Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - 9 METERS BIOPHARMA, INC. | ex322q12020.htm |
| EX-32.1 - EXHIBIT 32.1 - 9 METERS BIOPHARMA, INC. | ex321q12020.htm |
| EX-31.2 - EXHIBIT 31.2 - 9 METERS BIOPHARMA, INC. | ex312q12020.htm |
| EX-31.1 - EXHIBIT 31.1 - 9 METERS BIOPHARMA, INC. | ex311q12020.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-Q
(Mark One)
þ | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended March 31, 2020
OR
¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ________________ to ________________
Commission file number 001-37797
9 METERS BIOPHARMA, INC.
(Exact name of registrant as specified in its charter)
Delaware | 27-3948465 | |
(State or other jurisdiction of | (I.R.S. Employer | |
incorporation or organization) | Identification No.) | |
8480 Honeycutt Road, Suite 120
Raleigh, North Carolina 27615
(Address of principal executive offices, including zip code)
(919) 275-1933
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
Common Stock $0.0001 Par Value | NMTR | The Nasdaq Stock Market LLC |
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes þ No ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer | ¨ | Accelerated filer | ¨ | |
Non-accelerated filer | þ | Smaller reporting company | þ | |
Emerging growth company | þ | |||
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. þ
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
As of May 13, 2020, the registrant had 96,251,342 shares of common stock, par value $0.0001 per share, issued and outstanding and 382,783 shares of Series A Preferred Stock issued and outstanding
.
TABLE OF CONTENTS
Unaudited Condensed Balance Sheets as of March 31, 2020 and December 31, 2019 | ||
Unaudited Condensed Statements of Operations and Comprehensive Loss for the Three Months Ended March 31, 2020 and 2019 | ||
Unaudited Condensed Statements of Stockholders’ Equity (Deficit) for the Three Months Ended March 31, 2020 and 2019 | ||
Unaudited Condensed Statements of Cash Flows for the Three Months Ended March 31, 2020 and 2019 | ||
Notes to Unaudited Condensed Financial Statements | ||
UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS | ||
2
PART I – FINANCIAL INFORMATION
Item 1. Financial Statements
9 METERS BIOPHARMA, INC.
Condensed Balance Sheets
March 31, 2020 | December 31, 2019 | |||||||
Assets | (Unaudited) | |||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 2,667,743 | $ | 4,592,932 | ||||
Restricted deposit | 75,000 | 75,000 | ||||||
Prepaid expenses and other current assets | 488,211 | 555,052 | ||||||
Total current assets | 3,230,954 | 5,222,984 | ||||||
Property and equipment, net | 20,138 | 25,422 | ||||||
Right-of-use asset | 28,977 | 42,830 | ||||||
Other assets | 5,580 | 5,580 | ||||||
Total assets | $ | 3,285,649 | $ | 5,296,816 | ||||
Liabilities and Stockholders’ Deficit | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 5,075,478 | $ | 3,890,094 | ||||
Accrued expenses | 4,847,823 | 4,747,751 | ||||||
Convertible notes payable, net | 4,302,875 | 3,184,655 | ||||||
Derivative liabilities | 440,000 | 408,000 | ||||||
Warrant liabilities | 1,058,700 | 2,637,500 | ||||||
Accrued interest | 81,972 | — | ||||||
Lease liability, current portion | 28,977 | 42,830 | ||||||
Total current liabilities | 15,835,825 | 14,910,830 | ||||||
Commitments and contingencies (Note 7) | ||||||||
Stockholders’ deficit: | ||||||||
Preferred stock $0.0001 par value, 10,000,000 shares authorized; 0 shares issued and outstanding as of March 31, 2020 and December 31, 2019 | — | — | ||||||
Common stock - $0.0001 par value, 350,000,000 shares authorized; 41,324,976 and 39,477,667 shares issued and outstanding as of March 31, 2020 and December 31, 2019, respectively | 4,133 | 3,948 | ||||||
Additional paid-in capital | 61,613,470 | 60,946,816 | ||||||
Accumulated deficit | (74,167,779 | ) | (70,564,778 | ) | ||||
Total stockholders’ deficit | (12,550,176 | ) | (9,614,014 | ) | ||||
Total liabilities and stockholders’ deficit | $ | 3,285,649 | $ | 5,296,816 | ||||
See accompanying notes to these condensed financial statements.
3
9 METERS BIOPHARMA, INC.
Condensed Statements of Operations and Comprehensive Loss
(Unaudited)
Three Months Ended March 31, | ||||||||
2020 | 2019 | |||||||
Operating expenses: | ||||||||
Research and development | $ | 2,598,985 | $ | 1,198,315 | ||||
General and administrative | 1,669,807 | 3,114,495 | ||||||
Warrant inducement expense | 690,839 | — | ||||||
Total operating expenses | 4,959,631 | 4,312,810 | ||||||
Loss from operations | (4,959,631 | ) | (4,312,810 | ) | ||||
Other income (expense): | ||||||||
Interest income | 12,815 | 26,456 | ||||||
Interest expense | (572,985 | ) | (427,245 | ) | ||||
Loss on extinguishment of convertible note payable | — | (1,049,166 | ) | |||||
Change in fair value of derivative liability and extinguishment of derivative liability | 338,000 | 471,000 | ||||||
Change in fair value of warrant liabilities | 1,578,800 | 857,000 | ||||||
Total other income (expense), net | 1,356,630 | (121,955 | ) | |||||
Loss before income taxes | (3,603,001 | ) | (4,434,765 | ) | ||||
Benefit from income taxes | — | — | ||||||
Net loss | $ | (3,603,001 | ) | $ | (4,434,765 | ) | ||
Net loss per common share, basic and diluted | $ | (0.09 | ) | $ | (0.16 | ) | ||
Weighted-average common shares, basic and diluted | 41,162,296 | 27,252,278 | ||||||
See accompanying notes to these condensed financial statements.
4
9 METERS BIOPHARMA, INC.
Unaudited Condensed Statements of Stockholders’ Equity (Deficit)
Three Months Ended March 31, 2020 | |||||||||||||||||||
Common Stock Shares | Common Stock Amount | Additional Paid-in Capital | Accumulated Deficit | Total | |||||||||||||||
Balance as of December 31, 2019 | 39,477,667 | $ | 3,948 | $ | 60,946,816 | $ | (70,564,778 | ) | $ | (9,614,014 | ) | ||||||||
Warrant exchange | 1,847,309 | 185 | 690,654 | — | 690,839 | ||||||||||||||
Share-based compensation | — | — | 276,000 | — | 276,000 | ||||||||||||||
Stock issuance costs - Warrant exchange (FN-1) | — | — | (300,000 | ) | — | (300,000 | ) | ||||||||||||
Net loss | — | — | — | (3,603,001 | ) | (3,603,001 | ) | ||||||||||||
Balance as of March 31, 2020 | 41,324,976 | 4,133 | 61,613,470 | (74,167,779 | ) | (12,550,176 | ) | ||||||||||||
Three Months Ended March 31, 2019 | |||||||||||||||||||
Common Stock Shares | Common Stock Amount | Additional Paid-in Capital | Accumulated Deficit | Total | |||||||||||||||
Balance as of December 31, 2018 | 26,088,820 | $ | 2,609 | $ | 39,854,297 | $ | (43,515,970 | ) | $ | (3,659,064 | ) | ||||||||
Issuance of common stock and warrants | 4,886,782 | 489 | 11,474,766 | — | 11,475,255 | ||||||||||||||
Allocation of warrants to liabilities | — | — | (1,970,000 | ) | — | (1,970,000 | ) | ||||||||||||
Stock issuance costs | — | — | (319,819 | ) | — | (319,819 | ) | ||||||||||||
Share-based compensation | — | — | 526,000 | — | 526,000 | ||||||||||||||
Issuance of RSUs | 90,000 | 9 | (9 | ) | — | — | |||||||||||||
Net loss | — | — | — | (4,434,765 | ) | (4,434,765 | ) | ||||||||||||
Balance as of March 31, 2019 | 31,065,602 | $ | 3,107 | $ | 49,565,235 | $ | (47,950,735 | ) | $ | 1,617,607 | |||||||||
See accompanying notes to these condensed financial statements.
5
9 METERS BIOPHARMA, INC.
Unaudited Condensed Statements of Cash Flows
Three Months Ended March 31, | ||||||||
2020 | 2019 | |||||||
Cash flows from operating activities | ||||||||
Net loss | $ | (3,603,001 | ) | $ | (4,434,765 | ) | ||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
Share-based compensation | 276,000 | 526,000 | ||||||
Accrued interest on convertible notes | 81,972 | 35,139 | ||||||
Amortization of debt discount | 481,024 | 74,803 | ||||||
Depreciation | 5,284 | 5,139 | ||||||
Change in fair value of derivative liability | (338,000 | ) | (101,000 | ) | ||||
Change in fair value of warrant liability | (1,578,800 | ) | (857,000 | ) | ||||
Warrant inducement expense | 690,839 | — | ||||||
Extinguishment of derivative liability | — | (370,000 | ) | |||||
Loss on extinguishment of debt | — | 1,049,166 | ||||||
Changes in operating assets and liabilities: | ||||||||
Prepaid expenses and other assets | 66,841 | (298,096 | ) | |||||
Accounts payable | 1,185,384 | 163,564 | ||||||
Accrued expenses | 100,072 | 70,928 | ||||||
Accrued interest | — | (101,624 | ) | |||||
Net cash used in operating activities | (2,632,385 | ) | (4,237,746 | ) | ||||
Cash flows from investing activities | ||||||||
Net cash provided by investing activities | — | — | ||||||
Cash flows from financing activities | ||||||||
Borrowings from convertible notes | 2,500,000 | 5,000,000 | ||||||
Payments of convertible notes | (1,469,804 | ) | (6,245,833 | ) | ||||
Payments of debt issuance costs | (23,000 | ) | (57,000 | ) | ||||
Proceeds from issuance of common stock and warrants | — | 11,475,255 | ||||||
Payment of deferred offering costs | (300,000 | ) | (168,682 | ) | ||||
Net cash provided by financing activities | 707,196 | 10,003,740 | ||||||
Net (decrease) increase in cash and cash equivalents | (1,925,189 | ) | 5,765,994 | |||||
Cash and cash equivalents as of beginning of period | 4,592,932 | 5,728,900 | ||||||
Cash and cash equivalents as of end of period | $ | 2,667,743 | $ | 11,494,894 | ||||
Supplemental disclosure of cash flow information | ||||||||
Cash paid during the period for interest | $ | 54,578 | $ | 418,927 | ||||
Supplemental disclosure of non-cash financing activities | ||||||||
Non-cash addition of derivative liability | $ | 370,000 | $ | 1,281,000 | ||||
Non-cash addition of deferred offering costs | $ | — | $ | 151,137 | ||||
See accompanying notes to these condensed financial statements.
6
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
NOTE 1: SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business Description
As described in Note 8—Subsequent Events, Innovate Biopharmaceuticals, Inc. completed its merger with privately-held RDD Pharma, Ltd., an Israel corporation (“RDD”) on April 30, 2020 (the “RDD Merger”). Following the completion of the RDD Merger, Innovate Biopharmaceuticals, Inc. was renamed 9 Meters Biopharma, Inc. (the “Company” or “9 Meters”). The financial statements presented in this report cover periods that ended prior to the completion of the acquisition, and therefore only include the results of Innovate Biopharmaceuticals, Inc.
9 Meters is a clinical-stage biopharmaceutical company focused on orphan, rare and unmet needs in gastroenterology. The Company’s pipeline includes drug candidates for celiac disease, short bowel syndrome (SBS), nonalcoholic steatohepatitis (NASH), Crohn's disease, and ulcerative colitis.
Basis of Presentation
The unaudited condensed interim financial statements have been prepared by the Company pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”) for interim financial reporting. These financial statements are unaudited and, in the opinion of management, include all adjustments (consisting of normal recurring adjustments and accruals) necessary for a fair statement of the balance sheets, operating results, and cash flows for the periods presented in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”). Operating results for the three months ended March 31, 2020 are not necessarily indicative of the results that may be expected for the fiscal year ending December 31, 2020. Certain information and footnote disclosure normally included in the annual financial statements prepared in accordance with U.S. GAAP have been omitted in accordance with the SEC’s rules and regulations for interim reporting. The Company’s financial position, results of operations and cash flows are presented in U.S. Dollars. These financial statements and related notes should be read in conjunction with the audited financial statements and related notes thereto for the year ended December 31, 2019, included in the Company’s Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on March 20, 2020.
Except as noted below under the section entitled “Recently Issued Accounting Standards—Accounting Pronouncements Adopted,” there have been no material changes to the Company’s significant accounting policies during the three months ended March 31, 2020, as compared to the significant accounting policies disclosed in Note 1 of the Company’s financial statements for the years ended December 31, 2019 and 2018 included in the Company’s Annual Report on Form 10-K. However, the following accounting policies are the most critical in fully understanding the Company’s financial condition and results of operations.
Shelf Registration Filing
On March 15, 2018, the Company filed a shelf registration statement that was declared effective on July 13, 2018. Under the shelf registration statement, the Company may, from time to time, sell its common stock in one or more offerings up to an aggregate dollar amount of $175 million (of which up to an aggregate of $40 million could be sold in an “at-the-market” offering as defined in Rule 415 of the Securities Act; the use of this facility was terminated effective March 19, 2020). In addition, the selling stockholders included in the shelf registration statement may from time to time sell up to an aggregate amount of 13,990,403 shares of the Company’s common stock (including up to 2,051,771 shares issuable upon exercise of warrants) in one or more offerings.
March 2019 Offering
On March 17, 2019, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with SDS Capital Partners II, LLC and certain other accredited investors, pursuant to which the Company sold, on March 18, 2019, 4,181,068 shares of common stock and issued short-term warrants (the “Short-Term Warrants”) to purchase up to 4,181,068 shares of common stock, and long-term warrants (the “March Long-Term Warrants”) to purchase up to 2,508,634 shares of common stock. Pursuant to the Purchase Agreement, the Company issued the common stock and warrants at a purchase price of $2.33 per share for aggregate proceeds of approximately $9.7 million.
The March Long-Term Warrants issued will be exercisable for five years commencing on the six-month anniversary of March 18, 2019, have an initial exercise price of $2.56 per share, subject to certain adjustments, and have an expiration date of March 18,
7
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
2024. Any March Long-Term Warrant that has not been exercised by the expiration date shall be automatically exercised via cashless exercise. The Short-Term Warrants were originally exercisable for a period of one year from March 18, 2019, had an expiration date of March 18, 2020 and had an initial exercise price of $4.00 per share, subject to certain adjustments. If at any time after March 18, 2019, the weighted-average price of the Company’s common stock exceeds $5.25 for ten consecutive trading days, the Company may call the outstanding Short-Term Warrants and require that they be exercised in cash, except to the extent that such exercise would surpass the beneficial ownership limitations, as specified in the Purchase Agreement. If not previously exercised in full, at the expiration of their applicable terms, the warrants shall be automatically exercised via cashless exercise. The Short-Term Warrants and March Long-Term Warrants are classified as warrant liabilities on the accompanying condensed balance sheet.
On February 6, 2020, the Company and the holders of the Company’s outstanding Short-Term Warrants amended the Short-Term Warrants to extend the exercise period of each Short-Term Warrant by six months. The Short-Term Warrants, as amended, are exercisable for up to an aggregate of 4,181,068 shares of the Company’s common stock, par value $0.0001 per share, until September 18, 2020. In addition, on February 12, 2020, the Company offered to amend outstanding warrants, including the Short-Term Warrants, to (i) shorten the exercise period to expire concurrently with the closing of the RDD Merger, as defined in Note 8—Subsequent Events, on April 30, 2020 and (ii) reduce the exercise price to $0.10 per share (the “Offer to Amend and Exercise”). All other terms of each Short-Term Warrant remained in full force and effect and were not impacted by this amendment. See Note 8—Subsequent Events for additional details regarding the Offer to Amend and Exercise.
Additional Issuance of Warrants
On April 25, 2019, the Company entered into an amendment (the “Amendment”) to the Purchase Agreement dated as of March 17, 2019, between the Company and each purchaser party thereto. The Amendment (i) deleted Section 4.12 of the Purchase Agreement, which generally prohibited the Company from issuing, entering into agreements to issue, announcing proposed issuances, selling or granting certain securities between the date of the Purchase Agreement and the date that was 45 days following the closing date thereunder and (ii) gave each purchaser the right to purchase, for $0.125 per underlying share, an additional warrant to purchase shares of the Company’s common stock having an exercise price per share of $2.13 and otherwise having the terms of the March Long-Term Warrants (collectively, the “New Warrants”) pursuant to a securities purchase agreement to be entered into among the Company and each purchaser that desires to purchase the New Warrants. On May 17, 2019, the Company and each purchaser entered into such Securities Purchase Agreement (the “New Agreement”), and the Company issued New Warrants exercisable for an aggregate of 3,897,010 shares of the Company’s common stock.
The New Warrants are exercisable for five years beginning on the six-month anniversary of the date of issuance until the five-year anniversary of their date of issuance. The New Warrants have an initial exercise price equal to $2.13 per share, subject to certain adjustments. However, any holder may increase or decrease such percentage to any other percentage not in excess of 9.99% upon notice to the Company, provided that any increase in such percentage shall not be effective until 61 days after such notice. If not previously exercised in full, at the expiration of their applicable terms, the New Warrants will be automatically exercised via cashless exercise, in which case the holder would receive upon such exercise the net number of shares, if any, of common stock determined according to the formula set forth in the New Warrants. The New Warrants are classified as warrant liabilities on the accompanying condensed balance sheet. On February 12, 2020, the Company offered to amend the outstanding Long-Term Warrants in the Offer to Amend and Exercise. See Note 8—Subsequent Events for additional details regarding the Offer to Amend and Exercise.
April 2019 Offering
On April 29, 2019, the Company entered into a Securities Purchase Agreement (the “April Purchase Agreement”) with certain institutional and accredited investors providing for the sale by the Company of up to 4,318,272 shares of its common stock at a purchase price of $2.025 per share.
Pursuant to the April Purchase Agreement, the Company agreed to issue unregistered warrants (the “April Warrants”) to purchase up to 4,318,272 shares of common stock. Subject to certain ownership limitations, the April Warrants were exercisable beginning on the date of their issuance until the five-and-a-half-year anniversary of their date of issuance at an initial exercise price of $2.13 per share. The exercise price of the April Warrants was subject to adjustment for stock splits, reverse splits, and similar capital transactions as described in the April Warrants. If not previously exercised in full, at the expiration of their terms, the April Warrants would have been automatically exercised via cashless exercise.
The net proceeds from the offering and the private placement were approximately $7.9 million, after deducting commissions and estimated offering costs. The Company granted the placement agent warrants to purchase up to 215,914 shares of common stock (the “Placement Agent Warrants”). The Placement Agent Warrants had substantially the same terms as the April Warrants,
8
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
except that the Placement Agent Warrants had an exercise price of $2.53 per share and had a term of 5 years from the effective date of the offering. The Company also paid the placement agent a reimbursement for non-accountable expenses in the amount of $35,000 and a reimbursement for legal fees and expenses of the placement agent in the amount of $25,000. On December 19, 2019, the Company and each of the purchasers of the April Warrants and Placement Agent Warrants (collectively, the “Exchange Warrants”) entered into separate exchange agreements (the “Exchange Agreement”), pursuant to which the Company agreed to issue to the purchasers an aggregate of 5,441,023 shares of the Company’s common stock (the “Exchange Shares”), at a ratio of 1.2 Exchange Shares for each purchaser warrant in exchange for the cancellation and termination of all of the outstanding Exchange Warrants.
Business Risks
The Company faces risks, including those associated with biopharmaceutical companies whose products are in various stages of development. These risks include, among others, the Company’s need for additional financing to achieve key development milestones, the need to defend intellectual property rights, and the dependence on key members of management.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and disclosures made in the accompanying notes to the financial statements. Areas of the financial statements where estimates may have the most significant effect include accrued expenses, share-based compensation, valuation of the derivative liability and warrant liabilities, valuation allowance for income tax assets and management’s assessment of the Company’s ability to continue as a going concern. Changes in the facts or circumstances underlying these estimates could result in material changes and actual results could differ from these estimates.
Accrued Expenses
The Company incurs periodic expenses such as research and development, licensing fees, salaries and benefits, and professional fees. The Company is required to estimate its expenses resulting from obligations under contracts with clinical research organizations, vendors and consulting agreements that have been incurred by the Company prior to being invoiced. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred for the service when the Company has not yet been invoiced or otherwise notified of the actual cost. The majority of the Company’s service providers invoice monthly in arrears for services performed or when contractual milestones are met. The Company estimates accrued expenses as of each balance sheet date based on facts and circumstances known at that time.
Accrued expenses consisted of the following:
March 31, 2020 | December 31, 2019 | |||||||
Accrued compensation and benefits | $ | 530,760 | $ | 574,332 | ||||
Accrued clinical expenses | 4,284,110 | 4,143,269 | ||||||
Other accrued expenses | 32,953 | 30,150 | ||||||
Total | $ | 4,847,823 | $ | 4,747,751 | ||||
Derivative Liability
The Company accounts for derivative instruments in accordance with Accounting Standards Codification (“ASC”) 815, Derivative and Hedging, which establishes accounting and reporting standards for derivative instruments, including certain derivative instruments embedded in other financial instruments or contracts and requires recognition of all derivatives on the balance sheet at fair value. The Company’s derivative financial instruments consist of embedded options in the Company’s convertible notes. The embedded derivatives include provisions that provide the noteholder with certain conversion and put rights at various conversion or redemption values as well as certain call options for the Company. See Note 3—Debt for further details.
Warrant Liabilities
The warrants the Company issued during 2019 are freestanding financial instruments that contain net settlement options and may require the Company to settle these warrants in cash under certain circumstances. As such, the Company has classified these warrants as liabilities on the accompanying condensed balance sheets. The warrant liabilities are initially recorded at fair value on the date of issuance and will be subsequently re-measured to fair value at each balance sheet date until the warrant liabilities
9
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
are settled. Changes in the fair value of the warrants are recognized as a non-cash component of other income and expense in the accompanying condensed statements of operations and comprehensive loss.
Research and Development
Research and development expenses consist of costs incurred to further the Company’s research and development activities and include salaries and related employee benefits, manufacturing of pharmaceutical active ingredients and drug products, costs associated with clinical trials, nonclinical activities, regulatory activities, research-related overhead expenses and fees paid to expert consultants, external service providers and contract research organizations which conduct certain research and development activities on behalf of the Company. Costs incurred in the research and development of products are charged to research and development expense as incurred.
Costs for preclinical studies and clinical trial activities are recognized based on an evaluation of the vendors’ progress towards completion of specific tasks, using data such as patient enrollment, clinical site activations or information provided by vendors regarding their actual costs incurred. Payments for these activities are based on the terms of individual contracts and payment timing may differ significantly from the period in which the services were performed. The Company determines accrual estimates through reports from and discussions with applicable personnel and outside service providers as to the progress or state of completion of trials, or the services completed. The estimates of accrued expenses as of each balance sheet date are based on the facts and circumstances known at the time. Although the Company does not expect its estimates to be materially different from amounts incurred, the Company’s estimates and assumptions for clinical trial costs could differ significantly from actual costs incurred, which could result in increases or decreases in research and development expenses in future periods when actual results are known.
Nonrefundable advance payments for goods and services that will be used in future research and development activities are expensed when the goods have been received or when the activity is performed, rather than when payment is made.
Share-Based Compensation
The Company recognizes share-based compensation expense for grants of stock options based on the grant-date fair value of those awards using the Black-Scholes option-pricing model. Share-based compensation expense is generally recognized on a straight-line basis over the requisite service period for awards expected to vest.
Share-based compensation expense includes an estimate, which is made at the time of grant, of the number of awards that are expected to be forfeited. This estimate is revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Under the Black-Scholes option-pricing model, fair value is calculated based on assumptions with respect to:
• | Expected dividend yield. The expected dividend yield is assumed to be zero as the Company has never paid dividends and has no current plans to pay any dividends on the Company’s common stock. |
• | Expected stock-price volatility. Due to limited trading history as a public company, the expected volatility is derived from the average historical volatilities of publicly traded companies within the Company’s industry that the Company considers to be comparable to the Company’s business over a period approximately equal to the expected term. In evaluating comparable companies, the Company considers factors such as industry, stage of life cycle, financial leverage, size and risk profile. |
• | Risk-free interest rate. The risk-free interest rate is based on the U.S. Treasury yield in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to the expected term. |
• | Expected term. The expected term represents the period that the stock-based awards are expected to be outstanding. Due to limited history of stock option exercises, the Company estimates the expected term of employee stock options based on the simplified method, which calculates the expected term as the average of the time-to-vesting and the contractual life of the options. Pursuant to ASU-2018-07, the Company has elected to use the contractual life of the option as the expected term for non-employee options. |
Periodically, the Board may approve the grant of restricted stock units (“RSUs”) pursuant to the Company’s 2012 Omnibus Incentive Plan, as amended, which represent the right to receive shares of the Company’s common stock based on terms of the agreement. The fair value of RSUs is recognized as share-based compensation expense generally on a straight-line basis over the service period, net of estimated forfeitures. The grant date fair value of an RSU represents the closing price of the Company’s common stock on the date of grant.
10
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Fair Value of Financial Instruments
Fair value is defined as the price that would be received for sale of an asset or paid for transfer of a liability, in an orderly transaction between market participants at the measurement date. U.S. GAAP establishes a three-tier fair value hierarchy, which prioritizes the inputs used in measuring fair value. The hierarchy gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to unobservable inputs (Level 3 measurements). Financial instruments recorded in the accompanying condensed balance sheets are categorized based on the inputs to valuation techniques as follows:
•Level 1 - defined as observable inputs based on unadjusted quoted prices for identical instruments in active markets;
•Level 2 - defined as inputs other than Level 1 that are either directly or indirectly observable in the marketplace for identical or similar instruments in markets that are not active; and
•Level 3 - defined as unobservable inputs in which little or no market data exists where valuations are derived from techniques in which one or more significant inputs are unobservable.
The fair value of the embedded derivative issued in connection with the Unsecured Convertible Note and the Additional Note, further described in Note 3—Debt, was determined by using a Monte Carlo simulation technique (“MCS”) to value the embedded derivative associated with each note. As part of the MCS valuation, a discounted cash flow (“DCF”) model is used to value the debt on a stand-alone basis and determine the discount rate to utilize in both the DCF and MCS models. The significant estimates used in the DCF model include the time to maturity of the convertible debt and calculated discount rate, which includes an estimate of the Company’s specific risk premium. The MCS methodology calculates the theoretical value of an option based on certain parameters, including: (i) the threshold of exercising the option, (ii) the price of the underlying security, (iii) the time to expiration, or expected term, (iv) the expected volatility of the underlying security, (v) the risk-free rate and (vi) the number of paths.
These valuation techniques involve management’s estimates and judgment based on unobservable inputs and are classified in Level 3. The table below summarizes the valuation inputs into the MCS model for the derivative liability associated with the Unsecured Convertible Note and the Additional Convertible Note on their respective dates of issuance as of March 8, 2019 and January 10, 2020, respectively.
Derivative Liability | ||||||||||
March 31, | January 10, | March 8, | ||||||||
2020 | 2020 | 2019 | ||||||||
Expected dividend yield | ||||||||||
Discount rate | 28.2 | % | 21.6 | % | 29.3 | % | ||||
Expected stock price volatility | 96.9 | % | 103.9 | % | 101.1 | % | ||||
Risk-free interest rate | 0.2 | % | 1.6 | % | 2.5 | % | ||||
Expected term | 16 months | 24 months | 24 months | |||||||
Price of the underlying common stock | $ | 0.50 | $ | 0.65 | $ | 1.99 | ||||
The fair values of the warrants at their respective dates of issuance further described above in the sections entitled “March 2019 Offering,” “Additional Issuance of Warrants,” and “April 2019 Offering” were determined through the use of an MCS model. The MCS methodology calculates the theoretical value of an option based on certain parameters, including (i) the threshold of exercising the option, (ii) the price of the underlying security, (iii) the time to expiration, or expected term, (iv) the expected volatility of the underlying security, (v) the risk-free interest rate and (vi) the number of paths. Given the high level of the selected volatilities, the methodology selected simulates the Company’s market value of invested capital (“MVIC”) through the maturity date of the respective warrants (ranging from one year to five-and-a-half years). Further, the estimated future stock price of the Company is calculated by subtracting the debt plus accrued interest from the MVIC. The significant estimates used in the MCS model include management’s estimated probability of future financing and liquidation events.
Upon a fundamental transaction (as defined in the applicable warrant agreement), each holder of Short-Term Warrants and each holder of the March Long-Term Warrants and New Warrants (collectively, the “Long-Term Warrants”) can elect to require the Company or a successor entity to purchase such holder’s outstanding, unexercised warrants for a cash payment (or under certain circumstances other consideration) equal to the Black-Scholes value of the warrants on the date of consummation of the
11
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
fundamental transaction, calculated in accordance with the terms and using the assumptions specified in the applicable warrant agreement. Due to the RDD Merger, the Company entered into the Exchange Agreements with the holders of the Exchange Warrants, pursuant to which the Company agreed to issue the purchasers an aggregate of 5,441,023 shares in exchange for the cancellation and termination of the Exchange Warrants. On December 26, 2019, an aggregate of 2,994,762 warrants were exchanged for 3,593,714 shares of the Company’s common stock. During the three months ended March 31, 2020, 1,539,424 warrants were exchanged for 1,847,309 shares of the Company’s common stock. In addition, the Company amended the Short-Term Warrants and Long-Term Warrants in the Offer to Amend in Exercise on February 12, 2020. Immediately prior to the Offer to Amend and Exercise, the Company determined the fair value of the Short-Term Warrants and Long-Term Warrants as of February 11, 2020 and at period end on March 31, 2020. The Company recognized a gain in fair value of the Short-Term Warrants and Long-Term Warrants of approximately $1.2 million in the accompanying condensed statement of operations and comprehensive loss during the three months ended March 31, 2020. During the three months ended March 31, 2020, the Company recognized warrant inducement expense of approximately $0.7 million. There was no warrant inducement expense recognized during the three months ended March 31, 2019. The warrant inducement expense represents the accounting fair value of consideration issued to induce conversion of the Exchange Warrants at a ratio of 1.2 Exchange Shares for each purchaser warrant.
Management has assumed that the holders of the Short-Term Warrants and Long-Term Warrants would elect to receive cash payments under the respective warrant agreements following completion of the RDD Merger. As such, the Company determined the fair value of the Short-Term Warrants and Long-Term Warrants prior to the Offer to Amend and Exercise, for financial reporting purposes, through the use of the Black-Scholes model. Subsequent to the Offer to Amend and Exercise, the Company determined the fair value of the Short-Term Warrants and Long-Term Warrants using the reduced exercise price of $0.10 as of March 31, 2020. The estimates underlying the assumptions used in both the MCS model and Black-Scholes model are subject to risks and uncertainties and may change over time, and the assumptions used in both the MCS model and the Black-Scholes model for financial reporting purposes generally differ from the assumptions that would be applied in determining a payout under the applicable warrant agreements. These valuation techniques involve management’s estimates and judgment based on unobservable inputs and are classified in Level 3.
The table below summarizes the valuation inputs into the MCS model for the Short-Term Warrants and Long-Term Warrants at their respective dates of issuance.
Short-Term Warrants | Long-Term Warrants | ||||||||
March 18, 2019 | March 18, 2019 | May 17, 2019 | |||||||
Conversion price | $ | 4.00 | $ | 2.56 | $ | 2.13 | |||
Expected stock price volatility | 122.0 | % | 85.2 | % | 83.4 | % | |||
Risk-free interest rate | 2.5 | % | 2.2 | % | 2.2 | % | |||
Expected term | 1 year | 5 years | 5 years | ||||||
Price of the underlying common stock | $ | 2.48 | $ | 2.48 | $ | 1.58 | |||
The table below summarizes the range of valuation inputs into the Black-Scholes model for the Exchange Warrants on their date of issuance and immediately prior to the exchange.
Exchange Warrants | ||||||
May 1, 2019 | January 6, 2020 | |||||
Conversion price | $ 2.13 - $ 2.53 | $ | 2.13 | |||
Expected stock price volatility | 84.1 | % | 87.3 | % | ||
Risk-free interest rate | 2.2 | % | 1.7 | % | ||
Expected term | 5 - 5.5 years | 4.9 years | ||||
Price of the underlying common stock | $ | 1.54 | $ | 0.58 | ||
12
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
The table below summarizes the range of valuation inputs into the Black-Scholes model for the warrant liabilities as of February 11, 2020, immediately prior to the reduction in exercise price pursuant to the Offer to Amend and Exercise.
Short-Term Warrants | Long-Term Warrants | |||||
February 11, 2020 | ||||||
Conversion price | $ | 4.00 | $2.13 - $2.56 | |||
Expected stock price volatility | 97.1 | % | 87.9% - 89.2% | |||
Risk-free interest rate | 1.6 | % | 1.7 | % | ||
Expected term | 7 months | 4 years 2 months | ||||
Price of the underlying common stock | $ | 0.79 | $ | 0.79 | ||
The following table summarizes the fair value hierarchy of financial liabilities measured at fair value as of March 31, 2020 and December 31, 2019, respectively.
March 31, 2020 | ||||||||||||
Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | |||||||||
Derivative liability | $ | — | $ | — | $ | 440,000 | $ | 440,000 | ||||
Warrant liabilities | — | — | 1,058,700 | 1,058,700 | ||||||||
Total liabilities at fair value | $ | — | $ | — | $ | 1,498,700 | $ | 1,498,700 | ||||
December 31, 2019 | ||||||||||||
Quoted Prices in Active Markets for Identical Assets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | Total | |||||||||
Derivative liability | $ | — | $ | — | $ | 408,000 | $ | 408,000 | ||||
Warrant liabilities | — | — | 2,637,500 | 2,637,500 | ||||||||
Total liabilities at fair value | $ | — | $ | — | $ | 3,045,500 | $ | 3,045,500 | ||||
The following table summarizes the changes in fair value of the derivative liability and warrant liabilities classified in Level 3. Gains and losses reported in this table include changes in fair value that are attributable to unobservable inputs.
Three Months Ended March 31, 2020 | |||
Beginning balance as of December 31, 2019 | $ | 3,045,500 | |
Issuance of derivative liability (the Additional Note) | 370,000 | ||
Exchange of the April Warrants | (380,600 | ) | |
Change in fair value of warrant liabilities | (1,198,200 | ) | |
Change in fair value of derivative liability | (338,000 | ) | |
Ending balance as of March 31, 2020 | $ | 1,498,700 | |
The amount of total gain for the period included in earnings attributable to the change in unrealized gains relating to the fair value liabilities still held at the end of the period | $ | 1,536,200 | |
13
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
The cumulative unrealized gain relating to the change in fair value of the derivative liability and warrant liabilities of $1,536,200 and the gain on exchange of the April Warrants of $380,600 for the three months ended March 31, 2020 is included in other income (expense) in the condensed statements of operations and comprehensive loss.
ASC 820, Fair Value Measurement and Disclosures requires all entities to disclose the fair value of financial instruments, both assets and liabilities, for which it is practicable to estimate fair value. As of March 31, 2020 and December 31, 2019, the recorded values of cash and cash equivalents, restricted deposit, accounts payable, accrued expenses and convertible promissory notes approximate their fair values due to the short-term nature of the instruments.
Deferred Offering Costs
Deferred offering costs consist principally of legal, accounting and underwriters’ fees related to offerings or the Company’s shelf registration. Offering costs incurred prior to an offering are initially capitalized and then subsequently reclassified to additional paid-in capital upon completion of the offering. Deferred offering costs associated with the shelf registration will be charged to additional paid-in capital on a pro-rata basis in the event the Company completes an offering under the shelf registration.
Patent Costs
Costs associated with the submission of patent applications are expensed as incurred given the uncertainty of the future economic benefits of the patents. Patent and patent related legal and administrative costs included in general and administrative expenses were approximately $85,000 and $161,000 for the three months ended March 31, 2020 and 2019, respectively.
Net Loss Per Share
The Company calculates net loss per share as a measurement of the Company’s performance while giving effect to all potentially dilutive shares that were outstanding during the reporting period. Because the Company had a net loss for all periods presented, the inclusion of common stock options or other similar instruments would be anti-dilutive. Therefore, the weighted average shares outstanding used to calculate both basic and diluted net loss per share are the same. For the three months ended March 31, 2020 and 2019, 21.2 million and 15.8 million potentially dilutive securities related to warrants and stock options issued and outstanding have been excluded from the computation of diluted weighted average shares outstanding because the effect would be anti-dilutive. The potentially dilutive securities consisted of the following:
Three Months Ended March 31, | |||||||
2020 | 2019 | ||||||
Options outstanding under the Private Innovate Plan | 6,028,781 | 6,340,871 | |||||
Options outstanding under the Omnibus Plan | 2,717,870 | 841,131 | |||||
Warrants issued at a weighted-average exercise price of $55.31 | 154,403 | 154,403 | |||||
Warrants issued at an exercise price of $2.54 | 349,555 | 349,555 | |||||
Warrants issued at an exercise price of $3.18 | 1,410,358 | 1,410,358 | |||||
Short-term warrants issued at an exercise price of $4.00 | 4,181,068 | 4,181,068 | |||||
Long-term warrants issued at a weighted-average exercise price of $2.30 and $2.56, respectively | 6,405,644 | 2,508,634 | |||||
Total | 21,247,679 | 15,786,020 | |||||
Segments
Operating segments are defined as components of an enterprise engaging in business activities for which discrete financial information is available and regularly reviewed by the chief operating decision maker in deciding how to allocate resources and in assessing performance. The Company operates and manages its business as one operating segment and all of the Company’s operations are in North America.
14
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Recently Issued Accounting Standards
Accounting Pronouncements Adopted
In August 2018, the FASB issued ASU 2018-13, Fair Value Measurement (Topic 820): Disclosure Framework - Changes to the Disclosure Requirements for Fair Value Measurement. This standard no longer requires public companies to disclose transfers between Level 1 and 2 of the fair value hierarchy and adds additional disclosure requirements about the range and weighted average used to develop significant unobservable inputs for Level 3 fair value measurements. The Company adopted this guidance effective January 1, 2020 and the adoption of ASU 2018-13 did not have a material impact on the Company’s financial statements.
Accounting Pronouncements being Evaluated
In December 2019, the FASB issued ASU 2019-12, Income Taxes (Topic 740): Simplifying the Accounting for Income Taxes. ASU 2019-12 amends the accounting for income taxes by removing certain exceptions to the general principles in Topic 740 and improves consistent application of other areas of Topic 740 by clarifying and amending existing guidance. ASU 2019-12 is effective for fiscal years and interim periods within those fiscal years beginning after December 15, 2020. Early adoption is permitted and the Company is currently evaluating the impact this standard will have on the Company’s financial statements.
NOTE 2: LIQUIDITY AND GOING CONCERN
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. Although, the Company received gross proceeds of approximately $22.6 million upon completion of the RDD Merger Financing, further described in Note 8—Subsequent Events, the Company expects to incur substantial losses in the future as it conducts planned operating activities. Based on the Company’s limited operating history, recurring negative cash flows from operations, current plans and available resources, the Company will need substantial additional funding to support its planned and future operating activities, including progression of research and development programs. The Company has concluded that the prevailing conditions and ongoing liquidity risks faced by the Company raise substantial doubt about the Company’s ability to continue as a going concern for at least 1 year following the date these financial statements are issued.
The effect of the COVID-19 pandemic and its associated restrictions may increase the anticipated aggregate costs for the development of the Company's product candidates and may adversely impact the anticipated timelines for the development of the Company's product candidates by, among other things, causing disruptions in the supply chain for clinical supplies, delays in the timing and pace of subject enrollment in clinical trials and lower than anticipated subject enrollment and completion rates, delays in the review and approval of the Company’s regulatory submissions by the FDA and other agencies with respect to the Company's product candidates, and other unforeseen disruptions. The economic impact of the COVID-19 pandemic and its effect on capital markets and investor sentiment may adversely impact the Company's ability to raise capital when needed or on terms favorable to the Company and its stockholders to fund its development programs and operations. The Company does not yet know the full extent of potential delays or impacts on its business, clinical trial activities, ability to access capital or on healthcare systems or the global economy as a whole. However, these effects could have a material adverse impact on the Company's business and financial condition.
There can be no assurance that the Company will be able to obtain additional capital on terms acceptable to the Company, on a timely basis or at all. The failure to obtain sufficient additional funding or enter into strategic partnerships could adversely affect the Company’s ability to achieve its business objectives and product development timelines and could have a material adverse effect on the Company’s results of operations. The accompanying financial statements do not include any adjustments that might be necessary should the Company be unable to continue as a going concern.
NOTE 3: DEBT
Senior Convertible Note
The principal amount of the senior convertible note issued on October 4, 2018 (the “Senior Convertible Note”), was $5.2 million and bore interest at a rate of eight percent (8%) per annum payable quarterly in cash, with a scheduled maturity date of October 4, 2020. The interest rate would automatically increase to 18% per annum if there was an event of default during the period.
During January 2019, the noteholder issued a redemption notice to the Company requiring the Company to repay the noteholder $1,049,167 of principal and $1,399 of accrued interest. On January 7, 2019, the Company entered into an Option to Purchase
15
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Senior Convertible Note (the “Option Agreement”) with the noteholder. The Company paid the noteholder $250,000 in consideration for the noteholder entering into the Option Agreement with the Company, which was recorded as interest expense in the accompanying statements of operations and comprehensive loss. The Option Agreement provided the Company with the ability to repay (purchase) the outstanding principal and accrued interest of the Senior Convertible Note any time from January 7, 2019 until March 31, 2019 (“Option Period”).
During March 2019, the Company exercised its repurchase rights under the Option Agreement and paid the noteholder of the Senior Convertible Note approximately $5,200,000 in principal and $60,000 in interest, which was the full purchase amount of the Senior Convertible Note pursuant to the terms of the Option Agreement. There are no further amounts outstanding under the Senior Convertible Note and the Senior Convertible Note has been canceled. The Company accounted for the repayment of the Senior Convertible Note as a liability extinguishment in accordance with ASC 405, Extinguishments of Liabilities, which resulted in the Company recording a loss on extinguishment of debt of approximately $1.0 million in the accompanying statements of operations and comprehensive loss for the three months ended March 31, 2019.
Unsecured Convertible Promissory Note
On March 8, 2019, the Company entered into a Securities Purchase Agreement (the “Note Purchase Agreement”) with a purchaser (the “Convertible Noteholder”). Pursuant to the Note Purchase Agreement, the Company issued the Convertible Noteholder an unsecured Convertible Promissory Note (the “Unsecured Convertible Note”) in the principal amount of $5.5 million. The Convertible Noteholder may elect to convert all or a portion of the Unsecured Convertible Note at any time and from time to time into the Company’s common stock at a conversion price of $3.25 per share, subject to adjustment for stock splits, dividends, combinations and similar events. The Company may prepay all or a portion of the Unsecured Convertible Note at any time for an amount equal to 115% of then outstanding obligations or the portion of the obligations the Company is prepaying. The purchase price of the Unsecured Convertible Note was $5.0 million, and the Unsecured Convertible Note carries an original issuance discount (“OID”) of $0.5 million, which is included in the principal amount of the Unsecured Convertible Note. In addition, the Company agreed to pay $20,000 of transaction expenses, which were netted out of the purchase price of the Unsecured Convertible Note. The Company also incurred additional transaction costs of approximately $37,000, which were recorded as debt issuance costs. As a result of the redemption features of the Unsecured Convertible Note, further described below, the Company is amortizing the debt issuance costs and accreting the OID to interest expense over the estimated redemption period of 15 months, using the effective interest method.
The various conversion and redemption features contained in the Unsecured Convertible Note are embedded derivative instruments, which were recorded as a debt discount and derivative liability at the issuance date at their estimated fair value of $1.3 million. Amortization of debt discount and accretion of the OID for the Unsecured Convertible Note recorded as interest expense was approximately $0.4 million and $0.1 million for the three months ended March 31, 2020 and 2019, respectively.
The Unsecured Convertible Note bears interest at the rate of 10% (which will increase to 18% upon and during the continuance of an event of default) per annum, compounding on a daily basis. All principal and accrued interest on the Unsecured Convertible Note is due on the second-year anniversary of the Unsecured Convertible Note’s issuance. During the three months ended March 31, 2020, the Company made principal payments of $1.5 million on the Unsecured Convertible Note. There were no principal payments made on the Unsecured Convertible Note during the three months ended March 31, 2019.
At any time after the six-month anniversary of the issuance of the Unsecured Convertible Note, (i) if the average volume weighted average price over twenty trading dates exceeds $10.00 per share, the Company may generally require that the Unsecured Convertible Note convert into shares of its common stock at the $3.25 (as adjusted) conversion price, and (ii) the Convertible Noteholder may elect to require all or a portion of the Unsecured Convertible Note be redeemed by the Company. If the Convertible Noteholder requires a redemption, the Company, at its discretion, may pay the redeemed portion of the Unsecured Convertible Note in cash or in the Company’s common stock at a conversion rate equal to the lesser of (i) the $3.25 (as adjusted) conversion rate or (ii) 80% of the average of the five lowest volume weighted average price of the Company’s Common Stock over the preceding twenty trading days. The Convertible Noteholder may not redeem more than $500,000 per calendar month during the period between the six-month anniversary of the date of issuance until the first-year anniversary of the date of issuance and $750,000 per calendar month thereafter. See Note 8—Subsequent Events regarding a standstill agreement entered into on April 3, 2020 that temporarily suspends redemption payments. The obligation or right of the Company to deliver its shares upon the conversion or redemption of the Unsecured Convertible Note is subject to a 19.99% cap and subject to a floor price trading price of $3.25 (unless waived by the Company). Any amounts redeemed once the cap is reached or if the market price is less than the $3.25 floor price must be paid in cash.
If there is an Event of Default under the Unsecured Convertible Note, the Convertible Noteholder may accelerate the Company’s obligations or elect to increase the outstanding obligations under the Unsecured Convertible Note. The amount of the increase
16
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
ranges from 5% to 15% depending on the type of default (as defined in the Unsecured Convertible Note). In addition, the Unsecured Convertible Note obligations will be increased if there are delays in the Company’s delivery requirements for the shares or cash issuable upon the conversion or redemption of the Unsecured Convertible Note in certain circumstances.
If the Company issues convertible debt in the future with any terms, including conversion terms, that are more favorable to the terms of the Unsecured Convertible Note, the Convertible Noteholder may elect to incorporate the more favorable terms into the Unsecured Convertible Note.
Additional Note
On January 10, 2020, the Company entered into an additional securities purchase agreement and unsecured convertible promissory note with the Convertible Noteholder in the principal amount of $2,750,000 (the “Additional Note”). The Convertible Noteholder may elect to convert all or a portion of the Additional Note, at any time from time to time into the Company’s common stock at a conversion price of $3.25 per share, subject to adjustment for stock splits, dividends, combinations and similar events. The Company may prepay all or a portion of the Additional Note at any time for an amount equal to 115% of then outstanding obligations or the portion of the obligations we are prepaying. The purchase price of the Additional Note was $2.5 million and carries an original issuance discount of $250,000, which is included in the principal amount of the Additional Note.
The various conversion and redemption features contained in the Additional Note are embedded derivative instruments, which were recorded as a debt discount and derivative liability at the issuance date at their estimated fair value of $0.4 million. Amortization of debt discount and accretion of the OID for the Additional Note recorded as interest expense was approximately $0.1 million for the three months ended March 31, 2020.
The Additional Note bears interest at the rate of 10% (which will increase to 18% upon and during the continuance of an event of default) per annum, compounding on a daily basis. All principal and accrued interest on the Additional Note is due on the second anniversary of the date of the Additional Note’s issuance. There were no principal payments made on the Additional Note during the three months ended March 31, 2020.
At any time after the six-month anniversary of the issuance of the Additional Note, (i) if the average VWAP of the Company’s common stock over twenty trading dates exceeds $10.00 per share, the Company may generally require that the Additional Note convert into share of its common stock at the $3.25 (as adjusted) conversion rate or (ii) 80% of the average of the five lowest VWAP of the Company’s common stock over the preceding twenty trading days. The Convertible Noteholder may not redeem more than $500,000 per calendar month during the period between the six-month anniversary of the date of issuance until the first anniversary of the date of issuance and $750,000 per calendar month thereafter. The obligation or right of the Company to deliver its shares upon the conversion or redemption of the Additional Note is subject to a 19.99% cap and subject to a floor price of $3.25 (unless waived by the Company). Any amounts redeemed or converted once the cap is reached or if the market price is less than the $3.25 floor price must be paid in cash.
If there is an Event of Default under the Additional Note, the Convertible Noteholder may accelerate the Company’s obligations or the Convertible Noteholder may elect to increase the outstanding obligations under the Additional Note. The amount of the increase ranges from 15% for certain “Major Defaults,” 10% for failure to obtain the Convertible Noteholder’s approval for certain equity issuances with anti-dilution, price reset or variable pricing features of less than $2,500,000, and 5% for certain “Minor Defaults.” In addition, the Additional Note obligations will be increased if there are delays in the Company’s delivery requirements for the shares or cash issuable upon the conversion or redemption of the Additional Note in certain circumstances.
If the Company issues convertible debt in the future with any terms, including conversion terms, that are more favorable to the terms of the Additional Note, the Convertible Noteholder may elect to incorporate the more favorable terms into the Additional Note.
The convertible notes payable as of March 31, 2020 and December 31, 2019 consists of the following:
March 31, 2020 | December 31, 2019 | |||||
Convertible Notes | $ | 8,250,000 | $ | 5,500,000 | ||
Less: principal payments of debt | (3,014,528 | ) | (1,544,724 | ) | ||
Less: unamortized debt discount and OID accretion | (932,597 | ) | (770,621 | ) | ||
Total | $ | 4,302,875 | $ | 3,184,655 | ||
17
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
NOTE 4: LICENSE AGREEMENTS
During 2016, the Company entered into a license agreement (the “Alba License”) with Alba Therapeutics Corporation (“Alba”) to obtain the rights to certain intellectual property relating to larazotide acetate and related compounds. The Company’s initial area of focus for these assets relates to the treatment of celiac disease.
Upon execution of the Alba License, the Company paid Alba a non-refundable license fee of $0.5 million. In addition, the Company is required to make milestone payments to Alba upon the achievement of certain clinical and regulatory milestones totaling up to $1.5 million and payments upon regulatory approval and commercial sales of a licensed product totaling up to $150 million, which is based on sales ranging from $100 million to $1.5 billion.
Upon the Company paying Alba $2.5 million for the first commercial sale of a licensed product, the Alba License becomes perpetual and irrevocable. Upon the achievement of net sales in a year exceeding $1.5 billion, the Alba License also becomes free of milestone fees. The Alba License provides Alba with certain termination rights, including failure of the Company to use Commercially Reasonable Efforts to develop the licensed products.
During 2013, the Company entered into an exclusive license agreement with Seachaid Pharmaceuticals, Inc. (the “Seachaid Agreement”) to further develop and commercialize the licensed product, the compound known as APAZA. The agreement shall continue in effect on a country-by-country basis, unless terminated sooner in accordance with the termination provisions of the agreement, until the expiration of the royalty term for such product and such country. The royalty term for each such product and such country shall continue until the earlier of the expiration of certain patent rights (as defined in the agreement) or the date that the sales for one or more generic equivalents makes up a certain percentage of sales in an applicable country during a calendar year.
The Company was required to make an initial, non-refundable payment under the Seachaid Agreement in the amount of $0.2 million. The agreement also calls for milestone payments totaling up to $6.0 million to be paid when certain clinical and regulatory milestones are met. There are also commercialization milestone payments ranging from $1.0 million to $2.5 million depending on net sales of the products in a single calendar year, followed by royalty payments in the single digits based on net product sales.
During 2014, the Company entered into an Asset Purchase Agreement with Repligen Corporation (“Repligen”) to acquire Repligen’s RG-1068 program for the development of Secretin for the Pancreatic Imaging Market and Magnetic Resonance Cholangiopancreatography. As consideration for the Asset Purchase Agreement, the Company agreed to make a non-refundable cash payment on the date of the agreement and future royalty payments consisting of a percentage between five and fifteen of annual net sales, with the royalty payment percentage increasing as annual net sales increase. The royalty payments are made on a product-by-product and country-by-country basis and the obligation to make the payments expires with respect to each country upon the later of (i) the expiration of regulatory exclusivity for the product in that country or (ii) 10 years after the first commercial sale in that country. The royalty amount is subject to reduction in certain situations, such as the entry of generic competition in the market.
There were no milestone or royalty fees incurred during the three months ended March 31, 2020 and 2019.
NOTE 5: STOCKHOLDERS’ DEFICIT
The Company’s authorized capital stock consists of 360 million shares of capital stock, par value $0.0001 per share, of which 350 million shares are designated as common stock and 10 million shares are designated as preferred stock.
Preferred Stock
The Company’s amended and restated certificate of incorporation authorizes the Board to issue preferred stock in one or more classes or one or more series within any class from time to time. Voting powers, designations, preferences, qualifications, limitations, restrictions or other rights will be determined by the Board at that time. There were no shares of preferred stock issued and outstanding as of March 31, 2020 and December 31, 2019.
Common Stock
The holders of the Company’s common stock (i) have equal ratable rights to dividends from funds legally available therefore, when, as and if declared by the Board; (ii) are entitled to share in all the Company’s assets available for distribution to holders of common stock upon liquidation, dissolution or winding up of the Company’s affairs; (iii) do not have preemptive, subscription or
18
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
conversion rights (and there are no redemption or sinking fund provisions or rights); and (iv) are entitled to one non-cumulative vote per share on all matters on which stockholders may vote.
There were 41,324,976 and 39,477,667 shares of common stock outstanding as of March 31, 2020 and December 31, 2019, respectively. The Company had reserved shares of common stock for future issuance as follows:
March 31, | December 31, | ||||
2020 | 2019 | ||||
Outstanding stock options | 8,746,651 | 8,781,615 | |||
Warrants to purchase common stock | 12,501,028 | 14,040,452 | |||
Shares issuable upon conversion of convertible debt | 1,636,136 | 1,217,008 | |||
For possible future issuance under the Omnibus Plan | 3,076,622 | 1,102,739 | |||
Total common shares reserved for future issuance | 25,960,437 | 25,141,814 | |||
On December 19, 2019, the Company and each of the purchasers of the April Warrants and Placement Agent Warrants entered into the Exchange Agreements, pursuant to which the Company agreed to issue the purchasers an aggregate of 5,441,023 shares of Common Stock at a ratio of 1.2 Exchange Shares for each purchaser warrant in exchange for cancellation and termination of all of the outstanding April Warrants and Placement Agent Warrants. On December 26, 2019, an aggregate of 2,994,762 warrants were exchanged for 3,593,714 shares of the Company’s common stock. During the three months ended March 31, 2020, the Company issued 1,847,309 shares of common stock in exchange for cancellation and termination of the remaining outstanding Exchange Warrants. As of March 31, 2020, all of the April Warrants and Placement Agent Warrants were exchanged for Common Stock and there were no April Warrants or Placement Agent Warrants outstanding. See Note 1 - Summary of Significant Accounting Policies for further details.
On October 26, 2018, the Company entered into a common stock sales agreement with H.C. Wainwright & Co., LLC and Ladenburg Thalmann & Co., Inc. and filed a prospectus with the SEC relating to such offering. The Company previously filed a Form S-3 that became effective July 13, 2018 that included the registration of $40 million of its shares of common stock in connection with a potential ATM offering. Pursuant to the sales agreement, the Company could issue and sell shares having an aggregate gross sales price of up to $40 million and was required to pay the sales agents commissions of 3% of the gross sales price per share sold. During the three months ended March 31, 2019, the Company sold 705,714 shares under the ATM for total net proceeds of approximately $1,675,000. The Company voluntarily suspended the ATM facility as of June 24, 2019 and effective March 19, 2020, the Company terminated the ATM facility.
NOTE 6: SHARE-BASED COMPENSATION
The Company has two stock option plans in existence: the 2012 Omnibus Incentive Plan (the “Omnibus Plan”) and the Innovate 2015 Stock Incentive Plan (the “Private Innovate Plan”). The shares reserved for issuance under the Omnibus Plan automatically increase on the first day of each calendar year beginning in 2019 and ending in 2022 by an amount equal to the lesser of (i) five percent of the number of shares of common stock outstanding as of December 31st of the immediately preceding calendar year or (ii) such lesser number of shares of common stock as determined by the Board (the “Evergreen Provision”). On January 1, 2020 and 2019, the number of shares of common stock available under the Omnibus Plan automatically increased by 1,973,883 and 1,304,441 shares pursuant to the Evergreen Provision, respectively.
The terms of the option agreements are determined by the Board. The Company’s stock options vest based on the terms in the stock option agreements and typically vest over a period of three or four years. These stock options typically have a maximum term of ten years.
Private Innovate Plan
As of March 31, 2020, there were 6,028,781 stock options outstanding under the Private Innovate Plan. Since 2018, the Company has not issued, and does not intend to issue, any additional awards from the Private Innovate Plan.
The range of assumptions used in estimating the fair value of the options granted or re-measured under the Private Innovate Plan using the Black-Scholes option pricing model for the periods presented were as follows:
19
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Three Months Ended March 31, | ||||
2020 | 2019 | |||
Expected dividend yield | 0% | 0% | ||
Expected stock-price volatility | —% | 67% | ||
Risk-free interest rate | —% | 2.6% | ||
Expected term of options (in years) | 0 | 8.2 - 8.7 | ||
The following table summarizes stock option activity under the Private Innovate Plan:
Number of Shares | Weighted-Average Exercise Price | Aggregate Intrinsic Value | Weighted-Average Remaining Contractual Life (in years) | ||||||||||
Outstanding at December 31, 2019 | 6,063,745 | $ | 1.53 | $ | 496,275 | 5.4 | |||||||
Options granted | — | — | — | ||||||||||
Options forfeited | (34,964 | ) | 2.16 | — | |||||||||
Options exercised | — | — | — | ||||||||||
Outstanding at March 31, 2020 | 6,028,781 | 1.53 | 391,975 | 3.9 | |||||||||
Exercisable at March 31, 2020 | 5,762,005 | 1.50 | 391,975 | 3.8 | |||||||||
Vested and expected to vest at March 31, 2020 | 6,022,403 | $ | 1.53 | $ | 391,975 | 3.9 | |||||||
There were no options granted under the Private Innovate Plan during the three months ended March 31, 2020 and 2019.
The total fair value of stock option awards vested during the three months ended March 31, 2020 under the Private Innovate Plan was approximately $137,000. As of March 31, 2020, there was approximately $0.3 million of total unrecognized compensation cost related to unvested stock-based compensation arrangements under the Private Innovate Plan, which is expected to be recognized over a weighted average period of 1.2 years.
The Private Innovate Plan provides for accelerated vesting under certain change-of-control transactions, if approved by the Company’s board of directors.
Omnibus Plan
As of March 31, 2020, there were options to purchase 2,717,870 shares of the Company’s common stock outstanding under the Omnibus Plan and 3,076,622 shares available for future grants under the Omnibus Plan.
The range of assumptions used in estimating the fair value of the options granted under the Omnibus Plan using the Black-Scholes option pricing model for the periods presented were as follows:
Three Months Ended March 31, | ||||
2020 | 2019 | |||
Expected dividend yield | 0% | 0% | ||
Expected stock-price volatility | —% | 68% - 72% | ||
Risk-free interest rate | —% | 2.5% - 2.7% | ||
Expected term of options (in years) | 0 | 5.4 - 10.0 | ||
20
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
The following table summarizes stock option activity under the Amended Omnibus Plan:
Number of Shares | Weighted-Average Exercise Price | Aggregate Intrinsic Value | Weighted-Average Remaining Contractual Life (in years) | ||||||||||
Outstanding at December 31, 2019 | 2,717,870 | $ | 1.87 | $ | — | 9.4 | |||||||
Options granted | — | — | — | ||||||||||
Options forfeited | — | — | — | ||||||||||
Options exercised | — | — | — | ||||||||||
Outstanding at March 31, 2020 | 2,717,870 | 1.87 | — | 9.1 | |||||||||
Exercisable at March 31, 2020 | 1,532,242 | 2.05 | — | 9.1 | |||||||||
Vested and expected to vest at March 31, 2020 | 2,633,871 | $ | 1.86 | $ | — | 9.1 | |||||||
The weighted-average grant date fair value of options granted under the Omnibus Plan was $1.29 during the three months ended March 31, 2019. There were no options granted or exercised under the Omnibus Plan during the three months ended March 31, 2020.
The total fair value of stock option awards vested under the Omnibus Plan was approximately $110,000 during the three months ended March 31, 2020. As of March 31, 2020, there was approximately $1.1 million of total unrecognized compensation cost related to unvested stock-based compensation arrangements under the Omnibus Plan. This cost is expected to be recognized over a weighted average period of 2.5 years.
The Omnibus Plan provides for accelerated vesting under certain change-of-control transactions, if approved by the Company’s board of directors.
During the three months ended March 31, 2019, the board approved grants of 90,000 RSUs, which vest immediately upon the date of grant. The weighted-average fair value of RSUs granted during the three months ended March 31, 2019 was $2.12 and the Company recognized share-based compensation expense for the RSUs of approximately $17,000 and $190,000 during the three months ended March 31, 2020 and 2019, respectively. There were no RSUs granted during the three months ended March 31, 2020.
Total share-based compensation expense recognized in the accompanying statements of operations was as follows:
Three Months Ended March 31, | ||||||||
2020 | 2019 | |||||||
Research and development | $ | 128,000 | $ | 92,000 | ||||
General and administrative | 148,000 | 434,000 | ||||||
Total share-based compensation | $ | 276,000 | $ | 526,000 | ||||
NOTE 7: COMMITMENTS AND CONTINGENCIES
Clinical Trial Agreement
From time to time, the Company enters into agreements with contract research organizations and other service providers. In August 2018, the Company entered into such an agreement for its planned Phase 3 trial for the treatment of celiac disease. Under this agreement, the Company expects to pay approximately $1.1 million for data management over the course of the Phase 3 celiac disease trial for data management and biostatistics services.
Employment Agreements
The Company has entered into amended and restated executive employment agreements with the executives and new executive employment agreements with certain new executives (the “Executive Employment Agreements”). The Executive Employment
21
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Agreements provide an annual base salary and the opportunity to participate in the Company’s equity compensation, employee benefit and bonus plans once they are established and approved by the Board. The Executive Employment Agreements contain severance provisions if the executives are terminated under certain conditions that would provide the executive with 12 months of their base salary and up to 12 months of continuation of health insurance benefits.
In February 2019, the Company entered into a separation and general release agreement with a former executive of the Company that included separation benefits consistent with the former executive’s employment agreement. The Company recognized severance expense totaling $0.3 million during the three months ended March 31, 2019, which is being paid in equal installments over 12 months beginning February 2019. There was no severance expense recognized during the three months ended March 31, 2020. The accrued severance obligation in respect of the former executive has been fully paid as of March 31, 2020.
Office Lease
In October 2017, the Company entered into a three-year lease for office space that expires on September 30, 2020. Base annual rent is $60,000, or $5,000 per month. Monthly payments of $5,000 are due and payable over the 24-month term. A security deposit of $5,000 was paid in October 2017. The lease contains a two-year renewal option.
The Company estimated the present value of the lease payments over the remaining term of the lease using a discount rate of 12%, which represented the Company’s estimated incremental borrowing rate. The two-year renewal option was excluded from the lease payments as the Company concluded the exercise of this option was not considered reasonably certain.
Operating lease cost under ASC 842 was approximately $15,000 for the three months ended March 31, 2020 and 2019 and is included in general and administrative expenses on the accompanying condensed statement of operations and comprehensive loss. The total cash paid for amounts included in the measurement of the operating lease liability and reported within operating activities was less than $0.1 million during the three months ended March 31, 2020.
Future minimum payments under the Company’s lease liability were as follows:
Year ended December 31, | Operating Leases | ||
2020 Lease payments | 30,000 | ||
Less: imputed interest | (1,023 | ) | |
Total | $ | 28,977 | |
Legal
In prior periods, the Company reported a claim filed in the Superior Court of the State of Delaware regarding a former consultant of the Company who was compensated in cash and stock options for his services, demanding damages of up to approximately $3.6 million plus punitive damages in connection with a delay in such consultant’s ability and timing to exercise options and sell shares of the Company’s common stock related to past consulting services. The Company strongly denies any wrongdoing alleged in the threatened litigation and firmly believes the allegations in the complaint are entirely without merit and intends to defend against them vigorously. On October 15, 2019, the court granted the Company’s motion to dismiss and concluded the plaintiff failed to sufficiently assert claims. On May 5, 2020, the Supreme Court of the State of Delaware affirmed the dismissal granted by the Superior Court of the State of Delaware on October 15, 2019 with prejudice. As such, the Company has concluded this matter is closed.
On April 8, 2020, the Company received a summons and complaint that was filed in the Mecklenburg County Superior Court of North Carolina, regarding a vendor that alleges the Company owes approximately $1.7 million for services rendered prior to February 2019. The Company strongly denies any wrongdoing and firmly believes the allegations in the complaint are entirely without merit and intends to defend against them vigorously.
From time to time, the Company could become involved in other disputes and various litigation matters that arise in the normal course of business. These may include disputes and lawsuits related to intellectual property, licensing, contract law and employee relations matters. Periodically, the Company reviews the status of significant matters, if any exist, and assesses its potential financial exposure. If the potential loss from any claim or legal claim is considered probable and the amount can be estimated, the Company accrues a liability for the estimated loss. Legal proceedings are subject to uncertainties, and the outcomes are difficult to predict; therefore, accruals are based on the best information available at the time. As additional information becomes available, the Company reassesses the potential liability related to pending claims and litigation.
22
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Note 8: Subsequent Events
Offer to Amend and Exercise
On April 29, 2020, pursuant to the Offer to Amend and Exercise, warrants to purchase an aggregate of 12,230,418 shares of common stock were tendered, amended and exercised for aggregate gross proceeds of approximately $1.2 million. The total warrants exercised represent approximately 99% of the Company’s outstanding Original Warrants.
Agreement and Plan of Merger and Reorganization with RDD Pharma, Ltd.
On April 30, 2020, the RDD Merger was consummated in accordance with the terms of the RDD Merger Agreement, and all outstanding ordinary and preferred shares of RDD, nominal value of NIS 0.01 each, were converted into the right to receive shares of validly issued, fully paid and non-assessable shares of the Company’s common stock. Additionally, each outstanding RDD stock option was converted into and became an option exercisable for the Company’s common stock with the number and exercise price adjusted to be consistent with the merger consideration. Each outstanding RDD warrant was exercised or cancelled prior to the effective time of the RDD Merger. In connection with the RDD Merger, the Company changed its name from Innovate Biopharmaceuticals, Inc. to 9 Meters Biopharma, Inc.
As of April 30, 2020, following the completion of the RDD Merger, the pre-closing Innovate stockholders owned approximately 62.0% of the combined Company’s common stock and the former RDD shareholders owned approximately 38.0% of the combined Company’s common stock, on a fully diluted basis.
RDD Merger Financing
On April 29, 2020, the Company entered into a securities purchase agreement with various investors (the “Private Placement”) pursuant to which the Company agreed to issue and sell to the investors units consisting of one share of Series A Convertible Preferred Stock (the "Series A Preferred Stock") and one five-year warrant (the "Warrants") to purchase one share of Series A Preferred stock (the "Units"). On May 4, 2020, the Company closed the Private Placement with accredited investors pursuant to which the Company sold an aggregate of (i) 382,783 shares of Series A Convertible Preferred Stock, par value $0.0001 per share (the “Series A Preferred Stock”), which are convertible into 38,278,300 shares of common stock, and (ii) five-year warrants to purchase up to 382,783 shares of Series A Preferred Stock, which are convertible into 38,278,300 shares of common stock (the “Financing”). The exercise price of the warrants is $58.94 per share of Series A Preferred Stock, subject to adjustments as provided under the terms of the warrants. In addition, broker warrants covering 8,112 Units and broker warrants covering 10,899 shares of Series A Preferred Stock, which are convertible into 2,712,300 shares of common stock, were issued in connection with the Financing. Gross proceeds from the RDD Merger Financing were approximately $22.6 million with net proceeds of approximately $19.2 million after deducting commissions and estimated offering costs.
Naia Acquisition
On April 30, 2020, the Company entered into a two-step merger with Naia Rare Diseases, Inc. in accordance with the terms of an Agreement and Plan of Merger (the “Naia Acquisition”). The consideration for the Naia Acquisition at closing consisted of $2.1 million in cash and 4,835,438 shares of common stock, plus the pre-payment of certain operating costs on behalf of Naia totaling $0.1 million. Consideration for the Naia Acquisition also included future development and sales milestone payments worth up to $80.4 million and royalties on net sales of certain products to which Naia has exclusive rights by license.
As of May 6, 2020, following the completion of the Naia Acquisition, the pre-closing Company stockholders owned approximately 95.0% of the Company’s outstanding common stock and the former Naia shareholders owned approximately 5.0% of the Company’s outstanding common stock. As of May 13, 2020, after giving effect to the Offer to Amend and Exercise, closing of the RDD Merger and RDD Merger Financing, and Naia Acquisition, there were 96,251,342 shares of common stock outstanding and 382,783 shares of Series A Preferred Stock outstanding, which will be convertible into 38,278,300 shares of common stock. An aggregate of 10% of the shares of common stock issued to RDD was placed in escrow in accordance with an escrow agreement for a period of six months.
The issuance of the shares of common stock to the former shareholders of Naia in connection with the Merger and the related transactions did not require approval by Company stockholders. Prior to the consummation of the Merger, the shareholders of Naia approved the Merger Agreement at an extraordinary meeting of Naia shareholders.
23
9 METERS BIOPHARMA, INC.
NOTES TO UNAUDITED CONDENSED FINANCIAL STATEMENTS
Separation Agreements
In connection with the RDD Merger, the Company entered into separation agreements with the former executive officers of the Company in exchange for certain severance benefits. The Company recognized severance expense in May 2020 in connection with the former executive officers totaling $0.6 million which is being paid in monthly installments over 12 months beginning May 2020. In addition, the former executives’ received bonuses totaling $0.2 million which were paid in lump sum on May 4, 2020. The former executives will receive 12 months of COBRA supplement.
Employment Agreement
Effective upon the consummation of the RDD Merger, the Company entered into an employment agreement with Mr. Temperato for him to serve as the Company’s Chief Executive Officer (the “Employment Agreement”).
Pursuant to the Employment Agreement, Mr. Temperato began full-time employment with the Company upon the effective time of the RDD Merger on April 30, 2020, at an initial base salary of $450,000 per year, subject to review and adjustment by the Board from time to time. The Board approved an option grant to Mr. Temperato to purchase 1,000,000 shares of Common Stock, which will vest 25% upon grant, with the remainder vesting in 48 equal month installments, provided that Mr. Temperato remains an employee of the Company as of each such vesting date. Mr. Temperato will be eligible to receive a discretionary annual bonus with a target amount of 40% of his base salary, as determined by the Board in its sole discretion (and pro-rated for 2020). Mr. Temperato will also be eligible to participate in the Company’s other employee benefit plans in effect from time to time on the same basis as are generally made available to other senior executive employees of the Company.
If the employment of Mr. Temperato is terminated by the Company without “Cause” or by Mr. Temperato for “Good Reason” (each as defined in the Employment Agreement), in each case subject to Mr. Temperato entering into and not revoking a separation agreement, Mr. Temperato will be eligible to receive 12 months of his then-current base salary, the prorated amount of his target year-end bonus, and accelerated vesting of his unvested options and restricted stock unit awards that were scheduled to vest in the 12 months following termination.
Transaction Bonus
In connection with the consummation of the RDD Merger, the Board approved the grant of transaction bonuses payable to certain executive officers of the Company. The Company approved the grant of a transaction bonus for the executive’s efforts in assisting the Company to consummate the RDD Merger to each of the former Chief Executive Officer of the Company, in the amount of $212,438 and an option to purchase 389,294 shares of Common Stock, the Chief Financial Officer of the Company, in the amount of $213,750 and an option to purchase 176,156 shares of Common Stock, and the Company’s former President and Chief Business Officer, in the amount of $220,163 and an option to purchase 203,406 shares of Common Stock. Each transaction bonus option grant was immediately vested and has an exercise price of $0.60. Additionally, the Board approved a retention award for the Chief Financial Officer of an option exercisable for 125,000 shares of Common Stock, of which 25% shall be immediately vested and the remainder vests in 48 equal monthly installments, with an exercise price of $0.70.
Standstill Agreement
On April 3, 2020, the Company entered into a standstill agreement with the Convertible Noteholder (the “Standstill Agreement”). Pursuant to the Standstill Agreement, the Convertible Noteholder will not seek to redeem any portion of the Unsecured Convertible Note between April 1, 2020 and May 31, 2020. The outstanding balance of the Unsecured Convertible Note was increased by $150,000 on April 3, 2020 as consideration for the Standstill Agreement. All other terms of the Unsecured Convertible Note remain in full force and effect.
24
FORWARD-LOOKING STATEMENTS
This Quarterly Report on Form 10-Q includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). When used in this report, the words “believe,” “may,” “could,” “will,” “estimate,” “continue,” “anticipate,” “intend,” “expect,” “plan,” “indicate,” “seek,” “should,” “would” and similar expressions are intended to identify forward-looking statements, though not all forward-looking statements contain these identifying words. All statements other than statements of historical fact are statements that could be deemed forward-looking statements.
These forward-looking statements are based on our current expectations and beliefs and necessarily involve significant risks and uncertainties that may cause our actual results, performance, prospects and opportunities in the future to differ materially from those expressed or implied by such forward-looking statements. These risks and uncertainties include, among other things, risks related to our limited operating history; our need for substantial additional funding; the lengthy, expensive and uncertain nature of the clinical trials process; results of earlier studies and trials not being predictive of future trial results; our need to attract and retain senior management and key scientific personnel; our reliance on third parties; our ability to manage our growth; potential delays in commencement and completion of clinical studies; our ability to obtain and maintain effective intellectual property protection; risks associated with our merger with RDD Pharma Ltd. (the “RDD Merger”); and other risks described in greater detail in "Item 1A. Risk Factors" of the Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on March 20, 2020. These forward-looking statements are made as of the date of this Quarterly Report on Form 10-Q, and we assume no obligation to update or revise them to reflect new events or circumstances except as required by law.
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Except as otherwise noted or where the context otherwise requires, as used in this report, the words “we,” “us,” “our,” the “Company” and “9 Meters” refer to 9 Meters Biopharma, Inc. The following analysis includes historical results and periods that ended prior to the completion of the RDD Merger on April 30, 2020, and therefore only include the historical results of Innovate Biopharmaceuticals, Inc.
The following discussion of our financial condition and results of operations should be read in conjunction with our unaudited condensed financial statements and the related notes thereto included elsewhere in this Quarterly Report on Form 10-Q and our audited financial statements and related notes thereto for the year ended December 31, 2019, included in our Annual Report on Form 10-K, filed with the SEC on March 20, 2020.
Company Overview
9 Meters Biopharma, Inc.
9 Meters is a clinical-stage biopharmaceutical company focused on orphan, rare and unmet needs in gastroenterology. The Company’s pipeline includes drug candidates for celiac disease, short bowel syndrome (SBS) and two candidates for undisclosed rare and/or orphan diseases.
In 2019, we initiated a Phase 3 clinical trial for our lead drug candidate, larazotide acetate or larazotide, for the treatment of celiac disease. Larazotide has the potential to be a first-to-market therapeutic for celiac disease, an unmet medical need affecting an estimated 1% of the U.S. population or more than 3 million individuals. Patients with celiac disease have no treatment alternative other than a strict lifelong adherence to a gluten-free diet, which is difficult to maintain and can be deficient in key nutrients. In celiac disease, larazotide is the only drug which has successfully met its primary clinical efficacy endpoint with statistical significance in a Phase 2b efficacy trial, which was comprised of 342 patients. We completed the End of Phase 2 meeting with the FDA for the treatment of celiac disease with larazotide and received Fast Track designation. Larazotide has been shown to be safe and effective after being tested in several clinical trials involving nearly 600 patients, most recently in the Phase 2b trial for celiac disease. We have approximately 100 active clinical trial sites with three treatment groups, 0.25 mg of larazotide, 0.5 mg of larazotide and a placebo arm. Site activation and patient enrollment have recently been impacted by the announcement of the RDD Merger and the COVID-19 pandemic. We continue to monitor the evolving situation with COVID-19, which is likely to directly or indirectly impact the pace of enrollment over the next several months. We currently anticipate a readout from the trial in 2021.
NM-002 is a long-acting injectable GLP-1 analogue being developed for SBS. The compound links exenatide, a GLP-1 analogue to a long-acting linker technology and is designed specifically to address the gastric effects in SBS patients by slowing digestive transit time. The asset uses proprietary XTEN® technology to extend the half-life of exenatide, allowing for once-to twice-per-month dosing, thus potentially increasing convenience for patients and caregivers. NM-002 is patent-protected and has
25
received orphan drug designation by the FDA. The Company plans to start a phase 1b/2a study in 2020 in adult patients suffering from SBS. NM-003 is a proprietary long-acting GLP-2 agonist and NM-004 is a double-cleaved mesalamine with an immunomodulator. These two assets are being evaluated for development in rare and/or orphan indications. Our product development pipeline is currently positioned as described in the table below.
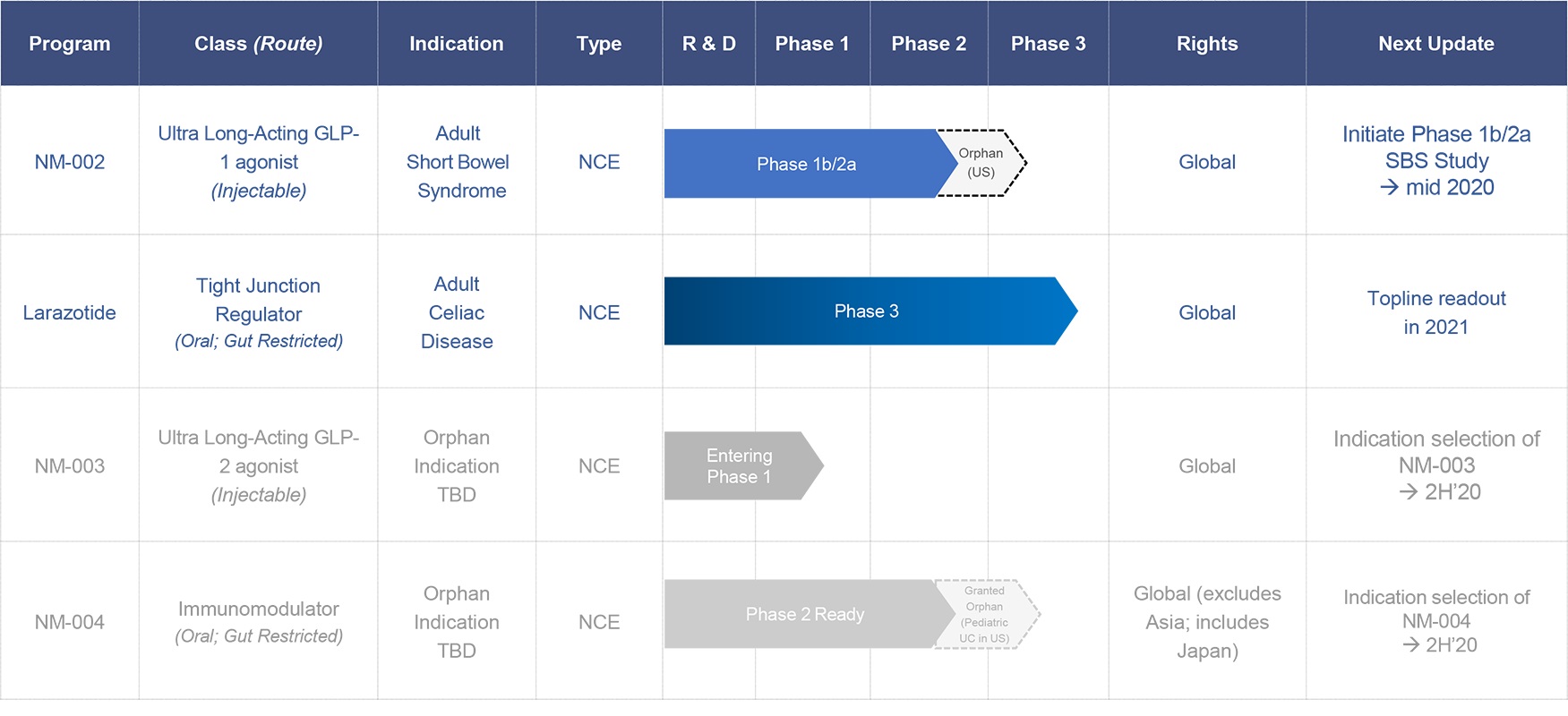
Agreement and Plan of Merger and Reorganization with RDD Pharma, Ltd.
On October 6, 2019, the Company entered into an Agreement and Plan of Merger and Reorganization pursuant to which the Company agreed to acquire all of the outstanding capital stock of privately-held RDD Pharma, Ltd., an Israel corporation (“RDD”), in exchange for common stock issued by the Company to the existing RDD shareholders (the “RDD Merger”). The RDD Merger closed on April 30, 2020. In connection with the RDD Merger, the Company changed its name from Innovate Biopharmaceuticals, Inc. to 9 Meters Biopharma, Inc. On April 30, 2020, the Board appointed John Temperato, the Chief Executive Officer of RDD, as Chief Executive Officer of the combined company, 9 Meters Biopharma, Inc. In connection with the RDD Merger, Jay P. Madan, Anthony E. Maida III, Ph.D., M.A., M.B.A., and Saira Ramasastry, M.S., M. Phil., resigned from the board and Mark Sirgo, Pharm.D., Nissim Darvish, M.D., Ph.D., and John Temperato were appointed to the Board.
On May 4, 2020, 9 Meters closed a private placement with accredited investors pursuant to which the Company sold an aggregate of (i) 382,783 shares of Series A Convertible Preferred Stock, par value $0.0001 per share (the “Series A Preferred Stock”), which are convertible into 38,278,300 shares of common stock, and (ii) five-year warrants to purchase up to 382,783 shares of Series A Preferred Stock, which are convertible into 38,278,300 shares of common stock (the “Financing”). The exercise price of the warrants is $58.94 per share of Series A Preferred Stock, subject to adjustments as provided under the terms of the warrants. In addition, broker warrants covering 8,112 units and 10,899 shares of Series A Preferred Stock, which are convertible into 2,712,300 shares of common stock, were issued in connection with the Financing. Gross proceeds from the RDD Merger Financing were approximately $22.6 million with net proceeds of approximately $19.2 million after deducting commission and offering costs.
In connection with the RDD Merger Financing, on April 29, 2020, the Company filed a Certificate of Designation of Preferences, Rights and Limitations of the Series A Convertible Preferred Stock with the Secretary of State of the State of Delaware creating a new series of authorized preferred stock of the Company designated as the “Series A Convertible Preferred Stock”.
Financial Overview
Since our inception, we have focused our efforts and resources on identifying and developing our research and development programs. We have not had any products approved for commercial sale and have incurred operating losses in each year since inception. Substantially all of our operating losses resulted from expenses incurred in connection with our research and development programs and from general and administrative costs associated with our operations.
26
As of March 31, 2020, we had an accumulated deficit of $74.2 million. We incurred net losses of $3.6 million and $4.4 million for the three months ended March 31, 2020 and 2019, respectively. We expect to continue to incur significant expenses and increase our operating losses for the foreseeable future, which may fluctuate significantly between periods. We anticipate that our expenses will increase substantially as and to the extent we:
•continue research and development, including preclinical and clinical development of our existing and future product candidates, including larazotide;
•complete integration of operations and personnel associated with the RDD Merger;
•potentially seek regulatory approval for our product candidates;
•commercialize any product candidates for which we obtain regulatory approval;
•maintain and protect our intellectual property rights;
•add operational, financial and management information systems and personnel; and
•continue to incur additional legal, accounting, regulatory, tax-related and other expenses required to operate as a public company.
As such, we will need substantial additional funding to support our operating activities. Adequate funding may not be available to us on acceptable terms, or at all. We currently anticipate that we will seek to fund our operations through equity or debt financings, strategic alliances or licensing arrangements, or other sources of financing. Our failure to obtain sufficient funds on acceptable terms could have a material adverse effect on our business, results of operations and financial condition.
Other Recent Developments
The effect of the COVID-19 pandemic and its associated restrictions may increase the anticipated aggregate costs for the development of our product candidates and may adversely impact the anticipated timelines for the development of our product candidates by, among other things, causing disruptions in the supply chain for clinical supplies, delays in the timing and pace of subject enrollment in clinical trials and lower than anticipated subject enrollment and completion rates, delays in the review and approval of our regulatory submissions by the FDA and other agencies with respect to our product candidates, and other unforeseen disruptions. The economic impact of the COVID-19 pandemic and its effect on capital markets and investor sentiment may adversely impact our ability to raise capital when needed or on terms favorable to us and our stockholders to fund our development programs and operations. We do not yet know the full extent of potential delays or impacts on our business, clinical trial activities, ability to access capital or on healthcare systems or the global economy as a whole. However, these effects could have a material adverse impact on our business and financial condition.
Naia Acquisition
On May 6, 2020, the Company entered into and consummated a two-step merger with Naia Rare Diseases, Inc. in accordance with the terms of an Agreement and Plan of Merger (the “Naia Acquisition”). The consideration for the Naia Acquisition at closing consisted of $2.1 million in cash and 4,835,438 shares of common stock, plus the pre-payment of certain operating costs on behalf of Naia totaling $0.1 million. Consideration for the Naia Acquisition also included potential future development and sales milestone payments worth up to $80.4 million and royalties on net sales of certain products to which Naia has exclusive rights by license.
Warrant Exchange
Pursuant to a purchase agreement dated April 29, 2019, we issued warrants to purchase up to 4,318,272 shares of our common stock (the “April Warrants”) and granted the placement agent warrants to purchase up to 215,914 shares of common stock (the “Placement Agent Warrants”). On December 19, 2019, we entered into separate exchange agreements with each of the purchasers of the April Warrants and the Placement Agent Warrants (the “Exchange Agreements”). Pursuant to the Exchange Agreements, we agreed to issue to the purchasers an aggregate of 5,441,023 shares of our common stock (the “Exchange Shares”) at a ratio of 1.2 Exchange Shares for each purchaser warrant in exchange for the cancellation and termination of all of the outstanding April Warrants and Placement Agent Warrants. On December 26, 2019, we issued 3,593,714 Exchange Shares in exchange for the cancellation and termination of Exchange Warrants to purchase 2,994,762 shares of common stock. During the three months ended March 31, 2020, we issued 1,847,309 Exchange Shares in exchange for the cancellation and termination of the remaining outstanding Exchange Warrants.
27
Warrant Extension and Offer to Amend and Exercise
Effective February 6, 2020, we entered into amendments with the holders of our outstanding short-term warrants originally issued March 18, 2019 (the “Short-Term Warrants”) to extend the exercise period of each Short-Term Warrant by six months. The Short-Term Warrants, as amended, are exercisable for up to an aggregate of 4,181,068 shares of our common stock, par value $0.0001 per share, until September 18, 2020. Except as specifically amended, the terms and conditions of each Short-Term Warrant remained in full force and effect and were not affected by this amendment. See “Note 1—Summary of Significant Accounting Policies” to the accompanying financial statements included in this Quarterly Report on Form 10-Q for additional terms of the Short-Term Warrants.
On February 12, 2020, we offered to amend outstanding warrants to purchase an aggregate of 12,346,631 shares of common stock (the “Original Warrants”) held by holders of certain outstanding warrants (the “Offer to Amend and Exercise”). The Original Warrants of eligible holders who elect to participate in the Offer to Amend and Exercise were amended to (i) shorten the exercise period so that they expired concurrently with the closing of the RDD Merger on April 30, 2020 and (ii) reduced the exercise price to $0.10 per share. The amended warrants were required to be exercised for cash, and any cashless exercise provisions in the Original Warrants were omitted. On April 29, 2020, warrants to purchase 12,230,418 shares of common stock were exercised in the Offer to Amend and Exercise for aggregate gross proceeds of approximately $1.2 million.
Amendment to the 2012 Omnibus Incentive Plan
On December 4, 2018, our stockholders approved an amendment to the 2012 Omnibus Incentive Plan (the “Omnibus Plan”) to provide for an additional 3,150,000 shares of common stock to be issued pursuant to the plan and an evergreen provision to automatically increase the number of shares issuable pursuant to the plan on an annual basis for the period commencing January 1, 2019 and ending on January 1, 2022. The plan will automatically terminate on April 30, 2022. Pursuant to the evergreen provision, on January 1, 2020 and 2019, the number of shares of common stock available under the Omnibus Plan automatically increased by 1,973,883 and 1,304,441 shares, respectively.
Research and Development Updates
During 2019, we dosed the first patient in our Phase 3 clinical trial for larazotide in adult patients with celiac disease. We have initiated over 100 clinical trial sites, and we are targeting 630 subjects in the first Phase 3 clinical trial, in three parallel treatment groups, 0.25 mg of larazotide tid, 0.5 mg of larazotide tid and a placebo arm. We currently anticipate a readout from the trial in 2021. In May 2020, we received a thorough QT (TQT) study waiver from the FDA for the Phase 3 trial of larazotide in celiac disease. The waiver supports larazotide’s strong precedent of safety and could potentially streamline the program’s timeline and cost effectiveness.
Recent research and development milestones include:
• | continued research collaboration with Institut Gustave Roussy to study regulation of intestinal permeability and the gut microbiota using larazotide in immuno-oncology checkpoint inhibitor failure preclinical models; |
• | continued research collaboration with Dr. Anthony Blikslager of North Carolina State University to explore life-cycle extension of our lead molecule larazotide acetate; |
• | initiated research collaboration with Dr. Younggeon Jin of University of Maryland, College Park, to understand tight junction biology; and |
• | continued research collaboration with Dr. James Nataro of the University of Virginia, Charlottesville to study larazotide’s effect on Environmental Enteric Dysfunction. |
Critical Accounting Policies and Use of Estimates
Use of Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of our financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other assumptions that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of
28
assets and liabilities that are not readily apparent from other sources. Changes in estimates are reflected in reported results for the period in which they become known. Actual results may differ materially from these estimates under different assumptions or conditions.
Critical Accounting Policies
Areas of the financial statements where estimates may have the most significant effect include fair value measurements, accrued expenses, share-based compensation, income taxes and management’s assessment of our ability to continue as a going concern. Changes in the facts or circumstances underlying these estimates could result in material changes and actual results could differ from these estimates. There have been no material changes to our critical accounting policies described in "Critical Accounting Policies and Use of Estimates" of the Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on March 20, 2020.
Recently Issued Accounting Pronouncements
For details of recent Accounting Standards Updates and our evaluation of their adoption on our condensed financial statements, see “Note 1—Summary of Significant Accounting Policies—Recent Accounting Pronouncements” to our condensed financial statements in "Part I. Financial Information - Item I. Financial Statements" included elsewhere in this Quarterly Report on Form 10-Q.
Results of Operations
Comparison of the Three Months Ended March 31, 2020 and 2019
The following table sets forth the key components of our results of operations for the three months ended March 31, 2020 and 2019:
Three Months Ended March 31, | |||||||||||||||
2020 | 2019 | $ Change | % Change | ||||||||||||
Operating expenses: | |||||||||||||||
Research and development | $ | 2,598,985 | $ | 1,198,315 | $ | 1,400,670 | 117 | % | |||||||
General and administrative | 1,669,807 | 3,114,495 | (1,444,688 | ) | (46 | )% | |||||||||
Warrant inducement expense | 690,839 | — | 690,839 | 100 | % | ||||||||||
Total operating expenses | 4,959,631 | 4,312,810 | 646,821 | 15 | % | ||||||||||
Loss from operations | (4,959,631 | ) | (4,312,810 | ) | (646,821 | ) | (15 | )% | |||||||
Total other income (expense), net | 1,356,630 | (121,955 | ) | 1,478,585 | 1,212 | % | |||||||||
Net loss | $ | (3,603,001 | ) | $ | (4,434,765 | ) | $ | 831,764 | 19 | % | |||||
Research and Development Expense
Research and development expense for the three months ended March 31, 2020, increased approximately $1.4 million, or 117%, as compared to the three months ended March 31, 2019. The increase of approximately $1.4 million was driven primarily by progress in our Phase 3 clinical trial in celiac disease during the three months ended March 31, 2020, including the addition of clinical trial consultants to manage the trial.
General and Administrative Expense
General and administrative expense for the three months ended March 31, 2020, decreased approximately $1.4 million, or 46%, as compared to the three months ended March 31, 2019. The decrease was primarily due to a decrease in compensation and personnel benefits of approximately $0.4 million and a decrease in costs associated with operating as a public company of approximately $0.4 million. In addition, non-cash share-based compensation expense decreased by approximately $0.3 million primarily due to restricted stock units issued during the three months ended March 31, 2019 that vested immediately. There were no new equity awards issued during the three months ended March 31, 2020. Business development, patent protection of our
29
intellectual property and other general corporate costs decreased by approximately $0.2 million and professional fees decreased by approximately $0.1 million.
Warrant Inducement Expense
During the three months ended March 31, 2020, we recognized warrant inducement expense of approximately $0.7 million. There was no warrant inducement expense during the three months ended March 31, 2019. The warrant inducement expense represents the accounting fair value of consideration issued to induce conversion of the April Warrants exchanged for 1.2 shares of our common stock per warrant, further described in “Note 1-Summary of Significant Accounting Policies” to the accompanying financial statements included in this Quarterly Report on Form 10-Q.
Other Income (Expense), Net
Other income (expense), net for the three months ended March 31, 2020, changed by approximately $1.5 million, or 1,212%, as compared to the three months ended March 31, 2019. Other expense decreased by approximately $1.3 million due to a one-time expense incurred for the extinguishment of debt during the three months ended March 31, 2019, including the option payment further described in Note 3—Debt. In addition, other income decreased by approximately $0.1 million due to the non-cash change in the fair value of derivative liabilities. These changes were offset by an increase of approximately $0.4 million in interest expense primarily due to the Additional Note issued in January 2020 and a full quarter of expense for the Unsecured Convertible Note issued in March 2019. The non-cash change in fair value of warrant liabilities increased approximately $0.7 million.
Liquidity and Capital Resources
Sources of Liquidity
As of March 31, 2020, we had cash and cash equivalents of approximately $2.7 million, compared to approximately $4.6 million as of December 31, 2019. The decrease in cash was primarily due to expenditures for business operations, research and development and clinical trial costs. These expenses were offset by the issuance of the Additional Note further described below.
On April 29, 2020, we entered into a securities purchase agreement with various investors (the “Private Placement”) pursuant to which we agreed to issue and sell to the investors units consisting of one share of Series A Convertible Preferred Stock (the "Series A Preferred Stock") and one five-year warrant (the "Warrants") to purchase one share of Series A Preferred stock (the "Units"). On May 4, 2020, upon closing of the RDD Merger Financing, we received net proceeds of $19.2 million after deducting placement agent fees and other offering expenses. We used $2.1 million to fund the Naia Merger and we plan to use the remaining funds to progress our current pipeline, including the ongoing Phase 3 clinical trial in celiac disease and initiation of a phase 1b/2a trial in SBS. We expect to incur substantial expenditures in the foreseeable future for the continued development and clinical trials of our product candidates. We will continue to require additional financing to develop our product candidates and fund operations for the foreseeable future. We plan to seek funds through debt or equity financings, strategic alliances and licensing arrangements, and other collaborations or sources of financing.
There can be no assurance that we will be able to raise the additional capital needed to continue our pipeline of research and development programs on terms acceptable to us, on a timely basis or at all. If we are unable to raise additional funds when needed, our ability to develop our product candidates will be impaired. We may also be required to delay, reduce or terminate some or all of our development programs and clinical trials.
Standstill Agreement
In order to preserve cash until completion of the RDD Merger, we entered into a standstill agreement on April 3, 2020, with the Convertible Noteholder (the “Standstill Agreement”). Pursuant to the Standstill Agreement, the Convertible Noteholder will not seek to redeem any portion of the Unsecured Convertible Note between April 1, 2020 and May 31, 2020. The outstanding balance of the Unsecured Convertible Note was increased by $150,000 as partial consideration for the Standstill Agreement. All other terms of the Unsecured Convertible Note remain in full force and effect.
Additional Note
On January 10, 2020, we entered into an additional securities purchase agreement and unsecured convertible promissory note with the Convertible Noteholder in the principal amount of $2,750,000 (the “Additional Note”). The Convertible Noteholder may elect to convert all or a portion of the Additional Note, at any time from time to time into our common stock at a conversion price
30
of $3.25 per share, subject to adjustment for stock splits, dividends, combinations and similar events. We may prepay all or a portion of the Additional Note at any time for an amount equal to 115% of then outstanding obligations or the portion of the obligations we are prepaying. The purchase price of the Additional Note was $2.5 million and carries an original issuance discount of $250,000, which is included in the principal amount of the Additional Note.
The Additional Note bears interest at the rate of 10% (which will increase to 18% upon and during the continuance of an event of default) per annum, compounding on a daily basis. All principal and accrued interest on the Additional Note is due on the second anniversary of the date of the Additional Note’s issuance.
At any time after the six-month anniversary of the issuance of the Additional Note, (i) if the average VWAP of our common stock over twenty trading dates exceeds $10.00 per share, we may generally require that the Additional Note convert into share of its common stock at the $3.25 (as adjusted) conversion rate or (ii) 80% of the average of the five lowest VWAP of our common stock over the preceding twenty trading days. The Convertible Noteholder may not redeem more than $500,000 per calendar month during the period between the six-month anniversary of the date of issuance until the first anniversary of the date of issuance and $750,000 per calendar month thereafter. Our obligation or right to deliver our shares upon the conversion or redemption of the Additional Note is subject to a 19.99% cap and subject to a floor price of $3.25 (unless waived by us). Any amounts redeemed or converted once the cap is reached or if the market price is less than the $3.25 floor price must be paid in cash.
If there is an Event of Default under the Additional Note, the Convertible Noteholder may accelerate our obligations or the Convertible Noteholder may elect to increase the outstanding obligations under the Additional Note. The amount of the increase ranges from 15% for certain “Major Defaults,” 10% for failure to obtain the Convertible Noteholder’s approval for certain equity issuances with anti-dilution, price reset or variable pricing features of less than $2,500,000, and 5% for certain “Minor Defaults.” In addition, the Additional Note obligations will be increased if there are delays in our delivery requirements for the shares or cash issuable upon the conversion or redemption of the Additional Note in certain circumstances.
If we issue convertible debt in the future with any terms, including conversion terms, that are more favorable to the terms of the Additional Note, the Convertible Noteholder may elect to incorporate the more favorable terms into the Additional Note. For further details regarding our current debt obligations, see “Note 3—Debt.”
Warrant Tender
On February 12, 2020, we offered to amend the Original Warrants to purchase an aggregate of 12,346,631 shares of common stock held by holders of certain outstanding warrants. The Original Warrants of eligible holders who elect to participate in the Offer to Amend and Exercise were amended to (i) shorten the exercise period to expire concurrently with the closing of the RDD Merger on April 30, 2020 and (ii) reduced the exercise price to $0.10 per share. The amended warrants are required to be exercised for cash, and any cashless exercise provisions in the Original Warrants have been omitted. On April 29, 2020, warrants to purchase 12,230,418 shares of common stock were exercised for aggregate gross proceeds of approximately $1.2 million.
Cash Flows
The following table sets forth the primary sources and uses of cash for the three months ended March 31, 2020 and 2019:
Three Months Ended March 31, | ||||||||
2020 | 2019 | |||||||
Net cash (used in) provided by: | ||||||||
Operating activities | $ | (2,632,385 | ) | $ | (4,237,746 | ) | ||
Investing activities | — | — | ||||||
Financing activities | 707,196 | 10,003,740 | ||||||
Net (decrease) increase in cash and cash equivalents | $ | (1,925,189 | ) | $ | 5,765,994 | |||
31
Operating Activities
For the three months ended March 31, 2020, our net cash used in operating activities of approximately $2.6 million primarily consisted of a net loss of $3.6 million, a non-cash gain of $1.6 million for the change in the fair value of the warrant liabilities and a non-cash gain of $0.3 million for the change in fair value of the convertible note derivative liabilities. These decreases were offset by adjustments for non-cash warrant inducement expense of $0.7 million, non-cash share-based compensation of approximately $0.3 million, non-cash interest expense of approximately $0.5 million and the net change in operating assets and liabilities of $1.4 million.
For the three months ended March 31, 2019, our net cash used in operating activities of approximately $4.2 million primarily consisted of a net loss of $4.4 million, a non-cash gain of $1.3 million for the extinguishment of the Senior Convertible Note derivative liability and changes in the fair value of the warrant liabilities and the Unsecured Convertible Note derivative liability and the net change in assets and liabilities of $0.2 million. These decreases were offset by adjustments for non-cash share-based compensation of approximately $0.5 million, a non-cash loss of $1.0 million on the extinguishment of debt and non-cash interest expense of approximately $0.1 million.
Investing Activities
We had no cash flows from investing activities for the three months ended March 31, 2020 or 2019.
Financing Activities
For the three months ended March 31, 2020, net cash provided by financing activities of approximately $0.7 million primarily consisted of the proceeds of $2.5 million from the issuance of the Additional Note. These increases were offset by approximately $1.5 million in debt repayments and $0.3 million in stock issuance costs associated with the Warrant Exchange.
For the three months ended March 31, 2019, net cash provided by financing activities of approximately $10.0 million primarily consisted of the proceeds of $11.5 million received from the sale of our common stock and warrants and $5.0 million from the issuance of the Unsecured Convertible Note. These increases were offset by approximately $6.2 million in debt repayments, $0.2 million in stock issuance costs and $0.1 million in debt issuance costs.
Capital Requirements
We have not generated any revenue from product sales or any other activities. We do not expect to generate significant revenue unless and until we obtain regulatory approval of and commercialize, or out-license, one or more of our product candidates and do not know when, or if, these will occur. In addition, we expect our expenses to increase in connection with our ongoing development activities, particularly as we continue the research, development and clinical trials of, and seek regulatory approval for, our product candidates. In addition, subject to obtaining regulatory approval of our product candidates, we expect to incur significant commercialization expenses for product sales, marketing, manufacturing and distribution. We anticipate that we will need substantial additional funding in connection with our continuing operations, including costs associated with being a public company and integrating the operations of RDD.
The accompanying financial statements have been prepared on a basis which assumes that we will continue as a going concern, which contemplates the realization of assets and the satisfaction of liabilities and commitments in the normal course of business. Based on our limited operating history and recurring operating losses, there is substantial doubt that we will continue as a going concern for at least one year following the date of this Quarterly Report on Form 10-Q, without additional financing. Management’s plans with regard to these matters include entering into strategic partnerships or seeking additional debt or equity financing arrangements or a combination of these activities. The failure to obtain sufficient financing or strategic partnerships could adversely affect our ability to achieve our business objectives and continue as a going concern. The accompanying financial statements do not include any adjustments that might be necessary should we be unable to continue as a going concern.
Contractual Obligations and Commitments
In October 2017, we entered into a three-year lease agreement for office space that expires on September 30, 2020 and includes a two-year renewal option. Base annual rent is $60,000. Monthly rent payments of $5,000 are due in advance of the first day of each month for the 24-month term. A security deposit of approximately $5,000 was paid in October 2017 and is included in other assets on the accompanying condensed balance sheets included in the condensed financial statements to this Quarterly Report on Form 10-Q.
32
We estimated the present value of the lease payments over the remaining term of the lease using a discount rate of 12%, which represented our estimated incremental borrowing rate. The two-year renewal option was excluded from the lease payments as we concluded the exercise of this option was not considered reasonably certain. See “Note 7—Commitments and Contingencies” in the accompanying financial statement included in this Quarterly Report on Form 10-Q for further details regarding accounting treatment of our lease agreement.
In February 2019, the Company entered into a separation and general release agreement with a former executive that included separation benefits consistent with the former executive’s employment agreement. We recognized severance expense totaling $0.3 million during the three months ended March 31, 2019 that is being paid in equal installments over 12 months beginning February 2019. The accrued severance obligation in respect of the former executive was fully paid as of March 31, 2020 and there were no accrued severance obligations outstanding as of March 31, 2020.
On April 30, 2020, in connection with the RDD Merger, we entered into separation agreements with our former executive officers of Innovate in exchange for certain severance benefits. We recognized severance expense in connection with the former executive officers totaling $0.6 million which is being paid in monthly installments over 12 months beginning May 2020.
We are obligated to make future payments to third parties under in-license agreements, including sublicense fees, royalties, and payments that become due and payable on the achievement of certain development and commercialization milestones. As the amount and timing of sub-license fees and the achievement and timing of these milestones are not probable and estimable, such commitments have not been included on the accompanying condensed balance sheets.
We also enter into agreements in the normal course of business with contract research organizations and other third parties with respect to services for clinical trials, clinical supply manufacturing and other operating purposes that are generally terminable by us with thirty to ninety days advance notice.
For further details, see “Note 7—Commitments and Contingencies” in the accompanying financial statements included in this Quarterly Report on Form 10-Q.
Off-Balance Sheet Arrangements
As of March 31, 2020, we had no off-balance sheet arrangements as defined in Item 303(a)(4) of Regulation S-K as promulgated by the SEC.
Item 3. Quantitative and Qualitative Disclosures About Market Risk
Not applicable.
Item 4. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our principal executive officer and principal financial officer, evaluated the effectiveness of our disclosure controls and procedures as of March 31, 2020. The term “disclosure controls and procedures,” as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act, means controls and other procedures of a company that are designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate, to allow timely decisions regarding required disclosure. Management identified a material weakness in internal control over financial reporting in connection with our audited financial statements for the year ended December 31, 2018, due to our inability to adequately segregate duties as a result of our limited number of accounting personnel. In an effort to remediate this material weakness during the year ended December 31, 2019, we added two full-time finance positions, a Chief Financial Officer who is serving as Principal Financial Officer and Principal Accounting Officer and a Controller. During the year ended December 31, 2019, we also enhanced our system of internal controls, including improving our segregation of duties. However, management concluded that as of March 31, 2020, our internal controls over financial reporting are ineffective.
Although we are committed to continuing to improve our internal control processes and intend to implement a plan to remediate our material weakness after completion of the RDD Merger, we cannot be certain of the effectiveness of such plan or that, in the future, additional material weaknesses or significant deficiencies will not exist or otherwise be discovered.
Changes in Internal Control Over Financial Reporting
As of March 31, 2020, there were no material changes in our internal control over financial reporting that materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
33
PART II -OTHER INFORMATION
Item 1. Legal Proceedings
Previously, we reported a claim filed in the Superior Court of the State of Delaware regarding a former consultant of the Company who was compensated in cash and stock options for his services, demanding damages of up to approximately $3.6 million plus punitive damages in connection with a delay in such consultant’s ability and timing to exercise options and sell shares of our common stock related to past consulting services. On October 15, 2019, the court granted our motion to dismiss and concluded the plaintiff failed to sufficiently assert claims. On May 5, 2020, the Supreme Court of the State of Delaware affirmed the dismissal granted by the Superior Court of the State of Delaware on October 15, 2019 with prejudice. As such, we have concluded this matter is closed.
On April 8, 2020, we received a summons and complaint that was filed in the Mecklenburg County Superior Court of North Carolina, regarding a vendor that alleges the Company owes approximately $1.7 million for services rendered prior to February 2019.
We strongly deny any wrongdoing and firmly believe the allegations in each complaint are entirely without merit and intend to defend against them vigorously.
Other than as described above, we are not currently a party to any legal or governmental regulatory proceedings, nor is our management aware of any pending or threatened legal or government regulatory proceedings proposed to be initiated against us that would have a material adverse effect on our business, financial condition or operating results.
Item 1A. Risk Factors.
There have been no material changes to the risk factors disclosed in "Item 1A. Risk Factors" of our Annual Report on Form 10-K for the year ended December 31, 2019, filed with the SEC on March 20, 2020, except that we have closed the RDD Merger, RDD Merger Financing and the Naia Acquisition.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds
None.
Item 3. Defaults Upon Senior Securities
Not applicable.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Other Information
On May 14, 2020, Roy Proujansky, M.D. notified the Company that he would not stand for re-election on the Board of Directors (the “Board”) and would therefore resign effective as of the date of the 2020 stockholder meeting, which is currently anticipated to be June 30, 2020. The resignation from the Board was not due to a disagreement with the Company on any matter relating to its operations, policies or practices.
On December 4, 2019, the Company received a notice from the staff of Nasdaq Market LLC (the “Staff”) it had failed to maintain a minimum market value of $35 million over the previous 30 consecutive business days as required by Nasdaq Listing Rule 5550(b)(2) (the “Rule”). On May 14, 2020, the Company received notice from the Staff that the Company had regained compliance with the Rule and this matter is now closed.
34
Item 6.
FILED | INCORPORATED BY REFERENCE | |||||||||
EXHIBIT NO. | DESCRIPTION | HEREWITH | FORM | EXHIBIT | FILING DATE | |||||
4.1 | 8-K | 4.1 | January 10, 2020 | |||||||
4.2 | 8-K | 4.1 | February 12, 2020 | |||||||
10.1 | 8-K | 10.1 | January 10, 2020 | |||||||
31.1 | X | |||||||||
31.2 | X | |||||||||
32.1 | X | |||||||||
32.2 | X | |||||||||
101.INS | XBRL Instance Document | X | ||||||||
101.SCH | XBRL Taxonomy Extension Schema Document | X | ||||||||
101.CAL | XBRL Taxonomy Extension Calculation Linkbase Document | X | ||||||||
101.DEF | XBRL Taxonomy Extension Definition Document | X | ||||||||
101.LAB | XBRL Taxonomy Extension Label Linkbase Document | X | ||||||||
101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document | X | ||||||||
35
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
9 METERS BIOPHARMA, INC. | |||
a Delaware corporation | |||
Date: | May 15, 2020 | By: | /s/ Edward J. Sitar |
Edward J. Sitar | |||
Chief Financial Officer (Principal Financial and Principal Accounting Officer) | |||
36
