Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Theravance Biopharma, Inc. | tm209740d1_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Theravance Biopharma, Inc. | tm209740d1_ex99-1.htm |
Exhibit 99.2

Fourth Quarter and Full Year 2019 Financial Highlights and Business Update February 24, 2020 THERAVANCE ® , the Cross/Star logo and MEDICINES THAT MAKE A DIFFERENCE® are registered trademarks of the Theravance Biopharma group of companies. All third party trademarks used herein are the property of their respective owners. © 2020 Theravance Biopharma. All rights reserved.

Forward looking statements 2 Under the safe harbor provisions of the U . S . Private Securities Litigation Reform Act of 1995 , the company cautions investors that any forward - looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward - looking statements or projections . Examples of forward - looking statements in this presentation may include the Company’s strategies, plans and objectives, the Company’s regulatory strategies and timing of clinical studies (including the data therefrom), the potential characteristics, benefits and mechanisms of action of the Company’s product and product candidates, the potential that the Company’s research programs will progress product candidates into the clinic, the Company’s expectations for product candidates through development, the Company's expectations regarding its allocation of resources, potential regulatory approval and commercialization (including their differentiation from other products or potential products), product sales or profit share revenue and the Company’s expectations for its 2019 operating loss, excluding share - based compensation and other financial results . The company’s forward - looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to potential future disagreements with Innoviva, Inc . and TRC LLC, the uncertainty of arbitration and litigation and the possibility that an arbitration award or litigation result could be adverse to the Company, delays or difficulties in commencing, enrolling or completing clinical studies, the potential that results from clinical or non - clinical studies indicate the Company’s compounds or product candidates are unsafe or ineffective, risks that product candidates do not obtain approval from regulatory authorities, the feasibility of undertaking future clinical trials for our product candidates based on policies and feedback from regulatory authorities, dependence on third parties to conduct clinical studies, delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with or relying on third parties to discover, develop, manufacture and commercialize products, and risks associated with establishing and maintaining sales, marketing and distribution capabilities with appropriate technical expertise and supporting infrastructure . Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Prospectus Supplement filed with the Securities and Exchange Commission (SEC) on February 12 , 2020 , Form 10 - Q filed with the SEC on November 8 , 2019 , and other periodic reports filed with the SEC .

Strategic objective 3 Transform the treatment of serious diseases through the discovery, development, and commercialization of organ - selective medicines designed to maximize patient benefit while minimizing patient risk

Creating transformational value for stakeholders 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties received pledged to service PhaRMA SM notes, 25% of royalties received retained by TBPH. All statements concerning TRELGY ELLIPTA based on publicly available information. 4 Innovative and productive research engine feeding pipeline of organ - selective assets Proven development expertise and established commercial infrastructure Strategic partnerships complement internal capabilities and balance technical, execution and financial risks Strong capital position augmented by TRELEGY ELLIPTA 1 royalties and YUPELRI ® launch Multiple milestones and value driving catalysts in 2020 and beyond

Program Indication Research Phase 1 Phase 2 Phase 3 Filed Marketed Collaborator Organ - selective YUPELRI® (revefenacin) LAMA COPD TD - 1473 GI JAKi UC CD TD - 8236 Inhaled JAKi Inflammatory lung diseases Wholly - owned TD - 5202 Irreversible JAK3i Inflammatory intestinal diseases New programs Multiple Wholly - owned Ampreloxetine (TD - 9855) NRI S ymptomatic neurogenic orthostatic hypotension Wholly - owned Program Indication Research Phase 1 Phase 2 Phase 3 Filed Marketed Rights Economic Interests TRELEGY ELLIPTA 1 FF/UMEC/VI COPD GSK & Innoviva, Inc. Asthma Skin - selective JAKi Dermatological diseases Key programs supported by proven development and commercial expertise 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties received pledged to service PhaRMA SM notes, 25% of royalties received retained by TBPH. All statements concerning TRELGY ELLIPTA based on publicly available information. FF/UMEC/VI: fluticasone furoate/umeclidinium/vilanterol; comprised of ICS, LAMA, and LABA, active comp one nts of Anoro (UMEC/VI). sNDA: supplemental new drug application.LAMA: long - acting muscarinic antagonist; COPD: chronic obstructive pulmonary disease; GI: gastrointestinal; JAKi: Janus kinase inhibitor ; N RI: norepinephrine reuptake inhibitor. 5 Phase 2 Phase 2 Phase 2b/3 Phase 1 Research Phase 3 Marketed Marketed Filed Research

TD - 1473 (JNJ - 8398) Oral gut - selective pan - JAK inhibitor Goal: Treat inflammatory intestinal diseases

TD - 1473: Gut - selective pan - JAK inhibitor LATE - STAGE STUDIES IN ULCERATIVE COLITIS AND CROHN’S DISEASE Phase 2 Crohn’s and Phase 2b/3 UC studies ongoing Phase 2 Crohn’s and Phase 2b UC data expected late - 2020 Global collaboration with JNJ leverages joint development expertise and provides significant economics to TBPH 4 1. Presented at the European Crohn’s and Colitis Organization meeting, March 8, 2019, Copenhagen, Denmark. 2. Component of total Mayo score clinical response. 3. Maintenance phase of the study will have induction responder patients re - randomized to active doses compared to placebo at 44 weeks. 4. Deal value up to $1B in payments to TBPH, including $100M upfront; profit - share in US (33% TBPH, 67% JNJ); double - digit royal ties to TBPH ex - US. Crohn’s disease Ulcerative colitis Phase 2: 12 weeks (N=160) Dose - finding induction Active treatment extension: 48 weeks Phase 2b/3: 8 weeks (N=240) Dose - finding induction Maintenance phase 3 : 44 weeks Phase 3: 8 weeks (N=640) Dose - confirming induction Responders 7

TD - 5202 Organ - gut selective irreversible JAK3 inhibitor Goal: Treat inflammatory intestinal diseases

TD - 5202 FIH Overall Results Summary ‣ No SAEs or severe AEs were reported ‣ All treatment - emergent AEs in TD - 5202 - treated subjects were mild in severity AE: adverse event; C max,ss : maximal steady - state concentration; ECG: electrocardiogram; JAK: Janus kinase; IC 50 : half - maximal inhibitory concentration; NK: natural killer. 9 TD - 5202: generally well - tolerated (single dose ≤2000 mg, multiple doses ≤1000 mg BID) for 10 consecutive days in healthy subjects ‣ Systemic exposures were dose proportional from 100 to 1000 mg BID ‣ Low steady - state systemic exposures: mean C max,ss ~11 - fold below the protein - adjusted JAK IC 50 at the highest tested dose (1000 mg BID), consistent with a gut - selective approach ‣ No clinically significant changes from baseline in vital signs and ECG assessments ‣ No clinically significant changes in chemistry or hematology parameters – No changes in NK cell count

Ampreloxetine (TD - 9855) Once - daily norepinephrine reuptake inhibitor for symptomatic neurogenic orthostatic hypotension

Potential to provide meaningful and durable symptom improvement to underserved patients Baseline OHSA #1 (Orthostatic Hypotension Symptom Assessment Question 1) >4 points. Negative change indicates improvement in symptoms; improvement of 1 point is defined as the MCID (minimal clinically importan t d ifference). ITT: intention - to - treat; SD: standard deviation. Mean (SD) change from baseline in OHSA #1 score n=17 n=13 n=7 n=6 Week Study 169: 4 weeks (N=188) Randomized, double - blind, placebo - controlled, parallel group Study 170: 22 weeks (N=254) Randomized 6 - week withdrawal phase Phase 3 registrational program ongoing; 4 - week efficacy data expected late 2020 11 Phase 3 Registrational Program Ampreloxetine Phase 2 data in nOH; 20 weeks of treatment Extension study: 3 years Completers: -7 -6 -5 -4 -3 -2 -1 0 1 2 3 0 4 8 12 16 20 24 Considered clinically meaningful Withdrawal Durability Efficacy

TD - 8236 Inhaled lung - selective pan - JAK inhibitor Goal: Treat moderate - to - severe asthma regardless of T2 phenotype

High medical and economic burden in uncontrolled asthma 1. 2018 DR/Decision Resources, LLC. All rights reserved. Reproduction, distribution, transmission or publication is prohibited ; reprinted with permission; 2. Sadatsafavi, M., et al. Can Respir J 2010;17:74 - 80; 3. Nurmagambetov T, et al. Ann Am Thorac Soc 2018;15:348 - 56. IFN: interferon; IL: interleukin; STAT: signal transducer and activator of transcription proteins; T2: type 2; TSLP: thymic s tro mal lymphopoietin. 13 Severe 14% Moderate 16% Moderate 25% 16M diagnosed asthma cases 1 Healthcare utilization 2 T2 - high T2 - low IL - 4 IL - 23 /IL - 12 IL - 13 IL - 6 IL - 5 IL - 27 TSLP IFN - γ Bold denotes biologics in development or approved JAK/STAT cytokines implicated in moderate to severe asthma Severe 61 % Inhaled pan - JAK inhibitor has the potential to address patient needs regardless of T2 phenotype Small portion of US patients cause ~$58B in medical costs

-16 -14 -12 -10 -8 -6 -4 -2 0 2 BL 1 2 3 4 5 6 7 TD - 8236: Lung - selective pan - JAK inhibitor PRELIMINARY POSITIVE FENO DATA IN MILD ASTHMATICS ‣ Phase 1 biomarker study in moderate to severe asthmatics ongoing; data expected mid - 2020 FeNO: fractional exhaled nitric oxide. 14 Phase 1 data; mild asthmatics (6 - Hour Post - Dose FeNO) Day 4000 µg 500 µg 1500 µg 150 µg Placebo - adjusted Change From Baseline (ppb)

TD - 8236: Lung - selective pan - JAK inhibitor PHASE 2 ALLERGEN CHALLENGE STUDY FeNO: fractional exhaled nitric oxide. 15 Phase 2 allergen challenge study underway Data expected 2020 TD - 8236 Phase 2 Lung Allergen Challenge 12 weeks (N=21) Dose characterization Randomized, double - blind, placebo - controlled, crossover study

Early - stage organ - selective programs
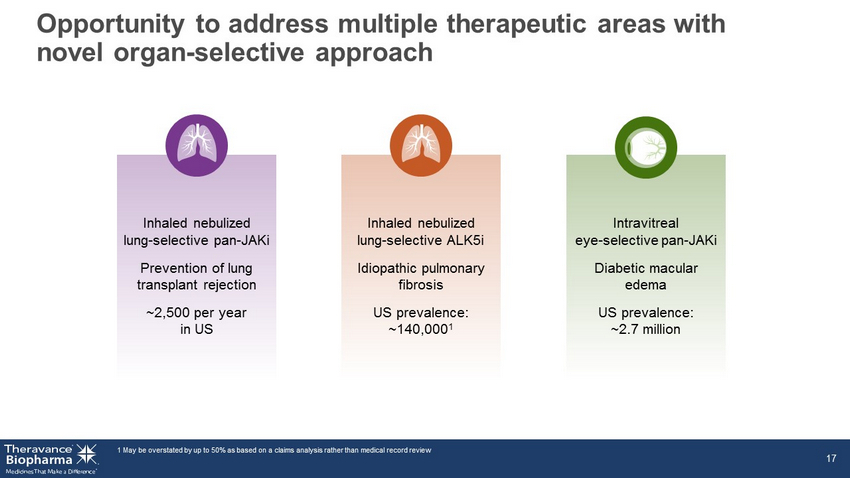
Opportunity to address multiple therapeutic areas with novel organ - selective approach 1. 1 May be overstated by up to 50% as based on a claims analysis rather than medical record review 17 Intravitreal eye - selective pan - JAKi Diabetic macular edema US prevalence: ~2.7 million Inhaled nebulized lung - selective ALK5i Idiopathic pulmonary fibrosis US prevalence: ~140,000 1 Inhaled nebulized lung - selective pan - JAKi Prevention of lung transplant rejection ~2,500 per year in US

YUPELRI ® (revefenacin) inhalation solution First and only once - daily, nebulized maintenance medicine for COPD

First and only once - daily, nebulized maintenance medicine for COPD YUPELRI ® (revefenacin) inhalation solution FDA - APPROVED FOR THE MAINTENANCE TREATMENT OF COPD 1. Global Strategy for Diagnosis, Management, and Prevention of COPD, 2018; 2. TBPH market research (N = 160 physicians); ref ers to US COPD patients 3. Loh CH, et al. Ann Am Thorac Soc. 2017 Aug;14:1305 - 11; 4. IMS Health information service: NSP for period MAT May, 2015. Excludes nebulized short - acting beta agonists. IMS expressly re serves all rights, including rights of copying, distribution and republication. LAMA, long - acting muscarinic antagonist; PIFR, peak inspiratory flow rate; LABA, long - acting beta agonist. 19 19 Once - daily LAMAs are first - line therapy for moderate to severe COPD 1 9% of COPD patients (~800,000) use nebulizers for ongoing maintenance therapy; 41% use nebulizers at least occasionally for bronchodilator therapy 2 Nebulized therapy associated with reduced hospital readmissions in low PIFR patients 3

Patient remains on YUPELRI ® as maintenance therapy Patients converted and discharged from hospital with prescription for YUPELRI ® Patients with worsening COPD symptoms present in hospital 1. For COPD and other respiratory diseases. TBPH is eligible to receive up to $259 million in development and sales milestone pa yments, as well as a profit//loss - sharing arrangement with MYL on US sales and double - digit royalties on ex - US sales, including China and adjacent territories. TBPH retains worldwide rights to revefenacin delivere d through other dosage forms (e.g. metered dose inhaler or dry powder inhaler). 20 + Companies copromote under US profit/loss share TBPH and MYL worldwide strategic collaboration to develop and commercialize nebulized YUPELRI ® (revefenacin) 1 65% 35% TBPH MYL YUPELRI ® commercial strategy COMBINED SALES INFRASTRUCTURES TARGET HCPS AT KEY INTERSECTIONS

Majority of YUPELRI® volume flows through durable medical equipment channel (approximately 3 month lag in data capture); rema ini ng volume flows through hospitals, retail and long - term care pharmacies. Wholesale acquisition cost (WAC): $1,066 per month (or ~$35 per day). 1. As of December 27, 2019. 2. TBPH estimate derived from integr ati ng multiple data sources. 3. For patients with supplemental insurance 4. Effective July 1, 2019. 21 85 wins (equates to 220 accounts) ~70 reviews scheduled (>400 potential accounts) 100% medical support requests fulfilled <30 days FORMULARY 1 Field force productivity goals exceeded ~30,000 patients 2 prescribed (through Q4 2019) PATIENT 100% Medicare Part B 3 ~50% commercial Permanent J - CODE issued 4 ACCESS YUPELRI ® launch metrics STRONG CUSTOMER ACCEPTANCE AND MARKET UPTAKE

1 Cash, cash equivalents and marketable securities. 2 Amounts include share - based compensation. 3 Derived from the audited consolidated financial statements included in the Company’s 2018 Form 10 - K. 22 Fourth Quarter 2019 Financial Highlights WELL CAPITALIZED WITH $285.8M 1 AS OF DECEMBER 31, 2019 ($, in thousands) (Unaudited) Product sales $ - $ 2,415 $ - $ 15,304 Collaboration revenue 9,584 10,047 31,250 41,791 Licensing revenue 10,000 - 28,500 - Mylan collaboration agreement 9,915 3,275 13,664 3,275 Total revenue 29,499 15,737 73,414 60,370 Cost of goods sold - 632 - 715 Research and development (2) 67,025 52,269 219,248 201,348 Selling, general and administrative (2) 33,046 25,457 106,081 97,058 Total costs and expenses 100,071 78,358 325,329 299,121 Loss from operations (70,572) (62,621) (251,915) (238,751) Share-based compensation expense: Research and development 10,615 5,806 28,953 25,563 Selling, general and administrative 13,297 5,908 31,497 25,750 Total share-based compensation expense 23,912 11,714 60,450 51,313 Operating loss excluding share-based compensation $ (46,660) $ (50,907) $ (191,465) $ (187,438) Year Ended December 31, 2019 2018 (3) (Unaudited) Three Months Ended December 31, 2019 2018

Economic interest GSK’s TRELEGY ELLIPTA (FF/UMEC/VI): First and only once - daily single inhaler triple therapy

Economic interest in GSK’s TRELEGY ELLIPTA UPWARD - TIERING ROYALTIES OF ~5.5% TO 8.5% OF WORLDWIDE NET SALES 1 Strongest US ELLIPTA launch to date ~31% share in class Marketed in 38 countries, including China launched in 4Q19 sNDA filed 2Q19 for mortality benefit compared with ANORO in COPD sNDA filed 3Q19 for use in asthma 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties pledged to service PhaRMA SM notes, 25% of royalties retained by TBPH. All statements concerning TRELGY ELLIPTA based on publicly available information. TRELEGY ELLIPTA is FF/UMEC/VI or fluticasone furoate/umeclidinium/vilanterol; comprised of ICS, LAMA, and LABA, act ive components of Anoro (UMEC/VI). COPD: chronic obstructive pulmonary disease; sNDA: supplemental new drug application. 24 20 40 60 80 100 120 140 160 180 200 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 TRx Volume (Thousands) Month Post Launch ANORO ARNUITY BREO INCRUSE Source: GSK, IQVIA NPA weekly TRx data. This information is an estimate derived from the use of information under license from the following IQVIA information service: NPA for the time period Sep 2013 through Nov 2019. IQVIA expressly reserves all rights, including rights of copying, distribution, and republication. TRELEGY Launched in US in November 2017

The Theravance Biopharma Difference

TRELEGY ELLIPTA 1 ‣ FDA decision for asthma and separately for mortality benefit vs. ANORO in COPD ‣ Upsizing of note facility 2 TD - 8236 ‣ Phase 1 Part C data in severe asthmatics ‣ Phase 2 allergen challenge data TD - 5202 ‣ Phase 1 topline data Ampreloxetine ‣ Phase 3 4 - week efficacy data TD - 1473 ‣ Phase 2b/3 ulcerative colitis topline data ‣ Phase 2 Crohn’s topline data Multiple potential milestones and value driving catalysts expected in 2020 and beyond Commercial progression of YUPELRI ® and TRELEGY ELLIPTA 1. TBPH holds 85% economic interest in upward - tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC Agreement over the next four fiscal quarters). 75% of royalties received pledged to service PhaRMA SM notes, 25% of royalties received retained by TBPH. All statements concerning TRELGY ELLIPTA based on publicly available infor mat ion. COPD: chronic obstructive pulmonary disease. 2. Recent note purchase agreement to, among other things, lend us $400MM on a non - recourse basis and notice of optiona l redemption to the existing note holders of the 2033 Notes contingent on the closing of the $400MM transaction. Refinanced Notes would bear interest at a slightly higher rate than the Non - Recourse 2033 Notes and have a term of at least 15 y ears. 26 2020 2021

Creating transformational value for stakeholders 27 Innovative research yielding organ - selective assets Proven development and commercial expertise Strategic partnerships Value driving catalysts Strong capital position

Strategic objective 28 Transform the treatment of serious diseases through the discovery, development, and commercialization of organ - selective medicines designed to maximize patient benefit while minimizing patient risk

About YUPELRI ® (revefenacin) inhalation solution YUPELRI ® (revefenacin) inhalation solution is a novel once - daily nebulized LAMA approved for the maintenance treatment of COPD in the US . Market research by Theravance Biopharma indicates approximately 9 % of the treated COPD patients in the US use nebulizers for ongoing maintenance therapy . 1 LAMAs are a cornerstone of maintenance therapy for COPD and YUPELRI is positioned as the first once - daily single - agent bronchodilator product for COPD patients who require, or prefer, nebulized therapy . YUPELRI’s stability in both metered dose inhaler and dry powder device formulations suggest that this LAMA could also serve as a foundation for novel handheld combination products . 1. TBPH market research (N=160 physicians); refers to US COPD patients. 29

YUPELRI ® (revefenacin) inhalation solution YUPELRI® inhalation solution is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD) . Important Safety Information (US) YUPELRI is contraindicated in patients with hypersensitivity to revefenacin or any component of this product . YUPELRI should not be initiated in patients during acutely deteriorating or potentially life - threatening episodes of COPD, or for the relief of acute symptoms, i . e . , as rescue therapy for the treatment of acute episodes of bronchospasm . Acute symptoms should be treated with an inhaled short - acting beta 2 - agonist . As with other inhaled medicines, YUPELRI can produce paradoxical bronchospasm that may be life - threatening . If paradoxical bronchospasm occurs following dosing with YUPELRI, it should be treated immediately with an inhaled, short - acting bronchodilator . YUPELRI should be discontinued immediately and alternative therapy should be instituted . YUPELRI should be used with caution in patients with narrow - angle glaucoma . Patients should be instructed to immediately consult their healthcare provider if they develop any signs and symptoms of acute narrow - angle glaucoma, including eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema . Worsening of urinary retention may occur . Use with caution in patients with prostatic hyperplasia or bladder - neck obstruction and instruct patients to contact a healthcare provider immediately if symptoms occur . Immediate hypersensitivity reactions may occur after administration of YUPELRI . If a reaction occurs, YUPELRI should be stopped at once and alternative treatments considered . The most common adverse reactions occurring in clinical trials at an incidence greater than or equal to 2 % in the YUPELRI group, and higher than placebo, included cough, nasopharyngitis, upper respiratory infection, headache and back pain . Coadministration of anticholinergic medicines or OATP 1 B 1 and OATP 1 B 3 inhibitors with YUPELRI is not recommended . YUPELRI is not recommended in patients with any degree of hepatic impairment . 30
