Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Avalo Therapeutics, Inc. | a8-kinvestorpresentation_j.htm |

Exhibit 99.1 Patient Inspired Science Establishing a leading, pediatric rare and orphan disease-focused biopharmaceutical company to deliver impactful new medicines to patients June | 2020 Corporate Highlights | Focus on COVID-19 ARDS

Forward-Looking Statements This presentation may include forward-looking statements made pursuant to the Private Securities Litigation Reform Act of 1995. Forward-looking statements are statements that are not historical facts. Such forward-looking statements are subject to significant risks and uncertainties that are subject to change based on various factors (many of which are beyond Cerecor, Inc. (“Cerecor”) control, which could cause actual results to differ from the forward-looking statements. Such statements may include, without limitation, statements with respect to Cerecor’s plans, objectives, projections, expectations and intentions and other statements identified by words such as “projects,” “may,” “might,” “will,” “could,” “would,” “should,” “continue,” “seeks,” “aims,” “predicts,” “believes,” “expects,” “anticipates,” “estimates,” “intends,” “plans,” “potential,” or similar expressions (including their use in the negative), or by discussions of future matters such as: our 2020 outlook; the development of product candidates or products; potential attributes and benefits of product candidates; strategic alternatives for neurological assets and Millipred; and other statements that are not historical. These statements are based upon the current beliefs and expectations of Cerecor’s management but are subject to significant risks and uncertainties, including: reliance on and integration of key personnel; drug development costs, timing and other risks, including reliance on investigators and enrollment of patients in clinical trials, which might be slowed by the COVID-19 pandemic; regulatory risks; Cerecor's cash position and the need for it to raise additional capital; risks related to potential strategic alternatives for our neurology assets and Millipred; general economic and market risks and uncertainties, including those caused by the COVID-19 pandemic and those other risks detailed in Cerecor’s filings with the Securities and Exchange Commission. Actual results may differ from those set forth in the forward-looking statements. Except as required by applicable law, Cerecor expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Cerecor’s expectations with respect thereto or any change in events, conditions or circumstances on which any statement is based. 2|
Highlights • Recent Merger of Cerecor and Aevi has created a rich pipeline of novel, 1st in class assets all with proven mechanistic rationale • Only known anti-LIGHT mAb in the clinic, offers potential to treat cytokine storm-induced COVID-19 ARDS in the near-term and broader ARDS indication beyond – CERC-002 will enter the clinic in June and is expected to deliver definitive topline POC data in Q4 2020 • CERC-007 (anti-IL-18 mAb), unique molecular target, is expected to deliver initial data in multiple myeloma 1Q 2021 and top line data 2Q 2021; initial data in adult onset Still’s Disease by 2Q 2021. • CERC-006 (dual mTOR inhibitor), topline data expected 2Q 2021 • CERC-800 series (substrate replacement therapy for congenital disorders of glycosylation, all orphan designated, Priority Review Vouchers eligible) will release data from CDG-FIRST 1H 2020 – CERC-801 pivotal trial expected start 4Q 2020, top line data expected 4Q 2021 – CERC-802 pivotal trial expected start 4Q 2020, top line data expected 3Q 2021 – CERC-803 pivotal trial expected start 1H 2021, top line data expected 2H 2021 3|
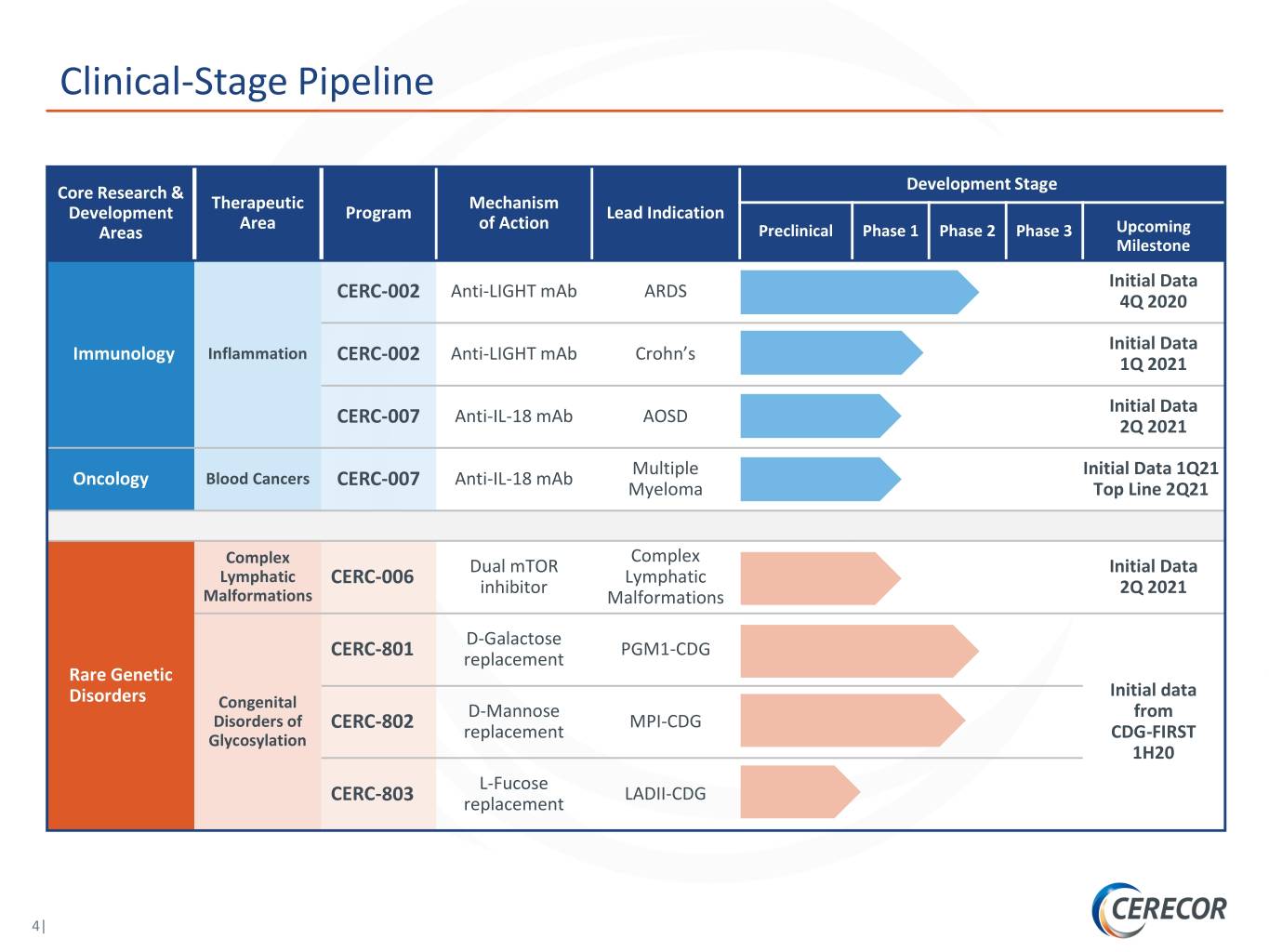
Clinical-Stage Pipeline Core Research & Development Stage Therapeutic Mechanism Development Program Lead Indication Area of Action Areas Preclinical Phase 1 Phase 2 Phase 3 Upcoming Milestone Initial Data Anti-LIGHT mAb ARDS CERC-002 4Q 2020 Initial Data Immunology Inflammation Anti-LIGHT mAb Crohn’s CERC-002 1Q 2021 Initial Data Anti-IL-18 mAb AOSD CERC-007 2Q 2021 Multiple Initial Data 1Q21 Oncology Blood Cancers Anti-IL-18 mAb CERC-007 Myeloma Top Line 2Q21 Complex Complex Dual mTOR Initial Data Lymphatic Lymphatic CERC-006 inhibitor 2Q 2021 Malformations Malformations D-Galactose PGM1-CDG CERC-801 replacement Rare Genetic Disorders Initial data Congenital D-Mannose from Disorders of CERC-802 MPI-CDG Glycosylation replacement CDG-FIRST 1H20 L-Fucose LADII-CDG CERC-803 replacement 4|

CERC-007 Phase 2-ready, anti-IL-18 monoclonal antibody for Multiple Myeloma & Adult Onset Still’s Disease
Phase II-Ready Asset for Multiple Myeloma (MM) and Adult Onset Still’s Disease (AOSD) First-in-class, only fully human anti-IL-18 mAb with potential to address multiple tumor types and inflammatory conditions Strong • Elevated IL-18 is correlated with poor survival in MM patients and disease Scientific Rationale severity in AOSD patients • A need for improved durability of response and treatment relapse rate in Need for multiple myeloma Novel MOAs • Currently there are no approved targeted therapies for AOSD in the U.S. Unique • IL-18 allows tumor to evade immune destruction, and is a driver of Mechanism tumor growth of Action • Demonstrated proof-of-concept with an IL-18 binding protein in AOSD • Unique MOA and safety profile makes it an ideal candidate for combination Clinical therapy in MM Differentiation • Completely new mechanism with strong correlation for disease severity in AOSD 6| Source: Nakamura Cancer Cell. 2018. 33(4):634-648.e5.; Kudela et al. (2019) BMC Rheumatol. 3:4
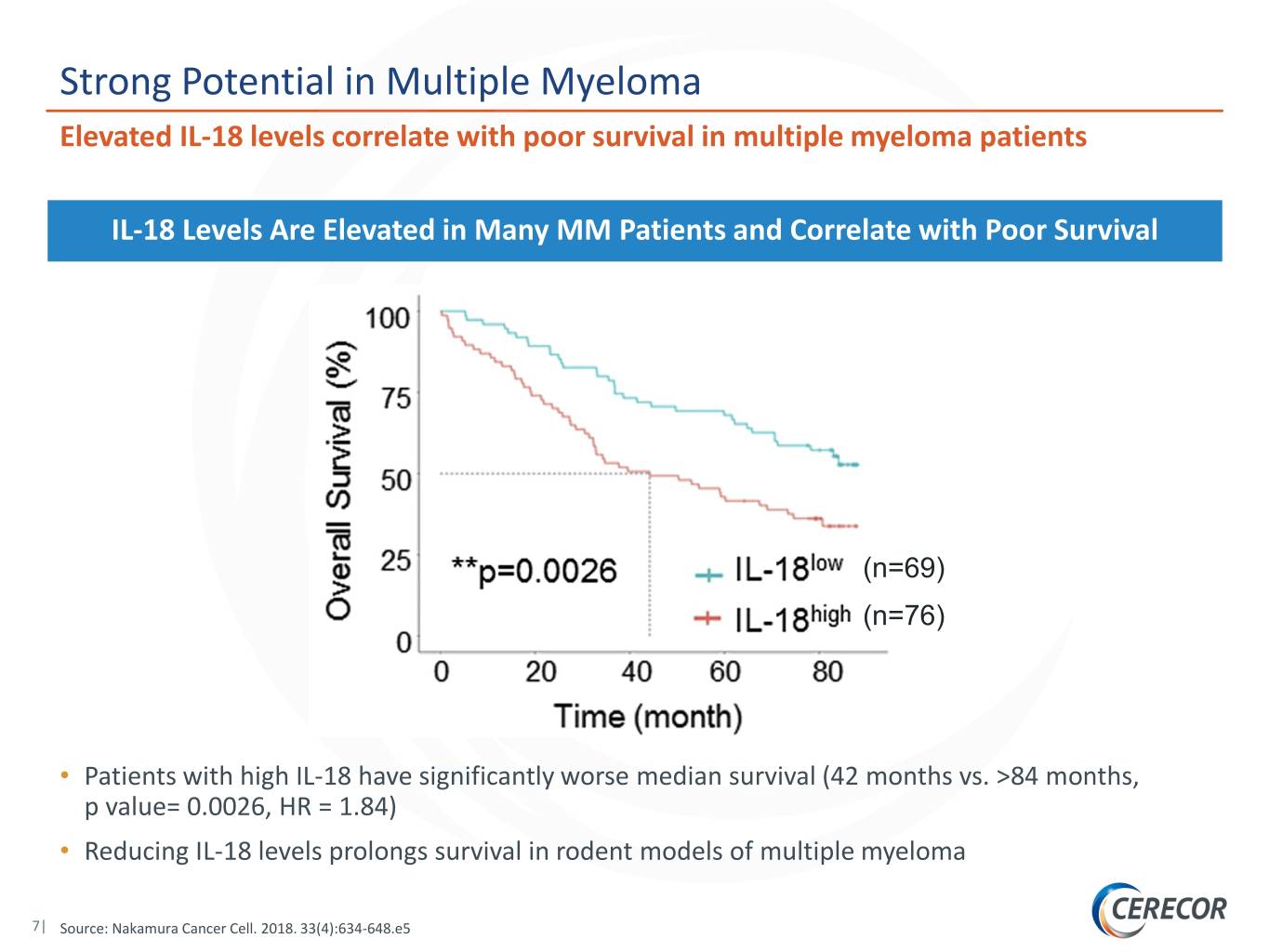
Strong Potential in Multiple Myeloma Elevated IL-18 levels correlate with poor survival in multiple myeloma patients IL-18 Levels Are Elevated in Many MM Patients and Correlate with Poor Survival (n=69) (n=76) • Patients with high IL-18 have significantly worse median survival (42 months vs. >84 months, p value= 0.0026, HR = 1.84) • Reducing IL-18 levels prolongs survival in rodent models of multiple myeloma 7| Source: Nakamura Cancer Cell. 2018. 33(4):634-648.e5
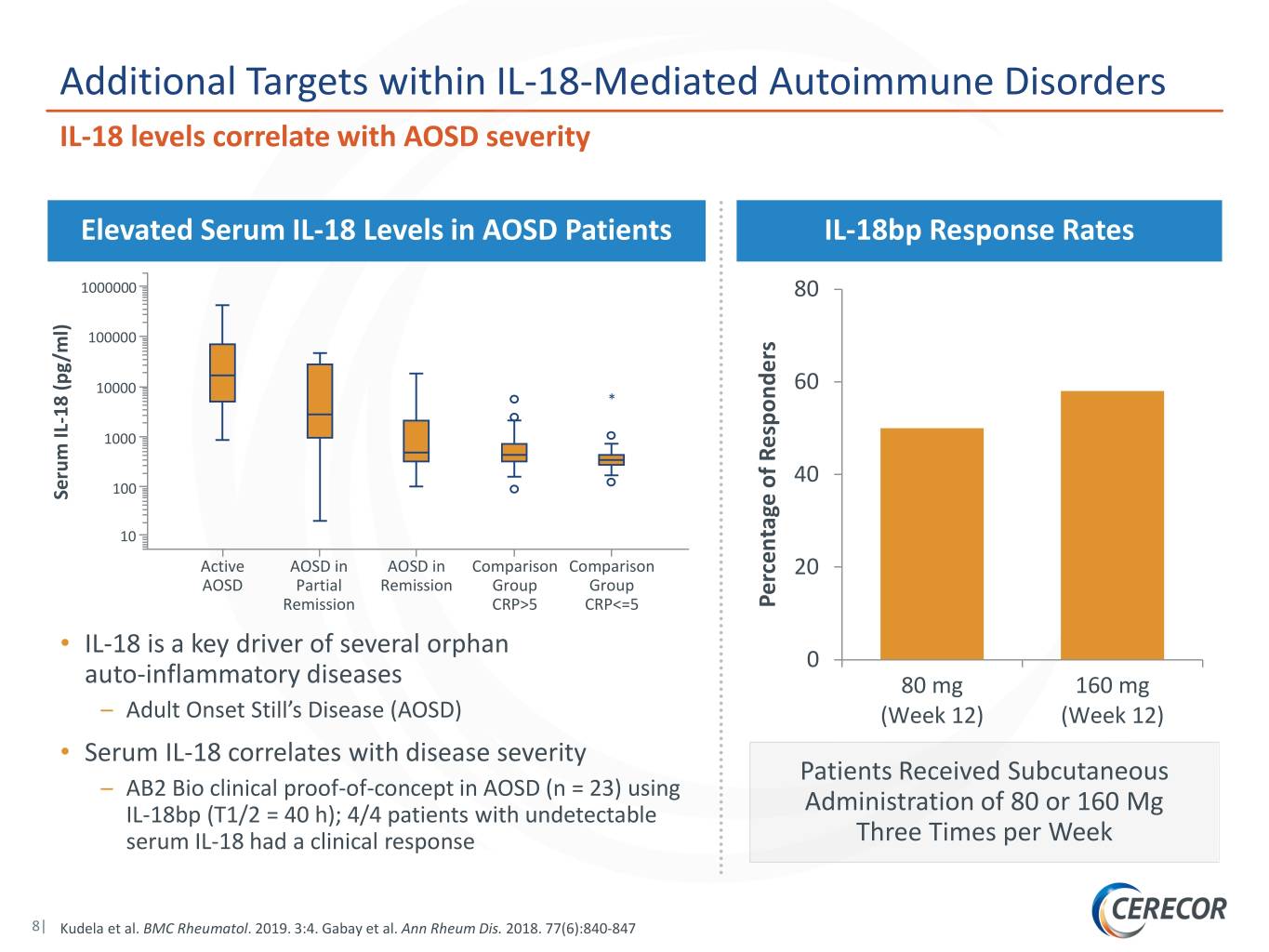
Additional Targets within IL-18-Mediated Autoimmune Disorders IL-18 levels correlate with AOSD severity Elevated Serum IL-18 Levels in AOSD Patients IL-18bp Response Rates 1000000 80 100000 10000 60 * 18 (pg/ml) 1000 40 100 Serum IL - 10 Active AOSD in AOSD in Comparison Comparison 20 AOSD Partial Remission Group Group Remission CRP>5 CRP<=5 of Responders Percentage • IL-18 is a key driver of several orphan 0 auto-inflammatory diseases 80 mg 160 mg – Adult Onset Still’s Disease (AOSD) (Week 12) (Week 12) • Serum IL-18 correlates with disease severity Patients Received Subcutaneous – AB2 Bio clinical proof-of-concept in AOSD (n = 23) using IL-18bp (T1/2 = 40 h); 4/4 patients with undetectable Administration of 80 or 160 Mg serum IL-18 had a clinical response Three Times per Week 8| Kudela et al. BMC Rheumatol. 2019. 3:4. Gabay et al. Ann Rheum Dis. 2018. 77(6):840-847
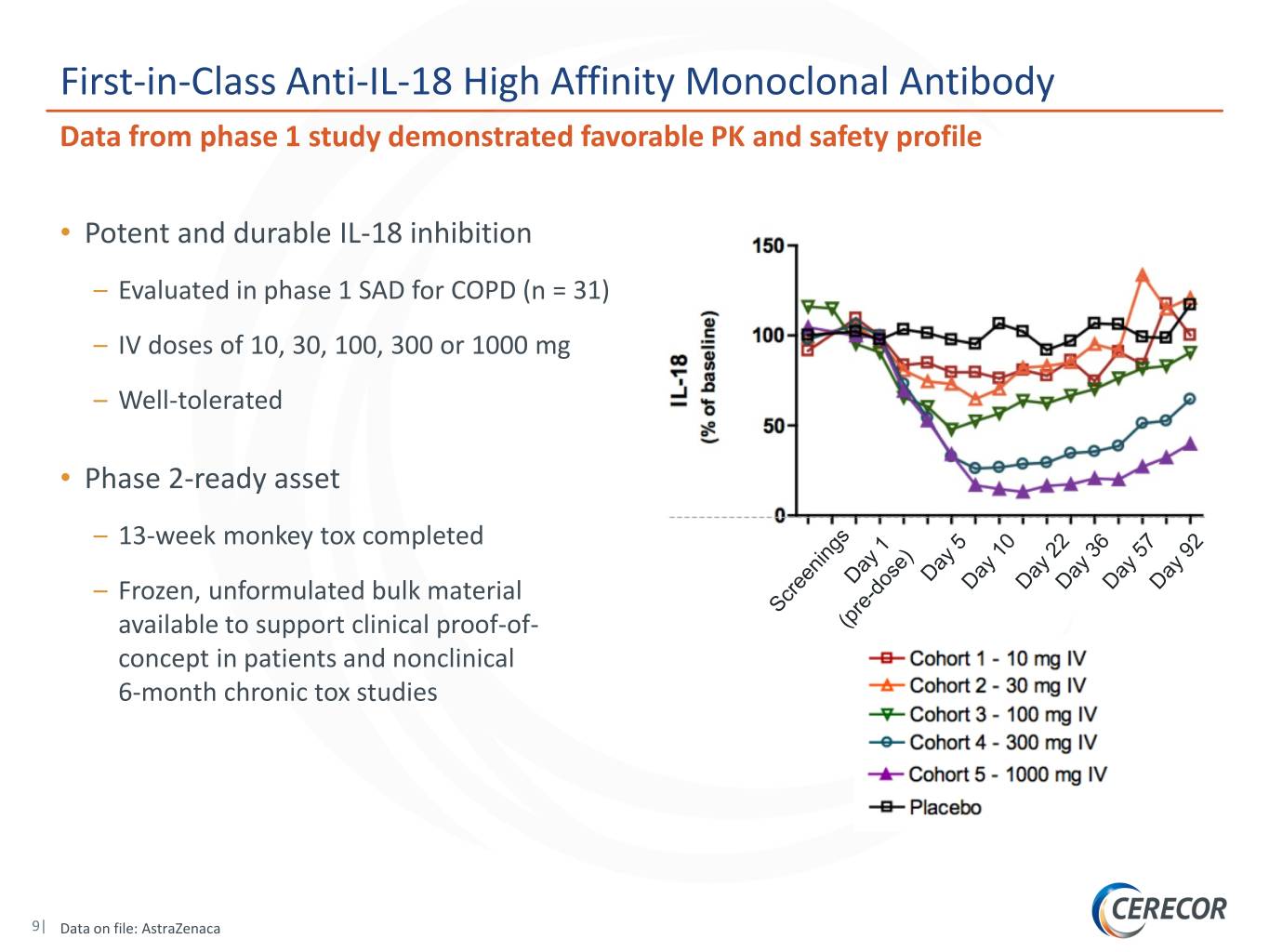
First-in-Class Anti-IL-18 High Affinity Monoclonal Antibody Data from phase 1 study demonstrated favorable PK and safety profile • Potent and durable IL-18 inhibition – Evaluated in phase 1 SAD for COPD (n = 31) – IV doses of 10, 30, 100, 300 or 1000 mg – Well-tolerated • Phase 2-ready asset – 13-week monkey tox completed – Frozen, unformulated bulk material available to support clinical proof-of- concept in patients and nonclinical 6-month chronic tox studies 9| Data on file: AstraZenaca

Program Update as of June 2020 Anticipate proof-of-concept trial initiation Q4 2020 in both MM and AOSD • Pre-IND meeting with the FDA completed, concurrence on high-level design • CRO under contract (PRA); sites selected; study start anticipated early 4Q 2020 Multiple Myeloma • Classic 3+3 dose escalation design to determine recommended Phase 2 dose (anticipated 1Q 2021) followed by a treatment expansion portion to establish response rate (anticipated 2Q 2021) • Pre-IND meeting with the FDA completed, working through design details AOSD • CRO under contract (PRA); study start anticipated 4Q 2020 • Preparing clinical supplies, expected 3Q 2020 CMC • Supply sufficient for multiple POC studies • CMO: Catalent Biologics 10|

CERC-006 Phase 2-ready, Dual mTORC 1/2 small molecule inhibitor for Complex Lymphatic Malformations
Phase II-Ready Asset for Complex Lymphatic Malformations Potential first-in-class potent inhibitor of mTORC1/mTORC2 High Unmet • Orphan disease(s) with combined US prevalence of 30 to 60k associated with Need high mortality rates of 20% to 50% over 3 to 7 years Demonstrated • Off label use of sirolimus (mTORC1 inhibitor) has demonstrated modest Proof of Concept efficacy, hampered by significant safety issues • Potent inhibitor of mTORC1/mTORC2 allowing for lower dosing to achieve Potent Dual efficacy and improve safety mTOR Inhibition • Dual inhibition may prevent upregulation of AKT and PI3K, potentially leading to less diabetes and mucositis Potential to Become • Potential to be the first pharmacologic therapy approved for complex Standard lymphatic malformations of Care 12| Source: ClearView market research
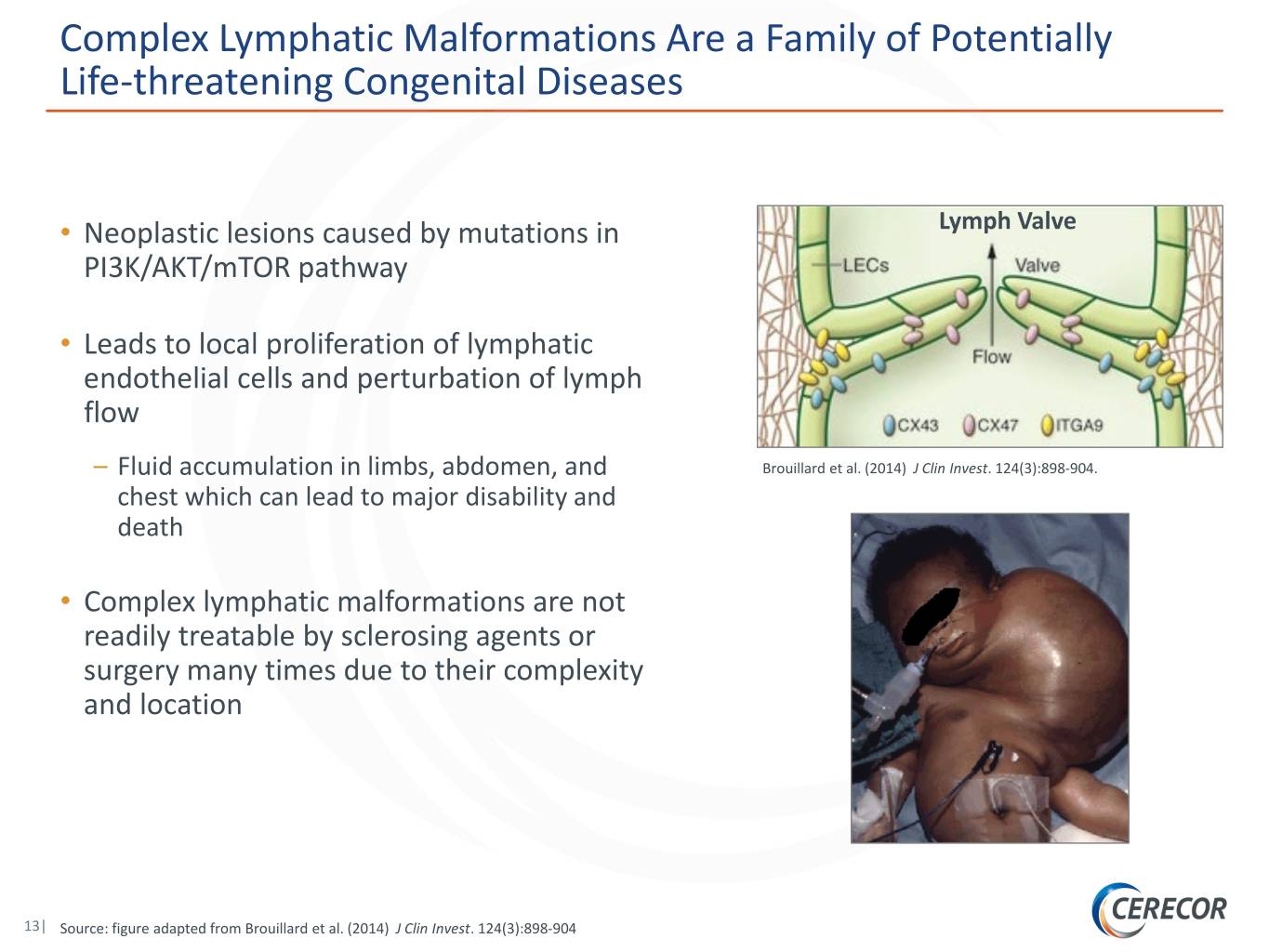
Complex Lymphatic Malformations Are a Family of Potentially Life-threatening Congenital Diseases • Neoplastic lesions caused by mutations in Lymph Valve PI3K/AKT/mTOR pathway • Leads to local proliferation of lymphatic endothelial cells and perturbation of lymph flow – Fluid accumulation in limbs, abdomen, and Brouillard et al. (2014) J Clin Invest. 124(3):898-904. chest which can lead to major disability and death • Complex lymphatic malformations are not readily treatable by sclerosing agents or surgery many times due to their complexity and location 13| Source: figure adapted from Brouillard et al. (2014) J Clin Invest. 124(3):898-904
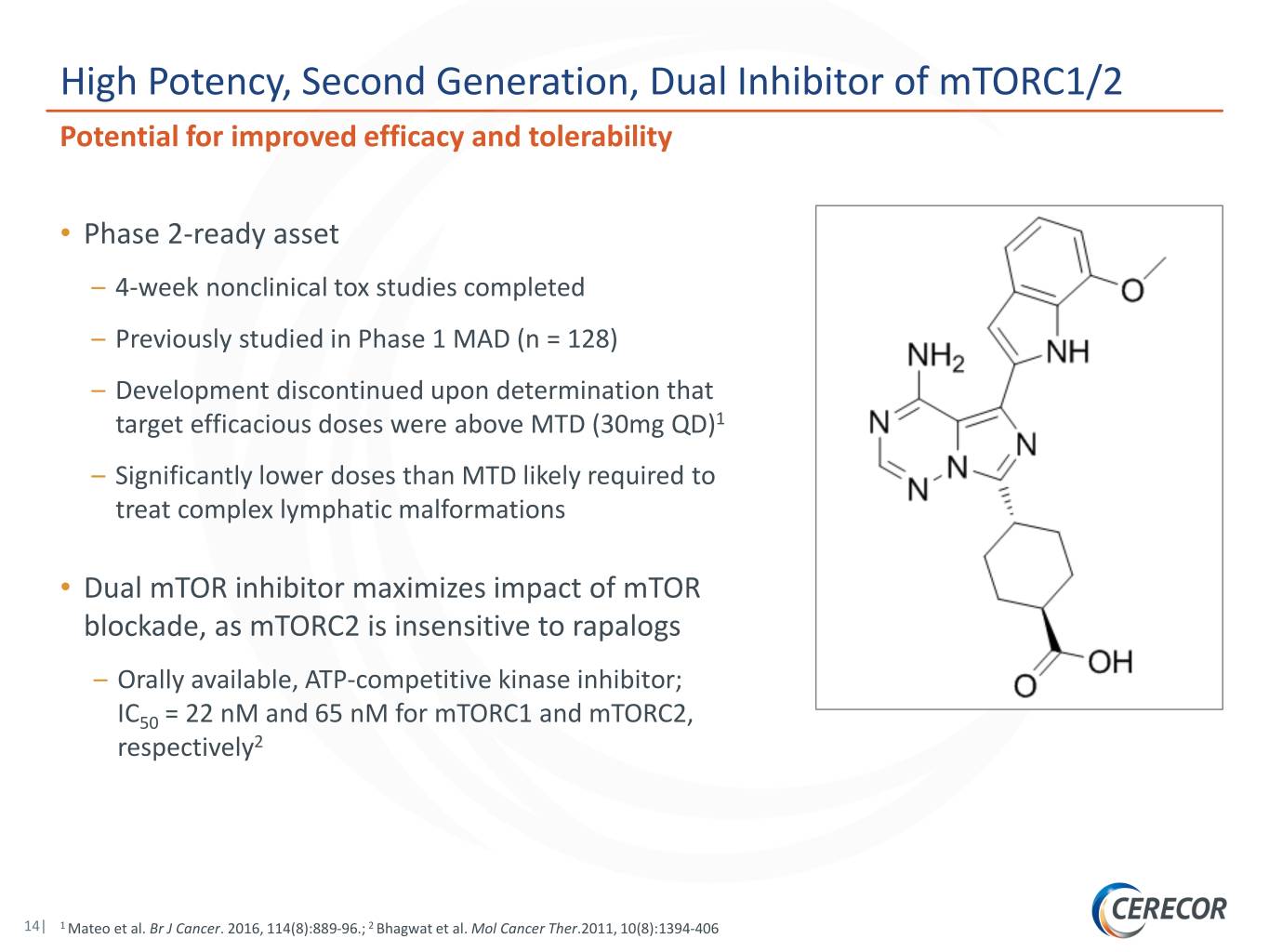
High Potency, Second Generation, Dual Inhibitor of mTORC1/2 Potential for improved efficacy and tolerability • Phase 2-ready asset – 4-week nonclinical tox studies completed – Previously studied in Phase 1 MAD (n = 128) – Development discontinued upon determination that target efficacious doses were above MTD (30mg QD)1 – Significantly lower doses than MTD likely required to treat complex lymphatic malformations • Dual mTOR inhibitor maximizes impact of mTOR blockade, as mTORC2 is insensitive to rapalogs – Orally available, ATP-competitive kinase inhibitor; IC50 = 22 nM and 65 nM for mTORC1 and mTORC2, respectively2 14| 1 Mateo et al. Br J Cancer. 2016, 114(8):889-96.; 2 Bhagwat et al. Mol Cancer Ther.2011, 10(8):1394-406
Program Update as of June 2020 Anticipate proof-of-concept trial initiation Q1 2021 • Pre-IND meeting with the FDA completed, concurrence on inclusion criteria and high-level design Complex Lymphatic • Key sites identified and KOLs engaged, study start anticipated 1Q 2021 Malformations • Orphan Drug and Rare Pediatric Disease Designation (PRV eligibility) application submission planned for June 2020 • Clinical supplies expected 4Q 2020 CMC • Manufacturing underway at Patheon 15|

CERC-800s Monosaccharide therapy for Congenital Disorders of Glycosylation (CDGs)
Treatment for Congenital Disorders of Glycosylation (CDGs) Monosaccharide therapy for PGM1-CDG, MPI-CDG and LADII High Patient • Ultra-rare Orphan diseases with an estimated 1,000 to 1,500 patients Unmet Need world-wide; no approved therapies to date Demonstrated • Data from the literature shows clinical and biomarker improvement when Proof of patients are treated with non-approved, non-GMP monosaccharides Concept (D-galactose / D-mannose / L-fucose) Efficient • Small prospective trials for each indication Development • Global engagement with KOLs helps identify patients and sites Approach • Potential Priority Review of NDAs (8-month review cycle) Rare Disease • All three programs have been orphan-designated and are PRV eligible Focus 17| Source: DelveInsight Syndicated Report
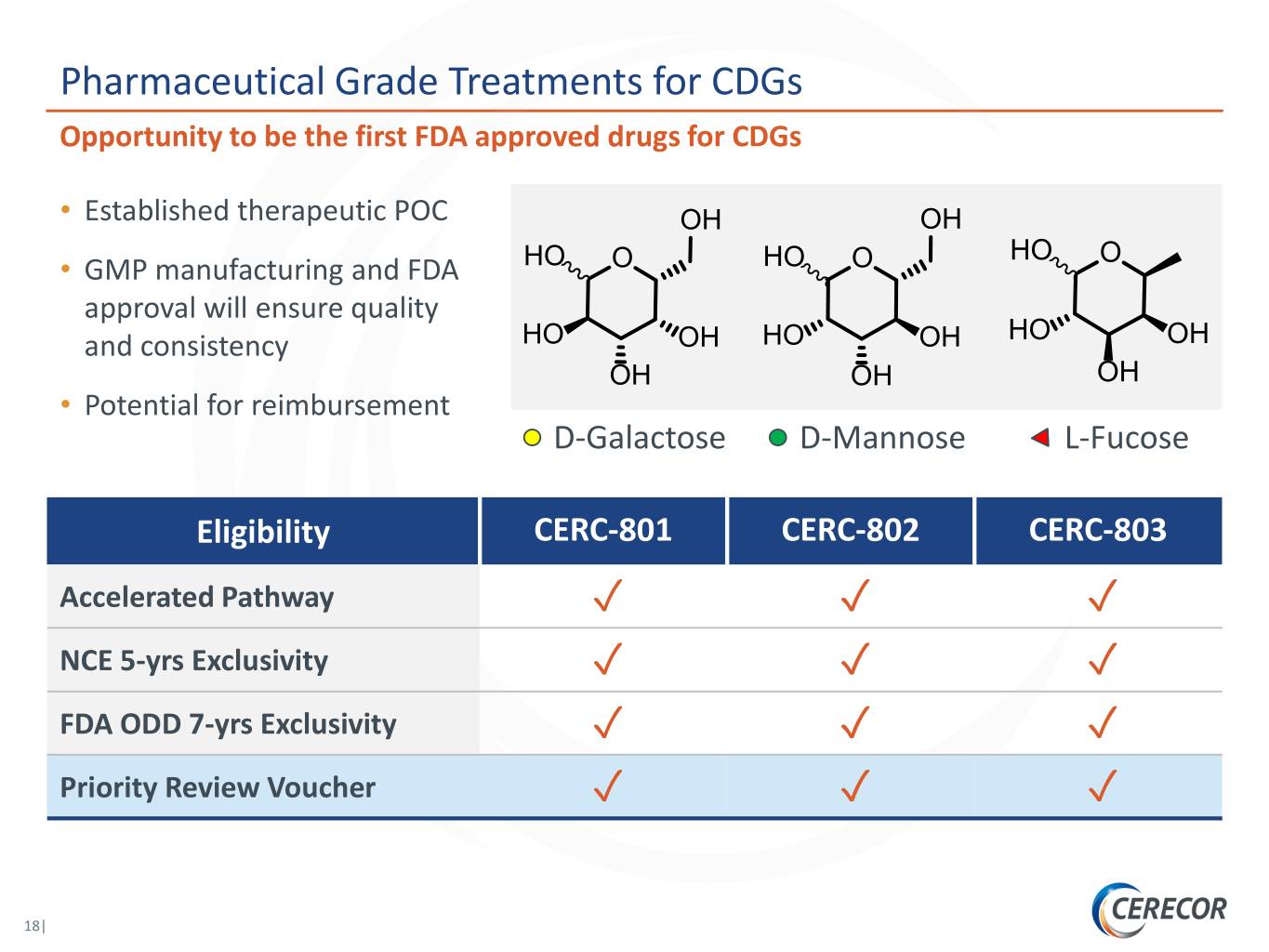
Pharmaceutical Grade Treatments for CDGs Opportunity to be the first FDA approved drugs for CDGs • Established therapeutic POC OH OH HO O • GMP manufacturing and FDA HO O HO O approval will ensure quality and consistency HO OH HO OH HO OH OH OH OH • Potential for reimbursement D-Galactose D-Mannose L-Fucose Eligibility CERC-801 CERC-802 CERC-803 Accelerated Pathway ✓ ✓ ✓ NCE 5-yrs Exclusivity ✓ ✓ ✓ FDA ODD 7-yrs Exclusivity ✓ ✓ ✓ Priority Review Voucher ✓ ✓ ✓ 18|
Program Update as of June 2020 All three programs on track • Natural history data is being provided by NIH funded Frontiers in Congenital Disorders of Glycosylation Consortium (FCDGC) Project in June 2020 • Pivotal trial will be run in collaboration with the Frontiers Project in 4Q 2020; CERC-801 trial design completed; Type C FDA meeting planned for 3Q 2020 • Sites and patients identified via the Frontiers Project • Estimated N=10; 6-month study with primary endpoint of surrogate biomarkers • Natural history data for MPI-CDG in press June 2020 • Sites and patients identified through engagement with KOLs CERC-802 • Type C FDA meeting planned for 3Q 2020; trial design completed; study start expected 4Q 2020 • Estimated N=5; 3-month study with primary endpoint: maintenance of antithrombin III • Ultra rare disease with multiple patients and sites identified CERC-803 • IND submission planned for 4Q 2020 CMC • Clinical supplies manufacturing on track for all three programs 19|

CERC-002 Anti-LIGHT monoclonal antibody in clinical studies for COVID-19 ARDS
The Impact of Cytokine Storm Induced COVID-19 ARDS The outbreak of Coronavirus Disease 2019 (COVID-19) has created a global health crisis The viral infection triggers a hyperactive Approximately 1,500* people in immune response leading to cytokine the United States die each day storm and Acute Respiratory Distress from COVID-19 Syndrome (ARDS), a leading cause of death in COVID-19 patients There is currently no effective Our data implicate the inflammatory cytokine, LIGHT, as a for treatment Cytokine Storm potential key driver of cytokine storm induced COVID-19 ARDS leading to ARDS and death We believe CERC-002 is the only known therapeutic currently in clinical development that inhibits the inflammatory cytokine LIGHT 21| *Data from Bloomberg COVID Tracker April 2020
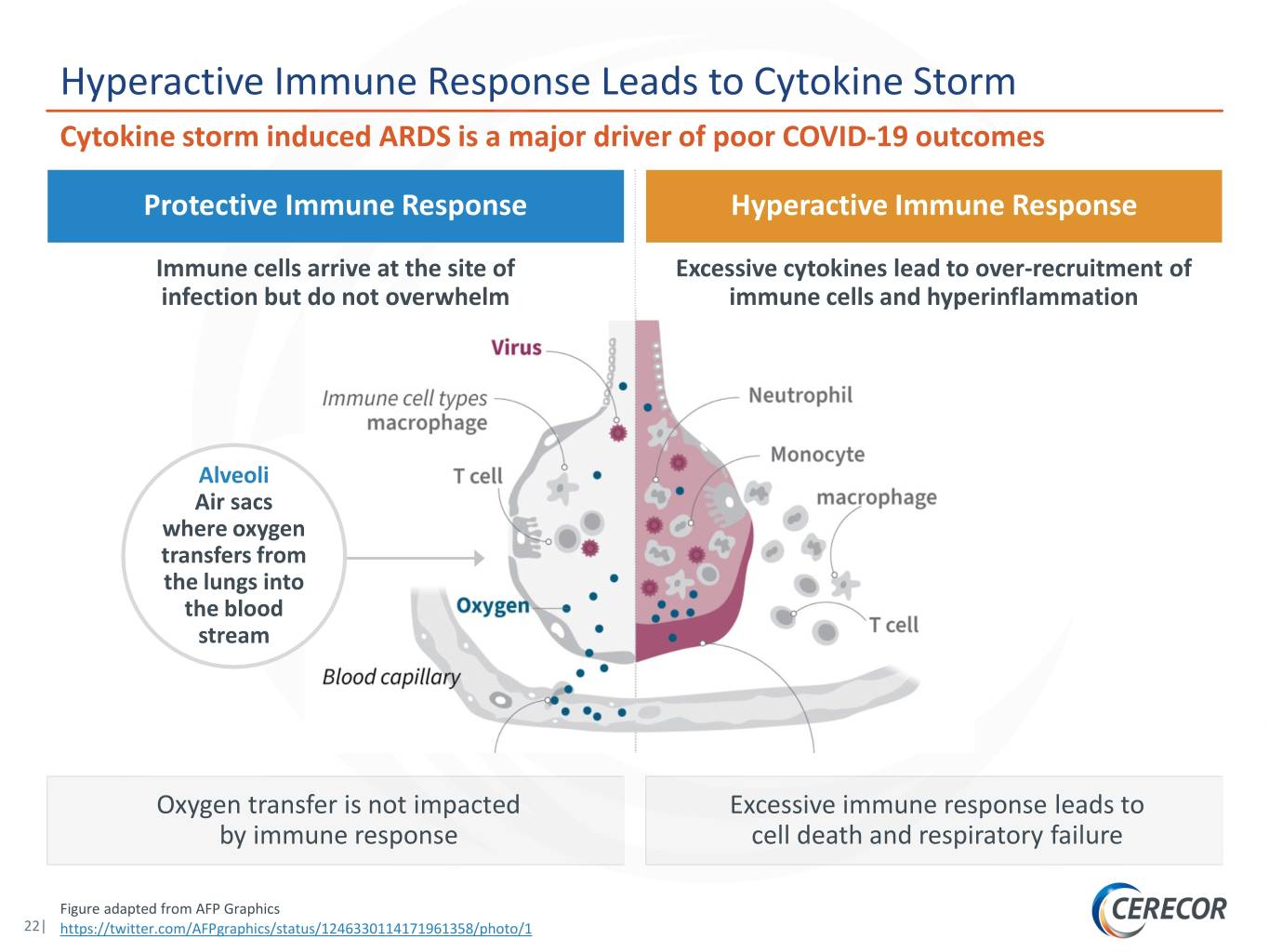
Hyperactive Immune Response Leads to Cytokine Storm Cytokine storm induced ARDS is a major driver of poor COVID-19 outcomes Protective Immune Response Hyperactive Immune Response Immune cells arrive at the site of Excessive cytokines lead to over-recruitment of infection but do not overwhelm immune cells and hyperinflammation Alveoli Air sacs where oxygen transfers from the lungs into the blood stream Oxygen transfer is not impacted Excessive immune response leads to by immune response cell death and respiratory failure Figure adapted from AFP Graphics 22| https://twitter.com/AFPgraphics/status/1246330114171961358/photo/1
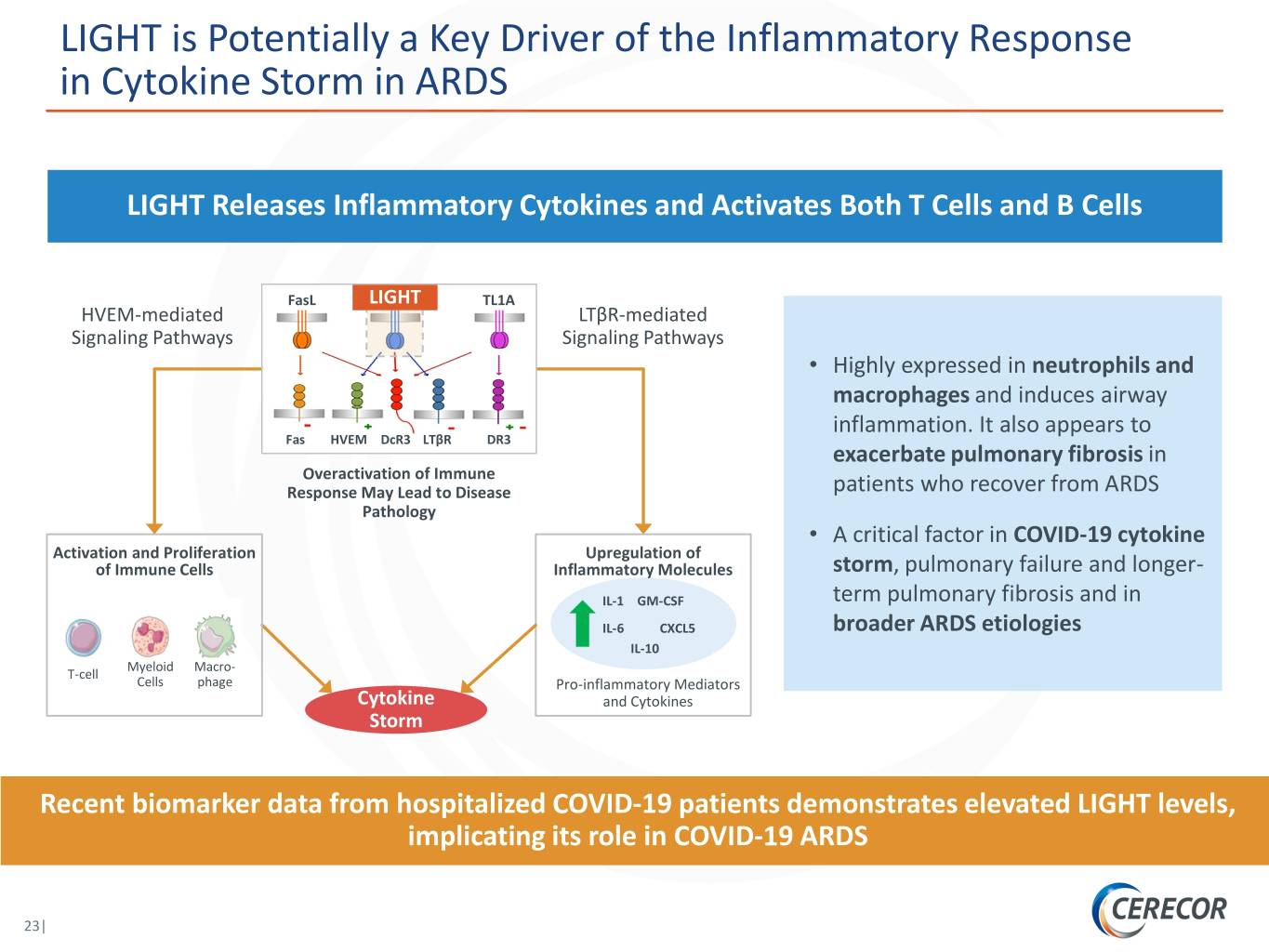
LIGHT is Potentially a Key Driver of the Inflammatory Response in Cytokine Storm in ARDS LIGHT Releases Inflammatory Cytokines and Activates Both T Cells and B Cells FasL LIGHT TL1A HVEM-mediated LTβR-mediated Signaling Pathways Signaling Pathways • Highly expressed in neutrophils and macrophages and induces airway inflammation. It also appears to Fas HVEM DcR3 LTβR DR3 exacerbate pulmonary fibrosis in Overactivation of Immune Response May Lead to Disease patients who recover from ARDS Pathology • A critical factor in COVID-19 cytokine Activation and Proliferation Upregulation of of Immune Cells Inflammatory Molecules storm, pulmonary failure and longer- IL-1 GM-CSF term pulmonary fibrosis and in IL-6 CXCL5 broader ARDS etiologies IL-10 Myeloid Macro- T-cell Cells phage Pro-inflammatory Mediators Cytokine and Cytokines Storm Recent biomarker data from hospitalized COVID-19 patients demonstrates elevated LIGHT levels, implicating its role in COVID-19 ARDS 23|
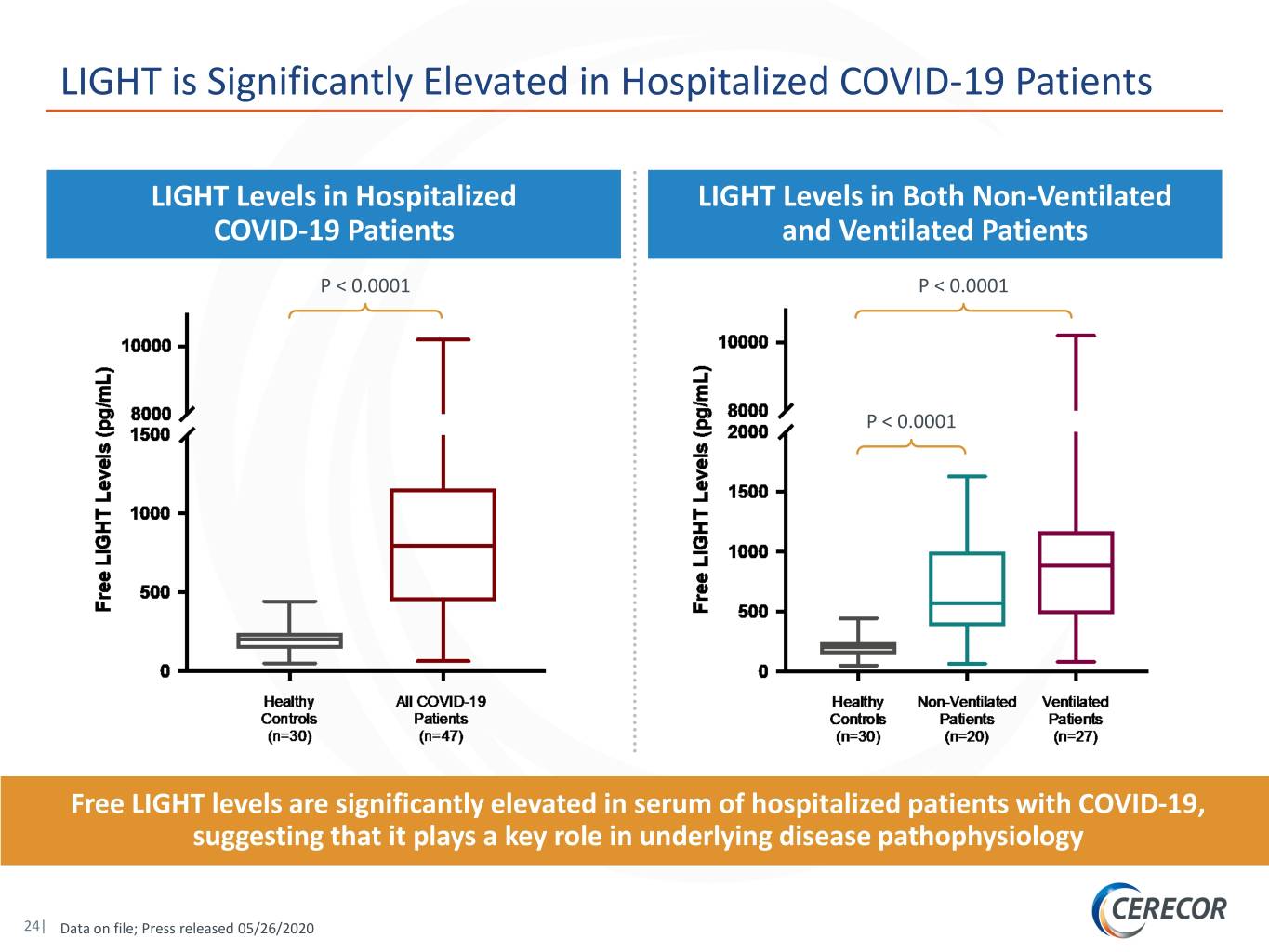
LIGHT is Significantly Elevated in Hospitalized COVID-19 Patients LIGHT Levels in Hospitalized LIGHT Levels in Both Non-Ventilated COVID-19 Patients and Ventilated Patients P < 0.0001 P < 0.0001 P < 0.0001 Free LIGHT levels are significantly elevated in serum of hospitalized patients with COVID-19, suggesting that it plays a key role in underlying disease pathophysiology 24| Data on file; Press released 05/26/2020
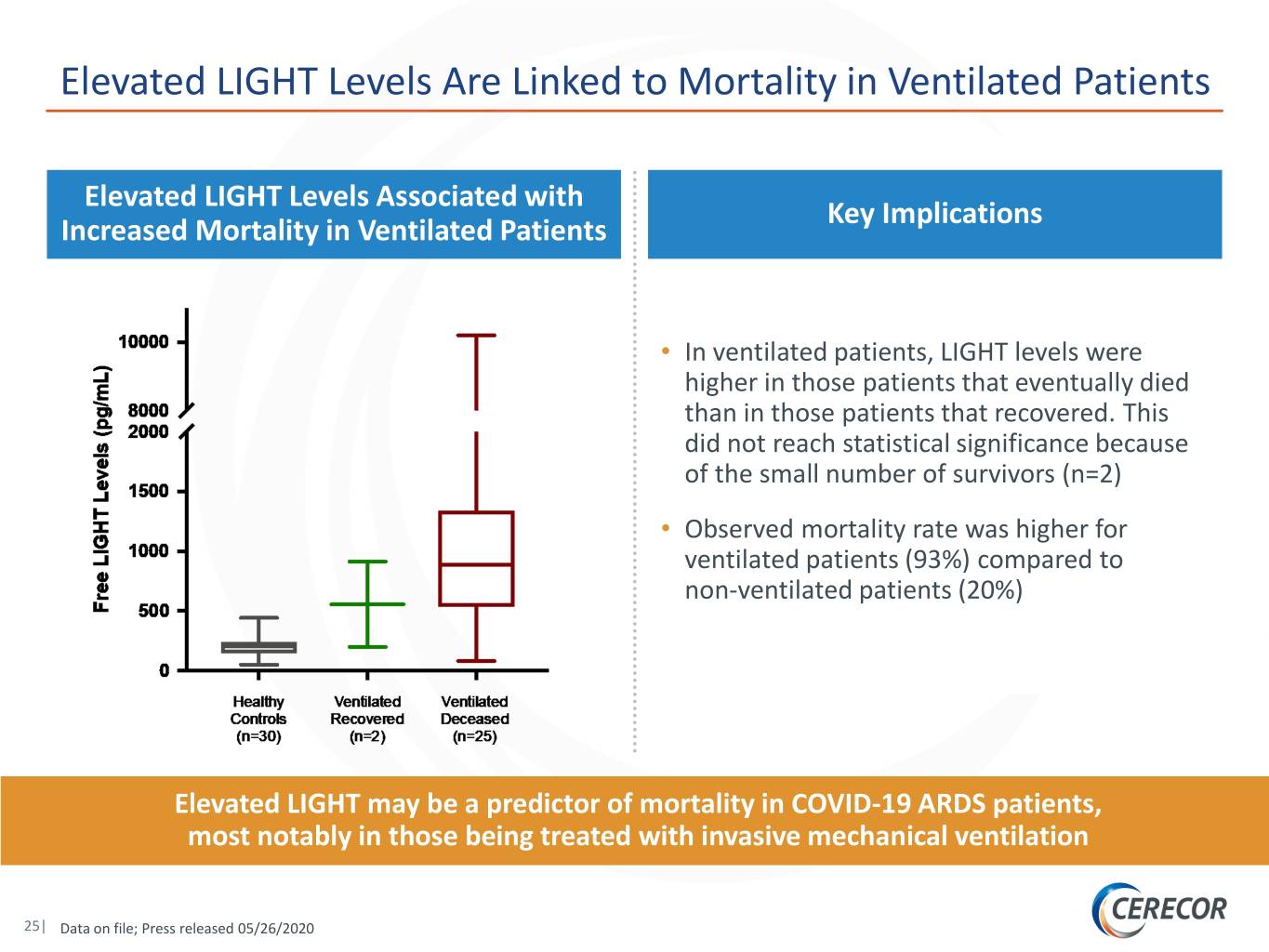
Elevated LIGHT Levels Are Linked to Mortality in Ventilated Patients Elevated LIGHT Levels Associated with Key Implications Increased Mortality in Ventilated Patients • In ventilated patients, LIGHT levels were higher in those patients that eventually died than in those patients that recovered. This did not reach statistical significance because of the small number of survivors (n=2) • Observed mortality rate was higher for ventilated patients (93%) compared to non-ventilated patients (20%) Elevated LIGHT may be a predictor of mortality in COVID-19 ARDS patients, most notably in those being treated with invasive mechanical ventilation 25| Data on file; Press released 05/26/2020
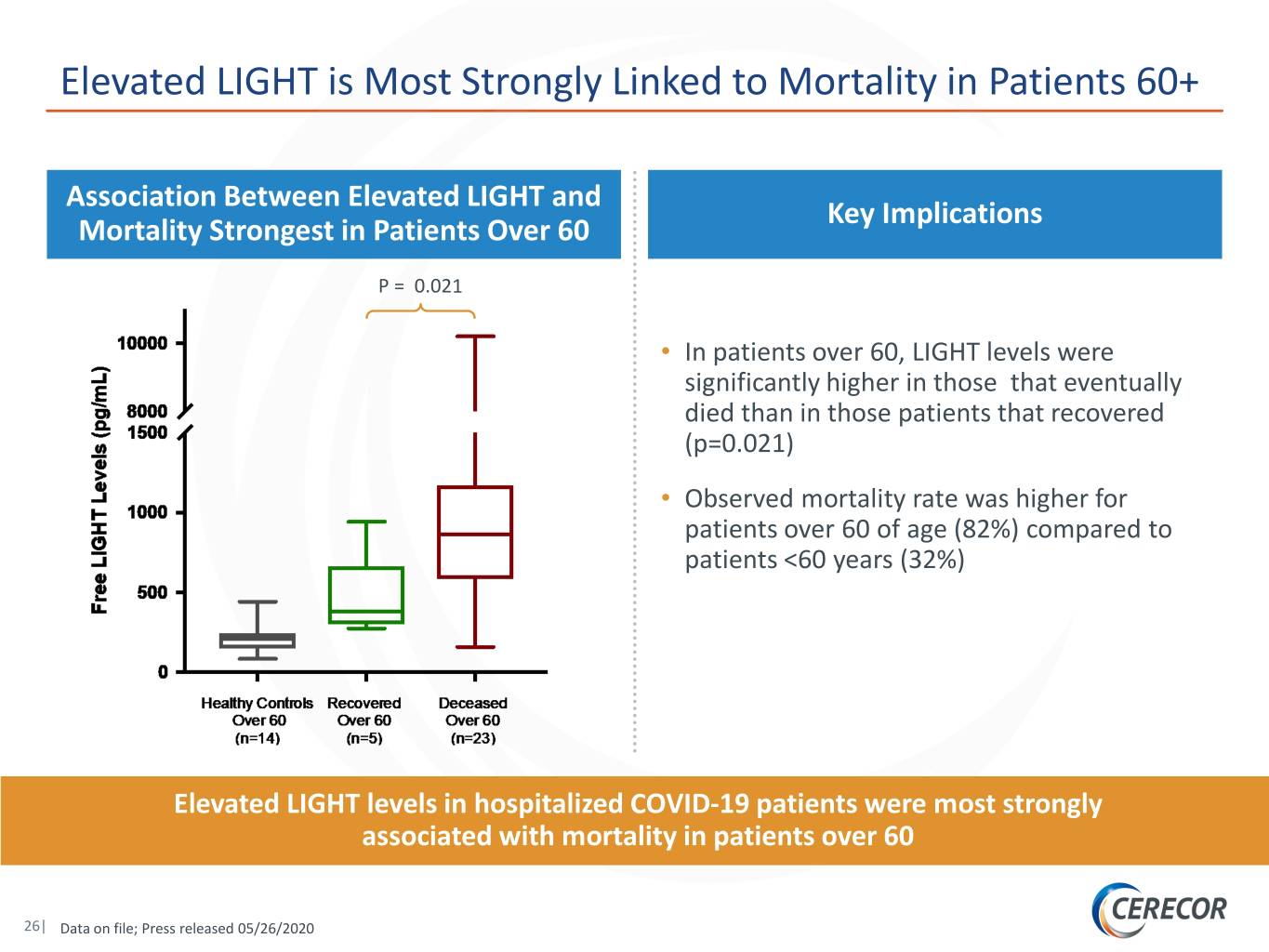
Elevated LIGHT is Most Strongly Linked to Mortality in Patients 60+ Association Between Elevated LIGHT and Key Implications Mortality Strongest in Patients Over 60 P = 0.021 • In patients over 60, LIGHT levels were significantly higher in those that eventually died than in those patients that recovered (p=0.021) • Observed mortality rate was higher for patients over 60 of age (82%) compared to patients <60 years (32%) Elevated LIGHT levels in hospitalized COVID-19 patients were most strongly associated with mortality in patients over 60 26| Data on file; Press released 05/26/2020
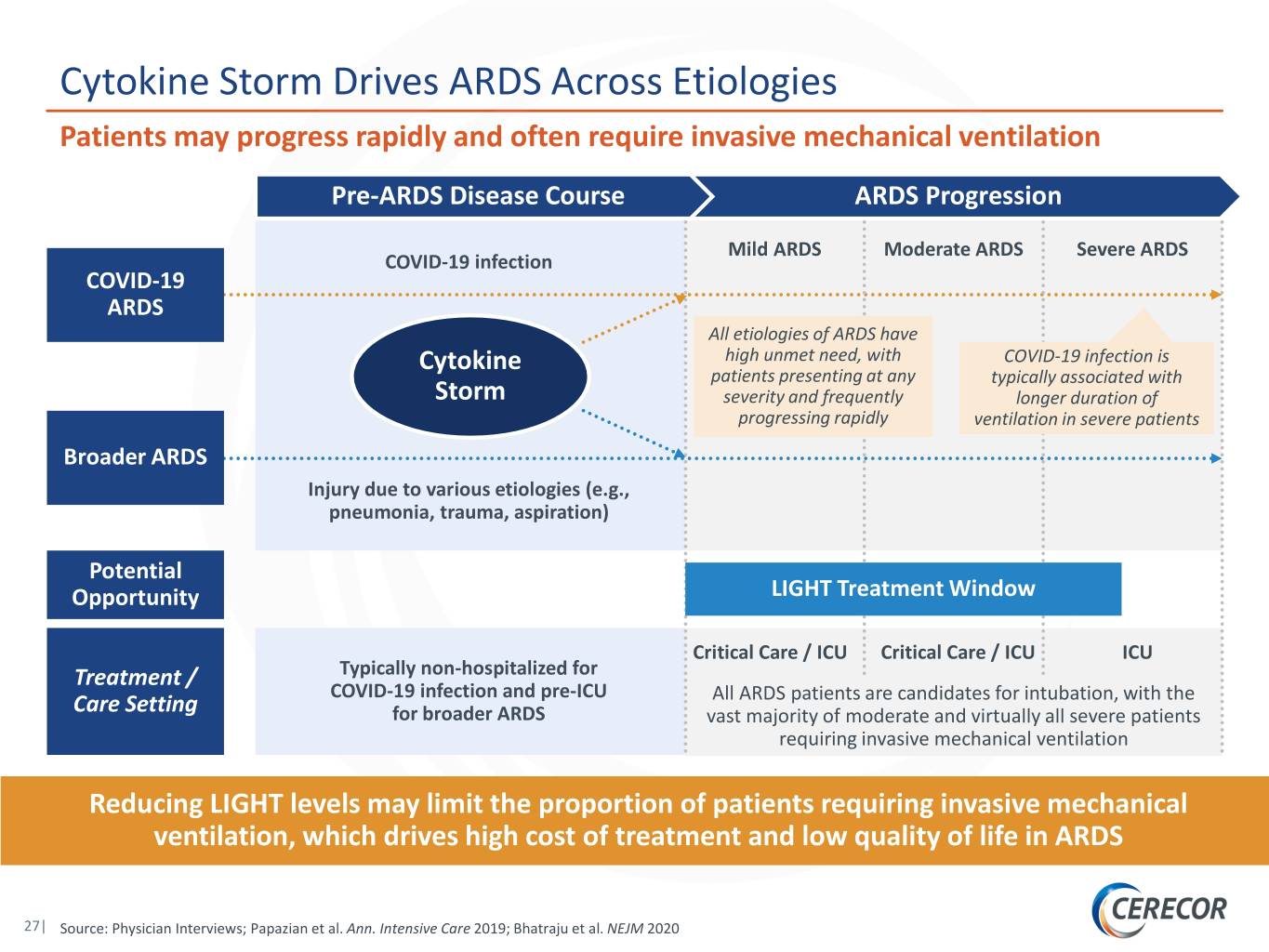
Cytokine Storm Drives ARDS Across Etiologies Patients may progress rapidly and often require invasive mechanical ventilation Pre-ARDS Disease Course ARDS Progression Mild ARDS Moderate ARDS Severe ARDS COVID-19 infection COVID-19 ARDS All etiologies of ARDS have high unmet need, with COVID-19 infection is Cytokine patients presenting at any typically associated with Storm severity and frequently longer duration of progressing rapidly ventilation in severe patients Broader ARDS Injury due to various etiologies (e.g., pneumonia, trauma, aspiration) Potential Opportunity LIGHT Treatment Window Critical Care / ICU Critical Care / ICU ICU Treatment / Typically non-hospitalized for COVID-19 infection and pre-ICU All ARDS patients are candidates for intubation, with the Care Setting for broader ARDS vast majority of moderate and virtually all severe patients requiring invasive mechanical ventilation Reducing LIGHT levels may limit the proportion of patients requiring invasive mechanical ventilation, which drives high cost of treatment and low quality of life in ARDS 27| Source: Physician Interviews; Papazian et al. Ann. Intensive Care 2019; Bhatraju et al. NEJM 2020
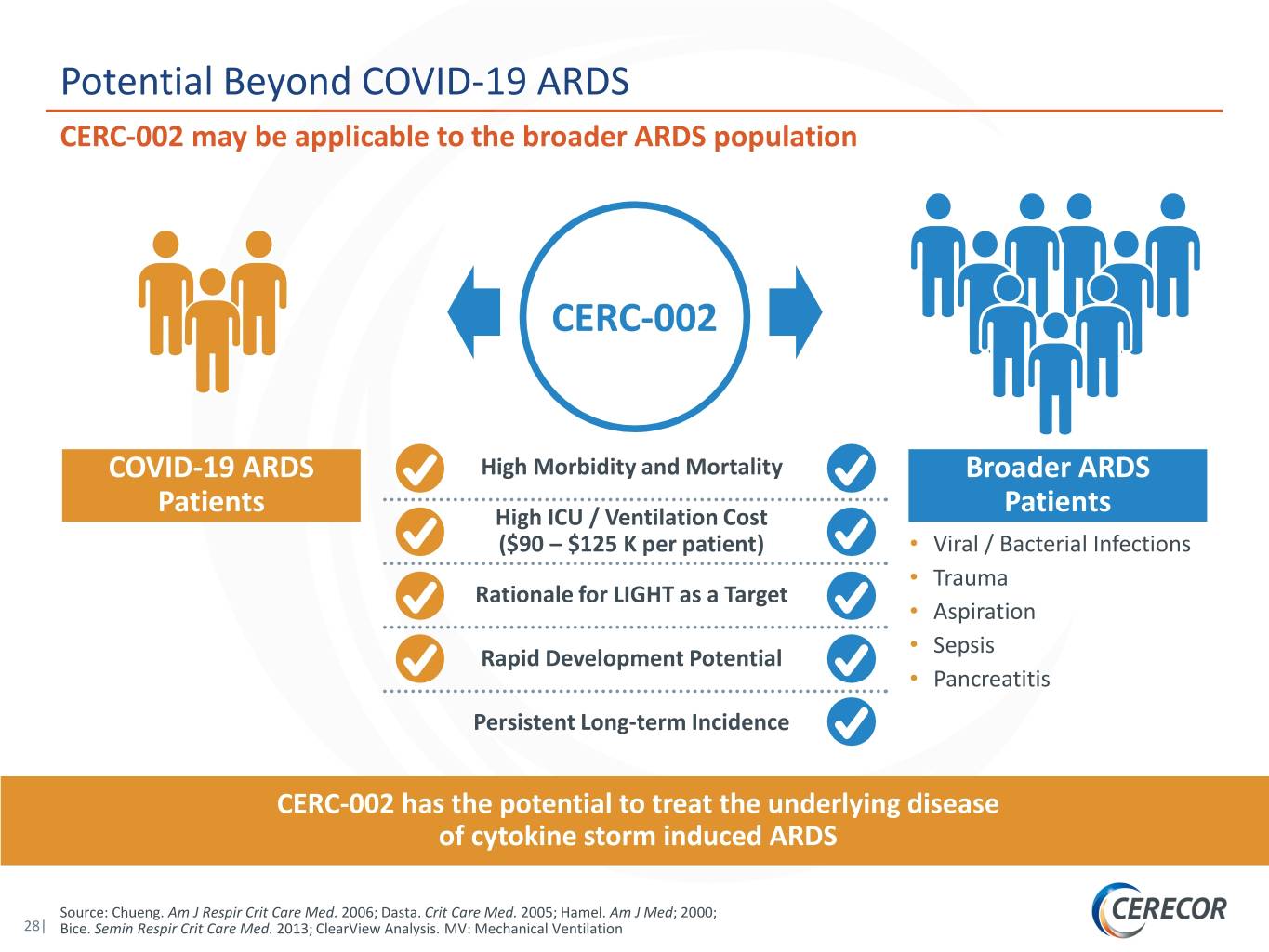
Potential Beyond COVID-19 ARDS CERC-002 may be applicable to the broader ARDS population CERC-002 COVID-19 ARDS High Morbidity and Mortality Broader ARDS Patients High ICU / Ventilation Cost Patients ($90 – $125 K per patient) • Viral / Bacterial Infections • Trauma Rationale for LIGHT as a Target • Aspiration • Sepsis Rapid Development Potential • Pancreatitis Persistent Long-term Incidence CERC-002 has the potential to treat the underlying disease of cytokine storm induced ARDS Source: Chueng. Am J Respir Crit Care Med. 2006; Dasta. Crit Care Med. 2005; Hamel. Am J Med; 2000; 28| Bice. Semin Respir Crit Care Med. 2013; ClearView Analysis. MV: Mechanical Ventilation
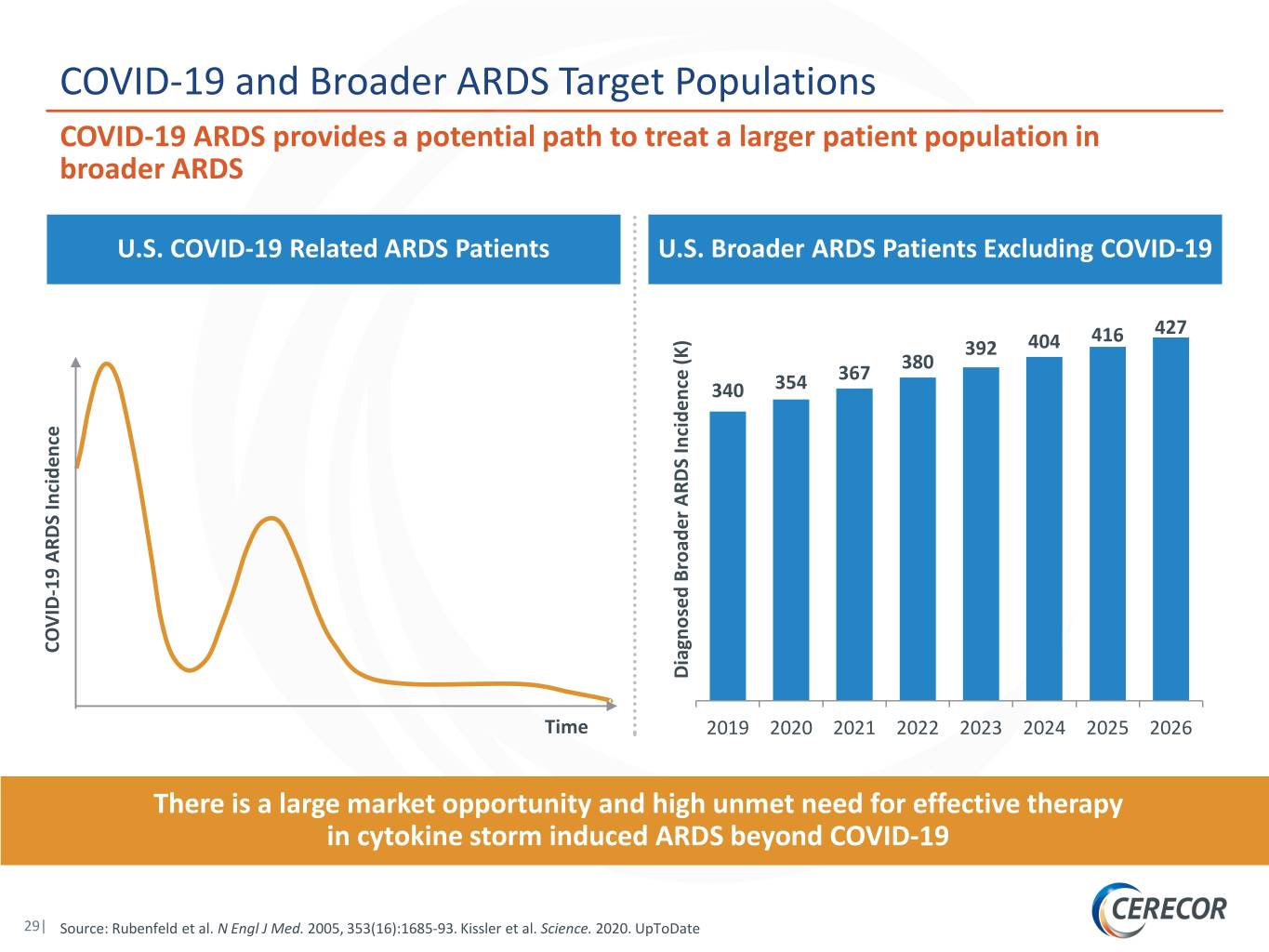
COVID-19 and Broader ARDS Target Populations COVID-19 ARDS provides a potential path to treat a larger patient population in broader ARDS U.S. COVID-19 Related ARDS Patients U.S. Broader ARDS Patients Excluding COVID-19 416 427 392 404 380 367 340 354 19 ARDS Incidence 19 ARDS - COVID Diagnosed Broader ARDS Incidence (K) Incidence ARDS Broader Diagnosed Time 2019 2020 2021 2022 2023 2024 2025 2026 There is a large market opportunity and high unmet need for effective therapy in cytokine storm induced ARDS beyond COVID-19 29| Source: Rubenfeld et al. N Engl J Med. 2005, 353(16):1685-93. Kissler et al. Science. 2020. UpToDate
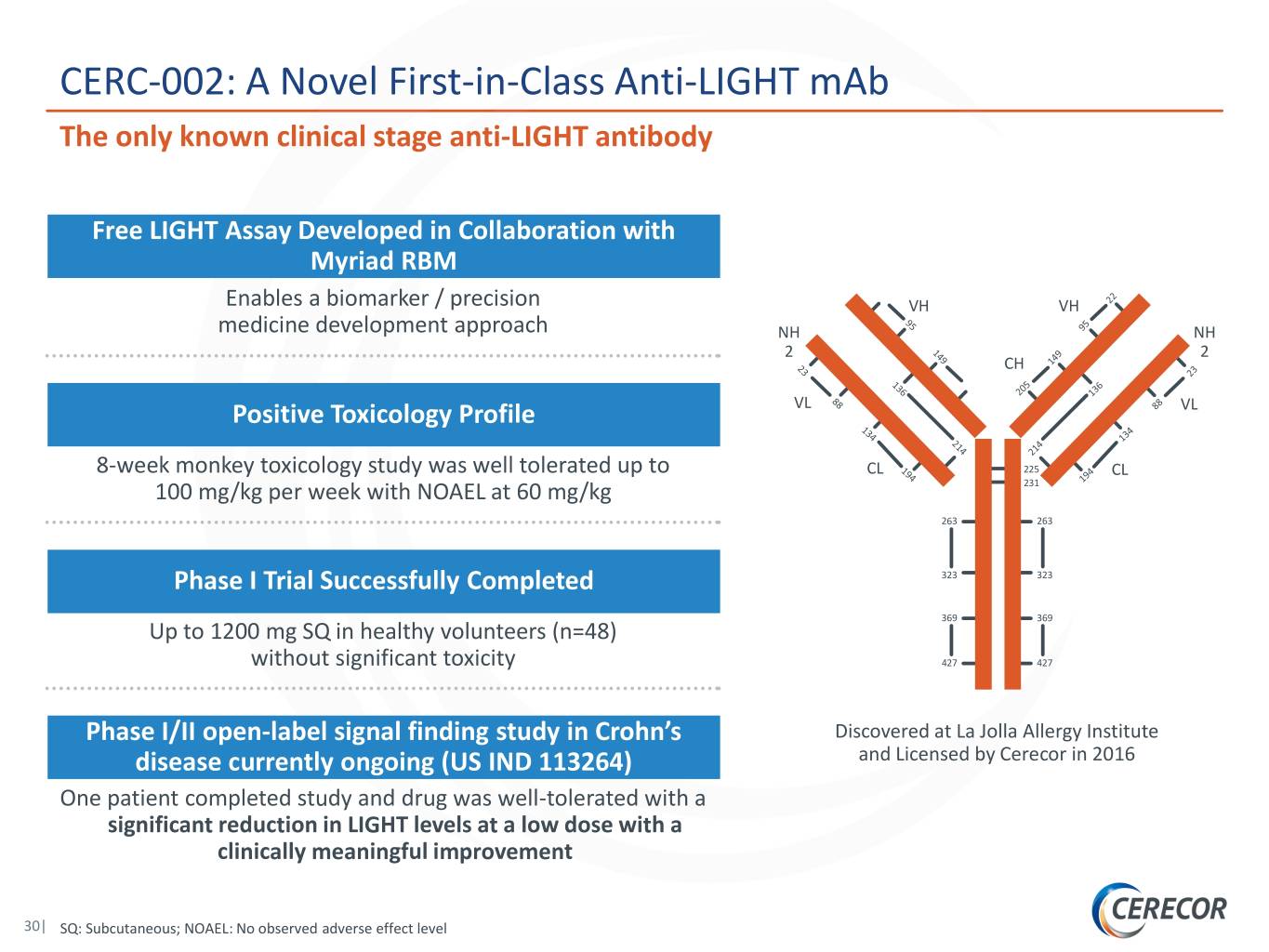
CERC-002: A Novel First-in-Class Anti-LIGHT mAb The only known clinical stage anti-LIGHT antibody Free LIGHT Assay Developed in Collaboration with Myriad RBM Enables a biomarker / precision VH VH medicine development approach NH NH 2 2 CH Positive Toxicology Profile VL VL 8-week monkey toxicology study was well tolerated up to CL 225 CL 100 mg/kg per week with NOAEL at 60 mg/kg 231 263 263 Phase I Trial Successfully Completed 323 323 369 369 Up to 1200 mg SQ in healthy volunteers (n=48) without significant toxicity 427 427 Phase I/II open-label signal finding study in Crohn’s Discovered at La Jolla Allergy Institute disease currently ongoing (US IND 113264) and Licensed by Cerecor in 2016 One patient completed study and drug was well-tolerated with a significant reduction in LIGHT levels at a low dose with a clinically meaningful improvement 30| SQ: Subcutaneous; NOAEL: No observed adverse effect level
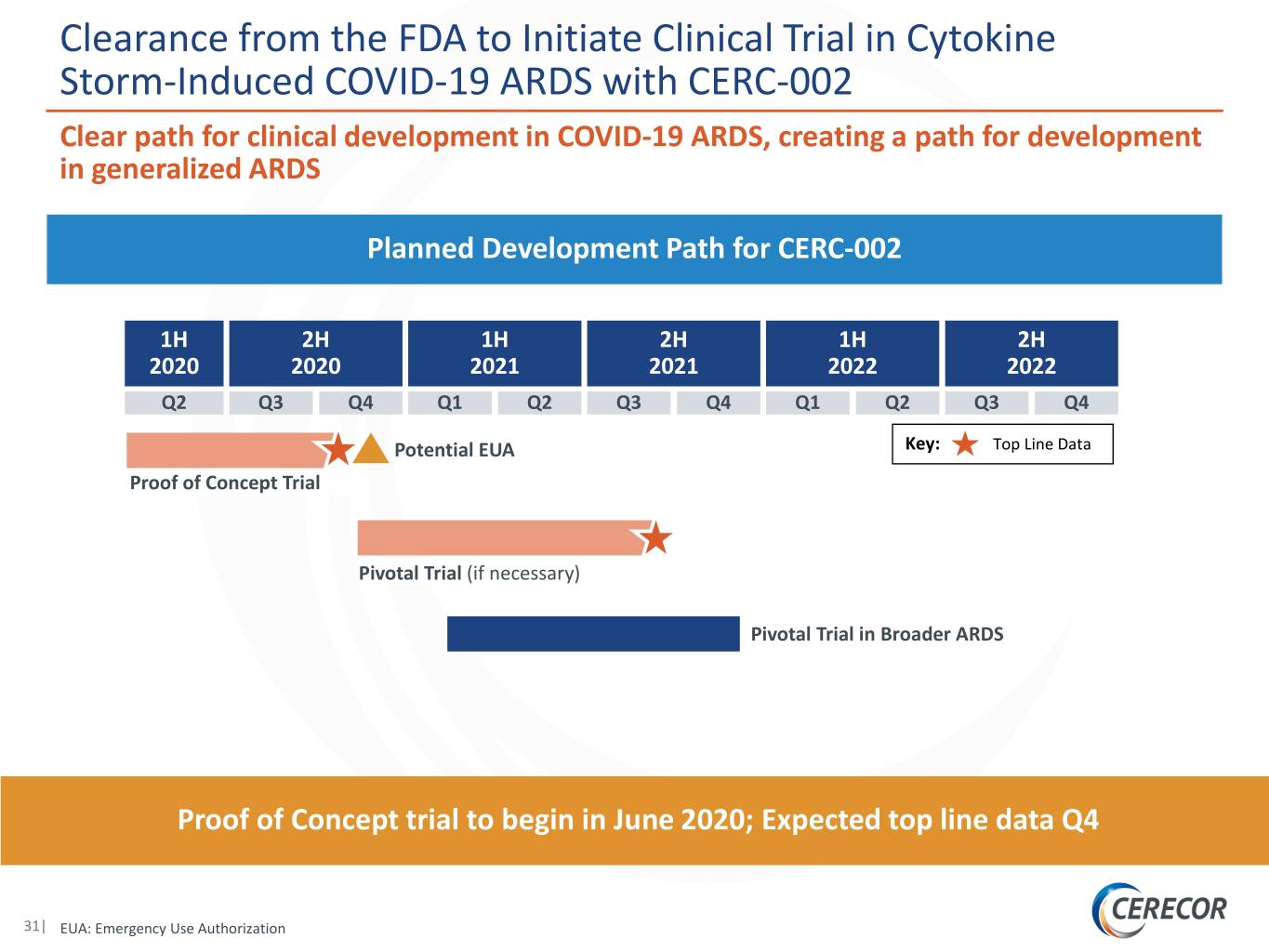
Clearance from the FDA to Initiate Clinical Trial in Cytokine Storm-Induced COVID-19 ARDS with CERC-002 Clear path for clinical development in COVID-19 ARDS, creating a path for development in generalized ARDS Planned Development Path for CERC-002 1H 2H 1H 2H 1H 2H 2020 2020 2021 2021 2022 2022 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Potential EUA Key: Top Line Data Proof of Concept Trial Pivotal Trial (if necessary) Pivotal Trial in Broader ARDS Proof of Concept trial to begin in June 2020; Expected top line data Q4 31| EUA: Emergency Use Authorization
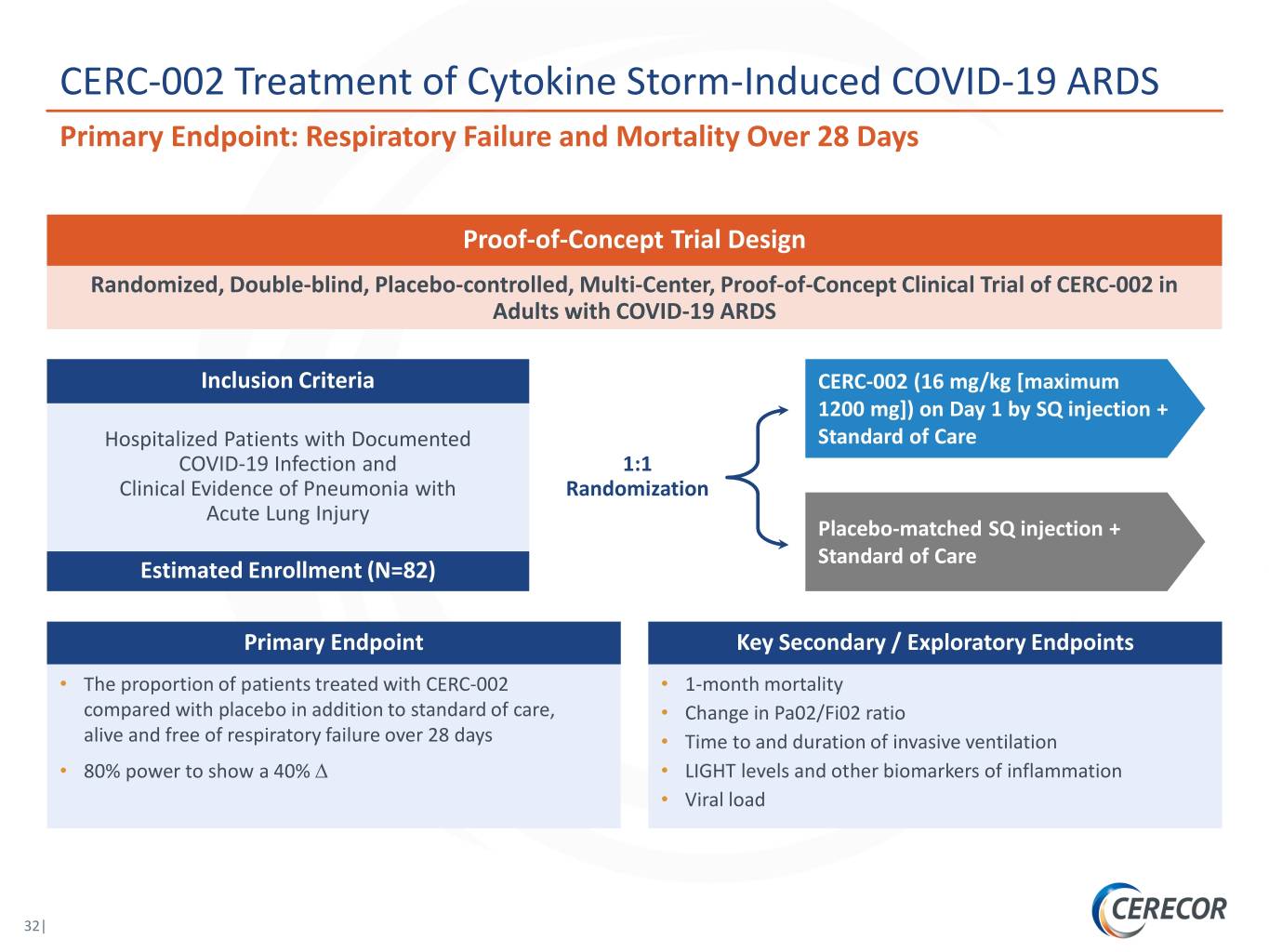
CERC-002 Treatment of Cytokine Storm-Induced COVID-19 ARDS Primary Endpoint: Respiratory Failure and Mortality Over 28 Days Proof-of-Concept Trial Design Randomized, Double-blind, Placebo-controlled, Multi-Center, Proof-of-Concept Clinical Trial of CERC-002 in Adults with COVID-19 ARDS Inclusion Criteria CERC-002 (16 mg/kg [maximum 1200 mg]) on Day 1 by SQ injection + Hospitalized Patients with Documented Standard of Care COVID-19 Infection and 1:1 Clinical Evidence of Pneumonia with Randomization Acute Lung Injury Placebo-matched SQ injection + Standard of Care Estimated Enrollment (N=82) Primary Endpoint Key Secondary / Exploratory Endpoints • The proportion of patients treated with CERC-002 • 1-month mortality compared with placebo in addition to standard of care, • Change in Pa02/Fi02 ratio alive and free of respiratory failure over 28 days • Time to and duration of invasive ventilation • 80% power to show a 40% ∆ • LIGHT levels and other biomarkers of inflammation • Viral load 32|
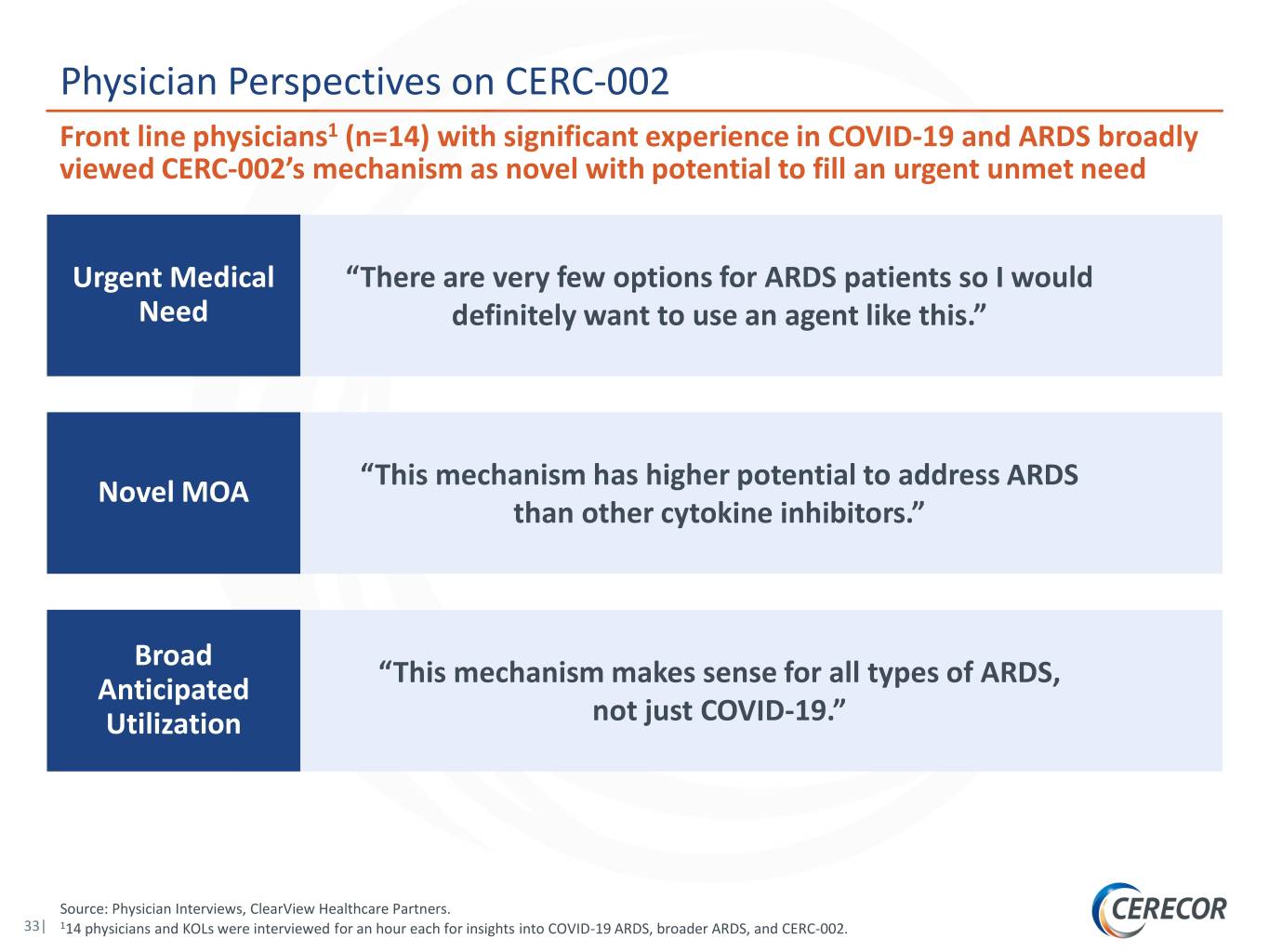
Physician Perspectives on CERC-002 Front line physicians1 (n=14) with significant experience in COVID-19 and ARDS broadly viewed CERC-002’s mechanism as novel with potential to fill an urgent unmet need Urgent Medical “There are very few options for ARDS patients so I would Need definitely want to use an agent like this.” “This mechanism has higher potential to address ARDS Novel MOA than other cytokine inhibitors.” Broad “This mechanism makes sense for all types of ARDS, Anticipated Utilization not just COVID-19.” Source: Physician Interviews, ClearView Healthcare Partners. 33| 114 physicians and KOLs were interviewed for an hour each for insights into COVID-19 ARDS, broader ARDS, and CERC-002.
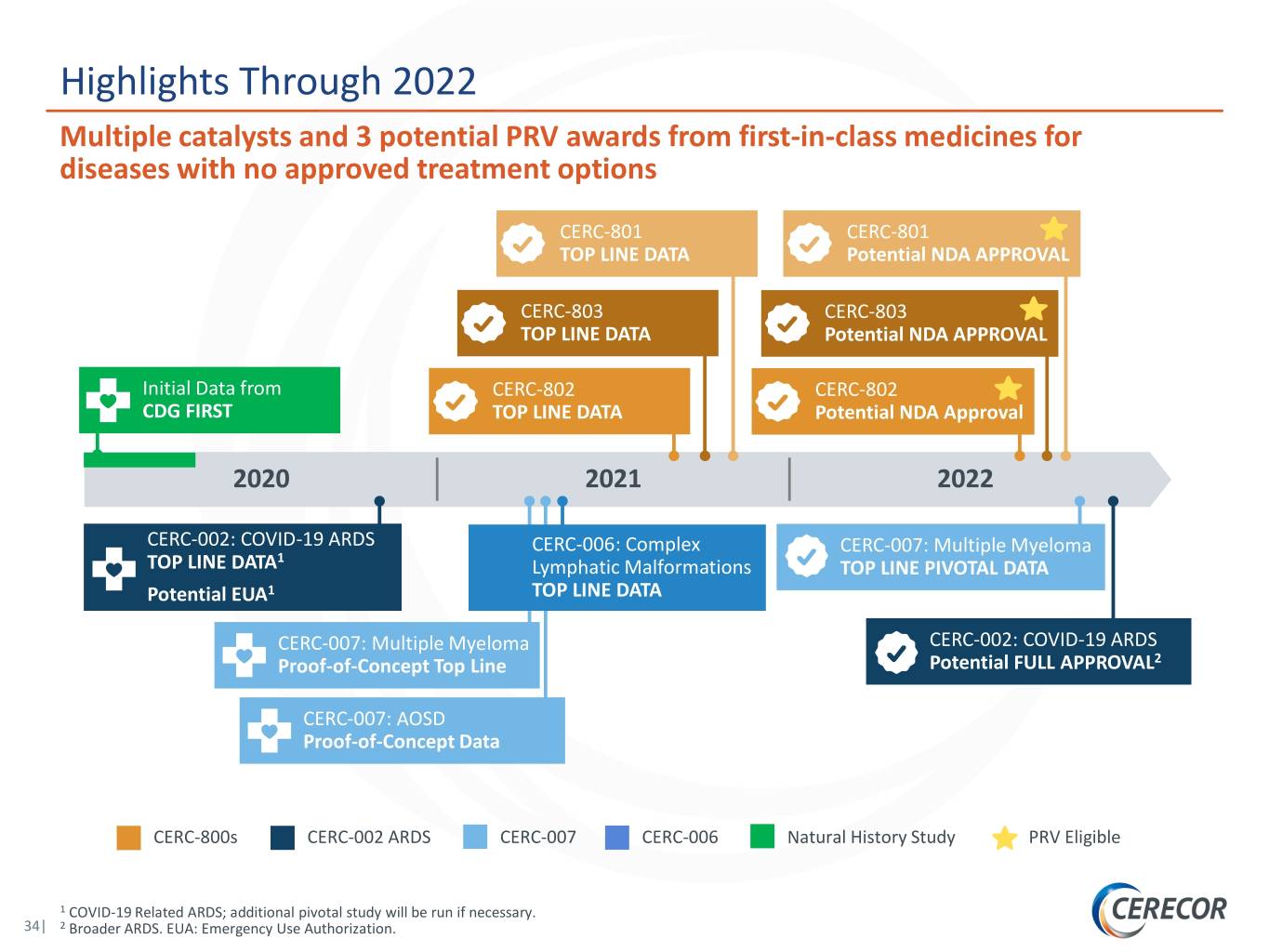
Highlights Through 2022 Multiple catalysts and 3 potential PRV awards from first-in-class medicines for diseases with no approved treatment options CERC-801 CERC-801 TOP LINE DATA Potential NDA APPROVAL CERC-803 CERC-803 TOP LINE DATA Potential NDA APPROVAL Initial Data from CERC-802 CERC-802 CDG FIRST TOP LINE DATA Potential NDA Approval 2020 2021 2022 CERC-002: COVID-19 ARDS CERC-006: Complex CERC-007: Multiple Myeloma 1 TOP LINE DATA Lymphatic Malformations TOP LINE PIVOTAL DATA Potential EUA1 TOP LINE DATA CERC-007: Multiple Myeloma CERC-002: COVID-19 ARDS Proof-of-Concept Top Line Potential FULL APPROVAL2 CERC-007: AOSD Proof-of-Concept Data CERC-800s CERC-002 ARDS CERC-007 CERC-006 Natural History Study PRV Eligible 1 COVID-19 Related ARDS; additional pivotal study will be run if necessary. 34| 2 Broader ARDS. EUA: Emergency Use Authorization.

NASDAQ:CERC www.cerecor.com

Select Board and Management Team Members Michael F. Cola Chief Executive Officer Garry A. Neil, MD Chief Scientific Officer Sol J. Barer, PhD Chairman 36|

References: CERC-002 • LIGHT: Lymphotoxin-like, exhibits Inducible expression, and competes with HSV Glycoprotein D for HVEM, a receptor expressed by T lymphocytes; encoded by TNFSF14 (Tumor Necrosis Factor Superfamily 14). • Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. • Bhatraju PK et al., Covid-19 in Critically Ill Patients in the Seattle Region — Case Series. NEJM. 2020. • Bice T, Cox CE, Carson SS. Cost and health care utilization in ARDS--different from other critical illness?. Semin Respir Crit Care Med. 2013;34(4):529‐536. • Cardinale CJ, Wei Z, Panossian S, et al. Targeted resequencing identifies defective variants of decoy receptor 3 in pediatric-onset inflammatory bowel disease. Genes Immun. 2013;14(7):447‐452. • Cheung AM, Tansey CM, Tomlinson G, et al. Two-year outcomes, health care use, and costs of survivors of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2006. • da Silva Antunes R, Madge L, Soroosh P, Tocker J, Croft M. The TNF Family Molecules LIGHT and Lymphotoxin αβ Induce a Distinct Steroid-Resistant Inflammatory Phenotype in Human Lung Epithelial Cells. J Immunol. 2015;195(5):2429‐2441. • da Silva Antunes R, Mehta AK, Madge L, Tocker J, Croft M. TNFSF14 (LIGHT) Exhibits Inflammatory Activities in Lung Fibroblasts Complementary to IL-13 and TGF-β. Front Immunol. 2018;9:576. • Daher P, Teixeira PG, Coopwood TB, et al. Mild to Moderate to Severe: What Drives the Severity of ARDS in Trauma Patients?. Am Surg. 2018;84(6):808‐812. • Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266‐1271. • Desai P, Tahiliani V, Hutchinson TE, et al. The TNF Superfamily Molecule LIGHT Promotes the Generation of Circulating and Lung-Resident Memory CD8 T Cells following an Acute Respiratory Virus Infection. J Immunol. 2018;200(8):2894‐2904. • Eworuke E, Major JM, Gilbert McClain LI. National incidence rates for Acute Respiratory Distress Syndrome (ARDS) and ARDS cause-specific factors in the United States (2006- 2014). J Crit Care. 2018;47:192‐197. • Hamel MB et al., Outcomes and cost-effectiveness of ventilator support and aggressive care for patients with acute respiratory failure due to pneumonia or acute respiratory distress syndrome. Am J Med. 2000. • Johnson ER, Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243‐252. • Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368(6493):860‐868. • Kugathasan S, Baldassano RN, Bradfield JP, et al. Loci on 20q13 and 21q22 are associated with pediatric-onset inflammatory bowel disease. Nat Genet. 2008;40(10):1211‐1215. • Papazan L et al., Formal guidelines: management of acute respiratory distress syndrome. Ann. Intensive Care. 2019. • Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685‐1693. • Ware CF. Targeting lymphocyte activation through the lymphotoxin and LIGHT pathways. Immunol Rev. 2008;223:186‐201. • Xu W, Xu Z, Huang L, et al. Transcriptome Sequencing Identifies Novel Immune Response Genes Highly Related to the Severity of Human Adenovirus Type 55 Infection. Front Microbiol. 2019;10:130. • Zhang M, Perrin L, Pardo P. A Randomized Phase 1 Study to Assess the Safety and Pharmacokinetics of the Subcutaneously Injected Anti-LIGHT Antibody, SAR252067. Clin Pharmacol Drug Dev. 2017;6(3):292‐301. 37|

References: CERC-006, CERC-007, CERC-800s • Adams DM, Trenor CC 3rd, Hammill AM, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics. 2016;137(2):e20153257. • Bhagwat SV, Gokhale PC, Crew AP, et al. Preclinical characterization of OSI-027, a potent and selective inhibitor of mTORC1 and mTORC2: distinct from rapamycin. Mol Cancer Ther. 2011;10(8):1394‐1406. • Brouillard P, Boon L, Vikkula M. Genetics of lymphatic anomalies. J Clin Invest. 2014;124(3):898‐904. • Croteau SE, Kozakewich HP, Perez-Atayde AR, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr. 2014;164(2):383‐388. • Gabay C, Fautrel B, Rech J, et al. Open-label, multicentre, dose-escalating phase II clinical trial on the safety and efficacy of tadekinig alfa (IL-18BP) in adult-onset Still's disease. Ann Rheum Dis. 2018;77(6):840‐847 • Kudela H, Drynda S, Lux A, Horneff G, Kekow J. Comparative study of Interleukin-18 (IL-18) serum levels in adult onset Still's disease (AOSD) and systemic onset juvenile idiopathic arthritis (sJIA) and its use as a biomarker for diagnosis and evaluation of disease activity. BMC Rheumatol. 2019;3:4. • Mateo J, Olmos D, Dumez H, et al. A first in man, dose-finding study of the mTORC1/mTORC2 inhibitor OSI-027 in patients with advanced solid malignancies. Br J Cancer. 2016;114(8):889‐896. • Nakamura K, Kassem S, Cleynen A, et al. Dysregulated IL-18 Is a Key Driver of Immunosuppression and a Possible Therapeutic Target in the Multiple Myeloma Microenvironment. Cancer Cell. 2018;33(4):634‐648.e5. • Ozeki M, Fujino A, Matsuoka K, Nosaka S, Kuroda T, Fukao T. Clinical Features and Prognosis of Generalized Lymphatic Anomaly, Kaposiform Lymphangiomatosis, and Gorham-Stout Disease. Pediatr Blood Cancer. 2016;63(5):832‐838. • Perkins JA, Manning SC, Tempero RM, et al. Lymphatic malformations: current cellular and clinical investigations. Otolaryngol Head Neck Surg. 2010;142(6):789‐794. • Radenkovic S, Bird MJ, Emmerzaal TL, et al. The Metabolic Map into the Pathomechanism and Treatment of PGM1-CDG. Am J Hum Genet. 2019;104(5):835‐846. • Wong SY, Beamer LJ, Gadomski T, et al. Defining the Phenotype and Assessing Severity in Phosphoglucomutase-1 Deficiency. J Pediatr. 2016;175:130‐136.e8. 38|

Appendix

Key Financial Information
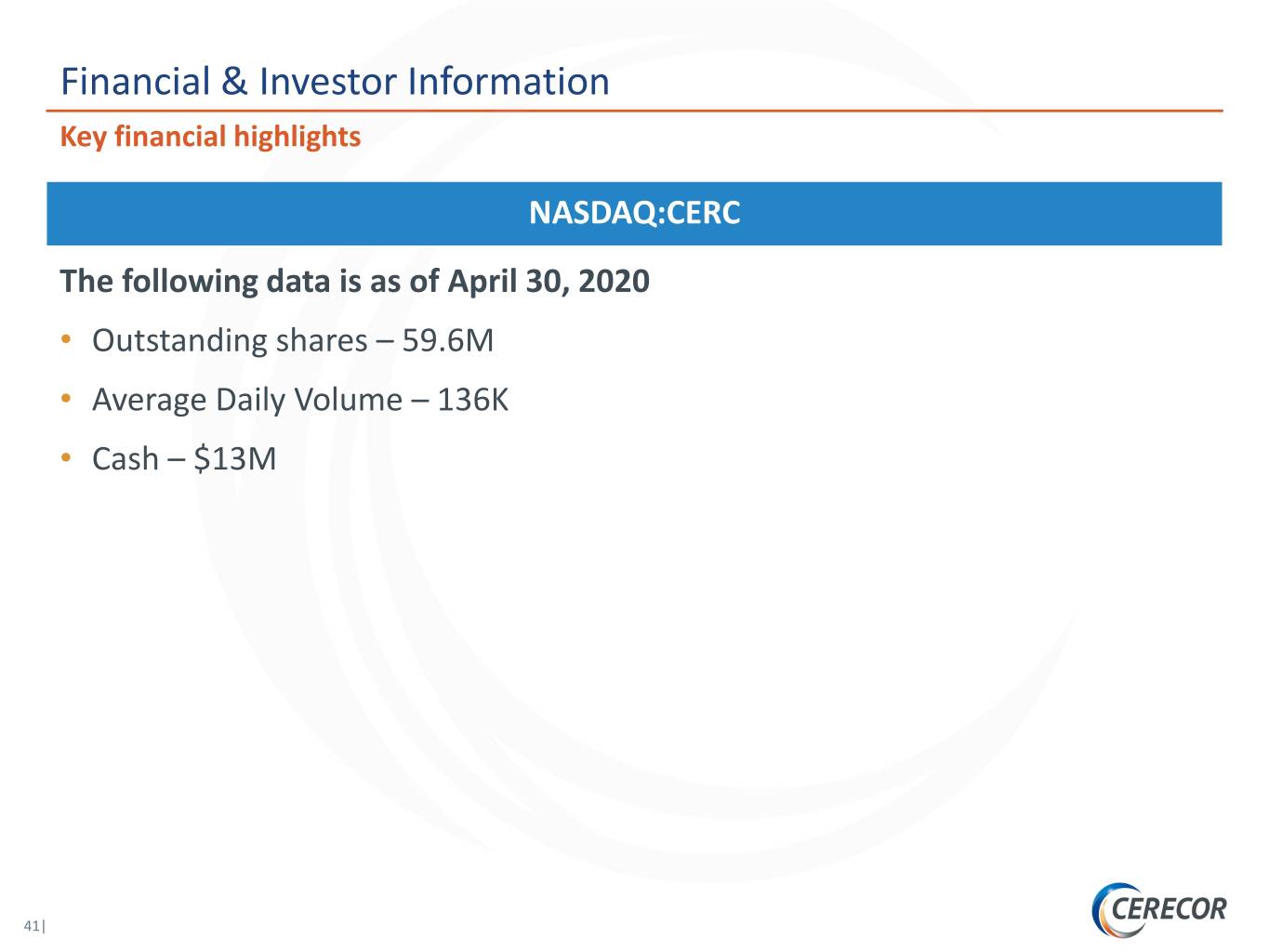
Financial & Investor Information Key financial highlights NASDAQ:CERC The following data is as of April 30, 2020 • Outstanding shares – 59.6M • Average Daily Volume – 136K • Cash – $13M 41|

CERC-002 Anti-LIGHT monoclonal antibody in clinical studies for Pediatric Crohn’s Disease
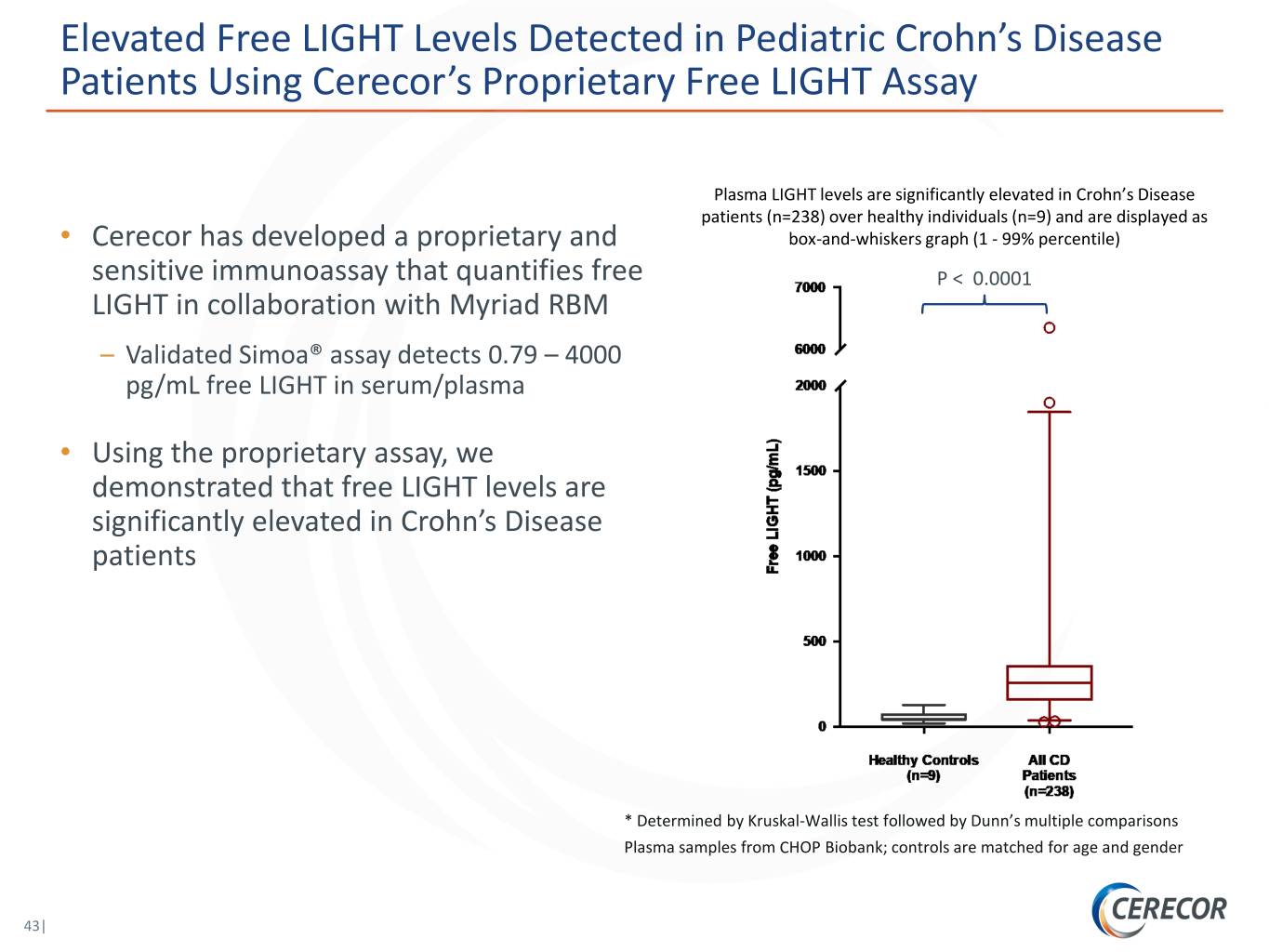
Elevated Free LIGHT Levels Detected in Pediatric Crohn’s Disease Patients Using Cerecor’s Proprietary Free LIGHT Assay Plasma LIGHT levels are significantly elevated in Crohn’s Disease patients (n=238) over healthy individuals (n=9) and are displayed as • Cerecor has developed a proprietary and box-and-whiskers graph (1 - 99% percentile) sensitive immunoassay that quantifies free P < 0.0001 LIGHT in collaboration with Myriad RBM – Validated Simoa® assay detects 0.79 – 4000 pg/mL free LIGHT in serum/plasma • Using the proprietary assay, we demonstrated that free LIGHT levels are significantly elevated in Crohn’s Disease patients * Determined by Kruskal-Wallis test followed by Dunn’s multiple comparisons Plasma samples from CHOP Biobank; controls are matched for age and gender 43|

CERC-006 Phase II-ready, Dual mTORC 1/2 small molecule inhibitor for Complex Lymphatic Malformations
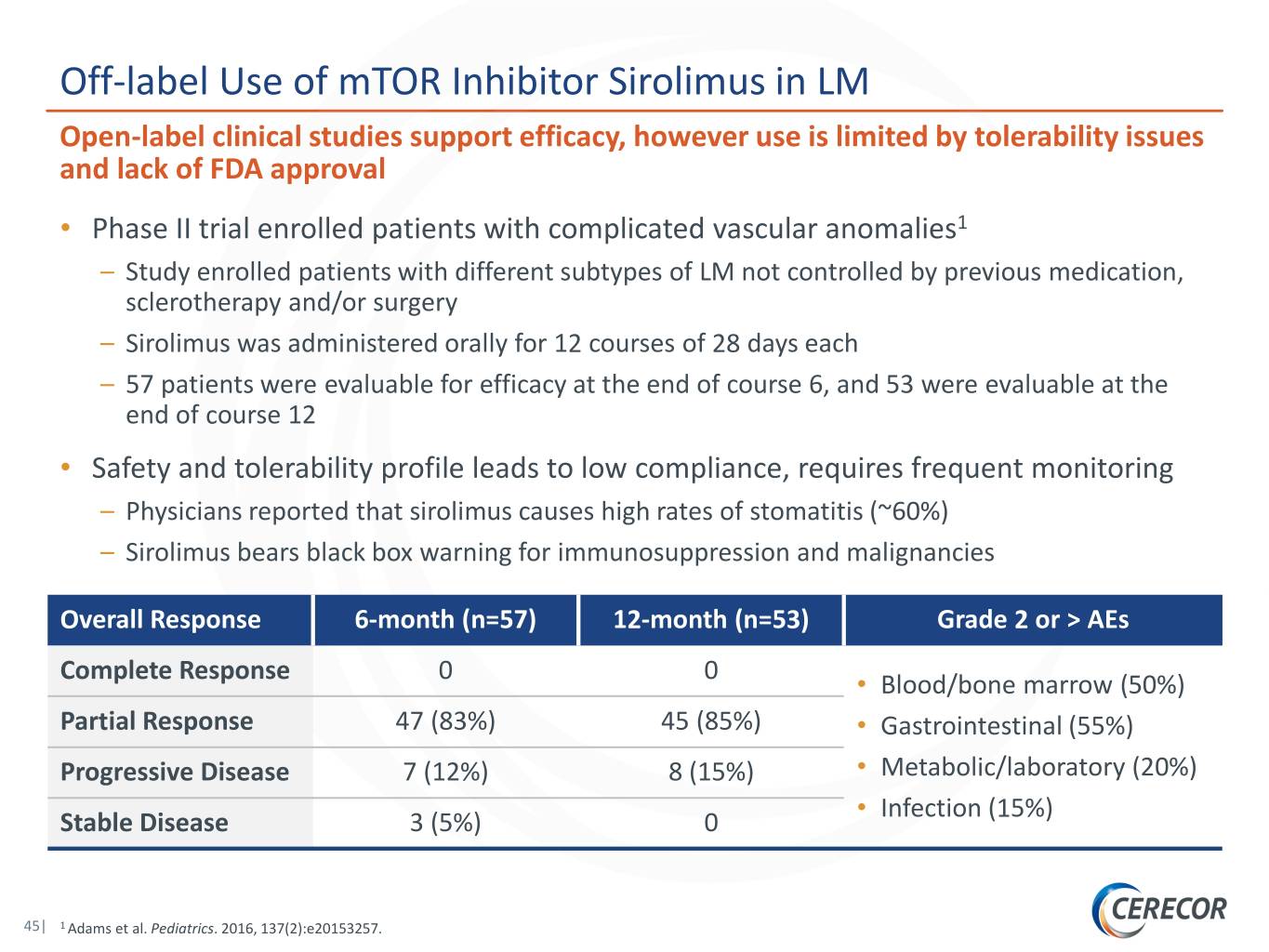
Off-label Use of mTOR Inhibitor Sirolimus in LM Open-label clinical studies support efficacy, however use is limited by tolerability issues and lack of FDA approval • Phase II trial enrolled patients with complicated vascular anomalies1 – Study enrolled patients with different subtypes of LM not controlled by previous medication, sclerotherapy and/or surgery – Sirolimus was administered orally for 12 courses of 28 days each – 57 patients were evaluable for efficacy at the end of course 6, and 53 were evaluable at the end of course 12 • Safety and tolerability profile leads to low compliance, requires frequent monitoring – Physicians reported that sirolimus causes high rates of stomatitis (~60%) – Sirolimus bears black box warning for immunosuppression and malignancies Overall Response 6-month (n=57) 12-month (n=53) Grade 2 or > AEs 0 0 Complete Response • Blood/bone marrow (50%) Partial Response 47 (83%) 45 (85%) • Gastrointestinal (55%) Progressive Disease 7 (12%) 8 (15%) • Metabolic/laboratory (20%) • Infection (15%) Stable Disease 3 (5%) 0 45| 1 Adams et al. Pediatrics. 2016, 137(2):e20153257.
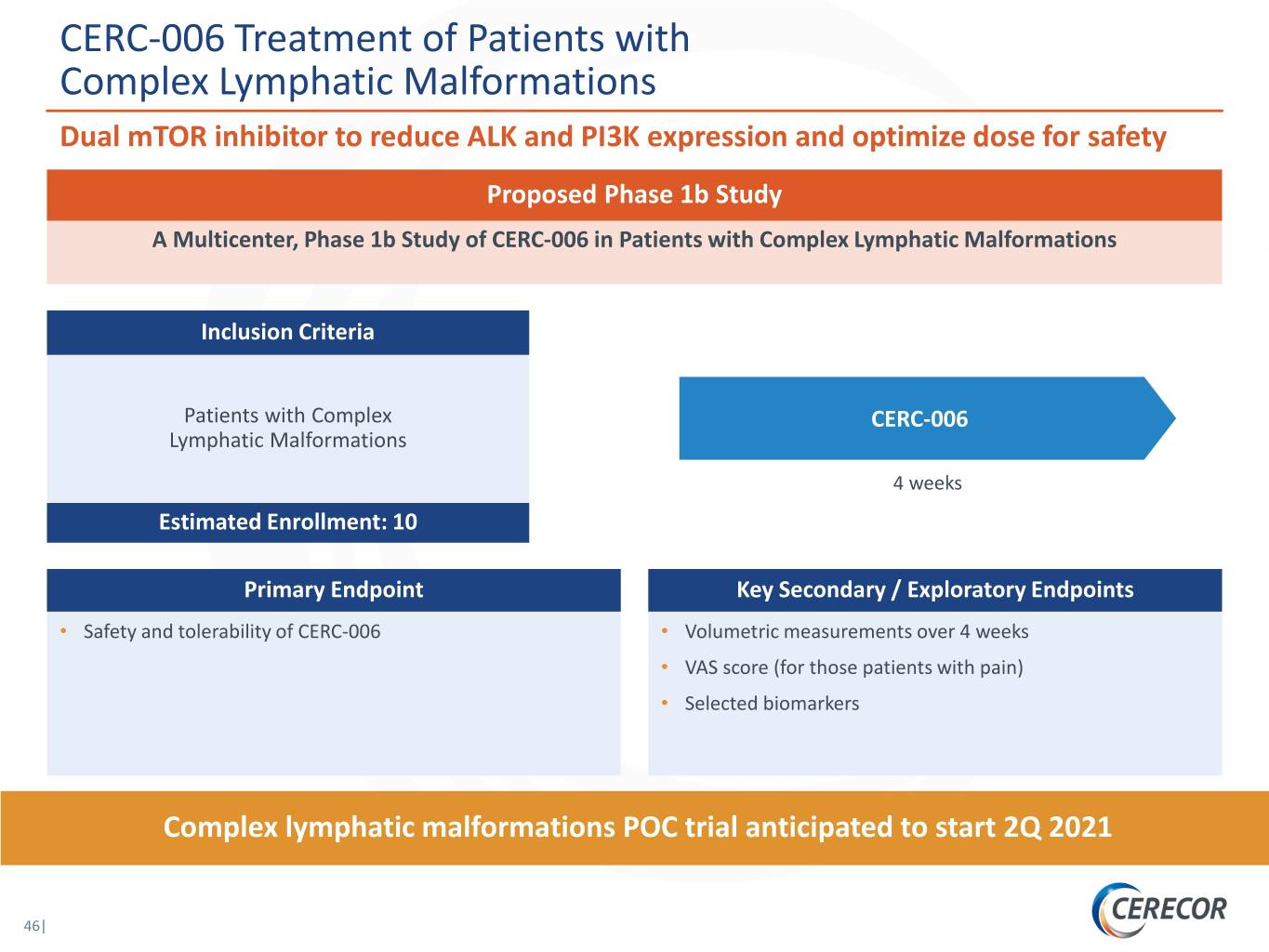
CERC-006 Treatment of Patients with Complex Lymphatic Malformations Dual mTOR inhibitor to reduce ALK and PI3K expression and optimize dose for safety Proposed Phase 1b Study A Multicenter, Phase 1b Study of CERC-006 in Patients with Complex Lymphatic Malformations Inclusion Criteria Patients with Complex CERC-006 Lymphatic Malformations 4 weeks Estimated Enrollment: 10 Primary Endpoint Key Secondary / Exploratory Endpoints • Safety and tolerability of CERC-006 • Volumetric measurements over 4 weeks • VAS score (for those patients with pain) • Selected biomarkers Complex lymphatic malformations POC trial anticipated to start 2Q 2021 46|

CERC-007 Phase 2-ready, anti-IL-18 monoclonal antibody for Multiple Myeloma
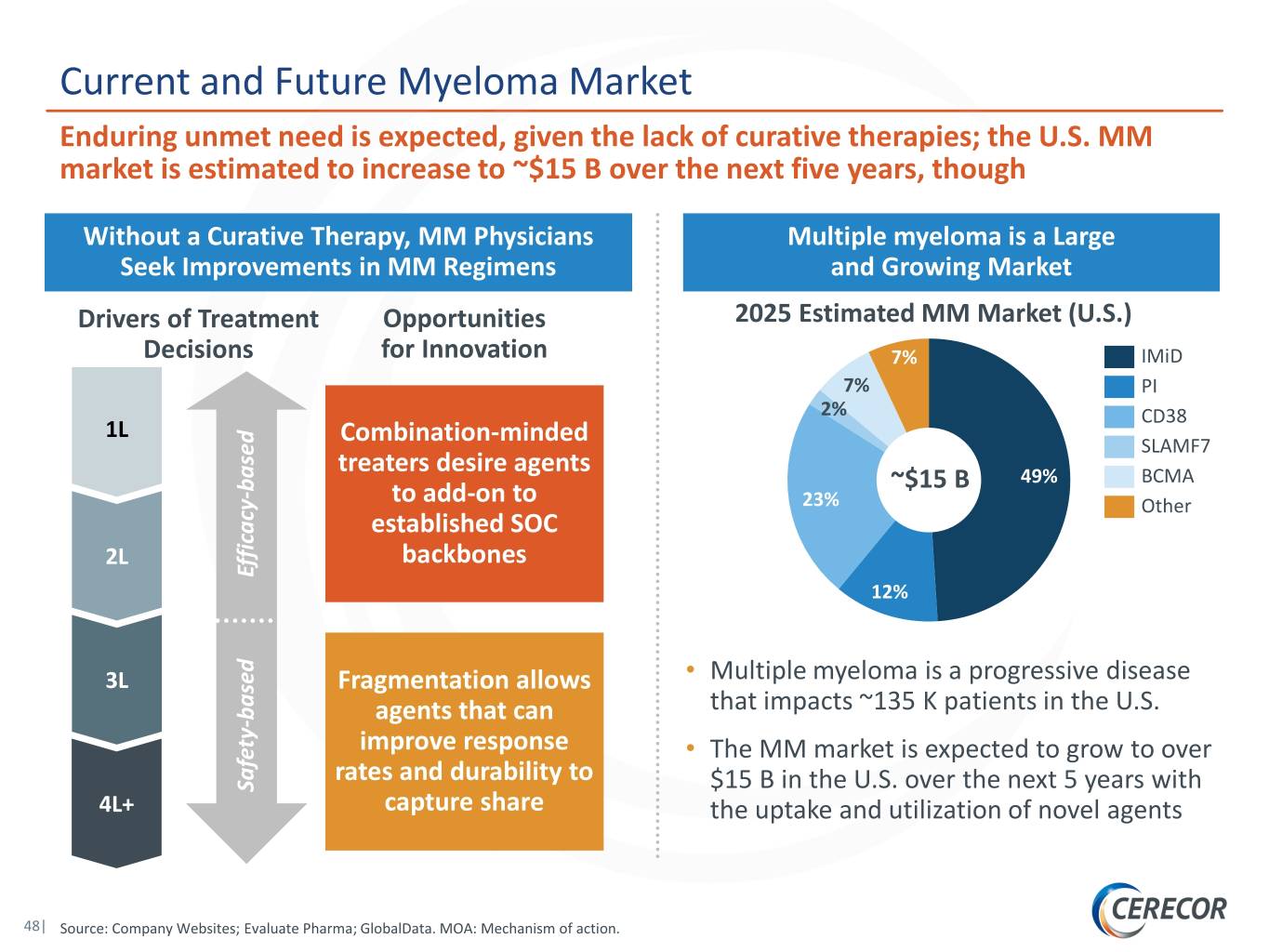
Current and Future Myeloma Market Enduring unmet need is expected, given the lack of curative therapies; the U.S. MM market is estimated to increase to ~$15 B over the next five years, though Without a Curative Therapy, MM Physicians Multiple myeloma is a Large Seek Improvements in MM Regimens and Growing Market Drivers of Treatment Opportunities 2025 Estimated MM Market (U.S.) Decisions for Innovation 7% IMiD 7% PI 2% CD38 1L Combination-minded SLAMF7 treaters desire agents 49% BCMA based ~$15 B - to add-on to 23% Other established SOC 2L backbones Efficacy 12% 3L Fragmentation allows • Multiple myeloma is a progressive disease that impacts ~135 K patients in the U.S. based - agents that can improve response • The MM market is expected to grow to over rates and durability to Safety $15 B in the U.S. over the next 5 years with 4L+ capture share the uptake and utilization of novel agents 48| Source: Company Websites; Evaluate Pharma; GlobalData. MOA: Mechanism of action.
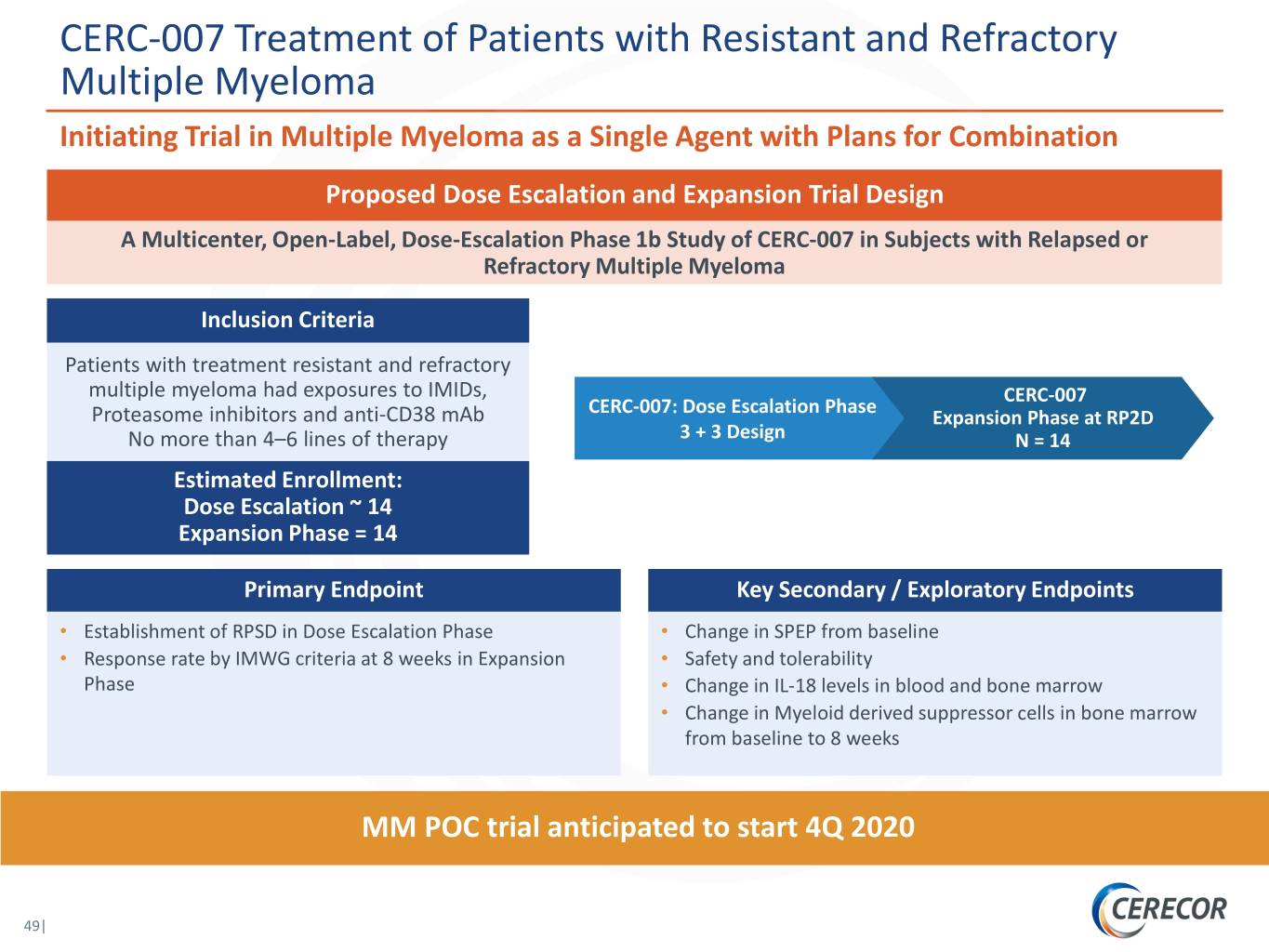
CERC-007 Treatment of Patients with Resistant and Refractory Multiple Myeloma Initiating Trial in Multiple Myeloma as a Single Agent with Plans for Combination Proposed Dose Escalation and Expansion Trial Design A Multicenter, Open-Label, Dose-Escalation Phase 1b Study of CERC-007 in Subjects with Relapsed or Refractory Multiple Myeloma Inclusion Criteria Patients with treatment resistant and refractory multiple myeloma had exposures to IMIDs, CERC-007 CERC-007: Dose Escalation Phase Proteasome inhibitors and anti-CD38 mAb Expansion Phase at RP2D No more than 4–6 lines of therapy 3 + 3 Design N = 14 Estimated Enrollment: Dose Escalation ~ 14 Expansion Phase = 14 Primary Endpoint Key Secondary / Exploratory Endpoints • Establishment of RPSD in Dose Escalation Phase • Change in SPEP from baseline • Response rate by IMWG criteria at 8 weeks in Expansion • Safety and tolerability Phase • Change in IL-18 levels in blood and bone marrow • Change in Myeloid derived suppressor cells in bone marrow from baseline to 8 weeks MM POC trial anticipated to start 4Q 2020 49|

CERC-007 Phase 2-ready, anti-IL-18 monoclonal antibody for Adult Onset Still’s Disease
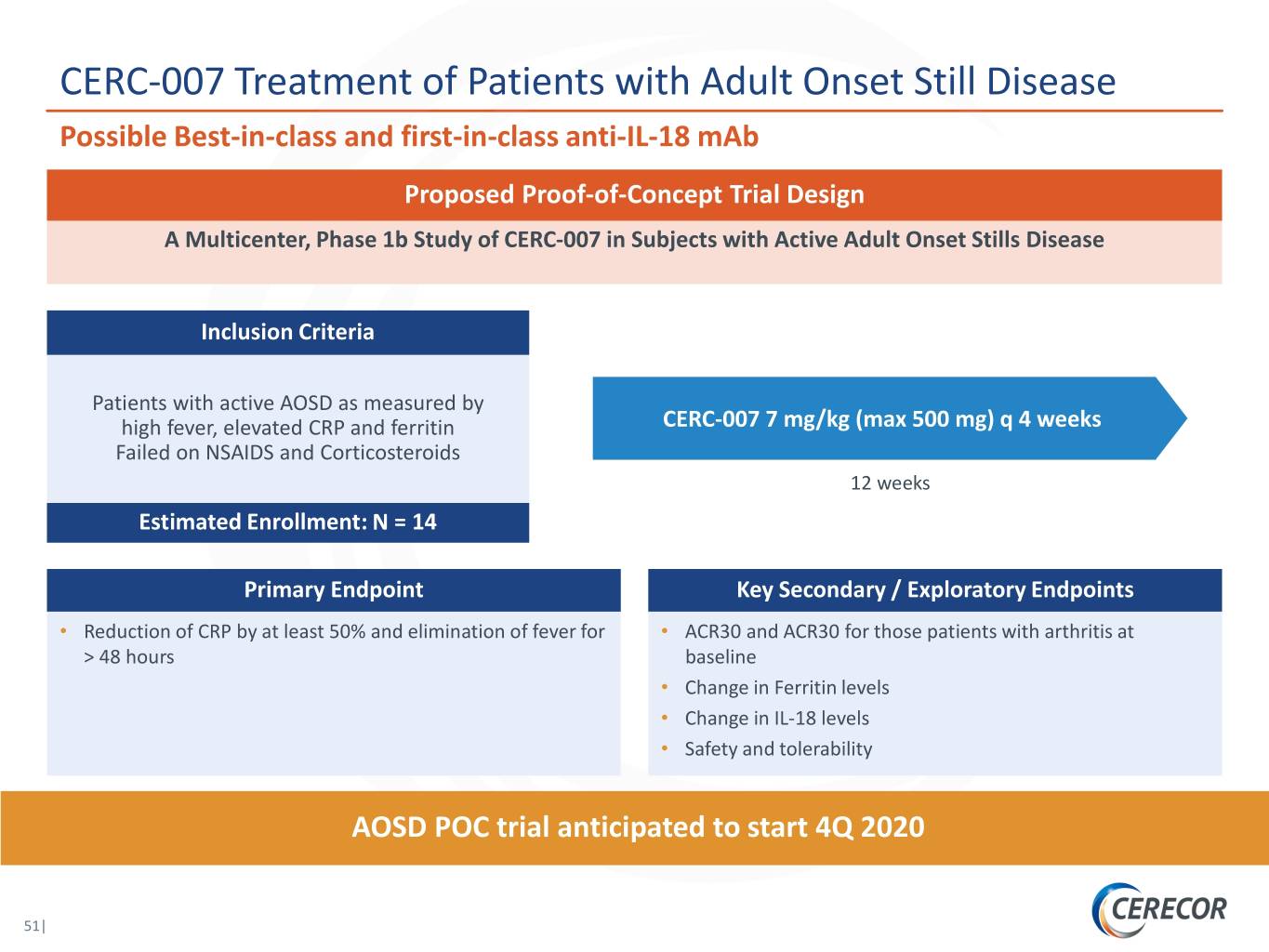
CERC-007 Treatment of Patients with Adult Onset Still Disease Possible Best-in-class and first-in-class anti-IL-18 mAb Proposed Proof-of-Concept Trial Design A Multicenter, Phase 1b Study of CERC-007 in Subjects with Active Adult Onset Stills Disease Inclusion Criteria Patients with active AOSD as measured by high fever, elevated CRP and ferritin CERC-007 7 mg/kg (max 500 mg) q 4 weeks Failed on NSAIDS and Corticosteroids 12 weeks Estimated Enrollment: N = 14 Primary Endpoint Key Secondary / Exploratory Endpoints • Reduction of CRP by at least 50% and elimination of fever for • ACR30 and ACR30 for those patients with arthritis at > 48 hours baseline • Change in Ferritin levels • Change in IL-18 levels • Safety and tolerability AOSD POC trial anticipated to start 4Q 2020 51|

CERC-800s Monosaccharide therapies for Congenital Disorders of Glycosylation (CDGs)
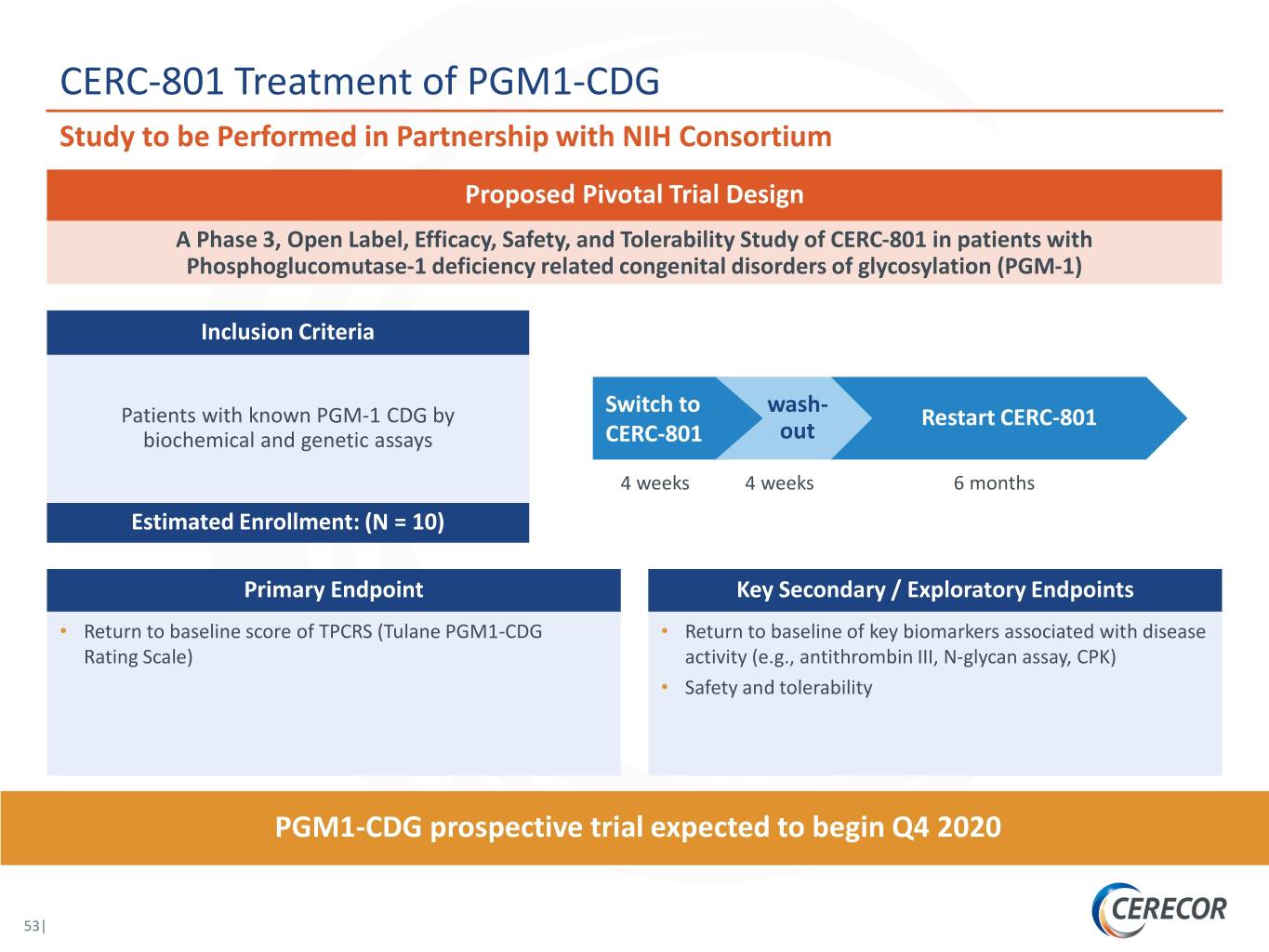
CERC-801 Treatment of PGM1-CDG Study to be Performed in Partnership with NIH Consortium Proposed Pivotal Trial Design A Phase 3, Open Label, Efficacy, Safety, and Tolerability Study of CERC-801 in patients with Phosphoglucomutase-1 deficiency related congenital disorders of glycosylation (PGM-1) Inclusion Criteria Switch to wash- Patients with known PGM-1 CDG by Restart CERC-801 biochemical and genetic assays CERC-801 out 4 weeks 4 weeks 6 months Estimated Enrollment: (N = 10) Primary Endpoint Key Secondary / Exploratory Endpoints • Return to baseline score of TPCRS (Tulane PGM1-CDG • Return to baseline of key biomarkers associated with disease Rating Scale) activity (e.g., antithrombin III, N-glycan assay, CPK) • Safety and tolerability PGM1-CDG prospective trial expected to begin Q4 2020 53|
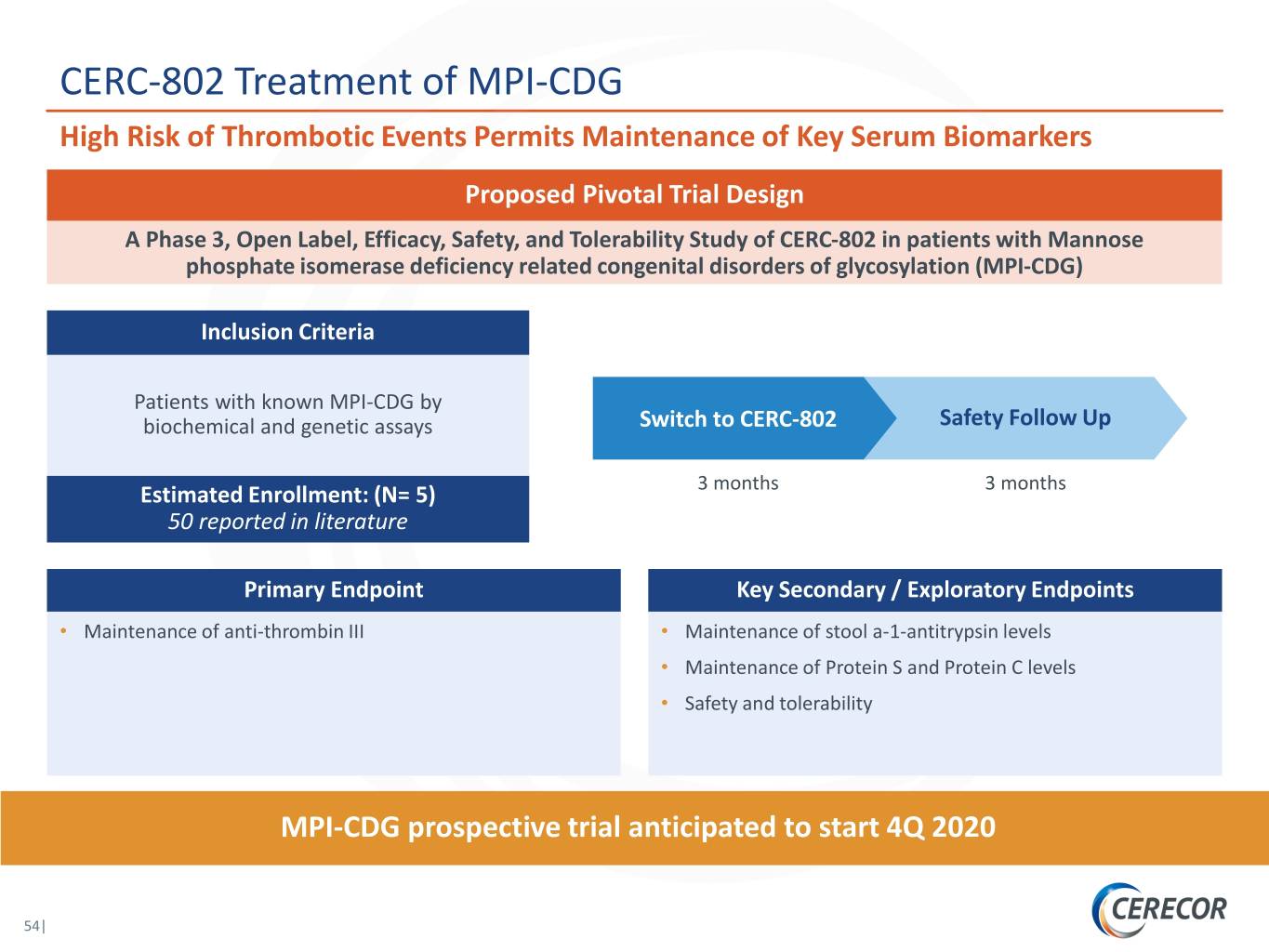
CERC-802 Treatment of MPI-CDG High Risk of Thrombotic Events Permits Maintenance of Key Serum Biomarkers Proposed Pivotal Trial Design A Phase 3, Open Label, Efficacy, Safety, and Tolerability Study of CERC-802 in patients with Mannose phosphate isomerase deficiency related congenital disorders of glycosylation (MPI-CDG) Inclusion Criteria Patients with known MPI-CDG by biochemical and genetic assays Switch to CERC-802 Safety Follow Up 3 months 3 months Estimated Enrollment: (N= 5) 50 reported in literature Primary Endpoint Key Secondary / Exploratory Endpoints • Maintenance of anti-thrombin III • Maintenance of stool a-1-antitrypsin levels • Maintenance of Protein S and Protein C levels • Safety and tolerability MPI-CDG prospective trial anticipated to start 4Q 2020 54|
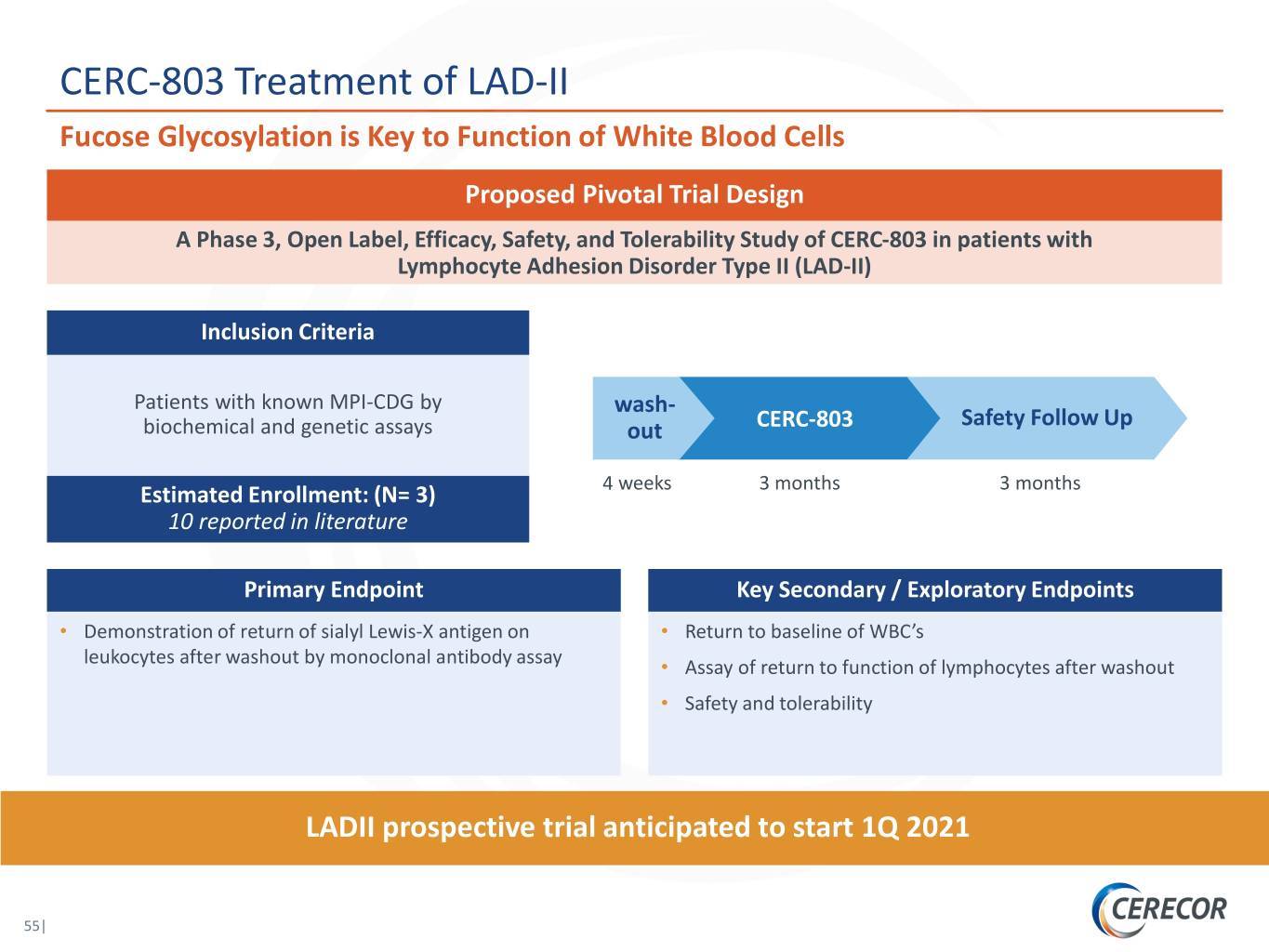
CERC-803 Treatment of LAD-II Fucose Glycosylation is Key to Function of White Blood Cells Proposed Pivotal Trial Design A Phase 3, Open Label, Efficacy, Safety, and Tolerability Study of CERC-803 in patients with Lymphocyte Adhesion Disorder Type II (LAD-II) Inclusion Criteria Patients with known MPI-CDG by wash- Safety Follow Up biochemical and genetic assays out CERC-803 4 weeks 3 months 3 months Estimated Enrollment: (N= 3) 10 reported in literature Primary Endpoint Key Secondary / Exploratory Endpoints • Demonstration of return of sialyl Lewis-X antigen on • Return to baseline of WBC’s leukocytes after washout by monoclonal antibody assay • Assay of return to function of lymphocytes after washout • Safety and tolerability LADII prospective trial anticipated to start 1Q 2021 55|

NASDAQ:CERC www.cerecor.com
