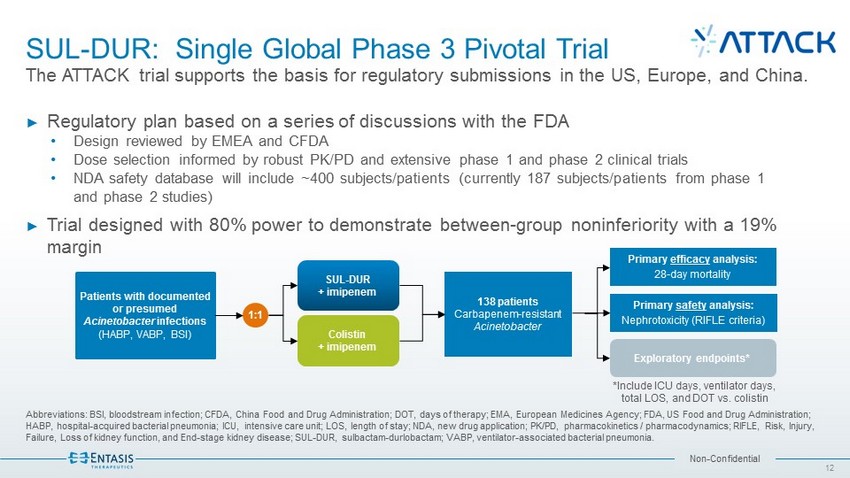
Non - Confidential SUL - DUR: Single Global Phase 3 Pivotal Trial The ATTACK trial supports the basis for regulatory submissions in the US, Europe, and China. Abbreviations: BSI, bloodstream infection; CFDA, China Food and Drug Administration; DOT, days of therapy; EMA, European Medi cin es Agency; FDA, US Food and Drug Administration; HABP, hospital - acquired bacterial pneumonia; ICU, intensive care unit; LOS, length of stay; NDA, new drug application; PK/PD, ph armacokinetics / pharmacodynamics; RIFLE, Risk, Injury, Failure, Loss of kidney function, and End - stage kidney disease; SUL - DUR, sulbactam - durlobactam; VABP, ventilator - associated bact erial pneumonia. 12 ► Regulatory plan based on a series of discussions with the FDA • Design reviewed by EMEA and CFDA • Dose selection informed by robust PK/PD and extensive phase 1 and phase 2 clinical trials • NDA safety database will include ~400 subjects/patients (currently 187 subjects/patients from phase 1 and phase 2 studies) ► Trial designed with 80% power to demonstrate between - group noninferiority with a 19% margin 138 patients Carbapenem - resistant Acinetobacter Primary efficacy analysis: 28 - day mortality SUL - DUR + imipenem Colistin + imipenem Patients with documented or presumed Acinetobacter infections (HABP, VABP, BSI) 1:1 Exploratory endpoints* Primary safety analysis: Nephrotoxicity (RIFLE criteria) *Include ICU days, ventilator days, total LOS, and DOT vs. colistin

Non - Confidential SUL - DUR: Single Global Phase 3 Pivotal Trial The ATTACK trial also includes a Part B to provide safety and supportive efficacy data. Abbreviations: AP, acute pyelonephritis; cUTI , complicated urinary tract infection; HABP, hospital - acquired bacterial pneumonia; SUL - DUR, sulbactam - durlobactam ; VABP, ventilator - associated bacterial pneumonia 13 ► Part B non - randomized cohort ~80 patients • Open - label arm with patients randomized to SUL - DUR plus imipenem • Supportive efficacy data not included in Part A primary analysis • Primary safety analysis will include patients in Parts A and B ► Part B patients are not eligible for Part A but will include: • HABP, VABP, and bacteremia due to colistin - resistant Acinetobacter • cUTI /AP, surgical, or traumatic wound infections due to colistin - resistant Acinetobacter • Patients with known intolerance to colistin SUL - DUR + imipenem Patients with documented or presumed Acinetobacter infections (not eligible for Part A) Safety and supportive efficacy Part B Non - Randomized Cohort: Study Design

Non - Confidential ATTACK Phase 3 Pivotal Trial As of July 2020, the ATTACK pivotal trial continues to progress despite the COVID - 19 pandemic. 14 ► The ATTACK Pivotal Phase 3 trial remains active in many countries and sites • As of July 2020, 87 clinical trial sites have been activated in 16 countries, including 17 sites in China • Intermittently, COVID - 19 has taken priority over ATTACK enrollment in many sites ► Carbapenem and colistin resistance rates have been higher than originally estimated • Carbapenem - resistant Acinetobacter rates are currently >90% vs. original global estimate of 60% • Colistin - resistant Acinetobacter also being seen; these patients are not eligible for Part A but are for Part B – Resistance to last available option reinforces the urgent need for new treatment options In July 2020, a pre - planned Data Safety Monitoring Board (DSMB) meeting advised Entasis to “continue the study without modification” (note: Entasis remains blinded to the results)
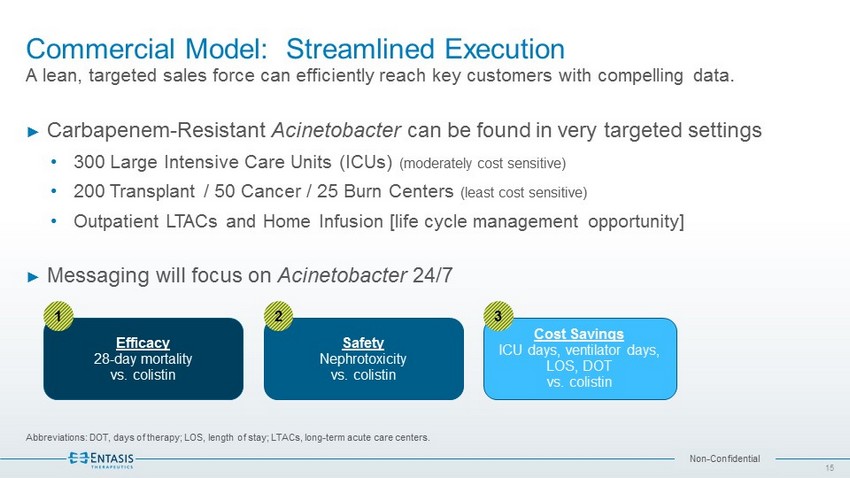
Non - Confidential Commercial Model: Streamlined Execution A lean, targeted sales force can efficiently reach key customers with compelling data. Abbreviations: DOT, days of therapy; LOS, length of stay; LTACs, long - term acute care centers. ► Carbapenem - Resistant Acinetobacter can be found in very targeted settings • 300 Large Intensive Care Units (ICUs) (moderately cost sensitive) • 200 Transplant / 50 Cancer / 25 Burn Centers (least cost sensitive) • Outpatient LTACs and Home Infusion [life cycle management opportunity] ► Messaging will focus on Acinetobacter 24/7 Efficacy 28 - day mortality vs. colistin 1 Safety Nephrotoxicity vs. colistin 2 Cost Savings ICU days, ventilator days, LOS, DOT vs. colistin 3 15

Non - Confidential Commercial Model: Product Positioning SUL - DUR aims to replace colistin and polypharmacy in carbapenem - resistant Acinetobacter. Current Treatment Protocols Future Treatment Protocols Nephrotoxic Neurotoxic Slow onset, complex pharmacodynamics Cumbersome dosing protocol and AE monitoring Dosing limited by safety Day 1: Carbapenem + Suspected Acinetobacter Days 2 - 14 or longer Colistin + Polypharmacy a Day 1: Carbapenem + Suspected Acinetobacter x No SAEs observed in phase 1 or 2 trials x Well tolerated x Well - understood pharmacodynamics x Easier dosing protocol x Dosing optimized for efficacy a For CRAB treatment, drugs used in combination with colistin come from the following classes: carbapenems, cephalosporins, tet rac yclines, penicillins , and aminoglycosides. Abbreviations: AE, adverse event; CRAB, carbapenem - resistant Acinetobacter ; SAEs, serious adverse events; SUL - DUR, sulbactam - durlobactam. Confirmed CRAB Confirmed CRAB Replaced by Days 2 - 14 or longer SUL - DUR 16

Non - Confidential Commercial Opportunity: Addressable Market At launch, annual worldwide addressable market for CRAB treatments should exceed $1 billion. Abbreviations: CRAB, carbapenem - resistant Acinetobacter; RoW , rest of world . Sources: 1. Data on file. Decision Resources. Note: Addressable market projections are for year 2022 based on Company estimates and assumptions, including estimated patie nt populations, market share and pricing, made as of the date of this presentation. These parameters could differ materially at the time of commercialization. 17 20,000 - 40,000 Annual CRAB Infections 1 Global Addressable Market $1B + 150,000 - 200,000 Annual CRAB Infections 1 U.S. RoW Pneumonia Bloodstream Skin and skin structure Urinary tract Wounds Inpatient Outpatient

Non - Confidential Commercial Opportunity: China Our Zai Lab partnership will help address CRAB in China. Abbreviations: CRAB, carbapenem - resistant Acinetobacter 18 ► CRAB presents a significant unmet medical need in China and Asia - Pacific territory ► Partnership with Zai Lab (Nasdaq: ZLAB) provides potential accelerated path for commercialization in China Co - enrollment of ATTACK pivotal phase 3 clinical trial If successful, provides path for regulatory approval Direct economic benefits to Entasis (milestone and royalty payments) in addition to partially offsetting phase 3 clinical trial costs ► Entasis maintains 100% economics outside Asia - Pacific, including North America and Europe

Non - Confidential Zoliflodacin Zoliflodacin For Gonorrhea Infections Partnered with 19

Non - Confidential Gonorrhea: Overview Only one drug remains effective for gonorrhea — intramuscular ceftriaxone. Sources: 1. Rowley J, et al. Bull World Health Organ . 2019;97:548 - 562P. 2. Unemo M, et al. Microbiol Spectr . 2016;4. 3. Gonorrhea - CDC fact sheet (detailed version). CDC website. https://www.cdc.gov/std/gonorrhea/stdfact - gonorrhea - detailed.htm. Reviewed October 25, 2016. Accessed September 23, 2019. 4. CDC Sexually Transmitted Disease Surveillance 2018, Table 1. 5. Unemo M, et al. Sex Health . 2019;16:412 - 425. *includes China and Australia 20 Currently, no oral antibiotics are recommended as an alternative to ceftriaxone for the treatment of N. gonorrhoeae infections ► The only recommended treatment option today is ceftriaxone (intramuscular [IM] injection), but resistance has emerged 5 Ceftriaxone 250mg administered via 21 - gauge 1.5 - inch needle ► 87 million worldwide annual cases 1 35.2 million in Western Pacific 2* 1.14 million in the United States 3 Growing >10% per year since 2009 4

Non - Confidential Gonorrhea: Overview The CDC has changed its STD Guidelines five times in the last 18 years 1 due to resistance. Sources: 1. CDC: Sexually Transmitted Diseases Treatment Guidelines, MMWR, May 10, 2002; Sexually Transmitted Diseases Treatm ent Guidelines, MMWR, August 4, 2006; Update to Sexually Transmitted Diseases Treatment Guidelines, 2006, MMWR, April 13, 2007; Sexually Transmitted Diseases Treatment Guid eli nes, 2010, MMWR, December 10, 2010; CDC No Longer Recommends Oral Drug for Gonorrhea Treatment, August 9, 2012; 2. CDC, Sexually Transmitted Disease Surveillance 2017, Fi gure 33 Abbreviations: STD, sexually - transmitted disease 21 Distribution of Primary Antimicrobial Drugs Used to Treat Gonorrhea Among Participants, 1988 – 2017 2
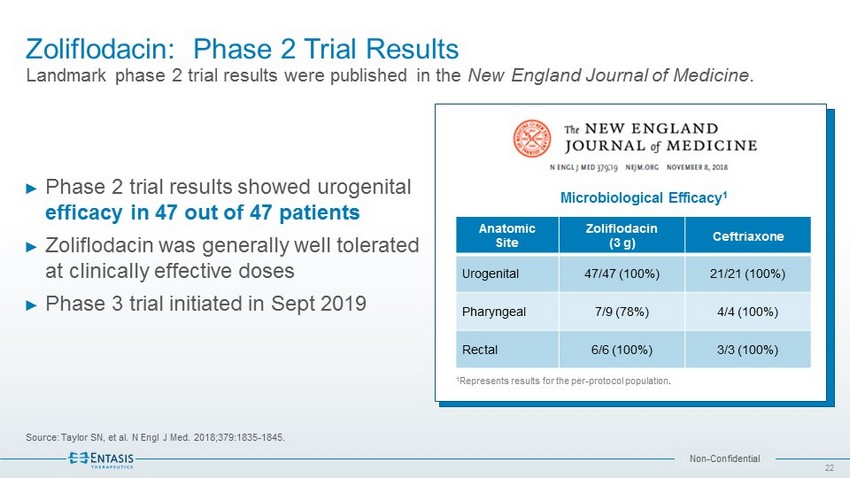
Non - Confidential Zoliflodacin: Phase 2 Trial Results Landmark phase 2 trial results were published in the New England Journal of Medicine . Source: Taylor SN, et al. N Engl J Med. 2018;379:1835 - 1845. ► Phase 2 trial results showed urogenital efficacy in 47 out of 47 patients ► Zoliflodacin was generally well tolerated at clinically effective doses ► Phase 3 trial initiated in Sept 2019 Anatomic Site Zoliflodacin (3 g) Ceftriaxone Urogenital 47/47 (100%) 21/21 (100%) Pharyngeal 7/9 (78%) 4/4 (100%) Rectal 6/6 (100%) 3/3 (100%) 1 Represents results for the per - protocol population . Microbiological Efficacy 1 22
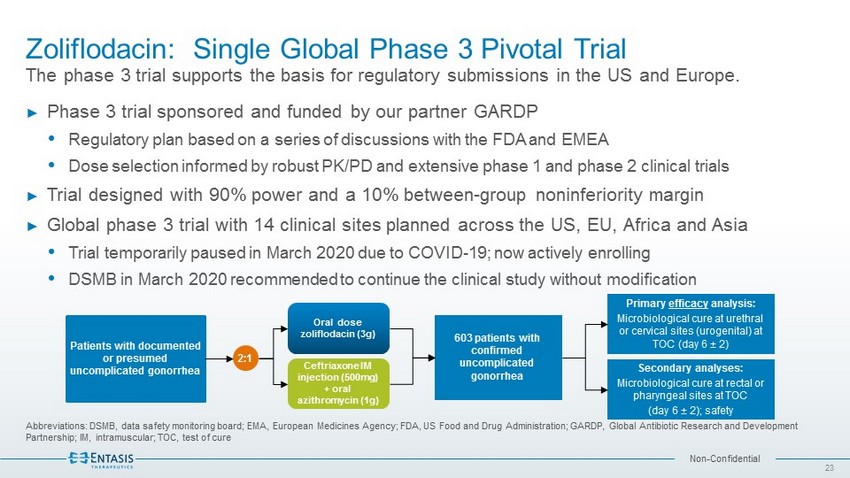
Non - Confidential Zoliflodacin : Single Global Phase 3 Pivotal Trial The phase 3 trial supports the basis for regulatory submissions in the US and Europe. Abbreviations: DSMB, data safety monitoring board; EMA, European Medicines Agency; FDA, US Food and Drug Administration; GARD P, Global Antibiotic Research and Development Partnership; IM, intramuscular; TOC, test of cure 23 ► Phase 3 trial sponsored and funded by our partner GARDP Regulatory plan based on a series of discussions with the FDA and EMEA Dose selection informed by robust PK/PD and extensive phase 1 and phase 2 clinical trials ► Trial designed with 90% power and a 10% between - group noninferiority margin ► Global phase 3 trial with 14 clinical sites planned across the US, EU, Africa and Asia Trial temporarily paused in March 2020 due to COVID - 19; now actively enrolling DSMB in March 2020 recommended to continue the clinical study without modification 603 patients with confirmed uncomplicated gonorrhea Primary efficacy analysis: Microbiological cure at urethral or cervical sites (urogenital) at TOC (day 6 “ 2) Oral dose zoliflodacin (3g) Ceftriaxone IM injection (500mg) + oral azithromycin (1g) Patients with documented or presumed u ncomplicated gonorrhea 2:1 Secondary analyses: Microbiological cure at rectal or pharyngeal sites at TOC (day 6 “ 2); safety

Non - Confidential Zoliflodacin: Commercial Opportunity Zoliflodacin has the potential to be the oral product of choice for gonorrhea. Abbreviations: MIC, minimum inhibitory concentration. a 49 states allow patients diagnosed with gonorrhea to provide medications to take to his/her partner without the health care p rov ider first examining the partner ( https://www.cdc.gov/std/ept/legal/default.htm ) Sources: 1. McLenon J, et al. J Adv Nurs . 2019;75:30 - 42; 2. Chisholm, J Antimicrob Chemother 2010; 65: 2141 – 2148. 24 Single - dose oral cure (oral suspension sachet) Alternative to intramuscular ceftriaxone 20% of the population are “needlephobes” 1 Ceftriaxone injection is notorious for its pain Increasing ceftriaxone MIC creep 2 Oral sachet allows “expedited partner therapy” (EPT) a

Non - Confidential Zoliflodacin: Partnership with GARDP The Phase 3 trial is sponsored by the Global Antibiotic Research & Development Partnership. Abbreviations: R&D, research and development Source: GARDP website, Entasis press release (July 6, 2017) ► Developed and fully funded in partnership with: ► GARDP is not - for - profit R&D organization that addresses global public health needs by developing and delivering new or improved antibiotic treatments while endeavoring to ensure their sustainable access ► GARDP will have zoliflodacin commercialization rights in most low - and select middle - income countries, while Entasis retains commercial rights in high - income markets 25

Non - Confidential Commercial Opportunity: Worldwide Addressable Market With over 85MM gonorrhea infections annually, the worldwide market could exceed $1 billion. Abbreviations: RoW , rest of world . Sources: 1. Centers for Disease Control and Prevention; 2. Rowley J, et al. Bull World Health Organ . 2019;97:548 - 562P Note: Addressable market projections are for year 2022 based on Company estimates and assumptions, including estimated patie nt populations, market share and pricing, made as of the date of this presentation. These parameters could differ materially at the time of commercialization. 26 1,000,000 Annual Gonorrhea Infections 1 Global Addressable Market $1B + >85,000,000 Annual Gonorrhea Infections 2 U.S. RoW
Non - Confidential ETX0282CPDP (ETX0282 + Cefpodoxime Proxetil ) for Complicated UTIs APPENDIX 27
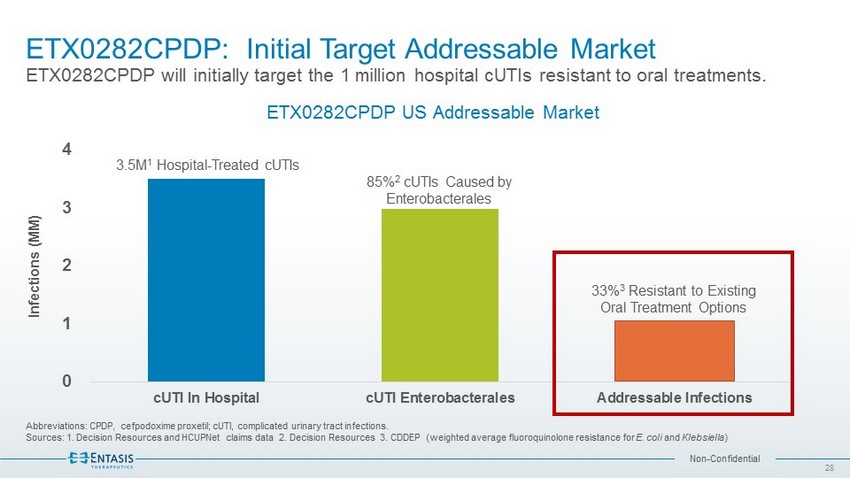
Non - Confidential ETX0282CPDP: Initial Target Addressable Market ETX0282CPDP will initially target the 1 million hospital cUTIs resistant to oral treatments. Abbreviations: CPDP, cefpodoxime proxetil ; cUTI , complicated urinary tract infections. Sources: 1. Decision Resources and HCUPNet claims data 2. Decision Resources 3. CDDEP (weighted average fluoroquinolone resistance for E. coli and Klebsiella ) 28 0 1 2 3 4 cUTI In Hospital cUTI Enterobacterales Addressable Infections Infections (MM) ETX0282CPDP US Addressable Market 85% 2 cUTIs Caused by Enterobacterales 33% 3 Resistant to Existing Oral Treatment Options 3.5M 1 Hospital - Treated cUTIs

Non - Confidential ETX0282CPDP ETX0282CPDP is a best - in - class oral β - lactamase inhibitor. Abbreviations: CPDP, cefpodoxime proxetil ; CRE, carbapenem - resistant Enterobacterales; ESBL, extended - spectrum β - lactamases; MDR, multidrug - resistant; UTIs, urinary tract infections. ► ETX0282CPDP could be the first oral therapeutic option for the treatment of complicated UTIs with broad coverage of MDR Enterobacterales including CRE Opportunity for expansion into the broader community setting ► ETX0282 is designed to have both high oral bioavailability and broad Class A and Class C β - lactamase inhibition No other orally bioavailable treatment covers both Class A and Class C β - lactamases, including ESBL - producing bacteria and CRE ► Initial phase 1 data trial in healthy volunteers supports further progression Investigated safety, pharmacokinetics, drug - drug interaction (DDI), and food effect Interim phase 1 data indicate ETX0282 is generally safe and well tolerated ► Potential for use in additional indications where multidrug - resistant Enterobacterales are commonly found 29

Non - Confidential ETX0282CPDP Oral ETX0282CPDP shows in vitro and in vivo activity against multidrug - resistant E. coli. a ETX0282CPDP is an oral prodrug which is metabolized into ETX1317, the active BLI, and cefpodoxime. The in vitro activity is o f E TX1317 + cefpodoxime. b ARC2687 = multidrug - resistant E. coli ( AmpC +; CTX - M - 14+), levofloxacin - resistant (MIC >4 mg/L), cefpodoxime - resistant (MIC >64 mg/L), cefpodoxime - ETX1317 – sensitive (MIC <0 .03 mg/L). c ETX0282 and cefpodoxime were dosed orally. Meropenem was dosed subcutaneously. Abbreviations: BLI, β - lactamase inhibitor; CFU, colony - forming unit; CPDP, cefpodoxime proxetil ; CRE, carbapenem - resistant Enterobacterales; IV, intravenous; MIC, minimal inhibitory concentration. 30 0% 20% 40% 60% 80% 100% 0.031 0.0625 0.125 0.25 0.5 1 2 4 8 16 32 64 Percentage of Strains Inhibited, % Concentration (mg/L) In Vitro Activity of ETX0282CPDP Against 54 Enterobacterales Strains, Including CRE a ETX0282CPDP Oral Agent 1 in Development Oral Agent 2 in Development Vabomere (IV) Avycaz (IV) 0 2 4 6 8 10 12 Pretreatment Vehicle Cefpodoxime 50 mg/kg ETX0282 10 mg/kg 10 mg/kg 25 mg/kg 100 mg/kg Meropenem 600 mg/kg Bacterial Load (Log CFU/g) In Vivo Activity of Oral ETX0282 + Cefpodoxime Proxetil in Mouse Thigh Model b,c 1 - Log Reduction 90% Inhibition Pretreatment Vehicle Cefpodoxime 50 mg/kg ETX0282 10 mg/kg 10 mg/kg 25 mg/kg 100 mg/kg Meropenem 600 mg/kg ETX0282 + Oral CPDP 50 mg/kg

Non - Confidential ETX0282CPDP Phase 1 Status Oral ETX0282CPDP has completed initial phase 1 testing. Abbreviations: CPDP, cefpodoxime proxetil 31 Fit - for - purpose formulation ► Drug substance in capsule (no excipients) First in Human study completed ► 99 healthy subjects were enrolled into 12 cohorts ► 26 subjects received a single dose of ETX0282 ► 51 received at least 2 doses of ETX0282 ► 22 received matching placebo Part A Single ascending dose, fasted Dose range: 100 mg - 800 mg Part B Food effect: fasted vs high fat meal Dose range: 100 mg - 300 mg Part C Multiple dose with regular meals Dose: 200 mg Part D Drug - drug interaction with regular meal Doses: ETX0282 400 mg and cefpodoxime proxetil 400 mg Part G Simulation of PK profile associated with high fat meal Dose range: 300 mg single dose vs 75 mg x 4 doses

Non - Confidential Key Pharmacokinetic (PK) and Safety Findings Oral ETX0282CPDP’s p lasma concentrations are in predicted therapeutic range. Abbreviations: C max , maximum plasma concentration; CPDP, cefpodoxime proxetil . 32 ► ETX0282 is rapidly converted to ETX1317 at all doses evaluated ► Plasma concentrations of ETX1317 are potentially in the desired therapeutic range ► Minimal accumulation of ETX1317 with multiple dosing ► No drug - drug interaction (either way) with cefpodoxime ► Mild - to - moderate emesis reported in 11 out of 77 receiving ETX0282 ► A high fat meal (but not normal diet) and Part G (75 mg x 4 doses ) both resulted in a modified PK profile with no emesis observed Decrease in peak concentration ( C max ) Decrease in absorption rate to peak concentration Broader exposure window Now progressing with development of an appropriate extended release clinical formulation to be evaluated in a future Phase 1 clinical trial to assess the safety and pharmacokinetic profile

Non - Confidential ETX0462 ETX0462 for Pseudomonas Infections APPENDIX 33

Non - Confidential P. aeruginosa Infections Pseudomonas infections remain a key priority due to high resistance rates and mortality. Sources: 1. ECDC/EMEA Joint Working Group. ECDC/EMEA Joint Technical Report. The bacterial challenge: time to react. https://www.ecdc.europa.eu/sites/default/files/media/en/publications/Publications/0909_TER_The_Bacterial_Challenge_Time_to_Re act .pdf. Published September 17, 2009. Accessed September 23, 2019. 2. Entasis triangulated estimate from Decision Resources, CDC, AMR, CDDEP, and literature reviews. Abbreviations: CDC, Centers for Disease Control and Prevention; CR, carbapenem - resistant; MDR, multidrug - resistant; WHO, World H ealth Organization. Note: Brand Resistant includes Avycaz, Zerbaxa, Vabomere, Recarbrio 34 ~10,000 extra deaths + ~800,000 extra hospital days caused by carbapenem - resistant P. aeruginosa 1 ~550,000 – 650,000 cases/year 2 ~30% MDR rate by 2040 WHO: Critical Priority 1 CDC: “Serious” Threat 120 90 35 0 20 40 60 80 100 120 140 MDR CR Brand Resistant Infections (000s) Estimated US Pseudomonas aeruginosa Infections by Resistance 2

Non - Confidential ETX0462 ETX0462 is a first of a novel class of PBP Inhibitors unaffected by all types of β - lactamases. Abbreviations: MDR, multidrug - resistant; MIC, minimal inhibitory concentration. 35 ► Penicillin Binding Protein inhibitors ( PBPi ) are β - lactam antibiotics representing some of the oldest and most widely used antibacterial agents but are vulnerable to degradation by β - lactamases ► Novel class of non – β - lactam PBPi (NBPs) is shown to be impervious to all classes of b - lactamases tested ► Excellent potency observed against multiple MDR Gram - negative pathogens with no β - lactamase inhibitor (BLI) needed, creating a potential new IV monotherapy Class A Class B Class C Class D Compound No β - lactamase CTX - M - 15 SHV - 2a KPC - 3 TEM - 1 PER - 1 VEB - 1 GES - 11 NDM - 1 VIM - 1 IMP - 1 AmpC P99 OXA - 1 OXA - 10 OXA - 48 Piperacillin 4 64 4 64 >64 8 32 4 >64 >64 >64 64 64 >64 >64 >64 Ceftazidime 0.5 32 16 16 1 64 >64 32 >64 >64 64 8 32 1 1 0.5 ETX0462 1 1 2 1 2 1 1 1 2 1 1 2 2 2 2 2 MIC (mg/L) Against E. coli Isogenic Strains Expressing Individual β - lactamases

Non - Confidential Entasis: A Leader in Targeted Antibiotics We have hit all major milestones in our first 5 years. Two phase 3 programs (SUL - DUR, zoliflodacin) Phase 1 data (ETX0282CPDP) Fourth candidate selected (ETX0462) Initial Public Offering Second partnership Spinout from AstraZeneca with $23.5M and 14 employees Two clinical programs (SUL - DUR, zoliflodacin) >$100M raised First partnership Phase 3 data on two lead programs (SUL - DUR, zoliflodacin) Commercial launch preparation Sustainable pipeline 2015 2016 - 17 2018 - 19 2021 and Beyond 36
