Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Milestone Pharmaceuticals Inc. | tm2013489d1_ex99-1.htm |
| 8-K - FORM 8-K - Milestone Pharmaceuticals Inc. | tm2013489-1_8k.htm |
Exhibit 99.2

NODE - 301 Topline Data Conference Call March 23, 2020

Disclaimers This Presentation contains forward - looking statements . Words such as "may," "will," "expect," "plan," "anticipate," "estimate," "intend" and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) are intended to identify these forward - looking statements . These forward - looking statements are based on Milestone's expectations and assumptions as of the date of this Presentation . Each of these forward - looking statements involves risks and uncertainties . Actual results may differ materially from these forward - looking statements . Forward - looking statements contained in this presentation include statements regarding (i) the design, progress, timing, scope and results of clinical trials, (ii) potential interactions with regulators, and (iii) the possibility that data will support future development . Important factors that could cause actual results to differ materially from those in the forward - looking statements include, but are not limited to, the risks inherent in biopharmaceutical product development and clinical trials, including the lengthy and uncertain regulatory approval process, uncertainties related to the timing of initiation, enrollment, completion and evaluation of clinical trials, and whether the clinical trials will validate the safety and efficacy of etripamil for PSVT or other indications, among others, as well as risks related to pandemics and public health emergencies, including those related to COVID - 19 , and risks related the sufficiency of our capital resources and our ability to raise additional capital . These and other risks are set forth in Milestone's filings with the U . S . Securities and Exchange Commission, including in its annual report on Form 10 - K for the year ended December 31 , 2019 , under the caption "Risk Factors . ” Except as required by law, Milestone assumes no obligation to update any forward - looking statements contained herein to reflect any change in expectations, even as new information becomes available . We may not actually achieve the plans, intentions or expectations disclosed in our forward - looking statements, and you should not place undue reliance on our forward - looking statements . Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward - looking statements we make . We undertake no obligation to update or revise any forward - looking statements, whether as a result of new information, the occurrence of certain events or otherwise . This Presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners . Certain information contained in this Presentation and statements made orally during this Presentation relate to or is based on studies, publications, surveys and other data obtained from third - party sources and the Company’s own internal estimates and research . While the Company believes these third - party studies, publications, surveys and other data to be reliable as of the date of the Presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources . In addition, no independent sources has evaluated the reasonableness or accuracy of the Company’s internal estimates or research and no reliance should be made on any information or statements made in this Presentation relating to or based on such internal estimates and research . 2

Call Participants Prepared Remarks • Joseph Oliveto, Chief Executive Officer Additional Q&A Participants • Amit Hasija, Chief Financial Officer • Lorenz Muller, Chief Commercial Officer • Jeff Nelson, Chief Operating Officer • Francis Plat, MD, Chief Medical Officer 3

Opportunity to Shift the Standard of Care out of the Acute - Care Setting for ~2 Million PSVT Patients Sources: Internal estimates based on market research and longitudinal analysis of Truven/ Marketscan and Medicare claims data; Page RL et al, 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: executive summary: a report of the ACC/AHA Task Force on Clinical Practice Gui delines and the Heart Rhythm Society. Circulation. 2016;133:e471 – e505 4 PSVT = Paroxysmal Supraventricular Tachycardia DC = Direct Current ED = Emergency Department Current acute treatment options are invasive, inconvenient, anxiety - provoking and/or costly • IV adenosine or DC cardioversion in the ED • >150K ED visits/hospital admissions per year • >600k health care claims every year • Many patients endure episodes when they occur Current Acute Treatment Options for PSVT A Paradigm - Changing Approach Opportunity to develop the first approved treatment to be used by patients wherever an episode occurs • Avoidance of ED visits/ hospital admissions • Less need for chronic medications • Alternative or bridge to ablation procedure

Overview NODE - 301 top - line: • Missed its primary endpoint over 5 hours • Showed clinically meaningful efficacy during the first 45 minutes consistent with the known pharmacology of etripamil • Human factors had little impact on study execution or results • Demonstrated a positive safety profile showing etripamil was well tolerated in the at - home setting Company continues to work with regulators to determine next steps 5
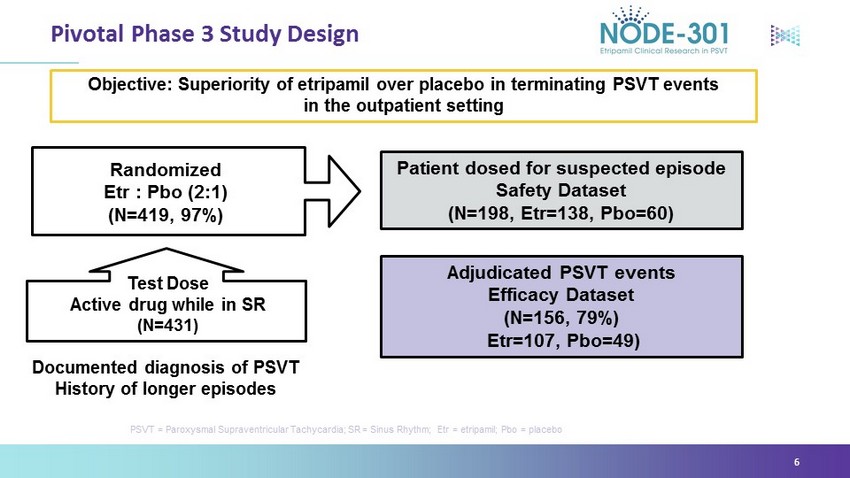
Pivotal Phase 3 Study Design PSVT = Paroxysmal Supraventricular Tachycardia; SR = Sinus Rhythm; Etr = etripamil; Pbo = placebo 6 Documented diagnosis of PSVT History of longer episodes A djudicated PSVT events Efficacy Dataset (N=156, 79%) Etr =107, Pbo =49) Objective: Superiority of etripamil over placebo in terminating PSVT events in the outpatient setting Test Dose Active drug while in SR (N=431) Randomized Etr : Pbo (2:1) (N=419, 97%) Patient dosed for suspected episode Safety Dataset (N=198, Etr =138, Pbo =60)
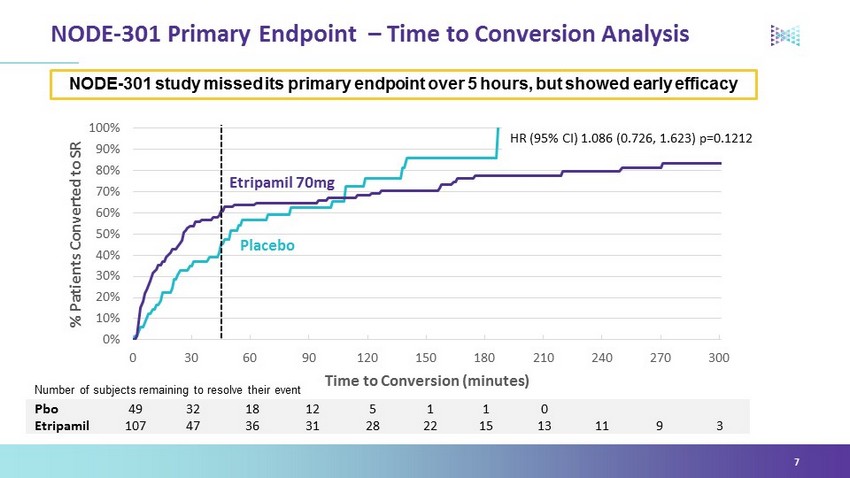
NODE - 301 Primary Endpoint – Time to Conversion Analysis 7 Pbo 49 32 18 12 5 1 1 0 Etripamil 107 47 36 31 28 22 15 13 11 9 3 NODE - 301 study missed its primary endpoint over 5 hours, but showed early efficacy Number of subjects remaining to resolve their event 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% 0 30 60 90 120 150 180 210 240 270 300 % Patients Converted to SR Time to Conversion (minutes) HR (95% CI) 1.086 ( 0.726 , 1.623) p=0.1212 Etripamil 70mg Placebo
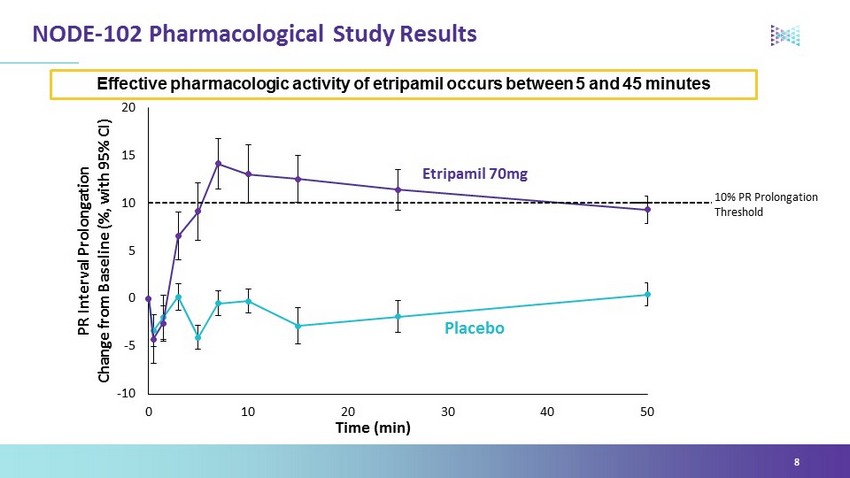
NODE - 102 Pharmacological Study Results 8 Effective pharmacologic activity of etripamil occurs between 5 and 45 minutes Placebo Etripamil 70mg -10 -5 0 5 10 15 20 0 10 20 30 40 50 PR Interval Prolongation Change from Baseline (%, with 95% CI) Time (min) 10% PR Prolongation Threshold
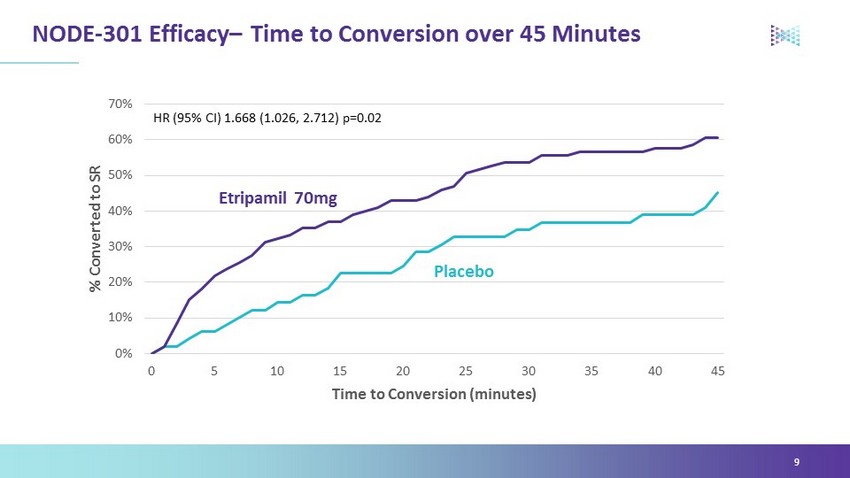
NODE - 301 Efficacy – Time to Conversion over 45 Minutes 9 0% 10% 20% 30% 40% 50% 60% 70% 0 5 10 15 20 25 30 35 40 45 % Converted to SR Time to Conversion (minutes) Placebo Etripamil 70mg HR (95% CI) 1.668 ( 1.026 , 2.712) p=0.02
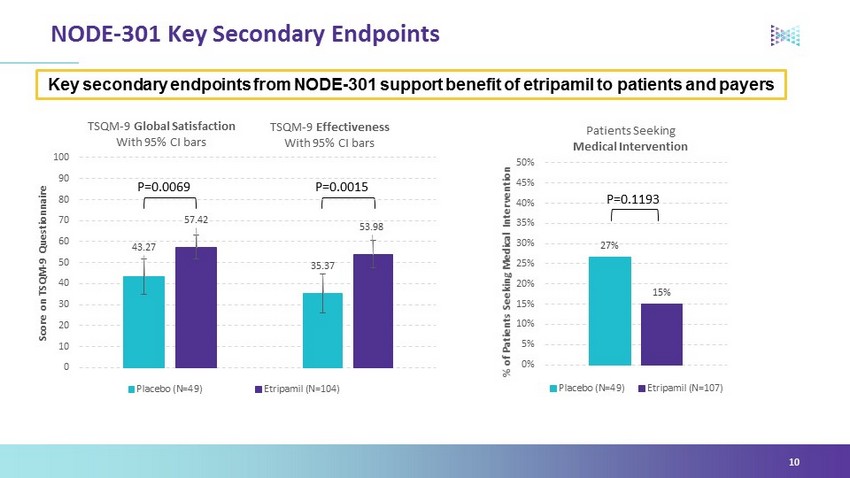
43.27 57.42 0 10 20 30 40 50 60 70 80 90 100 Score on TSQM - 9 Questionnaire TSQM - 9 Global Satisfaction With 95% CI bars Placebo (N=49) Etripamil (N=104) P=0.0069 P=0.0015 NODE - 301 Key Secondary Endpoints 10 Key secondary endpoints from NODE - 301 support benefit of etripamil to patients and payers 27% 15% 0% 5% 10% 15% 20% 25% 30% 35% 40% 45% 50% % of Patients Seeking Medical Intervention Patients Seeking Medical Intervention Placebo (N=49) Etripamil (N=107) P=0.1193 35.37 53.98 TSQM - 9 Effectiveness With 95% CI bars
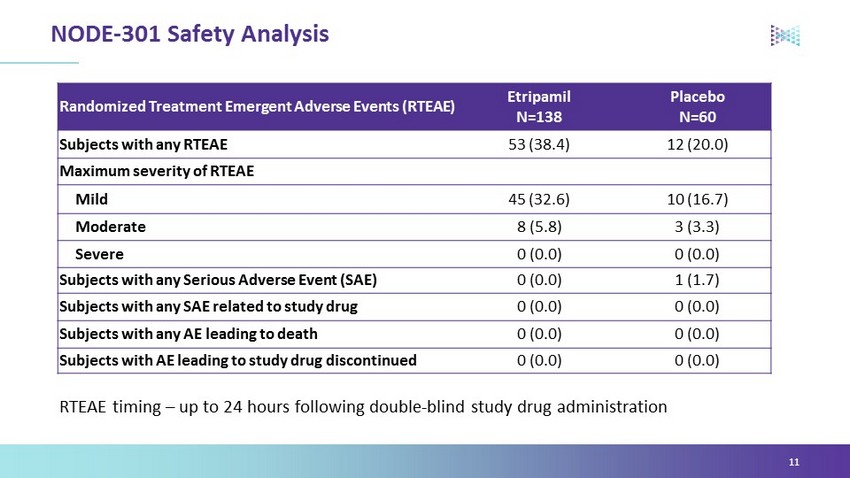
Randomized Treatment Emergent Adverse Events (RTEAE) Etripamil N=138 Placebo N=60 Subjects with any RTEAE 53 (38.4) 12 (20.0) Maximum severity of RTEAE Mild 45 (32.6) 10 (16.7) Moderate 8 (5.8) 3 (3.3) Severe 0 (0.0) 0 (0.0) Subjects with any Serious Adverse Event (SAE) 0 (0.0) 1 (1.7) Subjects with any SAE related to study drug 0 (0.0) 0 (0.0) Subjects with any AE leading to death 0 (0.0) 0 (0.0) Subjects with AE leading to study drug discontinued 0 (0.0) 0 (0.0) NODE - 301 Safety Analysis 11 RTEAE timing – up to 24 hours following double - blind study drug administration
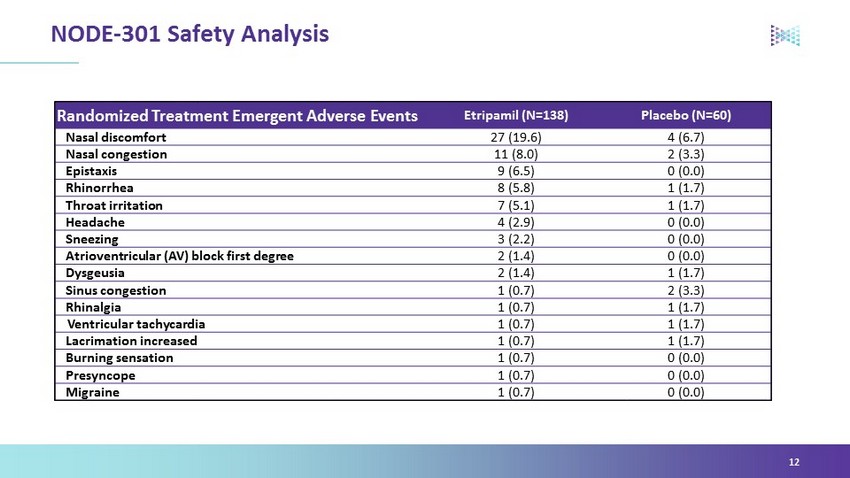
Randomized Treatment Emergent Adverse Events Etripamil (N=138) Placebo (N=60) Nasal discomfort 27 (19.6) 4 (6.7) Nasal congestion 11 (8.0) 2 (3.3) Epistaxis 9 (6.5) 0 (0.0) Rhinorrhea 8 (5.8) 1 (1.7) Throat irritation 7 (5.1) 1 (1.7) Headache 4 (2.9) 0 (0.0) Sneezing 3 (2.2) 0 (0.0) Atrioventricular (AV) block first degree 2 (1.4) 0 (0.0) Dysgeusia 2 (1.4) 1 (1.7) Sinus congestion 1 (0.7) 2 (3.3) Rhinalgia 1 (0.7) 1 (1.7) Ventricular tachycardia 1 (0.7) 1 (1.7) Lacrimation increased 1 (0.7) 1 (1.7) Burning sensation 1 (0.7) 0 (0.0) Presyncope 1 (0.7) 0 (0.0) Migraine 1 (0.7) 0 (0.0) NODE - 301 Safety Analysis 12

Etripamil PSVT Phase 3 Development Plan 2018 Efficacy & Safety Efficacy & Safety Safety NODE - 301 NODE - 301B NODE - 302 Open - Label Extension Safety NODE - 303 Open - Label Safety 2019 2020 2021 Data Primary Utility PSVT = Paroxysmal Supraventricular Tachycardia 13

Overview NODE - 301 top - line: • Missed its primary endpoint over 5 hours • Showed clinically meaningful efficacy during the first 45 minutes consistent with the known pharmacology of etripamil • Human factors had little impact on study execution or results • Demonstrated a positive safety profile showing etripamil was well tolerated in the at - home setting Company continues to work with regulators to determine next steps 14

Thank You 15
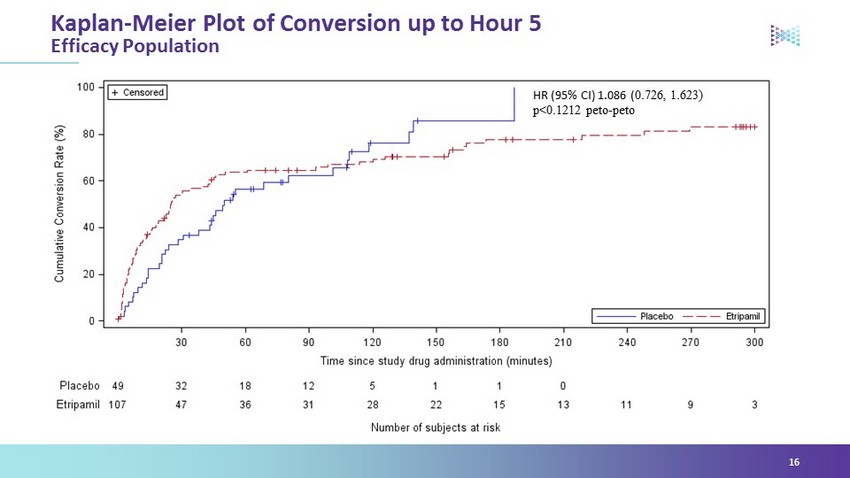
HR (95% CI) 1.086 ( 0.726 , 1.623) p<0.1212 peto - peto Kaplan - Meier Plot of Conversion up to Hour 5 Efficacy Population 16
