Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca-20200706.htm |

Better cancer immunotherapies through better antigen selection July 2020

This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, clinical trials and pre-clinical studies, regulatory approval of our product candidates, liquidity position and capital needs, financing plans, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “expects,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward- Disclaimer looking statements ultimately prove to be correct. Factors that may materially affect our results of operations include, among other things, our ability to progress product candidates in preclinical and clinical trials, the ability of ATLAS™ to identify promising oncology vaccine and immunotherapy product candidates, the scope, rate and progress of our preclinical and clinical trials and other research and development activities, anticipated timing of IND applications and new clinical trials, the amount of funds that we may require to conduct our clinical trials for our product candidates, the timing of, and ability to, obtain and maintain necessary regulatory approvals for our product candidates, and those listed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2019 and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2

Why Genocea? Revolutionary antigen discovery platform TARGETS MATTER PROPRIETARY PLATFORM GEN-009 GEN-011 Two differentiated, clinical stage programs Neoantigen Neoantigen vaccine cell therapy Q2 2020 IND Q3 2020 Significant near-term, Mid-2021 initial clinical data value-creating milestones initial clinical data 3

Why do targets matter? Lesson from infectious Lesson from cancer Optimized disease vaccines immunotherapy neoantigen Wrong antigens → no Responses to certain selection protection, even with neoantigens drives positive critical to strong immune responses checkpoint inhibitor (CPI), TIL therapy outcomes immunotherapy efficacy 4
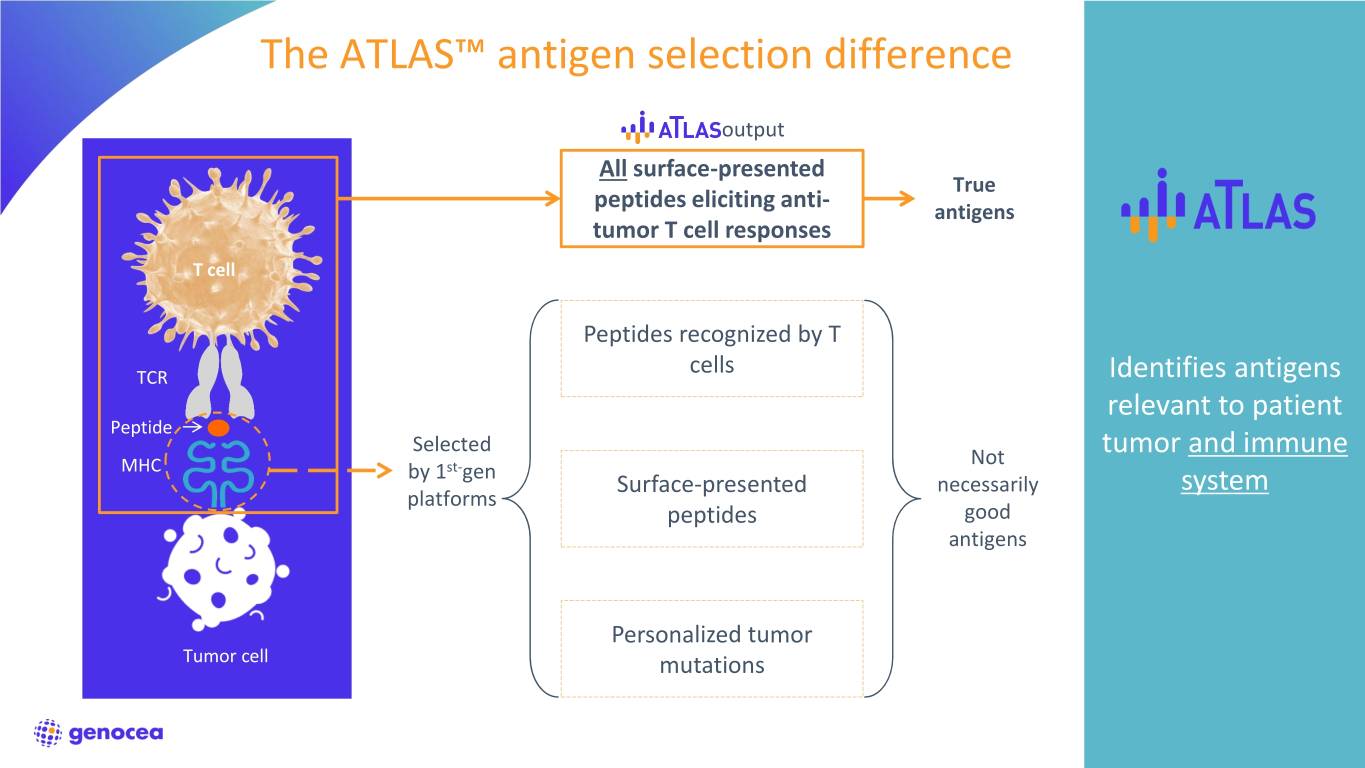
The ATLAS™ antigen selection difference output All surface-presented True peptides eliciting anti- antigens tumor T cell responses T cell Peptides recognized by T TCR cells Identifies antigens relevant to patient Peptide Selected Not tumor and immune MHC by 1st-gen Surface-presented necessarily system platforms peptides good antigens Personalized tumor Tumor cell mutations 5

ATLAS: high throughput platform harnessing the immune system for antigen selection Tumor NGS biopsy analysis Unique Bacterial plasmids for vectors Autologous every expressing each dendritic cell Comprehensive candidate candidate neoantigen profiling of patient- and tumor-relevant T cell responses Autologous T cell Blood sample 6

ATLAS identifies the tumor-specific, surface-presented neoantigens of both CD8+ and CD4+ T cell responses CD8+ T cells CD4+ T cells True neoantigens Irrelevant Concentration Concentration IFNg IFNg InhibigensTM NormalizedBackgroundto Control NormalizedBackgroundto Control Individual mutations Individual mutations Example: SCCHN patient; 76 mutations identified and profiled; IFNg responses depicted ATLAS antigen classification does not associate with TMB, expression levels, mutation type (truncal vs. other) 7

Inhibigen identification and exclusion is critical to ensure efficacy 1,600 Adjuvant only 1,000 ) 1,400 Protective vaccine Adjuvant only 3 ) 3 Inhibigens + Adjuvant m 1,200 Protective vaccine + Inhibigen m 800 m ( m ( e 1,000 e m 600 u m l 800 u o l v o r v 600 400 o r m o u 400 m T u 200 T 200 0 0 0 6 8 10 12 14 16 18 0 3 6 8 10 12 14 16 18 20 22 24 Day Day Therapeutic mouse vaccination with a pool of ATLAS-identified The presence of Inhibigens in an otherwise protective Inhibigens drives tumor hyperprogression mouse vaccine completely abrogates protection Inhibigen-specific T cells may be responsible for hyperprogression after CPI therapy1,2 Experiments run in B16F10 melanoma model 1. Champiat et al., Clin Can Res (2017) 2. Ferrara et al., JAMA (2018) 8

Antigen selection: Enabling differentiated immune responses Including only therapeutically relevant neoantigens Relevant both to tumor and patient immune system (surface-presented and immunogenic) Uniquely identifies Inhibigens for exclusion TARGETS MATTER Comprehensive Any patient, antigen, and cancer; Both CD4+ and CD8+ T cell antigens Well protected Issued global patent families protect into 2030s Substantial know-how 9

ATLAS drives emerging immunotherapy pipeline Discovery Pre-IND Phase 1/2a Pivotal Status & Anticipated Milestones GEN-009 – Neoantigen vaccine • ASCO 2019 Top 10 IO abstract • Initial clinical data in Q3 2020 GEN-011 – Neoantigen cell therapy • IND filed in Q2 2020 • Initial clinical data in mid-2021 Toolbox of novel assets to enable additional programs Shared neoantigens Tumor-associated antigens Viral cancer antigens 10

GEN-009 Unprecedented immune responses; Neoantigen Vaccine Emerging clinical efficacy | CONFIDENTIAL 11

Simple, scalable production and delivery 12

GEN-009 phase 1/2a trial design Part A • Vaccine monotherapy in patients with no evidence of disease • Multiple tumor types with CPI approval • Objectives: safety, immunogenicity • Status: all patients in long-term follow-up

Unparalleled immune responses in Part A Frequency Effector Central Memory % neoantigens with Function Response immune responses (ex vivo assay) (IVS assay) CD4+ CD8+ CD4+ CD8+ Best peer results3 60% 99% 51% GEN-0092 87% 41% 57% 57% 23% Best peer results1 GEN-0092 Achieved in combination Monotherapy 17% with CPI 0% Potential patient benefits Rapid tumor killing Durable efficacy Response breadth to prevent Potential patient tumor escape benefits 1 Sahin, et al., Nature 2017 (N=8); Ott, et al., Nature 2017 (N=8); Gritstone ESMO Immuno-Oncology Congress Presentation 2019 2 N=8 3 Sahin, et al., Nature 2017 (N=8); Ott, et al., Nature 2017 (N=8) • Results shown represent highest neoantigen vaccine percentage responses in peer publications • No comparison or head-to-head studies were performed between GEN-009 and the other product candidates noted above. Clinical trial criteria, including, without limitation, number of patients, inclusion and exclusion criteria, and primary endpoints were not necessarily the same 14
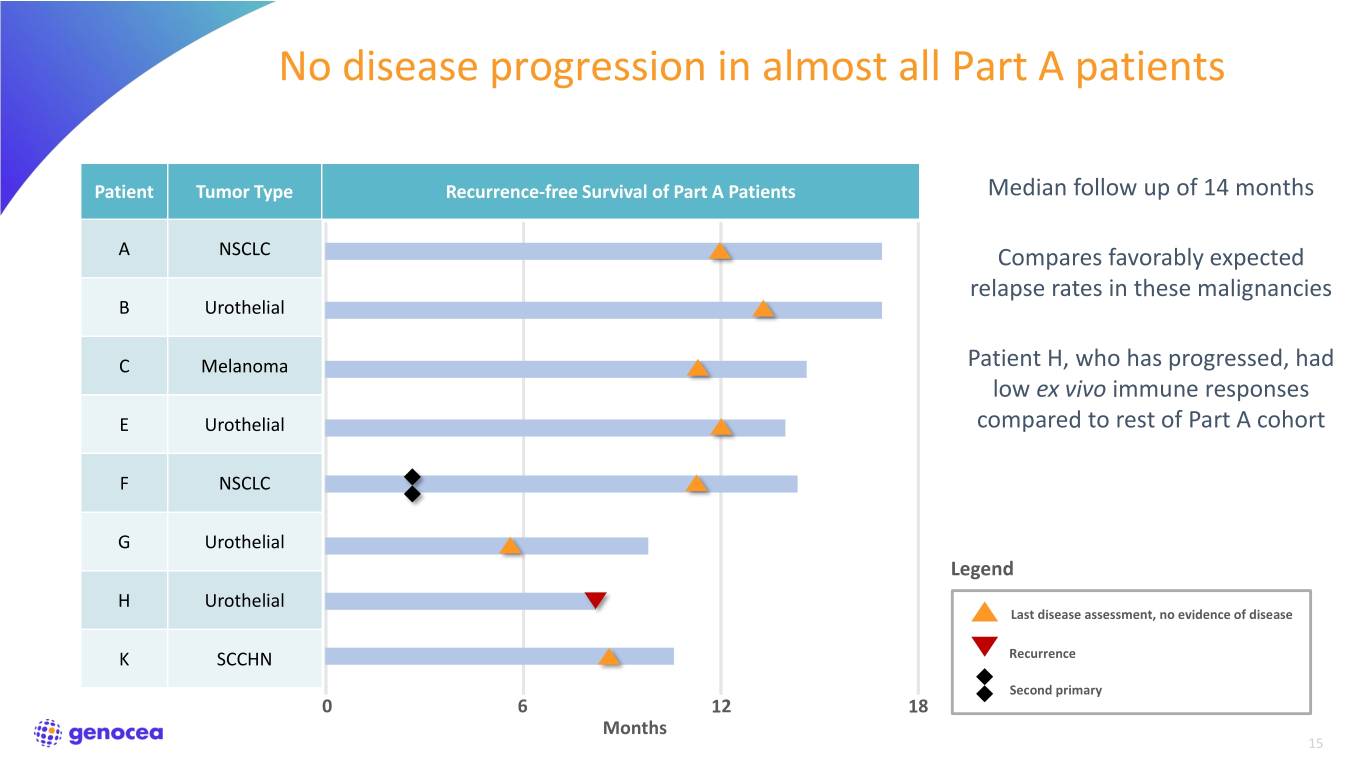
No disease progression in almost all Part A patients Patient Tumor Type Recurrence-free Survival of Part A Patients Median follow up of 14 months A NSCLC Compares favorably expected relapse rates in these malignancies B Urothelial C Melanoma Patient H, who has progressed, had low ex vivo immune responses E Urothelial compared to rest of Part A cohort F NSCLC G Urothelial Legend H Urothelial Last disease assessment, no evidence of disease K SCCHN Recurrence Second primary 0 6 12 18 Months 15

GEN-009 phase 1/2a trial design Part B • Combination of GEN-009 and standard-of-care PD-1-based regimen in patients with advanced disease • Tumor types: Melanoma, NSCLC, SCCHN, Urothelial, RCC • Objectives: safety, immunogenicity, efficacy • Vaccination contribution to be determined after CPI response established • Final clinical data from initial Part B cohort expected Q3 2020

Sample CPI spider plot: decreasing change in expected outcome over time Each patient can serve as own control Topalian et al., NEJM 366: 2443-54, 2012 17

GEN-011 Potential best-in-class solid tumor Neoantigen T cell Therapy adoptive T cell therapy | CONFIDENTIAL 18

GEN-011 – a new category of neoantigen T cell therapy: Peripheral blood-derived, ATLAS™-powered Peripheral blood (leukopak) GEN-011 Up to 30 neoantigens Neoantigen-specific cell expansion in fully closed single-use vessels Autologous, non-engineered 19

GEN-011: Embraces TIL advantages, overcomes limitations TIL limitations GEN-011 GEN-011 advantages Neoantigen • Targets up to 30 relevant neoantigens with • Limited tumor specificity selection via CD4+ and CD8+ memory T cells • Cannot avoid inhibitory, • Avoids pro-tumor Inhibigens that may pro-tumor responses be detrimental to clinical response • No extra surgery, or viable tumor, required • Requires sterile resection of tumors with sufficient T cells Peripheral blood lymphocytes (PBLs) • Billions of T cells to relevant tumor neoantigens, with proven cytolytic capacity • Expansion protocols exacerbate already exhausted TIL • Non-exhausted cells with potential for superior activity and persistence Potential for greater activity, for more patients, at a lower overall cost to the healthcare system 20

CD4+ and CD8 + memory T cells for anti-tumor efficacy, persistence and proliferation Pure T cell drug product Desired T cell memory phenotype Non-T cells 100 1% CD8+ y 75 c 35% n e u q 50 e CD4+ r F 65% T cells % 25 99% 0 Naive Terminal Memory memory (Cm+Em) (TEMRA) Data represent mean across cancer patients and healthy donor development runs 21

Billions of neoantigen-specific T cells Contains an average of Majority of T cells are specific for 3.3 billion T cells ATLAS-identified neoantigens 1010 Irrelevant antigens Neoantigens CD8+ 65% r e 5% b + m CD4 u 9 n 10 l l e c T Activation Marker ActivationMarker 2 ActivationMarker 2 108 Pre-expansion cells GEN-011 Activation Marker 1 Activation Marker 1 Mean across development runs with cancer patient and healthy donor material 22

GEN-011 T cells are highly functional and cytotoxic An average of 16,000 cells per T cells are potently million are secreting cytokines in cytolytic against target response to stimulation neoantigens CD8+ 100 Specific Neoantigens + 80 CD4 Unpulsed Control y t i c i 60 Negative x o t control o t y 40 C % 20 0 16:1 8:1 4:1 2:1 1:1 0.5:1 Neoantigen Effector:Target ratio pool 23

PBL-derived T cells exhibit greater neoantigen activity and potency compared to TIL T cells are activated appropriately Neoantigen-specific T cells upon neoantigen stimulation secrete abundant IFN + 80 CD8 25000 n o i t a l u 20000 g 60 e r p L u r 15000 m / e g k r 40 p a m N 10000 F n I o i t a 20 v i t 5000 c A % 0 0 1 GEN-011 TIL GEN-011 TIL2 Squares = healthy donor, viral antigen 1. Chandran Lancet 2017; Stevanovic JCO 2015; n=25 Circles = cancer patients, neoantigens 2. Ritthipichai, SITC 2017 poster N=5 24

Unparalleled breadth of neoantigen coverage GEN-011 targets up to 30 neoantigens Recent conventional TIL data show responses to an average 89% of 6% of the predicted neoantigens2 Recent selected TIL data show successful amplification to only 4% of antigens included in the process1 GEN-011 T cells are specific for 89% Recent engineered TCR-T data suggest only up to 3 of all intended neoantigen targets* neoantigen specificities targeted3 *Mean across development runs with cancer patient material 1. Samuel et al., AACR Presentation (April 2020) 3. Cristea et al., 2020 AACR Presentation (April 2020) 2. Creelan et al., AACR Presentation (April 2020) 25

Trial Objectives Phase 1/2 pilot study of GEN-011 in refractory solid tumors Targeted indications Cohort A Trial objectives Melanoma, NSCLC, SCLC, Multiple low dose • Safety SCCHN, UC, RCC, SqCC skin, (up to 12 patients) • Clinical activity SqCCAC • No lymphodepletion • ORR • Low dose IL-2 Tumors responsive to • DOR immunotherapy or virally- • GEN-011 proliferation associated without available and persistence disease control Cohort B • Tumor T cell Single high dose penetration (up to 12 patients) • Lymphodepletion • High dose IL-2 IND in Q2 2020 – Preliminary clinical data in mid-2021 26

Better immunotherapies through better antigen selection Revolutionary antigen discovery platform TARGETS MATTER PROPRIETARY PLATFORM GEN-009 GEN-011 Two differentiated, clinical stage programs Neoantigen Neoantigen vaccine cell therapy Q2 2020 IND Q3 2020 Significant near-term, Mid-2021 initial clinical data value-creating milestones initial clinical data 27

NASDAQ: GNCA 100 Acorn Park Drive Cambridge, MA 02140 USA +1 617.876.8191 www.genocea.com 28
