Attached files
| file | filename |
|---|---|
| EX-32.2 - eWELLNESS HEALTHCARE Corp | ex32-2.htm |
| EX-32.1 - eWELLNESS HEALTHCARE Corp | ex32-1.htm |
| EX-31.2 - eWELLNESS HEALTHCARE Corp | ex31-2.htm |
| EX-31.1 - eWELLNESS HEALTHCARE Corp | ex31-1.htm |
| EX-3.1A - eWELLNESS HEALTHCARE Corp | ex3-1a.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2019
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
Commission file number 000-55203

eWELLNESS HEALTHCARE CORPORATION
(Exact name of registrant as specified in its charter)
| Nevada | 90-1073143 | |
| (State
or other jurisdiction of incorporation or organization) |
(I.R.S.
Employer Identification No.) | |
| 333 Las Olas Way, Suite 100, Ft. Lauderdale, FL | 33301 | |
| (Address of principal executive offices) | (Zip Code) |
(855) 470-1700
(Registrant’s telephone number, including area code)
Securities registered under Section 12(b) of the Act: None
Securities registered under Section 12(g) of the Act: Common stock, $0.001 par value
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes [ ] No [X]
Indicate by check mark whether the issuer (1) filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes [X ] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer [ ] | Accelerated filer [ ] |
| Non-accelerated filer [ ] (Do not check if a smaller reporting company) | Smaller reporting company [X] |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ] No [X]
The aggregate market value of the 221,471,504 voting and non-voting common equity held by non-affiliates on June 30, 2019 (the last trading date in June 2019) computed by reference to the closing price of $199.75 on such date or the average bid and asked prices of such common equity as of the last business day of the registrant’s most recently completed second fiscal quarter was $44,238,932,924.
The number of shares of Common stock, $0.001 par value, outstanding on March 20, 2020 is 467,038,350.
DOCUMENTS INCORPORATED BY REFERENCE
None
eWellness Healthcare Corporation
Form 10-K
For the Year Ended December 31, 2019
Table of Contents
| 2 |
FORWARD-LOOKING STATEMENTS
This document contains “forward-looking statements”. All statements other than statements of historical fact are “forward-looking statements” for purposes of federal and state securities laws, including, but not limited to, any projections of earnings, revenue or other financial items; any statements of the plans, strategies and objections of management for future operations; any statements concerning proposed new services or developments; any statements regarding future economic conditions or performance; any statements of belief; and any statements of assumptions underlying any of the foregoing.
Forward-looking statements may include the words “may,” “could,” “estimate,” “intend,” “continue,” “believe,” “expect” or “anticipate” or other similar words. These forward-looking statements present our estimates and assumptions only as of the date of this report. Accordingly, readers are cautioned not to place undue reliance on forward-looking statements, which speak only as of the dates on which they are made.
Throughout this Annual Report references to “we”, “our”, “us”, “eWellness”, “the Company”, and similar terms refer to eWellness Healthcare Corporation and its wholly owned subsidiary.
eWellness Healthcare Corporation (f/k/a Dignyte, Inc.), (the “eWellness”, “Company”, “we”, “us”, “our”) was incorporated in the State of Nevada on April 7, 2011.
The Company has developed a new operating structure enabling it to operate in 48 states. The below noted chart illustrates the Company’s new operational structure that provides for three individual professional operating companies in California, New Jersey and most importantly Florida. With our Florida Professional Association (PA), we are able to provision our telehealth services in 46 states, (excluding: California, Delaware, Kansas and New Jersey). Thus, we formed two additional professional companies in California and New Jersey. Each professional company has executed a revocable operating agreement with the Company. These agreements are required by each individual state and states that Darwin Fogt, MPT, the sole officer, director and shareholder of each of the operating companies. All accounting services are supplied to these operating companies by the Company’s accounting team.

| 3 |
The Company and Nature of Business
eWellness Healthcare Corporation is a provider of the state of the art PHZIO platform for the physical therapy (“PT”) and telehealth markets and believes it is the first digital telehealth physical therapy company (“dtPT Company”) to offer real-time monitored physical therapy assessments and treatments to large-scale employers. The Company’s digital telehealth assessment and treatment platform (the “dtPT Platform” or “Platform”) has been designed to serve the $30 billion physical therapy market, the $4 billion musculoskeletal (“MSK”) market and the $8 billion corporate wellness market. Our dtPT Platform redefines the way physical therapy (“PT”) can be delivered. We believe that our Platform is able to transform the access, cost and quality dynamics of PT assessments and treatments. We began generating revenue during the fourth quarter of 2019.
We designed our Platform to enable its usage for all PT assessments and treatments by means of computer, smart phone and/or similar digital media devices (the “Access Devices”). This new approach will lower patient treatment costs, expand patient treatment access and improve patient compliance. Our dtPT Platform allows patients to avoid the time-consuming clinical experience to an immediate in-home PT experience. We believe that approximately 80% of all PT assessments and treatments can be performed using our Platform accessible via the Access Devices in the privacy of once home.
We believe that our innovative approach to solving the pervasive access, cost and quality challenges facing the current access to PT clinics, will lead to highly scalable and substantial growth in our revenues. The Company has signed 7 partnership and healthcare provider agreements to date. We believe that we are well positioned to participate in the rapidly evolving PT treatment market by introducing our innovative dtPT Platform enabling remote patient monitoring, post-discharge treatment plan adherence and in-home care. Our Platform incorporates research-based methods and focuses on, not only rehabilitation but also wellness, functional fitness, performance, and prevention.
Our dtPT Platform recognizes that the national healthcare industry (federal and private insurance) is moving toward a model of prevention and that physical therapy is expected to take a larger role in providing wellness services to patients. Due to the real-time patient monitoring feature, we believe that our dtPT Platform is reimbursable by insurance companies such as Anthem Blue Cross and Blue Shield.
The Company will initially rollout these new telehealth solutions within California, New Jersey, Georgia, Tennessee, Arizona and Canada, with plans to expand nationally over the next twelve months. With these new telehealth tools, eWellness will engage with the “At-Home” Physical Therapy and MSK treatment market. This market involves physical therapy practitioners treating patients in their home instead of a clinic. The “At-Home” market model when combined with the PHZIO and or MSK 360 offers patients and practitioners a means to receive and deliver PT and MSK services without having to leave work during normal business hours. Patients can receive physical therapy and MSK services at almost any hour of the day. A model that is not currently employed within traditional clinical settings.
During June 2019 the Company signed a Provider Service Agreement with CareIQ, a division of CorVel Healthcare Corporation, one of the largest Third-Party Insurance Administrators (“TPA”) in the U.S. with patients in all 50 states. https://www.corvel.com/about-us. Initially, PHZIO will be used to treat patients in five (5) states including: California, New Jersey, Georgia, Tennessee and Arizona. These initial states will be used to assess the effectiveness of the PHZIO digital physical therapy platform.
During October 2019, the Company introduced MSK 360 treatment platform as a new silo of business that focuses on the $4 Billion North American Musculoskeletal Treatment Market to address the global musculoskeletal diseases treatment market, that is expected to reach US$ 5.7 billion in 2025 from US $3.8 billion in 2017, according to a report by The Insight Partners. The musculoskeletal diseases treatment market is estimated to grow with a CAGR of 5.3% from 2018-2025. MSK disease affects the joints, bones and muscles and also back pain. More years are lived with musculoskeletal disability than any other long-term human condition.
Our PHZIO and MSK 360 platforms have been developed to significantly support us in becoming the leader in the new industry of digital telehealth in the MSK and PT markets. Our focus is to highlight that a majority of all future MSK PT treatments can be accomplished with a smart phone. This new digital adoption will lower employee treatment costs, expand employee treatment access and improve employee compliance. Our PHZIO and MSK 360 platform allows employees and PT’s to cut the cord from the old-school, wait in line, brick and mortar clinical experience to an immediate response digital, in-home PT experience. Nearly, 100% of all PT assessments and treatments can now be done on an employee’s smart phone in the privacy of their own home. Digital MSK treatments are clearly the next upgrade the industry needs to make.
| 4 |
The Company has created a strong path to initial revenue generation and substantial sales growth through executing on our Workers Compensation and MSK Sales Funnel. Our Workman’s Compensation and MSK Sales Funnel currently includes over 101 companies. Starting in the Summer of 2018 we pivoted our sales process to focus on the workman’s compensation PT industry. Additionally, we added the MSK market during the summer of 2019. Multiple agreements are anticipated to be executed from our workman’s compensation and MSK sales funnel through 2019 and beyond.
In October 2019 The Company signed a Direct to Consumer Marketing Agreement with Wosler Holdings, Inc., a Delaware Corporation d/b/a/ Slingshot Health (“Slingshot”) (https://www.slingshothealth.com), Through this agreement, Slingshot seeks to involve EWLL affiliated PT Providers, and EWLL seeks to gain their affiliated PT Providers access to the Slingshot consumer healthcare patients through the Slingshot platform. The Parties anticipate commencing these new direct to consumer sales and marketing efforts during the fourth quarter ended December 31, 2019. The Company believes that Slingshot Healthcare is one of the leading on-line platforms for digital healthcare to consumers. Slingshot Health is a healthcare marketplace connecting people to health and wellness providers, placing control directly in the hands of those seeking and delivering care. By removing layers of bureaucracy surrounding our healthcare system, Slingshot is achieving its mission of creating better access, more affordability and greater transparency in healthcare. Through Slingshot’s proprietary platform, consumers enter the services they want, their location, availability and the price they are willing to pay. Slingshot then matches them to a local provider who can deliver the service. Healthcare consumers receive high-quality, affordable services and providers earn more overall.
In October 2019, EWLL’s PHZIO Canada (“PHZIO Canada”) signed a one-year Pilot Program Agreement with C&C Insurance Consultants d/b/a/ StudentVIP.ca (“StudentVIP.ca”) (https://studentvip.ca/about-us/), Through this agreement, StudentVIP.ca seeks to market PHZIO.com services to its student health insurance clients. StudentVIP.ca is one of Canada’s largest student health insurance provider servicing over 100,000 college students. The Parties anticipate commencing these new direct to consumer sales and marketing efforts during the first quarter of 2020.
Our PHZIO and MSK 360 platforms completely disrupts the current in-clinic business model of the $30 billion PT industry, the 4 billion MSK market and the $8 billion corporate wellness industries. Innovators in other industries have solved access, cost and quality inefficiencies through the implementation of technology platforms and business models that deliver products and services on-demand and create new economies by connecting and empowering both consumers and businesses. We have taken the same approach to solving the pervasive access, cost and quality challenges facing the current access to PT and MSK clinics. eWellness’ underlying technology platform is complex, deeply integrated and purpose-built over the past five years for the evolving PT and MSK treatment marketplaces. eWellness’ PHZIO and MSK 360 platforms are highly scalable and can support substantial growth of third-party licensees. eWellness’ PHZIO and MSK 360 platforms provides for broad interconnectivity between PT practitioners and their patients, uniquely positioning the Company as a focal point in the rapidly evolving PT industry to introduce innovative, technology- based solutions, such as remote patient monitoring, post-discharge treatment plan adherence and in-home care.
Our Principal Products and Services
The principal features of our new digital telehealth physical therapy delivery system are as follows:
● ● |
SaaS technology platform solution for providers bundling rehabilitation services and employer wellness programs PTs can evaluate and screen patients and calculate joint angles using drawing tool | |
| ● | First real-time remote monitored one-to-many PT treatment platform for home use | |
| ● | Ability for PTs to observe multiple patients simultaneously in real-time | |
| ● | Solves what has been a structural problem and limitation in post-acute care practice growth. | |
| ● | PT practices can experience 20% higher adherence and compliance rates versus industry standards | |
| ● | Tracking to 30% increase in net income for a PT practice. |
| 5 |
We have commenced treating patients on various commercial contracts and started to generate revenues during the last quarter of the year ended December 31, 2019. We continue to train physical therapists on how to use our Platform, with many of these therapists treating various patients on our system on a complimentary basis. To date, our dtPT Platform has delivered over 4,000 PT assessments and treatments.
During the 2nd half of 2019, we intensified our focus on PT assessments and treatments covered under the Workers’ Compensation Insurance program which is a form of insurance providing wage replacement and medical benefits to employees injured in the course of employment. Changes in regulations related Workers’ Compensation Insurance have provided us with an opportunity to offer our MSK 360 Program as described below. Under the new regulation patients can choose to be treated in-clinic or through dtPT. Until recently, patients nearly all choose in-clinic treatment. In response to this change we developed our MSK360 Program.
We are in the final stage of developing a fourth program related to Rheumatoid Arthritis Exercises (“RA 360 Program”). We expect to make the RA 360 Program available during the third quarter of 2020.
To date, we have existing provider agreements with approximately 16 corporations based on which their employees can utilize our Platform. Additionally, we are actively pursuing as clients for our services numerus large corporate self-insured companies, TPA’s and insurance companies to sign provider agreements with us. We have historically had to devote up to one year in sales and marketing and sales activities and efforts to sign new provider agreements and to date we have executed and existing 16 provider agreements with the following companies that we expect to generate revenues during the first quarter of 2020 as follows:
Pepsi, Corvel, Imperial, Rogers, Manulife, CanadaLife, Navy & Stage Benefits, Health and Dental Plan, Slingshot Health, BBD Benefits By Design, Morneau Shapell, Green Shield Canada, Bruce Power.
Our dtPT Platform under the domain name PHZIO.com currently offers three treatment programs (i) PHIZO Program; (ii) MSK 360 Program and (iii) Pre-HabPT Program.
Background on our PHZIO Technology
The Company’s Chief Technology Officer (“CTO”), Curtis Hollister, oversees the operational aspects of the PHZIO platform via a team located in Ottawa, Canada. The below noted chart contains information on our PHZIO System.
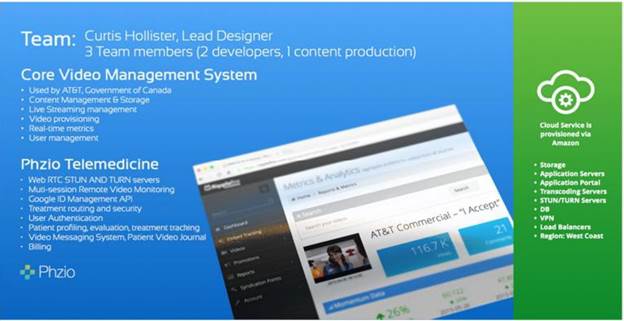
| 6 |
IP and Licensing
We have licensed our telemedicine platform from Bistromatics Inc., a company owned by our CTO, for perpetuity for any telemedicine application in any market worldwide. The below noted chart highlights what we have built to date.
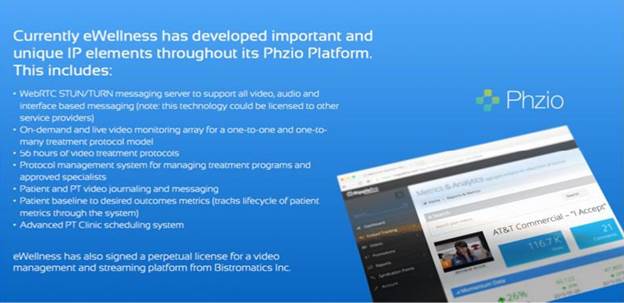
On November 12, 2016, the Company entered into a Services Agreement with Bistromatics, Inc. (the “Bistromatics Agreement”), a Company incorporated under the laws of Canada (“Bistromatics”). Pursuant to the Bistromatics Agreement, Bistromatics will provide operational oversight of the Company’s Phzio System including development, content editing, client on boarding, clinic training, support & maintenance, billing, hosting and oversight and support of CRM and helpdesk system. The Company has agreed to pay a monthly base fee of $50,000 monthly until Bistromatics has successfully signed and collected the first monthly service fee for 100 Physical Therapy Clinics to start using our Platform. When, and if, Bistromatics provides the Company with evidence of the 100 Physical Therapy Clinics, the monthly service fee will be $100,000. Bistromatics will have the ability to convert any outstanding amounts that fall in arrears for 60 days into common stock at the same terms as the next round of financing or the Company’s common stock market price, whichever is higher. Bistromatics will also have the right to appoint 40% of the directors.
Competition
We have identified multiple privately held telemedicine and exercise platform companies that utilize Avatar/Kinect-based telerehb platforms including: Reflexion Health, RespondWell, Physmodo, Jintronx, MotionCare 360 and Five Plus. Additionally, we have identified other video-based physical therapy solutions such as: Bluejay, PT Pal, VitalRock, Physiotech, SimplyTherapy and YouTube. Yet, none of these companies have real-time PT monitoring, one-to-many platform, treatments reimbursable by payors and strong program compliance and adherence by patients.
The PHZIO Program
Our PHZIO treatment enables patients to engage with live or on-demand video based dtPT assessments and treatments from their home or office. Following a physician’s exam and prescription for physical therapy to treat back, knee or hip pain, a patient can be examined by a physical therapist and if found appropriate inducted in our PHZIO program that includes a progressive 6-month telemedicine exercise program (including monthly in-clinic checkups). All PHZIO treatments are monitored by a licensed therapist that sees everything the patient is doing while providing their professional guidance and feedback in real-time. This ensures treatment compliance by the patient, maintains the safety and integrity of the prescribed exercises, tracks patient metrics and captures pre-and post-treatment evaluation data. This innovative assessment and treatment program enable any PT practice to be able to treat more patients while utilizing the same resources.
| 7 |
A Monitored In-office & Telemedicine Exercise Program: Our initial 6-month PHZIO exercise program has been designed to provide patients, who are accepted into the program, with traditional one-on-one PT evaluations, re-evaluations (one to four weeks throughout the PHZIO program depending on type of insurance), and after the conclusion of the program a Physical Performance Test. These PTs are known as Induction & Evaluation PTs (“IEPTs”). All patient medical data, information and records are retained in the files of the IEPT. The IEPT will also evaluate the progress of the patient’s participation in our PHZIO program.
| ● | Physician Diagnosis: Following a physician’s diagnosis of a patient with non-acute back pain, who is also likely overweight and pre-diabetic, a physician may prescribe the patient to participate in the eWellness PHZIO exercise program. | |
| ● | Enrollment Process: The accepted patients are assessed by a PT, located at a PT Licensee clinic and then enrolled in our PHZIO program by going online to our PHZIO program virtual private network (“VPN”) and creating a login name and password. The patient will then populate their calendar with planned times when they anticipate exercising. They will also be provided with a free exercise ball, resistance stretch bands, stretch strap and yoga mat at induction. | |
| ● | Exercising Begins: The day after the patient receives the equipment, the patient will log on to our VPN at least 3 times per week, to watch and follow the prescribed 40-minute on-line exercise program. The PHZIO platform also allows two-way communication (videoconferencing) with one of our licensee’s On-line PTs (“OLPT’s”), who is responsible for monitoring on-line patients. The OLPT’s are also available to answer patient’s questions. When available the patients exercise sessions are recorded and stored in our system as proof that they completed the prescribed exercises. There are 250 various 40-minute exercise videos that are viewed by our patients in successive order. | |
| ● | Driving Patients to work out between 6:00am-9:30am 5 days per week: Our PHZIO system has a calendar function so that patients can schedule when they will login to our PHZIO system. This calendar enables a PT Licensee to better spread the load of patients participating in any forty-minute on-line exercise program during our 15 hours of weekly operations, 6am through 9:30am Monday through Friday are to most optimal hours for patient engagement. Also, if the patient is not on-line at the planned exercise time, our system can send them an automated reminder, via text, voicemail and or e-mail messaging. |
Trackable Physical Therapy. The exercise PHZIO prescription and instruction will be delivered with a series of on-line videos easily accessed by each patient on the internet. Each video will be approximately 40 minutes in length with exercises, which will specifically address the common impairments associated with diabetes and/or obesity. Exercise programs will be able to be performed within each patient’s own home or work location without requiring standard gym equipment. Each patient will be required to log in to the system which will monitor performance automatically to ensure their compliance. Each patient will be required to follow up with their referring physician and PT at designated intervals and metrics such as blood pressure, blood sugars, BMI, etc. will be recorded to ensure success of the program.
Patient Program Goals. Our PHZIO program was designed so that the average patient is targeted to lose 2 pounds per week, totaling up to 48 pounds over the duration of the program to progress toward healthier defined BMI, reduction body fat percentage by at least 8%, reduced reliance on medication for blood glucose regulation and dosage or frequency and a goal of at least a 50% adherence to continuing the PHZIO program independently at conclusion of program.
Trackable Video Exercise Program. The PHZIO program video includes all aspects of wellness preventative care to ensure the best results: cardiovascular training, resistance training, flexibility, and balance and stabilization; research studies on all such distinct impairments have shown to provide effective treatment results. Each video integrates each of the four components to guarantee a comprehensive approach to the wellness program, but each video will specifically highlight one of the four components. All PHZIO Program videos can be viewed on the Access Devices.
| 8 |
Specific Video Programs. Each patient will receive a prescription for six months (26 week) of physical therapy and exercise that is provided by viewing on-line programs produced by us where the patient can do these exercises and stretching on their own at least 3 days per week for at least 40 minutes. To view the videos, the patient would log onto the Platform and would be directed to watch the appropriate video in sequence. As the patient is logged-in, the monitoring PT will be able to monitor how often and if the entire video session was viewed. This data would be captured and sent weekly to the prescribing physician and the monitoring PT for review. At all times, a licensed OLPT/PTA will have access to each patient utilizing the videos and will be able to communicate with a patient via videoconferencing and/or instant messaging. This will help improve adherence to the program as well as the success and safety of the patients’ treatments. A patient will also be instructed to walk or ride a bike at least 30 minutes three days per week in addition to participating in our program.
If the patient is not viewing the videos, then the prescribing physician and/or the monitoring PT would reach out to the patient by telephone and/or e-mail to encourage the patient to keep up their physical fitness regime. After each series, the patient returns for an office visit to the prescribing physician for blood tests, blood pressure and a weight management check- up as well as a follow-up visit with the PT for assessment of the patient’s progress toward established goals.
Exercise Patient Kits. Most patients will receive a home exercise tool kit which will include: an inflatable exercise ball, a hand pump, a yoga mat, a yoga strap and varying levels of resistance bands. Each of the PHZIO exercise videos will include exercises that incorporate the items in the tool kit. By using a bare minimum of equipment, patients should be able to participate more easily at home or at their workplace. Our estimated cost of the kit is $49, which we pay and factor into a PT licensees’ revenue stream and internal projections. The cost of the exercise kits may also be billed to the patients account.
MSK 360 Program
The musculoskeletal (MSK) system, which consists of our bones, muscles and joints, experience strain as we move. MSK related issues are a leading cause of absenteeism in the workplace and in many cases can lead to short- or long-term disability. These costs are a significant factor in any workplace and have cascading effects on employee productivity. We believe that to accelerate physical health, it is critical to prevent and address MSK timely to reduce future health costs.
Patients can receive virtual care through the MSK 360 Program with the guidance of a registered PT via our Platform through their Access Devices. As patients will not need to travel to their health appointments during the workday, telerehabilitation is a timesaver, and therefore a cost saver.
The employee will first be evaluated to determine the priority of patients’ treatments based on the severity of their condition if they are suitable for our MSK 360 Program. If a patient has experienced a major injury (e.g. fracture), he/she will be instructed to receive in-person PT care.
Any EPS and/or LW insureds may, after an in-home or in-office PT assessment, enroll in a 6-month comprehensive treatment program. The main treatment objective of our MSK360 Program is to graduate at least 60% of inducted patients through our 6-month program. Patients should expect to experience an average of a 20% reduction in BMI, a two-inch reduction in waist size, weight loss of at least 10 pounds, significant overall improvement in balance, coordination, flexibility, strength and lumbopelvic stability. Patients also should score better on Functional Outcomes Scales (Oswestry and LEFS) which indicates improved functional activity levels due to reduced low back, knee and hip pain.
PreHabPT Program
Any individuals covered by EPS and/or LW who are seeking non-emergency orthopedic surgery shall first receive an online consultation, in-home or in-office PT therapy evaluation and will be prescribed a four to eight-week pre-habilitation physical therapy (“PreHabPT”) exercise program prior to any surgery. Another in-home or in-office PT evaluation will be made following surgery and a treatment plan will be initiated. A PreHabPT Program is an eight-week physician to patient pre-surgical (Prehab) digital therapeutic exercise treatment for patients that anticipate having total join replacement (knee, hip and or shoulder) or back surgeries.
| 9 |
PurePT
PurePT is a patient and independent PT Program for connecting new patients to PT’s that are seeking to be treated with our PHZIO treatment system. Patient program assessments can be made in the privacy of a patient’s home or office. PurePT connects new patients to PT’s, particularly in states that have direct access rules where patient’s insurance will reimburse for treatment without requiring a physician’s prescription. PurePT puts the patient first.
Our Marketing Strategy
We pivoted and intensified our focus on generating revenues from PT assessments and treatments covered under the Workers’ Compensation Insurance program where changes in regulations related Workers’ Compensation Insurance have provided us with an opportunity to offer our MSK 360 Program as described above. Under the new regulation patients can choose to be treated in-clinic or through dtPT. Until recently, patients nearly all choose in-clinic treatment. In response to this change we developed our MSK360 Program.
Competition
The healthcare industry, including the physical therapy business, is highly competitive. The physical therapy business is highly fragmented with no company having a significant market share nationally. We believe that we are first dtPT Company to offer real-time monitored physical therapy assessments and treatments to large-scale employers.
The global Telehealth Market is likely to expand considerably with impetus from the ability of telehealth to serve rural populations. According to a report published on August 1, 2019 by Fortune Business Insights, titled “Telehealth Market: Global Market Analysis, Insights, and Forecast, 2019-2026,” the market was valued at US$ 49.8 Billion in 2018. Based on this report, the telehealth market will reach US$ 266.8 Billion by 2026.
Competitive factors affecting our business include quality of care, cost, treatment outcomes, convenience of treatment programs offered, and relationships with, and ability to meet the needs of, referral and payor sources. We compete, directly or indirectly, with many types of healthcare providers including the physical therapy departments of hospitals, private therapy clinics, physician-owned therapy clinics, and chiropractors. We may face more intense competition if consolidation of the therapy industry continues. We believe that our new approach to physical therapy will lower patient treatment costs, expand patient treatment access and improve patient compliance. Our dtPT Platform allows patients to avoid the time-consuming clinical experience to an immediate in-home PT experience. We believe that approximately 80% of all PT assessments and treatments can be performed using our Platform accessible via the Access Devices in the privacy of once home.
We believe that our business model based on digital telehealth physical therapy resulting in potential strategic competitive advantage We also believe that our competitive position is enhanced by our strategy by making physical therapy more easily accessible to patients. We offer convenient hours for the PT assessments and treatments. Our dtPT Platform allows patients to avoid the time-consuming clinical experience to an immediate in-home PT experience. We believe that approximately 80% of all PT assessments and treatments can be performed using our Platform accessible via the Access Devices in the privacy of once home. We have identified, as direct competitors, a number of privately held telemedicine and exercise platform companies as mentioned below:
Physera: Physera provides direct access to world-class physical therapists with personalized exercises programs for convenient and low-cost treatment of musculoskeletal pain. The Company has raised $10.8 million. Estimated revenues are less than $1 million in 2019. Post money valuation is currently $50 million.
Reflexion Health: Reflexion Health, Inc. develops and publishes a prescription software for medical professionals and their patients. It offers a rehab measurement tool to track patient adherence for the prescribed rehab plan. The company was incorporated in 2012 and is based in San Diego, California. The Company has raised $29.8 million. Estimated revenues are just over $1.3 million in 2019. Post money valuation is currently $100 million.
| 10 |
Hinge Health: Hinge Health focuses on musculoskeletal health. Hinge Health’s back and joint pain care pathways combine wearable sensor-guided exercise therapy with behavioral change through 1-on-1 health coaching and education. The Company has raised $36.1 million. Estimated revenues are just over $1.6 million in 2019. Post money valuation is currently $500 million.
Peerwell: PeerWell’s PreHab and ReHab app delivers customized daily lessons to those with scheduled surgery. The Company has raised $9.1 million. Estimated revenues are under $2.5 million in 2019. Post money valuation is currently $50 million.
Force Therapeutics: Force Therapeutics was founded in 2010 to transform the delivery of injury rehabilitation through web and mobile applications. In addition to its smart platform for mobile and web content delivery, FORCE Therapeutics produces high definition, evidenced based exercise videos for its applications. The Company has raised $25.7 million. Estimated revenues are under $1.6 million in 2019. Post money valuation is currently $100 million.
Respondwell: Respondwell offers a tele-rehabilitation platform that enables healthcare service providers to connect, monitor, and collect data about their patients. It provides its services for total joint replacement patients. Respondwell enables patients to access videos of physical therapies that are conducted by virtual instructors. The platform offers in-product rewards to increase patient engagement. It offers two online programs: Therapy@Home and Fitness@home. Estimated revenues are $5 million in 2019. The company is owned by Zimmer Biomet NYSE: ZBH.
We believe that none of the above direct competitors have real time patient monitoring, confirming patient adherence and compliance. We also believe that none of these companies offers an MSK Program, nor the depth of video exercise content and or abilities to monitor one-to-many-patient treatments as offered by our PHZIO Program.
Insurance/Reimbursement
Thus far in the state of California our initial licensee has successfully gained reimbursement from Blue Cross, Blue Shield and CIGNA insurance companies. The licensee receiver reimbursements that are equivalent to in-clinic patient reimbursements. For PT licensee patients, whose insurance companies provide little or no reimbursement for Physical Therapy Telemedicine Reimbursement, they may have higher co-payments for participating in the PHZIO program or be responsible to pay the full cost of such services.
Expansion into other markets where telemedicine has high support. On December 20, 2013, we executed a 25-year licensing agreement with a London, Ontario based telemedicine company Physical Relief Telemedicine Health Care Services (“PRTHCS”), pursuant to which we granted PRTHCS a limited, transferable right to use and promote our PHZIO Program within the province of Ontario; additional Canadian territories may be added at the parties’ mutual discretion. PRTHCS has a known track-record in the telemedicine industry in Canada. To date PRTHCS has been unsuccessful in licensing our PHZIO platform to any Canadian based PT clinics.
Our Planned Expansion into other States where Telemedicine has high support. The most common path being taken by states is to cover telemedicine services in their Medicaid program. 42 states now provide some form of Medicaid reimbursement for telemedicine services (mostly physician to physician consultations). More importantly 16 states have now expanded their definition of telemedicine to include physical therapy and have also required that state and private insurance plans cover telemedicine services. Those 16 states with the broadest telemedicine policies include: Alaska, Georgia, Hawaii, Louisiana, Maine, Maryland, Michigan, Mississippi, Missouri, Montana, New Mexico, Oklahoma, Oregon, Texas, Virginia and Vermont.
Intellectual Property
With adequate funding, we anticipate the patent protection and trademark protection associated with our technology platform and unique physical therapy treatments. We have licensed our telemedicine platform from Bistromatics Inc., a company owned by our CTO, for perpetuity for any telemedicine application in any market worldwide.
| 11 |
REGULATIONS AND HEALTHCARE REFORM
Numerous federal, state and local regulations regulate healthcare services and those who provide them. Some states into which we may expand have laws requiring facilities employing health professionals and providing health-related services to be licensed and, in some cases, to obtain a certificate of need (that is, demonstrating to a state regulatory authority the need for, and financial feasibility of, new facilities or the commencement of new healthcare services). Only one of the states in which we intend to roll out our services requires a certificate of need for the operation of our physical therapy business functions. Our therapists, however, are required to be licensed, as determined by the state in which they provide services. Failure to obtain or maintain any required certificates, approvals or licenses could have a material adverse effect on our business, financial condition and results of operations.
State Legislation
Insurance reimbursement for our PHZIO services is likely to improve in 2019 and beyond based upon current draft legislation in Congress that seeks to significantly expand Medicare’s reimbursement for telemedicine services including for physical therapy. If passed, this legislation would drive private healthcare insurers to also reimburse for physical therapy associated with telemedicine. We have received authorization from the California State Board of Physical Therapy (“CSBPT”) that we could operate our PHZIO platform and bill patients’ insurance within the Association’s rules in the state of California.
On July 21, 2017, bill SB 291 (now P.L.2017, c.117) became effective in New Jersey. The law establishes coverage of telemedicine and telehealth services, both under New Jersey Medicaid and commercial health insurance plans. The law does not explicitly impose a payment parity requirement (i.e., mandating that reimbursement for telemedicine and telehealth services be equal to reimbursement rates for identical in-person services). Instead the law sets the in-person reimbursement rate as the maximum ceiling for telemedicine and telehealth reimbursement rates.
On January 17, 2018 an amendment (“SB 1315”) to the New Jersey Physical Therapy Licensing Act of 1983 (“PT Licensing Act”), became effective. This law expands the scope of practice of PTs to include identification of balance disorders; wound debridement and care; utilization review; screening, examination, evaluation, and application of interventions for the promotion, improvement, and maintenance of fitness, health, wellness, and prevention services in populations of all ages exclusively related to physical therapy practice.
Stark Law
Provisions of the Omnibus Budget Reconciliation Act of 1993 (42 U.S.C. § 1395nn) (the “Stark Law”) prohibit referrals by a physician of “designated health services” which are payable, in whole or in part, by Medicare or Medicaid, to an entity in which the physician or the physician’s immediate family member has an investment interest or other financial relationship, subject to several exceptions. Unlike the Fraud and Abuse Law, the Stark Law is a strict liability statute. Proof of intent to violate the Stark Law is not required. Physical therapy services are among the “designated health services”. Further, the Stark Law has application to the Company’s management contracts with individual physicians and physician groups, as well as, any other financial relationship between us and referring physicians, including any financial transaction resulting from a clinic acquisition. The Stark Law also prohibits billing for services rendered pursuant to a prohibited referral. Several states have enacted laws like the Stark Law. These state laws may cover all (not just Medicare and Medicaid) patients. Many federal healthcare reform proposals in the past few years have attempted to expand the Stark Law to cover all patients as well. As with the Fraud and Abuse Law, we consider the Stark Law in planning our clinics, marketing and other activities, and believe that our operations follow the Stark Law. If we violate the Stark Law, our financial results and operations could be adversely affected. Penalties for violations include denial of payment for the services, significant civil monetary penalties, and exclusion from the Medicare and Medicaid programs.
| 12 |
HIPAA
To further combat healthcare fraud and protect patient confidentially, Congress included several anti-fraud measures in the Health Insurance Portability and Accountability Act of 1996 (“HIPAA”). HIPAA created a source of funding for fraud control to coordinate federal, state and local healthcare law enforcement programs, conduct investigations, provide guidance to the healthcare industry concerning fraudulent healthcare practices, and establish a national data bank to receive and report final adverse actions. HIPAA also criminalized certain forms of health fraud against all public and private insurers. Additionally, HIPAA mandates the adoption of standards regarding the exchange of healthcare information to ensure the privacy and electronic security of patient information and standards relating to the privacy of health information. Sanctions for failing to comply with HIPAA include criminal penalties and civil sanctions. In February of 2009, the American Recovery and Reinvestment Act of 2009 (“ARRA”) was signed into law. Title XIII of ARRA, the Health Information Technology for Economic and Clinical Health Act (“HITECH”), provided for substantial Medicare and Medicaid incentives for providers to adopt electronic health records (“EHRs”) and grants for the development of health information exchange (“HIE”). Recognizing that HIE and EHR systems will not be implemented unless the public can be assured that the privacy and security of patient information in such systems is protected, HITECH also significantly expanded the scope of the privacy and security requirements under HIPAA. Most notable are the new mandatory breach notification requirements and a heightened enforcement scheme that includes increased penalties, and which now apply to business associates as well as to covered entities. In addition to HIPAA, many states have adopted laws and/or regulations applicable in the use and disclosure of individually identifiable health information that can be more stringent than comparable provisions under HIPAA.
We believe that our current business operations are fully compliant with applicable standards for privacy and security of protected healthcare information. We cannot predict what negative effect, if any, HIPAA/HITECH or any applicable state law or regulation will have on our business.
Other Regulatory Factors
Political, economic and regulatory influences are fundamentally changing the healthcare industry in the United States. Congress, state legislatures and the private sector continue to review and assess alternative healthcare delivery and payment systems. Based upon newly finalized FDA rules, we believe that our PHZIO platform is exempt from Federal Drug Administration (“FDA”) regulation. Yet, in the unlikely event that these rules change in the future, the FDA could then require us to seek 510K approvals for our on-line services that could create delays in provisioning our PHZIO services. (See FDA ruling noted below) Also, potential alternative approaches could include mandated basic healthcare benefits, controls on healthcare spending through limitations on the growth of private health insurance premiums, the creation of large insurance purchasing groups, and price controls. Legislative debate is expected to continue in the future and market forces are expected to demand only modest increases or reduced costs. For instance, managed care entities are demanding lower reimbursement rates from healthcare providers and, in some case, are requiring or encouraging providers to accept capitated payments that may not allow providers to cover their full costs or realize traditional levels of profitability. We cannot reasonably predict what impact the adoption of any federal or state healthcare reform measures or future private sector reform may have on our business.
FDA Ruling: Examples of Mobile App’s which it Intends to Exclude from Regulation
On September 25, 2013, the FDA issued Finalized Guidance of medical mobile applications (“Apps”). The FDA has issued a ruling on Apps that may meet the definition of a medical device, but they have determined that they will not exercise enforcement on these Apps. The Guidance contains an appendix that provides examples of mobile apps that MAY meet the definition of medical device but for which FDA intends to exercise enforcement discretion. These mobile apps may be intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease. Even though these mobile apps may meet the definition of medical device, the FDA intends to exercise enforcement discretion for these mobile apps because they pose lower risk to the public. The FDA understands that there may be other unique and innovative mobile apps that may not be covered in this list that may also constitute healthcare related mobile apps. This list is not exhaustive; it is only intended to provide clarity and assistance in identifying the mobile apps that will not be subject to regulatory requirements at this time. Based on our understanding of the Guidance, although there can be no guarantee, we believe our PHZIO platform will not be subject to regulatory requirements because such services seem to fall within the statutory examples.
| 13 |
Employees
At the year ended December 31, 2019, we had 2 employees in the United States, approximately 7 employees through Bistromatics in Canada and various consultants. We utilize the services of consultants for safety testing, regulatory and legal compliance and other services.
Legal Proceedings
In June 2018, a settlement agreement was signed between the Company and holder of the note payable with the following terms for the cancellation of the note payable, accrued interest and all warrants granted relating to the various notes:
| 1. | The Company will issue 54,189 shares of commons stock that is immediately tradeable under Securities and Exchange Commission Rule 144, but subject to a daily trading limit of 500 shares per day; | |
| 2. | The Company will issue 25,811 shares of common stock that shall be subject to a 180-day holding period and are also subject to a daily trading limit of 25,000 shares per day; | |
| 3. | The holder of the note payable shall bear all fees and expenses, including attorneys’ fees, associated with the transfer and trading of the Company’s shares; | |
| 4. | Beyond issuing the shares noted above, the Company shall not take any additional action that would cause the note holder to incur tax consequence from the transfer or would affect the note holder’s tax consequences in any way. |
The Company issued the 80,000 shares of common stock on June 20, 2018. At December 31, 2019, the Company had no indebtedness to this holder of the note payable of principal or accrued interest or exercisable warrants relating to the note.
Transfer Agent
The transfer agent of the Company’s stock is VStock Transfer LLC, 18 Lafayette Place, Woodmere, NY 11598, (212) 828-8436.
NOTES REGARDING FORWARD-LOOKING STATEMENTS
The statements contained in this annual report are not purely historical are forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Exchange Act. These include statements about the Company’s expectations, beliefs, intentions or strategies for the future, which are indicated by words or phrases such as “anticipate,” “expect,” “intend,” “plan,” “will,” “the Company believes,” “management believes” and similar words or phrases. The forward-looking statements are based on the Company’s current expectations and are subject to certain risks, uncertainties and assumptions. The Company’s actual results could differ materially from results anticipated in these forward-looking statements. All forward-looking statements included in this document are based on information available to the Company on the date hereof, and the Company assumes no obligation to update any such forward-looking statements.
Investing in our securities involves a great deal of risk. Careful consideration should be made of the following factors as well as other information included in this Annual Report before deciding to purchase our common stock. Our business, financial condition or results of operations could be affected materially and adversely by any or all of these risks.
THE FOLLOWING MATTERS MAY HAVE A MATERIAL ADVERSE EFFECT ON OUR BUSINESS, FINANCIAL CONDITION, LIQUIDITY, RESULTS OF OPERATIONS OR PROSPECTS, FINANCIAL OR OTHERWISE. REFERENCE TO THIS CAUTIONARY STATEMENT IN THE CONTEXT OF A FORWARD-LOOKING STATEMENT OR STATEMENTS SHALL BE DEEMED TO BE A STATEMENT THAT ANY ONE OR MORE OF THE FOLLOWING FACTORS MAY CAUSE ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE IN SUCH FORWARD-LOOKING STATEMENT OR STATEMENTS.
| 14 |
Risks Related to our Financial Condition
Our Independent Registered Public Accounting Firm has expressed substantial doubt as to our ability to continue as a going concern.
The audited financial statements have been prepared assuming that we will continue as a going concern and do not include any adjustments that might result if we cease to continue as a going concern. We believe that to continue as a going concern we will need approximately $500,000 per year simply to cover the administrative, legal and accounting fees. We plan to fund these expenses primarily through cash flow, the sale of restricted shares of our common stock and the issuance of convertible notes.
Based on our financial statements for the years ended December 31, 2019 and 2018, our independent registered public accounting firm has expressed substantial doubt as to our ability to continue as a going concern. To date we have not generated any revenue.
Investing in our securities involves a great deal of risk. Careful consideration should be made of the following factors as well as other information included in this Prospectus before deciding to purchase our common stock. Our business, financial condition or results of operations could be affected materially and adversely by any or all these risks.
We may need to raise additional capital to fund continuing operations and an inability to raise the necessary capital or to do so on acceptable terms could threaten the success of our business.
To date, our operations have been funded entirely from the proceeds from equity and debt financings or loans from our management. We currently anticipate that our available capital resources will be insufficient to meet our expected working capital and capital expenditure requirements for the near future. We anticipate that we will require an additional $1.5 million during the next twelve months to fulfill our business plan. However, such resources may not be sufficient to fund the long-term growth of our business. If we determine that it is necessary to raise additional funds, we may choose to do so through strategic collaborations, licensing arrangements through our “White Labeling” strategy, public or private equity or debt financing, a bank line of credit, or other arrangements.
We cannot be sure that any additional funding will be available on terms favorable to us or at all. Any additional equity financing may be dilutive to our shareholders, new equity securities may have rights, preferences or privileges senior to those of existing holders of our shares of Common stock. Debt or equity financing may subject us to restrictive covenants and significant interest costs. If we obtain funding through a strategic collaboration or licensing arrangement, we may be required to relinquish our rights to our product or marketing territories. If we are unable to obtain the financing necessary to support our operations, we may be required to defer, reduce or eliminate certain planned expenditures or significantly curtail our operations.
We have a history of net losses; we may never achieve or sustain profitability or positive cash flow from operations.
We have incurred net losses in each fiscal year since our inception, including net losses of $9,460,785 for the year ended December 31, 2019 and $4,451,462 for the year ended December 31, 2018. As of the year ended December 31, 2019, we had an accumulated deficit of $30,862,019. We expect to continue to incur substantial expenditures to develop and market our services and could continue to incur losses and negative operating cash flow for the foreseeable future. We may never achieve profitability or positive cash flow in the future, and even if we do, we may not be able to continue being profitable.
We have a limited operating history under our current platform, it is difficult to evaluate our business and future prospects and increases the risks associated with investment in our securities.
We have operated our PHIZO platform since November 2014. As a result, our platform and business model have not been fully proven, and we have only a limited operating history on which to evaluate our business and future prospects. We have encountered, and will continue to encounter, risks and difficulties frequently experienced by growing companies in rapidly changing industries, including our ability to achieve market acceptance of our platform and attract, retain and incentivize clients to use our platform, as well as respond to competition and plan for and scale our operations to address future growth. We may not be successful in addressing these and other challenges we may face in the future, and our business and future prospects may be materially and adversely affected if we do not manage these and other risks successfully. Given our limited operating history, we may be unable to effectively implement our business plan which could materially harm our business or cause us to scale down or cease our operations.
| 15 |
Risks Related to our Platform and our Business
Our Platform may not be accepted in the marketplace.
Uncertainty exists as to whether our Platform will be accepted by the market without additional widespread PT or patient acceptance. Several factors may limit the market acceptance of our Platform, including the availability of alternative products and services as well as the price of our Platform services relative to alternative products. There is a risk that PT or patient acceptance will be encouraged to continue to use other products and/or methods instead of ours. We are assuming that, notwithstanding the fact that our Platform is new in the market, PT or patient acceptance will elect to use our Platform because it will permit to safe valuable PT’s time.
PT or patient needs to be persuaded that our Platform service is justified for the anticipated benefit, but there is no assurance that enough numbers of patients will be convinced to enable a successful market to develop for our Platform.
Our revenues will be dependent upon acceptance of our Platform product by the market. The failure of such acceptance will cause us to curtail or cease operations.
Our revenues are expected to come from our Platform. As a result, we will continue to incur operating losses until such time as revenues reach a mature level and we are able to generate enough revenues from our Platform to meet our operating expenses. There can be no assurance that PTs or patients will adopt our Platform. If we are not able to market and significantly increase the number of PTs or patients that use our Platform, or if we are unable to charge the necessary prices, our financial condition and results of operations will be materially and adversely affected.
Defects or malfunctions in our Platform could hurt our reputation, sales and profitability.
The acceptance of our Platform depends upon its effectiveness and reliability. Our Platform is complex and is continually being modified and improved, and as such may contain undetected defects or errors when first introduced or as new versions are released. To the extent that defects or errors cause our Platform to malfunction and our customers’ use of our Platform is interrupted, our reputation could suffer, and our potential revenues could decline or be delayed while such defects are remedied. We may also be subject to liability for the defects and malfunctions.
There can be no assurance that, despite our testing, errors will not be found in our Platform or new releases, resulting in loss of future revenues or delay in market acceptance, diversion of development resources, damage to our reputation, adverse litigation, or increased service, any of which would have a material adverse effect upon our business, operating results and financial condition.
Software failures, breakdowns in the operations of our servers and communications systems or the failure to implement system enhancements could harm our business.
Our success depends on the efficient and uninterrupted operation of our servers and communications systems. A failure of our network or data gathering procedures could impede services and could result in the loss of PT and patients. While our operations will have disaster recovery plans in place, they might not adequately protect us. Despite any precautions we take, damage from fire, floods, hurricanes, power loss, telecommunications failures, computer viruses, break-ins and similar events at our computer facilities could result in interruptions in the flow of data to our servers and from our servers to our clients. In addition, any failure by our computer environment to provide our required data communications capacity could result in interruptions in our service. In the event of a server failure, we could be required to transfer our client data collection operations to an alternative provider of server hosting services. Such a transfer could result in delays in our ability to deliver our products and services to our clients.
| 16 |
Additionally, significant delays in the planned delivery of system enhancements, improvements and inadequate performance of the systems once they are completed could damage our reputation and harm our business. Long-term disruptions in the infrastructure caused by events such as natural disasters, the outbreak of war, the escalation of hostilities and acts of terrorism, particularly involving cities in which we have offices, could adversely affect our businesses. Although, we plan to carry property and business interruption insurance for our business operations, our coverage might not be adequate to compensate us for all losses that may occur.
We face risks related to the storage of customers’ and their end users’ confidential and proprietary information.
Our Platform is designed to maintain the confidentiality and security of our patients’ confidential and proprietary data that are stored on our server systems, which may include sensitive personal data. However, any accidental or willful security breaches or other unauthorized access to these data could expose us to liability for the loss of such information, time-consuming and expensive litigation and other possible liabilities as well as negative publicity. Techniques used to obtain unauthorized access or to sabotage systems change frequently and generally are difficult to recognize and react to. We may be unable to anticipate these techniques or implement adequate preventative or reactionary measures.
We might incur substantial expense to further develop our Platform which may never become sufficiently successful.
Our growth strategy requires the successful launch of our Platform. Although management will take every precaution to ensure that our Platform will, with a high degree of likelihood, achieve commercial success, there can be no assurance that this will be the case. The causes for failure of our Platform once commercialized can be numerous, including:
| ● | market demand for our Platform proves to be smaller than we expect; | |
| ● | further Platform development turns out to be costlier than anticipated or takes longer; our Platform requires significant adjustment post commercialization, rendering the Platform uneconomic or extending considerably the likely investment return period; additional regulatory requirements may increase the overall costs of the development; patent conflicts or unenforceable intellectual property rights; and PTs and clients may be unwilling to adopt and/or use our Platform. | |
| ● | Compliance with changing regulations concerning corporate governance and public disclosure may result in additional expenses. |
We are required to comply with certain provisions of Section 404 of the Sarbanes-Oxley Act of 2002 and if we fail to comply in a timely manner, our business could be harmed, and our stock price could decline.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require an annual assessment of internal controls over financial reporting, and for certain issuers an attestation of this assessment by the issuer’s independent registered public accounting firm. The standards that must be met for management to assess the internal controls over financial reporting as effective are evolving and complex, and require significant documentation, testing, and possible remediation to meet the detailed standards.
We expect to incur expenses and to devote resources to Section 404 compliance on an ongoing basis. It is difficult for us to predict how long it will take or costly it will be to complete the assessment of the effectiveness of our internal control over financial reporting for each year and to remediate any deficiencies in our internal control over financial reporting. As a result, we may not be able to complete the assessment and remediation process on a timely basis. In addition, although attestation requirements by our independent registered public accounting firm are not presently applicable to us, we could become subject to these requirements in the future and we may encounter problems or delays in completing the implementation of any resulting changes to internal controls over financial reporting. If our Chief Executive Officer or Chief Financial Officer determine that our internal control over financial reporting is not effective as defined under Section 404, we cannot predict how the market prices of our shares will be affected; however, we believe that there is a risk that investor confidence and share value may be negatively affected.
| 17 |
These and other new or changed laws, rules, regulations and standards are, or will be, subject to varying interpretations in many cases due to their lack of specificity. As a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies, which could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. Our efforts to comply with evolving laws, regulations and standards are likely to continue to result in increased general and administrative expenses and a diversion of management time and attention from revenue-generating activities to compliance activities. Further, compliance with new and existing laws, rules, regulations and standards may make it more difficult and expensive for us to maintain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. Members of our board of directors and our principal executive officer and principal financial officer could face an increased risk of personal liability in connection with the performance of their duties. As a result, we may have difficulty attracting and retaining qualified directors and executive officers, which could harm our business. We continually evaluate and monitor regulatory developments and cannot estimate the timing or magnitude of additional costs we may incur as a result.
We cannot be certain that we will obtain patents for our Platform and technology or that such patents will protect us from competitors.
We believe that our success and competitive position will depend in part on our ability to obtain and maintain patents for our Platform, which is both costly and time consuming. We still are in the process to evaluate the patent potentials of our Platform. Patent Offices typically requires 12-24 months or more to process a patent application. There can be no assurance that any of our potential patent applications will be approved. However, we have decided to launch our Platform without patent protection. There can be no assurance that any potential patent issued or licensed to us will provide us with protection against competitive products, protect us against changes in industry trends which we have may not have anticipated or otherwise protect the commercial viability of our Platform, or that challenges will not be instituted against the validity or enforceability of any of our future patents or, if instituted, that such challenges will not be successful. The cost of litigation to uphold the validity of a patent and enforce it against infringement can be substantial. Even issued patents may later be modified or revoked by the Patent and Trademark Office or in legal proceedings. Patent applications in the United States are maintained in secrecy until the patent issues and, since publication of patents tends to lag behind actual discoveries, we cannot be certain that if we obtain patents for our product, we were the first creator of the inventions covered by a pending patent applications or the first to file patent applications on such inventions.
Liability issues are inherent in the Healthcare industry and insurance is expensive and difficult to obtain, we may be exposed to large lawsuits.
Our business exposes us to potential liability risks, which are inherent in the healthcare industry. While we will take precautions, we deem to be appropriate to avoid liability suits against us, there can be no assurance that we will be able to avoid significant liability exposure. Liability insurance for the healthcare industry is generally expensive. We have obtained professional indemnity insurance coverage for our Platform. There can be no assurance that we will be able to maintain such coverage on acceptable terms, or that any insurance policy will provide adequate protection against potential claims. A successful liability claim brought against us may exceed any insurance coverage secured by us and could have a material adverse effect on our results or ability to continue our Platform.
We depend upon reimbursement by third-party payers.
Substantially all revenues are anticipated to be derived from private third-party PT clinics that gain their revenue to pay our licensing fees from insurance payers. Initiatives undertaken by industry and government to contain healthcare costs affect the profitability of our licensee clinics. These payers attempt to control healthcare costs by contracting with healthcare providers to obtain services on a discounted basis. We believe that this trend will continue and may limit reimbursement for healthcare services. If insurers or managed care companies from whom we receive substantial payments were to reduce the amounts paid for services, our profit margins may decline, or we may lose PT licensees if they choose not to renew our contracts with these insurers at lower rates. In addition, in certain geographical areas, our operations may be approved as providers by key health maintenance organizations and preferred provider plans; failure to obtain or maintain these approvals would adversely affect our financial results. Although we created a business plan that will enable us to achieve revenue based on current reimbursement policies, if our belief that the insurance industry is poised for change, to offer more reimbursement for the services we seek to provide is not realized, we may not achieve the success we predict and we may not be able to carry out all the plans we disclose herein related to telemedicine. Ultimately, a shift in thinking and a willingness to adapt to new physical therapy telemedicine services and reimbursement thereof by healthcare providers is needed for the successful integration of our PHZIO telemedicine platform in mainstream healthcare environments.
| 18 |
We will need to increase the size of our organization and may experience difficulties in managing growth.
At present, we are a small company. We expect to experience a period of expansion in headcount, infrastructure and overhead and anticipate that further expansion will be required to address potential growth and market opportunities. Future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate new managers. Our future financial performance and its ability to compete effectively will depend, in part, on its ability to manage any future growth effectively.
Dependence on Key Existing and Future Personnel
Our success will depend, to a large degree, upon the efforts and abilities of our officers and key management employees. The loss of the services of one or more of our key employees could have a material adverse effect on our operations. In addition, as our business model is implemented, we will need to recruit and retain additional management and key employees in virtually all phases of our operations. Key employees will require a strong background in our industry. We cannot assure that we will be able to successfully attract and retain key personnel.
Currently, our management’s participation in our business and operations is limited
To date, we have been unable to offer cash compensation to our officers due to our lack of revenue. Accordingly, each of the Company’s executive officers maintain jobs outside of their position at eWellness. Although each of our executive officers have prepared to devote their efforts, on a full-time basis, towards our objectives once we can afford executive compensation commensurate with that being paid in the marketplace, until such time, our officers will not devote their full time and attention to the operations of the Company. None of our officers have committed a specific portion of their time or an approximate number of hours per week in writing to the objectives of the company and no assurances can be given as to when we will be financially able to engage our officers on a full-time basis and therefore, until such time, it is possible that the inability of such persons to devote their full-time attention to the Company may result in delays in progress toward implementing our business plan.
We operate in a highly competitive industry
Although we are not aware of any other Distance Monitored Physical Therapy Telemedicine Program precisely like ours, and targeting our specific population, we shall encounter competition from local, regional or national entities, some of which have superior resources or other competitive advantages in the larger physical therapy space. Intense competition may adversely affect our business, financial condition or results of operations. We may also experience competition from companies in the wellness space. These competitors may be larger and more highly capitalized, with greater name recognition. We will compete with such companies on brand name, quality of services, level of expertise, advertising, product and service innovation and differentiation of product and services. As a result, our ability to secure significant market share may be impeded. Although we believe our PHZIO services will enable us to service more patients than traditional physical therapy providers, if these more established offices or providers start offering similar services to ours, their name recognition or experience may enable them to capture a greater market share.
Limited product testing and operations
We have built out the technology platform and video library necessary to execute our planned business strategy. Of course, there may be other factors that prevent us from successfully marketing a product including, but not limited to, our limited cash resources. Further, our proposed reimbursement plan and the eventual operating results could susceptible to varying interpretations by scientists, medical personnel, regulatory personnel, statisticians and others, which may delay, limit or prevent our executing our proposed business plan.
| 19 |
We face substantial competition, and others may discover, develop, acquire or commercialize products before or more successfully than we do
We operate in a highly competitive environment. Our products compete with other products or treatments for diseases for which our products may be indicated. Other healthcare companies have greater clinical, research, regulatory and marketing resources than us. In addition, some of our competitors may have technical or competitive advantages for the development of technologies and processes. These resources may make it difficult for us to compete with them to successfully discover, develop and market new products.
We depend upon the cultivation and maintenance of relationships with the physicians in our markets.
Our success is dependent upon referrals from physicians in the communities that our PT licensees will service and their ability to maintain good relations with these physicians and other referral sources. Physicians referring patients to their clinics are free to refer their patients to other therapy providers or to their own physician owned therapy practice. If our PT licensees are unable to successfully cultivate and maintain strong relationships with physicians and other referral sources, our business may decrease, and our net operating revenues may decline.
We also depend upon our ability to recruit and retain experienced PTs
Our future revenue generation is dependent upon referrals from physicians in the communities our clinics serve, and our ability to maintain good relations with these physicians. Our PT licensees are the front line for generating these referrals and we are dependent on their talents and skills to successfully cultivate and maintain strong relationships with these physicians. If they cannot recruit and retain our base of experienced and clinically skilled therapists, our business may decrease, and our net operating revenues may decline.
Our revenues may fluctuate due to weather
We anticipate having a considerable number of PT licensees in locations in states that normally experience snow and ice during the winter months. Also, a considerable number of our clinics may be in states along the Gulf Coast and Atlantic Coast, which are subject to periodic winter storms, hurricanes and other severe storm systems. Periods of severe weather may cause physical damage to our facilities or prevent our staff or patients from traveling to our clinics, which may cause a decrease in our future net operating revenues.
We may incur closure costs and losses
The competitive, economic or reimbursement conditions in the markets in which we operate may require us to reorganize or to close certain clinical locations. In the event a clinic is reorganized or closed, we may incur losses and closure costs. The closure costs and losses may include, but are not limited to, lease obligations, severance, and write-down or write-off of intangible assets.
Certain of our internal controls, particularly as they relate to billings and cash collections, are largely decentralized at our clinic locations
Our future PT licensees’ operations are largely decentralized and certain of our internal controls, particularly the processing of billings and cash collections, occur at the clinic level. Taken as a whole, we believe our future internal controls for these functions at our PT licensees’ clinical facilities will be adequate. Our controls for billing and collections largely depend on compliance with our written policies and procedures and separation of functions among clinic personnel. We also intend to maintain corporate level controls, including an audit compliance program, that are intended to mitigate and detect any potential deficiencies in internal controls at the clinic level. The effectiveness of these controls to future periods are subject to the risk that controls may become inadequate because of changes in conditions or the level of compliance with our policies and procedures deteriorates.
| 20 |
Risks Related to Regulation
Our products may be subject to product liability legal claims, which could have an adverse effect on our business, results of operations and financial condition.
Certain of our products provide applications that relate to patient clinical information. Any failure by our products to provide accurate and timely information concerning patients, their medication, treatment and health status, generally, could result in claims against us which could materially and adversely impact our financial performance, industry reputation and ability to market new system sales. In addition, a court or government agency may take the position that our delivery of health information directly, including through licensed practitioners, or delivery of information by a third-party site that a consumer accesses through our websites, exposes us to assertions of malpractice, other personal injury liability, or other liability for wrongful delivery/handling of healthcare services or erroneous health information. We anticipate that in the future we will maintain insurance to protect against claims associated with the use of our products as well as liability limitation language in our end-user license agreements, but there can be no assurance that our insurance coverage or contractual language would adequately cover any claim asserted against us. A successful claim brought against us in excess of or outside of our insurance coverage could have an adverse effect on our business, results of operations and financial condition. Even unsuccessful claims could result in our expenditure of funds for litigation and management time and resources.
Certain healthcare professionals who use our Cloud-based products will directly enter health information about their patients including information that constitutes a record under applicable law that we may store on our computer systems. Numerous federal and state laws and regulations, the common law and contractual obligations, govern collection, dissemination, use and confidentiality of patient-identifiable health information, including:
| ● | state and federal privacy and confidentiality laws; | |
| ● | contracts with clients and partners; | |
| ● | state laws regulating healthcare professionals; | |
| ● | Medicaid laws; | |
| ● | the HIPAA and related rules proposed by the Health Care Financing Administration; and | |
| ● | Health Care Financing Administration standards for Internet transmission of health data. |
HIPAA establishes elements including, but not limited to, federal privacy and security standards for the use and protection of Protected Health Information. Any failure by us or by our personnel or partners to comply with applicable requirements may result in a material liability to us.
Although we have systems and policies in place for safeguarding Protected Health Information from unauthorized disclosure, these systems and policies may not preclude claims against us for alleged violations of applicable requirements. Also, third party sites and/or links that consumers may access through our web sites may not maintain adequate systems to safeguard this information or may circumvent systems and policies we have put in place. In addition, future laws or changes in current laws may necessitate costly adaptations to our policies, procedures, or systems.
There can be no assurance that we will not be subject to product liability claims, that such claims will not result in liability in excess of our insurance coverage, that our insurance will cover such claims or that appropriate insurance will continue to be available to us in the future at commercially reasonable rates. Such product liability claims could adversely affect our business, results of operations and financial condition.
There is significant uncertainty in the healthcare industry in which we operate, and we are subject to the possibility of changing government regulation, which may adversely impact our business, financial condition and results of operations.
The healthcare industry is subject to changing political, economic and regulatory influences that may affect the procurement processes and operation of healthcare facilities. During the past several years, the healthcare industry has been subject to an increase in governmental regulation of, among other things, reimbursement rates and certain capital expenditures.
| 21 |
Recently enacted public laws reforming the U.S. healthcare system may have an impact on our business. The Patient Protection and Affordable Care Act (H.R. 3590; Public Law 111-148) (“PPACA”) and The Health Care and Education Reconciliation Act of 2010 (H.R. 4872) (the “Reconciliation Act”), which amends the PPACA (collectively the “Health Reform Laws”), were signed into law in March 2010. The Health Reform Laws contain various provisions which may impact us and our patients. Some of these provisions may have a positive impact, while others, such as reductions in reimbursement for certain types of providers, may have a negative impact due to fewer available resources. Increases in fraud and abuse penalties may also adversely affect participants in the health care sector, including us.
Various legislators have announced that they intend to examine further proposals to reform certain aspects of the U.S. healthcare system. Healthcare providers may react to these proposals, and the uncertainty surrounding such proposals, by curtailing or deferring investments, including those for our systems and related services. Cost-containment measures instituted by healthcare providers as a result of regulatory reform or otherwise could result in a reduction of the allocation of capital funds. Such a reduction could have an adverse effect on our ability to sell our systems and related services. On the other hand, changes in the regulatory environment have increased and may continue to increase the needs of healthcare organizations for cost-effective data management and thereby enhance the overall market for healthcare management information systems. We cannot predict what effect, if any, such proposals or healthcare reforms might have on our business, financial condition and results of operations.
As existing regulations mature and become better defined, we anticipate that these regulations will continue to directly affect certain of our products and services, but we cannot fully predict the effect now. We have taken steps to modify our products, services and internal practices as necessary to facilitate our compliance with the regulations, but there can be no assurance that we will be able to do so in a timely or complete manner. Achieving compliance with these regulations could be costly and distract management’s attention and divert other company resources, and any noncompliance by us could result in civil and criminal penalties.
Developments of additional federal and state regulations and policies have the potential to positively or negatively affect our business. Our software is not anticipated to be considered a medical device by the FDA. Yet, if it were, it could be subject to regulation by the U.S. Food and Drug Administration (“FDA”) as a medical device. Such regulation could require the registration of the applicable manufacturing facility and software and hardware products, application of detailed record-keeping and manufacturing standards, and FDA approval or clearance prior to marketing. An approval or clearance requirement could create delays in marketing, and the FDA could require supplemental filings or object to certain of these applications, the result of which could adversely affect our business, financial condition and results of operations.
We may be subject to false or fraudulent claim laws
There are numerous federal and state laws that forbid submission of false information or the failure to disclose information in connection with submission and payment of physician claims for reimbursement. In some cases, these laws also forbid abuse of existing systems for such submission and payment. Any failure of our services to comply with these laws and regulations could result in substantial liability including, but not limited to, criminal liability, could adversely affect demand for our services and could force us to expend significant capital, research and development and other resources to address the failure. Errors by us or our systems with respect to entry, formatting, preparation or transmission of claim information may be determined or alleged to be in violation of these laws and regulations. Determination by a court or regulatory agency that our services violate these laws could subject us to civil or criminal penalties, invalidate all or portions of some of our client contracts, require us to change or terminate some portions of our business, require us to refund portions of our services fees, cause us to be disqualified from serving clients doing business with government payers and have an adverse effect on our business.
| 22 |
We are subject to the Stark Law, which may result in significant penalties
Provisions of the Omnibus Budget Reconciliation Act of 1993 (42 U.S.C. § 1395nn) (the “Stark Law”) prohibit referrals by a physician of “designated health services” which are payable, in whole or in part, by Medicare or Medicaid, to an entity in which the physician or the physician’s immediate family member has an investment interest or other financial relationship, subject to several exceptions. Unlike the Fraud and Abuse Law, the Stark Law is a strict liability statute. Proof of intent to violate the Stark Law is not required. Physical therapy services are among the “designated health services”. Further, the Stark Law has application to the Company’s management contracts with individual physicians and physician groups, as well as, any other financial relationship between us and referring physicians, including any financial transaction resulting from a clinic acquisition. The Stark Law also prohibits billing for services rendered pursuant to a prohibited referral. Several states have enacted laws similar to the Stark Law. These state laws may cover all (not just Medicare and Medicaid) patients. Many federal healthcare reform proposals in the past few years have attempted to expand the Stark Law to cover all patients as well. As with the Fraud and Abuse Law, we consider the Stark Law in planning our clinics, marketing and other activities and believe that our operations follow the Stark Law. If we violate the Stark Law, our financial results and operations could be adversely affected. Penalties for violations include denial of payment for the services, significant civil monetary penalties, and exclusion from the Medicare and Medicaid programs.
If our products fail to comply with evolving government and industry standards and regulations, we may have difficulty selling our products
We may be subject to additional federal and state statutes and regulations in connection with offering services and products via the Internet. On an increasingly frequent basis, federal and state legislators are proposing laws and regulations that apply to Internet commerce and communications. Areas being affected by these regulations include user privacy, pricing, content, taxation, copyright protection, distribution, and quality of products and services. To the extent that our products and services are subject to these laws and regulations, the sale of our products and services could be harmed.
We incur significant costs as a result of operating as a public company and our management will have to devote substantial time to public company compliance obligations
The Sarbanes-Oxley Act of 2002, as well as rules subsequently implemented by the Securities and Exchange Commission (“SEC”), and the stock exchange, has imposed various requirements on public companies, including requiring changes in corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance requirements and any new requirements that the Dodd-Frank Wall Street Reform and Consumer Protection Act of 2010 may impose on public companies. Moreover, these rules and regulations, along with compliance with accounting principles and regulatory interpretations of such principles, have increased and will continue to increase our legal, accounting and financial compliance costs and have made and will continue to make some activities more time consuming and costly. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced policy limits and coverage or incur substantial costs to maintain the same or similar coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors or our board committees, or as executive officers. We will evaluate the need to hire additional accounting and financial staff with appropriate public company experience and technical accounting and financial knowledge. We estimate the additional costs we expect to incur as a result of being a public company to be up to $500,000 annually.
Part of the requirements as a public company will be to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, which requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent registered public accounting firm addressing these assessments. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company.
Effective internal controls are necessary for us to provide reliable financial reports and to effectively prevent fraud. We maintain a system of internal control over financial reporting, which is defined as a process designed by, or under the supervision of, our principal executive officer and principal financial officer, or persons performing similar functions, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
| 23 |
We cannot assure you that we will not, in the future, identify areas requiring improvement in our internal control over financial reporting. We cannot assure that the measures we will take to remediate any areas in need of improvement will be successful or that we will implement and maintain adequate controls over our financial processes and reporting in the future as we continue our growth. If we are unable to maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, cause investors to lose confidence in our reported financial information and have a negative effect on the market price for shares of our common stock.
The regulatory framework for privacy and data protection is complex and evolving, and changes in laws or regulations relating to privacy or the protection or transfer of personal data, or any actual or perceived failure by us to comply with such laws and regulations, could adversely affect our business.
In the course of our day-to-day business operations we receive and use personal information and other user data. As the result, we are subject to numerous federal, state and local laws and regulations regarding privacy, data protection and protection of personal information. We are also subject to the terms of our privacy policies and obligations to third parties related to privacy, data protection, and information security. We strive to comply with applicable laws, regulations, policies, and other legal obligations relating to privacy, data protection, and information security to the extent possible. However, the regulatory framework for privacy and data protection in the United States is, and is likely to remain for the foreseeable future, uncertain and complex, is changing, and the interpretation and enforcement of the rules and regulations that form part of this regulatory framework may be inconsistent among jurisdictions, or conflict with other laws and regulations. Such laws and regulations as they apply to us may be interpreted and enforced in a manner that we do not currently anticipate. Any significant change in the applicable laws, regulations, or industry practices regarding the collection, use, retention, security, or disclosure of user data, or their interpretation, or any changes regarding the manner in which the express or implied consent of users for the collection, use, retention, or disclosure of such data must be obtained, could increase our costs and require us to modify our platform and our products and services, in a manner that could materially affect our business.
The laws, regulations, and industry standards concerning privacy, data protection, and information security also continue to evolve. For example, in June 2018, California passed the California Consumer Privacy Act (the “CCPA”), effective January 1, 2020, which requires companies that process personal information of California residents to make new disclosures to consumers about such companies’ data collection, use, and sharing practices and inform consumers of their personal information rights such as deletion rights, allows consumers to opt out of certain data sharing with third parties, and provides a new cause of action for data breaches. The State of Nevada has also passed a law, effective October 1, 2019, that amends the state’s online privacy law to allow consumers to submit requests to prevent websites and online service providers from selling personally identifiable information that they collect through a website or online service. The costs of compliance with, and other burdens imposed by, the privacy and data protection laws and regulations may limit the use and adoption of our services and could have a material adverse impact on our business. As a result, we may need to modify the way we treat such information.
Any failure or perceived failure by us to comply with any privacy and data protection policies, laws, rules, and regulations could result in proceedings or actions against us by individuals, consumer rights groups, governmental entities or agencies, or others. We could incur significant costs investigating and defending such claims and, if found liable, significant damages. Further, public scrutiny of or complaints about technology companies or their data handling or data protection practices, even if unrelated to our business, industry, or operations, may lead to increased scrutiny of technology companies, including us, and may cause government agencies to enact additional regulatory requirements, or to modify their enforcement or investigation activities, which may increase our costs and risks.
If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and the Sarbanes-Oxley Act which requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal control over financial reporting, we have expended, and anticipate that we will continue to expend, significant resources, including accounting-related costs and significant management oversight.
| 24 |
Our management concluded that our disclosure controls and procedures were not effective as of December 31, 2019. Any failure to develop or maintain effective controls or any difficulties encountered in their implementation or improvement could cause us to fail to meet our reporting obligations and may result in a restatement of our financial statements for prior periods. If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to produce timely and accurate financial statements or comply with applicable regulations could be impaired, which could result in loss of investor confidence and could have an adverse effect on our stock price.
Risks Relating to Our Securities
There is a limited market for our common stock, and there may never be, an active market for our common stock and we cannot assure you that the common stock will remain liquid or that it will continue to be listed on a securities exchange.
Our common stock is listed on the pink sheets and trades under the symbol “EWLLD”. An investor may find it difficult to obtain accurate quotations as to the market value of the common stock and trading of our common stock may be extremely sporadic. For example, several days may pass before any shares may be traded. A more active market for the common stock may never develop. In addition, if we fail to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling the common stock, which may further affect its liquidity. This would also make it more difficult for us to raise additional capital.
Until our common stock is listed on the NASDAQ or another stock exchange, we expect that our common stock will continue to be eligible to trade on the OTC Bulletin Board, another over-the-counter quotation system, or on the “pink sheets,” where our stockholders may find it more difficult to dispose of shares or obtain accurate quotations as to the market value of our common stock.
Our Common stock is subject to the “Penny Stock” rules of the SEC and the trading market in our stock is limited, which makes transactions in our stock cumbersome and may reduce the value of an investment.
The Securities and Exchange Commission has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| ● | That a broker or dealer approve a person’s account for transactions in penny stocks; and |
| ● | The broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
| ● | Obtain financial information and investment experience objectives of the person; and |
| ● | Make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. |
| 25 |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the Commission relating to the penny stock market, which, in highlight form:
| ● | Sets forth the basis on which the broker or dealer made the suitability determination; and |
| ● | That the broker or dealer received a signed, written agreement from the investor prior to the transaction. |
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of our Common stock and cause a decline in the market value of our stock.
Disclosure also must be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements must be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Financial Industry Regulatory Authority, Inc. (“FINRA”) sales practice requirements may limit a shareholder’s ability to buy and sell our common stock.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our Common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
Our common stock is thinly traded, sale of your holding may take a considerable amount of time.
Our shares of our common stock are thinly traded on the OTCQB Market, meaning that the number of persons interested in purchasing our common stock at or near bid prices at any given time may be relatively small or non-existent. Consequently, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained. Due to these conditions, we can give you no assurance that you will be able to sell your shares at or near bid prices or at all if you need money or otherwise desire to liquidate your shares.
Shares eligible for future sale may adversely affect the market.
From time to time, certain of our stockholders may be eligible to sell all or some of their shares of common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act, subject to certain limitations. In general, pursuant to amended Rule 144, non-affiliate stockholders may sell freely after six months subject only to the current public information requirement. Affiliates may sell after six months subject to the Rule 144 volume, manner of sale (for equity securities), current public information and notice requirements. Any substantial sales of our common stock pursuant to Rule 144 may have a material adverse effect on the market price of our common stock.
| 26 |
If we fail to maintain effective internal controls over financial reporting, the price of our common stock may be adversely affected.
Our internal control over financial reporting may have weaknesses and conditions that could require correction or remediation, the disclosure of which may have an adverse impact on the price of our common stock. We are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely affect our public disclosures regarding our business, prospects, financial condition or results of operations. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
Our share price could be volatile, and our trading volume may fluctuate substantially.
The price of our common shares has been and may in the future continue to be extremely volatile, with the sale price fluctuating from a low of $0.001 to a high of $200.00 since trading began in 2016. Many factors could have a significant impact on the future price of our common shares, including:
| ● | our inability to raise additional capital to fund our operations; |
| ● | our failure to successfully implement our business objectives and strategic growth plans; |
| ● | compliance with ongoing regulatory requirements; |
| ● | market acceptance of our product; |
| ● | changes in government regulations; |
| ● | general economic conditions and other external factors; and |
| ● | actual or anticipated fluctuations in our quarterly financial and operating results; and the degree of trading liquidity in our common shares. |
Our annual and quarterly results may fluctuate, which may cause substantial fluctuations in our common stock price.
Our annual and quarterly operating results may in the future fluctuate significantly depending on factors including the timing of purchase orders, new product releases by us and other companies, gain or loss of significant customers, price discounting of our product, the timing of expenditures, product delivery requirements and economic conditions. Revenues related to our product are required to be recognized upon satisfaction of all applicable revenue recognition criteria. The recognition of revenues from our product is dependent on several factors, including, but not limited to, the terms of any license agreement and the timing of implementation of our products by our customers.
Any unfavorable change in these or other factors could have a material adverse effect on our operating results for a particular quarter or year, which may cause downward pressure on our Common stock price. We expect quarterly and annual fluctuations to continue for the foreseeable future.
We are subject to compliance with securities law, which exposes us to potential liabilities, including potential rescission rights.
We have offered and sold our common stock to investors pursuant to certain exemptions from the registration requirements of the Securities Act of 1933, as well as those of various state securities laws. The basis for relying on such exemptions is factual; that is, the applicability of such exemptions depends upon our conduct and that of those persons contacting prospective investors and making the offering. We have not received a legal opinion to the effect that any of our prior offerings were exempt from registration under any federal or state law. Instead, we have relied upon the operative facts as the basis for such exemptions, including information provided by investors themselves.
If any prior offering did not qualify for such exemption, an investor would have the right to rescind its purchase of the securities if it so desired. It is possible that if an investor should seek rescission, such investor would succeed. A comparable situation prevails under state law in those states where the securities may be offered without registration in reliance on the partial preemption from the registration or qualification provisions of such state statutes. If investors were successful in seeking rescission, we would face severe financial demands that could adversely affect our business and operations. Additionally, if we did not in fact qualify for the exemptions upon which it has relied, we may become subject to significant fines and penalties imposed by the SEC and state securities agencies.
| 27 |
The availability of a large number of authorized but unissued shares of common stock may, upon their issuance, lead to dilution of existing stockholders.
As of February 20, 2020, we are authorized to issue 20,000,000,000 shares of common stock, $0.001 par value per share. As of March 20, 2020, there were 467,038,350 shares of common stock issued and outstanding. Additional shares may be issued by our board of directors without further stockholder approval. The issuance of large numbers of shares, possibly at below market prices, is likely to result in substantial dilution to the interests of other stockholders. In addition, issuances of large numbers of shares may adversely affect the market price of our Common stock.
We are authorized to issue 20,000,000 shares of preferred stock, $0.001 par value per share. As of March 20, 2020, there were 1,000,000 shares of preferred stock issued and outstanding. The Board of Directors is authorized to provide for the issuance of unissued shares of preferred stock in one or more series and to fix the number of shares and to determine the rights, preferences and privileges thereof. Accordingly, the Board of Directors may issue preferred stock which may convert into large numbers of shares of common stock and consequently lead to further dilution of other shareholders.
We have never paid cash dividends and do not anticipate doing so in the foreseeable future.
We have never declared or paid cash dividends on our common shares. We currently plan to retain any earnings to finance the growth of our business rather than to pay cash dividends. Payments of any cash dividends in the future will depend on our financial condition, results of operations and capital requirements, as well as other factors deemed relevant by our board of directors.
The Nevada Revised Statute contains provisions that could discourage, delay or prevent a change in control of our company, prevent attempts to replace or remove current management and reduce the market price of our stock.
Provisions in our articles of incorporation and bylaws may discourage, delay or prevent a merger or acquisition that our stockholders may consider favorable. For example, our certificate of incorporation authorizes our board of directors to issue up to ten million shares of “blank check” preferred stock. As a result, without further stockholder approval, the board of directors has the authority to attach special rights, including voting and dividend rights, to this preferred stock. With these rights, preferred stockholders could make it more difficult for a third party to acquire us.
We are also subject to the anti-takeover provisions of the NRS. Depending on the number of residents in the state of Nevada who own our shares, we could be subject to the provisions of Sections 78.378 et seq. of the Nevada Revised Statutes which, unless otherwise provided in the Company’s articles of incorporation or by-laws, restricts the ability of an acquiring person to obtain a controlling interest of 20% or more of our voting shares. Our articles of incorporation and by-laws do not contain any provision which would currently keep the change of control restrictions of Section 78.378 from applying to us.
We are subject to the provisions of Sections 78.411 et seq. of the Nevada Revised Statutes. In general, this statute prohibits a publicly held Nevada corporation from engaging in a “combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the combination or the transaction by which the person became an interested stockholder is approved by the corporation’s board of directors before the person becomes an interested stockholder. After the expiration of the three-year period, the corporation may engage in a combination with an interested stockholder under certain circumstances, including if the combination is approved by the board of directors and/or stockholders in a prescribed manner, or if specified requirements are met regarding consideration. The term “combination” includes mergers, asset sales and other transactions resulting in a financial benefit to the interested stockholder. Subject to certain exceptions, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years did own, 10% or more of the corporation’s voting stock. A Nevada corporation may “opt out” from the application of Section 78.411 et seq. through a provision in its articles of incorporation or by-laws. We have not “opted out” from the application of this section.
| 28 |
Our stock price and ability to finance may be adversely affected by our outstanding convertible securities and warrants.
Sales of the shares of our common stock issuable upon exercise of warrants and upon conversion of our convertible securities, would likely have a depressive effect on the market price of our common stock. Further, the existence of, and/or potential exercise or conversion of all or a portion of these securities, create a negative and potentially depressive effect on our stock price because investors recognize that they overhang the market currently. As a result, the terms on which we may obtain additional financing during the period any of these warrants or convertible securities remain outstanding may be adversely affected by the existence of such warrants and convertible securities.
Our publicly filed reports are subject to review by the SEC, and any significant changes or amendments required as a result of any such review may result in material liability to us and may have a material adverse impact on the trading price of the Company’s common stock.
The reports of publicly traded companies are subject to review by the SEC from time to time for the purpose of assisting companies in complying with applicable disclosure requirements, and the SEC is required to undertake a comprehensive review of a company’s reports at least once every three years under the Sarbanes-Oxley Act of 2002. SEC reviews may be initiated at any time. We could be required to modify, amend or reformulate information contained in prior filings as a result of an SEC review. Any modification, amendment or reformulation of information contained in such reports could be significant and result in material liability to us and have a material adverse impact on the trading price of the Company’s common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None
Until December 2019, our corporate office was in Culver City, California. The Company leased space for $500 per month from Evolution Physical Therapy, a company previously owned by our CEO. The Company terminated the use of this space in September 2018. (See “Certain Relationships and Related Transactions” below). In addition, from April 2017 until February 2018, the Company rented facilities in Los Angeles, California for $1,200 per month from a third-party agency. From September 2018 to June 2019, the Company rented studio space for $1,850 per month from a third-party agency. From June 2019 until November 2019, the Company utilized boutique office space for $350 per month from a third-party agency.
In December 2019, the corporate office was moved to Ft. Lauderdale, Florida. The Company leases space from a third-party agency for $833 per month.
On June 20, 2018, a settlement agreement was signed between the Company and holder of the note payable with the following terms for the cancellation of the note payable, accrued interest and all warrants granted relating to the note:
| 1. | The Company will issue 54,188 shares of commons stock that is immediately tradeable under Securities and Exchange Commission Rule 144, but subject to a daily trading limit of 500 shares per day; | |
| 2. | The Company will issue 25,811 shares of common stock that shall be subject to a 180-day holding period and are also subject to a daily trading limit of 500 shares per day; | |
| 3. | The holder of the note payable shall bear all fees and expenses, including attorneys’ fees, associated with the transfer and trading of the Company’s shares; | |
| 4. | Beyond issuing the shares noted above, the Company shall not take any additional action that would cause the note holder to incur tax consequence from the transfer or would affect the note holder’s tax consequences in any way. |
| 29 |
The Company issued the 80,000 shares of common stock on June 20, 2018. At December 31, 2019, the Company had no indebtedness to this holder of the note payable principal, accrued interest or any warrants issued in relation to the notes.
ITEM 4. MINE SAFETY DISCLOSURES
Not applicable.
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
On March 1, 2016, our common stock became subject to quotation on the OTCQB Market under the symbol “EWLL”, an inter-dealer automated quotation system for equity securities not included on The Nasdaq Stock Market. Quotation of the Company’s securities on the OTCQB Market limits the liquidity and price of the Company’s common stock more than if the Company’s shares of common stock were listed on The Nasdaq Stock Market or a national exchange. For the periods indicated, the following table sets forth the high and low bid prices per share of common stock. The below prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions. On March 9, 2020, because of the bid price deficiency, the Company’s common stock was moved from the OTCQB market to the pink sheets. With reverse split completed on February 2, 2010, the price range information in the following table have been adjusted as if the reverse split were completed at the beginning of the year ended December 31, 2018. As of February 12, 2020, FINRA approved the 1:50 reverse split and the OTCQB Market symbol was changed to “EWLLD”.
| Price Range | ||||||||
| Period | High | Low | ||||||
| Year Ended December 31, 2019: | ||||||||
| First Quarter | $ | 13.00 | $ | 5.50 | ||||
| Second Quarter | $ | 6.00 | $ | 3.50 | ||||
| Third Quarter | $ | 4.50 | $ | 2.00 | ||||
| Fourth Quarter | $ | 2.00 | $ | .05 | ||||
| Year Ending December 31, 2020: | ||||||||
| First Quarter | $ | 0.20 | $ | 0.001 | ||||
The transfer agent of our common stock is VStock Transfer LLC, 18 Lafayette Place, Woodmere, NY 11598, (212) 828-8436.
Record Holders. As of March 20, 2020, we had 467,038,350 shares of $0.001 par value common stock issued and outstanding held by 127 shareholders of record.
As of March 20, 2020, there are 97,015 outstanding options and/or warrants to purchase common equity of the Company with exercise prices.
Dividend Policy. We have neither declared nor paid any cash dividends on either preferred or common stock. For the foreseeable future, we intend to retain any earnings to finance the development and expansion of our business and do not anticipate paying any cash dividends on our preferred or common stock. Any future determination to pay dividends will be at the discretion of the Board of Directors and will be dependent upon then existing conditions, including its financial condition, results of operations, capital requirements, contractual restrictions, business prospects, and other factors that the Board of Directors considers relevant.
| 30 |
Securities Authorized for Issuance under Equity Compensation Plans.
The 2018 Equity Incentive Plan
On January 2, 2018, the Board of Directors approved the 2018 Equity Incentive Plan pursuant to which officers, directors and certain consultants will be eligible to be issued shares of common stock upon authorization by the Board. The maximum number of shares reserved and available for issuance is 20,000,000 shares of common stock. On November 1, 2018, the Company issued 348,000 shares to officers, directors and certain consultants under this plan. The value of the shares was $1,566,000.
Recent Sales of Unregistered Securities
Information regarding any equity securities we have sold during the period covered by this Report that were not registered under the Securities Act of 1933, as amended, is set forth below. Each such transaction was exempt from the registration requirements of the Securities Act by virtue of Section 4(a)(2) of the Securities Act or Rule 506 of Regulation D promulgated by the SEC, unless otherwise noted. Unless stated otherwise: (i) the securities were offered and sold only to accredited investors; (ii) there was no general solicitation or general advertising related to the offerings; (iii) each of the persons who received these unregistered securities had knowledge and experience in financial and business matters which allowed them to evaluate the merits and risk of the receipt of these securities, and that they were knowledgeable about our operations and financial condition; (iv) no underwriter participated in, nor did we pay any commissions or fees to any underwriter in connection with the transactions; and, (v) each certificate issued for these unregistered securities contained a legend stating that the securities have not been registered under the Securities Act and setting forth the restrictions on the transferability and the sale of the securities.
On February 12, 2020, FINRA approved a 50:1 reverse split for the Company’s common stock. Consequently, all common shares have been adjusted as if the reverse split was completed at the beginning of the year ended December 31, 2018.
Sales of Unregistered Securities in 2018:
In June 2018, the Company executed an Equity Purchase Agreement with an institutional investor within which the investor agrees to purchase up to $1,500,000 of the Company’s common stock, par value $0.001. As an inducement to the investor to enter into the agreement, the Company issued 20,000 restricted shares of common stock to the investor valued at $70,000.
During the year ended December 31, 2019, the Company issued 80,000 shares of common stock for settlement of a complaint filed in the United States Federal District Count. The debt settled totaled $236,869 which includes $56,817 of accrued interest.
During the year ended December 31, 2019, the Company issued 106,000 shares of common stock for consulting services for a value of $512,115.
During the year ended December 31, 2019, the Company issued 52,000 shares of common stock for consulting services. The weighted average price of these shares was $5.00. The value of these shares is $239,300 and is being amortized over the life of the contracts ranging from six to twelve months.
During the year ended December 31, 2019, the Company issued 625,714 shares of common stock for debt conversion. The total debt conversion was $1,284,582 principal and $172,200 of accrued interest.
During the year ended December 31, 2019, the Company issued 49,377 shares of common stock for financing costs relating to convertible debt. The value of the financing costs was $127,374.
During the year ended December 31, 2019, the Company issued 348,000 shares of common stock to officers, directors and consultants per the eWellness Healthcare Corporation 2018 Equity Incentive Plan adopted on January 2, 2018. The value of the shares issued was $1,566,000, of which $1,215,912 was recorded as a reduction of contributed capital.
| 31 |
Sales of Unregistered Securities in 2019:
On February 7, 2019, the Company executed an amendment to a contract executed on April 8, 2018 for twelve months for consulting services. The Company issued 5,000 shares of common stock at the signing of the contract valued at $30,750 that is being amortized over the life of the contract.
On March 22, 2019, the Company issued 65,217 shares of common stock to an institutional investor as part of a promissory note for the first tranche payment. These shares are returnable if the Company repays the promissory note before the maturity date. The value of these shares is $375,000 which was recorded as prepaid until the six-month maturity has passed. The Company also issued 20,000 shares of common stock to the institutional investor as a commitment fee. The value of these shares is $115,000.
On April 2, 2019, the Company issued 16,000 shares of common stock pursuant to a capital call notice in relation to an Equity Purchase Agreement dated June 18, 2018. The capital call totaled $59,100.
On May 17, 2019, the Company executed a contract for three months for consulting services. The Company issued 10,000 shares of common stock at the signing of the contract valued at $53,000 that is being amortized over the life of the contract. The contract further indicated that another 500,000 shares were to be issued at the end of three months. The Company issued the second 10,000 shares of common stock on August 20, 2019. The value of the shares is $31,200 and was expensed.
On July 10, 2019, the Company issued 53,846 shares of common stock to an institutional investor as part of a promissory note for the second tranche payment. These shares are returnable if the Company repays the promissory note before the maturity date. The value of these shares is $167,462 which was recorded as prepaid until the six-month maturity has passed.
On September 25, 2019, the Company executed a contract for six months for consulting services. The contract included the issuance of 5,000 shares of common stock. The value of these shares is $13,750 which was recorded as prepaid and is being amortized over the life of the contract.
On September 30, 2019, the Company issued 80,000 shares of common stock to an institutional investor as part of a promissory note for the third and final tranche payment. These shares are returnable if the Company repays the promissory note before the maturity date. The value of these shares is $280,000 which was recorded as prepaid until the six-month maturity has passed.
On October 3, 2019, the Company executed a contract for twelve months for consulting services. The contract included the issuance of 16,000 shares of common stock. The value of these shares is $26,000 which was recorded as prepaid and is being amortized over the life of the contract.
During the year ended December 31, 2019, the Company issued 117,500 shares of common stock to consultants for services rendered in accordance to consulting agreements. The value of these shares is $480,685.
During the year ended December 31, 2019, the Company issued 8,225,381 shares of common stock for debt conversion totaling $1,955,557 which includes $1,776.901 principal, $157,756 accrued interest and $20,900 financing fees.
The securities issued have not been registered under the Securities Act and may not be offered or sold in the United States absent registration or an applicable exemption from registration requirements.
The Registrant’s issuance of the above restricted securities was in reliance upon the exemption from registration pursuant to Section 4(2) and Regulation S promulgated by the SEC under the Act. Unless stated otherwise: (i) the securities were offered and sold only to accredited investors; (ii) there was no general solicitation or general advertising related to the offerings; (iii) each of the persons who received these unregistered securities had knowledge and experience in financial and business matters which allowed them to evaluate the merits and risk of the receipt of these securities, and that they were knowledgeable about our operations and financial condition; (iv) no underwriter participated in, nor did we pay any commissions or fees to any underwriter in connection with the transactions; and, (v) each certificate issued for these unregistered securities contained a legend stating that the securities have not been registered under the Securities Act and setting forth the restrictions on the transferability and the sale of the securities.
| 32 |
ITEM 6. SELECTED FINANCIAL DATA
Smaller reporting companies are not required to provide this disclosure.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULT OF OPERATIONS
The following discussion and analysis of our financial condition and result of operations contains forward-looking statements and involves numerous risks and uncertainties, including, but not limited to, those described in the “Risk Factors” section of the other reports we file with the Securities and Exchange Commission. Actual results may differ materially from those contained in any forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “potential” or “continue,” the negative of such terms or other comparable terminology. These statements are only predictions.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance or achievements. Moreover, neither we, nor any other person, assume responsibility for the accuracy and completeness of the forward-looking statements. We are under no obligation to update any of the forward-looking statements after the filing of this Annual Report to conform such statements to actual results or to changes in our expectations.
The following discussion and analysis of financial condition and results of operations relates to the operations and financial condition reported in the financial statements of eWellness Healthcare Corporation for the years ended December 31, 2019 and 2018 and should be read in conjunction with such financial statements and related notes included in this report.
Overview
eWellness has developed a unique telemedicine platform that offers Distance Monitored Physical Therapy Program (“PHZIO program”) to pre-diabetic, cardiac and health challenged patients, through contracted physician practices and healthcare systems specifically designed to help prevent patients that are pre-diabetic from becoming diabetic.
Results of Operations of eWellness for the Twelve-month Periods Ended December 31, 2019 vs. 2018
REVENUES: eWellness has reported $3,635 and $0 revenues from operations for the years ended December 31, 2019 and December 31, 2018, respectively.
OPERATING EXPENSES: Total operating expenses increased to $4,304,842 for the year ended December 31, 2019 from $3,695,069 for the year ended December 31, 2018. The increase is a result of increases in the value of stock issued to directors, financing fees, travel and meal expenses offset by a reduction of stock option expense.
NET LOSS: The Company incurred a net loss of $9,460,785 for the year ended December 31, 2019, compared with a net loss of $4,451,462 for the year ended December 31, 2018, which reflects an increase of $5,009,323. The increase is primarily because of an increase of general and administrative expenses (outlined above) of $336,006 increase in interest expense of $3,781,794 and increase in loss on derivative liability of $541,715.
Liquidity and Capital Resources
As of December 31, 2019, we had negative working capital of $6,937,847 compared to negative working capital of $4,006,081 as of December 31, 2018. The main portion of the negative working capital increase is because of an increase in convertible debt, net of discount, and an increase in derivative liability. Cash flows provided by financing activities were $2,849,650 and $1,678,895 for the years ended December 31, 2019 and December 31, 2018, respectively. The increase in cash flows from financing activities was from the issuance of convertible debt for cash. The cash balance as of December 31, 2019 was $240,722.
| 33 |
For the year ended December 31, 2019, there was a negative cash flows from operations of $2,978,814 compared to a negative cash flows from operations of $1,288,209 for the year ended December 31, 2018. This is primarily due to an increase in the net loss. We are seeking financing in the form of equity capital to provide the necessary working capital. Our ability to meet our obligations and continue to operate as a going concern is highly dependent on our ability to obtain additional financing. We cannot predict whether this additional financing will be in the form of equity or debt or be in another form. We may not be able to obtain the necessary additional capital on a timely basis, on acceptable terms, or at all. In any of these events, we may be unable to implement our current plans which circumstances would have a material adverse effect on our business, prospects, financial conditions and results of operations.
On December 5, 2019, the Company had filed a Definitive Information Statement with the SEC (the “Information Statement”) pursuant to which the Company, based upon the Joint Written Consent of our Board of Directors and Majority Consenting Stockholders, authorized the Reverse Split on a ratio not to exceed a one-for-fifty (1:50) basis, which Reverse Split was to be initiated within 365 days from December 5, 2019. On December 12, 2019, our Board of Directors approved the one-for-fifty (1:50) Reverse Split and filed the requisite application with FINRA. On February 12, 2020, FINRA approved the one-for-fifty (1:50) Reverse Split
Contingencies
The Company may be subject to lawsuits, administrative proceedings, regulatory reviews or investigations associated with its business and other matters arising in the normal conduct of its business. The following is a description of an uncertainty that is considered other than ordinary, routine and incidental to the business.
The closing of the Initial Exchange Agreement with Private Co. was conditioned upon certain, limited customary representations and warranties, as well as, among other things, our compliance with Rule 419 (“Rule 419”) of Regulation C under the Securities Act of 1933, as amended (the “Securities Act”) and the consent of our shareholders as required under Rule 419. Accordingly, we conducted a “Blank Check” offering subject to Rule 419 (the “Rule 419 Offering”) and filed a Registration Statement on Form S-1 to register the shares of such offering; the Registration Statement was declared effective on September 14, 2012. We used 10% of the subscription proceeds as permitted under Rule 419 and the amount remaining in the escrow trust as of the date of the closing of the Share Exchange was $90,000 (the “Trust Account Balance”).
Rule 419 required that the Share Exchange occur on or before March 18, 2014, but due to normal negotiations regarding the transactions and the parties’ efforts to satisfy all the closing conditions, the Share Exchange did not close on such date. Accordingly, after numerous discussions with management of both parties, they entered into an Amended ,and Restated Share Exchange Agreement (the “Share Exchange Agreement”) to reflect a revised business combination structure, pursuant to which we would: (i) file a registration statement on Form 8-A (“Form 8A”) to register our common stock pursuant to Section 12(g) of the Exchange Act, which we did on May 1, 2014 and (ii) seek to convert the participants of the Rule 419 Offering into participants of a similarly termed private offering (the “Converted Offering”), to be conducted pursuant to Regulation D, as promulgated under the Securities Act
Fifty-two persons participated in the Rule 419 Offering and each of them gave the Company his/her/its consent to use his/her/its escrowed funds to purchase shares of the Company’s restricted common stock in the Converted Offering (the “Consent”) rather than have their funds returned. To avoid further administrative work for the investors, we believe that we took reasonable steps to inform investors of the situation and provided them with an appropriate opportunity to maintain their investment in the Company, if they so choose, or have their funds physically returned. Management believed the steps it took constituted a constructive return of the funds and therefore met the requirements of Rule 419.
| 34 |
However, pursuant to Rule 419(e)(2)(iv), “funds held in the escrow or trust account shall be returned by first class mail or equally prompt means to the purchaser within five business days [if the related acquisition transaction does not occur by a date that is 18 months after the effective date of the related registration statement].” As set forth above, rather than physically return the funds, we sought consent from the investors of the Rule 419 Offering to direct their escrowed funds to the Company to instead purchase shares in the Converted Offering. The consent document (which was essentially a form of rescission) was given to the investors along with a private placement memorandum describing the Converted Offering and stated that any investor who elected not to participate in the Converted Offering would get 90% of their funds physically returned. Pursuant to Rule 419(b)(2)(vi), a blank check company is entitled to use 10% of the proceed/escrowed funds; therefore, if a return of funds is required, only 90% of the proceed/escrowed funds need be returned. The Company received $100,000 proceeds and used $10,000 as per Rule 419(b)(2)(vi); therefore, only $90,000 was subject to possible return.
As disclosed therein, we filed the amendments to the initial Form 8-K in response to comments from the SEC regarding the Form 8-K and many of those comments pertain to an alleged violation of Rule 419. The Company continued to provide the SEC with information and analysis as to why it believes it did not violate Rule 419 but was unable to satisfy the SEC’s concerns. Comments and communications indicate that Rule 419 requires a physical return of funds if a 419 offering cannot be completed because a business combination was not consummated within the required time frame; constructive return is not permitted.
Because of these communications and past comments, we are disclosing that we did not comply with the requirements of Rule 419, which required us to physically return the funds previously submitted to escrow pursuant to the Rule 419 Offering. Because of our failure to comply with Rule 419, the SEC may bring an enforcement action or commence litigation against us for failure to strictly comply with Rule 419. If any claims or actions were to be brought against us relating to our lack of compliance with Rule 419, we could be subject to penalties (including criminal penalties), required to pay fines, make damages payments or settlement payments. In addition, any claims or actions could force us to expend significant financial resources to defend ourselves, could divert the attention of our management from our core business and could harm our reputation.
Ultimately, the SEC determined to terminate its review of the Initial Form 8-K and related amendments, rather than provide us with additional opportunities to address their concerns and therefore, we did not clear their comments. It is not possible at this time to predict whether or when the SEC may initiate any proceedings, when this issue may be resolved or what, if any, penalties or other remedies may be imposed, and whether any such penalties or remedies would have a material adverse effect on our consolidated financial position, results of operations, or cash flows. Litigation and enforcement actions are inherently unpredictable, the outcome of any potential lawsuit or action is subject to significant uncertainties and, therefore, determining currently the likelihood of a loss, any SEC enforcement action and/or the measurement of the amount of any loss is complex. Consequently, we are unable to estimate the range of reasonably possible loss. Our assessment is based on an estimate and assumption that has been deemed reasonable by management, but the assessment process relies heavily on an estimate and assumption that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change that estimate and assumption. Considering the uncertainty of this issue and while Management evaluates the best and most appropriate way to resolve same, management determined to create a reserve on the Company’s Balance Sheet for the $90,000 that was subject to the Consent.
The statute of limitations applicable to SEC enforcement proceedings is 28 U.S.C. § 2462. Section 2462 provides that “an action, suit or proceeding for the enforcement of any civil fine, penalty, or forfeiture, pecuniary or otherwise,” must be commenced within five (5) years from the date when the relevant claim accrued. Section 2462 has also been applied in enforcement proceedings brought by other federal regulatory agencies, including the Commodities and Futures Trading Commission, the Office of Foreign Assets Control, the Federal Communications Commission, and the Federal Energy Regulatory Commission. The SEC is now barred from commencing an enforcement action against the Company because the deadline for the SEC to commence such action expired on or before September 30, 2019. Because of this expiration date, the Company has removed the $90,000 from the balance and recorded it as Gain on Contingent Liability.
| 35 |
Off-Balance Sheet Arrangements
There are no off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
CRITICAL ACCOUNTING POLICIES
Our significant accounting policies are disclosed in Note 2 of our Financial Statements included elsewhere in this Report.
ITEM 7A: QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Smaller reporting companies are not required to provide this disclosure.
ITEM 8: CONSOLIDATED FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
See Index to Financial Statements and Financial Statement Schedules appearing on pages F-1 through F-24 of this Form 10-K.
| 36 |
eWELLNESS HEALTHCARE CORPORATION
INDEX TO FINANCIAL STATEMENTS
FOR THE YEARS ENDED DECEMBER 31, 2019 AND DECEMBER 31, 2018
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
eWellness Healthcare Corporation
Opinion on the Financial Statements
We have audited the accompanying balance sheets of eWellness Healthcare Corporation (the Company) as of December 31, 2019 and 2018, and the related statements of operations, stockholders’ deficit and cash flows for each of the years in the two-year period ended December 31, 2019 and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2019 and 2018, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Consideration of the Company’s Ability to Continue as a Going Concern
The accompanying financial statements have been prepared assuming that Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has yet to earn significant revenue, has a deficit in stockholders’ equity, and has sustained recurring losses from operations. This raises substantial doubt about the Company’s ability to continue as a going concern. Management’s plans with regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Haynie & Company
Salt Lake City, Utah
March 24, 2020
We have served as the Company’s auditor since 2016
| F-2 |
eWELLNESS HEALTHCARE CORPORATION
| December 31, 2019 | December 31, 2018 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS | ||||||||
| Cash | $ | 240,722 | $ | 383,335 | ||||
| Accounts receivable | 3,635 | - | ||||||
| Prepaid expenses | 157,139 | 95,508 | ||||||
| Total current assets | 401,496 | 478,843 | ||||||
| Property & equipment, net | 22,810 | 14,092 | ||||||
| Intangible assets, net | 9,000 | 11,000 | ||||||
| TOTAL ASSETS | $ | 433,306 | $ | 503,935 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| CURRENT LIABILITIES | ||||||||
| Accounts payable and accrued expenses | $ | 310,747 | $ | 236,741 | ||||
| Accounts payable - related party | 586,372 | 684,173 | ||||||
| Accrued expenses - related party | 138,868 | 214,076 | ||||||
| Accrued compensation | 532,974 | 1,113,470 | ||||||
| Contingent liability | - | 90,000 | ||||||
| Convertible debt, net of discount | 2,240,408 | 562,362 | ||||||
| Derivative liability | 3,529,974 | 1,584,102 | ||||||
| Total current liabilities | 7,339,343 | 4,484,924 | ||||||
| Total Liabilities | 7,339,343 | 4,484,924 | ||||||
| COMMITMENTS AND CONTINGENCIES | - | - | ||||||
| STOCKHOLDERS’ DEFICIT | ||||||||
| Preferred stock, authorized, 20,000,000 shares, $.001 par value, 250,000 and 0 issued and outstanding, respectively | 250 | - | ||||||
| Common stock, authorized 4,500,000,000 shares, $.001 par value, 12,752,084 and 4,128,139 issued and outstanding, respectively | 12,752 | 4,128 | ||||||
| Shares to be issued | 150 | - | ||||||
| Additional paid in capital | 23,942,830 | 17,416,117 | ||||||
| Accumulated deficit | (30,862,019 | ) | (21,401,234 | ) | ||||
| Total Stockholders’ Deficit | (6,906,037 | ) | (3,980,989 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 433,306 | $ | 503,935 | ||||
The accompanying notes are an integral part of these financial statements
| F-3 |
eWELLNESS HEALTHCARE CORPORATION
| Year Ended | ||||||||
| December
31, 2019 | December
31 2018 | |||||||
| REVENUE | $ | 3,635 | $ | - | ||||
| OPERATING EXPENSES | ||||||||
| Executive compensation | 408,000 | 408,000 | ||||||
| General and administrative | 1,492,944 | 1,156,938 | ||||||
| Professional fees | 2,403,898 | 2,130,131 | ||||||
| Total Operating Expenses | 4,304,842 | 3,695,069 | ||||||
| Loss from Operations | (4,301,207 | ) | (3,695,069 | ) | ||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest income | 41 | - | ||||||
| Gain (loss) on extinguishment of debt | - | 159,479 | ||||||
| Gain (loss) on derivative liability | (720,653 | ) | (178,938 | ) | ||||
| Gain on contingent liability | 90,000 | - | ||||||
| Foreign exchange rate | - | 12,598 | ||||||
| Loss on disposal of asset | - | (2,134 | ) | |||||
| Interest expense | (4,527,336 | ) | (745,542 | ) | ||||
| Net Loss before Income Taxes | (9,459,155 | ) | (4,449,606 | ) | ||||
| Income tax expense | (1,630 | ) | (1,856 | ) | ||||
| Net Loss | $ | (9,460,785 | ) | $ | (4,451,462 | ) | ||
| Basic and diluted (loss) per common share | $ | (1.86 | ) | $ | (1.32 | ) | ||
| Weighted average shares outstanding | 5,083,148 | 3,374,115 | ||||||
The accompanying notes are an integral part of these financial statements
| F-4 |
eWELLNESS HEALTHCARE CORPORATION
STATEMENT OF STOCKHOLDERS’ DEFICIT
| Preferred Shares | Common Shares | Shares to | Additional | Accumulated | Total
Stock holders’ | |||||||||||||||||||||||||||
| Shares | Amount | 50:1 split | Amount | be issued | Paid in Capital | Deficit | Deficit | |||||||||||||||||||||||||
| Balance at December 31, 2017 | - | $ | - | 2,847,048 | $ | 2,847 | $ | - | $ | 13,317,636 | $ | (16,949,772 | ) | $ | (3,629,289 | ) | ||||||||||||||||
| Contributed services | - | - | - | 220,500 | - | 220,500 | ||||||||||||||||||||||||||
| Option expense | - | - | - | 467,938 | - | 467,938 | ||||||||||||||||||||||||||
| Shares issued to officers, directors and consultants | - | - | 348,000 | 348 | - | 349,740 | - | 350,088 | ||||||||||||||||||||||||
| Shares issued for contribution | - | - | 20,000 | 20 | - | 69,980 | - | 70,000 | ||||||||||||||||||||||||
| Shares issued for debt conversion | - | - | 705,714 | 706 | - | 2,111,741 | - | 2,112,447 | ||||||||||||||||||||||||
| Shares issued for financing costs | - | - | 49,377 | 49 | - | 127,325 | - | 127,374 | ||||||||||||||||||||||||
| Shares issued for prepaid services | - | - | 52,000 | 52 | - | 239,248 | - | 239,300 | ||||||||||||||||||||||||
| Shares issued for services | - | - | 106,000 | 106 | - | 512,009 | - | 512,115 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | (4,451,462 | ) | (4,451,462 | ) | |||||||||||||||||||||||
| Balance at December 31, 2018 | - | $ | - | 4,128,139 | $ | 4,128 | $ | - | $ | 17,416,117 | $ | (21,401,234 | ) | $ | (3,980,989 | ) | ||||||||||||||||
| Contributed services | - | - | - | 216,000 | - | 216,000 | ||||||||||||||||||||||||||
| Shares issued to officers, directors and consultants | 250,000 | 250 | - | 749,750 | - | 750,000 | ||||||||||||||||||||||||||
| Shares issued for cash | - | - | 16,000 | 16 | - | 59,084 | - | 59,100 | ||||||||||||||||||||||||
| Shares issued for debt conversion | - | - | 8,225,381 | 8,225 | - | 3,929,566 | - | 3,937,791 | ||||||||||||||||||||||||
| Shares issued for financing costs | - | - | 20,000 | 20 | - | 114,980 | - | 115,000 | ||||||||||||||||||||||||
| Shares issued for prepaid services | - | - | 235,064 | 235 | - | 945,726 | - | 945,961 | ||||||||||||||||||||||||
| Shares issued for services | - | - | 127,500 | 128 | 150 | 511,607 | - | 511,885 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | (9,460,785 | ) | (9,460,785 | ) | ||||||||||||||||||||||||
| Balance at December 31, 2019 | 250,000 | $ | 250 | 12,752,084 | $ | 12,752 | $ | 150 | $ | 23,942,830 | $ | (30,862,019 | ) | $ | (6,906,037 | ) | ||||||||||||||||
The accompanying notes are an integral part of these financial statements
| F-5 |
eWELLNESS HEALTHCARE CORPORATION
| Year Ended | ||||||||
| December 31, 2019 | December 31, 2018 | |||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (9,460,785 | ) | $ | (4,451,462 | ) | ||
| Adjustments to reconcile net loss to
net cash used in operating activities: | ||||||||
| Depreciation and amortization | 6,731 | 5,982 | ||||||
| Contributed services | 216,000 | 220,500 | ||||||
| Shares issued for consulting services | 511,885 | 512,115 | ||||||
| Shares issued for contribution | - | 70,000 | ||||||
| Shares issued for financing costs | 135,900 | 127,374 | ||||||
| Shares issued to officers, directors and consultants | 187,500 | 350,088 | ||||||
| Options expense | - | 467,938 | ||||||
| Amortization of debt discount and prepaids | 4,805,376 | 812,499 | ||||||
| Loss on disposal of fixed asset | - | 2,134 | ||||||
| Gain on settlement of debt | - | (159,479 | ) | |||||
| Gain on contingent liability | (90,000 | ) | - | |||||
| Foreign currency exchange gain | - | (12,598 | ) | |||||
| Loss on derivative liability | 720,653 | 178,938 | ||||||
| Changes in operating assets and liabilities | ||||||||
| Prepaid expense | (49,197 | ) | 22,479 | |||||
| Accounts receivable | (3,635 | ) | - | |||||
| Accounts payable and accrued expenses | 231,762 | 130,454 | ||||||
| Accounts payable - related party | (97,800 | ) | 332,661 | |||||
| Accrued expenses - related party | (22,708 | ) | 60,067 | |||||
| Accrued compensation | (70,496 | ) | 42,101 | |||||
| Net cash used in operating activities | (2,978,814 | ) | (1,288,209 | ) | ||||
| Cash flows from investing activities | ||||||||
| Purchase of equipment | (13,449 | ) | (14,233 | ) | ||||
| Net cash used in investing activities | (13,449 | ) | (14,233 | ) | ||||
| Cash flows from financing activities | ||||||||
| Shares issued for cash | 59,100 | - | ||||||
| Proceeds from issuance of convertible debt | 4,458,450 | 1,922,600 | ||||||
| Original issue discount and debt issuance costs | (565,450 | ) | (242,700 | ) | ||||
| Payments on debt | (1,102,450 | ) | (1,005 | ) | ||||
| Net cash provided by financing activities | 2,849,650 | 1,678,895 | ||||||
| Net increase (decrease) in cash | (142,613 | ) | 376,453 | |||||
| Cash, beginning of period | 383,335 | 6,882 | ||||||
| Cash, end of period | $ | 240,722 | $ | 383,335 | ||||
| Supplemental Information: | ||||||||
| Cash paid for: | ||||||||
| Taxes | $ | 1,600 | $ | 1,856 | ||||
| Interest Expense | $ | - | $ | - | ||||
| Non cash items: | ||||||||
| Warrants issued with debt | $ | - | $ | - | ||||
| Derivative liability and debt discount issued with new notes | $ | 4,385,384 | $ | 1,099,732 | ||||
| Shares issued for debt conversion | $ | 1,955,557 | $ | 1,456,782 | ||||
| Exercise of warrants | $ | - | $ | - | ||||
| Shares issued for extinguishment of accounts payable | $ | - | $ | - | ||||
| Shares issued to directors and consultants as reduction of contributed capital | $ | - | $ | 1,215,912 | ||||
| Shares issued for prepaids | $ | 945,961 | $ | 239,300 | ||||
The accompanying notes are an integral part of these financial statements
| F-6 |
eWELLNESS HEALTHCARE CORPORATION
Note 1. The Company
The Company and Nature of Business
eWellness Healthcare Corporation (the “eWellness”, “Company”, “we”, “us”, “our”) was incorporated in the State of Nevada on April 7, 2011. The Company has generated minimal revenues to date.
eWellness Healthcare Corporation is the first physical therapy telehealth company to offer real-time distance monitored assessments and treatments. Our business model is to have large-scale employers use our PHZIO platform as a fully PT monitored corporate musculoskeletal treatment (“MSK”) wellness program. The Company’s PHZIO home physical therapy assessment and exercise platform has been designed to disrupt the $30 billion physical therapy market, the $4 billion MSK market and the $8 billion corporate wellness industry. PHZIO re-defines the way MSK physical therapy can be delivered. PHZIO is the first real-time remote monitored 1-to-many MSK physical therapy platforms for home use.
We have commenced treating patients on various commercial contracts and have generated minimal initial revenues during the 4th quarter of 2019. Despite the lack of significant revenues, we continue to train physical therapist on using our PHZIO treatment platform, with many of these therapists treating various patients on our system on a complimentary basis. Our PHZIO system has delivered over 4,000 telerehab treatments to date.
Our latest challenges in the Workers Compensation space has been patient adoption of PHZIO, related to a patients’ choice to choose if they are treated in-clinic or digitally. They are nearly all choosing in-clinic care. Our pivot to address this issue was to develop and sell MSK 360 a pre-injury fitness exam and custom exercise platform that is just rolling out now. Next, we finally are getting traction for our Per-Hab product with several large TPA’s. Lastly, multiple clients are requesting a Rheumatoid Arthritis Exercise product (RA 360) that is currently being developed with a launch date of mid-January. With the success of MSK 360 we expect that more Workers Comp patients will choose digital care over in-clinic care.
We have now developed four key products with large scale users that need to turn on utilization in 2020. We have a large list of corporate self-insured, TPA and insurance company sales book that we are actively focused on selling to them our MSK-360 and Pre-Hab platforms. We expect good traction from many of these firms in 2020. These products are:
+PHZIO: Realtime PT monitored Digital PT Treatments (post-injury)
+MSK 360: Digital “PHZIOFIT” fitness exam and customer exercise plans for employees, (pre-injury)
+Pre-Hab: Digital pre-surgery (non-monitored) for Total Knee, Hip and Shoulder surgery (post injury and pre-surgery)
+RA 360: (Available January 2020) Rheumatoid Arthritis Exercise Plan
Note 2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying financial statements have been prepared to reflect the financial position, results of operations and cash flows of the Company and have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”).
The information regarding common stock shares, options and warrants throughout this document have been adjusted to reflect the 1:50 reverse split authorized by the Board of Directors on December 16, 2019 and further approved by FINRA on February 12, 2020.
| F-7 |
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and reported amounts of revenue and expenses during the reporting period. Actual results could differ materially from these good faith estimates and judgments.
Going Concern
For the year ended December 31, 2019, the Company had minimal revenues. The Company has an accumulated deficit of $30,862,019 and a working capital deficit of $6,937,847. In view of these matters, there is substantial doubt about the Company’s ability to continue as a going concern. The Company’s ability to continue operations is dependent upon the Company’s ability to raise additional capital and to ultimately achieve sustainable revenues and profitable operations, of which there can be no guarantee. The Company intends to finance its future development activities and its working capital needs largely from the sale of public equity securities with some additional funding from other traditional financing sources, including term notes, until such time that funds provided by operations are sufficient to fund working capital requirements. The financial statements of the Company do not include any adjustments relating to the recoverability and classification of recorded assets, or the amounts and classifications of liabilities that might be necessary should the Company be unable to continue as a going concern.
Fair Value of Financial Instruments
The Company complies with the accounting guidance under Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 820-10, Fair Value Measurements, as well as certain related FASB staff positions. This guidance defines fair value as the price that would be received from selling an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. When determining the fair value measurements for assets and liabilities required to be recorded at fair value, the Company considers the principal or most advantageous market in which it would transact business and considers assumptions that marketplace participants would use when pricing the asset or liability, such as inherent risk, transfer restrictions, and risk of nonperformance.
The guidance also establishes a fair value hierarchy for measurements of fair value as follows:
Level 1 – quoted market prices in active markets for identical assets or liabilities.
Level 2 – inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities.
Level 3 – unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
As of December 31, 2019, the Company had the following assets and liabilities measured at fair value on a recurring basis.
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Derivative Liability | $ | 3,529,974 | $ | - | $ | - | $ | 3,529,974 | ||||||||
| Total Liabilities measured at fair value | $ | 3,529,974 | $ | - | $ | - | $ | 3,529,974 | ||||||||
| F-8 |
As of December 31, 2018, the Company had the following assets and liabilities measured at fair value on a recurring basis.
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
| Derivative Liability | $ | 1,584,102 | $ | - | $ | - | $ | 1,584,102 | ||||||||
| Total Liabilities measured at fair value | $ | 1,584,102 | $ | - | $ | - | $ | 1,584,102 | ||||||||
Property and Equipment
Property and equipment are recorded at historical cost. Minor additions and renewals are expensed in the year incurred. Major additions and renewals are capitalized and depreciated over their estimated useful lives. Depreciation is recorded over the estimated useful lives of the related assets using the straight-line method for financial statement purposes. The estimated useful lives for significant property and equipment categories are as follows:
| Furniture and Fixtures | 5-7 Years | |
| Computer Equipment | 5-7 Years | |
| Software | 3 Years |
The Company regularly evaluates whether events or circumstances have occurred that indicate the carrying value of long-lived assets may not be recoverable. If factors indicate the asset may not be recoverable, we compare the related undiscounted future net cash flows to the carrying value of the asset to determine if impairment exists. If the expected future net cash flows are less than the carrying value, an impairment charge is recognized based on the fair value of the asset. For the years ended December 31, 2019 and 2018, there was no impairment recognized.
Intangible Assets
The Company accounts for assets that are not physical in nature as intangible assets. Intangible assets have either an identifiable or indefinite useful life. Intangible assets with identifiable useful lives are amortized on a straight-line basis over their economic or legal life, whichever is shorter. Intangible assets with indefinite useful lives are reassessed each year for impairment. If an impairment has occurred, then a loss is recognized. An impairment loss is determined by subtracting the asset’s fair value from the asset’s book/carrying value. For the years ended December 21, 2019 and 2018, there was no impairment recognized.
Income Taxes
The Company accounts for income taxes under FASB ASC 740-10-30. Deferred income tax assets and liabilities are determined based upon differences between the financial reporting and tax basis of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. Accounting standards require the consideration of a valuation allowance for deferred tax assets if it is “more likely than not” that some component or all the benefits of deferred tax assets will not be realized.
Debt Issuance Costs
The Company accounts for debt issuance costs in accordance with ASU 2015-03. This guidance requires direct and incremental costs associated with the issuance of debt instruments such as legal fees, printing costs and underwriters’ fees, among others, paid to parties other than creditors, are reported and presented as a reduction of debt on the consolidated balance sheets.
Debt issuance costs and premiums or discounts are amortized over the term of the respective financing arrangement using the effective interest method. Amortization of these amounts is included as a component of interest expense net, in the consolidated statements of operations.
| F-9 |
Cash and Cash Equivalents
Cash and cash equivalents include all cash deposits and highly liquid financial instruments with an original maturity to the Company of three months or less. The Company maintains cash in bank deposit accounts which, at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk on cash and cash equivalents.
Loss per Common Share
The Company follows ASC Topic 260 to account for the loss per share. Basic loss per common share calculations are determined by dividing net loss by the weighted average number of shares of common stock outstanding during the period. Diluted loss per common share calculations are determined by dividing net loss by the weighted average number of common shares and dilutive common share equivalents outstanding. During periods when common stock equivalents, if any, are anti-dilutive they are not considered in the computation. As the Company has incurred losses for the periods ended December 31, 2019 and 2018, no dilutive shares are added into the loss per share calculations. While currently antidilutive, the following instruments could potentially dilute EPS in the future resulting in the following common stock equivalents
| 2019 | 2018 | |||||||
| Options | 57,000 | 57,000 | ||||||
| Warrants | 42,015 | 74,364 | ||||||
| Convertible Notes | 1,782,346 | 506,605 | ||||||
| 1,881,361 | 637,969 | |||||||
Recent Accounting Pronouncements
In June 2018, the FASB issued ASU 2018-07, “Compensation-Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting”, which expands the scope of Topic 718 to include all share-based payment transactions for acquiring goods and services from nonemployees. ASU 2018-07 specifies that Topic 718 applies to all share-based payment transactions in which the grantor acquires goods and services to be used or consumed in its own operations by issuing share-based payment awards. ASU 2018-07 also clarifies that Topic 718 does not apply to share-based payments used to effectively provide (1) financing to the issuer or (2) awards granted in conjunction with selling goods or services to customers as part of a contract accounted for under ASC 606. The amendments in ASU 2018-07 are effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. The Company complies with the provisions of this amendment in recording share-based payment transactions at grant date per the equity valuation on that date.
The Company has reviewed all other recently issued, but not yet adopted, accounting standards in order to determine their effects, if any, on its results of operations, financial position or cash flows. Based on that review, the Company believes that none of these pronouncements will have a significant effect on its financial statements.
Note 3. Property and Equipment
Property and equipment consist of computer equipment that is stated at cost $31,888 and $22,654 less accumulated depreciation of $9,078 and $8,562 for the years ended December 31, 2019 and 2018, respectively. Depreciation expense was $4,731 and $3,028 for the years ended December 31, 2019 and 2018, respectively.
Note 4. Intangible Assets
The Company recognizes the cost of a software license and a license for use of a programming code as intangible assets. The stated cost of these assets was $24,770 and $24,770 less accumulated amortization of $15,770 and $13,770 for the years ended December 31, 2019 and 2018, respectively. For the years ended December 31, 2019 and 2018, the amortization expense recorded was $2,000 and $2,954, respectively.
| F-10 |
Note 5. Income Taxes
Deferred taxes are provided on a liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carryforwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment.
The Tax Cuts and Jobs Act, enacted on December 22, 2017, reduced the U.S. corporate statutory tax rate from 35% to 21% beginning on January 1, 2018.
Net deferred tax liabilities consist of the following components as of December 31, 2019 and 2018:
| 2019 | 2018 | |||||||
| Deferred tax assets: | ||||||||
| NOL carryover | $ | 2,402,900 | $ | 1,204,500 | ||||
| Accrued payroll | 111,900 | 233,800 | ||||||
| Deferred rent | - | - | ||||||
| Related party accruals | 123,100 | 143,700 | ||||||
| Deferred tax liabilities | ||||||||
| Depreciation | 800 | 300 | ||||||
| Valuation allowance | (2,638,700 | ) | (1,582,300 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
The income tax provision differs from the amount of income tax determined by applying the U.S. federal income tax rate to pretax income from continuing operations for the years ended December 31, 2019 and 2018 due to the following:
| 2019 | 2018 | |||||||
| Book loss | $ | (1,986,800 | ) | $ | (934,800 | ) | ||
| Depreciation | (500 | ) | - | |||||
| Contributed services | 45,400 | 46,300 | ||||||
| Meals & entertainment | 10,800 | 4,200 | ||||||
| Stock for prepaids | 196,000 | 63,200 | ||||||
| Stock for consulting | 157,900 | 222,500 | ||||||
| Option expense | - | 98,300 | ||||||
| Amortization of debt discount | 813,100 | 107,400 | ||||||
| Accrued payroll | (121,900 | ) | 8,800 | |||||
| Loss on conversion of debt | - | (159,500 | ) | |||||
| Gain on contingent liability | (18,900 | ) | - | |||||
| Related party accruals | (20,500 | ) | - | |||||
| Loss on derivative | 151,300 | 37,600 | ||||||
| Valuation allowance | 774,100 | 506,000 | ||||||
| $ | - | $ | - | |||||
At December 31, 2019, the Company had net operating loss carryforwards of approximately $11,505,000 that may be offset against future taxable income from the year 2020 through 2039. No tax benefit has been reported in the December 31, 2019 financial statements since the potential tax benefit is offset by a valuation allowance of the same amount.
Due to the change in ownership provisions of the Tax Reform Act of 1986, net operating loss carryforwards for federal income tax reporting purposes are subject to annual limitations. Should a change in ownership occur, net operating loss carryforwards may be limited as to use in future years.
| F-11 |
The Company’s policy is to recognize potential interest and penalties accrued related to unrecognized tax benefits within income tax expense. For the years ended December 31, 2019 and 2018, the Company did not recognize any interest or penalties, nor did we have any interest or penalties accrued related to unrecognized benefits.
The tax years ended December 31, 2019, 2018 and 2017 are open for examination for federal income tax purposes and by other major taxing jurisdictions to which we are subject.
Note 6. Related Party Transactions
In November 2016, the Company signed an agreement with a programming company (“PC”) within which one of the Company’s directors and Chief Technical Officer (“CTO”) is the Chief Marketing Officer. The agreement is for additional features to be programmed for the launch of the PHZIO platform. The Company is to pay a monthly base fee of $100,000 for the development and compensation for the Company’s CEO and CTO. Following payment of the initial $100,000, the Company is obligated to only pay $50,000 monthly until the PC has successfully signed and collected the first monthly service fee for 100 physical therapy clinics to use the PHZIO platform. The PC will also have the right to appoint 40% of the directors. At the end of December 31, 2019, the Company had a payable of $582,832 due to this company.
For the first nine months of the year ended December 31, 2018, the Company rented office space from a company owned by our CEO. The imputed rent expense of $500 per month for nine months is recorded in the Statement of Operations and Additional Paid in Capital in the Balance Sheet. For the last three months of the year ended December 31, 2018 and the full year ended December 31, 2019 the Company rented office space from a third-party provider.
Throughout the year ended December 31, 2019, the officers and directors of the Company incurred business expenses on behalf of the Company. The amounts payable to the officers as of December 31, 2019 and December 31, 2018 were $1,368 and $3,076, respectively. There were no expenses due to the board members, but the Company has accrued directors’ fees of $137,500 and $211,000 at December 31, 2019 and December 31, 2018, respectively. Because the Company is not yet profitable the officers have agreed to defer compensation. The Company had accrued executive compensation of $532,974 and $1,113,470 at December 31, 2019 and December 31, 2018 respectively.
Note 7. Convertible Notes Payable
Year Ended December 31, 2019
In January 2019, the Company received the third tranche of $60,000 relating to a note executed on July 13, 2018. During the year ending December 31, 2019, the Company accrued interest expense of $1,350. In July 2019, the Company prepaid this note of $60,000 plus accrued interest and a prepayment penalty of $30,000. At December 31, 2019, this note is fully paid.
In January 2019, the Company executed an 8% Convertible Promissory Notes payable to an institutional investor in the principal amount of $308,000. The note, which is due on January 8, 2020, has an original issue discount of $28,000 and transaction costs of $10,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to the 70% of the average of the two lowest per share trading prices for the twenty (20) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $20,466. During the year ended December 31, 2019, the investor converted $266,000 of principal and $17,672 of accrued interest for 1,409,860 shares of common stock prices ranging between $.05 and $1.75. At December 31, 2019, there is $42,000 principal outstanding.
In January 2019, the Company executed an 8% Convertible Promissory Notes payable to an institutional investor in the principal amount of $308,000 each. The note, which is due on January 8, 2020, has an original issue discount of $28,000 and transaction costs of $10,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to the 70% of the average of the two lowest per share trading prices for the twenty (20) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $18,535. During the year ended December 31, 2019, the investor converted $308,000 of principal and $18,535 of accrued interest for 839,210 shares of common stock for prices ranging from $.10 to $2.10. At December 31, 2019, this note is fully converted.
| F-12 |
In January 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $114,000. The note, which is due on October 30, 2019, has an original issue discount of $11,000 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to the 70% average of the two lowest per share trading prices for the ten (10) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $6,028. In July 2019, the Company prepaid this note of $114,000 plus accrued interest and a prepayment penalty of $42,010. At December 31, 2019, this note is fully paid.
In January 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $58,300. The note, which is due on November 15, 2019, has an original issue discount of $5,300 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to the 70% average of the two lowest per share trading prices for the ten (10) trading days prior to the conversion date. During the nine months ended December 31, 2019, the Company accrued interest of $2,753. In July 2019, the Company prepaid this note of $58,300 plus accrued interest and a prepayment penalty of $21,369. At December 31, 2019, this note is fully paid.
In February 2019, the Company received the fourth tranche of $30,000 relating to a note executed on July 13, 2018. During the year ending December 31, 2019, the Company accrued interest of $700. During the year ended December 31, 2019, the investor converted $29,504 of principal for 382,800 shares of common stock at prices ranging from $.05 and $1.50. At December 31, 2019, there is $496 principal and $700 accrued interest outstanding.
In March 2019, the Company executed a Securities Purchase Agreement for Convertible Debentures to an institutional investor in the principal amount of $365,000 to be funded in six tranches: $65,000 at signing, $100,000 forty-five (45) days after the signing date and $200,000 forty-five (45) days after the second closing date. The debentures, which are payable on March 18, 2022, have a 10% original issue discount and a commitment fee of $5,000 payable with the signing debenture. The debentures convert into common stock of the Company at a conversion price equal to the lesser of (i) $6.00 or (ii) seventy percent (70%) of the lowest traded price (as reported by Bloomberg LP) of the common stock for the ten (10) trading days prior to the conversion date. The first tranche of $65,000 was received in March 2019. In September 2019, the Company prepaid this note of $65,000 and a prepayment penalty of $19,500. At December 31, 2019, this note is fully paid.
In March 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $47,300. The note, which is payable on January 30, 2020, has an original issue discount of $4,300 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price equal to 70% of the average of the lowest two (2) trading prices during the ten (10) trading day period ending on the last complete trading day prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $3,226. In September 2019, the Company prepaid this note of $47,300 plus accrued interest and a prepayment penalty of $16,555. At December 31, 2019, this note is fully paid.
In March 2019, the Company executed a 3% Convertible Promissory Note payable to an institutional investor in the principal amount of $360,000. The note, which is payable twelve (12) months after each tranche is funded, has an original issue discount of $60,000. The original issue discount will be prorated with each tranche paid. The first tranche of $60,000 is due at signing date. The convertible note converts into common stock of the Company at a conversion price that shall be equal to 65% of the lesser of (i) lowest trading price or (ii) the lowest closing bid price on the OTCQB during the twenty-five (25) trading day period ending on the last complete trading day prior to the conversion date. The first tranche was received on March 29, 2019. The second tranche of $37,500 was received on July 19, 2019. During the year ended December 31, 2019, the Company accrued interest expense of $3.209. In September 2019, the Company prepaid the first tranche of $60,000 plus accrued interest and a prepayment penalty of $30,000. At December 31, 2019, only the second tranche of $37,500 is outstanding.
| F-13 |
In March 2019, the Company executed a 12% Convertible Promissory Note to an institutional investor in the principal amount of $1,500,000 to be funded over separate tranches; the first tranche to be funded on signing. The note, which is due and payable six (6) months after the funding date of each tranche, has an original issue discount of 10%. The Company issued 65,217 shares of restricted common stock on the closing date. These are deemed returnable shares which the investor must return if the Company repays the note prior to the maturity date. In addition, the Company issued 20,000 shares of restricted common stock as a commitment fee. The convertible note converts into common stock of the Company at a conversion price that shall be equal to 65% of the lowest trading price during the thirty (30) day trading period ending on the last complete trading day prior to the conversion date. The first tranche of $750,000 was received on March 25, 2019. The second tranche of $350,000 was received on July 12, 2019 and the Company issued 53,846 shares of restricted common stock. These shares are redeemable if the Company pays the note prior to the maturity date of January 20, 2020. The third and final tranche was received on September 9, 2019 and the Company issued 80,000 shares of restricted common stock. These shares are redeemable if the Company pays the note prior to the maturity date of March 12, 2020. During the year ended December 31, 2019, the Company accrued interest expense of $112,372. During the year ended December 31, 2019, the investor converted $393,647 of principal and $77,017 of accrued interest for 3,705,340 shares of common stock at prices ranging from $0.02 to $11.00. At the year ended December 31, 2019, there is $1,106,353 principal and $35,355 accrued interest outstanding.
In April 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $58,300. The note, which is payable on February 15, 2020, has an original issue discount of $5,300 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price equal to 70% of the average of the lowest two (2) trading prices during the ten (10) trading day period ending on the last complete trading day prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest of $3,811. In September 2019, the Company prepaid this note of $58,300 plus accrued interest and a prepayment penalty of $20,405. At December 31, 2019, this note is fully paid.
In May 2019, the Company executed a convertible note conversion period extension agreement on a note dated October 28, 2018, within which the period of conversion by note holder was extended to May 27, 2019. The Company paid $16,031 to note holder for this extension agreement. On May 28, 2019, the Company executed a second extension agreement on this note within which the period of conversion by note holder was extended to June 11, 2019. The Company paid $16,105 to note holder for this extension agreement. During the year ended December 31, 2019, the note holder converted the $308,000 note and accrued interest of $19,539 into 166,440 shares of common shares at prices ranging from $1.75 to $2.26. At December 31, 2019, this note has been fully converted.
In May 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $110,000. The note, which is due on February 13, 2020, has an original issue discount of $10,000 and transactions costs of $3,000. The convertible note converts into common stock of the Company at conversion price that shall be equal to the 65% of the lowest closing price for the twenty (20) trading days prior to the conversion date. During the years ended December 31, 2019, the Company accrued interest expense of $7,723. During the year ended December 31, 2019, the investor converted $91,500 of principal and $6,000 of accrued interest into 1,596,158 shares of common stock at prices ranging from $0.04 to $0.20. At the year ended December 31, 2019, there is $18,500 principal and $1,723 accrued interest outstanding.
In July 2019, two Back-End notes executed in October 2018 with an institutional investor was funded for $154,000 each. Each note, which is due on October 29, 2019, has an original issue discount of $14,000 and transaction costs of $2,500. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the 70% of the average of the two (2) lowest per share trading prices for the prior twenty (20) trading days including the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $6,143 for each note.
In July 2019, the Company signed an amendment to a convertible note issued on March 21, 2019 revising the conversion price from 75% to 65% of the lowest trading price during the thirty (30) trading days prior to the conversion date.
In July 2019 the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $140,800. The note, which is payable on April 30, 2020, has an original issue discount of $12,800 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price equal to 70% of the average of the lowest two (2) trading prices during the ten (10) trading day period ending on the last complete trading day prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest of $7,192.
| F-14 |
In July 2019, the Company executed a convertible note conversion period extension agreement on a note dated January 8, 2019 within which the period of conversion by note holder was extended to August 9, 2019. The Company paid $21,560 to note holder for this extension agreement.
In July 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $113,000. The note, which is due on July 9, 2020, has an original issue discount of $10,000 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to the 65% average of the lowest per share trading prices for the twenty (20) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $6,130.
In July 2019, the Company executed an 8% Convertible Promissory Note payable to an institutional investor in the principal amount of $235,200. The note, which is due on July 11, 2020, has an original issue discount of $25,200 and transaction costs of $10,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to the 65% average of the lowest closing bid price for the prior twenty (20) trading days including the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $8,351.
In July 2019, the Company executed a convertible note conversion period extension agreement on a note dated January 8, 2019 within which the period of conversion by note holder was extended to August 9, 2019. The Company paid $22,410 to note holder for this extension agreement.
In July 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $250,000. The note, which is due on April 19, 2020, has an original issue discount of $37,500 and transaction costs of $5,000. The convertible note converts into common stock of the Company at a conversion price that shall be equal to 65% of the average of the lowest per share trading prices for the twenty-five (25) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $12,986.
In July 2019, the Company executed two 12% Convertible Promissory Notes payable to two institutional investors in the principal amount of $38,500 each. Each note, which is due on April 30, 2020, has an original issue discount of $3,500 and transaction costs of $1,500. The convertible notes convert into common stock of the Company at a conversion price that shall be equal to the 65% of the lowest per share trading prices for the twenty (20) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $3,746 for the two notes.
In September 2019, the Company executed a 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $58,300. The note, which is payable on July 15, 2020, has an original issue discount of $5,300 and transaction costs of $3,000. The convertible note converts into common stock of the Company at a conversion price equal to 70% of the average of the lowest two (2) trading prices during the ten (10) trading day period ending on the last complete trading day prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest of $1,967.
In September 2019, a Back-End note executed in January 2019 with an institutional investor was funded for $154,000. The note, which is due on January 9, 2020, has an original issue discount of $14,000 and transaction costs of $5,000. The convertible note converts into common stock of the Company at conversion price that shall be equal to the 70% of the average of the two (2) lowest per share trading prices for the twenty (20) trading days prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $3,747.
In September 2019, two Back-End notes executed in January 2019 with an institutional investor was funded for $154,000 each. Each note, which is due on January 8, 2020, has an original issue discount of $14,000 and transactions costs of $5,000. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the 70% of the average of the two (2) lowest per share trading prices for the prior twenty (20) trading days including the conversion date. During the year ended December 31, 2019, the Company accrued interest expense of $3,476 for each note.
| F-15 |
In October 2019, the Company executed a 10% Convertible Promissory Note payable to an institutional investor in the principal amount of $57,750. The note, which is payable on October 2, 2020, has an original issue discount of $5,250 and transaction costs of $2,500. The convertible note converts into common stock of the Company at a conversion price equal to 65% of the lowest trading price during the twenty (20) trading days ending on the last complete trading day prior to the conversion date. During the year ended December 31, 2019, the Company accrued interest of $1,424.
Year Ended December 31, 2018
In January 2018, the Company executed an 8% Convertible Promissory Note payable to an institutional investor in the principal amount of $110,000. During the year ended December 31, 2018, the note, which was due on October 12, 2018, and accrued interest totaling $4,489 was fully converted into 48,257 shares of common stock at a price of $2.37 per share.
In January 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $91,300. During the year ended December 31, 2018, the note, which was due on October 30, 2018, and accrued interest totaling $4,980 was fully converted into 32,616 shares of common stock at prices ranging from $2.915 to $3.015.
In February 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $63,800. During year ended December 31, 2018, the note, which was due on November 30, 2018, and accrued interest totaling $3,480 was fully converted into 26,196 shares of common stock at prices ranging from $2.435 to $2.66.
In March 2018, the Company executed an 8% Convertible Promissory Note payable to an institutional investor in the principal amount of $77,000. As of September 30, 2018, the institutional investor exercised its MFN provision in Paragraph 4a increasing the OID from the stated in the note from 10% to 15% thus increasing the amount owed to $80,500. During the year ended December 31, 2018, the note, which was due on December 5, 2018, and accrued interest totaling $5,928 was fully converted into 48,049 shares of common stock at a price of $1.80.
In March 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $72,450. During the year ended December 31, 2018, the note, which was due on December 30, 2018, and accrued interest totaling $3,780 was fully converted into 37,556 shares of common stock at prices ranging from $1.965 to $2.185.
In May 2018, the Company executed an 8% Convertible Promissory Note payable to an institutional investor in the principal amount of $125,000. During the year ended December 31, 2018, the note, which is due on May 10, 2019, and accrued interest totaling $415 was fully converted into 32,525 shares of common stock at prices ranging from $3.14 to $5.16. At the year ended December 31, 2018, the Company is still liable for $5,288 of accrued interest that has not yet been converted.
In May 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $51,750. During the year ended December 31, 2018, the note, which is due on March 1, 2019, and accrued interest of $2,700 was fully converted into 13,174 shares of common stock at prices ranging from $4.05 and $4.25.
In July 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $56,500. The note, which is due on April 17, 2019, has an original issue discount of $6,500. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the lesser of: (i) $10.50 or (ii) 75% of the lowest per share trading price for the thirty (30) trading days before the issued date of this note. The Company issued 2,000 shares of common stock valued at $8,000 upon the execution of this note. During the year ended December 31, 2018, the Company recognized interest expense of $2,991.
| F-16 |
In July 2018, the Company executed an 3% Convertible Promissory Note payable to an institutional investor in the principal amount of $180,000 for funding in six tranches. The note, which is due twelve months from the date of each individual tranche, has an original issue discount of $10,000 per tranche. The convertible notes convert into common stock of the Company at conversion price that shall be equal to 75% of the market price which is lowest trading price during the twenty (20) trading day period ending on the last complete trading day prior to the conversion date. The trading price is the lesser of: (i) lowest traded price or (ii) the lowest closing bid price on the OTCQB. The first tranche of $60,000 was received in the month of July and second tranche of $30,000 was received in the month of August. During the year ended December 31, 2018, the Company recognized interest expense of $1,102.
In July 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $28,250. The note, which is due on April 17, 2019, has an original issue discount of $3,250. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the lesser of: (i) $10.50 or (ii) 75% of the lowest per share trading price for the thirty (30) trading days before the issued date of this note. The Company issued 1,000 shares of common stock valued at $4,000 upon the execution of this note. During the year ended December 31, 2018, the Company recognized interest expense of $1,495.
In July 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $77,000. As of September 30, 2018, the institutional investor exercised its MFN provision in Paragraph 4a increasing the OID from the stated in the note from 10% to 15% thus increasing the amount owed to $80,500. The note, which is due on April 5, 2019, has an original issue discount of $7,000. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the lesser of: (i) $3.00 or (ii) 75% of the lowest per share trading price for the ten (10) trading days before the conversion date. During the year ended December 31, 2018, the Company recognized interest expense of $4,870.
In July 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $60,950. The note, which is due on April 30, 2019, has an original issue discount of $7,950. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the lesser of: (i) $10.00 or (ii) variable conversion price which is 75% of the average of the lowest (2) VWAP for the ten (10) trading day period ending on the latest compete trading day prior to the conversion date. During the year ended December 31, 2018, the Company recognized interest expense of $3,647.
In August 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $58,300. The note, which is due on June 15, 2019, has an original issue discount of $5,300. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the lesser of: (i) $10.00 or (ii) variable conversion price which is 75% of the average of the two (2) lowest VWAP for the ten (10) trading day period ending on the latest compete trading day prior to the conversion date. During the year ended December 31, 2018, the Company recognized interest expense of $2,338.
In October 2018, the Company executed an 12% Convertible Promissory Note payable to an institutional investor in the principal amount of $47,300. The note, which is due on July 15, 2019, has an original issue discount of $7,300. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the variable conversion price which is 70% of the average of the two (2) lowest VWAP for the ten(10) trading day period ending on the latest compete trading day prior to the conversion date. During the year ended December 31, 2018, the Company recognized interest expense of $1,291.
In October 2018, the Company executed an 8% Convertible Promissory Note payable to an institutional investor in the principal amount of $165,000. The note, which is due on October 12, 2019, has an original issue discount of $15,000. The convertible notes convert into common stock of the Company at conversion price that shall be equal to 65% of the lowest per share closing price during the fifteen (15) trading days immediately preceding the date of the notice of conversion. The first tranche of $110,000 was received in the month of October and the second tranche of $55,000 was received in the month of November. During the year ended December 31, 2018, the Company recognized interest expense of $2,594.
In October 2018, the Company executed two 8% Convertible Promissory Notes payable to two institutional investors, each in the principal amount of $308,000. Each note, which is due on October 29, 2019, has an original issue discount of $33,000. The convertible notes convert into common stock of the Company at conversion price that shall be equal to the 70% of the average of the two (2) lowest per share trading prices for the twenty (20) trading days prior to the conversion date. During the year ended December 31, 2018, the Company recognized interest expense of $4,118 for each note.
| F-17 |
In November 2018, a Back-End note executed in May 2018 with an institutional investor was funded. The Back-End note is an 8% Convertible Promissory Note payable in the principal amount of $125,000. The note, which is due on May 10, 2019, has an original issue discount of $10,000. The convertible notes convert into common stock of the Company at conversion price that shall be equal to 72% of the lowest VWAP for the ten (10) trading days prior to and including the conversion date. Conversion into shares of common stock can commence following the 180thcalendar day after the Original Issue Date. During the year ended December 31, 2018, the Company recognized interest expense of $1,123.
As of December 31, 2019, all 2018 notes have been fully converted or paid. (See Note 8)
Note 8. Equity Transactions
Preferred Stock
The total number of shares of preferred stock which the Company shall have authority to issue is 20,000,000 shares with a par value of $0.001 per share. During the year ended December 31, 2019, the Company authorized the issuance of 1,000,000 shares of preferred stock to officers, directors and consultants as deferred compensation and/or expense. The shares are eligible for conversion after 24 months into 40 shares of common stock per each preferred share. The value of the issued shares was calculated on the basis of 40 shares per preferred share at the common share value on the date of issuance. The deferred compensation value of the shares will vest monthly at 1/24th of the calculated value of $3,000,000 and requisite expense or reduction of accrued compensation and/or accrued directors fees will be recorded. At the recording of the requisite vested share value, the corresponding number of preferred shares will be recorded as being issued. At the year ended December 31, 2019, there were 250,000 vested preferred shares and $510,000 was recorded to reduce accrued compensation; $52,500 was recorded to reduce accrued directors’ fees, and $187,500 was recorded as expense for a total of $750,000.
Common Stock
On July 9, 2019, the Company filed a Definitive Information Statement on Schedule 14C for the purpose of authorizing the increase in the number of authorized shares of Common Stock from four hundred million (400,000,000) shares of Common Stock to nine hundred million (900,000,000) shares of Common Stock (the “Authorized Common Stock Share Increase”). On July 9, 2019, the Company filed Articles of Amendment to the Company’s Articles of Incorporation to implement the Authorized Common Stock Share Increase with the State of Nevada.
On October 10, 2019, the Company filed a Definitive Information Statement on Schedule 14C for the purpose of authorizing the increase in the number of authorized shares of Common Stock from nine hundred million (900,000,000) shares of Common Stock to one billion nine hundred million (1,900,000,000) shares of Common Stock (the “Authorized Common Stock Share Increase”). On October 15, 2019, the Company filed Articles of Amendment to the Company’s Articles of Incorporation to implement the Authorized Common Stock Share Increase with the State of Nevada.
On December 6, 2019, the Corporation filed a Definitive Information Statement on Schedule 14C for the purpose of authorizing the implementation of a corporate action for a reverse stock split of the issued and outstanding shares of Common Stock, including shares of Common Stock reserved for issuance in a ratio and at a time and date to be determined by the Corporation’s Board of Directors, not to exceed a one-for-fifty (1:50) basis. On December 12, 2019, the Company’s Board of Directors authorized and approved the reverse stock on a one-for-fifty (1:50) basis. The Company subsequently filed with FINRA on December 20, 2019 for approval to implement this reverse stock split. FINRA approval was received on February 12, 2020. As of February 12, 2020, the Company’s stock began trading under the symbol of EWLLD. Throughout these financial statements, footnotes and elsewhere in the Form 10K for the years ended December 31, 2019 and 2018, the common shares outstanding and issued have been adjusted to reflect this reverse split.
| F-18 |
The Definitive Information Statement on Schedule 14C, noted above, was also filed for the purpose of authorizing the increase in the number of authorized shares of Common Stock one billion nine hundred million (1,900,000,000) shares of Common Stock to four billion five hundred million (4,500,000,000) shares of Common Stock (the “Authorized Common Stock Share Increase”). On December 9, 2019, the Company filed Articles of Amendment to the Company’s Articles of Incorporation to implement the Authorized Common Stock Share Increase with the State of Nevada.
Debt Conversion Shares
2019
During the year ended December 31, 2019, the Company issued a total of 8,225,381 shares of common stock per debt conversion of various convertible notes (See Note 7). The total of the debt conversion was for $1,776,901 of principal, $157,756 of accrued interest and $20.900 financing costs.
During the year ended December 31, 2019, the Company issued 219,064 shares of common stock for financing costs relating to convertible debt. The value of the financing costs was $937,462.
2018
During the year ended December 31, 2018, the Company issued a total of 625,714 shares of common stock per debt conversion of various convertible notes (See Note 7). The total of the debt conversion was $1,284,582 principal plus $172,200 accrued interest.
During the year ended December 31, 2018, the Company issued 49,377 shares of common stock for financing costs relating to convertible debt. The value of the financing costs was $127,374
Consultant Issued Shares
2019
During the year ended December 31, 2019, the Company issued 163,500 shares of common stock for marketing and consulting services valued at $635,385.
2018
During the year ended December 31, 2018, the Company issued 158,000 shares of common stock for marketing and consulting services valued at $751,415.
Institutional Investor Shares
2019
In April 2019, the Company issued 16,100 shares of common stock pursuant to a capital call notice in relation to an Equity Purchase Agreement dated June 18, 2018. The capital call totaled $59,100.
2018
During the year ended December 31, 2018, the Company issued 20,000 shares of common stock as an inducement per an Equity Purchase Agreement with an institutional investor within which the investor agrees to purchase up to $1,500,000 of the Company’s common stock, par value $0.001. The value of these shares is $70,000.
Stock Options
On August 6, 2015, the Board of Directors approved the 2015 Stock Option Plan, pursuant to which certain directors, officers, employees and consultants will be eligible for certain stock options and grants. The Plan is effective as of August 1, 2015 and the maximum number of shares reserved and available for granting awards under the Plan shall be an aggregate of 3,000,000 shares of common stock, provided however that on each January 1, starting with January 1, 2016, an additional number of shares equal to the lesser of (A) 2% of the outstanding number of shares (on a fully-diluted basis) on the immediately preceding December 31 and (B) such lower number of shares as may be determined by the Board or committee charged with administering the plan. This plan may be amended at any time by the Board or appointed plan Committee.
The following is a summary of the status of all Company’s stock options as of December 31, 2019 and changes during the periods ended on December 31, 2019 and 2018, respectively:
| Number | Weighted | ||||||||||||||||
| of Stock | Average | Remaining | Intrinsic | ||||||||||||||
| Options | Exercise Price | Life (yrs) | Value | ||||||||||||||
| Outstanding at January 1, 2018 | 400,000 | $ | 13.00 | 1.9 | $ | - | |||||||||||
| Granted | - | - | - | - | |||||||||||||
| Exercised | - | - | - | - | |||||||||||||
| Expired | (343,000 | ) | 3.50 | - | |||||||||||||
| Outstanding at December 31, 2018 | 57,000 | $ | 40.00 | 2.2 | $ | - | |||||||||||
| Granted | - | - | - | - | |||||||||||||
| Exercised | - | - | - | - | |||||||||||||
| Expired | - | - | - | - | |||||||||||||
| Outstanding at December 31, 2019 | 57,000 | 40.00 | 1.1 | $ | - | ||||||||||||
| Options exercisable at December 31, 2019 | 57,000 | $ | 40.00 | 1.1 | $ | - | |||||||||||
| F-19 |
The Company recognized stock option expense of $0 and $467,693 for the years ended December 31, 2019 and 2018, respectively.
Warrants
In March 2018, the Board of Directors, at the request and with the approval of the investors, determined that it was in the best interests of the Company and the Investors, based upon market price and relatively limited liquidity of the shares of common stock that the Company revised the expiration date and exercise price for 8,349 unexercised warrants granted on April 9, 2015. The original expiration date of April 9, 2018 was extended to April 9, 2019 and the original exercise price of $17.50 was reduced to $2.50. During the year ended December 31, 2019, these warrants expired.
Note 9. Commitments, Contingencies
The Company may be subject to lawsuits, administrative proceedings, regulatory reviews or investigations associated with its business and other matters arising in the normal conduct of its business. The following is a description of an uncertainty that is considered other than ordinary, routine and incidental to the business.
The closing of the Initial Exchange Agreement with Private Co. was conditioned upon certain, limited customary representations and warranties, as well as, among other things, our compliance with Rule 419 (“Rule 419”) of Regulation C under the Securities Act of 1933, as amended (the “Securities Act”) and the consent of our shareholders as required under Rule 419. Accordingly, we conducted a “Blank Check” offering subject to Rule 419 (the “Rule 419 Offering”) and filed a Registration Statement on Form S-1 to register the shares of such offering; the Registration Statement was declared effective on September 14, 2012. We used 10% of the subscription proceeds as permitted under Rule 419 and the amount remaining in the escrow trust as of the date of the closing of the Share Exchange was $90,000 (the “Trust Account Balance”).
| F-20 |
Rule 419 required that the Share Exchange occur on or before March 18, 2014, but due to normal negotiations regarding the transactions and the parties’ efforts to satisfy all the closing conditions, the Share Exchange did not close on such date. Accordingly, after numerous discussions with management of both parties, they entered into an Amended ,and Restated Share Exchange Agreement (the “Share Exchange Agreement”) to reflect a revised business combination structure, pursuant to which we would: (i) file a registration statement on Form 8-A (“Form 8A”) to register our common stock pursuant to Section 12(g) of the Exchange Act, which we did on May 1, 2014 and (ii) seek to convert the participants of the Rule 419 Offering into participants of a similarly termed private offering (the “Converted Offering”), to be conducted pursuant to Regulation D, as promulgated under the Securities Act
Fifty-two persons participated in the Rule 419 Offering and each of them gave the Company his/her/its consent to use his/her/its escrowed funds to purchase shares of the Company’s restricted common stock in the Converted Offering (the “Consent”) rather than have their funds returned. To avoid further administrative work for the investors, we believe that we took reasonable steps to inform investors of the situation and provided them with an appropriate opportunity to maintain their investment in the Company, if they so choose, or have their funds physically returned. Management believed the steps it took constituted a constructive return of the funds and therefore met the requirements of Rule 419.
However, pursuant to Rule 419(e)(2)(iv), “funds held in the escrow or trust account shall be returned by first class mail or equally prompt means to the purchaser within five business days [if the related acquisition transaction does not occur by a date that is 18 months after the effective date of the related registration statement].” As set forth above, rather than physically return the funds, we sought consent from the investors of the Rule 419 Offering to direct their escrowed funds to the Company to instead purchase shares in the Converted Offering. The consent document (which was essentially a form of rescission) was given to the investors along with a private placement memorandum describing the Converted Offering and stated that any investor who elected not to participate in the Converted Offering would get 90% of their funds physically returned. Pursuant to Rule 419(b)(2)(vi), a blank check company is entitled to use 10% of the proceed/escrowed funds; therefore, if a return of funds is required, only 90% of the proceed/escrowed funds need be returned. The Company received $100,000 proceeds and used $10,000 as per Rule 419(b)(2)(vi); therefore, only $90,000 was subject to possible return.
As disclosed therein, we filed the amendments to the initial Form 8-K in response to comments from the SEC regarding the Form 8-K and many of those comments pertain to an alleged violation of Rule 419. The Company continued to provide the SEC with information and analysis as to why it believes it did not violate Rule 419 but was unable to satisfy the SEC’s concerns. Comments and communications indicate that Rule 419 requires a physical return of funds if a 419 offering cannot be completed because a business combination was not consummated within the required time frame; constructive return is not permitted.
Because of these communications and past comments, we are disclosing that we did not comply with the requirements of Rule 419, which required us to physically return the funds previously submitted to escrow pursuant to the Rule 419 Offering. Because of our failure to comply with Rule 419, the SEC may bring an enforcement action or commence litigation against us for failure to strictly comply with Rule 419. If any claims or actions were to be brought against us relating to our lack of compliance with Rule 419, we could be subject to penalties (including criminal penalties), required to pay fines, make damages payments or settlement payments. In addition, any claims or actions could force us to expend significant financial resources to defend ourselves, could divert the attention of our management from our core business and could harm our reputation.
| F-21 |
Ultimately, the SEC determined to terminate its review of the Initial Form 8-K and related amendments, rather than provide us with additional opportunities to address their concerns and therefore, we did not clear their comments. It is not possible at this time to predict whether or when the SEC may initiate any proceedings, when this issue may be resolved or what, if any, penalties or other remedies may be imposed, and whether any such penalties or remedies would have a material adverse effect on our consolidated financial position, results of operations, or cash flows. Litigation and enforcement actions are inherently unpredictable, the outcome of any potential lawsuit or action is subject to significant uncertainties and, therefore, determining currently the likelihood of a loss, any SEC enforcement action and/or the measurement of the amount of any loss is complex. Consequently, we are unable to estimate the range of reasonably possible loss. Our assessment is based on an estimate and assumption that has been deemed reasonable by management, but the assessment process relies heavily on an estimate and assumption that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change that estimate and assumption. Considering the uncertainty of this issue and while Management evaluates the best and most appropriate way to resolve same, management determined to create a reserve on the Company’s Balance Sheet for the $90,000 that was subject to the Consent.
The statute of limitations applicable to SEC enforcement proceedings is 28 U.S.C. § 2462. Section 2462 provides that “an action, suit or proceeding for the enforcement of any civil fine, penalty, or forfeiture, pecuniary or otherwise,” must be commenced within five (5) years from the date when the relevant claim accrued. Section 2462 has also been applied in enforcement proceedings brought by other federal regulatory agencies, including the Commodities and Futures Trading Commission, the Office of Foreign Assets Control, the Federal Communications Commission, and the Federal Energy Regulatory Commission. The SEC is now barred from commencing an enforcement action against the Company because the deadline for the SEC to commence such action expired on or before September 30, 2019. Because of this expiration date, the Company has removed the $90,000 from the balance and recorded it as Gain on Contingent Liability.
From time to time the Company may become a party to litigation matters involving claims against the Company. Except as may be outlined above, the Company believes that there are no current matters that would have a material effect on the Company’s financial position or results of operations.
Note 10. Derivative Valuation
The Company evaluated the convertible debentures and associated warrants in accordance with ASC Topic 815, “Derivatives and Hedging,” and determined that the conversion feature of the convertible promissory notes was not afforded the exemption for conventional convertible instruments due to their variable conversion rates. The notes have no explicit limit on the number of shares issuable, so they did not meet the conditions set forth in current accounting standards for equity classification. In addition, the warrants have a Most Favored Nations clause resulting in the exercise price of the warrants also not being fixed. Therefore, these have been characterized as derivative instruments. We elected to recognize the notes under ASU paragraph 815-15-25-4, whereby there would be a separation into a host contract and derivative instrument. We elected to initially and subsequently measure the notes and warrants in their entirety at fair value, with changes in fair value recognized in earnings.
The debt discount is amortized over the life of the note and recognized as interest expense. For the years ended December 31, 2019 and 2018, the Company amortized the debt discount of $3,871,849 and $511,359, respectively, to interest expense.
During the years ended December 31, 2019 and 2018, the Company had the following activity in the derivative liability account:
| F-22 |
| Notes | Warrants | Total | ||||||||||
| Derivative liability at January 1, 2018 | $ | 365,591 | $ | 774,986 | $ | 1,140,577 | ||||||
| Addition of new conversion option derivatives | 1,243,333 | - | 1,243,333 | |||||||||
| Conversion of note derivatives | (429,927 | ) | - | (429,927 | ) | |||||||
| Changes in warrant derivatives | - | (202,610 | ) | (202,610 | ) | |||||||
| Change in fair value | 223,724 | (390,995 | ) | (167,271 | ) | |||||||
| Reclassification of derivative to gain on extinguishment of debt | - | - | - | |||||||||
| Derivative liability at December 31, 2018 | $ | 1,402,721 | $ | 181,381 | $ | 1,584,102 | ||||||
| Addition of new conversion option derivatives | 4,385,384 | - | 4,385,384 | |||||||||
| Conversion of note derivatives | (2,165,898 | ) | - | (2,165,898 | ) | |||||||
| Extinguishment due to note cancellations | - | |||||||||||
| Changes in warrant derivatives | - | - | - | |||||||||
| Change in fair value | (92,240 | ) | (181,374 | ) | (273,614 | ) | ||||||
| Reclassification of derivative to gain on extinguishment of debt | - | - | - | |||||||||
| Derivative liability at December 31, 2019 | $ | 3,529,967 | $ | 7 | $ | 3,529,974 | ||||||
For purposes of determining the fair market value of the derivative liability, the Company used Black Scholes option valuation model. The significant assumptions used in the Black Scholes valuation of the derivative are as follow:
| Stock price at valuation date | $ | .05-11.25 | ||
| Exercise price of warrants | $ | 12.50 | ||
| Conversion rate of convertible debt | $ | .0325 – 35.00 | ||
| Risk free interest rate | 1.48%-2.60 | % | ||
| Stock volatility factor | 103%-1468 | % | ||
| Years to Maturity | .02 – 1 | |||
| Expected dividend yield | None |
Note 11. Supplemental Cash Flow Information
During the year ended December 31, 2018 the Company had the following non-cash investing and financing activities:
| ● | Issued 49,377 shares of common stock for financing costs valued at $127,374 | |
| ● | Issued 20,000 shares of common stock as an inducement for an Equity Purchase Agreement valued at $70,000 | |
| ● | Issued 348,000 shares of common stock to officers, directors and certain consultants per the 2018 Equity Incentive Plan valued at $1,566,000 | |
| ● | Issued 80,000 shares of common stock for settlement of debt of $180,051 and accrued interest of $56,817 | |
| ● | Issued 52,000 shares of common stock valued at $239,300 which was recorded as a prepaid | |
| ● | Issued 106,000 shares of common stock valued at $512,115 for services. | |
| ● | Issued 625,714 shares of common stock for the extinguishment of $1,294,582 of debt and $172,00 of accrued interest |
| F-23 |
During the year ended December 31, 2019 the Company had the following non-cash investing and financing activities:
| ● | Issued 219,064 shares of common stock for financing costs valued at $937,462. | |
| ● | Issued 8,225,381 shares of common stock for settlement of debt of $1,776,900, accrued interest of $157,756 and $20,900 of financing costs. | |
| ● | Issued 36,000 shares of common stock valued at $123,500 which was recorded as a prepaid. | |
| ● | Issued 127,500 shares of common stock valued at $511,885 for services |
Note 12. Subsequent Events
On January 30, 2020, the Company executed a 12-month advisory services agreement. The Company is to issue 20,000 shares of common stock monthly. The Company issued 40,000 shares of common stock (for January and February) with a value of $118. In addition, the Company is to also pay the advisor a monthly fee of $2,500.
On February 12, 2020, FINRA approved a 1:50 reverse split of the Company’s common stock. As noted throughout this document, all common shares are stated as if the 1:50 reverse split had been completed as of the beginning of the year ended December 31, 2018. Following the approval, the Company’s stock began trading under the symbol “EWLLD”. Due to rounding issues for the reverse split, the Company issued 47,877 additional shares of common stock.
On February 19, 2020, the Board of Directors approved the increase of authorized common stock shares from 4,500,000,000 to 20,000,000,000. The number of authorized preferred shares remained at 20,000,000.
From January 1 until the filing of this report, the Company issued 454,143,389 shares of common stock for debt conversion totaling $251,040 which includes $338,510 principal, $43,248 accrued interest and $63,000 financing costs.
From January 1 until the filing of this report, the Company issued 55,000 shares of common stock to consultants for services rendered in accordance to consulting agreements. The value of these shares is $995.
| F-24 |
ITEM 9: CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
During the past two years, there was no disagreement of the type described in paragraph (a)(1)(iv) or any reportable event as described in paragraph (a)(1)(v) of Item 304 of Regulation S-K that is required to be disclosed under this Item 9.
ITEM 9A: CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
We maintain disclosure controls and procedures designed to provide reasonable assurance that material information required to be disclosed by us in the reports we file or submit under the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and that the information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure.
We performed an evaluation (“Evaluation”), under the supervision and with the participation of our management including our Chief Executive Officer (“CEO”) and Chief Financial Officer (“CFO”), of the effectiveness of the design and operation of our disclosure controls and procedures (“Disclosure Controls”) as of the end of the period covered by this Report pursuant to Rule 13a-15 of the Exchange Act. Based on this evaluation and the existence of the material weaknesses discussed below in “Management’s Report on Internal Control Over Financial Reporting,” our management, including our CEO and CFO, concluded that our disclosure controls and procedures were not effective at the reasonable assurance level as of the end of the period covered by this report.
We do not expect that our disclosure controls and procedures will prevent all errors and all instances of fraud. Disclosure controls and procedures, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the disclosure controls and procedures are met. Further, the design of disclosure controls and procedures must reflect the fact that there are resource constraints, and the benefits must be considered relative to their costs. Because of the inherent limitations in all disclosure controls and procedures, no evaluation of disclosure controls and procedures can provide absolute assurance that we have detected all our control deficiencies and instances of fraud, if any. The design of disclosure controls and procedures also is based partly on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our Board, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
| ● | pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| ● | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of our management and directors; and |
| ● | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
| 37 |
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Additionally, projections of any evaluation of effectiveness to future periods are subject to the risks that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management assessed the effectiveness of our internal control over financial reporting as of December 31, 2019. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control-Integrated Framework (2013). Based on this assessment, management concluded that our internal control over financial reporting was not effective as of December 31, 2019, due to the existence of the material weaknesses discussed below. A material weakness is a control deficiency (within the meaning of the Public Company Accounting Oversight Board (“PCAOB”) Auditing Standard No. 5), or combination of control deficiencies, that results in more than a remote likelihood that a material misstatement of the annual or interim financial statements will not be prevented or detected.
Management identified the following material weaknesses that have caused management to conclude that as of December 31, 2019, our internal control over financial reporting was not effective:
| ● | Our size has prevented us from being able to employ sufficient resources to enable us to have an adequate level of supervision and segregation of duties within our internal control system. Specifically, there is limited review of financial reporting and policies and procedures have not yet been implemented to analyze, document, monitor and report on non-routine and complex transactions that require management estimation or judgment. | |
| ● | The Company does not have adequate controls and procedures or an internal audit function to detect errors in accounting for certain of its financing transactions. | |
| ● | The lack of sufficient controls in place to ensure that all disclosures required were addressed in our financial statements, which may result in ineffective oversight in the establishment and monitoring of required internal controls and procedures. | |
| ● | Management determined that the Company does not have a sufficient complement of personnel with appropriate training and experience in U.S. generally accepted accounting principles (“GAAP”) and lacks certain subject matter expertise related to accounting for income taxes, complex debt and equity transactions, and disclosures. |
This Annual Report on Form 10-K does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting due to permanent exemptions for smaller reporting companies. Management’s report was not subject to such attestation pursuant to rules of the Securities and Exchange Commission that permits us to provide only management’s report in this Annual Report.
Remediation Plan for Material Weaknesses
While management believes that the Company’s financial statements previously filed in the Company’s SEC reports have been properly recorded and disclosed in accordance with GAAP, based on the control deficiencies identified above, we have begun taking steps and plan to take additional measures to remediate the underlying causes of the material weakness, primarily through the development and implementation of formal policies, improved processes and documented procedures, as well as the hiring of additional finance personnel. Management believes that the appointment of additional management personnel will lead to increased oversight over the accounting and reporting function. As soon as we can raise sufficient capital or our operations generate sufficient cash flow, we will hire additional personnel to handle our accounting and reporting functions. We also plan to supply enhanced training and education on principles related to accounting for financing transactions, when funds allow.
Management has reviewed the financial statements and underlying information included herein in detail and believes the procedures performed are adequate to fairly present our financial position, results of operations and cash flows for the periods presented in all material respects.
| 38 |
Changes in Internal Control Over Financial Reporting
Other than as described above, there have been no changes in our internal control over financial reporting during the year ended December 31, 2019 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting
None.
ITEM 10: DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE
Our directors were elected to serve until the next annual meeting of shareholders and until his respective successors will have been elected and will have qualified. The following table sets forth the name, age and position held with respect to our present executive officers and directors:
| Name | Age | Position(s) | ||
| Darwin Fogt | 43 | President, Chief Executive Officer and Member of the Board of Directors | ||
| David Markowski | 57 | Chief Financial Officer and Member of the Board of Directors | ||
| Douglas MacLellan | 64 | Chairman and Secretary | ||
| Curtis Hollister | 46 | Chief Technology Officer and Member of the Board of Directors | ||
| Douglas Cole | 64 | Member of the Board of Directors | ||
| Brandon Rowberry | 44 | Member of the Board of Directors | ||
| Rochelle Pleskow | 57 | Member of the Board of Directors |
Darwin Fogt, President, CEO & Director. Mr. Fogt has been CEO of eWellness Corporation since May 2013. From 2001 to 2018, he was founder, President and practicing therapist of Evolution Physical Therapy, Inc., a privately held company in Los Angeles, CA providing sports and orthopedic physical therapy services. From 2008 to 2018, Mr. Fogt was also founder and President of Bebe PT, a physical therapy practice specializing in perinatal rehabilitation and wellness. Additionally, from 2012 to 2018 Mr. Fogt was the founder and President of Evolution Fitness, a primarily cash-based fitness and rehabilitation center serving high level athletes and clients in Culver City, CA. Mr. Fogt has consulted with and been published by numerous national publications including Runner’s World, Men’s Health, Men’s Journal, and various Physical Therapy specific magazines; his 13 plus years of experience include rehabilitating the general population, as well as professional athletes, Olympic gold medalists, and celebrities. Mr. Fogt earned his B.S. in Exercise Science from the University of Southern California in 1996 and his MPT (Master of Physical Therapy) from California State University: Long Beach in 2001. He is currently working toward earning his DPT (Doctor of Physical Therapy) degree.
David Markowski, Chief Financial Officer & Director. Mr. Markowski has been CFO of eWellness Corporation since May 2013. From October 1997 to October 2002 he was CEO and Co-Founder of GFNN, Inc. From 2002 to 2013 Mr. Markowski has maintained various active roles within GFNN’s subsidiaries including Founder, Director and CEO positions. From October 2009 to December 2011, he was the Director of Corporate Development for Visualant, Inc. From June 2003 to 2010 he was President of Angel Systems, Inc. an independent consulting firm with competencies in strategic marketing and business development. From January 1998 to October 1998, Mr. Markowski served as the Vice President of Finance for Medcom USA, a NASDAQ listed company. Prior to that, he had a decade of investment banking experience on Wall Street involved in financing start-ups and public offerings. He is a business development specialist with accolades in INC Magazine and others. Mr. Markowski obtained a BA degree in Marketing from Florida State University in 1982.
| 39 |
Curtis Hollister, Chief Technology Officer & Director. Mr. Hollister has been a founder and CTO of eWellness since May 2013. From November 2008 to present he has been the founder and President of Social Pixels, a privately held Canadian company focused on helping companies apply online media and digital campaigning. From November 2008 to present he has been the founder and President of Ripplefire, a privately held Canadian company also specializing in the digital campaigning space. He is a global entrepreneur and innovator known for his ability to identify and capitalize on industry trends. His high-profile projects include such clients as Government of Canada, AT&T, Bell Canada, Microsoft, Nokia, Conversant IP and TD Bank. From 1998 to 2002 Mr. Hollister founded and operated TeamCast.com, a technology spin-off focusing on peer-to-peer networking. From 1997 to 2002 Mr. Hollister founded and operated Intrasoft Technologies, a technology start-up to capitalize on the emerging Intranet application market. From 1995 to 1997 Mr. Hollister founded and operated Intranet Technologies, the first successful Internet service provider in Ottawa, Canada’s capital city. Mr. Hollister graduated from Center Hastings Secondary in 1991 and from 1991 to 1995 attended Carleton University with a special focus on Economics.
Douglas MacLellan, Chairman of the Board. Mr. MacLellan currently serves as Chairman of the Board of eWellness Healthcare Corporation since May 2013. Mr. MacLellan is also an independent member of the board of directors of American Battery Metals Corporation (OTCQB: ABML), a development stage battery metals recycling and mining company since October 2017 to the present. From November 2009 to December 2017, Mr. MacLellan was an independent director of ChinaNet Online Holdings, Inc. (NASDAQ: CNET) a media development, advertising and communications company. From June 2011 to present Mr. MacLellan has been Chairman of Innovare Products, Inc., a privately held company that develops innovative consumer products. From May 2014 to October 2016, Mr. MacLellan was a member of the Board as an independent director of Jameson Stanford Resources Corporation (OTCBB: JMSN) an early stage mining company. From September 1992 through April 2014, Mr. MacLellan was Chairman and chief executive officer at Radient Pharmaceuticals Corporation. (OTCQB: RXPC.PK), a vertically integrated specialty pharmaceutical company. He also continues to serve as president and chief executive officer for the MacLellan Group, an international financial advisory firm since 1992. From August 2005 to May 2009, Mr. MacLellan was co-founder and vice chairman at Ocean Smart, Inc., a Canadian based aquaculture company. From February 2002 to September 2006, Mr. MacLellan served as chairman and cofounder at Broadband Access MarketSpace, Ltd., a China based IT advisory firm, and was also co-founder at Datalex Corp., a software and IT company specializing in mainframe applications, from February 1997 to May 2002. Mr. MacLellan was educated at the University of Southern California in economics and international relations.
Douglas Cole, Director. Mr. Cole has been a Director of the company since May 2014. Mr. Cole is also current Chairman & CEO of American Battery Metal Corporation (OTCQB: ABML). From 2005 to the present, Mr. Cole has been a Partner overseeing all ongoing deal activities with Objective Equity LLC, a boutique investment bank focused on the clean tech, mining and mineral sectors. From 2002 to 2005, Mr. Cole has played various executive roles as Executive Vice Chairman, Chief Executive Officer and President of TWL Corporation (TWLP.OB). From May 2000 to September 2005, he was also the Director of Lair of the Bear, The University of California Family Camp located in Pinecrest, California. During the period between 1991 and 1998, he was the CEO of HealthSoft, and he also founded and operated Great Bear Technology, which acquired Sony Image Soft and Starpress, then went public and eventually sold to Graphix Zone. In 1995 Mr. Cole was honored by NEA, a leading venture capital firm, as CEO of the year for his work in the Starpress integration. Since 1982 he has been very active with the University of California, Berkeley mentoring early-stage technology companies. Mr. Cole obtained his BA in Social Sciences from UC Berkeley in 1978.
Brandon Rowberry, Director. Mr. Rowberry has been a Director since June 2014. He is a well-known healthcare innovation executive. From 2010 to 201, he drove enterprise-wide Innovation/Venturing for United Health Group where in 2012 they were awarded the prestigious PDMA Outstanding Corporate Innovation Award. From 2012 to present, he has also been Managing Director of 7R Ventures an investment and advisory firm. From 2005 to 2009, he was Director of Strategy & Innovation at Circuit City. From 2001 to 2005, he was a Sr. Corporate Consultant focusing on Organizational Development and Innovation at Hallmark. From 2000 to 2001, he was a Manager of Organizational Development & Innovation at Honeywell. Mr. Rowberry has also been a frequent corporate innovation guest speaker on NBC, FOX, ABC. Mr. Rowberry obtained his Master of Organizational Behavior from Marriott School of Business, BYU in 2000.
| 40 |
Rochelle Pleskow, Director. From 2010 through 2014, Ms. Pleskow served as the Chief Healthcare Information Officer for Hewlett Packard. She developed the framework of healthcare analytics platform, which encompasses quality improvement, outcomes analysis, patient safety, operational analytics, clinical informatics, physician performance, and regulatory compliance monitoring for health plans, hospitals and physicians. From 2008 through 2010, she acted as a senior consultant to various companies on healthcare policy and procedures including acting as an advisor for ASP model start-up, whose business included a HIPAA/HL7 and PCI compliant processing tool, which verifies a patient’s insurance coverage, accurately calculates out-of-pocket costs, and processes payments in one system and at the time of service. This model improves revenue cycle management as it accelerates the collection of patient payments. From 2007 through 2008, she was Director of business Architecture for Blue Shield of California, where she developed the business framework and core elements of a large-scale IT systems implementation to increase competitive advantage for Blue Shield of California. Re-engineered core business processes in Health Services Division to modernize the technology.
Director Qualifications
We seek directors with established strong professional reputations and experience in areas relevant to the strategy and operations of our businesses. We also seek directors who possess the qualities of integrity and candor, who have strong analytical skills and who are willing to engage management and each other in a constructive and collaborative fashion, in addition to the ability and commitment to devote time and energy to service on the Board and its committees, as necessary. We believe that all our directors meet the foregoing qualifications.
The Board believes that the leadership skills and other experience of the Board members described below, in addition to each person’s experience set forth above in their respective biographies, provide the Company with a range of perspectives and judgment necessary to guide our strategies and monitor our executives’ business execution.
Darwin Fogt. Mr. Fogt is a co-founder of the Company and has been serving as a PT for over 12 years and has built three successful physical therapy practices. Mr. Fogt has contributed to the Board’s strong leadership and vision for the development of the Company’s innovative business model.
Douglas MacLellan. Mr. MacLellan is a co-founder of the Company and has been serving as an officer and/or director of various advance technology and high growth companies over the past 20 years. Mr. MacLellan has contributed to the Board’s strong leadership and vision for the development of the Company’s innovative business model.
Curtis Hollister. Mr. Hollister is a co-founder of the Company and has been serving in senior management positions in various advance technology, software and video content business over the past 20 years. He holds a wealth of experience in software development, video content management and network technology.
David Markowski. Mr. Markowski is a co-founder of the Company and has been serving in senior management positions in various companies over the past 20 years, with an emphasis on corporate finance, accounting, audit, financial modeling and marketing. He holds a wealth of experience in company management skills.
Doug Cole. Mr. Cole is an international business executive with over 20 years of active management and board roles in various software, educational and technology public and private companies.
Brandon Rowberry. Mr. Rowberry has held over 15 years in senior management positions as an innovation expert in various advance technology and healthcare industries. He is anticipated to greatly expand our industry relationships within healthcare insurers and the telemedicine industry.
Rochelle Pleskow. Ms. Pleskow holds a vast knowledge base on healthcare informatics and the scaling of various technology implementations at selected large-scale technology and healthcare companies and is anticipated to be a good addition to its board of directors as the Company implements its anticipated white label program to physical therapy clinics through the U.S. marketplace.
Involvement in Certain Legal Proceedings
To the best of the Company’s knowledge, none of the following events occurred during the past ten years that are material to an evaluation of the ability or integrity of any of our executive officers, directors or promoters:
| 41 |
(1) A petition under the Federal bankruptcy laws or any state insolvency law was filed by or against, or a receiver, fiscal agent or similar officer was appointed by a court for the business or property of such person, or any partnership in which he was a general partner at or within two years before the time of such filing, or any corporation or business association of which he was an executive officer at or within two years before the time of such filing;
(2) Convicted in a criminal proceeding or is a named subject of a pending criminal proceeding (excluding traffic violations and other minor offenses);
(3) Subject of any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from, or otherwise limiting, the following activities:
(i) Acting as a futures commission merchant, introducing broker, commodity trading advisor, commodity pool operator, floor broker, leverage transaction merchant, any other person regulated by the Commodity Futures Trading Commission, or an associated person of any of the foregoing, or as an investment adviser, underwriter, broker or dealer in securities, or as an affiliated person, director or employee of any investment company, bank, savings and loan association or insurance company, or engaging in or continuing any conduct or practice in connection with such activity;
(ii) Engaging in any type of business practice; or
(iii) Engaging in any activity in connection with the purchase or sale of any security or commodity or in connection with any violation of Federal or State securities laws or Federal commodities laws;
(4) Subject of any order, judgment or decree, not subsequently reversed, suspended or vacated, of any Federal or State authority barring, suspending or otherwise limiting for more than 60 days the right of such person to engage in any activity described in paragraph (3)(i) above, or to be associated with persons engaged in any such activity;
(5) Found by a court of competent jurisdiction in a civil action or by the Commission to have violated any Federal or State securities law, and the judgment in such civil action or finding by the Commission has not been subsequently reversed, suspended, or vacated;
(6) Found by a court of competent jurisdiction in a civil action or by the Commodity Futures Trading Commission to have violated any Federal commodities law, and the judgment in such civil action or finding by the Commodity Futures Trading Commission has not been subsequently reversed, suspended or vacated;
(7) Subject of, or a party to, any Federal or State judicial or administrative order, judgment, decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of:
(i) Any Federal or State securities or commodities law or regulation; or
(ii) Any law or regulation respecting financial institutions or insurance companies including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty or temporary or permanent cease-and-desist order, or removal or prohibition order; or
(iii) Any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or
(8) Subject of, or a party to, any sanction or order, not subsequently reversed, suspended or vacated, of any self-regulatory organization, any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member.
| 42 |
Promoters and Certain Control Persons
In light of the efforts and services they provided to the Private Co. prior to the Share Exchange, we believe that Douglas MacLellan and Darwin Fogt may be deemed “promoters” (within the meaning of Rule 405 under the Securities Act), since they took the initiative in the formation of our business and received 10% of our equity securities in exchange for the contribution of property or services, during the last five years. In addition, Gregg C. E. Johnson may be deemed a “promoter” of the Company as a result of his receipt of shares of our common stock at the time of completion of the Share Exchange.
Corporate Governance and Director Independence
Presently, we are not currently listed on a national securities exchange or in an inter-dealer quotation system and therefore are not required to comply with the director independence requirements of any securities exchange. In determining whether our directors are independent, however, we intend to comply with the rules of NASDAQ. The board of directors will also consult with counsel to ensure that the boards of director’s determinations are consistent with those rules and all relevant securities and other laws and regulations regarding the independence of directors, including those adopted under the Sarbanes-Oxley Act of 2002 with respect to the independence of audit committee members. Nasdaq Listing Rule 5605(a)(2) defines an “independent director” generally as a person other than an Executive Officer or employee of the Company or any other individual having a relationship which, in the opinion of the Company’s board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Our Board of Directors has determined that Douglas Cole, Mr. Rowberry and Ms. Pleskow would qualify as “independent” as that term is defined by Nasdaq Listing Rule 5605(a)(2). Further, Mr. Cole qualifies as “independent” under Nasdaq Listing Rules applicable to board committees.
Due to our lack of operations and size prior to the Share Exchange, we did not have an Audit Committee. For these same reasons, we did not have any other separate committees prior to the Share Exchange; all functions of a nominating committee, audit committee and compensation committee were performed by our sole director. Although, as stated above, we are not the subject of any listing requirements, in connection with the Share Exchange, our Board of Directors established several committees to assist it in carrying out its duties. In particular, committees shall work on key issues in greater detail than would be practical at a meeting of all the members of the Board of Directors; each committee reviews the results of its deliberations with the full Board of Directors.
The standing committees of the Board of Directors currently consist of the Audit Committee, the Compensation Committee and the Nominating and Corporate Governance Committee. Current copies of the charters for the Audit Committee, the Compensation Committee, and the Nominating and Corporate Governance Committee, as well as our Corporate Governance Guidelines, Code of Ethics and Business Conduct, may be found on our website at www.ewellnesshealth.com, under the heading “Corporate Information—Governance Documents.” Printed versions also are available to any stockholder who requests them by writing to our corporate Secretary at our corporate address. Our Board of Directors may, from time to time, establish certain other committees to facilitate our management.
The Board will consider appointing members to each of the Committees when enough independent directors are appointed to the Board or as otherwise determined by the Board. Until such time, the full board of directors will undertake the duties of the audit committee, compensation committee and nominating committee.
Compliance with Section 16(a) of the Securities Exchange Act of 1934
Section 16(a) of the Exchange Act, as amended, requires that our directors, executive officers and persons who own more than 10% of a class of our equity securities that are registered under the Exchange Act to file with the SEC initial reports of ownership and reports of changes of ownership of such registered securities.
Based solely upon a review of information furnished to the Company, to the Company’s knowledge, during the fiscal year ended December 31, 2017, all such forms were filed.
ITEM 11: EXECUTIVE COMPENSATION
For the fiscal years ended December 31, 2019 and 2018, we did not pay any compensation to our executive officers, nor did any other person receive a total annual salary and bonus exceeding $150,000. Prior to the Share Exchange, which closed in April 2014, we did not pay our sole officer any compensation nor did we have an employment agreement.
| 43 |
Following the Share Exchange, we do not currently have any formal employment salary arrangement with any of our new officers. However, the Board determined that the following salaries shall be recorded and accrued on a monthly basis as contributed capital and compensation for the following individuals for the services they provide to us:
After 1-1-14, but before profitability
| Monthly | Recognized | Contributed | Compensated | |||||||||
| Douglas MacLellan, Chairman | $ | 20,000 | $ | 11,000 | $ | 9,000 | ||||||
| Darwin Fogt, CEO/President | $ | 14,000 | $ | 7,000 | $ | 7,000 | ||||||
| David Markowski, CFO | $ | 14,000 | $ | 7,000 | $ | 7,000 | ||||||
| Curtis Hollister, CTO | $ | 14,000 | $ | 7,000 | $ | 7,000 | ||||||
At profitability and after
| Monthly | Recognized | Contributed | Compensated | |||||||||
| Douglas MacLellan, Chairman | $ | 20,000 | $ | 0 | $ | 20,000 | ||||||
| Darwin Fogt, CEO/President | $ | 14,000 | $ | 0 | $ | 14,000 | ||||||
| David Markowski, CFO | $ | 14,000 | $ | 0 | $ | 14,000 | ||||||
| Curtis Hollister, CTO | $ | 14,000 | $ | 0 | $ | 14,000 | ||||||
All of our current officers have agreed to defer their compensation until such time as we are cash flow positive; therefore, none of our officers have received any compensation as of the date of this Report. No retirement, pension, profit sharing or insurance programs or other similar programs have been adopted by the Company for the benefit of the Company’s employees. The Company has adopted a stock option plan for officers, directors and consultants.
Director’s Compensation
There is no formal or informal arrangements or agreements to compensate employee directors for service provided as a director; however, compensation for new non-employee directors is determined on an ad hoc basis by the existing members of the board of directors at the time a director is elected. The Company is accruing $2,000 per month for the non-employee directors. They are entitled to receive reimbursement of out-of-pocket expenses.
Compensation Policies and Practices as They Relate to the Company’s Risk Management
We believe that our compensation policies and practices for all employees, including executive officers, do not create risks that are reasonably likely to have a material adverse effect on us.
Employment Contracts
We do not have any formal employment agreement with any of the officers. Any future compensation will be determined by the Board of Directors, and, as appropriate, an employment agreement will be executed.
Outstanding Equity Awards
There were no equity awards outstanding as of the end the year ended December 31, 2019.
Option Grants
During the year ended December 31, 2019, there were no options granted.
| 44 |
Aggregated Option Exercises and Fiscal Year-End Option Value
There were no stock options exercised during the year ending December 31, 2019 and 2018 by our executive officers. During the year ending December 31, 2018, 343,000 options granted to officers, directors and specific consultants in 2016 expired.
Long-Term Incentive Plan (“LTIP”) Awards
There were no awards made to any named executive officers in the last completed fiscal year under any LTIP.
ITEM 12: SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth certain information regarding beneficial ownership of our common stock as of March 20, 2020 by (i) each person (or group of affiliated persons) who is known by us to own more than five percent (5%) of the outstanding shares of our common stock, (ii) each director, executive officer and director nominee, and (iii) all of our directors, executive officers and director nominees as a group.
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. For purposes of this table, a person or group of persons is deemed to have “beneficial ownership” of any shares of common stock that such person has the right to acquire within 60 days of the date of the respective table. For purposes of computing the percentage of outstanding shares of our common stock held by each person or group of persons named above, any shares that such person or persons has the right to acquire within 60 days of the date of the respective table is deemed to be outstanding for such person but is not deemed to be outstanding for the purpose of computing the percentage ownership of any other person. The inclusion herein of any shares listed as beneficially owned does not constitute an admission of beneficial ownership.
Except as otherwise indicated, the persons listed below have sole voting and investment power with respect to all shares of our common stock owned by them, except to the extent that power may be shared with a spouse.
As of March 20, 2020, we had 467,038,350 shares of common stock issued and outstanding.
| Amount and | ||||||||
| Nature of | ||||||||
| Beneficial | Percent of | |||||||
| Name of Beneficial Owner | Ownership | Class | ||||||
| Darwin Fogt | 148,000 | .04 | % | |||||
| Douglas MacLellan | 155,000 | .04 | % | |||||
| David Markowski | 62,000 | .02 | % | |||||
| Curtis Hollister | 534,958 | .12 | % | |||||
| Brandon Rowberry | 10,000 | 0.01 | % | |||||
| Doug Cole | 16,000 | 0.01 | % | |||||
| Rochelle Pleskow | 6,000 | 0.01 | % | |||||
| All officers and directors as a group | 931,958 | .25 | % | |||||
| 45 |
ITEM 13: CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Certain Related Party Transactions
Other than the relationships and transactions discussed below, we are not a party to, nor are we proposed to be a party, to any transaction during the last fiscal year involving an amount exceeding $120,000 and in which a related person, as such term is defined by Item 404 of Regulation S-K, had or will have a direct or indirect material interest.
Programming Agreement:
On November 11, 2016, the Company signed an agreement with a programming company (“PC”) within which the one of the Company’s directors and Chief Technical Officer is the Chief Marketing Officer. The agreement is for additional features to be programmed for the launch of the PHIZIO platform. The contract specifies that the Company’s CEO and CTO will retain their officer and director positions and retain their past due accrued compensation through June 30, 2016. The Company is to pay a monthly base fee of $100,000 for the development and compensation for the Company’s CEO and CTO. Following payment of the initial $100,000, the Company is obligated to only pay $50,000 monthly until the PC has successfully signed and collected the first monthly service fee for 100 physical therapy clinics to use the PHIZIO platform. The agreement establishes that the Company is indebted to the PC for $225,000 for past programming services. At the end of December 31, 2019, the Company had a payable of $582,832 due to this company.
Office Space: For the first nine months of the year ended December 31, 2018, the Company rented office space from a company owned by our CEO. The imputed rent expense of $500 per month for nine months is recorded in the Statement of Operations and Additional Paid in Capital in the Balance Sheet. From the end of September 2018 to the present, the Company has rented other office space from a third-party provider.
Indebtedness of Management
No officer, director or security holder known to us beneficially owns more than 5% of our Common stock or any member of the immediate family or sharing the household (other than a tenant or employee) of any of the foregoing persons is indebted to us in the years 2019 and 2018.
Review, Approval and Ratification of Related Party Transactions
Our Board of Directors conducts an appropriate review of and oversees all related-party transactions. We have not yet adopted formal standards in respect of the review and approval or ratification of related-party transactions; however, our board has conformed to the following standards: (i) all related-party transactions must be fair and reasonable to us and on terms comparable to those reasonably expected to be agreed to with independent third parties for the same goods and/or services at the time authorized by the board; and (ii) all related-party transactions must be authorized, approved or ratified by the affirmative vote of a majority of the directors who have no interest, either directly or indirectly, in any such related party transaction.
ITEM 14: PRINCIPAL ACCOUNTANT FEES AND SERVICES
The following table shows the fees that were billed for the audit and other services provided by Haynie & Company, our independent registered public accounting firm, for the fiscal years ended December 31, 2019 and 2018.
| 2019 | 2018 | |||||||
| Audit Fees | $ | 88,700 | $ | 80,000 | ||||
| Audit-Related Fees | 5,000 | 5,200 | ||||||
| Tax Fees | - | - | ||||||
| All Other Fees | - | - | ||||||
| Total | $ | 93,700 | $ | 85,200 | ||||
| 46 |
Audit Fees — This category includes the audit of our annual financial statements, review of financial statements included in our Annual Reports on Form 10-K and services that are normally provided by the independent registered public accounting firm in connection with engagements for those fiscal years. This category also includes advice on audit and accounting matters that arose during, or because of, the audit or the review of interim financial statements.
Audit-Related Fees — This category consists of assurance and related services by the independent registered public accounting firm that are reasonably related to the performance of the audit or review of our financial statements and are not reported above under “Audit Fees.” The services for the fees disclosed under this category include consultation regarding our correspondence with the Securities and Exchange Commission and other accounting consulting.
Tax Fees — This category consists of professional services rendered by our independent registered public accounting firm for tax compliance and tax advice. The services for the fees disclosed under this category include tax return preparation and technical tax advice.
All Other Fees — This category consists of fees for other miscellaneous items.
Preapproval Policy
Our Board of Directors reviews and approves audit and permissible non-audit services performed by its independent accountants, as well as the fees charged for such services. In its review of non-audit service fees and its appointment of Haynie & Company as our independent accountants, the Board of Directors considered whether the provision of such services is compatible with maintaining independence.
ITEM 15: EXHIBITS, CONSOLIDATED FINANCIAL STATEMENTS AND FINANCIAL STATEMENT SCHEDULES
(a)
| 1. | The financial statements listed in the “Index to Financial Statements” at page F-1 are filed as part of this report. | |
| 2. | Financial statement schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto. | |
| 3. | Exhibits included or incorporated herein: See index to Exhibits. |
(b) Exhibits
| 47 |
| 101.INS ** | XBRL Instance Document | |
| 101.SCH ** | XBRL Taxonomy Extension Schema Document | |
| 101.CAL ** | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF ** | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB ** | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE ** | XBRL Taxonomy Extension Presentation Linkbase Document |
** Filed herewith.
| 48 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| eWellness Healthcare Corporation | |||
| (Registrant) | |||
| By: | /s/ Darwin Fogt | Date: March 24, 2020 | |
| Darwin Fogt | |||
| President, CEO | |||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Darwin Fogt | Chief Executive Officer and Director | March 24, 2020 | ||
| Darin Fogt | (principal executive officer) | |||
| /s/ David Markowski | Chief Financial Officer | March 24, 2020 | ||
| David Markowski | (Principal Financial and Accounting Officer) | |||
| /s/ Brandon Rowberry | Director | March 24, 2020 | ||
| Brandon Rowberry | ||||
| /s/ Douglas Cole | Director | March 24, 2020 | ||
| Douglas Cole | ||||
| /s/ Curtis Hollister | Director | March 24, 2020 | ||
| Curtis Hollister | ||||
| /s/ Douglas MacLellan | Director | March 24, 2020 | ||
| Douglas MacLellan | ||||
| /s/ Rochelle Pleskow | Director | March 24, 2020 | ||
| Rochelle Pleskow |
| 49 |
