Attached files
| file | filename |
|---|---|
| 8-K - 8-K - BIOSPECIFICS TECHNOLOGIES CORP | form8k.htm |
Exhibit 99.1

Building a next-generation company on the foundation of our collagenase-based therapies MARCH 2020

Forward-Looking Statements This presentation includes “forward-looking statements” within the meaning
of, and made pursuant to the safe harbor provisions of, the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact, including statements regarding BioSpecifics Technologies Corp.’s (the
“Company”) strategy, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management and the Board of Directors, expected revenue growth, shareholder value, the timing and occurrence
of certain clinical trials, research and development plans, potential indications, FDA approvals and the timing thereof, future partnerships or acquisitions, and the assumptions underlying or relating to such statements, are “forward-looking
statements.” The forward-looking statements in this presentation may include statements concerning, among other things: the opportunity for minimally invasive non-surgical treatment for XIAFLEX® in potential pipeline indications; the expected
revenue growth for XIAFLEX® in 2020; the expansion of the market for XIAFLEX® for the treatment of Peyronie’s Disease and Dupuytren’s Contracture through future growth initiatives and the Company’s ability to achieve such initiatives; the
potential FDA approval of, and the potential commercial launch of XIAFLEX® for the treatment of cellulite; the occurrence, timing, and success of future clinical trials relating to additional indications for XIAFLEX®, including the treatment of
uterine fibroids with XIAFLEX®; the interest of Endo Pharmaceuticals, Inc. (“Endo”) in currently unlicensed indications of XIAFLEX®; the projected receipt of payments from Endo and the strength of the Company’s intellectual property portfolio.
In some cases, these statements can be identified by forward-looking words such as “expect,” “plan,” “anticipate,” “potential,” “estimate,” “can,” “will,” “continue,” “should,” “believe,” “schedule,” “intend,” the negative or plural of these
words, and other similar expressions. These forward-looking statements are predictions based on our current expectations and our projections about future events and various assumptions. There can be no assurance that we will realize our
expectations or that our beliefs will prove correct. These forward-looking statements involve known and unknown risks, uncertainties, and other factors, which may be beyond our control, and which may cause the actual results, performance, or
achievements of the Company to be materially different from future results, performance, or achievements expressed or implied by such forward-looking statements. There are a number of factors that could cause the Company’s actual results to
differ materially from those indicated by such forward-looking statements, including, without limitation: the timing of regulatory filings and action; the ability of Endo to achieve its objectives for XIAFLEX®; the market for XIAFLEX® in, and
timing, initiation, and outcome of clinical trials for, additional indications, which will determine the amount of milestone, royalty, mark-up on cost of goods sold, and license and sublicense income that the Company may receive; the potential
of XIAFLEX® to be used in additional indications; and Endo’s modification of its objectives or reallocation of its resources with respect to XIAFLEX®. All forward-looking statements included in this presentation are made as of the date hereof,
and are expressly qualified in their entirety by this cautionary notice, including, without limitation, those risks and uncertainties described in our Annual Report on Form 10-K for the year ended December 31, 2019, and otherwise in our filings
and reports filed with Securities and Exchange Commission. Except as may be required by law, we assume no obligation to update these forward-looking statements. 2
CCH Commercial and Development Pipeline Collagenases are naturally occurring enzymes responsible for
the breakdown of collagen 3 Biopharmaceutical company that originated and continues to develop, in collaboration with Endo Pharmaceuticals, a specific collagenase formulation, collagenase clostridium histolyticum (CCH) Collagen is the main
structural protein in the extracellular matrix in the various connective tissues of the body and is the most abundant protein in mammals Local accumulations of excess collagen are associated with a number of medical conditions

Multiple Conditions Associated with Collagen Accumulation Dupuytren’s contracturePeyronie’s
disease 4 Approved indications, marketed as XIAFLEX Cellulite Filed for FDA approval Adhesive capsulitis (frozen shoulder)Plantar fibromatosisUterine fibroids Potential future indications include

Profitable Company with Strong Balance Sheet to Support Future Growth Initiatives 5 Profitable with
lean corporate structure and strong balance sheet ~$106M in cash as of 12/31/19 $11.8M 4Q19 royalties received from Endo sales of XIAFLEX 0 Debt Seeking to partner with companies that possess interesting new technologies and products
in specialty markets

Diverse Development Pipeline 6 Indication Preclinical Phase 1 Phase 2 Phase 3 Marketed APPROVED
INDICATIONS Dupuytren’s Contracture Peyronie’s Disease FILED FOR APPROVAL Cellulite POTENTIAL FUTURE INDICATIONS Adhesive Capsulitis Uterine
Fibroids Plantar Fibromatosis Source: Endo International plc

Marketed XIAFLEX® Indications 7
Peyronie’s Disease 8 Can distort an erection and make sexual intercourse difficult or even
impossible in advanced cases In some mild cases, the plaque can resolve spontaneously without medical interventionIn severe cases, the penis can be bent at a 90-degree angle during erection Characterized by presence of a collagen plaque on the
shaft of the penis XIAFLEX is the first and only FDA-approved biologic therapy indicated for the treatment of Peyronie's disease in men with a palpable plaque and a curvature of 30 degrees or greater at the start of therapy.

Dupuytren’s Contracture 9 Onset is characterized by the formation of nodules at the juncture between
the fingers and palm that are composed primarily of collagenAs the disease progresses, collagen nodules begin to form a cord causing the patient’s finger(s) to contract, making it impossible to open the hand fully Deforming condition of the
hand in which one or more fingers contract toward the palm XIAFLEX is the first and only FDA-approved nonsurgical treatment for Dupuytren's contracture patients with a palpable cord

CCH Development Pipeline 10
Cellulite 11 Affects ~85-90% of post-pubertal femalesCurrently no FDA approved pharmaceutical
products to address fibrous septae primarily composed of collagen Skin dimpling occurring mainly on the buttocks, thighs and lower abdomen and arms FDA StatusEndo announced BLA accepted for review by FDA in November 2019July 2020 PDUFA
date
Adhesive Capsulitis 12 Common available treatment options are often painful and can require
anesthesiaLong-term intensive physical therapy, corticosteroids, manipulation under anesthesia and /or arthroscopic releaseCondition can last ~1 year to up to 3.5 years Inflammation and thickening of shoulder capsule due to collagen, limiting
the range of motion of the shoulder capsule Market OpportunityAffects 20-50 million people worldwide 300K cases diagnosed annually in the U.S.; 10% treated invasively Estimated to occur in 20% of diabeticsPotential to be only FDA
approved nonsurgical therapy
Plantar Fibromatosis 13 Formation of nodules or cords along tendons of the footPatients often have
Dupuytren’s disease, Peyronie’s disease and adhesive capsulitis Pain and disability caused by the thickening of the feet's deep connective tissue Market OpportunityAffects ~200,000 patients in the U.S. Current treatments include
orthotics and anti-inflammatory drugs in the early stages of the disease, steroid injections and surgery in advanced cases

Uterine Fibroids 14 Cause pelvic discomfort and pain, decreased fertility, pregnancy complications,
increased rate of miscarriage, uterine bleeding, prolonged menstrual bleeding and frequent urinationLeading cause of hysterectomies in the U.S., accounting for ~250,000 hysterectomies and 30,000 myomectomies each year Benign tumors in the
reproductive tract that contain large amounts of collagen Phase 1 data: safety and reductions in collagen content compared to control fibroids with a median reduction of 39 percent, as well as a 21 percent average reduction in density of
collagen bundlesAnalyzing the full Phase 1 data to guide the design of potential future studies of collagenase clostridium histolyticum (CCH) for the treatment of uterine fibroids.

BSTC Corporate Overview 15
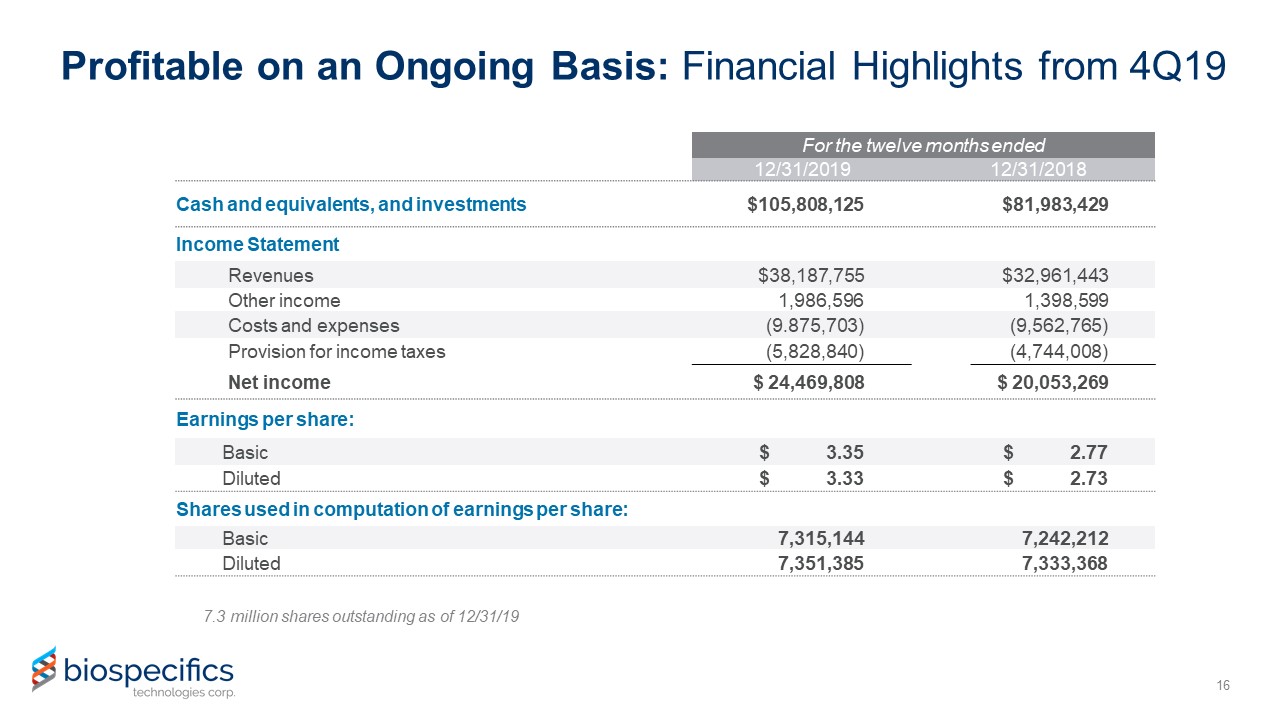
Profitable on an Ongoing Basis: Financial Highlights from 4Q19 16 For the twelve months
ended 12/31/2019 12/31/2018 Cash and equivalents, and investments $105,808,125 $81,983,429 Income Statement Revenues $38,187,755 $32,961,443 Other income 1,986,596 1,398,599
Costs and expenses (9.875,703) (9,562,765) Provision for income taxes (5,828,840) (4,744,008) Net income $ 24,469,808 $ 20,053,269 Earnings per share: Basic $ 3.35 $ 2.77 Diluted $
3.33 $ 2.73 Shares used in computation of earnings per share: Basic 7,315,144 7,242,212 Diluted 7,351,385 7,333,368 7.3 million shares outstanding as of 12/31/19
Corporate Outlook and Upcoming Milestones Continue to receive royalty revenues from partner Endo for
both marketed XIAFLEX indications; Peyronie’s disease and Dupuytren’s contracture Endo expects 2020 full year revenue growth to be ~20%IP through 2028 17 Opportunistic about Business Development opportunitiesExpanding BioSpecifics beyond
XIAFLEXPartner with companies that possess interesting new technologies and products in specialty markets Continue to advance diverse development pipelineBLA for cellulite accepted for FDA review in Nov. 2019, July 6, 2020 PDUFA date setNew
development plans in adhesive capsulitis and plantar fibromatosisContinue to analyze the full Phase 1 data to guide the design of potential future studies for uterine fibroids

Building a next-generation company on the foundation of our collagenase-based therapies MARCH 2020
