Attached files
| file | filename |
|---|---|
| 8-K - 8-K - aTYR PHARMA INC | life-8k_20200622.htm |
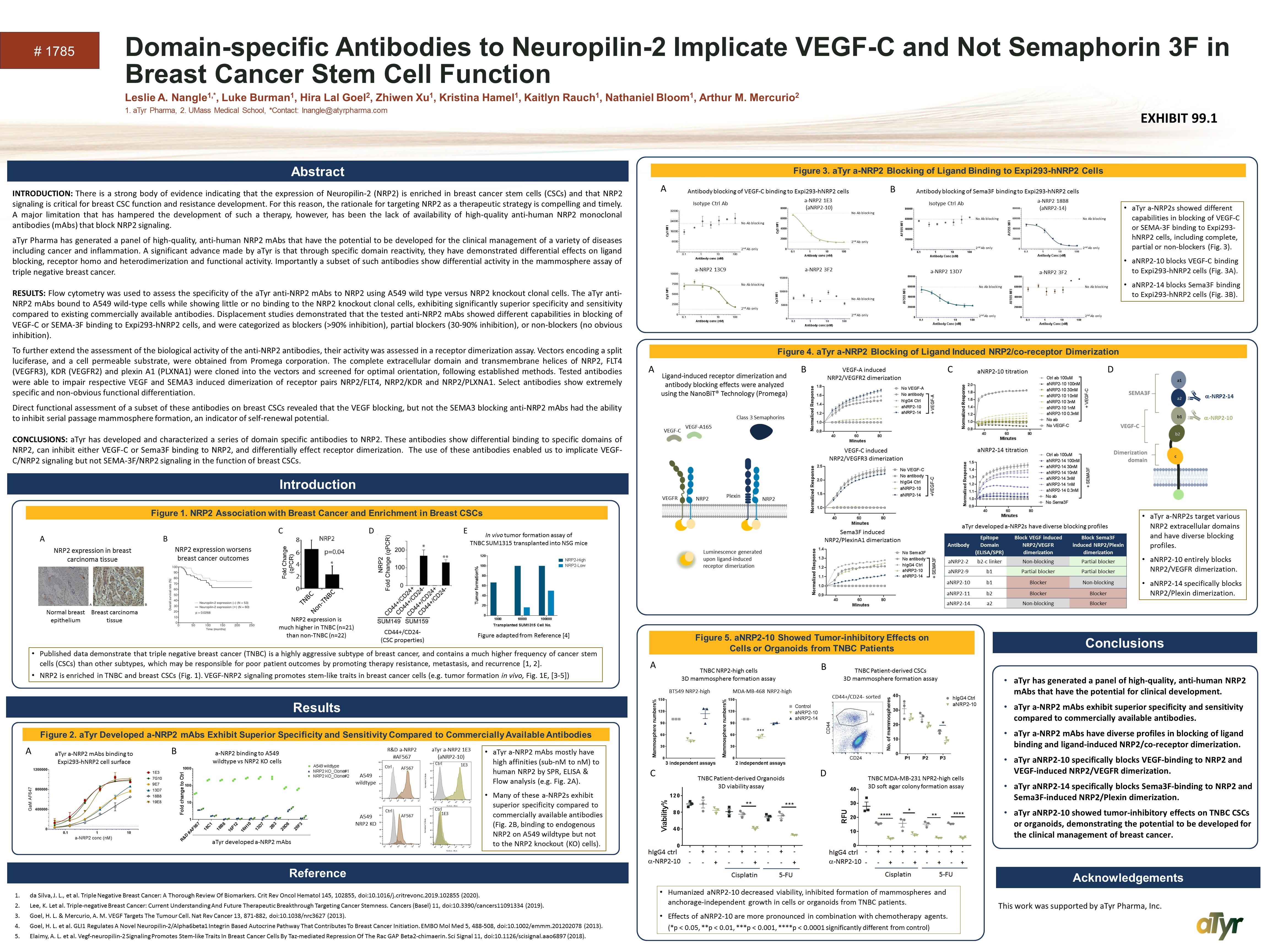
Domain-specific Antibodies to Neuropilin-2 Implicate VEGF-C and Not Semaphorin 3F in Breast Cancer Stem Cell Function Leslie A. Nangle1,*, Luke Burman1, Hira Lal Goel2, Zhiwen Xu1, Kristina Hamel1, Kaitlyn Rauch1, Nathaniel Bloom1, Arthur M. Mercurio2 1. aTyr Pharma, 2. UMass Medical School, *Contact: lnangle@atyrpharma.com # 1785 INTRODUCTION: There is a strong body of evidence indicating that the expression of Neuropilin-2 (NRP2) is enriched in breast cancer stem cells (CSCs) and that NRP2 signaling is critical for breast CSC function and resistance development. For this reason, the rationale for targeting NRP2 as a therapeutic strategy is compelling and timely. A major limitation that has hampered the development of such a therapy, however, has been the lack of availability of high-quality anti-human NRP2 monoclonal antibodies (mAbs) that block NRP2 signaling. aTyr Pharma has generated a panel of high-quality, anti-human NRP2 mAbs that have the potential to be developed for the clinical management of a variety of diseases including cancer and inflammation. A significant advance made by aTyr is that through specific domain reactivity, they have demonstrated differential effects on ligand blocking, receptor homo and heterodimerization and functional activity. Importantly a subset of such antibodies show differential activity in the mammosphere assay of triple negative breast cancer. RESULTS: Flow cytometry was used to assess the specificity of the aTyr anti-NRP2 mAbs to NRP2 using A549 wild type versus NRP2 knockout clonal cells. The aTyr anti-NRP2 mAbs bound to A549 wild-type cells while showing little or no binding to the NRP2 knockout clonal cells, exhibiting significantly superior specificity and sensitivity compared to existing commercially available antibodies. Displacement studies demonstrated that the tested anti-NRP2 mAbs showed different capabilities in blocking of VEGF-C or SEMA-3F binding to Expi293-hNRP2 cells, and were categorized as blockers (>90% inhibition), partial blockers (30-90% inhibition), or non-blockers (no obvious inhibition). To further extend the assessment of the biological activity of the anti-NRP2 antibodies, their activity was assessed in a receptor dimerization assay. Vectors encoding a split luciferase, and a cell permeable substrate, were obtained from Promega corporation. The complete extracellular domain and transmembrane helices of NRP2, FLT4 (VEGFR3), KDR (VEGFR2) and plexin A1 (PLXNA1) were cloned into the vectors and screened for optimal orientation, following established methods. Tested antibodies were able to impair respective VEGF and SEMA3 induced dimerization of receptor pairs NRP2/FLT4, NRP2/KDR and NRP2/PLXNA1. Select antibodies show extremely specific and non-obvious functional differentiation. Direct functional assessment of a subset of these antibodies on breast CSCs revealed that the VEGF blocking, but not the SEMA3 blocking anti-NRP2 mAbs had the ability to inhibit serial passage mammosphere formation, an indicator of self-renewal potential. CONCLUSIONS: aTyr has developed and characterized a series of domain specific antibodies to NRP2. These antibodies show differential binding to specific domains of NRP2, can inhibit either VEGF-C or Sema3F binding to NRP2, and differentially effect receptor dimerization. The use of these antibodies enabled us to implicate VEGF-C/NRP2 signaling but not SEMA-3F/NRP2 signaling in the function of breast CSCs. Introduction Published data demonstrate that triple negative breast cancer (TNBC) is a highly aggressive subtype of breast cancer, and contains a much higher frequency of cancer stem cells (CSCs) than other subtypes, which may be responsible for poor patient outcomes by promoting therapy resistance, metastasis, and recurrence [1, 2]. NRP2 is enriched in TNBC and breast CSCs (Fig. 1). VEGF-NRP2 signaling promotes stem-like traits in breast cancer cells (e.g. tumor formation in vivo, Fig. 1E, [3-5]) aTyr has generated a panel of high-quality, anti-human NRP2 mAbs that have the potential for clinical development. aTyr a-NRP2 mAbs exhibit superior specificity and sensitivity compared to commercially available antibodies. aTyr a-NRP2 mAbs have diverse profiles in blocking of ligand binding and ligand-induced NRP2/co-receptor dimerization. aTyr aNRP2-10 specifically blocks VEGF-binding to NRP2 and VEGF-induced NRP2/VEGFR dimerization. aTyr aNRP2-14 specifically blocks Sema3F-binding to NRP2 and Sema3F-induced NRP2/Plexin dimerization. aTyr aNRP2-10 showed tumor-inhibitory effects on TNBC CSCs or organoids, demonstrating the potential to be developed for the clinical management of breast cancer. da Silva, J. L., et al. Triple Negative Breast Cancer: A Thorough Review Of Biomarkers. Crit Rev Oncol Hematol 145, 102855, doi:10.1016/j.critrevonc.2019.102855 (2020). Lee, K. Let al. Triple-negative Breast Cancer: Current Understanding And Future Therapeutic Breakthrough Targeting Cancer Stemness. Cancers (Basel) 11, doi:10.3390/cancers11091334 (2019). Goel, H. L. & Mercurio, A. M. VEGF Targets The Tumour Cell. Nat Rev Cancer 13, 871-882, doi:10.1038/nrc3627 (2013). Goel, H. L. et al. GLI1 Regulates A Novel Neuropilin-2/Alpha6beta1 Integrin Based Autocrine Pathway That Contributes To Breast Cancer Initiation. EMBO Mol Med 5, 488-508, doi:10.1002/emmm.201202078 (2013). Elaimy, A. L. et al. Vegf-neuropilin-2 Signaling Promotes Stem-like Traits In Breast Cancer Cells By Taz-mediated Repression Of The Rac GAP Beta2-chimaerin. Sci Signal 11, doi:10.1126/scisignal.aao6897 (2018). A D This work was supported by aTyr Pharma, Inc. D CD44+/CD24- (CSC properties) NRP2 expression in breast carcinoma tissue Normal breast epithelium Breast carcinoma tissue A NRP2 expression worsens breast cancer outcomes B NRP2 expression is much higher in TNBC (n=21) than non-TNBC (n=22) C TNBC Non-TNBC E In vivo tumor formation assay of TNBC SUM1315 transplanted into NSG mice Figure adapted from Reference [4] Antibody Epitope Domain (ELISA/SPR) Block VEGF induced NRP2/VEGFR dimerization Block Sema3F induced NRP2/Plexin dimerization aNRP2-2 b2-c linker Non-blocking Partial blocker aNRP2-9 b1 Partial blocker Partial blocker aNRP2-10 b1 Blocker Non-blocking aNRP2-11 b2 Blocker Blocker aNRP2-14 a2 Non-blocking Blocker B VEGF-A induced NRP2/VEGFR2 dimerization VEGF-C induced NRP2/VEGFR3 dimerization Sema3F induced NRP2/PlexinA1 dimerization C aNRP2-10 titration aNRP2-14 titration aTyr a-NRP2s target various NRP2 extracellular domains and have diverse blocking profiles. aNRP2-10 entirely blocks NRP2/VEGFR dimerization. aNRP2-14 specifically blocks NRP2/Plexin dimerization. Luminescence generated upon ligand-induced receptor dimerization VEGF-A165 VEGF-C Class 3 Semaphorins VEGFR NRP2 Plexin NRP2 Ligand-induced receptor dimerization and antibody blocking effects were analyzed using the NanoBiT® Technology (Promega) aTyr developed a-NRP2s have diverse blocking profiles aTyr a-NRP2 mAbs binding to Expi293-hNRP2 cell surface A B aTyr developed a-NRP2 mAbs a-NRP2 binding to A549 wildtype vs NRP2 KO cells a-NRP2 3F2 Isotype Ctrl Ab a-NRP2 1E3 (aNRP2-10) 2nd Ab only No Ab blocking 2nd Ab only No Ab blocking 2nd Ab only No Ab blocking a-NRP2 13C9 2nd Ab only No Ab blocking A B Antibody blocking of VEGF-C binding to Expi293-hNRP2 cells Antibody blocking of Sema3F binding to Expi293-hNRP2 cells a-NRP2 18B8 (aNRP2-14) a-NRP2 13D7 a-NRP2 3F2 Isotype Ctrl Ab aTyr a-NRP2 mAbs mostly have high affinities (sub-nM to nM) to human NRP2 by SPR, ELISA & Flow analysis (e.g. Fig. 2A). Many of these a-NRP2s exhibit superior specificity compared to commercially available antibodies (Fig. 2B, binding to endogenous NRP2 on A549 wildtype but not to the NRP2 knockout (KO) cells). aTyr a-NRP2s showed different capabilities in blocking of VEGF-C or SEMA-3F binding to Expi293-hNRP2 cells, including complete, partial or non-blockers (Fig. 3). aNRP2-10 blocks VEGF-C binding to Expi293-hNRP2 cells (Fig. 3A). aNRP2-14 blocks Sema3F binding to Expi293-hNRP2 cells (Fig. 3B). Humanized aNRP2-10 decreased viability, inhibited formation of mammospheres and anchorage-independent growth in cells or organoids from TNBC patients. Effects of aNRP2-10 are more pronounced in combination with chemotherapy agents. (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 significantly different from control) BT549 NRP2-high MDA-MB-468 NRP2-high TNBC NRP2-high cells 3D mammosphere formation assay TNBC MDA-MB-231 NPR2-high cells 3D soft agar colony formation assay TNBC Patient-derived Organoids 3D viability assay A B C D TNBC Patient-derived CSCs 3D mammosphere formation assay CD44+/CD24- sorted * R&D a-NRP2 #AF567 aTyr a-NRP2 1E3 (aNRP2-10) Ctrl AF567 Ctrl AF567 Ctrl 1E3 Ctrl 1E3 A549 wildtype A549 NRP2 KO Results Reference Abstract Conclusions Acknowledgements Figure 1. NRP2 Association with Breast Cancer and Enrichment in Breast CSCs Figure 2. aTyr Developed a-NRP2 mAbs Exhibit Superior Specificity and Sensitivity Compared to Commercially Available Antibodies Figure 3. aTyr a-NRP2 Blocking of Ligand Binding to Expi293-hNRP2 Cells Figure 4. aTyr a-NRP2 Blocking of Ligand Induced NRP2/co-receptor Dimerization Figure 5. aNRP2-10 Showed Tumor-inhibitory Effects on Cells or Organoids from TNBC Patients 2nd Ab only No Ab blocking 2nd Ab only No Ab blocking 2nd Ab only No Ab blocking 2nd Ab only No Ab blocking EXHIBIT 99.1
