Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Axsome Therapeutics, Inc. | ex-99d1.htm |
| 8-K - 8-K - Axsome Therapeutics, Inc. | f8-k.htm |
Exhibit 99.2
|
|
NASDAQ: AXSM INTERCEPT Phase 3 Trial of AXS-07 Topline Results Conference Call in Migraine April 6, 2020 © Axsome Therapeutics, Inc. |
|
|
Overview AXS-07 in Migraine Acute Treatment INTERCEPT Phase 3 Trial Topline Results Clinical Development & Medical Affairs Dave Marek, Chief Commercial Officer © Axsome Therapeutics, Inc. 2 Introduction Mark Jacobson, Chief Operating Officer Overview and Summary Herriot Tabuteau, MD, Chief Executive Officer INTERCEPT Trial Design & Results Cedric O’Gorman, MD, Senior Vice President, Q&A Presenters, Nick Pizzie, Chief Financial Officer and Concluding Remarks Herriot Tabuteau, MD, Chief Executive Officer |
|
|
FLS Forward-Looking Statements & Safe Harbor Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials and the number or type of studies or nature of results necessary to support the filing of a new drug application for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates (including, but not limited to, FDA’s agreement with the Company’s plan to discontinue the bupropion treatment arm of the ADVANCE-1 study in accordance with the independent data monitoring committee’s recommendations); the Company’s ability to obtain additional capital necessary to fund its operations; the Company’s ability to generate revenues in the future; the potential for the MOMENTUM clinical trial to provide a basis for approval of AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment; the potential for the ASCEND clinical trial, combined with the GEMINI clinical trial results, to provide a basis for approval of AXS-05 for the treatment of major depressive disorder and accelerate its development timeline and commercial path to patients; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the enforceability of the Company’s license agreements; the acceptance by the market of the Company’s product candidates, if approved; the Company’s anticipated capital requirements, including the Company’s anticipated cash runway; unforeseen circumstances or other disruptions to normal business operations arising from or related to COVID-19; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. The data disclosed in this presentation are considered topline data and subject to further statistical review and the final results may vary. © Axsome Therapeutics, Inc. 3 |
|
|
Overview and Summary Herriot Tabuteau, MD CHIEF EXECUTIVE OFFICER AXSOME THERAPEUTICS, INC. © Axsome Therapeutics, Inc. 4 |
|
|
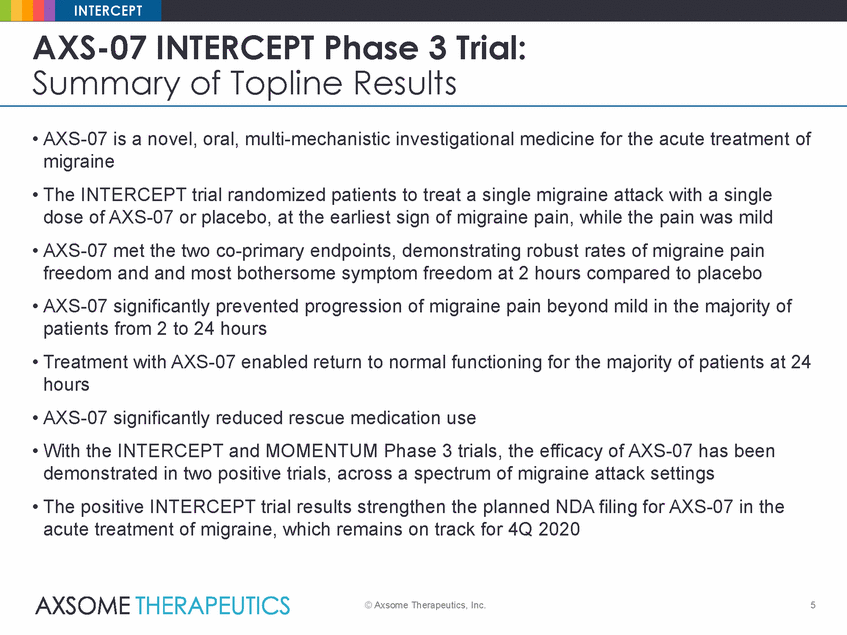
INTERCEPT AXS-07 INTERCEPT Phase 3 Trial: Summary of Topline Results • AXS-07 is a novel, oral, multi-mechanistic investigational medicine for the acute treatment of migraine • The INTERCEPT trial randomized patients to treat a single migraine attack with a single dose of AXS-07 or placebo, at the earliest sign of migraine pain, while the pain was mild • AXS-07 met the two co-primary endpoints, demonstrating robust rates of migraine pain freedom and and most bothersome symptom freedom at 2 hours compared to placebo • AXS-07 significantly prevented progression of migraine pain beyond mild in the majority of patients from 2 to 24 hours • Treatment with AXS-07 enabled return to normal functioning for the majority of patients at 24 hours • AXS-07 significantly reduced rescue medication use • With the INTERCEPT and MOMENTUM Phase 3 trials, the efficacy of AXS-07 has been demonstrated in two positive trials, across a spectrum of migraine attack settings • The positive INTERCEPT trial results strengthen the planned NDA filing for AXS-07 in the acute treatment of migraine, which remains on track for 4Q 2020 © Axsome Therapeutics, Inc. 5 |
|
|
INTERCEPT Migraine: Disabling Disease in Need of New Treatments • The World Health Organization classifies severe migraine attacks as among the most disabling illnesses, comparable to dementia, quadriplegia and active psychosis1,2 • Debilitating pain, and the often-constant fear of the next migraine attack, damage family life, social life and employment3 • Depression and anxiety are twice as common in people with migraine than in healthy individuals4 • Widespread misperception of the seriousness of migraine contributes to its under-recognition and under-treatment3 • Migraine treatment guidelines encourage rapid early treatment of migraines to limit reoccurance5 There is an urgent need for new treatments that provide improved efficacy for this serious neurological disease 1Menken et al. Arch Neurol. 2000;57:418-420. 2Shapiro and Goadsby. Cephalalgia. 2007;27:991-4. 3Global Burden of Disease Study. Lancet. 2017;390:1211-1259 4Antonaci et al. J Headache Pain. 2011;12:115–125. 5 Silberstein. Neurology. 2000 Sep 26;55(6):754-62. © Axsome Therapeutics, Inc. 6 |
|
|
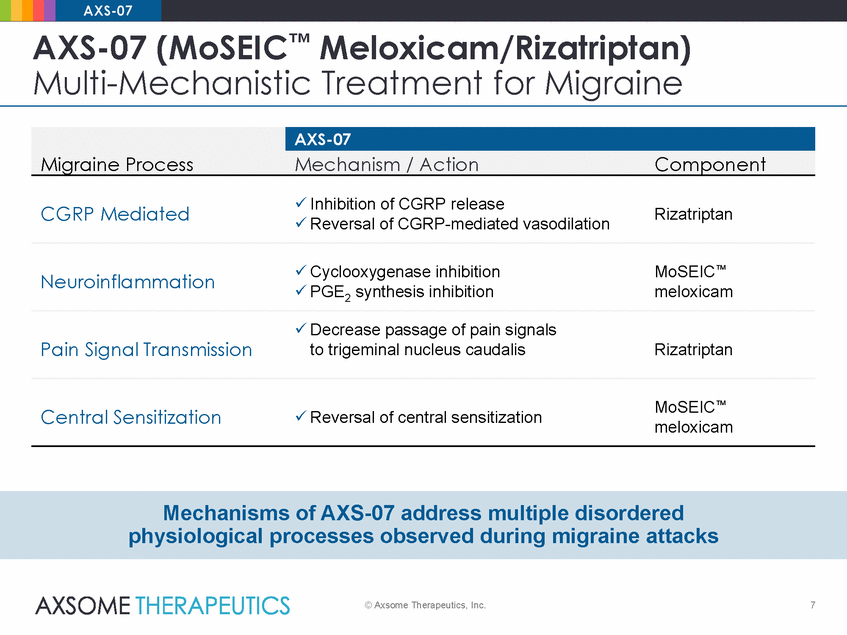
AXS-07 AXS-07 (MoSEIC™ Meloxicam/Rizatriptan) Multi-Mechanistic Treatment for Migraine Inhibition of CGRP release Reversal of CGRP-mediated vasodilation CGRP Mediated Rizatriptan Cyclooxygenase inhibition PGE2 synthesis inhibition MoSEIC™ meloxicam Neuroinflammation Decrease passage of pain signals to trigeminal nucleus caudalis Pain Signal Transmission Rizatriptan MoSEIC™ meloxicam Central Sensitization Reversal of central sensitization Mechanisms of AXS-07 address multiple disordered physiological processes observed during migraine attacks © Axsome Therapeutics, Inc. 7 Migraine Process AXS-07 Mechanism / ActionComponent |
|
|
INTERCEPT Phase 3 Trial Design & Results Cedric O’Gorman MD, MBA SENIOR VICE PRESIDENT, CLINICAL DEVELOPMENT AND MEDICAL AFFAIRS AXSOME THERAPEUTICS, INC. © Axsome Therapeutics, Inc. 8 |
|
|
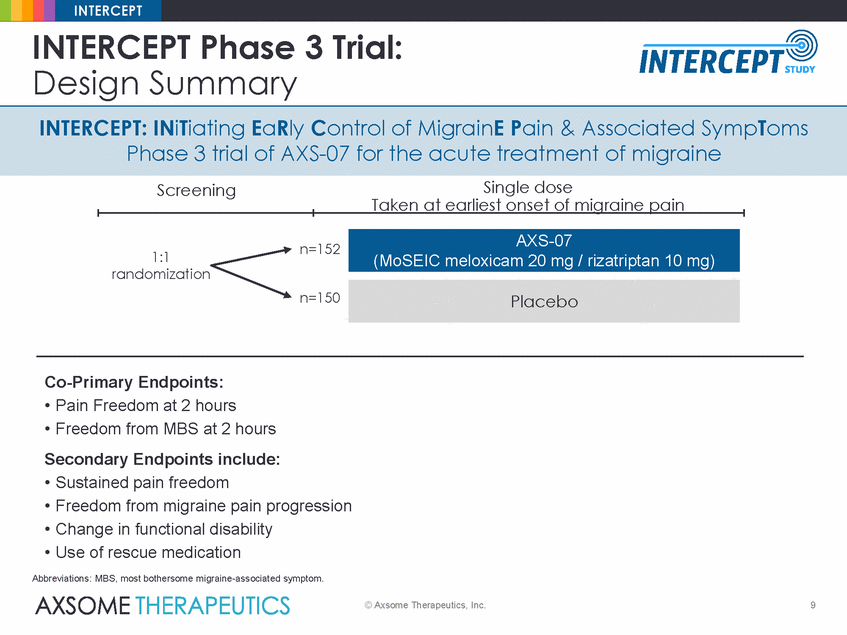
INTERCEPT INTERCEPT Phase 3 Trial: Design Summary INTERCEPT: INiTiating EaRly Control of MigrainE Pain & Associated SympToms Phase 3 trial of AXS-07 for the acute treatment of migraine Single dose Taken at earliest onset of migraine pain Screening n=152 1:1 randomization n=150 Co-Primary Endpoints: • Pain Freedom at 2 hours • Freedom from MBS at 2 hours Secondary Endpoints include: • Sustained pain freedom • Freedom from migraine pain progression • Change in functional disability • Use of rescue medication Abbreviations: MBS, most bothersome migraine-associated symptom. © Axsome Therapeutics, Inc. 9 Placebo AXS-07 (MoSEIC meloxicam 20 mg / rizatriptan 10 mg) |
|
|
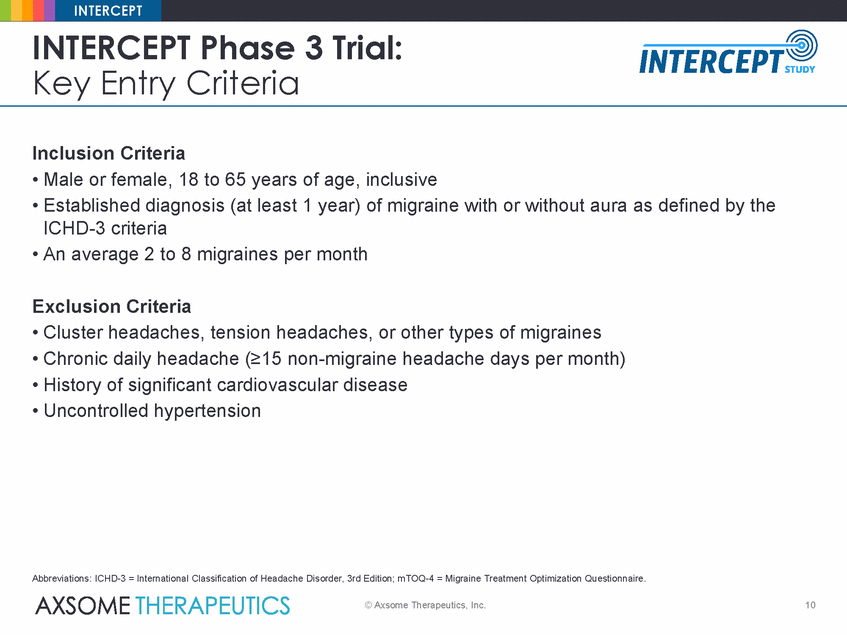
INTERCEPT INTERCEPT Phase 3 Trial: Key Entry Criteria Inclusion Criteria • Male or female, 18 to 65 years of age, inclusive • Established diagnosis (at least 1 year) of migraine with or without aura as defined by the ICHD-3 criteria • An average 2 to 8 migraines per month Exclusion Criteria • Cluster headaches, tension headaches, or other types of migraines • Chronic daily headache (≥15 non-migraine headache days per month) • History of significant cardiovascular disease • Uncontrolled hypertension Abbreviations: ICHD-3 = International Classification of Headache Disorder, 3rd Edition; mTOQ-4 = Migraine Treatment Optimization Questionnaire. © Axsome Therapeutics, Inc. 10 |
|
|
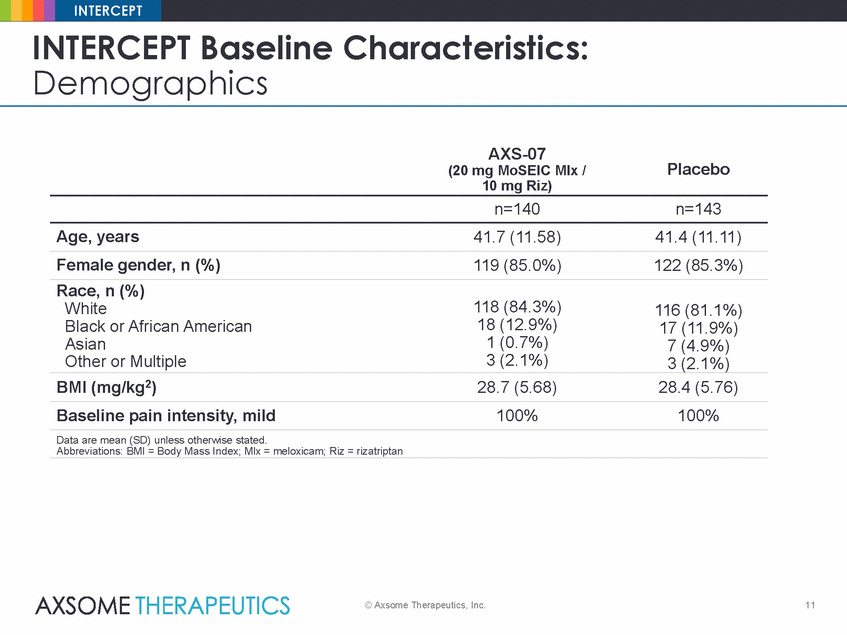
INTERCEPT INTERCEPT Baseline Demographics Characteristics: AXS-07 (20 mg MoSEIC Mlx / 10 mg Riz) Placebo n=140 n=143 Age, years 41.7 (11.58) 41.4 (11.11) Female gender, n (%) 119 (85.0%) 122 (85.3%) Race, n (%) White Black or African American Asian Other or Multiple 118 (84.3%) 18 (12.9%) 1 (0.7%) 3 (2.1%) 116 (81.1%) 17 (11.9%) 7 (4.9%) 3 (2.1%) BMI (mg/kg2) 28.7 (5.68) 28.4 (5.76) Baseline pain intensity, mild 100% 100% Data are mean (SD) unless otherwise stated. Abbreviations: BMI = Body Mass Index; Mlx = meloxicam; Riz = rizatriptan © Axsome Therapeutics, Inc. 11 |
|
|
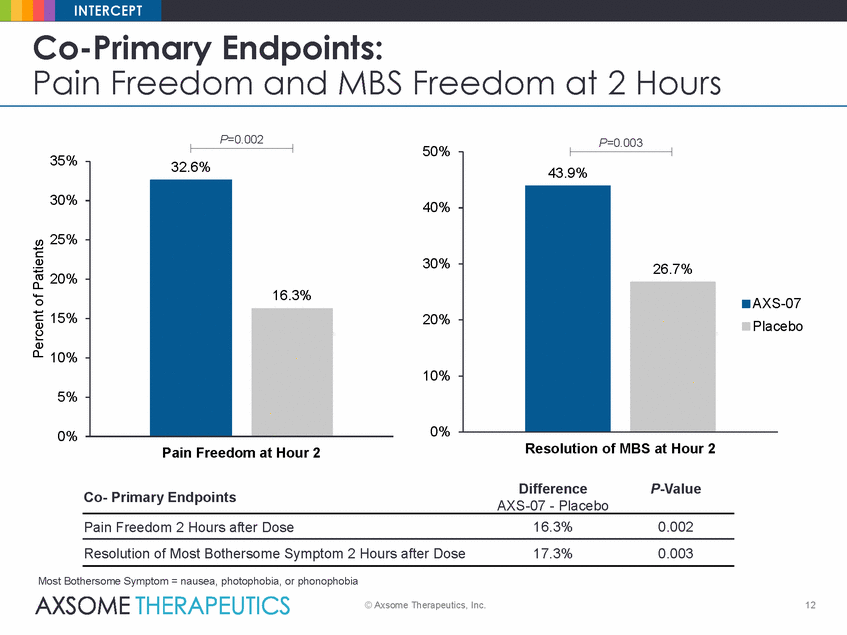
INTERCEPT Co-Primary Endpoints: Pain Freedom and MBS Freedom at 2 Hours P=0.002 P=0.003 50% 35% 30% 40% 25% 30% 20% S-07 acebo 15% 20% 10% 10% 5% 0% 0% Resolution of MBS at Hour 2 Pain Freedom at Hour 2 Difference P-Value Co-Primary Endpoints AXS-07 - Placebo 16.3% 0.002 Pain Freedom 2 Hours after Dose Resolution of Most Bothersome Symptom 2 Hours after Dose 17.3% 0.003 Most Bothersome Symptom = nausea, photophobia, or phonophobia © Axsome Therapeutics, Inc. 12 Percent of Patients 32.6% 16.3% 43.9% 26.7% AX Pl |
|
|
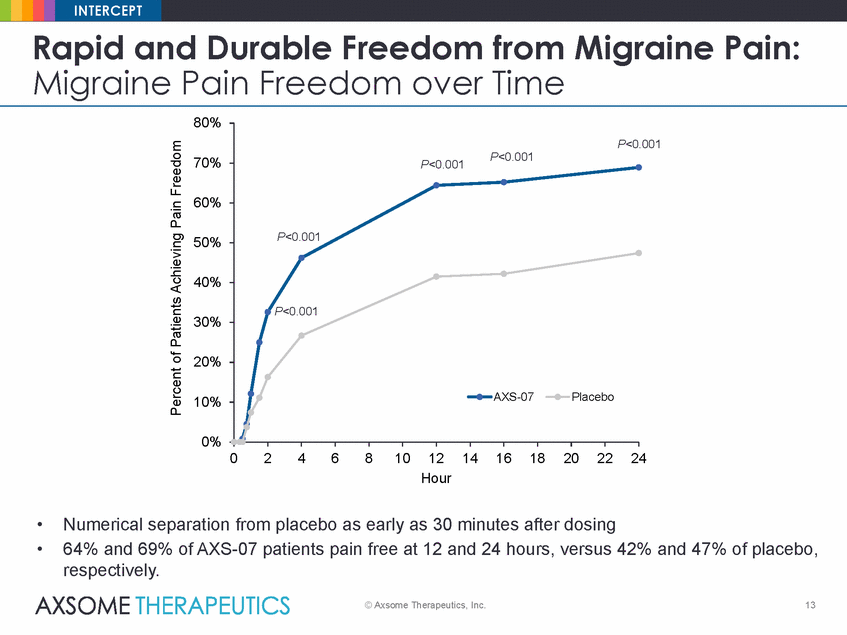
INTERCEPT Rapid and Durable Freedom from Time Migraine Pain: Migraine Pain Freedom over 80% .001 70% 60% 50% 40% 30% 20% 10% 0% 0 2 4 6 8 101214 Hour 16 18 20 22 24 • • Numerical separation from placebo as early as 30 minutes after dosing 64% and 69% of AXS-07 patients pain free at 12 and 24 hours, versus 42% and 47% of placebo, respectively. © Axsome Therapeutics, Inc. 13 Percent of Patients Achieving Pain Freedom P<0 P<0.001P<0.001 P<0.001 P<0.001 AXS-07Placebo |
|
|
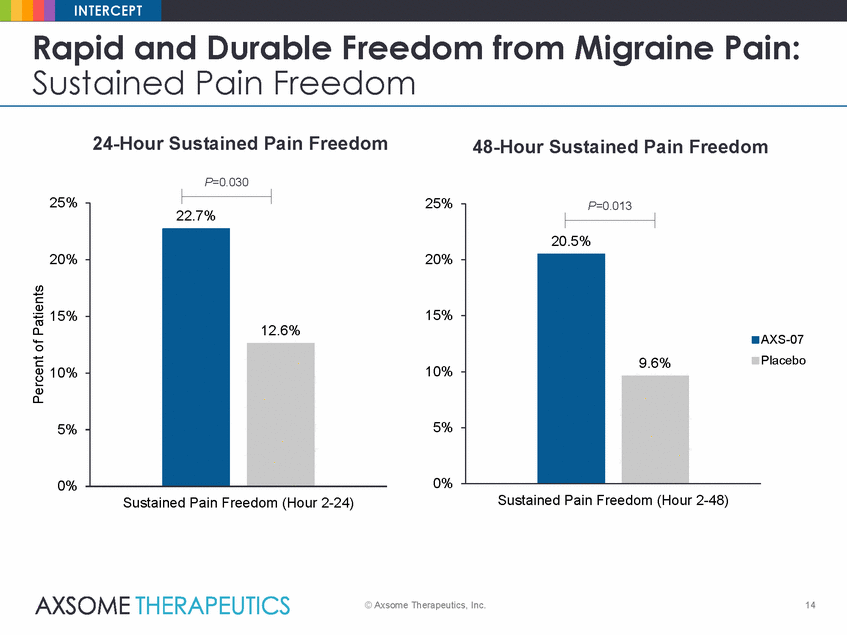
INTERCEPT Rapid and Durable Freedom from Migraine Pain: Sustained Pain Freedom 24-Hour Sustained Pain Freedom 48-Hour Sustained Pain Freedom P=0.030 25% 25% 20% 20% 15% 15% AXS-07 Placebo 10% 10% 5% 5% 0% 0% Sustained Pain Freedom (Hour 2-48) Sustained Pain Freedom (Hour 2-24) © Axsome Therapeutics, Inc. 14 Percent of Patients P=0.013 20.5% 9.6% 22.7% 12.6% |
|
|
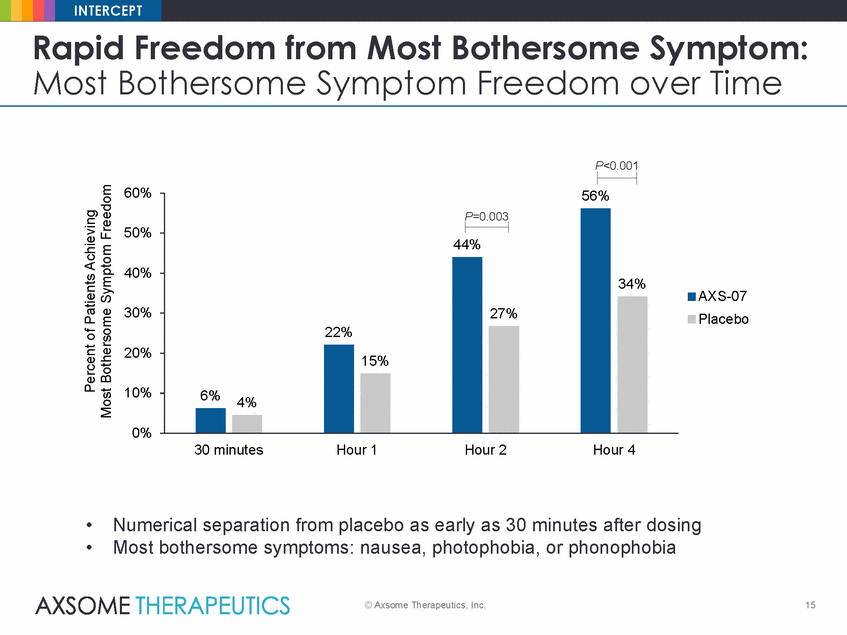
INTERCEPT Rapid Freedom from Most Bothersome Symptom: Most Bothersome Symptom Freedom over Time P<0.001 60% 50% 40% AXS-07 Placebo 30% 20% 10% 0% 30 minutes Hour 1 Hour 2 Hour 4 • • Numerical separation from placebo as early as 30 minutes after dosing Most bothersome symptoms: nausea, photophobia, or phonophobia © Axsome Therapeutics, Inc. 15 Percent of Patients Achieving Most Bothersome Symptom Freedom 56% P=0.003 44% 34% 22% 27% 6%4% 15% |
|
|
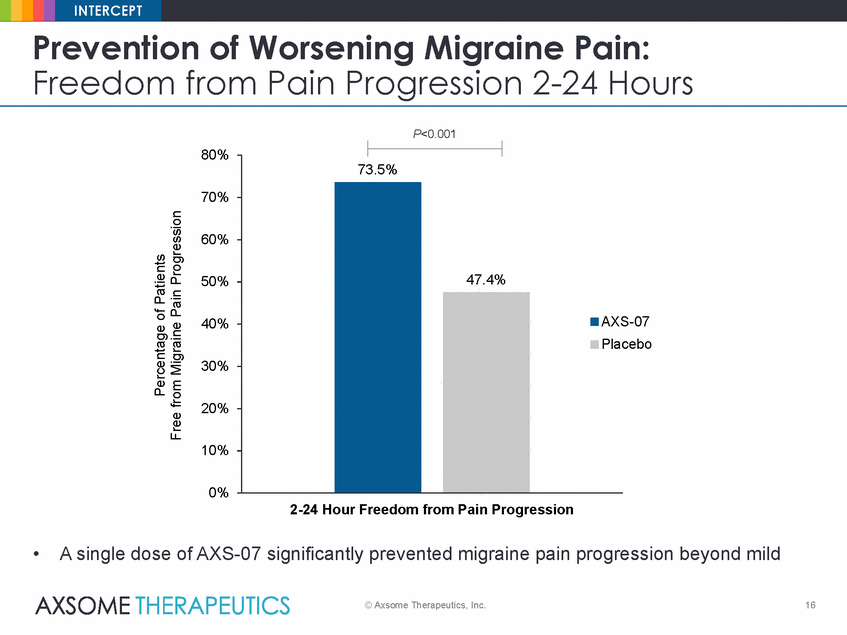
INTERCEPT Prevention of Worsening Migraine Pain: Freedom from Pain Progression 2-24 Hours P<0.001 80% 70% 60% 50% S-07 acebo 40% 30% 20% 10% 0% 2-24 Hour Freedom from Pain Progression • A single dose of AXS-07 significantly prevented migraine pain progression beyond mild © Axsome Therapeutics, Inc. 16 Percentage of Patients Free from Migraine Pain Progression 73.5% 47.4% AX Pl |
|
|
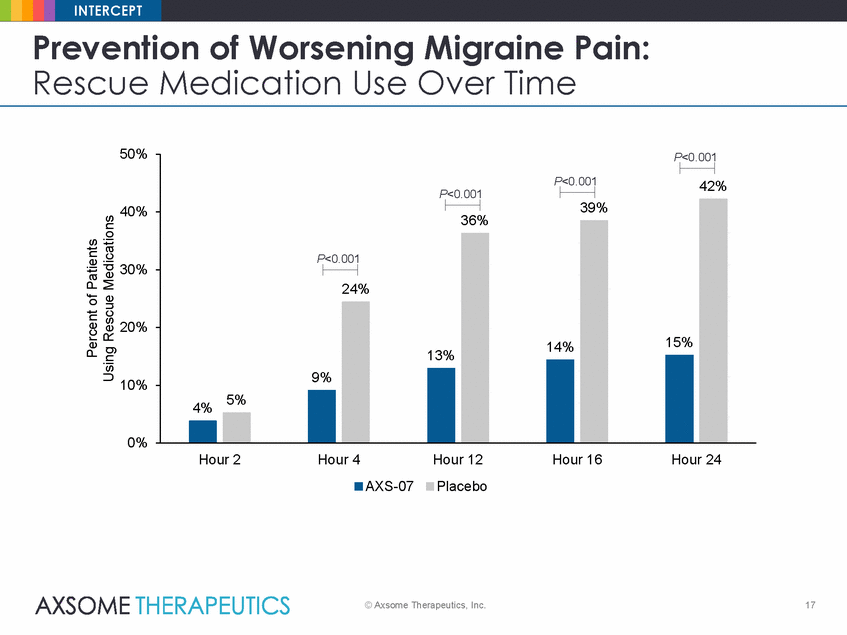
INTERCEPT Prevention of Worsening Migraine Pain: Rescue Medication Use Over Time 50% P<0.001 40% 36% 30% 20% 10% 0% Hour 2 Hour 4 Hour 12 Hour 16 Hour 24 AXS-07 Placebo © Axsome Therapeutics, Inc. 17 Percent of Patients Using Rescue Medications P<0.001 P<0.00142% 39% 15% P<0.001 24% 14% 9% 13% 4%5% |
|
|
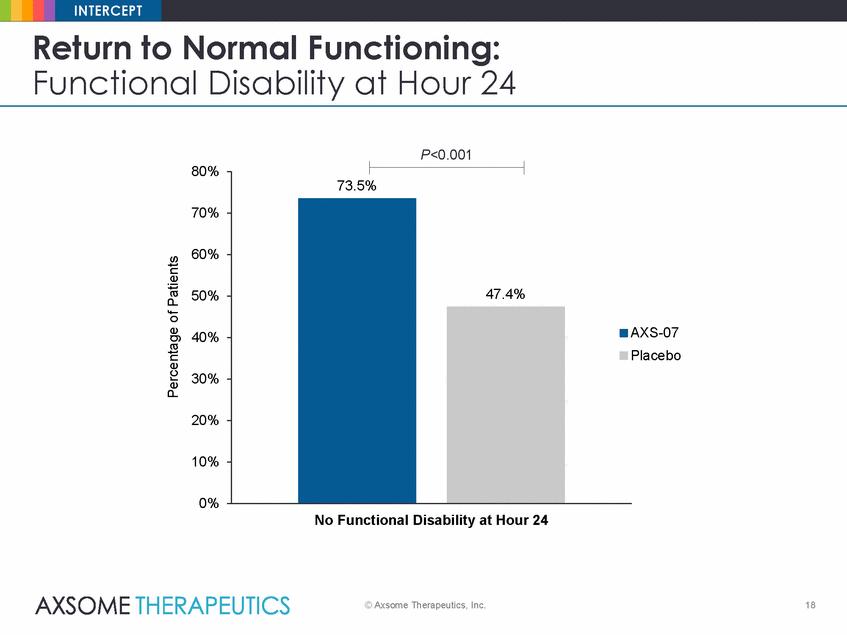
INTERCEPT Return to Normal Functioning: Functional Disability at Hour 24 P<0.001 80% 70% 60% 50% AXS-07 Placebo 40% 30% 20% 10% 0% No Functional Disability at Hour 24 © Axsome Therapeutics, Inc. 18 Percentage of Patients 73.5% 47.4% |
|
|
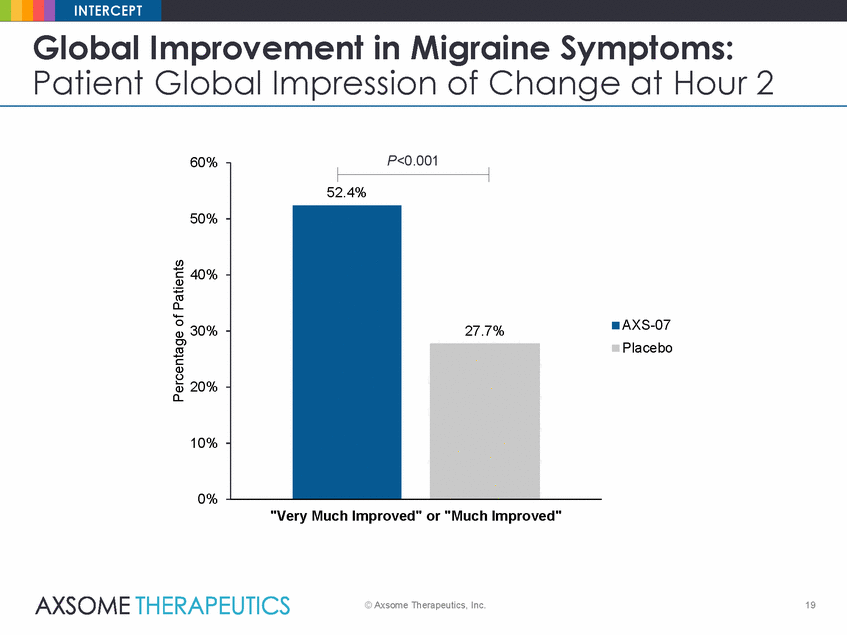
INTERCEPT Global Improvement in Migraine Symptoms: Patient Global Impression of Change at Hour 2 P<0.001 60% 50% 40% AXS-07 Placebo 30% 20% 10% 0% "Very Much Improved" or "Much Improved" © Axsome Therapeutics, Inc. 19 Percentage of Patients 52.4% 27.7% |
|
|
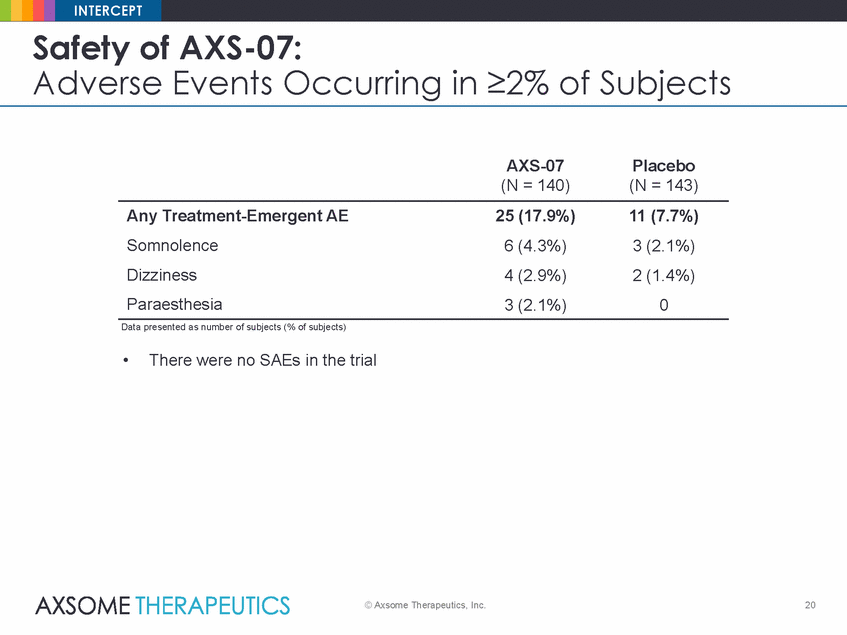
INTERCEPT Safety of AXS-07: Adverse Events Occurring in ≥2% of Subjects AXS-07 (N = 140) Placebo (N = 143) Any Treatment-Emergent AE Somnolence Dizziness Paraesthesia 25 (17.9%) 6 (4.3%) 4 (2.9%) 3 (2.1%) 11 (7.7%) 3 (2.1%) 2 (1.4%) 0 Data presented as number of subjects (% of subjects) • There were no SAEs in the trial © Axsome Therapeutics, Inc. 20 |
|
|
INTERCEPT INTERCEPT Phase 3 Trial Results: Summary • Treatment with AXS-07 resulted in rapid, sustained, and statistically significant efficacy as compared to placebo • Early treatment with AXS-07 resulted in significant prevention of migraine pain progression • Efficacy benefits of AXS-07 translated into significantly less use of rescue medication and return to normal functioning in the vast majority of AXS-07 treated patients • AXS-07 was generally safe and well tolerated in this study © Axsome Therapeutics, Inc. 21 |
|
|
Q&A © Axsome Therapeutics, Inc. 22 |
|
|
Concluding Remarks Herriot Tabuteau, MD CHIEF EXECUTIVE OFFICER AXSOME THERAPEUTICS, INC. © Axsome Therapeutics, Inc. 23 |
|
|
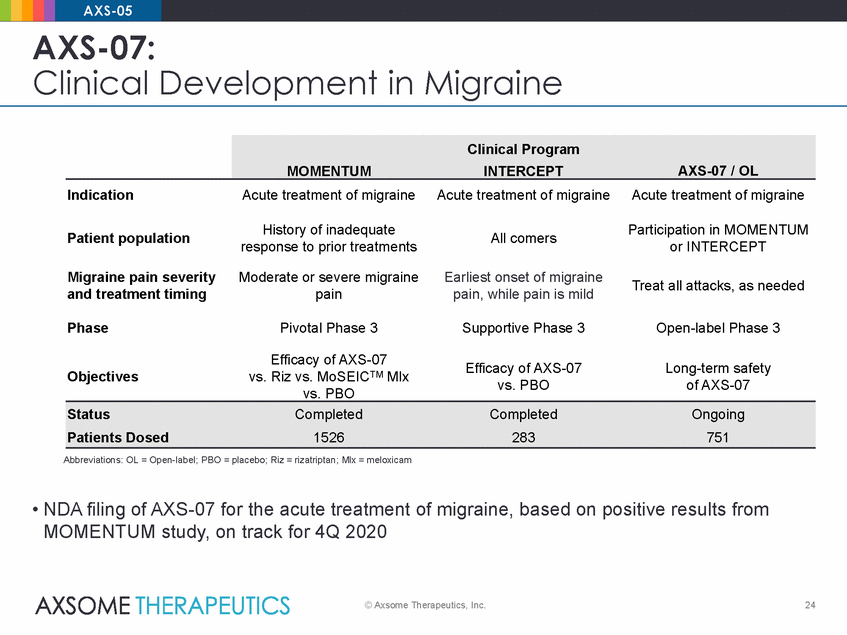
AXS-05 AXS-07: Clinical Development in Migraine Indication Acute treatment of migraine Acute treatment of migraine Acute treatment of migraine History of inadequate response to prior treatments Participation in MOMENTUM or INTERCEPT Patient population All comers Migraine pain severity and treatment timing Moderate or severe migraine pain Earliest onset of migraine pain, while pain is mild Treat all attacks, as needed Phase Pivotal Phase 3 Supportive Phase 3 Open-label Phase 3 Efficacy of AXS-07 vs. Riz vs. MoSEICTM Mlx vs. PBO Efficacy of AXS-07 vs. PBO Long-term safety of AXS-07 Objectives Abbreviations: OL = Open-label; PBO = placebo; Riz = rizatriptan; Mlx = meloxicam • NDA filing of AXS-07 for the acute treatment of migraine, based on positive results from MOMENTUM study, on track for 4Q 2020 © Axsome Therapeutics, Inc. 24 StatusCompletedCompletedOngoing Patients Dosed1526283751 Clinical Program MOMENTUMINTERCEPTAXS-07 / OL |
|
|
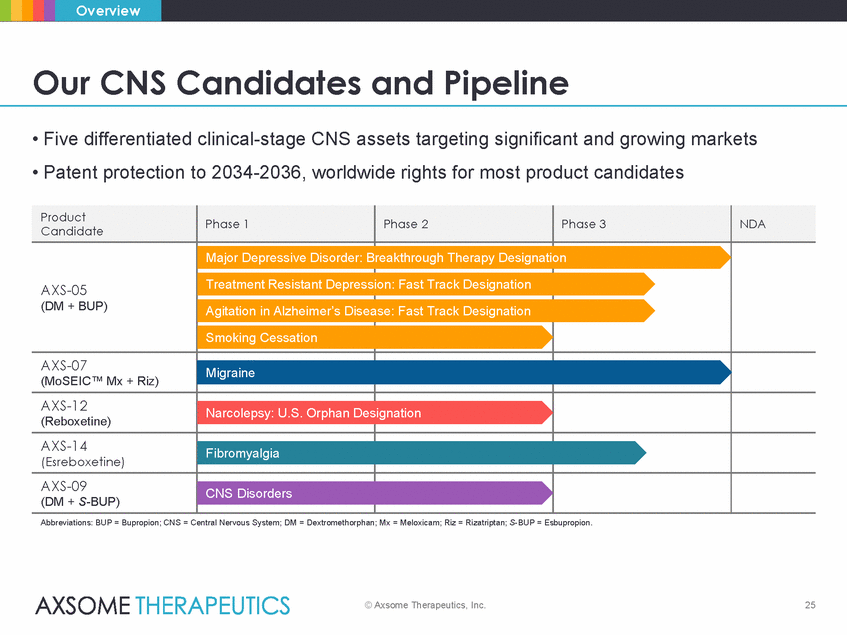
Overview Our CNS Candidates and Pipeline • Five differentiated clinical-stage CNS assets targeting significant and growing markets • Patent protection to 2034-2036, worldwide rights for most product candidates ease: Fast Track Designation Abbreviations: BUP = Bupropion; CNS = Central Nervous System; DM = Dextromethorphan; Mx = Meloxicam; Riz = Rizatriptan; S-BUP = Esbupropion. © Axsome Therapeutics, Inc. 25 Product Candidate Phase 1 Phase 2 Phase 3 NDA AXS-05 (DM + BUP) Major Depressive Disorder: B Treatment Resistant Depress Agitation in Alzheimer’s Dis Smoking Cessation reakthrough Therapy Designati ion: Fast Track Designation on AXS-07 (MoSEIC™ Mx + Riz) Migraine AXS-12 (Reboxetine) Narcolepsy: U.S. Orphan Des ignation AXS-14 (Esreboxetine) Fibromyalgia AXS-09 (DM + S-BUP) CNS Disorders |
|
|
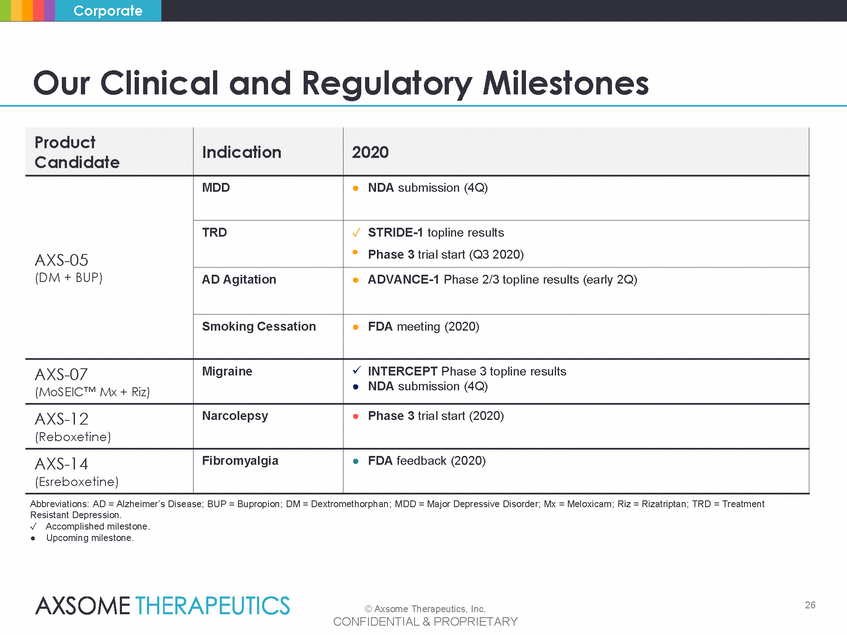
Corporate Our Clinical and Regulatory Milestones ● NDA submission (4Q) Abbreviations: AD = Alzheimer’s Disease; BUP = Bupropion; DM = Dextromethorphan; MDD = Major Depressive Disorder; Mx = Meloxicam; Riz = Rizatriptan; TRD = Treatment Resistant Depression. ✓ Accomplished milestone. ● Upcoming milestone. 26 © Axsome Therapeutics, Inc. CONFIDENTIAL & PROPRIETARY Product Candidate Indication 2020 AXS-05 (DM + BUP) MDD ● NDA submission (4Q) TRD ✓ STRIDE-1 topline results • Phase 3 trial start (Q3 2020) AD Agitation ● ADVANCE-1 Phase 2/3 topline results (early 2Q) Smoking Cessation ● FDA meeting (2020) AXS-07 (MoSEIC™ Mx + Riz) Migraine INTERCEPT Phase 3 topline results AXS-12 (Reboxetine) Narcolepsy ● Phase 3 trial start (2020) AXS-14 (Esreboxetine) Fibromyalgia ● FDA feedback (2020) |
|
|
For more information, please contact Mark Jacobson Chief Operating Officer 212-332-3243 mjacobson@Axsome.com axsome.com |